Abstract
The interaction between legume plants and soil bacteria rhizobia results in the formation of new organs on the plant roots, symbiotic nodules, where rhizobia fix atmospheric nitrogen. Symbiotic nodules represent a perfect model to trace how the pre-existing regulatory pathways have been recruited and modified to control the development of evolutionary “new” organs. In particular, genes involved in the early stages of lateral root development have been co-opted to regulate nodule development. Other regulatory pathways, including the players of the KNOX-cytokinin module, the homologues of the miR172-AP2 module, and the players of the systemic response to nutrient availability, have also been recruited to a unique regulatory program effectively governing symbiotic nodule development. The role of the NIN transcription factor in the recruitment of such regulatory modules to nodulation is discussed in more details.
1. Introduction
Plants have adapted to grow in the nutrient poor soil due to beneficial interactions with soil microorganisms, helping plants enhance nutrient acquisition. In particular, the symbiosis of legume plants with soil nitrogen-fixing bacteria rhizobia supplies plants with biologically available nitrogen. Rhizobia colonize the plant root and induce the formation of symbiotic organs on the root, the nodules, where they reside and differentiate into bacteroids to fix atmospheric nitrogen. The legume–rhizobia symbiosis evolved from a more ancient type of plant endosymbiosis with arbuscular mycorrhiza fungi, which form symbiotic associations with most land plants and help them to uptake more nutrients, in particular more phosphorus, from the soil [1]. In addition to legumes, the non-legume genus Parasponia (Cannabaceae) also forms symbiotic nodules with rhizobia [2]. A Parasponia–rhizobia symbiosis has been suggested to be evolutionary young and less specialized in comparison to the legume–rhizobia symbiosis. Parasponia nodules develop as modified lateral roots with a central vascular bundle and infected cells in the peripheral zone [2]. In addition, a set of species from the Fagales, Cucurbitales, and Rosales orders (collectively known as actinorhizal plants) are able to form the nitrogen-fixing symbiosis with the actinobacterial genus Frankia, and their symbiotic organs represent modified lateral roots (LRs) [3]. Plant species capable of forming the nitrogen-fixing symbiosis form so-called nitrogen-fixing clade [4].
In contrast to symbiotic nodules formed by Parasponia and actinorhizal plants, developing as modified lateral roots with centrally located vascular bundle, symbiotic nodules of legume plants have a more specialized structure. They represent spherical or cylindrical lateral organs with two or more peripheral vascular bundles converging towards the apical part of the nodule. Nevertheless, a set of data suggests that both symbiotic nodules and lateral roots share common regulators, and the players of the root developmental program have been recruited to control nodule organogenesis (for review see [5]). In addition, other regulatory pathways participate in symbiotic nodule development making the nodule a unique lateral organ. The NIN (NODULE INCEPTION) gene is specifically induced by rhizobia in inoculated plant roots. It encodes a key transcription factor activating diverse regulatory modules during nodulation [6,7]. In this review, we discuss recently published data on the regulatory modules which have been recruited to nodule organogenesis from other conserved developmental pathways. By “regulatory module”, we mean two or more interacting regulators, i.e., an activator/repressor and its target that could be considered as a single homologous “block” present in different regulatory pathways. Here, we provide a view on the program of symbiotic nodule development as a network of conserved regulatory modules, whose players have well-defined roles in other developmental pathways beyond nodulation. Our review focuses on some striking examples of such regulatory modules which include the players of the root developmental program, the homologues of shoot apical meristem regulators and regulators of flowering transition, as well as systemic regulators of nutrient responses. The recruitment of these pathways to nodulation during the evolution of legumes might have ensured the efficient and fine-tuned control of symbiotic nodule development by a host plant.
2. How Rhizobia Govern the Developmental Programs in Legumes: NIN as a Key Regulatory Hub in Nodulation
The formation of nodules is generally initiated by a signaling pathway triggered by Nod-factors—lipochito-oligosaccharide signal molecules secreted by rhizobia (see Figure 1). Perception of Nod-factors by plant receptors induces a signaling cascade, including a calcium-/calmodulin-dependent kinase (CCaMK) [8], which phosphorylates a coiled-coil transcription factor MtIPD3 (INTERACTING PROTEIN OF DMI3)/LjCYCLOPS [9,10,11,12]. In its turn, MtIPD3/LjCYCLOPS activates the expression of the NODULE INCEPTION (NIN) gene [13] (see Figure 1) which encodes a key transcription factor controlling the subsequent steps of nodulation by regulating both epidermal infection and nodule organogenesis (for review see [7]). A set of components of the Nod-factor signaling cascade, including CCaMK and MtIPD3/LjCYCLOPS, is involved in arbuscular mycorrhizal symbiosis establishment as well [10,11,12]. In this regard, they are considered to be part of a common symbiotic (SYM) signaling pathway [1]. Transcription factors (TFs) of the GRAS family, NSP1 and NSP2 (NODULATION SIGNALING PATHWAY) operate downstream of CCaMK and activate the expression of other genes necessary for the legume–rhizobia symbiosis [14,15] (Figure 1). A NSP1–NSP2 complex, where NSP1 is a DNA binding protein, whereas NSP2 lacks a DNA binding domain, binds to a specific promoter region of rhizobia-induced genes, present in the NIN gene, in particular [14]. In addition to their role in the legume–rhizobia symbiosis, NSP1 and NSP2 are also involved in the arbuscular mycorrhizal symbiosis [16,17], where they regulate strigolactone biosynthesis required for mycorrhization [18].
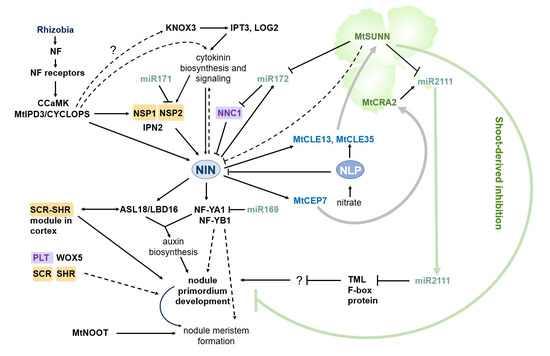
Figure 1.
Regulation of symbiotic nodule development in legumes. Rhizobia produce Nod-factors, which activate a signaling cascade leading to phosphorylation of the MtIPD3/LjCYCLOPS TF by CCaMK. MtIPD3/LjCYCLOPS and cytokinin-dependent pathway activate the expression of the NIN gene and other genes involved in the legume–rhizobia symbiosis. NSP1 and NSP2 TFs act downstream of MtIPD3/LjCYCLOPS, and NSP1 is able to bind to the promoter of the NIN gene. A MYB coiled-coil type transcription factor IPN2 (Interacting Protein of NSP2) interacts with NSP1 and NSP2 to activate NIN expression [23,24]. The NSP2 transcripts are post-transcriptionally regulated by miR171. The KNOX3 TF activates cytokinin biosynthesis genes, IPT3 and LOG2, during nodulation, and other factors contribute to activation of cytokinin biosynthesis at the very early stages of nodulation. The NIN TF directly induces the expression of NF-YA1 and NF-YB1 which regulate nodule development and emergence, as well as nodule meristem formation. NF-YA1 is targeted by miR169. NIN also activates the ASL18/LBD16 gene involved in the LR developmental program. ASL18/LBD16 forms complex with the NF-Y transcription factors to regulate both LR and nodule development by activating auxin biosynthesis. Other components of the root developmental program, including the PLT, SCR, SHR, and WOX5 TFs are also induced during nodule primordia development. MtNOOT1 and MtNOOT2 establish and maintain indeterminate nodule identity. NIN upregulates the level of miR172 which negatively regulates NNC1. NNC1 is а transcriptional repressor interacting with NIN and inhibit NIN activity. Both miR172 and NIN expression levels are negatively regulated by the autoregulation of nodulation (AON). NIN directly activates the expression of the CLE genes, including MtCLE13 in M. truncatula. In addition to NIN, the NLP TF activates the expression of the nitrate-induced CLE genes (MtCLE35 in M. truncatula) in response to the nitrate. The CLE peptides are produced in the root in response to rhizobia inoculation and the nitrate treatment and move to the shoot where they are recognized by their receptors, including the CLV1-like receptor kinase (MtSUNN in M. truncatula). Activation of the CLV1-like receptor kinase in the shoot triggers a negative feedback response, which inhibits subsequent nodulation on the root. MtCEP7 expression is also upregulated by NIN, and it positively regulates nodule development. The CEP peptides involved in nodulation are suggested to move from the root to the shoot, where they are recognized by their receptor (MtCRA2 in M. truncatula). MtCRA2-dependent signaling cascade upregulates and MtSUNN-dependent signaling cascade downregulates miR2111, which is a mobile miRNA transported from the shoot to the root. The TML transcripts, encoding an F-box-containing protein which negatively regulates nodulation, are the targets of miR2111 in the root. The GRAS TFs are highlighted by orange rectangles, the AP2/ERF TFs are highlighted by violet rectangles.
Activation of cytokinin biosynthesis genes and subsequent cytokinin accumulation are observed early in response to rhizobial inoculation/Nod-factor treatment [19,20,21]. Cytokinin is a key positive regulator of cortical cell division leading to nodule primordium development, and at the same time, it negatively regulates rhizobial infection (for review see [22]). Despite the fact that much knowledge has been accumulated on cytokinin biosynthesis and response gene action during nodulation in legume plants (see [22]), we still do not know definitively which factor activates cytokinin biosynthesis and response genes at the very early stages of Nod-factor-induced signaling cascade.
Downstream of Nod-factor-induced signaling cascade and cytokinin, the expression of the NIN gene is activated [14] (see Figure 1). NIN is a key TF in nodulation, and it acts as a regulatory hub coordinating different regulatory pathways in nodulation [6,7]. In general, two spatially distinct programs are activated after the initial step of Nod-factor recognition by plant receptors: an epidermal program associated with the rhizobial infection and nodule primordium formation from pericycle and root cortical cells (for review see [25]). NIN regulates both of these programs. The expression of NIN is observed in the epidermis, where infection takes place, and also in the inner cell layers, including the pericycle and cortex cells, where nodule primordium is initiated [26]. Spatiotemporal pattern of NIN expression is determined by the presence of different cis-regulatory elements in its promoter [13,26]. NIN expression in the epidermal cells is essential for infection thread formation [27]. In M. truncatula, the CYCLOPS-binding site in the NIN promoter located about 3 kb upstream of the start codon drives NIN expression in epidermis, and its deletion diminishes infection thread formation [26]. A distal promoter element including putative cytokinin response regulators (RRB) binding sites (named the CE region) is responsible for the induction of NIN expression in the inner cell layers necessary for nodule primordium formation is required [26]. The presence of the CE region in the NIN promoter was found to be specific for NIN-related genes in legumes, whereas the CYCLOPS-binding sites occur in the NIN promoters of legumes and non-legume nodulating plants, including Parasponia and actinorhizal plant Casuarina [7]. Cytokinin-dependent regulation of NIN expression is suggested to be legume-specific. Therefore, the occurrence of the CE region in the distal promoter of the NIN gene might be considered as an evolutionary important event leading to the legume–rhizobia symbiosis establishment [7,26].
Initially, NIN was identified as a nodulation-specific TF [27,28], and later the homologues of NIN belonging to a large family of NIN-like proteins (NLP) were described in both legume and non-legume plants as important regulators of nitrate-inducible gene expression [7,29]. NLP TFs possess the RWP-PK DNA binding domain in their C-terminal region which binds to NRE (nitrate-responsive cis-element) in the target genes [29]. The N-terminal region of the NLP proteins is responsible for the activation of gene expression in response to the nitrate [30]. In A. thaliana, AtNLP7 is accumulated in the nucleus in the presence of the nitrate, where it binds to NRE and activates the expression of nitrate-responsive target genes. Such activation and nuclear accumulation of AtNLP7 in response to the nitrate is mediated by phosphorylation of Ser-205 in AtNLP7 by Ca2+-sensor protein kinases (CPKs), which are induced by calcium signaling triggered by the nitrate [30,31]. In contrast to other NLP proteins, NIN does not respond to the nitrate signal, and such a loss of nitrate-responsiveness was suggested to be an important evolutionary event necessary for the establishment of the legume–rhizobia symbiosis [32]. According to phylogenetic and microsynteny analysis, NIN orthologues are present in all the nodulating species as well as in the genomes of species outside the nitrogen-fixing clade [33]. However, in the genomes of non-nodulating species from the nitrogen-fixing clade, multiple independent evolutionary events having led to the loss of NIN function in non-nodulating species were found [33]. This highlights the significance of NIN as a master regulator necessary for the successful nitrogen-fixing symbiosis establishment.
It was found that the NIN and NLP proteins are capable of binding to the same cis-regulatory elements in the target genes [34]. Moreover, NIN and NLP interact through their N-terminal PB1 domains to form heterodimers on cis-regulated DNA regions [34,35]. Despite of fact that that binding sites for the NIN and NLP TFs generally overlap and share a consensus sequence pattern with semi-palindromic structures, in L. japonicus, NIN and NLP have different DNA-binding specificities, where the NIN-specific binding sites were found to be less palindromic [34]. In L. japonicus, LjNLP4–LjNIN interaction reduces the chances of DNA binding by the LjNIN homodimers, and NLP could reduce the expression of the NIN-activated target genes. Therefore, in the presence of the nitrate, NLPs are accumulated in the nucleus and reduce NIN-mediated activation of symbiosis-related target genes, which could account for the nitrate-mediated inhibition of the symbiosis [34]. On the other hand, NIN alone has a weaker ability to induce the expression of nitrate-responsive genes activated by the NLP homodimers, whereas the formation of the NIN–NLP heterodimers reduced the induction level of nitrate-dependent gene expression brought by NLP [34]. However, among the NIN and NLP target genes, the CLE (CLV3/EMBRYO-SURROUNDING REGION) genes involved in autoregulation of nodulation contain both the NLP-specific and NIN-specific binding sites, and, therefore, they are upregulated by both the nitrate and rhizobia-induced signaling cascade (see Section 6). Therefore, the NIN-binding sites might have evolved as modified NRE, and the occurrence of new NIN-specific binding sites in the target gene promoters resulted in their recruitment to the nodulation program and accompanied the establishment of the legume–rhizobia symbiosis.
The NIN TF regulates the expression of a set of genes involved in nodule development (see Table 1, Figure 1). Among the direct targets of NIN, there are: RPG (RHIZOBIUM-DIRECTED POLAR GROWTH), NPL (NODULATION PECTATE LYASE1) and other genes involved in infection thread formation [6,36], the genes encoding the NF-YA1 and NF-YB1 (Nuclear Factor-Y A1 and B1) TFs which are essential for both infection and nodule primordia development [37,38], the CRE1 (CYTOKININ RESPONSE 1) gene encoding cytokinin receptor in M. truncatula [39], the LBD16 (LOB-DOMAIN PROTEIN 16) gene [40,41], as well as microRNA miR172c [42]. Moreover, NIN directly activates the expression of the CLE genes, encoding regulatory peptides inhibiting nodulation by a shoot-dependent negative feedback mechanism [43]. At the same time, NIN activates the expression of the MtCEP7 gene encoding a regulatory peptide of the CEP (C-TERMINALLY ENCODED PEPTIDEs) family that positively regulates nodule formation [44].

Table 1.
The targets of the NIN transcription factor involved in the control nodule organogenesis.
3. Players of the Root Developmental Networks in Nodule Organogenesis
Nodule primordium formation is initiated in the inner cell layers of the root. The cell layers where the initial cell divisions take place differ between the two types of symbiotic nodules: determinate and indeterminate ones (see [66]). In plants with indeterminate nodules (such as Medicago truncatula, pea and clover), initial cell divisions are observed in pericycle cells followed by the reactivation of the endodermal and inner cortex cells [67,68]. Specifically, in M. truncatula, the meristem of nodule primordia is derived from the third cortical cell layers, whereas the inner cortical cells, the endodermal, and pericycle cells contribute to the basal part of symbiotic nodule [68]. In plants with the determinate type of nodules (Lotus japonicus, soybean and common bean), cell divisions occur in the middle or outer cortex cells, leading to the formation of determinate nodules with a spherical shape that lack a persistent nodule meristem (see [66]).
Reactivation of cell divisions in the root also takes place during lateral root (LR) primordium development. In model species A. thaliana and other members of Brassicaceae family, only pericycle cells are mitotically activated during LR primordium formation. In the majority of other plant species, analyzed by Xiao et al., cortical and endodermal cell divisions were observed during LR formation [69]. Moreover, in M. truncatula, pea, and Cucurbitaceae species the cells derived from endodermis and cortex contribute to the LR primordium [69,70,71].
Auxin is a key hormone which triggers LR primordium formation. The LR founder cells, a group of the xylem pericycle cells where first divisions leading to LR primordium development occur, are characterized by an enhanced auxin maximum. Local auxin accumulation in the pericycle cells of Arabidopsis is required for LR formation [72]. Auxin activates the expression of key regulators of LR primordium initiation, including genes for the GATA23 and LBD16 TFs [73,74]. Genes from the auxin-regulated transcriptional network encoding WUSCHEL-RELATED HOMEOBOX 5 (WOX5) [75,76], the PLETHORA (PLT) TFs [77,78], and SHORTROOT (SHR) [79,80] known as regulators of the root apical meristem (RAM) are also induced during LR formation.
Auxin also was found to play a crucial role in nodulation by controlling both rhizobial infection and cell divisions during nodule primordia formation (for review, see [81]). Exogenous auxin treatment (specifically, 2,4-D) is able to induce nodule-like structures (NLS) in the root in both legume and non-legume plants [82,83,84,85]. Auxin-induced NLS in rice demonstrated the enhanced induction of the PLT, WOX5, and WOX11 genes involved in the lateral root developmental program, whereas rice orthologs of the nodulation-related NSP2 gene and other players of nodule symbiotic pathway were not activated in auxin-induced NLS in rice [86]. Therefore, auxin-induced NLS are common for both nodulating and non-nodulating species, and the ability of exogeneous auxin to induce nodule-like structure does not underlie the specific cellular response of legume plants to rhizobia.
3.1. Genes Controlling the Initiation of Lateral Root Development and Their Role in Nodule Organogenesis
In A. thaliana, a set of transcriptional regulators is required for the early steps of lateral root (LR) primordium initiation. Among them, there are the GATA23 and LATERAL ORGAN BOUNDARIES-DOMAIN 16 (LBD16)/ASYMMETRIC LEAVES2-LIKE 18 (ASL18) TFs, which are activated by the auxin signaling module in the LR founder cells [73,74]. The LBD/ASL proteins belong to the AS2 (ASYMMETRIC LEAVES2)/LOB (LATERAL ORGAN BOUNDARIES-DOMAIN) family, and they are involved in various aspects of plant development playing a crucial role in defining organ boundaries [87]. LBD16/ASL18 is activated specifically in the LR founder cells by AUXIN RESPONSE FACTOR (ARF) 7 and ARF19, and its activation is required for the establishment of the asymmetry in the LR founder cells before cell division [88].
Recently, the LBD16/ASL18 gene was found to be important for the early steps of nodule primordium formation in both M. truncatula and L. japonicus [40,41]. The expression of the LBD16/ASL18 gene was observed at the sites of both LR and nodule initiation, and its expression was induced by auxin [41]. In L. japonicus, knock-out of the LBD16/ASL18 gene resulted in lower LR densities and reduced number of nodules with reduced size [40]. In M. truncatula, roots overexpressing LBD16 demonstrated extensive root curling and initiation of ectopic root primordia [41]. Co-overexpression of the LjLBD16/LjASL18 and NF-Y subunit genes, which are also known as the direct targets of NIN, increased lateral root densities and also resulted in ectopic cell divisions in the root leading to bump formation [40]. LjLBD16/LjASL18 is able to interact with NF-Y transcription factors both in vivo and in planta [40], suggesting that LBD16/ASL18 could act in a complex with the NF-Y transcription factors to promote both lateral root and nodule development.
Initially, the MtNF-YA1 gene (previously known as MtHAP2-1) was described in M. truncatula as an essential regulator of nodule meristem development and rhizobial infection [38,46]. In L. japonicus, the roles of the NF-YA1 and NF-YB1 genes in nodule development have also been reported, and these genes regulate LR development as well [37]. LjNF-YA1 stimulates the expression of the SHORT INTERNODES/STYLISH (STY) genes encoding transcription factors which are required for nodule emergence and differentiation and which activate the expression of auxin biosynthesis genes [89,90]. Moreover, the miR169-regulated AtNF-YA2 and AtNF-YA10 genes in A. thaliana are involved in root growth and LR development, suggesting that the miR169-NF-YA module is a basic regulator of the root developmental program [47]. Interestingly, miR169 also might be involved in long-distant signaling in plant for reporting P or N status: miR169 was found in rapeseed (Brassica napus) phloem sap and its abundance decreased during N and P limitation and significantly increased during N-replete growth [91]. Therefore, it was suggested that miR169 could also be involved in the systemic regulation of nodulation depending on nitrogen availability (see also Section 6).
In L. japonicus and M. truncatula, the expression of LBD16/ASL18 in response to rhizobia inoculation is activated by the NIN TF, indicating that the NIN transcription factor is responsible for the recruitment of the LBD16/ASL18 gene to the nodule organogenesis program. Soyano et al. found the NIN-binding sites (NBS) located in the ASL18 introns (one NBS-S1 in the LjASL18b gene or both NBS-S1 and NBS-S2 in the LjASL18a gene) [40]. Importantly, NBS-S1 and its flanking sequences were found to be conserved in legume plants, but were not observed in the LBD16/ASL18 genes in non-legume plants [40]. Therefore, the occurrence of NBS in the LBD16/ASL18 genes in legume plants might play an important role during the evolution of the legume–rhizobia symbiosis, and recruitment of LBD16/ASL18, a LR developmental regulator, to nodule signaling pathway by the NIN TF is responsible for the activation of cell divisions in root cortex, which makes possible nodule primordia formation [40,41].
3.2. Genes Controlling Root Apical Meristem Development and Maintenance and Their Role in Nodule Organogenesis
In the developing and mature symbiotic nodules, the activity of regulators involved in root apical meristem (RAM) development and maintenance has also been reported [92,93,94,95]. In the RAM, the stem cell niche is maintained due to the activity of a set of TFs, which act in tight connection with auxin, including the WUSCHEL-RELATED HOMEOBOX 5 (WOX5), PLETHORA (PLT), SHORTROOT (SHR), and SCARECROW (SCR) proteins. The WOX5 gene, which encodes a homeodomain-containing TF, is expressed in the quiescent center of the RAM, and its activity is required for root stem cell maintenance and inhibition of stem cell differentiation [75]. In A. thaliana, WOX5 regulates auxin biosynthesis in the quiescent center [96], and auxin maximum in the RAM is known to determine the position of the stem cell niche [97]. Auxin also regulates the expression of four PLT genes encoding TFs that belong to the AINTEGUMENTA-LIKE (AIL) subgroup of the APETALA2/Ethylene Responsive Factor (AP2/ERF) family [77]. The PLT TFs are required for RAM maintenance and root stem cell niche formation. The PLT proteins form a gradient in the root apex, which is essential for proper cell proliferation and differentiation in the apical part of the root, and their highest concentration is observed in the root stem cell niche [98]. PLTs were shown to control the expression of the PIN genes, required for the proper auxin distribution in the root, as well as other regulators of RAM, including the SHR gene encoding a TF from the GRAS family [99]. SHR regulates the expression of the SCR gene, which also encodes a GRAS TF, and they both are required for RAM development and maintenance as well as for the proper radial patterning of the root [100].
In M. truncatula, homologues of the WOX5, PLT, SHR, and SCR TFs were shown to participate in the nodule developmental program [92,93,94,95]. The expression of the WOX5 gene was observed in the nodule primordia and, at the later stages, in the tips of vascular bundles of mature nodules [92]. Moreover, it was shown that the tips of the nodule vascular bundles also exhibit the maximum of auxin response visualized by DR5 reporter activity [101]. In addition, other homologues of RAM regulators, including MtPLT1 and MtPLT2, are also expressed in these sites [94]. The regions corresponding to the tips of vascular bundles in the nodule apex are referred to as the nodule vascular meristem (NVM) [94]. Based on the expression patterns of the specific markers of the “quiescence center”, it could be speculated that the NVM represents a domain similar to the organizing center of RAM. Interestingly, the RAM-specific nature of the NVM is also manifested by the phenotype of M. truncatula and pea mutants defective in the MtNOOT1,2/PsCOCH genes, which develop ectopic root from the NVM [102,103,104]. In M. truncatula, the Mtnoot1 noot2 double mutant demonstrated the complete loss of nodule identity with nodule-to-root homeosis [104]. Therefore, the MtNOOT1,2 and PsCOCH genes control the activity of the NVM and define nodule identity. In addition to its role in the NVM, the MtNOOT1 gene also controls the size of the RAM by defining the position of the boundary region between the apical meristem and differentiation zone [105]. The primary roots of the Mtnoot1 mutant demonstrated delayed xylem cell differentiation suggesting that MtNOOT1 promotes root vasculature differentiation [104].
The close homologues of the MtNOOT1,2/PsCOCH genes in A. thaliana are the BLADE-ON-PETIOLE 1 (BOP1) and BOP2 genes encoding co-transcriptional factors suppressing meristematic activity in the developing lateral organs as well as other developmental processes related to plant organ boundary regulation [106]. It was reported that BOP1 and BOP2 positively regulate the expression of the LBD/ASL genes and downregulate the expression of the KNOX genes in the developing leaf primordia, which encodes homeodomain transcription factors necessary for shoot meristem maintenance [106]. Interestingly, in M. truncatula, among three MtKNOX genes upregulated during nodulation, the expression levels of MtKNOX9 and MtKNOX3 were downregulated in the Mtnoot1/Mtnoot1 noot2 nodules, whereas MtKNOX5 expression was induced [104]. Therefore, like BOPs in A. thaliana, their homologues in M. truncatula also regulate the expression patterns of the KNOX genes. Since the homologues of the LBD/ASL genes are also involved in symbiotic nodule development, it would be interesting to study whether NOOT-BOP-COCH-LIKE (NBCL) could also regulate their expression in the developing nodule.
The involvement of root apical meristem-related genes in nodulation was shown for M. truncatula and pea, which form nodules of the indeterminate type with persistent meristem. The roles of these genes in the development of determinate nodules have not been studied yet. Interestingly, the mutation in LjNBCL1, an ortholog of the NBCL gene in L. japonicus, also results in ectopic roots arising from the NVM, suggesting that the NBCL genes function in both indeterminate and determinate nodules through the maintenance of nodule vascular bundle identity [107]. A similar phenotype was also observed when Phaseolus vulgaris plants, forming determinate nodules, were inoculated by rhizobia strains having mutations in the bacterial genes controlling lysine, purine, and pyrimidine biosynthesis [108]. Therefore, the suppression of the lateral root identity program in the cells of the NVM can be overpassed in both indeterminate and determinate types of nodules, which leads to the formation of ectopic roots from the developing nodule.
3.3. Endodermal and Cortical Cell Fate Regulators in Nodule Organogenesis
In addition to their role in root apical meristem, the SCR and SHR TFs are key regulators of the root radial patterning that are responsible for the cortical and endodermal cell differentiation in the root [100]. In Arabidopsis, the SHR gene is expressed in the stele cells of the root, and its protein product moves to the adjacent cell layers, including endodermis, quiescent center, the cortical endodermal initials, and cortical endodermal daughter cells, where the SHR TF activates the expression of the SCR gene. SHR together with SCR activates the expression of a D-type cyclin gene, CYCD6;1, which was shown to promote the asymmetric formative cell divisions separating the ground tissue into two different cell layers, the endodermal and cortical ones [109]. Ectopic SHR expression under the SCR promoter increased the number of endodermal cell layers in Arabidopsis [110]. Recently, it was shown that the SHR–SCR regulatory module is essential for legume-specific cortical cell divisions during nodulation [95].
In contrast to Arabisopsis, in M. truncatula and other legumes, the SCR gene is expressed not only in endodermis, but also in the root cortical cells and to a lesser degree in the root epidermal cells. Moreover, the accumulation of the SHR protein was also observed in the cortical cells in legumes [95]. The presence of SCR expression in the root cortex was associated with the occurrence of two cis-regulatory elements located close to each other only in the SCR promoters from legumes, but not in the ones from the species outside the nitrogen fixing clade. Furthermore, in M. truncatula the homologues of both the SHR and SCR genes are required for cortical cell divisions leading to nodule development, where the MtSHR1/2 protein levels are increased in the epidermal and cortical cells in response to rhizobial inoculation, and MtSHR1/2 subsequently activates the expression of the MtSCR gene [95]. MtSHR1 overexpression induces cortical cell divisions leading to the formation of nodule-like structures without rhizobial inoculation, which requires the functional MtSCR gene, but does not depend on the Nod-factor signaling components including NSP1, NSP2, and NIN. The SCR–SHR activity in the root cortex of legume plants was shown to be prerequisite for rhizobia-induced cortical cell divisions, as well as for the formation of nodule-like structures induced by cytokinin treatment or the ectopic expression of the NIN gene, suggesting that the SCR–SHR regulatory module acts downstream of cytokinin and NIN in nodulation (see Figure 1). These data suggest the involvement of the root-specific SCR–SHR regulatory module in the early steps of nodule primordium development which was recruited for the nodulation due to the broader patterns of SCR and SHR expression in legume roots encompassing the cortical and epidermal cells [95].
4. Cytokinin and the Homologues of Shoot Apical Meristem Regulators in Nodule Organogenesis
Cytokinin is as a key hormone stimulating shoot apical meristem development and maintenance (see [111]). In the shoot apical meristem, cytokinin amount is increased due to the local activation of the cytokinin biosynthesis genes by class I KNOX transcription factors [112,113]. Cytokinin is also a key positive regulator of nodule primordium development. Exogenously applied cytokinin stimulates the formation of nodule-like structures on the roots of legume plants [114,115]. In addition, a gain-of-function mutation in cytokinin receptor genes resulted in spontaneous nodule formation [116,117]. Cytokinin-response regulators activate the expression the NSP2 and NIN genes, which are also induced by the rhizobia-induced signaling cascade, suggesting the convergence of the cytokinin and symbiosis-induced pathway [26,118]. A key role of cytokinin in nodule organogenesis has been supported thoroughly by a set of genetic studies (for review see [22]).
At the same time, cytokinin is considered to be a negative regulator of LR development. Mutants with reduced cytokinin production and response possessed larger number of LRs [50,51], whereas exogenous cytokinin application represses LR initiation, influencing cell division pattern in the root [51,119]. Moreover, the increased cytokinin response is observed between developing LR primordia, suggesting that cytokinin is important for LR spacing along the main root, mediating lateral inhibition of new LR primordia emergence in the adjacent root pericycle cells [120]. However, in contrast to LR development and other developmental processes, such as shoot apical meristem development and maintenance where cytokinin and auxin act antagonistically [121], these two hormones cooperatively regulate nodule primordia development in legume plants.
Like auxin, exogenous cytokinin application was shown to induce nodule-like structures [114,115]. However, unlike auxin, the cytokinin application induces NLS only in nodulating species, including legume plants and Parasponia [122]. The ability of exogenously applied cytokinin (specifically, 6-BA) to induce cortical cell divisions was suggested to be an important feature underlying the ability of a plant to form root nodules in response to the signals produced by rhizobia [122]. In several studies, pseudonodules induced by cytokinin demonstrate the expression of the early nodulation genes, like ENOD40 [123,124] and NIN [115]. Moreover, L. japonicus mutants defective in NIN were not able to form pseudonodules in response to cytokinin treatment, which positions cytokinin action upstream of this symbiotic regulator in the control of NLS formation and highlights its requirement for the cytokinin-mediated activation of root cortical cells [115]. Therefore, cytokinin is an important positive regulator of nodule primordium development, and its action determines the specificity of the nodulation program in legume plants, whereas auxin and auxin-regulated transcriptional regulators are common for both lateral root and nodule primordium development.
Cytokinin biosynthesis gene LjIPT2 (ISOPENTENYL TRANSFERASE 2) and LjLOG4 (LONELY GUY 4), which regulate the production of active cytokinin bases from their nucleotide precursors, as well as MtIPT2 and MtIPT4 in M. truncatula, were found to be induced early in response to rhizobial inoculation/Nod-factor treatment [19,20,21]. In L. japonicus, LjIPT2 and LjLOG4 were found to contribute to the first rapid cytokinin accumulation in root cortex leading to nodule primordia development [21]. In addition to this first wave of cytokinin biosynthesis in the root cortex, activation of other members of IPT and LOG families occurs later during nodule primordium development [125,126,127], which is important for the subsequent stages of nodule organogenesis. Among these genes, there are MtIPT3 and MtLOG2 in M. truncatula, and for them the direct activation by the KNOX3 (KNOTTED1-LIKE HOMEOBOX 3) TF has been suggested in our previous studies [126,128]. The KNOX proteins are homeodomain containing TFs from the TALE (Three amino acid loop extension) superclass of homeobox proteins, which are subdivided into two classes, class I and class II [129]. The class I KNOX TFs are the key regulators of shoot apical meristem development and maintenance [130]. They maintain SAM (shoot apical meristem) activity by inducing the expression of the IPT genes [112,113]. This leads to a local increase of cytokinin in the SAM, where cytokinin is known as the key regulator of cell proliferation [131,132]. The class II KNOX genes are broadly expressed in differentiating tissues and mature organs, being suggested to play the roles antagonistic to the action of the class I KNOX genes [133]. MtKNOX3 is a member of the class II KNOX TFs in M. truncatula [134]. Previously, we found that it is involved in the symbiotic nodule development by stimulating the expression of cytokinin biosynthesis genes during nodulation [128]. Down-regulation of the MtKNOX3 gene by RNAi resulted in a decreased expression of MtLOG2 and MtIPT3, as well as the MtRR4 gene, a cytokinin response gene in M. truncatula [128]. Moreover, we found that the MtKNOX3 homeodomain directly bound to the MtIPT3 and MtLOG2 regulatory sequences in vitro [126], suggesting the direct activation of cytokinin biosynthesis genes by the MtKNOX3 TF. Together these findings suggest that MtKNOX3, a class II KNOX TF, is involved in the KNOX-cytokinin regulatory module as it was previously shown for the class I KNOX TFs acting in the SAM. Interestingly, the KNOX-IPT regulatory module was also found to be involved in sporophyte axis extension in the moss Physcomitrella patens, and it was suggested that such pre-existing KNOX-cytokinin regulatory module was recruited later into vascular plant shoot meristems during evolution [135]. In this connection, the KNOX3 TF with its IPT3 and LOG2 target genes involved in cytokinin biosynthesis provides an example of “shoot-related” conserved regulatory module that has been co-opted to regulate cytokinin biosynthesis in symbiotic nodules.
It should be mentioned that in contrast to our findings, Di Giacomo et al. proposed an alternative role of MtKNOX3 and other class II KNOX TFs in nodulation [136]. In the study by Di Giacomo et al., the simultaneous down-regulation of the MtKNOX3 gene and three other class II MtKNOXs (MtKNOX5, 9, 10) resulted in the decreased expression of cytokinin response gene MtRR4 [136], as it was observed for MtKNOX3-RNAi by Azarakhsh et al., 2015 [128]. However, in addition to this, the expression of the MtEFD (ETHYLENE RESPONSE FACTOR REQUIRED FOR NODULE DIFFERENTIATION) gene was also decreased in the transgenic roots with down-regulation of class II MtKNOXs. The MtEFD transcription factor is a negative regulator of nodulation known to be responsible for the activation of the MtRR4 gene [137]. MtRR4 is a primary cytokinin-activated gene, and at the same time, it is a member of the Response Regulator A (RRA) gene family, encoding negative regulators of cytokinin signaling [138]. This allowed the authors to suggest that MtKNOX3 together with other class II KNOX TFs inhibited cytokinin signaling through the activation of the EFD/RR4 regulatory module playing a negative role in nodulation [136]. However, the direct activation of the MtEFD gene by class II KNOX TFs has not been studied yet. Therefore, these data suggest a more complex role of MtKNOX3 and other class II MtKNOX TFs during nodulation. It should be noted that cytokinin itself have multiple functions in nodulation. In addition to its positive roles in nodule primordium development, cytokinin negatively regulates infection thread formation in the root epidermis [139] and is also involved in later stages of nodulation and nitrogen fixation [140,141]. Moreover, cytokinin was found to mediate shoot-derived inhibition of nodulation in L. japonicus [142].
Cytokinin was found to be important for NIN activation in legume plants, and the presence of the CE region in the NIN promoter was found to be specific for NIN-related genes in legumes [7,26]. In addition, it was found that NIN itself activates the cytokinin signaling in the root. NIN directly binds to the promoter of cytokinin receptor gene CRE1 and activates its expression in the cortex [39]. Therefore, NIN is also responsible for the increasing of cytokinin response in rhizobia-inoculated roots, which may account for the activation of cell divisions leading to nodule primordium development.
5. Homologues of Flowering-Related Genes and Their Role in Nodule Organogenesis
The NIN TF is also involved in the evolutionary conserved regulatory module known as NIN- miRNA172- NNC1 [42] (see Figure 1). NIN activates the expression of miR172c [42], a member of the evolutionary conserved microRNA family, which is known to target APETALA2 (AP2) and the homologous genes—well-known regulators of flowering time and flower development [53]. In soybean, miR172c is upregulated in response to rhizobia inoculation and nodule development, and its overexpression increased the number of the infection foci and the nodule primordia [42].
The target of miR172c is the NNC1 (NODULE NUMBER CONTROL 1) mRNA encoding an AP2-like transcriptional repressor. Its closest homologue in A. thaliana is TOE1 (TARGET OF EAT1), which represses the transcription of the FLOWERING LOCUS T (FT) gene (known as florigen) by binding to its promoter and inhibiting the action of its transcriptional activators [143]. NNC1 is a negative regulator of nodulation, and NNC1 knock-down by RNA-interference increased nodule number in soybean. Moreover, there is evidence that NNC1 directly interacts with NIN, thereby preventing the activation of its target genes [42].
Specifically, NNC1 represses the activity of the early nodulin ENOD40 gene, which is proposed to account for the NNC1 repressive effect on nodulation. Moreover, NNC1 also acts as a repressor of the GmRIC1 and GmRIC2 genes, encoding rhizobia-induced CLE peptides triggering AON in soybean (see Section 6). The NIN-binding sites (NBS) within the promoters of these genes were enriched for the NNC1 protein, and since NIN physically interacts with NNC1, it was proposed that NNC1 prevents activation of the GmRIC1 and GmRIC2 genes by competitive binding to NBS and by forming a complex with NIN [42]. In addition, AON activation inhibits the expression of NIN and miR172c by a negative feedback mechanism (see Figure 1). Therefore, the NMN regulatory module coordinates the nodulation and AON regulatory pathways in soybean [42].
Recently, it was shown that in addition to nodulation, the miR172c-NNC1 module also regulates root development in response to the salt stress [52]. NNC1 knockdown via RNAi resulted in the increased root tolerance to the salt stress, whereas NNC1 overexpression enhanced salt sensitivity of soybean roots, suggesting that NNC1 is a negative regulator of root tolerance to the salt stress in soybean. Therefore, the miR172-NNC1 regulatory module has a broad role in plant development and stress response, and it was recruited to nodulation program to orchestrate nodule development by repressing the activity of the NIN TF, a key integrator of different regulatory pathways in nodulation.
In addition to the evolutionary conserved miR172, another conserved microRNA, miR156, acts as its antagonist in the control of flowering transition in plants, as well as in other developmental processes [144]. The targets of miR156 are the transcripts of the genes encoding the SQUAMOSA promoter-binding protein-like (SPL) TFs, which are known as positive regulators of flowering [145]. In contrast to miR172, the amount of which increases with the age of a plant, miR156 level in plants is more abundant at the juvenile stage and decreases with plant aging [146]. Therefore, the level of these two miRNAs is considered to be a “molecular timer” of the age of a plant and determines developmental phase transitions in plants [146]. Interestingly, the role of miR156 in nodulation has also been reported [147,148]. In alfalfa, the effect of miR156 overexpression on nodulation was genotype-dependent: it either increased or had no effect on the number of nodules [148]. On the contrary, the ectopic expression of miR156 in L. japonicus reduced nodule numbers, as well as resulted in a decreased expression of a set of nodulation-related genes, including CYCLOPS, NSP1, and NIN [147]. Moreover, in soybean miR156 also reduced nodulation, and its level was negatively correlated with miR172 levels throughout nodule development, suggesting that miR156 might negatively regulate miR172 expression [149]. Therefore, in addition to flowering control, the antagonistic action of the two conserved miRNAs, miR172 and miRNA156, is also manifested in the regulation of symbiotic nodulation.
6. Players of Systemic Response to Nutrient Availability and Their Role in Nodulation
The development of symbiotic nodules is regulated by a systemic mechanism known as the autoregulation of nodulation (AON), which controls nodule number in legume plants. The key components of the AON are root-derived CLE (CLV3/EMBRYO-SURROUNDING REGION) peptides moving from the root through the xylem to the leaf where they activate a signaling cascade, which inhibits nodulation [54,55,56]. The CLE peptides represent a group of post-translationally modified regulatory peptides, which are important regulators of plant meristem maintenance, cell differentiation, early embryogenesis, and other developmental processes (for review, see [150]). Some members of the CLE peptide family are able to mediate systemic responses in plants via long-distance transport through the xylem ([54,56,151,152,153], reviewed in [154])
In AON, the CLE peptides are perceived by the CLAVATA1 like Leucine-rich repeat (LRR) receptor kinase (NODULE AUTOREGULATION RECEPTOR KINASE (NARK) in soybean, HYPERNODULATION ABERRANT ROOT FORMATION 1 (HAR1) in L. japonicus, SUPER NUMERIC NODULES (SUNN) in M. truncatula). Mutations in the corresponding genes cause a supernodulating phenotype [155,156,157]. The CLV1-like kinase genes involved in the AON are the closest relatives of the AtCLV1 kinase in A. thaliana which controls the stem cell maintenance in the SAM [158] by perceiving the CLAVATA3 (CLV3) signaling peptide, a member of the CLE peptide family [155,156,157]. In soybean, the GmNARK kinase involved in the AON is a paralogue of the GmCLV1A kinase, which acts in the SAM maintenance [158]. This suggests that the AON-related CLV1-kinases have evolved due to duplication and neodiversification of homologous CLV1-like kinases [159]. Accordingly, the genomic region where the MtSUNN gene is located is not syntenic with the region including the AtCLV1 gene, suggesting that MtSUNN is not a direct ortholog of the AtCLV1 kinase [157].
In addition to its function in the SAM, the CLV1 receptor kinase also functions in the primary root meristem as well as during LR formation, where CLV1 is able to interact with different co-receptors to perceive various CLE peptide ligands [61,160]. The CLE peptides triggering the AON belong to the group which also includes the AtCLE1-AtCLE7 peptides of A. thaliana [161,162]. It was found that AtCLE1/3/4/7 from these groups is activated under nitrate deficiency and the overexpression of these genes inhibits lateral root development [61]. Since such an inhibitory effect was abolished in the Atclv1 mutant, it was suggested that the AtCLE1/3/4/7 peptides inhibit LR development under nitrogen deficiency by acting through the AtCLV1 kinase in the root [61]. Therefore, the CLE peptides triggering the AON through the CLV1-like kinase in the shoot are homologous to the nitrate-regulated CLEs in A. thaliana, suggesting that the AON mechanisms might have evolved based on the system which controls root architecture in response to nitrate availability in plants.
Legume CLE genes involved in the AON differ in the way their expression is activated. In particular, in L. japonicus LjCLE-RS 1–3 (CLE-ROOT SIGNAL) are activated by rhizobia, whereas LjCLE-RS 2,3 are also activated in response to the nitrate treatment [56,163]. In M. truncatula, MtCLE12 and MtCLE13 are activated by rhizobia-induced signaling cascade [54] and are specific to nodulation, whereas MtCLE35 is induced by both rhizobia and the nitrate [58,59,60] (see Figure 1). All these CLE genes in L. japonicus and M. truncatula systemically inhibit nodulation when overexpressed [54,56,58]. It was found that the NIN TF directly activates the expression of rhizobia-induced CLE genes in L. japonicus and M. truncatula, suggesting that NIN is responsible for the induction of AON [43,44]. Moreover, in L. japonicus, the NIN gene itself is under the negative regulation by AON [43]. In this relation, the “NIN-CLE-СLV1-like kinase” regulatory module resembles the “WUS-CLV3-CLV1” feedback regulation, which acts in the SAM, where the WUS (WUSCHEL) TF directly activates the expression of CLV3 (encoding the CLE peptide) triggering signaling cascade to downregulate WUS expression by a feedback mechanism [164].
The nitrate-induced CLE genes in legumes were found to be activated by the NLP TFs [165,166], known as the master regulators of the nitrate-response in plants [31] (see also Section 2). Therefore, the activation of CLEs in response to rhizobia is mediated by NIN, whereas NLPs are responsible for the activation of CLE in response to the nitrate, mediating nitrate-mediated inhibition of the nodulation [43,44,166]. The presence of the NIN-specific binding sites together with NRE in the promoters of those genes which are activated by both NIN and NLP, brings together the rhizobia-induced and the nitrate-mediated pathways upregulating CLE expression and triggering the AON [166].
In addition to CLEs, NIN also activates the expression of the MtCEP7 gene encoding a CEP (C-TERMINALLY ENCODED PEPTIDE) peptide [44] (see Figure 1). Like the CLE peptides, the CEP peptides represent a group of post-translationally modified regulatory peptides [167]. The MtCEP1 and MtCEP7 genes were found to be the positive regulators of nodulation [44,62]. In A. thaliana, CEPs are activated under nitrate deficiency, and they mediate the inhibition of the root growth under low nitrate condition [64]. For CEPs in A. thaliana, both local and systemic action has been described: the AtCEP1 peptide systemically, through a shoot-acting receptor, AtCEPR (CEP RECEPTOR), activates the expression of nitrate transporter genes in the root [65], whereas AtCEP5 inhibits LR development locally through a root-acting receptor [63]. In M. truncatula, MtCEP1 systemically stimulates nodule development through its receptor MtCRA2 (COMPACT ROOT SYSTEM ARCHITECTURE 2) in the shoot, and inhibits LR development, which was found to be mediated by a local MtCRA2 activity in the root [62,168,169]. However, recently, it was found that MtCRA2 might be involved in a negative regulation of nodulation in the root, since grafting experiments showed that plants with the cra2 mutant roots had an increased nodule number at inhibitory nitrate levels [166]. Collectively, findings on CLE and CEP function in nodulation suggest that the systemic regulation of nodule number in legumes might have evolved based on the system that controls root architecture in plants in response to nitrogen availability.
Signaling cascades activated by the CEP and CLE peptides in the shoot were found to oppositely regulate a common downstream target, miRNA2111: CEP-activated signaling cascade activates miRNA2111 in the shoot, whereas the CLE peptides through shoot acting receptor decreases the abundance of miRNA2111 in the shoot [170,171] (see Figure 1). miRNA2111 is a mobile regulator of the symbiosis moving from the shoot to the root, where it activates nodulation by targeting the transcripts of the TOO MUCH LOVE (TML) gene [170]. TML encodes an F-box protein which negatively regulates nodulation in the root [172], and it might be responsible for the ubiquitin-mediate degradation of a positive regulator of the symbiosis. However, the protein targets of TML have not been described up to date. Interestingly, in sweet potato Ipomoea batatas, miRNA2111 was found to be involved in wound response, and a wound-inducible gene, IbFBK (F-box/kelch repeat protein), was identified as its target [173]. Moreover, it was found that the kelch-repeat domain of IbFBK interacts with IbCNR8 (CELL NUMBER REGULATOR 8), which was suggested to lead to the ubiquitination and degradation of IbCNR8. CNRs are transmembrane proteins known as the regulators of plant organ size (see [174]). In soybean, the member of the CNR protein family, GmFWL1, has been identified, which exclusively is expressed in the root hair cells in response to rhizobia and in the nodules. RNAi knockdown of the GmFWL1 gene resulted in a significant reduction in soybean nodule development, suggesting that it is a positive regulator of nodulation [175,176]. It is of great interest to learn if nodule-specific CNRs could interact with the F-box containing TML protein to be targeted for ubiquitin-dependent degradation, as it was suggested for FBK and its interacting protein of the CNR family in sweet potato.
It was also found that in rapeseed phloem sap miR2111 abundance was significantly increased under P starvation [91]. This suggests that long-acting miR2111 and its targets could play broader roles in plants, regulating root growth and architecture in response to nutrient availability. Moreover, the role of CLV1-like kinase and CLE peptides in phosphorus response was further shown in the arbuscular mycorrhizal symbiosis. In M.truncatula, the MtCLE53 and MtCLE33 peptides are induced by arbuscular mycorrhizal fungi and high phosphorus and negatively regulate root mycorrhization in MtSUNN-dependent manner, thereby mediating the autoregulation of mycorrhization [177,178]. Therefore, the CLV1-like kinase, encoded by the MtSUNN gene in M. truncatula, orchestrates different developmental responses in legumes by binding to different CLE peptide ligands.
7. Conclusions
The nitrogen-fixing symbiosis with rhizobia is a unique feature of legumes and Parasponia species from the Cannabaceae family. Regulatory pathways that are involved in the symbiotic nodule development have evolved based on the transcriptional regulators, many of which pre-exist in non-legume plants (see Figure 1). The close homologues of these regulators have also non-symbiotic function in plants and regulate different aspects of plant development, including LR development and root radial patterning, root and shoot meristem maintenance, flower transition, as well as the response to nutrient availability (see Table 1). Knowing which additional factors interact with such homologous regulatory modules in non-symbiotic condition will help to predict new players and new possible interactions in the regulation of the legume–rhizobia symbiosis.
Therefore, many of the regulators of nodule organogenesis also function in non-nodulating species, playing diverse roles in plant development. In this respect, what are the key features of legume-specific regulatory networks which control nodulation? NIN is a central regulator of the legume–rhizobia symbiosis, coordinating a set of conserved regulatory modules involved in nodule development. NIN itself has the homology with other NLP proteins. However, in the nodulating species, it lost nitrate-responsiveness and acquired cis-regulatory elements responsible for its expression in the root in response to rhizobia-induced and cytokinin-induced signaling pathways. Therefore, the NIN-coordinated regulatory network in nodulation might have evolved from the NLP-controlled network, which underlies nitrogen-dependent developmental processes in plants. In addition to this, the acquisition of the NIN-binding sites by a set of genes involved in rhizobial infection, LR development, and systemic response to nutrient availability made it possible to combine these regulatory modules in a single developmental program controlled by NIN, which has evolved to increase the specificity and effectiveness of symbiotic nodulation. Another set of evidence indicates that the nodulating species are characterized by a specific responsiveness of their root cortical cells to cytokinin. Recent findings suggest that a broader pattern of the SCH-SCR expression in the root cortex, due to the presence of the specific cis-regulatory elements in the SCRs promoters in legumes, could account for the ability of legume plants to activate cortical cell divisions in response to cytokinin treatment.
Therefore, during the evolution of legume plants, the changes in cis-regulatory elements in a set of genes, including SCR (broadening the expression pattern in the root cortex), NIN (acquisition of the rhizobia-induced and cytokinin-responsive elements (CE)), LBD16, and other targets of the NIN transcription factor (acquisition of the NIN-binding sites), might have been crucial evolutionary events necessary for the establishment of the nodule developmental program. Symbiotic nitrogen fixation is able to enrich agricultural ecosystems with a biologically available nitrogen and to minimize the application of nitrogen-containing fertilizers. The unraveling of evolutionary events underlying the establishment of the legume–rhizobia symbiosis is of great fundamental and practical interest, since it could help to introduce symbiotic features to non-legume species in the future.
Author Contributions
Writing—original draft preparation, M.L.; writing—review and editing, M.A., D.S., and M.L.; supervision, funding acquisition, L.L. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Russian Science Foundation Project No. 21-66-00012 to M.L., D.S. and L.L. (Sections 1, Sections 2, Sections 3, Section 5, Section 7), the grant from Saint-Petersburg State University ID: 73450407 to M.L. and D.S. (Section 6), Russia, as well as by the grant provided to M.A. (Section 4) by the Center for International Scientific Studies & Collaboration (CISSC), Ministry of Science Research and Technology, Iran.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parniske, M. Arbuscular Mycorrhiza: The Mother of Plant Root Endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Behm, J.E.; Geurts, R.; Kiers, E.T. Parasponia: A Novel System for Studying Mutualism Stability. Trends Plant Sci. 2014, 19, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, K.; Demchenko, K.N. The Diversity of Actinorhizal Symbiosis. Protoplasma 2012, 249, 967–979. [Google Scholar] [CrossRef]
- Huisman, R.; Geurts, R. A Roadmap toward Engineered Nitrogen-Fixing Nodule Symbiosis. Plant Commun. 2020, 1, 100019. [Google Scholar] [CrossRef]
- Soyano, T.; Liu, M.; Kawaguchi, M.; Hayashi, M. Leguminous Nodule Symbiosis Involves Recruitment of Factors Contributing to Lateral Root Development. Curr. Opin. Plant Biol. 2021, 59, 102000. [Google Scholar] [CrossRef]
- Liu, C.-W.; Breakspear, A.; Guan, D.; Cerri, M.R.; Jackson, K.; Jiang, S.; Robson, F.; Radhakrishnan, G.V.; Roy, S.; Bone, C.; et al. NIN Acts as a Network Hub Controlling a Growth Module Required for Rhizobial Infection. Plant Physiol. 2019, 179, 1704–1722. [Google Scholar] [CrossRef]
- Liu, J.; Bisseling, T. Evolution of NIN and NIN-like Genes in Relation to Nodule Symbiosis. Genes 2020, 11, 777. [Google Scholar] [CrossRef]
- Lévy, J.; Bres, C.; Geurts, R.; Chalhoub, B.; Kulikova, O.; Duc, G.; Journet, E.-P.; Ané, J.-M.; Lauber, E.; Bisseling, T.; et al. A Putative Ca2+ and Calmodulin-Dependent Protein Kinase Required for Bacterial and Fungal Symbioses. Science 2004, 303, 1361–1364. [Google Scholar] [CrossRef]
- Messinese, E.; Mun, J.-H.; Yeun, L.H.; Jayaraman, D.; Rougé, P.; Barre, A.; Lougnon, G.; Schornack, S.; Bono, J.-J.; Cook, D.R.; et al. A Novel Nuclear Protein Interacts with the Symbiotic DMI3 Calcium- and Calmodulin-Dependent Protein Kinase of Medicago Truncatula. Mol. Plant Microbe. Interact. 2007, 20, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Yoshida, S.; Müller, J.; Singh, S.; Banba, M.; Vickers, K.; Markmann, K.; White, C.; Schuller, B.; Sato, S.; et al. CYCLOPS, a Mediator of Symbiotic Intracellular Accommodation. Proc. Natl. Acad. Sci. USA 2008, 105, 20540–20545. [Google Scholar] [CrossRef] [PubMed]
- Horváth, B.; Yeun, L.H.; Domonkos, A.; Halász, G.; Gobbato, E.; Ayaydin, F.; Miró, K.; Hirsch, S.; Sun, J.; Tadege, M.; et al. Medicago Truncatula IPD3 Is a Member of the Common Symbiotic Signaling Pathway Required for Rhizobial and Mycorrhizal Symbioses. Mol. Plant Microbe. Interact. 2011, 24, 1345–1358. [Google Scholar] [CrossRef]
- Ovchinnikova, E.; Journet, E.-P.; Chabaud, M.; Cosson, V.; Ratet, P.; Duc, G.; Fedorova, E.; Liu, W.; den Camp, R.O.; Zhukov, V.; et al. IPD3 Controls the Formation of Nitrogen-Fixing Symbiosomes in Pea and Medicago spp. MPMI 2011, 24, 1333–1344. [Google Scholar] [CrossRef]
- Singh, S.; Katzer, K.; Lambert, J.; Cerri, M.; Parniske, M. CYCLOPS, a DNA-Binding Transcriptional Activator, Orchestrates Symbiotic Root Nodule Development. Cell Host Microbe 2014, 15, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; Kim, J.; Muñoz, A.; Heckmann, A.B.; Downie, J.A.; Oldroyd, G.E. GRAS Proteins Form a DNA Binding Complex to Induce Gene Expression during Nodulation Signaling in Medicago Truncatula. Plant Cell 2009, 21, 545–557. [Google Scholar] [CrossRef]
- Kaló, P.; Gleason, C.; Edwards, A.; Marsh, J.; Mitra, R.M.; Hirsch, S.; Jakab, J.; Sims, S.; Long, S.R.; Rogers, J.; et al. Nodulation Signaling in Legumes Requires NSP2, a Member of the GRAS Family of Transcriptional Regulators. Science 2005, 308, 1786–1789. [Google Scholar] [CrossRef]
- Delaux, P.; Bécard, G.; Combier, J. NSP 1 Is a Component of the Myc Signaling Pathway. New Phytol. 2013, 199, 59–65. [Google Scholar] [CrossRef]
- Takeda, N.; Tsuzuki, S.; Suzaki, T.; Parniske, M.; Kawaguchi, M. CERBERUS and NSP1 of Lotus Japonicus Are Common Symbiosis Genes That Modulate Arbuscular Mycorrhiza Development. Plant Cell Physiol. 2013, 54, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kohlen, W.; Lillo, A.; Op Den Camp, R.; Ivanov, S.; Hartog , M.; et al. Strigolactone Biosynthesis in Medicago Truncatula and Rice Requires the Symbiotic GRAS-Type Transcription Factors NSP1 and NSP2. Plant Cell 2011, 23, 3853–3865. [Google Scholar] [CrossRef]
- Van Zeijl, A.; Op den Camp, R.H.M.; Deinum, E.E.; Charnikhova, T.; Franssen, H.; Op den Camp, H.J.M.; Bouwmeester, H.; Kohlen, W.; Bisseling, T.; Geurts, R. Rhizobium Lipo-Chitooligosaccharide Signaling Triggers Accumulation of Cytokinins in Medicago Truncatula Roots. Mol. Plant 2015, 8, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Jardinaud, M.-F.; Boivin, S.; Rodde, N.; Catrice, O.; Kisiala, A.; Lepage, A.; Moreau, S.; Roux, B.; Cottret, L.; Sallet, E. A Laser Dissection-RNAseq Analysis Highlights the Activation of Cytokinin Pathways by Nod Factors in the Medicago Truncatula Root Epidermis. Plant Physiol. 2016, 171, 2256–2276. [Google Scholar] [CrossRef]
- Reid, D.; Nadzieja, M.; Novák, O.; Heckmann, A.B.; Sandal, N.; Stougaard, J. Cytokinin Biosynthesis Promotes Cortical Cell Responses during Nodule Development. Plant Physiol. 2017, 175, 361–375. [Google Scholar] [CrossRef]
- Gamas, P.; Brault, M.; Jardinaud, M.-F.; Frugier, F. Cytokinins in Symbiotic Nodulation: When, Where, What For? Trends Plant Sci. 2017, 22, 792–802. [Google Scholar] [CrossRef]
- Kang, H.; Chu, X.; Wang, C.; Xiao, A.; Zhu, H.; Yuan, S.; Yang, Z.; Ke, D.; Xiao, S.; Hong, Z.; et al. A MYB Coiled-Coil Transcription Factor Interacts with NSP2 and Is Involved in Nodulation in Lotus Japonicus. New Phytol. 2014, 201, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Xiao, A.; Yu, H.; Fan, Y.; Kang, H.; Ren, Y.; Huang, X.; Gao, X.; Wang, C.; Zhang, Z.; Zhu, H.; et al. Transcriptional Regulation of NIN Expression by IPN2 Is Required for Root Nodule Symbiosis in Lotus Japonicus. New Phytol. 2020, 227, 513–528. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.; Downie, J.A. Coordinating Nodule Morphogenesis with Rhizobial Infection in Legumes. Annu. Rev. Plant Biol. 2008, 59, 519–546. [Google Scholar] [CrossRef]
- Liu, J.; Rutten, L.; Limpens, E.; Van Der Molen, T.; Van Velzen, R.; Chen, R.; Chen, Y.; Geurts, R.; Kohlen, W.; Kulikova, O. A Remote Cis-Regulatory Region Is Required for NIN Expression in the Pericycle to Initiate Nodule Primordium Formation in Medicago Truncatula. Plant Cell 2019, 31, 68–83. [Google Scholar] [CrossRef]
- Schauser, L.; Roussis, A.; Stiller, J.; Stougaard, J. A Plant Regulator Controlling Development of Symbiotic Root Nodules. Nature 1999, 402, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.F.; Rakocevic, A.; Mitra, R.M.; Brocard, L.; Sun, J.; Eschstruth, A.; Long, S.R.; Schultze, M.; Ratet, P.; Oldroyd, G.E.D. Medicago Truncatula NIN Is Essential for Rhizobial-Independent Nodule Organogenesis Induced by Autoactive Calcium/Calmodulin-Dependent Protein Kinase. Plant Physiol. 2007, 144, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Konishi, M.; Yanagisawa, S. Arabidopsis NIN-like Transcription Factors Have a Central Role in Nitrate Signalling. Nature Commun. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Liu, K.; Niu, Y.; Konishi, M.; Wu, Y.; Du, H.; Chung, H.S.; Li, L.; Boudsocq, M.; McCormack, M.; Maekawa, S. Discovery of Nitrate–CPK–NLP Signalling in Central Nutrient–Growth Networks. Nature 2017, 545, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, F.; Crawford, N.M.; Wang, Y. Molecular Regulation of Nitrate Responses in Plants. Int. J.Mol. Sci. 2018, 19, 2039. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, W.; Konishi, M.; Yanagisawa, S. The Evolutionary Events Necessary for the Emergence of Symbiotic Nitrogen Fixation in Legumes May Involve a Loss of Nitrate Responsiveness of the NIN Transcription Factor. Plant Signal. Behav. 2013, 8, e25975. [Google Scholar] [CrossRef] [PubMed]
- Griesmann, M.; Chang, Y.; Liu, X.; Song, Y.; Haberer, G.; Crook, M.B.; Billault-Penneteau, B.; Lauressergues, D.; Keller, J.; Imanishi, L. Phylogenomics Reveals Multiple Losses of Nitrogen-Fixing Root Nodule Symbiosis. Science 2018, 361, eaat1743. [Google Scholar] [CrossRef]
- Nishida, H.; Nosaki, S.; Suzuki, T.; Ito, M.; Miyakawa, T.; Nomoto, M.; Tada, Y.; Miura, K.; Tanokura, M.; Kawaguchi, M. Different DNA-Binding Specificities of NLP and NIN Transcription Factors Underlie Nitrate-Induced Control of Root Nodulation. Plant Cell 2021, 33, 2340–2359. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Luo, Z.; Mysore, K.S.; Wen, J.; Xie, F. NIN Interacts with NLPs to Mediate Nitrate Inhibition of Nodulation in Medicago Truncatula. Nat. Plants 2018, 4, 942–952. [Google Scholar] [CrossRef] [PubMed]
- Xie, F.; Murray, J.D.; Kim, J.; Heckmann, A.B.; Edwards, A.; Oldroyd, G.E.D.; Downie, J.A. Legume Pectate Lyase Required for Root Infection by Rhizobia. Proc. Natl. Acad. Sci. USA 2012, 109, 633–638. [Google Scholar] [CrossRef]
- Soyano, T.; Kouchi, H.; Hirota, A.; Hayashi, M. Nodule Inception Directly Targets NF-Y Subunit Genes to Regulate Essential Processes of Root Nodule Development in Lotus Japonicus. PLoS Genet. 2013, 9, e1003352. [Google Scholar] [CrossRef]
- Laporte, P.; Lepage, A.; Fournier, J.; Catrice, O.; Moreau, S.; Jardinaud, M.-F.; Mun, J.-H.; Larrainzar, E.; Cook, D.R.; Gamas, P. The CCAAT Box-Binding Transcription Factor NF-YA1 Controls Rhizobial Infection. J. Exp. Bot. 2014, 65, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Vernié, T.; Kim, J.; Frances, L.; Ding, Y.; Sun, J.; Guan, D.; Niebel, A.; Gifford, M.L.; de Carvalho-Niebel, F.; Oldroyd, G.E. The NIN Transcription Factor Coordinates Diverse Nodulation Programs in Different Tissues of the Medicago Truncatula Root. Plant Cell 2015, 27, 3410–3424. [Google Scholar] [CrossRef]
- Soyano, T.; Shimoda, Y.; Kawaguchi, M.; Hayashi, M. A Shared Gene Drives Lateral Root Development and Root Nodule Symbiosis Pathways in Lotus. Science 2019, 366, 1021–1023. [Google Scholar] [CrossRef] [PubMed]
- Schiessl, K.; Lilley, J.L.S.; Lee, T.; Tamvakis, I.; Kohlen, W.; Bailey, P.C.; Thomas, A.; Luptak, J.; Ramakrishnan, K.; Carpenter, M.D.; et al. NODULE INCEPTION Recruits the Lateral Root Developmental Program for Symbiotic Nodule Organogenesis in Medicago Truncatula. Curr. Biol. 2019, 29, 3657–3668. [Google Scholar] [CrossRef]
- Wang, L.; Sun, Z.; Su, C.; Wang, Y.; Yan, Q.; Chen, J.; Ott, T.; Li, X. A GmNINa-MiR172c-NNC1 Regulatory Network Coordinates the Nodulation and Autoregulation of Nodulation Pathways in Soybean. Mol. Plant 2019, 12, 1211–1226. [Google Scholar] [CrossRef] [PubMed]
- Soyano, T.; Hirakawa, H.; Sato, S.; Hayashi, M.; Kawaguchi, M. NODULE INCEPTION Creates a Long-Distance Negative Feedback Loop Involved in Homeostatic Regulation of Nodule Organ Production. Proc. Natl. Acad. Sci. USA 2014, 111, 14607–14612. [Google Scholar] [CrossRef]
- Laffont, C.; Ivanovici, A.; Gautrat, P.; Brault, M.; Djordjevic, M.A.; Frugier, F. The NIN Transcription Factor Coordinates CEP and CLE Signaling Peptides That Regulate Nodulation Antagonistically. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Goh, T.; Joi, S.; Mimura, T.; Fukaki, H. The Establishment of Asymmetry in Arabidopsis Lateral Root Founder Cells Is Regulated by LBD16/ASL18 and Related LBD/ASL Proteins. Development 2012, 139, 883–893. [Google Scholar] [CrossRef]
- Combier, J.-P.; Frugier, F.; de Billy, F.; Boualem, A.; El-Yahyaoui, F.; Moreau, S.; Vernié, T.; Ott, T.; Gamas, P.; Crespi, M. MtHAP2-1 Is a Key Transcriptional Regulator of Symbiotic Nodule Development Regulated by MicroRNA169 in Medicago Truncatula. Genes Dev. 2006, 20, 3084–3088. [Google Scholar] [CrossRef]
- Sorin, C.; Declerck, M.; Christ, A.; Blein, T.; Ma, L.; Lelandais-Brière, C.; Njo, M.F.; Beeckman, T.; Crespi, M.; Hartmann, C. A Mi R 169 Isoform Regulates Specific NF-YA Targets and Root Architecture in A Rabidopsis. New Phytol. 2014, 202, 1197–1211. [Google Scholar] [CrossRef]
- Gonzalez-Rizzo, S.; Crespi, M.; Frugier, F. The Medicago Truncatula CRE1 Cytokinin Receptor Regulates Lateral Root Development and Early Symbiotic Interaction with Sinorhizobium Meliloti. Plant Cell 2006, 18, 2680–2693. [Google Scholar] [CrossRef] [PubMed]
- De León, B.G.-P.; Zorrilla, J.M.F.; Rubio, V.; Dahiya, P.; Paz-Ares, J.; Leyva, A. Interallelic Complementation at the Arabidopsis CRE1 Locus Uncovers Independent Pathways for the Proliferation of Vascular Initials and Canonical Cytokinin Signalling. Plant J. 2004, 38, 70–79. [Google Scholar] [CrossRef]
- Riefler, M.; Novak, O.; Strnad, M.; Schmülling, T. Arabidopsis Cytokinin Receptor Mutants Reveal Functions in Shoot Growth, Leaf Senescence, Seed Size, Germination, Root Development, and Cytokinin Metabolism. Plant Cell 2006, 18, 40–54. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmülling, T. Cytokinin-Deficient Transgenic Arabidopsis Plants Show Multiple Developmental Alterations Indicating Opposite Functions of Cytokinins in the Regulation of Shoot and Root Meristem Activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef] [PubMed]
- Sahito, Z.A.; Wang, L.; Sun, Z.; Yan, Q.; Zhang, X.; Jiang, Q.; Ullah, I.; Tong, Y.; Li, X. The MiR172c-NNC1 Module Modulates Root Plastic Development in Response to Salt in Soybean. BMC Plant Biol. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-H.; Helliwell, C.A. Regulation of Flowering Time and Floral Patterning by MiR172. J. Exp. Bot. 2011, 62, 487–495. [Google Scholar] [CrossRef]
- Mortier, V.; Den Herder, G.; Whitford, R.; Van de Velde, W.; Rombauts, S.; D’haeseleer, K.; Holsters, M.; Goormachtig, S. CLE Peptides Control Medicago Truncatula Nodulation Locally and Systemically. Plant Physiol. 2010, 153, 222–237. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.E.; Ferguson, B.J.; Gresshoff, P.M. Inoculation-and Nitrate-Induced CLE Peptides of Soybean Control NARK-Dependent Nodule Formation. Mol. Plant Microbe Interact. 2011, 24, 606–618. [Google Scholar] [CrossRef]
- Okamoto, S.; Ohnishi, E.; Sato, S.; Takahashi, H.; Nakazono, M.; Tabata, S.; Kawaguchi, M. Nod Factor/Nitrate-Induced CLE Genes That Drive HAR1-Mediated Systemic Regulation of Nodulation. Plant Cell Physiol. 2009, 50, 67–77. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, Y.W.; Lee, S.C.; Hwang, C.H. Nitrate Inhibits Soybean Nodulation by Regulating Expression of CLE Genes. Plant Sci. 2014, 229, 1–9. [Google Scholar] [CrossRef]
- Lebedeva, M.; Azarakhsh, M.; Yashenkova, Y.; Lutova, L. Nitrate-Induced CLE Peptide Systemically Inhibits Nodulation in Medicago Truncatula. Plants 2020, 9, 1456. [Google Scholar] [CrossRef] [PubMed]
- Mens, C.; Hastwell, A.H.; Su, H.; Gresshoff, P.M.; Mathesius, U.; Ferguson, B.J. Characterisation of Medicago Truncatula CLE34 and CLE35 in Nitrate and Rhizobia Regulation of Nodulation. New Phytol. 2021, 229, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Gautrat, P.; Frugier, F. Nitrate-Induced CLE35 Signaling Peptides Inhibit Nodulation through the SUNN Receptor and MiR2111 Repression. Plant Physiol. 2021, 185, 1216–1228. [Google Scholar] [CrossRef]
- Araya, T.; Miyamoto, M.; Wibowo, J.; Suzuki, A.; Kojima, S.; Tsuchiya, Y.N.; Sawa, S.; Fukuda, H.; Von Wirén, N.; Takahashi, H. CLE-CLAVATA1 Peptide-Receptor Signaling Module Regulates the Expansion of Plant Root Systems in a Nitrogen-Dependent Manner. Proc. Natl. Acad. Sci. USA 2014, 111, 2029–2034. [Google Scholar] [CrossRef]
- Imin, N.; Mohd-Radzman, N.A.; Ogilvie, H.A.; Djordjevic, M.A. The Peptide-Encoding CEP1 Gene Modulates Lateral Root and Nodule Numbers in Medicago Truncatula. J. Exp. Bot. 2013, 64, 5395–5409. [Google Scholar] [CrossRef] [PubMed]
- Roberts, I.; Smith, S.; Stes, E.; De Rybel, B.; Staes, A.; Van De Cotte, B.; Njo, M.F.; Dedeyne, L.; Demol, H.; Lavenus, J. CEP5 and XIP1/CEPR1 Regulate Lateral Root Initiation in Arabidopsis. J. Exp. Bot. 2016, 67, 4889–4899. [Google Scholar] [CrossRef]
- Delay, C.; Chapman, K.; Taleski, M.; Wang, Y.; Tyagi, S.; Xiong, Y.; Imin, N.; Djordjevic, M.A. CEP3 Levels Affect Starvation-Related Growth Responses of the Primary Root. J. Exp. Bot. 2019, 70, 4763–4774. [Google Scholar] [CrossRef] [PubMed]
- Tabata, R.; Sumida, K.; Yoshii, T.; Ohyama, K.; Shinohara, H.; Matsubayashi, Y. Perception of Root-Derived Peptides by Shoot LRR-RKs Mediates Systemic N-Demand Signaling. Science 2014, 346, 343–346. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Indrasumunar, A.; Hayashi, S.; Lin, M.; Lin, Y.; Reid, D.E.; Gresshoff, P.M. Molecular Analysis of Legume Nodule Development and Autoregulation. J. Integr. Plant Biol. 2010, 52, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Timmers, A.; Auriac, M.-C.; Truchet, G. Refined Analysis of Early Symbiotic Steps of the Rhizobium-Medicago Interaction in Relationship with Microtubular Cytoskeleton Rearrangements. Development 1999, 126, 3617–3628. [Google Scholar] [CrossRef]
- Xiao, T.T.; Schilderink, S.; Moling, S.; Deinum, E.E.; Kondorosi, E.; Franssen, H.; Kulikova, O.; Niebel, A.; Bisseling, T. Fate Map of Medicago Truncatula Root Nodules. Development 2014, 141, 3517–3528. [Google Scholar] [CrossRef]
- Xiao, T.T.; van Velzen, R.; Kulikova, O.; Franken, C.; Bisseling, T. Lateral Root Formation Involving Cell Division in Both Pericycle, Cortex and Endodermis Is a Common and Ancestral Trait in Seed Plants. Development 2019, 146. [Google Scholar] [CrossRef]
- Herrbach, V.; Remblière, C.; Gough, C.; Bensmihen, S. Lateral Root Formation and Patterning in Medicago Truncatula. J. Plant Physiol. 2014, 171, 301–310. [Google Scholar] [CrossRef]
- Ilina, E.L.; Kiryushkin, A.S.; Semenova, V.A.; Demchenko, N.P.; Pawlowski, K.; Demchenko, K.N. Lateral Root Initiation and Formation within the Parental Root Meristem of Cucurbita Pepo: Is Auxin a Key Player? Ann. Bot. 2018, 122, 873–888. [Google Scholar] [CrossRef]
- Dubrovsky, J.G.; Sauer, M.; Napsucialy-Mendivil, S.; Ivanchenko, M.G.; Friml, J.; Shishkova, S.; Celenza, J.; Benková, E. Auxin Acts as a Local Morphogenetic Trigger to Specify Lateral Root Founder Cells. Proc. Natl. Acad. Sci. 2008, 105, 8790–8794. [Google Scholar] [CrossRef]
- De Rybel, B.; Vassileva, V.; Parizot, B.; Demeulenaere, M.; Grunewald, W.; Audenaert, D.; Van Campenhout, J.; Overvoorde, P.; Jansen, L.; Vanneste, S. A Novel Aux/IAA28 Signaling Cascade Activates GATA23-Dependent Specification of Lateral Root Founder Cell Identity. Curr. Biol. 2010, 20, 1697–1706. [Google Scholar] [CrossRef]
- Okushima, Y.; Fukaki, H.; Onoda, M.; Theologis, A.; Tasaka, M. ARF7 and ARF19 Regulate Lateral Root Formation via Direct Activation of LBD/ASL Genes in Arabidopsis. Plant Cell 2007, 19, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Luijten, M.; Miyashima, S.; Lenhard, M.; Hashimoto, T.; Nakajima, K.; Scheres, B.; Heidstra, R.; Laux, T. Conserved Factors Regulate Signalling in Arabidopsis Thaliana Shoot and Root Stem Cell Organizers. Nature 2007, 446, 811–814. [Google Scholar] [CrossRef]
- Chen, S.-K.; Kurdyukov, S.; Kereszt, A.; Wang, X.-D.; Gresshoff, P.; Rose, R. The Association of Homeobox Gene Expression with Stem Cell Formation and Morphogenesis in Cultured Medicago Truncatula. Planta 2009, 230, 827–840. [Google Scholar] [CrossRef]
- Aida, M.; Beis, D.; Heidstra, R.; Willemsen, V.; Blilou, I.; Galinha, C.; Nussaume, L.; Noh, Y.-S.; Amasino, R.; Scheres, B. The PLETHORA Genes Mediate Patterning of the Arabidopsis Root Stem Cell Niche. Cell 2004, 119, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Scheres, B. PLETHORA Transcription Factors Orchestrate de Novo Organ Patterning during Arabidopsis Lateral Root Outgrowth. Proc. Natl. Acad. Sci. 2017, 114, 11709–11714. [Google Scholar] [CrossRef]
- Benfey, P.N.; Linstead, P.J.; Roberts, K.; Schiefelbein, J.W.; Hauser, M.-T.; Aeschbacher, R.A. Root Development in Arabidopsis: Four Mutants with Dramatically Altered Root Morphogenesis. Development 1993, 119, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.; Swarup, R.; Paponov, I.A.; Swarup, K.; Casimiro, I.; Lake, D.; Peret, B.; Zappala, S.; Mairhofer, S.; Whitworth, M. Short-Root Regulates Primary, Lateral, and Adventitious Root Development in Arabidopsis. Plant Physiol. 2011, 155, 384–398. [Google Scholar] [CrossRef]
- Kohlen, W.; Ng, J.L.P.; Deinum, E.E.; Mathesius, U. Auxin Transport, Metabolism, and Signalling during Nodule Initiation: Indeterminate and Determinate Nodules. J. Exp. Bot. 2018, 69, 229–244. [Google Scholar] [CrossRef]
- Hiltenbrand, R.; Thomas, J.; McCarthy, H.; Dykema, K.J.; Spurr, A.; Newhart, H.; Winn, M.E.; Mukherjee, A. A Developmental and Molecular View of Formation of Auxin-Induced Nodule-Like Structures in Land Plants. Front. Plant Sci. 2016, 7, 1692. [Google Scholar] [CrossRef]
- Hirsch, A.; Bhuvaneswari, T.; Torrey, J.; Bisseling, T. Early Nodulin Genes Are Induced in Alfalfa Root Outgrowths Elicited by Auxin Transport Inhibitors. Proc. Natl. Acad. Sci. 1989, 86, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Rightmyer, A.P.; Long, S.R. Pseudonodule Formation by Wild-Type and Symbiotic Mutant Medicago Truncatula in Response to Auxin Transport Inhibitors. Mol. Plant Microbe Interact. 2011, 24, 1372–1384. [Google Scholar] [CrossRef]
- Wu, C.; Dickstein, R.; Cary, A.J.; Norris, J.H. The Auxin Transport Inhibitor N-(1-Naphthyl) Phthalamic Acid Elicits Pseudonodules on Nonnodulating Mutants of White Sweetclover. Plant Physiol. 1996, 110, 501–510. [Google Scholar] [CrossRef]
- Thomas, J.; Hiltenbrand, R.; Bowman, M.J.; Kim, H.R.; Winn, M.E.; Mukherjee, A. Time-Course RNA-Seq Analysis Provides an Improved Understanding of Gene Regulation during the Formation of Nodule-like Structures in Rice. Plant Mol. Biol. 2020, 103, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Majer, C.; Hochholdinger, F. Defining the Boundaries: Structure and Function of LOB Domain Proteins. Trends Plant Sci. 2011, 16, 47–52. [Google Scholar] [CrossRef]
- Goh, T.; Kasahara, H.; Mimura, T.; Kamiya, Y.; Fukaki, H. Multiple AUX/IAA–ARF Modules Regulate Lateral Root Formation: The Role of Arabidopsis SHY2/IAA3-Mediated Auxin Signalling. Philos. Trans. R. Soc. B: Biol. Sci. 2012, 367, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Zhong, S.; Therrien, J.; Huebert, T.; Sato, S.; Mun, T.; Andersen, S.U.; Stougaard, J.; Lepage, A.; Niebel, A.; et al. Lotus Japonicus Nuclear Factor YA1, a Nodule Emergence Stage-Specific Regulator of Auxin Signalling. New Phytol. 2021, 229, 1535–1552. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Shrestha, A.; Zhong, S.; Miri, M.; Austin, R.S.; Sato, S.; Ross, L.; Huebert, T.; Tromas, A.; Torres-Jerez, I.; et al. Lotus Japonicus NF-YA1 Plays an Essential Role During Nodule Differentiation and Targets Members of the SHI/STY Gene Family. MPMI 2016, 29, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Pant, B.D.; Musialak-Lange, M.; Nuc, P.; May, P.; Buhtz, A.; Kehr, J.; Walther, D.; Scheible, W.-R. Identification of Nutrient-Responsive Arabidopsis and Rapeseed MicroRNAs by Comprehensive Real-Time Polymerase Chain Reaction Profiling and Small RNA Sequencing. Plant Physiol. 2009, 150, 1541–1555. [Google Scholar] [CrossRef] [PubMed]
- Osipova, M.A.; Mortier, V.; Demchenko, K.N.; Tsyganov, V.E.; Tikhonovich, I.A.; Lutova, L.A.; Dolgikh, E.A.; Goormachtig, S. Wuschel-Related Homeobox5 Gene Expression and Interaction of CLE Peptides with Components of the Systemic Control Add Two Pieces to the Puzzle of Autoregulation of Nodulation. Plant Physiol. 2012, 158, 1329–1341. [Google Scholar] [CrossRef]
- Roux, B.; Rodde, N.; Jardinaud, M.; Timmers, T.; Sauviac, L.; Cottret, L.; Carrère, S.; Sallet, E.; Courcelle, E.; Moreau, S. An Integrated Analysis of Plant and Bacterial Gene Expression in Symbiotic Root Nodules Using Laser-capture Microdissection Coupled to RNA Sequencing. Plant J. 2014, 77, 817–837. [Google Scholar] [CrossRef]
- Franssen, H.J.; Xiao, T.T.; Kulikova, O.; Wan, X.; Bisseling, T.; Scheres, B.; Heidstra, R. Root Developmental Programs Shape the Medicago Truncatula Nodule Meristem. Development 2015, 142, 2941–2950. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, Y.; Chang, H.; Wang, C.; Yang, J.; Shi, J.; Gao, J.; Yang, W.; Lan, L.; Wang, Y.; et al. An SHR–SCR Module Specifies Legume Cortical Cell Fate to Enable Nodulation. Nature 2020, 589, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wabnik, K.; Niu, T.; Li, H.; Yu, Q.; Pollmann, S.; Vanneste, S.; Govaerts, W.; Rolčík, J.; Geisler, M.; et al. WOX5–IAA17 Feedback Circuit-Mediated Cellular Auxin Response Is Crucial for the Patterning of Root Stem Cell Niches in Arabidopsis. Mol. Plant 2014, 7, 277–289. [Google Scholar] [CrossRef]
- Scheres, B.; Benfey, P.; Dolan, L. Root development. In The Arabidopsis Book/American Society of Plant Biologists, Publisher; American Society of Plant Biologists: Rockville, MD, USA, 2002; Volume 1. [Google Scholar]
- Galinha, C.; Hofhuis, H.; Luijten, M.; Willemsen, V.; Blilou, I.; Heidstra, R.; Scheres, B. PLETHORA Proteins as Dose-Dependent Master Regulators of Arabidopsis Root Development. Nature 2007, 449, 1053–1057. [Google Scholar] [CrossRef]
- Santuari, L.; Sanchez-Perez, G.F.; Luijten, M.; Rutjens, B.; Terpstra, I.; Berke, L.; Gorte, M.; Prasad, K.; Bao, D.; Timmermans-Hereijgers, J.L. The PLETHORA Gene Regulatory Network Guides Growth and Cell Differentiation in Arabidopsis Roots. Plant Cell 2016, 28, 2937–2951. [Google Scholar] [CrossRef]
- Sabatini, S.; Heidstra, R.; Wildwater, M.; Scheres, B. SCARECROW Is Involved in Positioning the Stem Cell Niche in the Arabidopsis Root Meristem. Genes Dev. 2003, 17, 354–358. [Google Scholar] [CrossRef]
- Couzigou, J.-M.; Mondy, S.; Sahl, L.; Gourion, B.; Ratet, P. To Be or Noot to Be: Evolutionary Tinkering for Symbiotic Organ Identity. Plant Signal. Behav. 2013, 8, 4498–4510. [Google Scholar] [CrossRef] [PubMed]
- Couzigou, J.-M.; Zhukov, V.; Mondy, S.; Abu el Heba, G.; Cosson, V.; Ellis, T.N.; Ambrose, M.; Wen, J.; Tadege, M.; Tikhonovich, I. NODULE ROOT and COCHLEATA Maintain Nodule Development and Are Legume Orthologs of Arabidopsis BLADE-ON-PETIOLE Genes. Plant Cell 2012, 24, 4498–4510. [Google Scholar] [CrossRef]
- Ferguson, B.J.; Reid, J.B. Cochleata: Getting to the Root of Legume Nodules. Plant Cell Physiol. 2005, 46, 1583–1589. [Google Scholar] [CrossRef]
- Magne, K.; Couzigou, J.-M.; Schiessl, K.; Liu, S.; George, J.; Zhukov, V.; Sahl, L.; Boyer, F.; Iantcheva, A.; Mysore, K.S.; et al. MtNODULE ROOT1 and MtNODULE ROOT2 Are Essential for Indeterminate Nodule Identity. Plant Physiol. 2018, 178, 295–316. [Google Scholar] [CrossRef]
- Shen, D.; Kulikova, O.; Guhl, K.; Franssen, H.; Kohlen, W.; Bisseling, T.; Geurts, R. The Medicago Truncatula Nodule Identity Gene MtNOOT1 Is Required for Coordinated Apical-Basal Development of the Root. BMC Plant Biol. 2019, 19, 571. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.M.; Jun, J.H.; Nam, H.G.; Fletcher, J.C. BLADE-ON-PETIOLE1 and 2 Control Arabidopsis Lateral Organ Fate through Regulation of LOB Domain and Adaxial-Abaxial Polarity Genes. Plant Cell 2007, 19, 1809–1825. [Google Scholar] [CrossRef]
- Magne, K.; George, J.; Tornero, A.B.; Broquet, B.; Madueño, F.; Andersen, S.U.; Ratet, P. Lotus Japonicus NOOT-BOP-COCH-LIKE1 Is Essential for Nodule, Nectary, Leaf and Flower Development. Plant J. 2018, 94, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Ferraioli, S.; Tatè, R.; Rogato, A.; Chiurazzi, M.; Patriarca, E.J. Development of Ectopic Roots from Abortive Nodule Primordia. MPMI 2004, 17, 1043–1050. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sozzani, R.; Cui, H.; Moreno-Risueno, M.; Busch, W.; Van Norman, J.; Vernoux, T.; Brady, S.; Dewitte, W.; Murray, J.A.H.; Benfey, P. Spatiotemporal Regulation of Cell-Cycle Genes by SHORTROOT Links Patterning and Growth. Nature 2010, 466, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Helariutta, Y.; Fukaki, H.; Wysocka-Diller, J.; Nakajima, K.; Jung, J.; Sena, G.; Hauser, M.-T.; Benfey, P.N. The SHORT-ROOT Gene Controls Radial Patterning of the Arabidopsis Root through Radial Signaling. Cell 2000, 101, 555–567. [Google Scholar] [CrossRef]
- Shani, E.; Yanai, O.; Ori, N. The Role of Hormones in Shoot Apical Meristem Function. Curr. Opin. Plant Biol. 2006, 9, 484–489. [Google Scholar] [CrossRef]
- Jasinski, S.; Piazza, P.; Craft, J.; Hay, A.; Woolley, L.; Rieu, I.; Phillips, A.; Hedden, P.; Tsiantis, M. KNOX Action in Arabidopsis Is Mediated by Coordinate Regulation of Cytokinin and Gibberellin Activities. Curr. Biol. 2005, 15, 1560–1565. [Google Scholar] [CrossRef]
- Yanai, O.; Shani, E.; Dolezal, K.; Tarkowski, P.; Sablowski, R.; Sandberg, G.; Samach, A.; Ori, N. Arabidopsis KNOXI Proteins Activate Cytokinin Biosynthesis. Curr. Biol. 2005, 15, 1566–1571. [Google Scholar] [CrossRef]
- Libbenga, K.R.; van Iren, F.; Bogers, R.J.; Schraag-Lamers, M.F. The Role of Hormones and Gradients in the Initiation of Cortex Proliferation and Nodule Formation in Pisum Sativum, L. Planta 1973, 114, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, A.B.; Sandal, N.; Bek, A.S.; Madsen, L.H.; Jurkiewicz, A.; Nielsen, M.W.; Tirichine, L.; Stougaard, J. Cytokinin Induction of Root Nodule Primordia in Lotus Japonicus Is Regulated by a Mechanism Operating in the Root Cortex. Mol. Plant Microbe Interact. 2011, 24, 1385–1395. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.D.; Karas, B.J.; Sato, S.; Tabata, S.; Amyot, L.; Szczyglowski, K. A Cytokinin Perception Mutant Colonized by Rhizobium in the Absence of Nodule Organogenesis. Science 2007, 315, 101–104. [Google Scholar] [CrossRef]
- Tirichine, L.; Sandal, N.; Madsen, L.H.; Radutoiu, S.; Albrektsen, A.S.; Sato, S.; Asamizu, E.; Tabata, S.; Stougaard, J. A Gain-of-Function Mutation in a Cytokinin Receptor Triggers Spontaneous Root Nodule Organogenesis. Science 2007, 315, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Ariel, F.; Brault-Hernandez, M.; Laffont, C.; Huault, E.; Brault, M.; Plet, J.; Moison, M.; Blanchet, S.; Ichanté, J.L.; Chabaud, M.; et al. Two Direct Targets of Cytokinin Signaling Regulate Symbiotic Nodulation in Medicago Truncatula. Plant Cell 2012, 24, 3838–3852. [Google Scholar] [CrossRef]
- Li, X.; Mo, X.; Shou, H.; Wu, P. Cytokinin-Mediated Cell Cycling Arrest of Pericycle Founder Cells in Lateral Root Initiation of Arabidopsis. Plant Cell Physiol. 2006, 47, 1112–1123. [Google Scholar] [CrossRef]
- Bielach, A.; Podlešáková, K.; Marhavý, P.; Duclercq, J.; Cuesta, C.; Müller, B.; Grunewald, W.; Tarkowski, P.; Benková, E. Spatiotemporal Regulation of Lateral Root Organogenesis in Arabidopsis by Cytokinin. Plant Cell 2012, 24, 3967–3981. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.-H.; Liu, Y.-B.; Zhang, X.-S. Auxin–Cytokinin Interaction Regulates Meristem Development. Mol. Plant 2011, 4, 616–625. [Google Scholar] [CrossRef] [PubMed]
- Gauthier-Coles, C.; White, R.G.; Mathesius, U. Nodulating Legumes Are Distinguished by a Sensitivity to Cytokinin in the Root Cortex Leading to Pseudonodule Development. Front. Plant Sci. 2019, 9, 1901. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Hirsch, A.M. Studying Early Nodulin Gene ENOD40 Expression and Induction by Nodulation Factor and Cytokinin in Transgenic Alfalfa. Plant Physiol. 1998, 116, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U.; Charon, C.; Rolfe, B.G.; Kondorosi, A.; Crespi, M. Temporal and Spatial Order of Events during the Induction of Cortical Cell Divisions in White Clover by Rhizobium Leguminosarum Bv. Trifolii Inoculation or Localized Cytokinin Addition. Mol. Plant Microbe Interact. 2000, 13, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, W.; Li, X.; Jiang, H.; Wu, P.; Xia, K.; Yang, Y.; Wu, G. Knockdown of LjIPT3 Influences Nodule Development in Lotus Japonicus. Plant Cell Physiol. 2014, 55, 183–193. [Google Scholar] [CrossRef]
- Azarakhsh, M.; Rumyantsev, A.M.; Lebedeva, M.A.; Lutova, L.A. Cytokinin Biosynthesis Genes Expressed during Nodule Organogenesis Are Directly Regulated by the KNOX3 Protein in Medicago Truncatula. PLoS ONE 2020, 15, e0232352. [Google Scholar] [CrossRef]
- Mortier, V.; Wasson, A.; Jaworek, P.; De Keyser, A.; Decroos, M.; Holsters, M.; Tarkowski, P.; Mathesius, U.; Goormachtig, S. Role of LONELY GUY Genes in Indeterminate Nodulation on Medicago Truncatula. New Phytologist 2014, 202, 582–593. [Google Scholar] [CrossRef]
- Azarakhsh, M.; Kirienko, A.; Zhukov, V.; Lebedeva, M.; Dolgikh, E.; Lutova, L. KNOTTED1-LIKE HOMEOBOX 3: A New Regulator of Symbiotic Nodule Development. J. Exp. Bot. 2015, 66, 7181–7195. [Google Scholar] [CrossRef]
- Kerstetter, R.; Vollbrecht, E.; Lowe, B.; Veit, B.; Yamaguchi, J.; Hake, S. Sequence Analysis and Expression Patterns Divide the Maize Knotted1-like Homeobox Genes into Two Classes. Plant Cell 1994, 6, 1877–1887. [Google Scholar] [CrossRef]
- Hay, A.; Tsiantis, M. KNOX Genes: Versatile Regulators of Plant Development and Diversity. Development 2010, 137, 3153–3165. [Google Scholar] [CrossRef]
- Kusaba, S.; Kano-Murakami, Y.; Matsuoka, M.; Tamaoki, M.; Sakamoto, T.; Yamaguchi, I.; Fukumoto, M. Alteration of Hormone Levels in Transgenic Tobacco Plants Overexpressing the Rice Homeobox Gene OSH1. Plant Physiol. 1998, 116, 471–476. [Google Scholar] [CrossRef]
- Frugis, G.; Giannino, D.; Mele, G.; Nicolodi, C.; Chiappetta, A.; Bitonti, M.B.; Innocenti, A.M.; Dewitte, W.; Van Onckelen, H.; Mariotti, D. Overexpression of KNAT1 in Lettuce Shifts Leaf Determinate Growth to a Shoot-like Indeterminate Growth Associated with an Accumulation of Isopentenyl-Type Cytokinins. Plant Physiol. 2001, 126, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Furumizu, C.; Alvarez, J.P.; Sakakibara, K.; Bowman, J.L. Antagonistic Roles for KNOX1 and KNOX2 Genes in Patterning the Land Plant Body Plan Following an Ancient Gene Duplication. PLoS Genet. 2015, 11, e1004980. [Google Scholar] [CrossRef]
- Di Giacomo, E.; Sestili, F.; Iannelli, M.A.; Testone, G.; Mariotti, D.; Frugis, G. Characterization of KNOX Genes in Medicago Truncatula. Plant Mol. Biol. 2008, 67, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Coudert, Y.; Novák, O.; Harrison, C.J. A KNOX-Cytokinin Regulatory Module Predates the Origin of Indeterminate Vascular Plants. Curr. Biol. 2019, 29, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, E.; Laffont, C.; Sciarra, F.; Iannelli, M.A.; Frugier, F.; Frugis, G. KNAT3/4/5-like Class 2 KNOX Transcription Factors Are Involved in Medicago Truncatula Symbiotic Nodule Organ Development. New Phytol. 2017, 213, 822–837. [Google Scholar] [CrossRef]
- Vernié, T.; Moreau, S.; de Billy, F.; Plet, J.; Combier, J.-P.; Rogers, C.; Oldroyd, G.; Frugier, F.; Niebel, A.; Gamas, P. EFD Is an ERF Transcription Factor Involved in the Control of Nodule Number and Differentiation in Medicago Truncatula. Plant Cell 2008, 20, 2696–2713. [Google Scholar] [CrossRef]
- Hwang, I.; Sheen, J.; Müller, B. Cytokinin Signaling Networks. Annu. Rev. Plant Biol. 2012, 63, 353–380. [Google Scholar] [CrossRef]
- Miri, M.; Janakirama, P.; Held, M.; Ross, L.; Szczyglowski, K. Into the root: How cytokinin controls rhizobial infection. Trends in plant science 2016, 21, 178–186. [Google Scholar] [CrossRef]
- Boivin, S.; Kazmierczak, T.; Brault, M.; Wen, J.; Gamas, P.; Mysore, K.S.; Frugier, F. Different Cytokinin Histidine Kinase Receptors Regulate Nodule Initiation as Well as Later Nodule Developmental Stages in Medicago Truncatula. Plant Cell Environ. 2016, 39, 2198–2209. [Google Scholar] [CrossRef]
- Dolgikh, E.A.; Kusakin, P.G.; Kitaeva, A.B.; Tsyganova, A.V.; Kirienko, A.N.; Leppyanen, I.V.; Dolgikh, A.V.; Ilina, E.L.; Demchenko, K.N.; Tikhonovich, I.A.; et al. Mutational Analysis Indicates That Abnormalities in Rhizobial Infection and Subsequent Plant Cell and Bacteroid Differentiation in Pea (Pisum Sativum) Nodules Coincide with Abnormal Cytokinin Responses and Localization. Ann. Bot. 2020, 125, 905–923. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Suzaki, T.; Soyano, T.; Kojima, M.; Sakakibara, H.; Kawaguchi, M. Shoot-Derived Cytokinins Systemically Regulate Root Nodulation. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, L.; Liu, B.; Hu, Y.; Cheng, F.; Liang, J.; Aarts, M.G.; Wang, X.; Wu, J. A Transposon Insertion in FLOWERING LOCUS T Is Associated with Delayed Flowering in Brassica Rapa. Plant Sci. 2015, 241, 211–220. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Abe, M. Regulation of Reproductive Development by Non-Coding RNA in Arabidopsis: To Flower or Not to Flower. J. Plant Res. 2012, 125, 693–704. [Google Scholar] [CrossRef]
- Wang, J.-W.; Czech, B.; Weigel, D. MiR156-Regulated SPL Transcription Factors Define an Endogenous Flowering Pathway in Arabidopsis Thaliana. Cell 2009, 138, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Huijser, P.; Schmid, M. The Control of Developmental Phase Transitions in Plants. Development 2011, 138, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.; Amyot, L.; Tian, L.; Xu, Z.; Gruber, M.Y.; Hannoufa, A. Ectopic Expression of MiR156 Represses Nodulation and Causes Morphological and Developmental Changes in Lotus Japonicus. Mol. Genet. Genom. 2015, 290, 471–484. [Google Scholar] [CrossRef]
- Aung, B.; Gruber, M.Y.; Amyot, L.; Omari, K.; Bertrand, A.; Hannoufa, A. Micro RNA 156 as a Promising Tool for Alfalfa Improvement. Plant Biotechnol. J. 2015, 13, 779–790. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Hossain, M.S.; Wang, J.; Valdés-López, O.; Liang, Y.; Libault, M.; Qiu, L.; Stacey, G. MiR172 Regulates Soybean Nodulation. Mol. Plant Microbe Interact. 2013, 26, 1371–1377. [Google Scholar] [CrossRef]
- Yamaguchi, Y.L.; Ishida, T.; Sawa, S. CLE Peptides and Their Signaling Pathways in Plant Development. J. Exp. Bot. 2016, 67, 4813–4826. [Google Scholar] [CrossRef]
- Takahashi, F.; Suzuki, T.; Osakabe, Y.; Betsuyaku, S.; Kondo, Y.; Dohmae, N.; Fukuda, H.; Yamaguchi-Shinozaki, K.; Shinozaki, K. A Small Peptide Modulates Stomatal Control via Abscisic Acid in Long-Distance Signalling. Nature 2018, 556, 235–238. [Google Scholar] [CrossRef]
- Okamoto, S.; Shinohara, H.; Mori, T.; Matsubayashi, Y.; Kawaguchi, M. Root-Derived CLE Glycopeptides Control Nodulation by Direct Binding to HAR1 Receptor Kinase. Nat. Commun. 2013, 4, 2191. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, S.; Suzuki, T.; Kawaguchi, M.; Higashiyama, T.; Matsubayashi, Y. A Comprehensive Strategy for Identifying Long-Distance Mobile Peptides in Xylem Sap. Plant J. 2015, 84, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, M.A.; Yashenkova, Y.S.; Dodueva, I.E.; Lutova, L.A. Molecular Dialog between Root and Shoot via Regulatory Peptides and Its Role in Systemic Control of Plant Development. Russ. J. Plant Physiol. 2020, 67, 985–1002. [Google Scholar] [CrossRef]
- Searle, I.R.; Men, A.E.; Laniya, T.S.; Buzas, D.M.; Iturbe-Ormaetxe, I.; Carroll, B.J.; Gresshoff, P.M. Long-Distance Signaling in Nodulation Directed by a CLAVATA1-like Receptor Kinase. Science 2003, 299, 109–112. [Google Scholar] [CrossRef]
- Nishimura, R.; Hayashi, M.; Wu, G.-J.; Kouchi, H.; Imaizumi-Anraku, H.; Murakami, Y.; Kawasaki, S.; Akao, S.; Ohmori, M.; Nagasawa, M. HAR1 Mediates Systemic Regulation of Symbiotic Organ Development. Nature 2002, 420, 426–429. [Google Scholar] [CrossRef]
- Schnabel, E.; Journet, E.-P.; de Carvalho-Niebel, F.; Duc, G.; Frugoli, J. The Medicago Truncatula SUNN Gene Encodes a CLV1-like Leucine-Rich Repeat Receptor Kinase That Regulates Nodule Number and Root Length. Plant Mol. Biol. 2005, 58, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.E.; Williams, R.W.; Meyerowitz, E.M. The CLAVATA1 gene Encodes a Putative Receptor Kinase That Controls Shoot and Floral Meristem Size in Arabidopsis. Cell 1997, 89, 575–585. [Google Scholar] [CrossRef]
- Mirzaei, S.; Batley, J.; El-Mellouki, T.; Liu, S.; Meksem, K.; Ferguson, B.J.; Gresshoff, P.M. Neodiversification of Homeologous CLAVATA1-like Receptor Kinase Genes in Soybean Leads to Distinct Developmental Outcomes. Sci. Rep. 2017, 7, 1–15. [Google Scholar]
- Stahl, Y.; Grabowski, S.; Bleckmann, A.; Kühnemuth, R.; Weidtkamp-Peters, S.; Pinto, K.G.; Kirschner, G.K.; Schmid, J.B.; Wink, R.H.; Hülsewede, A. Moderation of Arabidopsis Root Stemness by CLAVATA1 and ARABIDOPSIS CRINKLY4 Receptor Kinase Complexes. Curr. Biol. 2013, 23, 362–371. [Google Scholar] [CrossRef]
- Oelkers, K.; Goffard, N.; Weiller, G.F.; Gresshoff, P.M.; Mathesius, U.; Frickey, T. Bioinformatic Analysis of the CLE Signaling Peptide Family. BMC Plant Biol. 2008, 8, 1–15. [Google Scholar] [CrossRef]
- Hastwell, A.H.; Gresshoff, P.M.; Ferguson, B.J. Genome-Wide Annotation and Characterization of CLAVATA/ESR (CLE) Peptide Hormones of Soybean (Glycine Max) and Common Bean (Phaseolus Vulgaris), and Their Orthologues of Arabidopsis Thaliana. J. Exp. Bot. 2015, 66, 5271–5287. [Google Scholar] [CrossRef]
- Nishida, H.; Handa, Y.; Tanaka, S.; Suzaki, T.; Kawaguchi, M. Expression of the CLE-RS3 Gene Suppresses Root Nodulation in Lotus Japonicus. J. Plant Res. 2016, 129, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.C.; Brand, U.; Running, M.P.; Simon, R.; Meyerowitz, E.M. Signaling of Cell Fate Decisions by CLAVATA3 in Arabidopsis Shoot Meristems. Science 1999, 283, 1911–1914. [Google Scholar] [CrossRef]
- Nishida, H.; Suzaki, T. Nitrate-Mediated Control of Root Nodule Symbiosis. Curr. Opin. Plant Biol. 2018, 44, 129–136. [Google Scholar] [CrossRef]
- Luo, Z.; Lin, J.; Zhu, Y.; Fu, M.; Li, X.; Xie, F. NLP1 Reciprocally Regulates Nitrate Inhibition of Nodulation through SUNN-CRA2 Signaling in Medicago Truncatula. Plant Commun. 2021, 2, 100183. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Ogawa, M.; Matsubayashi, Y. Identification of a Biologically Active, Small, Secreted Peptide in Arabidopsis by in Silico Gene Screening, Followed by LC-MS-based Structure Analysis. Plant J. 2008, 55, 152–160. [Google Scholar] [CrossRef]
- Mohd-Radzman, N.A.; Laffont, C.; Ivanovici, A.; Patel, N.; Reid, D.; Stougaard, J.; Frugier, F.; Imin, N.; Djordjevic, M.A. Different Pathways Act Downstream of the CEP Peptide Receptor CRA2 to Regulate Lateral Root and Nodule Development. Plant Physiol. 2016, 171, 2536–2548. [Google Scholar] [CrossRef]
- Laffont, C.; Huault, E.; Gautrat, P.; Endre, G.; Kalo, P.; Bourion, V.; Duc, G.; Frugier, F. Independent Regulation of Symbiotic Nodulation by the SUNN Negative and CRA2 Positive Systemic Pathways1. Plant Physiol. 2019, 180, 559–570. [Google Scholar] [CrossRef]
- Tsikou, D.; Yan, Z.; Holt, D.B.; Abel, N.B.; Reid, D.E.; Madsen, L.H.; Bhasin, H.; Sexauer, M.; Stougaard, J.; Markmann, K. Systemic Control of Legume Susceptibility to Rhizobial Infection by a Mobile MicroRNA. Science 2018, 362, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Gautrat, P.; Laffont, C.; Frugier, F. Compact Root Architecture 2 Promotes Root Competence for Nodulation through the MiR2111 Systemic Effector. Curr. Biol. 2020, 30, 1339–1345. [Google Scholar] [CrossRef]
- Takahara, M.; Magori, S.; Soyano, T.; Okamoto, S.; Yoshida, C.; Yano, K.; Sato, S.; Tabata, S.; Yamaguchi, K.; Shigenobu, S. Too Much Love, a Novel Kelch Repeat-Containing F-Box Protein, Functions in the Long-Distance Regulation of the Legume–Rhizobium Symbiosis. Plant Cell Physiol. 2013, 54, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-T.; Kuo, Y.-W.; King, Y.-C.; Lin, H.-H.; Tu, P.-Y.; Tung, K.-S.; Jeng, S.-T. Regulation of MicoRNA2111 and Its Target IbFBK in Sweet Potato on Wounding. Plant Sci. 2020, 292, 110391. [Google Scholar] [CrossRef] [PubMed]
- Thibivilliers, S.; Farmer, A.; Libault, M. Biological and Cellular Functions of the Microdomain-Associated FWL/CNR Protein Family in Plants. Plants 2020, 9, 377. [Google Scholar] [CrossRef]
- Libault, M.; Zhang, X.; Govindarajulu, M.; Qiu, J.; Ong, Y.T.; Brechenmacher, L.; Berg, R.H.; Hurley-Sommer, A.; Taylor, C.G.; Stacey, G. A Member of the Highly Conserved FWL (Tomato FW2. 2-like) Gene Family Is Essential for Soybean Nodule Organogenesis. Plant J. 2010, 62, 852–864. [Google Scholar] [CrossRef]
- Qiao, Z.; Brechenmacher, L.; Smith, B.; Strout, G.W.; Mangin, W.; Taylor, C.; Russell, S.D.; Stacey, G.; Libault, M. The GmFWL1 (FW2-2-like) Nodulation Gene Encodes a Plasma Membrane Microdomain-associated Protein. Plant Cell Environ. 2017, 40, 1442–1455. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.M.; Harrison, M.J. Phytohormones, MiRNAs, and Peptide Signals Integrate Plant Phosphorus Status with Arbuscular Mycorrhizal Symbiosis. Curr. Opin. Plant Biol. 2019, 50, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Karlo, M.; Boschiero, C.; Landerslev, K.G.; Blanco, G.S.; Wen, J.; Mysore, K.S.; Dai, X.; Zhao, P.X.; de Bang, T.C. The CLE53–SUNN Genetic Pathway Negatively Regulates Arbuscular Mycorrhiza Root Colonization in Medicago Truncatula. J. Exp. Bot. 2020, 71, 4972–4984. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).