Insecticidal Effect of Wild-Grown Mentha pulegium and Rosmarinus officinalis Essential Oils and Their Main Monoterpenes against Culex pipiens (Diptera: Culicidae)
Abstract
:1. Introduction
2. Material and Methods
2.1. Material
2.2. Essential Oils Isolation and Chemical Analysis by GC-MS
2.3. Culex Pipiens Rearing Conditions
2.4. Fumigant Toxicity
2.5. Statistical Analyses
3. Results
3.1. Extraction Yield and Chemical Composition
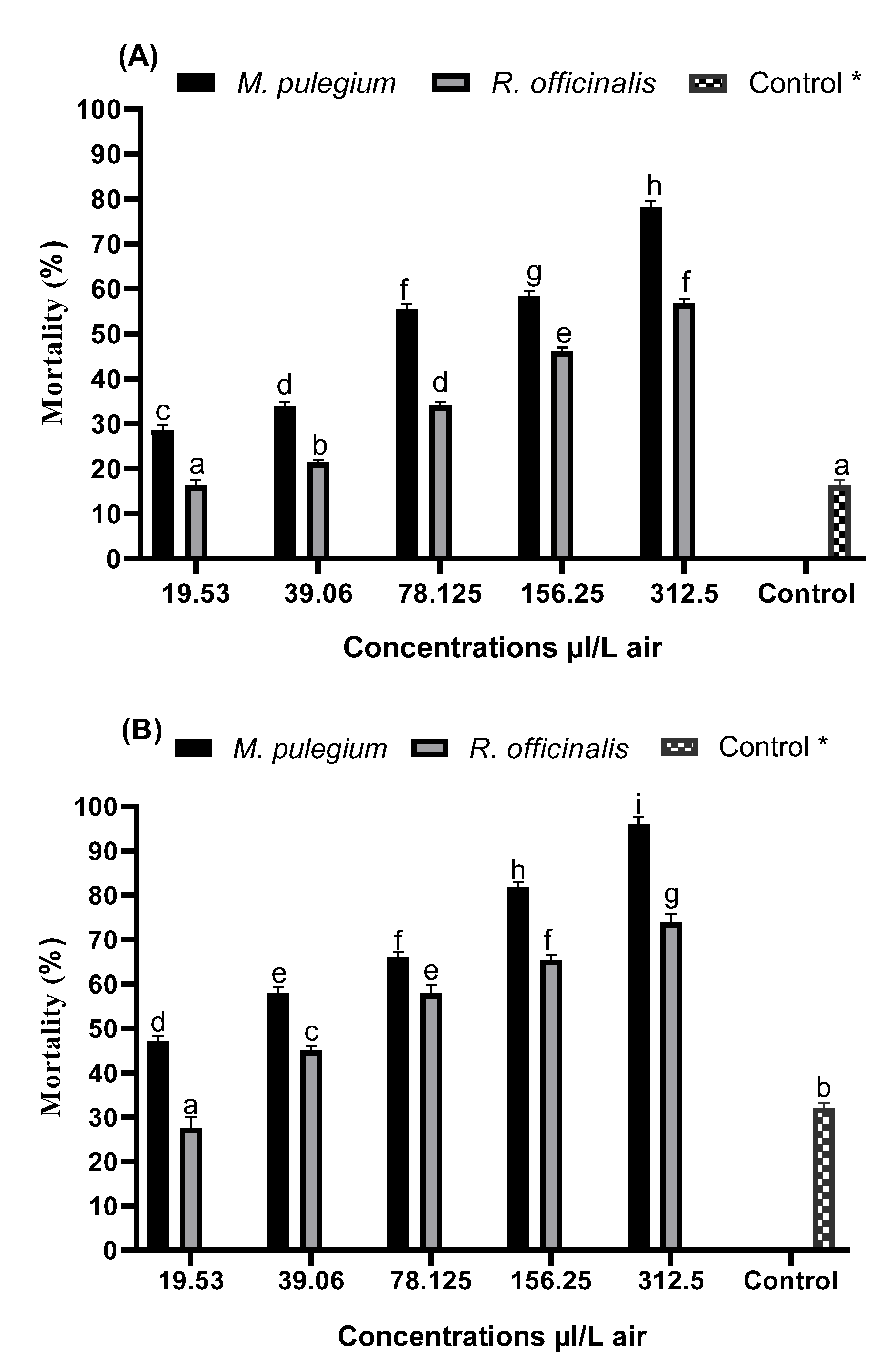
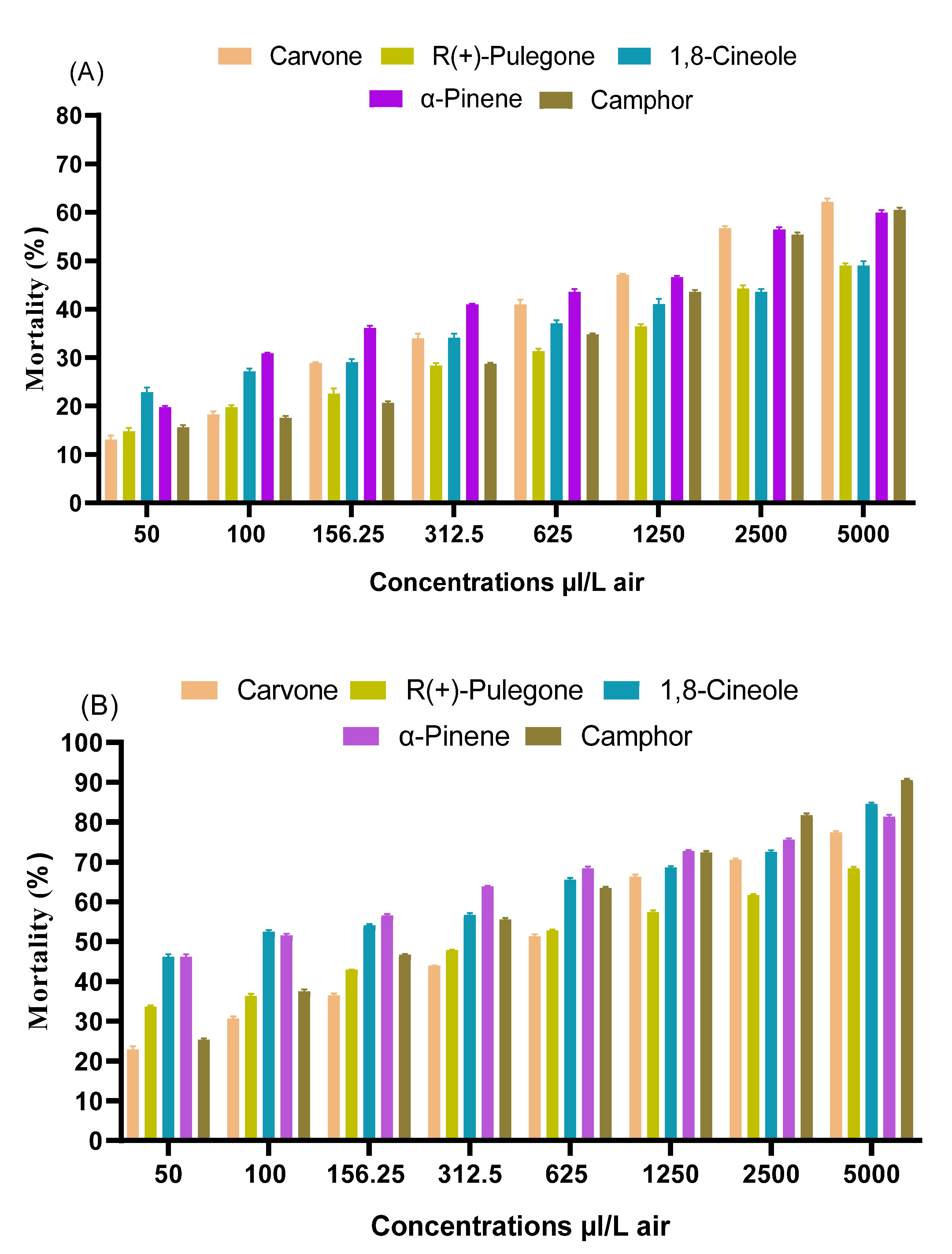
3.2. Comparative Toxicities of Both EOs and the Pure Compounds on C. pipiens Adults
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macêdo, N.S.; Silveira, Z.S.; Bezerra, A.H.; Costa, J.G.M.D.; Coutinho, H.D.M.; Romano, B.; Capasso, R.; Cunha, F.A.B.D.; da Silva, M.V. Caesalpinia ferrea C. Mart. (Fabaceae) Phytochemistry, Ethnobotany, and Bioactivities: A Review. Molecules 2020, 25, 3831. [Google Scholar] [CrossRef] [PubMed]
- Fahad, F.I.; Barua, N.; Islam, M.S.; Sayem, S.A.J.; Barua, K.; Uddin, M.J.; Chy, M.N.U.; Adnan, M.; Islam, M.N.; Sayeed, M.A.; et al. Investigation of the Pharmacological Properties of Lepidagathis hyalina Nees through Experimental Approaches. Life 2021, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Bouyahya, A.; Bakri, Y.; Et-Touys, A.; Talbaoui, A.; Khouchlaa, A.; El Idrissi, A.E.; Abrini, J.; Dakka, N. In vitro Screening of Antibacterial and Antioxidant Activities of Essential Oils from Four Moroccan Medicinal Plants. Microbiol. Res. J. Int. 2017, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Faustino, C.; Garcia, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An update review of its phytochemistry and biological activity. Future Sci. 2018, 4, FSO283. [Google Scholar] [CrossRef] [Green Version]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- Yakoubi, R.; Megateli, S.; Hadj Sadok, T.; Bensouici, C.; Bağci, E. A synergistic interactions of Algerian essential oils of Laurus nobilis L.; Lavandula stoechas L. and Mentha pulegium L. on anticholinesterase and antioxidant activities. Biocatal. Agric. Biotechnol. 2021, 31, 101891. [Google Scholar] [CrossRef]
- Sebai, E.; Serairi, R.; Saratsi, K.; Abidi, A.; Sendi, N.; Darghouth, M.A.; Wilson, M.S.; Sotiraki, S.; Akkari, H. Hydro-Ethanolic Extract of Mentha pulegium Exhibit Anthelmintic and Antioxidant Proprieties In Vitro and In Vivo. Acta Parasitol. 2020, 65, 375–387. [Google Scholar] [CrossRef]
- Rocha, D.; Novo, M.; Matos, O.C.; Figueiredo, A.; Delgado, M.D.; Cabral, M.; Liberato, M.; Moiteiro, C. Potential of Mentha pulegium for mosquito control. Rev. Ciênc. Agrár. 2015, 38, 155–165. [Google Scholar]
- Ahmed, A.; Ayoub, K.; Chaima, A.J.; Hanaa, L.; Abdelaziz, C. Effect of drying methods on yield, chemical composition and bioactivities of essential oil obtained from Moroccan Mentha pulegium L. Biocatal. Agric. Biotechnol. 2018, 16, 638–643. [Google Scholar] [CrossRef]
- Domingues, P.M.; Santos, L. Industrial Crops & Products Essential oil of pennyroyal ( Mentha pulegium ): Composition and applications as alternatives to pesticides—New tendencies. Ind. Crop. Prod. 2019, 139, 111534. [Google Scholar] [CrossRef]
- Chraibi, M.; Farah, A. Antimicrobial effcts of Moroccan rosemary essential oil, and its synergistic antimicrobial potential with carvacrol. J. Adv. Pharm. Technol. Res. 2020, 11, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mishra, S.; Malik, A.; Satya, S. Insecticidal properties of Mentha species: A review. Ind. Crop. Prod. 2011, 34, 802–817. [Google Scholar] [CrossRef]
- Pavela, R. Insecticidal activity of essential oils against cabbage aphid brevicoryne brassicae. J. Essent. Oil-Bearing Plants 2006, 9, 99–106. [Google Scholar] [CrossRef]
- Badreddine, B.S.; Olfa, E.; Samir, D.; Hnia, C.; Lahbib, B.J.M. Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae). Asian Pac. J. Trop. Med. 2015, 8, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Abdelli, M.; Moghrani, H.; Aboun, A.; Maachi, R. Algerian Mentha pulegium L. leaves essential oil : Chemical composition, antimicrobial, insecticidal and antioxidant activities. Ind. Crop. Prod. 2016, 94, 197–205. [Google Scholar] [CrossRef]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Mohammad Salamatullah, A.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A.; et al. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evidence-based Complement. Altern. Med. 2021, 2021, 1108133. [Google Scholar] [CrossRef]
- Salem, N.; Bachrouch, O.; Sriti, J.; Msaada, K.; Khammassi, S.; Hammami, M.; Selmi, S.; Boushih, E.; Koorani, S.; Abderraba, M.; et al. Fumigant and repellent potentials of Ricinus communis and Mentha pulegium essential oils against Tribolium castaneum and Lasioderma serricorne. Int. J. Food Prop. 2018, 20, S2899–S2913. [Google Scholar] [CrossRef] [Green Version]
- Koliopoulos, G.; Pitarokili, D.; Kioulos, E.; Michaelakis, A.; Tzakou, O. Chemical composition and larvicidal evaluation of Mentha, Salvia, and Melissa essential oils against the West Nile virus mosquito Culex pipiens. Parasitol. Res. 2010, 107, 327–335. [Google Scholar] [CrossRef]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef]
- Kazempour, S.; Shayeghi, M.; Abai, M.R.; Vatandoost, H.; Pirmohammadi, M. Larvicidal activities of essential oils of indigenous medicinal plants, Mentha pulegium L.; Satureja hortensis L.; and Thymus vulgaris L. against malaria vector, Anopheles stephensi. S. Afr. J. Bot. 2021, 139, 38–41. [Google Scholar] [CrossRef]
- Tine-Djebbar, F.; Guenez, R.; Soltani, N. Chemical composition and insecticide properties of the essential oils from mentha pulegium against aedes caspius (Diptera: Culicidae). Adv. Sci. Technol. Innov. 2018, 503–505. [Google Scholar] [CrossRef]
- Benelli, G. Plant-borne ovicides in the fight against mosquito vectors of medical and veterinary importance: A systematic review. Parasitol. Res. 2015, 114, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Zahran, H.E.D.M.; Abdelgaleil, S.A.M. Insecticidal and developmental inhibitory properties of monoterpenes on Culex pipiens L. (Diptera: Culicidae). J. Asia. Pac. Entomol. 2011, 14, 46–51. [Google Scholar] [CrossRef]
- Rozman, V.; Kalinovic, I.; Korunic, Z. Toxicity of naturally occurring compounds of Lamiaceae and Lauraceae to three stored-product insects. J. Stored Prod. Res. 2007, 43, 349–355. [Google Scholar] [CrossRef]
- Samir, A.M.; Al-Nagar, N.; Abou-Taleb, H.K.; Shawir, M.S. Effect of monoterpenes, phenylpropenes and sesquiterpenes on development, fecundity and fertility of Spodoptera littoralis (Boisduval). Int. J. Trop. Insect Sci. 2021, 40, 1–11. [Google Scholar] [CrossRef]
- Victor, A.; Brugman, L.M.; Hernández-Triana, J.M.; Medlock, A.R.; Fooks, S.C.; Nicholas, J. The Role of Culex pipiens L. (Diptera: Culicidae) in Virus Transmission in Europe. Int. J. Environ. Res. Public Health 2018, 15, 389. [Google Scholar] [CrossRef] [Green Version]
- García-Carrasco, J.M.; Muñoz, A.R.; Olivero, J.; Segura, M.; Real, R. Predicting the spatio-temporal spread of West Nile virus in Europe. PLoS Negl. Trop. Dis. 2021, 15, e0009022. [Google Scholar] [CrossRef]
- Jeffrey, G.S.; Melissa, H.; Yoshimizu, S.K. Pyrethroid resistance in Culex pipiens mosquitoes. Pestic. Biochem. Physiol. 2015, 120, 68–76. [Google Scholar]
- Tmimi, F.Z.; Faraj, C.; Bkhache, M.; Mounaji, K.; Failloux, A.B.; Sarih, M. Insecticide resistance and target site mutations (G119S ace-1 and L1014F kdr) of Culex pipiens in Morocco. Parasites Vectors 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Pub Corp: Carol Stream, IL, USA, 2007. [Google Scholar]
- Himmi, O.; Dakki, M.; Trari, B.; El Agbani, M.A. The Culicidae of Morocco: Identification KEYS with biological and Ecological Data (Work of the Scientific Institute); Zoology of series; Scientific Institute of Rabat: Rabat, Morocoo, 1995; p. 44.1-51. [Google Scholar]
- Brunhes, J.; Rhaim, A.; Geoffroy, B.; Hervy, J. P Mosquitoes of the Mediterranean Africa: Software Identification and Education, CD-ROM (Didactiques), IRDIRD editions; IRD & IPT: Paris, France, 2000. [Google Scholar]
- Finney, D. Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve; Cambridge University Press: Cambridge, UK, 1952. [Google Scholar]
- Babushok, V.I.; Linstrom, P.J.; Zenkevich, I.G. Retention Indices for Frequently Reported Compounds of Plant Essential Oils. J. Phys. Chem. Ref. Data 2011, 40, 043101. [Google Scholar] [CrossRef] [Green Version]
- Isman, M.B.; Miresmailli, S.; MacHial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Traboulsi, A.F.; Taoubi, K.; El-Haj, S.; Bessiere, J.M.; Rammal, S. Insecticidal properties of essential plant oils against the mosquito Culex pipiens molestus (Diptera: Culicidae). Pest Manag. Sci. 2002, 58, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Michaelakis, A.; Papachristos, D.; Kimbaris, A.; Koliopoulos, G.; Giatropoulos, A.; Polissiou, M.G. Citrus essential oils and four enantiomeric pinenes against Culex pipiens (Diptera: Culicidae). Parasitol. Res. 2009, 105, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.J.; Byun, S.G.; Cho, J.E.; Chung, K.; Ahn, Y.J. Larvicidal activity of Kaempferia galangal rhizome phenylpropanoids towards three mosquito species. Pest Manag. Sci. 2008, 64, 857–862. [Google Scholar]
- Chen, Y.; Luo, J.; Zhang, N.; Yu, W.; Jiang, J.; Dai, G. Insecticidal activities of Salvia hispanica L. essential oil and combinations of their main compounds against the beet armyworm Spodoptera exigua). Ind. Crop. Prod. 2021, 162, 113271. [Google Scholar] [CrossRef]
- Yang, P.; Ma, Y.; Zheng, S. Adulticidal activity of five essential oils against Culex pipiens quinquefasciatus. J. Pestic. Sci. 2005, 30, 84–89. [Google Scholar] [CrossRef] [Green Version]
- El-Aswad, A.F.; Abdelgaleil, S.A.M. Insecticidal, antifeedant and molluscicidal potential of essential oils extracted from Egyptian plants. J. Egypt. Soc. Toxicol. 2008, 38, 81–91. [Google Scholar]
- Al-Sarar, A.S.; Hussein, H.I.; Abobakr, Y. Chemical composition, adulticidal and repellent activity of essential oils from Mentha longifolia L. and Lavandula dentata L. against Culex pipiens L. J. Plant Prot. Path. Mansoura Univ. 2015, 5, 817–826. [Google Scholar] [CrossRef]
- Zahran, H.E.D.M.; Abou-Taleb, H.K.; Abdelgaleil, S.A.M. Adulticidal, larvicidal and biochemical properties of essential oils against Culex pipiens L. J. Asia. Pac. Entomol. 2017, 20, 133–139. [Google Scholar] [CrossRef]
- Erler, F.; Ulug, I.; Yalcinkaya, B. Repellent activity of five essential oils against Culex pipiens. Fitoterapia 2006, 77, 491–494. [Google Scholar] [CrossRef]
- Brahmi, F.; Abdenour, A.; Bruno, M.; Silvia, P.; Alessandra, P.; Danilo, F.; Drifa, Y.G.; Fahmi, E.M.; Khodir, M.; Mohamed, C. Chemical composition and in vitro antimicrobial, insecticidal and antioxidant activities of the essential oils of Mentha pulegium L. and Mentha rotundifolia (L.) Huds growing in Algeria. Ind. Crop. Prod. 2016, 88, 96–105. [Google Scholar] [CrossRef]
- Isikber, A.A.; Alma, M.H.; Kanat, M.; Karci, A. Fumigant toxicity of essential oils from Laurus nobilis and Rosmarinus officinalis against all life stages of Tribolium confusum. Phytoparasitica 2006, 34, 167–177. [Google Scholar] [CrossRef]
- Bin, M.W.; Feng, J.T.; Jiang, Z.L.; Wu, H.; Ma, Z.Q.; Zhang, X. Fumigant activity of eleven essential oil compounds and their selected binary mixtures against Culex pipiens pallens (Diptera: Culicidae). Parasitol. Res. 2014, 113, 3631–3637. [Google Scholar] [CrossRef]
- Rice, P.J.; Coats, J.R. Insecticidal properties of several monoterpenoids to the housefly (Diptera: Muscidae), red flour beetle (Coleoptera : Tenebrionidae) and southern corn root-worm (Coleoptera : Chrysomelidae). J. Econ. Entomol. 1994, 87, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Manoguerra, A.S.; Andrew, R.; Paul, M.W.; Lewis, S.N.; Caravati, E.M.; Cobaugh, D.J.; Chyka, P.A.; Olson, K.R.; Booze, L.L.; Woolf, A.D.; et al. Camphor Poisoning: An Evidence-Based Practice Guideline for Out-of-Hospital Management. Clin. Toxicol. 2008, 44, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A Fumigant during the Black Death and a Coveted Fragrant Wood in Ancient Egypt and Babylon—A Review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [Green Version]
- Hnin, K.; Weiss, D.; Nathan, G.; Hoffman, R.S.; Esteban-Cruciani, N.; Avner, J.R. A Cluster of Children with Seizures Caused by Camphor Poisoning. Pediatrics 2009, 123, 1269–1272. [Google Scholar] [CrossRef]
- Rahimi, M.; Shokri, F.; Moghaddam, H.H.; Zamani, N.; Pajoumand, A.; Shadnia, S. Severe camphor poisoning, a seven-year observational study. Environ. Toxicol. Pharmacol. 2017, 52, 8–13. [Google Scholar] [CrossRef]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the Lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef] [Green Version]
- Al-Harbi, N.A.; Al Attar, N.M.; Hikal, D.M.; Mohamed, S.E.; Abdel Latef, A.A.H.; Ibrahim, A.A.; Abdein, M.A. Evaluation of Insecticidal Effects of Plants Essential Oils Extracted from Basil, Black Seeds and Lavender against Sitophilus oryzae. Plants 2021, 10, 829. [Google Scholar] [CrossRef]
- Ramar, M.; Ignacimuthu, S.; Gabriel Paulraj, M. Mosquito knock-down and adulticidal activities of essential oils by vaporizer, impregnated filter paper and aerosol methods. Int. J. Mosq. Res. 2014, 1, 26–32. [Google Scholar]
- Trari, B. Mosquitoes (Insects, Diptera) from Morocco: Distribution Atlas and Epidemiological Studies. Ph.D. Thesis, Abdelmalek Essaâdi University, Tangier, Morocco, 1 April 2017. [Google Scholar] [CrossRef]
- Aboulfadl, S.; Mellouki, F.; Aouinty, B.; Faraj, C. Susceptibility status of Culex pipiens larvae (Diptera: Culicidae) to the main insecticides used in larval control in the regions of Rabat and Casablanca in Morocco. Int. J. Pest Manag. 2020. [Google Scholar] [CrossRef]
| Compound | RI (Exp) a | RI (Lit) b | M. pulegium* | R. officinalis* |
|---|---|---|---|---|
| α-Thujene | 931 | 924 | - | 0.48 |
| α-Pinene | 939 | 932 | 0.42 | 12.76 |
| Cyclohexanone-3-methyl | 952 | 945 | 0.16 | - |
| Camphene | 953 | 946 | - | 2.47 |
| β-Pinene | 976 | 974 | 0.33 | 2.89 |
| Unknown | 957 | - | - | t |
| Unknown | 958 | - | 0.08 | t |
| Unknown | 961 | - | 0.07 | t |
| Unknown | 985 | - | t | - |
| Myrcene | 993 | 988 | 0.16 | 2.54 |
| Octanol-3 | 993 | 995 | 1.32 | - |
| Unknown | 995 | - | - | t |
| δ-2-Carene | 1001 | 998 | 0.11 | - |
| Unknown | 1023 | - | 0.07 | 1.5 |
| Limonene | 1031 | 1029 | 1.34 | - |
| 1,8-Cineole | 1033 | 1033 | - | 29.31 |
| Unknown | 1035 | - | - | t |
| Unknown | 1040 | - | - | t |
| Unknown | 1064 | - | t | t |
| p-Mentha-3,8-diene | 1071 | 1072 | 2.04 | - |
| Unknown | 1075 | - | t | 1.03 |
| Unknown | 1100 | - | - | t |
| Camphor | 1143 | 1141 | - | 24.66 |
| Unknown | 1148 | - | - | t |
| Menthone | 1154 | 1148 | 0.13 | - |
| Borneol | 1165 | 1166 | - | 5.46 |
| Pinocarvone | 1168 | 1160 | 1.21 | - |
| Menthol | 1173 | 1167 | 0.45 | - |
| α-Terpineol | 1185 | 1186 | - | 0.63 |
| Dihydrocarvone | 1194 | 1191 | 3.66 | - |
| Myrtenol | 1195 | 1194 | - | 1.23 |
| Unknown | 1207 | - | 0.09 | - |
| Pulegone | 1238 | 1233 | 74.03 | - |
| Carvone | 1242 | 1239 | 5.45 | - |
| Unknown | 1245 | - | - | 0.2 |
| Peperitone | 1252 | 1249 | 1.12 | - |
| Unknown | 1265 | - | - | 0.2 |
| Myrtenyl acetate | 1322 | 1324 | - | 0.22 |
| α-Cubebene | 1351 | 1345 | - | 0.15 |
| α-Copaene | 1381 | 1374 | - | 0.10 |
| β-Bourbonene | 1384 | 1387 | - | 0.16 |
| Unknown | 1397 | - | 0.06 | - |
| Caryophyllene | 1419 | 1417 | 0.35 | - |
| γ-Gurjunene | 1473 | 1475 | - | 0.2 |
| Germacrene-D | 1480 | 1484 | 0.42 | |
| Ledene | 1493 | 1490 | - | 2.92 |
| α-Muurolene | 1499 | 1500 | - | 0.32 |
| γ-Cadinene | 1513 | 1513 | - | 0.8 |
| Caryophyllene oxide | 1581 | 1582 | - | 2.75 |
| Copaen-4-α-ol | 1584 | 1590 | - | 0.43 |
| Tetradecanal | 1611 | 1612 | - | 0,21 |
| γ-Eudesmol | 1630 | 1630 | 0.26 | - |
| τ.Cadinol | 1653 | 1654 | - | 0.19 |
| α-Eudesmol | 1649 | 1652 | 0.44 | - |
| Total identified compounds | 93.35 | 94.23 |
| EOs and Monoterpenes | Exposure Time (h) | LC50 a (µL/L Air) (95% Confidence Intervals) | Slope b | Intercept c | R2 | p-Value d |
|---|---|---|---|---|---|---|
| M. pulegium | 24 | 72.94 (60.34–83.32) | 1.146 ± 0.15 | 2.865 ± 0.30 | 0.94 | 0.005 |
| 48 | 25.43 (13.22–38.65) | 1.169 ± 0.18 | 3.357 ± 1.23 | 0.94 | 0.005 | |
| R. officinalis | 24 | 222.82 (210, 234.29) | 0.957 ± 0.11 | 2.753 ± 0.63 | 0.93 | 0.003 |
| 48 | 55.79 (50.77–60.81) | 1.093 ± 0.08 | 3.091 ± 1.18 | 0.93 | 0.003 | |
| Carvone | 24 | 1713.36 (1703.33–1723.36) | 0.774 ± 0.04 | 2.497 ± 0.12 | 0.96 | 0.000 |
| 48 | 538.96 (512.84–568.36) | 0.827 ± 0.02 | 2.741 ± 0.07 | 0.99 | 0.000 | |
| R(+)-Pulegone | 24 | 5395.58 (5295.58–5494.68) | 0.515 ± 0.01 | 3.078 ± 0.02 | 0.99 | 0.000 |
| 48 | 578.84 (578.24–597.64) | 0.577 ± 0.05 | 3.406 ± 0.14 | 0.93 | 0.000 | |
| 1,8-Cineole | 24 | 5395.65 (5282.45–5486.55) | 0.362 ± 0.01 | 3.649 ± 0.03 | 0.98 | 0.000 |
| 48 | 84.96 (75.8–95.26) | 0.537 ± 0.05 | 3.964 ± 0.14 | 0.90 | 0.000 | |
| Camphor | 24 | 2269.64 (2190.41–2354.45) | 0.663 ± 0.01 | 2.775 ± 0.04 | 0.98 | 0.000 |
| 48 | 205.38 (167.83–300.51) | 1. 027 ± 0.08 | 2. 6258 ± 0.22 | 0.94 | 0.000 | |
| α-Pinene | 24 | 1294.64 (1230–1345.14) | 0.642 ± 0.06 | 3.002 ± 0.16 | 0.92 | 0.000 |
| 48 | 85.74 (74.04–98.21) | 0.509 ± 0.01 | 4.016 ± 0.04 | 0.99 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramzi, A.; El Ouali Lalami, A.; Ez zoubi, Y.; Assouguem, A.; Almeer, R.; Najda, A.; Ullah, R.; Ercisli, S.; Farah, A. Insecticidal Effect of Wild-Grown Mentha pulegium and Rosmarinus officinalis Essential Oils and Their Main Monoterpenes against Culex pipiens (Diptera: Culicidae). Plants 2022, 11, 1193. https://doi.org/10.3390/plants11091193
Ramzi A, El Ouali Lalami A, Ez zoubi Y, Assouguem A, Almeer R, Najda A, Ullah R, Ercisli S, Farah A. Insecticidal Effect of Wild-Grown Mentha pulegium and Rosmarinus officinalis Essential Oils and Their Main Monoterpenes against Culex pipiens (Diptera: Culicidae). Plants. 2022; 11(9):1193. https://doi.org/10.3390/plants11091193
Chicago/Turabian StyleRamzi, Amal, Abdelhakim El Ouali Lalami, Yassine Ez zoubi, Amine Assouguem, Rafa Almeer, Agnieszka Najda, Riaz Ullah, Sezai Ercisli, and Abdellah Farah. 2022. "Insecticidal Effect of Wild-Grown Mentha pulegium and Rosmarinus officinalis Essential Oils and Their Main Monoterpenes against Culex pipiens (Diptera: Culicidae)" Plants 11, no. 9: 1193. https://doi.org/10.3390/plants11091193
APA StyleRamzi, A., El Ouali Lalami, A., Ez zoubi, Y., Assouguem, A., Almeer, R., Najda, A., Ullah, R., Ercisli, S., & Farah, A. (2022). Insecticidal Effect of Wild-Grown Mentha pulegium and Rosmarinus officinalis Essential Oils and Their Main Monoterpenes against Culex pipiens (Diptera: Culicidae). Plants, 11(9), 1193. https://doi.org/10.3390/plants11091193










