Seedling Selection in Olive Breeding Progenies
Abstract
1. Introduction
2. Results
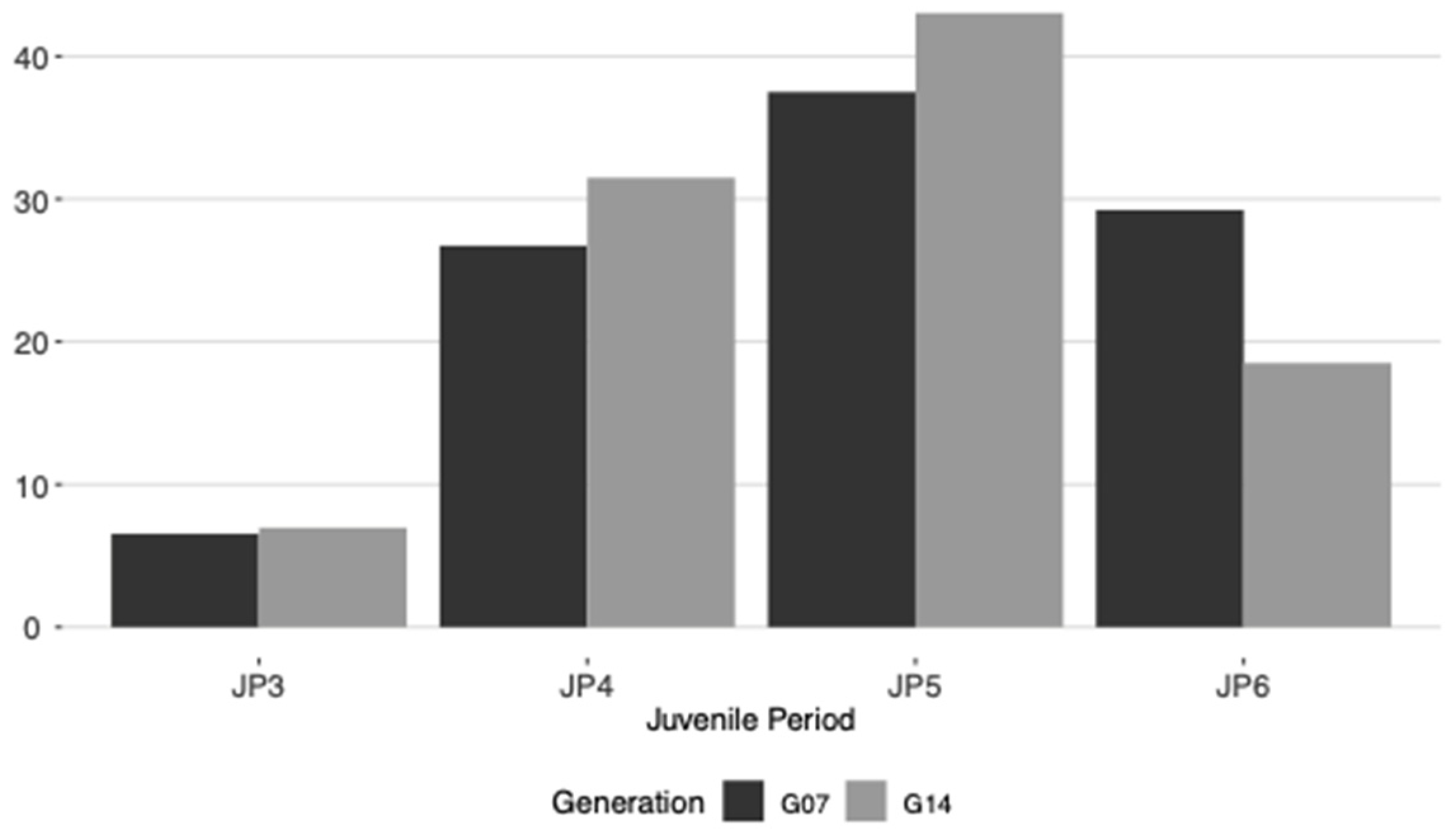
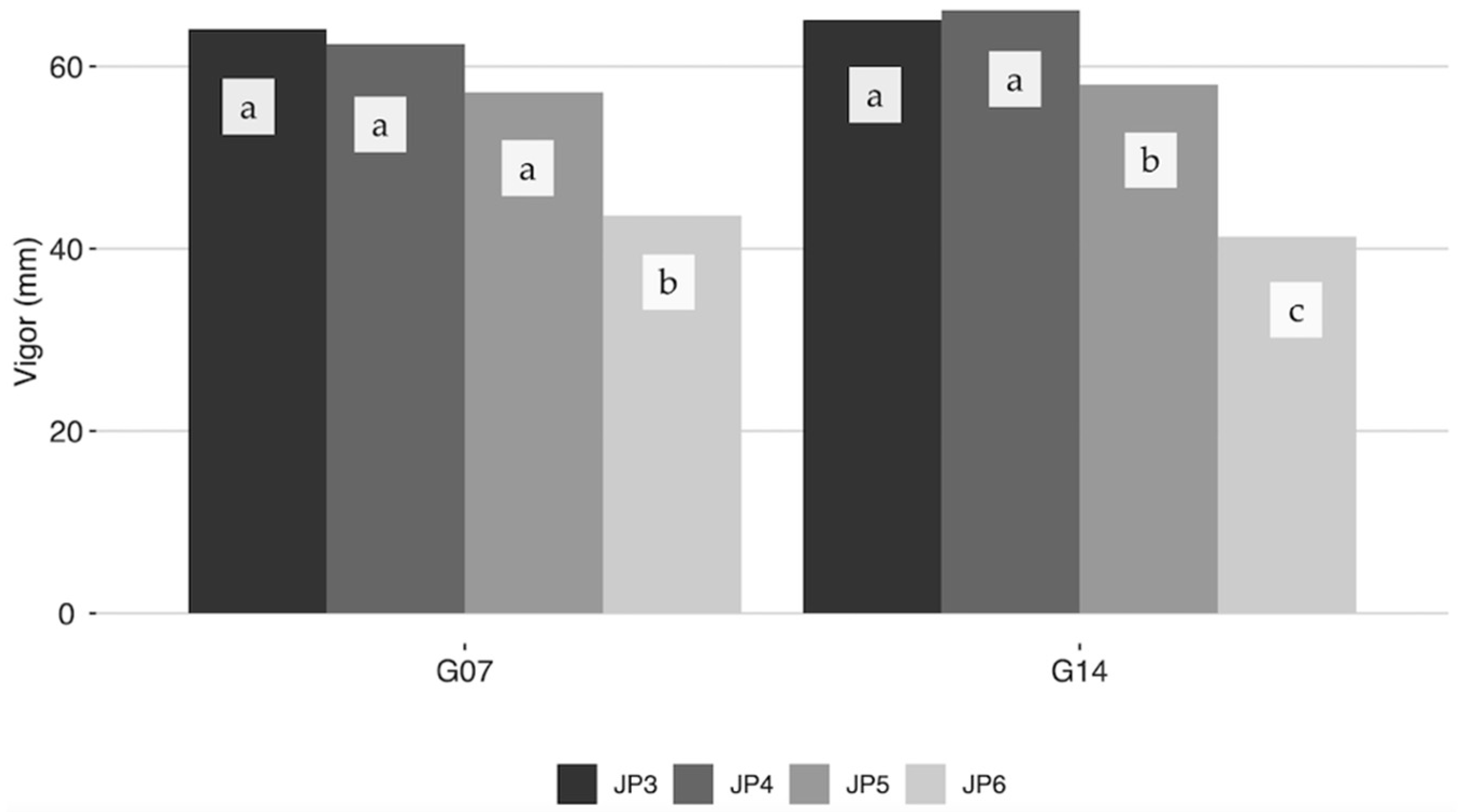
2.1. Vigor and Juvenile Period
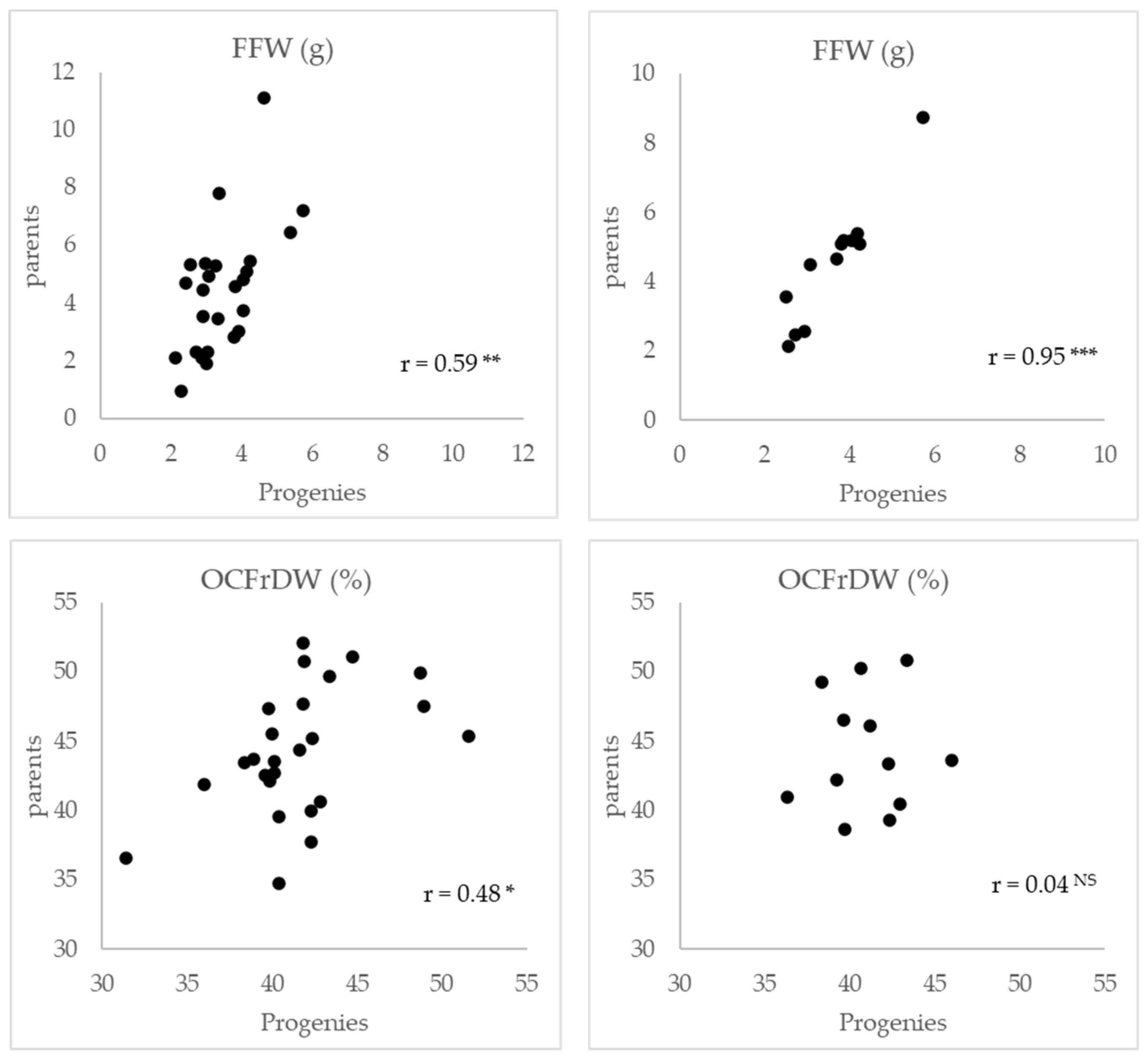
2.2. Variability for Fruit Fresh Weight and Oil Content of Fruit in Dry Weight
2.3. Selection Practise
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Traits Evaluated
4.3. Selection Practice
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Belaj, A.; Ninot, A.; Gómez-Gálvez, F.J.; El Riachy, M.; Gurbuz-Veral, M.; Torres, M.; Lazaj, A.; Klepo, T.; Paz, S.; Ugarte, J.; et al. Utility of EST-SNP Markers for Improving Management and Use of Olive Genetic Resources: A Case Study at the Worldwide Olive Germplasm Bank of Córdoba. Plants 2022, 11, 921. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, R.; Leon, L.; Guerrero, N.; Rallo, L.; and Barranco, D.; Barranco, D. Preliminary Results of an Olive Cultivar Trial at High Density. Aust. J. Agric. Res. 2007, 58, 392–395. [Google Scholar] [CrossRef]
- Lo Bianco, R.; Proietti, P.; Regni, L.; Caruso, T. Planting Systems for Modern Olive Growing: Strengths and Weaknesses. Agriculture 2021, 11, 494. [Google Scholar] [CrossRef]
- Fontanazza, G.; Bartolozzi, F.; Vergati, G. Fs-17. Riv. Fruttic. Ortofloric. 1998, 5, 61. [Google Scholar]
- Lavee, S.; Harshemesh, H.; Haskal, A.; Trapero, A.; Metzidakis, I.T.; Voyiatzis, D.G. “Maalot” a New Cultivar for Oil Extraction Resistant to Spilocaea Oleagina (Cast). Acta Hortic. 1999, 474, 125–128. [Google Scholar] [CrossRef]
- Bellini, E.; Giordani, E.; Parlati, M.V. Three New Olive Cultivars Obtained by Cross-Breeding. Acta Hortic. 2002, 586, 221–223. [Google Scholar] [CrossRef]
- Rallo, L.; Barranco, D.; de la Rosa, R.; León, L. ‘Chiquitita’ Olive. HortScience 2008, 43, 529–531. [Google Scholar] [CrossRef]
- Dridi, J.; Fendri, M.; Ayadi, M.; Jendoubi, F.; Msallem, M.; Larbi, A. Fruit and Oil Characteristics of Tunisian Olive Progenies Obtained by Controlled Crosses. J. New Sci. 2019, 62, 3914–3923. [Google Scholar]
- Amar, F.B.; Guellaoui, I.; Ayadi, M.; Elloumi, O.; Triki, M.A.; Boubaker, M. ‘Zeitoun Ennour’: A New Olive (Olea europaea L.) Cultivar in Tunisia with High Oil Quality. Genet. Resour. 2021, 2, 1–6. [Google Scholar] [CrossRef]
- Ozdemir, Y.; Yavas, H.; Zengin, M.; Akcay, M.E.; Aktepe Tangu, N.; Ercisli, S. Integrated Approach to Olive Oil Characteristics of Some Cultivar Candidates from Nutritional, Oxidative Stability and Sensory Perspective for Advanced Selection of Cross Breeding. Erwerbs-Obstbau 2021, 63, 193–200. [Google Scholar] [CrossRef]
- Hackett, W.P. Juvenility, Maturation, and Rejuvenation in Woody Plants. Hortic. Rev. 1985, 7, 109–155. [Google Scholar]
- Santos-antunes, F.; Alvarado, J.; Mohedo, A.; Trujillo, I.; Rallo, L. The Length of the Juvenile Period in Olive as In Fl Uenced by Vigor of the Seedlings and the Precocity of the Parents. HortScience 2005, 40, 1213–1215. [Google Scholar] [CrossRef]
- Aldwinckle, H.S. Early Flowering of Cultivated Apple Seedlings Forced in the Greenhouse. Acta Hortic. 1976, 56, 201–203. [Google Scholar] [CrossRef]
- Lavee, S. Aims, Methods and Advances in Breeding of New Olive (Olea europaea L.) Cultivars. Acta Hortic. 1989, 286, 23–40. [Google Scholar] [CrossRef]
- Moreno-Alías, I.; Rapoport, H.F.F.; León, L.; de la Rosa, R. Olive Seedling First-Flowering Position and Management. Sci. Hortic. 2010, 124, 74–77. [Google Scholar] [CrossRef]
- De La Rosa, R.; Kiran, A.I.I.; Barranco, D.; Leon, L. Seedling Vigour as a Preselection Criterion for Short Juvenile Period in Olive Breeding. Aust. J. Agric. Res. 2006, 57, 477–481. [Google Scholar] [CrossRef]
- Rallo, P.; Jiménez, R.; Ordovás, J.; Suárez, M.P. Possible Early Selection of Short Juvenile Period Olive Plants Based on Seedling Traits. Aust. J. Agric. Res. 2008, 59, 933–940. [Google Scholar] [CrossRef]
- Hammami, S.B.M.; León, L.; Rapoport, H.F.; de la Rosa, R. A New Approach for Early Selection of Short Juvenile Period in Olive Progenies. Sci. Hortic. 2021, 281, 109993. [Google Scholar] [CrossRef]
- Moral, J.; Diez, C.M.; Leon, L.; De la Rosa, R.; Santos-Antunes, F.; Barranco, D.; Rallo, L. Female Genitor Effect on the Juvenile Period of Olive Seedlings. Sci. Hortic. 2013, 156, 99–105. [Google Scholar] [CrossRef]
- Klepo, T.; Toumi, A.; De La Rosa, R.; León, L.; Belaj, A. Agronomic Evaluation of Seedlings from Crosses between the Main Spanish Olive Cultivar ‘Picual’ and Two Wild Olive Trees. J. Hortic. Sci. Biotechnol. 2014, 89, 508–512. [Google Scholar] [CrossRef]
- Bellini, E.; Giordani, E.; Rosati, A. Genetic Improvement of Olive from Clonal Selection to Cross-Breeding Programs. Adv. Hortic. Sci. 2008, 22, 73–86. [Google Scholar]
- Fernandez-Escobar, R.; de la Rosa, R.; Leon, L.; Gomez, J.A.; Testi, L.; Orgaz, F.; Gil-Ribes, J.A.; Quesada-moraga, E.; Trapero-Casas, A. Evolution and Sustainability of the Olive Production Systems. Options Méditerr. Sér. A Mediterr. Semin. 2013, 106, 11–41. [Google Scholar]
- Arias-Calderon, R.; Rouiss, H.; Rodriguez-Jurado, D.; de la Rosa, R.; Leon, L. Variability and Heritability of Fruit Characters in Olive Progenies from Open-Pollination. Sci. Hortic. 2014, 169, 94–98. [Google Scholar] [CrossRef]
- León, L.; Velasco, L.; De La Rosa, R. Initial Selection Steps in Olive Breeding Programs. Euphytica 2015, 201, 453–462. [Google Scholar] [CrossRef]
- Leon, L.; de la Rosa, R.; Barranco, D.; Rallo, L. Breeding for Early Bearing in Olive. HortScience 2007, 42, 499–502. [Google Scholar] [CrossRef]
- Serrano, A.; De la Rosa, R.; Sánchez-Ortiz, A.; Cano, J.; Pérez, A.G.; Sanz, C.; Arias-Calderón, R.; Velasco, L.; León, L. Chemical Components Influencing Oxidative Stability and Sensorial Properties of Extra Virgin Olive Oil and Effect of Genotype and Location on Their Expression. LWT 2021, 136, 110257. [Google Scholar] [CrossRef]
- Visser, T. Juvenile Phase and Growth of Apple and Pear Seedlings. Euphytica 1964, 13, 119–129. [Google Scholar] [CrossRef]
- Visser, T. The Relation between Growth, Juvenle Period and Fruiting of Apple Seedlings and Its Use to Improve Breeding Efficency. Euphytica 1970, 19, 293–302. [Google Scholar] [CrossRef]
- León, L.; Rallo, L.; Del Río, C.; Martín, L.M. Variability and Early Selection on the Seedling Stage for Agronomic Traits in Progenies from Olive Crosses. Plant Breed. 2004, 123, 73–78. [Google Scholar] [CrossRef]
- Leon, L.; Arias-Calderon, R.; de la Rosa, R.R.; Khadari, B.; Costes, E. Optimal Spatial and Temporal Replications for Reducing Environmental Variation for Oil Content Components and Fruit Morphology Traits in Olive Breeding. Euphytica 2016, 207, 675–684. [Google Scholar] [CrossRef]
- Lavee, S.; Avidan, B. Aspects of Female Heredity in the Breeding Progeny of Different Olive Cultivars. Acta Hortic. 2012, 949, 85–92. [Google Scholar] [CrossRef]
- Badenes, M.L.; Byrne, D.H. Fruit Breeding; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4419-0763-9. [Google Scholar]
- Bellini, E. Behaviour of Some Genetic Characters in Olive Seedlings Obtained by Cross-Breeding. Acta Hortic. 1992, 317, 197–208. [Google Scholar] [CrossRef]
- Ozdemir, Y.; Ozturk, A.; Guven, E.; Asan Nebioglu, M.; Aktepe Tangu, N.; Ackay, M.E.; Ercisli, S. Fruit and Oil Characteristics of Olive Candidate Cultivars from Turkey. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 147. [Google Scholar] [CrossRef][Green Version]
- Belaj, A.; de la Rosa, R.; Lorite, I.J.; Mariotti, R.; Cultrera, N.G.M.; Beuzón, C.R.; González-Plaza, J.J.; Muñoz-Mérida, A.; Trelles, O.; Baldoni, L. Usefulness of a New Large Set of High Throughput EST-SNP Markers as a Tool for Olive Germplasm Collection Management. Front. Plant Sci. 2018, 9, 215. [Google Scholar] [CrossRef] [PubMed]
| JP | TD | FFW | OCFrDW | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Progeny | Progeny | Parent | Progeny | Parent | Progeny | |||||
| Mean | Mean | Mean | Min | Max | Mean | Min | Max | |||
| “9–67” | 5.0 | 48.7 | 3.5 | 2.9 | 1.8 | 4.3 | 50.8 | 41.9 | 32.0 | 49.6 |
| “Arbequina” | 4.6 | 58.0 | 1.9 | 3.0 | 1.8 | 4.5 | 43.7 | 38.9 | 16.8 | 47.7 |
| “Blanqueta” | 4.9 | 55.1 | 2.1 | 2.9 | 1.0 | 5.9 | 37.8 | 42.3 | 35.0 | 52.5 |
| “Canetera” | 4.5 | 63.5 | 3.0 | 3.9 | 1.6 | 10.2 | 40.0 | 42.3 | 39.0 | 46.4 |
| “Chalkidiki” | 4.9 | 57.6 | 7.2 | 5.8 | 2.5 | 9.8 | 51.1 | 44.7 | 35.7 | 49.6 |
| “Changlot Real” | 5.1 | 50.7 | 3.8 | 4.0 | 2.4 | 5.9 | 47.7 | 41.8 | 31.4 | 53.6 |
| “Chorruo Castro Río” | 5.4 | 40.4 | 6.5 | 5.4 | 2.2 | 8.8 | 41.9 | 36.0 | 16.8 | 54.3 |
| “Empeltre” | 4.9 | 64.6 | 2.8 | 3.8 | 2.7 | 5.8 | 45.6 | 40.0 | 30.7 | 47.7 |
| “Frantoio” | 5.2 | 64.9 | 2.3 | 3.0 | 2.2 | 4.6 | 45.2 | 42.4 | 36.0 | 45.0 |
| “Hojiblanca” | 4.5 | 60.2 | 4.8 | 4.1 | 2.2 | 8.0 | 40.7 | 42.8 | 34.8 | 50.6 |
| “Koroneiki” | 4.6 | 59.0 | 1.0 | 2.3 | 1.2 | 3.9 | 43.6 | 40.2 | 28.1 | 48.2 |
| “Leccino” | 5.1 | 48.9 | 5.3 | 2.6 | 2.6 | 2.6 | 45.4 | 51.6 | 51.6 | 51.6 |
| “Lechín de Granada” | 5.2 | 47.9 | 2.1 | 2.1 | 1.1 | 3.4 | 36.6 | 31.4 | 7.8 | 43.0 |
| “Lechín de Sevilla” | 5.3 | 56.2 | 3.4 | 3.3 | 2.3 | 5.1 | 42.2 | 39.9 | 22.9 | 49.4 |
| “Manzanilla de Sevilla” | 4.5 | 53.3 | 5.5 | 4.3 | 2.5 | 6.2 | 49.9 | 48.7 | 42.9 | 51.3 |
| “Manzanilla del Piquito” | 4.8 | 55.7 | 5.3 | 3.3 | 2.0 | 5.1 | 39.6 | 40.4 | 23.3 | 46.6 |
| “Meski” | 5.5 | 47.9 | 7.5 | 47.3 | ||||||
| “Morona” | 4.8 | 56.8 | 5.4 | 3.0 | 2.1 | 3.9 | 42.7 | 40.2 | 30.6 | 49.2 |
| “Negrillo de Arjona” | 5.2 | 56.5 | 5.1 | 4.2 | 3.2 | 5.1 | 47.5 | 48.9 | 41.7 | 56.1 |
| “Ocal” | 4.9 | 49.2 | 7.8 | 3.4 | 1.5 | 7.1 | 52.1 | 41.9 | 27.4 | 53.4 |
| “Picual” | 4.7 | 59.4 | 4.5 | 2.9 | 0.5 | 5.0 | 47.4 | 39.8 | 14.1 | 49.7 |
| “Picudo” | 4.7 | 63.6 | 4.9 | 3.1 | 1.8 | 4.0 | 42.6 | 39.6 | 31.8 | 47.5 |
| “Sikitita” | 5.1 | 38.2 | 2.3 | 2.7 | 1.8 | 4.2 | 44.4 | 41.6 | 36.9 | 47.7 |
| “Tanche” | 4.8 | 60.7 | 4.7 | 2.4 | 1.6 | 4.3 | 43.4 | 38.4 | 27.9 | 46.7 |
| “Toffahi” | 4.5 | 58.7 | 11.1 | 4.6 | 3.1 | 5.9 | 34.8 | 40.4 | 28.5 | 48.1 |
| “Trylia” | 5.0 | 53.7 | 4.6 | 3.8 | 2.5 | 5.1 | 49.7 | 43.4 | 35.5 | 48.6 |
| JP | TD | FFW | OCFrDW | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Progeny | Progeny | Parent | Progeny | Parent | Progeny | |||||
| Mean | Mean | Mean | Min | Max | Mean | Min | Max | |||
| “Acebuchera” | 4.5 | 57.3 | 2.9 | 1.5 | 4.1 | 43.5 | 27.1 | 49.8 | ||
| “Aloreña de Iznalloz” | 4.9 | 54.3 | 5.1 | 3.8 | 1.7 | 7.0 | 50.3 | 40.6 | 24.6 | 51.8 |
| “Askal” | 4.2 | 63.5 | 2.1 | 2.6 | 1.0 | 5.4 | 43.7 | 46.0 | 28.0 | 58.5 |
| “Barnea” | 4.3 | 68.1 | 2.6 | 2.9 | 1.4 | 5.2 | 46.1 | 41.2 | 30.6 | 55.5 |
| “Bodoquera” | 4.7 | 62.1 | 5.2 | 4.0 | 1.8 | 7.6 | 43.4 | 42.2 | 25.2 | 56.6 |
| “Cordobes Arroyo Luz” | 5.4 | 55.5 | 4.7 | 3.7 | 1.3 | 5.1 | 40.5 | 43.0 | 30.1 | 50.5 |
| “Cordovil de Serpa-130” | 4.6 | 55.4 | 3.6 | 2.5 | 1.0 | 5.1 | 41.0 | 36.3 | 22.6 | 47.2 |
| “Cornezuelo de Jaen” | 4.7 | 52.0 | 5.4 | 4.2 | 1.5 | 6.4 | 50.8 | 43.4 | 26.3 | 53.8 |
| “Dulzal” | 4.7 | 54.4 | 4.5 | 3.1 | 1.7 | 5.8 | 39.3 | 42.3 | 33.1 | 52.1 |
| “Grosal Vimbodi” | 4.9 | 59.2 | 2.5 | 2.7 | 1.2 | 6.5 | 42.3 | 39.3 | 28.5 | 52.5 |
| “Mahati-1010” | 4.8 | 56.5 | 8.7 | 5.7 | 2.2 | 8.9 | 38.7 | 39.7 | 21.1 | 46.4 |
| “Morisca” | 5.2 | 57.0 | 5.2 | 3.9 | 1.5 | 6.4 | 49.3 | 38.4 | 25.8 | 47.4 |
| “Nevadillo de Sant Pto” | 4.9 | 53.4 | 5.1 | 4.2 | 0.8 | 8.4 | 46.6 | 39.7 | 25.1 | 53.1 |
| Mean | CV | Min | Max | |||
|---|---|---|---|---|---|---|
| FFW (g) | G07 | Parents | 4.56 | 49.34 | 0.97 | 11.12 |
| Progenies | 3.49 | 43.55 | 0.51 | 10.19 | ||
| G14 | Parents | 4.55 | 39.12 | 2.12 | 8.74 | |
| Progenies | 3.35 | 43.28 | 0.82 | 8.93 | ||
| OCFrDW (%) | G07 | Parents | 44.37 | 10.25 | 34.78 | 52.14 |
| Progenies | 40.94 | 17.24 | 7.8 | 56.12 | ||
| G14 | Parents | 44.31 | 9.64 | 38.66 | 50.84 | |
| Progenies | 41.75 | 16.86 | 21.08 | 58.46 |
| Variance Components | FFW (%) | OCFrDW (%) | |
|---|---|---|---|
| G07 | VB | 35.78 | 20.54 |
| VW | 64.22 | 79.46 | |
| G14 | VB | 32.81 | 14.78 |
| VW | 67.19 | 85.22 |
| Progenies | No of Genotypes | SI1 | % | SI2 | % | |
|---|---|---|---|---|---|---|
| G07 | TOTAL | 520 | 77 | 15 | 69 | 13 |
| “9-67” | 21 | 2 | 10 | 2 | 10 | |
| “Arbequina” | 20 | 4 | 20 | 4 | 20 | |
| “Blanqueta” | 21 | 4 | 19 | 4 | 19 | |
| “Canetera” | 20 | 7 | 35 | 5 | 25 | |
| “Chalkidiki” | 20 | 5 | 25 | 5 | 25 | |
| “Changlot Real” | 21 | 3 | 14 | 2 | 10 | |
| “Chorruo de Castro del Río” | 21 | - | - | - | - | |
| “Empeltre” | 21 | 3 | 14 | 3 | 14 | |
| “Frantoio” | 21 | 1 | 5 | 1 | 5 | |
| “Hojiblanca” | 20 | 5 | 25 | 4 | 20 | |
| “Koroneiki” | 18 | 3 | 17 | 3 | 17 | |
| “Leccino” | 15 | 1 | 7 | 1 | 7 | |
| “Lechín de Granada” | 18 | - | - | - | - | |
| “Lechín de Sevilla” | 21 | 1 | 5 | 1 | 5 | |
| “Manzanilla de Sevilla” | 20 | 8 | 40 | 8 | 40 | |
| “Manzanilla del Piquito” | 21 | 2 | 10 | 2 | 10 | |
| “Meski” | 18 | - | - | - | - | |
| “Morona” | 21 | 3 | 14 | 2 | 10 | |
| “Negrillo de Arjona” | 20 | 1 | 5 | 1 | 5 | |
| “Ocal” | 21 | 3 | 14 | 3 | 14 | |
| “Sikitita” | 19 | 2 | 11 | 2 | 11 | |
| “Picual” | 20 | 4 | 20 | 3 | 15 | |
| “Picudo” | 21 | 3 | 14 | 2 | 10 | |
| “Tanche” | 20 | 2 | 10 | 3 | 15 | |
| “Toffahi” | 20 | 6 | 30 | 4 | 20 | |
| “Trylia” | 21 | 4 | 19 | 4 | 19 | |
| G14 | TOTAL | 1048 | 156 | 15 | 145 | 14 |
| “Acebuchera” | 76 | 9 | 12 | 9 | 12 | |
| “Aloreña de Iznalloz” | 67 | 9 | 13 | 7 | 10 | |
| “Askal” | 96 | 35 | 36 | 35 | 36 | |
| “Barnea” | 100 | 20 | 20 | 17 | 17 | |
| “Bodoquera” | 88 | 20 | 23 | 17 | 19 | |
| “Cordobes Arroyo Luz” | 81 | 3 | 4 | 3 | 4 | |
| “Cordovil de Serpa-130” | 78 | 4 | 5 | 4 | 5 | |
| “Cornezuelo de Jaen” | 88 | 20 | 23 | 19 | 22 | |
| “Dulzal” | 81 | 11 | 14 | 10 | 12 | |
| “Grosal Vimbodi” | 76 | 3 | 4 | 3 | 4 | |
| “Mahati-1010” | 71 | 12 | 17 | 11 | 15 | |
| “Morisca” | 80 | 3 | 4 | 3 | 4 | |
| “Nevadillo de Sant Pto” | 66 | 7 | 11 | 7 | 11 | |
| TOTAL | 1568 | 233 | 15 | 214 | 14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yılmaz-Düzyaman, H.; de la Rosa, R.; León, L. Seedling Selection in Olive Breeding Progenies. Plants 2022, 11, 1195. https://doi.org/10.3390/plants11091195
Yılmaz-Düzyaman H, de la Rosa R, León L. Seedling Selection in Olive Breeding Progenies. Plants. 2022; 11(9):1195. https://doi.org/10.3390/plants11091195
Chicago/Turabian StyleYılmaz-Düzyaman, Hande, Raúl de la Rosa, and Lorenzo León. 2022. "Seedling Selection in Olive Breeding Progenies" Plants 11, no. 9: 1195. https://doi.org/10.3390/plants11091195
APA StyleYılmaz-Düzyaman, H., de la Rosa, R., & León, L. (2022). Seedling Selection in Olive Breeding Progenies. Plants, 11(9), 1195. https://doi.org/10.3390/plants11091195









