Abstract
Background: Aneurysmal subarachnoid hemorrhage (aSAH) is notoriously known for its high mortality and morbidity. Approximately one-third of the patients who survive aneurysm rupture are reported to develop delayed cerebral ischemia (DCI), which contributes to a poor clinical outcome. Currently, there are no biomarkers for identifying which aSAH patients are at risk of developing DCI. We aimed to determine the feasibility of cerebrospinal fluid (CSF) exosomal microRNAs (miRNAs) for predicting DCI post-aSAH. Methods: aSAH patients were prospectively enrolled, and CSF samples were collected at two time points (<24 h and 72 h post-aSAH) from individuals undergoing external ventricular drainage. Exosomal miRNAs were isolated from the CSF for analysis. In the initial group of patients (discovery cohort), an exploratory analysis was conducted using a CSF panel containing 84 miRNAs, assessed by quantitative real-time PCR (RT-qPCR). Based on this analysis, 27 miRNAs were selected for further evaluation in a second group of patients (validation cohort). Among these, 10 miRNAs had previously been reported in SAH-related CSF studies, supporting their relevance for continued investigation. Results: In this study, RT-qPCR analysis of 84 miRNAs in CSF samples from aSAH patients (n = 10 DCI, n = 16 no DCI) and non-aSAH controls (n = 5) identified 9 upregulated and 13 downregulated miRNAs in the DCI group, and 7 upregulated and 18 downregulated miRNAs in the no-DCI group, compared to the controls. When comparing DCI to no-DCI patients, 13 miRNAs were found to be upregulated in the DCI group. Additionally, seven miRNAs showed temporal upregulation in DCI patients between early (<24 h/T1) and later (72 h/T3) time points across both discovery and validation cohorts. However, no miRNAs were uniquely expressed in either DCI or no-DCI groups, limiting their potential as specific biomarkers for DCI. Conclusions: Despite analyses in both the discovery and validation phases, no miRNAs emerged as consistent and reliable biomarkers for distinguishing DCI from no-DCI patients. However, the identified miRNAs are involved in the key KEGG pathways that regulate vascular integrity, neuronal survival, and inflammatory processes central to DCI pathophysiology. These findings highlight the complexity of miRNA regulation following aSAH, as reflected by the variability in differentially expressed miRNAs between cohorts. This variability may be influenced by factors such as limited sample size, patient heterogeneity, individual biological differences, and experimental variability. Comprehensive profiling using larger, well-characterized cohorts, along with rigorous validation, is essential to determine the predictive value and mechanistic significance of candidate miRNAs in DCI.
1. Introduction
Aneurysmal subarachnoid hemorrhage (aSAH) is a global health burden that accounts for 5% of all strokes and is notoriously known for its high fatality and permanent disability rates [,]. While aSAH leads to pathophysiological events which contribute to early brain injury [], it is delayed cerebral ischemia (DCI) that leads to significant morbidity and mortality []. DCI typically occurs between 3 and 14 days after the initial hemorrhage and is characterized by new focal neurological deficits or a decline in consciousness [,]. Its multifactorial pathophysiology includes cerebral vasospasm, microthrombosis, cortical spreading depolarizations, and impaired autoregulation, all contributing to secondary ischemic injury [,]. Approximately one third of aSAH patients who survive aneurysm rupture develop DCI []; thus, preventing or treating DCI after aSAH is of paramount significance as DCI leads to progressive brain damage, cognitive decline, and poor outcomes.
Currently, there is no reliable biomarker to accurately identify patients at risk of developing DCI following aSAH. Several studies have investigated serum biomarkers for inflammation [,] and platelet factors [,]. However, one limitation of serum biomarkers is that their levels can be affected by infection [,] and comorbidities []. Cerebrospinal fluid (CSF), which is in close contact with the extracellular space of the brain, has an intricate relationship with the brain, blood vessels, and blood products of aneurysmal rupture, meaning that it may be a better fluid to detect DCI biomarkers.
MicroRNAs (miRNAs) are small single-stranded molecules comprising 17–25 nucleotides, secreted by cells into the extravascular space and then circulated in the blood or CSF [,,,]. Physiologically, miRNAs promote degradation or interfere with the translation of mRNA and therefore regulate a broad range of cellular processes from normal development, homeostasis, and regeneration to apoptosis [,]. As of now, in humans, over 2000 miRNAs have been discovered and identified as specific for certain organs, tissue, or cells [,,,,,]. Brain-specific miRNAs that are enriched in neurites and synapses have also been identified [,,,,,,].
Since exosomal miRNAs are released by cells and participate in signaling, rather than disposed of during cell death [], exosomal miRNAs might be involved in the mechanisms responding to injuries. Our study aims to investigate exosomal miRNAs from CSF at two time points post-aSAH to evaluate their potential as predictive markers for the development of DCI. Therefore, we hypothesized that exosomal miRNAs in CSF could serve as potential biomarkers for identifying aSAH patients at risk of developing DCI.
2. Methods
2.1. Patient Enrollment
Under Institutional Review Board (IRB) of UTHealth approval (IRB Number: HSC-MS-22-0425, approved on 18 February 2023), patients admitted with aneurysmal subarachnoid hemorrhage (aSAH) were enrolled for the prospective collection of CSF and clinical data. Written informed consent was obtained from all participants or their legally authorized representatives prior to enrollment. The inclusion criteria were age ≥18 years and radiologically confirmed aneurysmal SAH. Patients with traumatic or non-aneurysmal SAH, malignancy, or systemic inflammatory disease were excluded. Per protocol, data were de-identified and stored in a prospective database. Age, sex, comorbidities, Glasgow Coma Scale (GCS), and Hunt–Hess score were collected and compared among the groups using Wilcoxon’s test or Fisher’s exact test for continuous and categorical variables, respectively. The aneurysmal source of bleeding was confirmed using computed tomography angiography (CTA). CSF samples were also collected from control patients undergoing lumbar puncture for neurological symptoms who had no history or radiographic evidence of any form of stroke. Controls were included if they were ≥18 years old and had no intracranial hemorrhage or CNS infection and were excluded if they had any prior cerebrovascular disease, CNS malignancy, or infection. A diagnosis of delayed cerebral ischemia (DCI), adjudicated prospectively by the NeuroICU faculty at a scheduled weekly meeting, was defined as a drop of 2 or more in GCS.
2.2. Sample Details
External ventricular drains (EVDs) were placed as standard care for SAH patients and used for serial CSF collection. CSF samples were collected at <24 (T1) and 72 h (T2) post-SAH, de-identified, and stored at −80 °C until analysis. All CSF samples underwent standard quality assessment prior to downstream processing, including visual inspection to ensure clarity and absence of visible blood contamination. Specimen collection and analysis occurred in two phases: discovery (26 aSAH patients, 5 controls) and validation (26 aSAH patients).
In the discovery cohort, CSF samples were obtained from 6 controls and 26 aSAH patients (10 DCI, 16 no DCI). Due to limited CSF availability in 8 no-DCI patients, samples from 2 patients were pooled, resulting in a total of 12 samples for the statistical analysis, comprising 8 individual and 4 pooled samples. Similarly, to address low CSF volume in some control samples, 1 pool consisting of 2 control samples was created, resulting in 4 individual controls and 1 pooled sample, for a total of 5 control samples. In the validation cohort, CSF was collected from 26 aSAH patients (12 no DCI, 14 DCI), all with sufficient volume for individual analysis.
The two-phase design allowed for an initial broad screening of miRNA expression changes in the discovery phase to generate hypotheses and identify candidate miRNAs potentially associated with aSAH and DCI. The validation phase then assessed the reproducibility and consistency of these candidates in an independent cohort. This approach balances sensitivity and specificity, helping to distinguish consistent miRNA alterations from random variation or cohort-specific effects, thereby laying the foundation for future biomarker studies.
2.3. Exosomal RNA Isolation and Quantification
The isolation and analysis of exosomes have been described previously []. Exosomes and RNA were isolated from 1 mL of CSF using the exoRNeasy Midi Kit (Qiagen, Hilden, Germany; Cat. No. 77144) and eluted to a final volume of 10 µL with nuclease-free water, following the manufacturer’s protocol. Reverse transcription of the isolated RNA was performed using the miRCURY LNA RT Kit (Qiagen; Cat. No. 339340). Real-time quantification assays were performed using the miRCURY LNA miRNA SYBR green PCR Assay Kit (Qiagen; Cat. No. 339346), which was optimized with a 1:10 dilution of cDNA.
In the discovery phase, RT-qPCR was performed using the LightCycler 480 instrument (Roche, Basel, Switzerland) on a 384-well plate with a Qiagen CSF miRNA panel, which includes pre-defined assays for 84 miRNAs expressed in exosomes from CSF (Table S1). After data analysis, 27 miRNAs with p < 0.1 in class comparisons (DCI vs. no DCI and T1 vs. T2) were selected for further validation in a new validation cohort (Table S2). Of these, 10 miRNAs had previously been reported in SAH-related CSF studies [,,]. In the validation phase, RT-qPCR was conducted using Quant Studio 3 (Applied Biosystems, Waltham, MA, USA) to assess the selected miRNAs. In the validation phase, RT-qPCR was carried out using the QuantStudio 3 system (Applied Biosystems) with ABI-compatible 96-well plates (EarthOx, Chiyoda, Tokyo, Cat. No. PCRPH96). For reverse transcription, we used the Qiagen miRCURY LNA RT Kit (Hilden, Germany; Cat. No. 339340), and for amplification, the miRCURY LNA SYBR Green PCR Kit (Hilden, Germany; Cat. No. 339346) was used, following the manufacturer’s instructions. Based on Qiagen’s recommendations, hsa-miR-191-5p and hsa-miR-103a-3p were used as the controls for data normalization.
2.4. Statistical Analysis
We conducted linear regression analysis using GraphPad Prism (v10.0.2) to examine the demographic characteristics of the samples. Prior to statistical analysis with BRB-Array Tools (v4.6.2) [], miRNA Ct values were transformed and normalized to prepare the dataset. For transformation, we subtracted each miRNA’s Ct value from a custom constant value of 50. This constant was selected because it exceeded the Ct values of the non-template controls (NTCs) in both cohorts, ensuring that all transformed values were positive. This transformation was applied to approximate a normal distribution, enhancing data stability and compatibility with BRB-Array Tools. Following transformation, the data were normalized to the average geometric mean of the control miRNAs to account for technical variation. We then used BRB-Array Tools (developed by Dr. Richard Simon and the BRB-Array Tools Development Team) to assess statistical differences in miRNA expression across the various group comparisons. A p-value threshold of <0.10 (raw p-values) was applied in both the discovery and validation phases to allow for the inclusion of miRNAs with potential biological relevance, minimizing the risk of false negatives in this CSF-based exploratory study, while validation in an independent cohort served to support the robustness of the findings. Additionally, pathway enrichment analysis for DCI-associated miRNAs was performed using the online tool miRPath v4.0.3.
3. Results
3.1. Baseline Characteristics of the Samples
The demographics were compared among the groups. Overall, there were no significant differences in age, gender, or comorbidities between the SAH and control groups. However, the GCS scores differed significantly between the DCI and no-DCI groups within the validation cohort (p = 0.038). Additionally, the GCS scores also showed a significant difference between the discovery and validation aSAH groups (p = 0.023). In the subgroup analysis, hyperlipidemia (HLD) differed significantly between the discovery and validation cohorts within the no-DCI group (p = 0.044). Apart from these findings, no other significant differences were observed (Table 1). Hierarchical clustering of miRNAs in the discovery and validation cohorts is visualized using a heatmap (Figure 1).

Table 1.
Demographic and clinical characteristics of study participants across discovery and validation cohorts. The table summarizes the demographic and clinical variables of the participants in the discovery and validation cohorts, including age, gender, comorbidities such as hypertension (HTN), hyperlipidemia (HLD), and diabetes mellitus (DM), and Glasgow Coma Scale (GCS) scores.
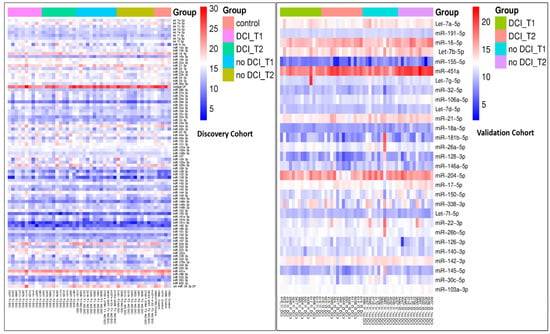
Figure 1.
Heatmap showing clustering of miRNAs in the discovery and validation cohorts. The expression values within each group were represented, where red indicates higher expression and blue indicates lower expression. Overlap with previously reported SAH-associated miRNAs.
Among the 27 miRNAs selected for validation (p < 0.1), several had been previously identified in CSF from SAH patients in independent studies, supporting the relevance of our candidate selection. Specifically, let-7a-5p, let-7d-5p, hsa-miR-155-5p, and hsa-miR-451a were reported by Pedrosa []; hsa-miR-22-3p, hsa-miR-140-3p, hsa-miR-191-5p, and let-7b-5p by Kikkawa []; and hsa-miR-142-3p, hsa-miR-146a-5p, hsa-miR-150-5p, hsa-miR-451a, and let-7b-5p by Wang []. Notably, let-7b-5p and hsa-miR-451a were consistently reported across multiple studies.
3.2. Differential miRNA Expression Pattern Between Control and aSAH
In the discovery phase of this study, we performed RT-qPCR using a Qiagen panel of 84 miRNAs on exosomes of CSF samples from 26 aSAH patients (n = 10 DCI, n = 16 no DCI) and 6 non-aSAH controls. Following statistical analysis, significant miRNAs with a p-value less than 0.10 (p < 0.10) were selected. We observed 9 upregulated and 13 downregulated miRNAs in the DCI group compared to the controls and 7 upregulated and 18 downregulated miRNAs in the no-DCI group compared to the controls.
When comparing significant miRNAs between the controls vs. DCI/no-DCI groups, four miRNAs were uniquely upregulated and two were downregulated (out of six in DCI group vs. control), whereas two miRNAs were upregulated and six were downregulated (out of eight in no-DCI group vs. control) (Figure 2).
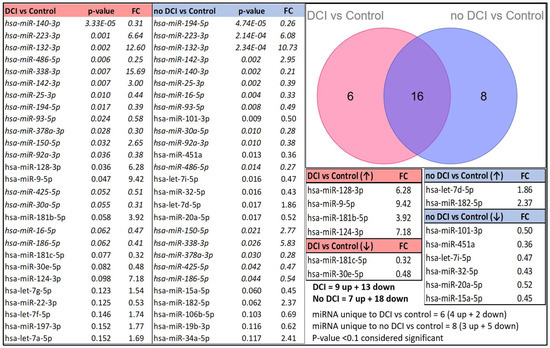
Figure 2.
Differential miRNA expression patterns between the control and aSAH groups (DCI and no-DCI groups) are presented, sorted by p-values (p < 0.10). miRNAs that appear in both tables are italicized. A Venn diagram illustrates the number of unique and overlapping miRNAs in the DCI and no-DCI groups compared to the controls.
3.3. Differential miRNA Expression Pattern for DCI vs. No-DCI Groups
We aimed to identify a CSF exosome miRNA profile specific to the DCI cohort that could serve as a diagnostic or prognostic marker for individuals at risk of DCI. To achieve this, we conducted a comparative analysis between the DCI and no-DCI groups in both the discovery and validation cohorts. However, despite analyzing both cohorts, we were unable to confirm any miRNAs as reliable predictors of DCI, even though hsa-miR-128-3p and hsa-miR-106a-5p were present in both cohorts but exhibited opposite patterns of regulation in the discovery and validation phases (Table 2).

Table 2.
List of differential miRNA expression between DCI vs. no-DCI groups sorted based on p-values less than 0.10. miRNAs that appear in both tables are italicized. FC: fold change from the no-DCI value.
3.4. Temporal Changes in CSF Exosome miRNA Expression in Cohorts with and Without DCI
Our next goal was to examine temporal changes in miRNA expression in the DCI of both the discovery and validation cohorts between 24 h (T1) and 72 h (T2). Our analysis revealed that 13 miRNAs were upregulated between T1 and T2 in the discovery cohorts and 7 in the validation cohorts. Four miRNAs, let-7a-5p, hsa-miR-126-3p, hsa-miR-146a-5p, and hsa-miR-26a-5p, were common in both.
Next, we compared miRNA expression at T1 and T2 in the no-DCI groups and identified three upregulated and one downregulated miRNAs in the discovery cohort. Upon validation, two miRNAs, hsa-miR-16-5p and hsa-miR-146a-5p, exhibited the same trend. Specifically, miRNA hsa-miR-16-5p was downregulated and miRNA hsa-miR-146a-5p was upregulated in both the discovery and validation cohorts (Figure 3).
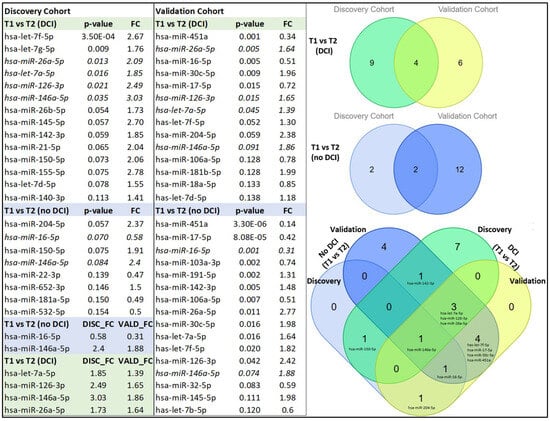
Figure 3.
Temporal expression changes in exosomal miRNA in DCI and non-DCI cohorts (p < 0.10). miRNAs present in both tables are italicized. A Venn diagram depicts the number of overlapping miRNAs between T1 (<24 h) and T2 (72 h) within the DCI and non-DCI groups, with shades of blue and green indicating different categories. Additionally, a complex Venn diagram illustrates the overlap between the DCI and non-DCI groups, with miRNAs distinctly marked for clarity. FC: fold change from the (72 h) value.
The temporal miRNAs identified in the DCI group—let-7a-5p, hsa-miR-126-3p, hsa-miR-146a-5p, and hsa-miR-26a-5p—were also present in the non-DCI group. Likewise, temporal miRNAs specific to the non-DCI group, such as miR-16-5p and 146a-5p, exhibited similar regulatory patterns in DCI cases. As a result, their reliability as distinct biomarkers for DCI or non-DCI could not be validated. Although these miRNAs lack specificity as exclusive DCI biomarkers, their involvement in key signaling pathways (Figure 4) suggests potential roles in vascular integrity, neuroinflammation, and blood–brain barrier stability. To explore their functional relevance, we performed KEGG and Gene Ontology Biological Process (GOBP) enrichment analyses. While GOBP enrichment offered limited insight and is included as a Supplementary Figure (Figure S1), KEGG pathway analysis provided more informative direction, supporting the relevance of these miRNAs in broader mechanisms of neurovascular dysfunction and repair in DCI.
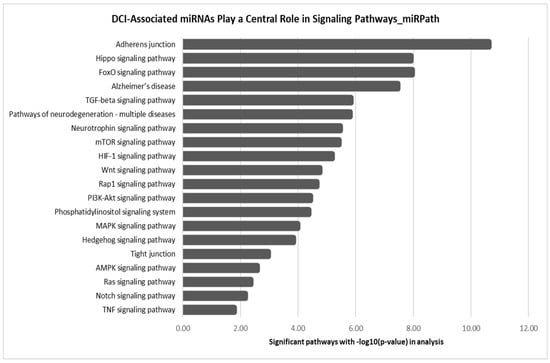
Figure 4.
KEGG pathway enrichment analysis of DCI-associated miRNAs (hsa-let-7a-5p, hsa-miR-126-3p, hsa-miR-146a-5p, and hsa-miR-26a-5p), showing their involvement in key signaling pathways related to vascular integrity, neuroinflammation, and blood–brain barrier stability.
4. Discussion
Circulating miRNAs, known for their disease-specific alterations, have been investigated as diagnostic biomarkers for SAH and intracranial aneurysms, with their expression levels correlating with disease severity []. Among them, exosomal miRNAs have drawn particular interest for their potential to diagnose, predict, and monitor SAH prognosis []. However, their role in tracking DCI progression remains under-validated. In our study, we analyzed differentially regulated miRNAs in CSF samples from aSAH patients to identify molecular distinctions between the DCI and no-DCI groups. The observed differences in miRNA expression among the DCI, no-DCI, and non-aSAH control groups suggest that exosomal miRNA profiles are likely modulated by the aSAH condition, rather than implying a direct mechanistic role in DCI pathogenesis. miRNAs are associated with broader aSAH-related pathophysiological processes, such as inflammation, oxidative stress, and blood–brain barrier disruption [,,], which are expected to be common in the DCI and no-DCI patient groups. There are some shared miRNAs between the DCI and no-DCI groups that underscore the need for further validation studies to refine the specificity of these candidates.
Our study aimed to identify a specific miRNA profile in CSF exosomes that could differentiate DCI from no-DCI cohorts, with the potential to serve as diagnostic or prognostic markers for managing individuals at risk of DCI. The contrasting expression patterns of hsa-miR-128-3p and hsa-miR-106a-5p with apparent upregulation in DCI patients in the discovery cohort but downregulation in the validation cohort warrant further investigation to clarify their roles in aSAH. For instance, hsa-miR-128-3p has been implicated in neuronal function and inflammatory responses [,], while hsa-miR-106a-5p is known to be involved in oxidative stress modulation and brain injury repair after ICH, particularly by influencing the activation of the Nrf2/ARE pathway []. The opposing expression patterns observed (Table 1) suggest that these miRNAs may have context-dependent roles, which may not be directly linked to DCI pathogenesis. Alternatively, their differential expression could reflect physiological variations rather than a specific mechanistic role in DCI. Further studies are needed to determine whether these miRNAs contribute to DCI development or are merely indicative of broader neurovascular changes.
The consistent upregulation of let-7a-5p, hsa-miR-126-3p, hsa-miR-146a-5p, and hsa-miR-26a-5p at critical time points (<24 h and 72 h) in both discovery and validation cohorts of DCI, as well as in the validation cohort of no-DCI patients (Figure 3), suggests their potential involvement in the molecular response to aSAH.
Among those potentially contributing to pathological processes, let-7a-5p has been implicated in inflammatory and immune responses and previously detected in plasma following aSAH []. Similarly, hsa-miR-146a-5p, a known regulator of inflammatory signaling cascades [,], may contribute to sustained neuroinflammation, which is a hallmark of DCI. Its elevated expression in DCI cohorts could reflect the amplification of inflammatory injury. hsa-miR-26a-5p, associated with cellular stress, apoptosis, and inflammation [,], may also reflect broader cell injury responses and ongoing pathological stress in the brain after SAH.
In contrast, hsa-miR-126-3p, which is strongly associated with endothelial function and vascular integrity [], may represent a protective or compensatory response to vascular injury. Its upregulation may indicate attempts to preserve cerebrovascular stability in the face of early ischemic or inflammatory insults []. The presence of upregulated hsa-miR-126-3p and other miRNAs in the no-DCI group further supports the notion of shared, early adaptive mechanisms following aSAH, irrespective of subsequent DCI development.
The analysis of temporal changes in miRNA expression in the no-DCI group exhibited consistent expression patterns with hsa-miR-16-5p and hsa-miR-146a-5p, with the former being downregulated and the later one upregulated over time, possibly suggestive of their involvement in post-aSAH responses not leading to DCI. The downregulation of hsa-miR-16-5p, a known regulator of cellular stress, apoptosis, and inflammation [], may indicate a protective or adaptive response aimed at mitigating pro-inflammatory signaling and fostering a more stable post-aSAH environment. Conversely, like that observed temporally in DCI, the upregulation of hsa-miR-146a-5p suggests that inflammatory processes remain active in SAH patients, contributing to cerebrovascular stability. However, the observation that hsa-miR-16-5p and hsa-miR-146a-5p exhibit similar temporal regulation in the DCI vs. no-DCI groups limits their specificity as biomarkers to identify aSAH patients at risk of DCI.
Despite the lack of differential expression, DCI miRNAs are involved in key KEGG pathways associated with cerebrovascular and neuroinflammatory processes. Pathways such as TGF-β, FoxO, mTOR, HIF-1, Wnt, and PI3K-Akt signaling are critical for maintaining vascular integrity, neuronal survival, and inflammation resolution, all central to DCI pathophysiology. Their involvement in pathways linked to adherens and tight junctions, Hippo, and MAPK signaling further suggests a role in blood–brain barrier stability and endothelial function, both of which are vital in post-SAH complications. Although their specificity as DCI biomarkers remains inconclusive, their regulation in neurodegeneration-related pathways, such as those linked to Alzheimer’s disease, highlights their broader relevance to neurovascular dysfunction. Rather than serving as exclusive biomarkers, these miRNAs may reflect overarching mechanisms of vascular stress, neuroinflammation, and repair responses, contributing to a deeper understanding of DCI pathogenesis. To refine their utility as biomarkers and therapeutic targets, future studies should investigate the mechanistic roles of these miRNAs in endothelial dysfunction, inflammation, and neuronal stress, exploring their differential regulation in DCI versus no-DCI groups.
5. Limitations
Our study highlights the inherent challenges in discovering robust biomarkers for predicting DCI, reflecting the complex and multifactorial nature of the condition. The variability in the number of differentially expressed miRNAs between discovery and validation cohort points to the complexity of miRNA regulation and the influence of various factors, including sample size, patient heterogeneity, individual patient variability, and the dynamic nature of miRNA regulation post-aSAH. Future studies with larger and more diverse patient populations, as well as more frequent sampling across multiple time points of blood and CSF, will be necessary to confirm these findings and fully elucidate the temporal dynamics of miRNA expression in DCI.
6. Conclusions
Our investigation identified a consistent upregulation of hsa-let-7a-5p, hsa-miR-126-3p, hsa-miR-146a-5p, and hsa-miR-26a-5p at critical time points (<24 h and 72 h) in DCI patients across both the discovery and validation cohorts, and notably also in the no-DCI validation group. These patterns suggest a shared early molecular response following aSAH. While no single miRNA reliably distinguished DCI from no-DCI patients, the repeated elevation of select miRNAs highlights their potential relevance in the post-SAH inflammatory or injury cascade. These findings support the idea that a combinatorial or pathway-based approach, possibly integrating multiple miRNA signatures and clinical variables, may be more effective in predicting DCI. Our results underscore the need for continued, large-scale profiling and validation to refine the predictive utility of miRNA biomarkers in aSAH.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom15081161/s1, Figure S1. Top-10 enriched Gene Ontology Biological Process (GOBP) terms for predicted targets of DCI-associated miRNAs. Table S1. List of 84 miRNAs included in the Qiagen. Table S2. Exosomal miRNAs selected for validation.
Author Contributions
Conceptualization, D.W.M., S.L.B. and P.K.T.; Methodology, C.M.S., D.W.M., S.L.B. and P.K.T.; Validation, C.M.S. and D.W.M.; Formal analysis, C.M.S., D.W.M., Y.Y., H.A.Z., J.P.H., J.P.S., A.D., S.L.B. and P.K.T.; Investigation, C.M.S., D.W.M., S.L.B. and P.K.T.; Resources, H.A.C., S.L.B. and P.K.T.; Writing—original draft, C.M.S. and D.W.M.; Writing—review & editing, D.W.M., Y.Y., H.A.Z., J.P.H., H.A.C., J.P.S., A.D., S.L.B. and P.K.T.; Visualization, C.M.S.; Supervision, S.L.B. and P.K.T.; Project administration, S.L.B. and P.K.T.; Funding acquisition, S.L.B. and P.K.T. All authors have read and agreed to the published version of the manuscript.
Funding
NIH/NINDS-R01 NS121339-01, R21 RNS135176A, Brain Aneurysm foundation.
Institutional Review Board Statement
The study protocol was reviewed and approved by the Institutional Review Board and Safety Committees at UTHealth (Approval Number: HSC-MS-22-0425) approved on 18 February 2023.
Informed Consent Statement
Human Subjects: All participants provided written informed consent prior to their inclusion in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- D’Souza, S. Aneurysmal Subarachnoid Hemorrhage. J. Neurosurg. Anesthesiol. 2015, 27, 222–240. [Google Scholar] [CrossRef] [PubMed]
- Van Gijn, J.; Kerr, R.S.; Rinkel, G.J.E. Subarachnoid haemorrhage. Lancet 2007, 369, 306–318. [Google Scholar] [CrossRef]
- Sabri, M.; Lass, E.; Macdonald, R.L. Early brain injury: A common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Res. Treat. 2013, 2013, 394036. [Google Scholar] [CrossRef]
- Laskowitz, D.T.; Kolls, B.J. Neuroprotection in subarachnoid hemorrhage. Stroke 2010, 41 (Suppl. S10), S79–S84. [Google Scholar] [CrossRef]
- Østergaard, L.; Aamand, R.; Karabegovic, S.; Tietze, A.; Blicher, J.U.; Mikkelsen, I.K.; Iversen, N.K.; Secher, N.; Engedal, T.S.; Anzabi, M.; et al. The role of the microcirculation in delayed cerebral ischemia and chronic degenerative changes after subarachnoid hemorrhage. J. Cereb. Blood Flow Metab. 2013, 33, 1825. [Google Scholar] [CrossRef]
- Abdulazim, A.; Heilig, M.; Rinkel, G.; Etminan, N. Diagnosis of Delayed Cerebral Ischemia in Patients with Aneurysmal Subarachnoid Hemorrhage and Triggers for Intervention. Neurocritical Care 2023, 39, 311. [Google Scholar] [CrossRef]
- Etminan, N.; DI Vergouwen, M.; Ilodigwe, D.; Macdonald, R.L. Effect of pharmaceutical treatment on vasospasm, delayed cerebral ischemia, and clinical outcome in patients with aneurysmal subarachnoid hemorrhage: A systematic review and meta-analysis. J. Cereb. Blood Flow Metab. 2011, 31, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Geraghty, J.R.; Testai, F.D. Delayed Cerebral Ischemia after Subarachnoid Hemorrhage: Beyond Vasospasm and Towards a Multifactorial Pathophysiology. Curr. Atheroscler. Rep. 2017, 19, 50. [Google Scholar] [CrossRef]
- Megjhani, M.; Terilli, K.; Weiss, M.; Savarraj, J.; Chen, L.H.; Alkhachroum, A.; Roh, D.J.; Agarwal, S.; Connolly, E.S.; Velazquez, A.; et al. Dynamic Detection of Delayed Cerebral Ischemia: A Study in 3 Centers. Stroke 2021, 52, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.-H.; Burkett, A.; Paz, A.; Savarraj, J.P.; Hinds, S.; Hergenroeder, G.; Gusdon, A.M.; Ren, X.; Hong, J.-H.; Choi, H.A. Systemic inflammatory markers of persistent cerebral edema after aneurysmal subarachnoid hemorrhage. J. Neuroinflammation 2022, 19, 199. [Google Scholar] [CrossRef]
- Chou, S.H.-Y.; Feske, S.K.; Atherton, J.; Konigsberg, R.G.; De Jager, P.L.; Du, R.; Ogilvy, C.S.; Lo, E.H.; Ning, M. Early Elevation of Serum Tumor Necrosis Factor-α is Associated with Poor Outcome in Subarachnoid Hemorrhage. J. Investig. Med. 2012, 60, 1054–1058. [Google Scholar] [CrossRef]
- Hirashima, Y.; Nakamura, S.; Endo, S.; Kuwayama, N.; Naruse, Y.; Takaku, A. Elevation of platelet activating factor, inflammatory cytokines, and coagulation factors in the internal jugular vein of patients with subarachnoid hemorrhage. Neurochem. Res. 1997, 22, 1249–1255. [Google Scholar] [CrossRef]
- Perez, P.; Lukaszewicz, A.-C.; Lenck, S.; Nizard, R.; Drouet, L.; Payen, D. Platelet activation and aggregation after aneurysmal subarachnoid hemorrhage. BMC Neurol. 2018, 18, 57. [Google Scholar] [CrossRef]
- Bogossian, E.G.; Attanasio, L.; Creteur, J.; Grimaldi, D.; Schuind, S.; Taccone, F.S. The Impact of Extracerebral Infection After Subarachnoid Hemorrhage: A Single-Center Cohort Study. World Neurosurg. 2020, 144, e883–e897. [Google Scholar] [CrossRef]
- Sarrafzadeh, A.; Schlenk, F.; Meisel, A.; Dreier, J.; Vajkoczy, P.; Meisel, C. Immunodepression after aneurysmal subarachnoid hemorrhage. Stroke 2011, 42, 53–58. [Google Scholar] [CrossRef]
- Burzyńska, M.; Uryga, A.; Woźniak, J.; Załuski, R.; Robba, C.; Goździk, W. The Role of Early Serum Biomarkers and Clinical Rating Scales in the Prediction of Delayed Cerebral Ischaemia and Short-Term Outcome after Aneurysmal Subarachnoid Haemorrhage: Single Centre Experience. J. Clin. Med. 2023, 12, 5614. [Google Scholar] [CrossRef]
- Burgos, K.; Malenica, I.; Metpally, R.; Courtright, A.; Rakela, B.; Beach, T.; Shill, H.; Adler, C.; Sabbagh, M.; Villa, S.; et al. Profiles of extracellular miRNA in cerebrospinal fluid and serum from patients with Alzheimer’s and Parkinson’s diseases correlate with disease status and features of pathology. PLoS ONE 2014, 9, e94839. [Google Scholar] [CrossRef] [PubMed]
- Di Ieva, A.; Butz, H.; Niamah, M.; Rotondo, F.; De Rosa, S.; Sav, A.; Yousef, G.M.; Kovacs, K.; Cusimano, M.D. MicroRNAs as biomarkers in pituitary tumors. Neurosurgery 2014, 75, 181–189; discussion 188–189. [Google Scholar] [CrossRef] [PubMed]
- Sheinerman, K.S.; Umansky, S.R. Circulating cell-free microRNA as biomarkers for screening, diagnosis and monitoring of neurodegenerative diseases and other neurologic pathologies. Front. Cell. Neurosci. 2013, 7, 150. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J.; Niu, W.; Guo, W.; Song, H.; Li, H.; Fan, H.; Zhao, L.; Zhong, A.; Dai, Y.; et al. A preliminary analysis of microRNA as potential clinical biomarker for schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. Off. Publ. Int. Soc. Psychiatr. Genet. 2015, 168, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.-J.; Tang, Z.-Y.; Tu, K.; Zhu, L.; Li, Y.-X.; Xie, L.; Xiao, H.-S. Identification and target prediction of miRNAs specifically expressed in rat neural tissue. BMC Genom. 2009, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Maki, M.; Ding, R.; Yang, Y.; Zhang, B.; Xiong, L. Genome-wide survey of tissue-specific microRNA and transcription factor regulatory networks in 12 tissues. Sci. Rep. 2014, 4, 5150. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef]
- Lee, E.J.; Baek, M.; Gusev, Y.; Brackett, D.J.; Nuovo, G.J.; Schmittgen, T.D. Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors. RNA 2008, 14, 35–42. [Google Scholar] [CrossRef]
- Liang, Y.; Ridzon, D.; Wong, L.; Chen, C. Characterization of microRNA expression profiles in normal human tissues. BMC Genom. 2007, 8, 166. [Google Scholar] [CrossRef]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef]
- Bicker, S.; Lackinger, M.; Weiß, K.; Schratt, G. MicroRNA-132, -134, and -138: A microRNA troika rules in neuronal dendrites. Cell. Mol. Life Sci. CMLS 2014, 71, 3987–4005. [Google Scholar] [CrossRef]
- Cougot, N.; Bhattacharyya, S.N.; Tapia-Arancibia, L.; Bordonné, R.; Filipowicz, W.; Bertrand, E.; Rage, F. Dendrites of mammalian neurons contain specialized P-body-like structures that respond to neuronal activation. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 13793–13804. [Google Scholar] [CrossRef]
- Kye, M.-J.; Liu, T.; Levy, S.F.; Xu, N.L.; Groves, B.B.; Bonneau, R.; Lao, K.; Kosik, K.S. Somatodendritic microRNAs identified by laser capture and multiplex RT-PCR. RNA 2007, 13, 1224–1234. [Google Scholar] [CrossRef] [PubMed]
- Lugli, G.; Torvik, V.I.; Larson, J.; Smalheiser, N.R. Expression of microRNAs and their precursors in synaptic fractions of adult mouse forebrain. J. Neurochem. 2008, 106, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Schratt, G. microRNAs at the synapse. Nat. Rev. Neurosci. 2009, 10, 842–849. [Google Scholar] [CrossRef]
- Schratt, G.M.; Tuebing, F.; Nigh, E.A.; Kane, C.G.; Sabatini, M.E.; Kiebler, M.; Greenberg, M.E. A brain-specific microRNA regulates dendritic spine development. Nature 2006, 439, 283–289. [Google Scholar] [CrossRef]
- Smalheiser, N.R. The RNA-centred view of the synapse: Non-coding RNAs and synaptic plasticity. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2014, 369, 20130504. [Google Scholar] [CrossRef]
- Nail, H.M.; Chiu, C.-C.; Leung, C.-H.; Ahmed, M.M.M.; Wang, H.-M.D. Exosomal miRNA-mediated intercellular communications and immunomodulatory effects in tumor microenvironments. J. Biomed. Sci. 2023, 30, 69. [Google Scholar] [CrossRef]
- López-Pérez, Ó.; Sanz-Rubio, D.; Hernaiz, A.; Betancor, M.; Otero, A.; Castilla, J.; Andréoletti, O.; Badiola, J.J.; Zaragoza, P.; Bolea, R.; et al. Cerebrospinal Fluid and Plasma Small Extracellular Vesicles and miRNAs as Biomarkers for Prion Diseases. Int. J. Mol. Sci. 2021, 22, 6822. [Google Scholar] [CrossRef] [PubMed]
- Kikkawa, Y.; Ogura, T.; Nakajima, H.; Ikeda, T.; Takeda, R.; Neki, H.; Kohyama, S.; Yamane, F.; Kurogi, R.; Amano, T.; et al. Altered Expression of MicroRNA-15a and Kruppel-Like Factor 4 in Cerebrospinal Fluid and Plasma After Aneurysmal Subarachnoid Hemorrhage. World Neurosurg. 2017, 108, 909–916.e3. [Google Scholar] [CrossRef]
- Pedrosa, L.; Hoyos, J.; Reyes, L.; Llull, L.; Santana, D.; de Riva, N.; Mellado, R.; Sala, X.; Rodríguez-Hernández, A.; Enseñat, J.; et al. MicroRNA cerebrospinal fluid profile during the early brain injury period as a biomarker in subarachnoid hemorrhage patients. Front. Cell. Neurosci. 2022, 16, 1016814. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-X.; Springer, J.E.; Xie, K.; Fardo, D.W.; Hatton, K.W. A Highly Predictive MicroRNA Panel for Determining Delayed Cerebral Vasospasm Risk Following Aneurysmal Subarachnoid Hemorrhage. Front. Mol. Biosci. 2021, 8, 657258. [Google Scholar] [CrossRef]
- Simon, R.; Lam, A.; Li, C.; Ngan, M.; Menenzes, S.; Zhao, Y. Analysis of Gene Expression Data Using BRB-Array Tools. Cancer Inform. 2007, 3, 117693510700300022. [Google Scholar] [CrossRef]
- Gareev, I.; Beylerli, O.; Yang, G.; Izmailov, A.; Shi, H.; Sun, J.; Zhao, B.; Liu, B.; Zhao, S. Diagnostic and prognostic potential of circulating miRNAs for intracranial aneurysms. Neurosurg. Rev. 2021, 44, 2025–2039. [Google Scholar] [CrossRef]
- Liao, L.; Wang, H.; Wei, D.; Yi, M.; Gu, Y.; Zhang, M.; Wang, L. Exosomal microRNAs: Implications in the pathogenesis and clinical applications of subarachnoid hemorrhage. Front. Mol. Neurosci. 2023, 16, 1300864. [Google Scholar] [CrossRef]
- Das, K.; Rao, L.V.M. The Role of microRNAs in Inflammation. Int. J. Mol. Sci. 2022, 23, 15479. [Google Scholar] [CrossRef]
- Saha, S. Role of microRNA in Oxidative Stress. Stresses 2024, 4, 269–281. [Google Scholar] [CrossRef]
- Wang, J.; Xu, F.; Zhu, X.; Li, X.; Li, Y.; Li, J. Targeting microRNAs to Regulate the Integrity of the Blood-Brain Barrier. Front. Bioeng. Biotechnol. 2021, 9, 673415. [Google Scholar] [CrossRef]
- Kiel, K.; Król, S.K.; Bronisz, A.; Godlewski, J. MiR-128-3p—A gray eminence of the human central nervous system. Mol. Ther. Nucleic Acids 2024, 35, 102141. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Chen, F.; Fang, B.; Zhang, Z.; Dong, Y.; Tong, X.; Ma, H. MiR-128-3p Alleviates Spinal Cord Ischemia/Reperfusion Injury Associated Neuroinflammation and Cellular Apoptosis via SP1 Suppression in Rat. Front. Neurosci. 2020, 14, 609613. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yan, B.; Tang, Y.; Zhou, X.; Ji, Z.; Xu, F. Baicalein ameliorates oxidative stress and brain injury after intracerebral hemorrhage by activating the Nrf2/ARE pathway via miR-106a-5p/PHLPP2 axis. Int. J. Neurosci. 2023, 133, 1380–1393. [Google Scholar] [CrossRef]
- Hou, J.; Deng, Q.; Deng, X.; Zhong, W.; Liu, S.; Zhong, Z. MicroRNA-146a-5p alleviates lipopolysaccharide-induced NLRP3 inflammasome injury and pro-inflammatory cytokine production via the regulation of TRAF6 and IRAK1 in human umbilical vein endothelial cells (HUVECs). Ann. Transl. Med. 2021, 9, 1433. [Google Scholar] [CrossRef]
- Zhang, Z.; Zou, X.; Zhang, R.; Xie, Y.; Feng, Z.; Li, F.; Han, J.; Sun, H.; Ouyang, Q.; Hua, S.; et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging 2021, 13, 3060–3079. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.E.; Kim, S.W.; Jeong, S.; Moon, H.; Choi, W.S.; Lim, S.; Lee, S.; Hwang, K.-C.; Choi, J.-W. MicroRNA-26a/b-5p promotes myocardial infarction-induced cell death by downregulating cytochrome c oxidase 5a. Exp. Mol. Med. 2021, 53, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Lafourcade, C.A.; Fernández, A.; Ramírez, J.P.; Corvalán, K.; Carrasco, M.Á.; Iturriaga, A.; Bátiz, L.F.; Luarte, A.; Wyneken, U. A Role for mir-26a in Stress: A Potential sEV Biomarker and Modulator of Excitatory Neurotransmission. Cells 2020, 9, 1364. [Google Scholar] [CrossRef]
- Wang, S.; Aurora, A.B.; Johnson, B.A.; Qi, X.; McAnally, J.; Hill, J.A.; Richardson, J.A.; Bassel-Duby, R.; Olson, E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell 2008, 15, 261–271. [Google Scholar] [CrossRef]
- Ebrahimi, V.; Rastegar-Moghaddam, S.H.; Mohammadipour, A. Therapeutic Potentials of MicroRNA-126 in Cerebral Ischemia. Mol. Neurobiol. 2023, 60, 2062–2069. [Google Scholar] [CrossRef] [PubMed]
- Toro, R.; Pérez-Serra, A.; Mangas, A.; Campuzano, O.; Sarquella-Brugada, G.; Quezada-Feijoo, M.; Ramos, M.; Alcalá, M.; Carrera, E.; García-Padilla, C.; et al. miR-16-5p Suppression Protects Human Cardiomyocytes against Endoplasmic Reticulum and Oxidative Stress-Induced Injury. Int. J. Mol. Sci. 2022, 23, 1036. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).