Bullous Pemphigoid Develops Independently of DAP12
Abstract
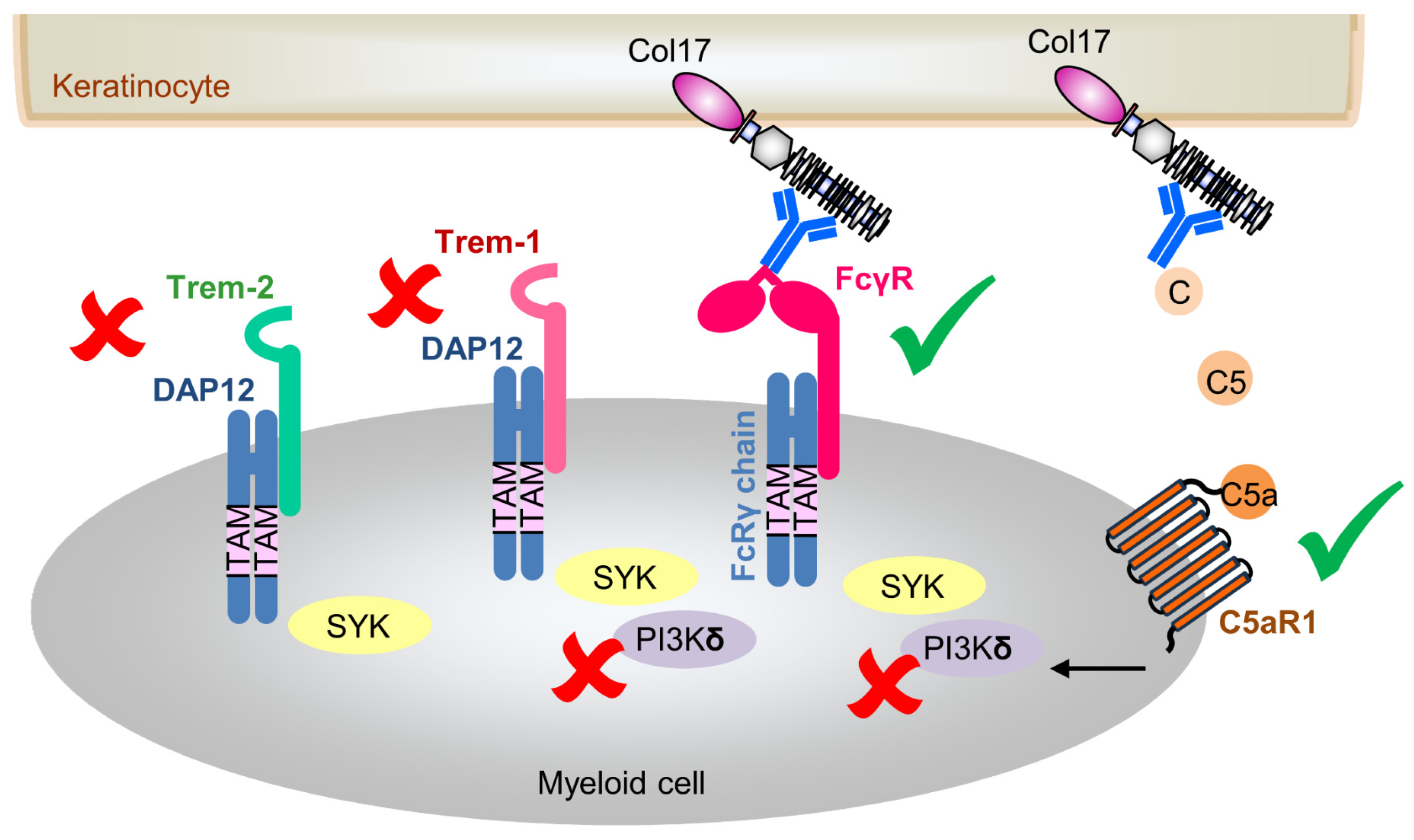
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Mice
2.3. Antibody Transfer-Induced BP Mouse Model and Treatment
2.4. Direct and Indirect Immunofluorescence (IF) Staining
2.5. Histological Analysis
2.6. Flow Cytometry
2.7. Statistical Analyses
3. Results
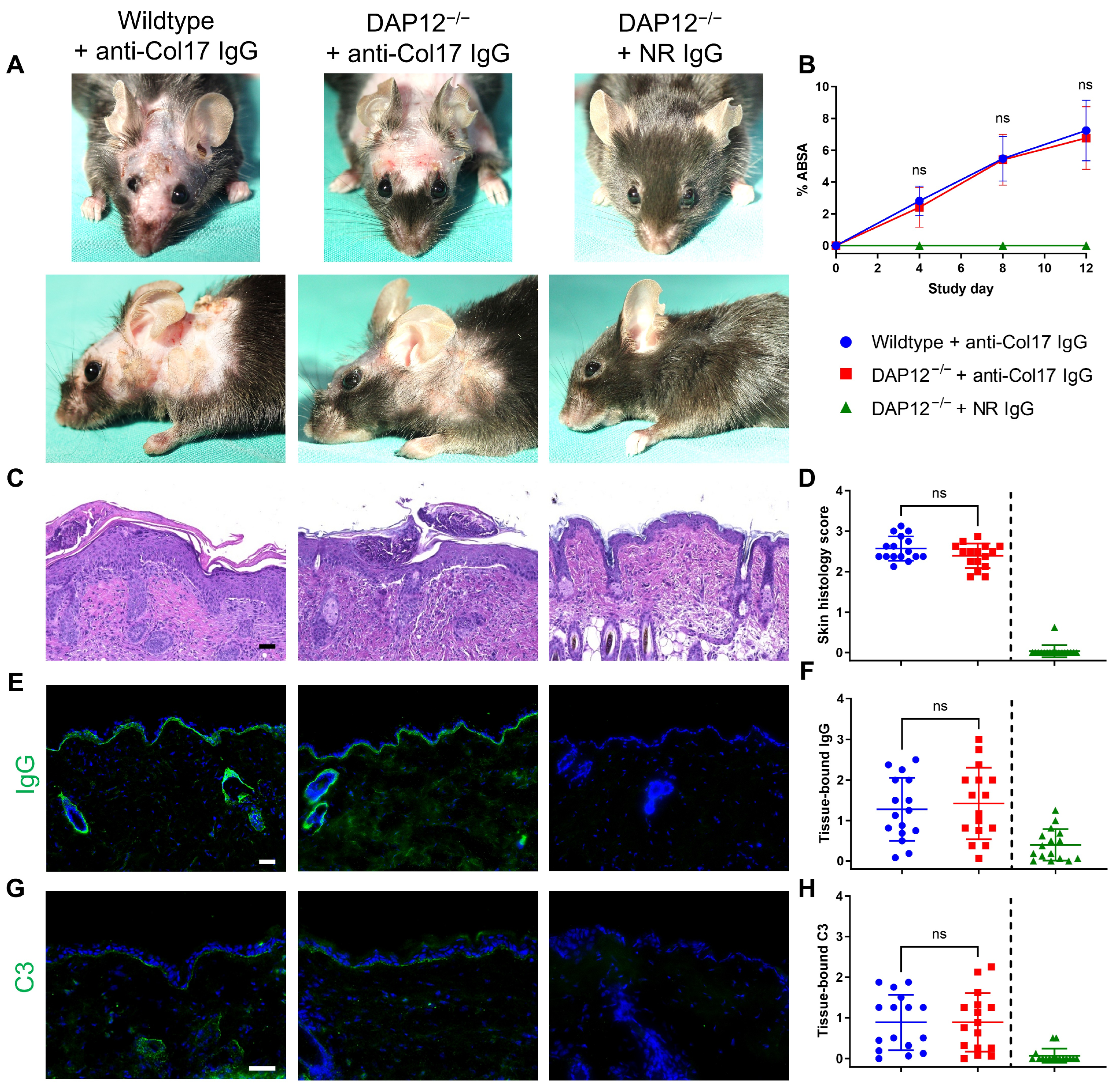
3.1. Loss of DAP12 Does Not Impact Disease Activity in Anti-Col17 IgG-Induced BP
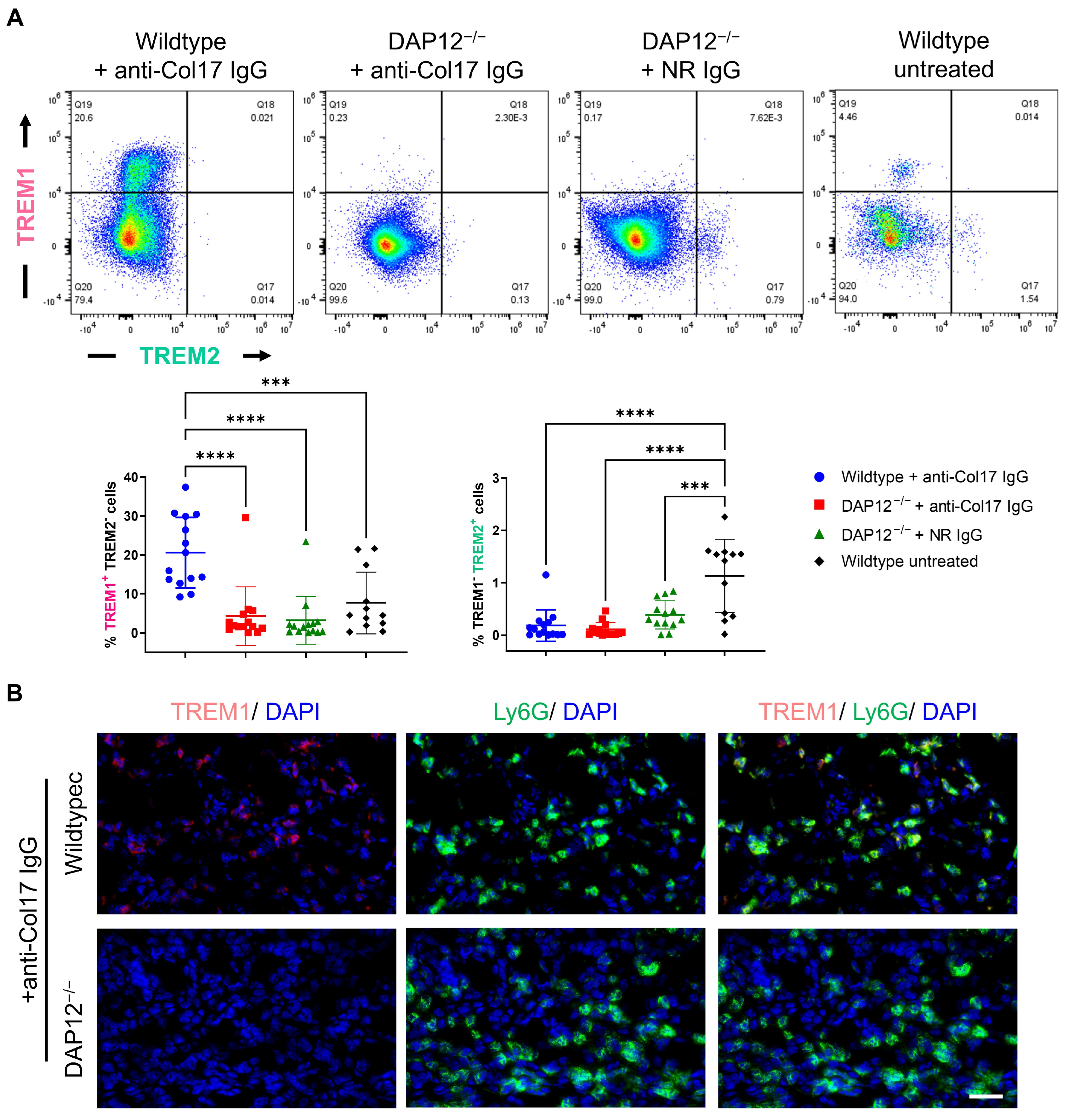
3.2. Dysregulation of DAP12-Associating Receptors TREM1 and TREM2 Has No Impact on BP Pathogenesis
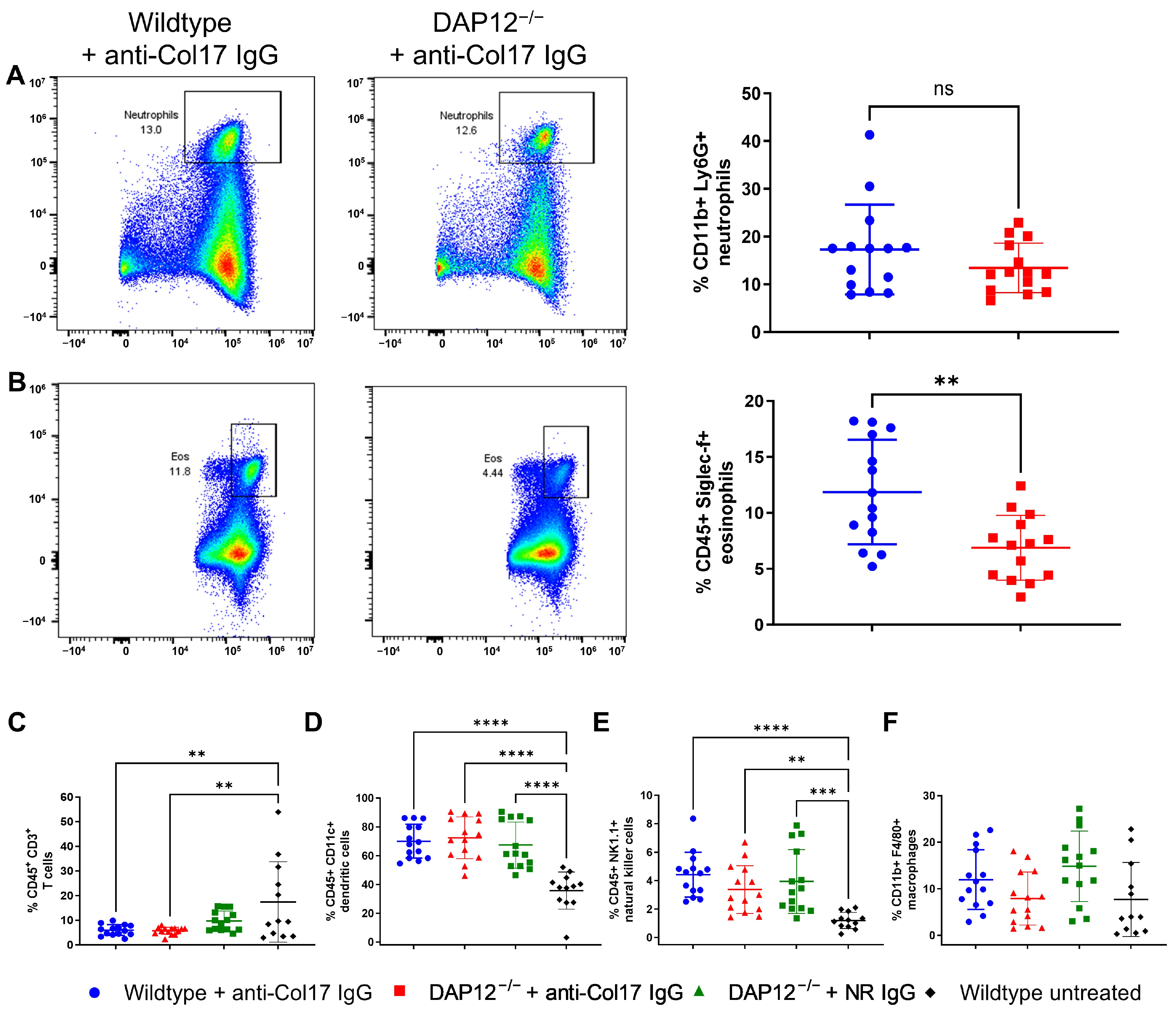
3.3. Changes in the Inflammatory Infiltrate Composition in DAP12-Deficient Skin Do Not Affect BP
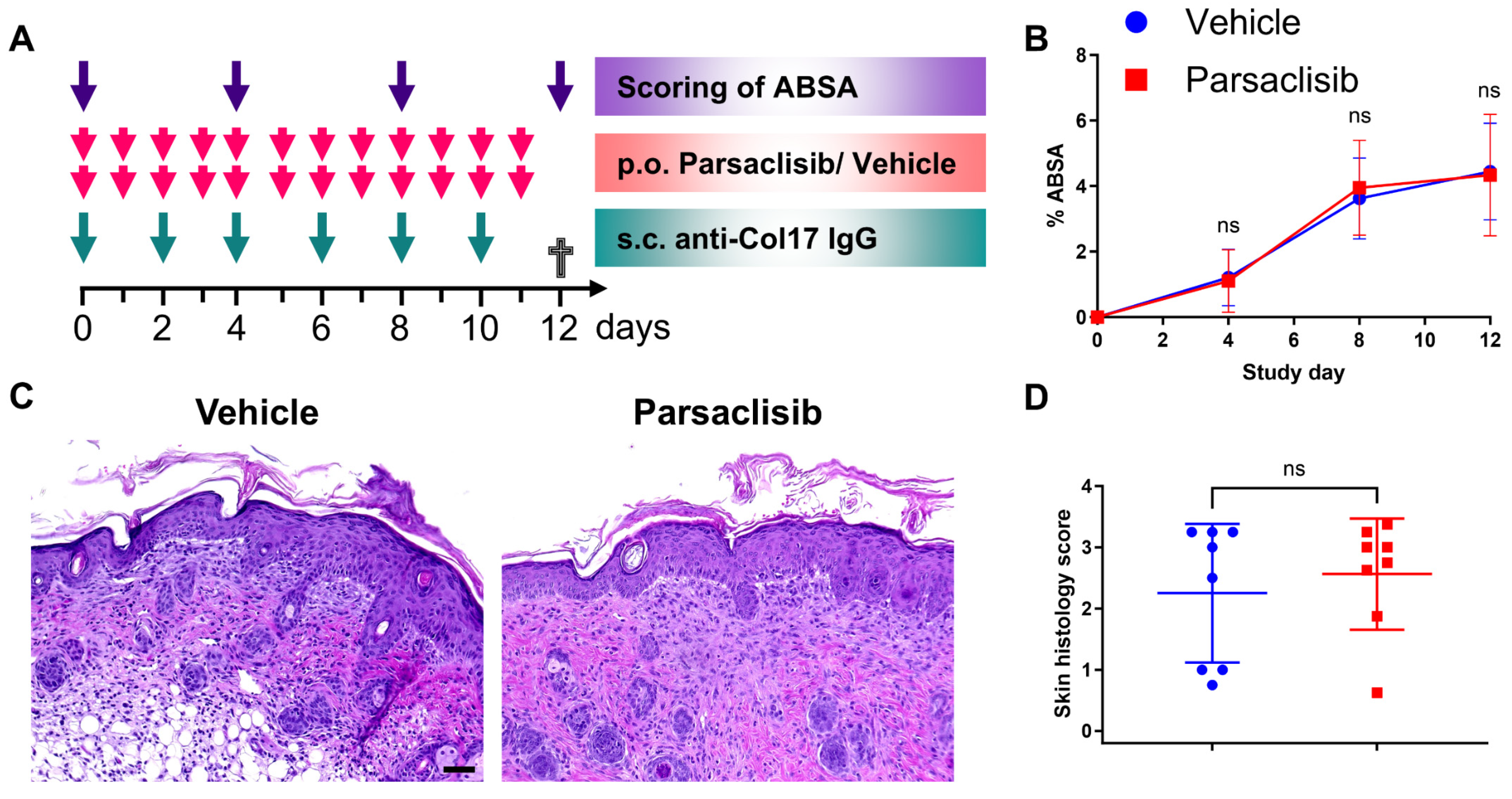
3.4. Pharmacological Modulation of PI3Kδ Does Not Affect Disease Activity in Anti-Col17 IgG-Induced BP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BP | bullous pemphigoid |
| Col17 | type XVII collagen |
| DAP12 | DNAX-activating protein of 12 kDa |
| EBA | epidermolysis bullosa acquisita |
| FcγR | Fc gamma receptor |
| IF | immunofluorescence |
| ITAM | immunoreceptor tyrosine-based activation motif |
| i.p. | intraperitoneal |
| mTOR | mechanisitic target of rapamycin |
| NC | noncollagenous |
| PI3K | phosphatidylinositol 3-kinase |
| s.c. | subcutaneous |
| SYK | spleen tyrosine kinase |
| TREMs | triggering receptors expressed on myeloid cells |
References
- Schmidt, E.; Zillikens, D. Pemphigoid diseases. Lancet 2013, 381, 320–332. [Google Scholar] [CrossRef]
- Persson, M.S.M.; Harman, K.E.; Vinogradova, Y.; Langan, S.M.; Hippisley-Cox, J.; Thomas, K.S.; Gran, S. Incidence, prevalence and mortality of bullous pemphigoid in England 1998–2017: A population-based cohort study. Br. J. Dermatol. 2021, 184, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Akbarialiabad, H.; Schmidt, E.; Patsatsi, A.; Lim, Y.L.; Mosam, A.; Tasanen, K.; Yamagami, J.; Daneshpazhooh, M.; De, D.; Cardones, A.R.G.; et al. Bullous pemphigoid. Nat. Rev. Dis. Primers 2025, 11, 12. [Google Scholar] [CrossRef]
- van Beek, N.; Weidinger, A.; Schneider, S.W.; Kleinheinz, A.; Glaser, R.; Holtsche, M.M.; von Georg, A.; Hammers, C.M.; Hubner, F.; Lima, A.L.; et al. Incidence of pemphigoid diseases in Northern Germany in 2016—First data from the Schleswig-Holstein Registry of Autoimmune Bullous Diseases. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Kridin, K.; Ludwig, R.J. The Growing Incidence of Bullous Pemphigoid: Overview and Potential Explanations. Front. Med. 2018, 5, 220. [Google Scholar] [CrossRef]
- Opelka, B.; Schmidt, E.; Goletz, S. Type XVII collagen: Relevance of distinct epitopes, complement-independent effects, and association with neurological disorders in pemphigoid disorders. Front. Immunol. 2022, 13, 948108. [Google Scholar] [CrossRef] [PubMed]
- Stander, S.; Hammers, C.M.; Vorobyev, A.; Schmidt, E.; Zillikens, D.; Ghorbanalipoor, S.; Bieber, K.; Ludwig, R.J.; Kridin, K. The impact of lesional inflammatory cellular infiltrate on the phenotype of bullous pemphigoid. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1702–1711. [Google Scholar] [CrossRef]
- Borradori, L.; Van Beek, N.; Feliciani, C.; Tedbirt, B.; Antiga, E.; Bergman, R.; Bockle, B.C.; Caproni, M.; Caux, F.; Chandran, N.S.; et al. Updated S2 K guidelines for the management of bullous pemphigoid initiated by the European Academy of Dermatology and Venereology (EADV). J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1689–1704. [Google Scholar] [CrossRef]
- Boch, K.; Zirpel, H.; Thaci, D.; Mruwat, N.; Zillikens, D.; Ludwig, R.J.; Kridin, K. Mortality in eight autoimmune bullous diseases: A global large-scale retrospective cohort study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, e535–e537. [Google Scholar] [CrossRef]
- Turnbull, I.R.; Colonna, M. Activating and inhibitory functions of DAP12. Nat. Rev. Immunol. 2007, 7, 155–161. [Google Scholar] [CrossRef]
- Tomasello, E.; Vivier, E. KARAP/DAP12/TYROBP: Three names and a multiplicity of biological functions. Eur. J. Immunol. 2005, 35, 1670–1677. [Google Scholar] [CrossRef]
- Futosi, K.; Mocsai, A. Tyrosine kinase signaling pathways in neutrophils. Immunol. Rev. 2016, 273, 121–139. [Google Scholar] [CrossRef]
- Paradowska-Gorycka, A.; Jurkowska, M. Structure, expression pattern and biological activity of molecular complex TREM-2/DAP12. Hum. Immunol. 2013, 74, 730–737. [Google Scholar] [CrossRef]
- Hamerman, J.A.; Ni, M.; Killebrew, J.R.; Chu, C.L.; Lowell, C.A. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol. Rev. 2009, 232, 42–58. [Google Scholar] [CrossRef]
- Lanier, L.L. DAP10- and DAP12-associated receptors in innate immunity. Immunol. Rev. 2009, 227, 150–160. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, Z.L.; Li, X.; Liao, C.; Mou, P.; Wang, T.; Wang, Z.; Wang, Z.; Wei, M.; Xu, H.; et al. TREM2/DAP12 Complex Regulates Inflammatory Responses in Microglia via the JNK Signaling Pathway. Front. Aging Neurosci. 2017, 9, 204. [Google Scholar] [CrossRef]
- Colonna, M. The biology of TREM receptors. Nat. Rev. Immunol. 2023, 23, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanalipoor, S.; Emtenani, S.; Parker, M.; Kamaguchi, M.; Osterloh, C.; Pigors, M.; Gross, N.; Khil’chenko, S.; Kasprick, A.; Patzelt, S.; et al. Cutaneous kinase activity correlates with treatment outcomes following PI3K delta inhibition in mice with experimental pemphigoid diseases. Front. Immunol. 2022, 13, 865241. [Google Scholar] [CrossRef]
- Kridin, K.; Kowalski, E.H.; Kneiber, D.; Laufer-Britva, R.; Amber, K.T. From bench to bedside: Evolving therapeutic targets in autoimmune blistering disease. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2239–2252. [Google Scholar] [CrossRef] [PubMed]
- Pigors, M.; Patzelt, S.; Reichhelm, N.; Dworschak, J.; Khil’chenko, S.; Emtenani, S.; Bieber, K.; Hofrichter, M.; Kamaguchi, M.; Goletz, S.; et al. Bullous pemphigoid induced by IgG targeting type XVII collagen non-NC16A/NC15A extracellular domains is driven by Fc gamma receptor- and complement-mediated effector mechanisms and is ameliorated by neonatal Fc receptor blockade. J. Pathol. 2024, 262, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.B.; Hoek, R.M.; Cerwenka, A.; Blom, B.; Lucian, L.; McNeil, T.; Murray, R.; Phillips, L.H.; Sedgwick, J.D.; Lanier, L.L. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity 2000, 13, 345–353. [Google Scholar] [CrossRef]
- Yue, E.W.; Li, Y.L.; Douty, B.; He, C.; Mei, S.; Wayland, B.; Maduskuie, T.; Falahatpisheh, N.; Sparks, R.B.; Polam, P.; et al. INCB050465 (Parsaclisib), a Novel Next-Generation Inhibitor of Phosphoinositide 3-Kinase Delta (PI3Kdelta). ACS Med. Chem. Lett. 2019, 10, 1554–1560. [Google Scholar] [CrossRef]
- Bieber, K.; Koga, H.; Nishie, W. In vitro and in vivo models to investigate the pathomechanisms and novel treatments for pemphigoid diseases. Exp. Dermatol. 2017, 26, 1163–1170. [Google Scholar] [CrossRef]
- Vafia, K.; Groth, S.; Beckmann, T.; Hirose, M.; Dworschak, J.; Recke, A.; Ludwig, R.J.; Hashimoto, T.; Zillikens, D.; Schmidt, E. Pathogenicity of autoantibodies in anti-p200 pemphigoid. PLoS ONE 2012, 7, e41769. [Google Scholar] [CrossRef]
- Schulze, F.S.; Beckmann, T.; Nimmerjahn, F.; Ishiko, A.; Collin, M.; Kohl, J.; Goletz, S.; Zillikens, D.; Ludwig, R.; Schmidt, E. Fcgamma receptors III and IV mediate tissue destruction in a novel adult mouse model of bullous pemphigoid. Am. J. Pathol. 2014, 184, 2185–2196. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Sun, Y.; Wang, H. Protocol for Flow Cytometric Detection of Immune Cell Infiltration in the Epidermis and Dermis of a Psoriasis Mouse Model. STAR Protoc. 2020, 1, 100115. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.S.; Roediger, B.; Tong, P.L.; Tikoo, S.; Weninger, W. The Skin-Resident Immune Network. Curr. Dermatol. Rep. 2014, 3, 13–22. [Google Scholar] [CrossRef]
- Radonjic-Hoesli, S.; Bruggen, M.C.; Feldmeyer, L.; Simon, H.U.; Simon, D. Eosinophils in skin diseases. Semin. Immunopathol. 2021, 43, 393–409. [Google Scholar] [CrossRef]
- Chen, X.; Eksioglu, E.A.; Carter, J.D.; Fortenbery, N.; Donatelli, S.S.; Zhou, J.; Liu, J.; Yang, L.; Gilvary, D.; Djeu, J.; et al. Inactivation of DAP12 in PMN inhibits TREM1-mediated activation in rheumatoid arthritis. PLoS ONE 2015, 10, e0115116. [Google Scholar] [CrossRef] [PubMed]
- Piccio, L.; Buonsanti, C.; Mariani, M.; Cella, M.; Gilfillan, S.; Cross, A.H.; Colonna, M.; Panina-Bordignon, P. Blockade of TREM-2 exacerbates experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2007, 37, 1290–1301. [Google Scholar] [CrossRef]
- Hall, H.T.L.; Sjölin, H.; Brauner, H.; Tomasello, E.; Dalod, M.; Vivier, E.; Höglund, P. Increased diabetes development and decreased function of CD4 D25 Treg in the absence of a functional DAP12 adaptor protein. Eur. J. Immunol. 2008, 38, 3191–3199. [Google Scholar] [CrossRef] [PubMed]
- Kaifu, T.; Nakahara, J.; Inui, M.; Mishima, K.; Momiyama, T.; Kaji, M.; Sugahara, A.; Koito, H.; Ujike-Asai, A.; Nakamura, A.; et al. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 2003, 111, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Paloneva, J.; Kestila, M.; Wu, J.; Salminen, A.; Bohling, T.; Ruotsalainen, V.; Hakola, P.; Bakker, A.B.; Phillips, J.H.; Pekkarinen, P.; et al. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 2000, 25, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Heinz, R.; Schneider, U.C. TLR4-Pathway-Associated Biomarkers in Subarachnoid Hemorrhage (SAH): Potential Targets for Future Anti-Inflammatory Therapies. Int. J. Mol. Sci. 2022, 23, 12618. [Google Scholar] [CrossRef]
- Genua, M.; Rutella, S.; Correale, C.; Danese, S. The triggering receptor expressed on myeloid cells (TREM) in inflammatory bowel disease pathogenesis. J. Transl. Med. 2014, 12, 293. [Google Scholar] [CrossRef]
- Drager, S.; Kalies, K.; Sidronio, T.B.; Witte, M.; Ludwig, R.J.; Bieber, K. Increased TREM-1 expression in inflamed skin has no functional impact on the pathogenesis of cutaneous disorders. J. Dermatol. Sci. 2017, 88, 152–155. [Google Scholar] [CrossRef]
- Weber, B.; Schuster, S.; Zysset, D.; Rihs, S.; Dickgreber, N.; Schürch, C.; Riether, C.; Siegrist, M.; Schneider, C.; Pawelski, H.; et al. TREM-1 Deficiency Can Attenuate Disease Severity without Affecting Pathogen Clearance. PLoS Pathog. 2014, 10, e1003900. [Google Scholar] [CrossRef]
- Do, T.H.; Ma, F.; Andrade, P.R.; Teles, R.; de Andrade Silva, B.J.; Hu, C.; Espinoza, A.; Hsu, J.E.; Cho, C.S.; Kim, M.; et al. TREM2 macrophages induced by human lipids drive inflammation in acne lesions. Sci. Immunol. 2022, 7, eabo2787. [Google Scholar] [CrossRef]
- Wang, E.C.E.; Dai, Z.; Ferrante, A.W.; Drake, C.G.; Christiano, A.M. A Subset of TREM2+ Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth. Cell Stem Cell 2019, 24, 654–669.e656. [Google Scholar] [CrossRef]
- Li, Q.; Ujiie, H.; Shibaki, A.; Wang, G.; Moriuchi, R.; Qiao, H.J.; Morioka, H.; Shinkuma, S.; Natsuga, K.; Long, H.A.; et al. Human IgG1 monoclonal antibody against human collagen 17 noncollagenous 16A domain induces blisters via complement activation in experimental bullous pemphigoid model. J. Immunol. 2010, 185, 7746–7755. [Google Scholar] [CrossRef]
- Oswald, E.; Sesarman, A.; Franzke, C.W.; Wolfle, U.; Bruckner-Tuderman, L.; Jakob, T.; Martin, S.F.; Sitaru, C. The flavonoid luteolin inhibits Fcgamma-dependent respiratory burst in granulocytes, but not skin blistering in a new model of pemphigoid in adult mice. PLoS ONE 2012, 7, e31066. [Google Scholar] [CrossRef]
- Konrad, S.; Ali, S.R.; Wiege, K.; Syed, S.N.; Engling, L.; Piekorz, R.P.; Hirsch, E.; Nurnberg, B.; Schmidt, R.E.; Gessner, J.E. Phosphoinositide 3-kinases gamma and delta, linkers of coordinate C5a receptor-Fcgamma receptor activation and immune complex-induced inflammation. J. Biol. Chem. 2008, 283, 33296–33303. [Google Scholar] [CrossRef]
- Morris, A.C.; Brittan, M.; Wilkinson, T.S.; McAuley, D.F.; Antonelli, J.; McCulloch, C.; Barr, L.C.; McDonald, N.A.; Dhaliwal, K.; Jones, R.O.; et al. C5a-mediated neutrophil dysfunction is RhoA-dependent and predicts infection in critically ill patients. Blood 2011, 117, 5178–5188. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J.T.; Vassallo, A.; Summers, C.; Chilvers, E.R.; Conway-Morris, A. C5a anaphylatoxin and its role in critical illness-induced organ dysfunction. Eur. J. Clin. Invest. 2018, 48, e13028. [Google Scholar] [CrossRef] [PubMed]
- Alflen, A.; Stadler, N.; Aranda Lopez, P.; Teschner, D.; Theobald, M.; Hess, G.; Radsak, M.P. Idelalisib impairs TREM-1 mediated neutrophil inflammatory responses. Sci. Rep. 2018, 8, 5558. [Google Scholar] [CrossRef] [PubMed]
- Koga, H.; Kasprick, A.; Lopez, R.; Auli, M.; Pont, M.; Godessart, N.; Zillikens, D.; Bieber, K.; Ludwig, R.J.; Balague, C. Therapeutic Effect of a Novel Phosphatidylinositol-3-Kinase delta Inhibitor in Experimental Epidermolysis Bullosa Acquisita. Front. Immunol. 2018, 9, 1558. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pigors, M.; Patzelt, S.; Brudey, M.; Emtenani, S.; Khil’chenko, S.; Kamaguchi, M.; Reichhelm, N.; Parker, M.; Bieber, K.; Ludwig, R.J.; et al. Bullous Pemphigoid Develops Independently of DAP12. Biomolecules 2025, 15, 1549. https://doi.org/10.3390/biom15111549
Pigors M, Patzelt S, Brudey M, Emtenani S, Khil’chenko S, Kamaguchi M, Reichhelm N, Parker M, Bieber K, Ludwig RJ, et al. Bullous Pemphigoid Develops Independently of DAP12. Biomolecules. 2025; 15(11):1549. https://doi.org/10.3390/biom15111549
Chicago/Turabian StylePigors, Manuela, Sabrina Patzelt, Maëlys Brudey, Shirin Emtenani, Stanislav Khil’chenko, Mayumi Kamaguchi, Niklas Reichhelm, Melissa Parker, Katja Bieber, Ralf J. Ludwig, and et al. 2025. "Bullous Pemphigoid Develops Independently of DAP12" Biomolecules 15, no. 11: 1549. https://doi.org/10.3390/biom15111549
APA StylePigors, M., Patzelt, S., Brudey, M., Emtenani, S., Khil’chenko, S., Kamaguchi, M., Reichhelm, N., Parker, M., Bieber, K., Ludwig, R. J., & Schmidt, E. (2025). Bullous Pemphigoid Develops Independently of DAP12. Biomolecules, 15(11), 1549. https://doi.org/10.3390/biom15111549






