Abstract
Dengue caused by dengue virus (DENV) is a mosquito-borne disease. Dengue exhibits a wide range of symptoms, ranging from asymptomatic to flu-like illness, and a few symptomatic cases may develop into severe dengue, leading to death. However, there are no effective and safe therapeutics for DENV infections. We have previously reported that cytokine expression, especially inflammatory cytokines, was altered in patients with different severities of dengue. Antrodia cinnamomea (A. cinnamomea) is a precious and endemic medical mushroom in Taiwan. It contains unique chemical components and exhibits biological activities, including suppressing effects on inflammation and viral infection-related diseases. According to previous studies, megakaryocytes can support DENV infection, and the number of megakaryocytes is positively correlated with the viral load in the serum of acute dengue patients. In the study, we investigated the anti-DENV effects of two ethanolic extracts (ACEs 1–2) and three isolated compounds (ACEs 3–5) from A. cinnamomea on DENV infection in Meg-01 cells. Our results not only demonstrated that ACE-3 and ACE-4 significantly suppressed DENV infection, but also reduced interleukin (IL)-6 and IL-8 levels. Moreover, the level of the antiviral cytokine interferon (IFN)-α was also increased by ACE-3 and ACE-4 in Meg-01 cells after DENV infection. Here, we provide new insights into the potential use of A. cinnamomea extracts as therapeutic agents against DENV infection. However, the detailed mechanisms underlying these processes require further investigation.
1. Introduction
Dengue virus (DENV), a single-stranded RNA virus, belongs to the family Flaviviridae and genus Flavivirus [1,2]. There are four serotypes of DENV (DENV-1, DENV-2, DENV-3, and DENV-4) that can be distinguished by neutralization assay data [3]. Different serotypes may circulate in different areas and can be attributed to variations in severity. However, due to global warming, the increase in long-distance travel, population growth, and urbanization, cases of dengue have occurred in an increasing number of countries [4,5,6]. Dengue caused by DENV infection is one of the most important mosquito-borne diseases, with approximately four billion people living under the threat of dengue worldwide [1]. The clinical manifestations of dengue range from asymptomatic to flu-like, and some symptomatic cases develop severe dengue, including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [7]. Severe dengue is life-threatening to patients, resulting in plasma leakage, organ failure, severe bleeding, cytokine storm, and even death [8,9]. In addition, we have previously reported that the expression of cytokines changes with different severities of dengue [10]. Many of the studies have also indicated that the levels of pro-inflammatory cytokines are elevated in severe dengue patients, such as interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, and IL-1β, which may result in cytokine storm and increase the severity of dengue in patients [11,12,13,14]. In contrast, type I interferon (IFN), including IFN-α and IFN-β, which are not only important human antiviral responses against pathogens but also the key factors linking the innate and adaptive immune systems, are suppressed by DENV and contribute to the production of DENV replication in host cells and evasion of antiviral immunity [15,16,17].
DENV has been around for many decades since 1943; however, there are no effective therapeutic agents against DENV infection. Currently, preventive medicine and supportive care are the main treatments administered to dengue patients [18]. Therefore, safe and efficient therapeutics against DENV infection are urgently required for the prevention and control of dengue. Dengvaxia, a live-attenuated dengue vaccine, is the first licensed dengue vaccine, but is recommended by the Food and Drug Administration (FDA) to be used only for children aged 9–16 years and who have been infected by DENV previously due to the vaccinated individuals who have not been infected by DENV, leading to an increased risk of developing severe dengue [19,20,21,22]. Although there are some candidates for antiviral agents for dengue that have entered clinical trials, none of them have been used to treat dengue patients [23].
A. cinnamomea is a fungal parasite in the inner cavity of the endemic species Cinnamomum kanehirae (bull camphor tree) Hayata (Lauraceae). The host plant is a large evergreen broad-leaved tree that grows only in Taiwan and is distributed over broad-leaved forests at altitudes of 200–2000 m [24]. It is an edible Taiwanese mushroom that is regarded as a precious Chinese herbal medicine in Taiwan [25]. Many studies have reported that A. cinnamomea and its special components possess many pharmacological activities, including antihypertensive, neuroprotective, anticancer, and antimicrobial activities [26,27,28,29]. A recent study indicated that A. cinnamomea could inhibit angiotensin-converting enzyme 2 (ACE2), which supports severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) entry and disease onset [30]. Moreover, numerous studies have indicated that A. cinnamomea can inhibit the secretion of inflammatory cytokines, especially IL-6 or TNF-α, and suppress the surface antigens of the hepatitis B virus [31,32,33]. The ethanol extract of A. cinnamomea effectively suppressed hepatoma migration through downregulation of MAPK signaling, which exhibited potent DPPH radical- and superoxide dismutase (SOD)-like scavenging activities [34,35]. Furthermore, the anti-inflammatory compounds such as zhankuic acid C (ergostane-type triterpenoids), 4,7-dimethoxy-5-methyl-1,3-benzodioxole (benzenoids), and dehydrosulfurenic acid (lanostane-type triterpenoids) have been isolated and identified from the fruiting bodies and solid-state cultivated products of A. cinnamomea [24]. Active components isolated from A. cinnamomea have been demonstrated to be the major contributors to various medical benefits. Dehydroeburicoic acid can modulate glycolysis to prevent type 2 diabetes [36]. Zhankuic acids display an immunosuppressive effect on immune cells and could be used as a potential treatment for chronic inflammation [37,38]. 4,7-dimethoxy-5-methyl-1,3-benzodioxole induced cell apoptosis, resulting in decreased colon cancer cell growth and reduced pro-inflammatory cytokines by suppressing the nuclear factor-κβ (NF-κβ) signaling pathway [28,39,40]. Overall, A. cinnamomea is a potential source for novel strategies for developing new treatments for dengue.
Bone marrow (BM) suppression is a common phenomenon in dengue patients and leads to many hematological disorders such as leukopenia, thrombocytopenia, and atypical lymphocytes [41,42,43]. Furthermore, DENV directly infects and reduces the proliferative capacity of hematopoietic stem progenitor cells, which are BM progenitor cells, during DENV infection [44]. We have also demonstrated that megakaryocytes isolated from human BM are the dominant cells supporting DENV infection, and the number of megakaryocyte precursors was positively correlated with the viral load in the serum of patients with acute dengue [45]. Bone marrow-derived megakaryoblastic cells, Meg-01 cells, which are permissive for DENV infection, also show phenotypic properties similar to megakaryoblasts in the BM [46,47,48]. Hence, the hypothesis of this study was that the human megakaryocytic cell line Meg-01 as a cell model to explore the bioactivities of A. cinnamomea extracts against DENV infection.
2. Results
2.1. Cell Viability of the Five A. cinnamomea Extracts in Meg-01 Cells
Information on the five A. cinnamomea extracts is presented in Table 1. The WST-1 cell proliferation assay kit was used to assess the viability of Meg-01 cells after treatment with A. cinnamomea extracts. The results showed that each A. cinnamomea extract caused concentration-dependent cytotoxicity in Meg-01 cells (Figure 1). The values of CC10 and CC50 values were calculated to select an appropriate concentration of the A. cinnamomea extract for the following antiviral activity test (Table 1). Of the five A. cinnamomea samples, ACE-4 demonstrated the lowest cytotoxicity, whereas ACE-1, ACE-2 and ACE-5 induced higher levels of cell death in Meg-01 cells. The values of CC10 were between 3.84 and 5.68 μg/mL, and the values of CC50 were between 35.67 and 55.96 μg/mL.

Table 1.
The inhibition effects of prepared two ethanolic extracts and isolated three compounds from A. cinnamomea on Meg-01 cells.
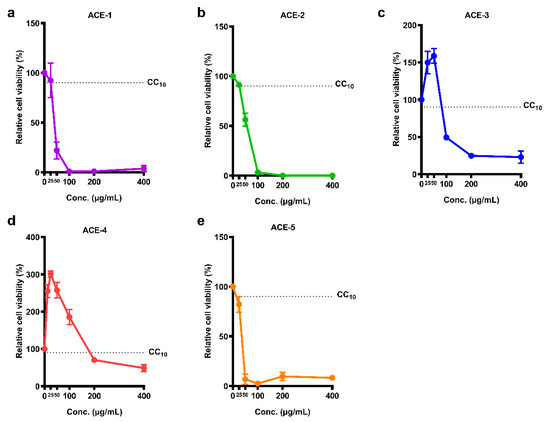
Figure 1.
Cell viability of the five A. cinnamomea extracts in Meg-01 cells. Meg-01 cells were cultured with different concentrations of A. cinnamomea extracts, (a) ACE-1, (b) ACE-2, (c) ACE-3, (d) ACE-4, and (e) ACE-5, for 24 h followed by reacting with WST-1 reagent. All the values represent as mean ± SEM of three experiments. The dotted line represents the value of CC10.
2.2. Inhibitory Effects of the A. cinnamomea Extracts against DENV
Meg-01 cells were treated with different A. cinnamomea extracts after DENV infection, and the viral titers of DENV were evaluated using a plaque assay (Figure 2). The concentrations of ACE-1–ACE-5 were used in viral titer, which are 3.84, 5.67, 91.68, 176.51, 5.68 μg/mL, respectively. The replication curve for DENV in Meg-01 cells was calculated. The viral titer peak was observed on day 5 post-infection and remained steady until day 7 (Figure 2a). The antiviral effects of A. cinnamomea extracts on DENV were determined by performing a plaque assay with the supernatants collected on different days post-infection. From the results, it was observed that the viral titers of DENV decreased in both ACE-3 and ACE-4 treatments during DENV infection as of day 2. Moreover, a significant reduction in DENV resulting from ACE-3 and ACE-4 treatments was also observed at days 5 and 7 post-infection (Figure 2b). Although there was a visible decrease in the viral titer of DENV at 24 h after DENV infection in ACE-1, ACE-2, and ACE-3 treatments, the difference was not statistically significant.
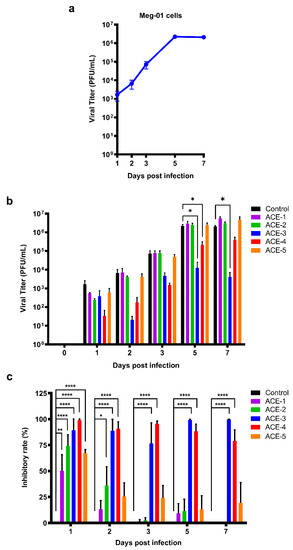
Figure 2.
Both ACE-3 and ACE-4 could significantly inhibit DENV replication. (a) Replication curve of DENV in Meg-01 cells, and the peak of the titer was showed at day 5 post infection. (b) The viral titers of DENV in the A. cinnamomea extracts-treated Meg-01 cells. (c) The inhibitory rate of the A. cinnamomea extracts against DENV infection in Meg-01 cells. All the values represent as mean ± SEM of three experiments. p values were calculated using two-way ANOVA, statistically significant difference * p < 0.05, ** p < 0.01, **** p < 0.0001.
2.3. Anti-Inflammatory Effects of the A. cinnamomea Extracts
Disorder in cytokine expression is one of the clinical manifestations of dengue. To date, much of the evidence suggests that this dysfunction is correlated with disease severity. Therefore, we evaluated the ability of A. cinnamomea extract to regulate cytokine expression. The supernatants of A. cinnamomea extract-treated Meg-01 cells after DENV infection were collected to analyze the secretion patterns of inflammatory cytokines, such as IL-6, IL-8, and TNF-α. The results indicated that the expression of IL-6 and IL-8 in control group which was Meg-01 cells without A. cinnamomea extract treatment was associated with the dynamics of dengue viremia. Notably, after treatment with the A. cinnamomea extracts, ACE-3 and ACE-4, the expression of both IL-6 and IL-8 was significantly decreased after DENV infection (Figure 3). As a result, ACE-3 and ACE-4 efficiently reduced the inflammatory cytokines IL-6 and IL-8, in Meg-01 cells against DENV infection.
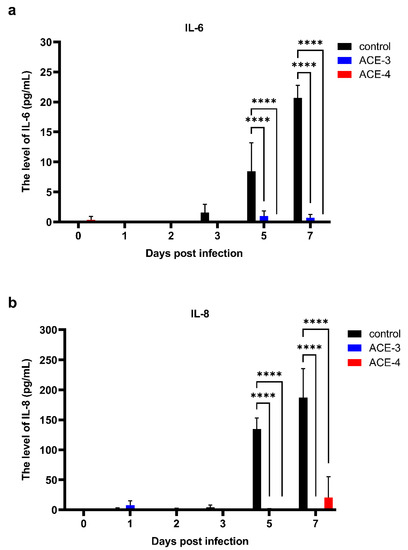
Figure 3.
ACE-3 and ACE-4 suppressed the expression of the IL-6 and IL-8 after DENV infection. The levels of (a) IL-6 and (b) IL-8, in Meg-01 cells treated with the A. cinnamomea extracts after DENV infection. All the values represent as mean ± SEM of three experiments. p values were calculated using two-way ANOVA, p < 0.0001 **** compared to the control.
2.4. Antiviral Activities of the A. cinnamomea Extracts
As mentioned previously, DENV can suppress the antiviral activities of the host, especially impeding the signaling response of type I IFN, to promote viral replication. Therefore, we analyzed the production of type I IFN, IFN-α, and IFN-β. Increased levels of IFN-α were observed in Meg-01 cells treated with A. cinnamomea extracts after DENV infection when compared to the control, which were only infected with DENV (Figure 4a). Additionally, the level of IFN-β fluctuated and had no relationship with the viral titers or A. cinnamomea extract treatment (Figure 4b). Taken together, A. cinnamomea extracts, ACE-3, and ACE-4, increased the secretion of IFN-α to suppress DENV replication.
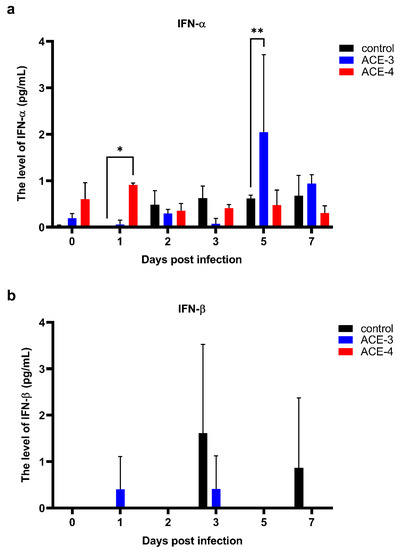
Figure 4.
ACE-3 and ACE-4 may inhibit DENV replication by enhancing the secretion of IFN-α. The levels of antiviral cytokines, (a) IFN-α and (b) IFN-β, in Meg-01 cells treated with A. cinnamomea extracts after DENV infection. All the values represent as mean ± SEM of three experiments. p values were calculated using two-way ANOVA, statistically significant difference * p < 0.05, ** p < 0.01 compared to the control.
3. Discussion
In recent years, some investigational drugs against DENV infection entered into the clinical development phase but failed during or after the clinical trials [23]. Although there are many standard operating procedures that may mitigate the prevalence and occurrence of dengue fever, the healthcare costs involved are high. Therefore, there is an increasing requirement for dengue therapeutics and an impetus for antiviral drug and vaccine development in this realm. In the past century, natural components such as plants and microorganisms, which have biological activities, have emerged as one of the major sources for developing new pharmaceuticals [49,50,51].
Herbal medicine refers to botanicals that have been modified and successfully applied to treat many diseases [52,53,54]. According to the WHO, herbal medicine is widely used for health care, disease prevention, and treatment in many countries [55]. A. cinnamomea has been revealed to have diverse activities, and the active components in A. cinnamomea are important ingredients used in the production of health food products [56,57].
In this study, we focused on the antiviral and anti-inflammatory effects of A. cinnamomea in DENV infection. We found that ACE-3 and ACE-4 have potential therapeutic effects against DENV by enhancing the antiviral cytokine expression and suppressing the inflammatory cytokine secretion to inhibit DENV replication in Meg-01 cells. These protective effects of A. cinnamomea were examined by pre-treating Meg-01 cells with A. cinnamomea extract before DENV infection. Moreover, it was found that the activity of type I interferons increased early during DENV infection when Meg-01 cells were treated with ACE-3 or ACE-4, in comparison to that without treatment. This result implied that the components of A. cinnamomea appeared to repair IFN damage. DENV life cycle can be divided into three stages: entry, replication, and release. To investigate the potent inhibitory mechanism of A. cinnamomea extract against DENV infection, Meg-01 cells were treated with A. cinnamomea extract and DENV at various incubation periods and temperatures in a previous study to find out the target molecule or the signaling pathway altered by the A. cinnamomea extract [58].
ACE-3 (Zhankuic acid C), one of the major components ofthe fruiting bodies of A. cinnamomea, has been isolated from the extract by using HPLC in the study. It has been reported to have anti-inflammatory effects in neutrophils and dendritic cells, thereby treating chronic inflammation and auto immune diseases [37]. ACE-4 (4,7-dimethoxy-5-methyl-1,3-benzodioxole), which was also isolated from the fruiting bodies of A. cinnamomea and found to display a remarkable inhibitory effect in LPS-induced inflammation [39]. In the past, the fruiting bodies of A. cinnamomea have been used for the prevention or treatment of numerous diseases including liver diseases, food and drug intoxication, diarrhea, abdominal pain, hypertension, itchy skin, and tumorigenic disease [24]. However, the presence of any anti-viral effect of A. cinnamomea extract has barely been explored. Likewise, the relevance of A. cinnamomea components’ function to DENV has not been evaluated to date. The discovery of IFN damage repair by A. cinnamomea components was a momentous breakthrough in the development of anti-DENV drugs.
4. Materials and Methods
4.1. Cells and Viruses
Baby hamster kidney fibroblast cells (BHK-21) (ATCC, Manassas, VT, USA) and African green monkey kidney epithelial cells (Vero) (ATCC, Manassas, VT, USA) were cultured and maintained in Dulbecco’s modified Eagle´s medium (DMEM) (Cytiva, Chicago, IL, USA) supplemented with 5% heat-inactivated fetal bovine serum (FBS) (Gibco, Waltham, MA, USA). Philadelphia chromosome-positive chronic myelogenous leukemia bone marrow cells (Meg-01) (ATCC, Manassas, VT, USA) were cultured and maintained in RPMI-1640 medium (RPMI) (Cytiva, Chicago, IL, USA) supplemented with 10% heat-inactivated FBS. All the cells were grown at 37 °C with 5% CO2. Subculturing procedures were performed according to the guidelines provided by ATCC. Dengue virus serotype 2 (DENV, 16681 strain) was used in these experiments [59]. DENV was propagated in Vero cells, and the viruses were titrated by plaque assay and used for the following experiments.
4.2. A. cinnamomea Extracts Preparation
Fruiting bodies of A. cinnamomea were collected from Yuli, Hualien County (23°24′32.5″ N, 121°24′49.1″ E), Taiwan, in December 2007. The extracts were prepared as follows: crude extracts of A. cinnamomea: 20 g of ground A. cinnamomea (passed through a 30 mesh screen) was mixed in 50% ethanol solution and 95% ethanol solution and stirred at 125 rpm for 2 days at room temperature. The extracts were then filtered using medium-grade filter papers and concentrated under reduced pressure at 35 °C to give a dark brown syrup.
ACE-3, ACE-4, and ACE-5 isolation: The dried and pulverized A. cinnamomea fruiting body samples were extracted thrice with 95% ethanol at a ratio of 1:10 (w/v) for 2 days at room temperature under constant stirring at 125 rpm. After two days, the ethanol solution was filtered and concentrated to dryness in vacuo. The crude extract was suspended in deionized water and successively partitioned with ethyl acetate (EtOAc). The concentrated EtOAc layer was chromatographed on a silica gel column by elution with an n-hexane/EtOAc gradient with increasing polarity, yielding five fractions. The fractions were then analyzed using high-performance liquid chromatography to obtain ACE-3, ACE-4 and ACE-5, respectively. The structures of the compounds (ACE-3, ACE-4, and ACE-5) were determined by 1H and 13C nuclear magnetic resonance spectroscopy and by comparison of the spectral data with published values [60,61,62,63]. A summary of the current protocol is shown in Figure 5.
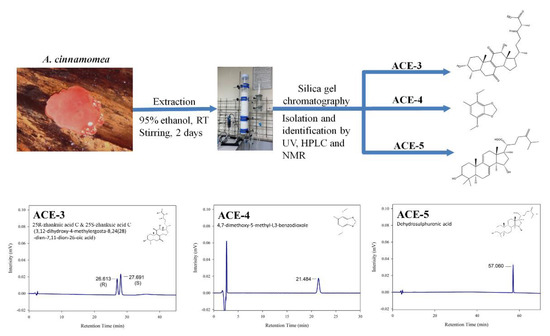
Figure 5.
Methods for isolation and identification ofactive compounds from A. cinnamomea.
4.3. Cell Viability Test
Cell viability was measured using Cell Proliferation Reagent WST-1 (Roche, Basel, BS, Switzerland). In brief, Meg-01 cells were seeded at a density of 1000 cells per well in 100 μL culture medium containing gradient concentrations of A. cinnamomea extracts in 96-well plates. Cells cultured in a medium without A. cinnamomea extract were used as control cells. The plates were incubated at 37 °C in an incubator with 5% CO2 for 7 days. After incubation, 10 μL of WST-1 assay reagent was added to each well and incubated for another 2 h. Absorbance at 440 nm was determined using theenzyme-linked immunosorbent assay (ELISA) reader (Multiskan SkyHigh Microplate Spectrophotometer, Thermo Fisher Scientific, Waltham, MA, USA). All experiments were performed in duplicate three times. According previous studies described [64,65,66], the values of CC10 and CC50 (cytotoxicity concentration that causes a 10% and 50% reduction in cell numbers compared to the control) were calculated a 4-parameter logistic (4PL) model and were regarded as the working concentration of the A. cinnamomea extracts for the virus inhibition assay.
4.4. Virus Inhibition Assay
Meg-01 cells were transferred into the flow tube at a density of 2 × 105 per tube in 1 mL culture, followed by DENV infection at a multiplicity of infection (MOI) of 1. The tubes were incubated at 37 °C incubator with 5% CO2. After incubation, the cells were washed with fresh culture medium and resuspended in a culture medium containing different A. cinnamomea extracts. Cells resuspended in the culture medium without A. cinnamomea extract were used as control cells. The supernatants were collected 1, 2, 3, 5, and 7 days post infection and stored at −80 °C for subsequent titration by plaque assay.
4.5. Plaque Assay
BHK-21 cells were seeded at a density of 5 × 105 cells/well in 1 mL of culture medium in 6-well plates. Serial dilution (10−1–10−6) was performed by adding 100 μL supernatants or the virus to 900 μL DMEM supplemented with 2% FBS to make a 1 to 10 dilution. After the cells were attached, the medium was removed, and 400 μL of the serial dilutions was added to each well. The plates were incubated at 37 °C and 5% CO2 for 2 h and shaken every 15 min to prevent the cells from drying. After the incubation, supernatants were removed, and 2 mL 1% methyl cellulose (Sigma-Aldrich, St. Louis, MO, USA) medium containing with 1% L-glutamine (Cytiva, Chicago, IL, USA), 1%sodium pyruvate (Cytiva, Chicago, IL, USA), 1%sodium bicarbonate (Cytiva, Chicago, IL, USA), 20 mL FBS, 1% HEPES (Cytiva, Chicago, IL, USA) was added to each well. Seven days after incubation, the cells were stained with crystal violet and the plaques were counted to determine the viral titers. The viral titers were calculated using the following formula:
4.6. Detection of Inflammatory Cytokines
The supernatants collected from the virus inhibition assay were analyzed using enzyme-linked immunosorbent assay (ELISA) to detect the expression of cytokines, including IL-6 (Invitrogen, Waltham, MA, USA), IL-8 (Invitrogen, Waltham, MA, USA), TNF-α (Invitrogen, Waltham, MA, USA), IFN-α (Invitrogen, Waltham, MA, USA), and IFN-β (Invitrogen, Waltham, MA, USA). The assay was performed according to the manufacturer’s instructions.
4.7. Statistic
The results are presented as the mean standard error of the mean (mean ± SEM). Statistical analyses were performed using analysis of variance (ANOVA) or the Mann–Whitney test, and the significant differences were marked as * (p < 0.05), ** (p < 0.01), *** (p < 0.001), and **** (p < 0.0001), respectively. All results and statistical calculations were performed using Microsoft Excel 2019 and GraphPad Prism version 9.0.
5. Conclusions
We can conclude that A. cinnamomea may be a potential source for developing antiviral drugs or therapeutics for dengue fever by decreasing the inflammatory response and enhancing antiviral cytokine secretion. These findings provide new insights into the possible use of A. cinnamomea extract as a therapeutic agent against DENV infections.
Author Contributions
Conceptualization, C.-C.C., G.-C.P. and H.-M.L.; methodology, Y.-J.C., Y.-C.T., T.-C.H. and G.-C.P.; validation, T.-C.H., I.P. and G.-C.P.; formal analysis, Y.-J.C., Y.-C.T. and T.-C.H.; investigation, Y.-J.C. and Y.-C.T.; resources, C.-C.C. and H.-M.L.; writing—original draft preparation, Y.-J.C.; writing—review and editing, I.P., G.-C.P. and H.-M.L.; visualization, Y.-J.C., Y.-C.T., T.-C.H., I.P. and C.-C.C.; supervision, G.-C.P. and H.-M.L.; project administration, C.-C.C., G.-C.P. and H.-M.L.; funding acquisition: C.-C.C., G.-C.P. and H.-M.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Science and Technology (MOST), Taiwan, Republic of China (MOST 107-2314-B-006-063-MY3). The funders had no role in the study design, data collection and analysis, decision topublish, or manuscript preparation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Syiherb Biological Technology Co. Ltd., to provide the Antrodia cinnamomea extracts used in this study.
Conflicts of Interest
The authors have declared that no competing interests exist.
References
- Simmons, C.P.; Farrar, J.J.; Nguyenv, V.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Murray, N.E.; Quam, M.B.; Wilder-Smith, A. Epidemiology of dengue: Past, present and future prospects. Clin. Epidemiol. 2013, 5, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Yung, C.-F.; Lee, K.-S.; Thein, T.-L.; Tan, L.-K.; Gan, V.C.; Wong, J.G.; Lye, D.C.; Ng, L.-C.; Leo, Y.-S. Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, Singapore. Am. J. Trop. Med. Hyg. 2015, 92, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Messina, J.P.; Brady, O.J.; Golding, N.; Kraemer, M.U.G.; Wint, G.R.W.; Ray, S.E.; Pigott, D.M.; Shearer, F.M.; Johnson, K.; Earl, L.; et al. The current and future global distribution and population at risk of dengue. Nat. Microbiol. 2019, 4, 1508–1515. [Google Scholar] [CrossRef]
- Ebi, K.L.; Nealon, J. Dengue in a changing climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Hales, S.; de Wet, N.; Maindonald, J.; Woodward, A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 2002, 360, 830–834. [Google Scholar] [CrossRef]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The global distribution and burden of dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Almas, A.; Parkash, O.; Akhter, J. Clinical factors associated with mortality in dengue infection at a tertiary care center. Southeast Asian J. Trop Med. Public Health 2010, 41, 333–340. [Google Scholar]
- Kalayanarooj, S. Clinical Manifestations and Management of Dengue/DHF/DSS. Trop Med. Health 2011, 39, 83–87. [Google Scholar] [CrossRef]
- Puc, I.; Ho, T.C.; Yen, K.L.; Vats, A.; Tsai, J.J.; Chen, P.L.; Chien, Y.W.; Lo, Y.C.; Perng, G.C. Cytokine Signature of Dengue Patients at Different Severity of the Disease. Int. J. Mol. Sci. 2021, 22, 2879. [Google Scholar] [CrossRef]
- Imad, H.A.; Phumratanaprapin, W.; Phonrat, B.; Chotivanich, K.; Charunwatthana, P.; Muangnoicharoen, S.; Khusmith, S.; Tantawichien, T.; Phadungsombat, J.; Nakayama, E.; et al. Cytokine Expression in Dengue Fever and Dengue Hemorrhagic Fever Patients with Bleeding and Severe Hepatitis. Am. J. Trop. Med. Hyg. 2020, 102, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A.; Mathew, A.; Rothman, A.L. Immune-mediated cytokine storm and its role in severe dengue. Semin. Immunopathol. 2017, 39, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Malavige, G.N.; Jeewandara, C.; Ogg, G.S. Dysfunctional Innate Immune Responses and Severe Dengue. Front. Cell. Infect. Microbiol. 2020, 10, 590004. [Google Scholar] [CrossRef] [PubMed]
- Masood, K.I.; Jamil, B.; Rahim, M.; Islam, M.; Farhan, M.; Hasan, Z. Role of TNF α, IL-6 and CXCL10 in Dengue disease severity. Iran. J. Microbiol. 2018, 10, 202–207. [Google Scholar] [PubMed]
- Tough, D.F. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk Lymphoma 2004, 45, 257–264. [Google Scholar] [CrossRef]
- Castillo Ramirez, J.A.; Urcuqui-Inchima, S. Dengue Virus Control of Type I IFN Responses: A History of Manipulation and Control. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2015, 35, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Upasani, V.; Scagnolari, C.; Frasca, F.; Smith, N.; Bondet, V.; Vanderlinden, A.; Lay, S.; Auerswald, H.; Heng, S.; Laurent, D.; et al. Decreased Type I Interferon Production by Plasmacytoid Dendritic Cells Contributes to Severe Dengue. Front. Immunol. 2020, 11, 605087. [Google Scholar] [CrossRef]
- World Health Organization. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; World Health Organization: Geneva, Switzerland, 2009. [Google Scholar]
- Paz-Bailey, G.; Adams, L.; Wong, J.M.; Poehling, K.A.; Chen, W.H.; McNally, V.; Atmar, R.L.; Waterman, S.H. Dengue Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2021. MMWR. Recomm. Rep. Morb. Mortal. Wkly. Report. Recomm. Rep. 2021, 70, 1–16. [Google Scholar] [CrossRef]
- Sridhar, S.; Luedtke, A.; Langevin, E.; Zhu, M.; Bonaparte, M.; Machabert, T.; Savarino, S.; Zambrano, B.; Moureau, A.; Khromava, A.; et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N. Engl. J. Med. 2018, 379, 327–340. [Google Scholar] [CrossRef]
- Halstead, S.B. Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 2017, 35, 6355–6358. [Google Scholar] [CrossRef]
- World Health Organization. Dengue vaccines: WHO position paper—September 2018. Wkly. Epidemiol. Rec. 2018, 93, 457–476. [Google Scholar]
- Troost, B.; Smit, J.M. Recent advances in antiviral drug development towards dengue virus. Curr. Opin. Virol. 2020, 43, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Geethangili, M.; Tzeng, Y.M. Review of Pharmacological Effects of Antrodia camphorata and Its Bioactive Compounds. Evid. -Based Complementary Altern. Med. ECAM 2011, 2011, 212641. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.C.; El-Shazly, M.; Wu, T.Y.; Du, Y.C.; Chang, T.T.; Chen, C.F.; Hsu, Y.M.; Lai, K.H.; Chiu, C.P.; Chang, F.R.; et al. Recent research and development of Antrodia cinnamomea. Pharmacol. Ther. 2013, 139, 124–156. [Google Scholar] [CrossRef]
- Chang, J.-S.; Kuo, H.-P.; Chang, K.L.B.; Kong, Z.-L. Apoptosis of hepatocellular carcinoma cells induced by nanoencapsulated polysaccharides extracted from Antrodia camphorata. PLoS ONE 2015, 10, e0136782. [Google Scholar] [CrossRef]
- Yi, Z.; Liu, X.; Liang, L.; Wang, G.; Xiong, Z.; Zhang, H.; Song, X.; Ai, L.; Xia, Y. Antrodin A from Antrodia camphorata modulates the gut microbiome and liver metabolome in mice exposed to acute alcohol intake. Food Funct. 2021, 12, 2925–2937. [Google Scholar] [CrossRef]
- Lien, H.-M.; Kuo, P.-T.; Huang, C.-L.; Kao, J.-Y.; Lin, H.; Yang, D.-Y.; Lai, Y.-Y. Study of the Anti-Proliferative Activity of 5-Substituted 4,7-Dimethoxy-1,3-Benzodioxole Derivatives of SY-1 from Antrodia camphorata on Human COLO 205 Colon Cancer Cells. Evid. -Based Complementary Altern. Med. Ecam 2011, 2011, 450529. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Cheng, K.-C.; Wang, H.-T.; Hsieh, C.-W.; Lai, Y.-J. Extracts of Antrodia cinnamomea mycelium as a highly potent tyrosinase inhibitor. J. Cosmet. Dermatol. 2021, 20, 2341–2349. [Google Scholar] [CrossRef]
- Senthil Kumar, K.J.; Gokila Vani, M.; Hsieh, H.-W.; Lin, C.-C.; Wang, S.-Y. Antcins from Antrodia cinnamomea and Antrodia salmonea Inhibit Angiotensin-Converting Enzyme 2 (ACE2) in Epithelial Cells: Can Be Potential Candidates for the Development of SARS-CoV-2 Prophylactic Agents. Plants 2021, 10, 1736. [Google Scholar] [CrossRef]
- Lee, I.H.; Huang, R.L.; Chen, C.T.; Chen, H.C.; Hsu, W.C.; Lu, M.K. Antrodia camphorata polysaccharides exhibit anti-hepatitis B virus effects. FEMS Microbiol. Lett. 2002, 209, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.-C.; Tung, Y.-T.; Kuo, Y.-H.; Liao, J.-W.; Tsai, H.-C.; Chong, K.-Y.; Chen, H.-L.; Chen, C.-M. Anti-inflammatory effects of Antrodia camphorata, a herbal medicine, in a mouse skin ischemia model. J. Ethnopharmacol. 2015, 159, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, N.; Baskaran, R.; Velmurugan, B.K.; Thanh, N.C. Antrodia cinnamomea—An updated minireview of its bioactive components and biological activity. J. Food Biochem. 2019, 43, e12936. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; Liu, F.C.; Wu, T.S.; Sheu, M.J. Antrodia cinnamomea Inhibits Migration in Human Hepatocellular Carcinoma Cells: The Role of ERp57 and PGK-1. Am. J. Chin. Med. 2015, 43, 1671–1696. [Google Scholar] [CrossRef]
- Wang, J.J.; Wu, C.C.; Lee, C.L.; Hsieh, S.L.; Chen, J.B.; Lee, C.I. Antimelanogenic, Antioxidant and Antiproliferative Effects of Antrodia camphorata Fruiting Bodies on B16-F0 Melanoma Cells. PLoS ONE 2017, 12, e0170924. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H.; Lin, C.H.; Shih, C.C. Dehydroeburicoic Acid from Antrodia camphorata Prevents the Diabetic and Dyslipidemic State via Modulation of Glucose Transporter 4, Peroxisome Proliferator-Activated Receptor α Expression and AMP-Activated Protein Kinase Phosphorylation in High-Fat-Fed Mice. Int. J. Mol. Sci. 2016, 17, 872. [Google Scholar] [CrossRef]
- Shen, Y.C.; Wang, Y.H.; Chou, Y.C.; Chen, C.F.; Lin, L.C.; Chang, T.T.; Tien, J.H.; Chou, C.J. Evaluation of the anti-inflammatory activity of zhankuic acids isolated from the fruiting bodies of Antrodia camphorata. Planta Med. 2004, 70, 310–314. [Google Scholar] [CrossRef]
- Lin, M.K.; Lee, M.S.; Chang, W.T.; Chen, H.Y.; Chen, J.F.; Li, Y.R.; Lin, C.C.; Wu, T.S. Immunosuppressive effect of zhankuic acid C from Taiwanofungus camphoratus on dendritic cell activation and the contact hypersensitivity response. Bioorganic Med. Chem. Lett. 2015, 25, 4637–4641. [Google Scholar] [CrossRef]
- Shie, P.H.; Wang, S.Y.; Lay, H.L.; Huang, G.J. 4,7-Dimethoxy-5-methyl-1,3-benzodioxole from Antrodia camphorata inhibits LPS-induced inflammation via suppression of NF-κB and induction HO-1 in RAW264.7 cells. Int. Immunopharmacol. 2016, 31, 186–194. [Google Scholar] [CrossRef]
- Wu, K.-H.; Lee, W.-J.; Cheng, T.-C.; Chang, H.-W.; Chen, L.-C.; Chen, C.-C.; Lien, H.-M.; Lin, T.-N.; Ho, Y.-S. Study of the antitumor mechanisms of apiole derivatives (AP-02) from Petroselinum crispum through induction of G0/G1 phase cell cycle arrest in human COLO 205 cancer cells. BMC Complementary Altern. Med. 2019, 19, 188. [Google Scholar] [CrossRef]
- Bierman, H.R.; Nelson, E.R. Hematodepressive Virus Diseases of Thailand. Ann. Intern. Med. 1965, 62, 867–884. [Google Scholar] [CrossRef]
- Oliveira, E.C.; Pontes, E.R.; Cunha, R.V.; Fróes, I.B.; Nascimento, D. Hematological abnormalities in patients with dengue. Rev. Soc. Bras. Med. Trop 2009, 42, 682–685. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-J.; Liu, L.-T.; Chang, K.; Wang, S.-H.; Hsiao, H.-M.; Clark, K.B.; Perng, G.C. The importance of hematopoietic progenitor cells in dengue. Ther. Adv. Hematol. 2012, 3, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Jain, P.; Gangodkar, S.V.; Shetty, S.; Ghosh, K. Dengue 2 virus inhibits in vitro megakaryocytic colony formation and induces apoptosis in thrombopoietin-inducible megakaryocytic differentiation from cord blood CD34+ cells. FEMS Immunol. Med. Microbiol. 2008, 53, 46–51. [Google Scholar] [CrossRef]
- Hsu, A.Y.; Wu, S.R.; Tsai, J.J.; Chen, P.L.; Chen, Y.P.; Chen, T.Y.; Lo, Y.C.; Ho, T.C.; Lee, M.; Chen, M.T.; et al. Infectious dengue vesicles derived from CD61+ cells in acute patient plasma exhibited a diaphanous appearance. Sci. Rep. 2015, 5, 17990. [Google Scholar] [CrossRef] [PubMed]
- Nakao, S.; Lai, C.J.; Young, N.S. Dengue virus, a flavivirus, propagates in human bone marrow progenitors and hematopoietic cell lines. Blood 1989, 74, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Clark, K.B.; Noisakran, S.; Onlamoon, N.; Hsiao, H.-M.; Roback, J.; Villinger, F.; Ansari, A.A.; Perng, G.C. Multiploid CD61+ cells are the pre-dominant cell lineage infected during acute dengue virus infection in bone marrow. PLoS ONE 2012, 7, e52902. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Morishima, Y.; Ohno, R.; Kato, Y.; Hirabayashi, N.; Nagura, H.; Saito, H. Establishment of a novel human megakaryoblastic leukemia cell line, MEG-01, with positive Philadelphia chromosome. Blood 1985, 66, 1384–1392. [Google Scholar] [CrossRef]
- Hausheer, F.H.; Kochat, H.; Parker, A.R.; Ding, D.; Yao, S.; Hamilton, S.E.; Petluru, P.N.; Leverett, B.D.; Bain, S.H.; Saxe, J.D. New approaches to drug discovery and development: A mechanism-based approach to pharmaceutical research and its application to BNP7787, a novel chemoprotective agent. Cancer Chemother Pharm. 2003, 52 (Suppl. 1), S3–S15. [Google Scholar] [CrossRef]
- Baker, D.D.; Chu, M.; Oza, U.; Rajgarhia, V. The value of natural products to future pharmaceutical discovery. Nat. Prod. Rep. 2007, 24, 1225–1244. [Google Scholar] [CrossRef]
- Li, J.W.; Vederas, J.C. Drug discovery and natural products: End of an era or an endless frontier? Science 2009, 325, 161–165. [Google Scholar] [CrossRef]
- Nowak, A.; Kojder, K.; Zielonka-Brzezicka, J.; Wróbel, J.; Bosiacki, M.; Fabiańska, M.; Wróbel, M.; Sołek-Pastuszka, J.; Klimowicz, A. The Use of Ginkgo Biloba, L. as a Neuroprotective Agent in the Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 775034. [Google Scholar] [CrossRef] [PubMed]
- Zheng, E.X.; Navarro, V.J. Liver Injury from Herbal, Dietary, and Weight Loss Supplements: A Review. J. Clin. Transl. Hepatol. 2015, 3, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Alostad, A.H.; Steinke, D.T.; Schafheutle, E.I. International Comparison of Five Herbal Medicine Registration Systems to Inform Regulation Development: United Kingdom, Germany, United States of America, United Arab Emirates and Kingdom of Bahrain. Pharmaceut. Med. 2018, 32, 39–49. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Programme on Traditional, M. WHO Traditional Medicine Strategy 2002–2005; World Health Organization: Geneva, Switzerland, 2002. [Google Scholar]
- Wang, H.-C.; Chu, F.-H.; Chien, S.-C.; Liao, J.-W.; Hsieh, H.-W.; Li, W.-H.; Lin, C.-C.; Shaw, J.-F.; Kuo, Y.-H.; Wang, S.-Y. Establishment of the Metabolite Profile for an Antrodia cinnamomea Health Food Product and Investigation of Its Chemoprevention Activity. J. Agric. Food Chem. 2013, 61, 8556–8564. [Google Scholar] [CrossRef]
- Lin, C.-C.; Kumar, K.J.S.; Liao, J.-W.; Kuo, Y.-H.; Wang, S.-Y. Genotoxic, teratotoxic and oral toxic assessments of Antrodia cinnamomea health food product (Leader Deluxe Antrodia cinnamomea®). Toxicol. Rep. 2015, 2, 1409–1417. [Google Scholar] [CrossRef]
- Diamond, M.S.; Zachariah, M.; Harris, E. Mycophenolic Acid Inhibits Dengue Virus Infection by Preventing Replication of Viral RNA. Virology 2002, 304, 211–221. [Google Scholar] [CrossRef]
- Russell, P.K.; Nisalak, A.; Sukhavachana, P.; Vivona, S. A plaque reduction test for dengue virus neutralizing antibodies. J. Immunol. 1967, 99, 285–290. [Google Scholar]
- Chen, C.-H.; Yang, S.-W.; Shen, Y.-C. New Steroid Acids from Antrodia cinnamomea, a Fungal Parasite of Cinnamomum micranthum. J. Nat. Prod. 1995, 58, 1655–1661. [Google Scholar] [CrossRef]
- Hung-Chen, C.; De-Peng, W.; Cherng, I.W.; Chuen-Her, U. A sesquiterpene lactone, phenyl and biphenyl compounds from Antrodia cinnamomea. Phytochemistry 1995, 39, 613–616. [Google Scholar] [CrossRef]
- Yang, S.-W.; Shen, Y.-C.; Chen, C.-H. Steroids and triterpenoids of Antodia cinnamomea—A fungus parasitic on Cinnamomum micranthum. Phytochemistry 1996, 41, 1389–1392. [Google Scholar] [CrossRef]
- Shen, C.-C.; Kuo, Y.-C.; Huang, R.-L.; Lin, L.-C.; Don, M.-J.; Chang, T.-T.; Chou, C.-J. New Ergostane and Lanostane from Antrodia Camphorata. J. Chin. Med. 2003, 14, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Agassi, S.F.T.; Yeh, T.M.; Chang, C.D.; Hsu, J.L.; Shih, W.L. Potentiation of Differentiation and Apoptosis in a Human Promyelocytic Leukemia Cell Line by Garlic Essential Oil and Its Organosulfur Compounds. Anticancer. Res. 2020, 40, 6345–6354. [Google Scholar] [CrossRef] [PubMed]
- Leneva, I.; Kartashova, N.; Poromov, A.; Gracheva, A.; Korchevaya, E.; Glubokova, E.; Borisova, O.; Shtro, A.; Loginova, S.; Shchukina, V.; et al. Antiviral Activity of Umifenovir In Vitro against a Broad Spectrum of Coronaviruses, Including the Novel SARS-CoV-2 Virus. Viruses 2021, 13, 1665. [Google Scholar] [CrossRef] [PubMed]
- Theerawatanasirikul, S.; Lueangaramkul, V.; Thangthamniyom, N.; Chankeeree, P.; Semkum, P.; Lekcharoensuk, P. Andrographolide and Deoxyandrographolide Inhibit Protease and IFN-Antagonist Activities of Foot-and-Mouth Disease Virus 3C(pro). Animals 2022, 12, 1995. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).