Abstract
Calcific aortic valve disease (CAVD) is a progressive disorder where molecular alterations occur long before visible calcification, making early biomarkers essential. Extracellular vesicles (EVs) have gained attention as stable biomarkers due to their lipid bilayer, which protects proteins, lipids, and RNAs, ensuring reliable detection even in archived samples. This review highlights the role of EVs as biomarkers and delivery tools in CAVD. EVs derived from valvular endothelial, interstitial, and immune cells carry disease-specific signatures, including osteogenic proteins (BMP-2, Annexins), inflammatory miRNAs (miR-30b, miR-122-5p), and lipid mediators. These reflect early pathogenic processes before macroscopic calcification develops. Their presence in minimally invasive samples such as blood, urine, or saliva facilitates diagnosis, while their stability supports long-term monitoring of disease progression and therapeutic response. Advances in purification and single-EV analysis increase specificity, though challenges remain in standardizing methods and distinguishing CAVD-derived EVs from those in atherosclerosis. Beyond diagnostics, engineered EVs show promise as therapeutic carriers. Delivery of anti-calcific miRNAs or combined RNA cargos has reduced calcification and inflammation in preclinical models. Overall, EVs act as molecular mirrors of CAVD, enabling early diagnosis, risk stratification, and novel therapeutic strategies. Yet, clinical translation requires technical refinement and validation of the disease-specific signatures.
1. Introduction
Calcific aortic valve disease (CAVD) is increasingly recognized as the main valvular condition among the aging population, with a prevalence approaching 2% in individuals over 65 years old. The disease progresses through fibrotic thickening and calcium buildup within the aortic valve, leading to clinically apparent aortic stenosis (AS) [1]. Without prompt valve replacement, patients with severe AS have a significantly reduced life expectancy, often less than two years [2]. Despite its clinical importance, no effective pharmacological treatments are available at present, partly due to limited understanding of the molecular pathways involved in early aortic sclerosis, the asymptomatic precursor stage. This knowledge gap also hinders the development of early-stage biomarkers that can reliably detect disease onset or progression.
Extracellular vesicles (EVs) have emerged as powerful biomarkers in calcific aortic valve disease (CAVD) due to their ability to encapsulate and transport molecular cargo reflective of disease pathogenesis [3]. These membrane-bound nanoparticles, released by valvular endothelial cells (VECs), interstitial cells (VICs), and infiltrating immune cells, carry a dynamic repertoire of proteins, RNAs (e.g., microRNAs, mRNAs), and lipids that mirror the pathological state of the valve tissue. For instance, EVs derived from calcified aortic valves are enriched in osteogenic proteins such as BMP-2 and Annexins, which drive calcification via activation of Wnt/β-catenin and Notch pathways [4,5]. Proteomic analyses have identified CAVD-specific EV signatures, including WNT5A and amyloid precursor protein, which are absent in healthy valves, highlighting their potential as disease-specific fingerprints [4,6]. Furthermore, EV-associated microRNAs like miR-30b are downregulated in CAVD, and miR-125b leads to depression of osteogenic transcription factors (e.g., RUNX2) and exacerbating calcification [7,8,9]. These findings underscore the role of EVs as molecular snapshots of valve pathology, capturing real-time changes in cellular activity during disease progression.
The lipidomic profile of EVs further enhances their diagnostic utility. Oxidized phospholipids and sphingolipids within EVs correlate with CAVD severity, triggering inflammatory cascades via TLR4/NF-κB signaling in recipient cells [10,11]. Other studies have reported that CD144+ endothelial-derived EVs are elevated in acute myocardial infarction, supporting their potential as candidate biomarkers for detecting valvular dysfunction [12]. Similarly, macrophage-derived EVs carrying miR-122-5p are linked to inflammation-driven calcification, offering a mechanistic link between immune activation and osteogenic transformation [13,14]. The stability of EV cargo, particularly RNA, allows for robust biomarker analysis even in archived samples, overcoming limitations of free circulating nucleic acids [15,16].
Despite these advances, challenges remain in standardizing EV isolation and characterizing disease-specific subpopulations. For example, techniques such as ultracentrifugation (UC) and size-exclusion chromatography (SEC) yield variable EV subsets, which affect the reproducibility of cargo profiles [17]. Future studies must prioritize validation of CAVD-specific EV markers (e.g., valve-derived collagen fragments) to distinguish them from atherosclerotic contaminants [18]. Integrating multi-omics approaches—such as proteomics and metabolomics—will further refine EV-based diagnostics, enabling personalized risk stratification and early therapeutic intervention in CAVD [19,20].
2. Structural Stability of Extracellular Vesicles in CAVD
The structural stability of EVs represents one of their most valuable attributes for clinical application, particularly in diseases characterized by chronic and progressive tissue remodeling, such as calcification [21]. The defining lipid bilayer envelope of EVs functions as a robust shield that protects encapsulated biomolecules—including microRNAs (miRNAs), messenger RNAs (mRNAs), long non-coding RNAs, and proteins—from degradation in the hostile extracellular environment [22,23,24]. By contrast, as shown in Table 1, the vesicular encapsulation of RNAs significantly prolongs their half-life. Studies have shown that EV-encapsulated miRNAs exhibit up to a tenfold greater stability compared to free-circulating RNAs in serum, retaining integrity even in archived samples that have undergone repeated freeze–thaw cycles [25,26]. This intrinsic protection not only facilitates biomarker discovery but also supports therapeutic applications, since engineered RNA cargos maintain structural fidelity until released in target cells.
The biochemical composition of the EV membrane further strengthens this resilience [27]. Lipid species such as cholesterol, ceramide, phosphatidylserine, and sphingolipids are enriched in EV bilayers, conferring resistance to osmotic stress, oxidative damage, and shear forces experienced during circulation in the cardiovascular system [28]. This composition, similar to the lipid rafts, allows vesicles to preserve their morphology and cargo under mechanical strain such as blood flow turbulence in the aortic valve [29]. This is particularly relevant in CAVD, where the pathological microenvironment is characterized by chronic inflammation, oxidative stress, and the deposition of hydroxyapatite, all of which would typically destabilize synthetic nanoparticles [30]. Nevertheless, EVs maintain their structure, ensuring faithful signal or therapeutic molecule delivery [31].
Pathophysiologically, this structural stability is crucial in CAVD because vesicles secreted by VECs and VICs transport disease-reflective miRNAs, which regulate calcification and osteogenic differentiation [32]. Remarkably, these vesicle-bound miRNAs are detectable in circulation without significant degradation [33,34]. This durability provides a reliable source of disease-associated nucleic acids, supporting their translational value both as biomarkers and therapeutic carries [35,36].

Table 1.
Comparative Advantages Over Free Circulating Biomarkers.
Table 1.
Comparative Advantages Over Free Circulating Biomarkers.
| Parameter | EV-Encapsulated RNA | Free Circulating RNA | Reference |
|---|---|---|---|
| RNase Resistance | High (lipid bilayer protection) | Low (direct exposure) | [25] |
| Stability in Storage | >6 months at −80 °C | Degrades within weeks | [26] |
| Signal-to-Noise Ratio | High (enriched cargo) | Low (diluted in biofluid) | [25] |
| Disease Specificity | Cell-of-origin signatures | Non-specific degradation products | [37] |
EVs protect RNA through lipid bilayers, optimizing their use as biomarkers in CAVD [38].
3. Extracellular Vesicles Across Biofluids Enhance Diagnostic Accessibility
Another key advantage of EVs is their broad distribution across diverse biological fluids, which significantly enhances diagnostic accessibility in cardiovascular disease [39]. Unlike tissue biopsies, which are invasive and impractical for routine monitoring, EVs can be isolated from minimally invasive sources such as blood, urine, saliva, and even cerebrospinal fluid [40]. Importantly, their encapsulated cargos maintain stability for prolonged periods under storage and handling conditions that would rapidly degrade free-circulating molecules [41]. In blood plasma, for example, EV-associated RNAs remain stable for more than 72 h at 4 °C, whereas free RNAs degrade within hours due to RNase activity [42,43]. This stability facilitates sample handling in clinical and multicenter studies [44].
For CAVD specifically, the capacity to detect and analyze EVs in serum or plasma provides a non-invasive “liquid biopsy” reflecting molecular processes within the aortic valve [45]. Several studies have confirmed that serum-derived EVs from CAVD patients contain calcification-associated miRNAs, including miR-16, miR-24, miR-451, and miR-181a, which remain detectable with minimal degradation even after prolonged storage [43,46]. This consistent preservation enhances diagnostic reliability and enables retrospective analyses in archived biobanks.
The diagnostic accessibility of EVs extends beyond CAVD and finds parallels in other cardiovascular conditions. In myocardial infarction and heart failure, plasma EVs have been used to identify stress- and apoptosis-associated miRNAs [47,48], while in other diseases [49,50], urine-derived EVs have been explored as markers of extracellular matrix degradation [51]. In CAVD, the minimally invasive detection of vesicular cargo opens new opportunities for early diagnosis and risk stratification, which are currently lacking in clinical practice. Traditional imaging modalities such as echocardiography and CT only detect advanced structural changes, whereas EV-based biomarkers may reveal earlier molecular perturbations [52,53]. Indeed, recent studies have demonstrated that systemic EV signatures correlate with the extent of valve calcification and predict disease progression [4,54].
Taken together, the cross-fluid availability and inherent cargo stability of EVs position them as highly promising diagnostic tools in CAVD. They offer a minimally invasive, reproducible, and biologically meaningful window into disease biology, capable of complementing existing imaging-based assessments.
4. Extracellular Vesicles as Robust Biomarkers in CAVD
As mentioned, the physical resilience of EVs enables stringent processing in biomarker workflows, overcoming key limitations of traditional circulating biomarkers [55]. High-resolution purification techniques such as density gradient UC and SEC effectively isolate EVs while excluding contaminating proteins and lipoproteins that interfere with downstream RNA analysis [56]. These methods preserve EV integrity and enhance specificity, as demonstrated in studies optimizing SEC for cardiovascular applications [57,58,59]. Recent advancements in dual-mode SEC + UC further improve EV purity by depleting abundant plasma proteins, enabling deeper proteomic and transcriptomic analysis of EV-derived biomarkers [58].
EVs facilitate sensitive detection of disease-specific molecules because their encapsulated RNAs are protected from degradation and enriched in pathological signatures [60]. For example, macrophage-derived EVs carrying miR-122-5p or antiosteogenic miRNAs like miR-30b can be detected at lower concentrations than free RNA via qPCR or sequencing, offering superior signal-to-noise ratios in CAVD diagnostics [33,61]. Multiplexed techniques, such as single-EV analysis, enhance detection by profiling multiple biomarkers simultaneously, revealing heterogeneity in EV subpopulations correlated with disease stages [62].
The stability of EV cargo supports longitudinal monitoring of CAVD progression. For instance, Annexin V+-EVs, which reflect calcification activity, can be tracked over time to assess therapeutic response or disease advancement [3,63,64,65]. Emerging technologies like multiphoton microscopy and fluorescence lifetime imaging enable non-invasive, spatiotemporal tracking of EV-mediated processes, such as oxidative stress and matrix remodeling, in preclinical models [66].
Free RNAs are vulnerable to rapid clearance by nucleases and bind inconsistently to carrier proteins (e.g., Ago2), causing variability in measurements. In contrast, EV RNAs reflect parent-cell biology with high fidelity, as demonstrated in CAVD studies where EV miR-30b levels inversely correlate with osteogenic gene expression in valve tissue [67].
These membrane-bound nanoparticles carry a diverse cargo of proteins and lipids that act as disease-specific fingerprints, capturing pathological processes in a minimally invasive manner [54]. As detailed in Table 2, specific EV-associated proteins, such as osteopontin (OPN) and matrix metalloproteinase-9 (MMP-9), along with distinct lipid species including oxidized phospholipids, have been linked to inflammatory and osteogenic signaling pathways in the valve. While these cargos provide valuable insights, their presence alone may not be entirely specific to CAVD, as similar molecules can also be detected in other inflammatory or cardiovascular conditions, emphasizing the need for integrated analysis [33].

Table 2.
Extracellular Vesicle-Associated Proteins and Lipids in CAVD.
Building on this, the selective encapsulation of molecular cargo within EVs further enhances their diagnostic potential. As illustrated in Figure 1, proteins such as BMP-2, annexin V and miRNA 30b when enclosed within EVs, are protected from degradation, allowing robust detection in blood samples [63]. By preserving the integrity of bioactive molecules and restricting their release to EVs, these vesicles act as highly informative carriers, capturing subtle cellular alterations in valvular tissue that may otherwise go unnoticed.
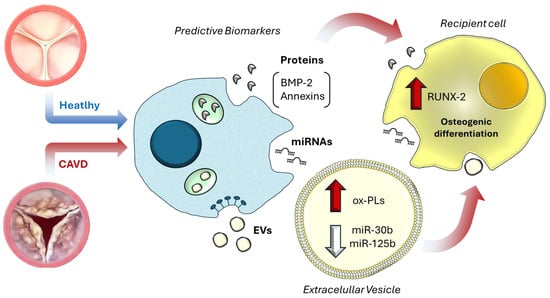
Figure 1.
Extracellular vesicle-mediated molecular mirrors in CAVD. In CAVD, extracellular vesicles released from valvular cells carry disease-specific molecular cargo, including oxidized phospholipids (ox-PLs), pro-osteogenic proteins such as bone morphogenetic protein 2 (BMP-2) and Annexin V, and regulatory microRNAs (miR-30b, miR-125b). These molecules can be detected both freely circulating in plasma and encapsulated within EVs, acting as “molecular mirrors” that reflect ongoing pathological processes. Beyond their biomarker potential, EV cargo exerts functional effects on recipient cells, such as upregulation of runt-related transcription factor 2 (RUNX2), thereby promoting osteogenic differentiation and contributing to valve calcification [9].
Moreover, EVs enriched with inflammation-related miRNAs, such as miR-30b, miR-125b, and miR-122-5p, have been detected in the blood of CAVD patients [4,33]. The structural stability of EVs combined with minimally invasive collection from blood, positions EVs as superior to traditional circulating biomarkers for early CAVD detection [4].
However, beyond their diagnostic utility, EVs also provide valuable prognostic information. Quantitative changes in specific EV subpopulations—for example, elevated CD144+ endothelial-derived EVs or CD14+ monocyte-derived EVs—have been linked to rapid hemodynamic progression and adverse clinical outcomes in CAVD patients [9,68,69]. Moreover, certain EV cargos, including GDF-15 and PON3, offer superior predictive value for major adverse cardiac events compared to conventional plasma biomarkers, highlighting the translational potential of integrating protein and lipid analyses within EVs for comprehensive patient monitoring [4,70,71,72]. Longitudinal tracking of these EV subpopulations and cargos allows dynamic assessment of disease progression and therapeutic response, capturing information that static imaging or conventional biomarkers cannot provide [63,69].
Finally, as described in Table 3, EV-encapsulated miRNAs offer exceptional potential for differentiating stages of CAVD. Specific miRNAs, such as miR-30b, miR-125b, and miR-122-5p, correlate with osteogenic reprogramming and inflammatory activation in VICs and VECs. Because miRNAs often reflect the cellular origin of EVs, their profiles provide precise molecular signatures capable of distinguishing early, intermediate, and advanced disease stages. Combined with the intrinsic stability of EVs and the minimally invasive nature of blood collection, these small RNAs position EVs as highly versatile biomarkers for both early detection and stage-specific assessment of CAVD [4,33].
Comparative studies further show that EV lipidomic and miRNA profiles mirror key pathological processes in the valve: for instance, oxidized phospholipids indicate oxidative stress, while downregulation of miR-30b reflects osteogenic reprogramming of VICs [7,16,73,74]. Emerging technologies, including microfluidic chips and electrochemical biosensors, are enhancing the sensitivity and specificity of EV-based diagnostics, paving the way for point-of-care applications and real-time monitoring of disease mechanisms [75,76].

Table 3.
Extracellular vesicle-associated microRNAs across stages of CAVD.
Table 3.
Extracellular vesicle-associated microRNAs across stages of CAVD.
| CAVD Stage | miRNA | Expression in EVs | Biological Role | Clinical Utility | Refs. |
|---|---|---|---|---|---|
| Early Stage (Aortic Sclerosis) | miR-30b | ↓ in valve-derived EVs | Inhibits inflammation and osteogenic differentiation | Predicts early calcification risk; inversely correlates with Agatston scores | [77] |
| miR-125b | ↓ in plasma EVs | Suppresses VIC activation via TRAF6/NF-κB inhibition | Low levels linked to faster hemodynamic progression | [9,33] | |
| miR-146a | ↑ in macrophage EVs | Anti-inflammatory; targets TRAF6/IL-1R to reduce inflammation | Potential therapeutic target | [78,79] | |
| Intermediate Stage (Fibrosis/Calcification) | miR-214 | ↑ in VIC-derived EVs | Promotes calcification by inhibiting ATF4, an osteoclast activator | Correlates with ECM remodeling and valve stiffness | [80,81] |
| miR-122-5p | ↑ in VEC-derivedEVs | Drives inflammation via TLR4 signaling in VICs and cardiomyocytes | Elevated in early CAVD plasma EVs; predicts subclinical inflammation | [82,83] | |
| miR-148a | ↓ in circulating EVs | Normally inhibits osteogenic transition via Wnt/β-catenin suppression | Loss correlates with accelerated calcification and AS | [84,85] | |
| Advanced Stage (Severe Stenosis) | miR-21 | ↑ in platelet EVs | Promotes fibrosis via PTEN suppression and MMP-9 activation | Associated with the need for valve replacement | [86] |
| miR-221 | ↑ in endothelial EVs | Enhances angiogenesis and osteogenesis via p27/CDKN1B inhibition | Linked to adverse post-TAVR outcomes (e.g., paravalvular leaks) | [87,88] | |
| miR-155 | ↑ inflammatory EVs | Drives macrophage polarization to pro-calcific (M1) phenotype | Predicts MACE in CAVD patients (e.g., post-AVR heart failure) | [86,89,90] |
Summary of selected miRNAs identified in EVs from different cellular origins across CAVD progression stages. Biological roles were assigned based on experimental studies, and clinical utility reflects reported diagnostic, prognostic, or therapeutic relevance. EV origins include VIC—valve interstitial cell; VEC—valve endothelial cell; macrophage EVs; platelet EVs; and circulating/plasma EVs. AS: aortic stenosis; TRAF6: TNF receptor-associated factor 6; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; IL-1R: interleukin-1 receptor; ATF4: activating transcription factor 4; TLR4: toll-like receptor 4; PTEN: phosphatase and tensin homolog; MMP-9: matrix metalloproteinase 9; CDKN1B: cyclin-dependent kinase inhibitor 1B; MACE: major adverse cardiovascular events ↑ indicates enrichment or increased release, whereas ↓ denotes reduction or decreased release.
5. Bioengineering of EVs in CAVD
Extracellular vesicles are naturally occurring nanoscale carriers that facilitate intercellular communication through the transport of bioactive molecules such as proteins, peptides, lipids, and nucleic acids [65,91,92]. Recent advances in bioengineering have enabled the functional modification of EVs to enhance their therapeutic potential, particularly in cardiovascular diseases like CAVD [93]. Bioengineering strategies aim to improve cargo specificity, cellular targeting, and biological activity, making EVs an attractive platform for precision therapy [94].
During CAVD, VICs undergo activation, osteogenic differentiation, and extracellular matrix remodeling, leading to progressive calcification and stenosis [95]. To address these pathological processes, EVs can be engineered to carry specific miRNAs that regulate key signaling pathways. For instance, MSC-derived exosomes enriched with miR-146a have been shown to mitigate calcification in vascular smooth muscle cells exposed to advanced glycation end products, downregulating pro-osteogenic markers such as RUNX2 and BMP2 [96]. Similarly, telocyte-derived EVs carrying miR-30b inhibit calcification in VICs via the miR-30b/Runx2/Wnt/β-catenin axis, highlighting the potential of EVs as gene modulators in valvular pathology [33].
Surface engineering further enhances EV specificity and uptake by target cells [97]. Conjugation of valve-targeting peptides, including elastin-binding sequences derived from valvular extracellular matrix, enables selective delivery to aortic valves while minimizing off-target effects [98]. Additionally, modifications such as pH-sensitive fusogenic peptides allow EVs to release their cargo preferentially in acidic microenvironments characteristic of calcifying valves [99,100,101]. This bioengineering approach not only improves therapeutic efficacy but also reduces potential systemic toxicity.
Comparative studies in other cardiovascular diseases provide valuable insights. In atherosclerosis, EVs derived from endothelial or immune cells can either propagate inflammation or confer protective effects depending on their cargo [102,103,104]. Engineering these EVs with anti-inflammatory miRNAs such as miR-145 or miR-21-5p has been shown to stabilize plaques, reduce endothelial activation, and restore autophagic flux [105]. In aortic pathologies, including aneurysms and dissections, MSC-derived EVs carrying miR-146a and angiogenic factors have demonstrated protective effects by attenuating endothelial senescence and promoting vascular repair [106]. These parallels underscore the versatility of EV bioengineering and suggest that strategies effective in one cardiovascular context may be adapted for CAVD.
In addition to nucleic acids, EVs can be loaded with therapeutic peptides and proteins [97,107]. Anti-inflammatory cytokines or matrix metalloproteinase inhibitors can be incorporated to modulate local inflammatory responses and extracellular matrix remodeling [108]. Such multifunctional bioengineered EVs can simultaneously address multiple pathogenic mechanisms, positioning them as a promising regenerative approach for CAVD and related cardiovascular diseases [109].
6. EVs as Drug Delivery Systems in CAVD
EVs, including exosomes and microvesicles, have also emerged as promising drug delivery systems in cardiovascular medicine due to their biological properties (Figure 2). Biological barrier penetration is facilitated by the EV lipid bilayer, which traverses endothelial barriers impermeable to synthetic nanoparticles [109,110].
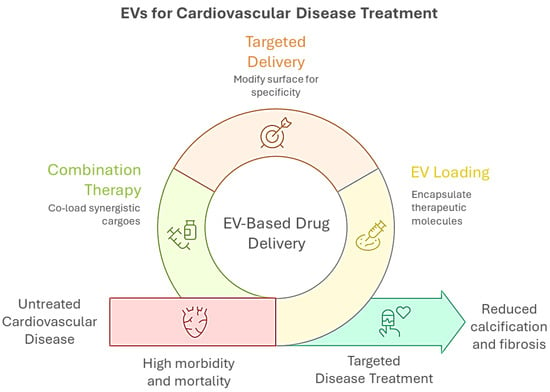
Figure 2.
Graphical overview of extracellular vesicle (EV)-based strategies for CVD treatment. EVs can be engineered for drug delivery through surface modification for targeted delivery, encapsulation of therapeutic molecules (EV loading), and co-loading of synergistic agents (combination therapy). These approaches aim to overcome the limitations of untreated CVD, which is associated with high morbidity and mortality, by enabling targeted interventions that reduce calcification and fibrosis, ultimately improving disease outcomes.
In the context of CAVD, EVs offer a versatile platform for delivering therapeutic agents directly to diseased valvular tissue, potentially mitigating calcification and fibrosis while minimizing the systemic toxicity associated with conventional pharmacological interventions [108,111]. EVs can be loaded with microRNAs, siRNAs, mRNAs, peptides, or proteins, thereby modulating the activity of VICs, endothelial cells, and inflammatory cells [39,112]. For instance, mesenchymal stromal cell-derived EVs carrying miR-146a have been shown to attenuate osteogenic differentiation and calcification in vascular smooth muscle cells, whereas telocyte-derived EVs enriched with miR-30b inhibit VIC calcification through the Runx2/Wnt/β-catenin pathway [33,113].
For example, EVs loaded with miR-148a (an osteoclast activator) were shown to cross the valvular endothelium in human explants, reprogramming myofibroblasts to suppress hydroxyapatite crystallization [97]. This delivery exploits endogenous trafficking mechanisms, such as clathrin-mediated endocytosis, which is upregulated in CAVD-affected valves [114]. EVs can also transport bioactive proteins and peptides, including anti-inflammatory cytokines or matrix-modulating enzymes that reduce macrophage-driven fibrosis and regulate extracellular matrix remodeling [115,116]. Moreover, surface engineering approaches—for instance, conjugating valve-targeting peptides or elastin-binding motifs derived from the extracellular matrix [117]—enhance the specificity of EV homing to the aortic valve, thereby limiting off-target effects.
Combination therapies represent another promising avenue. Co-loading EVs with synergistic cargoes, such as RUNX2 siRNA (targeting osteogenesis) [118,119] and IL-10 mRNA (anti-inflammatory) [120], has been shown to halt CAVD progression by simultaneously inhibiting calcification and macrophage-driven fibrosis.
The therapeutic potential of EVs as drug delivery systems also extends beyond CAVD to other aortic pathologies, including abdominal aortic aneurysms (AAAs) and aortic dissections [121,122,123]. In AAAs, M2 macrophage-derived EVs delivering miR-221-5p promote anti-inflammatory macrophage polarization, reduce oxidative stress, and preserve vascular smooth muscle cell viability [124,125,126]. Likewise, mesenchymal stem cell-derived EVs attenuate neutrophil extracellular trap-mediated inflammation and elastin degradation, both critical drivers of aneurysm progression [49,127]. In parallel, platelet-derived EVs, which naturally contribute to intercellular vascular signaling, can be engineered to deliver anti-inflammatory molecules or matrix-stabilizing proteins, enhancing tissue repair and reducing rupture risk [128]. Innovative targeting strategies—such as hybrid EVs incorporating monocyte or platelet membranes [129]—further refine localization by directing vesicles specifically to the aneurysmal intraluminal thrombus, thereby improving therapeutic effectiveness [130,131].
Similarly, EV-based approaches are being explored for other aortic conditions, including aortic dissection and coarctation. In dissections, EVs carrying inhibitors of matrix metalloproteinases or collagen-synthesis modulators could stabilize the aortic wall and prevent further tissue degradation [132]. In congenital or acquired coarctation, EVs may deliver agents that modulate endothelial function and smooth muscle cell proliferation [133], counteracting pathological remodeling induced by abnormal shear stress [126]. These studies highlight the versatility of EVs as therapeutic carriers capable of addressing complex, multifactorial vascular pathologies through targeted and multi-modal interventions.
7. Clinical Translation of Extracellular Vesicles in CAVD
Despite their promise, several challenges must be addressed for clinical translation of EV-based therapies. Standardization of isolation and characterization techniques is essential to ensure reproducibility and batch-to-batch consistency [134]. Optimizing circulation time and minimizing rapid clearance by the mononuclear phagocyte system are also critical considerations [135]. Additionally, efficient targeting remains a central challenge, requiring ongoing development of ligands or surface modifications that direct EVs to specific tissues [136]. Finally, regulatory pathways for EV-based therapeutics are still evolving, and comprehensive preclinical studies are necessary to evaluate safety, immunogenicity, and long-term effects.
Overall, EVs represent a unique and highly adaptable platform for drug delivery in CAVD and other aortic diseases [137]. Their ability to encapsulate a wide range of molecular cargos, navigate biological barriers, and selectively target diseased tissues positions them at the forefront of emerging cardiovascular therapies [137]. Continued research and optimization of bioengineering strategies will be pivotal in translating these promising preclinical findings into effective clinical interventions capable of addressing the complex pathophysiology of valvular calcification, aneurysm formation, and related vascular disorders.
While EVs carry rich molecular information reflective of CAVD pathogenesis, translating these signatures into clinical biomarkers is challenged by systemic conditions that alter EV release and composition [138]. Chronic kidney disease (CKD), for example, profoundly modifies the EV landscape through uremic toxins, oxidative stress, and impaired renal clearance, resulting in vesicles enriched with pro-inflammatory and matrix-remodeling cargo that may mimic valvular disease signals [139]. Similarly, systemic inflammation and atherosclerosis induce widespread endothelial activation and vesiculation, releasing EVs carrying oxidized phospholipids, annexins, osteopontin, or MMP-9—molecules also implicated in CAVD pathogenesis [138,139]. Because CAVD shares mechanistic pathways with these conditions—including endothelial dysfunction, lipid oxidation, and extracellular matrix remodeling—plasma-derived EVs often represent a composite vascular signal rather than a purely valvular one, particularly in elderly patients or those with CKD, diabetes, or systemic inflammatory conditions [140].
Distinguishing valve-derived EVs from systemic or renal sources is feasible but requires rigorous methodological strategies [140]. Paired valve–plasma studies and multi-omics analyses have identified candidate valve-enriched cargos, such as NOTCH1 and WNT pathway modulators, as well as specific ECM fragments less abundant in atherosclerotic or renal EVs [141]. Incorporating these markers, together with proteomic, lipidomic, and non-coding RNA profiling, can enhance diagnostic specificity [4]. Additionally, stratification by CKD stage, assessment of systemic inflammatory activity (e.g., CRP, IL-6), and immuno-enrichment of valve-endothelial EV subsets may further mitigate confounding signals [142]. From a clinical perspective, EV-based screening could target high-risk populations—older adults (>65 years) with CKD or metabolic syndrome—where early, non-invasive detection would be most impactful. Longitudinal EV profiling, integrated with imaging and clinical metrics, could refine the timing of intervention and improve patient stratification.
Despite these challenges, several human studies are advancing EV-based diagnostics and therapeutics toward clinical translation. The phase I trial NCT05774509 evaluates cardiovascular progenitor cell-derived EVs in non-ischemic dilated cardiomyopathy [143], while the SEAL-HF study (NCT06169540) investigates salivary and plasma EV-associated long non-coding RNAs as biomarkers in acute and chronic heart failure [144]. The EVOC trial (NCT06408961) examines adipose-derived EVs in obesity and cardiometabolic disease, providing mechanistic insights relevant to vascular inflammation and myocardial remodeling [145]. Additional trials, including EASY-AS (NCT04204915) [146] and NCT06002841, explore EVs in early valve replacement and acute respiratory failure, whereas NCT04897841 investigates mesenchymal stem cell therapy in cardiovascular disease, contributing valuable translational data even if not EV-specific [147,148]. Collectively, these studies bridge preclinical discovery and clinical application, establishing safety frameworks, bioprocessing standards, and biomarker validation pipelines relevant to CAVD.
However, critical challenges remain. The mechanistic overlap between CAVD, CKD, and atherosclerosis complicates the identification of truly CAVD-specific EV biomarkers, as systemic vesicles may mimic valvular signatures [138,140,149]. In addition, the absence of unified protocols affects reproducibility, particularly for CAVD-specific markers like NOTCH1 fragments. International initiatives, such as the MISEV guidelines (2023) [150], advocate for orthogonal characterization—combining nanoparticle tracking analysis (NTA) with Western blot—to validate EV isolates. For CAVD, integrating valve-specific markers with universal tetraspanins improves disease specificity. Recent frameworks propose standardized panels (e.g., CD63 + NOTCH1+ EVs) to distinguish pathological valvular signals from atherosclerotic noise [56,151].
Inter-individual variability, comorbidities, and technical heterogeneity in EV isolation and characterization further limit reproducibility and clinical adoption. Isolation variability remains a primary concern, as traditional ultracentrifugation methods induce mechanical damage to EV membranes, leading to cargo degradation and aggregation, while polymer-based precipitation co-isolates contaminants like chylomicrons and lipoproteins [152]. This variability skews quantification of low-abundance CAVD-specific biomarkers, complicating diagnostic accuracy [64]. Emerging solutions leverage microfluidic technologies with immunoaffinity capture, utilizing anti-tetraspanin antibodies (e.g., CD9/CD63) to isolate EVs with >95% purity from plasma [153]. These platforms minimize shear stress and reduce processing time, enhancing yield for downstream CAVD biomarker analysis [26,154,155].
Recent advancements in EV-based diagnostics are reshaping the detection and monitoring landscape of CAVD, particularly by enhancing sensitivity, reproducibility, and applicability at the point of care. A notable development in this area is the adoption of silicon nanowire (SiNW) biosensors, which facilitate ultrasensitive and label-free detection of miRNAs—including those encapsulated in EVs—with remarkable specificity [156,157,158]. Additionally, luminescent SiNW optical biosensors have demonstrated the ability to isolate and quantify EVs—marked with tetraspanin proteins like CD81—with a limit of detection around 2 × 105 small EVs per mL, using minimal sample volume [159,160]. These breakthroughs forgo extensive sample processing and instead favor rapid, high-fidelity analyses.
Complementing these technological strides are efforts to improve standardization and cross-study reproducibility through the use of synthetic EV mimics [157]. These engineered nanoparticles, spiked with calcification-relevant RNA cargos such as RUNX2 siRNA, serve as reference materials to benchmark and calibrate EV isolation kits and detection platforms [161,162]. When used in comparative studies across laboratories, these mimics enable harmonization of protocols and improve the reliability of CAVD biomarker pipelines. Together, these innovations address key bottlenecks in the translational pipeline for EV-based diagnostics—namely, variability in sample processing and limited access to real-time assays—while paving the way for more robust and disease-specific applications in cardiovascular medicine.
8. Conclusions
In summary, the combination of valve-specific markers, multi-omics integration, standardized protocols, and carefully designed cohorts will be essential to ensure that EV-derived biomarkers reliably reflect CAVD pathology, enabling early detection, risk stratification, and monitoring of therapeutic interventions.
Author Contributions
Conceptualization, M.S.; writing—original draft preparation M.S., A.C.-C., M.D.-M. and B.F.-R.; review and editing, A.C.-C., review and supervision C.Z.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Junta de Comunidades de Castilla-La Mancha, Spain, grant number SBPLY/23/180225/000081, through the Research and Innovation Agency of Castilla-La Mancha and co-funded by the European Regional Development Fund (ERDF); and by the Comunidad de Madrid, Spain, under the I+D Biomedicine Activities Program 2022, grant number P2022/BMD-7223 CIFRA_COR_CM. María Delgado-Marín holds an FPI fellowship from the University of Alcalá (UAH).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AAAs | Abdominal Aortic Aneurysms |
| Ago2 | Argonaute 2 |
| APP | Amyloid Precursor Protein |
| AS | Aortic Stenosis |
| ATF4 | Activating Transcription Factor 4 |
| AVR | Aortic Valve Replacement |
| BMP-2 | Bone Morphogenetic Protein 2 |
| CAVD | Calcific Aortic Valve Disease |
| CDKN1B | Cyclin Dependent Kinase Inhibitor 1B |
| CVD | Cardiovascular Disease |
| ECM | Extracellular Matrix |
| EV(s) | Extracellular Vesicle(s) |
| FLIM | Fluorescence Lifetime Imaging Microscopy |
| GDF-15 | Growth Differentiation Factor 15 |
| IL-1R | Interleukin-1 Receptor |
| MACE | Major Adverse Cardiac Events |
| MISEV | Minimal Information for Studies of Extracellular Vesicles |
| miRNA(s) | microRNA(s) |
| mRNA(s) | messenger RNA(s) |
| MMP-9 | Matrix Metalloproteinase 9 |
| MPM | Multiphoton Microscopy |
| MSC(s) | Mesenchymal Stem Cell(s) |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NTA | Nanoparticle Tracking Analysis |
| OPN | Osteopontin |
| ox-PL(s) | Oxidized Phospholipid(s) |
| PON3 | Paraoxonase 3 |
| PTEN | Phosphatase and Tensin Homolog |
| RNA(s) | Ribonucleic Acid(s) |
| RNase(s) | Ribonuclease(s) |
| RUNX2 | Runt-related Transcription Factor 2 |
| SEC | Size-Exclusion Chromatography |
| SiNW | Silicon Nanowire |
| TAVR | Transcatheter Aortic Valve Replacement |
| TLR4 | Toll-like Receptor 4 |
| UC | Ultracentrifugation |
| VEC(s) | Valvular Endothelial Cell(s) |
| VIC(s) | Valvular Interstitial Cell(s) |
| VSMC(s) | Vascular Smooth Muscle Cell(s) |
References
- Blaser, M.C.; Bäck, M.; Lüscher, T.F.; Aikawa, E. Calcific aortic stenosis: Omics-based target discovery and therapy development. Eur. Heart J. 2025, 46, 620–634. [Google Scholar] [CrossRef]
- Hernandez-Vaquero, D.; Diaz, R.; Alperi, A.; Almendarez, M.G.; Escalera, A.; Cubero-Gallego, H.; Avanzas, P.; Moris, C.; Pascual, I. Life expectancy of patients undergoing surgical aortic valve replacement compared with that of the general population. Interact. Cardiovasc. Thorac. Surg. 2020, 30, 394–399. [Google Scholar] [CrossRef]
- Ragni, E. Extracellular Vesicles: Recent Advances and Perspectives. Front. Biosci. 2025, 30, 36405. [Google Scholar] [CrossRef]
- Blaser, M.C.; Buffolo, F.; Halu, A.; Turner, M.E.; Schlotter, F.; Higashi, H.; Pantano, L.; Clift, C.L.; Saddic, L.A.; Atkins, S.K.; et al. Multiomics of Tissue Extracellular Vesicles Identifies Unique Modulators of Atherosclerosis and Calcific Aortic Valve Stenosis. Circulation 2023, 148, 661–678. [Google Scholar] [CrossRef]
- Di Vito, A.; Donato, A.; Presta, I.; Mancuso, T.; Brunetti, F.S.; Mastroroberto, P.; Amorosi, A.; Malara, N.; Donato, G. Extracellular Matrix in Calcific Aortic Valve Disease: Architecture, Dynamic and Perspectives. Int. J. Mol. Sci. 2021, 22, 913. [Google Scholar] [CrossRef] [PubMed]
- Sivan, S.; Vijayakumar, G.; Pillai, I.C. Non-coding RNAs mediating the regulation of genes and signaling pathways in aortic valve calcification. Gene 2025, 936, 149117. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, X.; Zhang, X.; Song, Z.; Han, L.; He, Y.; Xu, Z. MicroRNA-30b is a multifunctional regulator of aortic valve interstitial cells. J. Thorac. Cardiovasc. Surg. 2014, 147, 1073–1080.e2. [Google Scholar] [CrossRef]
- Martin, P.J.; Haren, N.; Ghali, O.; Clabaut, A.; Chauveau, C.; Hardouin, P.; Broux, O. Adipogenic RNAs are transferred in osteoblasts via bone marrow adipocytes-derived extracellular vesicles (EVs). BMC Cell Biol. 2015, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Cheng, N.; Han, Y.; Zhang, C.; Zhang, H. miRNA Expression Profiling Uncovers a Role of miR-139-5p in Regulating the Calcification of Human Aortic Valve Interstitial Cells. Front. Genet. 2021, 12, 722564. [Google Scholar] [CrossRef] [PubMed]
- Resch, C.; Stamenkovic, A.; Surendran, A.; Zhang, A.; Oudit, G.Y.; Shah, A.; Ravandi, A. Valvular oxidized phospholipids correlate with severity of human aortic valvular stenosis. Free Radic. Biol. Med. 2025, 239, 219–229. [Google Scholar] [CrossRef]
- Niu, W.; Sun, B.; Li, M.; Cui, J.; Huang, J.; Zhang, L. TLR-4/microRNA-125a/NF-κB signaling modulates the immune response to Mycobacterium tuberculosis infection. Cell Cycle 2018, 17, 1931–1945. [Google Scholar] [CrossRef]
- Moreno, A.; Alarcón-Zapata, P.; Guzmán-Gútierrez, E.; Radojkovic, C.; Contreras, H.; Nova-Lampeti, E.; A Zúñiga, F.; Rodriguez-Alvárez, L.; Escudero, C.; Lagos, P.; et al. Changes in the Release of Endothelial Extracellular Vesicles CD144+, CCR6+, and CXCR3+ in Individuals with Acute Myocardial Infarction. Biomedicines 2024, 12, 2119. [Google Scholar] [CrossRef]
- Ji, J.; He, Q.; Xia, Y.; Sha, X.; Liang, Q.; Xu, Y.; Chen, P.; Dong, C.; Zhao, R.; Yang, J.; et al. Circulating plasma derived exosomes from systemic lupus erythematosus aggravate lupus nephritis through miR-122-5p/FOXO3-mediated macrophage activation. J. Nanobiotechnol. 2024, 22, 779. [Google Scholar] [CrossRef]
- Lyu, J.; Sheng, M.; Cao, Y.; Jia, L.; Zhang, C.; Weng, Y.; Yu, W. Ischemia and reperfusion-injured liver-derived exosomes elicit acute lung injury through miR-122-5p regulated alveolar macrophage polarization. Int. Immunopharmacol. 2024, 131, 111853. [Google Scholar] [CrossRef] [PubMed]
- Hermann, S.; Grätz, C.; Kirchner, B.; Pfaffl, M.W. Extracellular vesicle-derived microRNA biomarkers: Goals and pitfalls. Trillium Exctracell. Vesicles 2020, 2, 42–47. [Google Scholar]
- Bernáth-Nagy, D.; Kalinyaprak, M.S.; Giannitsis, E.; Ábrahám, P.; Leuschner, F.; Frey, N.; Krohn, J.B. Circulating extracellular vesicles as biomarkers in the diagnosis, prognosis and therapy of cardiovascular diseases. Front. Cardiovasc. Med. 2024, 11, 1425159. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Tong, L.; Hu, L.; Hong, Y.; Zhou, R.; Li, Z.; Dong, M.; Hou, J.; Xu, T. Systematic Evaluation of Isolation Techniques and Freeze-Thaw Effects on Plasma Extracellular Vesicle Heterogeneity and Subpopulation Profiling. J. Extracell. Biol. 2025, 4, e70058. [Google Scholar] [CrossRef]
- Newman, L.; Rowland, A. Detection and Isolation of Tissue-Specific Extracellular Vesicles From the Blood. J. Extracell. Biol. 2025, 4, e70059. [Google Scholar] [CrossRef] [PubMed]
- Blaser, M.C.; Kraler, S.; Lüscher, T.F.; Aikawa, E. Multi-Omics Approaches to Define Calcific Aortic Valve Disease Pathogenesis. Circ. Res. 2021, 128, 1371–1397. [Google Scholar] [CrossRef]
- Reventun, P.; Sánchez-Esteban, S.; Cook-Calvete, A.; Delgado-Marín, M.; Roza, C.; Jorquera-Ortega, S.; Hernandez, I.; Tesoro, L.; Botana, L.; Zamorano, J.L.; et al. Endothelial ILK induces cardioprotection by preventing coronary microvascular dysfunction and endothelial-to-mesenchymal transition. Basic Res. Cardiol. 2023, 118, 28. [Google Scholar]
- Viegas, C.; Carreira, J.; Maia, T.M.; Macedo, A.L.; Matos, A.P.; Neves, J.; Simes, D. Gla Rich Protein (GRP) Mediates Vascular Smooth Muscle Cell (VSMC) Osteogenic Differentiation, Extracellular Vesicle (EV) Calcification Propensity, and Immunomodulatory Properties. Int. J. Mol. Sci. 2024, 25, 12406. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles. 2022, 11, e12238. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538. [Google Scholar] [CrossRef]
- Vago, R.; Radano, G.; Zocco, D.; Zarovni, N. Urine stabilization and normalization strategies favor unbiased analysis of urinary EV content. Sci. Rep. 2022, 12, 17663. [Google Scholar] [CrossRef]
- Sun, D.S.; Chang, H.H. Extracellular vesicles: Function, resilience, biomarker, bioengineering, and clinical implications. Tzu Chi Med. J. 2024, 36, 251–259. [Google Scholar]
- Kumari, S.; Lausted, C.; Scherler, K.; Ng, A.H.C.; Lu, Y.; Lee, I.; Hood, L.; Wang, K. Approaches and Challenges in Characterizing the Molecular Content of Extracellular Vesicles for Biomarker Discovery. Biomolecules 2024, 14, 1599. [Google Scholar] [CrossRef] [PubMed]
- Ghadami, S.; Dellinger, K. The lipid composition of extracellular vesicles: Applications in diagnostics and therapeutic delivery. Front. Mol. Biosci. 2023, 10, 1198044. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.H.; Reed, J.H. Extracellular vesicles as next-generation therapeutics and biomarkers in amyloidosis: A new frontier. Front. Biomater. Sci. 2024, 2, 1343658. [Google Scholar] [CrossRef]
- Skotland, T.; Sagini, K.; Sandvig, K.; Llorente, A. An emerging focus on lipids in extracellular vesicles. Adv. Drug Deliv. Rev. 2020, 159, 308–321. [Google Scholar] [CrossRef]
- Tanase, D.M.; Valasciuc, E.; Gosav, E.M.; Floria, M.; Costea, C.F.; Dima, N.; Tudorancea, I.; Maranduca, M.A.; Serban, I.L. Contribution of Oxidative Stress (OS) in Calcific Aortic Valve Disease (CAVD): From Pathophysiology to Therapeutic Targets. Cells 2022, 11, 2663. [Google Scholar] [CrossRef]
- Fu, E.; Pan, K.; Li, Z. Engineering extracellular vesicles for targeted therapeutics in cardiovascular disease. Front. Cardiovasc. Med. 2024, 11, 1503830. [Google Scholar] [CrossRef]
- Sánchez-Esteban, S.; Castro-Pinto, M.; Cook-Calvete, A.; Reventún, P.; Delgado-Marín, M.; Benito-Manzanaro, L.; Hernandez, I.; López-Menendez, J.; Zamorano, J.L.; Zaragoza, C.; et al. Integrin-Linked Kinase Expression in Human Valve Endothelial Cells Plays a Protective Role in Calcific Aortic Valve Disease. Antioxidants 2022, 11, 1736. [Google Scholar] [CrossRef]
- Yang, R.; Tang, Y.; Chen, X.; Yang, Y. Telocytes-derived extracellular vesicles alleviate aortic valve calcification by carrying miR-30b. ESC Heart Fail. 2021, 8, 3935–3946. [Google Scholar] [CrossRef]
- Wang, L.; Wang, X.; Chen, Q.; Wei, Z.; Xu, X.; Han, D.; Zhang, Y.; Chen, Z.; Liang, Q. MicroRNAs of extracellular vesicles derived from mesenchymal stromal cells alleviate inflammation in dry eye disease by targeting the IRAK1/TAB2/NF-κB pathway. Ocul. Surf. 2023, 28, 131–140. [Google Scholar] [CrossRef]
- Mori, M.A.; Ludwig, R.G.; Garcia-Martin, R.; Brandão, B.B.; Kahn, C.R. Extracellular miRNAs: From Biomarkers to Mediators of Physiology and Disease. Cell Metab. 2019, 30, 656–673. [Google Scholar] [CrossRef]
- Barile, L.; Vassalli, G. Exosomes: Therapy delivery tools and biomarkers of diseases. Pharmacol. Ther. 2017, 174, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Assunção, R.R.S.; Santos, N.L.; de Sousa Andrade, L.N. Extracellular vesicles as cancer biomarkers and drug delivery strategies in clinical settings: Advances, perspectives, and challenges. Clinics 2025, 80, 100635. [Google Scholar] [CrossRef]
- Novoa-Herrán, S. Retos y oportunidades en el estudio de vesículas extracelulares: Contexto institucional a nivel mundial y situación actual en Colombia. Biomédica 2021, 41, 555–589. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Cheng, Q.; Bai, L.; Huang, S.; Gao, J. Extracellular Vesicles in Cardiovascular Diseases: Diagnosis and Therapy. Front. Cell Dev. Biol. 2022, 10, 875376. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Lucien, F.; Gustafson, D.; Lenassi, M.; Li, B.; Teske, J.J.; Boilard, E.; von Hohenberg, K.C.; Falcón-Perez, J.M.; Gualerzi, A.; Reale, A.; et al. MIBlood-EV: Minimal information to enhance the quality and reproducibility of blood extracellular vesicle research. J. Extracell. Vesicles 2023, 12, e12385. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, K.; Wang, Z.; Wang, Y.; Liu, J.; Lin, L.; Shao, Y.; Gao, L.; Yin, H.; Cui, C.; et al. DNA in serum extracellular vesicles is stable under different storage conditions. BMC Cancer 2016, 16, 753. [Google Scholar] [CrossRef]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in Plasma Exosome is Stable under Different Storage Conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef]
- Poupardin, R.; Wolf, M.; Strunk, D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv. Drug Deliv. Rev. 2021, 176, 113872. [Google Scholar] [CrossRef]
- Kumar, A.; Nader, M.A.; Deep, G. Emergence of Extracellular Vesicles as “Liquid Biopsy” for Neurological Disorders: Boom or Bust. Pharmacol. Rev. 2024, 76, 199–227. [Google Scholar] [CrossRef]
- Ma, C.; Ding, R.; Hao, K.; Du, W.; Xu, L.; Gao, Q.; Yu, C. Storage Stability of Blood Samples for miRNAs in Glycosylated Extracellular Vesicles. Molecules 2023, 29, 103. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Dai, Y.; Han, Y.; Wei, X.; Wei, G.; Chen, W.; Kong, S.; He, Y.; Liu, H.; et al. Neutrophil N1 polarization induced by cardiomyocyte-derived extracellular vesicle miR-9-5p aggravates myocardial ischemia/reperfusion injury. J. Nanobiotechnol. 2024, 22, 632. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cao, J.; Li, S.; Cui, T.; Ni, J.; Zhang, H.; Zhu, Y.; Mao, J.; Gao, X.; Midgley, A.C.; et al. M2 Macrophage-Derived sEV Regulate Pro-Inflammatory CCR2 + Macrophage Subpopulations to Favor Post-AMI Cardiac Repair. Adv. Sci. 2023, 10, 2202964. [Google Scholar] [CrossRef] [PubMed]
- Dang, G.; Li, T.; Yang, D.; Yang, G.; Du, X.; Yang, J.; Miao, Y.; Han, L.; Ma, X.; Song, Y.; et al. T lymphocyte-derived extracellular vesicles aggravate abdominal aortic aneurysm by promoting macrophage lipid peroxidation and migration via pyruvate kinase muscle isozyme 2. Redox Biol. 2022, 50, 102257. [Google Scholar] [CrossRef]
- Saxena, S.; Volpe, M.C.; Agostinis, C.; Vodret, S.; Ring, N.A.R.; Colliva, A.; Vuerich, R.; Braga, L.; Cook-Calvete, A.; Romano, F.; et al. Anti-miRNA therapeutics for uterine fibroids. Biomed. Pharmacother. 2025, 185, 117946. [Google Scholar] [CrossRef] [PubMed]
- Steiner, L.; Eldh, M.; Offens, A.; Veerman, R.E.; Johansson, M.; Hemdan, T.; Netterling, H.; Huge, Y.; Abdul-Sattar Aljabery, F.; Alamdari, F.; et al. Protein profile in urinary extracellular vesicles is a marker of malignancy and correlates with muscle invasiveness in urinary bladder cancer. Cancer Lett. 2025, 609, 217352. [Google Scholar] [CrossRef]
- Lapitz, A.; Azkargorta, M.; Milkiewicz, P.; Olaizola, P.; Zhuravleva, E.; Grimsrud, M.M.; Schramm, C.; Arbelaiz, A.; O’Rourke, C.J.; La Casta, A.; et al. Liquid biopsy-based protein biomarkers for risk prediction, early diagnosis, and prognostication of cholangiocarcinoma. J. Hepatol. 2023, 79, 93–108. [Google Scholar] [CrossRef]
- Su, Y.; Chen, M.; Xu, W.; Gu, P.; Fan, X. Advances in Extracellular-Vesicles-Based Diagnostic and Therapeutic Approaches for Ocular Diseases. ACS Nano 2024, 18, 22793–22828. [Google Scholar] [CrossRef]
- Krohn, J.B.; Aikawa, E.; Aikawa, M.; Hutcheson, J.D.; Sahoo, S.; Fish, J.E. Editorial: Extracellular vesicles in cardiovascular inflammation and calcification. Front. Cardiovasc. Med. 2022, 9, 1077124. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; Carter, D.R.F.; Clayton, A.; Lambert, D.W.; Raposo, G.; Vader, P. Challenges and directions in studying cell–cell communication by extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2022, 23, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Singh, M.; Tiwari, P.K.; Kashyap, V.; Kumar, S. Proteomics of Extracellular Vesicles: Recent Updates, Challenges and Limitations. Proteomes 2025, 13, 12. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular vesicle isolation methods: Rising impact of size-exclusion chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef]
- Zhao, Z.; Wijerathne, H.; Godwin, A.K.; Soper, S.A. Isolation and analysis methods of extracellular vesicles (EVs). Extracell. Vesicles Circ. Nucl. Acids 2021, 2, 80–103. [Google Scholar]
- Yuan, F.; Li, Y.M.; Wang, Z. Preserving extracellular vesicles for biomedical applications: Consideration of storage stability before and after isolation. Drug Deliv. 2021, 28, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Di, K.; Fan, B.; Wu, J.; Gu, X.; Sun, Y.; Khan, A.; Li, P.; Li, Z. MicroRNAs in extracellular vesicles: Sorting mechanisms, diagnostic value, isolation, and detection technology. Front. Bioeng. Biotechnol. 2022, 10, 948959. [Google Scholar] [CrossRef]
- Spitzberg, J.D.; Ferguson, S.; Yang, K.S.; Peterson, H.M.; Carlson, J.C.T.; Weissleder, R. Multiplexed analysis of EV reveals specific biomarker composition with diagnostic impact. Nat. Commun. 2023, 14, 1239. [Google Scholar] [CrossRef]
- Botha, J.; Handberg, A.; Simonsen, J.B. Lipid-based strategies used to identify extracellular vesicles in flow cytometry can be confounded by lipoproteins: Evaluations of annexin V, lactadherin, and detergent lysis. J. Extracell. Vesicles 2022, 11, e12200. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, M.C.; Quarto, R.; Tasso, R. Extracellular Vesicles as Biomarkers and Therapeutic Tools: From Pre-Clinical to Clinical Applications. Biology 2021, 10, 359. [Google Scholar] [CrossRef]
- Krohn, J.B.; Hutcheson, J.D.; Martínez-Martínez, E.; Aikawa, E. Extracellular vesicles in cardiovascular calcification: Expanding current paradigms. J. Physiol. 2016, 594, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Tandon, I.; Quinn, K.P.; Balachandran, K. Label-Free Multiphoton Microscopy for the Detection and Monitoring of Calcific Aortic Valve Disease. Front. Cardiovasc. Med. 2021, 8, 688513. [Google Scholar] [CrossRef] [PubMed]
- Veziroglu, E.M.; Mias, G.I. Characterizing Extracellular Vesicles and Their Diverse RNA Contents. Front. Genet. 2020, 11, 700. [Google Scholar] [CrossRef]
- Diehl, P.; Nagy, F.; Sossong, V.; Helbing, T.; Beyersdorf, F.; Olschewski, M.; Bode, C.; Moser, M. Increased levels of circulating microparticles in patients with severe aortic valve stenosis. Thromb. Haemost. 2008, 99, 711–719. [Google Scholar] [CrossRef]
- Lorite, P.; Domínguez, J.N.; Palomeque, T.; Torres, M.I. Extracellular Vesicles: Advanced Tools for Disease Diagnosis, Monitoring, and Therapies. Int. J. Mol. Sci. 2024, 26, 189. [Google Scholar] [CrossRef]
- Hulanicka, M.; Garncarz, M.; Parzeniecka-Jaworska, M.; Jank, M. Plasma miRNAs as potential biomarkers of chronic degenerative valvular disease in Dachshunds. BMC Vet. Res. 2014, 10, 205. [Google Scholar] [CrossRef]
- Jung, R.G.; Duchez, A.-C.; Simard, T.; Dhaliwal, S.; Gillmore, T.; Di Santo, P.; Labinaz, A.; Ramirez, F.D.; Rasheed, A.; Robichaud, S.; et al. Plasminogen Activator Inhibitor-1–Positive Platelet-Derived Extracellular Vesicles Predicts MACE and the Proinflammatory SMC Phenotype. JACC Basic Transl. Sci. 2022, 7, 985–997. [Google Scholar] [CrossRef]
- Verwer, M.C.; Mekke, J.; Timmerman, N.; Waissi, F.; Boltjes, A.; Pasterkamp, G.; de Borst, G.J.; de Kleijn, D.P.V. Comparison of cardiovascular biomarker expression in extracellular vesicles, plasma and carotid plaque for the prediction of MACE in CEA patients. Sci. Rep. 2023, 13, 1010. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liang, J.; Witwer, K.W.; Zhang, Y.; Wang, Q.; Yin, H. Extracellular vesicle-associated microRNA-30b-5p activates macrophages through the SIRT1/ NF-κB pathway in cell senescence. Front. Immunol. 2022, 13, 955175. [Google Scholar] [CrossRef]
- Li, X.; Chen, J.; Yang, Y.; Cai, H.; Ao, Z.; Xing, Y.; Li, K.; Yang, K.; Guan, W.; Friend, J.; et al. Extracellular vesicle-based point-of-care testing for diagnosis and monitoring of Alzheimer’s disease. Microsyst. Nanoeng. 2025, 11, 65. [Google Scholar] [CrossRef]
- Siavashy, S.; Soltani, M.; Rahimi, S.; Hosseinali, M.; Guilandokht, Z.; Raahemifar, K. Recent advancements in microfluidic-based biosensors for detection of genes and proteins: Applications and techniques. Biosens. Bioelectron. X 2024, 19, 100489. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, R.; Wang, B.; He, C.; Bai, S.; Yan, H.; Yu, J.; Li, H.; Peng, B.; Gao, Z.; et al. Electrochemical detection of extracellular vesicles for early diagnosis: A focus on disease biomarker analysis. Extracell. Vesicles Circ. Nucl. Acids 2024, 5, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zheng, M.; Xiao, Q.; Wang, H.; Chi, C.; Lin, T.; Wang, Y.; Yi, X.; Zhu, L. Recent Advances in Microfluidic-Based Extracellular Vesicle Analysis. Micromachines 2024, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, X.; Ha, T.; Gao, M.; Liu, L.; Wang, R.; Yu, K.; Kalbfleisch, J.H.; Kao, R.L.; Williams, D.L.; et al. MicroRNA-125b Prevents Cardiac Dysfunction in Polymicrobial Sepsis by Targeting TRAF6-Mediated Nuclear Factor κB Activation and p53-Mediated Apoptotic Signaling. J. Infect. Dis. 2016, 214, 1773–1783. [Google Scholar] [CrossRef]
- Jiang, W.; Kong, L.; Ni, Q.; Lu, Y.; Ding, W.; Liu, G.; Pu, L.; Tang, W.; Kong, L. miR-146a Ameliorates Liver Ischemia/Reperfusion Injury by Suppressing IRAK1 and TRAF6. PLoS ONE 2014, 9, e101530. [Google Scholar] [CrossRef]
- Wu, H.; Fan, H.; Shou, Z.; Xu, M.; Chen, Q.; Ai, C.; Dong, Y.; Liu, Y.; Nan, Z.; Wang, Y.; et al. Extracellular vesicles containing miR-146a attenuate experimental colitis by targeting TRAF6 and IRAK1. Int. Immunopharmacol. 2019, 68, 204–212. [Google Scholar] [CrossRef]
- Li, N.; Bai, Y.; Zhou, G.; Ma, Y.; Tan, M.; Qiao, F.; Li, X.; Shen, M.; Song, X.; Zhao, X.; et al. miR-214 Attenuates Aortic Valve Calcification by Regulating Osteogenic Differentiation of Valvular Interstitial Cells. Mol. Ther. Nucl. Acids 2020, 22, 971–980. [Google Scholar] [CrossRef]
- Salim, M.T.; Esmerats, J.F.; Arjunon, S.; Villa-Roel, N.; Nerem, R.M.; Jo, H.; Yoganathan, A.P. miR-214 is Stretch-Sensitive in Aortic Valve and Inhibits Aortic Valve Calcification. Ann. Biomed. Eng. 2019, 47, 1106–1115. [Google Scholar] [CrossRef]
- Hosen, M.R.; Goody, P.R.; Zietzer, A.; Xiang, X.; Niepmann, S.T.; Sedaghat, A.; Tiyerili, V.; Chennupati, R.; Moore, J.B.; Boon, R.A.; et al. Circulating MicroRNA-122-5p Is Associated with a Lack of Improvement in Left Ventricular Function After Transcatheter Aortic Valve Replacement and Regulates Viability of Cardiomyocytes Through Extracellular Vesicles. Circulation 2022, 146, 1836–1854. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, J.; López, B.; Hermida, N.; Schroen, B.; José, G.S.; Heymans, S.; Valencia, F.; Gómez-Doblas, J.J.; De Teresa, E.; Díez, J.; et al. microRNA-122 down-regulation may play a role in severe myocardial fibrosis in human aortic stenosis through TGF-β1 up-regulation. Clin. Sci. 2014, 126, 497–506. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, H.; Yue, Y.; Li, S.; Zhang, D.; He, R. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/β-catenin in ox-LDL -stimulated vascular smooth muscle cells. J. Biomed. Sci. 2018, 25, 11. [Google Scholar] [CrossRef]
- Yu, F.; Duan, Y.; Liu, C.; Huang, H.; Xiao, X.; He, Z. Extracellular vesicles in atherosclerosis and vascular calcification: The versatile non-coding RNAs from endothelial cells and vascular smooth muscle cells. Front. Med. 2023, 10, 1193660. [Google Scholar] [CrossRef] [PubMed]
- Mollajan, E.; Yazdani, S.; Ghasemzadeh, M.; Mozhgani, S.H. miR-21 in cardiovascular disease: New insights and emerging therapeutic potential. Discov. Appl. Sci. 2025, 7, 447. [Google Scholar] [CrossRef]
- Verjans, R.; Peters, T.; Beaumont, F.J.; van Leeuwen, R.; van Herwaarden, T.; Verhesen, W.; Munts, C.; Bijnen, M.; Henkens, M.; Diez, J.; et al. MicroRNA-221/222 Family Counteracts Myocardial Fibrosis in Pressure Overload–Induced Heart Failure. Hypertension 2018, 71, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Qiao, E.; Huang, Z.; Wang, W. Exploring potential genes and pathways related to calcific aortic valve disease. Gene 2022, 808, 145987. [Google Scholar] [CrossRef]
- Chen, A.; Wen, J.; Lu, C.; Lin, B.; Xian, S.; Huang, F.; Wu, Y.; Zeng, Z. Inhibition of miR 155 5p attenuates the valvular damage induced by rheumatic heart disease. Int. J. Mol. Med. 2019, 45, 429–440. [Google Scholar] [CrossRef]
- Cable, J.; Witwer, K.W.; Coffey, R.J.; Milosavljevic, A.; von Lersner, A.K.; Jimenez, L.; Pucci, F.; Barr, M.M.; Dekker, N.; Barman, B.; et al. Exosomes, microvesicles, and other extracellular vesicles—A Keystone Symposia report. Ann. N. Y. Acad. Sci. 2023, 1523, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Bavafa, A.; Izadpanahi, M.; Hosseini, E.; Hajinejad, M.; Abedi, M.; Forouzanfar, F.; Sahab-Negah, S. Exosome: An overview on enhanced biogenesis by small molecules. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 6473–6508. [Google Scholar] [CrossRef]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef]
- Chae, C.W.; Choi, G.; Yoon, T.; Kwon, Y.W. Exosome-Based Therapy in Cardiovascular Diseases: A New Frontier in Cardiovascular Disease Treatment. Korean Circ. J. 2025, 55, 461–480. [Google Scholar] [CrossRef]
- Ramasubramanian, L.; Du, S.; Gidda, S.; Bahatyrevich, N.; Hao, D.; Kumar, P.; Wang, A. Bioengineering Extracellular Vesicles for the Treatment of Cardiovascular Diseases. Adv. Biol. 2022, 6, e2200087. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.; Lincoln, J. Calcific Aortic Valve Disease: A Developmental Biology Perspective. Curr. Cardiol. Rep. 2018, 20, 21. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.Q.; Zhu, Y.; Han, X.Q.; Liu, N. Exosomes Derived from Mesenchymal Stromal Cells Pretreated with Advanced Glycation End Product-Bovine Serum Albumin Inhibit Calcification of Vascular Smooth Muscle Cells. Front. Endocrinol. 2018, 9, 524. [Google Scholar] [CrossRef]
- Mathew, B.; Ravindran, S.; Liu, X.; Torres, L.; Chennakesavalu, M.; Huang, C.C.; Feng, L.; Zelka, R.; Lopez, J.; Sharma, M.; et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials 2019, 197, 146–160. [Google Scholar] [CrossRef]
- Nannan, L.; Decombis, S.; Terryn, C.; Audonnet, S.; Michel, J.; Brassart-Pasco, S.; Gsell, W.; Himmelreich, U.; Brassart, B. Dysregulation of intercellular communication in vitro and in vivo via extracellular vesicles secreted by pancreatic duct adenocarcinoma cells and generated under the influence of the AG9 elastin peptide-conditioned microenvironment. J. Extracell. Biol. 2024, 3, e145. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dong, S.; Grippin, A.J.; Teng, L.; Lee, A.S.; Kim, B.Y.S.; Jiang, W. Engineering therapeutical extracellular vesicles for clinical translation. Trends Biotechnol. 2025, 43, 61–82. [Google Scholar] [CrossRef]
- Nakase, I.; Futaki, S. Combined treatment with a pH-sensitive fusogenic peptide and cationic lipids achieves enhanced cytosolic delivery of exosomes. Sci. Rep. 2015, 5, 10112. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar]
- Jiang, F.; Chen, Q.; Wang, W.; Ling, Y.; Yan, Y.; Xia, P. Hepatocyte-derived extracellular vesicles promote endothelial inflammation and atherogenesis via microRNA-1. J. Hepatol. 2020, 72, 156–166. [Google Scholar] [CrossRef]
- Patel, S.; Guo, M.K.; Abdul Samad, M.; Howe, K.L. Extracellular vesicles as biomarkers and modulators of atherosclerosis pathogenesis. Front. Cardiovasc. Med. 2023, 10, 1202187. [Google Scholar] [CrossRef]
- Bhattacharya, P.; Dhawan, U.K.; Hussain, M.T.; Singh, P.; Bhagat, K.K.; Singhal, A.; Austin-Williams, S.; Sengupta, S.; Subramanian, M. Efferocytes release extracellular vesicles to resolve inflammation and tissue injury via prosaposin-GPR37 signaling. Cell Rep. 2023, 42, 112808. [Google Scholar] [CrossRef]
- Goody, P.R.; Christmann, D.; Goody, D.; Hildebrand, S.; Billig, H.; Nehl, D.; Chennupati, R.; Gladka, M.; Wilhelm-Jüngling, K.; Uchida, S.; et al. Calcific aortic valve disease augments vesicular microRNA-145-5p to regulate the calcification of valvular interstitial cells via cellular crosstalk. Basic Res. Cardiol. 2025, 120, 991–1010. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, M.; Heydarkhan-Hagvall, S.; Tangruksa, B.; Garibotti, H.G.; Jing, Y.; Maugeri, M.; Kohl, F.; Hultin, L.; Reyahi, A.; Camponeschi, A.; et al. Lipid Nanoparticles Deliver the Therapeutic VEGFA mRNA In Vitro and In Vivo and Transform Extracellular Vesicles for Their Functional Extensions. Adv. Sci. 2023, 10, 202206187. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.C.; Jayasinghe, M.K.; Pham, T.T.; Yang, Y.; Wei, L.; Usman, W.M.; Chen, H.; Pirisinu, M.; Gong, J.; Kim, S.; et al. Covalent conjugation of extracellular vesicles with peptides and nanobodies for targeted therapeutic delivery. J. Extracell. Vesicles 2021, 10, e12057. [Google Scholar] [CrossRef]
- Park, D.J.; Duggan, E.; Ho, K.; Dorschner, R.A.; Dobke, M.; Nolan, J.P.; Eliceiri, B.P. Serpin-loaded extracellular vesicles promote tissue repair in a mouse model of impaired wound healing. J. Nanobiotechnol. 2022, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Iannotta, D.; Amruta, A.; Kijas, A.W.; Rowan, A.E.; Wolfram, J. Entry and exit of extracellular vesicles to and from the blood circulation. Nat. Nanotechnol. 2024, 19, 13–20. [Google Scholar] [CrossRef]
- Gupta, D.; Wiklander, O.P.B.; Wood, M.J.A.; El-Andaloussi, S. Biodistribution of therapeutic extracellular vesicles. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 170–190. [Google Scholar] [CrossRef]
- Miceli, R.T.; Chen, T.; Nose, Y.; Tichkule, S.; Brown, B.; Fullard, J.F.; Saulsbury, M.D.; Heyliger, S.O.; Gnjatic, S.; Kyprianou, N.; et al. Extracellular vesicles, RNA sequencing, and bioinformatic analyses: Challenges, solutions, and recommendations. J. Extracell. Vesicles 2024, 13, e70005. [Google Scholar] [CrossRef]
- Cecchin, R.; Troyer, Z.; Witwer, K.; Morris, K.V. Extracellular vesicles: The next generation in gene therapy delivery. Mol. Ther. 2023, 31, 1225–1230. [Google Scholar] [CrossRef]
- Liu, M.; Sun, Y.; Zhang, Q. Emerging Role of Extracellular Vesicles in Bone Remodeling. J. Dent. Res. 2018, 97, 859–868. [Google Scholar] [CrossRef]
- Blaser, M.C.; Aikawa, E. Roles and Regulation of Extracellular Vesicles in Cardiovascular Mineral Metabolism. Front. Cardiovasc. Med. 2018, 5, 187. [Google Scholar] [CrossRef]
- Li, Y.; Xing, L.; Zhu, M.; Li, X.; Wei, F.; Sun, W.; Jia, Y. HPMA Copolymers: A Versatile Platform for Targeted Peptide Drug Delivery. Biomolecules 2025, 15, 596. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Fan, X.; Wang, Y.; Shen, M.; Zheng, Y.; Zhao, S.; Yang, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles in Liver Immunity and Therapy. Front. Immunol. 2022, 13, 833878. [Google Scholar] [CrossRef]
- Trębacz, H.; Barzycka, A. Mechanical Properties and Functions of Elastin: An Overview. Biomolecules 2023, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Zhu, X.; Liu, J.; Zhou, J. Biofunctionalized Decellularized Tissue-Engineered Heart Valve with Mesoporous Silica Nanoparticles for Controlled Release of VEGF and RunX2-siRNA against Calcification. Bioengineering 2023, 10, 859. [Google Scholar] [CrossRef]
- Voicu, G.; Mocanu, C.A.; Safciuc, F.; Anghelache, M.; Deleanu, M.; Cecoltan, S.; Pinteala, M.; Uritu, C.M.; Droc, I.; Simionescu, M.; et al. Nanocarriers of shRNA-Runx2 directed to collagen IV as a nanotherapeutic system to target calcific aortic valve disease. Mater. Today Bio 2023, 20, 100620. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Li, Z.; Hou, Y.; Sun, W.; Zhang, R.; Zhao, L.; Wei, M.; Yang, G.; Yuan, L. Exosome-mediated delivery of inflammation-responsive Il-10 mRNA for controlled atherosclerosis treatment. Theranostics 2021, 11, 9988–10000. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Y.; Wang, Z.; Zhang, L.; Xu, Y.; Li, Y.; Zhang, L.; Wang, G.; Yang, S.; Xue, G. Mesenchymal stem cell-derived extracellular vesicles protect against abdominal aortic aneurysm formation by inhibiting NET-induced ferroptosis. Exp. Mol. Med. 2023, 55, 939–951. [Google Scholar] [CrossRef]
- Ouyang, Y.; Hong, Y.; Mai, C.; Yang, H.; Wu, Z.; Gao, X.; Zeng, W.; Deng, X.; Liu, B.; Zhang, Y.; et al. Transcriptome analysis reveals therapeutic potential of NAMPT in protecting against abdominal aortic aneurysm in human and mouse. Bioact. Mater. 2024, 34, 17–36. [Google Scholar] [CrossRef]
- Stavrou, A.; Ortiz, A. Extracellular Vesicles: A Novel Tool in Nanomedicine and Cancer Treatment. Cancers 2022, 14, 4450. [Google Scholar] [CrossRef]
- Wang, G.; Ma, X.; Huang, W.; Wang, S.; Lou, A.; Wang, J.; Tu, Y.; Cui, W.; Zhou, W.; Zhang, W.; et al. Macrophage biomimetic nanoparticle-targeted functional extracellular vesicle micro-RNAs revealed via multiomics analysis alleviate sepsis-induced acute lung injury. J. Nanobiotechnol. 2024, 22, 362. [Google Scholar] [CrossRef]
- Lee, Y.J.; Park, M.; Kim, H.Y.; Kim, J.K.; Kim, W.K.; Lim, S.C.; Kang, K.W. Circulating small extracellular vesicles promote proliferation and migration of vascular smooth muscle cells via AXL and MerTK activation. Acta Pharmacol. Sin. 2023, 44, 984–998. [Google Scholar]
- Wang, Z.; Zhu, D.; Zhang, Y.; Xia, F.; Zhu, J.; Dai, J.; Zhuge, X. Extracellular vesicles produced by avian pathogenic Escherichia coli (APEC) activate macrophage proinflammatory response and neutrophil extracellular trap (NET) formation through TLR4 signaling. Microb. Cell Factories 2023, 22, 177. [Google Scholar] [CrossRef] [PubMed]
- Ortmann, W.; Such, A.; Cichon, I.; Baj-Krzyworzeka, M.; Weglarczyk, K.; Kolaczkowska, E. Large extracellular vesicle (EV) and neutrophil extracellular trap (NET) interaction captured in vivo during systemic inflammation. Sci. Rep. 2024, 14, 4680. [Google Scholar] [CrossRef]
- Johnson, J.; Law, S.Q.K.; Shojaee, M.; Hall, A.S.; Bhuiyan, S.; Lim, M.B.L.; Silva, A.; Kong, K.J.W.; Schoppet, M.; Blyth, C.; et al. First-in-human clinical trial of allogeneic, platelet-derived extracellular vesicles as a potential therapeutic for delayed wound healing. J. Extracell. Vesicles 2023, 12, e12332. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Chen, J.; Hu, H.; Zhu, Y.; Wang, X.; Zhou, S.; Wang, F.; Xiang, M. Engineered M2 macrophage-derived extracellular vesicles with platelet membrane fusion for targeted therapy of atherosclerosis. Bioact. Mater. 2024, 35, 447–460. [Google Scholar] [CrossRef]
- Li, Q.; Song, Y.; Wang, Q.; Chen, J.; Gao, J.; Tan, H.; Li, S.; Wu, Y.; Yang, H.; Huang, H.; et al. Engineering extracellular vesicles with platelet membranes fusion enhanced targeted therapeutic angiogenesis in a mouse model of myocardial ischemia reperfusion. Theranostics 2021, 11, 3916–3931. [Google Scholar] [CrossRef]
- Rodríguez, D.A.; Vader, P. Extracellular Vesicle-Based Hybrid Systems for Advanced Drug Delivery. Pharmaceutics 2022, 14, 267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, D.; Choi, J.C.; Minard, C.G.; Hou, X.; Coselli, J.S.; Shen, Y.H.; LeMaire, S.A. Matrix metalloproteinase levels in chronic thoracic aortic dissection. J. Surg. Res. 2014, 189, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ballantyne, L.L.; Yu, Y.; Funk, C.D. Perivascular adipose tissue–derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB J. 2019, 33, 12704–12722. [Google Scholar] [CrossRef]
- Gupta, D.; Zickler, A.M.; El Andaloussi, S. Dosing extracellular vesicles. Adv. Drug Deliv. Rev. 2021, 178, 113961. [Google Scholar] [CrossRef]
- Coumans, F.A.W.; Brisson, A.R.; Buzas, E.I.; Dignat-George, F.; Drees, E.E.E.; El-Andaloussi, S.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Methodological Guidelines to Study Extracellular Vesicles. Circ. Res. 2017, 120, 1632–1648. [Google Scholar] [CrossRef]
- Furmanik, M.; Chatrou, M.; van Gorp, R.; Akbulut, A.; Willems, B.; Schmidt, H.; Emanueli, C.; Gasecka, A.; Hendrix, A.; Hill, A.F.; et al. Reactive Oxygen-Forming Nox5 Links Vascular Smooth Muscle Cell Phenotypic Switching and Extracellular Vesicle-Mediated Vascular Calcification. Circ. Res. 2020, 127, 911–927. [Google Scholar] [CrossRef]
- Muskan, M.; Abeysinghe, P.; Cecchin, R.; Branscome, H.; Morris, K.V.; Kashanchi, F. Therapeutic potential of RNA-enriched extracellular vesicles: The next generation in RNA delivery via biogenic nanoparticles. Mol. Ther. 2024, 32, 2939–2949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, W.; Wu, Z.; Chen, Y. Diversity of extracellular vesicle sources in atherosclerosis: Role and therapeutic application. Angiogenesis 2025, 28, 34. [Google Scholar] [CrossRef]
- Fang, F.; Yang, H.; Wang, X.; Zhao, T.; Zhao, P.; Liu, X. Extracellular Vesicles in Atherosclerosis: From Pathogenesis to Theranostic Applications. Small 2025, 21, e2504761. [Google Scholar] [CrossRef] [PubMed]
- Favretto, G.; da Cunha, R.S.; Flores Santos, A.; Leitolis, A.; Schiefer, E.M.; Gregório, P.C.; Franco, C.R.C.; Massy, Z.; Dalboni, M.A.; Stinghen, A.E.M. Uremic endothelial-derived extracellular vesicles: Mechanisms of formation and their role in cell adhesion, cell migration, inflammation, and oxidative stress. Toxicol. Lett. 2021, 347, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Jiapaer, Z.; Li, C.; Yang, X.; Sun, L.; Chatterjee, E.; Zhang, L.; Lei, J.; Li, G. Extracellular Non-Coding RNAs in Cardiovascular Diseases. Pharmaceutics 2023, 15, 155. [Google Scholar] [CrossRef]
- Gąsecka, A.; Szolc, P.; van der Pol, E.; Niewiara, Ł.; Guzik, B.; Kleczyński, P.; Tomaniak, M.; Figura, E.; Zaremba, M.; Grabowski, M.; et al. Endothelial Cell-Derived Extracellular Vesicles Allow to Differentiate Between Various Endotypes of INOCA: A Multicentre, Prospective, Cohort Study. J. Cardiovasc. Transl. Res. 2025, 18, 305–315. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Extracellular Vesicles from Cardiovascular Progenitor Cells in the Treatment of Non-Ischemic Dilated Cardiomyopathy. Identifier NCT05774509. Available online: https://clinicaltrials.gov/ct2/show/NCT05774509 (accessed on 22 October 2025).
- ClinicalTrials.gov. Salivary and Plasma Extracellular Vesicle-Associated Long Non-Coding RNAs in Acute and Chronic Heart Failure (SEAL-HF). Identifier NCT06169540. Available online: https://clinicaltrials.gov/ct2/show/NCT06169540 (accessed on 22 October 2025).
- ClinicalTrials.gov. Extracellular Vesicles in Obesity and Cardiometabolic Disease (EVOC). Identifier NCT06408961. Available online: https://clinicaltrials.gov/ct2/show/NCT06408961 (accessed on 22 October 2025).
- ClinicalTrials.gov. Early Valve Replacement in Severe Asymptomatic Aortic Stenosis (EASY-AS). Identifier NCT04204915. Available online: https://clinicaltrials.gov/ct2/show/NCT04204915 (accessed on 22 October 2025).
- ClinicalTrials.gov. Extracellular Vesicles from Mesenchymal Cells in the Treatment of Acute Respiratory Failure Syndrome. Identifier NCT06002841. Available online: https://clinicaltrials.gov/ct2/show/NCT06002841 (accessed on 22 October 2025).
- ClinicalTrials.gov. Safety and Efficacy of Mesenchymal Stem Cell Therapy in Patients with Cardiovascular Disease. Identifier NCT04897841. Available online: https://clinicaltrials.gov/ct2/show/NCT04897841 (accessed on 22 October 2025).
- Düsing, P.; Zietzer, A.; Goody, P.R.; Hosen, M.R.; Kurts, C.; Nickenig, G.; Jansen, F. Vascular pathologies in chronic kidney disease: Pathophysiological mechanisms and novel therapeutic approaches. J. Mol. Med. 2021, 99, 335–348. [Google Scholar] [CrossRef]
- Small, A.; Kiss, D.; Giri, J.; Anwaruddin, S.; Siddiqi, H.; Guerraty, M.; Chirinos, J.A.; Ferrari, G.; Rader, D.J. Biomarkers of Calcific Aortic Valve Disease. Arter. Thromb. Vasc. Biol. 2017, 37, 623–632. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Ji, X.; Tan, Z.; Lubman, D.M. Column-based Technology for CD9-HPLC Immunoaffinity Isolation of Serum Extracellular Vesicles. J. Proteome Res. 2021, 20, 4901–4911. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Luo, S.; Lu, P.; Cai, C.; Li, W.; Li, C. Composition, functions, and applications of exosomal membrane proteins. Front. Immunol. 2024, 15, 1408415. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Tang, S.; Lin, S.; Liao, M.; Chen, H.; Zhou, J. The role of extracellular vesicles in renal fibrosis. Cell Death Dis. 2019, 10, 367. [Google Scholar] [CrossRef]
- Havers, M.; Broman, A.; Lenshof, A.; Laurell, T. Advancement and obstacles in microfluidics-based isolation of extracellular vesicles. Anal. Bioanal. Chem. 2023, 415, 1265–1285. [Google Scholar] [CrossRef]
- Chen, S.; Tang, R.; Liu, B. Current Understanding of Cardiovascular Calcification in Patients with Chronic Kidney Disease. Int. J. Mol. Sci. 2024, 25, 10225. [Google Scholar] [CrossRef]
- Qin, M.; Hu, J.; Li, X.; Liu, J.; Jiang, R.; Shi, Y.; Wang, Z.; Zhang, L.; Zhao, Y.; Gao, H.; et al. Exosomal membrane proteins analysis using a silicon nanowire field effect transistor biosensor. Talanta 2024, 278, 126534. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hu, J.; Liu, J.; Li, X.; Sun, S.; Luan, X.; Zhao, Y.; Wei, S.; Li, M.; Zhang, Q.; et al. Si nanowire Bio-FET for electrical and label-free detection of cancer cell-derived exosomes. Microsyst. Nanoeng. 2022, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.J. Silicon Nanowire Biosensor for Ultrasensitive and Label-Free Direct Detection of miRNAs. Methods Mol. Biol. 2011, 67, 111–121. [Google Scholar]
- Lo Faro, M.; Leonardi, A.; Priolo, F.; Fazio, B.; Irrera, A. Future Prospects of Luminescent Silicon Nanowires Biosensors. Biosensors 2022, 12, 1052. [Google Scholar] [CrossRef]
- Mishra, S.; Vaughn, A.D.; Devore, D.I.; Roth, C.M. Delivery of siRNA silencing Runx2 using a multifunctional polymer-lipid nanoparticle inhibits osteogenesis in a cell culture model of heterotopic ossification. Integr. Biol. 2012, 4, 1498–1507. [Google Scholar] [CrossRef][Green Version]
- Voicu, G.; Rebleanu, D.; Constantinescu, C.A.; Fuior, E.V.; Ciortan, L.; Droc, I.; Uritu, C.M.; Pinteala, M.; Manduteanu, I.; Simionescu, M.; et al. Nano-Polyplexes Mediated Transfection of Runx2-shRNA Mitigates the Osteodifferentiation of Human Valvular Interstitial Cells. Pharmaceutics 2020, 12, 507. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).