Abstract
KMT2C (histone lysine N-methyltransferase 2C, also known as MML3, myeloid/lymphoid or mixed-lineage leukemia 3) is a causal gene for Kleefstra syndrome 2, a rare neurodevelopmental disorder. Recent human genetic studies have identified it as a high-risk gene for autism spectrum disorder (ASD), with 79% of patients harboring KMT2C variants having ASD. However, the causal link between KMT2C haploinsufficiency and ASD remains unclear. KMT2C/MLL3 encodes a histone methyltransferase, a core protein of the KMT2C/D COMPASS (complex proteins associated with Set1) complex, which plays fundamental roles in chromatin modification, occupancy, and gene expression. The expression of KMT2C/Kmt2c peaks during the developmental period in the human/mouse brain, which indicates the critical roles of KMT2C/Kmt2c in neurodevelopment. Here, we investigated the impact of germline Kmt2c haploinsufficiency on autism-like behavioral deficits in mice, which modeled humans carrying diverse KMT2C variants. Compared with Kmt2c+/+ controls, Kmt2c haploinsufficiency mice had normal motor function without anxiety-like behaviors. Notably, Kmt2c haploinsufficiency mice exhibited autism-like social deficits and increased self-grooming in both males and females, which recapitulated the core phenotypes of ASD patients. Novel object recognition and spatial memory deficits were observed in male and female Kmt2c haploinsufficiency mice. This study reveals a causal link between Kmt2c haploinsufficiency and ASD-like behavioral deficits. These germline Kmt2c haploinsufficiency mice can be used for further studying the molecular mechanisms and developing therapeutic interventions for KMT2C haploinsufficiency-associated behavioral deficits.
1. Introduction
Autism spectrum disorder (ASD) is a complex developmental disorder with unclear etiology. Growing evidence suggests that the interaction between genetic and environmental factors contributes to the occurrence of ASD [1,2]. Meta-analysis from human studies shows that genetic factors play major roles in ASD, given that ASD has a higher concordance in twins [3,4].
Human genetic studies have identified risk genes for ASD, which are enriched in gene expression or neuronal communication (Simons Foundation Autism Research Initiative, SFARI) [5,6,7]. KMT2C (histone lysine N-methyltransferase 2C, also known as MML3, myeloid/lymphoid or mixed-lineage leukemia 3) located on human chromosomal band 7q36.1, is a causal gene for Kleefstra syndrome 2 (a rare neurodevelopmental disorder) and a risk gene for ASD. Humans carrying KMT2C variants have a spectrum of clinical manifestations named KMT2C-related syndrome, including neurodevelopmental delay (NDD), intellectual disability (ID), schizophrenia, seizures, and ASD [5,6,8,9,10,11,12]. Despite the discovery of KMT2C as a top-ranking ASD risk gene, the causal link between KMT2C haploinsufficiency and ASD remains unclear, and little is known about the molecular mechanisms underlying the behavioral deficits.
KMT2C encodes a histone methyltransferase, a core protein of the KMT2C/D COMPASS (complex proteins associated with Set1) complex, which plays fundamental roles in chromatin modification, occupancy, and gene expression via catalyzing histone 3 lysine (K) 4 (H3K4) methylation in the promoters and enhancers [13,14,15]. KMT2C is also a member of the ASC-2/NCOA6 complex (ASCOM), which involves transcriptional coactivation [16]. As a tumor suppressor, KMT2C/MLL3 is linked to various cancers, including leukemia, breast, and bladder cancer [17,18,19]. Recent studies showed that mice with brain-specific knockout of Kmt2c or a heterozygous frameshift mutation of Kmt2c displayed autism-like behaviors [20,21]. Here, we characterized the impact of Kmt2c haploinsufficiency on autism-like behavioral deficits by using a different global Kmt2c haploinsufficiency mouse model, which modeled individuals with KMT2C haploinsufficiency caused by inherited, de novo mutations, or deletions. The latest data from the CDC (Centers for Disease Control and Prevention) show that ASD affects 1 in 32 children in the US. Males are more vulnerable to having ASD than females. Therefore, sex as a biological variable was incorporated in this study [22].
2. Materials and Methods
2.1. Protein–Protein Interaction (PPI) Network and Functional Annotation Analysis
The PPI network associated with KMT2C was constructed using the STRING database (version 12.0). The first shell interactors in the PPI network were used for biological process (gene ontology) enrichment analysis.
2.2. Animal Care and Husbandry
The use of animals and procedures performed was approved by the Institutional Animal Care and Use Committee of Sanford School of Medicine, University of South Dakota. Kmt2c haploinsufficiency (Kmt2c+/−) mice were a kind of gift from Dr. Jeffrey Magee (Washington University School of Medicine at St. Louis) [14]. A germline premature stop codon was produced in exon 14 due to a 5-base pair deletion (CATGG). Animals were group-housed (n = 4–5) in standard cages and were kept on a 12 h light-dark cycle in a temperature-controlled room. Food and water were available ad libitum. Male and female Kmt2c+/− mice and sex- and age-matched Kmt2c+/+ littermates were used (6–7 weeks old), which were derived from Kmt2c+/− breeding pairs. All behavioral assays were carried out by investigators in a blind fashion (with no prior knowledge of genotypes).
2.3. Behavioral Tests
Hindlimb clasping test is a behavioral assessment to evaluate motor function. In brief, mice were gently lifted by the tails, and the hindlimb posture was observed for 30 s. A scoring scale was used to quantify the degree of clasping, as described [23]. Other behavioral tests were performed as in our previous studies [24,25,26]. See Supplementary Materials for details regarding other behavioral tests.
2.4. Statistical Analysis
The sample size of each group was calculated to detect behavioral differences based on predicting detectable differences to reach a power of 0.80 at a significance level of 0.05 by power analyses in G*Power software 3.1.9.7. GraphPad software Prism 10.0 (GraphPad Software, La Jolla, CA, USA) was used for statistical comparisons. Differences between more than two groups were assessed with two-way or three-way ANOVA, followed by post hoc Bonferroni tests for multiple comparisons. Data were presented as the mean ± SEM.
3. Results
3.1. KMT2C/Kmt2c Is Spatiotemporally Expressed in the Human/Mouse Brain
To determine the expression of KMT2C in the human brain, we searched it in the Human Brain Transcriptome (HBT) dataset (https://hbatlas.org, accessed on 20 September 2025) [27,28]. As shown in Figure 1A, KMT2C is spatiotemporally expressed in the brain, including the neocortex (NCX), hippocampus (HIP), striatum (STR), amygdala (AMY), mediodorsal nucleus of the thalamus (MD), and cerebellar cortex (CBC). The peak level of KMT2C is found during the early fetal to late mid-fetal stage in fetal development. To determine the expression of Kmt2c in the mouse brain, we searched it in the Allen Developing Mouse Brain Atlas (https://developingmouse.brain-map.org, accessed on 20 September 2025). Kmt2c is spatiotemporally expressed in the mouse brain. The peak level of Kmt2c is found in P4 mice (Figure 1B). These data indicate the critical roles of KMT2C/Kmt2c in neurodevelopment.
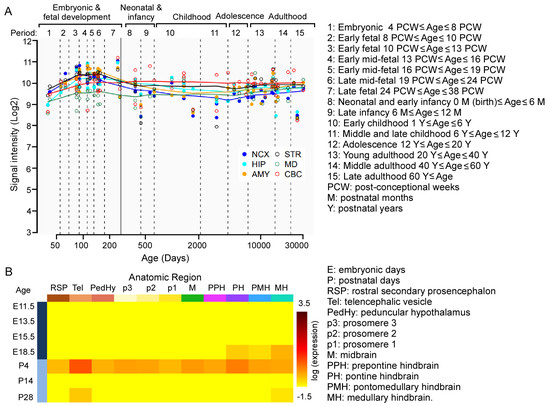
Figure 1.
The spatiotemporal expression of KMT2C/Kmt2c in the human/mouse brain. (A) The spatiotemporal expression of KMT2C in the human brain. Reprinted and modified from the Human Brain Transcriptome dataset (https://hbatlas.org, accessed on 20 September 2025). (B) The spatiotemporal expression of Kmt2c in the mouse brain. Reprinted from the Allen Developing Mouse Brain Atlas (https://developingmouse.brain-map.org, accessed on 20 September 2025).
As shown in Figure 2, human KMT2C/MLL3 encodes a protein of 4911 amino acids with several key domains, including AT-hook, PHD (plant homologous), SET, and post-SET domains, which are involved in binding to the minor groove of DNA, recognition of unmodified or methylated lysine in histone 3, and methylation of histones on lysine to modify chromatin for transcription [29]. In the SFARI database, there are 10 types of KMT2C variants reported, including copy number gain, copy number loss, frameshift variant, intron variant, missense variant, splice region variant, splice site variant, stop gained variant, synonymous variant, and translocation. Among them, the majority are frameshift variants (41), missense variants (59), and stop gained variants (32) in the coding region.
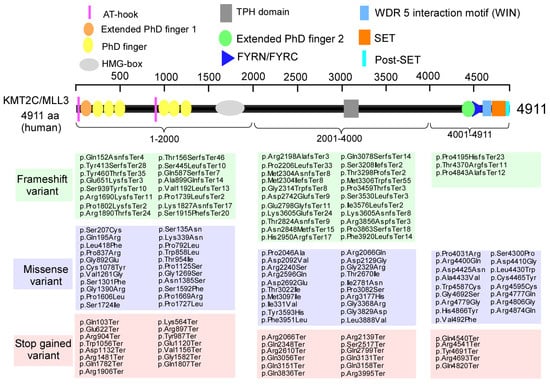
Figure 2.
KMT2C variants are identified in humans. Domain organization of human KMT2C. AT-hook: adenosine-thymidine-hook; PHD: plant homeodomain; HMG: high-mobility group: TPH: Trichohyalin-plectin-homology; FYRN/FYRC, phenylalanine and tyrosine rich region (N- and C-terminal); SET, Su(var)3–9, Enhancer-of-zeste and Trithorax; Post-SET, C-terminal of SET. Modified from the SFARI dataset (https://gene.sfari.org, accessed on 20 September 2025).
3.2. The Biological Roles of KMT2C in the Brain
To determine the biological roles of KMT2C and its mechanistic link to brain disorders, we did a PPI network analysis using the STRING database. The minimum required interaction score was set at highest confidence (0.9). As shown in Figure 3A, the PPI network identified the maximal first shell interactors with KMT2C. The number of nodes was 23, and the number of edges (both functional and physical protein associations) was 163 (PPI enrichment p-value: <1.0 × 10−16). The top proteins with higher scores included RBBP5, ASH2L, DPY30, and NCOA6, which are the members of the KMT2C/D COMPASS or ASC-2/NCOA6 complex. The second shell interactors with KMT2C were not shown. Biological process (gene ontology) analysis revealed that the first shell interactors were mostly enriched in H3-K4 methylation, chromatin assembly, nucleosome assembly, and chromatin remodeling (Figure 3B). These results suggest a multifaceted role for KMT2C in the regulation of transcription.
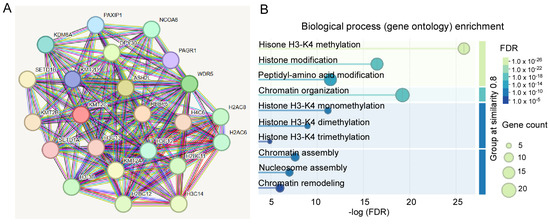
Figure 3.
PPI network analysis of KMT2C. (A) The PPI network analysis showing the first shell interactors with KMT2C. (B) Biological process (gene ontology) enrichment analysis of first shell interactors. Reprinted and modified from the STRING database (https://string-db.org, accessed on 20 September 2025).
3.3. Kmt2c Haploinsufficiency Mice Have Normal Motor Function Without Anxiety-like Behaviors
87% of individuals carrying KMT2C variants displayed gross motor delay; however, all individuals older than 3.5 years of age achieved independent walking [12]. The impact of Kmt2c haploinsufficiency on motor function in mice was examined at 6–7 weeks old, which is equivalent to a human in teenage years [30]. As shown in Figure 4A, in a hindlimb clasping test, male and female Kmt2c+/+ mice showed normal extension reflexes in the hindlimbs. Hindlimb clasping was not observed in male and female Kmt2c+/− mice, indicating normal motor function. Then, we examined the basic locomotion function, the ability of mice to freely move. Male and female Kmt2c+/− mice showed similar distance traveled (F Genotype (1, 46) = 0.003, p = 0.95; F Sex (1, 46) = 0.0004, p = 0.98) and speed (F Genotype (1, 46) = 0.003, p = 0.95; F Sex (1, 46) = 0.0004, p = 0.98) with male and female Kmt2c+/+ mice in the open field test (Figure 4B,C). To further examine whether Kmt2c haploinsufficiency affects motor coordination, we performed a rotarod test. Male and female Kmt2c+/- mice had similar latency to fall (F Genotype (1, 46) = 0.11, p = 0.74; F Sex (1, 46) = 0.0007, p = 0.98) (Figure 4D), demonstrating that Kmt2c haploinsufficiency does not affect motor function.
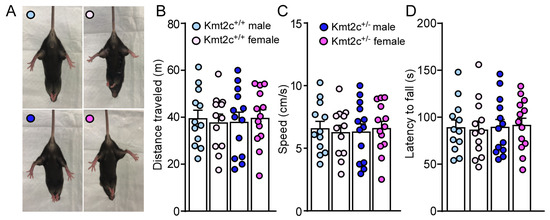
Figure 4.
Kmt2c haploinsufficiency mice have normal motor function. (A) Representative photos showing the hindlimb postures of male and female Kmt2c+/+ and Kmt2c+/− mice in the hindlimb clasping test. Bar graphs showing total distance traveled (B), speed (C) during the open field test and latency to fall (D) during the rotarod test of male and female Kmt2c+/+ and Kmt2c+/− mice. (B–D): two-way ANOVA. Male Kmt2c+/+ mice: n = 12; Female Kmt2c+/+ mice: n = 12; Male Kmt2c+/− mice: n = 13; Female Kmt2c+/− mice: n = 13.
To determine the impact of Kmt2c haploinsufficiency on anxiety, a comorbidity of ASD, we did an open-field test. Kmt2c+/− mice displayed a similar time (F Genotype (1, 46) = 0.0005, p = 0.98; F Sex (1, 46) = 0.22, p = 0.64) spent and number of entries (F Genotype (1, 46) = 0.37, p = 0.54; F Sex (1, 46) = 0.15, p = 0.70) in the center (Figure 5A,B), compared to Kmt2c+/+ mice. The similar anxiety index in Kmt2c+/+ and Kmt2c+/− mice (F Genotype (1, 46) = 0.0005, p = 0.98; F Sex (1, 46) = 0.22, p = 0.64) (Figure 5C,D) indicates that Kmt2c haploinsufficiency does not induce anxiety-like behaviors under a mild stress condition. To further examine if Kmt2c haploinsufficiency induces anxiety-like behaviors under a relatively high stress condition, we performed an elevated plus maze (EPM) test [31]. Kmt2c+/− mice spent a similar time (F Genotype (1, 46) = 0.05, p = 0.82, F Sex (1, 46) = 0.02, p = 0.89) and number of entries (F Genotype (1, 46) = 0.08, p = 0.78, F Sex (1, 46) = 1.17, p = 0.29) in the open arms with Kmt2c+/+ mice (Figure 5E,F). Kmt2c+/+ and Kmt2c+/− mice had similar anxiety index in the EPM test (F Genotype (1, 46) = 0.008, p = 0.93; F Sex (1, 46) = 0.04, p = 0.84) (Figure 5G,H), which suggests that Kmt2c haploinsufficiency has no effects on anxiety-like behaviors under a relatively high stress condition.
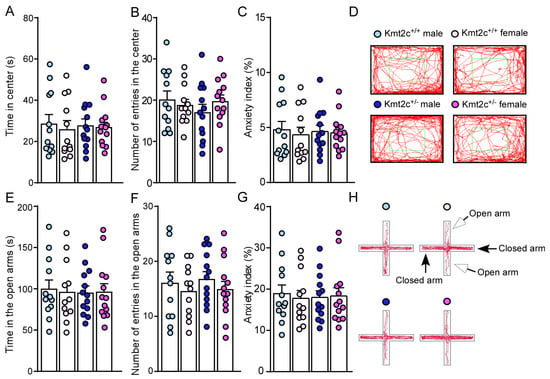
Figure 5.
Kmt2c haploinsufficiency mice do not have anxiety-like behaviors. Bar graphs showing time spent (A), number of entries (B) in the center, (C) anxiety index, and representative trajectory diagrams (D) of Kmt2c+/+ and Kmt2c+/− mice in the open field test. The anxiety index in the open field test was calculated as: (time spent in the center)/(total time) × 100%. Bar graphs showing time spent (E), number of entries (F) in the open arms, (G) anxiety index, and representative trajectory diagrams (H) of Kmt2c+/+ and Kmt2c+/− mice in the EPM test. The anxiety index in the EPM test was calculated as: (time spent in the open arms)/(total time spent in the open and closed arms) × 100%. (A–C) and (E–G): two-way ANOVA. Male Kmt2c+/+ mice: n = 12; Female Kmt2c+/+ mice: n = 12; Male Kmt2c+/− mice: n = 13; Female Kmt2c+/− mice: n = 13.
3.4. Kmt2c Haploinsufficiency Mice Exhibit Autism-like Behavioral Deficits
79% of patients with KMT2C-related syndrome had autism [12]. To determine whether Kmt2c haploinsufficiency causes autism-like social deficits, male and female Kmt2c+/− and Kmt2c+/+ mice were subjected to the three-chamber social interaction assay [24,32]. As shown in Figure 6A, Kmt2c+/+ mice spent significantly more time exploring the social stimulus over the non-social object, while Kmt2c+/− displayed reduced preference for the social stimulus (Male Kmt2c+/+: social: 133.3 ± 8.8 s, nonsocial: 43.4 ± 3.0 s, n = 12; Female Kmt2c+/+: social: 132.2 ± 7.9 s, nonsocial: 44.7 ± 3.7 s, n = 12; Male Kmt2c+/−: social: 97.1 ± 4.9 s, nonsocial: 68.7 ± 4.1 s, n = 13; Female Kmt2c+/−: social: 95.2 ± 6.2 s, nonsocial: 67 ± 4.6 s, n = 13, F Soc vs. NS × Genotype (1, 92) = 56.1, p < 0.0001). Consistently, male and female Kmt2c+/− exhibited a significantly reduced social preference index compared to Kmt2c+/+ mice (Male Kmt2c+/+: 49.5% ± 4.2%, n = 12; Female Kmt2c+/+: 49.2% ± 3.6%, n = 12; Male Kmt2c+/−: 17.3% ± 3.2%, n = 13; Female Kmt2c+/−: 17.2% ± 2.7%, n = 13. F Genotype (1, 46) = 87.5, p < 0.0001, F Sex (1, 46) = 0.005, p = 0.95) (Figure 6B,C). The lower time exploring the social stimulus and social preference index in Kmt2c+/− mice suggests that Kmt2c haploinsufficiency causes autism-like social deficits in male and female mice.
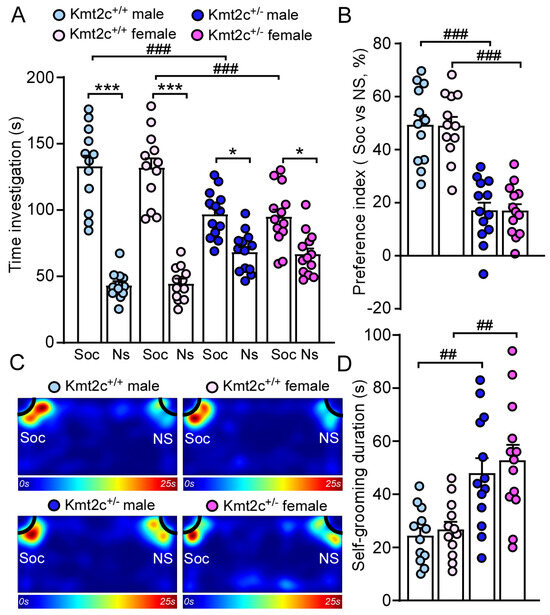
Figure 6.
Kmt2c haploinsufficiency mice exhibit autism-like behavioral deficits. Bar graphs showing the time spent investigating social (Soc) or non-social (NS) stimulus (A) and social preference index (B) in the three-chamber sociability test of Kmt2c+/+ and Kmt2c+/− mice. The preference index was calculated as: [time spent on social stimulus (Soc) − time spent on non-social stimulus (NS)]/[total time exploring the social and non-social stimuli (Soc + NS)] × 100%. (C) Representative heatmaps illustrating the time spent in different locations of the three chambers. Bar graphs showing the time spent self-grooming (D) in male and female Kmt2c+/+ and Kmt2c+/− mice. * p < 0.05, *** p < 0.001, Soc versus NS; ## p < 0.01, ### p < 0.001, Kmt2c+/− versus Kmt2c+/+. (A): three-way ANOVA; (B,D): two-way ANOVA. Male Kmt2c+/+ mice: n = 12; Female Kmt2c+/+ mice: n = 12; Male Kmt2c+/− mice: n = 13; Female Kmt2c+/− mice: n = 13.
Self-grooming is an innate behavior in rodents and is used as an indication of compulsive and repetitive behavior [33]. Male and female Kmt2c+/− mice spent significantly more time engaged in self-grooming compared to male and female Kmt2c+/+ mice (Male Kmt2c+/+: 24.6 ± 3.0 s, n = 12; Female Kmt2c+/+: 26.9 ± 3.1 s, n = 12; male Kmt2c+/−: 48.2 ± 5.8 s, n = 13, Female Kmt2c+/−: 52.9 ± 6.1 s, n = 13. F Genotype (1, 46) = 26.2, p < 0.0001, F Sex (1, 46) = 0.5, p = 0.47) (Figure 6D). These results demonstrated that Kmt2c haploinsufficiency induces autism-like repetitive & restrictive behaviors in males and females.
3.5. Kmt2c Haploinsufficiency Mice Display Cognitive Deficits
86% of patients with KMT2C-related syndrome had intellectual disability with varied severity [12]. To assess the impact of Kmt2c haploinsufficiency on cognition, we first did a novel object recognition (NOR) test to examine whether Kmt2c haploinsufficiency could impair novel object recognition memory. 5 min after initial familiarization with two identical objects in the habituated arena, the mice were allowed to explore the same arena in the presence of a familiar object and a novel object. As shown in Figure 7A, male and female Kmt2c+/+ mice spent significantly more time exploring the novel object over the familiar object, while male and female Kmt2c+/− mice lacked a preference for the novel object (Male Kmt2c+/+: novel object: 34.9 ± 7.2 s, familiar object: 10.8 ± 1.1 s, n = 12; Female Kmt2c+/+: novel object: 33.4 ± 4.2 s, familiar object: 13.3 ± 2.8 s, n = 12; Male Kmt2c+/−: novel object: 21.9 ± 3.4 s, familiar object: 14.9 ± 1.6 s, n = 13; Female Kmt2c+/−: novel object: 22.8 ± 2.9 s, familiar object: 15.6 ± 1.4 s, n = 13, F Object × Genotype (1, 92) = 9.0, p = 0.0035). Male and female Kmt2c+/− mice showed similar entry times to the novel object or the familiar object and distance traveled with male and female Kmt2c+/+ mice (Figure 7B,C). The lower discrimination index in male and female Kmt2c+/− mice indicates Kmt2c haploinsufficiency impairs novel object recognition memory (Male Kmt2c+/+: 0.44 ± 0.06, n = 12; Female Kmt2c+/+: 0.44 ± 0.06, n = 12; Male Kmt2c+/−: 0.16 ± 0.05, n = 13; Female Kmt2c+/−: 0.15 ± 0.05, n = 13. F Genotype (1, 46) = 26.5, p < 0.001; F Sex (1, 46) = 0.02, p = 0.89) (Figure 7D,E).
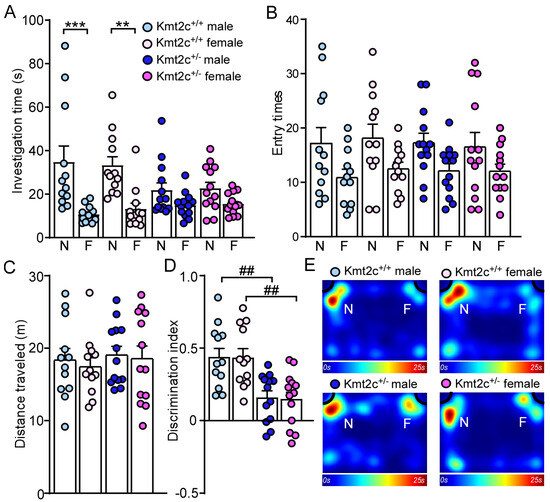
Figure 7.
Kmt2c haploinsufficiency mice display novel object recognition deficits. Bar graphs showing the time spent (A) and entry times (B) to either novel object (N) or familiar object (F), total distance traveled (C), and discrimination index (D) in the NOR test of Kmt2c+/+ and Kmt2c+/− mice. The discrimination index was calculated as: [time spent on novel object (N) − time spent on familiar object (F)]/[total time exploring both objects (N + F)] for the test session. (E) Representative heatmaps showing the time spent exploring the familiar and novel object during the NOR test. ** p < 0.01, *** p < 0.0001, novel object versus familiar object. ## p < 0.01, Kmt2c+/− versus Kmt2c+/+. (A,B): three-way ANOVA; (C,D): two-way ANOVA. Male Kmt2c+/+ mice: n = 12; Female Kmt2c+/+ mice: n = 12; Male Kmt2c+/− mice: n = 13; Female Kmt2c+/− mice: n = 13.
To examine whether Kmt2c haploinsufficiency affects spatial memory, we performed a Barnes maze test [24,34]. 15 min after two learning phases finding the correct hole and entering the escape box (information acquisition), the mice were allowed to explore the same platform in the absence of the escape box under the correct hole in the memory phase (information retention and retrieval). There were no differences in total investigation time (T1 + T2), distance traveled, and entry times to the correct hole between Kmt2c+/+ and Kmt2c+/− mice (Figure 8A–C). Kmt2c+/− mice displayed significantly lower spatial memory index (T1/T2) (F Genotype (1, 46) = 32.3, p < 0.001; F Sex (1, 46) = 0.02, p = 0.89), compared with Kmt2c+/+ mice (Figure 8D,E), which demonstrates that Kmt2c haploinsufficiency impairs spatial memory.
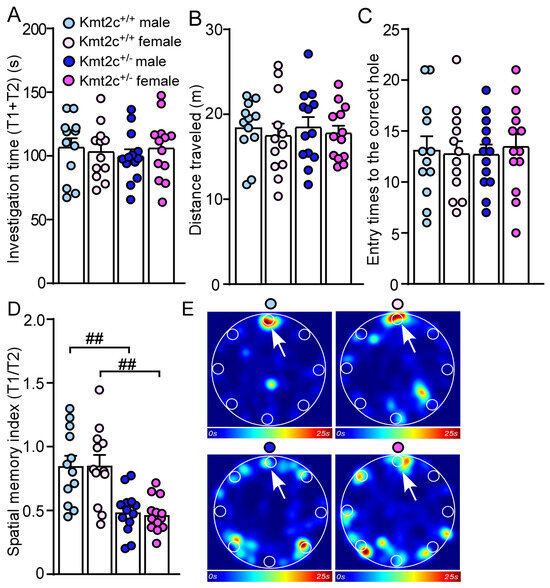
Figure 8.
Kmt2c haploinsufficiency mice display spatial memory deficits. Bar graphs showing the total time spent investigating the correct hole and incorrect holes (A), distance traveled (B), entry time to the correct hole (C), and spatial memory index (T1/T2) (D) in the Barnes maze test of Kmt2c+/+ and Kmt2c+/− mice. (E) Representative heatmaps illustrating the time spent in different locations of the arena during the memory phase (escape box removed). T1: time spent investigating the correct hole; T2: time spent investigating the other seven incorrect holes. The correct hole is pointed to by the arrow. ## p < 0.01, Kmt2c+/− versus Kmt2c+/+. (A–D): two-way ANOVA. Male Kmt2c+/+ mice: n = 12; Female Kmt2c+/+ mice: n = 12; Male Kmt2c+/− mice: n = 13; Female Kmt2c+/− mice: n = 13.
4. Discussion
In this study, we characterized the autism-like behavioral deficits in a germline Kmt2c haploinsufficiency mouse model. Kmt2c haploinsufficiency mice exhibited autism-like social deficits and increased self-grooming, which recapitulated the core phenotypes of ASD patients carrying KMT2C variants and confirmed the causal link between KMT2C haploinsufficiency and ASD. Furthermore, we identified that Kmt2c haploinsufficiency caused cognitive impairments in mice, confirming that ID is a key symptom of Kleefstra syndrome 2 and a major comorbidity of ASD [35].
The P4 mice are still in the developmental stage, which corresponds to the late stage of the second trimester in human gestation, at around the 21st–24th week of gestation [30,36]. Consistently, transcriptomic studies from humans and mice showed the peak expression of KMT2C/Kmt2c in the brain during the similar developmental time window. The perinatal lethality of Kmt2c-/- mice further confirmed the essential role of KMT2C in brain development [14,37,38]. H3K4 methylation is related to transcriptional activation via three types of modifications: mono-, di-, and trimethylation (H3K4me1, H3K4me2, and H3K4me3). Dysregulation of H3K4 methylation associated with genetic risks is implicated in neurodevelopmental disorders, including ASD [39,40]. KMT2C is well known for transcriptional regulation via catalyzing H3K4me1 and H3K4me2 [38]. Recent chromatin immunoprecipitation followed by sequencing (ChIP-seq) analysis showed that KMT2C peaks colocalized with H3K4me3 peaks [21], which is consistent with the gene ontology analysis of the first shell interactors of KMT2C. Neuronal-specific H3K4me3 peaks in human PFC are enriched in synaptic function and conserved in chimpanzee, macaque, and mouse [41,42], suggesting the key role of KMT2C in transcriptional regulation of genes involved in neuronal communications.
The phenotypes caused by KMT2C mutations are extensive heterogeneity [12]. Male and female Kmt2c+/− mice had normal motor function without anxiety-like behaviors. ASD is characterized by persistent deficits in social communication & interaction, and restricted & repetitive patterns of behavior, interests, or activities. Male and female Kmt2c+/− mice displayed significantly decreased time spent with social stimulus, diminished social preference index, and increased self-grooming, which are consistent with the reports from other groups by using different Kmt2c deficiency mouse models [20,21]. Even though the prevalence of ASD is higher in males than in females [43,44], the similar social behavioral deficits and self-grooming in male and female Kmt2c+/− mice indicate KMT2C haploinsufficiency affects both males and females. ASD patients have impaired communication as early as age 1–2, preceding clinical diagnosis at about 4–5 years old. The limitation of this study is the focus on one time window but not developmental trajectories. Further longitudinal characterization of behaviors would help identify the impact of Kmt2c haploinsufficiency on developmental milestones.
ID is one of the key features of KMT2C-related syndrome [12], and 70% of ASD patients have ID [35]. Trithorax-related (trr) is the KMT2C ortholog in Drosophila (fruit fly). Knockdown of trr in the mushroom body (MB), the learning and memory center of the fly brain, impaired short-term memory, indicating KMT2C/MLL3 are required for short-term memory. The lack of gross morphological defects in the MB upon trr knockdown suggested that trr-mediated transcriptional activation related to MB neuronal functions was diminished [8]. Kmt2c+/− mice displayed decreased discrimination index in the novel object recognition test and spatial memory index in the Barnes maze test, which indicates that Kmt2c haploinsufficiency affects the brain regions related to object recognition memory formation, such as the perirhinal cortex [45], and spatial memory, such as the hippocampus and PFC. Lysine-specific histone demethylase 1 (LSD1) inhibitors are developed for cancer therapies. A recent study showed that vafidemstat, an LSD1 inhibitor, could ameliorate impairments in sociality but not working memory in a Kmt2c+/fs mouse model [21]. However, transitional antineoplastic drugs to children with ASD may cause untoward effects.
The prefrontal cortex (PFC) is a hub brain region for “high level” executive functions [46,47] such as social behaviors, emotion, and cognition, which is impaired in ASD patients and mouse models of autism [25,48,49]. Hyperactivity of the PFC has been thought of as a key pathogenesis of social deficits in ASD [50,51,52,53]. In a rat cortical neuronal culture study, knockdown of Kmt2c increased neuronal excitability via altered intrinsic property, excitatory and inhibitory synaptic inputs. RNA sequencing of Kmt2c-deficient neuronal networks at DIV 20 (days in vitro) showed that the differentially expressed genes (most of them were downregulated genes) were enriched in ion transmembrane transport and chemical synaptic transmission [54]. However, Nakamura et al. performed RNA-seq of mice bulk adult forebrain, mainly including the prefrontal cortex, and found that Kmt2c haploinsufficiency upregulated genes were enriched in synapse, ion transport, cell projection and morphogenesis, while Kmt2c haploinsufficiency downregulated genes were associated with ribosome and cell death [21]. PFC sends out glutamatergic and GABAergic transmission to downstream targets [55,56] and receives ascending synaptic inputs from multiple brain regions via bottom–up innervation [57,58,59,60]. The striatum is a brain region important for stereotypic behaviors, which was found to be defective in Shank3-deficient autism mouse models [61,62,63]. The neural mechanisms that drive social deficits and increased self-grooming in Kmt2c+/− mice at cellular and circuit levels need to be further investigated.
5. Conclusions
In summary, Kmt2c haploinsufficiency resulted in autistic-like behaviors in both males and females. The face validity of these mice builds a strong basis for deep mechanistic studies and developing novel, safe therapeutic interventions for ASD patients with KMT2C variants.
Supplementary Materials
The supplementary methods for behavioral tests can be downloaded at https://www.mdpi.com/article/10.3390/biom15111547/s1.
Author Contributions
K.M.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing—review & editing. M.W.: Writing—review and editing. H.H.: Writing—review & editing. L.Q.: Conceptualization, Funding acquisition, Data curation, Formal analysis, Investigation, Methodology, Resources, Project administration, Supervision, Validation, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work is supported by startup funding from Sanford School of Medicine, University of South Dakota (L.Q.), Sanford School of Medicine Faculty Research Grant (L.Q.), South Dakota Board of Regents Competitive Research Grant (L.Q.), NIH R21 MH134106-01 (L.Q.), and NIH P30 GM154633-01 (L.Q.).
Institutional Review Board Statement
Animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Sanford School of Medicine, University of South Dakota (Protocol Number: 01-03-25-28D; Approval date: 4 March 2025).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article, further inquiries can be directed to the corresponding author.
Acknowledgments
We are grateful to Jeffrey A. Magee for the Kmt2c haploinsufficiency mice. We thank Jamie Scholl for the behavioral core support. All behavioral tests were performed at the behavioral core facility in the Sanford School of Medicine, University of South Dakota.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ASD | Autism spectrum disorder |
| NDD | Neurodevelopmental delay |
| ID | Intellectual disability |
| CDC | Centers for Diseases Control and Prevention ear dichroism |
| PFC | Prefrontal cortex |
| DIV | Days in vitro |
| PPI | Protein–protein interaction |
| SFARI | Simons Foundation Autism Research Initiative |
References
- Matelski, L.; Van de Water, J. Risk factors in autism: Thinking outside the brain. J. Autoimmun. 2016, 67, 1–7. [Google Scholar] [CrossRef]
- Love, C.; Sominsky, L.; O’Hely, M.; Berk, M.; Vuillermin, P.; Dawson, S.L. Prenatal environmental risk factors for autism spectrum disorder and their potential mechanisms. BMC Med. 2024, 22, 393. [Google Scholar] [CrossRef]
- Bailey, A.; Le Couteur, A.; Gottesman, I.; Bolton, P.; Simonoff, E.; Yuzda, E.; Rutter, M. Autism as a strongly genetic disorder: Evidence from a British twin study. Psychol. Med. 1995, 25, 63–77. [Google Scholar] [CrossRef]
- Tick, B.; Bolton, P.; Happé, F.; Rutter, M.; Rijsdijk, F. Heritability of autism spectrum disorders: A meta-analysis of twin studies. J. Child Psychol. Psychiatry 2016, 57, 585–595. [Google Scholar] [CrossRef]
- Satterstrom, F.K.; Kosmicki, J.A.; Wang, J.; Breen, M.S.; De Rubeis, S.; An, J.Y.; Peng, M.; Collins, R.; Grove, J.; Klei, L.; et al. Large-Scale Exome Sequencing Study Implicates Both Developmental and Functional Changes in the Neurobiology of Autism. Cell 2020, 180, 568–584.e523. [Google Scholar] [CrossRef]
- De Rubeis, S.; He, X.; Goldberg, A.P.; Poultney, C.S.; Samocha, K.; Cicek, A.E.; Kou, Y.; Liu, L.; Fromer, M.; Walker, S.; et al. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 2014, 515, 209–215. [Google Scholar] [CrossRef]
- Wang, T.; Guo, H.; Xiong, B.; Stessman, H.A.; Wu, H.; Coe, B.P.; Turner, T.N.; Liu, Y.; Zhao, W.; Hoekzema, K.; et al. De novo genic mutations among a Chinese autism spectrum disorder cohort. Nat. Commun. 2016, 7, 13316. [Google Scholar] [CrossRef]
- Koemans, T.S.; Kleefstra, T.; Chubak, M.C.; Stone, M.H.; Reijnders, M.R.F.; de Munnik, S.; Willemsen, M.H.; Fenckova, M.; Stumpel, C.; Bok, L.A.; et al. Functional convergence of histone methyltransferases EHMT1 and KMT2C involved in intellectual disability and autism spectrum disorder. PLoS Genet. 2017, 13, e1006864. [Google Scholar] [CrossRef]
- Stessman, H.A.; Xiong, B.; Coe, B.P.; Wang, T.; Hoekzema, K.; Fenckova, M.; Kvarnung, M.; Gerdts, J.; Trinh, S.; Cosemans, N.; et al. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 2017, 49, 515–526. [Google Scholar] [CrossRef]
- Chen, C.H.; Huang, A.; Huang, Y.S.; Fang, T.H. Identification of a Rare Novel KMT2C Mutation That Presents with Schizophrenia in a Multiplex Family. J. Pers. Med. 2021, 11, 1254. [Google Scholar] [CrossRef]
- Whitford, W.; Taylor, J.; Hayes, I.; Smith, W.; Snell, R.G.; Lehnert, K.; Jacobsen, J.C. A novel 11 base pair deletion in KMT2C resulting in Kleefstra syndrome 2. Mol. Genet. Genom. Med. 2023, e2350. [Google Scholar] [CrossRef]
- Rots, D.; Choufani, S.; Faundes, V.; Dingemans, A.J.M.; Joss, S.; Foulds, N.; Jones, E.A.; Stewart, S.; Vasudevan, P.; Dabir, T.; et al. Pathogenic variants in KMT2C result in a neurodevelopmental disorder distinct from Kleefstra and Kabuki syndromes. Am. J. Hum. Genet. 2024, 111, 1626–1642. [Google Scholar] [CrossRef]
- Lavery, W.J.; Barski, A.; Wiley, S.; Schorry, E.K.; Lindsley, A.W. KMT2C/D COMPASS complex-associated diseases [K(CD)COM-ADs]: An emerging class of congenital regulopathies. Clin. Epigenetics 2020, 12, 10. [Google Scholar] [CrossRef]
- Chen, R.; Okeyo-Owuor, T.; Patel, R.M.; Casey, E.B.; Cluster, A.S.; Yang, W.; Magee, J.A. Kmt2c mutations enhance HSC self-renewal capacity and convey a selective advantage after chemotherapy. Cell Rep. 2021, 34, 108751. [Google Scholar] [CrossRef]
- Sze, C.C.; Shilatifard, A. Mll3/Mll4/Compass Family on Epigenetic Regulation of Enhancer Function and Cancer. Cold Spring Harb. Perspect. Med. 2016, 6, a026427. [Google Scholar] [CrossRef]
- Lee, S.; Roeder, R.G.; Lee, J.W. Roles of Histone H3-Lysine 4 Methyltransferase Complexes in NR-Mediated Gene Transcription. Prog. Mol. Biol. Transl. Sci. 2009, 87, 343–382. [Google Scholar]
- Gala, K.; Li, Q.; Sinha, A.; Razavi, P.; Dorso, M.; Sanchez-Vega, F.; Chung, Y.R.; Hendrickson, R.; Hsieh, J.J.; Berger, M.; et al. KMT2C Mediates the Estrogen Dependence of Breast Cancer Through Regulation of ERα Enhancer Function. Oncogene 2018, 37, 4692–4710. [Google Scholar] [CrossRef]
- Rampias, T.; Karagiannis, D.; Avgeris, M.; Polyzos, A.; Kokkalis, A.; Kanaki, Z.; Kousidou, E.; Tzetis, M.; Kanavakis, E.; Stravodimos, K.; et al. The Lysine-Specific Methyltransferase KMT2C/MLL3 Regulates DNA Repair Components in Cancer. EMBO Rep. 2019, 20, e46821. [Google Scholar] [CrossRef]
- Jiao, Y.; Lv, Y.; Liu, M.; Liu, Y.; Han, M.; Xiong, X.; Zhou, H.; Zhong, J.; Kang, X.; Su, W. The Modification Role and Tumor Association with a Methyltransferase: KMT2C. Front. Immunol. 2024, 15, 1444923. [Google Scholar] [CrossRef]
- Brauer, B.; Merino-Veliz, N.; Ahumada-Marchant, C.; Arriagada, G.; Bustos, F.J. KMT2C Knockout Generates ASD-Like Behaviors in Mice. Front. Cell Dev. Biol. 2023, 11, 1227723. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshihara, T.; Tanegashima, C.; Kadota, M.; Kobayashi, Y.; Honda, K.; Ishiwata, M.; Ueda, J.; Hara, T.; Nakanishi, M.; et al. Transcriptomic Dysregulation and Autistic-Like Behaviors in Kmt2c Haploinsufficient Mice Rescued by an LSD1 Inhibitor. Mol. Psychiatry 2024, 29, 2888–2904. [Google Scholar] [CrossRef]
- Shansky, R.M.; Woolley, C.S. Considering Sex as a Biological Variable Will Be Valuable for Neuroscience Research. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 11817–11822. [Google Scholar] [CrossRef]
- Zhu, J.W.; Li, Y.F.; Wang, Z.T.; Jia, W.Q.; Xu, R.X. Toll-Like Receptor 4 Deficiency Impairs Motor Coordination. Front. Neurosci. 2016, 10, 33. [Google Scholar] [CrossRef]
- Ma, K.; Taylor, C.; Williamson, M.; Newton, S.S.; Qin, L. Diminished Activity-Dependent Bdnf Signaling Differentially Causes Autism-Like Behavioral Deficits in Male and Female Mice. Front. Psychiatry 2023, 14, 1182472. [Google Scholar] [CrossRef]
- Ma, K.; McDaniel, K.; Zhang, D.; Webb, M.; Qin, L. Chemogenetic Inhibition of Prefrontal Cortex Ameliorates Autism-Like Social Deficits and Absence-Like Seizures in a Gene-Trap Ash1l Haploinsufficiency Mouse Model. Genes 2024, 15, 1619. [Google Scholar] [CrossRef]
- Ma, K.; Qin, L.; Matas, E. Histone Deacetylase Inhibitor MS-275 Restores Social and Synaptic Function in a Shank3-Deficient Mouse Model of Autism. Neuropsychopharmacology 2018, 43, 1779–1788. [Google Scholar] [CrossRef]
- Kang, H.J.; Kawasawa, Y.I.; Cheng, F.; Zhu, Y.; Xu, X.; Li, M.; Sousa, A.M.; Pletikos, M.; Meyer, K.A.; Sedmak, G.; et al. Spatio-temporal Transcriptome of the Human Brain. Nature 2011, 478, 483–489. [Google Scholar] [CrossRef]
- Johnson, M.B.; Kawasawa, Y.I.; Mason, C.E.; Krsnik, Z.; Coppola, G.; Bogdanović, D.; Geschwind, D.H.; Mane, S.M.; State, M.W.; Sestan, N. Functional and Evolutionary Insights into Human Brain Development Through Global Transcriptome Analysis. Neuron 2009, 62, 494–509. [Google Scholar] [CrossRef]
- Herz, H.M.; Garruss, A.; Shilatifard, A. Set for Life: Biochemical Activities and Biological Functions of Set Domain-Containing Proteins. Trends Biochem. Sci. 2013, 38, 621–639. [Google Scholar] [CrossRef]
- Cottam, N.C.; Ofori, K.; Stoll, K.T.; Bryant, M.; Rogge, J.R.; Hekmatyar, K.; Sun, J.; Charvet, C.J. From Circuits to Lifespan: Translating Mouse and Human Timelines with Neuroimaging-Based Tractography. J. Neurosci. Off. J. Soc. Neurosci. 2025, 45, e1429242025. [Google Scholar] [CrossRef]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated Plus Maze for Mice. J. Vis. Exp. JoVE 2008, 22, 1088. [Google Scholar]
- Qin, L.; Williams, J.B.; Tan, T.; Liu, T.; Cao, Q.; Ma, K.; Yan, Z. Deficiency of autism risk factor ASH1L in prefrontal cortex induces epigenetic aberrations and seizures. Nat. Commun. 2021, 12, 6589. [Google Scholar] [CrossRef]
- Kim, H.; Lim, C.-S.; Kaang, B.-K. Neuronal mechanisms and circuits underlying repetitive behaviors in mouse models of autism spectrum disorder. Behav. Brain Funct. 2016, 12, 3. [Google Scholar] [CrossRef]
- Pitts, M.W. Barnes Maze Procedure for Spatial Learning and Memory in Mice. Bio. Protoc. 2018, 8, e2744. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Schwartz, C.E. Intellectual Disability and Autism Spectrum Disorders: Causal Genes and Molecular Mechanisms. Neurosci. Biobehav. Rev. 2014, 46, 161–174. [Google Scholar] [CrossRef]
- Charvet, C.J. Closing the Gap from Transcription to the Structural Connectome Enhances the Study of Connections in the Human Brain. Dev. Dyn. 2020, 249, 1047–1061. [Google Scholar] [CrossRef]
- Ashokkumar, D.; Zhang, Q.; Much, C.; Bledau, A.S.; Naumann, R.; Alexopoulou, D.; Dahl, A.; Goveas, N.; Fu, J.; Anastassiadis, K.; et al. MLL4 is Required After Implantation, Whereas Mll3 Becomes Essential During Late Gestation. Development 2020, 147, dev186999. [Google Scholar] [CrossRef]
- Xie, G.; Lee, J.E.; Senft, A.D.; Park, Y.K.; Jang, Y.; Chakraborty, S.; Thompson, J.J.; McKernan, K.; Liu, C.; Macfarlan, T.S.; et al. Mll3/Mll4 Methyltransferase Activities Control Early Embryonic Development and embryonic Stem Cell Differentiation in a Lineage-Selective Manner. Nat. Genet. 2023, 55, 693–705. [Google Scholar] [CrossRef]
- Vallianatos, C.N.; Iwase, S. Disrupted Intricacy of Histone H3K4 Methylation in neurodevelopmental Disorders. Epigenomics 2015, 7, 503–519. [Google Scholar] [CrossRef]
- Wang, H.; Helin, K. Roles of H3k4 Methylation in Biology and Disease. Trends Cell Biol. 2025, 35, 115–128. [Google Scholar] [CrossRef]
- Cheung, I.; Shulha, H.P.; Jiang, Y.; Matevossian, A.; Wang, J.; Weng, Z.; Akbarian, S. Developmental Regulation and Individual Differences of Neuronal H3K4me3 Epigenomes in the Prefrontal Cortex. Proc. Natl. Acad. Sci. USA 2010, 107, 8824–8829. [Google Scholar]
- Dincer, A.; Gavin, D.P.; Xu, K.; Zhang, B.; Dudley, J.T.; Schadt, E.E.; Akbarian, S. Deciphering H3K4me3 Broad Domains Associated with Gene-Regulatory Networks and Conserved Epigenomic Landscapes in the Human Brain. Transl. Psychiatry 2015, 5, e679. [Google Scholar] [CrossRef]
- Werling, D.M.; Geschwind, D.H. Sex Differences in Autism Spectrum Disorders. Curr. Opin. Neurol. 2013, 26, 146–153. [Google Scholar] [CrossRef]
- Loomes, R.; Hull, L.; Mandy, W.P.L. What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 466–474. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The Novel Object Recognition Memory: Neurobiology, Test Procedure, and Its Modifications. Cogn. Process 2012, 13, 93–110. [Google Scholar] [CrossRef]
- Arnsten, A.F.; Rubia, K. Neurobiological Circuits Regulating Attention, Cognitive Control, Motivation, and Emotion: Disruptions in Neurodevelopmental Psychiatric Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2012, 51, 356–367. [Google Scholar] [CrossRef]
- Fortier, A.V.; Meisner, O.C.; Nair, A.R.; Chang, S.W.C. Prefrontal Circuits Guiding Social Preference: Implications in Autism Spectrum Disorder. Neurosci. Biobehav. Rev. 2022, 141, 104803. [Google Scholar] [CrossRef]
- Wang, X.; Bey, A.L.; Katz, B.M.; Badea, A.; Kim, N.; David, L.K.; Duffney, L.J.; Kumar, S.; Mague, S.D.; Hulbert, S.W.; et al. Altered mGluR5-Homer Scaffolds and Corticostriatal Connectivity in a Shank3 Complete Knockout Model of Autism. Nat. Commun. 2016, 7, 11459. [Google Scholar] [CrossRef]
- Duffney, L.J.; Zhong, P.; Wei, J.; Matas, E.; Cheng, J.; Qin, L.; Ma, K.; Dietz, D.M.; Kajiwara, Y.; Buxbaum, J.D.; et al. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep. 2015, 11, 1400–1413. [Google Scholar] [CrossRef]
- Lee, E.; Lee, J.; Kim, E. Excitation/Inhibition Imbalance in Animal Models of Autism Spectrum Disorders. Biol. Psychiatry 2016, 81, 838–847. [Google Scholar] [CrossRef]
- Gao, R.; Penzes, P. Common Mechanisms of Excitatory and Inhibitory Imbalance in Schizophrenia and Autism Spectrum Disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; O’Shea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical Excitation/Inhibition Balance in Information Processing and Social Dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef]
- Spratt, P.W.E.; Ben-Shalom, R.; Keeshen, C.M.; Burke, K.J., Jr.; Clarkson, R.L.; Sanders, S.J.; Bender, K.J. The Autism-Associated Gene Scn2a Contributes to Dendritic Excitability and Synaptic Function in the Prefrontal Cortex. Neuron 2019, 103, 673–685.e675. [Google Scholar] [CrossRef] [PubMed]
- Frega, M.; Selten, M.; Mossink, B.; Keller, J.M.; Linda, K.; Moerschen, R.; Qu, J.; Koerner, P.; Jansen, S.; Oudakker, A.; et al. Distinct Pathogenic Genes Causing Intellectual Disability and Autism Exhibit a Common Neuronal Network Hyperactivity Phenotype. Cell Rep. 2020, 30, 173–186.e176. [Google Scholar] [CrossRef]
- Murugan, M.; Jang, H.J.; Park, M.; Miller, E.M.; Cox, J.; Taliaferro, J.P.; Parker, N.F.; Bhave, V.; Hur, H.; Liang, Y.; et al. Combined Social and Spatial Coding in a Descending Projection from the Prefrontal Cortex. Cell 2017, 171, 1663–1677.e1616. [Google Scholar] [CrossRef]
- Ferguson, B.R.; Gao, W.J. PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front. Neural Circuits 2018, 12, 37. [Google Scholar] [CrossRef]
- Zhong, P.; Cao, Q.; Yan, Z. Selective Impairment of Circuits Between Prefrontal Cortex Glutamatergic Neurons and Basal Forebrain Cholinergic Neurons in a Tauopathy Mouse Model. Cereb. Cortex 2022, 32, 5569–5579. [Google Scholar] [CrossRef]
- Zhong, P.; Qin, L.; Yan, Z. Dopamine Differentially Regulates Response Dynamics of Prefrontal Cortical Principal Neurons and Interneurons to Optogenetic Stimulation of Inputs from Ventral Tegmental Area. Cereb. Cortex 2020, 30, 4402–4409. [Google Scholar] [CrossRef]
- Ferguson, B.R.; Gao, W.J. Thalamic Control of Cognition and Social Behavior Via Regulation of Gamma-Aminobutyric Acidergic Signaling and Excitation/Inhibition Balance in the Medial Prefrontal Cortex. Biol. Psychiatry 2018, 83, 657–669. [Google Scholar] [CrossRef]
- Gunaydin, L.A.; Grosenick, L.; Finkelstein, J.C.; Kauvar, I.V.; Fenno, L.E.; Adhikari, A.; Lammel, S.; Mirzabekov, J.J.; Airan, R.D.; Zalocusky, K.A.; et al. Natural Neural Projection Dynamics Underlying Social Behavior. Cell 2014, 157, 1535–1551. [Google Scholar] [CrossRef]
- Langen, M.; Bos, D.; Noordermeer, S.D.; Nederveen, H.; van Engeland, H.; Durston, S. Changes in the Development of Striatum are Involved in repetitive Behavior in Autism. Biol. Psychiatry 2014, 76, 405–411. [Google Scholar] [CrossRef]
- Peça, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 Mutant Mice Display Autistic-Like Behaviours and Striatal Dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, C.; Chen, Q.; van der Goes, M.S.; Hawrot, J.; Yao, A.Y.; Gao, X.; Lu, C.; Zang, Y.; Zhang, Q.; et al. Striatopallidal Dysfunction Underlies Repetitive Behavior in Shank3-Deficient Model of Autism. J. Clin. Investig. 2017, 127, 1978–1990. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).