Abstract
Type I interferons (IFN-I) are pivotal effectors of innate immunity and constitute a central axis of host defense against pathogens. Sensing of exogenous or endogenous nucleic acids by pattern-recognition receptors—exemplified by Toll-like receptors—triggers transcriptional induction of IFN-I. Engagement of the heterodimeric IFN-I receptor on nucleated cells reprograms cellular states via canonical Janus kinase–signal transducer and activator of transcription (JAK–STAT) signaling as well as STAT-independent, noncanonical pathways. This axis is tempered by multilayered regulatory mechanisms, including epigenetic remodeling, and important aspects remain incompletely defined. Dysregulation of IFN-I activity underlies diverse autoimmune disorders, notably systemic lupus erythematosus, wherein IFN-responsive gene signatures stratify disease endotypes, reflect disease activity trajectories, and predict therapeutic responsiveness. In recent years, therapeutic strategies targeting this pathway are now available: anti-IFN-I receptor therapy for systemic lupus erythematosus (SLE) and JAK inhibition for rheumatoid arthritis (RA) and giant cell arteritis (GCA). Altogether, a refined understanding of the IFN-I axis furnishes a pragmatic framework for patient stratification, response prediction, and mechanism-informed therapy design across immune-mediated diseases.
1. Introduction
Type I interferons (IFN-I) constitute a cytokine family encompassing IFN-α and IFN-β that orchestrate innate immunity by governing antiviral responses and shaping the activation of adaptive immunity [1,2]. IFN was first identified as a factor produced by influenza virus-infected chick embryo cells that conferred resistance to infection by both homologous and heterologous viruses [3]. Early reports characterized IFN as a wholly non-toxic antiviral with no effects in uninfected cells; however, by the mid-1970s, evidence had already emerged indicating that IFN can exert deleterious effects in vivo [4]. Contemporaneously, Skurkovich et al. demonstrated that administration of anti-IFN serum reduces both cell-mediated and humoral immunity in mice [5]. They subsequently hypothesized that IFN is a central driver of immune dysregulation across diverse autoimmune diseases, advocated the therapeutic potential of IFN neutralization, and established a conceptual framework for IFN biology and its clinical targeting in autoimmunity [6,7].
Over the past five decades, converging evidence has implicated dysregulated IFN-I activity as a principal pathogenic driver in systemic autoimmune diseases, including systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and various forms of vasculitis [8,9,10,11]. Among these conditions, SLE is characteristically marked by a persistent “type I interferon signature,” defined by sustained upregulation of IFN-stimulated genes in peripheral blood and affected tissues [12]. In RA, a subset of patients displays an interferon-inducible molecular phenotype that prognosticates therapeutic response and disease trajectory [13]. Similarly, in giant cell arteritis and other primary vasculitis, recent transcriptomic analyses and serological evidence substantiate a contributory role for IFN-I in vascular inflammation and tissue injury [14,15]. Moreover, dysregulation of the type I interferon system has been documented in several other autoimmune rheumatic diseases, including primary Sjögren’s syndrome, systemic sclerosis, and dermatomyositis [16,17,18]. Although the reasons why elevated IFN-I activity is observed across these clinically divergent conditions remain incompletely defined, they can be rationalized within a unifying framework in which heterogeneous upstream triggers converge on a conserved axis that begins with the sensing of nucleic acids and proceeds through the IFN-I receptor (IFNAR) and the Janus kinase–signal transducer and activator of transcription (JAK–STAT) pathway. This conceptual view has, in turn, catalyzed efforts to therapeutically modulate the interferon pathway. Notably, the recent approval of a monoclonal antibody targeting IFNAR in SLE [19], together with the expanding deployment of JAK–STAT inhibitors that attenuate signaling downstream of IFN-I [20], marks a substantive advance toward precision medicine by enabling patient stratification and individualized therapeutic selection. In autoimmune diseases, JAK inhibitors, originally developed for rheumatoid arthritis, are now being investigated across a wide range of autoimmune disorders, underscoring both their robust therapeutic efficacy and the common interferon-driven pathogenic mechanisms [21]. This review delineates the IFN-I signaling axis, elucidates the contributions of IFN-I to the pathogenesis of RA, SLE, and vasculitis, and surveys therapeutic strategies that directly or indirectly target the IFN-I system.
2. Type I Interferon Biology and Signaling Architecture
2.1. Molecular Diversity and Subtype Characteristics
IFN-I are pivotal initiators of innate immune defenses against a broad spectrum of pathogens [22]. The IFN-I family comprises IFN-α, IFN-β, IFN-δ, IFN-ε, IFN-κ, IFN-ω, and IFN-ζ, with IFN-α represented by 13 distinct homologs in humans and 14 in mice. Among these subtypes, humans express IFN-α, IFN-β, IFN-ε, IFN-κ, and IFN-ω [23].
IFN-I, particularly IFN-β, can be produced by most cell types; however, antiviral IFN-I production by blood cells predominantly relies on nucleic acid sensing via Toll-like receptor (TLR)7 and TLR9 in plasmacytoid dendritic cells (pDCs) [24,25]. Additionally, tissue macrophages, epithelial cells, and fibroblasts contribute to IFN-I production in a context-dependent manner [26,27].
IFN-I are constitutively expressed under physiological conditions, sustaining tissue homeostasis and maintaining a poised antiviral state [28]. Upon detection of pathogen-associated molecular patterns (PAMPs) derived from viral or bacterial nucleic acids, or damage-associated molecular patterns (DAMPs) originating from tissue injury, by pattern-recognition receptors (PRRs), diverse cell types—including pDCs—induce IFN-I production [29]. The response unfolds in two phases: an initial, IFN regulatory factor 3 (IRF3)-dominant wave, followed by IRF7 activation that establishes a positive feedback loop and amplifies IFN-I secretion [23,30,31]. Secreted IFN-I amplifies IFNAR signaling through autocrine and paracrine mechanisms, thereby promoting antigen presentation, chemokine production, and the coordinated reprogramming of innate and adaptive immunity via broad induction of interferon-stimulated genes (ISGs) [29,32,33]. Conversely, negative regulators such as suppressor of cytokine signaling 1 (SOCS1) and ubiquitin-specific protease 18 (USP18) [34], together with cytokine milieu-dependent thresholds [32,35], finely modulate the magnitude and duration of this response.
2.2. Upstream Sensing Pathways: TLRs, RLRs, and cGAS–STING
The initiation of IFN-I production is governed by PRRs that sense exogenous or endogenous nucleic acids [36]. These include plasma membrane TLRs such as TLR4, endosomal TLRs such as TLR7 and TLR9, and cytosolic sensors such as retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated gene 5 (MDA5) [37,38,39,40] (Figure 1). These pathways can also be engaged by endogenous ligands released from injured or apoptotic cells, indicating that the detection of self-nucleic acids is pivotal in sterile inflammation and autoimmunity [41].
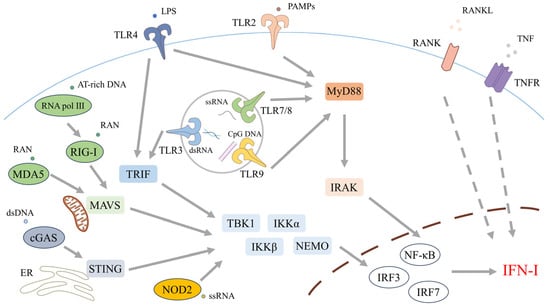
Figure 1.
Pattern-recognition receptor (PRR) pathways that initiate type I interferon (IFN-I) production. IFN-I induction is triggered when PRRs sense exogenous or endogenous nucleic acids. At the plasma membrane, Toll-like receptor (TLR)4 recognizes lipopolysaccharide (LPS) from Gram-negative bacteria, while TLR2—typically in heterodimers with TLR1 or TLR6—detects a broad range of pathogen-associated molecular patterns (PAMPs) from bacteria, fungi, parasites, and viruses. Endosomal TLRs chiefly sense nucleic acids: TLR3 detects double-stranded RNA (dsRNA), TLR7 and TLR8 recognize single-stranded RNA (ssRNA), and TLR9 recognizes unmethylated CpG DNA. TLR signaling proceeds via MyD88-dependent and MyD88-independent branches. All TLRs except TLR3 employ MyD88 to activate NF-κB via IRAK family kinases, whereas TLR3 and TLR4 engage TRIF (with TRAM for TLR4) to promote TRAF3-dependent activation of TBK1/IKKε, culminating in IRF3 phosphorylation and dimerization. In the cytosol, the RIG-I-like receptors (RLRs) RIG-I and MDA5 sense viral RNA and signal via the adaptor MAVS to TBK1/IKKε, thereby activating IRF3 and IRF7. In addition, AT-rich DNA can be transcribed by RNA polymerase III into 5′-triphosphate RNA that serves as a RIG-I agonist. The cGAS–STING pathway detects cytosolic double-stranded DNA (dsDNA): cGAS generates cyclic GMP–AMP (cGAMP), which binds STING on the endoplasmic reticulum (ER) to recruit and activate TBK1, driving IRF3 nuclear translocation and IFN-I gene induction. NOD-like receptors NOD1 and NOD2, which sense bacterial and viral signatures, can further contribute to IFN-I responses. Collectively, phosphorylated IRF3/IRF7 together with NF-κB bind IFN-I promoters to initiate transcription of IFN-I and ISGs. Beyond PRRs, members of the tumor necrosis factor receptor superfamily—including RANK—can reinforce ISG expression through paracrine and autocrine signaling.
Ten TLRs have been identified in humans. Cell surface TLRs (TLR1, TLR2, TLR4, TLR5, TLR6, TLR10) primarily detect microbial membrane components and elicit proinflammatory responses, whereas intracellular TLRs (TLR3, TLR7, TLR8, TLR9) principally recognize microbial nucleic acids of bacterial or viral origin, triggering both IFN-I production and inflammatory signaling [42].
The cell surface receptor TLR4, a founding member of the Toll-like receptor family, senses bacterial lipopolysaccharide (LPS), a constituent of the outer membrane of Gram-negative bacteria [43]. TLR2 participates in the recognition of a broad spectrum of PAMPs derived from bacteria, fungi, parasites, and viruses, typically forming heterodimers with TLR1 or TLR6 [43,44,45]. TLR5 recognizes flagellin, the principal protein component of bacterial flagella [43]. Non-TLR membrane receptors implicated in these pathways include members of the tumor necrosis factor (TNF) receptor superfamily and the receptor activator of nuclear factor κB (RANK) [46,47,48]. Both TNF and receptor activator of NF-κB ligand (RANKL) have been implicated in promoting sustained expression of ISGs through paracrine and autocrine signaling mechanisms [32,41].
By contrast, intracellular TLRs are predominantly localized to endosomes [41]. TLR3 senses double-stranded ribonucleic acid (RNA), whereas TLR7 and TLR8 detect single-stranded RNA [49,50]. TLR9 recognizes unmethylated cytosine–phosphate–guanine (CpG) motifs within deoxyribonucleic acid (DNA) [51].
TLR signaling is broadly categorized into two pathways: the myeloid differentiation primary response 88 (MyD88)-dependent pathway and the MyD88-independent pathway [42]. MyD88, the first identified Toll/interleukin-1 receptor (TIR) domain-containing adaptor protein is employed by all TLRs except TLR3 and principally activates NF-κB signaling [42]. Upon ligand engagement, MyD88 associates with the receptor via its TIR domain, recruits the serine/threonine interleukin (IL)-1 receptor-associated kinases (IRAKs), and nucleates a signaling complex with IRAK family members known as the myddosome [52,53,54]. Activated IRAKs engage TNF receptor-associated factor 6 (TRAF6), leading to activation of the IκB kinase complex [55]. The inhibitor of κB kinase (IKK) complex phosphorylates IκB, triggering its degradation and permitting the transcription factor NF-κB to translocate into the nucleus [56].
By contrast, TLR3 and TLR4 signal through a MyD88-independent pathway. Upon activation, TIR domain-containing adaptor protein-inducing IFN-β (TRIF) engages these receptors via its TIR domain, promoting TRAF3-dependent activation of the IKK-related kinase TANK-binding kinase 1 (TBK1). TRAF3 activates TBK1 and IKK—often within complexes containing NF-κB essential modulator (NEMO)—culminating in IRF3 phosphorylation and dimerization. The IRF3 homodimer then translocates to the nucleus, where it drives transcription of IFN-I genes and ISGs [57,58]. The MyD88-independent arm of TLR4 signaling additionally requires the TRIF-related adaptor molecule (TRAM) [59].
Following the discovery of TLRs, multiple classes of cytosolic PRRs have been delineated, notably the RIG-I–like receptors (RLRs) and the nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) [42].
The RLR family comprises three members— RIG-I, MDA5, and LGP2 (protein named “laboratory of genetics and physiology 2”)—which serve as the principal cytosolic sensors of RNA and detect RNA viruses [60,61,62,63]. In addition, RIG-I and MDA5 contain two N-terminal caspase activation and recruitment domains (CARDs) that mediate downstream signaling. Downstream signaling is transmitted via mitochondrial antiviral signaling protein (MAVS) to TBK1 and IKKε, which in turn activate IRF3 and IRF7; together with NF-κB, these factors drive transcription of IFN-I and ancillary antiviral genes [64]. Moreover, AT-rich DNA can be transcribed by RNA polymerase III into 5′-triphosphorylated RNA that functions as a RIG-I agonist [23].
Cyclic GMP–AMP synthase (cGAS) is activated upon binding double-stranded DNA (dsDNA), catalyzing the conversion of adenosine triphosphate (ATP) and guanosine triphosphate (GTP) into cyclic GMP–AMP (cGAMP). cGAMP, together with other cyclic dinucleotides (CDNs), engages STING on the endoplasmic reticulum (ER). Following activation, STING translocates from the ER to the ER–Golgi intermediate compartment (ERGIC) and recruits TBK1. TBK1-mediated phosphorylation of STING and IRF3 drives IRF3 nuclear translocation, thereby inducing IFN-I production [59,65].
Additional cytosolic DNA motifs are sensed by diverse receptors—including DNA-dependent activators of interferon regulatory factors (DAIs) and DEAD- and DEAH-box (DExD/H-box) helicases—all of which have been implicated in IFN-I induction [61,66,67]. Moreover, the cytosolic pattern-recognition receptor NOD1 initiates IFN-I signaling upon sensing γ-D-glutamyl–meso-diaminopimelic acid (iE-DAP), a peptidoglycan motif characteristic of Gram-negative and select Gram-positive bacteria. By contrast, NOD2 recognizes muramyl dipeptide (MDP), a conserved peptidoglycan fragment present in both Gram-positive and Gram-negative bacteria, and also responds to viral ssRNA [68,69,70,71]. These receptors recruit receptor-interacting serine/threonine kinase (RICK) via CARDs. RICK activates the IKK complex through NEMO, enabling NF-κB nuclear translocation [72].
Thus, induced phosphorylated IRF3/IRF7 and NF-κB bind to the promoter regions of the IFN-I gene family, thereby regulating IFN-I expression [73,74].
2.3. Canonical IFNAR–JAK–STAT Signaling Cascade
All nucleated cells express functional transmembrane IFN-I receptors, which are generally composed of IFNAR1 and IFNAR2 heterodimers [75,76].
IFN-I signals through IFNAR1/IFNAR2, which couple to TYK2 and JAK1, respectively; ligand engagement induces receptor rearrangement and JAK activation, generating phosphotyrosine docking sites for STATs [23] (Figure 2). Phosphorylated STAT1 and STAT2 assemble with IRF9 to form interferon-stimulated gene factor 3 (ISGF3), which translocates to the nucleus, binds interferon-stimulated response elements (ISREs), and induces ISGs—for example, myxovirus resistance 1 (MX1), 2′–5′-oligoadenylate synthetase 1 (OAS1), and members of the interferon-induced proteins with tetratricopeptide repeats (IFIT) family—thereby establishing antiviral and immunoregulatory programs [77,78]. In specific contexts, STAT1 or STAT3 homodimers engage gamma-activated sequence (GAS) elements to drive distinct ISG modules [79,80]; unphosphorylated STATs also contribute to transcriptional regulation [81].
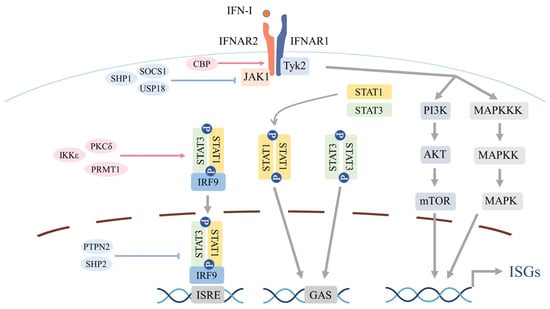
Figure 2.
Canonical and noncanonical signaling downstream of the type I interferon (IFN-I) receptor. All nucleated cells express the transmembrane IFN-I receptor, typically a heterodimer of IFNAR1 and IFNAR2. Ligand engagement brings together IFNAR1-associated TYK2 and IFNAR2-associated JAK1, driving receptor rearrangement and activation of the receptor-bound Janus kinases. This leads to phosphorylation of tyrosine residues on IFNAR and subsequent phosphorylation of STAT1 and STAT2. Phosphorylated STAT1 and STAT2 dimerize and, with IRF9, form the interferon-stimulated gene factor 3 (ISGF3) complex, which translocates to the nucleus, binds interferon-stimulated response elements (ISREs), and induces ISGs. Under specific contexts, STAT1 or STAT3 homodimers bind gamma-activated sequence (GAS) to drive distinct ISG modules. In parallel, IFNAR activation can signal through STAT-independent, noncanonical cascades—including phosphoinositide 3-kinase (PI3K)–AKT and mitogen-activated protein kinase (MAPK) pathways—to amplify cellular responses. Multiple regulatory checkpoints tune pathway amplitude and duration. Proximally, SOCS family proteins restrain JAK1/TYK2 and limit STAT phosphorylation, while USP18 associates with IFNAR2 to competitively limit JAK1 recruitment. By contrast, PKCδ-mediated STAT1 Ser727 phosphorylation and IKKε-mediated STAT1 Ser708 phosphorylation enhance transcriptional output, and protein arginine methyltransferase 1 (PRMT1) augments STAT1 DNA binding and transactivation. Within the nucleus, phosphatases such as PTPN2 and SHP2 (PTPN11) dephosphorylate STATs to temper signaling. Chromatin-level control includes context-dependent histone acetylation by CBP and GCN5 at ISG loci. Beyond these mechanisms, IFN-I signaling is further modulated by ubiquitination and ubiquitin-like modifications (e.g., SUMOylation, ISGylation).
2.4. Noncanonical Signaling Routes and Crosstalk
Beyond the canonical JAK–STAT cascade, IFNAR1/IFNAR2 engagement activates STAT-independent PI3K and MAPK pathways, thereby broadening IFN-I effector outputs [77] (Figure 2). In the PI3K–AKT axis, IFN-I–induced phosphorylation of insulin receptor substrate 1 (IRS1) recruits the p85 regulatory subunit of PI3K and activates the p110 catalytic subunit [82]. PI3K converts phosphatidylinositol 4,5-bisphosphate (PIP2) into phosphatidylinositol 3,4,5-trisphosphate (PIP3) [83], which co-localizes 3-phosphoinositide–dependent protein kinase 1 (PDK1) and AKT at the plasma membrane; PDK1 subsequently phosphorylates AKT at Thr308 [84,85], propagating signaling to the mechanistic target of rapamycin (mTOR), a central regulator of protein translation and cellular metabolism [86]. Within the MAPK arm, JAK phosphorylates VAV and other guanine-nucleotide-exchange factors (GEFs), leading to RAC1 activation and sequential activation of MAPK kinase kinase (MAPKKK) and MAPK kinase (MAPKK), most prominently MKK3/6, which in turn activate p38 MAPK [87,88]. Activated p38 signals to MAPK-activated protein kinase (MAPKAPK)2/3, mitogen- and stress-activated kinase 1 (MSK1), and MAPK-interacting protein kinase 1 (MNK1) [89,90]; in mammals, ERK and c-Jun N-terminal kinase (JNK) can also contribute [78].
2.5. Regulatory Checkpoints of IFN-I Activity
Although the IFN-I response is highly potent, excessive or sustained activation can precipitate autoimmunity and tissue injury. Accordingly, cells finely calibrate its amplitude, duration, and gene selectivity through multiple regulatory layers [91,92]. Proximally, receptor- and JAK-level feedback, phosphatase activity, and post-translational modifications limit signaling strength and timing. At the epigenetic and RNA-mediated layer, chromatin remodeling, histone/DNA modifications, and non-coding RNAs shape ISG accessibility, transcriptional elongation, and persistence. We summarize these mechanisms and their net effects in Table 1. IFN-induced epigenetic alterations can persist for days to weeks as transcriptional memory, sustaining ISG expression after upstream JAK–STAT activity has waned. Even non-ISGs undergo bookmarking, which reprograms responsiveness to subsequent stimuli [93,94].

Table 1.
Positive and Negative Regulators of IFN-I Signaling.
3. Functional Landscape of Type I Interferon Across the Immune Systems
IFN-I reprogram functional states across multiple compartments of innate and adaptive immunity, with an early antiviral program at the core during initial infection. Their effects are bidirectional—potentiating or suppressive—depending on phase (acute versus chronic), concentration, receptor composition, cytokine milieu, and tissue niche; even within a single cell, outcomes can invert according to context.
3.1. B Cells
IFN-I modulate B-cell survival, differentiation, and antibody production in a context-dependent fashion. Notably, strain background influences these effects: in C57BL/6 mice, Ifnar1 deficiency yields minimal changes in peritoneal and splenic B-cell subsets, whereas in Ifnar1-deficient mice on the 129Sv background, alterations in the immature bone marrow B-cell repertoire have been reported, accompanied by differential susceptibility to B cell receptor (BCR)-dependent blockade of terminal differentiation under identical conditions [132]. Functionally, IL-6 costimulation promotes plasma-cell differentiation [133]. By contrast, reports on survival and proliferation are inconsistent, describing both enhancement and inhibition [134], a discrepancy sometimes attributed to dose dependence, whereby low IFN-I doses augment immunoglobulin production while high doses suppress it [135].
3.2. T Cells
The canonical pathway of Th1 differentiation entails IL-12 engagement of its receptor, activation of JAK2 and TYK2, phosphorylation of STAT4, and consequent induction of T-bet [136]. By contrast, IFN-α/β can acutely phosphorylate STAT4, but in human CD4+ T cells this activation is transient and insufficient to drive robust Th1 differentiation or IFN-γ production [137]. Nevertheless, in vivo evidence indicates that IFN-α/β contributes to Th1 establishment in pathogen-responsive contexts [138]. Moreover, Th1 cells can arise in IL-12-deficient settings, implicating alternative or cooperative pathways [139].
Th2 differentiation is driven primarily by IL-4, with the transcription factor GATA3 promoting transcription across the IL-4/IL-5/IL-13 locus while concurrently repressing IL-12Rβ2 to inhibit Th1 commitment. IL-33 and thymic stromal lymphopoietin (TSLP) function as costimulatory inputs, and GATA3 sustains Th2 identity through a self-reinforcing circuit [140,141]. In contrast, IFN-I exerts suppressive effects on the Th2 axis: early studies demonstrated inhibition of IL-5 secretion and eosinophil recruitment in allergic inflammation [142,143]. More recent work indicates that, in humans, IFN-α/β robustly and selectively inhibits IL-4–dependent Th2 differentiation and destabilizes committed Th2 cells, whereas in mice it does not exert comparably strong inhibition [144].
Th17 cells are induced by cooperative signaling from transforming growth factor-β (TGF-β), IL-6, IL-23, and IL-1β, which activates RORγt and drives production of IL-17A, IL-17F, and IL-22, thereby contributing to diverse inflammatory processes [145]. IFN-I suppress Th17 differentiation in mice [146], and this inhibition extends to human Th17 cells [147]. Clinically, although IFN-β therapy is employed in multiple sclerosis, certain patient subsets exhibit limited efficacy or worsening of Th17-dominant inflammation [148]. A contributory mechanism involves IFN-β–induced C-C motif chemokine ligand (CCL)2 production [149], which recruits Th17.1 cells and inflammatory monocytes into the central nervous system; these monocytes upregulate IL-1β and locally differentiate into dendritic cells, thereby further amplifying Th17 responses [150,151].
3.3. DCs
DCs initiate antiviral T-cell responses [152]. IFN-I promotes DC maturation, augmenting major histocompatibility complex (MHC) class II and costimulatory molecule expression and enhancing antigen-presenting capacity; they also drive differentiation from plasmacytoid to myeloid DCs, thereby strengthening T-cell activation [152,153]. Conversely, during persistent IFN-I signatures, myeloid differentiation and DC proliferation are suppressed, yielding an inhibitory phenotype characterized by reduced splenic CD11c+ cells and elevated PD-L1 and IL-10 expression [154,155,156]. Moreover, whereas TLR stimulation rapidly diminishes CIITA and de novo MHC-II synthesis alongside antigen processing during maturation, IFN-I elicit a distinct maturation program that preserves CIITA and MHC-II expression while simultaneously increasing surface MHC-II and sustaining intracellular antigen processing [152,153].
3.4. Monocytes, Macrophages, and NK Cells
Inflammatory monocytes are rapidly mobilized to sites of infection, where they augment the antiviral functions of local and neighboring immune cells, amplify inflammation, and differentiate into macrophages and DCs [157]. Conversely, IFN-I have also been reported to suppress the activity of proinflammatory NOS2+ Ly6C− monocytes, modulating both the magnitude and quality of the monocyte response through dual mechanisms of enhanced recruitment and functional inhibition [158].
IFN-I generally exerts inhibitory effects on macrophages. Notably, they downregulate IFN-γ receptor expression and attenuate cellular sensitivity to IFN-γ stimulation [159]. Accordingly, in certain bacterial infections, such as tuberculosis, IFN-I signaling has repeatedly been implicated in adverse host outcomes, in part due to impaired macrophage activation arising from dampened IFN-γ-dependent pathways [160,161,162].
IFN-I orchestrate the activation and regulation of natural killer (NK)-cell function. The IFNAR–STAT axis underpins cytotoxicity and IFN-γ production; STAT1-deficient mice display reduced NK-cell cytotoxicity and heightened virus-induced mortality [154]. Conversely, IFN-I acts as a biphasic regulator: during chronic lymphocytic choriomeningitis virus (LCMV) infection, IFNAR blockade restores NK-cell IFN-γ production, whereas sustained IFN-I drives an inhibitory phenotype via PD-L1 and IL-10 induction [154]. Clinically, pegylated IFN-α2 administration enhances NK-cell activation and TRAIL/CD107a expression, while potentially diminishing IFN-γ–positive NK cells [163,164]. A timing-dependent effect is also evident: early responses are dominated by STAT4-mediated IFN-γ induction, but with prolonged IFN-I exposure the balance shifts toward STAT1-dependent suppression, ultimately curtailing IFN-γ production—a transcription-factor switch that has been proposed mechanistically [165,166,167].
4. Type I Interferon in Systemic Lupus Erythematosus
SLE is a chronic, multisystem inflammatory disorder that manifests in distinct clinical trajectories, including chronic persistent, relapsing–remitting, and long-term remission phenotypes [168,169]. Its pathogenesis centers on aberrant immune activation arising from impaired clearance of apoptotic cells, loss of self-tolerance, T- and B-cell dysfunction, and dysregulated cytokine networks [170]. The IFN-I pathway contributes to SLE susceptibility through both genetic predisposition and perturbations of innate immune regulation [171]. Although not a singular etiologic driver in all patients, IFN-I signaling is thought to underlie a substantial component of the disease’s pathophysiological heterogeneity [172].
Both clinical and experimental data implicate IFN-I in disease initiation. De novo SLE has been reported in patients receiving recombinant IFN-α therapy, with symptom improvement following discontinuation [11,173]. Elevated circulating interferon activity is frequently observed in unaffected relatives and exhibits familial correlation [174,175], while longitudinal analyses reveal a steep increase in interferon activity approximately one year before diagnosis [176]. Historically, exogenous IFN induction accelerated disease in NZB/NZW F1 mice [8]. In humans, hundreds of ISGs are markedly upregulated in peripheral blood, establishing IFN-I signaling as the most prominently activated molecular pathway in SLE [177,178].
4.1. Biomarker Landscape: Blood and Tissue Interferon Signatures
In SLE, IFN-α is the predominant IFN-I; however, its activity varies widely among patients, with serum IFN-α within the normal range in 40–50% of cases [174]. Thus, IFN-I are unlikely to be a singular causal driver in all patients and may instead constitute a key determinant of the disease’s heterogeneity [172]. Recent studies indicate that elevated baseline IFN-α levels correlate with adverse renal outcomes, including recurrent nephropathy, and may serve as a biomarker for identifying high-risk individuals preemptively [179]. In cohorts with heightened IFN-I activity, strong associations are observed with autoantibodies such as anti-dsDNA and anti-Ro, and interferon signatures are detected across ancestral populations [172]. By contrast, in African Americans, the signature has been reported to depend on anti-RNP antibodies [172,180]. Serum IFN-α levels also exhibit an inverse correlation with complement C3/C4 [181].
Furthermore, peripheral blood interferon signatures correlate with disease severity and organ involvement. Cross-sectional analyses link these signatures to the number of diagnostic criteria fulfilled and to renal, central nervous system (CNS), and hematologic manifestations [182]. Longitudinally, however, interferon signatures are relatively stable and exhibit limited predictive power for relapse [183,184]. By contrast, interferon- and cytokine-inducible chemokines such as C-X-C motif chemokine ligand (CXCL)10, CCL2, and CCL19 correlate with disease activity and may aid in forecasting long-term relapse risk [185]. At the transcriptomic level, non–interferon modules—most notably the plasmablastic signature—often track disease activity more closely [186], suggesting that IFN-I may exert its greatest influence during the early phase of disease around onset.
Concordant findings emerge from tissue and single-cell analyses. Nearly all peripheral immune cell populations in SLE display IFN-I response, with particularly pronounced activation in monocytes [187]. Robust expression of IFN-inducible genes is also observed in synovial tissue from patients with arthritis and in renal biopsies from class IV lupus nephritis [188,189]. Single-cell RNA-seq of kidney biopsies detects interferon signatures across multiple subsets of infiltrating leukocytes and resident tissue cells [190,191] (Figure 3) (Box 1).
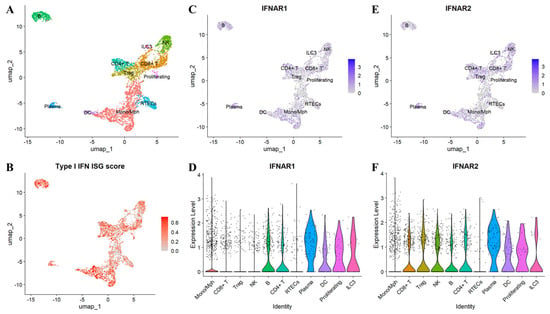
Figure 3.
Interferon-stimulated gene (ISG) signature and IFNAR1/2 expression in renal tissue from patients with systemic lupus erythematosus (SLE). Single-cell RNA sequencing data from the Accelerating Medicines Partnership (AMP) RA/SLE Network [190] were re-analyzed to characterize ISG activity and IFNAR1/2 expression in lupus nephritis kidney tissue. (A) Annotated UMAP plot showing major renal and immune cell populations. (B) UMAP feature plot depicting the aggregated ISG signature across single cells. (C) UMAP feature plot of IFNAR1 transcript abundance. (D) Violin plot showing IFNAR1 expression by annotated cell type. (E) UMAP feature plot of IFNAR2 transcript abundance. (F) Violin plot showing IFNAR2 expression by annotated cell type. RTECs, Renal tubular epithelial cells.
Box 1. Single-cell reanalysis of AMP RA/SLE datasets.
Analyses were performed in R 4.4.2 using Seurat 5.3.0. Raw UMI matrices were imported as sparse objects; cell metadata were merged; where available, CITE-seq ADT data were linked per cell and included as a separate assay. RNA counts were normalized, variable features selected (VST, 2000 genes), scaled, and reduced by PCA; samples were integrated with Seurat’s CCA-based workflow (IntegrateLayers; k.weight = 30), followed by graph construction, Leiden clustering (resolution = 0.5), and UMAP (dims 1–30). ADT was CLR-normalized. Cluster identities were assigned by canonical RNA markers with ADT support. IFN-stimulated genes (ISG) activity was quantified per cell using a six-gene signature (IFI27, IFI44L, IFIT1, ISG15, RSAD2, SIGLEC1); scoring used UCell.
4.2. Genetics, Pathways, and Epigenomic Imprinting of the IFN Program
SLE susceptibility is linked to single-nucleotide polymorphisms (SNPs) within the IFN-I axis, including IRF5/7/8, STAT4, and TYK2 [192,193]. The IRF family constitutes a core set of transcription factors that regulate IFN-I and ISGs transcription in a cell type-specific manner [194]. IRF5, in particular, governs inflammatory cytokine production and IFN-I induction in innate immune cells and modulates B-cell responses downstream of TLR stimulation [194]. In SLE, IRF5 risk alleles associate with elevated circulating IFN-I activity in a manner contingent upon anti-RNA-binding protein (anti-RBP) or anti-dsDNA autoantibodies [195,196]. Moreover, the IRF5 risk haplotype correlates with autoantibody production even in healthy individuals, suggesting a potential feedforward loop encompassing enhanced B-cell TLR signaling, nucleic acid-containing immune complex formation, innate-cell TLR activation, and excessive interferon production [197].
Genetic variants in IRF7 and IRF8 likewise confer increased SLE risk and are associated with altered IFN-I responses in patients [194]. Beyond the IRF family, numerous genes that modulate interferon pathway function—including STAT4, MAVS, IFIH1, and PTPN22—also contribute to susceptibility, underscoring IFN activity in SLE as a polygenic trait [198]. Moreover, genome-wide association studies (GWAS) stratified by circulating IFN-α levels have identified loci such as PRKG1, PNP, and ANKS1A—difficult to detect in conventional case–control designs—as associated with elevated interferon activity, implicating functional effects in dendritic cells and NK cells [175]. Notably, the repertoire of peripherally overexpressed ISGs does not necessarily overlap with genetic risk loci, and these risk effects may be further modulated by epigenetic regulation [75,199]. Recently, among ~200 loci identified by GWAS and exome studies, increasing attention has focused on the contribution of noncoding enhancers/super-enhancers and three-dimensional genome architecture (e.g., CTCF-dependent loops, Z-DNA/RNA) [200].
Inputs that drive interferon production converge on two principal routes: endosomal TLRs and cytoplasmic nucleic acid sensors. Induction of IFN-α by immune complexes—including autoantibodies to RNA-binding proteins (e.g., Ro/Sm), nucleic acids, and products of necrotic or apoptotic cells—correlates strongly with the interferon signature [201,202,203]. Genetic data implicating IRF5 (downstream of TLR7) together with pharmacologic inhibition of TLR7 reinforce the view that TLR7 access to RNA-containing immune complexes is a primary driver [204]. Additional candidate inputs for endosomal TLR activation include neutrophil extracellular traps (NETs), DNA-bearing microparticles, and circulating mitochondrial DNA [205,206,207,208]. Conversely, insights from interferonopathies show that mutations affecting RIG-I/MDA5, the cGAS–STING pathway, and endogenous nucleic-acid–regulatory factors can generate IFN-I signatures. In SLE, oxidized mitochondrial DNA and DNA/RNA derived from endogenous retroelements are plausible inducers of STING-dependent interferon production [209,210,211]. The relative contributions of endosomal TLRs versus cytoplasmic sensors in sporadic SLE—and their roles in monogenic variants—remain to be elucidated.
The tissue niche also modulates interferon outputs. Polymorphisms in IFNK (IFN-κ) have been implicated in cutaneous lupus erythematosus and exhibit sex-dependent effects [212]. Although IFN-κ is not the principal source of circulating IFN-I [174,212], it can amplify local cutaneous inflammation by promoting pDC-dependent IFN production and keratinocyte IL-6 overproduction—processes enhanced by TLR stimulation or UVB exposure in an IFN-κ–dependent manner [213].
4.3. Cellular Circuits Executing the IFN Program
4.3.1. DCs
pDCs are regarded as the principal source of IFN-I in SLE, with their output finely tuned by signals from diverse immune cells [214]. In SLE-prone mice, IFN-I–treated DCs exhibit relative resistance to apoptosis, implicating prolonged survival of activated DCs in disease initiation [215]. In lupus-prone mice, pDC depletion mitigates disease severity, and in humans, administration of an anti-BDCA2 antibody—selectively inhibiting pDC function—significantly attenuates the peripheral IFN-I signature [216,217,218]. In SLE patients with cutaneous involvement, anti-BDCA2 therapy markedly reduces IFN-α and MxA within lesional skin, changes that correlate with improved clinical scores and implicate pDC dominance within the cutaneous niche [218]. Nevertheless, the interferon milieu in SLE is not exclusively pDC-dependent: monocytes and conventional DCs (cDCs) also contribute, predominantly via IFN-β production, thereby reinforcing the IFN network through interactions among multiple immune cell types [219,220].
As upstream inputs to pDC activation, exosome-delivered microRNAs have emerged as potent activators, directly stimulating pDCs to augment IFN-I secretion [221]. Moreover, the late endosomal transport inhibitor EGA reduces IFN-α production and release in TLR7-activated pDCs, concurrently decreasing pro-TNF–expressing pDCs and suppressing TNF-α production and release in the R837 stimulation system [222]. In addition, integrin αvβ3, acting as a receptor for apoptotic cell debris, modulates TLR signaling—suppressing autoreactive B-cell activation in lupus models while providing essential contextual cues to pDCs that constrain excessive responses to self nucleic acids [223]. Thus, pharmacological control of the pDC–IFN-I axis constitutes a significant therapeutic objective in SLE [224].
4.3.2. T Cells
T cells undergo positive and negative selection in the thymus and exit to the periphery as naïve cells with defined epitope specificity [225]. Following antigen encounter, in parallel with effector differentiation, a subset acquires regulatory T-cell (Treg) or memory T-cell fates [225]. In SLE, single-cell RNA-seq and clinical studies demonstrate an inverse correlation between IFN-I activity and peripheral lymphocyte or naïve T-cell abundance [226,227]. Although Th1–Th2 disequilibrium is observed in SLE, it is largely contextualized by IFN-γ biology, with comparatively limited delineation of IFN-I-specific effects [228]. In inflamed tissues, increased Th17 and decreased Treg frequencies are reported [229]. IFN-I upregulates Radical S-adenosyl methionine domain-containing protein 2 (RSAD2) in naïve T cells, thereby promoting Th17 and follicular helper T-cell (Tfh) differentiation and contributing to inflammatory cytokine production and disease initiation [230].
Tfh cells—characterized by CXCR5, PD-1, BCL6, BTLA, ICOS, IL-21, and SH2D1A expression—correlate with SLE activity and organ damage and drive autoantibody production through aberrant germinal center (GC) responses [231]. IFN-I enhance STAT4 phosphorylation in Tfh cells, inducing dysregulated IL-21/IFN-γ production [232]. Elevated IFN-I further hyperactivates mTOR, contributing to lymphopenia [233]. In addition, IFN-α upregulates CD25 on Tfh cells, augmenting IL-2–STAT5 signaling; STAT5 then suppresses H3K4me3 at the BCL6 locus and competes with STAT1, reducing BCL6 expression, shifting PD-1+CXCR5+ Tfh-like cells toward PD-1+CXCR5− Tph-like cells and destabilizing the Tfh phenotype [234]. IFN-I also shields Tfh cells from NK cell-mediated cytotoxicity [235].
T peripheral helper (Tph) cells, originally defined in RA, localize to tertiary lymphoid structures and contribute to SLE pathology [236]. Their frequencies increase in active disease and lupus nephritis (LN) [237], and IFN-α-induced Tph-like cells robustly drive B cells toward CD38hiCD27hi plasma cells [234]. Blockade of IFN-α may suppress Tph-like differentiation and plasmacytogenesis [238].
Conversely, SLE is characterized by Treg dysfunction and/or depletion [239]. Although IFN-β can directly promote Treg induction through STAT1/P300-dependent Foxp3 acetylation, IFN-γ-centered pathways remain predominant in the SLE literature [240,241,242].
In LN, CD8+ T cells accumulate in periglomerular regions, and their density correlates with disease severity. IFN-I promote CD8+ T-cell differentiation, cytotoxicity, and chemotaxis through upregulation of ISGs [243]. In IFNAR-deficient mice, effector CD8+ T-cell accumulation is diminished and Treg frequencies increase [244]. Transcriptomically, SLE CD8+ T cells differ from healthy controls by aberrations in interferon-stimulated and mitochondrial pathways, and IFN-α has been shown to enhance apoptosis in effector memory CD8+ T cells [245].
4.3.3. B Cells
IFN-I upregulate TLR7 on naïve B cells, heightening sensitivity to RNA-associated antigens, while increasing costimulatory molecule expression to augment antigen presentation, T-cell help acquisition, and participation in GC reactions [246,247]. B cells from SLE patients display a pronounced IFN-I–related transcriptional profile, and human B cells can express IFN-I genes in response to IFN-λ stimulation [248].
During this susceptibility phase, IFN-I directly promotes differentiation into CD138+ plasmablasts/plasma cells and antibody production in a BCR- and CD40-dependent manner [246]. In vivo, IFN-I fosters B-cell-to-plasmablast transitions, thereby amplifying inflammation and tissue injury [249,250]. In SLE, not only is the mature B-cell pool expanded, but antigen-presenting cells (APC)-like B cells are also enriched within the mature naïve compartment, increasing overall antigen-presenting capacity [251]. Elevated IFN-α augments sensitivity to IL-21 and endosomal TLR ligands, facilitating the emergence of autoreactive age-associated/atypical B cells (ABCs) [252]. IFN-α further enhances BCR signaling, activation, and differentiation, and can partially substitute for B-cell activating factor (BAFF) during the T1-to-T2 transition [252]. Moreover, IFN-I elevate serum BAFF, promoting B-cell proliferation and erosion of immune tolerance [253]. With respect to memory fates, IL-4R and IFN-I receptor signaling regulate the developmental trajectory from transitional B cells to DN1 and classical memory (cMEM) B cells [254].
Moreover, IFN-I expand antibody-forming cells (AFCs) and ICOShi ExFO- T helper cells, which are critical for the emergence of autoantibody responses [246]. In a subset of SLE patients, the plasmablast-to-memory (PB/M) ratio is markedly elevated and correlates with disease activity (SLEDAI). In this high PB/M group, CD21^low activated switched memory B cells are increased and pre-plasmablasts accumulate. Single-cell transcriptomics of PBs reveals strong expression of proliferation-associated genes and interferon-stimulated genes (e.g., IFI6, IFITM1), indicating activation of IFN receptor pathways centered on IRF7/ISRE and supporting interferon-dependent plasmablast differentiation [255]. Repertoire analyses further show IgG1 dominance with polyclonal expansion, and somatic hypermutation levels comparable to those of memory B cells; serologically, this associates with increased IgG, decreased IgM (a high IgG/IgM ratio), and persistent hypergammaglobulinemia [255]. Notably, anti-Sm/RNP positivity is strikingly frequent, and given their interferon inducibility, a positive feedback circuit linking IFN, plasmablast differentiation, and anti-Sm/RNP production is inferred [255]. Conversely, observations that the presence of anti-IFN-α autoantibodies associates with reduced IFN-I activity and improved clinical indices suggest that long-lived plasma cells sustain their production and that targeted suppression of the IFN axis may ameliorate clinical parameters [256].
This B-cell circuit is also intertwined with metabolic and stress-response pathways. IFN-α enhances mitochondrial function in human CD19+ B cells while promoting plasma cell–like differentiation and antibody production [257]. In SLE B cells, the DNA damage response (DDR) is excessively activated, with IFN-I inducing the ataxia telangiectasia mutated and Rad3 related Checkpoint kinase 1 (ATR–Chk1) pathway [258]. Because ATR inhibition suppresses B-cell activation, plasmacytoma formation, antibody production, and inflammatory responses, the metabolic/DDR node of the IFN-dependent circuit represents a viable therapeutic target [258].
Finally, pathogenic B cells contribute to disease through autoantibody production, inflammatory cytokine secretion, and self-antigen presentation [259]. The resulting autoantibodies re-stimulate pDCs as immune complexes (ICs); notably, IgE–ICs amplify IFN-I production by pDCs and exacerbate disease activity [260].
4.3.4. NK Cells, Neutrophils, Monocytes and Macrophages
The innate immune circuitry in SLE is driven by IFN-I and comprises a multilayered feedback system that amplifies these signals. Within the NK cell–DC axis, NK cells have been shown to cooperate with dendritic cells to induce IFN-α production [261].
Neutrophils are a principal source of endogenous antigenic triggers in SLE [262], exhibiting heightened sensitivity to IFN-I and a strong propensity for NET formation [263]. NETs potentiate autoantibody responses by promoting pDC activation and IFN-I secretion [264,265], while the transfer RNA-derived fragment tRF-His-GTG-1 augments IFN-α via TLR8–IRF7 activation [266]. At the clinical genomics level, transcriptomic markers associated with IFN-α/ω inhibition (e.g., JNJ-55920839) have been detected in whole blood, underscoring the pharmacologic plasticity of interferon-pathway targets [267]. Phenotypically, low-density granulocytes (LDGs) display elevated IFN-I production and inflammatory cytokine output and are linked to vascular dysfunction [268]. Conventional neutrophils likewise exhibit increased production of a proliferation-inducing ligand (APRIL), IL-21, and CXCL10 [269]. Collectively, neutrophils contribute to SLE through a triad of antigen provision, interferon amplification, and end-organ injury [270].
Macrophages (Mφ) exhibit M1/M2 plasticity, and in SLE, M2 polarization is diminished, with IRF4 shaping macrophage fate decisions [271,272]. In TLR7-stimulated bone marrow-derived Mφ, aconitate decarboxylase 1 (ACOD1) is induced via IFNAR signaling, promoting immunoregulation and vasoprotection [273]. Conversely, the MyD88-like adaptor protein Mal mediates TLR9-dependent IFN-β/TNF-α transcription through ERK1/2 activation in HSV-1-infected Mφ, engaging noncanonical NF-κB [274]. Systems-level transcriptomics has identified macrophage activation syndrome (MAS) clusters involving IFN-I [275,276]. Metabolically, in SLE patient peripheral blood mononuclear cells (PBMCs), spermine directly binds JAKs, suppressing JAK1 phosphorylation and coordinately restraining IFN-I/II and IL-2/IL-6 pathways [277]. In the kidney, LN macrophages transition dynamically from an inflammatory patrolling state to a phagocytic phenotype and subsequently to an antigen-presenting phenotype [278]. Conversely, plasma 7α,25-dihydroxycholesterol is elevated in SLE and binds Epstein–Barr virus-induced gene 2 (EBI2) to suppress IFN-I responses in Mφ [279]. Collectively, these findings position macrophages as regulatory nodes spanning both the accelerating and braking phases of interferon signaling.
Monocytes comprise chemically distinct “classical” Ly6Chi (CM) and “non-classical” Ly6Clo (NCM) subsets, with CMs identified as the principal source of IFN-I in pristane-induced lupus [280,281]. In SLE, NCMs exhibit defects in DNA repair, heightened cell-cycle and interferon signaling, and skewing toward an M1-like phenotype [282]. SLE monocytes also display a senescent program marked by increased cyclin-dependent kinase inhibitor 2A (CDKN2A), which induces GATA-binding protein 4 (GATA4) and augments IFN-α production via activation of the cGAS–STING pathway, thereby linking cellular aging to inflammation [283]. Clinically, IL-10 and IFN-γ drive CD64 upregulation on monocytes, which correlates with SLEDAI, BUN, and anti-Sm antibodies [284]. Collectively, these findings position monocytes as amplifiers of the IFN-I axis while simultaneously embodying pathological features associated with senescence, defective DNA repair, and altered metabolism.
5. Type I Interferon in Rheumatoid Arthritis
5.1. Interferon Signatures in Rheumatoid Arthritis: Biomarkers of Therapeutic Response and Subtype Bias
RA is a chronic autoimmune disorder characterized by pain, stiffness, and deformity driven by erosion of articular cartilage and bone [285]. In RA, IFN-I–related markers have emerged as candidate predictors of responsiveness to biologic agents. Pre-treatment interferon gene signature (IGS) positivity in peripheral blood correlates with favorable responses to B-cell depletion therapy with rituximab [286]. By contrast, elevated IFN-I signatures are associated with non-response to anti–TNF therapy [287]. Moreover, the IFN-β–to–IFN-α activity ratio (IFN-β: IFN-α) inversely correlates with responsiveness to anti–TNF therapy; in an independent cohort, a high IFN-β: IFN-α ratio strongly predicted nonresponse to TNF inhibitors [288,289]. Although the biological basis for circulating subtype ratios remains unresolved, cross-disease comparisons indicate IFN-α predominance in SLE and relative IFN-β predominance in RA, suggesting that IFN-β bias in RA may shape therapeutic responsiveness [174,289,290]. Functionally, while early clinical studies posited anti-inflammatory effects of IFN-β, trials of recombinant IFN-β were unsuccessful [291,292,293,294]. This apparent inconsistency with the observation of elevated circulating IFN-β in TNF inhibitor nonresponders likely reflects the substantial plasticity of IFN-I signaling, which is contingent on dose, duration, anatomical site of production, and the surrounding inflammatory milieu [77,289].
5.2. Determinants of IFN-I Activity in RA—IGS Dynamics, Susceptibility Loci, Epigenetic Remodeling, and Nucleic-Acid Triggers
Expression of ISG in the synovium was first documented nearly four decades ago, and multiple studies—including recent RNA-seq analyses—have reaffirmed robust IGS expression in synovial tissue [9,295,296,297] (Figure 4) (Box 1). IGS is detectable in peripheral blood and are present even in the preclinical phase [298,299]. Although circulating ISG levels are generally lower than in conditions such as SLE, several interferon-pathway genes are linked to RA risk and may aid in disease prediction [298,300,301,302]. Stage dependence is also evident: elevated IGS (e.g., MxA, OAS1, ISG15, IFI44L, IFI6) are more frequent in early RA and decline with treatment initiation, whereas in established RA the association between IGS and disease activity is inconsistent [303,304,305]. Ontology and network analyses further identify IGS as discriminating DMARD-naïve early arthritis patients who progress to persistent inflammatory arthritis from those who undergo spontaneous remission [306]. In preclinical cohorts, IGS correlates with anti-citrullinated protein antibodies (ACPA) positivity, which associates with imminent RA onset [307,308,309]. By contrast, in established RA, relationships between IGS/IFN-I signaling and autoantibodies (ACPA, rheumatoid factor [RF]) are conflicting, likely reflecting differences in ISG selection, biospecimen matrices, and disease stage [303,304,305,310,311]. Collectively, the interferon program appears phase-dependent: it exhibits strong predictive and stratification value from the preclinical through early stages, with attenuated disease association once RA is established.
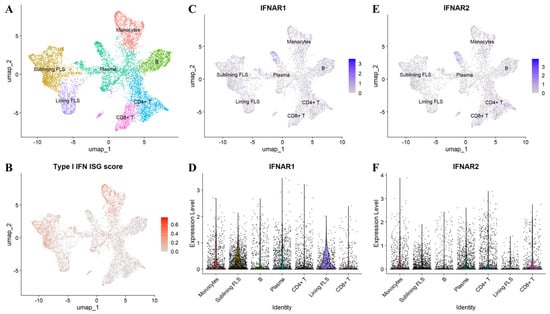
Figure 4.
Interferon-stimulated gene (ISG) signature and IFNAR1/2 expression in rheumatoid arthritis (RA) synovial tissue. Single-cell RNA sequencing data from the Accelerating Medicines Partnership (AMP) RA/SLE Network [297] were re-analyzed to profile ISG activity and IFNAR1/2 expression in inflamed synovium from patients with RA. (A) UMAP embedding with annotated major synovial cell populations. (B) UMAP feature plot of the aggregated ISG score across single cells. (C) UMAP feature plot of IFNAR1 transcript abundance. (D) Violin plot showing IFNAR1 expression by annotated cell type. (E) UMAP feature plot of IFNAR2 transcript abundance. (F) Violin plot showing IFNAR2 expression by annotated cell type.
GWAS have identified numerous SNPs associated with RA risk, many clustering in genes within the IFN-I response pathway—including DNA sensors, Toll-like receptors, and JAK–STAT mediators. Although the functional consequences of these variants are only partially understood, risk alleles in IRF5, STAT4, and PTPN22—linked to heightened IFN-I signaling in SLE—are likewise associated with RA [198]. Notably, IRF5 polymorphisms correlate with severe, erosive disease, consonant with observations that higher IGS scores in early RA predict refractory phenotypes [304,312].
Epigenetic remodeling (CpG methylation and chromatin reconfiguration) emerges early in RA and varies by cell subset [313]. In untreated early disease, methylation differences have been associated with initial methotrexate responsiveness and with responses to specific biologics in established RA, highlighting epigenetics as a modifier of clinical course and phenotype [313,314,315,316].
Although the drivers of elevated IGS/IFN-α in RA remain incompletely defined, triggers such as viral infections and microbial DNA/antigen fragments have been repeatedly detected in rheumatoid joints [317,318,319]. Noncoding DNA derived from ancient transposable elements (retroelements) may activate intracellular viral sensors and promote localized IFN-I production [320]. Increased retroelement expression has been reported in RA synovium, and in some patients, viral-like transcriptional profiles and IFN-I signaling correlate with high ACPA titers [188,321].
Cell-free nucleic acids (cfDNA) also merit attention. Mice with defects in DNA clearance develop chronic polyarthritis resembling human RA, and elevated cfDNA levels are reproducibly detected in both peripheral blood and synovial fluid in patients with RA [322,323,324].
5.3. Cellular and Tissue Drivers of Type I Interferon Signaling in Rheumatoid Arthritis
Neutrophil NETosis—abundant in the synovium—constitutes a potential trigger of IFN-I. NET-derived DNA forms complexes with LL37, secretory leukocyte protease inhibitor (SLPI), or immunoglobulins to activate pDC TLR7/9, thereby inducing IFN-α production [262,325,326,327]. In RA, associations between NETs and ACPA support a vicious cycle in which TNF-α, IL-17A, and even IFN-α itself promote NETosis [325,328].
Regarding lifestyle and environmental modifiers, physical activity inversely correlates with IFN-I signaling; exercise downregulates TLR and IL-17R pathways, reducing production of inflammatory cytokines, including IFN-I [329].
DCs enhance maturation and antigen-presenting capacity by upregulating human leukocyte antigen (HLA)-DR, CD40, CD80, and CD86 in response to IFN-I [330]. This increase in costimulatory molecules enables presentation of self-antigens to low-affinity autoreactive T cells and may contribute to autoimmunity in predisposed individuals [23]. In RA, pDCs are present within the synovium, and expression of ISGs, IFN-α, and IFN-β has been documented, although it remains uncertain whether pDCs constitute the principal source of IFN-α [331,332,333,334,335]. In established RA, synovial pDCs increase while peripheral pDCs decrease; the remaining, albeit immature, pDCs exhibit enhanced IFN-I-producing capacity [336]. Synovial pDCs can produce robust IFN-α, and persistent arthritis has been induced by intra-articular transfer of IFN-I-producing DCs [337,338]; conversely, in other models, pDC deficiency exacerbates arthritis, and disease activity improves with pDC mobilization/activation via TLR7 agonists [339,340,341]. Thus, pDC function appears dualistic, varying with local microenvironment, disease stage, and subset. Moreover, in untreated early RA, whole-blood IGS correlates negatively with CD14+ DC frequency—but not with circulating CD1c+ DCs or pDCs—highlighting the likelihood that IFN-I signaling drivers differ among DC subsets [340].
Synovial fibroblasts (FLS) are resident stromal cells of the joint that acquire an activated phenotype characterized by apoptosis resistance, heightened proliferation, and increased production of inflammatory mediators [342,343]. In early RA, IFN-α concentrations are comparable in serum and synovial fluid, and chromatin architectural alterations at the IFNAR2 locus correlate with poor prognosis [304,344]. Upregulation of ISGs in FLS is driven not only by direct IFN-I signaling but also by TNF-α–induced autocrine IFN-β production via the mTOR pathway, activating the IRF1–IFN-β–IFNAR–JAK–STAT1 axis; this may underlie the high IRG expression observed in pathogenic sublining FLS (THY1+HLA-DRhigh) [297]. Functionally, IFN-α correlates positively with TLR3/7 expression in the synovial lining and sublining and augments downstream IL-6 and TNF production. Moreover, IFN-α markedly enhances TLR4-dependent IL-1β/IL-18 production in synovial cells, whereas IFN-β exerts anti-inflammatory effects by suppressing IL-1β and TNF in PBMCs while dose-dependently increasing IL-1 receptor antagonist (IL-1Ra) [291]. IFN-β also elevates IL-1Ra secretion in FLS and amplifies IL-1β–induced IL-1Ra production in both FLS and chondrocytes [292]. Consistent with these preclinical data, IFN-β administration ameliorated arthritis in collagen-induced models [293,345]; however, in a multicenter, randomized, double-blind phase II trial, subcutaneous recombinant IFN-β failed to demonstrate efficacy in active RA [294].
Neutrophils play a pivotal role in RA pathogenesis and associate with heightened clinical disease activity and exacerbated inflammatory status [346]. Polymorphonuclear granulocytes (PMNs) exhibit higher IFNAR expression than PBMCs from either healthy controls or RA patients and constitute major contributors to the whole-blood IGS in RA [347]. Next-generation sequencing of circulating neutrophils from RA patients demonstrates significantly increased IRG expression relative to healthy controls [348]. Nonetheless, the peripheral interferon signature in RA is generally weaker than in SLE, necessitating attention to disease-specific differences in signal intensity [349].
Classical and nonclassical monocytes contribute to RA pathogenesis [350]. Although the in vivo impact of the IGS on monocyte function remains uncertain, in vitro exposure to IFN-I upregulates TLR7 and IRF expression in monocytes, heightening their responsiveness to subsequent immunostimulatory ligands [351]. In parallel, CD40, CD80, CD86, and HLA-DR are increased, promoting differentiation into highly antigen-presenting monocyte-derived dendritic cells (moDCs), which are expanded in RA synovium and can drive Th17 differentiation [330,352]. These effects are subset dependent; for instance, in murine models, inflammatory monocytes may secondarily augment IFN-α responsiveness via increased IFNAR expression, indicating that behavior varies with the in vivo context [219].
IFN-I broadly augments B-cell activity by inducing BAFF production from monocytes, directly stimulating B cells, and indirectly supporting B-cell survival via T-cell and DC activation [353]. Consequently, IFN-I promote long-term B-cell survival, facilitates differentiation into memory and plasma cells, drives isotype switching, and enhances autoantibody formation, while shifting the plasma-cell transcriptome toward a proinflammatory state [354,355]. Mechanistically, IFNAR-mediated modulation of BCR signaling can potentiate pathways underpinning antibody formation and germinal center responses, as shown in murine models [250]. In parallel, IFN-I skew CD4+ T cells toward Th1 differentiation—thereby amplifying B-cell activation—and enhances CD8+ T-cell survival and cytotoxicity, sustaining proliferation and expansion of antigen-specific CD8+ T cells by limiting apoptosis [356,357,358]. Within RA synovium, CD8+ T cells express IFN-γ more frequently than CD4+ T cells, indicating that they constitute the principal intratissue source of IFN-γ and a pathogenic subset capable of driving the local interferon signature [359].
6. Type I Interferon in Vasculitis
The contribution of IFN-I to vasculitis varies substantially across disease entities. In a cross-sectional cohort, interferon signatures did not reliably distinguish patients with giant cell arteritis (GCA) or polymyalgia rheumatica (PMR) from controls, and IFN-I–inducible serum markers were uninformative for initial diagnosis or monitoring [360]. By contrast, multiple tissue-level studies demonstrate heightened IFN-I activity in affected vessels: MxA—a prototypic IFN-I-induced protein—is detected in temporal artery biopsies, and aortic transcriptomes show robust induction of type I/II interferon programs [10,14,361]. Network analyses place IFN–JAK–STAT nodes—including STAT3, IRF7, STAT1, and IRF1—at the core, implicating interferon signaling as a primary pathway in arteritis [14]. Experimental models recapitulate increased type I/II interferon transcripts in human temporal artery transplant systems [361]. Clinically, the reduction in ISG expression following prednisolone therapy suggests that interferon responses are already active at disease onset [362]. In some series, pDCs are not detected on temporal artery biopsy (TAB), implicating non-pDC sources—such as macrophages—as producers of IFN-I [10,363].
In GCA, IL-6 and IFN-γ are major disease drivers, both signaling via JAK–STAT [364]; the incomplete suppression of inflammation by IFN-γ blockade alone suggests a contributory role for IFN-I [364]. Corroborating this, baricitinib has shown efficacy in relapsing GCA, and upadacitinib demonstrated efficacy and subsequently received FDA approval for GCA (April 2025) [20,365,366].
Reports implicating IFN-I in vasculitis other than GCA are limited. Although IFN-I responses are not considered primary drivers in human ANCA-associated vasculitis, a murine model shows that cGAS–STING activation worsens disease, with IFN-β produced by monocyte-derived macrophages acting as a decisive effector [367,368]. In Takayasu arteritis (TAK), current evidence likewise suggests that IFN-I are not a major pathogenic driver [369].
7. Therapeutic Implications and Stratification Strategies
Therapeutics targeting the IFN-I axis can be organized into three domains: inhibition of IFN-I production, neutralization of IFN-I itself, and blockade of downstream signaling (Table 2).
7.1. Upstream Strategies to Limit Type I Interferon Production: Receptor Blockade, pDC Targeting, and Sensor/Transcriptional Modulation
Strategies to curb IFN-I production proceed sequentially from receptor-level interception to depletion of producing cells and inhibition of intracellular sensors/transcriptional programs. Classically, hydroxychloroquine (HCQ) impedes endosomal acidification and TLR7/9 signaling, reducing IFN-α and TNF production by pDCs upon TLR7/9 stimulation in SLE [370]. Clinically, HCQ is recommended for all patients with SLE—where not contraindicated—to reduce flare frequency and severity and prevent disability, and it is widely employed as part of combination therapy in RA [371,372].
Direct pDC targeting includes the anti-BDCA2 antibody litifilimab, which suppresses pDCs and diminishes IFN production in cutaneous lupus lesions, and anti-CD123 antibodies that similarly attenuate IFN-I via pDC engagement [224,373]. Along the intracellular sensor axis, cordycepin may temper IFN-I excess by promoting autophagy-mediated degradation of STING after DNA stimulation [374]. The CXCR4 agonist clobenpropit robustly suppresses IRF7 phosphorylation, lowers IFN production, and reduces inflammatory cytokines in a lupus model [375]. At the transcriptional tier, HDAC10 inhibits IRF3 phosphorylation; in SLE, enhanced autophagic degradation of HDAC10 suggests a mechanistic link with the clinical use of autophagy inhibitors (chloroquine/HCQ) [376]. Upstream receptor blockade includes oral TLR7/8 inhibitors (enpatoran, afimetoran), now in phase II trials for SLE [377,378,379]. Modulation of circulating immune-cell trafficking with the selective S1P receptor modulator cenerimod reduces interferon-related biomarkers [380,381]. Additionally, methylprednisolone pulse therapy promotes Treg differentiation by inducing CD4+ T-cell apoptosis and enhancing monocyte TGF-β production, thereby fostering an immunoregulatory milieu that suppresses CD4+ proliferation and IFN-γ production [382].
Collectively, a multilayered program—spanning the TLR–pDC–STING–IRF continuum and incorporating HCQ, pDC-directed antibodies, TLR7/8 antagonists, STING degradation, inhibition of IRF phosphorylation, and S1P-pathway modulation—is being implemented. Precise, upstream inhibition tailored to disease- and tissue-specific IFN drivers will be pivotal going forward.
7.2. Neutralizing IFN-α and Blocking IFNAR
Neutralization of IFN-α with monoclonal antibodies has shown mixed results: sifalimumab improved disease activity and reduced the interferon signature in SLE, whereas rontalizumab did not meet its primary endpoint but suggested efficacy in patients with low pretreatment interferon signatures [383,384,385]. In addition, IFN-α quinones have been reported to elicit polyclonal anti–IFN-α humoral responses with potential clinical benefit [386]. In idiopathic inflammatory myopathies, sifalimumab similarly reduced the circulating interferon signature [387].
Receptor-level blockade offers broader coverage: anifrolumab (anti-IFNAR1) inhibits signaling downstream of both IFN-α and IFN-β, suppresses ISGs, and confers multidomain clinical improvement in SLE, leading to regulatory approval [19,169,266,388]. In RA, a small pilot study enriched for high-IGS cases showed a preliminary efficacy signal, warranting further validation [389,390]. QX006N, which similarly targets the SD3 domain of IFNAR1 to prevent receptor complex formation, is also in clinical development [391]. Across trials, elevated pretreatment interferon signatures have repeatedly emerged as candidate predictors of therapeutic responsiveness, underscoring the value of biomarker-based stratification across diseases [383,385,392].
7.3. Downstream Attenuation of IFN-I Signaling
Downstream of IFNAR, the JAK–STAT pathway constitutes the central signaling node, with TYK2 integrating both IFN-I and IL-12/23 pathways [393]. In SLE, IL-12 stimulation coactivates STAT1 and STAT4, expanding Tfh–Th1–like populations and underscoring the therapeutic appeal of this axis [394]. JAK/TYK2 inhibition attenuates interferon signaling: the JAK1/2-selective inhibitor baricitinib improves cutaneous and articular manifestations, and the JAK1-selective filgotinib likewise ameliorates skin disease [395,396,397]. Upadacitinib has progressed to phase III evaluation, with ongoing confirmation of efficacy [398,399]. TYK2 inhibitors may suppress the interferon signature and the IL-12 axis while preserving IL-2-dependent Treg differentiation [400]; the highly selective inhibitor deucravacitinib potently abrogated IFN-α-induced lymphopenia [401]. The JAK2 inhibitor ruxolitinib also reduces ISG expression and JAK–STAT activity [402]. Mechanistically, JAK blockade limits STAT phosphorylation, thereby diminishing IRG expression, and inhibits IFN-I-dependent plasmacytoid differentiation, synovial BAFF production, CD80/CD86 upregulation on moDCs, and Th1/Th17 differentiation [198,403,404,405]. Notably, secondary analyses of phase II SLE trials with baricitinib indicate that clinical efficacy is independent of reductions in the interferon gene signature [406].
IFN-I induce the B-cell activating factor BAFF; accordingly, belimumab, which disrupts this downstream axis, has established efficacy in SLE and, when added for active lupus nephritis, increases remission rates and reduces relapse [407]. Moreover, pretreatment interferon signature scores have been reported to predict 12-month SRI responses to belimumab [408]. Preclinical data further suggest that belimumab mitigates lupus-like pathology by modulating the V-domain immunoglobulin suppressor of T-cell activation (VISTA) pathway and thereby regulating IFN-I/noncanonical NF-κB signaling [409]. In addition, small molecules targeting the upstream TBK1/IKKε node (selective JAK3/JAK1/TBK1 inhibitors) suppress IFN-I production and osteoclastogenesis in mice, with efficacy demonstrated in autoimmune arthritis models [410,411,412,413].
From a safety standpoint, JAK inhibitors—which attenuate the JAK–STAT pathway integral to Th1 maturation—are associated with increased risk of intracellular infections such as herpes zoster and tuberculosis, both theoretically and in clinical practice [414,415,416]. Pre-initiation vaccination is recommended [414]. More broadly, a two-tiered therapeutic paradigm—comprising IFN receptor blockade and attenuation of downstream signaling—may facilitate more refined stratification by disease subtype, biomarker profile, and infection risk.sectio.

Table 2.
IFN-I Axis Therapies.
Table 2.
IFN-I Axis Therapies.
| Class | Agent | Target | Outcome | Safety | Refs. |
|---|---|---|---|---|---|
| Upstream modulators | Hydroxychloroquine (HCQ) | Endosomal pH increase; TLR7/9 inhibition in pDCs; autophagy/HDAC10 linkage in SLE | First-line in SLE; flares/severity reduced; used in RA combinations | Ocular toxicity | [370,371,372,376,417] |
| Litifilimab; anti-CD123 | pDC targeting; IFN production reduced | IFN signature reduced in cutaneous lupus lesions | Infection risk | [224,373,418] | |
| Clobenpropit | CXCR4 agonism; IRF7 phosphorylation reduced; IFN production lowered | IFN and inflammatory cytokines reduced in lupus model | Preclinical | [375] | |
| Enpatoran; Afimetoran | Selective oral TLR7/8 inhibitors | Rapid suppression of IFN-I gene signature with early clinical signals in CLE/SLE | Phase 2 programs ongoing; safety profile still being defined | [377,378,379] | |
| Cenerimod | Selective S1P receptor modulator | IFN-associated proteins and IFN-1/IFN-γ/plasma-cell signatures reduced; larger effect at 4 mg | Dose-related lymphopenia | [380,381] | |
| IFN-I–directed agents | Sifalimumab; Rontalizumab | Anti–IFN-α mAbs | Sifalimumab: disease activity and IFN signature reduced; Rontalizumab: primary endpoint not met overall (signal in low IFN-signature subgroup) | Infections | [383,384,385] |
| Anifrolumab | IFNAR1 blockade; ISGs reduced | Multidomain clinical improvement; regulatory approval in SLE | Herpes zoster and other infections | [19,169,266,388,389,390] | |
| QX006N | IFNAR1 SD3 binding; receptor complex formation prevented | In clinical development | — | [391] | |
| Downstream modulators | Baricitinib | JAK1/2 inhibition | Improves cutaneous/articular disease; efficacy not strictly tied to IFN-signature reduction | Infections | [395,396] |
| Filgotinib; Upadacitinib | JAK1 inhibition | Filgotinib: skin disease improvement; Upadacitinib: phase III ongoing | Infections | [397,398,399] | |
| Ruxolitinib | JAK2 inhibition | ISG expression and JAK–STAT activity reduced; signal in IFN-driven states | Infections | [402] | |
| Deucravacitinib | Selective allosteric TYK2 inhibitor | Higher SRI-4 and secondary responses vs. placebo | Infections | [401] | |
| Belimumab | BAFF neutralization | Established efficacy in SLE; added to active LN increases remission and reduces relapse | infections | [407,408,409] |
IFN, interferon; IFN-I, type I interferon; IFN-γ, interferon-gamma (type II interferon); IFNAR1, interferon-α/β receptor 1; ISG(s), interferon-stimulated gene(s); JAK, Janus kinase; JAK–STAT, Janus kinase–signal transducer and activator of transcription pathway; TYK2, tyrosine kinase 2; pDC(s), plasmacytoid dendritic cell(s); TLR7/9, Toll-like receptor 7/9; CXCR4, C-X-C chemokine receptor 4; IRF7, interferon regulatory factor 7; S1P/S1P1, sphingosine-1-phosphate/S1P receptor 1; BAFF, B-cell activating factor; HCQ, hydroxychloroquine; HDAC10, histone deacetylase 10; mAb, monoclonal antibody; SLE, systemic lupus erythematosus; CLE, cutaneous lupus erythematosus; RA, rheumatoid arthritis; LN, lupus nephritis.
8. Conclusions
IFN-I occupy a pivotal position at the interface of antiviral defense and breakdown of self-tolerance, with effects that appear highly context dependent across diseases, tissues, and disease stages. Convergent nucleic-acid sensing and IFNAR–JAK–STAT signaling, modulated by post-transcriptional control, epigenetic modifications, and non-coding RNAs, yield interferon-stimulated gene signatures that are typically more pronounced in systemic lupus erythematosus and more heterogeneous across rheumatoid arthritis and vasculitis. Therapeutic strategies that target the IFN-I axis may mitigate aberrant inflammation; however, broader implementation is constrained by incomplete mechanistic delineation, limited high-quality preclinical and clinical evidence, and the challenge of selectively modulating inflammation without compromising host defense. Further work is warranted to map IFN-I regulatory networks across disease trajectories, refine and validate biomarker panels for patient stratification and monitoring, and design trial frameworks aligned to mechanistic endotypes.
Author Contributions
R.I., writing—original draft preparation; R.W., writing—review and editing; M.S., creating figures; Y.F., M.K., K.F. and S.Y. provided general support for the study; M.H., supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This manuscript received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The AMP RA/SLE Network data used for this publication are available at https://arkportal.synapse.org/Explore/Programs/DetailsPage?Program=AMP-RA/SLE (accessed on 30 September 2025).
Conflicts of Interest
R.W. received a research grant from AbbVie and speaker’s fee from AbbVie, Asahi Kasei, AstraZeneca, Chugai, Eli Lilly, GSK, and Taisho. MH received research grants and/or speaker fee from Asahi Kasei, Astellas, AstraZeneca, Ayumi Pharma, Brystol Meyers, Chugai, Eisai, Eli Lilly, Gilead Sciences Japan, Janssen pharma, Ono Pharma, Taisho Pharma, Tanabe Mitsubishi, UCB Japan. R.I., M.S., Y.F., M.K., K.F., S.Y declare no conflicts of interest.
Glossary
| ABC | age-associated/atypical B cell |
| ACOD1 | aconitate decarboxylase 1 |
| APC | antigen-presenting cell |
| ATP | adenosine triphosphate |
| BAFF | B-cell activating factor |
| BCR | B cell receptor |
| BRD | bromodomain-containing protein |
| CARDs | caspase activation and recruitment domains |
| CBP | CREB-binding protein |
| CCL | C-C motif chemokine ligand |
| cDNs | cyclic dinucleotides |
| cfDNA | cell-free DNA |
| cGAMP | cyclic GMP–AMP |
| cGAS | cyclic GMP–AMP synthase |
| CLE | cutaneous lupus erythematosus |
| CNS | central nervous system |
| CpG | cytosine–phosphate–guanine |
| CXCL | C-X-C motif chemokine ligand |
| CXCR | C-X-C motif chemokine receptor |
| DAI | DNA-dependent activators of interferon regulatory factor |
| DAMPs | damage-associated molecular patterns |
| DC | dendritic cells |
| DNA | deoxyribonucleic acid |
| dsDNA | double-stranded DNA |
| ER | endoplasmic reticulum |
| ERGIC | ER–Golgi intermediate compartment |
| GAS | gamma-activated sequence |
| GC | germinal center |
| GCA | giant cell arteritis |
| GCN5 | general control nonderepressible 5 |
| GEF | guanine-nucleotide-exchange factors |
| GTP | guanosine triphosphate |
| HAT | histone acetyltransferase |
| HCQ | hydroxychloroquine |
| HDAC | histone deacetylase |
| iE-DAP | γ-D-glutamyl–meso-diaminopimelic acid |
| IFIT | interferon-induced proteins with tetratricopeptide repeat |
| IFN-I | type I interferon |
| IFNAR | interferon-α/β receptor |
| IGS | interferon gene signature |
| IKK | inhibitor of κB kinase |
| IKKε- | IκB kinase epsilon |
| IL | interleukin |
| IRAK | IL-1 receptor associated kinase |
| IRS | insulin receptor substrate |
| IRF | IFN regulatory factor |
| ISG | interferon-stimulated gene |
| ISRE | interferon-stimulated response element |
| JAK | Janus kinase |
| JNK | c-Jun N-terminal kinase |
| LCMV | lymphocytic choriomeningitis virus |
| LDG | low-density granulocyte |
| LGP2 | laboratory of genetics and physiology 2 |
| LN | lupus nephritis |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MAPKAPK | MAPK-activated protein kinase |
| MAVS | mitochondrial antiviral signaling protein |
| MDA5 | melanoma differentiation–associated gene 5 |
| MDP | muramyl dipeptide |
| MNK1 | MAPK-interacting protein kinase 1 |
| MSK1 | mitogen- and stress-activated kinase 1 |
| MHC | major histocompatibility complex |
| mTOR | mechanistic target of rapamycin |
| MyD88 | myeloid differentiation primary response 88 |
| MX1 | myxovirus resistance 1 |
| NEMO | NF-κB essential modulator |
| NETs | neutrophil extracellular traps |
| NK | natural killer |
| NKLAM | natural killer lytic-associated molecule |
| NLRs | NOD–like receptors |
| NOD | nucleotide-binding oligomerization domain |
| OAS1 | 2′–5′-oligoadenylate synthetase 1 |
| pDC | plasmacytoid dendritic cell |
| PAMPs | pathogen-associated molecular patterns |
| PDK | 3-phosphoinositide–dependent protein kinase |
| PIAS1 | protein inhibitor of activated STAT1 |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PIP3 | phosphatidylinositol 3,4,5-trisphosphate |
| PI3K | including phosphoinositide 3-kinase |
| PLZF | promyelocytic leukemia zinc finger |
| PRMT1 | protein arginine methyltransferase 1 |
| PRRs | pattern-recognition receptors |
| P-TEFb | positive transcription elongation factor b |
| PTPN | protein tyrosine phosphatase non-receptor type |
| RA | rheumatoid arthritis |
| RANK | receptor activator of nuclear factor κB |
| RANKL | receptor activator of NF-κB ligand |
| RBP | RNA-binding protein |
| RICK | receptor-interacting serine/threonine kinase |
| RIG-I | retinoic acid–inducible gene I |
| RLRs | RIG-I–like receptors |
| RNA | ribonucleic acid |
| RSAD2 | Radical S-adenosyl methionine domain-containing protein 2 |
| S1P | sphingosine-1-phosphate |
| S1P1 | S1P receptor 1 |
| SHP | Src homology region 2 domain-containing phosphatase |
| SLE | systemic lupus erythematosus |
| SLIM | STAT-interacting LIM protein |
| SMURF | SMAD-specific E3 ubiquitin protein ligase |
| SNP | single-nucleotide polymorphism |
| SOCS | suppressor of cytokine signaling |
| STAT | signal transducer and activator of transcription |
| TAK | Takayasu arteritis |
| TBK1 | TANK-binding kinase 1 |
| Tfh | follicular helper T-cell |
| TGF-β | transforming growth factor-β |
| TIR | Toll/interleukin-1 receptor |
| TLR | Toll-like receptor |
| TNF | tumor necrosis factor |
| Tph | T peripheral helper |
| TRAF | TNF receptor–associated factor |
| TRAM | TRIF-related adaptor molecule |
| Treg | regulatory T-cell |
| TRIF | TIR domain-containing adaptor protein-inducing IFN-β |
| TSLP | thymic stromal lymphopoietin |
| TYK2 | tyrosine kinase 2 |
| USP18 | ubiquitin-specific protease 18 |
References
- Piperakis, A.; Galani, I.E.; Andreakos, E. Type III interferons in innate and adaptive immunity in the respiratory tract. Curr. Opin. Immunol. 2024, 87, 102430. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef]
- Isaacs, A.; Lindenmann, J. Virus interference. I. The interferon. Proc. R. Soc. Lond. B. Biol. Sci. 1957, 147, 258–267. [Google Scholar] [CrossRef]
- Gresser, I.; Tovey, M.G.; Maury, C.; Chouroulinkov, I. Lethality of interferon preparations for newborn mice. Nature 1975, 258, 76–78. [Google Scholar] [CrossRef]
- Skurkovich, S.V.; Klinova, E.G.; Eremkina, E.I.; Levina, N.V. Immunosuppressive effect of an anti-interferon serum. Nature 1974, 247, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Skurkovich, S.; Skurkovich, B.; Bellanti, J.A. A unifying model of the immunoregulatory role of the interferon system: Can interferon produce disease in humans? Clin. Immunol. Immunopathol. 1987, 43, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Skurkovich, S.; Skurkovich, B. Anticytokine therapy, especially anti-interferon-gamma, as a pathogenetic treatment in TH-1 autoimmune diseases. Ann. N. Y. Acad. Sci. 2005, 1051, 684–700. [Google Scholar] [CrossRef]
- Steinberg, A.D.; Baron, S.; Talal, N. The pathogenesis of autoimmunity in New Zealand mice, I. Induction of antinucleic acid antibodies by polyinosinic-polycytidylic acid. Proc. Natl. Acad. Sci. USA 1969, 63, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Jahn, B.; Rohwer, P.; Zacher, J.; Winchester, R.J.; Kalden, J.R. Differential expression of Ia antigens by rheumatoid synovial lining cells. J. Clin. Investig. 1987, 80, 595–604. [Google Scholar] [CrossRef]
- van Nieuwland, M.; Esen, I.; Reitsema, R.D.; Abdulahad, W.H.; van Sleen, Y.; Jiemy, W.F.; Sandovici, M.; Brouwer, E.; van Bon, L. Evidence for increased interferon type I activity in CD8+ T cells in giant cell arteritis patients. Front. Immunol. 2023, 14, 1197293. [Google Scholar] [CrossRef]
- Rönnblom, L.E.; Alm, G.V.; Oberg, K.E. Possible induction of systemic lupus erythematosus by interferon-alpha treatment in a patient with a malignant carcinoid tumour. J. Intern. Med. 1990, 227, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Postal, M.; Vivaldo, J.F.; Fernandez-Ruiz, R.; Paredes, J.L.; Appenzeller, S.; Niewold, T.B. Type I interferon in the pathogenesis of systemic lupus erythematosus. Curr. Opin. Immunol. 2020, 67, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; Ballina-García, F.J.; Suárez, A. Heterogeneity of the Type I Interferon Signature in Rheumatoid Arthritis: A Potential Limitation for Its Use As a Clinical Biomarker. Front. Immunol. 2017, 8, 2007. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.; Régnier, P.; Maciejewski-Duval, A.; Le Joncour, A.; Darasse-Jèze, G.; Rosenzwajg, M.; Klatzmann, D.; Cacoub, P.; Saadoun, D. Interferon signature in giant cell arteritis aortitis. J. Autoimmun. 2022, 127, 102796. [Google Scholar] [CrossRef]
- Brilland, B.; Despré, M.; Khatri, R.; Quéméneur, T.; Vandenbussche, C.; Merillon, N.; Boizard-Moracchini, A.; Roy, M.; Preisser, L.; Riou, J.; et al. A stronger type I interferon signature distinguishes ANCA-associated vasculitis phenotypes and predicts kidney prognosis. Kidney Int. 2025, in press. [CrossRef]
- Nezos, A.; Gravani, F.; Tassidou, A.; Kapsogeorgou, E.K.; Voulgarelis, M.; Koutsilieris, M.; Crow, M.K.; Mavragani, C.P. Type I and II interferon signatures in Sjogren’s syndrome pathogenesis: Contributions in distinct clinical phenotypes and Sjogren’s related lymphomagenesis. J. Autoimmun. 2015, 63, 47–58. [Google Scholar] [CrossRef]
- Hinchcliff, M.; Khanna, D.; De Lorenzis, E.; Di Donato, S.; Carriero, A.; Ross, R.L.; Huang, S.; Aren, K.A.; Bernstein, E.J.; Carns, M.; et al. Serum type I interferon score as a disease activity biomarker in patients with diffuse cutaneous systemic sclerosis: A retrospective cohort study. Lancet Rheumatol. 2025, 7, e403–e414. [Google Scholar] [CrossRef]
- Qian, J.; Li, R.; Chen, Z.; Cao, Z.; Lu, L.; Fu, Q. Type I interferon score is associated with the severity and poor prognosis in anti-MDA5 antibody-positive dermatomyositis patients. Front. Immunol. 2023, 14, 1151695. [Google Scholar] [CrossRef]
- Kalunian, K.C.; Furie, R.; Morand, E.F.; Bruce, I.N.; Manzi, S.; Tanaka, Y.; Winthrop, K.; Hupka, I.; Zhang, L.J.; Werther, S.; et al. A Randomized, Placebo-Controlled Phase III Extension Trial of the Long-Term Safety and Tolerability of Anifrolumab in Active Systemic Lupus Erythematosus. Arthritis Rheumatol. 2023, 75, 253–265. [Google Scholar] [CrossRef]
- Blockmans, D.; Penn, S.K.; Setty, A.R.; Schmidt, W.A.; Rubbert-Roth, A.; Hauge, E.M.; Keen, H.I.; Ishii, T.; Khalidi, N.; Dejaco, C.; et al. A Phase 3 Trial of Upadacitinib for Giant-Cell Arteritis. N. Engl. J. Med. 2025, 392, 2013–2024. [Google Scholar] [CrossRef]
- Bonelli, M.; Kerschbaumer, A.; Kastrati, K.; Ghoreschi, K.; Gadina, M.; Heinz, L.X.; Smolen, J.S.; Aletaha, D.; O’Shea, J.; Laurence, A. Selectivity, efficacy and safety of JAKinibs: New evidence for a still evolving story. Ann. Rheum. Dis. 2024, 83, 139–160. [Google Scholar] [CrossRef] [PubMed]
- Kalliolias, G.D.; Ivashkiv, L.B. Overview of the biology of type I interferons. Arthritis Res. Ther. 2010, 12 (Suppl. S1), S1. [Google Scholar] [CrossRef]
- González-Navajas, J.M.; Lee, J.; David, M.; Raz, E. Immunomodulatory functions of type I interferons. Nat. Rev. Immunol. 2012, 12, 125–135. [Google Scholar] [CrossRef]
- Swiecki, M.; Colonna, M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015, 15, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Reizis, B. Plasmacytoid Dendritic Cells: Development, Regulation, and Function. Immunity 2019, 50, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Swiecki, M.; Colonna, M. Type I interferons: Diversity of sources, production pathways and effects on immune responses. Curr. Opin. Virol. 2011, 1, 463–475. [Google Scholar] [CrossRef]
- Perez-Shibayama, C.; Islander, U.; Lütge, M.; Cheng, H.W.; Onder, L.; Ring, S.S.; De Martin, A.; Novkovic, M.; Colston, J.; Gil-Cruz, C.; et al. Type I interferon signaling in fibroblastic reticular cells prevents exhaustive activation of antiviral CD8(+) T cells. Sci. Immunol. 2020, 5, eabb7066. [Google Scholar] [CrossRef]
- Ivashkiv, L.B. IFNγ: Signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 545–558. [Google Scholar] [CrossRef]
- Donnelly, C.R.; Chen, O.; Ji, R.R. How Do Sensory Neurons Sense Danger Signals? Trends Neurosci. 2020, 43, 822–838. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’Garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Marchetti, M.; Monier, M.N.; Fradagrada, A.; Mitchell, K.; Baychelier, F.; Eid, P.; Johannes, L.; Lamaze, C. Stat-mediated signaling induced by type I and type II interferons (IFNs) is differentially controlled through lipid microdomain association and clathrin-dependent endocytosis of IFN receptors. Mol. Biol. Cell. 2006, 17, 2896–2909. [Google Scholar] [CrossRef] [PubMed]
- Arimoto, K.I.; Miyauchi, S.; Stoner, S.A.; Fan, J.B.; Zhang, D.E. Negative regulation of type I IFN signaling. J. Leukoc. Biol. 2018, 103, 1099–1116. [Google Scholar] [CrossRef]
- Meyer, O. Interferons and autoimmune disorders. Jt. Bone Spine 2009, 76, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.; Schneider, W.M.; Hoffmann, H.H.; Yarden, G.; Busetto, A.G.; Manor, O.; Sharma, N.; Rice, C.M.; Schreiber, G. Multifaceted activities of type I interferon are revealed by a receptor antagonist. Sci. Signal. 2014, 7, ra50. [Google Scholar] [CrossRef]
- Henig, N.; Avidan, N.; Mandel, I.; Staun-Ram, E.; Ginzburg, E.; Paperna, T.; Pinter, R.Y.; Miller, A. Interferon-beta induces distinct gene expression response patterns in human monocytes versus T cells. PLoS ONE 2013, 8, e62366. [Google Scholar] [CrossRef]
- Ranf, S. Sensing of molecular patterns through cell surface immune receptors. Curr. Opin. Plant Biol. 2017, 38, 68–77. [Google Scholar] [CrossRef]
- Stros, M. HMGB proteins: Interactions with DNA and chromatin. Biochim. Biophys. Acta 2010, 1799, 101–113. [Google Scholar] [CrossRef]
- Hemmi, H.; Takeuchi, O.; Kawai, T.; Kaisho, T.; Sato, S.; Sanjo, H.; Matsumoto, M.; Hoshino, K.; Wagner, H.; Takeda, K.; et al. A Toll-like receptor recognizes bacterial DNA. Nature 2000, 408, 740–745. [Google Scholar] [CrossRef]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef]
- Kang, D.C.; Gopalkrishnan, R.V.; Wu, Q.; Jankowsky, E.; Pyle, A.M.; Fisher, P.B. mda-5: An interferon-inducible putative RNA helicase with double-stranded RNA-dependent ATPase activity and melanoma growth-suppressive properties. Proc. Natl. Acad. Sci. USA 2002, 99, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Barrat, F.J.; Elkon, K.B.; Fitzgerald, K.A. Importance of Nucleic Acid Recognition in Inflammation and Autoimmunity. Annu. Rev. Med. 2016, 67, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.G.; Lee, H.; Lee, J.O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef]
- Kang, J.Y.; Nan, X.; Jin, M.S.; Youn, S.J.; Ryu, Y.H.; Mah, S.; Han, S.H.; Lee, H.; Paik, S.G.; Lee, J.O. Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 2009, 31, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Yarilina, A.; Park-Min, K.H.; Antoniv, T.; Hu, X.; Ivashkiv, L.B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat. Immunol. 2008, 9, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Kim, S.; Matsuo, K.; Suzuki, H.; Suzuki, T.; Sato, K.; Yokochi, T.; Oda, H.; Nakamura, K.; Ida, N.; et al. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature 2002, 416, 744–749. [Google Scholar] [CrossRef]
- Sakaniwa, K.; Fujimura, A.; Shibata, T.; Shigematsu, H.; Ekimoto, T.; Yamamoto, M.; Ikeguchi, M.; Miyake, K.; Ohto, U.; Shimizu, T. TLR3 forms a laterally aligned multimeric complex along double-stranded RNA for efficient signal transduction. Nat. Commun. 2023, 14, 164. [Google Scholar] [CrossRef]
- Schoenemeyer, A.; Barnes, B.J.; Mancl, M.E.; Latz, E.; Goutagny, N.; Pitha, P.M.; Fitzgerald, K.A.; Golenbock, D.T. The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J. Biol. Chem. 2005, 280, 17005–17012. [Google Scholar] [CrossRef]
- Moynagh, P.N. TLR signalling and activation of IRFs: Revisiting old friends from the NF-kappaB pathway. Trends Immunol. 2005, 26, 469–476. [Google Scholar] [CrossRef]
- Lin, S.C.; Lo, Y.C.; Wu, H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature 2010, 465, 885–890. [Google Scholar] [CrossRef]
- Motshwene, P.G.; Moncrieffe, M.C.; Grossmann, J.G.; Kao, C.; Ayaluru, M.; Sandercock, A.M.; Robinson, C.V.; Latz, E.; Gay, N.J. An oligomeric signaling platform formed by the Toll-like receptor signal transducers MyD88 and IRAK-4. J. Biol. Chem. 2009, 284, 25404–25411. [Google Scholar] [CrossRef] [PubMed]
- Bonham, K.S.; Orzalli, M.H.; Hayashi, K.; Wolf, A.I.; Glanemann, C.; Weninger, W.; Iwasaki, A.; Knipe, D.M.; Kagan, J.C. A promiscuous lipid-binding protein diversifies the subcellular sites of toll-like receptor signal transduction. Cell 2014, 156, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Wang, C.; Spencer, E.; Yang, L.; Braun, A.; You, J.; Slaughter, C.; Pickart, C.; Chen, Z.J. Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 2000, 103, 351–361. [Google Scholar] [CrossRef]
- Iguchi, Y.; Takahashi, Y.; Li, J.; Araki, K.; Amakusa, Y.; Kawakami, Y.; Kobayashi, K.; Yokoi, S.; Katsuno, M. IκB kinase phosphorylates cytoplasmic TDP-43 and promotes its proteasome degradation. J. Cell Biol. 2024, 223, e202302048. [Google Scholar] [CrossRef]
- McWhirter, S.M.; Fitzgerald, K.A.; Rosains, J.; Rowe, D.C.; Golenbock, D.T.; Maniatis, T. IFN-regulatory factor 3-dependent gene expression is defective in Tbk1-deficient mouse embryonic fibroblasts. Proc. Natl. Acad. Sci. USA 2004, 101, 233–238. [Google Scholar] [CrossRef]
- Sharma, S.; tenOever, B.R.; Grandvaux, N.; Zhou, G.P.; Lin, R.; Hiscott, J. Triggering the interferon antiviral response through an IKK-related pathway. Science 2003, 300, 1148–1151. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Yoneyama, M.; Fujita, T. RNA recognition and signal transduction by RIG-I-like receptors. Immunol. Rev. 2009, 227, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Goubau, D.; Deddouche, S.; Reis e Sousa, C. Cytosolic sensing of viruses. Immunity 2013, 38, 855–869. [Google Scholar] [CrossRef]
- Lim, Y.J.; Koo, J.E.; Hong, E.H.; Park, Z.Y.; Lim, K.M.; Bae, O.N.; Lee, J.Y. A Src-family-tyrosine kinase, Lyn, is required for efficient IFN-β expression in pattern recognition receptor, RIG-I, signal pathway by interacting with IPS-1. Cytokine 2015, 72, 63–70. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Kawai, T.; Takahashi, K.; Sato, S.; Coban, C.; Kumar, H.; Kato, H.; Ishii, K.J.; Takeuchi, O.; Akira, S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005, 6, 981–988. [Google Scholar] [CrossRef]
- Ishikawa, H.; Barber, G.N. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 2008, 455, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Paludan, S.R.; Bowie, A.G. Immune sensing of DNA. Immunity 2013, 38, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Oshiumi, H.; Sakai, K.; Matsumoto, M.; Seya, T. DEAD/H BOX 3 (DDX3) helicase binds the RIG-I adaptor IPS-1 to up-regulate IFN-beta-inducing potential. Eur. J. Immunol. 2010, 40, 940–948. [Google Scholar] [CrossRef]
- Chamaillard, M.; Hashimoto, M.; Horie, Y.; Masumoto, J.; Qiu, S.; Saab, L.; Ogura, Y.; Kawasaki, A.; Fukase, K.; Kusumoto, S.; et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat. Immunol. 2003, 4, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Girardin, S.E.; Boneca, I.G.; Viala, J.; Chamaillard, M.; Labigne, A.; Thomas, G.; Philpott, D.J.; Sansonetti, P.J. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 2003, 278, 8869–8872. [Google Scholar] [CrossRef]
- Sabbah, A.; Chang, T.H.; Harnack, R.; Frohlich, V.; Tominaga, K.; Dube, P.H.; Xiang, Y.; Bose, S. Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 2009, 10, 1073–1080. [Google Scholar] [CrossRef]
- Moreira, L.O.; Zamboni, D.S. NOD1 and NOD2 Signaling in Infection and Inflammation. Front. Immunol. 2012, 3, 328. [Google Scholar] [CrossRef]
- Strober, W.; Murray, P.J.; Kitani, A.; Watanabe, T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat. Rev. Immunol. 2006, 6, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kimbrel, E.A.; Kenan, D.J.; McDonnell, D.P. Direct interactions between corepressors and coactivators permit the integration of nuclear receptor-mediated repression and activation. Mol. Endocrinol. 2002, 16, 1482–1491. [Google Scholar] [CrossRef]
- Leung, T.H.; Hoffmann, A.; Baltimore, D. One nucleotide in a kappaB site can determine cofactor specificity for NF-kappaB dimers. Cell 2004, 118, 453–464. [Google Scholar] [CrossRef]
- Gallucci, S.; Meka, S.; Gamero, A.M. Abnormalities of the type I interferon signaling pathway in lupus autoimmunity. Cytokine 2021, 146, 155633. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Cassol, E. Role of cellular metabolism in regulating type I interferon responses: Implications for tumour immunology and treatment. Cancer Lett. 2017, 409, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef]
- Platanias, L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat. Rev. Immunol. 2005, 5, 375–386. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Cao, X. Regulation of type I interferon signaling in immunity and inflammation: A comprehensive review. J. Autoimmun. 2017, 83, 1–11. [Google Scholar] [CrossRef]
- Tsai, M.H.; Pai, L.M.; Lee, C.K. Fine-Tuning of Type I Interferon Response by STAT3. Front. Immunol. 2019, 10, 1448. [Google Scholar] [CrossRef]
- Cheon, H.; Stark, G.R. Unphosphorylated STAT1 prolongs the expression of interferon-induced immune regulatory genes. Proc. Natl. Acad. Sci. USA 2009, 106, 9373–9378. [Google Scholar] [CrossRef]
- Fritsch, R.; de Krijger, I.; Fritsch, K.; George, R.; Reason, B.; Kumar, M.S.; Diefenbacher, M.; Stamp, G.; Downward, J. RAS and RHO families of GTPases directly regulate distinct phosphoinositide 3-kinase isoforms. Cell 2013, 153, 1050–1063. [Google Scholar] [CrossRef]
- Tian, L.Y.; Smit, D.J.; Jücker, M. The Role of PI3K/AKT/mTOR Signaling in Hepatocellular Carcinoma Metabolism. Int. J. Mol. Sci. 2023, 24, 2652. [Google Scholar] [CrossRef] [PubMed]
- Stokoe, D.; Stephens, L.R.; Copeland, T.; Gaffney, P.R.; Reese, C.B.; Painter, G.F.; Holmes, A.B.; McCormick, F.; Hawkins, P.T. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 1997, 277, 567–570. [Google Scholar] [CrossRef]
- Alessi, D.R.; Andjelkovic, M.; Caudwell, B.; Cron, P.; Morrice, N.; Cohen, P.; Hemmings, B.A. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996, 15, 6541–6551. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 169, 361–371. [Google Scholar] [CrossRef]
- Feller, S.M. Crk family adaptors-signalling complex formation and biological roles. Oncogene 2001, 20, 6348–6371. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Batra, S.; Sassano, A.; Majchrzak, B.; Levy, D.E.; Gaestel, M.; Fish, E.N.; Davis, R.J.; Platanias, L.C. Activation of mitogen-activated protein kinase kinase (MKK) 3 and MKK6 by type I interferons. J. Biol. Chem. 2005, 280, 10001–10010. [Google Scholar] [CrossRef]
- Uddin, S.; Majchrzak, B.; Woodson, J.; Arunkumar, P.; Alsayed, Y.; Pine, R.; Young, P.R.; Fish, E.N.; Platanias, L.C. Activation of the p38 mitogen-activated protein kinase by type I interferons. J. Biol. Chem. 1999, 274, 30127–30131. [Google Scholar] [CrossRef]
- Soloaga, A.; Thomson, S.; Wiggin, G.R.; Rampersaud, N.; Dyson, M.H.; Hazzalin, C.A.; Mahadevan, L.C.; Arthur, J.S. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J. 2003, 22, 2788–2797. [Google Scholar] [CrossRef]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type I Interferon in Chronic Virus Infection and Cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef]
- Lukhele, S.; Boukhaled, G.M.; Brooks, D.G. Type I interferon signaling, regulation and gene stimulation in chronic virus infection. Semin. Immunol. 2019, 43, 101277. [Google Scholar] [CrossRef]
- Ostuni, R.; Piccolo, V.; Barozzi, I.; Polletti, S.; Termanini, A.; Bonifacio, S.; Curina, A.; Prosperini, E.; Ghisletti, S.; Natoli, G. Latent enhancers activated by stimulation in differentiated cells. Cell 2013, 152, 157–171. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, K.; Giannopoulou, E.; Qiao, Y.; Kang, K.; Kim, G.; Park-Min, K.H.; Ivashkiv, L.B. Type I interferons and the cytokine TNF cooperatively reprogram the macrophage epigenome to promote inflammatory activation. Nat. Immunol. 2017, 18, 1104–1116. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK-STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Fenner, J.E.; Starr, R.; Cornish, A.L.; Zhang, J.G.; Metcalf, D.; Schreiber, R.D.; Sheehan, K.; Hilton, D.J.; Alexander, W.S.; Hertzog, P.J. Suppressor of cytokine signaling 1 regulates the immune response to infection by a unique inhibition of type I interferon activity. Nat. Immunol. 2006, 7, 33–39. [Google Scholar] [CrossRef]
- Skaug, B.; Chen, Z.J. Emerging role of ISG15 in antiviral immunity. Cell 2010, 143, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Struckhoff, J.J.; Schneider, J.; Martinez-Sobrido, L.; Wolff, T.; García-Sastre, A.; Zhang, D.E.; Lenschow, D.J. Mice lacking the ISG15 E1 enzyme UbE1L demonstrate increased susceptibility to both mouse-adapted and non-mouse-adapted influenza B virus infection. J. Virol. 2009, 83, 1147–1151. [Google Scholar] [CrossRef]
- Mustelin, T.; Vang, T.; Bottini, N. Protein tyrosine phosphatases and the immune response. Nat. Rev. Immunol. 2005, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.R.; Hong, Y.K.; Wang, X.D.; Ling, M.Y.; Dragoi, A.M.; Chung, A.S.; Campbell, A.G.; Han, Z.Y.; Feng, G.S.; Chin, Y.E. SHP-2 is a dual-specificity phosphatase involved in Stat1 dephosphorylation at both tyrosine and serine residues in nuclei. J. Biol. Chem. 2002, 277, 47572–47580. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Sassano, A.; Deb, D.K.; Verma, A.; Majchrzak, B.; Rahman, A.; Malik, A.B.; Fish, E.N.; Platanias, L.C. Protein kinase C-δ (PKC-δ) is activated by type I interferons and mediates phosphorylation of Stat1 on serine 727. J. Biol. Chem. 2002, 277, 14408–14416. [Google Scholar] [CrossRef]
- Tenoever, B.R.; Ng, S.L.; Chua, M.A.; McWhirter, S.M.; García-Sastre, A.; Maniatis, T. Multiple functions of the IKK-related kinase IKKepsilon in interferon-mediated antiviral immunity. Science 2007, 315, 1274–1278. [Google Scholar] [CrossRef]
- Mowen, K.A.; Tang, J.; Zhu, W.; Schurter, B.T.; Shuai, K.; Herschman, H.R.; David, M. Arginine methylation of STAT1 modulates IFNalpha/beta-induced transcription. Cell 2001, 104, 731–741. [Google Scholar] [CrossRef]
- Tang, X.; Gao, J.S.; Guan, Y.J.; McLane, K.E.; Yuan, Z.L.; Ramratnam, B.; Chin, Y.E. Acetylation-dependent signal transduction for type I interferon receptor. Cell 2007, 131, 93–105. [Google Scholar] [CrossRef]
- Paulson, M.; Press, C.; Smith, E.; Tanese, N.; Levy, D.E. IFN-Stimulated transcription through a TBP-free acetyltransferase complex escapes viral shutoff. Nat. Cell Biol. 2002, 4, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Krämer, O.H.; Knauer, S.K.; Greiner, G.; Jandt, E.; Reichardt, S.; Gührs, K.H.; Stauber, R.H.; Böhmer, F.D.; Heinzel, T. A phosphorylation-acetylation switch regulates STAT1 signaling. Genes Dev. 2009, 23, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chen, Z.J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 2011, 12, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liu, B.; Li, Z.; Wu, H.; Wang, P.; Zhao, K.; Jiang, G.; Zhang, L.; Gao, C. E3 ubiquitin ligase RNF128 promotes innate antiviral immunity through K63-linked ubiquitination of TBK1. Nat. Immunol. 2016, 17, 1342–1351. [Google Scholar] [CrossRef]
- Kumar, K.G.; Tang, W.; Ravindranath, A.K.; Clark, W.A.; Croze, E.; Fuchs, S.Y. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. EMBO J. 2003, 22, 5480–5490. [Google Scholar] [CrossRef]
- Tanaka, T.; Soriano, M.A.; Grusby, M.J. SLIM is a nuclear ubiquitin E3 ligase that negatively regulates STAT signaling. Immunity 2005, 22, 729–736. [Google Scholar] [CrossRef]
- Yuan, C.; Qi, J.; Zhao, X.; Gao, C. Smurf1 protein negatively regulates interferon-γ signaling through promoting STAT1 protein ubiquitination and degradation. J. Biol. Chem. 2012, 287, 17006–17015. [Google Scholar] [CrossRef]
- Lawrence, D.W.; Kornbluth, J. E3 ubiquitin ligase NKLAM ubiquitinates STAT1 and positively regulates STAT1-mediated transcriptional activity. Cell. Signal. 2016, 28, 1833–1841. [Google Scholar] [CrossRef]
- Ungureanu, D.; Vanhatupa, S.; Grönholm, J.; Palvimo, J.J.; Silvennoinen, O. SUMO-1 conjugation selectively modulates STAT1-mediated gene responses. Blood 2005, 106, 224–226. [Google Scholar] [CrossRef]
- Patel, M.C.; Debrosse, M.; Smith, M.; Dey, A.; Huynh, W.; Sarai, N.; Heightman, T.D.; Tamura, T.; Ozato, K. BRD4 coordinates recruitment of pause release factor P-TEFb and the pausing complex NELF/DSIF to regulate transcription elongation of interferon-stimulated genes. Mol. Cell. Biol. 2013, 33, 2497–2507. [Google Scholar] [CrossRef]
- Chang, H.M.; Paulson, M.; Holko, M.; Rice, C.M.; Williams, B.R.; Marié, I.; Levy, D.E. Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc. Natl. Acad. Sci. USA 2004, 101, 9578–9583. [Google Scholar] [CrossRef]
- Gao, B.; Duan, Z.; Xu, W.; Xiong, S. Tripartite motif-containing 22 inhibits the activity of hepatitis B virus core promoter, which is dependent on nuclear-located RING domain. Hepatology 2009, 50, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Fang, T.C.; Schaefer, U.; Mecklenbrauker, I.; Stienen, A.; Dewell, S.; Chen, M.S.; Rioja, I.; Parravicini, V.; Prinjha, R.K.; Chandwani, R.; et al. Histone H3 lysine 9 di-methylation as an epigenetic signature of the interferon response. J. Exp. Med. 2012, 209, 661–669. [Google Scholar] [CrossRef]
- Kroetz, D.N.; Allen, R.M.; Schaller, M.A.; Cavallaro, C.; Ito, T.; Kunkel, S.L. Type I Interferon Induced Epigenetic Regulation of Macrophages Suppresses Innate and Adaptive Immunity in Acute Respiratory Viral Infection. PLoS Pathog. 2015, 11, e1005338. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.J.; Thillainadesan, G.; Yousef, A.F.; Ablack, J.N.; Mossman, K.L.; Torchia, J.; Mymryk, J.S. Adenovirus evasion of interferon-mediated innate immunity by direct antagonism of a cellular histone posttranslational modification. Cell Host Microbe 2012, 11, 597–606. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, D.; Roswit, W.T.; Jin, X.; Patel, A.C.; Patel, D.A.; Agapov, E.; Wang, Z.; Tidwell, R.M.; Atkinson, J.J.; et al. PARP9-DTX3L ubiquitin ligase targets host histone H2BJ and viral 3C protease to enhance interferon signaling and control viral infection. Nat. Immunol. 2015, 16, 1215–1227. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, Y. Reversing DNA methylation: Mechanisms, genomics, and biological functions. Cell 2014, 156, 45–68. [Google Scholar] [CrossRef]
- Kohli, R.M.; Zhang, Y. TET enzymes, TDG and the dynamics of DNA demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, K.; Shen, Q.; Han, Y.; Gu, Y.; Li, X.; Zhao, D.; Liu, Y.; Wang, C.; Zhang, X.; et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 2015, 525, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Qu, C.; Zhou, Y.; Konkel, J.E.; Shi, S.; Liu, Y.; Chen, C.; Liu, S.; Liu, D.; Chen, Y.; et al. Hydrogen Sulfide Promotes Tet1- and Tet2-Mediated Foxp3 Demethylation to Drive Regulatory T Cell Differentiation and Maintain Immune Homeostasis. Immunity 2015, 43, 251–263. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.S.; Dooley, J.; Linterman, M.A.; Pierson, W.; Ucar, O.; Kyewski, B.; Zuklys, S.; Hollander, G.A.; Matthys, P.; Gray, D.H.; et al. The thymic epithelial microRNA network elevates the threshold for infection-associated thymic involution via miR-29a mediated suppression of the IFN-α receptor. Nat. Immunol. 2011, 13, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Hou, J.; Lin, L.; Wang, C.; Liu, X.; Li, D.; Ma, F.; Wang, Z.; Cao, X. Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1. J. Immunol. 2010, 185, 6226–6233. [Google Scholar] [CrossRef]
- Wang, P.; Xue, Y.; Han, Y.; Lin, L.; Wu, C.; Xu, S.; Jiang, Z.; Xu, J.; Liu, Q.; Cao, X. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science 2014, 344, 310–313. [Google Scholar] [CrossRef]
- Josset, L.; Tchitchek, N.; Gralinski, L.E.; Ferris, M.T.; Eisfeld, A.J.; Green, R.R.; Thomas, M.J.; Tisoncik-Go, J.; Schroth, G.P.; Kawaoka, Y.; et al. Annotation of long non-coding RNAs expressed in collaborative cross founder mice in response to respiratory virus infection reveals a new class of interferon-stimulated transcripts. RNA Biol. 2014, 11, 875–890. [Google Scholar] [CrossRef]
- Gomez, J.A.; Wapinski, O.L.; Yang, Y.W.; Bureau, J.F.; Gopinath, S.; Monack, D.M.; Chang, H.Y.; Brahic, M.; Kirkegaard, K. The NeST long ncRNA controls microbial susceptibility and epigenetic activation of the interferon-γ locus. Cell 2013, 152, 743–754. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhu, X.; Chen, Y.; Wei, H.; Chen, Q.; Chi, X.; Qi, B.; Zhang, L.; Zhao, Y.; Gao, G.F.; et al. NRAV, a long noncoding RNA, modulates antiviral responses through suppression of interferon-stimulated gene transcription. Cell Host Microbe 2014, 16, 616–626. [Google Scholar] [CrossRef]
- Vasconcellos, R.; Braun, D.; Coutinho, A.; Demengeot, J. Type I IFN sets the stringency of B cell repertoire selection in the bone marrow. Int. Immunol. 1999, 11, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Jego, G.; Palucka, A.K.; Blanck, J.P.; Chalouni, C.; Pascual, V.; Banchereau, J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 2003, 19, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Daugan, M.; Murira, A.; Mindt, B.C.; Germain, A.; Tarrab, E.; Lapierre, P.; Fritz, J.H.; Lamarre, A. Type I Interferon Impairs Specific Antibody Responses Early during Establishment of LCMV Infection. Front. Immunol. 2016, 7, 564. [Google Scholar] [CrossRef]
- Peters, M.; Ambrus, J.L.; Zheleznyak, A.; Walling, D.; Hoofnagle, J.H. Effect of interferon-alpha on immunoglobulin synthesis by human B cells. J. Immunol. 1986, 137, 3153–3157. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, K.A.; Schulze, L.L.; Paap, E.M.; Müller, T.M.; Neurath, M.F.; Zundler, S. Immunology of IL-12: An update on functional activities and implications for disease. EXCLI J. 2020, 19, 1563–1589. [Google Scholar] [CrossRef]
- Davis, A.M.; Hagan, K.A.; Matthews, L.A.; Bajwa, G.; Gill, M.A.; Gale, M., Jr.; Farrar, J.D. Blockade of virus infection by human CD4+ T cells via a cytokine relay network. J. Immunol. 2008, 180, 6923–6932. [Google Scholar] [CrossRef]
- Way, S.S.; Havenar-Daughton, C.; Kolumam, G.A.; Orgun, N.N.; Murali-Krishna, K. IL-12 and type-I IFN synergize for IFN-gamma production by CD4 T cells, whereas neither are required for IFN-gamma production by CD8 T cells after Listeria monocytogenes infection. J. Immunol. 2007, 178, 4498–4505. [Google Scholar] [CrossRef]
- Schijns, V.E.; Haagmans, B.L.; Wierda, C.M.; Kruithof, B.; Heijnen, I.A.; Alber, G.; Horzinek, M.C. Mice lacking IL-12 develop polarized Th1 cells during viral infection. J. Immunol. 1998, 160, 3958–3964. [Google Scholar] [CrossRef]
- Ziegler, S.F. The role of thymic stromal lymphopoietin (TSLP) in allergic disorders. Curr. Opin. Immunol. 2010, 22, 795–799. [Google Scholar] [CrossRef]
- Hegazy, A.N.; Peine, M.; Helmstetter, C.; Panse, I.; Fröhlich, A.; Bergthaler, A.; Flatz, L.; Pinschewer, D.D.; Radbruch, A.; Löhning, M. Interferons direct Th2 cell reprogramming to generate a stable GATA-3(+)T-bet(+) cell subset with combined Th2 and Th1 cell functions. Immunity 2010, 32, 116–128. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, R.M.; Lawrence, W.D. Interferon-alpha for the hypereosinophilic syndrome. Ann. Intern. Med. 1990, 113, 716–718. [Google Scholar] [CrossRef] [PubMed]
- Schandené, L.; Del Prete, G.F.; Cogan, E.; Stordeur, P.; Crusiaux, A.; Kennes, B.; Romagnani, S.; Goldman, M. Recombinant interferon-alpha selectively inhibits the production of interleukin-5 by human CD4+ T cells. J. Clin. Investig. 1996, 97, 309–315. [Google Scholar] [CrossRef]
- Huber, J.P.; Ramos, H.J.; Gill, M.A.; Farrar, J.D. Cutting edge: Type I IFN reverses human Th2 commitment and stability by suppressing GATA3. J. Immunol. 2010, 185, 813–817. [Google Scholar] [CrossRef]
- Gong, J.; Zhan, H.; Liang, Y.; He, Q.; Cui, D. Role of Th22 Cells in Human Viral Diseases. Front. Med. 2021, 8, 708140. [Google Scholar] [CrossRef]
- Harrington, L.E.; Hatton, R.D.; Mangan, P.R.; Turner, H.; Murphy, T.L.; Murphy, K.M.; Weaver, C.T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005, 6, 1123–1132. [Google Scholar] [CrossRef]
- Moschen, A.R.; Geiger, S.; Krehan, I.; Kaser, A.; Tilg, H. Interferon-alpha controls IL-17 expression in vitro and in vivo. Immunobiology 2008, 213, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Axtell, R.C.; de Jong, B.A.; Boniface, K.; van der Voort, L.F.; Bhat, R.; De Sarno, P.; Naves, R.; Han, M.; Zhong, F.; Castellanos, J.G.; et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat. Med. 2010, 16, 406–412. [Google Scholar] [CrossRef]
- Szczuciński, A.; Losy, J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol. Scand. 2007, 115, 137–146. [Google Scholar] [CrossRef]
- Ifergan, I.; Kébir, H.; Bernard, M.; Wosik, K.; Dodelet-Devillers, A.; Cayrol, R.; Arbour, N.; Prat, A. The blood-brain barrier induces differentiation of migrating monocytes into Th17-polarizing dendritic cells. Brain 2008, 131, 785–799. [Google Scholar] [CrossRef]
- Tosello Boari, J.; Acosta Rodriguez, E.V. IL-1β/CD14 pathway induces IL-17 production: Dendritic cells activated with IL-1β set Th17 cells on fire by CD14-mediated mechanisms. Immunol. Cell Biol. 2016, 94, 903–904. [Google Scholar] [CrossRef]
- Kurche, J.S.; Haluszczak, C.; McWilliams, J.A.; Sanchez, P.J.; Kedl, R.M. Type I IFN-dependent T cell activation is mediated by IFN-dependent dendritic cell OX40 ligand expression and is independent of T cell IFNR expression. J. Immunol. 2012, 188, 585–593. [Google Scholar] [CrossRef]
- Simmons, D.P.; Wearsch, P.A.; Canaday, D.H.; Meyerson, H.J.; Liu, Y.C.; Wang, Y.; Boom, W.H.; Harding, C.V. Type I IFN drives a distinctive dendritic cell maturation phenotype that allows continued class II MHC synthesis and antigen processing. J. Immunol. 2012, 188, 3116–3126. [Google Scholar] [CrossRef]
- Teijaro, J.R.; Ng, C.; Lee, A.M.; Sullivan, B.M.; Sheehan, K.C.; Welch, M.; Schreiber, R.D.; de la Torre, J.C.; Oldstone, M.B. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science 2013, 340, 207–211. [Google Scholar] [CrossRef]
- Wilson, E.B.; Yamada, D.H.; Elsaesser, H.; Herskovitz, J.; Deng, J.; Cheng, G.; Aronow, B.J.; Karp, C.L.; Brooks, D.G. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science 2013, 340, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.T.; Oldstone, M.B. Infected CD8α- dendritic cells are the predominant source of IL-10 during establishment of persistent viral infection. Proc. Natl. Acad. Sci. USA 2012, 109, 14116–14121. [Google Scholar] [CrossRef] [PubMed]
- Iijima, N.; Mattei, L.M.; Iwasaki, A. Recruited inflammatory monocytes stimulate antiviral Th1 immunity in infected tissue. Proc. Natl. Acad. Sci. USA 2011, 108, 284–289. [Google Scholar] [CrossRef]
- Stifter, S.A.; Bhattacharyya, N.; Pillay, R.; Flórido, M.; Triccas, J.A.; Britton, W.J.; Feng, C.G. Functional Interplay between Type I and II Interferons Is Essential to Limit Influenza A Virus-Induced Tissue Inflammation. PLoS Pathog. 2016, 12, e1005378. [Google Scholar] [CrossRef]
- Eshleman, E.M.; Delgado, C.; Kearney, S.J.; Friedman, R.S.; Lenz, L.L. Down regulation of macrophage IFNGR1 exacerbates systemic L. monocytogenes infection. PLoS Pathog. 2017, 13, e1006388. [Google Scholar] [CrossRef] [PubMed]
- McNab, F.W.; Ewbank, J.; Howes, A.; Moreira-Teixeira, L.; Martirosyan, A.; Ghilardi, N.; Saraiva, M.; O’Garra, A. Type I IFN induces IL-10 production in an IL-27-independent manner and blocks responsiveness to IFN-γ for production of IL-12 and bacterial killing in Mycobacterium tuberculosis-infected macrophages. J. Immunol. 2014, 193, 3600–3612. [Google Scholar] [CrossRef]
- Novikov, A.; Cardone, M.; Thompson, R.; Shenderov, K.; Kirschman, K.D.; Mayer-Barber, K.D.; Myers, T.G.; Rabin, R.L.; Trinchieri, G.; Sher, A.; et al. Mycobacterium tuberculosis triggers host type I IFN signaling to regulate IL-1β production in human macrophages. J. Immunol. 2011, 187, 2540–2547. [Google Scholar] [CrossRef]
- Henry, T.; Kirimanjeswara, G.S.; Ruby, T.; Jones, J.W.; Peng, K.; Perret, M.; Ho, L.; Sauer, J.D.; Iwakura, Y.; Metzger, D.W.; et al. Type I IFN signaling constrains IL-17A/F secretion by gammadelta T cells during bacterial infections. J. Immunol. 2010, 184, 3755–3767. [Google Scholar] [CrossRef]
- Ahlenstiel, G.; Edlich, B.; Hogdal, L.J.; Rotman, Y.; Noureddin, M.; Feld, J.J.; Holz, L.E.; Titerence, R.H.; Liang, T.J.; Rehermann, B. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology 2011, 141, 1231–1239.e2. [Google Scholar] [CrossRef][Green Version]
- Werner, J.M.; Serti, E.; Chepa-Lotrea, X.; Stoltzfus, J.; Ahlenstiel, G.; Noureddin, M.; Feld, J.J.; Liang, T.J.; Rotman, Y.; Rehermann, B. Ribavirin improves the IFN-γ response of natural killer cells to IFN-based therapy of hepatitis C virus infection. Hepatology 2014, 60, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Gil, M.P.; Wang, X.; Louten, J.; Chu, W.M.; Biron, C.A. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J. Exp. Med. 2007, 204, 2383–2396. [Google Scholar] [CrossRef]
- Lee, A.J.; Chen, B.; Chew, M.V.; Barra, N.G.; Shenouda, M.M.; Nham, T.; van Rooijen, N.; Jordana, M.; Mossman, K.L.; Schreiber, R.D.; et al. Inflammatory monocytes require type I interferon receptor signaling to activate NK cells via IL-18 during a mucosal viral infection. J. Exp. Med. 2017, 214, 1153–1167. [Google Scholar] [CrossRef]
- Mack, E.A.; Kallal, L.E.; Demers, D.A.; Biron, C.A. Type 1 interferon induction of natural killer cell gamma interferon production for defense during lymphocytic choriomeningitis virus infection. mBio 2011, 2, e00169-11. [Google Scholar] [CrossRef] [PubMed]
- Accapezzato, D.; Caccavale, R.; Paroli, M.P.; Gioia, C.; Nguyen, B.L.; Spadea, L.; Paroli, M. Advances in the Pathogenesis and Treatment of Systemic Lupus Erythematosus. Int. J. Mol. Sci. 2023, 24, 6578. [Google Scholar] [CrossRef]
- Gensous, N.; Lazaro, E.; Blanco, P.; Richez, C. Anifrolumab: First biologic approved in the EU not restricted to patients with a high degree of disease activity for the treatment of moderate to severe systemic lupus erythematosus. Expert Rev. Clin. Immunol. 2024, 20, 21–30. [Google Scholar] [CrossRef]
- Crow, M.K. Pathogenesis of systemic lupus erythematosus: Risks, mechanisms and therapeutic targets. Ann. Rheum. Dis. 2023, 82, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Tsao, B.P. Genetic susceptibility to systemic lupus erythematosus in the genomic era. Nat. Rev. Rheumatol. 2010, 6, 683–692. [Google Scholar] [CrossRef]
- Weckerle, C.E.; Franek, B.S.; Kelly, J.A.; Kumabe, M.; Mikolaitis, R.A.; Green, S.L.; Utset, T.O.; Jolly, M.; James, J.A.; Harley, J.B.; et al. Network analysis of associations between serum interferon-α activity, autoantibodies, and clinical features in systemic lupus erythematosus. Arthritis Rheum. 2011, 63, 1044–1053. [Google Scholar] [CrossRef]
- Niewold, T.B.; Swedler, W.I. Systemic lupus erythematosus arising during interferon-alpha therapy for cryoglobulinemic vasculitis associated with hepatitis C. Clin. Rheumatol. 2005, 24, 178–181. [Google Scholar] [CrossRef]
- Niewold, T.B.; Hua, J.; Lehman, T.J.; Harley, J.B.; Crow, M.K. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007, 8, 492–502. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, S.N.; Franek, B.S.; Kumar, A.A.; Arrington, J.; Mikolaitis, R.A.; Utset, T.O.; Jolly, M.; Crow, M.K.; Skol, A.D.; Niewold, T.B. Trait-stratified genome-wide association study identifies novel and diverse genetic associations with serologic and cytokine phenotypes in systemic lupus erythematosus. Arthritis Res. Ther. 2010, 12, R151. [Google Scholar] [CrossRef] [PubMed]
- Munroe, M.E.; Lu, R.; Zhao, Y.D.; Fife, D.A.; Robertson, J.M.; Guthridge, J.M.; Niewold, T.B.; Tsokos, G.C.; Keith, M.P.; Harley, J.B.; et al. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann. Rheum. Dis. 2016, 75, 2014–2021. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.K.; Wohlgemuth, J. Microarray analysis of gene expression in lupus. Arthritis Res. Ther. 2003, 5, 279–287. [Google Scholar] [CrossRef][Green Version]
- Catalina, M.D.; Bachali, P.; Geraci, N.S.; Grammer, A.C.; Lipsky, P.E. Gene expression analysis delineates the potential roles of multiple interferons in systemic lupus erythematosus. Commun. Biol. 2019, 2, 140. [Google Scholar] [CrossRef]
- Whittall Garcia, L.P.; Gladman, D.D.; Urowitz, M.; Bonilla, D.; Schneider, R.; Touma, Z.; Wither, J. Interferon-α as a biomarker to predict renal outcomes in lupus nephritis. Lupus Sci. Med. 2024, 11, e001347. [Google Scholar] [CrossRef]
- Ko, K.; Koldobskaya, Y.; Rosenzweig, E.; Niewold, T.B. Activation of the Interferon Pathway is Dependent Upon Autoantibodies in African-American SLE Patients, but Not in European-American SLE Patients. Front. Immunol. 2013, 4, 309. [Google Scholar] [CrossRef]
- Paradowska-Gorycka, A.; Wajda, A.; Stypinska, B.; Walczuk, E.; Rzeszotarska, E.; Walczyk, M.; Haladyj, E.; Romanowska-Prochnicka, K.; Felis-Giemza, A.; Lewandowska, A.; et al. Variety of endosomal TLRs and Interferons (IFN-α, IFN-β, IFN-γ) expression profiles in patients with SLE, SSc and MCTD. Clin. Exp. Immunol. 2021, 204, 49–63. [Google Scholar] [CrossRef]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.M.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef]
- Landolt-Marticorena, C.; Bonventi, G.; Lubovich, A.; Ferguson, C.; Unnithan, T.; Su, J.; Gladman, D.D.; Urowitz, M.; Fortin, P.R.; Wither, J. Lack of association between the interferon-alpha signature and longitudinal changes in disease activity in systemic lupus erythematosus. Ann. Rheum. Dis. 2009, 68, 1440–1446. [Google Scholar] [CrossRef]
- Petri, M.; Singh, S.; Tesfasyone, H.; Dedrick, R.; Fry, K.; Lal, P.; Williams, G.; Bauer, J.; Gregersen, P.; Behrens, T.; et al. Longitudinal expression of type I interferon responsive genes in systemic lupus erythematosus. Lupus 2009, 18, 980–989. [Google Scholar] [CrossRef]
- Bauer, J.W.; Petri, M.; Batliwalla, F.M.; Koeuth, T.; Wilson, J.; Slattery, C.; Panoskaltsis-Mortari, A.; Gregersen, P.K.; Behrens, T.W.; Baechler, E.C. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: A validation study. Arthritis Rheum. 2009, 60, 3098–3107. [Google Scholar] [CrossRef] [PubMed]
- Banchereau, R.; Hong, S.; Cantarel, B.; Baldwin, N.; Baisch, J.; Edens, M.; Cepika, A.M.; Acs, P.; Turner, J.; Anguiano, E.; et al. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell 2016, 165, 1548–1550. [Google Scholar] [CrossRef] [PubMed]
- Labonte, A.C.; Kegerreis, B.; Geraci, N.S.; Bachali, P.; Madamanchi, S.; Robl, R.; Catalina, M.D.; Lipsky, P.E.; Grammer, A.C. Identification of alterations in macrophage activation associated with disease activity in systemic lupus erythematosus. PLoS ONE 2018, 13, e0208132. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; Sagalovskiy, I.; Guo, Q.; Nezos, A.; Kapsogeorgou, E.K.; Lu, P.; Liang Zhou, J.; Kirou, K.A.; Seshan, S.V.; Moutsopoulos, H.M.; et al. Expression of Long Interspersed Nuclear Element 1 Retroelements and Induction of Type I Interferon in Patients With Systemic Autoimmune Disease. Arthritis Rheumatol. 2016, 68, 2686–2696. [Google Scholar] [CrossRef]
- Nzeusseu Toukap, A.; Galant, C.; Theate, I.; Maudoux, A.L.; Lories, R.J.; Houssiau, F.A.; Lauwerys, B.R. Identification of distinct gene expression profiles in the synovium of patients with systemic lupus erythematosus. Arthritis Rheum. 2007, 56, 1579–1588. [Google Scholar] [CrossRef]
- Arazi, A.; Rao, D.A.; Berthier, C.C.; Davidson, A.; Liu, Y.; Hoover, P.J.; Chicoine, A.; Eisenhaure, T.M.; Jonsson, A.H.; Li, S.; et al. The immune cell landscape in kidneys of patients with lupus nephritis. Nat. Immunol. 2019, 20, 902–914. [Google Scholar] [CrossRef]
- Der, E.; Suryawanshi, H.; Morozov, P.; Kustagi, M.; Goilav, B.; Ranabothu, S.; Izmirly, P.; Clancy, R.; Belmont, H.M.; Koenigsberg, M.; et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 2019, 20, 915–927. [Google Scholar] [CrossRef]
- Nian, Z.; Mao, Y.; Xu, Z.; Deng, M.; Xu, Y.; Xu, H.; Chen, R.; Xu, Y.; Huang, N.; Mao, F.; et al. Multi-omics analysis uncovered systemic lupus erythematosus and COVID-19 crosstalk. Mol. Med. 2024, 30, 81. [Google Scholar] [CrossRef]
- Ding, X.; Cai, M.; Wang, S.; Yang, Q.; Zheng, X.; Zuo, X.; Liu, S. Gene-based association analysis identified a novel gene associated with systemic lupus erythematosus. Ann. Hum. Genet. 2021, 85, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.A.; Niewold, T.B. Interferon regulatory factors: Critical mediators of human lupus. Transl. Res. 2015, 165, 283–295. [Google Scholar] [CrossRef]
- Niewold, T.B.; Kelly, J.A.; Flesch, M.H.; Espinoza, L.R.; Harley, J.B.; Crow, M.K. Association of the IRF5 risk haplotype with high serum interferon-alpha activity in systemic lupus erythematosus patients. Arthritis Rheum. 2008, 58, 2481–2487. [Google Scholar] [CrossRef] [PubMed]
- Niewold, T.B.; Kelly, J.A.; Kariuki, S.N.; Franek, B.S.; Kumar, A.A.; Kaufman, K.M.; Thomas, K.; Walker, D.; Kamp, S.; Frost, J.M.; et al. IRF5 haplotypes demonstrate diverse serological associations which predict serum interferon alpha activity and explain the majority of the genetic association with systemic lupus erythematosus. Ann. Rheum. Dis. 2012, 71, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Cherian, T.S.; Kariuki, S.N.; Franek, B.S.; Buyon, J.P.; Clancy, R.M.; Niewold, T.B. Brief Report: IRF5 systemic lupus erythematosus risk haplotype is associated with asymptomatic serologic autoimmunity and progression to clinical autoimmunity in mothers of children with neonatal lupus. Arthritis Rheum. 2012, 64, 3383–3387. [Google Scholar] [CrossRef]
- Muskardin, T.L.W.; Niewold, T.B. Type I interferon in rheumatic diseases. Nat. Rev. Rheumatol. 2018, 14, 214–228. [Google Scholar] [CrossRef]
- Kariuki, S.N.; Ghodke-Puranik, Y.; Dorschner, J.M.; Chrabot, B.S.; Kelly, J.A.; Tsao, B.P.; Kimberly, R.P.; Alarcón-Riquelme, M.E.; Jacob, C.O.; Criswell, L.A.; et al. Genetic analysis of the pathogenic molecular sub-phenotype interferon-alpha identifies multiple novel loci involved in systemic lupus erythematosus. Genes Immun. 2015, 16, 15–23. [Google Scholar] [CrossRef]
- Ghodke-Puranik, Y.; Olferiev, M.; Crow, M.K. Systemic lupus erythematosus genetics: Insights into pathogenesis and implications for therapy. Nat. Rev. Rheumatol. 2024, 20, 635–648. [Google Scholar] [CrossRef]
- Barrat, F.J.; Meeker, T.; Gregorio, J.; Chan, J.H.; Uematsu, S.; Akira, S.; Chang, B.; Duramad, O.; Coffman, R.L. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005, 202, 1131–1139. [Google Scholar] [CrossRef]
- Hua, J.; Kirou, K.; Lee, C.; Crow, M.K. Functional assay of type I interferon in systemic lupus erythematosus plasma and association with anti-RNA binding protein autoantibodies. Arthritis Rheum. 2006, 54, 1906–1916. [Google Scholar] [CrossRef]
- Lövgren, T.; Eloranta, M.L.; Båve, U.; Alm, G.V.; Rönnblom, L. Induction of interferon-alpha production in plasmacytoid dendritic cells by immune complexes containing nucleic acid released by necrotic or late apoptotic cells and lupus IgG. Arthritis Rheum. 2004, 50, 1861–1872. [Google Scholar] [CrossRef]
- Barrat, F.J.; Meeker, T.; Chan, J.H.; Guiducci, C.; Coffman, R.L. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 2007, 37, 3582–3586. [Google Scholar] [CrossRef] [PubMed]
- Caielli, S.; Athale, S.; Domic, B.; Murat, E.; Chandra, M.; Banchereau, R.; Baisch, J.; Phelps, K.; Clayton, S.; Gong, M.; et al. Oxidized mitochondrial nucleoids released by neutrophils drive type I interferon production in human lupus. J. Exp. Med. 2016, 213, 697–713. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Ries, M.; Schuster, P.; Thomann, S.; Donhauser, N.; Vollmer, J.; Schmidt, B. Identification of novel oligonucleotides from mitochondrial DNA that spontaneously induce plasmacytoid dendritic cell activation. J. Leukoc. Biol. 2013, 94, 123–135. [Google Scholar] [CrossRef]
- Sisirak, V.; Sally, B.; D’Agati, V.; Martinez-Ortiz, W.; Özçakar, Z.B.; David, J.; Rashidfarrokhi, A.; Yeste, A.; Panea, C.; Chida, A.S.; et al. Digestion of Chromatin in Apoptotic Cell Microparticles Prevents Autoimmunity. Cell 2016, 166, 88–101. [Google Scholar] [CrossRef]
- Zhou, Y.; Song, H.M. Type I interferon pathway in pediatric systemic lupus erythematosus. World J. Pediatr. 2024, 20, 653–668. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, N.; Mertens, C.; Zillinger, T.; Wenzel, J.; Bald, T.; Zahn, S.; Tüting, T.; Hartmann, G.; Barchet, W. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity 2013, 39, 482–495. [Google Scholar] [CrossRef] [PubMed]
- Stetson, D.B.; Ko, J.S.; Heidmann, T.; Medzhitov, R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell 2008, 134, 587–598. [Google Scholar] [CrossRef]
- Harley, I.T.; Niewold, T.B.; Stormont, R.M.; Kaufman, K.M.; Glenn, S.B.; Franek, B.S.; Kelly, J.A.; Kilpatrick, J.R.; Hutchings, D.; Divers, J.; et al. The role of genetic variation near interferon-kappa in systemic lupus erythematosus. J. Biomed. Biotechnol. 2010, 2010, 706825. [Google Scholar] [CrossRef] [PubMed]
- Stannard, J.N.; Reed, T.J.; Myers, E.; Lowe, L.; Sarkar, M.K.; Xing, X.; Gudjonsson, J.E.; Kahlenberg, J.M. Lupus Skin Is Primed for IL-6 Inflammatory Responses through a Keratinocyte-Mediated Autocrine Type I Interferon Loop. J. Investig. Dermatol. 2017, 137, 115–122. [Google Scholar] [CrossRef]
- Davison, L.M.; Jorgensen, T.N. New Treatments for Systemic Lupus Erythematosus on the Horizon: Targeting Plasmacytoid Dendritic Cells to Inhibit Cytokine Production. J. Clin. Cell. Immunol. 2017, 8, 6. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Y.H.; Wang, Y.; Huang, L.; Sandoval, H.; Liu, Y.J.; Wang, J. Dendritic cell apoptosis in the maintenance of immune tolerance. Science 2006, 311, 1160–1164. [Google Scholar] [CrossRef]
- Rowland, S.L.; Riggs, J.M.; Gilfillan, S.; Bugatti, M.; Vermi, W.; Kolbeck, R.; Unanue, E.R.; Sanjuan, M.A.; Colonna, M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J. Exp. Med. 2014, 211, 1977–1991. [Google Scholar] [CrossRef] [PubMed]
- Sisirak, V.; Ganguly, D.; Lewis, K.L.; Couillault, C.; Tanaka, L.; Bolland, S.; D’Agati, V.; Elkon, K.B.; Reizis, B. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 2014, 211, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Furie, R.; Werth, V.P.; Merola, J.F.; Stevenson, L.; Reynolds, T.L.; Naik, H.; Wang, W.; Christmann, R.; Gardet, A.; Pellerin, A.; et al. Monoclonal antibody targeting BDCA2 ameliorates skin lesions in systemic lupus erythematosus. J. Clin. Investig. 2019, 129, 1359–1371. [Google Scholar] [CrossRef]
- Han, S.; Zhuang, H.; Lee, P.Y.; Li, M.; Yang, L.; Nigrovic, P.A.; Reeves, W.H. Differential Responsiveness of Monocyte and Macrophage Subsets to Interferon. Arthritis Rheumatol. 2020, 72, 100–113. [Google Scholar] [CrossRef]
- Khiewkamrop, P.; Kaewraemruaen, C.; Manipuntee, C.; Saengruengrit, C.; Insin, N.; Leelahavanichkul, A.; Kaewduangduen, W.; Sonpoung, O.; Ariya-Anandech, K.; Hirankarn, N.; et al. Immunosuppressive Polymeric Nanoparticles Targeting Dendritic Cells Alleviate Lupus Disease in Fcgr2b-/- Mice by Mediating Antigen-Specific Immune Tolerance. Int. J. Mol. Sci. 2023, 24, 8313. [Google Scholar] [CrossRef] [PubMed]
- Salvi, V.; Gianello, V.; Busatto, S.; Bergese, P.; Andreoli, L.; D’Oro, U.; Zingoni, A.; Tincani, A.; Sozzani, S.; Bosisio, D. Exosome-delivered microRNAs promote IFN-α secretion by human plasmacytoid DCs via TLR7. JCI Insight 2018, 3, e98204. [Google Scholar] [CrossRef]
- Wiest, M.J.; Baert, L.; Gu, C.; Gayler, K.M.; Ham, H.; Gorvel, L.; Keddis, M.T.; Griffing, L.W.; Joo, H.; Gorvel, J.P.; et al. Endosomal trafficking inhibitor EGA can control TLR7-mediated IFNα expression by human plasmacytoid dendritic cells. Front. Immunol. 2023, 14, 1202197. [Google Scholar] [CrossRef] [PubMed]
- Lorant, A.K.; Yoshida, A.E.; Gilbertson, E.A.; Chu, T.; Stefani, C.; Acharya, M.; Hamerman, J.A.; Lacy-Hulbert, A. Integrin αvβ3 Limits Cytokine Production by Plasmacytoid Dendritic Cells and Restricts TLR-Driven Autoimmunity. J. Immunol. 2024, 212, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, K.A.; Hoi, A.; Gamell, C.; Tai, T.Y.; Linggi, B.; Jordan, J.; Cesaroni, M.; Sato, T.; Ng, M.; Oon, S.; et al. CSL362 potently and specifically depletes pDCs invitro and ablates SLE-immune complex-induced IFN responses. iScience 2023, 26, 107173. [Google Scholar] [CrossRef]
- Sun, L.; Su, Y.; Jiao, A.; Wang, X.; Zhang, B. T cells in health and disease. Signal Transduct. Target. Ther. 2023, 8, 235. [Google Scholar] [CrossRef]
- Oke, V.; Gunnarsson, I.; Dorschner, J.; Eketjäll, S.; Zickert, A.; Niewold, T.B.; Svenungsson, E. High levels of circulating interferons type I, type II and type III associate with distinct clinical features of active systemic lupus erythematosus. Arthritis Res. Ther. 2019, 21, 107. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.K.; Gordon, M.G.; Subramaniam, M.; Kim, M.C.; Hartoularos, G.C.; Targ, S.; Sun, Y.; Ogorodnikov, A.; Bueno, R.; Lu, A.; et al. Single-cell RNA-seq reveals cell type-specific molecular and genetic associations to lupus. Science 2022, 376, eabf1970. [Google Scholar] [CrossRef]
- Dai, X.; Fan, Y.; Zhao, X. Systemic lupus erythematosus: Updated insights on the pathogenesis, diagnosis, prevention and therapeutics. Signal Transduct. Target. Ther. 2025, 10, 102. [Google Scholar] [CrossRef]
- Alunno, A.; Bartoloni, E.; Bistoni, O.; Nocentini, G.; Ronchetti, S.; Caterbi, S.; Valentini, V.; Riccardi, C.; Gerli, R. Balance between regulatory T and Th17 cells in systemic lupus erythematosus: The old and the new. Clin. Dev. Immunol. 2012, 2012, 823085. [Google Scholar] [CrossRef]
- Xin, Y.; He, Z.; Mei, Y.; Li, X.; Zhao, Z.; Zhao, M.; Yang, M.; Wu, H. Interferon-α regulates abnormally increased expression of RSAD2 in Th17 and Tfh cells in systemic lupus erythematosus patients. Eur. J. Immunol. 2023, 53, e2350420. [Google Scholar] [CrossRef]
- Huang, X.; Wu, H.; Qiu, H.; Yang, H.; Deng, Y.; Zhao, M.; Luo, H.; Zhou, X.; Xie, Y.; Chan, V.; et al. The expression of Bcl-6 in circulating follicular helper-like T cells positively correlates with the disease activity in systemic lupus erythematosus. Clin. Immunol. 2016, 173, 161–170. [Google Scholar] [CrossRef]
- Dong, X.; Antao, O.Q.; Song, W.; Sanchez, G.M.; Zembrzuski, K.; Koumpouras, F.; Lemenze, A.; Craft, J.; Weinstein, J.S. Type I Interferon-Activated STAT4 Regulation of Follicular Helper T Cell-Dependent Cytokine and Immunoglobulin Production in Lupus. Arthritis Rheumatol. 2021, 73, 478–489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Qi, H.; Li, M.; Li, Y.; Zhu, X.; Amin, S.; Alexander, M.; Diadhiou, C.; Davidson, A.; Zeng, H. mTORC2 contributes to systemic autoimmunity. Immunology 2023, 168, 554–568. [Google Scholar] [CrossRef]
- Jiang, Q.; Wang, J.; Jiang, H.; Li, W.; Sun, Y.; Shan, Y.; Wei, T.; Chi, X.; Yu, S.; Ma, X. Competitive binding of transcription factors underlies flexibility of T peripheral helper cells and T follicular helper cells in SLE. Rheumatology 2022, 61, 4547–4557. [Google Scholar] [CrossRef]
- Klarquist, J.; Cantrell, R.; Lehn, M.A.; Lampe, K.; Hennies, C.M.; Hoebe, K.; Janssen, E.M. Type I IFN Drives Experimental Systemic Lupus Erythematosus by Distinct Mechanisms in CD4 T Cells and B Cells. Immunohorizons 2020, 4, 140–152. [Google Scholar] [CrossRef]
- Rao, D.A.; Gurish, M.F.; Marshall, J.L.; Slowikowski, K.; Fonseka, C.Y.; Liu, Y.; Donlin, L.T.; Henderson, L.A.; Wei, K.; Mizoguchi, F.; et al. Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis. Nature 2017, 542, 110–114. [Google Scholar] [CrossRef]
- Shan, Y.; Nakayamada, S.; Nawata, A.; Yamagata, K.; Sonomoto, K.; Tanaka, H.; Satoh-Kanda, Y.; Nguyen, M.P.; Todoroki, Y.; Nagayasu, A.; et al. TGF-β3 in differentiation and function of Tph-like cells and its relevance to disease activity in patients with systemic lupus erythematosus. Rheumatology 2023, 62, 2464–2474. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, S.; Seki, N.; Tsujimoto, H.; Saito, S.; Kikuchi, J.; Sugahara, K.; Yoshimoto, K.; Suzuki, K.; Kaneko, Y.; Chiba, K.; et al. Role of interferons (IFNs) in the differentiation of T peripheral helper (Tph) cells. Int. Immunol. 2022, 34, 533–544. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, S.; Wang, S.; Xie, J.; Cui, D. mTOR signaling: A pivotal player in Treg cell dysfunction in systemic lupus erythematosus. Clin. Immunol. 2022, 245, 109153. [Google Scholar] [CrossRef] [PubMed]
- Fava, A.; Buyon, J.; Mohan, C.; Zhang, T.; Belmont, H.M.; Izmirly, P.; Clancy, R.; Trujillo, J.M.; Fine, D.; Zhang, Y.; et al. Integrated urine proteomics and renal single-cell genomics identify an IFN-γ response gradient in lupus nephritis. JCI Insight 2020, 5, e138345. [Google Scholar] [CrossRef]
- Blanco, L.P.; Patino-Martinez, E.; Nakabo, S.; Zhang, M.; Pedersen, H.L.; Wang, X.; Carmona-Rivera, C.; Claybaugh, D.; Yu, Z.X.; Desta, E.; et al. Modulation of the Itaconate Pathway Attenuates Murine Lupus. Arthritis Rheumatol. 2022, 74, 1971–1983. [Google Scholar] [CrossRef] [PubMed]
- Kailashiya, V.; Singh, U.; Kailashiya, J. CTLA4 Gene Polymorphism and its Association with Disease Occurrence, Clinical Manifestations, Serum Markers and Cytokine Levels in SLE Patients from North India. Indian J. Dermatol. 2022, 67, 311. [Google Scholar] [CrossRef]
- Huang, B.; Li, H.; Jiang, Q.; Li, Y.; Jiang, Z.; Cao, H.; Wang, S.; Wang, X.; Li, J.; Li, G. Elevated type I IFN signalling directly affects CD8(+) T-cell distribution and autoantigen recognition of the skeletal muscles in active JDM patients. J. Autoimmun. 2024, 146, 103232. [Google Scholar] [CrossRef]
- Keller, E.J.; Dvorina, N.; Jørgensen, T.N. Spontaneous CD4+ T Cell Activation and Differentiation in Lupus-Prone B6.Nba2 Mice Is IFNAR-Independent. Int. J. Mol. Sci. 2022, 23, 874. [Google Scholar] [CrossRef]
- Radziszewska, A.; Peckham, H.; Restuadi, R.; Kartawinata, M.; Moulding, D.; de Gruijter, N.M.; Robinson, G.A.; Butt, M.; Deakin, C.T.; Wilkinson, M.G.L.; et al. Type I interferon and mitochondrial dysfunction are associated with dysregulated cytotoxic CD8+ T cell responses in juvenile systemic lupus erythematosus. Clin. Exp. Immunol. 2025, 219, uxae127. [Google Scholar] [CrossRef]
- Soni, C.; Perez, O.A.; Voss, W.N.; Pucella, J.N.; Serpas, L.; Mehl, J.; Ching, K.L.; Goike, J.; Georgiou, G.; Ippolito, G.C.; et al. Plasmacytoid Dendritic Cells and Type I Interferon Promote Extrafollicular B Cell Responses to Extracellular Self-DNA. Immunity 2020, 52, 1022–1038.e7. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.H.; Smith, F.L.; Baumgarth, N. B Cell Activation and Response Regulation During Viral Infections. Viral Immunol. 2020, 33, 294–306. [Google Scholar] [CrossRef]
- Barnas, J.L.; Albrecht, J.; Meednu, N.; Alzamareh, D.F.; Baker, C.; McDavid, A.; Looney, R.J.; Anolik, J.H. B Cell Activation and Plasma Cell Differentiation Are Promoted by IFN-λ in Systemic Lupus Erythematosus. J. Immunol. 2021, 207, 2660–2672. [Google Scholar] [CrossRef]
- Dahlgren, M.W.; Plumb, A.W.; Niss, K.; Lahl, K.; Brunak, S.; Johansson-Lindbom, B. Type I Interferons Promote Germinal Centers Through B Cell Intrinsic Signaling and Dendritic Cell Dependent Th1 and Tfh Cell Lineages. Front. Immunol. 2022, 13, 932388. [Google Scholar] [CrossRef]
- Care, M.A.; Stephenson, S.J.; Barnes, N.A.; Fan, I.; Zougman, A.; El-Sherbiny, Y.M.; Vital, E.M.; Westhead, D.R.; Tooze, R.M.; Doody, G.M. Network Analysis Identifies Proinflammatory Plasma Cell Polarization for Secretion of ISG15 in Human Autoimmunity. J. Immunol. 2016, 197, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Quách, T.D.; Manjarrez-Orduño, N.; Adlowitz, D.G.; Silver, L.; Yang, H.; Wei, C.; Milner, E.C.; Sanz, I. Anergic responses characterize a large fraction of human autoreactive naive B cells expressing low levels of surface IgM. J. Immunol. 2011, 186, 4640–4648. [Google Scholar] [CrossRef] [PubMed]
- Ferri, D.M.; Nassar, C.; Manion, K.P.; Kim, M.; Baglaenko, Y.; Muñoz-Grajales, C.; Wither, J.E. Elevated Levels of Interferon-α Act Directly on B Cells to Breach Multiple Tolerance Mechanisms Promoting Autoantibody Production. Arthritis Rheumatol. 2023, 75, 1542–1555. [Google Scholar] [CrossRef]
- Liu, Z.; Davidson, A. IFNα Inducible Models of Murine SLE. Front. Immunol. 2013, 4, 306. [Google Scholar] [CrossRef]
- Gao, M.; Liu, S.; Chatham, W.W.; Mountz, J.D.; Hsu, H.C. IL-4-Induced Quiescence of Resting Naive B Cells Is Disrupted in Systemic Lupus Erythematosus. J. Immunol. 2022, 209, 1513–1522. [Google Scholar] [CrossRef]
- van Dooren, H.J.; Atisha-Fregoso, Y.; Dorjée, A.L.; Huizinga, T.W.J.; Mackay, M.; Aranow, C.; Toes, R.E.M.; Diamond, B.; Suurmond, J. Interferon signatures fuel B cell hyperactivity and plasmablast expansion in systemic lupus erythematosus. J. Autoimmun. 2025, 154, 103438. [Google Scholar] [CrossRef]
- Bradford, H.F.; Haljasmägi, L.; Menon, M.; McDonnell, T.C.R.; Särekannu, K.; Vanker, M.; Peterson, P.; Wincup, C.; Abida, R.; Gonzalez, R.F.; et al. Inactive disease in patients with lupus is linked to autoantibodies to type I interferons that normalize blood IFNα and B cell subsets. Cell Rep. Med. 2023, 4, 100894. [Google Scholar] [CrossRef]
- Sumikawa, M.H.; Iwata, S.; Zhang, M.; Miyata, H.; Ueno, M.; Todoroki, Y.; Nagayasu, A.; Kanda, R.; Sonomoto, K.; Torimoto, K.; et al. An enhanced mitochondrial function through glutamine metabolism in plasmablast differentiation in systemic lupus erythematosus. Rheumatology 2022, 61, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Manolakou, T.; Nikolopoulos, D.; Gkikas, D.; Filia, A.; Samiotaki, M.; Stamatakis, G.; Fanouriakis, A.; Politis, P.; Banos, A.; Alissafi, T.; et al. ATR-mediated DNA damage responses underlie aberrant B cell activity in systemic lupus erythematosus. Sci. Adv. 2022, 8, eabo5840. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C.; Lo, M.S.; Costa Reis, P.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef]
- Henault, J.; Riggs, J.M.; Karnell, J.L.; Liarski, V.M.; Li, J.; Shirinian, L.; Xu, L.; Casey, K.A.; Smith, M.A.; Khatry, D.B.; et al. Self-reactive IgE exacerbates interferon responses associated with autoimmunity. Nat. Immunol. 2016, 17, 196–203. [Google Scholar] [CrossRef]
- Hagberg, N.; Berggren, O.; Leonard, D.; Weber, G.; Bryceson, Y.T.; Alm, G.V.; Eloranta, M.L.; Rönnblom, L. IFN-α production by plasmacytoid dendritic cells stimulated with RNA-containing immune complexes is promoted by NK cells via MIP-1β and LFA-1. J. Immunol. 2011, 186, 5085–5094. [Google Scholar] [CrossRef]
- Lande, R.; Ganguly, D.; Facchinetti, V.; Frasca, L.; Conrad, C.; Gregorio, J.; Meller, S.; Chamilos, G.; Sebasigari, R.; Riccieri, V.; et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra19. [Google Scholar] [CrossRef] [PubMed]
- Wigerblad, G.; Kaplan, M.J. Neutrophil extracellular traps in systemic autoimmune and autoinflammatory diseases. Nat. Rev. Immunol. 2023, 23, 274–288. [Google Scholar] [CrossRef]
- Yan, M.; Gu, Y.; Sun, H.; Ge, Q. Neutrophil extracellular traps in tumor progression and immunotherapy. Front. Immunol. 2023, 14, 1135086. [Google Scholar] [CrossRef]
- Matta, B.; Barnes, B.J. Coordination between innate immune cells, type I IFNs and IRF5 drives SLE pathogenesis. Cytokine 2020, 132, 154731. [Google Scholar] [CrossRef] [PubMed]
- Casey, K.A.; Guo, X.; Smith, M.A.; Wang, S.; Sinibaldi, D.; Sanjuan, M.A.; Wang, L.; Illei, G.G.; White, W.I. Type I interferon receptor blockade with anifrolumab corrects innate and adaptive immune perturbations of SLE. Lupus Sci. Med. 2018, 5, e000286. [Google Scholar] [CrossRef]
- Seridi, L.; Cesaroni, M.; Orillion, A.; Schreiter, J.; Chevrier, M.; Marciniak, S.; Migone, T.S.; Stohl, W.; Chatham, W.W.; Furie, R.A.; et al. Novel signatures associated with systemic lupus erythematosus clinical response to IFN-α/-ω inhibition. Lupus 2021, 30, 795–806. [Google Scholar] [CrossRef]
- Denny, M.F.; Yalavarthi, S.; Zhao, W.; Thacker, S.G.; Anderson, M.; Sandy, A.R.; McCune, W.J.; Kaplan, M.J. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010, 184, 3284–3297. [Google Scholar] [CrossRef] [PubMed]
- Jog, N.R.; Wagner, C.A.; Aberle, T.; Chakravarty, E.F.; Arriens, C.; Guthridge, J.M.; James, J.A. Neutrophils isolated from systemic lupus erythematosus patients exhibit a distinct functional phenotype. Front. Immunol. 2024, 15, 1339250. [Google Scholar] [CrossRef]
- Ma, S.; Jiang, W.; Zhang, X.; Liu, W. Insights into the pathogenic role of neutrophils in systemic lupus erythematosus. Curr. Opin. Rheumatol. 2023, 35, 82–88. [Google Scholar] [CrossRef]
- Leitinger, N.; Schulman, I.G. Phenotypic polarization of macrophages in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1120–1126. [Google Scholar] [CrossRef]
- Xiao, Z.X.; Liang, R.; Olsen, N.; Zheng, S.G. Roles of IRF4 in various immune cells in systemic lupus erythematosus. Int. Immunopharmacol. 2024, 133, 112077. [Google Scholar] [CrossRef]
- Patiño-Martinez, E.; Nakabo, S.; Jiang, K.; Carmona-Rivera, C.; Tsai, W.L.; Claybaugh, D.; Yu, Z.X.; Romero, A.; Bohrnsen, E.; Schwarz, B.; et al. The Aconitate Decarboxylase 1/Itaconate Pathway Modulates Immune Dysregulation and Associates with Cardiovascular Disease Markers and Disease Activity in Systemic Lupus Erythematosus. J. Immunol. 2024, 213, 419–434. [Google Scholar] [CrossRef]
- Zyzak, J.; Mitkiewicz, M.; Leszczyńska, E.; Reniewicz, P.; Moynagh, P.N.; Siednienko, J. HSV-1/TLR9-Mediated IFNβ and TNFα Induction Is Mal-Dependent in Macrophages. J. Innate Immun. 2020, 12, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, T.; Kurasawa, K.; Hasegawa, A.; Miyao, T.; Tanaka, A.; Arai, S.; Arima, M.; Maezawa, R. Differences and similarities in cytokine profiles of macrophage activation syndrome in systemic lupus erythematosus and adult-onset Still’s disease. Clin. Exp. Med. 2023, 23, 3407–3416. [Google Scholar] [CrossRef]
- Qian, Y.; Lu, M.; Zheng, Q. IL-10 and IFN-γ as markers for early recognition of pediatric systemic lupus erythematosus complicated with macrophage activation syndrome. Rheumatology 2025, 64, 235–241. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, X.; Wang, X.; Li, B.; Yu, H.; Quan, Y.; Jiang, Y.; You, Y.; Wang, Y.; Wen, M.; et al. Cellular spermine targets JAK signaling to restrain cytokine-mediated autoimmunity. Immunity 2024, 57, 1796–1811.e8. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Shen, H.; Zhang, Y.; Liu, C.; Li, S.; Yao, J.; Jin, Z.; Yu, H. Integrative analysis of single-cell and bulk transcriptome data reveal the significant role of macrophages in lupus nephritis. Arthritis Res. Ther. 2024, 26, 84. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, B.; Ding, H.; Li, X.; Wang, X.; Zhang, X.; Liu, Q.; Feng, Q.; Han, M.; Chen, L.; et al. The Oxysterol Receptor EBI2 Links Innate and Adaptive Immunity to Limit IFN Response and Systemic Lupus Erythematosus. Adv. Sci. 2023, 10, e2207108. [Google Scholar] [CrossRef] [PubMed]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef]
- Reeves, W.H.; Lee, P.Y.; Weinstein, J.S.; Satoh, M.; Lu, L. Induction of autoimmunity by pristane and other naturally occurring hydrocarbons. Trends Immunol. 2009, 30, 455–464. [Google Scholar] [CrossRef]
- Stergioti, E.M.; Manolakou, T.; Sentis, G.; Samiotaki, M.; Kapsala, N.; Fanouriakis, A.; Boumpas, D.T.; Banos, A. Transcriptomic and proteomic profiling reveals distinct pathogenic features of peripheral non-classical monocytes in systemic lupus erythematosus. Clin. Immunol. 2023, 255, 109765. [Google Scholar] [CrossRef]
- Kuga, T.; Chiba, A.; Murayama, G.; Hosomi, K.; Nakagawa, T.; Yahagi, Y.; Noto, D.; Kusaoi, M.; Kawano, F.; Yamaji, K.; et al. Enhanced GATA4 expression in senescent systemic lupus erythematosus monocytes promotes high levels of IFNα production. Front. Immunol. 2024, 15, 1320444. [Google Scholar] [CrossRef]
- Chen, K.L.; Patel, J.; Zeidi, M.; Wysocka, M.; Bashir, M.M.; Patel, B.; Maddukuri, S.; White, B.; Werth, V.P. Myeloid Dendritic Cells Are Major Producers of IFN-β in Dermatomyositis and May Contribute to Hydroxychloroquine Refractoriness. J. Investig. Dermatol. 2021, 141, 1906–1914.e2. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Raterman, H.G.; Vosslamber, S.; de Ridder, S.; Nurmohamed, M.T.; Lems, W.F.; Boers, M.; van de Wiel, M.; Dijkmans, B.A.; Verweij, C.L.; Voskuyl, A.E. The interferon type I signature towards prediction of non-response to rituximab in rheumatoid arthritis patients. Arthritis Res. Ther. 2012, 14, R95. [Google Scholar] [CrossRef]
- Iwasaki, T.; Watanabe, R.; Ito, H.; Fujii, T.; Okuma, K.; Oku, T.; Hirayama, Y.; Ohmura, K.; Murata, K.; Murakami, K.; et al. Dynamics of Type I and Type II Interferon Signature Determines Responsiveness to Anti-TNF Therapy in Rheumatoid Arthritis. Front. Immunol. 2022, 13, 901437. [Google Scholar] [CrossRef] [PubMed]
- Mavragani, C.P.; La, D.T.; Stohl, W.; Crow, M.K. Association of the response to tumor necrosis factor antagonists with plasma type I interferon activity and interferon-beta/alpha ratios in rheumatoid arthritis patients: A post hoc analysis of a predominantly Hispanic cohort. Arthritis Rheum. 2010, 62, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Wampler Muskardin, T.; Vashisht, P.; Dorschner, J.M.; Jensen, M.A.; Chrabot, B.S.; Kern, M.; Curtis, J.R.; Danila, M.I.; Cofield, S.S.; Shadick, N.; et al. Increased pretreatment serum IFN-β/α ratio predicts non-response to tumour necrosis factor α inhibition in rheumatoid arthritis. Ann. Rheum. Dis. 2016, 75, 1757–1762. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.D.; Vosslamber, S.; Mantel, E.; de Ridder, S.; Wesseling, J.G.; van der Pouw Kraan, T.C.; Leurs, C.; Hegen, H.; Deisenhammer, F.; Killestein, J.; et al. Physiological evidence for diversification of IFNα- and IFNβ-mediated response programs in different autoimmune diseases. Arthritis Res. Ther. 2016, 18, 49. [Google Scholar] [CrossRef]
- Coclet-Ninin, J.; Dayer, J.M.; Burger, D. Interferon-beta not only inhibits interleukin-1beta and tumor necrosis factor-alpha but stimulates interleukin-1 receptor antagonist production in human peripheral blood mononuclear cells. Eur. Cytokine Netw. 1997, 8, 345–349. [Google Scholar]
- Palmer, G.; Mezin, F.; Juge-Aubry, C.E.; Plater-Zyberk, C.; Gabay, C.; Guerne, P.A. Interferon beta stimulates interleukin 1 receptor antagonist production in human articular chondrocytes and synovial fibroblasts. Ann. Rheum. Dis. 2004, 63, 43–49. [Google Scholar] [CrossRef]
- van Holten, J.; Reedquist, K.; Sattonet-Roche, P.; Smeets, T.J.; Plater-Zyberk, C.; Vervoordeldonk, M.J.; Tak, P.P. Treatment with recombinant interferon-beta reduces inflammation and slows cartilage destruction in the collagen-induced arthritis model of rheumatoid arthritis. Arthritis Res. Ther. 2004, 6, R239. [Google Scholar] [CrossRef] [PubMed]
- van Holten, J.; Pavelka, K.; Vencovsky, J.; Stahl, H.; Rozman, B.; Genovese, M.; Kivitz, A.J.; Alvaro, J.; Nuki, G.; Furst, D.E.; et al. A multicentre, randomised, double blind, placebo controlled phase II study of subcutaneous interferon beta-1a in the treatment of patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2005, 64, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chakravarty, S.D.; Ivashkiv, L.B. Regulation of interferon and Toll-like receptor signaling during macrophage activation by opposing feedforward and feedback inhibition mechanisms. Immunol. Rev. 2008, 226, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Orange, D.E.; Agius, P.; DiCarlo, E.F.; Robine, N.; Geiger, H.; Szymonifka, J.; McNamara, M.; Cummings, R.; Andersen, K.M.; Mirza, S.; et al. Identification of Three Rheumatoid Arthritis Disease Subtypes by Machine Learning Integration of Synovial Histologic Features and RNA Sequencing Data. Arthritis Rheumatol. 2018, 70, 690–701. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef]
- Lübbers, J.; Brink, M.; van de Stadt, L.A.; Vosslamber, S.; Wesseling, J.G.; van Schaardenburg, D.; Rantapää-Dahlqvist, S.; Verweij, C.L. The type I IFN signature as a biomarker of preclinical rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 776–780. [Google Scholar] [CrossRef]
- Barrat, F.J.; Crow, M.K.; Ivashkiv, L.B. Interferon target-gene expression and epigenomic signatures in health and disease. Nat. Immunol. 2019, 20, 1574–1583. [Google Scholar] [CrossRef]
- Higgs, B.W.; Liu, Z.; White, B.; Zhu, W.; White, W.I.; Morehouse, C.; Brohawn, P.; Kiener, P.A.; Richman, L.; Fiorentino, D.; et al. Patients with systemic lupus erythematosus, myositis, rheumatoid arthritis and scleroderma share activation of a common type I interferon pathway. Ann. Rheum. Dis. 2011, 70, 2029–2036. [Google Scholar] [CrossRef]
- Dieguez-Gonzalez, R.; Calaza, M.; Perez-Pampin, E.; de la Serna, A.R.; Fernandez-Gutierrez, B.; Castañeda, S.; Largo, R.; Joven, B.; Narvaez, J.; Navarro, F.; et al. Association of interferon regulatory factor 5 haplotypes, similar to that found in systemic lupus erythematosus, in a large subgroup of patients with rheumatoid arthritis. Arthritis Rheum. 2008, 58, 1264–1274. [Google Scholar] [CrossRef] [PubMed]
- Han, T.U.; Cho, S.K.; Kim, T.; Joo, Y.B.; Bae, S.C.; Kang, C. Association of an activity-enhancing variant of IRAK1 and an MECP2-IRAK1 haplotype with increased susceptibility to rheumatoid arthritis. Arthritis Rheum. 2013, 65, 590–598. [Google Scholar] [CrossRef]
- Cooles, F.A.H.; Anderson, A.E.; Lendrem, D.W.; Norris, J.; Pratt, A.G.; Hilkens, C.M.U.; Isaacs, J.D. The interferon gene signature is increased in patients with early treatment-naive rheumatoid arthritis and predicts a poorer response to initial therapy. J. Allergy Clin. Immunol. 2018, 141, 445–448.e4. [Google Scholar] [CrossRef]
- Cooles, F.A.H.; Tarn, J.; Lendrem, D.W.; Naamane, N.; Lin, C.M.; Millar, B.; Maney, N.J.; Anderson, A.E.; Thalayasingam, N.; Diboll, J.; et al. Interferon-α-mediated therapeutic resistance in early rheumatoid arthritis implicates epigenetic reprogramming. Ann. Rheum. Dis. 2022, 81, 1214–1223. [Google Scholar] [CrossRef]
- de Jong, T.D.; Blits, M.; de Ridder, S.; Vosslamber, S.; Wolbink, G.; Nurmohamed, M.T.; Verweij, C.L. Type I interferon response gene expression in established rheumatoid arthritis is not associated with clinical parameters. Arthritis Res. Ther. 2016, 18, 290. [Google Scholar] [CrossRef]
- Seyhan, A.A.; Gregory, B.; Cribbs, A.P.; Bhalara, S.; Li, Y.; Loreth, C.; Zhang, Y.; Guo, Y.; Lin, L.L.; Feldmann, M.; et al. Novel biomarkers of a peripheral blood interferon signature associated with drug-naïve early arthritis patients distinguish persistent from self-limiting disease course. Sci. Rep. 2020, 10, 8830. [Google Scholar] [CrossRef]
- Chalan, P.; Bijzet, J.; van den Berg, A.; Kluiver, J.; Kroesen, B.J.; Boots, A.M.; Brouwer, E. Analysis of serum immune markers in seropositive and seronegative rheumatoid arthritis and in high-risk seropositive arthralgia patients. Sci. Rep. 2016, 6, 26021. [Google Scholar] [CrossRef]
- Macías-Segura, N.; Castañeda-Delgado, J.E.; Bastian, Y.; Santiago-Algarra, D.; Castillo-Ortiz, J.D.; Alemán-Navarro, A.L.; Jaime-Sánchez, E.; Gomez-Moreno, M.; Saucedo-Toral, C.A.; Lara-Ramírez, E.E.; et al. Transcriptional signature associated with early rheumatoid arthritis and healthy individuals at high risk to develop the disease. PLoS ONE 2018, 13, e0194205. [Google Scholar] [CrossRef] [PubMed]
- Brink, M.; Lundquist, A.; Alexeyenko, A.; Lejon, K.; Rantapää-Dahlqvist, S. Protein profiling and network enrichment analysis in individuals before and after the onset of rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 288. [Google Scholar] [CrossRef] [PubMed]
- Castañeda-Delgado, J.E.; Bastián-Hernandez, Y.; Macias-Segura, N.; Santiago-Algarra, D.; Castillo-Ortiz, J.D.; Alemán-Navarro, A.L.; Martínez-Tejada, P.; Enciso-Moreno, L.; Garcia-De Lira, Y.; Olguín-Calderón, D.; et al. Type I Interferon Gene Response Is Increased in Early and Established Rheumatoid Arthritis and Correlates with Autoantibody Production. Front. Immunol. 2017, 8, 285. [Google Scholar] [CrossRef]
- Reynolds, J.A.; Briggs, T.A.; Rice, G.I.; Darmalinggam, S.; Bondet, V.; Bruce, E.; Khan, M.; Haque, S.; Chinoy, H.; Herrick, A.L.; et al. Type I interferon in patients with systemic autoimmune rheumatic disease is associated with haematological abnormalities and specific autoantibody profiles. Arthritis Res. Ther. 2019, 21, 147. [Google Scholar] [CrossRef]
- Dawidowicz, K.; Allanore, Y.; Guedj, M.; Pierlot, C.; Bombardieri, S.; Balsa, A.; Westhovens, R.; Barrera, P.; Alves, H.; Teixeira, V.H.; et al. The interferon regulatory factor 5 gene confers susceptibility to rheumatoid arthritis and influences its erosive phenotype. Ann. Rheum. Dis. 2011, 70, 117–121. [Google Scholar] [CrossRef]
- Adams, C.; Nair, N.; Plant, D.; Verstappen, S.M.M.; Quach, H.L.; Quach, D.L.; Carvidi, A.; Nititham, J.; Nakamura, M.; Graf, J.; et al. Identification of Cell-Specific Differential DNA Methylation Associated With Methotrexate Treatment Response in Rheumatoid Arthritis. Arthritis Rheumatol. 2023, 75, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Nair, N.; Plant, D.; Verstappen, S.M.; Isaacs, J.D.; Morgan, A.W.; Hyrich, K.L.; Barton, A.; Wilson, A.G. Differential DNA methylation correlates with response to methotrexate in rheumatoid arthritis. Rheumatology 2020, 59, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Gosselt, H.R.; van Zelst, B.D.; de Rotte, M.; Hazes, J.M.W.; de Jonge, R.; Heil, S.G. Higher baseline global leukocyte DNA methylation is associated with MTX non-response in early RA patients. Arthritis Res. Ther. 2019, 21, 157. [Google Scholar] [CrossRef]
- Yang, C.; Li, D.; Teng, D.; Zhou, Y.; Zhang, L.; Zhong, Z.; Yang, G.J. Epigenetic Regulation in the Pathogenesis of Rheumatoid Arthritis. Front. Immunol. 2022, 13, 859400. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Chen, B.; Li, S.; Yang, L.; Zhu, D.; Wang, Y.; Wang, H.; Wang, T.; Shi, B.; Gai, Z.; et al. Detection and characterization of bacterial nucleic acids in culture-negative synovial tissue and fluid samples from rheumatoid arthritis or osteoarthritis patients. Sci. Rep. 2018, 8, 14305. [Google Scholar] [CrossRef]
- van der Heijden, I.M.; Wilbrink, B.; Tchetverikov, I.; Schrijver, I.A.; Schouls, L.M.; Hazenberg, M.P.; Breedveld, F.C.; Tak, P.P. Presence of bacterial DNA and bacterial peptidoglycans in joints of patients with rheumatoid arthritis and other arthritides. Arthritis Rheum. 2000, 43, 593–598. [Google Scholar] [CrossRef]
- Chen, T.; Rimpiläinen, M.; Luukkainen, R.; Möttönen, T.; Yli-Jama, T.; Jalava, J.; Vainio, O.; Toivanen, P. Bacterial components in the synovial tissue of patients with advanced rheumatoid arthritis or osteoarthritis: Analysis with gas chromatography-mass spectrometry and pan-bacterial polymerase chain reaction. Arthritis Rheum. 2003, 49, 328–334. [Google Scholar] [CrossRef]
- Kassiotis, G. The Immunological Conundrum of Endogenous Retroelements. Annu. Rev. Immunol. 2023, 41, 99–125. [Google Scholar] [CrossRef]
- van der Pouw Kraan, T.C.; van Baarsen, L.G.; Wijbrandts, C.A.; Voskuyl, A.E.; Rustenburg, F.; Baggen, J.M.; Dijkmans, B.A.; Tak, P.P.; Verweij, C.L. Expression of a pathogen-response program in peripheral blood cells defines a subgroup of rheumatoid arthritis patients. Genes Immun. 2008, 9, 16–22. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kawane, K.; Ohtani, M.; Miwa, K.; Kizawa, T.; Kanbara, Y.; Yoshioka, Y.; Yoshikawa, H.; Nagata, S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature 2006, 443, 998–1002. [Google Scholar] [CrossRef]
- Hashimoto, T.; Yoshida, K.; Hashimoto, N.; Nakai, A.; Kaneshiro, K.; Suzuki, K.; Kawasaki, Y.; Shibanuma, N.; Hashiramoto, A. Circulating cell free DNA: A marker to predict the therapeutic response for biological DMARDs in rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 722–730. [Google Scholar] [CrossRef]
- Dong, C.; Liu, Y.; Sun, C.; Liang, H.; Dai, L.; Shen, J.; Wei, S.; Guo, S.; Leong, K.W.; Chen, Y.; et al. Identification of Specific Joint-Inflammatogenic Cell-Free DNA Molecules From Synovial Fluids of Patients With Rheumatoid Arthritis. Front. Immunol. 2020, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ye, J.; Pan, N.; Tan, C.; Herrmann, M. Neutrophil Extracellular Traps Tied to Rheumatoid Arthritis: Points to Ponder. Front. Immunol. 2020, 11, 578129. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Carmona-Rivera, C.; Smith, C.K.; Kaplan, M.J. Neutrophil extracellular trap-associated protein activation of the NLRP3 inflammasome is enhanced in lupus macrophages. J. Immunol. 2013, 190, 1217–1226. [Google Scholar] [CrossRef] [PubMed]
- Guiducci, C.; Tripodo, C.; Gong, M.; Sangaletti, S.; Colombo, M.P.; Coffman, R.L.; Barrat, F.J. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 2010, 207, 2931–2942. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, X.; Zhang, S.; Deng, C.; Zhao, L.; Wang, M.; Wu, Q.; Yang, H.; Zhou, J.; Peng, L.; et al. The potential roles of type I interferon activated neutrophils and neutrophil extracellular traps (NETs) in the pathogenesis of primary Sjögren’s syndrome. Arthritis Res. Ther. 2022, 24, 170. [Google Scholar] [CrossRef]
- Patterson, S.L.; Sun, S.; Rychkov, D.; Katz, P.; Tsitsiklis, A.; Nakamura, M.C.; Serpa, P.H.; Langelier, C.R.; Sirota, M. Physical Activity Associates With Lower Systemic Inflammatory Gene Expression in Rheumatoid Arthritis. J. Rheumatol. 2022, 49, 1320–1327. [Google Scholar] [CrossRef]
- Baccala, R.; Hoebe, K.; Kono, D.H.; Beutler, B.; Theofilopoulos, A.N. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat. Med. 2007, 13, 543–551. [Google Scholar] [CrossRef]
- Lande, R.; Giacomini, E.; Serafini, B.; Rosicarelli, B.; Sebastiani, G.D.; Minisola, G.; Tarantino, U.; Riccieri, V.; Valesini, G.; Coccia, E.M. Characterization and recruitment of plasmacytoid dendritic cells in synovial fluid and tissue of patients with chronic inflammatory arthritis. J. Immunol. 2004, 173, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, L.L.; Boyce, A.; Smith, L.; Padmanabha, J.; Filgueira, L.; Pietschmann, P.; Thomas, R. Rheumatoid arthritis synovium contains plasmacytoid dendritic cells. Arthritis Res. Ther. 2005, 7, R230. [Google Scholar] [CrossRef]
- van Holten, J.; Smeets, T.J.; Blankert, P.; Tak, P.P. Expression of interferon beta in synovial tissue from patients with rheumatoid arthritis: Comparison with patients with osteoarthritis and reactive arthritis. Ann. Rheum. Dis. 2005, 64, 1780–1782. [Google Scholar] [CrossRef]
- Roelofs, M.F.; Wenink, M.H.; Brentano, F.; Abdollahi-Roodsaz, S.; Oppers-Walgreen, B.; Barrera, P.; van Riel, P.L.; Joosten, L.A.; Kyburz, D.; van den Berg, W.B.; et al. Type I interferons might form the link between Toll-like receptor (TLR) 3/7 and TLR4-mediated synovial inflammation in rheumatoid arthritis (RA). Ann. Rheum. Dis. 2009, 68, 1486–1493. [Google Scholar] [CrossRef]
- Kwok, S.K.; Lee, J.Y.; Park, S.H.; Cho, M.L.; Min, S.Y.; Park, S.H.; Kim, H.Y.; Cho, Y.G. Dysfunctional interferon-alpha production by peripheral plasmacytoid dendritic cells upon Toll-like receptor-9 stimulation in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2008, 10, R29. [Google Scholar] [CrossRef]
- Jongbloed, S.L.; Lebre, M.C.; Fraser, A.R.; Gracie, J.A.; Sturrock, R.D.; Tak, P.P.; McInnes, I.B. Enumeration and phenotypical analysis of distinct dendritic cell subsets in psoriatic arthritis and rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R15. [Google Scholar] [CrossRef]
- Lebre, M.C.; Jongbloed, S.L.; Tas, S.W.; Smeets, T.J.; McInnes, I.B.; Tak, P.P. Rheumatoid arthritis synovium contains two subsets of CD83-DC-LAMP- dendritic cells with distinct cytokine profiles. Am. J. Pathol. 2008, 172, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Narendra, S.C.; Chalise, J.P.; Höök, N.; Magnusson, M. Dendritic cells activated by double-stranded RNA induce arthritis via autocrine type I IFN signaling. J. Leukoc. Biol. 2014, 95, 661–666. [Google Scholar] [CrossRef]
- Nehmar, R.; Alsaleh, G.; Voisin, B.; Flacher, V.; Mariotte, A.; Saferding, V.; Puchner, A.; Niederreiter, B.; Vandamme, T.; Schabbauer, G.; et al. Therapeutic Modulation of Plasmacytoid Dendritic Cells in Experimental Arthritis. Arthritis Rheumatol. 2017, 69, 2124–2135. [Google Scholar] [CrossRef] [PubMed]
- Cooles, F.A.H.; Anderson, A.E.; Skelton, A.; Pratt, A.G.; Kurowska-Stolarska, M.S.; McInnes, I.; Hilkens, C.M.U.; Isaacs, J.D. Phenotypic and Transcriptomic Analysis of Peripheral Blood Plasmacytoid and Conventional Dendritic Cells in Early Drug Naïve Rheumatoid Arthritis. Front. Immunol. 2018, 9, 755. [Google Scholar] [CrossRef]
- Yamada, S.; Nagafuchi, Y.; Wang, M.; Ota, M.; Hatano, H.; Takeshima, Y.; Okubo, M.; Kobayashi, S.; Sugimori, Y.; Masahiro, N.; et al. Immunomics analysis of rheumatoid arthritis identified precursor dendritic cells as a key cell subset of treatment resistance. Ann. Rheum. Dis. 2023, 82, 809–819. [Google Scholar] [CrossRef]
- Németh, T.; Nagy, G.; Pap, T. Synovial fibroblasts as potential drug targets in rheumatoid arthritis, where do we stand and where shall we go? Ann. Rheum. Dis. 2022, 81, 1055–1064. [Google Scholar] [CrossRef]
- Mousavi, M.J.; Karami, J.; Aslani, S.; Tahmasebi, M.N.; Vaziri, A.S.; Jamshidi, A.; Farhadi, E.; Mahmoudi, M. Transformation of fibroblast-like synoviocytes in rheumatoid arthritis; from a friend to foe. Autoimmuity Highlights 2021, 12, 3. [Google Scholar] [CrossRef]
- Carini, C.; Hunter, E.; Ramadass, A.S.; Green, J.; Akoulitchev, A.; McInnes, I.B.; Goodyear, C.S. Chromosome conformation signatures define predictive markers of inadequate response to methotrexate in early rheumatoid arthritis. J. Transl. Med. 2018, 16, 18. [Google Scholar] [CrossRef]
- Triantaphyllopoulos, K.A.; Williams, R.O.; Tailor, H.; Chernajovsky, Y. Amelioration of collagen-induced arthritis and suppression of interferon-gamma, interleukin-12, and tumor necrosis factor alpha production by interferon-beta gene therapy. Arthritis Rheum. 1999, 42, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Li, J.; Wu, R. Neutrophils in Rheumatoid Arthritis Synovium: Implications on Disease Activity and Inflammation State. J. Inflamm. Res. 2025, 18, 4741–4753. [Google Scholar] [CrossRef] [PubMed]
- de Jong, T.D.; Lübbers, J.; Turk, S.; Vosslamber, S.; Mantel, E.; Bontkes, H.J.; van der Laken, C.J.; Bijlsma, J.W.; van Schaardenburg, D.; Verweij, C.L. The type I interferon signature in leukocyte subsets from peripheral blood of patients with early arthritis: A major contribution by granulocytes. Arthritis Res. Ther. 2016, 18, 165. [Google Scholar] [CrossRef]
- Wright, H.L.; Thomas, H.B.; Moots, R.J.; Edwards, S.W. Interferon gene expression signature in rheumatoid arthritis neutrophils correlates with a good response to TNFi therapy. Rheumatology 2015, 54, 188–193. [Google Scholar] [CrossRef]
- Rönnblom, L.; Eloranta, M.L. The interferon signature in autoimmune diseases. Curr. Opin. Rheumatol. 2013, 25, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Wampler Muskardin, T.L.; Fan, W.; Jin, Z.; Jensen, M.A.; Dorschner, J.M.; Ghodke-Puranik, Y.; Dicke, B.; Vsetecka, D.; Wright, K.; Mason, T.; et al. Distinct Single Cell Gene Expression in Peripheral Blood Monocytes Correlates With Tumor Necrosis Factor Inhibitor Treatment Response Groups Defined by Type I Interferon in Rheumatoid Arthritis. Front. Immunol. 2020, 11, 1384. [Google Scholar] [CrossRef]
- Obermoser, G.; Pascual, V. The interferon-alpha signature of systemic lupus erythematosus. Lupus 2010, 19, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Coutant, F. Shaping of Monocyte-Derived Dendritic Cell Development and Function by Environmental Factors in Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 13670. [Google Scholar] [CrossRef]
- Seyler, T.M.; Park, Y.W.; Takemura, S.; Bram, R.J.; Kurtin, P.J.; Goronzy, J.J.; Weyand, C.M. BLyS and APRIL in rheumatoid arthritis. J. Clin. Investig. 2005, 115, 3083–3092. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.W.; Kolhatkar, N.S.; Rawlings, D.J. B cells take the front seat: Dysregulated B cell signals orchestrate loss of tolerance and autoantibody production. Curr. Opin. Immunol. 2015, 33, 70–77. [Google Scholar] [CrossRef]
- Litinskiy, M.B.; Nardelli, B.; Hilbert, D.M.; He, B.; Schaffer, A.; Casali, P.; Cerutti, A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002, 3, 822–829. [Google Scholar] [CrossRef]
- Jarry, A.; Malard, F.; Bou-Hanna, C.; Meurette, G.; Mohty, M.; Mosnier, J.F.; Laboisse, C.L.; Bossard, C. Interferon-Alpha Promotes Th1 Response and Epithelial Apoptosis via Inflammasome Activation in Human Intestinal Mucosa. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 72–81. [Google Scholar] [CrossRef]
- Eisenbarth, S.C.; Baumjohann, D.; Craft, J.; Fazilleau, N.; Ma, C.S.; Tangye, S.G.; Vinuesa, C.G.; Linterman, M.A. CD4(+) T cells that help B cells—A proposal for uniform nomenclature. Trends Immunol. 2021, 42, 658–669. [Google Scholar] [CrossRef]
- Kolumam, G.A.; Thomas, S.; Thompson, L.J.; Sprent, J.; Murali-Krishna, K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J. Exp. Med. 2005, 202, 637–650. [Google Scholar] [CrossRef]
- Firestein, G.S.; Zvaifler, N.J. Peripheral blood and synovial fluid monocyte activation in inflammatory arthritis. II. Low levels of synovial fluid and synovial tissue interferon suggest that gamma-interferon is not the primary macrophage activating factor. Arthritis Rheum. 1987, 30, 864–871. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwland, M.; Mulder, A.H.L.; Colin, E.M.; Alves, C.; van Bon, L.; Brouwer, E. Investigating interferon type I responses in patients with suspected giant cell arteritis and polymyalgia rheumatica. Clin. Exp. Immunol. 2024, 218, 308–313. [Google Scholar] [CrossRef]
- Zhang, H.; Watanabe, R.; Berry, G.J.; Tian, L.; Goronzy, J.J.; Weyand, C.M. Inhibition of JAK-STAT Signaling Suppresses Pathogenic Immune Responses in Medium and Large Vessel Vasculitis. Circulation 2018, 137, 1934–1948. [Google Scholar] [CrossRef] [PubMed]
- Estupiñán-Moreno, E.; Ortiz-Fernández, L.; Li, T.; Hernández-Rodríguez, J.; Ciudad, L.; Andrés-León, E.; Terron-Camero, L.C.; Prieto-González, S.; Espígol-Frigolé, G.; Cid, M.C.; et al. Methylome and transcriptome profiling of giant cell arteritis monocytes reveals novel pathways involved in disease pathogenesis and molecular response to glucocorticoids. Ann. Rheum. Dis. 2022, 81, 1290–1300. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Berry, G.J.; Liang, D.H.; Goronzy, J.J.; Weyand, C.M. Cellular Signaling Pathways in Medium and Large Vessel Vasculitis. Front. Immunol. 2020, 11, 587089. [Google Scholar] [CrossRef]
- Corbera-Bellalta, M.; Planas-Rigol, E.; Lozano, E.; Terrades-García, N.; Alba, M.A.; Prieto-González, S.; García-Martínez, A.; Albero, R.; Enjuanes, A.; Espígol-Frigolé, G.; et al. Blocking interferon γ reduces expression of chemokines CXCL9, CXCL10 and CXCL11 and decreases macrophage infiltration in ex vivo cultured arteries from patients with giant cell arteritis. Ann. Rheum. Dis. 2016, 75, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- AbbVie Inc. Rinvoq (Upadacitinib) Receives U.S. FDA Approval for Giant Cell Arteritis [News Release]. 29 April 2025. Available online: https://news.abbvie.com/2025-04-29-RINVOQ-R-upadacitinib-Receives-U-S-FDA-Approval-for-Giant-Cell-Arteritis-GCA (accessed on 29 September 2025).
- Koster, M.J.; Crowson, C.S.; Giblon, R.E.; Jaquith, J.M.; Duarte-García, A.; Matteson, E.L.; Weyand, C.M.; Warrington, K.J. Baricitinib for relapsing giant cell arteritis: A prospective open-label 52-week pilot study. Ann. Rheum. Dis. 2022, 81, 861–867. [Google Scholar] [CrossRef]
- Batten, I.; Robinson, M.W.; White, A.; Walsh, C.; Fazekas, B.; Wyse, J.; Buettner, A.; D’Arcy, S.; Greenan, E.; Murphy, C.C.; et al. Investigation of type I interferon responses in ANCA-associated vasculitis. Sci. Rep. 2021, 11, 8272. [Google Scholar] [CrossRef] [PubMed]
- Kessler, N.; Viehmann, S.F.; Krollmann, C.; Mai, K.; Kirschner, K.M.; Luksch, H.; Kotagiri, P.; Böhner, A.M.C.; Huugen, D.; de Oliveira Mann, C.C.; et al. Monocyte-derived macrophages aggravate pulmonary vasculitis via cGAS/STING/IFN-mediated nucleic acid sensing. J. Exp. Med. 2022, 219, e20220759. [Google Scholar] [CrossRef]
- Uslu, E.; İlbay, A.; Göveç Giynaş, N.; Gaydan, A.; Ateş, E.S.; Dalva, K.; Yüksel, M.; Sezer, S.; Yayla, M.E.; Turgay, T.M.; et al. ABS0942 Type I Interferon Signature in Large Vessel Vasculitis; Present or Imaginary. Ann. Rheum. Dis. 2025, 84, 2349. [Google Scholar] [CrossRef]
- Sacre, K.; Criswell, L.A.; McCune, J.M. Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res. Ther. 2012, 14, R155. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Andersen, J.; Aringer, M.; Arnaud, L.; Bae, S.C.; Boletis, J.; Bruce, I.N.; Cervera, R.; Doria, A.; et al. EULAR recommendations for the management of systemic lupus erythematosus: 2023 update. Ann. Rheum. Dis. 2024, 83, 15–29. [Google Scholar] [CrossRef]
- Takei, H.; Takanashi, S.; Otomo, K.; Hanaoka, H.; Kikuchi, J.; Yamaoka, K.; Yoshimoto, K.; Abe, T.; Takeuchi, T.; Kaneko, Y. Clinical and immunological effects of hydroxychloroquine in patients with active rheumatoid arthritis despite antirheumatic treatment. Mod. Rheumatol. 2023, 34, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, Y.; Li, B.; Li, J.; Qiu, Y.; Zhu, Z.; Hua, H. An immunomodulatory antibody-drug conjugate targeting BDCA2 strongly suppresses plasmacytoid dendritic cell function and glucocorticoid responsive genes. Rheumatology 2024, 63, 242–250. [Google Scholar] [CrossRef]
- Yang, D.; Peng, N.; Zhang, H.; Qiu, Z.; Xu, L.; Pan, M. Cordycepin ameliorates autoimmunity by promoting STING degradation via autophagy pathway. Br. J. Pharmacol. 2025, 182, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Bekaddour, N.; Smith, N.; Caspar, B.; Grinberg, S.; Giorgiutti, S.; Rodeschini, V.; Dupuy, S.; Leboulanger, N.; Duffy, D.; Soulas-Sprauel, P.; et al. The histamine analogue clobenpropit modulates IRF7 phosphorylation and interferon production by targeting CXCR4 in systemic lupus erythematosus models. Front. Immunol. 2024, 15, 1490593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wang, J.; Wang, X.; Wang, B.; Zhao, Z.; Fu, J.; Wang, Y.; Zhang, X.; Zhu, P.; Jiang, M.; et al. Degradation of HDAC10 by autophagy promotes IRF3-mediated antiviral innate immune responses. Sci. Signal. 2022, 15, eabo4356. [Google Scholar] [CrossRef]
- Deshmukh, A.; Pereira, A.; Geraci, N.; Tzvetkov, E.; Przetak, M.; Catalina, M.D.; Morand, E.F.; Bender, A.T.; Vaidyanathan, B. Preclinical Evidence for the Glucocorticoid-Sparing Potential of a Dual Toll-Like Receptor 7/8 Inhibitor in Autoimmune Diseases. J. Pharmacol. Exp. Ther. 2024, 388, 751–764. [Google Scholar] [CrossRef]
- Witte, T.; Fernandez-Ruiz, R.; Abramova, N.; Weinelt, D.; Moreau, F.; Klopp-Schulze, L.; Shaw, J.; Denis, D.; Wenzel, J. Enpatoran, a first-in-class, selective, orally administered toll-like receptor 7/8 inhibitor, in systemic and cutaneous lupus erythematosus: Results from a randomised, placebo-controlled phase Ib study. Lupus Sci Med. 2025, 12, e001705. [Google Scholar] [CrossRef]
- Hosein, F.; Ignatenko, S.; Chadwick, K.D.; Zhu, L.; Baribaud, F.; Saini, J.; Bach, T.; Aras, U.; Zhang, W.; Karabeber, H.; et al. Safety, Tolerability, Efficacy, Pharmacokinetics, and Pharmacodynamics of Afimetoran, a Toll-Like Receptor 7 and 8 Inhibitor, in Patients With Cutaneous Lupus Erythematosus: A Phase 1b Randomized, Double-Blind, Placebo-Controlled Study. ACR Open Rheumatol. 2025, 7, e70059. [Google Scholar] [CrossRef]
- Suffiotti, M.; Brazauskas, P.; Keller, M.P.; Berkani, O.; Seifer, G.; Cornelisse, P.; Murphy, M.J.; Strasser, D.S. Pharmacodynamics of the S1P(1) receptor modulator cenerimod in a phase 2b randomised clinical trial in patients with moderate to severe SLE. Ann. Rheum. Dis. 2025, 84, 284–293. [Google Scholar] [CrossRef]
- Askanase, A.D.; D’Cruz, D.; Kalunian, K.; Merrill, J.T.; Navarra, S.V.; Cahuzac, C.; Cornelisse, P.; Murphy, M.J.; Strasser, D.S.; Trokan, L.; et al. Cenerimod, a sphingosine-1-phosphate receptor modulator, versus placebo in patients with moderate-to-severe systemic lupus erythematosus (CARE): An international, double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Rheumatol. 2025, 7, e21–e32. [Google Scholar] [CrossRef]
- Sun, J.L.; Lyu, T.B.; Chen, Z.L.; Lian, C.F.; Liu, S.Y.; Shao, T.H.; Zhang, S.; Zhao, L.L.; Liu, J.J.; Peng, L.Y.; et al. Methylprednisolone pulse therapy promotes the differentiation of regulatory T cells by inducing the apoptosis of CD4(+) T cells in patients with systemic lupus erythematosus. Clin. Immunol. 2022, 241, 109079. [Google Scholar] [CrossRef] [PubMed]
- Khamashta, M.; Merrill, J.T.; Werth, V.P.; Furie, R.; Kalunian, K.; Illei, G.G.; Drappa, J.; Wang, L.; Greth, W. Sifalimumab, an anti-interferon-α monoclonal antibody, in moderate to severe systemic lupus erythematosus: A randomised, double-blind, placebo-controlled study. Ann. Rheum. Dis. 2016, 75, 1909–1916. [Google Scholar] [CrossRef]
- Petri, M.; Wallace, D.J.; Spindler, A.; Chindalore, V.; Kalunian, K.; Mysler, E.; Neuwelt, C.M.; Robbie, G.; White, W.I.; Higgs, B.W.; et al. Sifalimumab, a human anti-interferon-α monoclonal antibody, in systemic lupus erythematosus: A phase I randomized, controlled, dose-escalation study. Arthritis Rheum. 2013, 65, 1011–1021. [Google Scholar] [CrossRef]
- Kalunian, K.C.; Merrill, J.T.; Maciuca, R.; McBride, J.M.; Townsend, M.J.; Wei, X.; Davis, J.C., Jr.; Kennedy, W.P. A Phase II study of the efficacy and safety of rontalizumab (rhuMAb interferon-α) in patients with systemic lupus erythematosus (ROSE). Ann. Rheum. Dis. 2016, 75, 196–202. [Google Scholar] [CrossRef]
- Lauwerys, B.R.; Hachulla, E.; Spertini, F.; Lazaro, E.; Jorgensen, C.; Mariette, X.; Haelterman, E.; Grouard-Vogel, G.; Fanget, B.; Dhellin, O.; et al. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis Rheum. 2013, 65, 447–456. [Google Scholar] [CrossRef]
- Higgs, B.W.; Zhu, W.; Morehouse, C.; White, W.I.; Brohawn, P.; Guo, X.; Rebelatto, M.; Le, C.; Amato, A.; Fiorentino, D.; et al. A phase 1b clinical trial evaluating sifalimumab, an anti-IFN-α monoclonal antibody, shows target neutralisation of a type I IFN signature in blood of dermatomyositis and polymyositis patients. Ann. Rheum. Dis. 2014, 73, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Mosca, M.; Emmas, C.; Nekeman-Nan, C.; Stirnadel-Farrant, H.; Chen, S.; Carty, L.; Waratani, M.; Seo, C.; Chen, S.; Sorrentino, A. Anifrolumab Study for Treatment Effectiveness in the Real World (ASTER) among patients with systemic lupus erythematosus: Protocol for an international observational effectiveness study. BMJ Open 2024, 14, e086055. [Google Scholar] [CrossRef] [PubMed]
- Karonitsch, T.; Yeghiazaryan, L.; Lackner, A.; Brezinsek, H.P.; Stamm, T.A.; König, F.; Aletaha, D.; Smolen, J.S. Targeting type I interferon (IFN) signalling in patients with RA with a high type I IFN gene signature. RMD Open 2022, 8, e002525. [Google Scholar] [CrossRef] [PubMed]
- Morand, E.F.; Furie, R.; Tanaka, Y.; Bruce, I.N.; Askanase, A.D.; Richez, C.; Bae, S.C.; Brohawn, P.Z.; Pineda, L.; Berglind, A.; et al. Trial of Anifrolumab in Active Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 382, 211–221. [Google Scholar] [CrossRef]
- Chen, X.; Ke, H.; Li, W.; Yin, L.; Chen, W.; Chen, T.; Wu, Y.; Qiu, J.; Feng, W. Structural basis for the recognition of IFNAR1 by the humanized therapeutic monoclonal antibody QX006N for the treatment of systemic lupus erythematosus. Int. J. Biol. Macromol. 2024, 268, 131721. [Google Scholar] [CrossRef]
- Furie, R.; Khamashta, M.; Merrill, J.T.; Werth, V.P.; Kalunian, K.; Brohawn, P.; Illei, G.G.; Drappa, J.; Wang, L.; Yoo, S. Anifrolumab, an Anti-Interferon-α Receptor Monoclonal Antibody, in Moderate-to-Severe Systemic Lupus Erythematosus. Arthritis Rheumatol. 2017, 69, 376–386. [Google Scholar] [CrossRef]
- Fang, Z.; Sun, H.; Wang, Y.; Sun, Z.; Yin, M. Discovery of WD-890: A novel allosteric TYK2 inhibitor for the treatment of multiple autoimmune diseases. Biomed. Pharmacother. 2023, 167, 115611. [Google Scholar] [CrossRef]
- Ma, X.; Nakayamada, S.; Kubo, S.; Sakata, K.; Yamagata, K.; Miyazaki, Y.; Yoshikawa, M.; Kitanaga, Y.; Zhang, M.; Tanaka, Y. Expansion of T follicular helper-T helper 1 like cells through epigenetic regulation by signal transducer and activator of transcription factors. Ann. Rheum. Dis. 2018, 77, 1354–1361. [Google Scholar] [CrossRef]
- Yin, J.; Hou, Y.; Wang, C.; Qin, C. Clinical outcomes of baricitinib in patients with systemic lupus erythematosus: Pooled analysis of SLE-BRAVE-I and SLE-BRAVE-II trials. PLoS ONE 2025, 20, e0320179. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, S.M.M.; Ahmed Fatmi, S.A.; Ashraf, M.H.; Waheed, F.; Jawwad, M.; Hai, A.A.; Ahmed, S.A. Efficacy of baricitinib in the treatment of Systemic lupus erythematosus (SLE): A Systemic review and meta-analysis. Heliyon 2023, 9, e22643. [Google Scholar] [CrossRef] [PubMed]
- Werth, V.P.; Fleischmann, R.; Robern, M.; Touma, Z.; Tiamiyu, I.; Gurtovaya, O.; Pechonkina, A.; Mozaffarian, A.; Downie, B.; Matzkies, F.; et al. Filgotinib or lanraplenib in moderate to severe cutaneous lupus erythematosus: A phase 2, randomized, double-blind, placebo-controlled study. Rheumatology 2022, 61, 2413–2423. [Google Scholar] [CrossRef]
- Merrill, J.T.; Saxena, A.; Aringer, M.; Tanaka, Y.; Zeng, X.; Cheng, L.; Doan, T.T.; D’Cruz, D.; Masri, K.R.; D’Silva, K.M. Efficacy and safety of upadacitinib as monotherapy or combined with elsubrutinib for the treatment of systemic lupus erythematosus: Results through 104 weeks in a long-term extension study. RMD Open 2025, 11, e005742. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ning, W.; Tang, H.; Mu, C.; Huang, X. Efficacy and safety study of targeted small-molecule drugs in the treatment of systemic lupus erythematosus. Arthritis Res. Ther. 2024, 26, 98. [Google Scholar] [CrossRef]
- Satoh-Kanda, Y.; Nakayamada, S.; Tanaka, Y. Fine-tuning SLE treatment: The potential of selective TYK2 inhibition. RMD Open 2024, 10, e005072. [Google Scholar] [CrossRef]
- Morand, E.; Pike, M.; Merrill, J.T.; van Vollenhoven, R.; Werth, V.P.; Hobar, C.; Delev, N.; Shah, V.; Sharkey, B.; Wegman, T.; et al. Deucravacitinib, a Tyrosine Kinase 2 Inhibitor, in Systemic Lupus Erythematosus: A Phase II, Randomized, Double-Blind, Placebo-Controlled Trial. Arthritis Rheumatol. 2023, 75, 242–252. [Google Scholar] [CrossRef]
- Wenzel, J.; van Holt, N.; Maier, J.; Vonnahme, M.; Bieber, T.; Wolf, D. JAK1/2 Inhibitor Ruxolitinib Controls a Case of Chilblain Lupus Erythematosus. J. Investig. Dermatol. 2016, 136, 1281–1283. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Sakata, K.; Kitanaga, Y.; Ma, X.; Lee, S.; Ishii, A.; Yamagata, K.; Nakano, K.; Tanaka, Y. Janus Kinase Inhibitor Baricitinib Modulates Human Innate and Adaptive Immune System. Front. Immunol. 2018, 9, 1510. [Google Scholar] [CrossRef]
- Kubo, S.; Yamaoka, K.; Kondo, M.; Yamagata, K.; Zhao, J.; Iwata, S.; Tanaka, Y. The JAK inhibitor, tofacitinib, reduces the T cell stimulatory capacity of human monocyte-derived dendritic cells. Ann. Rheum. Dis. 2014, 73, 2192–2198. [Google Scholar] [CrossRef]
- Bonelli, M.; Dalwigk, K.; Platzer, A.; Olmos Calvo, I.; Hayer, S.; Niederreiter, B.; Holinka, J.; Sevelda, F.; Pap, T.; Steiner, G.; et al. IRF1 is critical for the TNF-driven interferon response in rheumatoid fibroblast-like synoviocytes: JAKinibs suppress the interferon response in RA-FLSs. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Dörner, T.; Tanaka, Y.; Petri, M.; Smolen, J.S.; Dow, E.R.; Higgs, R.E.; Benschop, R.J.; Abel, A.; Silk, M.E.; Bono, S.d.; et al. 185 Baricitinib-associated changes in type I interferon gene signature during a 24-week phase 2 clinical SLE trial. Lupus Sci. Med. 2019, 6. [Google Scholar] [CrossRef]
- Itotagawa, E.; Tomofuji, Y.; Kato, Y.; Konaka, H.; Tsujimoto, K.; Park, J.; Nagira, D.; Hirayama, T.; Jo, T.; Hirano, T.; et al. SLE stratification based on BAFF and IFN-I bioactivity for biologics and implications of BAFF produced by glomeruli in lupus nephritis. Rheumatology 2023, 62, 1988–1997. [Google Scholar] [CrossRef]
- Li, H.; Ju, B.; Luo, J.; Zhu, L.; Zhang, J.; Hu, N.; Mo, L.; Wang, Y.; Tian, J.; Li, Q.; et al. Type I interferon-stimulated genes predict clinical response to belimumab in systemic lupus erythematosus. Eur. J. Pharmacol. 2025, 987, 177204. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, T.; Wang, P.; Chen, W.; Liu, W.; He, X.; Zhang, Y.; Jin, S.; Luo, Z.; Zhang, Z.; et al. Imatinib and M351-0056 enhance the function of VISTA and ameliorate the development of SLE via IFN-I and noncanonical NF-κB pathway. Cell Biol. Toxicol. 2023, 39, 3287–3304. [Google Scholar] [CrossRef]
- Shan, S.; Zhou, Y.; Yu, J.; Yang, Q.; Pan, D.; Wang, Y.; Li, L.; Zhu, J.; Zhang, Y.; Huang, S.; et al. Therapeutic treatment of a novel selective JAK3/JAK1/TBK1 inhibitor, CS12192, in rat and mouse models of rheumatoid arthritis. Int. Immunopharmacol. 2019, 77, 105914. [Google Scholar] [CrossRef] [PubMed]
- Louis, C.; Ngo, D.; D’Silva, D.B.; Hansen, J.; Phillipson, L.; Jousset, H.; Novello, P.; Segal, D.; Lawlor, K.E.; Burns, C.J.; et al. Therapeutic Effects of a TANK-Binding Kinase 1 Inhibitor in Germinal Center-Driven Collagen-Induced Arthritis. Arthritis Rheumatol. 2019, 71, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Hu, Y.; Dai, J.; He, L.; He, J.; Xu, B.; Han, X.; Zhong, F.; Lan, H.; Wang, Q. CS12192, a Novel JAK3/JAK1/TBK1 Inhibitor, Synergistically Enhances the Anti-Inflammation Effect of Methotrexate in a Rat Model of Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 13394. [Google Scholar] [CrossRef] [PubMed]
- Hammaker, D.; Boyle, D.L.; Firestein, G.S. Synoviocyte innate immune responses: TANK-binding kinase-1 as a potential therapeutic target in rheumatoid arthritis. Rheumatology 2012, 51, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Torigo, S.; Ihara, T.; Kamiya, H. IL-12, IFN-gamma, and TNF-alpha released from mononuclear cells inhibit the spread of varicella-zoster virus at an early stage of varicella. Microbiol. Immunol. 2000, 44, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef]
- van Panhuys, N.; Tang, S.C.; Prout, M.; Camberis, M.; Scarlett, D.; Roberts, J.; Hu-Li, J.; Paul, W.E.; Le Gros, G. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proc. Natl. Acad. Sci. USA 2008, 105, 12423–12428. [Google Scholar] [CrossRef]
- Marmor, M.F.; Kellner, U.; Lai, T.Y.; Melles, R.B.; Mieler, W.F. Recommendations on Screening for Chloroquine and Hydroxychloroquine Retinopathy (2016 Revision). Ophthalmology 2016, 123, 1386–1394. [Google Scholar] [CrossRef]
- Werth, V.P.; Furie, R.A.; Romero-Diaz, J.; Navarra, S.; Kalunian, K.; van Vollenhoven, R.F.; Nyberg, F.; Kaffenberger, B.H.; Sheikh, S.Z.; Radunovic, G.; et al. Trial of Anti-BDCA2 Antibody Litifilimab for Cutaneous Lupus Erythematosus. N. Engl. J. Med. 2022, 387, 321–331. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).