Biodegradable Flame Retardants for Biodegradable Polymer
Abstract
1. Introduction
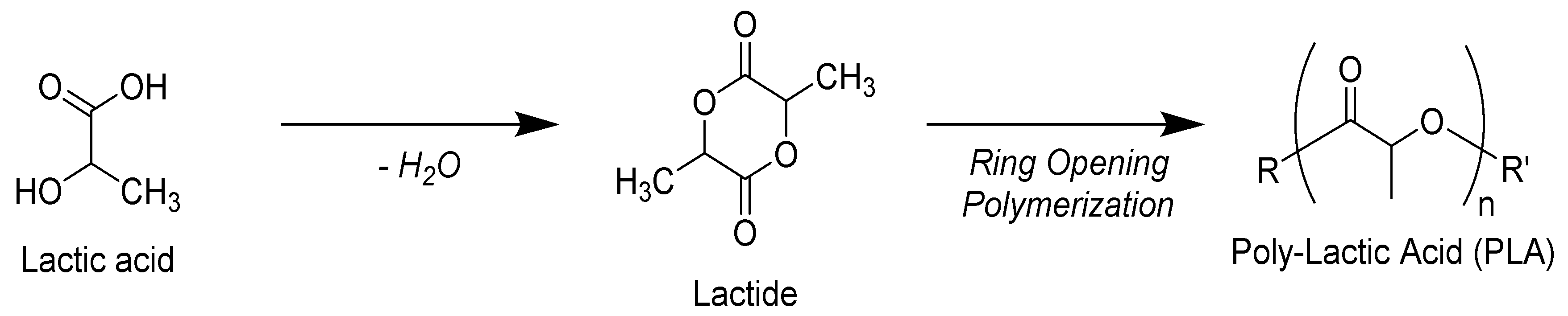
2. Polylactic Acid (PLA)
3. Flame Retardants
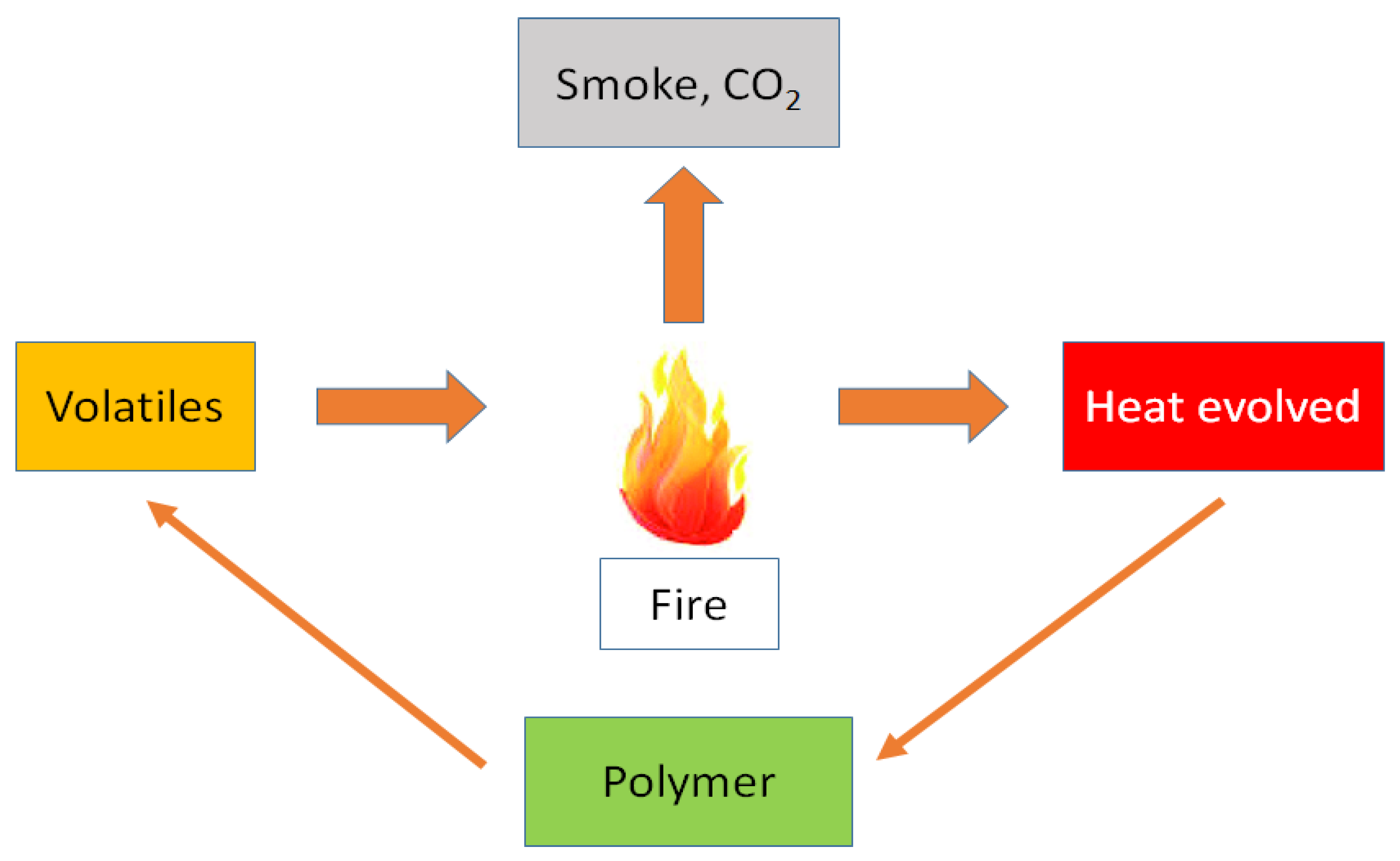
4. Action Mechanism of Flame Retardants
4.1. Dilution of Volatile Products
4.2. Inhibition of Vapor Phase Combustion
4.3. Removing Heat of Combustion
4.4. Smoke Suppressants
4.5. Char Formation
5. Intumescent Flame Retardants
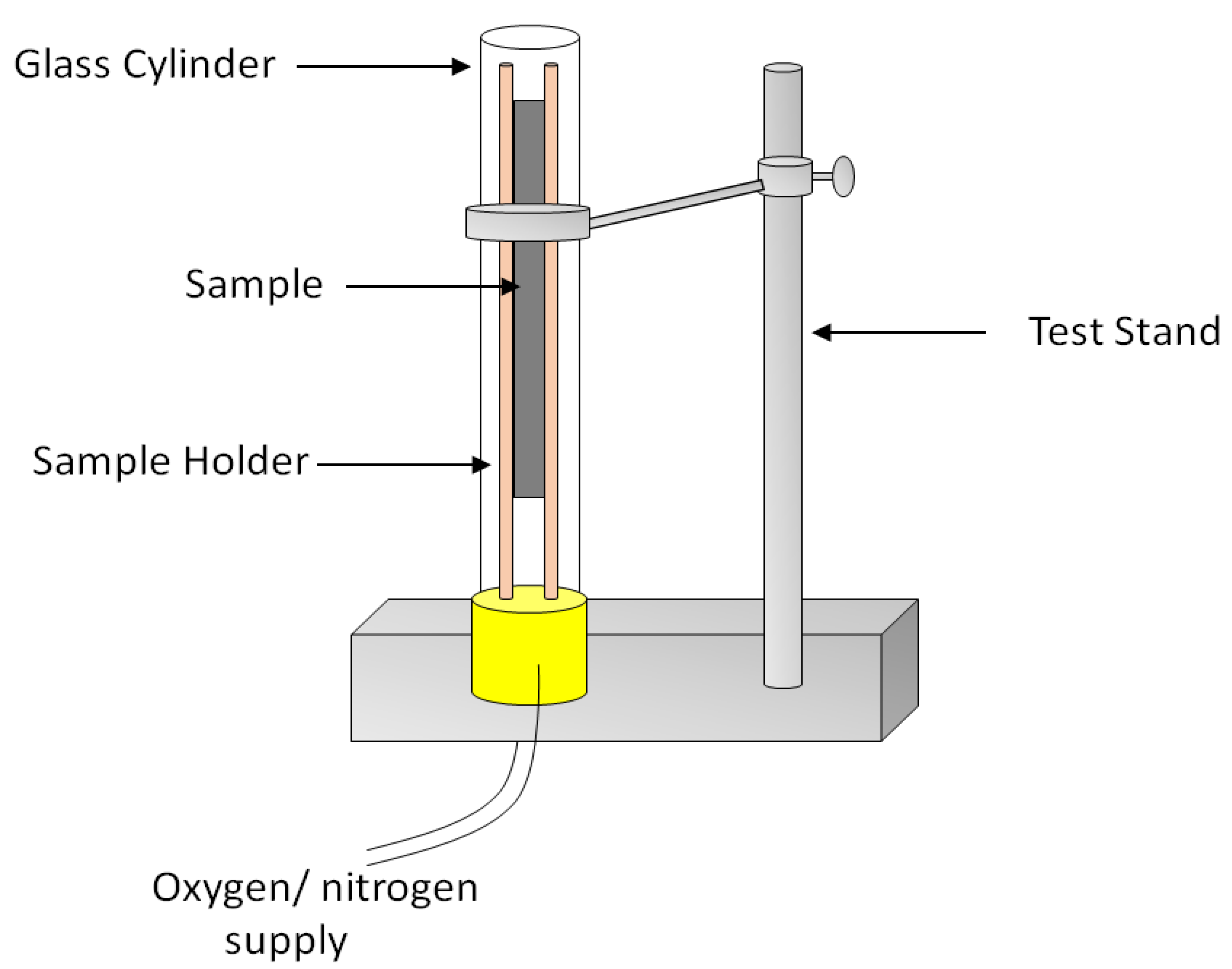
6. Fire Testing Methods
7. Potential Bio-Based and Biodegradable Carbonization Agents
7.1. Cellulose
7.2. Starch
7.3. Chitosan
7.4. Alginates
7.5. Lignin
8. Approaches Used to Make PLA Flame-Retardant: State of the Art
9. Prospects of Bio-Based Flame Retardants for the Industrial Scale-Up
9.1. Health and Environmental Impact
9.2. Fire Performance
9.3. Economic Efficiency
10. Future Work
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, Y.; Mo, X.; Pang, J.; Yang, F. Effects of silica on the morphology, structure, and properties of thermoplastic cassava starch/poly(vinyl alcohol) blends. J. Appl. Polym. Sci. 2016, 44020, 1–9. [Google Scholar] [CrossRef]
- Vothi, H.; Nguyen, C.; Lee, K.; Kim, J. Thermal stability and flame retardancy of novel phloroglucinol based organo phosphorus compound. Polym. Degrad. Stab. 2010, 95, 1092–1098. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Ruan, F.; Liu, J.; Li, N.; Li, X. Effects of a Macromolecule Spirocyclic Inflatable Flame Retardant on the Thermal and Flame Retardant Properties of Epoxy Resin. Polymers 2020, 12, 132. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, W.; Meng, W.; Xie, W.; Cui, Y.; Xu, J. Core-Shell Graphitic Carbon Nitride/Zinc Phytate as a Novel Efficient Flame Retardant for Fire Safety and Smoke Suppression in Epoxy Resin. Polymers 2020, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ji, Q.; Shen, X.; Xia, Y.; Tan, L.; Kong, Q. Pyrolysis products and thermal degradation mechanism of intrinsically flame-retardant calcium alginate fibre. Polym. Degrad. Stab. 2011, 96, 936–942. [Google Scholar] [CrossRef]
- Menard, R.; Negrell, C.; Ferry, L.; Sonnier, R.; David, G. Synthesis of biobased phosphorus-containing flame retardants for epoxy thermosets comparison of additive and reactive approaches. Polym. Degrad. Stab. 2015, 120, 300–312. [Google Scholar] [CrossRef]
- Menard, R.; Negrell, C.; Fache, M.; Ferry, L.; Sonnier, R.; David, G. From a bio-based phosphorus-containing epoxy monomer to fully bio-based flame-retardant thermosets. RSC Adv. 2015, 5, 70856–70867. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Zhu, P.; Zhao, J.; Zhang, C.; Guo, Y.; Cui, L. Thermal degradation properties of biobased iron alginate film. J. Anal. Appl. Pyrolysis 2016, 1–10. [Google Scholar] [CrossRef]
- Laachachi, A.; Cochez, M.; Leroy, E.; Gaudon, P.; Ferriol, M.; Cuesta, J.M.L. Effect of Al2O3 and TiO2 nanoparticles and APP on thermal stability and flame retardance of PMMA. Polym. Adv. Technol. 2006, 17, 327–334. [Google Scholar] [CrossRef]
- Bourbigot, S.; Fontaine, G. Flame retardancy of polylactide: An overview. Polym. Chem. 2010, 1, 1413–1422. [Google Scholar] [CrossRef]
- Murariu, M.; Bonnaud, L.; Yoann, P.; Fontaine, G.; Bourbigot, S.; Dubois, P. New trends in polylactide (PLA)-based materials: “Green” PLA-Calcium sulfate (nano)composites tailored with flame retardant properties. Polym. Degrad. Stab. 2010, 95, 374–381. [Google Scholar] [CrossRef]
- Chapple, S.; Anandjiwala, R.; Ray, S.S. Mechanical, thermal, and fire properties of polylactide/starch blend/clay composites. J. Therm. Anal. Calorim. 2013, 113, 703–712. [Google Scholar] [CrossRef]
- Gui, H.; Xu, P.; Hu, Y.; Wang, J.; Yang, X.; Bahader, A.; Ding, Y. Synergistic effect of graphene and an ionic liquid containing phosphonium on the thermal stability and flame retardancy of polylactide. RSC Adv. 2015, 5, 27814–27822. [Google Scholar] [CrossRef]
- Zhou, R.; Ming, Z.; He, J.; Ding, Y.; Jiang, J. Effect of Magnesium Hydroxide and Aluminum Hydroxide on the Thermal Stability, Latent Heat and Flammability Properties of Paraffin/ HDPE Phase. Polymers 2020, 12, 180. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.N.; Rigout, M.; Yeates, S.G.; Carr, C. Surface chemical analysis of the effect of curing conditions on the properties of thermally-cured pigment printed poly (lactic acid) fabrics. Dye. Pigment. 2014, 103, 168–174. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, B.; Shi, Y.; Mao, L.; Xie, J.; Pan, H.; Liu, Y. Insight into Hyper-Branched Aluminum Phosphonate in Combination with Multiple Phosphorus Synergies for Fire-Safe Epoxy Resin Composites. Polymers 2020, 12, 64. [Google Scholar] [CrossRef]
- Suardana, N.; Kyoo, M. Effects of diammonium phosphate on the flammability and mechanical properties of bio-composites. Mater. Des. 2011, 32, 1990–1999. [Google Scholar] [CrossRef]
- Teoh, E.L.; Mariatti, M.; Chow, W.S. Thermal and Flame Resistant Properties of Poly (Lactic Acid)/Poly (Methyl Methacrylate) Blends Containing Halogen-free Flame Retardant. Procedia Chem. 2016, 19, 795–802. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, G.; Zhang, Y.; Wu, L.; Jia, Y.; Tan, Y.; Liu, J. Enhancement of Flame Retardancy of Colorless and Transparent Semi-Alicyclic Polyimide Film from Hydrogenated-BPDA and 4,4′-oxydianiline via the Incorporation of Phosphazene Oligomer. Polymers 2020, 12, 90. [Google Scholar]
- Huang, R.; Guo, X.; Ma, S.; Xie, J.; Xu, J.; Ma, J. Novel Phosphorus-Nitrogen-Containing Ionic Liquid Modified Metal-Organic Framework as an Effective Flame Retardant for Epoxy Resin. Polymers 2020, 12, 108. [Google Scholar] [CrossRef]
- Lim, L.; Auras, R.; Rubino, M. Processing technologies for poly (lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Pla, P.; Raquez, J.; Habibi, Y.; Murariu, M.; Dubois, P. Polylactide (PLA) based nanocomposites. Prog. Polym. Sci. 2013, 38, 1504–1542. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Kim, S.H. Surface Alkylation of Cellulose Nanocrystals to Enhance Their Compatibility with Polylactide. Polymers 2020, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Praglowska, J.; Janus, L.; Piatkowski, M.; Bogdal, D.; Matysek, D. Hybrid Bilayer PLA/Chitosan Nanofibrous Scaffolds Doped with ZnO, Fe3O4, and Au Nanoparticles with Bioactive Properties for Skin Tissue Engineering. Polymers 2020, 12, 159. [Google Scholar]
- Nampoothiri, K.M.; Nair, N.R.; John, R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010, 101, 8493–8501. [Google Scholar] [CrossRef] [PubMed]
- Pilla, S.; Kramschuster, A. Microcellular processing of polylactide—Hyperbranched polyester—Nanoclay composites. J. Mater. Sci. 2010, 45, 2732–2746. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Anjum, N. Preparation of Poly (lactic acid) Fiber by Dry – Jet – Wet Spinning. II. Effect of Process Parameters on Fiber Properties. J. Appl. Polym. Sci. 2006, 101, 3774–3780. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Development of biobased socks from sustainable polymer and statistical modeling of their thermo-physiological properties. J. Clean. Prod. 2018, 197, 170–177. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly (lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Liu, H.; Xie, F.; Yu, L.; Chen, L.; Li, L. Thermal processing of starch-based polymers. Prog. Polym. Sci. 2009, 34, 1348–1368. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Statistical modeling of thermal properties of biobased compostable gloves developed from sustainable polymer. Fibers Polym. 2018, 19, 1094–1101. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modi fi cations: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N. Poly (lactic acid) fiber: An overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Wischke, C.; Schwendeman, S.P. Principles of encapsulating hydrophobic drugs in PLA/PLGA microparticles. Int. J. Pharm. 2008, 364, 298–327. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Geng, A.; Wu, Y.; Wang, L.; Fang, X.; Xu, L. Antibacterial, Flexible, and Conductive Membrane Based on MWCNTs/Ag Coated Electro-Spun PLA Nanofibrous Scaffolds as Wearable Fabric for Body Motion Sensing. Polymers 2020, 12, 120. [Google Scholar] [CrossRef]
- Alongi, J.; Andrea, R.; Bosco, F.; Carosio, F.; Di, A.; Cuttica, F.; Antonucci, V.; Giordano, M.; Malucelli, G. Caseins and hydrophobins as novel green flame retardants for cotton fabrics. Polym. Degrad. Stab. 2014, 99, 111–117. [Google Scholar] [CrossRef]
- Anastasakis, K.; Ross, A.B.; Jones, J.M. Pyrolysis behaviour of the main carbohydrates of brown macro-algae. Fuel 2011, 90, 598–607. [Google Scholar] [CrossRef]
- Dorez, G.; Ferry, L.; Sonnier, R.; Taguet, A. Effect of cellulose, hemicellulose and lignin contents on pyrolysis and combustion of natural fibers. J. Anal. Appl. Pyrolysis 2014, 107, 323–331. [Google Scholar] [CrossRef]
- De Chirico, A.; Armanini, M.; Chini, P.; Cioccolo, G. Flame retardants for polypropylene based on lignin. Polym. Degrad. Stab. 2003, 79, 139–145. [Google Scholar] [CrossRef]
- Carosio, F.; Fontaine, G.; Alongi, J.; Bourbigot, S. Starch-Based Layer by Layer Assembly: Efficient and Sustainable Approach to Cotton Fire Protection. Appl. Mater. Interfaces 2015. [Google Scholar] [CrossRef]
- Carosio, F.; Alongi, J.; Malucelli, G. Layer by Layer ammonium polyphosphate-based coatings for flame retardancy of polyester—Cotton blends. Carbohydr. Polym. 2012, 88, 1460–1469. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Cortemiglia, M. Overview of Fire Retardant Mechanisms. Polym. Degrad. Stab. 1991, 33, 131–154. [Google Scholar] [CrossRef]
- Alongi, J.; Cuttica, F.; Bourbigot, S.; Malucelli, G. Thermal and flame retardant properties of ethylene vinyl acetate copolymers containing deoxyribose nucleic acid or ammonium polyphosphate. J. Therm. Anal. Calorim. 2015. [Google Scholar] [CrossRef]
- Qian, W.; Li, X.; Wu, Z.; Liu, Y.; Fang, C.; Meng, W. Formulation of Intumescent Flame Retardant Coatings Containing Natural-Based Tea Saponin. J. Agric. Food Chem. 2015. [Google Scholar] [CrossRef] [PubMed]
- Tondi, G.; Wieland, S.; Wimmer, T. Tannin-boron preservatives for wood buildings: Mechanical and fire properties. Eur. J. Wood Prod. Prod. 2012, 70, 689–696. [Google Scholar] [CrossRef]
- Pan, Y.; Zhan, J.; Pan, H.; Wang, W.; Tang, G.; Song, L.; Hu, Y. Effect of Fully Biobased Coatings Constructed via Layer-by-Layer Assembly of Chitosan and Lignosulfonate on the Thermal, Flame Retardant, and Mechanical Properties of Flexible Polyurethane Foam. Sustain. Chem. Eng. 2015, 4–11. [Google Scholar] [CrossRef]
- Soares, J.P.; Santos, J.E.; Chierice, G.O.; Cavalheiro, E.T.G. Thermal behavior of alginic acid and its sodium salt. Eclet. Quim. 2004, 29, 53–56. [Google Scholar] [CrossRef]
- Mihaela, A.; Moldoveanu, C.; Odochian, L.; Maria, C.; Apostolescu, N.; Neculau, R. Study on the thermal behavior of casein under nitrogen and air atmosphere by means of the TG-FTIR technique. Thermochim. Acta 2012, 546, 120–126. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-cuesta, J.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Illy, N.; Fache, M.; Menard, R.; Negrell, C.; Caillol, S.; David, G. Phosphorylation of bio-based compounds: State of the art. Polym. Chem. 2015, 1–32. [Google Scholar] [CrossRef]
- Enescu, D.; Alongi, J.; Frache, A.; Chimica, I.; Torino, P.; Branch, A.; Michel, V.T. Evaluation of Nonconventional Additives as Fire Retardants on Polyamide 6,6: Phosphorous-Based Master Batch, a-Zirconium Dihydrogen Phosphate, and b-Cyclodextrin Based Nanosponges. J. Appl. Polym. Sci. 2011, 123, 3545–3555. [Google Scholar] [CrossRef]
- Carosio, F.; Laufer, G.; Alongi, J.; Camino, G.; Grunlan, J.C. Layer-by-layer assembly of silica-based flame retardant thin film on PET fabric. Polym. Degrad. Stab. 2011, 96, 745–750. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B.; Fichera, M.A.; Ja, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6, 6. Polym. Degrad. Stab. 2007, 92, 1528–1545. [Google Scholar] [CrossRef]
- Bocz, K.; Domonkos, M.; Igricz, T.; Kmetty, Á.; Bárány, T.; Marosi, G. Flame retarded self-reinforced poly (lactic acid) composites of outstanding impact resistance. Compos. PART A 2015, 70, 27–34. [Google Scholar] [CrossRef]
- Jimenez, M.; Guin, T.; Bellayer, S.; Dupretz, R.; Bourbigot, S.; Grunlan, J.C. Microintumescent mechanism of flame-retardant water-based chitosan–ammonium polyphosphate multilayer nanocoating on cotton fabric. J. Appl. Polym. Sci. 2016, 43783, 1–12. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Investigation of the Flammability and Thermal Stability of Halogen-Free Intumescent System in Biopolymer Composites Containing Biobased Carbonization Agent and Mechanism of Their Char Formation. Polymers 2018, 11, 48. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, Q.; Li, J.; Tao, K.; Xue, L.; Yan, Q. Synergistic effect between expandable graphite and ammonium polyphosphate on flame retarded polylactide. Polym. Degrad. Stab. 2011, 96, 183–189. [Google Scholar] [CrossRef]
- Kotresh, T.M.; Indushekar, R.; Subbulakshmi, M.S.; Vijayalakshmi, S.N.; Prasad, A.S.K.; Agrawal, A.K. Evaluation of commercial flame retardant polyester curtain fabrics in the cone calorimeter. J. Ind. Text. 2006, 36, 47–58. [Google Scholar] [CrossRef]
- Qian, Y.; Wei, P.; Jiang, P.; Li, Z.; Yan, Y.; Ji, K. Aluminated mesoporous silica as novel high-effective flame retardant in polylactide. Compos. Sci. Technol. 2013, 82, 1–7. [Google Scholar] [CrossRef]
- Wang, K.; Wang, J.; Zhao, D.; Zhai, W. Preparation of microcellular poly(lactic acid) composites foams with improved flame retardancy. J. Cell. Plast. 2017, 53, 45–63. [Google Scholar] [CrossRef]
- Depeng, L.; Chixiang, L.; Xiulei, J.; Tao, L.; Ling, Z. Synergistic effects of intumescent flame retardant and nano-CaCO 3 on foamability and flame-retardant property of polypropylene composites foams. J. Cell. Plast. 2017, 1–17. [Google Scholar] [CrossRef]
- Vahabi, H.; Ferry, L.; Longuet, C.; Otazaghine, B.; Negrell-guirao, C.; David, G.; Lopez-cuesta, J. Combination effect of polyhedral oligomeric silsesquioxane (POSS) and a phosphorus modified PMMA, flammability and thermal stability properties. Mater. Chem. Phys. 2012, 136, 762–770. [Google Scholar] [CrossRef]
- Zhan, J.; Song, L.; Nie, S.; Hu, Y. Combustion properties and thermal degradation behavior of polylactide with an effective intumescent flame retardant. Polym. Degrad. Stab. 2009, 94, 291–296. [Google Scholar] [CrossRef]
- Maqsood, M.; Langensiepen, F.; Seide, G. The Efficiency of Biobased Carbonization Agent and Intumescent Flame Retardant on Flame Retardancy of Biopolymer Composites and Investigation of their melt spinnability. Molecules 2019, 24, 1513. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, D.; Chen, L.; Wang, X.; Wang, Y. Inherent flame retardation of bio-based poly (lactic acid) by incorporating phosphorus linked pendent group into the backbone. Polym. Degrad. Stab. 2011, 96, 1669–1675. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.; Zhang, R.; Wang, B.; Hong, N.; Hu, Y.; Song, L.; Gong, X. Effect of rare earth hypophosphite salts on the fire performance of biobased polylactide composites. Ind. Eng. Chem. Res. 2013, 1–10. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.; Xing, W.; Zhang, P.; Wang, B.; Hong, N.; Yang, W.; Hu, Y.; Song, L. Thermal Degradation and Flame Retardance of Biobased Polylactide Composites Based on Aluminum Hypophosphite. Ind. Eng. Chem. Res. 2012, 51, 12009–12016. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N. Pyrolysis combustion flow calorimetry. J. Anal. Appl. Pyrolysis 2004, 71, 27–46. [Google Scholar] [CrossRef]
- Murariu, M.; Laure, A.; Bonnaud, L.; Paint, Y.; Gallos, A.; Fontaine, G.; Bourbigot, S.; Dubois, P. The production and properties of polylactide composites filled with expanded graphite. Polym. Degrad. Stab. 2010, 95, 889–900. [Google Scholar] [CrossRef]
- Matusinovic, Z.; Wilkie, C.A. Fire retardancy and morphology of layered double hydroxide nanocomposites: A review. J. Mater. Chem. 2012, 22, 18701–18704. [Google Scholar] [CrossRef]
- Cheng, X.W.; Guan, J.P.; Tang, R.C.; Liu, K.Q. Improvement of flame retardancy of poly(lactic acid) nonwoven fabric with a phosphorus- containing flame retardant. J. Ind. Text. 2016, 46, 914–928. [Google Scholar] [CrossRef]
- Murariu, M.; Dechief, A.L.; Paint, Y.; Peeterbroeck, S.; Bonnaud, L.; Dubois, P. Polylactide (PLA)-Halloysite Nanocomposites: Production, Morphology and Key-Properties. J. Polym. Environ. 2012, 20, 932–943. [Google Scholar] [CrossRef]
- Avinc, O.; Day, R.; Carr, C.; Wilding, M. Effect of combined flame retardant, liquid repellent and softener finishes on poly(lactic acid) (PLA) fabric performance. Text. Res. J. 2012, 82, 975–984. [Google Scholar] [CrossRef]
- Lin, H.; Han, L.; Dong, L. Thermal degradation behavior and gas phase flame-retardant mechanism of polylactide/PCPP blends. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Katsoulis, C.; Kandare, E.; Kandola, B.K. The combined effect of epoxy nanocomposites and phosphorus flame retardant additives on thermal and fire reaction properties of fiber-reinforced composites. J. Fire Sci. 2011, 29, 361–383. [Google Scholar] [CrossRef]
- Laachachi, A.; Cochez, M.; Leroy, E.; Ferriol, M.; Lopez-cuesta, J.M.; Verlaine-metz, P.; Demange, V.; Cedex, S. Fire retardant systems in poly (methyl methacrylate): Interactions between metal oxide nanoparticles and phosphinates. Polym. Degrad. Stab. 2007, 92, 61–69. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- Petrovic, Z. Polyurethanes from Vegetable Oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Malucelli, G. Layer by layer complex architectures based on ammonium polyphosphate, chitosan and silica on polyester-cotton blends: Flammability and combustion behaviour. Cellulose 2012, 19, 1041–1050. [Google Scholar] [CrossRef]
- Bozic, M.; Liu, P.; Mathew, A.; Kokol, V. Enzymatic phosphorylation of cellulose nanofibers to new highly-ions adsorbing, flame-retardant and hydroxyapatite-growth induced natural nanoparticles. Cellulose 2014. [Google Scholar] [CrossRef]
- Gan, L.; Liao, J.; Lin, N.; Hu, C.; Wang, H.; Huang, J. Focus on Gradientwise Control of the Surface Acetylation of Cellulose Nanocrystals to Optimize Mechanical Reinforcement for Hydrophobic Polyester-Based Nanocomposites. ACS Omega 2017, 2, 4725–4736. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Wang, B.; Qian, X.; Shi, Y.; Yu, B.; Hong, N.; Song, L.; Hu, Y. Cyclodextrin microencapsulated ammonium polyphosphate: Preparation and its performance on the thermal, flame retardancy and mechanical properties of ethylene vinyl acetate copolymer. Compos. Part. B 2015, 69, 22–30. [Google Scholar] [CrossRef]
- Pack, S.; Bobo, E.; Muir, N.; Yang, K.; Swaraj, S.; Ade, H.; Cao, C.; Korach, C.S.; Kashiwagi, T.; Rafailovich, M.H. Engineering biodegradable polymer blends containing flame retardant-coated starch/nanoparticles. Polymer 2012, 53, 4787–4799. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Improved Thermal Processing of Polylactic Acid/Oxidized Starch Composites and Flame-Retardant Behavior of Intumescent Non-Wovens. Coatings 2020, 10, 291. [Google Scholar] [CrossRef]
- Zhang, T.; Yan, H.; Shen, L.; Fang, Z.; Zhang, X.; Wang, J.; Zhang, B. Chitosan/phytic acid polyelectrolyte complex: A green and renewable intumescent flame retardant system for ethylene-vinyl acetate copolymer. Ind. Eng. Chem. Res. 2014, 53, 19199–19207. [Google Scholar] [CrossRef]
- Logithkumar, R.; Keshavnarayan, A.; Dhivya, S.; Chawla, A.; Saravanan, S.; Selvamurugan, N. A Review of Chitosan and its Derivatives in Bone Tissue Engineering. Carbohydr. Polym. 2016. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Peng, Y.; Wang, D.; Yang, L.; Peng, H.; Zhu, P.; Wang, D. Effect of reactive time on flame retardancy and thermal degradation behavior of bio-based zinc alginate film. Polym. Degrad. Stab. 2016, 1–12. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Aguedo, M.; Richel, A.; Brohez, S.; Delvosalle, C.; Dubois, P. Phosphorus and nitrogen derivatization as efficient route for improvement of lignin flame retardant action in PLA. Eur. Polym. J. 2016, 84, 652–667. [Google Scholar] [CrossRef]
- Ding, P.; Kang, B.; Zhang, J.; Yang, J.; Song, N.; Tang, S.; Shi, L. Phosphorus-containing flame retardant modified layered double hydroxides and their applications on polylactide film with good transparency. J. Colloid Interface Sci. 2015, 440, 46–52. [Google Scholar] [CrossRef]
- Lin, H.J.; Liu, S.R.; Han, L.J.; Wang, X.M.; Bian, Y.J.; Dong, L.S. Effect of a phosphorus-containing oligomer on flame-retardant, rheological and mechanical properties of poly (lactic acid). Polym. Degrad. Stab. 2013, 98, 1389–1396. [Google Scholar] [CrossRef]
- Maqsood, M.; Langensiepen, F.; Seide, G. Investigation of melt spinnability of plasticized polylactic acid biocomposites-containing intumescent flame retardant. J. Therm. Anal. Calorim. 2019, 1–14. [Google Scholar] [CrossRef]
- Maqsood, M.; Seide, G. Novel Bicomponent Functional Fibers with Sheath/Core Configuration Containing Intumescent Flame-Retardants for Textile Applications. Materials 2019, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Lligadas, G.; Callau, L.; Ronda, J.; Galia, M.; Cadiz, V. Novel Organic—Inorganic Hybrid Materials from Renewable Resources: Hydrosilylation of Fatty Acid Derivatives. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6295–6307. [Google Scholar] [CrossRef]
- Kimura, K.; Horikoshi, Y. Bio-based polymers. Fujitsu Sci. Tech. J. 2005, 41, 173–180. [Google Scholar] [CrossRef]
- Nishida, H.; Fan, Y.; Mori, T.; Oyagi, N.; Shirai, Y.; Endo, T. Feedstock Recycling of Flame-Resisting Poly (lactic acid)/ Aluminum Hydroxide Composite to L, L -lactide. Ind. Eng. Chem. Res. 2005, 44, 1433–1437. [Google Scholar] [CrossRef]
- Yanagisawa, T. Enhanced flame retardancy of polylactic acid with aluminum tri-hydroxide and phenolic resins. Kobunshi Ronbunshu 2009, 66, 49–54. [Google Scholar] [CrossRef]
- Kubokawa, H.; Hatakeyama, T. Thermal decomposition behavior of polylactide fabrics treated with flame retardants. Sen’i Gakkaishi 1999, 55, 349–355. [Google Scholar] [CrossRef][Green Version]
- Wang, D.Y.; Song, Y.P.; Lin, L.; Wang, X.L.; Wang, Y.Z. A novel phosphorus-containing poly(lactic acid) toward its flame retardation. Polymer 2011, 52, 233–238. [Google Scholar] [CrossRef]
- Wei, L.L.; Wang, D.Y.; Chen, H.B.; Chen, L.; Wang, X.L.; Wang, Y.Z. Effect of a phosphorus-containing flame retardant on the thermal properties and ease of ignition of poly(lactic acid). Polym. Degrad. Stab. 2011, 96, 1557–1561. [Google Scholar] [CrossRef]
- Bourbigot, S.; Duquesne, S.; Fontaine, G.; Bellayer, S.; Turf, T.; Samyn, F. Characterization and Reaction to Fire of Polymer Nanocomposites with and without Conventional Flame Retardants. Mol. Cryst. Liq. Cryst. 2008, 486, 37–41. [Google Scholar] [CrossRef]
- Solarski, S.; Mahjoubi, F.; Ferreira, M.; Devaux, E.; Bachelet, P.; Bourbigot, S.; Delobel, R.; Coszach, P.; Murariu, M.; Da Silva Ferreira, A.; et al. (Plasticized) Polylactide/clay nanocomposite textile: Thermal, mechanical, shrinkage and fire properties. J. Mater. Sci. 2007, 42, 5105–5117. [Google Scholar] [CrossRef]
- Wang, N.; Hu, L.; Vignesh, H. Effect of tea saponin-based intumescent flame retardant on thermal stability, mechanical property and flame retardancy of natural rubber composites. J. Therm. Anal. Calorim. 2016. [Google Scholar] [CrossRef]
- Mngomezulu, M.E.; Luyt, A.S.; John, M.J. Morphology, thermal and dynamic mechanical properties of poly(lactic acid)/expandable graphite (PLA/EG) flame retardant composites. J. Thermoplast. Compos. Mater. 2017, 1–19. [Google Scholar] [CrossRef]
- Mauldin, T.; Zammarano, M.; Gilman, J.; Shields, J.; Boday, J. Synthesis and characterization of isosorbide-based polyphosphonates as biobased flame-retardants. Polym. Chem. 2014, 5, 5139–5146. [Google Scholar] [CrossRef]
- Fox, D.M.; Lee, J.; Citro, C.J.; Novy, M. Flame retarded poly(lactic acid) using POSS-modified cellulose. 1. Thermal and combustion properties of intumescing composites. Polym. Degrad. Stab. 2013, 98, 590–596. [Google Scholar] [CrossRef]
- Wan, J.; Gan, B.; Li, C. A novel biobased epoxy resin with high mechanical stiffness and low flammability: Synthesis, characterization and properties. J. Mater. Chem. A 2015. [Google Scholar] [CrossRef]
- De Britto, D.; Campana-filho, S.P. Kinetics of the thermal degradation of chitosan. Thermochim. Acta 2007, 465, 73–82. [Google Scholar] [CrossRef]
- González, A.; Dasari, A.; Herrero, B.; Plancher, E.; Santarén, J.; Esteban, A.; Lim, S. Fire retardancy behavior of PLA based nanocomposites. Polym. Degrad. Stab. 2012, 97, 248–256. [Google Scholar] [CrossRef]
- Fontaine, G.; Bourbigot, S. Intumescent Polylactide: A Nonflammable Material. J. Appl. Polym. Sci. 2009, 113, 3860–3865. [Google Scholar] [CrossRef]
- Didane, N.; Giraud, S.; Devaux, E.; Lemort, G. A comparative study of POSS as synergists with zinc phosphinates for PET fire retardancy. Polym. Degrad. Stab. 2012, 97, 383–391. [Google Scholar] [CrossRef]
- Sorbo, P.; Girardot, J.; Dau, F.; Iordanoff, I. Numerical investigations on a yarn structure at the microscale towards scale transition. Compos. Struct. 2018, 183, 489–498. [Google Scholar] [CrossRef]

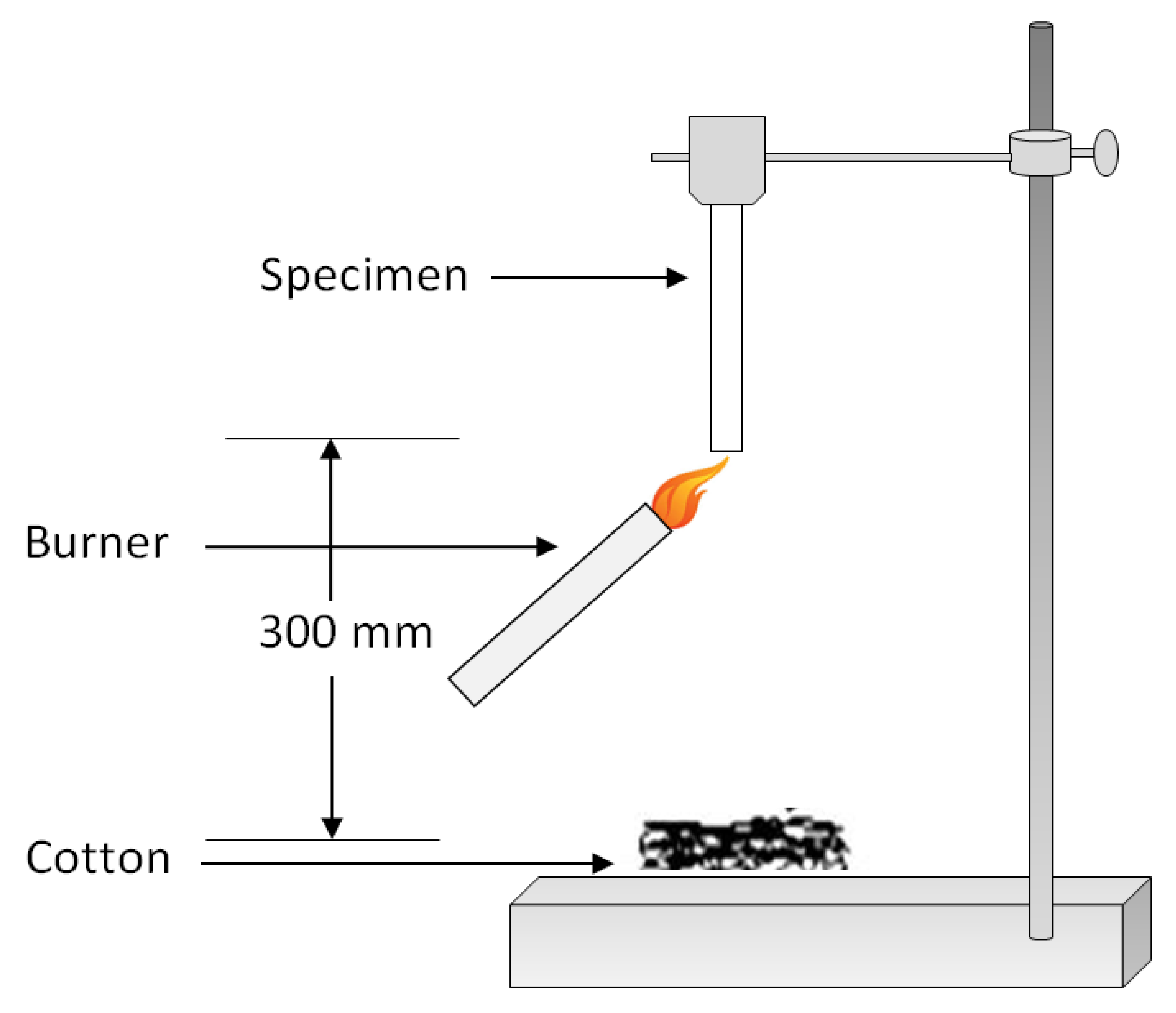

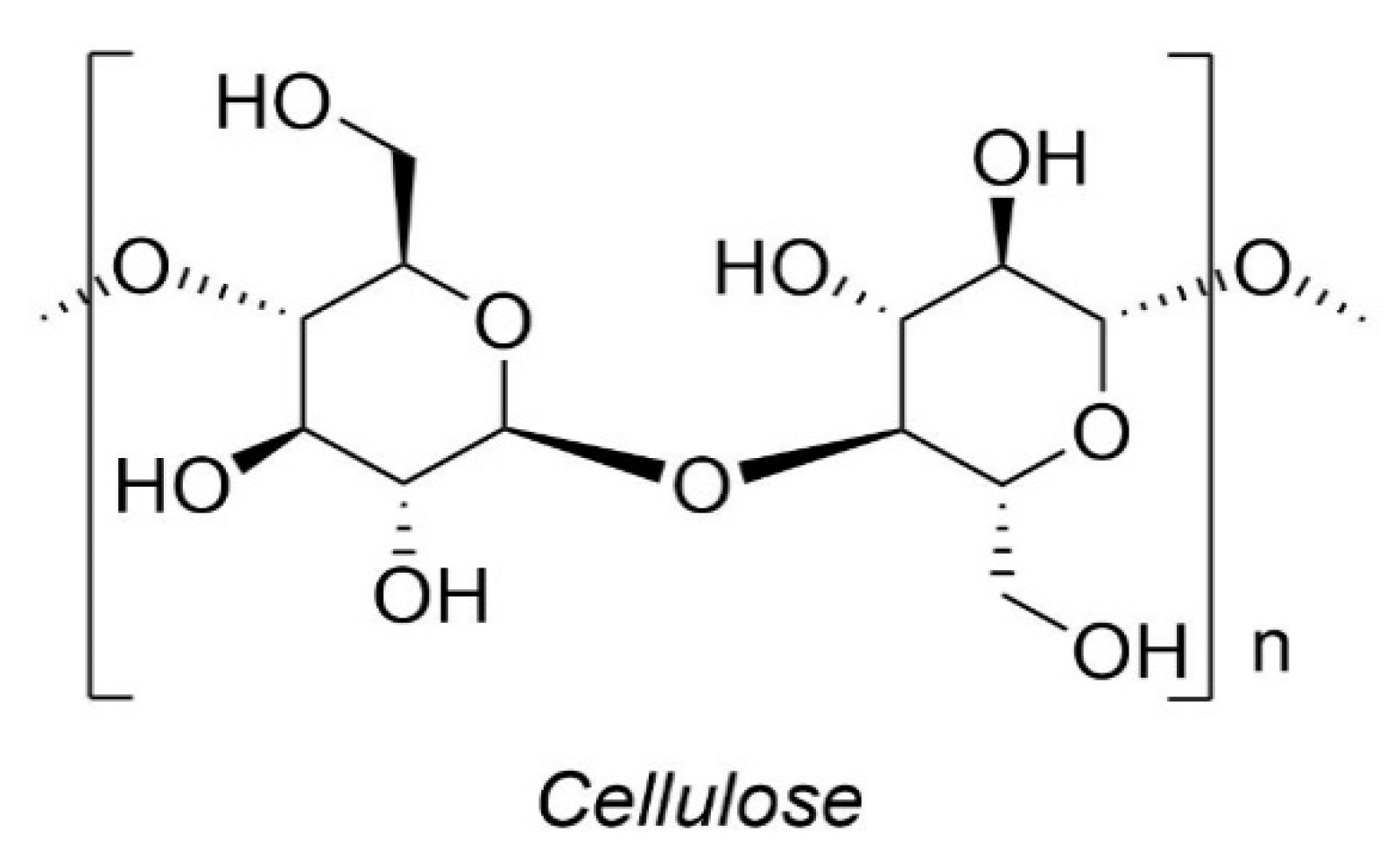

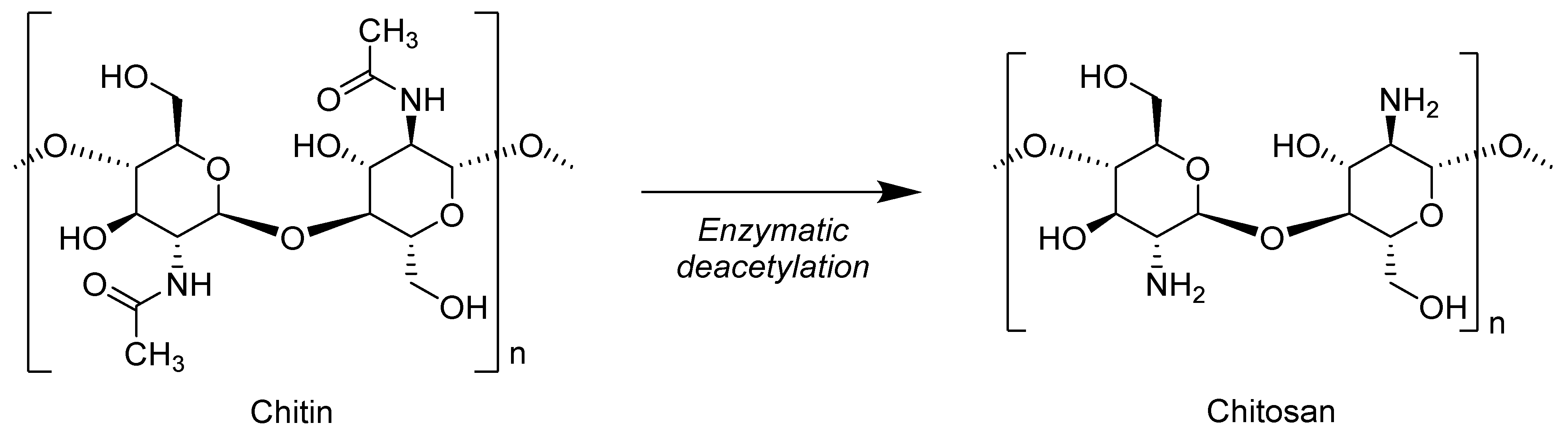
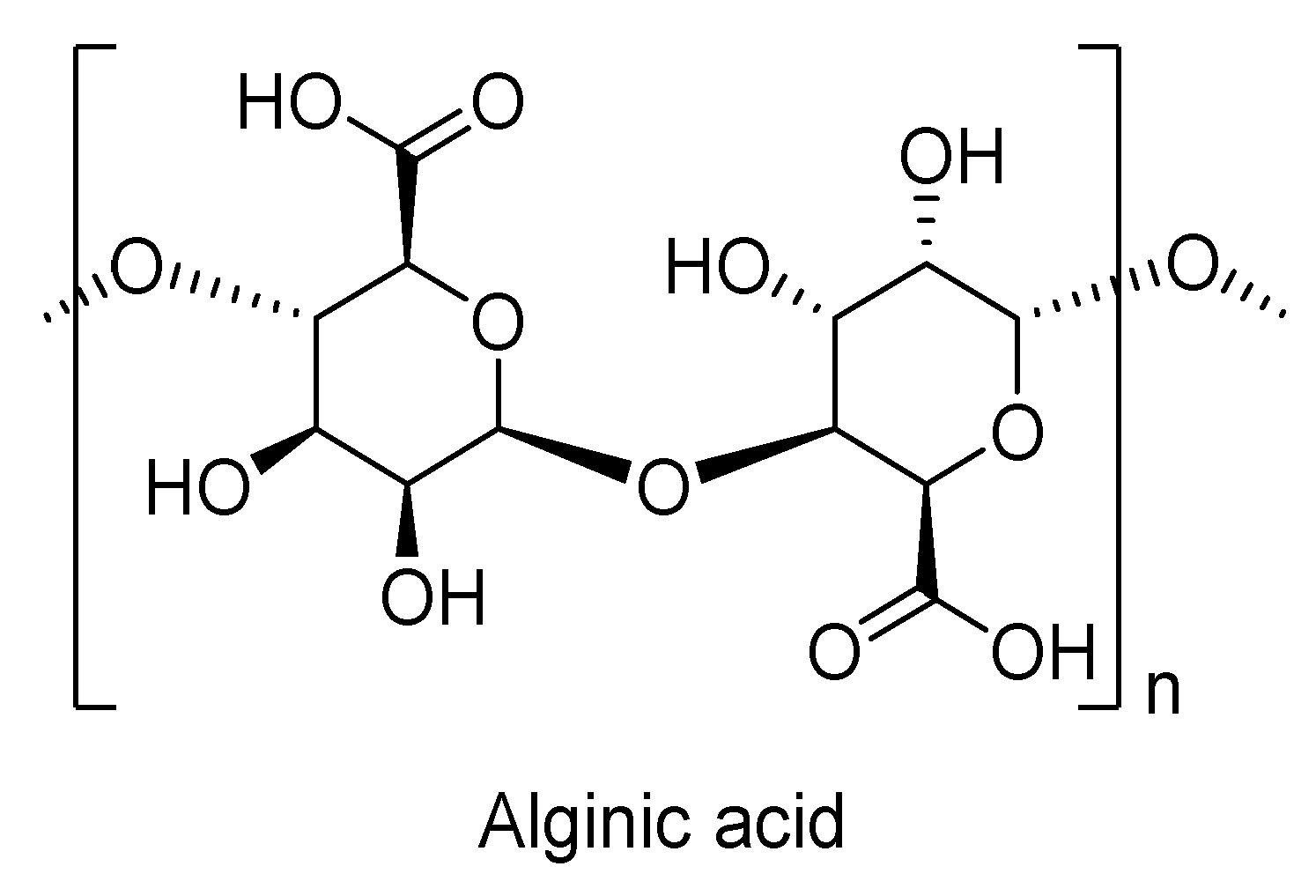
| Properties | Unit | PLA | PET | PA6 | PP |
|---|---|---|---|---|---|
| Density | g/cm3 | 1.24 | 1.38 | 1.14 | 0.92 |
| Melting temperature | °C | 170–180 | 255–260 | 210–220 | 155–165 |
| Glass transition temperature | °C | 55–60 | 70–80 | 57–65 | −10 |
| Time to ignition | s | 58 | 46 | 48 | 42 |
| Smoke generation | m2/kg | 63 | 394 | 195 | 142 |
| Limiting oxygen index | % | 24 | 19 | 18 | 20 |
| Peak heat release rate | kW/m2 | 425–450 | 625–640 | 575–590 | 495–510 |
| Effective heat of combustion | kJ/g | 18 | 24 | 19 | 21 |
| Source | Authors | Short Description | Findings |
|---|---|---|---|
| [95] | Kimura and Horikoshi | Tried to improve flame retardancy of PLA by blending with virgin polycarbonate (PC), but results showed not very significant improvement in flame retardancy of the blend. | To achieve better results, silicon-comprising PC was blended with PLA, but still only a V-2 rating could be achieved in UL-94 vertical burning test. |
| [96] | Nishida et al. | Studied flame-retardant properties of PLA by compounding Alumina trihydrate (ATH) in the polymer. | However, they found that, in order to get superior results, a relatively higher amount of ATH (about 40 to 50%) needs to be added to the PLA matrix. |
| [97] | Yanagisawa et al. | Incorporated ATH together with phenolic resins in PLA matrix to improve its flame retardancy for electronic applications. A significant char formation during combustion on the surface of the composite was observed. | The addition of phenolic resin not only resulted in improved flame retardancy, reinforced by alumina from ATH on the surface of composite, but also reduced the loading content of ATH to 35% (w/w). |
| [98] | Kubokawa et al. | Determined flame retardancy of PLA based fabrics by using bromine containing additives, and tri-phenyl phosphates and tested LOI of the fabrics. | Limiting oxygen index (LOI) of untreated fabrics was 24%; however, after treatment with FR additives, LOI of the fabrics increased to 28% with tri-phenyl phosphate and to 26% with bromine-containing FR additives. |
| [99] | Wang et al. | Prepared composites containing pentaerythritol (PER) and melamine cyanurate (MC) by controlling the weight ratio of 2:2:1 and organo-modified zinc-aluminum-layered double hydroxide (Zn-Al-LDH). | Microscale combustion calorimeter (MCC) and cone calorimetry results revealed substantial progresses in flame retardancy of the nanocomposites. A significant reduction in heat release rate and total heat release was observed. |
| [100] | Wei et al. | Investigated the effect of aryl poly-phenyl phosphonate together with PLA. LOI, UL-94 vertical burning and cone calorimetry tests were carried out together with the investigation of thermal and mechanical behavior of the composites. | PLA composites containing 7 wt % and 10 wt % of poly phenyl phosphonate achieved V-0 rating in UL-94 vertical burning test. However, not much improvement in HRR and THR of the FR composites were observed in comparison to neat PLA. |
| [101] | Bourbigot et al. | Studied flame-retardancy of different polymer nanocomposites such as polylactides, polyurethane and polyamides. Different nano-fillers such as carbon nanotubes and organoclay were incorporated in these polymers and their flame retardancy was investigated. | Found that nano-dispersion play a vital role in improving flame retardancy of nanocomposites and nano-fillers give better results when they are used in combination with inorganic flame retardants due to better synergistic effects. |
| [102] | Solarski et al. | Developed PLA/clay nanocomposites and studied their thermal and fire properties. Four different formulations ranging from 1 wt % to 4 wt % of the organo clay (C30B) were prepared. The nanocomposites were melt spun to produce multifilament yarns. | Yarns with better mechanical properties were used to produce knitted structures. The fire properties of the knitted fabrics were tested by cone calorimetry. It was observed that pHHR of the fabrics containing only 2 wt % of the clay were reduced up to 38%. |
| [17] | Suardana et al. | Prepared bio-composites containing natural fibers together with di-ammonium phosphate and investigated their mechanical and fire related properties. | By increasing wt % of di-ammonium phosphate, fire properties, flexural modulus, and weight loss rate of the composites were improved; however, tensile and flexural strengths of the composites were reduced. |
| [59] | Qian et al. | Prepared aluminated mesoporous silica and used it as an FR additive in PLA resin. Different formulations of fumed silica and aluminated mesoporous silica were compounded in PLA resin. | Achieved UL-94 V-0 rating and LOI value also increased quite significantly. pHHR of FR composites decreased to about 15% compared to neat PLA by the addition of only 0.5 wt % of aluminated mesoporous silica. |
| [103] | Wang et al. | Developed an inherently fire-resistant PLA polymer using a chain-extending procedure of pre-polylactic acid (PPLA). PPLA was produced by direct condensation reaction of L-lactic acid and its fire properties were tested. | Here, 5 wt % of PPLA in PLA polymer would be sufficient to achieve remarkable FR properties as LOI value of 35% and UL-94 V-0 rating was achieved with delayed ignition time compared to pure PLA. |
| [104] | Mngomezulu et al. | Investigated FR properties of PLA and expandable graphite (PLA/EG) composites. EG was compounded in PLA with different wt % to produce PLA/EG composites and their surface morphology, filler dispersion, dynamic mechanical behavior and crystallization rate were studied. | The presence of graphite layers with not so good filler dispersion resulted in poor bonding between PLA resin and EG. The crystallization rate of PLA was increased with an increase in the glass transition temperature. Furthermore, lower modulus of the composites with higher wt % of EG was observed. |
| [105] | Shumao et al. | Used ramie fibers together with PLA to reinforce the polymer and to enhance the mechanical properties. Used different formulations together with ramie fibers. | At 40 wt %, loading the LOI value could only be reached at 30%. Resulted phosphoric acid contributed in intermolecular dehydration of ramie fibers followed by dehydrogenation. |
| [63] | Zhan et al. | Investigated the combustion and thermal degradation behavior of PLA with a special flame-retardant consisting of spirocyclic-pentaerythritol bisphosphorate disphosphoryl melamine (SPDPM) and melt compounded with different wt % in PLA. | Attained UL-94 V-0 rating at 25 wt % loading and LOI value was 38%. A significant reduction in weight loss rate of PLA was observed after addition of SPDPM in PLA as confirmed by thermogravimetric analysis. |
| [106] | Fox et al. | Polyoligomeric Silsesquioxane (POSS) modified cellulose was melt blended with PLA to prepare PLA/POSS composites. Thermal and fire related properties of as prepared composites were tested by thermogravimetric analysis, dynamic mechanical analysis and cone calorimetry. | pHHR of the composites was reduced to 45% by the introduction of 15 wt % of modified cellulose in comparison to pHHR of neat PLA. Total heat release (THR) was also reduced to 20% and lesser smoke generation was observed in comparison to non-modified cellulose. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maqsood, M.; Seide, G. Biodegradable Flame Retardants for Biodegradable Polymer. Biomolecules 2020, 10, 1038. https://doi.org/10.3390/biom10071038
Maqsood M, Seide G. Biodegradable Flame Retardants for Biodegradable Polymer. Biomolecules. 2020; 10(7):1038. https://doi.org/10.3390/biom10071038
Chicago/Turabian StyleMaqsood, Muhammad, and Gunnar Seide. 2020. "Biodegradable Flame Retardants for Biodegradable Polymer" Biomolecules 10, no. 7: 1038. https://doi.org/10.3390/biom10071038
APA StyleMaqsood, M., & Seide, G. (2020). Biodegradable Flame Retardants for Biodegradable Polymer. Biomolecules, 10(7), 1038. https://doi.org/10.3390/biom10071038






