Inflammaging and Senescence-Driven Extracellular Matrix Remodeling in Age-Associated Cardiovascular Disease
Abstract
1. Introduction
2. Chronic Low-Grade Inflammation (Inflammaging) in Cardiovascular Aging
3. Cellular Senescence in the Cardiovascular System
4. Extracellular Matrix Remodeling Mediated by Inflammation and Senescence
5. Clinical Implications in Major Cardiovascular Diseases
6. Sex Differences in Cardiovascular Aging and the Impact of Comorbidities
7. Therapeutic Opportunities and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DALYs | Disability-adjusted life years |
| IHD | Ischemic Heart Disease |
| COPD | Chronic obstructive pulmonary disease |
| CVD | Cardiovascular disease |
| AHA | American Heart Association |
| TNF-α | Tumor necrosis factor alpha |
| IL-1β | Interleukin 1 beta |
| CRP | C-reactive protein |
| LPS | Lipopolysaccharide |
| TLRs | Toll-like receptors |
| NLRP3 | Nod-like receptor pyrin domain-containing 3 |
| HFpEF | Heart failure with preserved ejection fraction |
| ROS | Reactive oxygen species |
| mtDNA | Mitochondrial DNA |
| DAMP | Damage-associated molecular pattern |
| NO | Nitric Oxide |
| hsCRP | High-sensitivity C-reactive protein |
| AMPK | 5′ AMP-activated protein kinas |
| Nrf2 | Nuclear factor erythroid 2–related factor 2 |
| SASP | Senescence-associated secretory phenotype |
| DDR | DNA damage response |
| ER | Endoplasmic reticulum |
| VSMCs | Vascular smooth muscle cells |
| RAAS | Renin–angiotensin–aldosterone system |
| ECM | The extracellular matrix |
| MMPs | Matrix metalloproteinases |
| LOX | Lysyl oxidase |
| LOXL | LOX-like |
| EMT | Epithelial-mesenchymal transition |
| IPF | Idiopathic pulmonary fibrosis |
| TIMPs | Tissue inhibitors of metalloproteinases |
| MLR | Media-to-Lumen Ratio |
| WLR | Wall-to-Lumen Ratio |
| VEGF | Vascular Endothelial Growth Factor |
| AF | Atrial Fibrillation |
| OR | Odds Ratio |
| PFDR | P-value adjusted for False Discovety Rate |
| HR | Hazard Ratio |
| TCFA | Thin-Cap Fibroatheroma |
| HF | Heart failure |
| TGF- β | Transforming Growth Factor Beta |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| RNA-seq | RNA sequencing |
| ACLY | ATP-Citrate Lyase |
| LV | Left Ventricle |
| MI | Myocardial Infarction |
| CHD | Coronary heart Disease |
| CKD | Chronic Kidney Disease |
| eNOS | Endothelial Nitric Oxide Synthase |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| SC | Senescent Cells |
| SCAPs | Senescent Cell Anti-Apoptotic Pathways |
| STEMI | ST-segment Elevation Myocardial Infarction |
| siRNA | Small Interfering RNA |
| mRNA | Messenger RNA |
References
- Ismail, Z.; Ahmad, W.I.W.; Hamjah, S.H.; Astina, I.K. The Impact of Population Ageing: A Review. Iran. J. Public. Health 2021, 50, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.; Walker, R. Global Ageing: Successes, Challenges and Opportunities. Br. J. Hosp. Med. 2020, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kanasi, E.; Ayilavarapu, S.; Jones, J. The Aging Population: Demographics and the Biology of Aging. Periodontol. 2000 2016, 72, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.J.; Wu, F.; Guo, Y.; Gutierrez Robledo, L.M.; O’Donnell, M.; Sullivan, R.; Yusuf, S. The Burden of Disease in Older People and Implications for Health Policy and Practice. Lancet 2015, 385, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Mattiuzzi, C.; Lippi, G. Worldwide Disease Epidemiology in the Older Persons. Eur. Geriatr. Med. 2020, 11, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, X. Editorial: Aging and Chronic Disease: Public Health Challenge and Education Reform. Front. Public. Health 2023, 11, 1175898. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef]
- Curtis, A.B.; Karki, R.; Hattoum, A.; Sharma, U.C. Arrhythmias in Patients ≥80 Years of Age: Pathophysiology, Management, and Outcomes. J. Am. Coll. Cardiol. 2018, 71, 2041–2057. [Google Scholar] [CrossRef]
- North, B.J.; Sinclair, D.A. The Intersection between Aging and Cardiovascular Disease. Circ. Res. 2012, 110, 1097–1108. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Newman, A.B. The Burden of Cardiovascular Disease in the Elderly: Morbidity, Mortality, and Costs. Clin. Geriatr. Med. 2009, 25, 563–577. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, E.; García-Ortiz, L.; Gómez-Marcos, M.A.; Recio-Rodríguez, J.I.; Mora-Simón, S.; Pérez-Arechaederra, D.; Agudo-Conde, C.; Escribano-Hernández, A.; Patino-Alonso, M.C. Prevalencia de Enfermedades Cardiovasculares y de Factores de Riesgo Cardiovascular En Mayores de 65 Años de Un Área Urbana: Estudio DERIVA. Atención Primaria 2013, 45, 349–357. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of Aging: An Expanding Universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Aging Hallmarks and Progression and Age-Related Diseases: A Landscape View of Research Advancement. ACS Chem. Neurosci. 2024, 15, 1–30. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Song, S.; Tchkonia, T.; Jiang, J.; Kirkland, J.L.; Sun, Y. Targeting Senescent Cells for a Healthier Aging: Challenges and Opportunities. Adv. Sci. 2020, 7, 2002611. [Google Scholar] [CrossRef]
- Schmauck-Medina, T.; Molière, A.; Lautrup, S.; Zhang, J.; Chlopicki, S.; Madsen, H.B.; Cao, S.; Soendenbroe, C.; Mansell, E.; Vestergaard, M.B.; et al. New Hallmarks of Ageing: A 2022 Copenhagen Ageing Meeting Summary. Aging 2022, 14, 6829–6839. [Google Scholar] [CrossRef]
- Müller, L.; Di Benedetto, S. Inflammaging, Immunosenescence, and Cardiovascular Aging: Insights into Long COVID Implications. Front. Cardiovasc. Med. 2024, 11, 1384996. [Google Scholar] [CrossRef]
- Liberale, L.; Montecucco, F.; Tardif, J.-C.; Libby, P.; Camici, G.G. Inflamm-Ageing: The Role of Inflammation in Age-Dependent Cardiovascular Disease. Eur. Heart J. 2020, 41, 2974–2982. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic Inflammation in Ageing, Cardiovascular Disease, and Frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Ajoolabady, A.; Pratico, D.; Vinciguerra, M.; Lip, G.Y.H.; Franceschi, C.; Ren, J. Inflammaging: Mechanisms and Role in the Cardiac and Vasculature. Trends Endocrinol. Metab. 2023, 34, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Barcena, M.L.; Aslam, M.; Pozdniakova, S.; Norman, K.; Ladilov, Y. Cardiovascular Inflammaging: Mechanisms and Translational Aspects. Cells 2022, 11, 1010. [Google Scholar] [CrossRef]
- Puspitasari, Y.M.; Ministrini, S.; Schwarz, L.; Karch, C.; Liberale, L.; Camici, G.G. Modern Concepts in Cardiovascular Disease: Inflamm-Aging. Front. Cell Dev. Biol. 2022, 10, 882211. [Google Scholar] [CrossRef]
- Conway, J.; A Duggal, N. Ageing of the Gut Microbiome: Potential Influences on Immune Senescence and Inflammageing. Ageing Res. Rev. 2021, 68, 101323. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Jeong, J.-J.; Yoo, S.-Y.; Kim, D.-H. Gut Microbiota Lipopolysaccharide Accelerates Inflamm-Aging in Mice. BMC Microbiol. 2016, 16, 9. [Google Scholar] [CrossRef] [PubMed]
- Caetano-Silva, M.E.; Shrestha, A.; Duff, A.F.; Kontic, D.; Brewster, P.C.; Kasperek, M.C.; Lin, C.-H.; Wainwright, D.A.; Hernandez-Saavedra, D.; Woods, J.A.; et al. Aging Amplifies a Gut Microbiota Immunogenic Signature Linked to Heightened Inflammation. Aging Cell 2024, 23, e14190. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, T.; Lin, Y.-N.; Ibrahim, A. Chronic Low-Grade Inflammation in Heart Failure with Preserved Ejection Fraction. Aging Cell 2021, 20, e13453. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bagi, Z.; Feher, A.; Recchia, F.A.; Sonntag, W.E.; Pearson, K.; de Cabo, R.; Csiszar, A. Resveratrol Confers Endothelial Protection via Activation of the Antioxidant Transcription Factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H18–H24. [Google Scholar] [CrossRef]
- Tang, X.; Li, P.-H.; Chen, H.-Z. Cardiomyocyte Senescence and Cellular Communications Within Myocardial Microenvironments. Front. Endocrinol. 2020, 11, 280. [Google Scholar] [CrossRef]
- Suda, M.; Paul, K.H.; Minamino, T.; Miller, J.D.; Lerman, A.; Ellison-Hughes, G.M.; Tchkonia, T.; Kirkland, J.L. Senescent Cells: A Therapeutic Target in Cardiovascular Diseases. Cells 2023, 12, 1296. [Google Scholar] [CrossRef]
- Mao, Z.; Ke, Z.; Gorbunova, V.; Seluanov, A. Replicatively Senescent Cells Are Arrested in G1 and G2 Phases. Aging 2012, 4, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Cellular Senescence: A Translational Perspective. EBioMedicine 2017, 21, 21–28. [Google Scholar] [CrossRef]
- Demaria, M.; Ohtani, N.; Youssef, S.A.; Rodier, F.; Toussaint, W.; Mitchell, J.R.; Laberge, R.-M.; Vijg, J.; Van Steeg, H.; Dollé, M.E.T.; et al. An Essential Role for Senescent Cells in Optimal Wound Healing through Secretion of PDGF-AA. Dev. Cell 2014, 31, 722–733. [Google Scholar] [CrossRef]
- Jun, J.-I.; Lau, L.F. Cellular Senescence Controls Fibrosis in Wound Healing. Aging 2010, 2, 627–631. [Google Scholar] [CrossRef]
- Storer, M.; Mas, A.; Robert-Moreno, A.; Pecoraro, M.; Ortells, M.C.; Di Giacomo, V.; Yosef, R.; Pilpel, N.; Krizhanovsky, V.; Sharpe, J.; et al. Senescence Is a Developmental Mechanism That Contributes to Embryonic Growth and Patterning. Cell 2013, 155, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Zhu, Y.; van Deursen, J.; Campisi, J.; Kirkland, J.L. Cellular Senescence and the Senescent Secretory Phenotype: Therapeutic Opportunities. J. Clin. Investig. 2013, 123, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.J.; Zhao, J.; Bukata, C.; Wade, E.A.; McGowan, S.J.; Angelini, L.A.; Bank, M.P.; Gurkar, A.U.; McGuckian, C.A.; Calubag, M.F.; et al. Tissue Specificity of Senescent Cell Accumulation during Physiologic and Accelerated Aging of Mice. Aging Cell 2020, 19, e13094. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Lee, R.T.; Garbern, J.C. Senescence Mechanisms and Targets in the Heart. Cardiovasc. Res. 2021, 118, 1173–1187. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Jia, C.; Sowers, J.R. Endothelial Cell Senescence in Aging-Related Vascular Dysfunction. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 1802–1809. [Google Scholar] [CrossRef]
- Saucerman, J.J.; Tan, P.M.; Buchholz, K.S.; McCulloch, A.D.; Omens, J.H. Mechanical Regulation of Gene Expression in Cardiac Myocytes and Fibroblasts. Nat. Rev. Cardiol. 2019, 16, 361–378. [Google Scholar] [CrossRef]
- Zhu, F.; Li, Y.; Zhang, J.; Piao, C.; Liu, T.; Li, H.-H.; Du, J. Senescent Cardiac Fibroblast Is Critical for Cardiac Fibrosis after Myocardial Infarction. PLoS ONE 2013, 8, e74535. [Google Scholar] [CrossRef]
- Cui, S.; Xue, L.; Yang, F.; Dai, S.; Han, Z.; Liu, K.; Liu, B.; Yuan, Q.; Cui, Z.; Zhang, Y.; et al. Postinfarction Hearts Are Protected by Premature Senescent Cardiomyocytes Via GATA4—Dependent CCN1 Secretion. J. Am. Heart Assoc. 2018, 7, e009111. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Chen, Q.; Hoppel, C.L. Mitochondrial Metabolism in Aging Heart. Circ. Res. 2016, 118, 1593–1611. [Google Scholar] [CrossRef] [PubMed]
- Vasileiou, P.V.S.; Evangelou, K.; Vlasis, K.; Fildisis, G.; Panayiotidis, M.I.; Chronopoulos, E.; Passias, P.-G.; Kouloukoussa, M.; Gorgoulis, V.G.; Havaki, S. Mitochondrial Homeostasis and Cellular Senescence. Cells 2019, 8, 686. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.; Haile, B.; Arif, M.; Pandey, R.; Rokvic, M.; Nieman, M.; Maliken, B.D.; Paul, A.; Wang, Y.; Sadayappan, S.; et al. Inhibition of Senescence—Associated Genes Rb1 and Meis2 in Adult Cardiomyocytes Results in Cell Cycle Reentry and Cardiac Repair Post–Myocardial Infarction. J. Am. Heart Assoc. 2019, 8, e012089. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Wang, S.; Guo, H.; Tan, Y.; Liang, Y.; Feng, A.; Liu, Q.; Damodaran, C.; Zhang, Z.; Keller, B.B.; et al. Inhibition of P53 Prevents Diabetic Cardiomyopathy by Preventing Early-Stage Apoptosis and Cell Senescence, Reduced Glycolysis, and Impaired Angiogenesis. Cell Death Dis. 2018, 9, 82. [Google Scholar] [CrossRef]
- Anderson, R.; Lagnado, A.; Maggiorani, D.; Walaszczyk, A.; Dookun, E.; Chapman, J.; Birch, J.; Salmonowicz, H.; Ogrodnik, M.; Jurk, D.; et al. Length-independent Telomere Damage Drives Post-mitotic Cardiomyocyte Senescence. EMBO J. 2019, 38, e100492. [Google Scholar] [CrossRef]
- Boon, R.A.; Iekushi, K.; Lechner, S.; Seeger, T.; Fischer, A.; Heydt, S.; Kaluza, D.; Tréguer, K.; Carmona, G.; Bonauer, A.; et al. MicroRNA-34a Regulates Cardiac Ageing and Function. Nature 2013, 495, 107–110. [Google Scholar] [CrossRef]
- van Almen, G.C.; Verhesen, W.; van Leeuwen, R.E.W.; van de Vrie, M.; Eurlings, C.; Schellings, M.W.M.; Swinnen, M.; Cleutjens, J.P.M.; van Zandvoort, M.A.M.J.; Heymans, S.; et al. MicroRNA-18 and microRNA-19 Regulate CTGF and TSP-1 Expression in Age-Related Heart Failure. Aging Cell 2011, 10, 769–779. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular Smooth Muscle Cell Death, Autophagy and Senescence in Atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Basatemur, G.L.; Jørgensen, H.F.; Clarke, M.C.H.; Bennett, M.R.; Mallat, Z. Vascular Smooth Muscle Cells in Atherosclerosis. Nat. Rev. Cardiol. 2019, 16, 727–744. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, S.D.; Fiedler, J.; Bauersachs, J.; Thum, T.; Sedding, D.G. Senescence-Induced Inflammation: An Important Player and Key Therapeutic Target in Atherosclerosis. Eur. Heart J. 2020, 41, 2983–2996. [Google Scholar] [CrossRef] [PubMed]
- Lacolley, P.; Regnault, V.; Segers, P.; Laurent, S. Vascular Smooth Muscle Cells and Arterial Stiffening: Relevance in Development, Aging, and Disease. Physiol. Rev. 2017, 97, 1555–1617. [Google Scholar] [CrossRef] [PubMed]
- Nakano-Kurimoto, R.; Ikeda, K.; Uraoka, M.; Nakagawa, Y.; Yutaka, K.; Koide, M.; Takahashi, T.; Matoba, S.; Yamada, H.; Okigaki, M.; et al. Replicative Senescence of Vascular Smooth Muscle Cells Enhances the Calcification through Initiating the Osteoblastic Transition. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1673–H1684. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; An, J.; Zou, M.-H. Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells 2020, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Monnerat, G.; Alarcón, M.L.; Vasconcellos, L.R.; Hochman-Mendez, C.; Brasil, G.; Bassani, R.A.; Casis, O.; Malan, D.; Travassos, L.H.; Sepúlveda, M.; et al. Macrophage-Dependent IL-1β Production Induces Cardiac Arrhythmias in Diabetic Mice. Nat. Commun. 2016, 7, 13344. [Google Scholar] [CrossRef]
- Zlatanova, I.; Pinto, C.; Bonnin, P.; Mathieu, J.R.R.; Bakker, W.; Vilar, J.; Lemitre, M.; Voehringer, D.; Vaulont, S.; Peyssonnaux, C.; et al. Iron Regulator Hepcidin Impairs Macrophage-Dependent Cardiac Repair After Injury. Circulation 2019, 139, 1530–1547. [Google Scholar] [CrossRef]
- Kamo, T.; Akazawa, H.; Komuro, I. Cardiac Nonmyocytes in the Hub of Cardiac Hypertrophy. Circ. Res. 2015, 117, 89–98. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Xia, N.; Zhou, S.-F.; Tang, T.-T.; Yan, X.-X.; Lv, B.-J.; Nie, S.-F.; Wang, J.; Iwakura, Y.; Xiao, H.; et al. IL-17A Contributes to Myocardial Ischemia/Reperfusion Injury by Regulating Cardiomyocyte Apoptosis and Neutrophil Infiltration. J. Am. Coll. Cardiol. 2012, 59, 420–429. [Google Scholar] [CrossRef]
- Ngkelo, A.; Richart, A.; Kirk, J.A.; Bonnin, P.; Vilar, J.; Lemitre, M.; Marck, P.; Branchereau, M.; Le Gall, S.; Renault, N.; et al. Mast Cells Regulate Myofilament Calcium Sensitization and Heart Function after Myocardial Infarction. J. Exp. Med. 2016, 213, 1353–1374. [Google Scholar] [CrossRef] [PubMed]
- He, A.; Fang, W.; Zhao, K.; Wang, Y.; Li, J.; Yang, C.; Benadjaoud, F.; Shi, G.-P. Mast Cell-Deficiency Protects Mice from Streptozotocin-Induced Diabetic Cardiomyopathy. Transl. Res. 2019, 208, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kvakan, H.; Kleinewietfeld, M.; Qadri, F.; Park, J.-K.; Fischer, R.; Schwarz, I.; Rahn, H.-P.; Plehm, R.; Wellner, M.; Elitok, S.; et al. Regulatory T Cells Ameliorate Angiotensin II-Induced Cardiac Damage. Circulation 2009, 119, 2904–2912. [Google Scholar] [CrossRef] [PubMed]
- Zacchigna, S.; Martinelli, V.; Moimas, S.; Colliva, A.; Anzini, M.; Nordio, A.; Costa, A.; Rehman, M.; Vodret, S.; Pierro, C.; et al. Paracrine Effect of Regulatory T Cells Promotes Cardiomyocyte Proliferation during Pregnancy and after Myocardial Infarction. Nat. Commun. 2018, 9, 2432. [Google Scholar] [CrossRef] [PubMed]
- Horio, T.; Maki, T.; Kishimoto, I.; Tokudome, T.; Okumura, H.; Yoshihara, F.; Suga, S.; Takeo, S.; Kawano, Y.; Kangawa, K. Production and Autocrine/Paracrine Effects of Endogenous Insulin-like Growth Factor-1 in Rat Cardiac Fibroblasts. Regul. Pept. 2005, 124, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Urbanczyk, M.; Layland, S.L.; Schenke-Layland, K. The Role of Extracellular Matrix in Biomechanics and Its Impact on Bioengineering of Cells and 3D Tissues. Matrix Biol. 2020, 85–86, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Cox, T.R.; Bird, D.; Baker, A.-M.; Barker, H.E.; Ho, M.W.-Y.; Lang, G.; Erler, J.T. LOX-Mediated Collagen Crosslinking Is Responsible for Fibrosis-Enhanced Metastasis. Cancer Res. 2013, 73, 1721–1732. [Google Scholar] [CrossRef]
- Levi, N.; Papismadov, N.; Solomonov, I.; Sagi, I.; Krizhanovsky, V. The ECM Path of Senescence in Aging: Components and Modifiers. FEBS J. 2020, 287, 2636–2646. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Sun, H.; Zhang, Y.; Zou, D. New Insights into Fibrosis from the ECM Degradation Perspective: The Macrophage-MMP-ECM Interaction. Cell Biosci. 2022, 12, 117. [Google Scholar] [CrossRef]
- Musale, V.; Wasserman, D.H.; Kang, L. Extracellular Matrix Remodelling in Obesity and Metabolic Disorders. Life Metab. 2023, 2, load021. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and Homeostasis of the Extracellular Matrix: Implications for Fibrotic Diseases and Cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Schuppan, D.; Ruehl, M.; Somasundaram, R.; Hahn, E.G. Matrix as a Modulator of Hepatic Fibrogenesis. Semin. Liver Dis. 2001, 21, 351–372. [Google Scholar] [CrossRef]
- Selman, M.; Pardo, A. Fibroageing: An Ageing Pathological Feature Driven by Dysregulated Extracellular Matrix-Cell Mechanobiology. Ageing Res. Rev. 2021, 70, 101393. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.-N.; Luo, G.; Gao, W.-J.; Yang, S.-J.; Zhou, H. miR-29 Family: A Potential Therapeutic Target for Cardiovascular Disease. Pharmacol. Res. 2021, 166, 105510. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Fear, M.W.; Suk Choi, Y.; Wood, F.M.; Allahham, A.; Mutsaers, S.E.; Prêle, C.M. The Extracellular Matrix and Mechanotransduction in Pulmonary Fibrosis. Int. J. Biochem. Cell Biol. 2020, 126, 105802. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.G.; Andriotis, O.G.; Roberts, J.J.; Lunn, K.; Tear, V.J.; Cao, L.; Ask, K.; Smart, D.E.; Bonfanti, A.; Johnson, P.; et al. Nanoscale Dysregulation of Collagen Structure-Function Disrupts Mechano-Homeostasis and Mediates Pulmonary Fibrosis. Elife 2018, 7, e36354. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef]
- Broder, C.; Arnold, P.; Vadon-Le Goff, S.; Konerding, M.A.; Bahr, K.; Müller, S.; Overall, C.M.; Bond, J.S.; Koudelka, T.; Tholey, A.; et al. Metalloproteases Meprin α and Meprin β Are C- and N-Procollagen Proteinases Important for Collagen Assembly and Tensile Strength. Proc. Natl. Acad. Sci. USA 2013, 110, 14219–14224. [Google Scholar] [CrossRef] [PubMed]
- Tanase, D.M.; Valasciuc, E.; Anton, I.-B.; Gosav, E.M.; Dima, N.; Cucu, A.I.; Costea, C.F.; Floria, D.E.; Hurjui, L.L.; Tarniceriu, C.C.; et al. Matrix Metalloproteinases: Pathophysiologic Implications and Potential Therapeutic Targets in Cardiovascular Disease. Biomolecules 2025, 15, 598. [Google Scholar] [CrossRef] [PubMed]
- Williamson, R.A.; Marston, F.A.; Angal, S.; Koklitis, P.; Panico, M.; Morris, H.R.; Carne, A.F.; Smith, B.J.; Harris, T.J.; Freedman, R.B. Disulphide Bond Assignment in Human Tissue Inhibitor of Metalloproteinases (TIMP). Biochem. J. 1990, 268, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Bjørnstad, J.L.; Neverdal, N.O.; Vengen, O.A.; Knudsen, C.W.; Husebye, T.; Pepper, J.; Lie, M.; Christensen, G.; Tønnessen, T. Alterations in Circulating Activin A, GDF-15, TGF-Beta3 and MMP-2, -3, and -9 during One Year of Left Ventricular Reverse Remodelling in Patients Operated for Severe Aortic Stenosis. Eur. J. Heart Fail. 2008, 10, 1201–1207. [Google Scholar] [CrossRef]
- Onal, I.K.; Altun, B.; Onal, E.D.; Kirkpantur, A.; Gul Oz, S.; Turgan, C. Serum Levels of MMP-9 and TIMP-1 in Primary Hypertension and Effect of Antihypertensive Treatment. Eur. J. Intern. Med. 2009, 20, 369–372. [Google Scholar] [CrossRef]
- de Castro Brás, L.E.; Frangogiannis, N.G. Extracellular Matrix-Derived Peptides in Tissue Remodeling and Fibrosis. Matrix Biol. 2020, 91–92, 176–187. [Google Scholar] [CrossRef]
- Barallobre-Barreiro, J.; Loeys, B.; Mayr, M.; Rienks, M.; Verstraeten, A.; Kovacic, J.C. Extracellular Matrix in Vascular Disease, Part 2/4: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 2189–2203. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Bennett, M.R. Vascular Smooth Muscle Cells in Atherosclerosis: Time for a Re-Assessment. Cardiovasc. Res. 2021, 117, 2326–2339. [Google Scholar] [CrossRef] [PubMed]
- De Bartolo, A.; Angelone, T.; Rocca, C. Elucidating Emerging Signaling Pathways Driving Endothelial Dysfunction in Cardiovascular Aging. Vasc. Pharmacol. 2025, 158, 107462. [Google Scholar] [CrossRef] [PubMed]
- Rizzoni, D.; Agabiti-Rosei, C.; De Ciuceis, C. State of the Art Review: Vascular Remodeling in Hypertension. Am. J. Hypertens. 2023, 36, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Gao, H.; He, C.; Xin, S.; Wang, B.; Zhang, S.; Gao, L.; Tao, Q.; Wu, W.; Sun, F.; et al. An Emerging View on Vascular Fibrosis Molecular Mediators and Relevant Disorders: From Bench to Bed. Front. Cardiovasc. Med. 2023, 10, 1273502. [Google Scholar] [CrossRef]
- Harvey, A.; Montezano, A.C.; Lopes, R.A.; Rios, F.; Touyz, R.M. Vascular Fibrosis in Aging and Hypertension: Molecular Mechanisms and Clinical Implications. Can. J. Cardiol. 2016, 32, 659–668. [Google Scholar] [CrossRef]
- Totoń-Żurańska, J.; Mikolajczyk, T.P.; Saju, B.; Guzik, T.J. Vascular Remodelling in Cardiovascular Diseases: Hypertension, Oxidation, and Inflammation. Clin. Sci. 2024, 138, 817–850. [Google Scholar] [CrossRef]
- Sharma, N.; Khatib, M.N.; Roopashree, R.; Kaur, M.; Srivastava, M.; Barwal, A.; Siva Prasad, G.V.; Rajput, P.; Syed, R.; Kundra, K.; et al. Association between Vascular Endothelial Growth Factor and Atrial Fibrillation: A Systematic Review. BMC Cardiovasc. Disord. 2025, 25, 5. [Google Scholar] [CrossRef]
- Babapoor-Farrokhran, S.; Gill, D.; Alzubi, J.; Mainigi, S.K. Atrial Fibrillation: The Role of Hypoxia-Inducible Factor-1-Regulated Cytokines. Mol. Cell. Biochem. 2021, 476, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Xue, L.; Chen, C.; Xie, J.; Kong, F.; Zhang, F. Causal Effect of Vascular Endothelial Growth Factor on the Risk of Atrial Fibrillation: A Two-Sample Mendelian Randomization Study. Front. Cardiovasc. Med. 2024, 11, 1416412. [Google Scholar] [CrossRef] [PubMed]
- Berntsson, J.; Smith, J.G.; Johnson, L.S.B.; Söderholm, M.; Borné, Y.; Melander, O.; Orho-Melander, M.; Nilsson, J.; Engström, G. Increased Vascular Endothelial Growth Factor D Is Associated with Atrial Fibrillation and Ischaemic Stroke. Heart 2019, 105, 553–558. [Google Scholar] [CrossRef] [PubMed]
- Freestone, B.; Chong, A.Y.; Lim, H.S.; Blann, A.; Lip, G.Y.H. Angiogenic Factors in Atrial Fibrillation: A Possible Role in Thrombogenesis? Ann. Med. 2005, 37, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Corban, M.T.; Toya, T.; Ahmad, A.; Lerman, L.O.; Lee, H.-C.; Lerman, A. Atrial Fibrillation and Endothelial Dysfunction: A Potential Link? Mayo Clin. Proc. 2021, 96, 1609–1621. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation during the Life Cycle of the Atherosclerotic Plaque. Cardiovasc. Res. 2021, 117, 2525–2536. [Google Scholar] [CrossRef] [PubMed]
- Pandit, R.; Yurdagul, A. The Atherosclerotic Plaque Microenvironment as a Therapeutic Target. Curr. Atheroscler. Rep. 2025, 27, 47. [Google Scholar] [CrossRef]
- Bu, L.-L.; Yuan, H.-H.; Xie, L.-L.; Guo, M.-H.; Liao, D.-F.; Zheng, X.-L. New Dawn for Atherosclerosis: Vascular Endothelial Cell Senescence and Death. Int. J. Mol. Sci. 2023, 24, 15160. [Google Scholar] [CrossRef]
- van Veelen, A.; van der Sangen, N.M.R.; Delewi, R.; Beijk, M.A.M.; Henriques, J.P.S.; Claessen, B.E.P.M. Detection of Vulnerable Coronary Plaques Using Invasive and Non-Invasive Imaging Modalities. J. Clin. Med. 2022, 11, 1361. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, P. Novel Ultrasound Techniques in the Identification of Vulnerable Plaques—An Updated Review of the Literature. Front. Cardiovasc. Med. 2023, 10, 1069745. [Google Scholar] [CrossRef]
- Rao, W.; Li, D.; Zhang, Q.; Liu, T.; Gu, Z.; Huang, L.; Dai, J.; Wang, J.; Hou, X. Complex Regulation of Cardiac Fibrosis: Insights from Immune Cells and Signaling Pathways. J. Transl. Med. 2025, 23, 242. [Google Scholar] [CrossRef]
- Lanzer, J.D.; Wienecke, L.M.; Ramirez Flores, R.O.; Zylla, M.M.; Kley, C.; Hartmann, N.; Sicklinger, F.; Schultz, J.-H.; Frey, N.; Saez-Rodriguez, J.; et al. Single-Cell Transcriptomics Reveal Distinctive Patterns of Fibroblast Activation in Heart Failure with Preserved Ejection Fraction. Basic. Res. Cardiol. 2024, 119, 1001–1028. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Das, M.; Ghosh, A.; Rana, S. Molecular and Cellular Pathophysiology of Circulating Cardiomyocyte-Specific Cell Free DNA (cfDNA): Biomarkers of Heart Failure and Potential Therapeutic Targets. Genes. Dis. 2023, 10, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Harrington, A.; Moore-Morris, T. Cardiac Fibroblasts in Heart Failure and Regeneration. Front. Cell Dev. Biol. 2024, 12, 1388378. [Google Scholar] [CrossRef] [PubMed]
- Kararigas, G.; Fliegner, D.; Forler, S.; Klein, O.; Schubert, C.; Gustafsson, J.-Å.; Klose, J.; Regitz-Zagrosek, V. Comparative Proteomic Analysis Reveals Sex and Estrogen Receptor β Effects in the Pressure Overloaded Heart. J. Proteome Res. 2014, 13, 5829–5836. [Google Scholar] [CrossRef] [PubMed]
- Appiah, D.; Schreiner, P.J.; Demerath, E.W.; Loehr, L.R.; Chang, P.P.; Folsom, A.R. Association of Age at Menopause With Incident Heart Failure: A Prospective Cohort Study and Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e003769. [Google Scholar] [CrossRef] [PubMed]
- Yusifov, A.; Woulfe, K.C.; Bruns, D.R. Mechanisms and Implications of Sex Differences in Cardiac Aging. J. Cardiovasc. Aging 2022, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Papamitsou, T.; Barlagiannis, D.; Papaliagkas, V.; Kotanidou, E.; Dermentzopoulou-Theodoridou, M. Testosterone-Induced Hypertrophy, Fibrosis and Apoptosis of Cardiac Cells—An Ultrastructural and Immunohistochemical Study. Med. Sci. Monit. 2011, 17, BR266–BR273. [Google Scholar] [CrossRef]
- Lu, Y.; Kiechl, S.J.; Wang, J.; Xu, Q.; Kiechl, S.; Pechlaner, R. Global Pulse Wave Velocity Study Group Global Distributions of Age- and Sex-Related Arterial Stiffness: Systematic Review and Meta-Analysis of 167 Studies with 509,743 Participants. EBioMedicine 2023, 92, 104619. [Google Scholar] [CrossRef]
- Ruperez, C.; Madeo, F.; de Cabo, R.; Kroemer, G.; Abdellatif, M. Obesity Accelerates Cardiovascular Ageing. Eur. Heart J. 2025, 46, 2161–2185. [Google Scholar] [CrossRef]
- Zhang, Q.; Lu, C.; Lu, F.; Liao, Y.; Cai, J.; Gao, J. Challenges and Opportunities in Obesity: The Role of Adipocytes during Tissue Fibrosis. Front. Endocrinol. 2024, 15, 1365156. [Google Scholar] [CrossRef]
- Zhang, Z.; He, X.; Sun, Y.; Li, J.; Sun, J. Type 2 Diabetes Mellitus: A Metabolic Model of Accelerated Aging—Multi-Organ Mechanisms and Intervention Approaches. Aging Dis. 2025. [Google Scholar] [CrossRef]
- Muniyappa, R.; Sowers, J.R. Role of Insulin Resistance in Endothelial Dysfunction. Rev. Endocr. Metab. Disord. 2013, 14, 5–12. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic Effects of Statins on the Cardiovascular System. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.H.; Genest, J.; Gotto, A.M.; Kastelein, J.J.P.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; et al. Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Qiu, Y.; Wu, L.; Wang, H.; Wu, L.; Zhao, L.; Xie, D. Statins Have an Anti-Inflammation in CKD Patients: A Meta-Analysis of Randomized Trials. Biomed. Res. Int. 2022, 2022, 4842699. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, M.; Amiri, S.; Pecic, S.; Machaj, F.; Rosik, J.; Łos, M.J.; Alizadeh, J.; Mahdian, R.; da Silva Rosa, S.C.; Schaafsma, D.; et al. Pleiotropic Effects of Statins: A Focus on Cancer. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165968. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The Role of Nrf2 in Cardiovascular Function and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Gao, L.; Zimmerman, M.C.; Zucker, I.H. Myocardial Infarction-Induced microRNA-Enriched Exosomes Contribute to Cardiac Nrf2 Dysregulation in Chronic Heart Failure. Am. J. Physiol.-Heart Circ. Physiol. 2018, 314, H928–H939. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, W.; Du, B.; Miao, X.; Bai, Y.; Xin, Y.; Tan, Y.; Cui, W.; Liu, B.; Cui, T.; et al. Therapeutic Effect of MG-132 on Diabetic Cardiomyopathy Is Associated with Its Suppression of Proteasomal Activities: Roles of Nrf2 and NF-κB. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H567–H578. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, S.A.; Shanley, L.C.; Dunne, A. The Nrf2-HO-1 System and Inflammaging. Front. Immunol. 2024, 15, 1457010. [Google Scholar] [CrossRef]
- Ridker, P.M.; Libby, P.; MacFadyen, J.G.; Thuren, T.; Ballantyne, C.; Fonseca, F.; Koenig, W.; Shimokawa, H.; Everett, B.M.; Glynn, R.J. Modulation of the Interleukin-6 Signalling Pathway and Incidence Rates of Atherosclerotic Events and All-Cause Mortality: Analyses from the Canakinumab Anti-Inflammatory Thrombosis Outcomes Study (CANTOS). Eur. Heart J. 2018, 39, 3499–3507. [Google Scholar] [CrossRef] [PubMed]
- Georgakis, M.K.; Malik, R.; Li, X.; Gill, D.; Levin, M.G.; Vy, H.M.T.; Judy, R.; Ritchie, M.; Verma, S.S.; Regeneron Genetics Center; et al. Genetically Downregulated Interleukin-6 Signaling Is Associated With a Favorable Cardiometabolic Profile. Circulation 2021, 143, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Senolytic Drugs: From Discovery to Translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef] [PubMed]
- Lizotte-Waniewski, M.; Brew, K.; Hennekens, C.H. Hypothesis: Metalloproteinase Inhibitors Decrease Risks of Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 368–371. [Google Scholar] [CrossRef]
- Yaras, N.; Sariahmetoglu, M.; Bilginoglu, A.; Aydemir-Koksoy, A.; Onay-Besikci, A.; Turan, B.; Schulz, R. Protective Action of Doxycycline against Diabetic Cardiomyopathy in Rats. Br. J. Pharmacol. 2008, 155, 1174–1184. [Google Scholar] [CrossRef]
- Hudson, M.P.; Armstrong, P.W.; Ruzyllo, W.; Brum, J.; Cusmano, L.; Krzeski, P.; Lyon, R.; Quinones, M.; Theroux, P.; Sydlowski, D.; et al. Effects of Selective Matrix Metalloproteinase Inhibitor (PG-116800) to Prevent Ventricular Remodeling after Myocardial Infarction: Results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) Trial. J. Am. Coll. Cardiol. 2006, 48, 15–20. [Google Scholar] [CrossRef]
- Tayebjee, M.H.; Nadar, S.; Blann, A.D.; Beevers, D.G.; MacFadyen, R.J.; Lip, G.Y.H. Matrix Metalloproteinase-9 and Tissue Inhibitor of Metalloproteinase-1 in Hypertension and Their Relationship to Cardiovascular Risk and Treatment*: A Substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Am. J. Hypertens. 2004, 17, 764–769. [Google Scholar] [CrossRef]
- Kazemi, A.; Fathy, M.; Jahanian, A.; Khanali, J.; Ostadi, Y.; Babajani, A.; Tayebi, T.; Niknejad, H. The Role of MMPs and TIMPs in Regenerative Medicine: From Pathological ECM Remodeling to Therapeutic Applications. Biomed. Pharmacother. 2025, 191, 118457. [Google Scholar] [CrossRef]
- Liu, Q.; Schwartz, J.B.; Slattum, P.W.; Lau, S.W.J.; Guinn, D.; Madabushi, R.; Burckart, G.; Califf, R.; Cerreta, F.; Cho, C.; et al. Roadmap to 2030 for Drug Evaluation in Older Adults. Clin. Pharmacol. Ther. 2022, 112, 210–223. [Google Scholar] [CrossRef]
- Dzau, V.J.; Hodgkinson, C.P. RNA Therapeutics for the Cardiovascular System. Circulation 2024, 149, 707–716. [Google Scholar] [CrossRef]
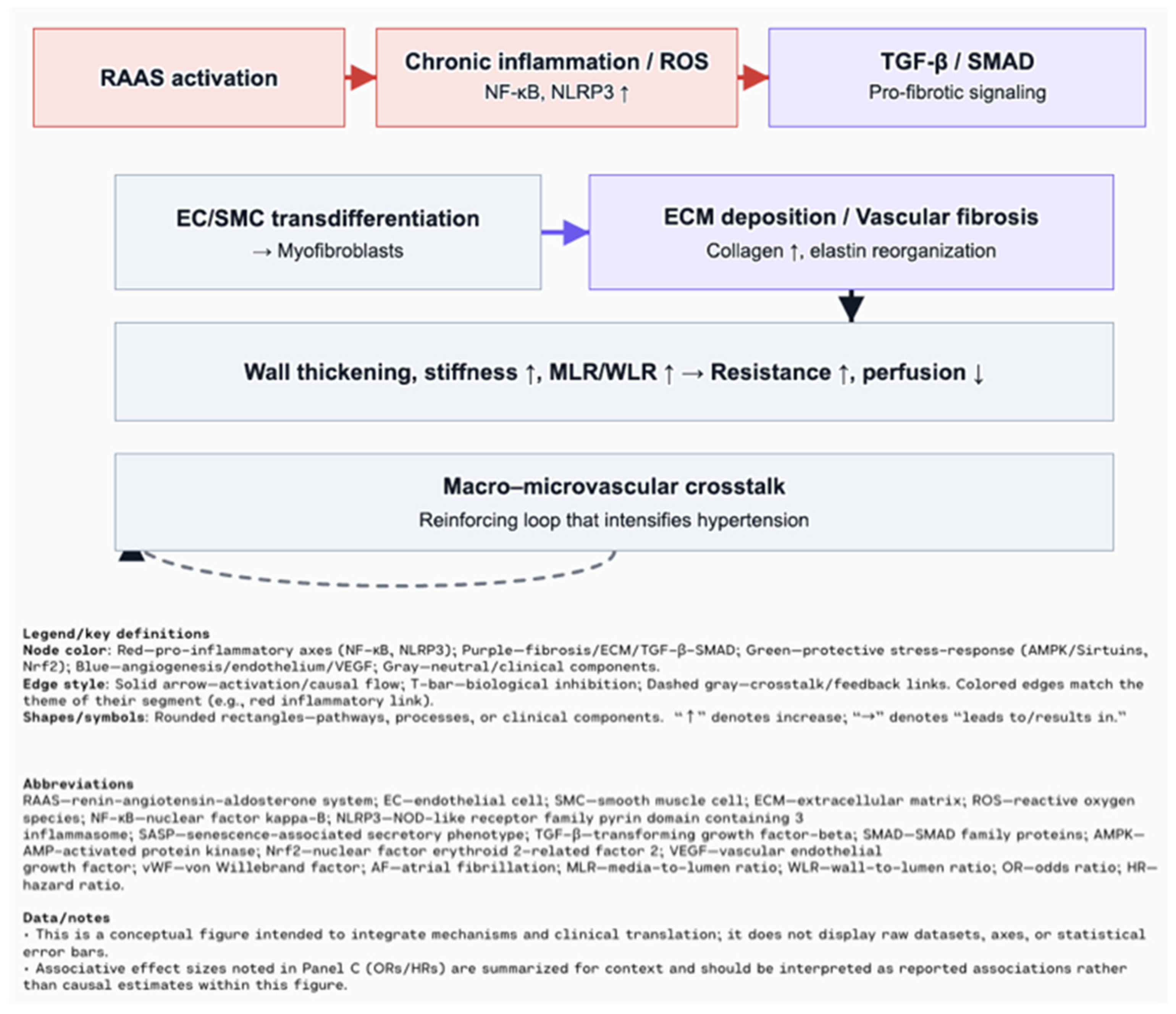
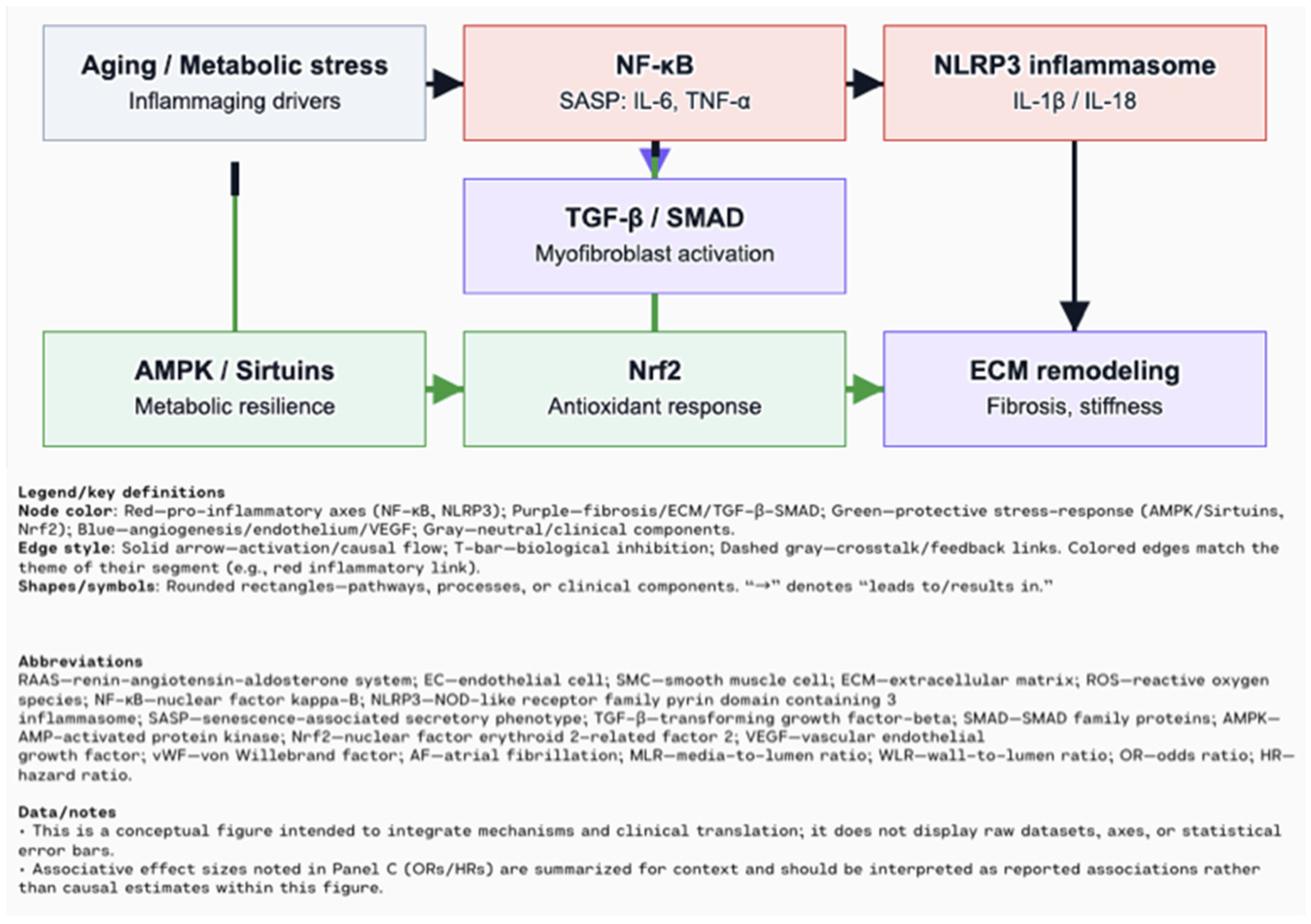
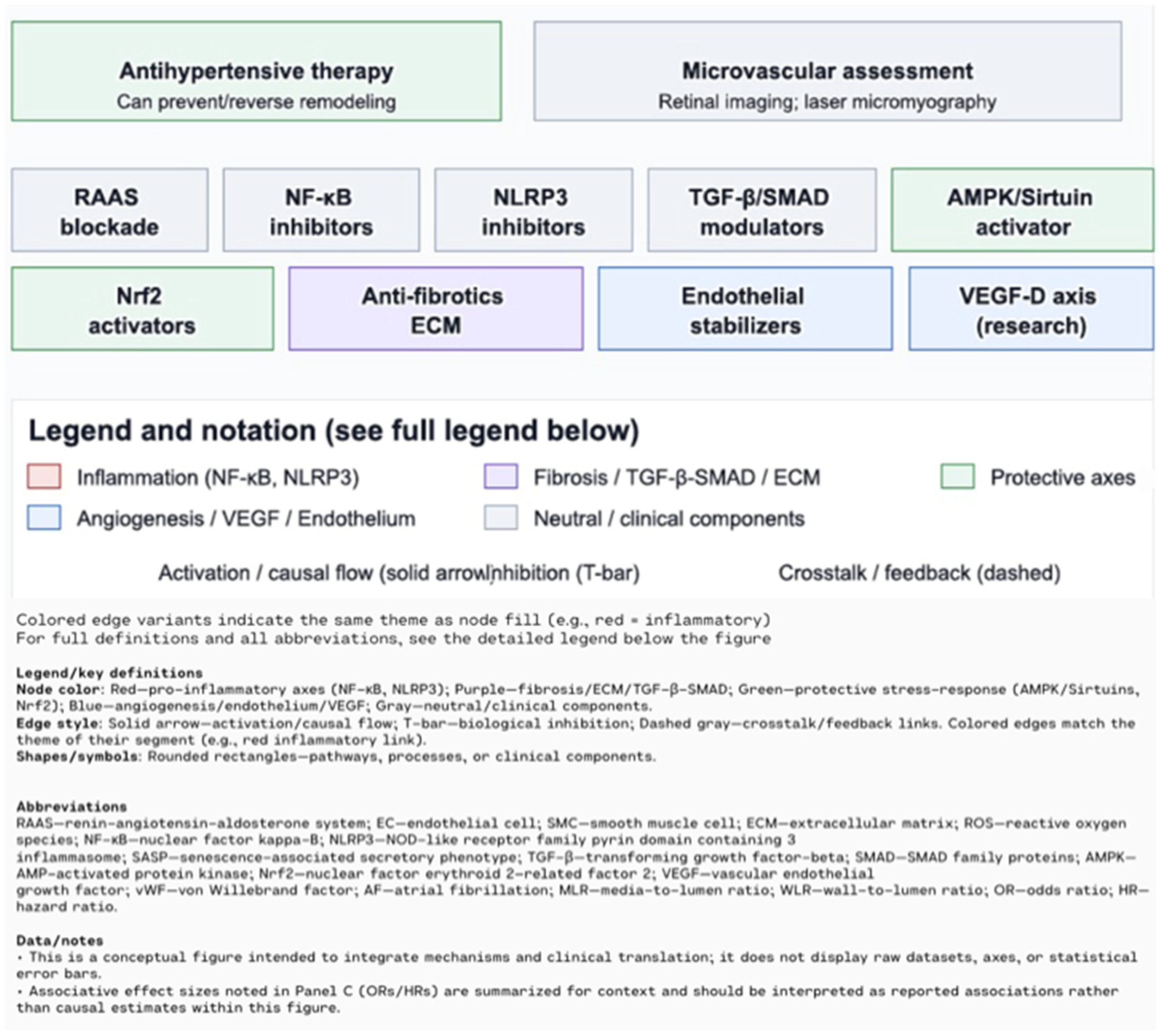
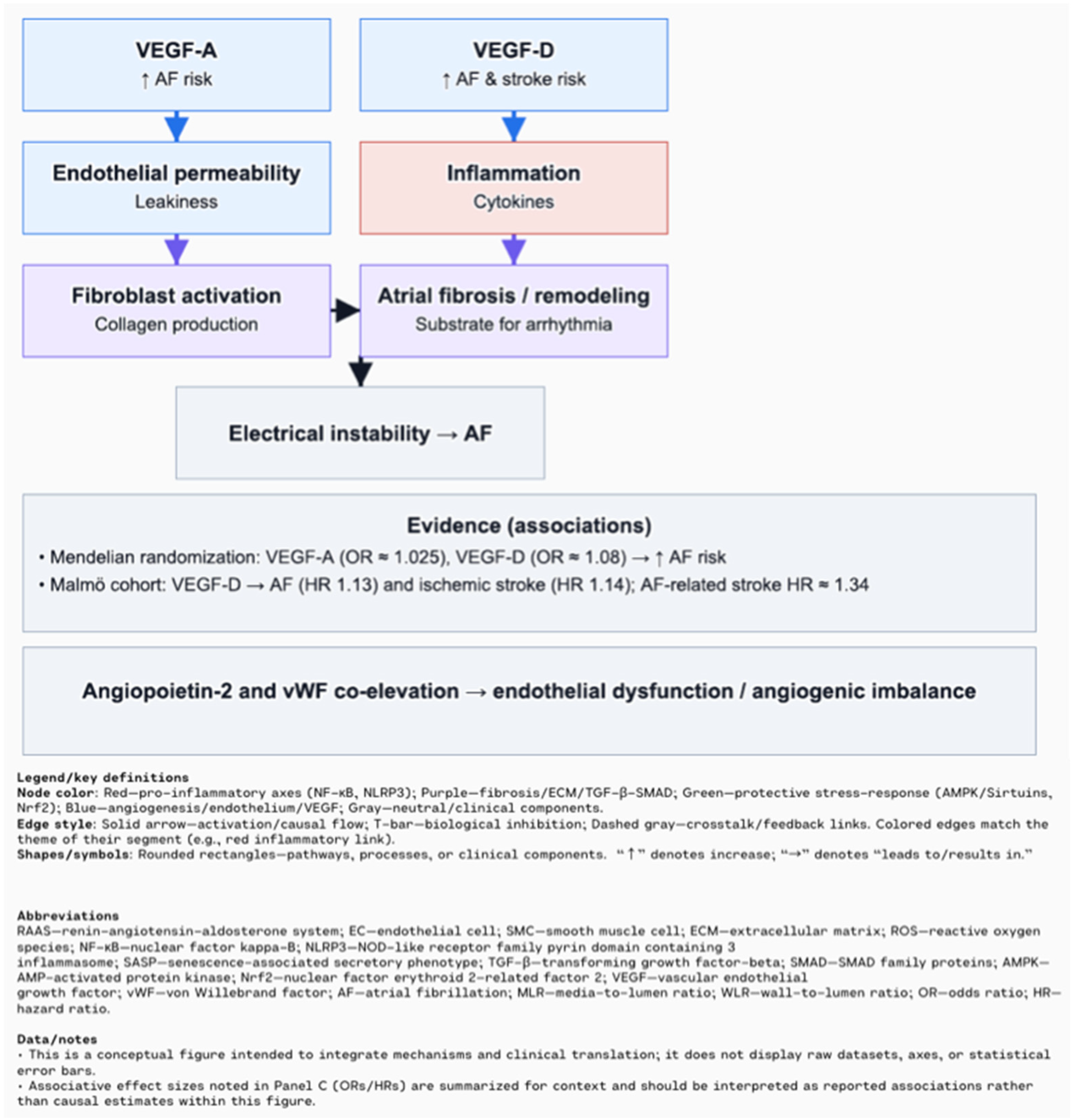
| Hallmark | Meaning |
|---|---|
| Genomic instability | Accumulation of DNA damage and mutations with age, increasing risk of cancer and neurological diseases |
| Telomere attrition | Telomere shortening during cell division, leading to cellular senescence |
| Epigenetic alterations | Age-related changes in gene expression not involving DNA sequence, contributing to disease |
| Loss of proteostasis | Decline in the cell’s ability to fold and clear proteins, promoting aging |
| Dysregulated nutrient sensing | Impaired response to nutrient levels, linked to metabolic disorders (e.g., diabetes) |
| Mitochondrial dysfunction | Reduced mitochondrial efficiency, causing energy loss and oxidative stress |
| Cellular senescence | Permanent cell cycle arrest, driving aging processes |
| Stem cell exhaustion | Decrease in stem cell number and function, limiting tissue repair |
| Altered intercellular communication | Disruption of hormonal and cellular signaling, affecting tissue function |
| Compromised autophagy | Impaired clearance of damaged proteins, linked to neurodegeneration and immune aging |
| Microbiome disturbances | Loss of gut microbial diversity, contributing to inflammation |
| Splicing dysregulation | Misregulation of RNA splicing, fostering cellular aging |
| Chronic low-level inflammation (inflammaging) | Persistent mild inflammation, increasing disease risk in the elderly |
| Mechanical properties alterations | Stiffening and cross-linking of tissues (e.g., collagen), leading to conditions like hypertension |
| General features | SENESCENCE-ASSOCIATED SECRETORY PHENOTYPE (SASP) is a heterogeneous collection of secreted factors produced by senescent cells: · pro-inflammatory cytokines: IL-1α/β, IL-6, TNF-α, IL-8 · fibrotic and pro-hypertrophic factors: TGF-β, activin A, GDF15, CCN1 · chemokines: CCL2/MCP-1, CXCL1, CXCL8, attracting monocytes, neutrophils, T and B cells · damage-associated molecular patterns (DAMPs) · proteolytic enzymes: MMPs, elastases, cathepsins · hemostatic and vasoactive mediators: PAI-1, PAI-2, prostanoids, bradykinins, endothelin-1 | ||||
| Cell- type specific aspects | Senescent cardiomyocytes | Senescent fibroblast | Senescent endothelial cells | Senescent VSMCs | Immune cells |
| IL-1, IL-6, TNF-α, TGF-β, GDF15 | IL-1, IL-6, TNF-α, TGF-β, CCN1. | Cytokines + reduced angiogenic factors. | IL-1β, IL-6, TNF-α, MMPs. | Macrophages, T cells neutrophils, mast cells, NK cells. | |
| DDR activation, ER stress, mitochondrial dysfunction, elevated ROS levels contractile abnormalities, epigenetic dysregulation (miRNAs), promotes fibroblast activation: inflammation, fibrosis, hypertrophy. | Central role in ECM remodeling; senescent by p53/p-21, drive paracrine hypertrophy, fibrosis. | NO, EDHF, mitochondrial dysfunction, RAAS activation, oxidative stress, impaired angiogenesis, promote vascular inflammation. | Telomere attrition, DNA damage, oxidative stress, defective autophagy; induce immune recruitment, matrix degradation, osteogenic shift: vascular stiffening, calcification. | Respond to SASP signals, further shaping inflammation, senescence propagation, or clearance. | |
| Functional roles in CVS | Acute → immune cell recruitment, clearance of senescent cells, wound healing, regeneration. Chronic → Persistent inflammation, impaired stem cell function, fibrosis, hypertrophy, ECM degradation, vascular dysfunction and progression of age-related cardiac diseases: HFpEF, atherosclerosis, atrial fibrillation, diabetic cardiomyopathy. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młynarska, E.; Kowalik, A.; Krajewska, A.; Krupińska, N.; Marcinkowska, W.; Motor, J.; Przybylak, A.; Tłustochowicz, K.; Rysz, J.; Franczyk, B. Inflammaging and Senescence-Driven Extracellular Matrix Remodeling in Age-Associated Cardiovascular Disease. Biomolecules 2025, 15, 1452. https://doi.org/10.3390/biom15101452
Młynarska E, Kowalik A, Krajewska A, Krupińska N, Marcinkowska W, Motor J, Przybylak A, Tłustochowicz K, Rysz J, Franczyk B. Inflammaging and Senescence-Driven Extracellular Matrix Remodeling in Age-Associated Cardiovascular Disease. Biomolecules. 2025; 15(10):1452. https://doi.org/10.3390/biom15101452
Chicago/Turabian StyleMłynarska, Ewelina, Adrianna Kowalik, Agnieszka Krajewska, Natalia Krupińska, Weronika Marcinkowska, Jakub Motor, Aleksandra Przybylak, Katarzyna Tłustochowicz, Jacek Rysz, and Beata Franczyk. 2025. "Inflammaging and Senescence-Driven Extracellular Matrix Remodeling in Age-Associated Cardiovascular Disease" Biomolecules 15, no. 10: 1452. https://doi.org/10.3390/biom15101452
APA StyleMłynarska, E., Kowalik, A., Krajewska, A., Krupińska, N., Marcinkowska, W., Motor, J., Przybylak, A., Tłustochowicz, K., Rysz, J., & Franczyk, B. (2025). Inflammaging and Senescence-Driven Extracellular Matrix Remodeling in Age-Associated Cardiovascular Disease. Biomolecules, 15(10), 1452. https://doi.org/10.3390/biom15101452









