Evaluation of the Emulsification Properties of Marine-Derived Rhamnolipids for Encapsulation: A Comparison with Commercial Surfactants
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of Rhamnolipids Mixtures
2.2. Evaluation of Surface Tension and Critical Micelle Concentration
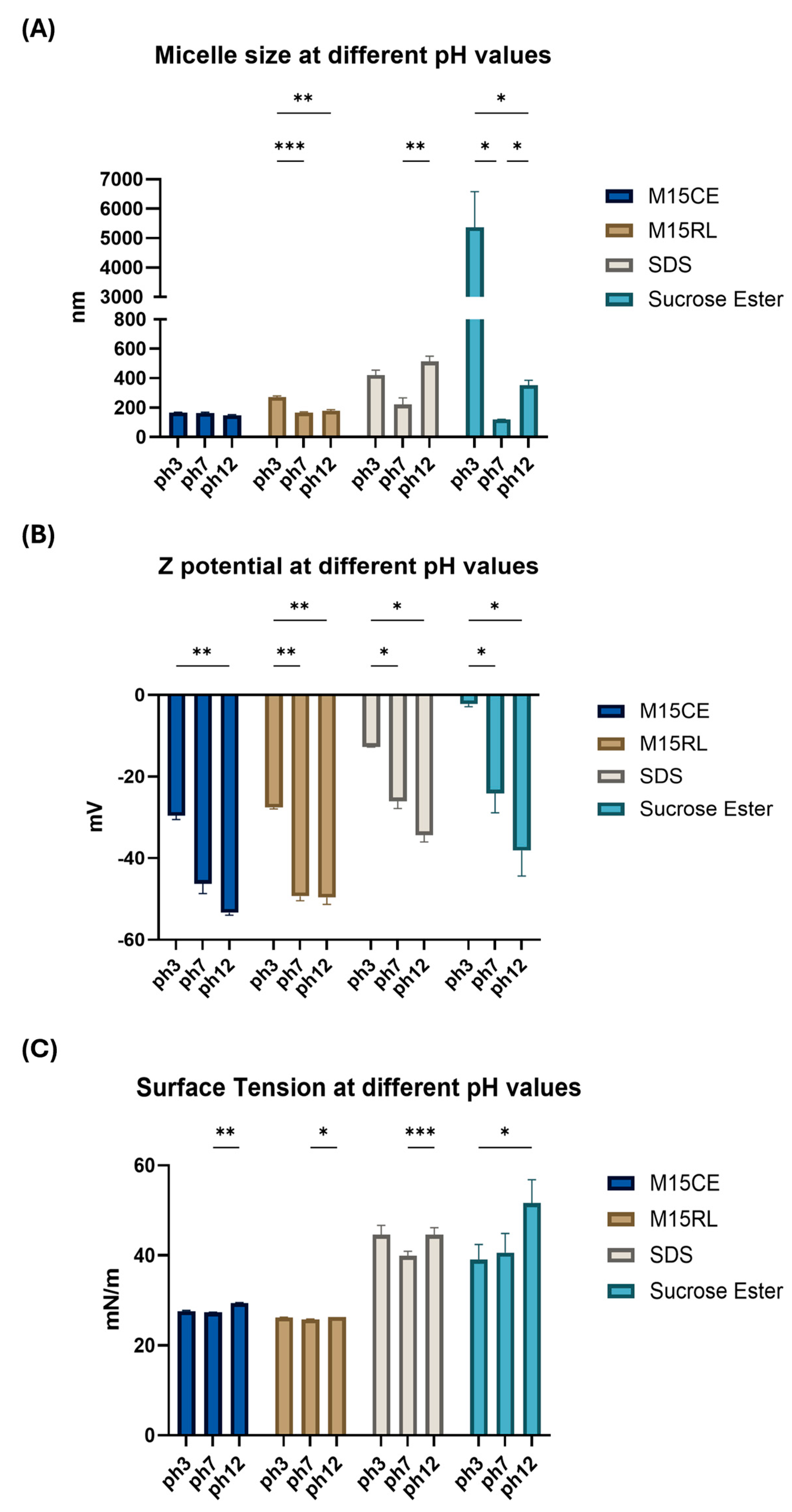
2.3. Surface Activity at Different pH
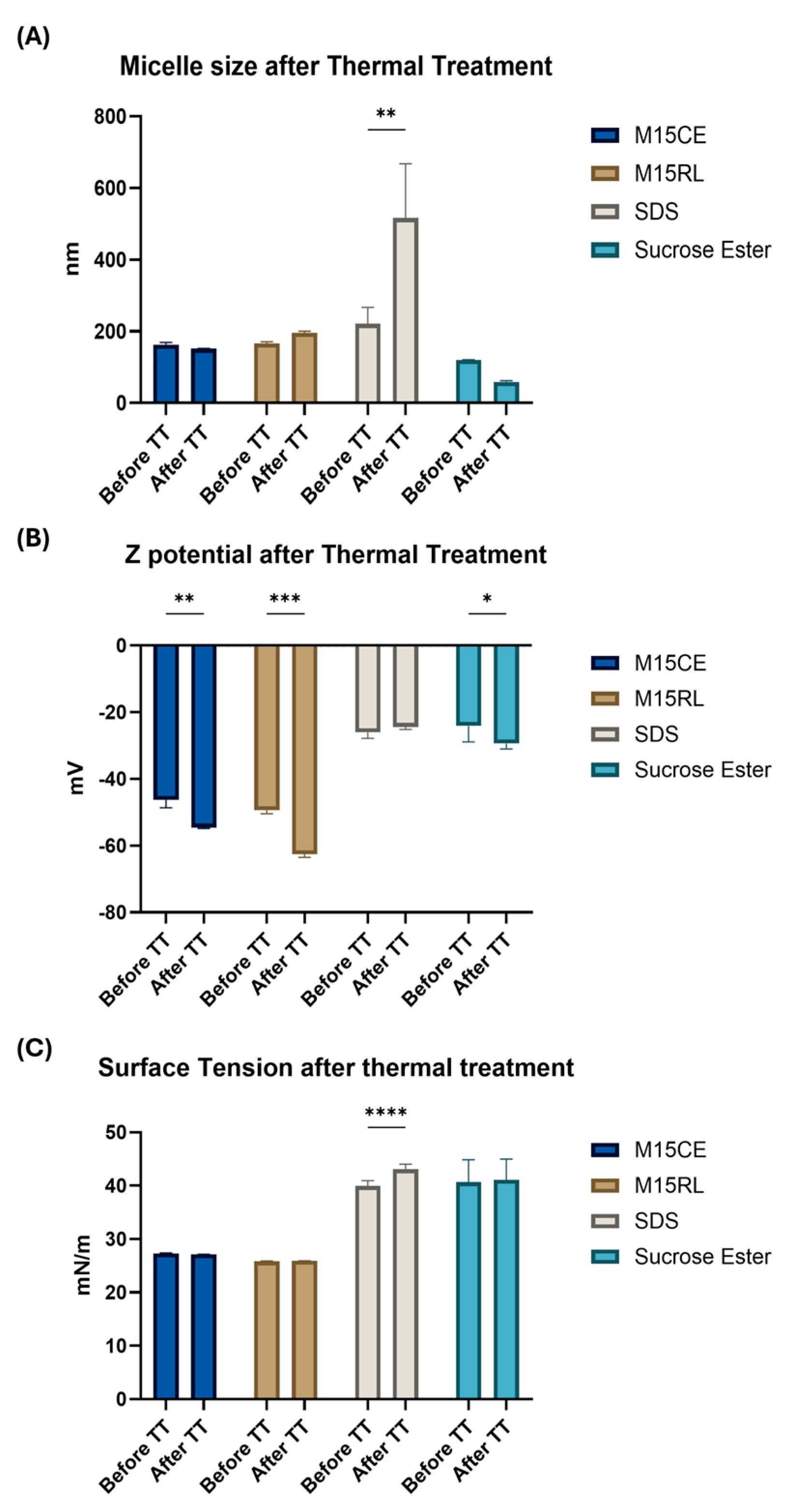
2.4. Thermal Treatment
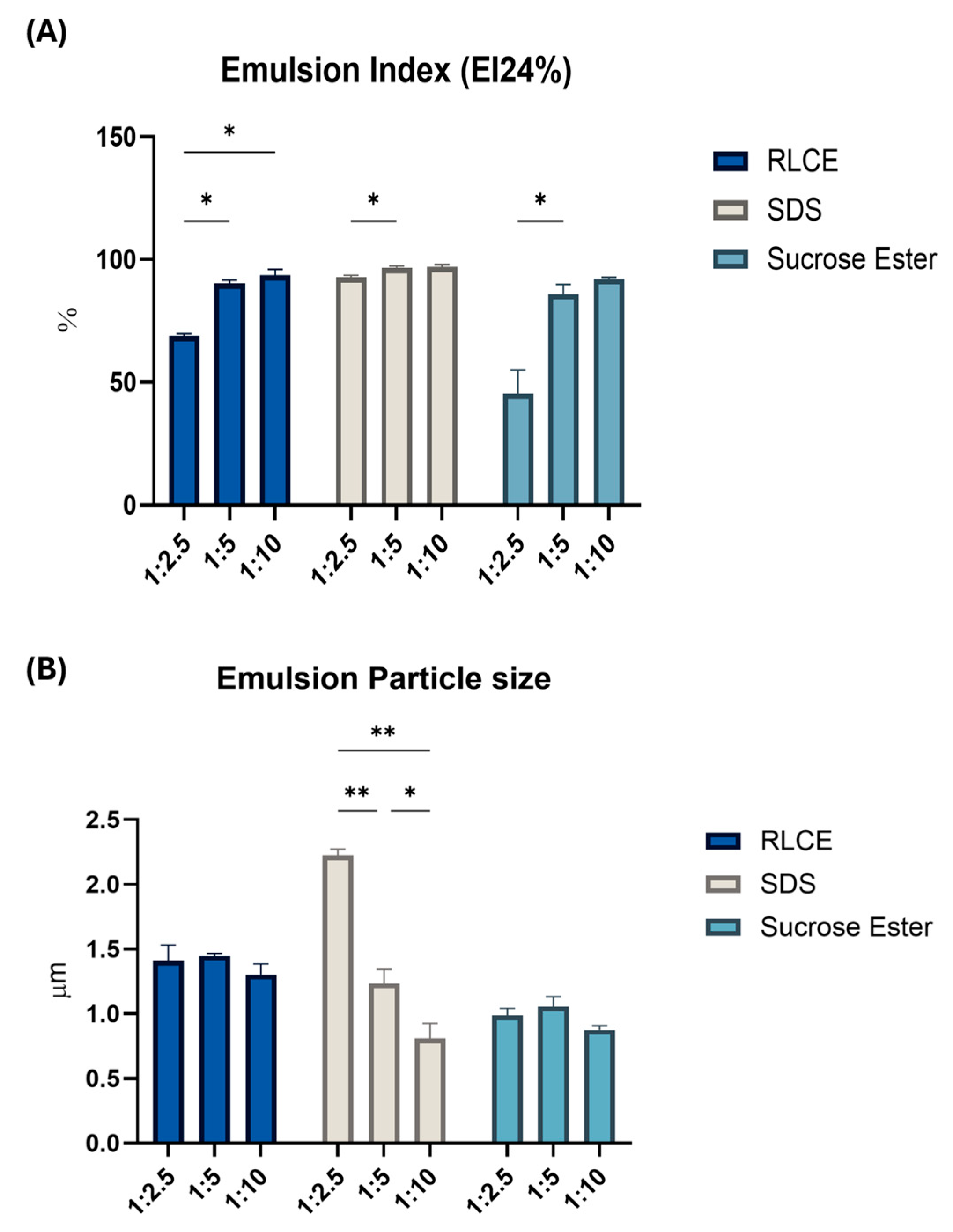
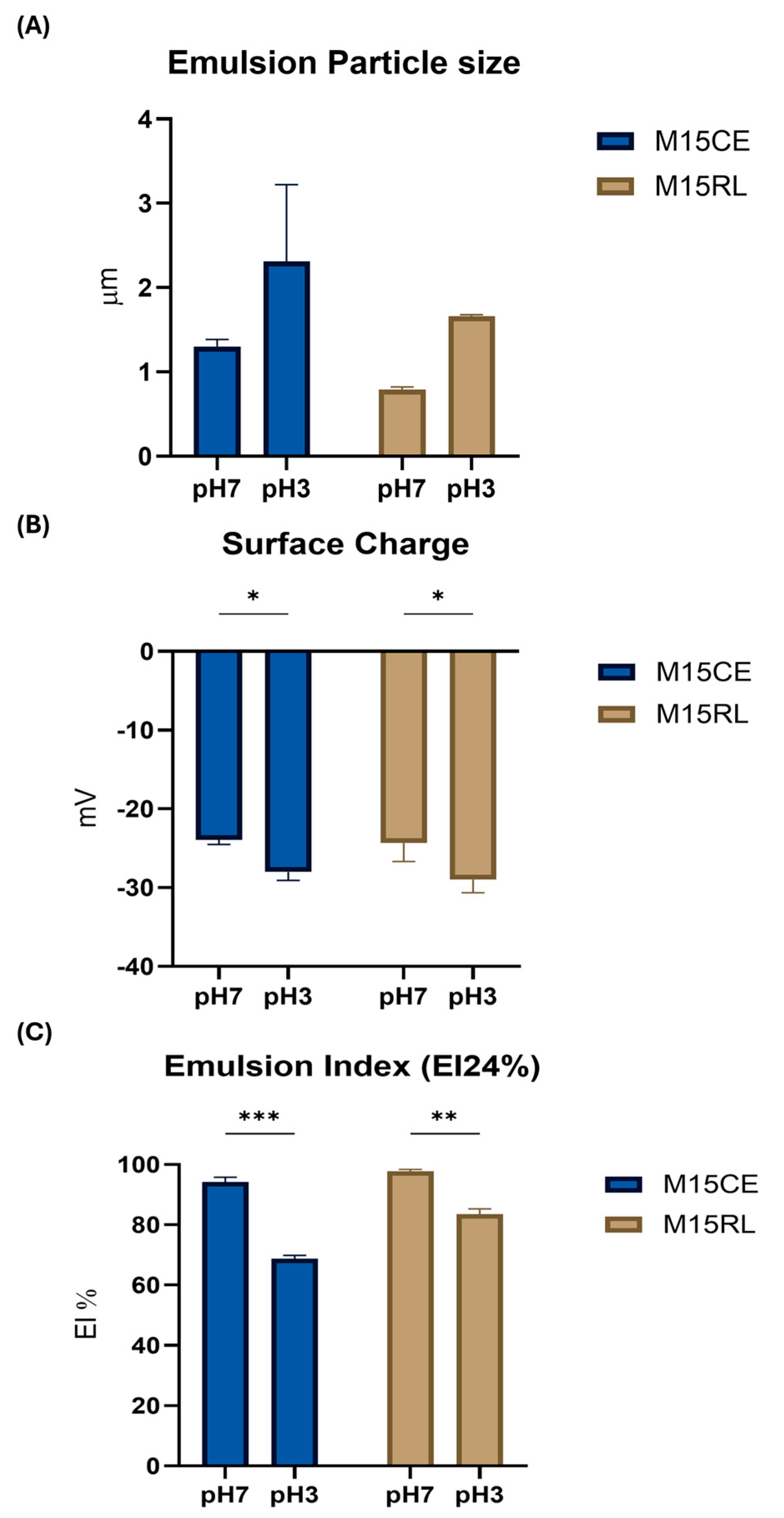
2.5. Emulsifying Activity
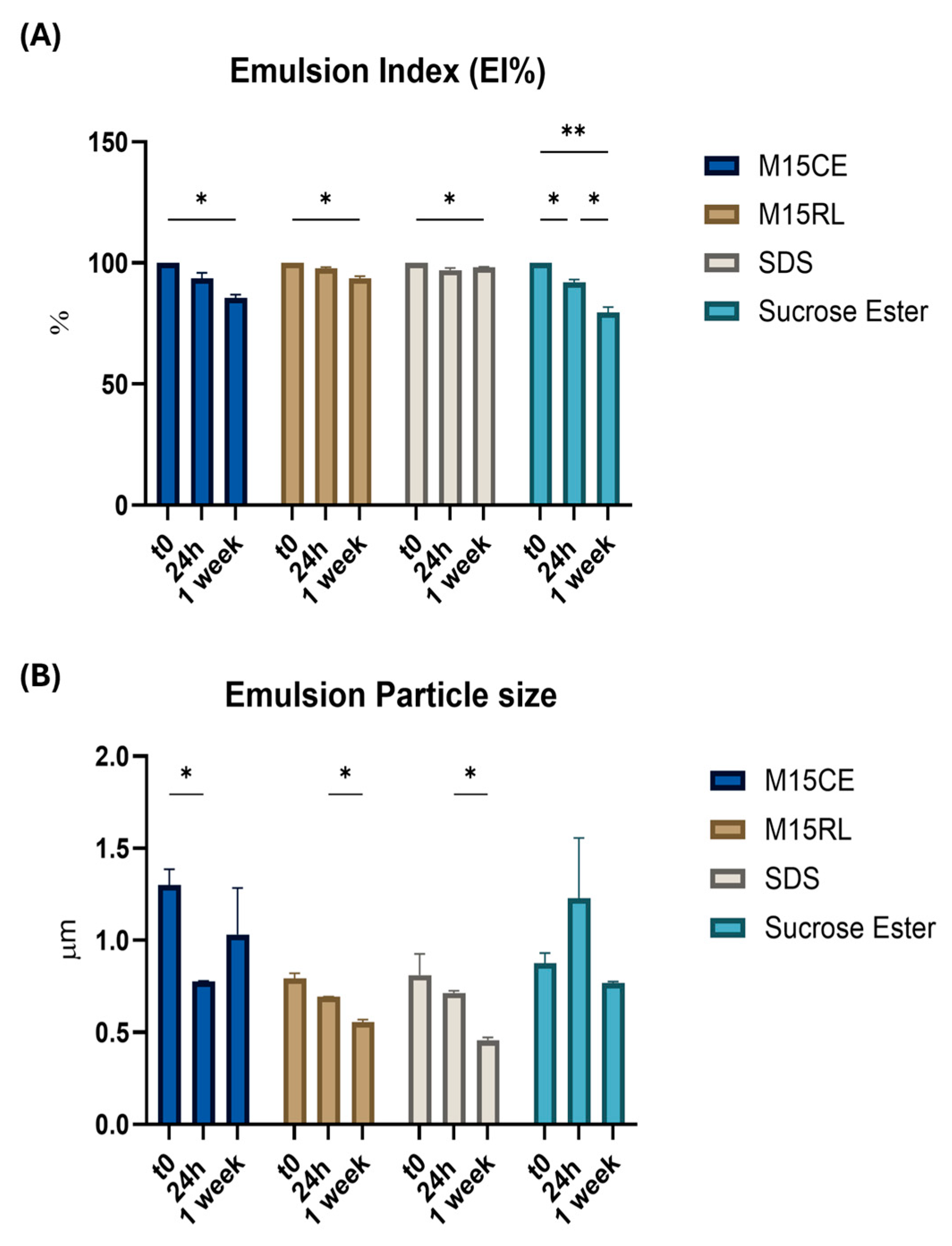
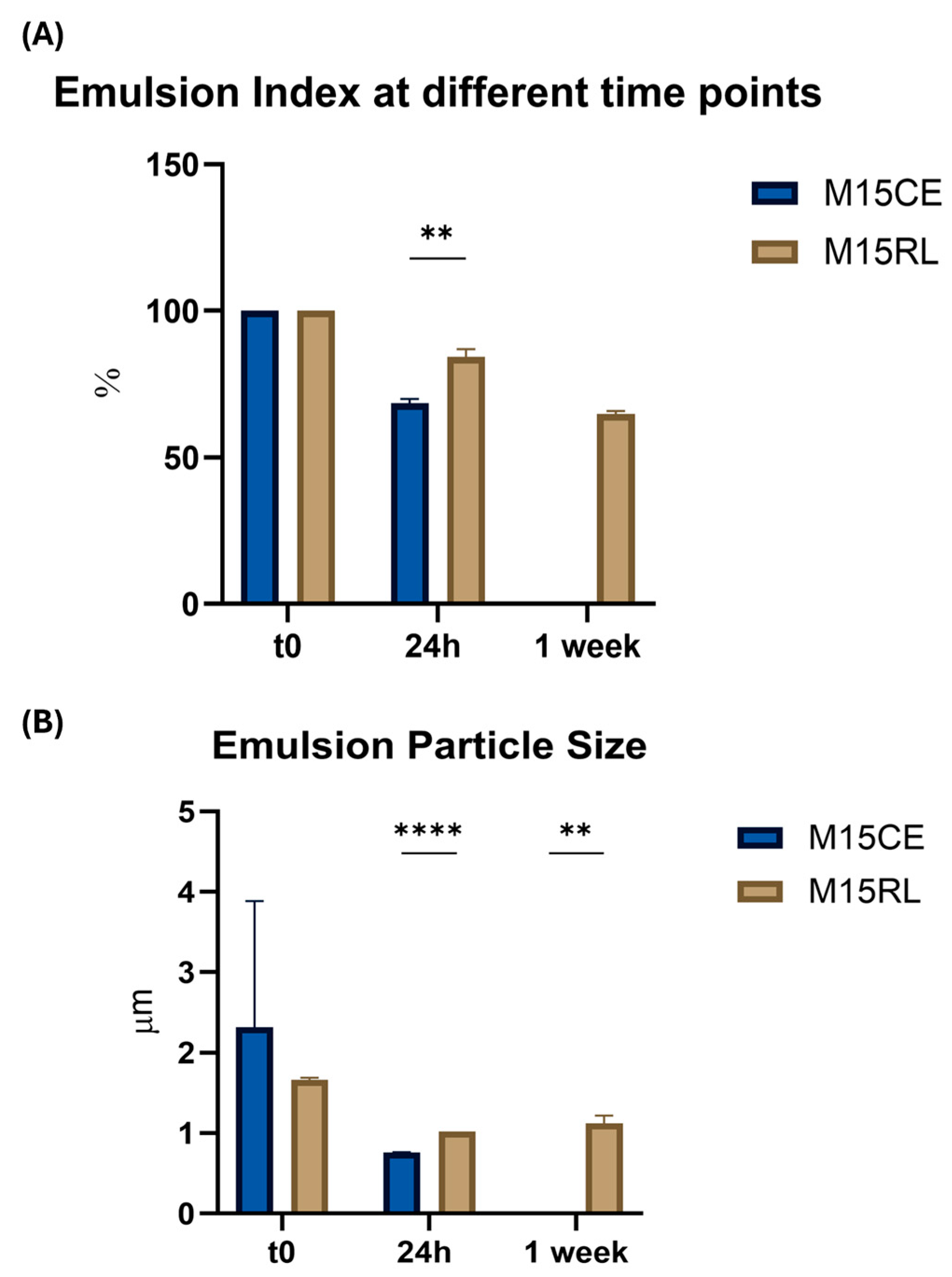
2.6. Rhamnolipids Crude Extract and Purified Mixture Emulsion Stability
2.7. Efficiency of Coenzyme Q10 Encapsulation
3. Materials and Methods
3.1. Chemicals
3.2. Production of P. gessardii M15 RLs Mixture
3.3. Surface Tension and Critical Micelle Concentration
3.4. Particle Size, Polydispersity Index, and Surface Charge
3.5. Mass Spectrometry of M15CE and M15RL Mixtures
3.6. Influence of pH and Thermal Treatment
3.7. O/W Emulsion Preparation
3.8. Emulsion Stability
3.9. Emulsion Characterization
3.9.1. Emulsion Index Assay
3.9.2. Emulsion Particle Size
3.10. Coenzyme Q10 Encapsulation and Encapsulation Efficiency
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SPE | Solid-phase-extraction |
| LC-HRMS2 | Directory of open access journals |
| BSs | Biosurfactants |
| RLs | Rhamnolipids |
| M15CE | M15 crude extract |
| M15RL | M15 purified fraction |
| PoC | Proof of Concept |
| Q10 | Coenzyme Q10 |
| CMC | Critical micelle concentration |
| SDS | Sodium dodecyl sulfate |
| SE | Sucrose Ester |
| ST | Surface tension |
| TT | Thermal treatment |
| DLS | Dynamic laser scattering |
| PDI | Polydispersity index |
| EI | Emulsion index |
| o/w | Oil in water emulsion |
References
- McClements, D.J.; Gumus, C.E. Natural emulsifiers—Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Adv. Colloid Interface Sci. 2016, 234, 3–26. [Google Scholar] [CrossRef]
- Henkel, M.; Müller, M.M.; Kügler, J.H.; Lovaglio, R.B.; Contiero, J.; Syldatk, C.; Hausmann, R. Rhamnolipids as biosurfactants from renewable resources: Concepts for next-generation rhamnolipid production. Process Biochem. 2012, 47, 1207–1219. [Google Scholar] [CrossRef]
- Moldes, A.; Vecino, X.; Rodríguez-López, L.; Rincón-Fontán, M.; Cruz, J.M. Chapter 8—Biosurfactants: The use of biomolecules in cosmetics and detergents. In New and Future Developments in Microbial Biotechnology and Bioengineering; Rodrigues, A.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 163–185. [Google Scholar]
- Bai, L.; McClements, D.J. Formation and stabilization of nanoemulsions using biosurfactants: Rhamnolipids. J. Colloid Interface Sci. 2016, 479, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.; Trybala, A.; Starov, V.; Pinfield, V.J. Effect of synthetic surfactants on the environment and the potential for substitution by biosurfactants. Adv. Colloid Interface Sci. 2021, 288, 102340. [Google Scholar] [CrossRef]
- Stubbs, S.; Yousaf, S.; Khan, I. A review on the synthesis of bio-based surfactants using green chemistry principles. DARU J. Pharm. Sci. 2022, 30, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: Properties, applications and current developments. Bioresour. Technol. 2021, 330, 124963. [Google Scholar] [CrossRef]
- Buonocore, C.; Tedesco, P.; Vitale, G.A.; Esposito, F.P.; Giugliano, R.; Monti, M.C.; D’Auria, M.V.; de Pascale, D. Characterization of a New Mixture of Mono-Rhamnolipids Produced by Pseudomonas gessardii Isolated from Edmonson Point (Antarctica). Mar. Drugs 2020, 18, 269. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, C.; Giugliano, R.; Della Sala, G.; Palma Esposito, F.; Tedesco, P.; Folliero, V.; Galdiero, M.; Franci, G.; de Pascale, D. Evaluation of Antimicrobial Properties and Potential Applications of Pseudomonas gessardii M15 Rhamnolipids towards Multiresistant Staphylococcus aureus. Pharmaceutics 2023, 15, 700. [Google Scholar] [CrossRef]
- da Silva, N.G.; Colodi, F.G.; Pedrão, M.R.; Celligoi, M.A.P.C. Encapsulating Biosurfactants in Biopolymers for Innovative Applications in the Food Industry. ACS Food Sci. Technol. 2024, 4, 2528–2549. [Google Scholar] [CrossRef]
- Amani, H.; Müller, M.M.; Syldatk, C.; Hausmann, R. Production of Microbial Rhamnolipid by Pseudomonas Aeruginosa MM1011 for Ex Situ Enhanced Oil Recovery. Appl. Biochem. Biotechnol. 2013, 170, 1080–1093. [Google Scholar] [CrossRef]
- Müller, F.; Hönzke, S.; Luthardt, W.-O.; Wong, E.L.; Unbehauen, M.; Bauer, J.; Haag, R.; Hedtrich, S.; Rühl, E.; Rademann, J. Rhamnolipids form drug-loaded nanoparticles for dermal drug delivery. Eur. J. Pharm. Biopharm. 2017, 116, 31–37. [Google Scholar] [CrossRef]
- Haba, E.; Bouhdid, S.; Torrego-Solana, N.; Marqués, A.M.; Espuny, M.J.; García-Celma, M.J.; Manresa, A. Rhamnolipids as emulsifying agents for essential oil formulations: Antimicrobial effect against Candida albicans and methicillin-resistant Staphylococcus aureus. Int. J. Pharm. 2014, 476, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhou, H.; Wei, Y.; Gao, Y.; McClements, D.J. Curcumin encapsulation in zein-rhamnolipid composite nanoparticles using a pH-driven method. Food Hydrocoll. 2019, 93, 342–350. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, D.; Park, E.; Jang, S.-y.; Cheon, S.Y.; Han, S.; Koo, H. Rhamnolipid-coated W/O/W double emulsion nanoparticles for efficient delivery of doxorubicin/erlotinib and combination chemotherapy. J. Nanobiotechnol. 2021, 19, 411. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, K.; Kolli, H.B.; Killingmoe Christensen, M.; Bore, S.L.; Diezemann, G.; Gauss, J.; Milano, G.; Lund, R.; Cascella, M. Supramolecular Packing Drives Morphological Transitions of Charged Surfactant Micelles. Angew. Chem. 2020, 59, 18591–18598. [Google Scholar] [CrossRef]
- Pagureva, N.; Cholakova, D.; Mitrinova, Z.; Hristova, M.; Burdzhiev, N.; Tcholakova, S. Temperature response of sucrose palmitate solutions: Role of ratio between monoesters and diesters. J. Colloid Interface Sci. 2024, 674, 209–224. [Google Scholar] [CrossRef]
- McClements, D.J. Critical review of techniques and methodologies for characterization of emulsion stability. Crit. Rev. Food Sci. Nutr. 2007, 47, 611–649. [Google Scholar] [CrossRef]
- Rocha, V.A.L.; de Castilho, L.V.A.; de Castro, R.P.V.; Teixeira, D.B.; Magalhães, A.V.; Gomez, J.G.C.; Freire, D.M.G. Comparison of mono-rhamnolipids and di-rhamnolipids on microbial enhanced oil recovery (MEOR) applications. Biotechnol. Prog. 2020, 36, e2981. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Yang, X.-P.; Ma, L.Z. Analysis of biosurfactants from industrially viable Pseudomonas strain isolated from crude oil suggests how rhamnolipids congeners affect emulsification property and antimicrobial activity. Front. Microbiol. 2014, 5, 696. [Google Scholar] [CrossRef]
- Rikalovic, M.G.; Abdel-Mawgoud, A.M.; Déziel, E.; Gojgic-Cvijovic, G.D.; Nestorovic, Z.; Vrvic, M.M.; Karadzic, I.M. Comparative Analysis of Rhamnolipids from Novel Environmental Isolates of Pseudomonas aeruginosa. J. Surfactants Deterg. 2013, 16, 673–682. [Google Scholar] [CrossRef]
- Fauvel, M.; Trybala, A.; Tseluiko, D.; Starov, V.M.; Bandulasena, H.C.H. Stability of Two-Dimensional Liquid Foams under Externally Applied Electric Fields. Langmuir 2022, 38, 6305–6321. [Google Scholar] [CrossRef]
- Pelekh-Bondaruk, I.; Revyatskyy, I.; Bilous, S. Modelling of the composition of emulsion medicines and cosmetics stabilized by a biocomplex of surfactant substances based on rhamnolipids Pseudomonas sp. PS-17. Sci. Pharm. Sci. 2023, 3, 31–38. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.; Aboulwafa, M.M.; Hassouna, N.A.-H. Characterization of Rhamnolipid Produced by Pseudomonas aeruginosa Isolate Bs20. Appl. Biochem. Biotechnol. 2009, 157, 329–345. [Google Scholar] [CrossRef]
- Kłosowska-Chomiczewska, I.E.; Mędrzycka, K.; Hallmann, E.; Karpenko, E.; Pokynbroda, T.; Macierzanka, A.; Jungnickel, C. Rhamnolipid CMC prediction. J. Colloid Interface Sci. 2017, 488, 10–19. [Google Scholar] [CrossRef]
- Cholakova, D.; Pagureva, N.; Hristova, M.; Tcholakova, S. Salt-induced gelation of nonionic sucrose ester dispersions. J. Colloid Interface Sci. 2025, 693, 137610. [Google Scholar] [CrossRef]
- El-Housseiny, G.S.; Aboshanab, K.M.; Aboulwafa, M.M.; Hassouna, N.A. Structural and Physicochemical Characterization of Rhamnolipids produced by Pseudomonas aeruginosa P6. AMB Express 2020, 10, 201. [Google Scholar] [CrossRef]
- Varjani, S.J.; Upasani, V.N. Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: Production, characterization and surface active properties of biosurfactant. Bioresour. Technol. 2016, 221, 510–516. [Google Scholar] [CrossRef]
- Al-Sakkaf, M.K.; Onaizi, S.A. Crude oil/water nanoemulsions stabilized by rhamnolipid biosurfactant: Effects of acidity/basicity and salinity on emulsion characteristics, stability, and demulsification. Fuel 2023, 344, 128052. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A. Pickering emulsions in food and nutraceutical technology: From delivering hydrophobic compounds to cutting-edge food applications. Explor. Foods Foodomics 2024, 2, 408–442. [Google Scholar] [CrossRef]
- Kong, S.; Shen, C.; Li, Y.; Meng, Q. Rhamnolipids Sustain Unchanged Surface Activities during Decomposition in Alkaline Solutions. ACS Omega 2021, 6, 15750–15755. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-m.; Lai, L.; Lu, Q.; Mei, P.; Wang, Y.-q.; Cheng, L.; Liu, Y. Comparative studies on the surface/interface properties and aggregation behavior of mono-rhamnolipid and di-rhamnolipid. Colloids Surf. B Biointerfaces 2019, 181, 593–601. [Google Scholar] [CrossRef]
- Li, Z.; Dai, L.; Wang, D.; Mao, L.; Gao, Y. Stabilization and Rheology of Concentrated Emulsions Using the Natural Emulsifiers Quillaja Saponins and Rhamnolipids. J. Agric. Food Chem. 2018, 66, 3922–3929. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Baek, Y.; Jeong, E.W.; Lee, H.G. Effect of coenzyme Q10 encapsulation with different sterols on stability, antioxidant activity, and cellular properties of nanoliposomes. Food Biosci. 2023, 56, 103179. [Google Scholar] [CrossRef]
- Uner, B.; Celik, A.; Ergin, A.D.; Altay Benetti, A.; Benetti, C. Enhancement of in-vivo cellular uptake of Coenzyme Q10 using saponin derivatives in rTALAP transgenic mice model. J. Drug Deliv. Sci. Technol. 2024, 96, 105636. [Google Scholar] [CrossRef]
- Ragozzino, C.; Palma Esposito, F.; Buonocore, C.; Tedesco, P.; Coppola, D.; Paccagnella, D.; Ziemert, N.; Della Sala, G.; de de Pascale, D. Integrated genome and metabolome mining unveiled structure and biosynthesis of novel lipopeptides from a deep-sea Rhodococcus. Microb. Biotechnol. 2024, 17, e70011. [Google Scholar] [CrossRef]
- Tiab, D.; Donaldson, E.C. Chapter 6—Wettability. In Petrophysics, 4th ed.; Tiab, D., Donaldson, E.C., Eds.; Gulf Professional Publishing: Boston, MA, USA, 2016; pp. 319–357. [Google Scholar]
- Abruzzo, A.; Giordani, B.; Parolin, C.; Vitali, B.; Protti, M.; Mercolini, L.; Cappelletti, M.; Fedi, S.; Bigucci, F.; Cerchiara, T.; et al. Novel mixed vesicles containing lactobacilli biosurfactant for vaginal delivery of an anti-Candida agent. Eur. J. Pharm. Sci. 2018, 112, 95–101. [Google Scholar] [CrossRef]
- Cooper, D.G.; Goldenberg, B.G. Surface-Active Agents from Two Bacillus Species. Appl. Environ. Microbiol. 1987, 53, 224–229. [Google Scholar] [CrossRef]

| Rt (min.) | Rhamnolipids 1 | Base Peak | M15CE (%) | M15RL (%) |
|---|---|---|---|---|
| 16.14 | Rha-C12:1 | 359.2075 | 0.5% | - |
| 17.35 | Rha-C12 | 361.2231 | 1.8% | - |
| 18.92 | Rha-C14:1 | 387.2388 | 0.8% | - |
| 22.03 | Rha-C14 | 389.2544 | 0.8% | - |
| 23.61 | Rha-C16:1 | 415.2701 | 0.1% | - |
| 25.08 | Rha-C8-C10 | 475.2907 | 10.4% | 2.14% |
| 27.59 | Rha-C8-C11 | 489.3068 | 0.2% | 0.21% |
| 28.16 | Rha-C9-C10 | 489.3066 | 0.7% | 0.85% |
| 28.77 | Rha-C8-C12:1 | 501.3068 | 0.2% | 0.47% |
| 29.10 | Rha-C16:1 | 417.2900 | - | 0.10% |
| 29.37 | Rha-C12:1-C8 | 501.3067 | 0.2% | 0.51% |
| 31.57 | Rha-C10-C10 | 503.3223 | 22.8% | 33.44% |
| 32.82 | Rha-C11:1-C10 | 503.3223 | 0.2% | 0.81% |
| Rha-C12:1-C9 | ||||
| 33.03 | Rha-C12:1-C10:1 | 527.3224 | 0.1% | 0.40% |
| 33.82 | Rha-C12:1-C10 | 529.3380 | 0.7% | 2.39% |
| 34.27 | Rha-C11-C10 | 517.3380 | 0.7% | 2.34% |
| 35.15 | Rha-C11-C10 | 517.3380 | 4.5% | 9.49% |
| 35.43 | Rha-C10-C12:1 | 529.3379 | 1.1% | 2.44% |
| 36.43 | Rha-C12:1-C10 | 529.3381 | 29.2% | 27.43% |
| 38.80 | Rha-C12-C10 | 531.3538 | 17.6% | 13.00% |
| 39.55 | Rha-C12:1-C12:1 | 555.3538 | 2.2% | 2.08% |
| 40.42 | Rha-C14:1-C10 | 557.3694 | 2.6% | 1.79% |
| 41.20 | Rha-C12-C11 | 545.3695 | 0.3% | 0.22% |
| 41.94 | Rha-C12-C12:1 | 557.3694 | 0.9% | 0.84% |
| 42.17 | Rha-C13-C10 | 545.3697 | 0.4% | 0.39% |
| 43.28 | Rha-C12:1-C12 | 557.3694 | 0.4% | 0.36% |
| 45.20 | Rha-C12-C12 | 559.3850 | 0.2% | 0.18% |
| 45.56 | Rha-C14-C10 | 559.3851 | 0.2% | 0.15% |
| 45.99 | Rha-C16:1-C10 | 585.4008 | 0.1% | 0.10% |
| 53.87 | Rha-C16-C10 | 587.4166 | 0.1% | - |
| Sample | Oil (%) | GA (%) | Surfactant (%) | Q10 (%) |
|---|---|---|---|---|
| M15CE | 11.1 | 20 | 0.2 | 1.11 |
| SDS | 11.1 | 20 | 0.2 | 1.11 |
| M15CE_B | 11.1 | 20 | 0.6 | 1.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorrieri, S.; Buonocore, C.; Donà, G.; Pezzoli, C.; Vakarelova, M.; Coppola, D.; Palma Esposito, F.; de Pascale, D.; Della Sala, G.; Zanoni, F.; et al. Evaluation of the Emulsification Properties of Marine-Derived Rhamnolipids for Encapsulation: A Comparison with Commercial Surfactants. Biomolecules 2025, 15, 1451. https://doi.org/10.3390/biom15101451
Gorrieri S, Buonocore C, Donà G, Pezzoli C, Vakarelova M, Coppola D, Palma Esposito F, de Pascale D, Della Sala G, Zanoni F, et al. Evaluation of the Emulsification Properties of Marine-Derived Rhamnolipids for Encapsulation: A Comparison with Commercial Surfactants. Biomolecules. 2025; 15(10):1451. https://doi.org/10.3390/biom15101451
Chicago/Turabian StyleGorrieri, Sara, Carmine Buonocore, Giulia Donà, Chiara Pezzoli, Martina Vakarelova, Daniela Coppola, Fortunato Palma Esposito, Donatella de Pascale, Gerardo Della Sala, Francesca Zanoni, and et al. 2025. "Evaluation of the Emulsification Properties of Marine-Derived Rhamnolipids for Encapsulation: A Comparison with Commercial Surfactants" Biomolecules 15, no. 10: 1451. https://doi.org/10.3390/biom15101451
APA StyleGorrieri, S., Buonocore, C., Donà, G., Pezzoli, C., Vakarelova, M., Coppola, D., Palma Esposito, F., de Pascale, D., Della Sala, G., Zanoni, F., & Tedesco, P. (2025). Evaluation of the Emulsification Properties of Marine-Derived Rhamnolipids for Encapsulation: A Comparison with Commercial Surfactants. Biomolecules, 15(10), 1451. https://doi.org/10.3390/biom15101451











