Abstract
Erectile dysfunction (ED) is a common yet frequently underrecognized microvascular complication of diabetes, affecting up to three out of four individuals. Key contributing factors include advancing age, long-standing disease duration, and suboptimal glycemic control, as well as insulin resistance and androgen deficiency—the latter being particularly common in men with type 2 diabetes (T2D) and obesity. While numerous studies have investigated the effects of various antidiabetic therapies on diabetes-related ED, the results remain inconsistent, limiting definitive conclusions. In recent years, increasing attention has focused on a novel class of antidiabetic medications, namely glucagon-like peptide-1 receptor agonists (GLP-1 RAs). These agents have become central to the treatment of T2D due to their potent glucose-lowering properties and well-documented benefits on cardiovascular outcomes, and weight loss. Given these pleiotropic effects, GLP-1 RAs have been presumed to positively influence erectile function—a hypothesis supported by a growing body of experimental and clinical research. However, preliminary reports have also raised concerns about a possible association between GLP-1 RA use and ED. This narrative review aims to synthesize current evidence regarding the impact of GLP-1 RAs on erectile function, providing a platform for future research in this evolving field.
1. Introduction
Erectile function is a cornerstone of male sexual health, and its impairment can negatively affect overall quality of life (QoL), emotional intimacy, and relationship satisfaction. Erectile dysfunction (ED) is defined as the persistent inability to achieve or maintain an erection sufficient for satisfactory sexual performance and can significantly diminish sexual well-being [1,2]. The diagnosis of ED relies on a comprehensive clinical approach, including detailed medical and sexual history, physical examination, laboratory evaluations, and standardized assessment questionnaires [1,3]. Among the most widely used instruments is the 15-item International Index of Erectile Function (IIEF), while its abridged 5-item version (IIEF-5) provides a practical alternative for clinical settings. In addition to facilitating diagnosis, the IIEF-5 enables classification of ED severity, with scores ranging from severe ED (5–7) to no ED (22–25) [4]. First-line treatment for ED typically involves oral phosphodiesterase type 5 (PDE5) inhibitors, such as sildenafil, tadalafil, vardenafil, and avanafil. These agents act by inhibiting the PDE5 enzyme, thereby increasing cyclic guanosine monophosphate (cGMP) levels. Elevated cGMP promotes smooth muscle relaxation and enhances blood flow to the corpus cavernosum during sexual arousal, thereby facilitating penile erection [5].
ED is particularly prevalent among men with diabetes and has also been reported in individuals with prediabetes [6]. Hyperglycemia, insulin resistance (IR) and hypogonadism, particularly in the presence of obesity, constitutes the core pathophysiological triad underlying diabetes-induced ED [7]. Men with diabetes are approximately 3.5 times more likely to experience ED compared to their non-diabetic counterparts [8]. Although numerous epidemiological studies have sought to quantify the prevalence of diabetes-related ED, reported rates vary considerably, ranging from 35% to 75% [9]. A recent umbrella review encompassing data from 108,030 men with diabetes estimated a global pooled prevalence of 65.8% [10]. Notably, prevalence appears to be lower among men with type 1 diabetes (T1D), with reported rates up to 42.5% [11,12].
Several risk factors have been implicated in the development of diabetes-associated ED. These factors encompass advancing age, long-standing disease duration, and poor glycemic control as captured by high glycated hemoglobin (HbA1c) levels, and glycemic variability detected via continuous glucose monitoring (CGM). Additional risk factors include cardiometabolic comorbidities, including hypercholesterolemia, hypertension, cardiovascular disease (CVD), and obesity, as well as diabetes-related microvascular complications [11,12,13,14]. Notably, a recent study introduced a predictive model with strong performance (concordance index [C-index] = 0.827, 95% confidence interval [CI] = 0.772–0.882) for early detection of ED in subjects with type 2 diabetes (T2D), incorporating variables such as diabetes duration, carotid intima-media thickness (CIMT), and low-density lipoprotein cholesterol (LDL-C) levels [15].
Research has also explored the impact of various antidiabetic therapies on erectile function; however, the current evidence remains limited and ambiguous. In this context, increasing attention has been directed toward the potential effect of newer incretin-based therapies on erectile health, particularly glucagon-like peptide-1 receptor agonists (GLP-1 RAs) [16]. GLP-1 RAs have transformed the landscape of diabetes care by providing not only effective glycemic control but also substantial cardiovascular (CV) protection and significant weight loss [17,18]. Given that endothelial integrity and body weight are critical determinants of erectile function, it is plausible to hypothesize that these therapeutic advantages might extend to this domain as well. But does the available evidence truly support this assumption?
This narrative review examines the current evidence regarding the influence of GLP-1 RAs on erectile function. It begins with a discussion of the physiology of penile erection, and the pathophysiological mechanisms underlying diabetes-related ED, followed by a summary of the pharmacological and clinical characteristics of GLP-1 RAs. Subsequently, it presents the available preclinical and clinical data regarding their role in modulating erectile physiology. Finally, the review considers the impact of other antidiabetic drug classes on erectile function, offering a comparative perspective with GLP-1 RAs. Limitations of the existing literature are discussed, along with current challenges and future directions for research aimed at elucidating the therapeutic potential of GLP-1 RAs in this context.
2. Physiological Mechanisms Underlying Male Sexual Arousal
Sexual desire may be elicited by a variety of stimuli, including visual, auditory, tactile, or cognitive cues, which activate neural pathways that facilitate the relaxation of vascular and cavernosal smooth muscle. This relaxation allows for increased arterial inflow into the corpora cavernosa, a process governed by neurovascular mechanisms originating from hypothalamic centers and the sacral spinal cord (SSC) [19].
Central to this process is the neurotransmitter nitric oxide (NO), which is synthesized and released by two main categories of neurons. Non-adrenergic, non-cholinergic (NANC) neurons located in the pelvic plexus and pelvic nerves express neuronal nitric oxide synthase (nNOS) and release NO directly into adjacent vascular smooth muscle cells (VSMCs). In parallel, cholinergic parasympathetic neurons, whose cell bodies reside in the sacral parasympathetic nucleus (S2–S4) and project to the pelvic plexus and corpora cavernosa, release acetylcholine. Acetylcholine acts on M3 muscarinic receptors expressed on endothelial cells, activating endothelial nitric oxide synthase (eNOS) and promoting NO production [20,21]. Once produced, NO diffuses into neighboring VSMCs, where it stimulates soluble guanylyl cyclase (sGC), catalyzing the conversion of guanosine triphosphate (GTP) into cGMP. Elevated intracellular cGMP levels activate protein kinase G type 1 (PKG-1), which lowers cytosolic calcium concentrations and activates calcium-dependent potassium (KCa) channels, favoring VSMC hyperpolarization and smooth muscle relaxation [22,23].
Testosterone secreted by the testes exerts both genomic and non-genomic effects [24]. Through its interaction with nuclear androgen receptors (ARs) in endothelial and VSMCs, the hormone modulates gene expression to support vascular integrity. In addition, testosterone exerts non-genomic actions via membrane-bound androgen receptors (mARs) that function independently of classical AR activation [25]. These mechanisms collectively enhance endothelial NO production and stimulate the release of endothelium-derived hyperpolarizing factors (EDHFs), such as epoxyeicosatrienoic acids, hydrogen peroxide, and potassium ions, which further contribute to vasodilation within the erectile tissue [26].
As the corpora cavernosa fill with blood and expand, the resulting increase in intracavernosal pressure compresses the subtunical venous plexus beneath the tunica albuginea, restricting venous outflow and helping to maintain the erection. Additionally, contraction of pelvic floor muscles, such as the bulbospongiosus and ischiocavernosus further compresses the corpora cavernosa, enhancing penile rigidity and erection stability [27]. Figure 1 illustrates the main pathophysiological mechanisms driving male erection.
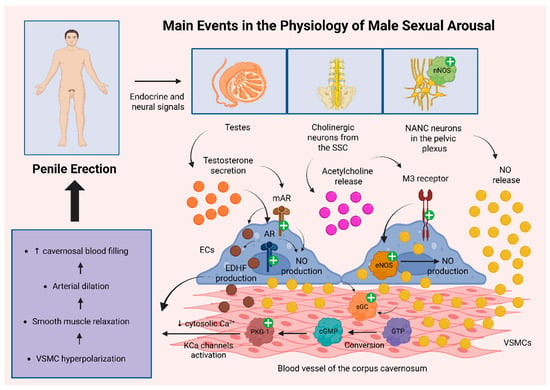
Figure 1.
Key pathophysiological mechanisms underlying penile erection. NO plays a central role in the regulation of male erection, with two primary sources: (1) activation of nNOS in NANC neurons of the pelvic plexus, leading to direct NO release into VSMCs, and (2) activation of eNOS in endothelial cells upon stimulation of muscarinic M3 receptors by Ach released from cholinergic neurons in the SSC, with NO subsequently diffusing to VSMCs. In both cases, NO activates sGC in VSMCs, catalyzing the conversion of GTP to cGMP. Elevated cGMP levels activate PKG-1, which in turn decreases cytosolic calcium concentrations and activates KCa channels, resulting in VSMC hyperpolarization. Testosterone also enhances NO production via activation of androgen receptors (ARs and mARs), contributing to both nNOS and eNOS pathways. Additionally, testosterone stimulates the production of EDHFs, which further support VSMC hyperpolarization. The resulting hyperpolarization induces smooth muscle relaxation and vasodilation, leading to increased cavernosal blood flow and penile erection [20,21,22,23,24,25,26]. Abbreviations: Ach: acetylcholine; AR: androgen receptor; cGMP: cyclic guanosine monophosphate; ECs: endothelial cells; EDHF: endothelium-derived hyperpolarizing factor; eNOS: endothelial nitric oxide synthase; GTP: guanosine triphosphate; KCa: calcium-dependent potassium; M3 receptor: muscarinic M3 receptor; mAR: membrane-bound androgen receptor; NANC: non-adrenergic, non-cholinergic; nNOS: neuronal nitric oxide synthase; NO: nitric oxide; PKG-1: protein kinase G type 1; sGC: soluble guanylyl cyclase; SSC: sacral spinal cord; VSMC: vascular smooth muscle cell; +: activation; ↑: increase; ↓: decrease. Created in BioRender. Kounatidis, D. (2025) https://BioRender.com/rykt1qj (assessed on 27 August 2025).
3. Pathophysiology of Erectile Dysfunction in Patients with Diabetes
3.1. Hyperglycemia
Poor glycemic control is a major contributing factor to the development of ED, regardless of diabetes type. Persistent hyperglycemia contributes to ED through several interconnected mechanisms, primarily driven by the generation of advanced glycation end products (AGEs) and reactive oxygen species (ROS) [28]. AGEs constitute a heterogeneous group of compounds formed via non-enzymatic reactions between reducing sugars (e.g., glucose) and proteins, lipids, or nucleic acids. Prolonged hyperglycemia accelerates this glycation process, resulting in the accumulation of AGEs both endogenously through metabolic pathways and exogenously from dietary sources. Over time, AGEs undergo further irreversible modifications that disrupt cellular and tissue function. These effects are mainly mediated through their interaction with the receptor for advanced glycation end products (RAGE) [29].
AGEs exacerbate ED by promoting oxidative stress and attenuating NO bioavailability, thereby impairing the relaxation of cavernosal smooth muscle [30]. This dysfunction is further exacerbated by the decreased expression and activity of both nNOS and eNOS, leading to impaired neurovascular regulation of penile erection [31,32,33]. Additionally, in diabetes, a state of NO resistance emerges, characterized by diminished responsiveness of cavernosal smooth muscle cells to NO signaling. This resistance is primarily driven by increased oxidative stress, and desensitization of the sGC–cGMP–PKG signaling cascade [34]. Moreover, hyperglycemia impairs maximal cavernosal smooth muscle relaxation in response to both acetylcholine and nitrergic nerve stimulation. This dysfunction is linked to enhanced activation of key signaling pathways and molecules, including Rho-associated kinase 2 (ROCK2), p38 mitogen-activated protein kinase (p38 MAPK), and arginase II [35]. ROCK2 signaling is critical in regulating vascular tone, cytoskeletal dynamics, intercellular adhesion, and endothelial barrier function [36]. Research has shown that in diabetic mice with heterozygous deficiency of ROCK2, these pathological changes are significantly reduced or even absent [35].
Beyond impairments in cavernosal relaxation, ROS accumulation and oxidative stress linked to AGE formation promote reductions in antioxidant responses, lipid peroxidation, DNA damage, and oxidative protein modifications [37,38]. Nitration of tyrosine residues to form 3-nitrotyrosine (3-NT) alters protein structure and function, disrupting enzymatic activity and intracellular signaling. These reactions, mediated by reactive nitrogen species (RNS), further reduce NO availability, ultimately worsening endothelial dysfunction and impairing vasorelaxation [39]. Interestingly, recent evidence supports that the combined accumulation of AGEs and ROS triggers various forms of programmed cell death, including apoptosis, pyroptosis, and ferroptosis, resulting in ED aggravation [40].
Low-intensity pulsed ultrasound (LIPA) may exert protective effects against AGEs-induced endothelial injury in the corpus cavernosum by promoting mitophagy, thereby preserving mitochondrial function and cellular homeostasis [41]. Furthermore, sustained high glucose levels might facilitate corpus cavernosum fibrosis via the transforming growth factor beta 1/SMAD family proteins/connective tissue growth factor (TGF-β1/Smad/CTGF) signaling pathway [42]. Epidemiological evidence indicates an association between diabetes and Peyronie’s disease, with diabetes recognized as a risk factor for its development [43].
The frequent coexistence of comorbidities in diabetes can intensify ED by amplifying the aforementioned pathophysiological processes. For instance, the coexistence of diabetes and hypertension might exhibit a synergistic negative effect on erectile function by enhancing oxidative stress, inflammation, and apoptosis [44]. Notably, ED may persist or even worsen despite appropriate antihypertensive treatment, as certain medications, especially thiazide diuretics, beta-adrenergic blockers, and aldosterone antagonists, have been linked to detrimental effects [45]. Moreover, hypercholesterolemia may further impair NO signaling by promoting the overactivation of nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) oxidase subunits, leading to elevated superoxide production and eNOS uncoupling [46].
3.2. Insulin Resistance
IR may contribute to ED development, primarily through its noxious impact on endothelial function. By impairing endothelial NO synthesis and attenuating insulin-mediated vasodilation, IR disrupts the vascular mechanisms essential for erection [47]. In a model of insulin-resistant, obese mice, short-term pharmacological improvement of IR was sufficient to restore erectile function. This restoration was attributed to the normalization of both endothelium-dependent and nerve-mediated corpus cavernosum relaxation, along with reactivation of the cGMP signaling pathway and attenuation of cavernosal hypercontractility [48]. Clinical research further supports this association. In a prospective study involving 283 men with ED lasting at least six months, IR was identified in nearly half of the participants. Notably, IR was independently associated not only with the presence of ED but also with its severity [49]. Several studies, including analyses from large-scale datasets such as the National Health and Nutrition Examination Survey (NHANES), have identified surrogate markers related to IR that could potentially facilitate early risk assessment for ED. These indices include the homeostasis model assessment of insulin resistance (HOMA-IR), the triglyceride-glucose (TyG) index, as well as the metabolic score for insulin resistance (METS-IR). However, considerable heterogeneity among studies and variability in the diagnostic performance of these indices currently limit their clinical utility as predictive tools for ED [50,51,52].
3.3. Hypogonadism
Hypogonadism, defined by impaired testosterone production due to the dysfunction of the testes, hypothalamus, or pituitary gland, serves as a key metabolic disturbance linking diabetes and ED [53]. Both reduced circulating testosterone levels and diminished concentrations of its primary plasma binding protein, sex hormone-binding globulin (SHBG), have been identified as independent predictors of T2D development [54,55]. When present alongside obesity, androgen deficiency augments IR, creating a bidirectional loop that worsens both T2D and ED [56]. Although obesity is commonly associated with T2D, it is increasingly observed in T1D, giving rise to the concept of ‘’double diabetes’’ [57]. Moreover, hypertension and other comorbidities may further compound testosterone-driven impairments in erectile function [58].
Androgen deprivation disrupts vascular homeostasis by promoting vasoconstriction, VSMC apoptosis, adipocyte infiltration, and fibrosis of the tunica albuginea [59,60,61,62]. Within the corpora cavernosa, the absence of adequate androgen signaling leads to reduced expression of eNOS and PDE5 mRNA, with the severity of the functional consequences correlating with the extent of enzymatic downregulation [63]. Persistent high glucose levels have been linked to decreased testosterone levels, in part due to the downregulation of vascular endothelial growth factor (VEGF), which compromises testicular microcirculation, leading to Leydig cell dysfunction and blunted testosterone synthesis [64]. On the other hand, disrupted insulin signaling mitigates hypothalamic kisspeptin expression, perturbing the hypothalamic–pituitary–gonadal (HPG) axis via altered mammalian target of rapamycin (mTOR)–kisspeptin pathway activity and impaired gonadotropin-releasing hormone (GnRH) secretion [65,66].
The HPG axis is further suppressed in obesity, through increased aromatase activity in adipose depots, driving peripheral conversion of androgens to estrogens. Elevated estradiol activates hypothalamic estrogen receptors, triggering a negative feedback on gonadotropin release and further lowering testosterone output [67]. This androgen deficiency skews stromal progenitor cells (SPCs) toward adipogenic differentiation, causing ectopic lipid accumulation within erectile tissue and worsening structural dysfunction [68]. Interestingly, a recent transcriptomic analysis has identified aberrant expression patterns of adiponectin receptors in men with ED [69].
Collectively, the combined effects of prolonged hyperglycemia, obesity-driven hormonal imbalance, and androgen deprivation promote the development of a chronic pro-inflammatory milieu that favors progressive endothelial dysfunction [70]. Figure 2 presents key pathophysiological events and mechanisms underlying ED in individuals with diabetes.
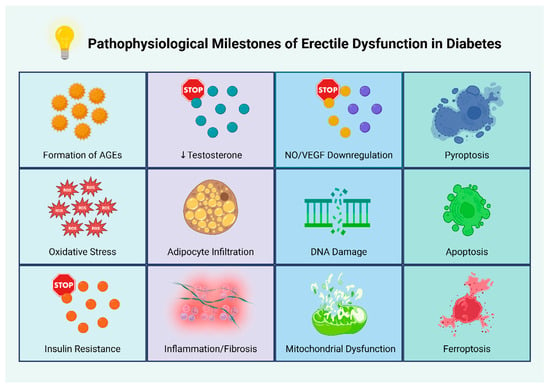
Figure 2.
Pathophysiological milestones of erectile dysfunction in diabetes. Abbreviations: AGEs: advanced glycation end products; NO: nitric oxide; ROS: reactive oxygen species; VEGF: vascular endothelial growth factor [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69]. Created in BioRender. Kounatidis, D. (2025) https://BioRender.com/ebz5ezk (assessed on 28 July 2025).
4. GLP-1 Receptor Agonists: Therapeutic Impact and Safety Considerations
4.1. Pharmacological Profile and Clinical Benefits of GLP-1 Receptor Agonists
GLP-1 RAs constitute a novel class of antidiabetic medications that have reshaped the management of T2D. These agents function by mimicking the actions of endogenous GLP-1, binding to its receptor to enhance glucose-dependent insulin secretion from pancreatic β-cells while suppressing glucagon release from α-cells. This glucose-dependent mechanism offers the benefit of avoiding hypoglycemia, while simultaneously contributing to effective glycemic control [71]. GLP-1 RAs are classified into short-acting and long-acting agents based on their pharmacokinetic properties. Short-acting agents, such as exenatide twice-daily and lixisenatide, primarily target postprandial glucose excursions by delaying gastric emptying. In contrast, long-acting GLP-1 RAs, including exenatide long-acting release (exenatide LAR), liraglutide, dulaglutide, and semaglutide, provide more sustained reductions in fasting plasma glucose (FPG) by continuously enhancing insulin secretion and suppressing glucagon release [72]. In contemporary clinical practice, once-weekly injectable formulations, particularly semaglutide are preferred, due to their superior efficacy and ease of use [71].
Beyond glucose control, GLP-1 RAs have gained prominence for their CV benefits, especially in individuals with established CVD. These benefits are not limited to patients with T2D but also extend to those with obesity, even in the absence of T2D [73]. The landmark LEADER (Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results) trial, which enrolled 9340 individuals with T2D at high CV risk, demonstrated that liraglutide significantly reduced the incidence of major adverse cardiovascular events (MACE) compared with placebo over a median follow-up of 3.8 years (Hazard Ratio [HR]: 0.87; 95% CI: 0.78–0.97; p = 0.01 for superiority; p < 0.001 for noninferiority) [74]. Similar CV benefits were observed in the SUSTAIN-6 trial for semaglutide. This study included 3297 patients with T2D at high CV risk, 83% of whom had established CVD, chronic kidney disease (CKD), or both. Once-weekly semaglutide significantly reduced the incidence of the primary composite outcome (first occurrence of CV death, nonfatal myocardial infarction, or nonfatal stroke) compared with placebo (6.6% vs. 8.9%; HR: 0.74; 95% CI: 0.58–0.95; p < 0.001 for noninferiority [75]. Prescription of semaglutide may also provide renal protective benefits in patients with T2D and CKD. Recent evidence indicated that its use reduced the risk of a composite kidney-specific outcome, including progression to kidney failure or a sustained ≥50% decline in estimated glomerular filtration rate (eGFR), by 21% (HR: 0.79; 95% CI: 0.66–0.94) and attenuated the annual decline in eGFR by 1.16 mL/min/1.73 m2 (p < 0.001) [76].
One of the most impactful therapeutic benefits of GLP-1 RAs is their capacity to induce substantial and sustained weight loss, particularly for liraglutide and semaglutide. Although the precise mechanisms underlying this effect are not fully elucidated, it is thought to involve central appetite suppression, enhanced satiety, and delayed gastric emptying [77]. High-dose formulations of these agents, specifically liraglutide at 3.0 mg and semaglutide at 2.4 mg, have been approved for the treatment of obesity, independent of glycemic status [78]. More recently, tirzepatide, a dual glucose-dependent insulinotropic polypeptide (GIP)/GLP-1 receptor agonist, has demonstrated superior weight-reducing effects compared with both placebo and semaglutide, particularly in the SURMOUNT trials, with these benefits sustained for up to 72 weeks [79,80,81].
GLP-1 RAs have also shown efficacy in metabolic dysfunction-associated steatotic liver disease (MASLD) and are now recommended as a first-line antidiabetic treatment for patients with metabolic dysfunction-associated steatohepatitis (MASH) and diabetes [82]. In a 24-week trial, dulaglutide administration produced a 26.4% relative decrease in liver fat content alongside significant reductions in gamma-glutamyl transpeptidase (GGT) levels [83]. Semaglutide has yielded even greater benefits, with its maximal dosage over 72 weeks increasing the rate of MASH resolution without fibrosis progression (59% vs. 17% with placebo; p < 0.001) [84]. The wide-ranging biological effects of GLP-1 RAs have further prompted investigation into other conditions, including Alzheimer’s disease (AD), where their impact appears favorable, although the current evidence remains preliminary [85].
4.2. Adverse Effects and Safety Profile of GLP-1 Receptor Agonists
The most frequently reported adverse effects of GLP-1 RAs are gastrointestinal, including nausea, vomiting, diarrhea, and constipation. These events are typically dose-dependent and occur most often during treatment initiation or dose escalation. They are attributed to mechanisms overlapping with the weight-reducing properties of GLP-1 RAs, particularly delayed gastric emptying, which is considered the predominant factor [86,87]. A modest increase in heart rate has also been observed, although current evidence does not support an increased risk of arrhythmia [86].
GLP-1 RAs have also been associated with pancreatitis, which has predominantly been linked to gallbladder disease as a secondary effect of weight loss [88]. In addition, evidence suggests that GLP-1 RAs may increase pancreatic enzyme secretion, while the expression and subsequent stimulation of GLP-1 receptors in pancreatic islet and exocrine duct cells have been correlated with overgrowth of the ductal epithelial cells, resulting in hyperplasia and chronic low-grade or acute inflammation, potentially leading to pancreatitis [89]. Nevertheless, the true incidence appears to be low, and several studies have questioned the existence of a causal relationship [90,91,92]. Earlier experimental data are consistent with this view, showing that although GLP-1 receptor activation can increase pancreatic mass and alter gene expression, it does not confer susceptibility to pancreatitis [93]. GLP-1 RAs have also been associated with an increased risk of pancreatic cancer, given the established link between pancreatitis and pancreatic malignancy. Nevertheless, recent evidence largely supports the safety profile of GLP-1 RAs. Regarding other malignancies, preclinical and clinical studies suggest potential anti-cancer properties through suppression of tumor initiation and progression. An important exception includes individuals with a personal or family history of medullary thyroid carcinoma (MTC), where GLP-1 RAs are contraindicated. This recommendation is primarily based on rodent studies demonstrating increased calcitonin secretion and C-cell hyperplasia, although only minimal GLP-1 receptor expression has been reported in human C-cells [94].
With the increasing adoption of GLP-1 RAs, less common adverse effects are receiving increased attention. Recent pharmacovigilance data have implicated GLP-1 RAs, particularly semaglutide, in the development of androgenetic alopecia [95,96]. The precise mechanisms remain insufficiently defined, with available data relying largely on speculations. The leading hypothesis posits that rapid weight loss may induce metabolic and nutrient alterations that trigger telogen effluvium [97]. Ocular safety has also emerged as a key consideration in GLP-1 RA therapy, encompassing worsening of diabetic retinopathy (DR), particularly in semaglutide users with pre-existing DR at baseline, as well as ischemic optic neuropathy (ION) [75,98,99]. Current evidence suggests that these effects are more likely attributable to rapid improvements in glycemic control rather than a direct pharmacological action of GLP-1 RAs. In contrast, other investigations have not confirmed this association, while preclinical research has suggested a potential protective role through modulation of the ROCK2 pathway within the retinal microvasculature [100,101,102,103]. Of note, a very recent in vitro study demonstrated that semaglutide exerted potent antioxidative and cytoprotective effects in retinal endothelial cells, thereby facilitating wound healing [104]. Figure 3 summarizes the main advantages and disadvantages of GLP-1 RA prescription.
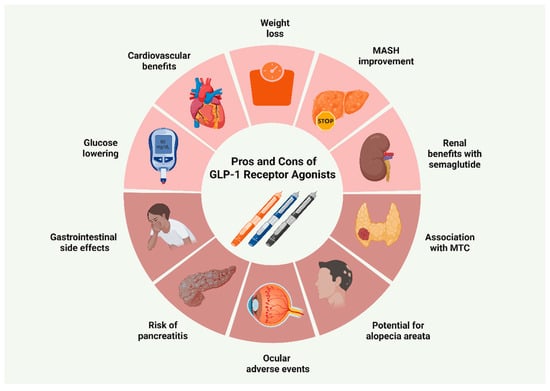
Figure 3.
Benefits and drawbacks of GLP-1 receptor agonist use. Abbreviations: GLP-1: glucagon-like peptide-1; MASH: metabolic dysfunction-associated steatohepatitis; MTC: medullary thyroid carcinoma [71,73,76,77,82,86,90,94,95,96,98,99]. Created in BioRender. Kounatidis, D. (2025) https://BioRender.com/ez8dhoa (assessed on 28 July 2025).
The broader implications of GLP-1 RAs on diabetic microvascular disease, including ED, remain only partially understood. Both animal and human studies increasingly support the beneficial impact of GLP-1 RAs on erectile performance, primarily by improving penile vascular health and endothelial function, as well as through their weight-reducing properties [105,106]. Nevertheless, a limited number of studies have reported potential deterioration in erectile function following GLP-1 RA initiation [107]. Given these contradictory findings, the subsequent sections will shed light on the existing literature to determine whether current evidence permits any definitive clinical conclusions regarding the impact of GLP-1 RAs on erectile function.
5. Cardiovascular and Potential Erectile Implications of GLP-1 Receptor Agonists: Insights from Preclinical Research
5.1. Cardiovascular Effects of GLP-1 Receptor Agonists
A significant body of preclinical research has illuminated the beneficial impact of GLP-1 RAs on endothelial function, largely attributed to the widespread distribution of GLP-1 receptors on endothelial cells throughout different anatomical regions [108,109]. For instance, GLP-1 receptor expression has been identified in human umbilical vein endothelial cells (HUVECs), where GLP-1 appears to exert direct anti-inflammatory effects. These actions are primarily mediated via suppression of RAGE expression, through activation of the cyclic adenosine monophosphate (cAMP) signaling pathway [110]. Additional studies have indicated that GLP-1 enhances endothelial function in diabetic vasculature by reducing endothelial permeability and contractility, while also offering protection against oxidative stress and autophagic processes [111,112].
Endothelium-protective effects have been observed with both short- and long-acting GLP-1 RAs. For instance, exenatide has been shown to ameliorate endothelial dysfunction induced by hyperglycemia and hyperlipidemia through direct activation of eNOS via the AMP-activated protein kinase (AMPK) pathway [113]. Lixisenatide not only upregulates eNOS but may also promote VEGF expression, with evidence suggesting that some of its vascular effects may occur independently of GLP-1 receptor activation [114]. In the case of liraglutide, research demonstrated enhanced endothelial function via stimulation of the mammalian target of rapamycin complex 2/protein kinase B (mTORC2)/Akt pathway, resulting in increased NO production, elevated telomerase activity, and anti-apoptotic effects [115]. Liraglutide has also been reported to attenuate VSMC proliferation and atherosclerotic progression through AMPK activation and cell cycle arrest mechanisms [116]. Interestingly, in endothelial cells derived from individuals with diabetes, liraglutide alleviated endoplasmic reticulum stress, reduced c-Jun N-terminal kinase (JNK) activity, and restored insulin-mediated eNOS activation [117].
Once-weekly formulations have also produced favorable outcomes. Dulaglutide has been shown to reduce hyperglycemia-induced endothelial injury, potentially by preserving sirtuin 1 (SIRT1) levels and curbing mitochondrial fission [118], as well as via the downregulation of the NLR family pyrin domain containing 3 (NLRP3) inflammasome [119]. On the other hand, semaglutide has exhibited vascular protective effects by mitigating inflammatory damage to endothelial progenitor cells (EPCs), likely through suppression of microRNA-155 (MiR-155) in macrophage-derived exosomes [120]. It may also contribute to the maintenance of endothelial barrier integrity by limiting extracellular matrix components associated with heightened permeability [121]. Further findings revealed that semaglutide reversed cerebral microvascular rarefaction induced by high-fat diet and supported the structural coherence of the neurovascular unit [122]. Notably, the drug appears to outperform dietary interventions in improving endothelial and cardiac function in obesity-associated heart failure, possibly via modulation of inflammatory pathways, immune cell responses, and adipose tissue remodeling [123].
GLP-1 RAs may also confer indirect benefits on endothelial function through their weight-reducing properties. Weight loss not only improves glycemic control but also reduces ectopic adipose tissue depots, such as perivascular and epicardial fat, that exert direct deleterious effects on the vascular endothelium [124]. Weight loss also contributes to improved insulin sensitivity. Beyond these indirect effects, GLP-1 RAs have been shown to exert direct actions on insulin signaling, including enhanced glucose uptake, reduced oxidative stress, and improved lipid metabolism [125]. These mechanisms collectively underscore the central role of GLP-1 RAs in promoting CV health—mechanisms that may likewise translate to improved erectile function, given the shared pathophysiology between CVD and ED [126]. However, dedicated studies specifically evaluating the effects of GLP-1 RAs on erectile function remain scarce.
5.2. Potential Impact of GLP-1 Receptor Agonists on Erectile Function
Existing data point to a positive role of exenatide in facilitating NO–dependent smooth muscle relaxation within the corpus cavernosum, likely mediated by reduced NADPH oxidase activity. In a rat model of cavernosal dysfunction, Dalaklioglu et al. reported that exenatide significantly improved erectile function, correlating with restoration of endothelium-dependent and neurogenic relaxation, diminished oxidative stress and apoptosis, and normalization of signaling within the eNOS and ROCK2 pathways [127]. Similar effects have been reported for liraglutide, which targets overlapping molecular pathways involved in smooth muscle tone regulation. Beyond its vasodilatory properties, liraglutide has been shown to promote structural remodeling of penile tissue by enhancing autophagic activity, thereby facilitating tissue regeneration independently of its glucose-lowering or weight-reducing effects [128]. Furthermore, liraglutide may enhance the Akt/eNOS signaling pathway by promoting Akt-dependent phosphorylation of eNOS, a crucial process for maintaining NO bioavailability and supporting endothelial function in penile erection [129,130]. Indirect mechanisms have also been proposed, particularly in the context of androgen deficiency. In an orchiectomized rat model, liraglutide was found to improve metabolic disturbances, such as increased adiposity and impaired glucose regulation [131]. By contrast, to the best of our knowledge, there is currently no direct experimental evidence exploring the effects of dulaglutide or semaglutide on erectile function.
Emerging evidence indicates that GLP-1 RAs may have therapeutic potential in diabetes-related ED through a mitochondria-targeted, piezoelectric nanosystem. In a diabetic mouse model of ED, ultrasound-activated barium titanate (BaTiO3)-based nanosystem (BaTCG) generated localized piezoelectric currents that consumed protons (H+) on the mitochondrial outer membrane. This process reduced mitochondrial matrix H+ availability, collapsed the mitochondrial membrane potential, and triggered mitophagy, thereby attenuating oxidative stress, inflammation, and apoptosis. Simultaneously, the nanosystem enabled sustained release of long-acting GLP-1 RAs, which activated pancreatic β-cell receptors to enhance insulin secretion and improve glycemic control. The combined effects of restored metabolic balance, reduced mitochondrial injury, and suppressed inflammation promoted tissue regeneration within the corpus cavernosum, ultimately improving erectile function. Figure 4 provides a schematic representation of this nanosystem, highlighting the role of GLP-1 RA-mediated glycemic regulation in mitochondrial recovery and erectile tissue repair [132]. Several similar nanoplatforms have demonstrated promise in optimizing antidiabetic therapy; however, their clinical application remains investigational [133].
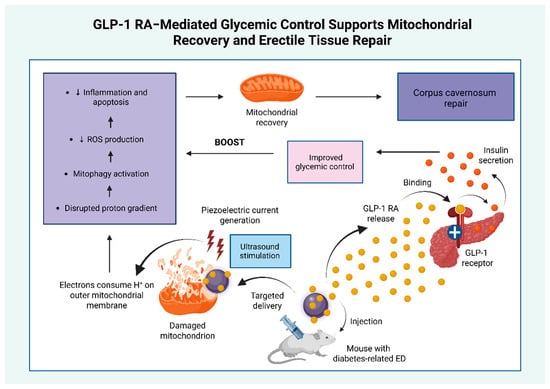
Figure 4.
Nanotechnology-based GLP-1 RA delivery system supports mitochondrial recovery and erectile tissue repair. This figure illustrates how the improvement in glycemic control mediated by the action of GLP-1 RAs enhanced the anti-oxidant, anti-inflammatory, and anti-apoptotic effects of a mitochondria-targeted piezoelectric nanosystem, providing an additional benefit to the overall amelioration of erectile dysfunction [132]. Abbreviations: ED: erectile dysfunction; GLP-1: glucagon-like peptide 1; GLP-1 RA: glucagon-like peptide 1 receptor agonist; H+: protons; ROS: reactive oxygen species; +: activation. ↓: reduction. Created in BioRender. Kounatidis, D. (2025) https://BioRender.com/agueqv0 (assessed on 20 July 2025).
6. GLP-1 Receptor Agonists and Erectile Function: Insights from Clinical Research
6.1. Evidence Supporting a Beneficial Effect of GLP-1 Receptor Agonists on Erectile Function
While high-quality clinical data remain limited, accumulating evidence increasingly supports a potential therapeutic role for GLP-1 RAs in the management of ED. A small prospective, randomized, open-label study involving men with obesity-related functional hypogonadism unresponsive to lifestyle modification examined the effects of liraglutide (3 mg daily) and testosterone replacement therapy (TRT). Both interventions led to significant increases in total testosterone (TT) (+2.6 ± 3.5 nmol/L with liraglutide vs. +5.9 ± 7.2 nmol/L with TRT), with comparable improvements in sexual function, including libido and erectile performance. Furthermore, 16 weeks of liraglutide treatment outperformed TRT in promoting weight loss (−7.9 ± 3.8 kg vs. −0.9 ± 4.5 kg with TRT, p < 0.001), while a marked differential effect on the hypothalamic–pituitary–testicular (HPT) axis emerged, as TRT induced suppression of gonadotropins, whereas liraglutide produced a significant increase (p < 0.001) [134]. On the other hand, a subsequent prospective comparative study involving young men (aged 18–35) with metabolic hypogonadism, randomized to gonadotropins (Group A), liraglutide (Group B), or TRT (Group C), demonstrated the superiority of liraglutide in improving sexual function. After 4 months, Group B demonstrated significantly greater improvements in erectile function, with IIEF-5 rising from 4 ± 2 to 21 ± 4 (p < 0.05), while PDE5 inhibitor use declined most notably in Group B (Group A: 48%, Group B: 31%, Group C: 45%). Liraglutide treatment was associated with improved sperm quality, accompanied by a significant increase in TT of 192.9% (1.4 ± 0.6 vs. 4.1 ± 0.5 ng/mL, p < 0.05) and an elevation in SHBG of 157.1% (14.0 ± 3.0 vs. 36.0 ± 4.0 nmol/L, p < 0.05), both relative to baseline and in comparison with the other two groups at the end of treatment. In addition, patients receiving liraglutide exhibited significantly higher gonadotropin concentrations compared with the other groups, with FSH (Group A: 0.9 ± 0.2 IU/L vs. Group B: 2.6 ± 0.2 IU/L vs. Group C: 0.2 ± 0.1 IU/L, p < 0.05) and LH (Group A: 1.0 ± 0.3 IU/L vs. Group B: 3.2 ± 0.2 IU/L vs. Group C: 0.3 ± 0.1 IU/L, p < 0.05) [135].
The therapeutic benefits of GLP-1 RAs have also been reported when used as an adjunct to metformin. In a retrospective one-year cohort study involving male outpatients with T2D, Lisco et al. found that the combination of GLP-1 RAs with metformin, compared with metformin monotherapy, produced marked increases in TT (+41.41 ± 6.11 ng/dL, p < 0.0001) and free testosterone (fT) (+0.44 ± 0.09 ng/dL, p < 0.0001) levels. These hormonal changes were accompanied by a significant rise in IIEF-5 score (+2.26 ± 0.26, p < 0.0001). Interestingly, the magnitude of benefit was greater in men with higher baseline IIEF-5 score (p = 0.045), those presenting with carotid artery stenosis (p = 0.045), and those who experienced weight loss during treatment (p = 0.013) [106]. These results corroborate an earlier observational study by the same research group, which further demonstrated that the addition of liraglutide or dulaglutide to metformin enhanced erectile function, irrespective of baseline gonadal status (p < 0.01) [136].
Liraglutide also appears to provide additional benefits when used alongside TRT and metformin in men with T2D, obesity, hypogonadism, and recent-onset ED. After one year of combined TRT and metformin, patients experienced significant improvements in erectile function (p < 0.01), although none reached the glycemic target (HbA1c < 7.5%). Among the 43 participants, 26 showed a limited glycemic response and were considered poor responders. These individuals received liraglutide at 1.2 mg daily during the second year, while the remaining 17 continued on TRT and metformin combination. At the end of the second year, patients who remained on TRT and metformin exhibited no further gains in IIEF scores and experienced significant increases in HbA1c (p < 0.05) and body weight (p < 0.04). In contrast, subjects receiving liraglutide not only reached glycemic targets and lost weight (p < 0.01) but also showed additional increases in SHBG (p < 0.05) and TT (p < 0.01), with concurrent marked improvements in erectile function [137]. Genetic data provide further support for a causal link between GLP-1 RA use and reduced ED risk. A Mendelian randomization study found genetically proxied GLP-1 RA exposure to be inversely associated with ED (OR: 0.493; 95% CI: 0.430–0.565; p < 0.001), with mediation analysis indicating that conventional risk factors such as T2D, obesity, hypertension, and CVD explained only a small portion of the observed effect [138].
To date, the main randomized control trial (RCT) addressing erectile outcomes with GLP-1 RA therapy is an exploratory analysis of the REWIND (Researching Cardiovascular Events With a Weekly INcretin in Diabetes) trial. Among 5312 men with T2D, 3725 completed baseline and follow-up IIEF assessments. Dulaglutide treatment was associated with a modest but significant reduction in moderate-to-severe ED compared with placebo (21.3 vs. 22.0 per 100 person-years; HR: 0.92, 95% CI: 0.85–0.99; p = 0.021). Dulaglutide also attenuated erectile function decline over time, with a least squares (LS) mean difference in erectile function subscore of 0.61 (95% CI: 0.18–1.05; p = 0.006) [139].
6.2. Evidence Suggesting a Potential Adverse Impact of GLP-1 Receptor Agonists on Erectile Function
Despite increasing evidence supporting the beneficial effects of GLP-1 RAs on erectile function, a limited number of studies have raised concerns regarding potential adverse sexual outcomes. A retrospective cohort study using the TriNetX Research database evaluated the incidence of ED in 3094 non-diabetic men with obesity (aged 18–50) prescribed semaglutide for weight management, matched to an equal number of semaglutide-naïve men with obesity. After excluding patients with prior ED, T2D, or confounding pharmacologic treatments, semaglutide use was associated with a higher incidence of newly diagnosed ED or initiation of PDE5 inhibitor therapy (1.47% vs. 0.32%; RR: 4.5). Reported rates of testosterone deficiency were also higher among semaglutide users (1.53% vs. 0.80%; RR: 1.9). While ED and testosterone deficiency were uncommon in younger men, non-diabetic men with obesity on semaglutide showed a higher risk. Thus, the authors emphasized the importance of informing patients, particularly those at elevated risk, about this potential adverse effect, while also highlighting the need for further research to elucidate underlying mechanisms and optimize therapeutic benefits [140].
Additional signals have emerged from a cross-sectional analysis of the FDA Adverse Event Reporting System (FAERS) database (2003–2024). This pharmacovigilance study detected 182 GLP-1 RA-related sexual adverse events, extending beyond ED, to include reduced libido and orgasmic disorders. Most reports involved men aged 40–60 with T2D being the main indication of use (43.9%). Exenatide (24.2%) and semaglutide (21.4%) were the most frequently implicated agents, followed by liraglutide (20.9%) and dulaglutide (18.7%). Among the different types of sexual dysfunction identified, ED accounted for the majority, representing 71.4% of cases. Although the analysis revealed a statistically significant association (p < 0.0001), the study had important limitations, including those inherent to the FAERS database. Furthermore, the signal strength was weak, insufficient to indicate a significant clinical risk, highlighting the need for cautious interpretation of the study results [107]. Table 1 summarizes the main characteristics and findings of clinical studies evaluating the impact of GLP-1 RAs on erectile function.

Table 1.
Main clinical studies assessing the impact of GLP-1 receptor agonists on erectile function.
7. The Influence of Other Antidiabetic Drugs on Erectile Function: Comparative Evidence with GLP-1 Receptor Agonists
7.1. Erectile Effects of Other Antidiabetic Therapies: Mechanistic Insights and Clinical Evidence
Multiple studies have examined the effects of antidiabetic therapies on erectile function, but findings remain inconsistent and at times contradictory. Metformin, for example, has demonstrated both beneficial and adverse effects. In a preclinical model of angiotensin II–induced ED, metformin normalized corpus cavernosum contractility and enhanced smooth muscle relaxation through increased eNOS phosphorylation [141]. When combined with antioxidants, it improved intracavernosal pressure and eNOS activity, while reducing mitochondrial autophagy, AGE generation, and the collagen-to-smooth muscle ratio in erectile tissue [142]. Conversely, short-term metformin use has been linked to reduced testosterone and libido, potentially blunting the testosterone rise typically associated with improved glycemic control [143,144].
Pioglitazone, an insulin sensitizer that functions as a peroxisome proliferator-activated receptor gamma (PPAR-γ) agonist, may exert protective effects against ED [145,146]. PPAR-γ is expressed in VSMCs, where its activation reduces oxidative stress and suppresses pro-inflammatory signaling [147]. Pioglitazone also attenuated endothelin-1–induced vasoconstriction by downregulating cyclooxygenase-2 (COX-2) via activator protein-1 (AP-1) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), while enhancing NO bioavailability [148]. In a rat model of bilateral cavernous nerve injury, it restored erectile function via insulin-like growth factor-1 (IGF-1) signaling activation [149]. Clinically, pioglitazone has been shown to improve erectile response in men with sildenafil-resistant ED [150].
Insulin therapy, particularly when combined with antioxidants, has been shown to mitigate ED by inhibiting the AGEs–RAGE–oxidative stress axis [151]. It may also promote recovery by modulating apoptotic protein expression and by restoring androgen receptor expression in penile tissue [152,153]. Evidence on insulin secretagogues such as SUs indicates mixed effects. Genetic studies suggest an increased ED risk with gliclazide [154], possibly due to SU-mediated inhibition of ATP-sensitive potassium channels essential for prostaglandin E1 (PGE1)- and cAMP-dependent vasodilation [155]. Conversely, SUs have been associated with increased testosterone, enhanced libido, and improved erectile function [156]. Notably, adulterated sexual enhancement products containing glibenclamide or glyburide have caused outbreaks of severe hypoglycemia [157,158].
The role of dipeptidyl peptidase-4 inhibitors (DPP-4is) in erectile performance remains uncertain, although preliminary evidence suggests vascular benefits, including endothelial repair via endothelial progenitor cells (EPCs) mobilization [159]. Saxagliptin has been demonstrated to improve ED by upregulating stromal cell-derived factor 1 (SDF-1), which enhances EPC homing, and by activating phosphoinositide 3-kinase/protein kinase B (PI3K)/Akt signaling, thereby diminishing oxidative stress and apoptosis [160]. Additional research has revealed that toleragliptin improved erectile responses in a vincristine-induced ED model via increased nNOS expression and endothelial signaling [161]. Conversely, the VILDA study reported a 22.8% incidence of ED over two years with vildagliptin use [162].
Sodium-glucose cotransporter-2 inhibitors (SGLT-2is) are well recognized for their CV benefits, including improved arterial stiffness and endothelial function [163]; however, their effects on sexual health remain ambiguous. Dapagliflozin and empagliflozin have been shown to restore NO availability under tumor necrosis factor-alpha (TNF-α)-induced endothelial inflammation through ROS reduction. Interestingly, these effects appear independent of eNOS activity or barrier integrity [164,165]. Data on canagliflozin are limited, though one study suggested potential interaction with tadalafil metabolism via cytochrome P450 3A4 inhibition, warranting caution in coadministration [166]. Clinically, dapagliflozin improved erectile function and enhanced tadalafil responsiveness in men with T2D [167]. In contrast, a large observational study reported increased incident ED risk with SGLT-2i therapy (adjusted HR: 1.55; 95% CI: 1.40–1.72) [168].
7.2. Potential Comparative Advantage of GLP-1 Receptor Agonists in Erectile Function
Although limited, comparative evidence suggests that GLP-1 RAs may exert superior effects on erectile function relative to other antidiabetic agents. In a trial comparing exenatide plus metformin with glimepiride plus metformin, TT increases over 3 months were significantly greater with exenatide (121.72 ± 56.73 ng/dL vs. 34.67 ± 16.30 ng/dL) [169]. Another study reported higher IIEF-5 scores in men treated with GLP-1 RAs versus insulin (16.7 ± 4.7 vs. 12.9 ± 6.2; p = 0.02), a difference that remained significant after adjustment for age and diabetes duration (p = 0.01). No differences in erectile function were observed between metformin, SUs, DPP-4is, or SGLT-2is [170].
The comparative advantage of GLP-1 RAs has also been supported by recent systematic reviews and meta-analyses. A meta-analysis by Yang et al. demonstrated superiority of GLP-1 RAs over metformin in improving erectile function (p = 0.02), with greater benefits in men with BMI ≥ 30 kg/m2 (p = 0.02) [171]. Similarly, a meta-analysis by Rastrelli et al., including 4112 patients, showed that observational data supported improvements in erectile health with GLP-1 RAs, SGLT-2is, and metformin, though not superior to lifestyle interventions. In contrast, RCTs demonstrated significant IIEF improvements with metformin, pioglitazone, and dulaglutide. The authors suggested that GLP-1 RAs and SGLT-2is may be particularly effective in advanced metabolic dysfunction, whereas metformin may remain preferable for less complex cases [172]. The same research group also found that GLP-1 RA therapy significantly increased TT, fT, SHBG, and gonadotropin levels, with concurrent improvements in erectile function. Meta-regression analysis demonstrated a strong correlation between GLP-1 RA–induced weight loss and TT elevation, implicating weight reduction as a principal mediator (p < 0.0001) [173].
These findings are further supported by two additional systematic reviews and meta-analyses, both highlighting the favorable impact of GLP-1 RAs on functional hypogonadism, largely attributable to weight loss [174,175]. Van Cauwenberghe et al. [174] reported minimal or no hormonal effects from other antidiabetic agents. Notably, an individual study within this review yielded insights such as the neutral impact of exenatide on serum testosterone in men with T2D [176]. In contrast, Salvio et al. found that while androgenic effects of GLP-1 RAs were comparable to those of other antidiabetic therapies, they offered superior benefits in terms of BMI reduction, increased gonadotropin levels, and improved erectile function scores [175]. Table 2 presents the data, findings, and conclusions of these meta-analyses.

Table 2.
Evidence from recently published meta-analyses on the comparative role of GLP-1 receptor agonists in erectile dysfunction relative to other antidiabetic therapies.
8. Research Gaps, Challenges, Future Perspectives, and Conclusions
8.1. Evaluating Mechanistic and Clinical Evidence Supporting GLP-1 Receptor Agonists–Mediated Erectile Benefits
ED is recognized as an early and independent predictor of CVD, with evidence indicating a 45% increased risk among individuals with ED [177]. The two conditions share overlapping pathophysiological mechanisms, including systemic inflammation, endothelial dysfunction, and atherosclerosis [178]. Current experimental data on the role of GLP-1 RAs in ED remains limited, primarily focusing on exenatide and liraglutide, with an emphasis on vascular outcomes [127,128,129,130]. On the other hand, understanding the mechanisms through which GLP-1 RAs exert their beneficial effects on CVD provides a theoretical framework for their potential effects on erectile function.
Potential benefits on erectile performance may arise from glycemic control–related mechanisms, including inhibition of AGE generation and reduction of oxidative stress [179], as well as preservation of mitochondrial integrity via modulation of programmed cell death pathways, such as pyroptosis and ferroptosis [180,181]. Furthermore, GLP-1 RAs may exert synergistic anti-inflammatory and antioxidant effects through reduced neutrophil extracellular trap (NET) formation and inhibition of NLRP3 inflammasome activation [182]. In diabetic ED models, a transcriptomic analysis demonstrated downregulation of genes involved in smooth muscle contractility and mitochondrial function, alongside upregulation of Wnt signaling and extracellular matrix remodeling [183].
Additional mechanisms may involve the modulation of key signaling pathways. The protein kinase A (PKA) pathway supports NO–mediated smooth muscle relaxation via cAMP signaling [184], whereas the Hippo–YAP1 axis regulates endothelial integrity, glucose metabolism, and fibrosis [185]. GLP-1 RA–mediated modulation of these pathways has shown benefits in other models and may potentially extent to erectile health [186,187]. Beyond endothelial dysfunction, pericyte impairment has been identified as a contributor to diabetic ED [188]. Given that GLP-1 receptors are expressed on cerebral pericytes, where they mediate vascular stabilization, similar effects in penile pericytes are plausible [189]. Moreover, the detection of GLP-1 receptors in testicular tissue raises the possibility of effects on gonadal function, mediated not only through hormonal pathways [190]. Although preclinical models frequently employ dosing regimens that limit direct translational applicability, they remain essential for delineating pharmacodynamic mechanisms, including those underlying the well-established CV benefits of GLP-1 RAs, which could also have implications for ED [191].
On the clinical front, an increasing number of studies in recent years have suggested favorable effects of GLP-1 RAs on erectile and sexual function, as well as on reproductive outcomes in both men and women [192]. However, these observations are subject to significant methodological constraints. Clinical trials investigating the effects of GLP-1 RAs on erectile function are typically limited by small sample sizes, short follow-up durations, and lack of evaluation of dose–response and exposure–response relationships. Furthermore, most available studies are observational in design and display considerable heterogeneity. To date, no RCT has been conducted with erectile function as a predefined primary endpoint [106,134,135,136,137,138,139].
These limitations also apply to comparative analyses of GLP-1 RAs versus other antidiabetic agents, for which the impact on erectile function remains uncertain. A plausible explanation for the apparent advantage of GLP-1 RAs may involve their superior weight-loss efficacy relative to other antidiabetic drugs, particularly SGLT-2is, which themselves confer substantial CV benefits. Few studies have specifically evaluated symptoms of androgen deficiency, and reported changes in testosterone levels are generally modest and subject to variability in study design [169,170,171,172,173,174,175]. Finally, the reliability and validity of instruments used to assess ED, such as the IIEF, remain debated, with evidence suggesting important limitations [193].
8.2. Assessing Emerging Concerns and Theoretical Risks of GLP-1 Receptor Agonists on Erectile Function
While the preponderance of available evidence suggests a beneficial effect of GLP-1 RAs on erectile function, rare reports of potential harm warrant careful consideration, even though the underlying studies face significant constraints, restricting causal inference. For instance, Able et al. relied on claims-based International Classification of Diseases, Tenth Revision (ICD-10), without verification of medication adherence. Lack of confirmation of actual drug intake limits the ability to draw conclusions about long-term effects on sexual health. Additional confounders included baseline BMI differences and the inability to distinguish between specific forms of sexual dysfunction [140]. On the other hand, although the FAERS analysis identified a statistically significant association between GLP-1 RA use and sexual dysfunction, the signal was weak, and inherent database limitations, including reporting bias and residual confounding, were present [107]. Combining the results of these two studies suggests that, while the overall risk of ED appears low or even uncertain, the increasing use of GLP-1 RAs highlights the need for ongoing clinical vigilance [107,140,194]. Of note, a recent U.S. surveillance study of emergency department visits for semaglutide-related adverse effects reported no cases of ED, though such visits are inherently uncommon [195,196].
To date, no experimental studies have demonstrated a harmful effect of GLP-1 RAs on erectile function. Consequently, the mechanisms through which GLP-1 RAs might negatively influence sexual health remain uncertain. It has been proposed that GLP-1 receptors in brain regions regulating appetite and reward may modulate neurotransmitters, such as dopamine and serotonin, potentially influencing sexual arousal [107,197]. Additional hypotheses include the rapid correction of glycemia following GLP-1 RA administration, similar to the exacerbation of DR, as well as long-term effects of prior hyperglycemia via persistent changes in endothelial gene expression, often referred to as “glucose memory” [198,199]. Other potential mechanisms involve altered testosterone pulsatility and GLP-1–mediated smooth muscle relaxation in penile tissue [140,200]. The development of ED may also be linked to the weight-lowering features of GLP-1 RAs, which can cause significant lean body mass loss, occasionally progressing to sarcopenia, a factor associated with ED [201,202]. On the other hand, in normal-weight individuals, caloric restriction has been linked to reduced testosterone levels, suggesting that GLP-1 RA use in such T2D patients may contribute to ED [203,204].
Overall, although caution is warranted, the aforementioned data should not dissuade clinicians from prescribing GLP-1 RAs, as such an association is not firmly established, and even in cases with semaglutide, the incidence was particularly low, approximately 1.4% [140,205]. In instances where ED develops following GLP-1 RA therapy, discontinuation is indicated, accompanied by a thorough urological evaluation. According to the 2025 European Association of Urology (EAU) guidelines, this evaluation should include a comprehensive medical and sexual history as well as early-morning fasting TT measurement. PDE5 inhibitors constitute the first-line treatment, while adjunctive TRT may be considered for hypogonadal non-responders, with careful attention to potential side effects, such as gastrointestinal symptoms, increased blood pressure, and thrombosis [206]. From a diabetology perspective, a reasonable approach involves substituting GLP-1 RA therapy with an alternative antidiabetic agent. If erectile function and testosterone levels improve but GLP-1 RA therapy remains clinically necessary, re-initiation at a previously tolerated lower dose or switching to a different GLP-1 RA may be considered, with close monitoring and informed consent. Notably, the timeline for testosterone recovery remains uncertain and may be prolonged [207]. Among available GLP-1 RAs, dulaglutide may represent the safest option, with even favorable effects [139], further supported by a recent RCT showing that in eugonadal normal-weight men, dulaglutide did not adversely affect sexual desire, the HPG axis, or sperm parameters compared with placebo. However, the study included a small number of participants and had a limited follow-up of only one month [208]. Despite these various hypotheses and therapeutic considerations, it is clear that well-designed, long-term RCTs are needed to provide definitive answers.
8.3. Comparing GLP-1 Receptor Agonists with Other Weight Loss Interventions
Assessment of the impact of GLP-1 RAs on erectile function is further limited by the lack of comparative data with other weight loss interventions. Theoretically, tirzepatide may confer additional erectile health benefits through its GIP component. However, the vascular effects of GIP remain poorly defined due to inconsistent GIP receptor (GIPR) expression across different endothelial beds [209]. Furthermore, while both GIP and GLP-1 exert protective effects on the vasculature under normal glucose levels, only GLP-1 enhances endothelial function in states of hyperglycemia. This occurs via increased eNOS expression and subsequent NO production through cAMP signaling [210]. This divergence may have implications for diabetes-associated ED, where NO bioavailability is essential. Moreover, although GIPR is expressed in the testes in preclinical models, its role in human reproductive physiology appears limited based on gene expression data [211]. On the other hand, tirzepatide may offer superior benefits over GLP-1 RAs due to its greater efficacy in weight loss. In a pilot study involving men with obesity and metabolic hypogonadism, tirzepatide improved both erectile function and hormonal profiles (p < 0.05), outperforming lifestyle modification and TRT [212]. It is worth noting that pharmacovigilance data, which linked GLP-1 RA administration with ED development, reported tirzepatide in 14.3% of cases—a lower proportion compared to liraglutide, dulaglutide, and semaglutide. Nevertheless, the annual distribution of adverse event reports revealed that tirzepatide exceeded semaglutide in 2023 and early 2024, consistent with its rising clinical use [107].
Relevant insights also arise from non-pharmacological weight loss interventions, such as metabolic bariatric surgery (MBS). Evidence suggests that MBS improves erectile function through mechanisms related to weight loss and T2D remission. On the contrary, although postoperative testosterone levels increase, they do not seem to independently improve ED [213]. Notably, metabolomic studies indicate that GLP-1 RAs induce modest metabolic changes primarily related to glycemic control and appetite, whereas MBS triggers broader shifts in gut hormone signaling and nutrient sensing [214,215]. Despite emerging evidence, comparative data evaluating the effects of GLP-1 RAs, tirzepatide, and MBS on erectile function remain lacking.
In conclusion, ED represents a common yet frequently underrecognized microvascular complication of diabetes, influenced by a broad spectrum of risk factors. Emerging preclinical and clinical evidence suggests that GLP-1 RAs may exert beneficial effects on erectile function, potentially exceeding those of other antidiabetic therapies. Nevertheless, conclusions remain premature. Concurrently, recent reports have raised concerns regarding possible ED development among GLP-1 RA users. However, these observations are limited by methodological shortcomings, and the strength of the association appears weak. Further mechanistic investigations are warranted to delineate the underlying biological pathways, while rigorously designed, long-term RCTs with erectile function as a predefined primary endpoint are essential. Until such data become available, clinicians should remain vigilant for symptoms of ED following the initiation or dose titration of GLP-1 RAs and be prepared to implement appropriate therapeutic strategies. Figure 5 summarizes the key features of diabetes-related ED and outlines the current evidence regarding the effects of GLP-1 RAs on erectile function.

Figure 5.
Diabetes-associated erectile dysfunction and the role of GLP-1 receptor agonists: An evidence-based snapshot. Abbreviations: AGEs: advanced glycation end products; CVD: cardiovascular disease; ED: erectile dysfunction; GLP-1 RA: glucagon-like peptide-1 receptor agonist; RCT: randomized controlled trial. Created in BioRender. Kounatidis, D. (2025) https://BioRender.com/21i8b6j (assessed on 27 August 2025).
Author Contributions
D.K. and N.G.V. conceived the idea of the manuscript and organized its plan. D.K. wrote the whole manuscript; E.R. and K.V. performed the literature search and were responsible for the references and the tables; D.K. was responsible for the figures; N.G.V., E.D., K.M. and N.T. reviewed, edited and supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
3-NT: 3-nitrotyrosine; Ach: acetylcholine; AD: Alzheimer’s disease; AGEs: advanced glycation end products; Akt: protein kinase B; AMPK: AMP-activated protein kinase; AP-1: activator protein-1; AR: androgen receptor; BaTCG: barium titanate-based composite; BMI: body mass index; cAMP: cyclic adenosine monophosphate; CGM: continuous glucose monitoring; cGMP: cyclic guanosine monophosphate; CI: 95% confidence interval; C-index: concordance index; CIMT: carotid intima-media thickness; CKD: chronic kidney disease; COX-2: cyclooxygenase-2; CTGF: connective tissue growth factor; CV: cardiovascular; CVD: cardiovascular disease; DPP-4i: dipeptidyl peptidase-4 inhibitor; EAU: European Association of Urology; ED: erectile dysfunction; EDHF: endothelium-derived hyperpolarizing factor; eGFR: estimated glomerular filtration rate; eNOS: endothelial nitric oxide synthase; EPC: endothelial progenitor cell; Exenatide LAR: exenatide long-acting release; FAERS: FDA Adverse Event Reporting System; FPG: fasting plasma glucose; FSH: follicle-stimulating hormone; fT: free testosterone; GGT: gamma-glutamyl transferase; GIP: gastric inhibitory polypeptide; GIPR: GIP receptor; GLP-1 RA: glucagon-like peptide-1 receptor agonist; GnRH: gonadotropin-releasing hormone; GTP: guanosine triphosphate; HbA1c: glycated hemoglobin; hCG: human chorionic gonadotropin; HOMA-IR: homeostasis model assessment of insulin resistance; HPG: hypothalamic-pituitary-gonadal; HR: hazard ratio; HUVEC: human umbilical vein endothelial cell; ICD-10: International Classification of Diseases, Tenth Revision; IGF-1: insulin-like growth factor-1; IIEF: International Index of Erectile Function; ION: ischemic optic neuropathy; IR: insulin resistance; JNK: c-Jun N-terminal kinase; KCa: calcium-dependent potassium; LDL-C: low-density lipoprotein cholesterol; LEADER: Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results; LH: luteinizing hormone; LIPA: low-intensity pulsed ultrasound; LS: least squares; M3 receptor: muscarinic M3 receptor; MACE: major cardiovascular events; mAR: membrane-bound androgen receptor; MASH: metabolic dysfunction-associated steatohepatitis; MBS: metabolic bariatric surgery; MetS: metabolic syndrome; METS-IR: metabolic score for insulin resistance; MiR-155: microRNA-155; mTORC2: mammalian target of rapamycin complex 2; MTC: medullary thyroid carcinoma; mTOR: mammalian target of rapamycin; NADPH: nicotinamide adenine dinucleotide phosphate; NANC: non-adrenergic, non-cholinergic; NET: neutrophil extracellular trap; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NHANES: National Health and Nutrition Examination Survey; NLRP3: NLR family pyrin domain containing 3; nNOS: neuronal nitric oxide synthase; NO: nitric oxide; p38 MAPK: p38 mitogen-activated protein kinase; PDE5: phosphodiesterase type 5; PGE1: prostaglandin E1; PI3K: phosphoinositide 3-kinase; PKA: protein kinase A; PPAR-γ: peroxisome proliferator-activated receptor gamma; PKG-1: protein kinase G type 1; Q1: first quartile; QoL: quality of life; RAGE: receptor for advanced glycation end products; RCT: randomized controlled trial; REWIND: Researching Cardiovascular Events With a Weekly INcretin in Diabetes; RNS: reactive nitrogen species; ROCK2: Rho-associated kinase 2; ROS: reactive oxygen species; sGC: soluble guanylyl cyclase; SDF-1: stromal cell-derived factor 1; SGLT-2i: sodium–glucose cotransporter-2 inhibitor; SHBG: sex hormone-binding globulin; SIRT1: sirtuin 1; Smad: SMAD family proteins; SPC: stromal progenitor cell; SSC: sacral spinal cord; SU: sulfonylurea; T1D: type 1 diabetes; T2D: type 2 diabetes; TGF-β1; transforming growth factor beta 1; TNFα: tumor necrosis factor-alpha; TRT: testosterone replacement therapy; TT: total testosterone; TyG: triglyceride-glucose; TZD: thiazolidinedione; VEGF: vascular endothelial growth factor; VSMC: vascular smooth muscle cell; WC: waist circumference.
References
- Yafi, F.A.; Jenkins, L.; Albersen, M.; Corona, G.; Isidori, A.M.; Goldfarb, S.; Maggi, M.; Nelson, C.J.; Parish, S.; Salonia, A.; et al. Erectile dysfunction. Nat. Rev. Dis. Primers 2016, 2, 16003. [Google Scholar] [CrossRef] [PubMed]
- Obi, P.C.; Njoku, G.H.; Mbaike, A.C.; Ihim, A.C.; Nwako, O.F.; Anyanwu, A.C.; Ohiri, J.U.; Nwazor, E.; Ubani, B.C.; Ogbonna, S.U.; et al. Quality of Sexual Life and its Correlates among Men with Diabetes Mellitus and Erectile dysfunction attending a tertiary hospital in Owerri, Nigeria. Niger. Med. J. 2025, 66, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pang, K.; Pan, D.; Xu, H.; Ma, Y.; Wang, J.; Xu, P.; Wang, H.; Zang, G. Advances in physical diagnosis and treatment of male erectile dysfunction. Front. Physiol. 2023, 13, 1096741. [Google Scholar] [CrossRef]
- Rhoden, E.L.; Telöken, C.; Sogari, P.R.; Vargas Souto, C.A. The use of the simplified International Index of Erectile Function (IIEF-5) as a diagnostic tool to study the prevalence of erectile dysfunction. Int. J. Impot. Res. 2002, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Pyrgidis, N.; Mykoniatis, I.; Haidich, A.B.; Tirta, M.; Talimtzi, P.; Kalyvianakis, D.; Ouranidis, A.; Hatzichristou, D. Effect of phosphodiesterase-type 5 inhibitors on erectile function: An overview of systematic reviews and meta-analyses. BMJ Open 2021, 11, e047396. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, R.K.; Patel, N.P.; Goutam, P. Evaluation of Erectile Dysfunction in Prediabetic Males at Tertiary Care Center in Central India. J. Assoc. Physicians India 2024, 72, e10–e13. [Google Scholar] [CrossRef]
- Dandona, P.; Dhindsa, S. Update: Hypogonadotropic hypogonadism in type 2 diabetes and obesity. J. Clin. Endocrinol. Metab. 2011, 96, 2643–2651. [Google Scholar] [CrossRef]
- Kouidrat, Y.; Pizzol, D.; Cosco, T.; Thompson, T.; Carnaghi, M.; Bertoldo, A.; Solmi, M.; Stubbs, B.; Veronese, N. High prevalence of erectile dysfunction in diabetes: A systematic review and meta-analysis of 145 studies. Diabet. Med. 2017, 34, 1185–1192. [Google Scholar] [CrossRef]
- Chu, N.V.; Edelman, S.V. Erectile dysfunction and diabetes. Curr. Diabetes Rep. 2002, 2, 60–66. [Google Scholar] [CrossRef]
- Kitaw, T.A.; Abate, B.B.; Tilahun, B.D.; Yilak, G.; Rede, M.B.; Getie, A.; Haile, R.N. The global burden of erectile dysfunction and its associated risk factors in diabetic patients: An umbrella reviews. BMC Public Health 2024, 24, 2816. [Google Scholar] [CrossRef]
- Maiorino, M.I.; Bellastella, G.; Della Volpe, E.; Casciano, O.; Scappaticcio, L.; Cirillo, P.; Giugliano, D.; Esposito, K. Erectile dysfunction in young men with type 1 diabetes. Int. J. Impot. Res. 2017, 29, 17–22. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Z.; Tang, G. Global prevalence of erectile dysfunction and its associated risk factors among men with type 1 diabetes: A systematic review and meta-analysis. Int. J. Impot. Res. 2024, 36, 365–374. [Google Scholar] [CrossRef]
- Yao, S.; Xing, S.; Shan, X.; Dai, W.; Cao, Y.; Hu, B. Importance of assessing erectile dysfunction in patients with type 2 diabetes mellitus based on glucose fluctuation: A Cross-Sectional study. Endocrine 2025, 88, 650–659. [Google Scholar] [CrossRef]
- Dilixiati, D.; Waili, A.; Tuerxunmaimaiti, A.; Tao, L.; Zebibula, A.; Rexiati, M. Risk factors for erectile dysfunction in diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 2024, 15, 1368079. [Google Scholar] [CrossRef]
- Lu, M.; Ni, D.; Wu, W.; Xu, C.; Zhang, Y. Analysis of Risk Factors Associated with Organic Erectile Dysfunction in Patients with Type 2 Diabetes Mellitus and Erectile Dysfunction. Discov. Med. 2025, 37, 141–151. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.A.; D’Oria, R.; Giordano, F.; Caruso, I.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Role of Glucose-Lowering Medications in Erectile Dysfunction. J. Clin. Med. 2021, 10, 2501. [Google Scholar] [CrossRef]
- Westermeier, F.; Fisman, E.Z. Glucagon like peptide-1 (GLP-1) agonists and cardiometabolic protection: Historical development and future challenges. Cardiovasc. Diabetol. 2025, 24, 44. [Google Scholar] [CrossRef]
- Pantea, I.; Repanovici, A.; Andreescu, O. The Influence of GLP1 on Body Weight and Glycemic Management in Patients with Diabetes—A Scientometric Investigation and Visualization Study. Medicina 2024, 60, 1761. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Cacciola, A.; Bruschetta, D.; Milardi, D.; Quattrini, F.; Sciarrone, F.; la Rosa, G.; Bramanti, P.; Anastasi, G. Neuroanatomy and function of human sexual behavior: A neglected or unknown issue? Brain Behav. 2019, 9, e01389. [Google Scholar] [CrossRef]
- Azadzoi, K.M.; Yang, J.; Siroky, M.B. Neural regulation of sexual function in men. World J. Clin. Urol. 2013, 2, 32–41. [Google Scholar] [CrossRef]
- Liu, X.; Yu, Y.; Zhang, H.; Zhang, M.; Liu, Y. The Role of Muscarinic Acetylcholine Receptor M3 in Cardiovascular Diseases. Int. J. Mol. Sci. 2024, 25, 7560. [Google Scholar] [CrossRef]
- Lincoln, T.M.; Dey, N.; Sellak, H. Invited review: cGMP-dependent protein kinase signaling mechanisms in smooth muscle: From the regulation of tone to gene expression. J. Appl. Physiol. 2001, 91, 1421–1430. [Google Scholar] [CrossRef]
- Morgado, M.; Cairrão, E.; Santos-Silva, A.J.; Verde, I. Cyclic nucleotide-dependent relaxation pathways in vascular smooth muscle. Cell. Mol. Life Sci. 2012, 69, 247–266. [Google Scholar] [CrossRef]
- Lucas-Herald, A.K.; Alves-Lopes, R.; Montezano, A.C.; Ahmed, S.F.; Touyz, R.M. Genomic and non-genomic effects of androgens in the cardiovascular system: Clinical implications. Clin. Sci. 2017, 131, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Foradori, C.D.; Weiser, M.J.; Handa, R.J. Non-genomic actions of androgens. Front. Neuroendocrinol. 2008, 29, 169–181. [Google Scholar] [CrossRef]
- Goto, K.; Ohtsubo, T.; Kitazono, T. Endothelium-Dependent Hyperpolarization (EDH) in Hypertension: The Role of Endothelial Ion Channels. Int. J. Mol. Sci. 2018, 19, 315. [Google Scholar] [CrossRef]
- Prieto, D. Physiological regulation of penile arteries and veins. Int. J. Impot. Res. 2008, 20, 17–29. [Google Scholar] [CrossRef]
- Ma, J.; Chen, Y.; Si, Y.; Qian, J.; Wang, C.; Jin, J.; He, Q. The multifaceted nature of diabetic erectile dysfunction: Uncovering the intricate mechanisms and treatment strategies. Front. Endocrinol. 2024, 15, 1460033. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef]
- Castela, Â.; Costa, C. Molecular mechanisms associated with diabetic endothelial-erectile dysfunction. Nat. Rev. Urol. 2016, 13, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Cartledge, J.J.; Eardley, I.; Morrison, J.F. Advanced glycation end-products are responsible for the impairment of corpus cavernosal smooth muscle relaxation seen in diabetes. BJU Int. 2001, 87, 402–407. [Google Scholar] [CrossRef]
- Kaltsas, A.; Zikopoulos, A.; Dimitriadis, F.; Sheshi, D.; Politis, M.; Moustakli, E.; Symeonidis, E.N.; Chrisofos, M.; Sofikitis, N.; Zachariou, A. Oxidative Stress and Erectile Dysfunction: Pathophysiology, Impacts, and Potential Treatments. Curr. Issues Mol. Biol. 2024, 46, 8807–8834. [Google Scholar] [CrossRef]
- Cai, Z.; Yuan, S.; Zhong, Y.; Deng, L.; Li, J.; Tan, X.; Feng, J. Amelioration of Endothelial Dysfunction in Diabetes: Role of Takeda G Protein-Coupled Receptor 5. Front. Pharmacol. 2021, 12, 637051. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Kashfi, K.; Ghasemi, A. Vascular nitric oxide resistance in type 2 diabetes. Cell Death Dis. 2023, 14, 410. [Google Scholar] [CrossRef]
- Toque, H.A.; Nunes, K.P.; Yao, L.; Liao, J.K.; Webb, R.C.; Caldwell, R.B.; Caldwell, R.W. Activated Rho kinase mediates diabetes-induced elevation of vascular arginase activation and contributes to impaired corpora cavernosa relaxation: Possible involvement of p38 MAPK activation. J. Sex. Med. 2013, 10, 1502–1515. [Google Scholar] [CrossRef]
- Zhou, M.; Li, G.; Zhu, L.; Zhou, H.; Lu, L. Arctiin attenuates high glucose-induced human retinal capillary endothelial cell proliferation by regulating ROCK1/PTEN/PI3K/Akt/VEGF pathway in vitro. J. Cell. Mol. Med. 2020, 24, 5695–5706. [Google Scholar] [CrossRef]
- Kilarkaje, N.; Yousif, M.H.; El-Hashim, A.Z.; Makki, B.; Akhtar, S.; Benter, I.F. Role of angiotensin II and angiotensin-(1-7) in diabetes-induced oxidative DNA damage in the corpus cavernosum. Fertil. Steril. 2013, 100, 226–233. [Google Scholar] [CrossRef]
- Deng, W.; Bivalacqua, T.J.; Champion, H.C.; Hellstrom, W.J.; Murthy, S.N.; Kadowitz, P.J. Superoxide dismutase—A target for gene therapeutic approach to reduce oxidative stress in erectile dysfunction. Methods Mol. Biol. 2010, 610, 213–227. [Google Scholar] [CrossRef]
- Radi, R. Protein tyrosine nitration: Biochemical mechanisms and structural basis of functional effects. Acc. Chem. Res. 2013, 46, 550–559. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, S.; Mao, J.; Liu, X.; Wang, T.; Liu, J.; Song, X.; Song, W. The role of pogrammed cell death in diabetes mellitus-induced erectile dysfunction: From mechanisms to targeted therapy. Reprod. Biol. Endocrinol. 2025, 23, 32. [Google Scholar] [CrossRef]
- Chen, Y.; Xiao, M.; Zhao, L.; Huang, Y.; Lin, Y.; Xie, T.; Tian, J.; Wang, Q.; Tang, Y.; Su, Z. Low-Intensity Pulsed Ultrasound Counteracts Advanced Glycation End Products-Induced Corpus Cavernosal Endothelial Cell Dysfunction via Activating Mitophagy. Int. J. Mol. Sci. 2022, 23, 14887. [Google Scholar] [CrossRef]
- Qabazard, B.; Yousif, M.; Mousa, A.; Phillips, O.A. GYY4137 attenuates functional impairment of corpus cavernosum and reduces fibrosis in rats with STZ-induced diabetes by inhibiting the TGF-β1/Smad/CTGF pathway. Biomed. Pharmacother. 2021, 138, 111486. [Google Scholar] [CrossRef]
- Kadioglu, A.; Dincer, M.; Salabas, E.; Culha, M.G.; Akdere, H.; Cilesiz, N.C. A Population-Based Study of Peyronie’s Disease in Turkey: Prevalence and Related Comorbidities. Sex. Med. 2020, 8, 679–685. [Google Scholar] [CrossRef]
- Yang, C.C.; Liao, P.H.; Cheng, Y.H.; Chien, C.Y.; Cheng, K.H.; Chien, C.T. Diabetes associated with hypertension exacerbated oxidative stress-mediated inflammation, apoptosis and autophagy leading to erectile dysfunction in rats. J. Chin. Med. Assoc. 2022, 85, 346–357. [Google Scholar] [CrossRef]
- Chrysant, S.G. Antihypertensive therapy causes erectile dysfunction. Curr. Opin. Cardiol. 2015, 30, 383–390. [Google Scholar] [CrossRef]
- Musicki, B.; Liu, T.; Lagoda, G.A.; Strong, T.D.; Sezen, S.F.; Johnson, J.M.; Burnett, A.L. Hypercholesterolemia-induced erectile dysfunction: Endothelial nitric oxide synthase (eNOS) uncoupling in the mouse penis by NAD(P)H oxidase. J. Sex. Med. 2010, 7, 3023–3032. [Google Scholar] [CrossRef]
- Potenza, M.A.; Addabbo, F.; Montagnani, M. Vascular actions of insulin with implications for endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 568–577. [Google Scholar] [CrossRef]
- Silva, F.H.; Alexandre, E.C.; Calmasini, F.B.; Calixto, M.C.; Antunes, E. Treatment with Metformin Improves Erectile Dysfunction in a Murine Model of Obesity Associated with Insulin Resistance. Urology 2015, 86, 423.e1-6. [Google Scholar] [CrossRef]
- Chen, S.; Wu, R.; Huang, Y.; Zheng, F.; Ou, Y.; Tu, X.; Zhang, Y.; Gao, Y.; Chen, X.; Zheng, T.; et al. Insulin resistance is an independent determinate of ED in young adult men. PLoS ONE 2013, 8, e83951. [Google Scholar] [CrossRef]
- Jalali, S.; Zareshahi, N.; Behnoush, A.H.; Azarboo, A.; Shirinezhad, A.; Hosseini, S.Y.; Javidan, A.; Ghaseminejad-Raeini, A. Association of insulin resistance surrogate indices and erectile dysfunction: A systematic review and meta-analysis. Reprod. Biol. Endocrinol. 2024, 22, 148. [Google Scholar] [CrossRef]
- Wu, S.; Xia, W. Association between different insulin resistance surrogates and erectile dysfunction in non-diabetic men: A large population-based study. BMC Public Health 2025, 25, 1949. [Google Scholar] [CrossRef]
- Sun, C.; Gao, Y.; Liang, Z.; Liu, C.; Chen, M. Association of METS-IR index with prevalence of erectile dysfunction in US adults: A cross-sectional study. Int. Urol. Nephrol. 2024, 56, 2157–2164. [Google Scholar] [CrossRef]
- De Silva, N.L.; Papanikolaou, N.; Grossmann, M.; Antonio, L.; Quinton, R.; Anawalt, B.D.; Jayasena, C.N. Male hypogonadism: Pathogenesis, diagnosis, and management. Lancet Diabetes Endocrinol. 2024, 12, 761–774. [Google Scholar] [CrossRef]
- Ding, E.L.; Song, Y.; Manson, J.E.; Hunter, D.J.; Lee, C.C.; Rifai, N.; Buring, J.E.; Gaziano, J.M.; Liu, S. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N. Engl. J. Med. 2009, 361, 1152–1163. [Google Scholar] [CrossRef]
- Khalil, S.H.A.; Dandona, P.; Osman, N.A.; Assaad, R.S.; Zaitoon, B.T.A.; Almas, A.A.; Amin, N.G. Diabetes surpasses obesity as a risk factor for low serum testosterone level. Diabetol. Metab. Syndr. 2024, 16, 143. [Google Scholar] [CrossRef]
- Grossmann, M. Hypogonadism and male obesity: Focus on unresolved questions. Clin. Endocrinol. 2018, 89, 11–21. [Google Scholar] [CrossRef]
- Infante, M.; Silvestri, F.; Padilla, N.; Pacifici, F.; Pastore, D.; Pinheiro, M.M.; Caprio, M.; Tesauro, M.; Fabbri, A.; Novelli, G.; et al. Unveiling the Therapeutic Potential of the Second-Generation Incretin Analogs Semaglutide and Tirzepatide in Type 1 Diabetes and Latent Autoimmune Diabetes in Adults. J. Clin. Med. 2025, 14, 1303. [Google Scholar] [CrossRef]
- Mulligan, T.; Frick, M.F.; Zuraw, Q.C.; Stemhagen, A.; McWhirter, C. Prevalence of hypogonadism in males aged at least 45 years: The HIM study. Int. J. Clin. Pract. 2006, 60, 762–769. [Google Scholar] [CrossRef]
- Traish, A.M. Androgens play a pivotal role in maintaining penile tissue architecture and erection: A review. J. Androl. 2009, 30, 363–369. [Google Scholar] [CrossRef]
- Cai, J.J.; Wen, J.; Jiang, W.H.; Lin, J.; Hong, Y.; Zhu, Y.S. Androgen actions on endothelium functions and cardiovascular diseases. J. Geriatr. Cardiol. 2016, 13, 183–196. [Google Scholar] [CrossRef]
- Traish, A.M.; Galoosian, A. Androgens modulate endothelial function and endothelial progenitor cells in erectile physiology. Korean J. Urol. 2013, 54, 721–731. [Google Scholar] [CrossRef]
- Varlamov, O.; White, A.E.; Carroll, J.M.; Bethea, C.L.; Reddy, A.; Slayden, O.; O’Rourke, R.W.; Roberts, C.T., Jr. Androgen effects on adipose tissue architecture and function in nonhuman primates. Endocrinology 2012, 153, 3100–3110. [Google Scholar] [CrossRef] [PubMed]
- Schardein, J.N.; Hotaling, J.M. The Impact of Testosterone on Erectile Function. Androgens 2022, 3, 113–124. [Google Scholar] [CrossRef]
- Long, L.; Qiu, H.; Cai, B.; Chen, N.; Lu, X.; Zheng, S.; Ye, X.; Li, Y. Hyperglycemia induced testicular damage in type 2 diabetes mellitus rats exhibiting microcirculation impairments associated with vascular endothelial growth factor decreased via PI3K/Akt pathway. Oncotarget 2018, 9, 5321–5336. [Google Scholar] [CrossRef]
- Schoeller, E.L.; Schon, S.; Moley, K.H. The effects of type 1 diabetes on the hypothalamic, pituitary and testes axis. Cell Tissue Res. 2012, 349, 839–847. [Google Scholar] [CrossRef]
- Noori, T.; Sureda, A.; Shirooie, S. Role of natural mTOR inhibitors in treatment of diabetes mellitus. Fundam. Clin. Pharmacol. 2023, 37, 461–479. [Google Scholar] [CrossRef]
- Kuryłowicz, A. Estrogens in Adipose Tissue Physiology and Obesity-Related Dysfunction. Biomedicines 2023, 11, 690. [Google Scholar] [CrossRef]
- Traish, A.M.; Toselli, P.; Jeong, S.J.; Kim, N.N. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: A potential mechanism for veno-occlusive dysfunction in androgen deficiency. J. Androl. 2005, 26, 242–248. [Google Scholar] [CrossRef]
- Begum, M.; Choubey, M.; Tirumalasetty, M.B.; Arbee, S.; Sadik, S.; Mohib, M.M.; Srivastava, S.; Minhaz, N.; Alam, R.; Mohiuddin, M.S. Exploring the Molecular Link Between Diabetes and Erectile Dysfunction Through Single-Cell Transcriptome Analysis. Genes 2024, 15, 1596. [Google Scholar] [CrossRef]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef]
- Müller, T.D.; Finan, B.; Bloom, S.R.; D’Alessio, D.; Drucker, D.J.; Flatt, P.R.; Fritsche, A.; Gribble, F.; Grill, H.J.; Habener, J.F.; et al. Glucagon-like peptide 1 (GLP-1). Mol. Metab. 2019, 30, 72–130. [Google Scholar] [CrossRef]
- Yuan, X.; Gao, Z.; Hao, Z.; Ma, H.; Duan, K.; Yang, C. Effect of long-acting versus short-acting glucagon-like peptide-1 receptor agonists on improving body weight and related metabolic parameters in type 2 diabetes: A head-to-head meta-analysis. Medicine 2023, 102, e35739. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Brown-Frandsen, K.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Esbjerg, S.; Hardt-Lindberg, S.; Hovingh, G.K.; Kahn, S.E.; Kushner, R.F.; et al. Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes. N. Engl. J. Med. 2023, 389, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef]
- Perkovic, V.; Tuttle, K.R.; Rossing, P.; Mahaffey, K.W.; Mann, J.F.E.; Bakris, G.; Baeres, F.M.M.; Idorn, T.; Bosch-Traberg, H.; Lausvig, N.L.; et al. Effects of Semaglutide on Chronic Kidney Disease in Patients with Type 2 Diabetes. N. Engl. J. Med. 2024, 391, 109–121. [Google Scholar] [CrossRef]
- Moiz, A.; Filion, K.B.; Tsoukas, M.A.; Yu, O.H.; Peters, T.M.; Eisenberg, M.J. Mechanisms of GLP-1 Receptor Agonist-Induced Weight Loss: A Review of Central and Peripheral Pathways in Appetite and Energy Regulation. Am. J. Med. 2025, 138, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Herrera, M.A.; Mera-Carreiro, S.; Sánchez-Pernaute, A.; Ramos-Levi, A.M. Impact of Treatment with GLP1 Receptor Agonists, Liraglutide 3.0 mg and Semaglutide 1.0 mg, While on a Waiting List for Bariatric Surgery. Biomedicines 2023, 11, 2785. [Google Scholar] [CrossRef]
- Jastreboff, A.M.; Aronne, L.J.; Ahmad, N.N.; Wharton, S.; Connery, L.; Alves, B.; Kiyosue, A.; Zhang, S.; Liu, B.; Bunck, M.C.; et al. Tirzepatide Once Weekly for the Treatment of Obesity. N. Engl. J. Med. 2022, 387, 205–216. [Google Scholar] [CrossRef]
- Garvey, W.T.; Frias, J.P.; Jastreboff, A.M.; le Roux, C.W.; Sattar, N.; Aizenberg, D.; Mao, H.; Zhang, S.; Ahmad, N.N.; Bunck, M.C.; et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT-2): A double-blind, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2023, 402, 613–626. [Google Scholar] [CrossRef]
- Aronne, L.J.; Horn, D.B.; le Roux, C.W.; Ho, W.; Falcon, B.L.; Gomez Valderas, E.; Das, S.; Lee, C.J.; Glass, L.C.; Senyucel, C.; et al. Tirzepatide as Compared with Semaglutide for the Treatment of Obesity. N. Engl. J. Med. 2025, 393, 26–36. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD). J. Hepatol. 2024, 81, 492–542. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Krishan, S.; Mishra, S.K.; Choudhary, N.S.; Singh, M.K.; Wasir, J.S.; Kaur, P.; Gill, H.K.; Bano, T.; Farooqui, K.J.; et al. Effect of dulaglutide on liver fat in patients with type 2 diabetes and NAFLD: Randomised controlled trial (D-LIFT trial). Diabetologia 2020, 63, 2434–2445. [Google Scholar] [CrossRef]
- Newsome, P.N.; Buchholtz, K.; Cusi, K.; Linder, M.; Okanoue, T.; Ratziu, V.; Sanyal, A.J.; Sejling, A.S.; Harrison, S.A.; NN9931-4296 Investigators. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2021, 384, 1113–1124. [Google Scholar] [CrossRef]
- Fessel, J. All GLP-1 Agonists Should, Theoretically, Cure Alzheimer’s Dementia but Dulaglutide Might Be More Effective Than the Others. J. Clin. Med. 2024, 13, 3729. [Google Scholar] [CrossRef] [PubMed]
- Reiss, A.B.; Gulkarov, S.; Lau, R.; Klek, S.P.; Srivastava, A.; Renna, H.A.; De Leon, J. Weight Reduction with GLP-1 Agonists and Paths for Discontinuation While Maintaining Weight Loss. Biomolecules 2025, 15, 408. [Google Scholar] [CrossRef]
- Wan, J.; Ferrari, C.; Tadros, M. GLP-1RA Essentials in Gastroenterology: Side Effect Management, Precautions for Endoscopy and Applications for Gastrointestinal Disease Treatment. Gastroenterol. Insights 2024, 15, 191–212. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Ping, F.; Yang, N.; Huang, J.; Li, Y.; Xu, L.; Li, W.; Zhang, H. Association of Glucagon-Like Peptide-1 Receptor Agonist Use with Risk of Gallbladder and Biliary Diseases: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Intern. Med. 2022, 182, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ernst, S.A.; Heidenreich, K.; Williams, J.A. Glucagon-like peptide-1 receptor is present in pancreatic acinar cells and regulates amylase secretion through cAMP. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 310, G26–G33. [Google Scholar] [CrossRef]
- Guo, H.; Guo, Q.; Li, Z.; Wang, Z. Association between different GLP-1 receptor agonists and acute pancreatitis: Case series and real-world pharmacovigilance analysis. Front. Pharmacol. 2024, 15, 1461398. [Google Scholar] [CrossRef]
- Ayoub, M.; Chela, H.; Amin, N.; Hunter, R.; Anwar, J.; Tahan, V.; Daglilar, E. Pancreatitis Risk Associated with GLP-1 Receptor Agonists, Considered as a Single Class, in a Comorbidity-Free Subgroup of Type 2 Diabetes Patients in the United States: A Propensity Score-Matched Analysis. J. Clin. Med. 2025, 14, 944. [Google Scholar] [CrossRef] [PubMed]
- Lomeli, L.D.; Kodali, A.M.; Tsushima, Y.; Mehta, A.E.; Pantalone, K.M. The incidence of acute pancreatitis with GLP-1 receptor agonist therapy in individuals with a known history of pancreatitis. Diabetes Res. Clin. Pract. 2024, 215, 111806. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.A.; Baggio, L.L.; Lamont, B.J.; Ali, S.; Drucker, D.J. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes 2009, 58, 2148–2161. [Google Scholar] [CrossRef]
- Kounatidis, D.; Vallianou, N.G.; Karampela, I.; Rebelos, E.; Kouveletsou, M.; Dalopoulos, V.; Koufopoulos, P.; Diakoumopoulou, E.; Tentolouris, N.; Dalamaga, M. Anti-Diabetic Therapies and Cancer: From Bench to Bedside. Biomolecules 2024, 14, 1479. [Google Scholar] [CrossRef]
- Burke, O.; Sa, B.; Cespedes, D.A.; Sechi, A.; Tosti, A. Glucagon-like peptide-1 receptor agonist medications and hair loss: A retrospective cohort study. J. Am. Acad. Dermatol. 2025, 92, 1141–1143. [Google Scholar] [CrossRef]
- Godfrey, H.; Leibovit-Reiben, Z.; Jedlowski, P.; Thiede, R. Alopecia associated with the use of semaglutide and tirzepatide: A disproportionality analysis using the FDA adverse event reporting system (FAERS) from 2022 to 2023. J. Eur. Acad. Dermatol. Venereol. 2025, 39, e153–e154. [Google Scholar] [CrossRef]
- Buontempo, M.G.; Santos, B.T. Exploring the hair loss risk in glucagon-like peptide-1 agonists: Emerging concerns and clinical implications. J. Eur. Acad. Dermatol. Venereol. 2025, 39, 263–264. [Google Scholar] [CrossRef]
- Sharma, A.; Parachuri, N.; Kumar, N.; Saboo, B.; Tripathi, H.N.; Kuppermann, B.D.; Bandello, F. Semaglutide and the risk of diabetic retinopathy-current perspective. Eye 2022, 36, 10–11. [Google Scholar] [CrossRef]
- Lakhani, M.; Kwan, A.T.H.; Mihalache, A.; Popovic, M.M.; Nanji, K.; Xie, J.S.; Feo, A.; Rabinovitch, D.; Shor, R.; Sadda, S.; et al. Association of Glucagon-Like Peptide-1 Receptor Agonists with Optic Nerve and Retinal Adverse Events: A Population-Based Observational Study Across 180 Countries. Am. J. Ophthalmol. 2025, 277, 148–168. [Google Scholar] [CrossRef]
- Cigrovski Berkovic, M.; Strollo, F. Semaglutide-eye-catching results. World J. Diabetes. 2023, 14, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Sharma, N.; Shaia, J.; Wu, A.K.; Skugor, M.; Singh, R.P.; Rachitskaya, A.V. The Effect of Glucagon-Like Peptide-1 Receptor Agonists on Diabetic Retinopathy at a Tertiary Care Center. Ophthalmol. Sci. 2024, 4, 100547. [Google Scholar] [CrossRef]
- Wang, F.; Mao, Y.; Wang, H.; Liu, Y.; Huang, P. Semaglutide and Diabetic Retinopathy Risk in Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trials. Clin. Drug Investig. 2022, 42, 17–28. [Google Scholar] [CrossRef]
- Wei, L.; Mo, W.; Lan, S.; Yang, H.; Huang, Z.; Liang, X.; Li, L.; Xian, J.; Xie, X.; Qin, Y.; et al. GLP-1 RA Improves Diabetic Retinopathy by Protecting the Blood-Retinal Barrier through GLP-1R-ROCK-p-MLC Signaling Pathway. J. Diabetes Res. 2022, 2022, 1861940. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, I.A.; Tentolouris, A.; Sarantis, P.; Katsaouni, A.; Rebelos, E.; Mourouzis, I.; Pantos, C.; Tentolouris, N. Semaglutide Enhances Cellular Regeneration in Skin and Retinal Cells In Vitro. Pharmaceutics 2025, 17, 1115. [Google Scholar] [CrossRef]
- Varnum, A.A.; Pozzi, E.; Deebel, N.A.; Evans, A.; Eid, N.; Sadeghi-Nejad, H.; Ramasamy, R. Impact of GLP-1 Agonists on Male Reproductive Health-A Narrative Review. Medicina 2023, 60, 50. [Google Scholar] [CrossRef]
- Lisco, G.; Bartolomeo, N.; De Tullio, A.; De Pergola, G.; Guastamacchia, E.; Jirillo, E.; Piazzolla, G.; Triggiani, V.; Giagulli, V.A. Long-acting glucagon-like peptide 1 receptor agonists boost erectile function in men with type 2 diabetes mellitus complaining of erectile dysfunction: A retrospective cohort study. Andrology 2024, 12, 633–642. [Google Scholar] [CrossRef]
- Pourabhari Langroudi, A.; Chen, A.L.; Basran, S.; Sommer, E.R.; Stinson, J.; Cheng, Y.S.; Del Giuduce, F.; Scott, M.; Eisenberg, M.L. Male sexual dysfunction associated with GLP-1 receptor agonists: A cross-sectional analysis of FAERS data. Int. J. Impot. Res. 2025, 37, 661–667. [Google Scholar] [CrossRef]
- Helmstädter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kröller-Schön, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide In Mice with Experimental Arterial Hypertension. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 145–158. [Google Scholar] [CrossRef]
- Fu, Z.; Gong, L.; Liu, J.; Wu, J.; Barrett, E.J.; Aylor, K.W.; Liu, Z. Brain Endothelial Cells Regulate Glucagon-Like Peptide 1 Entry Into the Brain via a Receptor-Mediated Process. Front. Physiol. 2020, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Takeuchi, M.; Yamagishi, S. Glucagon-like peptide-1 (GLP-1) inhibits advanced glycation end product (AGE)-induced up-regulation of VCAM-1 mRNA levels in endothelial cells by suppressing AGE receptor (RAGE) expression. Biochem. Biophys. Res. Commun. 2010, 391, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.T.; Tang, H.Q.; Su, H.; Wang, Y.; Zhou, Q.; Zhang, Q.; Wang, Y.; Zhu, H.Q. Glucagon-like peptide-1 attenuates endothelial barrier injury in diabetes via cAMP/PKA mediated down-regulation of MLC phosphorylation. Biomed. Pharmacother. 2019, 113, 108667. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; She, M.; Xu, M.; Chen, H.; Li, J.; Chen, X.; Zheng, D.; Liu, J.; Chen, S.; Zhu, J.; et al. GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. Int. J. Biol. Sci. 2018, 14, 1696–1708. [Google Scholar] [CrossRef]
- Koska, J.; Sands, M.; Burciu, C.; D’Souza, K.M.; Raravikar, K.; Liu, J.; Truran, S.; Franco, D.A.; Schwartz, E.A.; Schwenke, D.C.; et al. Exenatide Protects Against Glucose- and Lipid-Induced Endothelial Dysfunction: Evidence for Direct Vasodilation Effect of GLP-1 Receptor Agonists in Humans. Diabetes 2015, 64, 2624–2635. [Google Scholar] [CrossRef]
- Abdel-Latif, R.G.; Heeba, G.H.; Taye, A.; Khalifa, M.M.A. Lixisenatide ameliorates cerebral ischemia-reperfusion injury via GLP-1 receptor dependent/independent pathways. Eur. J. Pharmacol. 2018, 833, 145–154. [Google Scholar] [CrossRef]
- Wu, H.; Xiao, C.; Zhao, Y.; Yin, H.; Yu, M. Liraglutide Improves Endothelial Function via the mTOR Signaling Pathway. J. Diabetes Res. 2021, 2021, 2936667. [Google Scholar] [CrossRef]
- Jojima, T.; Uchida, K.; Akimoto, K.; Tomotsune, T.; Yanagi, K.; Iijima, T.; Suzuki, K.; Kasai, K.; Aso, Y. Liraglutide, a GLP-1 receptor agonist, inhibits vascular smooth muscle cell proliferation by enhancing AMP-activated protein kinase and cell cycle regulation, and delays atherosclerosis in ApoE deficient mice. Atherosclerosis 2017, 261, 44–51. [Google Scholar] [CrossRef]
- Bretón-Romero, R.; Weisbrod, R.M.; Feng, B.; Holbrook, M.; Ko, D.; Stathos, M.M.; Zhang, J.Y.; Fetterman, J.L.; Hamburg, N.M. Liraglutide Treatment Reduces Endothelial Endoplasmic Reticulum Stress and Insulin Resistance in Patients with Diabetes Mellitus. J. Am. Heart Assoc. 2018, 7, e009379. [Google Scholar] [CrossRef]
- Mei, X.; Li, Y.; Wu, J.; Liao, L.; Lu, D.; Qiu, P.; Yang, H.L.; Tang, M.W.; Liang, X.Y.; Liu, D. Dulaglutide restores endothelial progenitor cell levels in diabetic mice and mitigates high glucose-induced endothelial injury through SIRT1-mediated mitochondrial fission. Biochem. Biophys. Res. Commun. 2024, 716, 150002. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Hu, Y.; He, S.; Ye, Q.; Lv, Z.; Liu, J.; Chen, X. Dulaglutide inhibits high glucose- induced endothelial dysfunction and NLRP3 inflammasome activation. Arch. Biochem. Biophys. 2019, 671, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Yang, L.; Wang, S.; Liu, Y.; Yue, L.; Chen, S. Semaglutide alleviates inflammation-Induced endothelial progenitor cells injury by inhibiting MiR-155 expression in macrophage exosomes. Int. Immunopharmacol. 2023, 119, 110196. [Google Scholar] [CrossRef]
- Yue, L.; Chen, S.; Ren, Q.; Niu, S.; Pan, X.; Chen, X.; Li, Z.; Chen, X. Effects of semaglutide on vascular structure and proteomics in high-fat diet-induced obese mice. Front. Endocrinol. 2022, 13, 995007. [Google Scholar] [CrossRef] [PubMed]
- Estato, V.; Obadia, N.; Chateaubriand, P.H.; Figueiredo, V.; Curty, M.; Costa Silva, M.; Ferreira, R.G.L.; Santa-Ritta, J.; Campos Baroni, M.; Aragão, A.; et al. Semaglutide restores astrocyte-vascular interactions and blood-brain barrier integrity in a model of diet-induced metabolic syndrome. Diabetol. Metab. Syndr. 2025, 17, 2. [Google Scholar] [CrossRef] [PubMed]
- Withaar, C.; Meems, L.M.G.; Nollet, E.E.; Schouten, E.M.; Schroeder, M.A.; Knudsen, L.B.; Niss, K.; Madsen, C.T.; Hoegl, A.; Mazzoni, G.; et al. The Cardioprotective Effects of Semaglutide Exceed Those of Dietary Weight Loss in Mice with HFpEF. JACC Basic Transl. Sci. 2023, 8, 1298–1314. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Barchuk, M.; Lobo, M.; Nogueira, J.P. Effect of glucagon-like peptide-1 (GLP-1) analogues on epicardial adipose tissue: A meta-analysis. Diabetes Metab. Syndr. 2022, 16, 102562. [Google Scholar] [CrossRef]
- Bednarz, K.; Kowalczyk, K.; Cwynar, M.; Czapla, D.; Czarkowski, W.; Kmita, D.; Nowak, A.; Madej, P. The Role of Glp-1 Receptor Agonists in Insulin Resistance with Concomitant Obesity Treatment in Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2022, 23, 4334. [Google Scholar] [CrossRef]
- Randrup, E.; Baum, N.; Feibus, A. Erectile dysfunction and cardiovascular disease. Postgrad. Med. 2015, 127, 166–172. [Google Scholar] [CrossRef]
- Dalaklioglu, S.; Tasatargil, A.; Kuscu, N.; Celik, S.; Celik-Ozenci, C.; Ozdem, S.; Barutcigil, A.; Kucukcetin, I. Protective effect of exendin-4 treatment on erectile dysfunction induced by chronic methylglyoxal administration in rats. Peptides 2018, 106, 1–8. [Google Scholar] [CrossRef]
- Yuan, P.; Ma, D.; Gao, X.; Wang, J.; Li, R.; Liu, Z.; Wang, T.; Wang, S.; Liu, J.; Liu, X. Liraglutide Ameliorates Erectile Dysfunction via Regulating Oxidative Stress, the RhoA/ROCK Pathway and Autophagy in Diabetes Mellitus. Front. Pharmacol. 2020, 11, 1257. [Google Scholar] [CrossRef]
- Yue, L.; Xu, J.L.; Dong, J.; Xjang, G.D.; Xiang, L.; Zhao, L.S.; Zhang, J.X.; Zhai, Z.Y.; Zhu, G.P.; Liu, M.; et al. Regulatory effect of liraglutide on the expression of eNOS in the corpus cavernosum of diabetic rats. Zhonghua Nan Ke Xue 2016, 22, 212–218. [Google Scholar]
- Hurt, K.J.; Musicki, B.; Palese, M.A.; Crone, J.K.; Becker, R.E.; Moriarity, J.L.; Snyder, S.H.; Burnett, A.L. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc. Natl. Acad. Sci. USA 2002, 99, 4061–4066. [Google Scholar] [CrossRef]
- Model, J.F.A.; Lima, M.V.; Ohlweiler, R.; Sarapio, E.; Vogt, É.L.; Rocha, D.S.; de Souza, S.K.; Vinagre, A.S. Liraglutide treatment counteracts alterations in adipose tissue metabolism induced by orchiectomy in rats. Life Sci. 2021, 278, 119586. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Zang, Z.; Liang, X.; Jia, B.; Ye, T.; Lan, Y.; Shi, X. A Mitochondrion-Targeting Piezoelectric Nanosystem for the Treatment of Erectile Dysfunction via Autophagy Regulation. Adv. Mater. 2025, 37, e2413287. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Dalamaga, M.; Pavlou, A.; Rebelos, E.; Karamanolis, N.N.; Papachristoforou, E.; Mavrothalassitis, E.; Eleftheriadou, I.; Tentolouris, N.; Kounatidis, D. The Transformative Role of Nanotechnology in the Management of Diabetes Mellitus: Insights from Current Research. Biomolecules 2025, 15, 653. [Google Scholar] [CrossRef]
- Jensterle, M.; Podbregar, A.; Goricar, K.; Gregoric, N.; Janez, A. Effects of liraglutide on obesity-associated functional hypogonadism in men. Endocr. Connect. 2019, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Condorelli, R.A.; Calogero, A.E.; Cannarella, R.; Aversa, A. Sexual and Reproductive Outcomes in Obese Fertile Men with Functional Hypogonadism after Treatment with Liraglutide: Preliminary Results. J. Clin. Med. 2023, 12, 672. [Google Scholar] [CrossRef] [PubMed]
- Lisco, G.; Ramunni, M.I.; De Pergola, G.; Jirillo, E.; Guastamacchia, E.; Triggiani, V.; Giagulli, V.A. GLP-1 Receptor Agonists and Erectile Dysfunction in Diabetic Men with and without Hypogonadism: A 1-Year Retrospective Observational Study. Endocr. Abstr. 2022, 81, P355. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Carbone, M.D.; Ramunni, M.I.; Licchelli, B.; De Pergola, G.; Sabbà, C.; Guastamacchia, E.; Triggiani, V. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology 2015, 3, 1094–1103. [Google Scholar] [CrossRef]
- An, H.; Xie, K.; Gan, H. Glucagon-like peptide-1 receptor agonists and the risk of erectile dysfunction: A drug target Mendelian randomization study. Front. Endocrinol. 2024, 15, 1448394. [Google Scholar] [CrossRef]
- Bajaj, H.S.; Gerstein, H.C.; Rao-Melacini, P.; Basile, J.; Colhoun, H.; Conget, I.; Cushman, W.C.; Dagenais, G.R.; Franek, E.; Hanefeld, M.; et al. Erectile function in men with type 2 diabetes treated with dulaglutide: An exploratory analysis of the REWIND placebo-controlled randomised trial. Lancet Diabetes Endocrinol. 2021, 9, 484–490. [Google Scholar] [CrossRef]
- Able, C.; Liao, B.; Saffati, G.; Maremanda, A.; Applewhite, J.; Nasrallah, A.A.; Sonstein, J.; Alzweri, L.; Kohn, T.P. Prescribing semaglutide for weight loss in non-diabetic, obese patients is associated with an increased risk of erectile dysfunction: A TriNetX database study. Int. J. Impot. Res. 2025, 37, 315–319. [Google Scholar] [CrossRef]
- Labazi, H.; Wynne, B.M.; Tostes, R.; Webb, R.C. Metformin treatment improves erectile function in an angiotensin II model of erectile dysfunction. J. Sex. Med. 2013, 10, 2154–2164. [Google Scholar] [CrossRef]
- Defeudis, G.; Mazzilli, R.; Tenuta, M.; Rossini, G.; Zamponi, V.; Olana, S.; Faggiano, A.; Pozzilli, P.; Isidori, A.M.; Gianfrilli, D. Erectile dysfunction and diabetes: A melting pot of circumstances and treatments. Diabetes Metab. Res. Rev. 2022, 38, e3494. [Google Scholar] [CrossRef]
- Cai, T.; Hu, Y.; Ding, B.; Yan, R.; Liu, B.; Cai, L.; Jing, T.; Jiang, L.; Xie, X.; Wang, Y.; et al. Effect of Metformin on Testosterone Levels in Male Patients with Type 2 Diabetes Mellitus Treated with Insulin. Front. Endocrinol. 2021, 12, 813067. [Google Scholar] [CrossRef]
- Hu, Y.; Ding, B.; Shen, Y.; Yan, R.N.; Li, F.F.; Sun, R.; Jing, T.; Lee, K.O.; Ma, J.H. Rapid Changes in Serum Testosterone in Men with Newly Diagnosed Type 2 Diabetes with Intensive Insulin and Metformin. Diabetes Care 2021, 44, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Kounatidis, D.; Vallianou, N.G.; Rebelos, E.; Kouveletsou, M.; Kontrafouri, P.; Eleftheriadou, I.; Diakoumopoulou, E.; Karampela, I.; Tentolouris, N.; Dalamaga, M. The Many Facets of PPAR-γ Agonism in Obesity and Associated Comorbidities: Benefits, Risks, Challenges, and Future Directions. Curr. Obes. Rep. 2025, 14, 19. [Google Scholar] [CrossRef]
- Idris-Khodja, N.; Ouerd, S.; Trindade, M.; Gornitsky, J.; Rehman, A.; Barhoumi, T.; Offermanns, S.; Gonzalez, F.J.; Neves, M.F.; Paradis, P.; et al. Vascular smooth muscle cell peroxisome proliferator-activated receptor γ protects against endothelin-1-induced oxidative stress and inflammation. J. Hypertens. 2017, 35, 1390–1401. [Google Scholar] [CrossRef]
- Hamblin, M.; Chang, L.; Fan, Y.; Zhang, J.; Chen, Y.E. PPARs and the cardiovascular system. Antioxid. Redox Signal. 2009, 11, 1415–1452. [Google Scholar] [CrossRef]
- Palacios-Ramírez, R.; Hernanz, R.; Martín, A.; Pérez-Girón, J.V.; Barrús, M.T.; González-Carnicero, Z.; Aguado, A.; Jaisser, F.; Briones, A.M.; Salaices, M.; et al. Pioglitazone Modulates the Vascular Contractility in Hypertension by Interference with ET-1 Pathway. Sci. Rep. 2019, 9, 16461. [Google Scholar] [CrossRef]
- Heidenberg, D.J.; Haney, N.M.; Rezk, B.M.; Talwar, S.; Okpechi, S.C.; Srivastav, S.K.; Honda, M.; Song, B.; Swan, K.; Awadallah, S.; et al. Pioglitazone’s beneficial effects on erectile function preservation after cavernosal nerve injury in the rat are negated by inhibition of the insulin-like growth factor-1 receptor: A preclinical study. Int. J. Impot. Res. 2019, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gholamine, B.; Shafiei, M.; Motevallian, M.; Mahmoudian, M. Effects of pioglitazone on erectile dysfunction in sildenafil poor-responders: A randomized, controlled study. J. Pharm. Pharm. Sci. 2008, 11, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, Y.; Li, H.; Lei, H.; Guan, R.; Gao, Z.; Xin, Z. Antioxidant icariside II combined with insulin restores erectile function in streptozotocin-induced type 1 diabetic rats. J. Cell. Mol. Med. 2015, 19, 960–969. [Google Scholar] [CrossRef]
- Yamanaka, M.; Shirai, M.; Shiina, H.; Tanaka, Y.; Tsujimura, A.; Matsumiya, K.; Okuyama, A.; Dahiya, R. Diabetes induced erectile dysfunction and apoptosis in penile crura are recovered by insulin treatment in rats. J. Urol. 2003, 170, 291–297. [Google Scholar] [CrossRef]
- Shirai, M.; Yamanaka, M.; Shiina, H.; Igawa, M.; Ogishima, T.; Fujime, M.; Ishii, N.; Okuyama, A.; Lue, T.F.; Dahiya, R. Androgen, estrogen, and progesterone receptor gene regulation during diabetic erectile dysfunction and insulin treatment. Urology 2004, 64, 1244–1249. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Jinhua, W.; Shulin, G.; Jiangping, X.; Zhongxiang, L.; Xiaohong, L. Causal association between antidiabetic drugs and erectile dysfunction: Evidence from Mendelian randomization. Front. Endocrinol. 2024, 15, 1414958. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Rubio, J.L.; Hernández, M.; Rivera de los Arcos, L.; Benedito, S.; Recio, P.; García, P.; García-Sacristán, A.; Prieto, D. Role of ATP-sensitive K+ channels in relaxation of penile resistance arteries. Urology 2004, 63, 800–805. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Erectile Dysfunction and Low Sex Drive in Men with Type 2 DM: The Potential Role of Diabetic Pharmacotherapy. J. Clin. Diagn. Res. 2016, 10, FC21–FC26. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y. Outbreaks of severe hypoglycaemia due to illegal sexual enhancement products containing undeclared glibenclamide. Pharmacoepidemiol. Drug Saf. 2009, 18, 1250–1251. [Google Scholar] [CrossRef]
- Kao, S.L.; Chan, C.L.; Tan, B.; Lim, C.C.; Dalan, R.; Gardner, D.; Pratt, E.; Lee, M.; Lee, K.O. An unusual outbreak of hypoglycemia. N. Engl. J. Med. 2009, 360, 734–736. [Google Scholar] [CrossRef]
- Altabas, V.; Altabas, K. DPP-4 inhibition improves a sexual condition? Med. Hypotheses 2015, 85, 124–126. [Google Scholar] [CrossRef]
- Sun, T.; Xu, W.; Wang, J.; Wang, T.; Wang, S.; Liu, K.; Liu, J. Saxagliptin alleviates erectile dysfunction through increasing stromal cell-derived factor-1 in diabetes mellitus. Andrology 2023, 11, 295–306. [Google Scholar] [CrossRef]
- Kataoka, T.; Sanagawa, A.; Hotta, Y.; Kimura, K. Effect of DPP-4 Inhibitors on Erectile Dysfunction Caused by Vincristine in Rats. J. Sex. Med. 2022, 19, S141. [Google Scholar] [CrossRef]
- Simon, D.; Detournay, B.; Eschwege, E.; Bouée, S.; Bringer, J.; Attali, C.; Dejager, S. Use of Vildagliptin in Management of Type 2 Diabetes: Effectiveness, Treatment Persistence and Safety from the 2-Year Real-Life VILDA Study. Diabetes Ther. 2014, 5, 207–224. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, D.M.; Battson, M.L.; Jarrell, D.K.; Hou, S.; Ecton, K.E.; Weir, T.L.; Gentile, C.L. SGLT2 inhibition via dapagliflozin improves generalized vascular dysfunction and alters the gut microbiota in type 2 diabetic mice. Cardiovasc. Diabetol. 2018, 17, 62. [Google Scholar] [CrossRef]
- Uthman, L.; Homayr, A.; Juni, R.P.; Spin, E.L.; Kerindongo, R.; Boomsma, M.; Hollmann, M.W.; Preckel, B.; Koolwijk, P.; van Hinsbergh, V.W.M.; et al. Empagliflozin and Dapagliflozin Reduce ROS Generation and Restore NO Bioavailability in Tumor Necrosis Factor α-Stimulated Human Coronary Arterial Endothelial Cells. Cell. Physiol. Biochem. 2019, 53, 865–886. [Google Scholar] [CrossRef]
- Assaly, R.; Gorny, D.; Compagnie, S.; Mayoux, E.; Bernabe, J.; Alexandre, L.; Giuliano, F.; Behr-Roussel, D. The Favorable Effect of Empagliflozin on Erectile Function in an Experimental Model of Type 2 Diabetes. J. Sex. Med. 2018, 15, 1224–1234. [Google Scholar] [CrossRef]
- Ali, A.B.H.; Abdel-Aal, F.A.M.; Rageh, A.H.; Mohamed, A.I. Cytochrome P450 3A4-mediated pharmacokinetic interaction study between tadalafil and canagliflozin using high-performance thin-layer chromatography. J. Sep. Sci. 2022, 45, 4187–4197. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Leanza, C.; Garofalo, V.; Aversa, A.; Papa, G.; Calogero, A.E.; La Vignera, S. Dapagliflozin improves erectile dysfunction in patients with type 2 diabetes mellitus: An open-label, non-randomized pilot study. Diabet. Med. 2024, 41, e15217. [Google Scholar] [CrossRef]
- Hu, W.S.; Lin, C.L. Erectile Dysfunction Risk Among Patients with Diabetes Mellitus Using Sodium-Glucose Cotransporter 2 Inhibitors. J. Cardiovasc. Pharmacol. 2024, 84, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Shao, N.; Yu, X.Y.; Yu, Y.M.; Li, B.W.; Pan, J.; Wu, W.H.; Zhang, H.J.; Ma, X.F.; Hao, M.; Kuang, H.Y. Short-term combined treatment with exenatide and metformin is superior to glimepiride combined metformin in improvement of serum testosterone levels in type 2 diabetic patients with obesity. Andrologia 2018, 50, e13039. [Google Scholar] [CrossRef] [PubMed]
- Defeudis, G.; Di Tommaso, A.M.; Di Rosa, C.; Cimadomo, D.; Khazrai, Y.M.; Faggiano, A.; Cincione, R.I.; Napoli, N.; Mazzilli, R. The Role of Antihyperglycemic Drugs and Diet on Erectile Function: Results from a Perspective Study on a Population with Prediabetes and Diabetes. J. Clin. Med. 2022, 11, 3382. [Google Scholar] [CrossRef]
- Yang, B.; Cheng, H.; Hu, Y.; Chen, Y.; Xu, Y.; Huang, W.; Long, Y.; Gao, C. Effects of Anti-Diabetic Drugs on Erectile Dysfunction: A Systematic Review and Meta-Analysis. Diabetes Metab. Syndr. Obes. 2025, 18, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Rastrelli, G.; Corona, G.; Sparano, C.; Vignozzi, L.; Sforza, A.; Maggi, M. Impact of Diabetic Medications on Erectile Function: A Meta-Analysis. J. Sex. Med. 2025, 22 (Suppl. 2), qdaf077.056. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Sparano, C.; Vignozzi, L.; Sforza, A.; Maggi, M. Role of Glucagon-Like Peptide Type 1 Analogues on Hypothalamus-Pituitary-Testis Axis and Sexual Function in Men: A Systematic Review and Meta-Analysis. J. Sex. Med. 2025, 22 (Suppl. 2), qdaf077.063. [Google Scholar] [CrossRef]
- Van Cauwenberghe, J.; De Block, C.; Vanderschueren, D.; Antonio, L. Effects of treatment for diabetes mellitus on testosterone concentrations: A systematic review. Andrology 2023, 11, 225–233. [Google Scholar] [CrossRef]
- Salvio, G.; Ciarloni, A.; Ambo, N.; Bordoni, M.; Perrone, M.; Rossi, S.; Balercia, G. Effects of glucagon-like peptide 1 receptor agonists on testicular dysfunction: A systematic review and meta-analysis. Andrology, 2025; Ahead of print. [Google Scholar] [CrossRef]
- Graybill, S.; Hatfield, J.; Kravchenko, M.; Beckman, D.; Tate, J.; Beauvais, A.; Clerc, P.; Davila, D.; Forbes, W.; Wardian, J.; et al. Neutral effect of exenatide on serum testosterone in men with type 2 diabetes mellitus: A prospective cohort. Andrology 2021, 9, 792–800. [Google Scholar] [CrossRef]
- Mostafaei, H.; Mori, K.; Hajebrahimi, S.; Abufaraj, M.; Karakiewicz, P.I.; Shariat, S.F. Association of erectile dysfunction and cardiovascular disease: An umbrella review of systematic reviews and meta-analyses. BJU Int. 2021, 128, 3–11. [Google Scholar] [CrossRef]
- Nguyen Ngoc Dang, H.; Viet Luong, T.; Kiem Pham, A.; Trung Le, T.; Duc Le, N.; Minh Nguyen, H.; Anh Hoang, T.; Anh Ho, B. Exploring the bidirectional link between erectile dysfunction and 10-year cardiovascular risk in men with diabetes and hypertension. Sci. Rep. 2024, 14, 28816. [Google Scholar] [CrossRef]
- Peng, W.; Zhou, R.; Sun, Z.F.; Long, J.W.; Gong, Y.Q. Novel Insights into the Roles and Mechanisms of GLP-1 Receptor Agonists against Aging-Related Diseases. Aging Dis. 2022, 13, 468–490. [Google Scholar] [CrossRef]
- Lu, C.; Xu, C.; Li, S.; Ni, H.; Yang, J. Liraglutide and GLP-1(9-37) alleviated hepatic ischemia-reperfusion injury by inhibiting ferroptosis via GSK3β/Nrf2 pathway and SMAD159/Hepcidin/FTH pathway. Redox Biol. 2025, 79, 103468. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.; Qi, J.; Zhang, K.; Jiang, Y.; Feng, B.; Lv, T.; Yang, L.; Yang, Q.; Zhao, M.; et al. Glucagon-like Peptide 1 Receptor Activation Inhibits Microglial Pyroptosis via Promoting Mitophagy to Alleviate Depression-like Behaviors in Diabetic Mice. Nutrients 2022, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Rroji, M.; Spahia, N.; Figurek, A.; Spasovski, G. Targeting Diabetic Atherosclerosis: The Role of GLP-1 Receptor Agonists, SGLT2 Inhibitors, and Nonsteroidal Mineralocorticoid Receptor Antagonists in Vascular Protection and Disease Modulation. Biomedicines 2025, 13, 728. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Peng, Y.; Gu, J.; Li, T.; Wang, Q.; Qi, X.; Wei, A. Single-cell RNA sequencing reveals critical modulators of extracellular matrix of penile cavernous cells in erectile dysfunction. Sci. Rep. 2024, 14, 5886. [Google Scholar] [CrossRef]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef]
- Wei, L.; Gao, J.; Wang, L.; Tao, Q.; Tu, C. Hippo/YAP signaling pathway: A new therapeutic target for diabetes mellitus and vascular complications. Ther. Adv. Endocrinol. Metab. 2023, 14, 20420188231220134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Zong, Y.; Ma, Y.; Tian, Y.; Pang, Y.; Zhang, C.; Gao, J. Glucagon-like peptide-1 receptor: Mechanisms and advances in therapy. Signal Transduct. Target Ther. 2024, 9, 234. [Google Scholar] [CrossRef]
- Li, Y.; Du, J.; Zhu, E.; Zhang, J.; Han, J.; Zhao, W.; Sun, B.; Tian, D. Liraglutide suppresses proliferation and induces adipogenic differentiation of 3T3-L1 cells via the Hippo-YAP signaling pathway. Mol. Med. Rep. 2018, 17, 4499–4507. [Google Scholar] [CrossRef]
- Bae, S.G.; Yin, G.N.; Ock, J.; Suh, J.K.; Ryu, J.K.; Park, J. Single-cell transcriptome analysis of cavernous tissues reveals the key roles of pericytes in diabetic erectile dysfunction. elife 2024, 12, RP88942. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Coucha, M.; Bolduc, D.R.; Burnett, F.N.; Barrett, A.C.; Ghaly, M.; Abdelsaid, M. GLP-1 receptor nitration contributes to loss of brain pericyte function in a mouse model of diabetes. Diabetologia 2022, 65, 1541–1554. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, J.; Pan, Y.; Lv, J.; Liu, Y.; Yu, S.; Zhang, Y. Glucagon-like peptide-1 receptor expression and its functions are regulated by androgen. Biomed. Pharmacother. 2019, 120, 109555. [Google Scholar] [CrossRef]
- Dudal, S.; Bissantz, C.; Caruso, A.; David-Pierson, P.; Driessen, W.; Koller, E.; Krippendorff, B.F.; Lechmann, M.; Olivares-Morales, A.; Paehler, A.; et al. Translating pharmacology models effectively to predict therapeutic benefit. Drug Discov. Today 2022, 27, 1604–1621. [Google Scholar] [CrossRef]
- Jensterle, M.; Janez, A.; Fliers, E.; DeVries, J.H.; Vrtacnik-Bokal, E.; Siegelaar, S.E. The role of glucagon-like peptide-1 in reproduction: From physiology to therapeutic perspective. Hum. Reprod. Update 2019, 25, 504–517. [Google Scholar] [CrossRef]
- Neijenhuijs, K.I.; Holtmaat, K.; Aaronson, N.K.; Holzner, B.; Terwee, C.B.; Cuijpers, P.; Verdonck-de Leeuw, I.M. The International Index of Erectile Function (IIEF)-A Systematic Review of Measurement Properties. J. Sex. Med. 2019, 16, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Morris, R.; Bu, K.; Zhu, T.; Huang, H.; Cheng, F. Analysis of literature-derived duplicate records in the FDA Adverse Event Reporting System (FAERS) database. Can. J. Physiol. Pharmacol. 2025, 103, 56–69. [Google Scholar] [CrossRef]
- Lovegrove, M.C.; Stone, N.D.; Geller, A.I.; Weidle, N.J.; Lind, J.N.; Cohen, P.A. U.S. Emergency Department Visits Attributed by Clinicians to Semaglutide Adverse Events, 2022–2023. Ann. Intern. Med. 2025, 178, 898–900. [Google Scholar] [CrossRef]
- Hollander, J.E. Emergency department considerations regarding erectile dysfunction. Am. J. Cardiol. 2005, 96, 73M–75M. [Google Scholar] [CrossRef] [PubMed]
- Arillotta, D.; Floresta, G.; Papanti Pelletier, G.D.; Guirguis, A.; Corkery, J.M.; Martinotti, G.; Schifano, F. Exploring the Potential Impact of GLP-1 Receptor Agonists on Substance Use, Compulsive Behavior, and Libido: Insights from Social Media Using a Mixed-Methods Approach. Brain Sci. 2024, 14, 617. [Google Scholar] [CrossRef] [PubMed]
- Bain, S.C.; Klufas, M.A.; Ho, A.; Matthews, D.R. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: A review. Diabetes Obes. Metab. 2019, 21, 454–466. [Google Scholar] [CrossRef]
- Pitocco, D.; Zaccardi, F.; Di Stasio, E.; Romitelli, F.; Santini, S.A.; Zuppi, C.; Ghirlanda, G. Oxidative stress, nitric oxide, and diabetes. Rev. Diabet. Stud. 2010, 7, 15–25. [Google Scholar] [CrossRef]
- Jeibmann, A.; Zahedi, S.; Simoni, M.; Nieschlag, E.; Byrne, M.M. Glucagon-like peptide-1 reduces the pulsatile component of testosterone secretion in healthy males. Eur. J. Clin. Investig. 2005, 35, 565–572. [Google Scholar] [CrossRef]
- Memel, Z.; Gold, S.L.; Pearlman, M.; Muratore, A.; Martindale, R. Impact of GLP- 1 Receptor Agonist Therapy in Patients High Risk for Sarcopenia. Curr. Nutr. Rep. 2025, 14, 63. [Google Scholar] [CrossRef]
- Viken, A.F.; Siiak, S.P.; Schlünssen, V.; Thorarinsdottir, E.H.; Skulstad, S.M.; Gyawali, S.; Bertelsen, R.J.; Real, F.G. Muscle Strength and Male Sexual Function. J. Clin. Med. 2024, 13, 426. [Google Scholar] [CrossRef]
- Schulte, D.M.; Hahn, M.; Oberhäuser, F.; Malchau, G.; Schubert, M.; Heppner, C.; Müller, N.; Güdelhöfer, H.; Faust, M.; Krone, W.; et al. Caloric restriction increases serum testosterone concentrations in obese male subjects by two distinct mechanisms. Horm. Metab. Res. 2014, 46, 283–286. [Google Scholar] [CrossRef]
- Smith, S.J.; Teo, S.Y.M.; Lopresti, A.L.; Heritage, B.; Fairchild, T.J. Examining the effects of calorie restriction on testosterone concentrations in men: A systematic review and meta-analysis. Nutr. Rev. 2022, 80, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Kohn, T.P.; Lipshultz, L.I. Comment on: Male sexual dysfunction associated with GLP-1 receptor agonists: A cross-sectional analysis of FAERS data. Int. J. Impot. Res. 2025; Ahead of print. [Google Scholar] [CrossRef]
- Salonia, A.; Capogrosso, P.; Boeri, L.; Cocci, A.; Corona, G.; Dinkelman-Smit, M.; Falcone, M.; Jensen, C.F.; Gül, M.; Kalkanli, A.; et al. European Association of Urology Guidelines on Male Sexual and Reproductive Health: 2025 Update on Male Hypogonadism, Erectile Dysfunction, Premature Ejaculation, and Peyronie’s Disease. Eur. Urol. 2025, 88, 76–102. [Google Scholar] [CrossRef] [PubMed]
- Bong, G.W.; Clarke, H.S., Jr.; Hancock, W.C.; Keane, T.E. Serum testosterone recovery after cessation of long-term luteinizing hormone-releasing hormone agonist in patients with prostate cancer. Urology 2008, 71, 1177–1180. [Google Scholar] [CrossRef]
- Lengsfeld, S.; Probst, L.; Emara, Y.; Werlen, L.; Vogt, D.R.; Bathelt, C.; Baur, F.; Caviezel, B.; Vukajlovic, T.; Fischer, M.; et al. Effects of the glucagon-like peptide-1 receptor agonist dulaglutide on sexuality in healthy men: A randomised, double-blind, placebo-controlled crossover study. EBioMedicine 2024, 107, 105284. [Google Scholar] [CrossRef]
- Zhong, Q.; Bollag, R.J.; Dransfield, D.T.; Gasalla-Herraiz, J.; Ding, K.H.; Min, L.; Isales, C.M. Glucose-dependent insulinotropic peptide signaling pathways in endothelial cells. Peptides 2000, 21, 1427–1432. [Google Scholar] [CrossRef]
- Lim, D.M.; Park, K.Y.; Hwang, W.M.; Kim, J.Y.; Kim, B.J. Difference in protective effects of GIP and GLP-1 on endothelial cells according to cyclic adenosine monophosphate response. Exp. Ther. Med. 2017, 13, 2558–2564. [Google Scholar] [CrossRef]
- Killion, E.A.; Hussien, R.; Shkumatov, A.; Davies, R.; Lloyd, D.J.; Véniant, M.M.; Lebrec, H.; Fort, M.M. GIPR gene expression in testis is mouse specific and can impact male mouse fertility. Andrology 2022, 10, 789–799. [Google Scholar] [CrossRef] [PubMed]
- La Vignera, S.; Cannarella, R.; Garofalo, V.; Crafa, A.; Barbagallo, F.; Condorelli, R.A.; Calogero, A.E. Short-term impact of tirzepatide on metabolic hypogonadism and body composition in patients with obesity: A controlled pilot study. Reprod. Biol. Endocrinol. 2025, 23, 92. [Google Scholar] [CrossRef]
- Małczak, P.; Wysocki, M.; Kawa, I.; Wikar, T.; Pisarska-Adamczyk, M.; Pędziwiatr, M.; Major, P. Improved erectile function after bariatric surgery: Role of testosterone and other factors-a cohort prospective study. Surg. Obes. Relat. Dis. 2025, 21, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, I.A.; Kounatidis, D.; Honka, M.-J.; Vallianou, N.G.; Rebelos, E.; Karamanolis, N.N.; Dalamaga, M.; Pantos, C.; Mourouzis, I. Metabolomic Alterations in Patients with Obesity and the Impact of Metabolic Bariatric Surgery: Insights for Future Research. Metabolites 2025, 15, 434. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Sands, C.; Alexiadou, K.; Minnion, J.; Tharakan, G.; Behary, P.; Ahmed, A.R.; Purkayastha, S.; Lewis, M.R.; Bloom, S.; et al. The Metabolomic Effects of Tripeptide Gut Hormone Infusion Compared to Roux-en-Y Gastric Bypass and Caloric Restriction. J. Clin. Endocrinol. Metab. 2022, 107, e767–e782. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).