In Situ Synthesis of Bacterial Cellulose-Supported CoAl-Layered Double Hydroxide as a Peroxymonosulfate Activator for Enhancing the Removal of Tetracycline
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
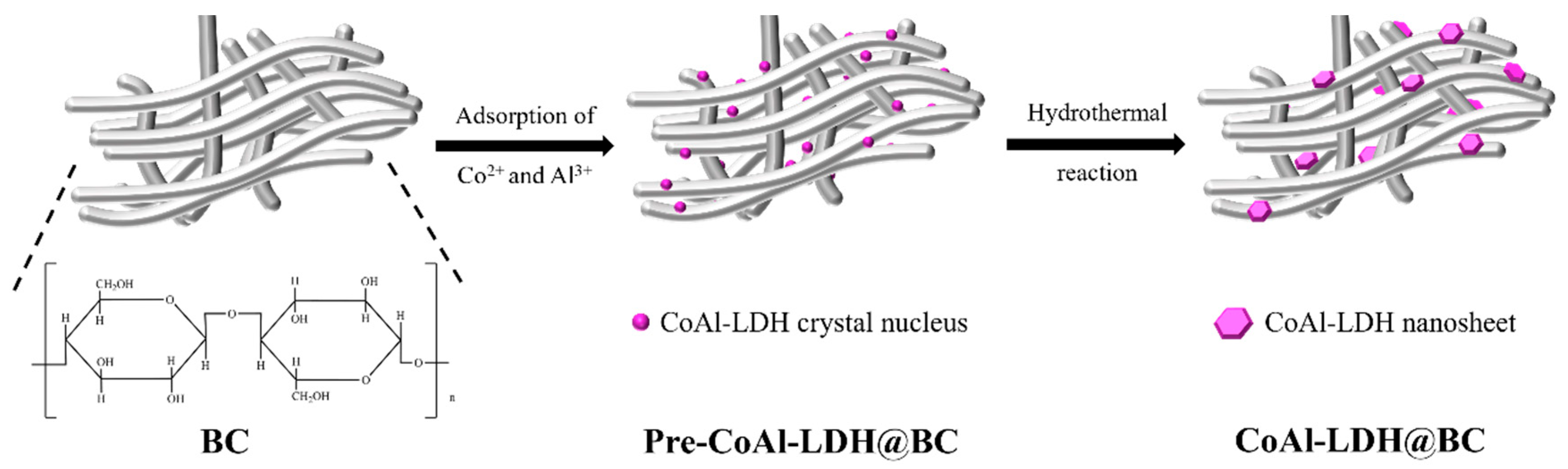
2.2. Preparation of CoAl-LDH@BC
2.3. Catalyst Characterization
2.4. Experimental Method
2.5. Effects of TC Degraded Products on Plant Growth
3. Results
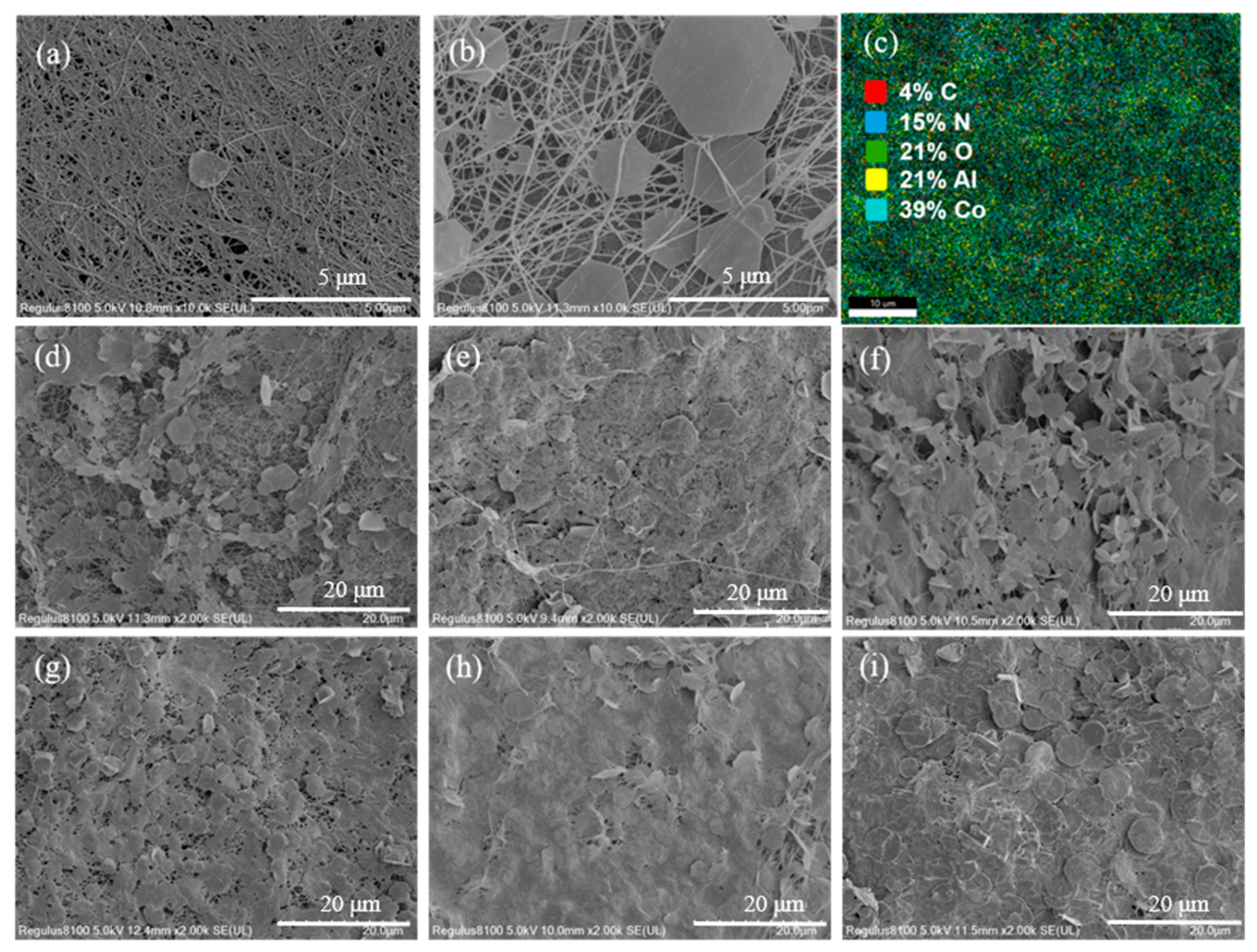
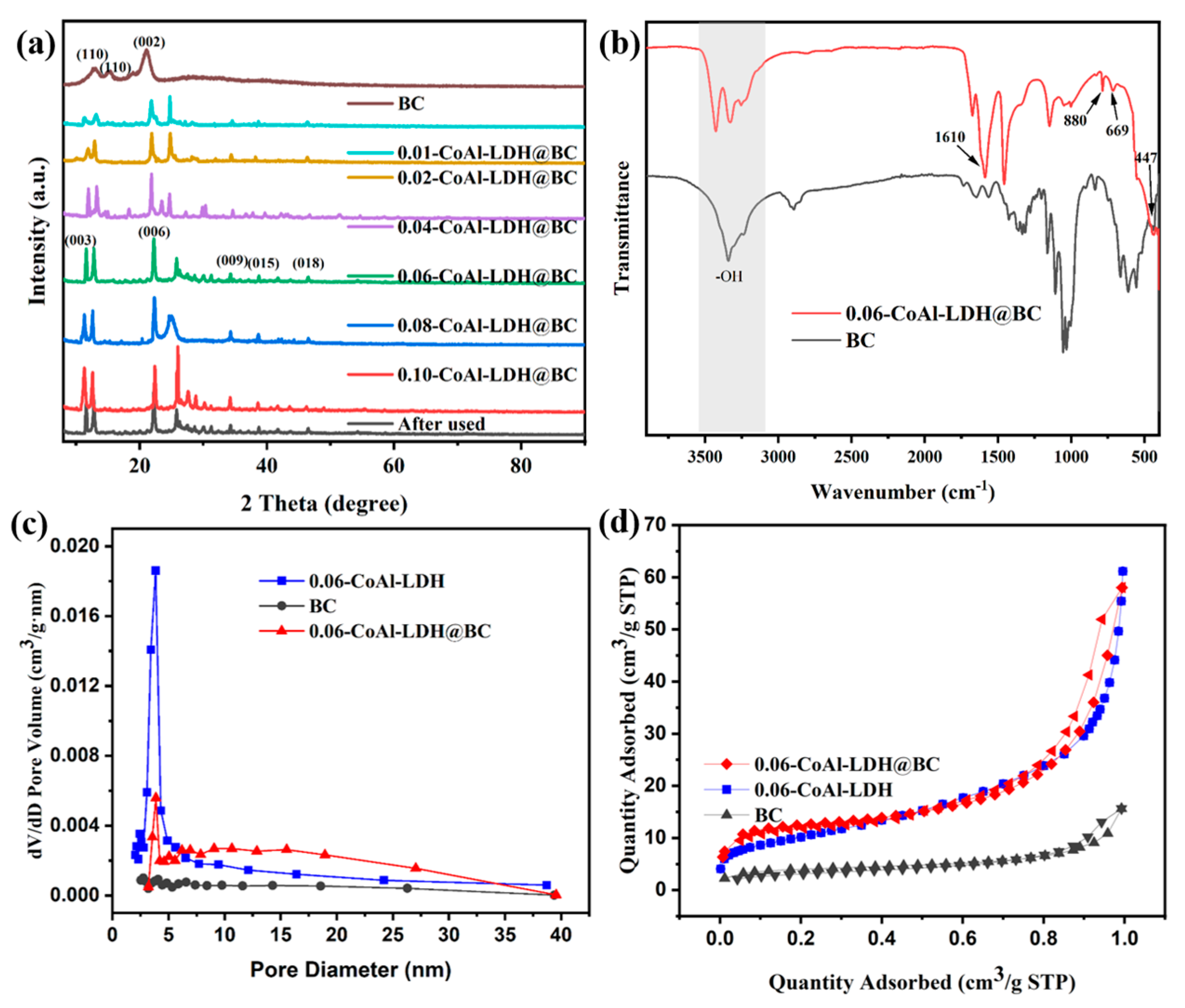
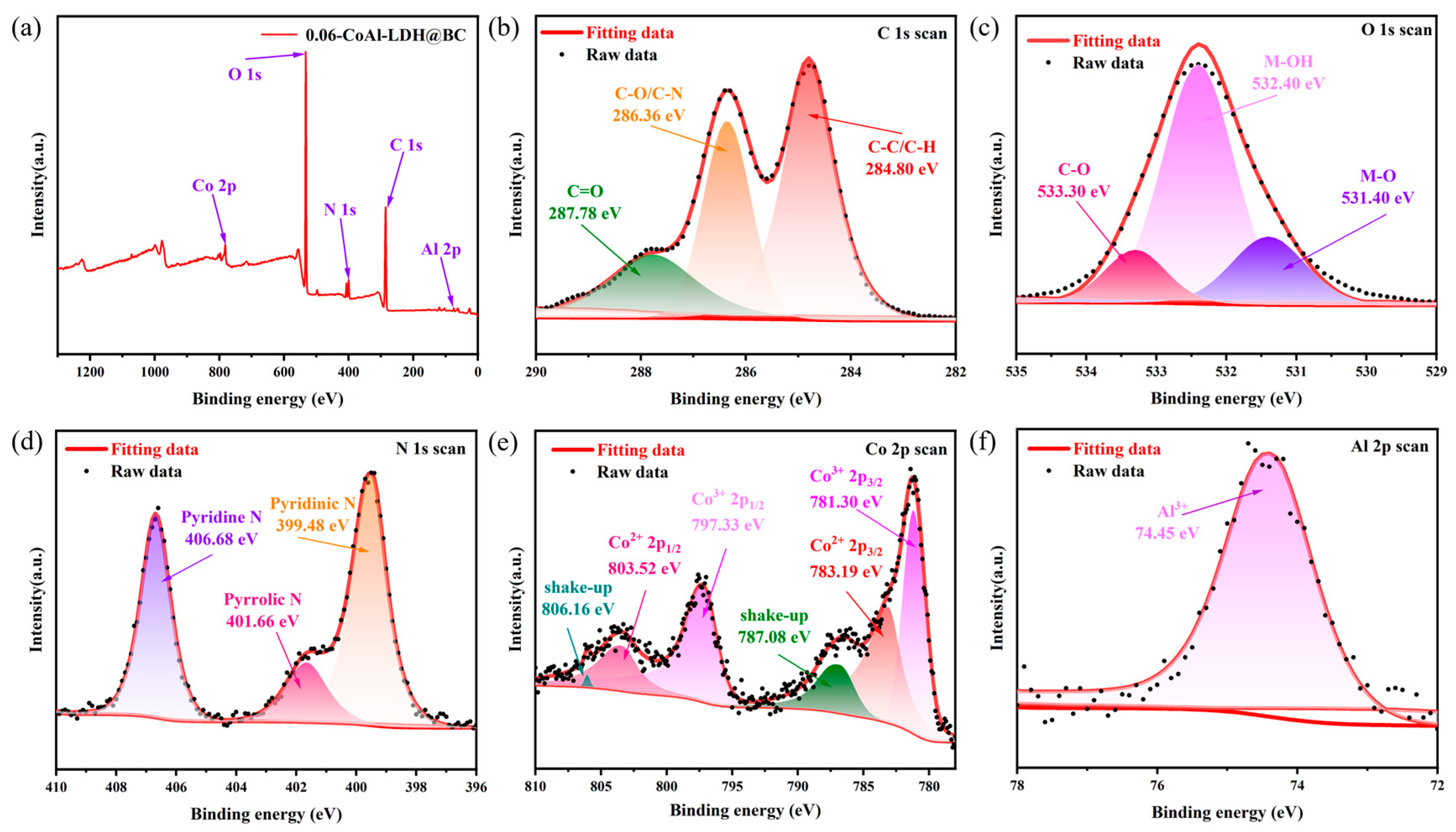
3.1. Characterization
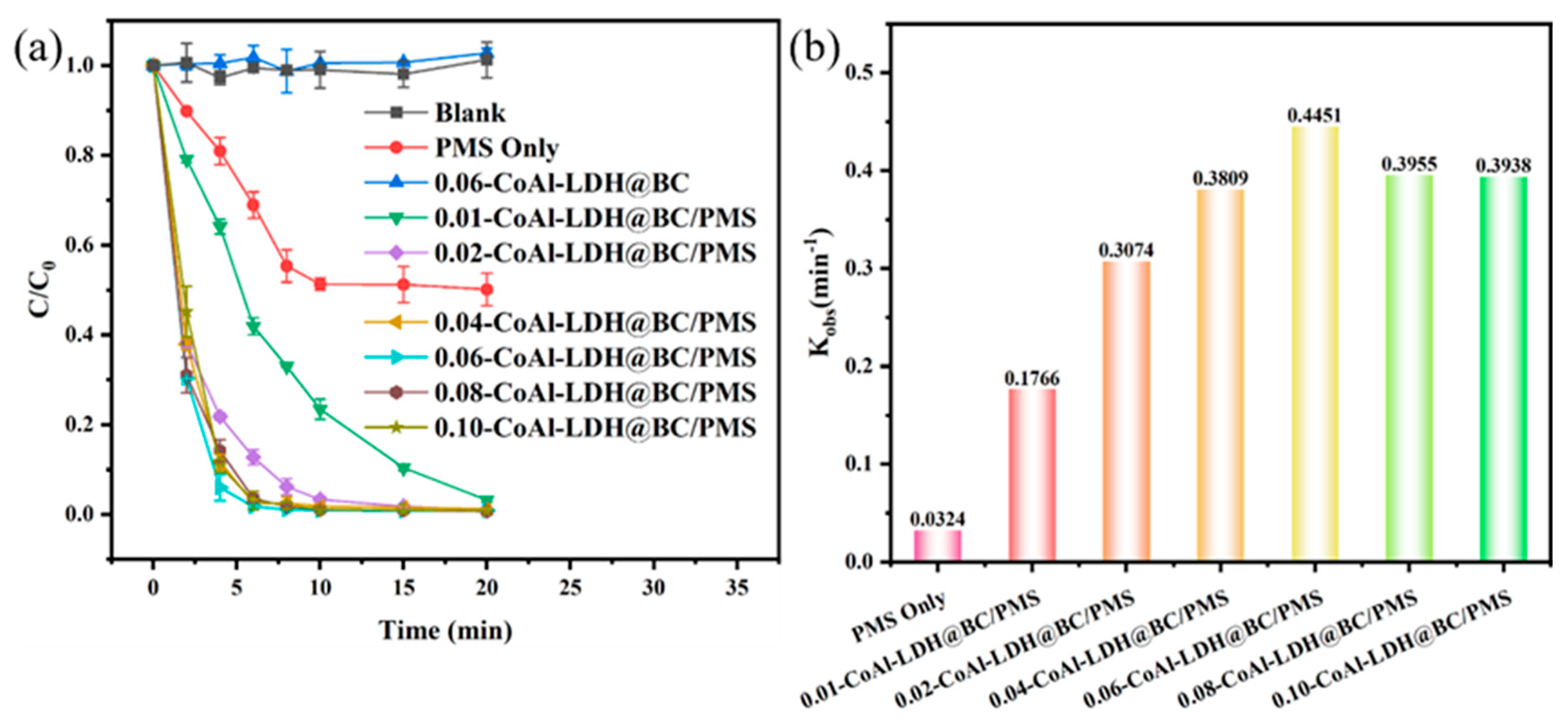
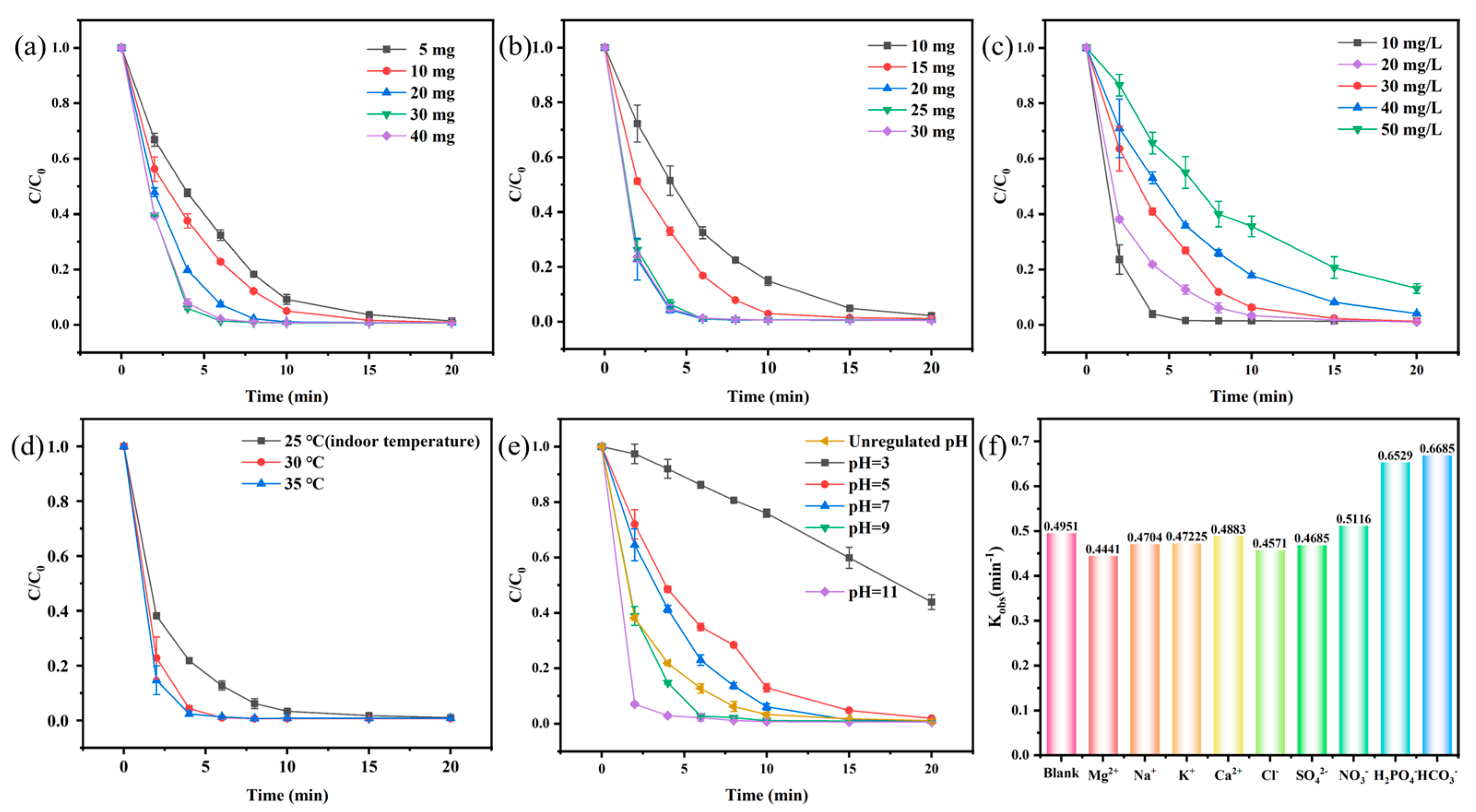
3.2. Catalytic Performance
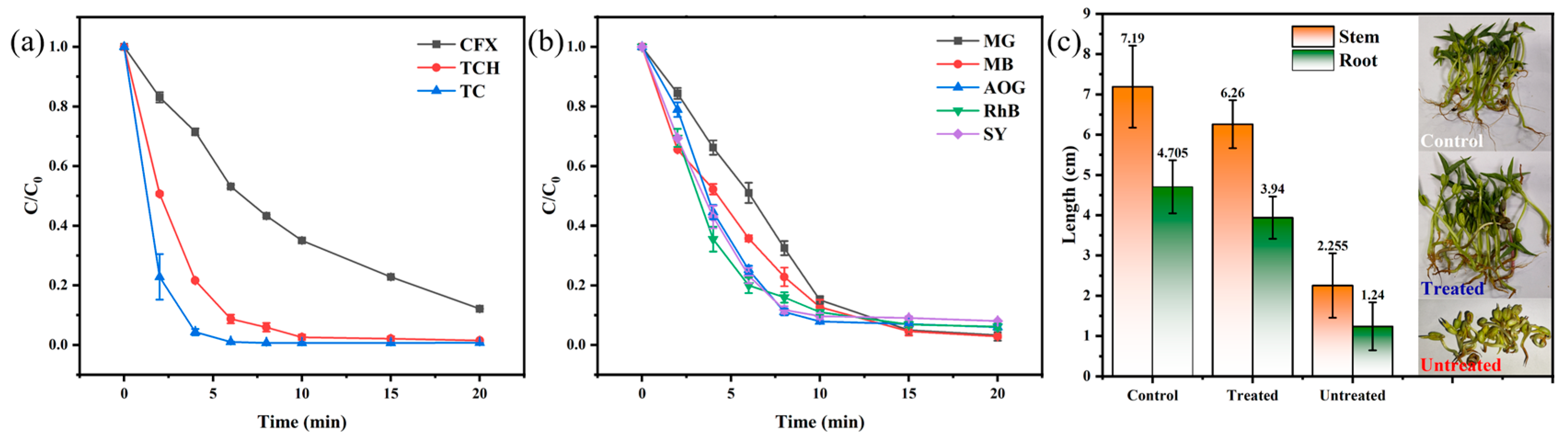
3.3. Toxic Test
3.4. Possible Degradation Pathway of TC
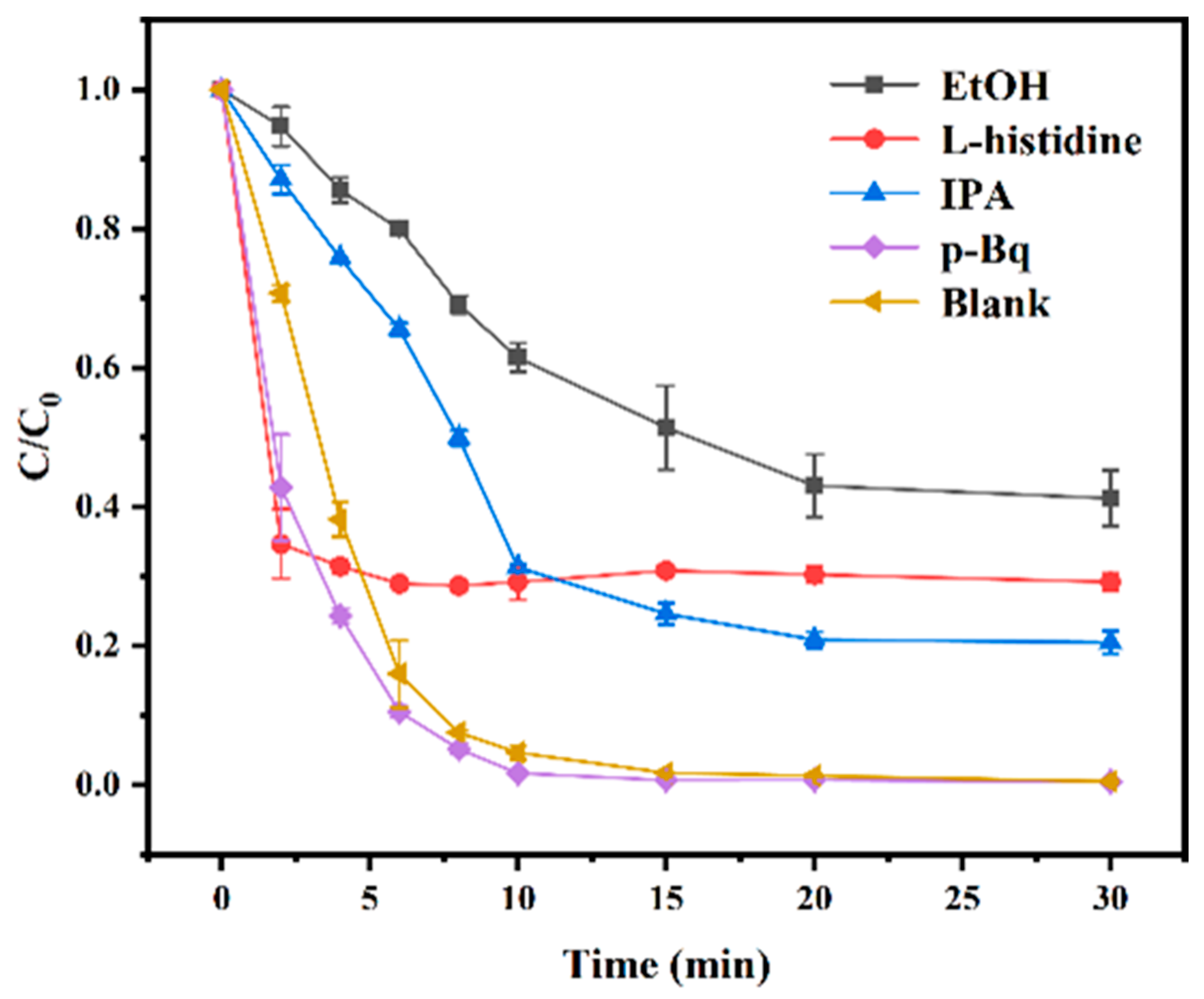
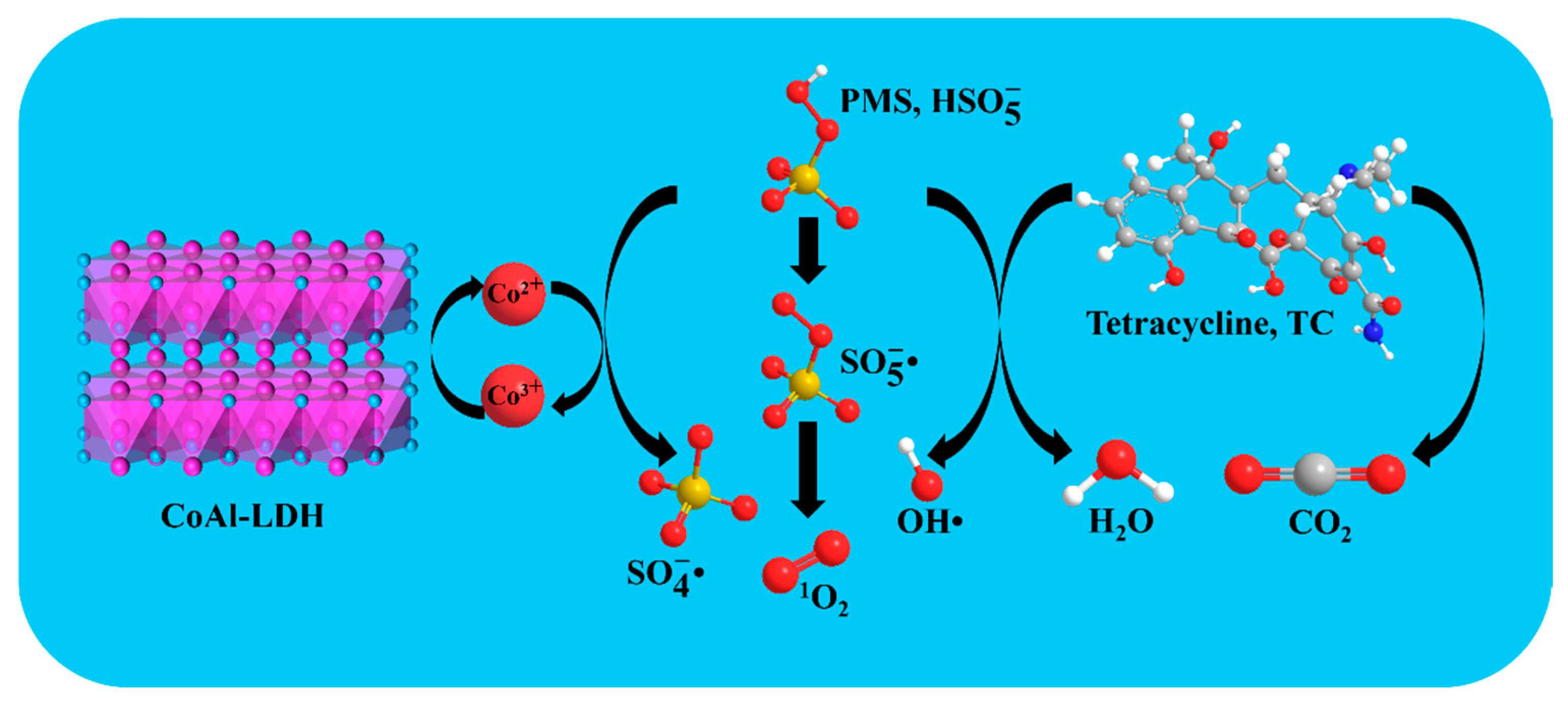
3.5. Catalytic Mechanisms
3.6. Reusability of CoAl-LDH@BC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Chen, X.-T.; Ying, Y.-M.; Wen, J.-L.; Wang, H.-N.; Le, C.; Qu, Y.-S.; Li, M.; Wu, J.; Fu, Z.-G. Metal sulfide-based electrocatalysts for water splitting: From rational design to advanced applications. Fuel 2026, 404, 136208. [Google Scholar] [CrossRef]
- Javan, K.; Darestani, M.; Ibrar, I.; Pignatta, G. Interrelated issues within the Water-Energy-Food nexus with a focus on environmental pollution for sustainable development: A review. Environ. Pollut. 2025, 368, 125706. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.-H.; Hao, W.-L.; Zhang, H.-N.; Miao, J.-J.; Shi, H.-G. Pollution status and spatial distribution of antibiotic resistance genes in urban surface water and surrounding soil media. J. Environ. Chem. Eng. 2024, 12, 113856. [Google Scholar] [CrossRef]
- Li, N.; Zhu, X.-C.; Miao, Y.-H.; Wang, Z.-Y.; Ki, C.-S.; Li, C. Meta-analysis and empirical research on the effectiveness of biochar in remediating tetracyclines pollution in water bodies. Bioresour. Technol. 2025, 435, 132917. [Google Scholar] [CrossRef]
- Wu, H.; Lv, H.-G.; Yu, Y.-X.; Du, Y.-G.; Du, D.-Y. Ammonium persulfate-triggered modified chitosan biochar for co-adsorption of Cr(VI) and tetracycline antibiotics: Behavior and mechanisms. Int. J. Biol. Macromol. 2025, 311, 143432. [Google Scholar] [CrossRef]
- Rajguru, P.; Dihingia, K.D.; Konwar, A.; Bora, P.; Bhuyan, C.; Saha, S.; Sastry, G.N.; Hazarika, S. Carbon nanotube/Fe2O3 nanocomposites for optimizing membrane-based separation of antibiotics: Experimental and computational approach. Mater. Today Commun. 2025, 44, 111951. [Google Scholar] [CrossRef]
- Feng, Q.-Y.; Guo, K.-Y.; Liu, B.-B.; Wang, C.-X.; Yan, B.-J.; Yue, Q.-Y.; Gao, Y.; Gao, B. Recent advances and prospects in antibiotics removal by coagulation technique: A systematic review. J. Clean. Prod. 2025, 514, 145825. [Google Scholar] [CrossRef]
- Ren, H.; Pan, Y.-W.; Zhong, J.-H.; Wang, J.-Y.; Lu, Z.-X.; He, Q.; Zhou, S.-Y.; Liao, X.-P.; Liu, Y.-H.; An, T.-C.; et al. An antibiotic-destructase-activated Fenton-like catalyst for synergistic removal of tetracycline residues from aquatic environment. Chem. Eng. J. 2023, 459, 141576. [Google Scholar] [CrossRef]
- Li, K.-Q.; Zhang, X.-P.; Wang, J.; Guo, L.-X.; Deng, X.-D.; Xie, T.; Wang, J.-L.; Zhu, G. Hetero-metal MOF derivatives anchored on porous carbonized wood as photo-Fenton catalysts for ultrafast organic pollutant removal. J. Photochem. Photobiol. A Chem. 2025, 470, 116616. [Google Scholar] [CrossRef]
- You, X.-Y.; Wang, C.-H.; Wang, C.-Q.; Xu, X.; Hu, Y.-Y.; Li, N.; Hu, F.-P.; Liu, W.; Peng, X.-M. Unveiling the synergistic mechanism of Fe-Cu bimetallic catalysts for degradation of tetracycline antibiotic with ultralow peroxymonosulfate dosage consumption: Key contribution of electron transfer process. J. Colloid Interface Sci. 2025, 689, 137277. [Google Scholar] [CrossRef]
- Liu, M.-F.; Cui, X.-Q.; Wang, Y.-N.; Sun, Y.-J.; Wang, H.-W. Sponge-loaded biogenic manganese oxide (BMO@Sponge) activated peroxymonosulfate for tetracycline degradation in wastewater: Efficacy and mechanism. J. Water Process Eng. 2025, 69, 106779. [Google Scholar] [CrossRef]
- Xia, X.-Y.; Gao, Q.-Y.; Ji, X.-X.; Wan, J.-Q.; Wang, Y.; Ma, Y.-W. Novel cobalt-doped CuFe2O4 spinel catalyst for enhanced peroxymonosulfate activation to achieve rapid degradation of tetracycline hydrochloride: Dominant roles of O2 and 1O2 in the oxidation process. J. Water Process Eng. 2025, 69, 106638. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Yu, X.-T.; Zhao, W.-Y.; Bao, S.-Q.; Wang, Y.-N.; Xu, L. Efficient degradation of antibiotic mixtures by light-assisted Co-NiFe-LDHs activation of peroxymonosulfate: Performance, mechanism, and degradation pathways. J. Solid State Chem. 2025, 350, 125491. [Google Scholar] [CrossRef]
- Rezaee, R.; Faraji, A.; Ashouri, F. Dendritic magnetic polymeric core-shell and cobalt-wastewater as an efficient peroxymonosulfate activator for degradation of tetracycline antibiotic and methylene blue dye. Inorg. Chem. Commun. 2022, 146, 110184. [Google Scholar] [CrossRef]
- Ding, Q.-H.; Guo, L.; Xu, T.-T.; Sun, Y.; Chen, S.-W.; Jiang, Y.; Jia, Y.-H.; Li, H.; Mao, N.; Li, X.-L. Cobalt-loaded biochar composites as effective sonocatalysts for degradation of tetracycline in water under activation of peroxymonosulfate. J. Water Process Eng. 2025, 76, 108302. [Google Scholar] [CrossRef]
- Zhang, Q.-E.; Hu, C.-C.; Guo, S.-Q.; Liu, J.-W.; Hu, S.-C.; Xin, Y.-X.; Yu, J.-G.; Zhao, G.-Q.; Lu, L.-M. Design and synthesis of uniform defective carbon nanosheets via CoAl-layered double hydroxide interlayer confinement for boosting peroxymonosulfate activation with highly efficient degradation of antibiotics contaminants. J. Environ. Chem. Eng. 2025, 13, 115601. [Google Scholar] [CrossRef]
- Kim, C.; Park, C.; Song, J.; Jeong, Y.; Senthamaraikannan, T.G.; Lim, D.H.; Hong, H.J. Mechanistic investigation of NiAl-layered double hydroxide activated peroxymonosulfate for tetracycline degradation: Feasibility of an integrated ultrafiltration system. Environ. Res. 2025, 284, 122218. [Google Scholar] [CrossRef]
- Fang, Q.-Z.; Liu, N.; Gu, Y.-L.; Yang, H.-L.; Ye, S.-J.; Yang, Z.-Z.; Chen, G.-B.; Tan, X.-F.; Hu, X.-J. Mechanism exploration in tetracycline degradation by Ni-Fe layered double hydroxide-biochar/peroxymonosulfate system: Nonradical-dominated oxidation process. Sep. Purif. Technol. 2024, 335, 126122. [Google Scholar] [CrossRef]
- Pedrosa, M.F.F. An overview of bio-cellulose derived materials for catalytic water treatment. Int. J. Biol. Macromol. 2024, 258, 128789. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Wang, Z.-Y.; Zhao, M.-J.; Xiao, F.; Zhang, L.-Y.; Yang, L.; Wu, Z.-J.; Bai, J.-W.; He, P. MOF/bacterial cellulose derived octahedral MnO/carbon nanofiber network: A hybrid for peroxymonosulfate activation toward degradation of tetracycline. J. Alloys Compd. 2023, 968, 171896. [Google Scholar] [CrossRef]
- Kaitheri, A.; Padmanabhan, S.K.; Pal, S.; Corato, R.D.; Neeleman, K.; Einarsrud, M.A.; Licciulli, A. Synthesis and characterisation of titania supported on bacterial cellulose films and evaluation of photocatalytic activity under solar irradiation. J. Environ. Chem. Eng. 2025, 13, 116976. [Google Scholar] [CrossRef]
- Rosson, L.; Tan, B.; Best, W.; Byrne, N. Applications of regenerated bacterial cellulose: A review. Cellulose 2024, 31, 10165–10190. [Google Scholar] [CrossRef]
- Ni, J.; Wang, J.; Zhao, S.-J.; Zhong, F.; Qu, T.; Hu, F.-Q.; Liu, H.; Gong, C.-L.; Wen, S. LDH nanosheets anchored on bacterial cellulose-based composite anion exchange membranes for significantly enhanced strength and ionic conductivity. Appl. Clay Sci. 2022, 217, 106391. [Google Scholar] [CrossRef]
- Abd El-Monaem, E.M.; Hosny, M.; Eltaweil, A.S. Synergistic effect between adsorption and Fenton-like degradation on CoNiFe-LDH/ZIF-8 composite for efficient degradation of doxycycline. Chem. Eng. Sci. 2024, 287, 119707. [Google Scholar] [CrossRef]
- Xie, M.; Liang, M.; Liu, C.; Xu, Z.; Yu, Y.; Xu, J.; You, S.; Wang, D.; Rad, S. Peroxymonosulfate activation by CuMn-LDH for the degradation of bisphenol A: Effect, mechanism, and pathway. Ecotox. Environ. Saf. 2024, 270, 115929. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhou, J.; Rafiq, M.I.; Dai, S.; Tang, J.; Tang, W.-H. Growth of Ni-Mn layered double hydroxide and polypyrene on bacterial cellulose nanofibers for efficient supercapacitors. Electrochim. Acta 2019, 295, 82–91. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.-R.; Zhou, R.-S.; Lin, S.; Zhou, L. High-efficiency carbamazepine degradation using a Ni/Co-LDH as the peroxymonosulfate activator: Performance, mechanism and degradation pathway. Appl. Surf. Sci. 2022, 574, 151580. [Google Scholar] [CrossRef]
- Wang, L.-L.; Wang, D.; Zheng, L.-L.; Song, X.-R.; Yan, Y.; Li, J.-H.; Tian, S.-H.; Wang, M.-L.; Peng, M.; Yin, Z.-H.; et al. Construction of 3D hollow NiCo-layered double hydroxide nanostructures for high-performance industrial overall seawater electrolysis. Nano Res. 2024, 17, 9472–9482. [Google Scholar] [CrossRef]
- Chen, X.-B.; Yang, X.-F.; Guo, P.-Y.; Chen, Y.-W.; He, Z.; Li, Y.; Xu, W.-Y.; Hou, Z.-H.; Zhou, M.-J.; He, B.-H. Boosting oxygen evolution reaction via oxygen vacancies and phase engineering on CoFe-layered double hydroxide nanoflowers. J. Mater. Res. 2025, 40, 1098–1107. [Google Scholar] [CrossRef]
- Qi, L.-Y.; Ma, G.-F.; Kou, R.-H.; Wang, Z.-Y.; Zhang, H.-L.; Yang, Y.-H. Novel Fenton-like degradation via generation of hydrogen peroxide using CoAl-LDH/porous g-C3N4 catalyst. J. Water Process Eng. 2025, 70, 107018. [Google Scholar] [CrossRef]
- Mosaffa, E.; Oroujzadeh, M.; Ramsheh, N.A.; Jamshidi, E.; Patel, H.; Parekh, K.; Manteghi, F.; Banerjee, A. Bioinspired chitosan/PVA beads cross-linked with LTH-doped bacterial cellulose hydrochar for high-efficiency removal of antibiotics. Int. J. Biol. Macromol. 2025, 306, 141522. [Google Scholar] [CrossRef]
- Gu, J.; Yi, W.-B.; Liu, X.-R.; Ru, Y.; Tan, L.-P.; Liu, T.-J. Synthesis of highly porous ferric hydroxide-bacterial cellulose nanocomposites via in-situ mineralization for efficient glyphosate removal. Cellulose 2024, 31, 8041–8053. [Google Scholar] [CrossRef]
- Aita, S.A.; Mahmoud, R.; Hafez, S.H.M.; Zaher, A. Investigating adsorption of aqueous heavy metals through isotherms and kinetics with Zn-Co-Fe/LDH for remarkable removal efficiency. Appl. Water Sci. 2025, 15, 75. [Google Scholar] [CrossRef]
- Aghaziarati, M.; Sereshti, H. A facile in-situ ultrasonic-assisted synthesis of terephthalic acid-layered double hydroxide/bacterial cellulose: Application for eco-friendly analysis of multi-residue pesticides in vineyard soils. Microchem. J. 2024, 202, 110761. [Google Scholar] [CrossRef]
- Ma, R.; Chen, Z.-J.; Xu, W.-H.; Yu, R.; Zhang, Y.-C.; Chen, F.; Peng, X.-M.; Ni, B.-J.; Qian, J. Fe-MOFs/CuS nanocomposite-mediated peroxymonosulfate activation for tetracycline degradation: Boosted dual redox cycles. J. Clean. Prod. 2024, 442, 140885. [Google Scholar] [CrossRef]
- Moradi, M.; Afkhami, A.; Madrakian, T.; Moazami, H.R.; Tirandaz, A. Partial hydrothermal sulfidation of electrosynthesized Co-Mn layered-double-hydroxide as an active material for supercapacitor applications. J. Power Sources 2025, 629, 235993. [Google Scholar] [CrossRef]
- Yang, M.-X.; Li, Y.-J.; Jin, Z.-L. In Situ XPS proved graphdiyne (CnH2n−2)-based CoFe LDH/CuI/GD double S-scheme heterojunction photocatalyst for hydrogen evolution. Sep. Purif. Technol. 2023, 311, 123229. [Google Scholar] [CrossRef]
- Yang, W.-S.; Luo, M.-S.; Yang, Z.; Rahman, R.; Li, Z.-Y.; Cui, X.-T.; Xia, L.-M. Synthesis gas to alcohols over noble metal promoted FeCu-LDH nano catalyst. Appl. Catal. A-Gen. 2025, 699, 120280. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Ye, S.; Cheng, L.; Sun, L.-X.; Yan, P.-X. In-situ forming of bacterial cellulose nanofiber aerogel loaded with hierarchical structured Ru-Co(OH)2 nanosheets in hydrogen generation via NaBH4 hydrolysis. Fuel 2025, 388, 134493. [Google Scholar] [CrossRef]
- Xu, Y.-Q.; Chen, R.-R.; Li, C.-J.; Wang, H.; Ma, S.-Z.; Wang, L.-J. Engineered active Co sites in ternary NiFeCo-layered double hydroxide nanoarrays for highly-efficient photocathodic protection of 304 stainless steel in the dark. Surf. Interfaces 2025, 58, 105894. [Google Scholar] [CrossRef]
- Xia, K.-D.; Cheng, Y.-F.; Zhang, H.; Han, F.; Duan, L.-Y.; Liu, X. Highly microporous nitrogen-doped carbon derived from silicon oxycarbide ceramics for supercapacitor application. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2023–2024. [Google Scholar] [CrossRef]
- Faheem, M.; Saddique, Z.; Akhter, T.; Wang, K.; Qian, J.; Gu, W.-Y.; Afzal, A.; Long, L.-L. High-performance disposable electrochemical sensors for creatinine derived from hollow CoNi-LDH@Creatinine-imprinted Poly(methacrylic acid) (i-PMA) composites. Anal. Chim. Acta 2025, 1346, 343768. [Google Scholar] [CrossRef]
- Mi, X.-H.; Ma, R.; Pu, X.-C.; Fu, X.-Y.; Geng, M.-Q.; Qian, J. FeNi-layered double hydroxide (LDH)@biochar composite for, activation of peroxymonosulfate (PMS) towards enhanced degradation of doxycycline (DOX): Characterizations of the catalysts, catalytic performances, degradation pathways and mechanisms. J. Clean. Prod. 2022, 378, 134514. [Google Scholar] [CrossRef]
- Wang, X.-Z.; Zhu, S.-B.; Dai, A.-T.; Lin, Z.-Y.; Xie, Y.; Zhou, Q.; Qiu, Y.-Q.; Zhang, C.; Yi, X.-H.; Huang, M.-Z. Enhancement of the radical pathway-dominated degradation of imidacloprid in PMS-AOP by 1T-MoS2-based dual-atom catalyst: Synergistic catalysis by Fe and Ni atoms. Chem. Eng. J. 2025, 512, 162063. [Google Scholar] [CrossRef]
- Li, Y.-L.; Ren, J.-Y.; Ding, J.; Wang, Y.-X.; Yan, H.; Liu, F.-Y.; Wei, J.; Zhai, X.-D.; Al-Anazi, A.; Falaras, P. Revealing the enhanced role of hydroxylamine and bimetals in the CuFe2O4/PMS/HA system towards effective degradation of organic contaminants. J. Hazard. Mater. 2025, 488, 137312. [Google Scholar] [CrossRef]
- Lu, H.; Feng, W.-H.; Li, Q.-P. Degradation efficiency analysis of sulfadiazine in water by ozone/persulfate advanced oxidation process. Water 2022, 14, 162476. [Google Scholar] [CrossRef]
- Yang, J.-C.E.; Zhu, M.-P.; Dionysiou, D.D.; Yuan, B.-L.; Fu, M.-L. Interplay of bicarbonate and the oxygen-containing groups of carbon nanotubes dominated the metal-free activation of peroxymonosulfate. Chem. Eng. J. 2022, 430, 133102. [Google Scholar] [CrossRef]
- Palas, B. Catalytic performance of FeCo2O4 spinel cobaltite for degradation of ethylparaben in a peroxymonosulfate activation process: Response surface optimization, reaction kinetics and cost estimation. J. Mol. Struct. 2025, 1322, 140340. [Google Scholar] [CrossRef]
- Ashraf, G.A.; Al-Sulaimi, S.; Hassan, N.; Ajmal, Z.; Mahmood, S.; Rasool, R.T.; Anwar, M.T.; Husnain, N.; Alotaibi, N.H.; Alsoqair, H.-Y. Mesoporous Carbon@ZnCuFeS nanoparticles for photocatalytic degradation of organic pollutants via peroxymonosulfate activation. J. Organomet. Chem. 2025, 1032, 123624. [Google Scholar]
- Wu, Z.-J.; Zou, J.; Li, S.; He, L.-F.; Li, Q.-S.; Zhou, Z.-M.; Huang, Y.; Yang, Z.-M.; Ma, J. Strong enhancement on tetrabromobisphenol A removal in natural water matrix by adding cysteine into Cu(II)/peroxymonosulfate system over the wide pH range of 4–12: Efficiency and mechanism. Chem. Eng. J. 2024, 501, 157548. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.-W.; Zhang, Z.-L.; Xu, Y.-S.; Yang, N.; Wu, Z.-Z.; Lin, J.-H.; Shi, J.; Deng, H.-P. Cu-O-Fe boosts electronic transport for efficient peroxymonosulfate activation over a wide range of pH. J. Hazard. Mater. 2025, 492, 138118. [Google Scholar] [CrossRef]
- Sun, J.; Yan, B.-Z.; Li, Y.; Sun, H.-X.; Wang, Y.-H.; Chen, J.-Q. Characterization and cause analysis of shallow groundwater hydrochemistry in the plains of Henan Province, China. Sustainability 2025, 13, 12586. [Google Scholar] [CrossRef]
- Wang, J.-S.; Yi, X.-H.; Xu, X.-T.; Ji, H.-D.; Alanazi, A.M.; Wang, C.-C.; Zhao, C.; Kaneti, Y.V.; Wang, P.; Liu, W.; et al. Eliminating tetracycline antibiotics matrix via photoactivated sulfate radical-based advanced oxidation process over the immobilized MIL-88A: Batch and continuous experiments. Chem. Eng. J. 2022, 431, 133213. [Google Scholar] [CrossRef]
- Chuang, Y.-H.; Tzou, Y.-M.; Wang, M.-K.; Liu, C.-H.; Chiang, P.-N. Removal of 2-chlorophenol from aqueous solution by Mg/Al layered double hydroxide (LDH) and modified LDH. Ind. Eng. Chem. Res. 2008, 47, 3813–3819. [Google Scholar] [CrossRef]
- Aurelio-Soria, D.; Rodriguez, J.A.; Paez-Hernandez, M.E.; Perez-Silva, I.; Flores-Aguilar, J.F.; Ibarra, I.S. Development of a dispersive solid phase microextraction method based on the application of MgAl, NiAl, and CoAl-layered double hydroxides for the efficient removal of α- and β-naphthol isomers from water samples by capillary electrophoresis. J. Chromatogr. A 2024, 1731, 465174. [Google Scholar] [CrossRef]
- Jiang, H.-T.; Ma, J.-M.; Zhang, M.; Dai, Y.-J.; Wang, Y.-X.; Huang, H.; Yuan, S.-C.; Lia, K.-P.; Zhou, T.; Lv, R.-B.; et al. Calcium carbonate self fixed crayfish shell composite biochar for removing tetracycline from water. Colloid. Surf. A 2025, 711, 136371. [Google Scholar] [CrossRef]
- Thamilselvan, A.; Dang, V.D.; Doong, R.A. Ni-Co bimetallic decorated dodecahedral ZIF as an efficient catalyst for photoelectrochemical degradation of sulfamethoxazole coupled with hydrogen production. Sci. Total Environ. 2023, 873, 162208. [Google Scholar] [CrossRef]
- Trujillo-Reyes, Á.; Purswani, J.; Hueso, R.; Calvo, C.; Serrano, A.; Aranda, E. Chemical and bio-hazard assessment of swine manure valorisation: Antibiotic and antibiotic-resistant bacteria screening. Environ. Res. 2025, 13, 122614. [Google Scholar] [CrossRef]
- Kayani, K.-F.; Mohammed, S.-J.; Mustafa, M.-S.; Aziz, S.-B. Dyes and their toxicity: Removal from wastewater using carbon dots/metal oxides as hybrid materials: A review. Mater. Adv. 2025, 6, 5391–5409. [Google Scholar] [CrossRef]
- Chen, H.-B.; Yu, Z.-Y.; Sun, W.-K.; Li, T.; Zhang, J.; Qiu, Z.-M.; Younas, M. Degradation of Tetracycline through peroxymonosulfate activation with Co/Fe-LDH modified magnetic hydrochar: Synergistic effect and low toxicity. Sep. Purif. Technol. 2024, 351, 128023. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, A.; Wang, S.-M.; Long, Y.; Fan, G.-Y.; Yu, X.-J. Efficient tetracycline degradation via peroxymonosulfate activation by magnetic Co/N co-doped biochar: Emphasizing the important role of biochar graphitization. Chem. Eng. J. 2022, 450, 138428. [Google Scholar] [CrossRef]
- Xu, M.-Y.; Deng, Y.-C.; Li, S.-H.; Zheng, J.-Y.; Liu, J.-Y.; Tremblay, P.L.; Zhang, T. Bacterial cellulose fakes loaded with Bi2MoO6 nanoparticles and quantum dots for the photodegradation of antibiotic and dye pollutants. Chemosphere 2023, 312, 137249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xie, J.-F.; Yang, J.-C.E.; Li, D.; Zhong, L.-B.; Zheng, Y.-M. Ultra-fast degradation of ciprofloxacin by the peroxymonosulfate activation using a Co/Al-LDH decorated magnetic hydrochar: Structural design, catalytic performance and synergistic effects. Chem. Eng. J. 2023, 477, 146961. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Do, Q.-H.; Chen, C.-W.; Chen, W.-H.; Bui, X.-T.; Dong, C.-D. Decoration of marigold flower-like CoMo LDH on reinforced cow manure-derived biochar as an effective peroxymonosulfate activator for degradation of Bisphenol A in water. J. Environ. Chem. Eng. 2024, 12, 114699. [Google Scholar] [CrossRef]
- Ni, Q.-Q.; Ke, X.; Qian, W.; Yan, Z.; Luan, J.; Liu, W. Insight into tetracycline photocatalytic degradation mechanism in a wide pH range on BiOI/BiOBr: Coupling DFT/QSAR simulations with experiments. Appl. Catal. B Environ. 2024, 340, 123226. [Google Scholar] [CrossRef]


| BET Surface Area (m2 g−1) | Pore Size (nm) | Vc t (cm3 g−1) | |
|---|---|---|---|
| 0.06-CoAl-LDH | 36.5975 | 9.3998 | 0.0954 |
| BC | 14.6398 | 12.4906 | 0.0221 |
| 0.06-CoAl-LDH@BC | 48.1334 | 13.2620 | 0.0816 |
| Oxidizing Reagent | Pollutant | Experimental Condition | Degradation Rate (%) | Reference |
|---|---|---|---|---|
| N-BC@Co3O4 | TC | [TC] = 10 mg L−1; [TC vol.] = 50 mL; catalyst = 10 mg; [PMS] = 10 mmol L−1; nature pH; time = 60 min | 98.2 | [15] |
| CoAl-LDH/porous g-C3N4 | RhB | [RhB] = 5 mg L−1; [RhB vol.] = 50 mL; catalyst = 400 mg L−1; [H2O2] = 1.5 mL; nature pH; time = 75 min | 94.5 | [30] |
| BiOI/BiOBr | TC | [TC] = 10 mg L−1; [TC vol.] = 160 mL; catalyst = 0.06 g; Light= Xe lamp 300 W; pH = 8.5; time = 90 min | Completely remove | [65] |
| CoAl-LDH@BC | TC | [TC] = 20 mg L−1; [TC vol.] = 100 mL; catalyst = 30 mg; [PMS] = 20 mg; nature pH; time = 15 min | 99.9 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, X.; Du, Y.; Liu, Z.; Cheng, J.; Yang, J.; Li, Y. In Situ Synthesis of Bacterial Cellulose-Supported CoAl-Layered Double Hydroxide as a Peroxymonosulfate Activator for Enhancing the Removal of Tetracycline. Biomolecules 2025, 15, 1283. https://doi.org/10.3390/biom15091283
Bai X, Du Y, Liu Z, Cheng J, Yang J, Li Y. In Situ Synthesis of Bacterial Cellulose-Supported CoAl-Layered Double Hydroxide as a Peroxymonosulfate Activator for Enhancing the Removal of Tetracycline. Biomolecules. 2025; 15(9):1283. https://doi.org/10.3390/biom15091283
Chicago/Turabian StyleBai, Xiuzhi, Yongsheng Du, Zhongxiang Liu, Jing Cheng, Jie Yang, and Ying Li. 2025. "In Situ Synthesis of Bacterial Cellulose-Supported CoAl-Layered Double Hydroxide as a Peroxymonosulfate Activator for Enhancing the Removal of Tetracycline" Biomolecules 15, no. 9: 1283. https://doi.org/10.3390/biom15091283
APA StyleBai, X., Du, Y., Liu, Z., Cheng, J., Yang, J., & Li, Y. (2025). In Situ Synthesis of Bacterial Cellulose-Supported CoAl-Layered Double Hydroxide as a Peroxymonosulfate Activator for Enhancing the Removal of Tetracycline. Biomolecules, 15(9), 1283. https://doi.org/10.3390/biom15091283





