Abstract
This study focused on the measurement of anthropogenic radionuclides such as americium (Am) and plutonium (Pu) in environmental samples. Plutonium isotopes, particularly , , and , originated from nuclear weapons testing, nuclear power plants, and accidents like Chernobyl and Fukushima Daiichi. Accurate measurement of these isotopes, considering their half-lives and trace concentrations, provides critical information about their persistence and environmental transport. Using the 1 MV Tandetron accelerator, we expanded the measurement capabilities to include , . Chemical separation of these isotopes was achieved through ion chromatography, employing reference isotopes and for method validation. Certified reference materials, including IAEA-410 (Bikini Atoll sediment) and Sample 05, were analyzed to ensure accuracy. We validated the / ratio in an Am standard (IFIN-STD-Am, our laboratory produced standard for Am), achieving a measured value of 0.158 at·at−1 (3%), in good agreement with the nominal value of 0.154 at·at−1. Additionally, we determined the + ratio in the ColPuS standard to be equal to 0.029 at ·at−1 (7%). These results demonstrate the potential of AMS for improved detection of actinides at low concentrations and contribute to understanding the behavior of Pu and Am isotopes.
1. Introduction
Large amounts of anthropogenic plutonium were released into the environment during the 20th century through nuclear weapons testing, accidents, and waste disposal. Atmospheric nuclear tests dispersed long-lived plutonium isotopes, especially ( = 24,109 y) and ( = 6562 y) [1], which were deposited globally as fallout. For instance, plays a key role in source identification, as the /() activity ratio is commonly used in alpha spectrometry to distinguish between different contamination events. Global fallout is typically characterized by an activity ratio of /() of around 0.03, and an atomic ratio of / of 0.180 ± 0.014 [2]. Global fallout from atmospheric nuclear tests showed plutonium isotope ratios with a activity ratio of between 13 and 15 in 1963, and a atom ratio of 0.18. High-yield US thermonuclear tests in the 1950s produced elevated activity ratios from 20 to 30, higher atom ratios of around 0.3, and lower activity ratios below 0.01. Nuclear accidents such as Chernobyl and Fukushima released plutonium with significantly higher activity ratios of 0.5 and 1.2, respectively, elevated activity ratios of 85 and 108, and atom ratios of 0.41 and between 0.30 and 0.33 [3]. These values highlight the variability of isotopic ratios based on the source. Plutonium predominantly remains in soil and sediments due to its chemical properties, posing a long-term environmental hazard because of these extensive half-lives.
Building on this context, it is important to note that ( = 14.4 y) has a much shorter half-life than its decay product, ( = 432.2 y), and it plays a significant role in the environmental radiological profile due to its transformation into . Unlike other isotopes, which are directly deposited, mainly results from the gradual in-growth of over time [4]. However, for more recent releases of , such as those from nuclear reprocessing plants or nuclear accidents, the decay to is less pronounced in the short term. Given the relatively short half-life of , significant amounts can remain in the environment for several decades, continuing to contribute to radiological hazards. As a result, the long-term environmental impact of is closely linked to both its initial release and the gradual accumulation of over time. This transformation not only alters the isotopic composition of plutonium in the environment but also amplifies the long-term radiological impact due to the persistence and mobility of americium isotopes. However, the assumption that americium is universally more mobile than plutonium does not always hold true and is strongly dependent on the environmental matrix. For example, in seawater, has been found to be more particle-reactive than plutonium, with suspended particulate matter showing significant enrichment in relative to by a factor of 8 [5]. In terrestrial environments, recent studies have shown that americium and plutonium exhibit similar migration rates in various soil types, indicating comparable long-term mobility [6]. These findings highlight the importance of considering matrix-specific geochemical behavior when evaluating the environmental transport of transuranic radionuclides. The increasing presence of over time due to decay highlights the importance of monitoring these isotopes for environmental safety. Knowing how much plutonium is present and where it has spread is essential for managing contaminated sites, evaluating public health risks, and ensuring safe handling of radioactive materials [7].
In contrast, the measurement of and its decay product does not directly date the sediments but rather provides an estimate of the time since the last plutonium irradiation. Since is produced solely through the decay of , its presence can be used to trace the timing of plutonium introduction into the environment. This method, therefore, informs us about the history of anthropogenic deposition events, rather than the sediment age itself [8].
Determination of radionuclide concentrations and isotopic compositions in environmental samples can be performed using several methods, two of the most prominent being alpha spectrometry and inductively coupled plasma sector field mass spectrometry (ICP-SFMS). Alpha spectrometry is used to measure radionuclide activities, such as and , but it is limited by long analysis times and the difficulty in distinguishing closely related isotopes, like and . It is important to note that alpha spectrometry can only yield precise results when concentrations are high enough to ensure reliable counting statistics. Furthermore, the lengthy analysis times make it less suitable for routine environmental monitoring. ICP-SFMS allows for rapid analysis and the simultaneous determination of multiple radionuclides, providing detailed isotopic characterization. Despite these advantages, these techniques are limited by molecular or atomic isobaric interferences, such as U when measuring , or the isobaric background like in the determination of . Accelerator Mass Spectrometry (AMS) is an ultra-sensitive technique for detecting radionuclides at ultra-trace levels. Unlike ICP-MS and TIMS, AMS uses a particle accelerator and a stripping gas to eliminate molecular interferences and improve isotope separation, especially for actinides. While AMS involves certain sample preparation steps, it is a highly effective tool for precise isotopic analysis, particularly in environmental and radiological research. The main advantage of AMS over traditional mass spectrometry is its simplified chemical procedures and its ability to suppress molecular isobars, which makes it particularly advantageous for the analysis of actinides and other challenging isotopic systems. In the past, the measurement of heavy isotopes was performed using AMS at high-energy accelerator facilities. However, over the last two decades, research has focused on utilizing lower-energy accelerators (0.3–1 MV), which offer advantages such as compact size and reduced maintenance costs, although they may require different configurations to achieve the same sensitivity for certain isotopes. For example, the 1MV AMS facility at IFIN-HH in Romania is part of the first generation of compact AMS systems. These systems have demonstrated sensitivities comparable to those of conventional high-voltage AMS facilities. They are capable of measuring a wide range of radionuclides, including , , , , , , and . Detailed information and further explanations about the facility in Bucharest, including its capabilities and experimental setup, are provided in [9,10].
This paper introduces a method for the chemical separation of plutonium and americium isotopes, tested on materials with well-known isotopic ratios and activities. The approach utilizes ion chromatography to separate these isotopes from sediment and water samples. Due to the absence of stable isotopes for normalization, reference isotopes such as and are employed for both method development and validation. This paper is structured as follows: the Introduction, which provides the context and outlines the main objectives; Materials and Methods, detailing the chemical preparation process and the use of accelerator mass spectrometry (AMS) for isotope analysis; Results, which present the findings obtained through the proposed method; and Conclusions.
2. Materials and Methods
2.1. Samples
To test and validate the determination of plutonium and americium in environmental matrices using the 1 MV accelerator at IFIN-HH, a variety of sample types were analyzed, including procedural blanks, certified reference materials (CRMs), and standard materials.
Procedural blanks consisted of aliquots taken from the reference isotope solutions, which were subjected to the same chemical procedures as the actual samples.
The sediment sample IAEA-410, collected offshore Bikini Atoll in November 1997, was impacted by the historical nuclear tests conducted in the region. These tests, carried out between 1946 and 1958, resulted in the release of significant levels of anthropogenic radionuclides, including plutonium and americium isotopes [11]. Following the procedure described in [12], aliquots of approximately 0.5 g were prepared and loaded into individual cathodes for AMS measurements.
Sample 05 consisted of drinking water collected in Seibersdorf, Austria. It was acidified with approximately 0.05 M to ensure radionuclide stability, then gravimetrically spiked with a certified standard solution containing a mixture of radionuclides. The added plutonium and americium isotopes were present at concentrations higher than those typically found in environmental samples, enabling the development and validation of the radiochemical separation and AMS measurement protocols [13].
IFIN-STD-Am is our laboratory-produced standard for americium, prepared by mixing the reference isotope with Sample 02, an artificial water sample spiked with [13].
2.2. Sample Preparation
For accurate isotopic analysis, reference isotopes (NPL, UK, A170459, = 375,000 y) and (NPL, UK, A12291, = 7345 y) are added at the beginning of the procedure [14,15,16,17]. Approximately 2 pg of each isotope are spiked into every sample to ensure precise recovery monitoring and enable isotopic dilution analysis during AMS measurements.
The environmental sediment sample (IAEA-410) is prepared by drying the sediment in a muffle furnace and calcinating it at 550 °C for 8 h to remove organic material. The calcined residue is transferred to a Teflon beaker, where it is treated with 20 mL of concentrated and 10 mL of concentrated HCl. The beaker is heated on a hotplate close to its boiling point for 3 h. The solution, along with undissolved solids, is transferred to polypropylene centrifuge tubes and centrifuged at 4000 rpm for 5 min. The supernatant is separated and stored, while the residue is returned to the original beaker.
To further dissolve the residue, 20 mL of concentrated and 15 mL of concentrated HF are added and evaporated to dryness on a hotplate. HF is used to break down silicate minerals in the sediment matrix, ensuring complete digestion of refractory materials such as quartz. This step is repeated twice to guarantee complete decomposition of the sample. Subsequently, 10 mL of 7% is added to oxidize residual fluorides and transform them into nitrates, which are more soluble and compatible with subsequent resin separations. The sample is then evaporated to dryness. The stored supernatant is reintroduced into the beaker, and the combined solution is evaporated to dryness again. A total of 10 mL of concentrated are added and evaporated twice more to ensure removal of residual organic material and prepare the sample for resin separation. Finally, the residue is dissolved in a solution of 10 mL of warm 3 M –0.25 M H3BO3, 14 mL of 7 M , and 14 mL of 2 M aluminum nitrate (Al(NO3)3).
Boric acid is included in the load solution to bind fluoride ions, thereby preventing the formation of stable fluoride complexes with plutonium that could interfere with their retention on the selective resin. Excess phosphate in the sample can reduce actinide recovery. The addition of aluminum ions (), which have a stronger affinity for phosphate than actinides, helps to mitigate this issue.
Artificial water samples, including Sample 05 and IFIN-STD-Am, are prepared by evaporating the solutions to dryness and dissolving them in the same conditioning solutions used for sediment samples. The ColPuS plutonium standard, spiked with , is prepared by directly introducing the reference isotopes before proceeding to the separation step.
Prior to separation, the valence state of plutonium in the sample solution is adjusted to ensure optimal retention on the TEVA® resin. Plutonium is reduced to by adding 1 mL of 1.5 M sulfamic acid (H3NSO3) and 0.5 mg of Fe as iron nitrate. After 3 min, 1.25 mL of 1.5 M ascorbic acid (C6H8O6) is added to maintain reducing conditions. Finally, 1 mL of 3.5 M sodium nitrite () is introduced to oxidize to , the valence state required for binding to the TEVA® resin. The sample solution at this stage is maintained in 3 M HNO3 to ensure the chemical environment is suitable for resin interaction and to prevent reoxidation or reduction of the actinides.
The separation of plutonium and americium is performed using Eichrom TEVA® (TE-R50-S) and TRU® (TR-R50-S) resin cartridges. Each cartridge contains 2 mL of resin with a particle size of 50–100 µm, providing a high surface area for selective binding of actinides. TEVA® resin is specifically designed to retain tetravalent actinides, such as , while TRU® resin is optimized for retaining trivalent actinides, such as Am3+. This selective affinity allows for efficient separation of these actinides in a single step. The resin cartridges are arranged in a stacked configuration, with TEVA® on top and TRU® below, to allow for sequential retention of plutonium and americium. Both resins are preconditioned with 5 mL of 3 M HNO3 to ensure optimal retention.
The sample solutions are passed through the stacked resins at a flow rate of 1–2 mL/min using an Eichrom vacuum extraction box. After loading the sample, the stacked columns are rinsed with 20 mL of 3 M HNO3 to remove matrix interferences while preserving the actinides on the resins.
Following the loading and rinsing steps, the TEVA® and TRU® cartridges are separated, and the fractions are processed individually. Plutonium retained on the TEVA® resin is first treated with 9 M HCl to elute thorium, which is discarded. An additional 5 mL of 3 M HNO3 is used to clean the resin, minimizing potential resin bleed. Plutonium is then eluted using 20 mL of a solution containing 0.1 M HCl and 0.05 M HF. Americium retained on the TRU® resin is eluted with 15 mL of 4 M HCl, and the fractions are collected in separate vials.
The plutonium and americium fractions are processed separately to produce distinct cathodes for AMS. To each fraction, 1 mL of an iron nitrate solution (1 mg Fe/mL) is added, and iron hydroxides are co-precipitated with ammonium hydroxide (NH4OH). The precipitate is recovered via centrifugation, washed with ethanol, and dried on a hotplate at 60 °C. The dried material is calcinated at 800 °C for 4 h to produce PuO2 and AmO2 dispersed in Fe2O3. Finally, 3 mg of niobium powder is added to each fraction to improve the thermal and electrical properties of the targets.
This procedure provides separation and purification of plutonium and americium, resulting in two distinct cathodes that are suitable for AMS analysis.
2.3. Accelerator Mass Spectrometry
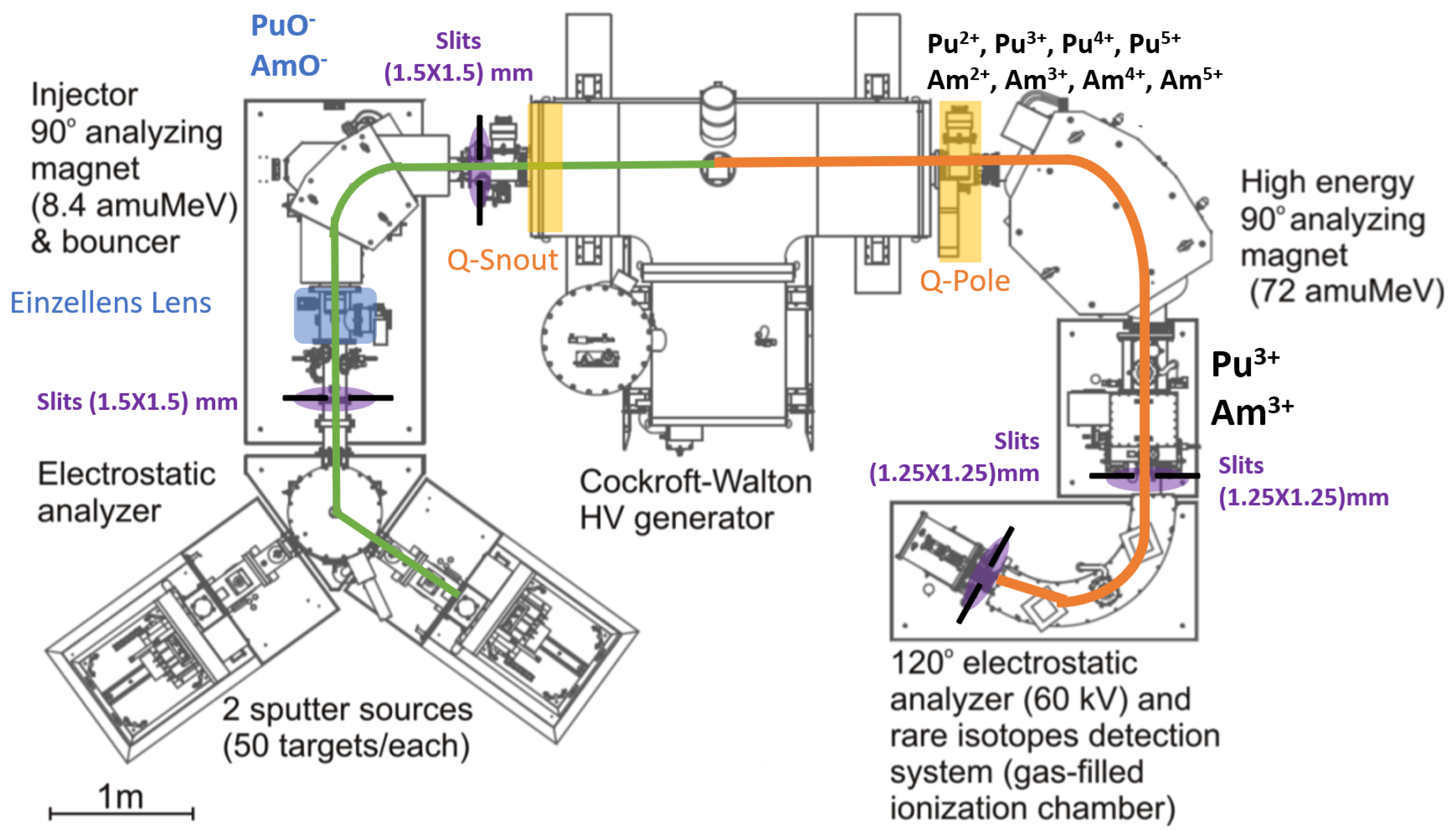
The AMS measurements were conducted using the 1 MV Tandetron system at IFIN-HH [18,19,20,21]. A similar AMS facility, part of the first-generation compact AMS systems and also based on a 1 MV Tandetron accelerator developed by High Voltage Engineering Europe (HVEE), is operated at the Centro Nacional de Aceleradores (CNA) in Seville, Spain [22,23,24]. The existence of such comparable systems highlights the reliability and widespread use of compact 1 MV AMS setups for high-sensitivity isotope measurements. Building on this established configuration, the system includes a negative ion source (SO-110B), a low-energy injection magnet (LEM), a 1 MV accelerator operated with positive high voltage (), a high-energy analyzing magnet (HEM), an electrostatic analyzer (HE ESA), and a detection system, as shown in Figure 1. The isotopes of interest were extracted from the Cs-sputter ion source as negative oxide molecules.

Figure 1.
Schematic diagram of the 1 MV Tandetron Accelerator (HVE, Amersfoort, The Netherlands) [10].
At the center of the acceleration tubes, a gas stripping channel filled with argon is used. Here, molecular ions are dissociated via collisions with gas atoms, and negative ions are stripped of electrons to form positive ions in various charge states. Argon was used as the stripping gas at a pressure of 4 × 10−3 mbar, enabling efficient molecular dissociation and production of positive ions. The ion transmission through the accelerator and the charge state yield was approximately 10 % for uranium. Although the transmission for other actinides could not be directly measured, it is expected to be similar in magnitude, considering comparable behavior during acceleration and charge state formation [25].
On the high-energy side, positive ions in the 3+ charge state were further accelerated and selected based on their mass-to-charge ratio using a 90° dipole magnet and a 120° HE ESA. Actinide ions were detected and counted individually using a double-anode gas ionization chamber (GIC) filled with isobutane at a pressure of 6 mbar. The GIC is equipped with a silicon nitride window measuring 8 × 8 mm2 and 100 nm in thickness, ensuring detection based on the total energy of the ions.
The isotope was used as a pilot beam for determining ion transport parameters, as its abundance is sufficient to allow current measurements in a Faraday cup. Table 1 presents the main measurement conditions for the 238U pilot beam, as well as the optimized parameters subsequently used for the analysis of plutonium and americium isotopes.

Table 1.
Experimental values for U (pilot beam) and Pu and Am (rare isotopes counted in GIC).
Precise beam transmission is essential for maintaining stable magnetic fields and minimizing magnetic hysteresis. In our 1 MV AMS system—used to measure actinide isotopes such as 239Pu, 240Pu, 241Pu, 242Pu, and 243Am—these adjustments are performed automatically by the Slow Sequential Injection (SSI) mode of the newly installed ARGUS control software. The SSI mode preserves constant magnetic rigidity before and after acceleration by dynamically setting the low-energy magnet’s bouncer voltage, the terminal voltage, and the high-energy electrostatic analyzer field.
The measurement time was 10 s for , 20 s for , 35 s for mass 241 (covering both and ), and 5 s each for and . A unified measurement sequence was applied to all target samples, resulting in the acquisition of plutonium signals even in americium-only samples, and vice versa. Each isotope was measured six times consecutively before switching to the next target sample. This entire measurement cycle was repeated five times, ensuring consistent and reproducible data across all isotopes and target samples.
3. Results
An important aspect of quantifying different isotopes using AMS is the investigation of the procedural blank, which reflects the level of contamination introduced during the chemical processing and measurement steps. The blank values are subtracted from the sample results to correct for this contamination. Additionally, procedural blanks are used to obtain information about the chemical recovery of the resin separation. We measured roughly 25 cps·pg−1 for and about 20 cps·pg−1 for , confirming satisfactory recovery. In the sediment sample, we measured 22 cps · pg−1 for , whereas could not be quantified because of molecular interferences (see the discussion below). For the water sample, the count rates were 23 cps · pg−1 for and 25 cps · pg−1 for . These results point to good chemical recovery of plutonium from both matrices. Americium recovery, however, was satisfactory only for the water sample, whose simpler composition allowed for efficient separation of both elements.
Table 2 presents a comparison of AMS measurement parameters and blank levels for plutonium and americium isotopes across different studies. In our work, two approaches were investigated: with and without chemical separation. Chemically processed samples exhibited elevated blank values (e.g., fg for ), which are likely attributable to a combination of incomplete purification, residual stable elements causing potential interferences, molecular species such as overlapping with , and laboratory-derived contamination. These factors are currently under investigation and will be addressed in future work to optimize the analytical protocol.

Table 2.
Comparison of AMS measurement parameters and results of Pu and Am.
In contrast, procedural blanks without chemical treatment yielded substantially lower values— fg for , fg for , fg for , and fg for —primarily reflecting isotopic impurities present in the reference solutions.
When compared to the lower blank levels achieved in other laboratories, such as ETH ( fg for and fg for ) and AEL-AMS ( fg for and fg for ), our findings highlight that while the method is operational, further refinement in chemical purification and contamination control is necessary to reduce background levels, improve detection limits, and enhance overall measurement precision.
In the measurements presented in Table 2, we observe that the AEL-AMS study used a significantly higher terminal voltage of 2.4 MV, compared to the lower values of 0.65 MV employed in both our study and the ETH study. This difference in terminal voltage directly influences the energy of the accelerated particles.
At higher terminal voltages, such as in the AEL-AMS setup, particles have higher kinetic energy and interact more extensively with the detector gas. In this case, both the energy loss () and the residual energy () are measured using a two-anode detection system.
A key difference between the methodologies lies in the choice of resins used for the separation of plutonium and americium. In our study, TEVA® and TRU® resins were employed in a stacked configuration to facilitate the sequential retention of and . This setup enables efficient separation in a single step, with TEVA® resin selectively binding tetravalent actinides, while TRU® resin enhances retention of trivalent actinides. A critical part of this process is the valence adjustment of plutonium prior to separation: is oxidized to to ensure its effective retention on the TEVA® resin.
In contrast, one of the comparative studies utilizes DGA® resin, which exhibits strong affinity for Am(III) and Pu(VI) in concentrated nitric acid [28]. In this method, Pu is maintained in the tetravalent state using sodium nitrite to ensure selective retention on the DGA® resin column. The separation is achieved by first eluting Am with hydrochloric acid, followed by the reduction of Pu(VI) to Pu(III) using before final elution. While effective, this approach requires strict control of Pu oxidation states and introduces additional chemical handling steps, which may impact overall efficiency and reproducibility.
The other comparative study follows a different strategy, employing an anion exchange chromatography column loaded with AGMP-1M resin. This method allows for the selective removal of interfering species prior to the final elution of Pu [26].
Overall, while all three methods aim to achieve effective separation of Pu and Am, the choice of resin strategies significantly influence efficiency, selectivity, and procedural complexity. Our approach minimizes chemical handling and enables sequential separation in a single pass. However, the alternative methods, though more chemically involved, report lower uncertainties and improved precision. This indicates that further refinement of our separation protocol is needed to enhance reproducibility and reduce procedural variability.
The detection of species using beam optics is governed by the mass-to-charge ratio, which allows for the identification of additional species such as , , or molecular ions with equivalent mass and charge. In the spectra of and , the low intensities of and ions do not result in significant interference. However, for , the high abundance of ions poses a potential source of contamination, requiring the use of pile-up rejection functionality to minimize their impact.
Additional interferences in actinide measurements at the charge state can arise from residual molecular ions such as , which can interfere with the detection of [29].
Another source of interference occurs when uranium-containing samples are introduced into the ion source. Charge exchange and dispersive processes in the high-energy region may allow molecular fragments of to reach the detector simultaneously with , due to insufficient separation by the high-energy spectrometer or the HE ESA positioned before the detector. This overlap can be mitigated by carefully tuning the HE ESA settings and applying effective chemical separation techniques, particularly when analyzing plutonium from real samples [25].
These potential interferences and contamination sources underscore the importance of using procedural blanks to ensure the accuracy and reliability of actinide measurements by AMS.
In AMS measurements, both procedural blanks and standard materials are essential to ensure data quality and analytical reliability. Although blanks help quantify background contributions and contamination introduced during chemical processing and measurement, standard materials provide a reference framework for calibration, normalization, and accuracy verification. Together, they allow for meaningful interpretation of isotope ratios and detection limits in environmental matrices.
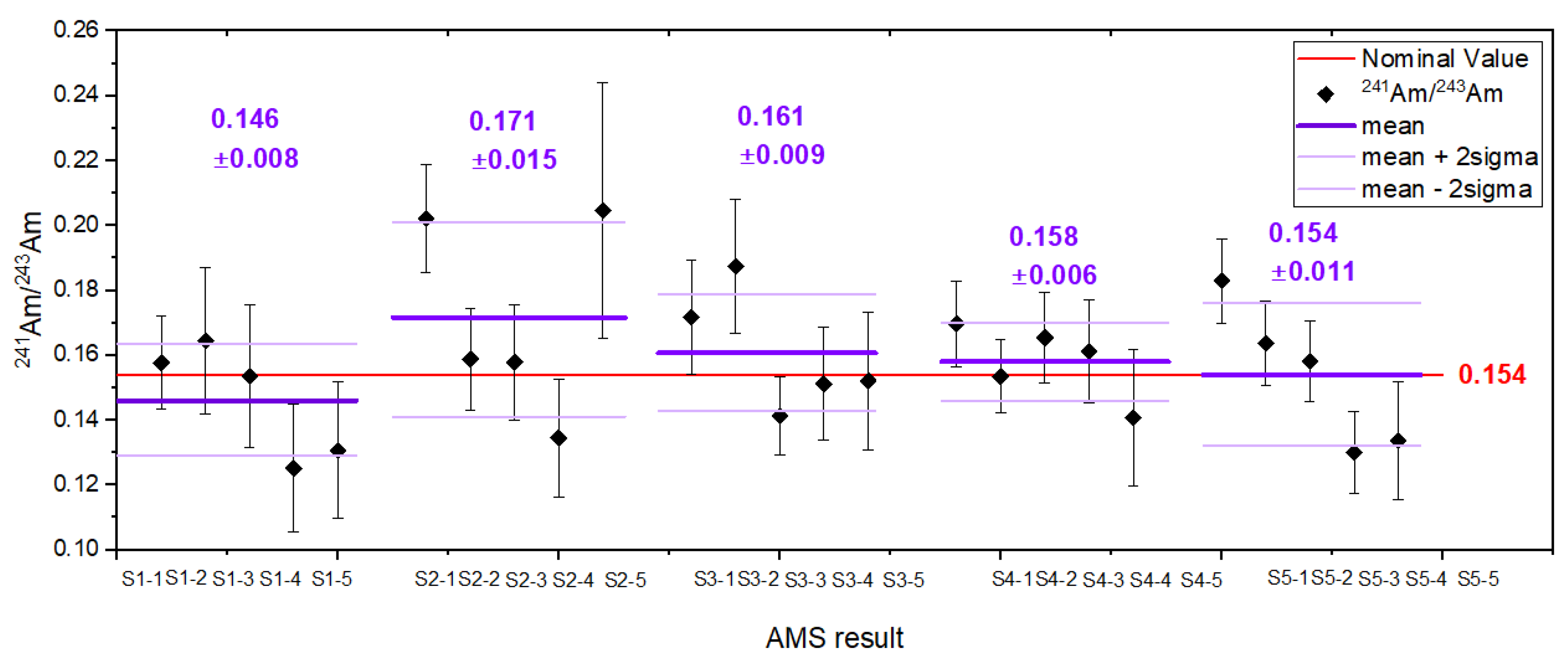
The first standard material evaluated in this study was the americium standard. Its atom ratio, /, was determined from the gravimetric composition and isotopic ratios of the mixed components, yielding a value of 0.154 ± 0.007 at·at−1. Targets prepared from this standard enable correction for potential fractionation effects occurring at any stage of the analytical procedure. Figure 2 presents results for five targets, each gravimetrically prepared from separate aliquots of the standard solution. The measured ratios are in very good agreement with the nominal value, confirming both the accuracy and reproducibility of the applied measurement protocol.
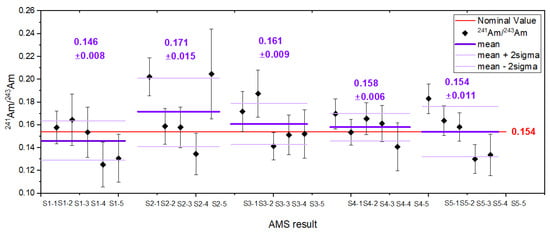
Figure 2.
The isotopic ratios for the laboratory prepared standard. Results are shown for five individual cathodes, along with the corresponding average value and associated uncertainty. The nominal (expected) value is indicated in red.
To assess the accuracy and precision of plutonium isotopic ratio measurements, the Plutonium Standard ColPuS [30] (approximately 1 pg Pu/g solution) was used. This standard is critical for AMS-based measurements of plutonium isotopes.
Although this standard has been extensively studied in our laboratory in the past [25], with measurements of , , , and , we only recently became aware that it also contains isotopes with mass 241. Consequently, it was included in the same measurement campaign as the americium standard to evaluate the quantification.
The results of the experiment are shown in Table 3, which illustrates the consistency between the AMS measurements and the nominal values of the standard. The AMS results that were not normalized to any secondary standard closely align with the nominal reference values, with minor discrepancies observed within the range of uncertainty. Table 3 lists the nominal values of the isotopic ratios and their corresponding reference source, serving as a basis for the validation and calibration of measurements.

Table 3.
Plutonium isotope ratios of the ColPuS standard material as calculated from the AMS measurements and the associated uncertainties. The samples labeled with TEVA® were subjected to the chemical procedure used to separate Pu from Am.
To further investigate the composition of this standard, it was subjected to the chemical separation procedure designed to isolate plutonium from americium. As shown in Table 3, the samples labeled with “TEVA”—indicating separation via TEVA® resin—show a lower value for the isotopic ratio . This suggests that the major contribution to mass 241 in this standard arises from rather than . This distinction is important, as americium and plutonium may behave differently during the ionization process in AMS, potentially affecting measurement accuracy.
The consistency observed in the concentrations of three plutonium isotopes throughout the analytical procedure indicates the absence of significant isotopic fractionation under the applied measurement conditions. Given the well-established chemical homogeneity among plutonium isotopes, it is reasonable to infer that similarly experienced no fractionation. Moreover, americium was efficiently separated prior to analysis, ensuring that the measured activity originates solely from plutonium isotopes.
The ColPuS standard was prepared by mixing monoisotopic plutonium solutions. A critical consideration when using such standards is the control of isotopic impurities, as these can influence the reproducibility and accuracy of analytical measurements. The study by Dittmann et al. [31] provides detailed information on the preparation and characterization of these monoisotopic solutions.
According to that study, the monoisotopic solutions—IRMM-081a () [32], IRMM-083 () [33], IRMM-043 (), and IRMM-042a () [34]—were supplied by JRC IRMM and carefully characterized to ensure high isotopic purity. Nonetheless, trace levels of secondary isotopes are present in these materials—for example, 2.202% of in IRMM-081a and 8.528% of in IRMM-043—contributing to isotopic impurities, including the presence of .
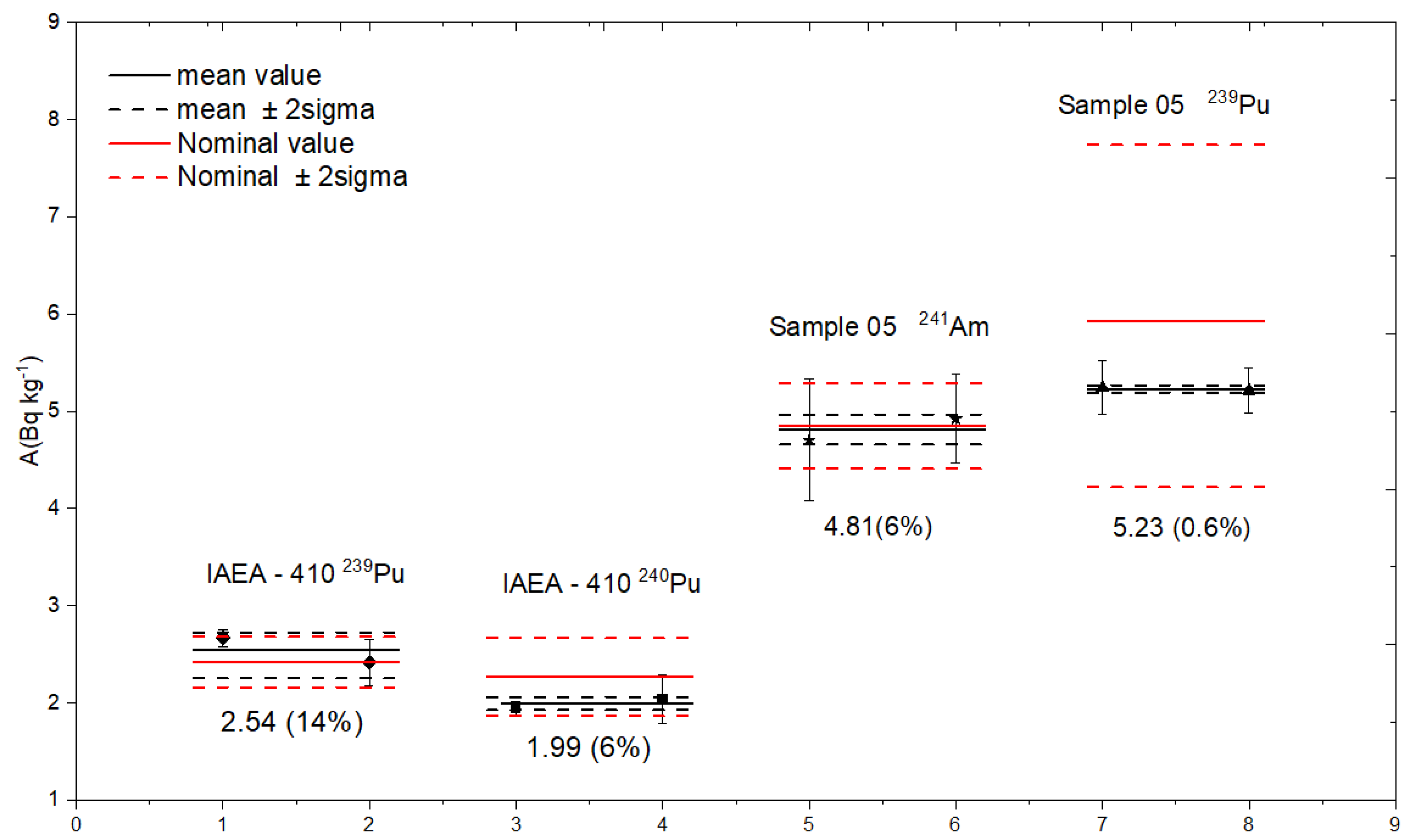
Figure 3 presents the activity concentrations of , , and measured in the certified reference materials IAEA-410 (marine sediment from Bikini Atoll) and Sample 05 (environmental water). The chemical digestion and separation were carried out using the procedure described earlier in this work. For IAEA-410, the measured activity concentration for was 2.54 ± 0.16 Bq·kg−1, in good agreement with the certified value of 2.42 ± 0.26 Bq·kg−1. Similarly, the measured value for was 1.99 ± 0.16 Bq·kg−1, compared to the nominal value of 2.27 ± 0.40 Bq·kg−1. For Sample 05, the activity concentration was determined to be 4.81 ± 0.54 Bq·kg−1, closely matching the certified value of 4.12 ± 0.28 Bq·kg−1. In the same water sample, was also measured at 5.23 ± 0.03 Bq·kg−1, in good agrement with the nominal value of 5.93 ± 2.27 Bq·kg−1, with a relative uncertainty of only 0.6%, demonstrating high precision.

Figure 3.
The concentration obtained for , from IAEA-410 and , from Sample 05 at 1MV AMS facility at IFIN-HH.
These results confirm the reliability and accuracy of the developed radiochemical separation and AMS measurement procedure. In the case of in IAEA-410 sediment, this isotope could not be quantified due to its low abundance relative to the background; only an upper limit of 6.52 Bq·kg−1 (as of January 2024) could be estimated. Moreover, could not be reliably quantified in the sediment due to interference from lanthanide elements, particularly dysprosium. This interference arises from molecular fragments (e.g., ) that can lead to pile-up events in the detector. To address this issue, it is necessary to include an additional TEVA® resin purification step prior to AMS measurement, as also recommended in [35].
In contrast, for Sample 05, the water sample, where only and were artificially added, both isotopes were successfully measured. The absence of naturally occurring lanthanides in this matrix confirms the effectiveness of the separation procedure for americium and plutonium under clean conditions. However, when analyzing sediment samples with higher dysprosium content, additional purification steps are essential to avoid spectral interferences and ensure accurate quantification.
4. Conclusions
This study demonstrates an efficient and precise method for the chemical separation and analysis of plutonium and americium isotopes in environmental samples, utilizing ion chromatography and accelerator mass spectrometry (AMS). The results, validated using certified reference materials such as Bikini Atoll sediments (IAEA-410) and spiked water, confirmed the accuracy and reliability of the proposed method. For example, for IAEA-410, the measured activity concentrations for the isotope were 2.54 ± 0.16 Bq·kg−1, which is in agreement with the certified value of 2.42 ± 0.26 Bq·kg−1, and for , the obtained value was 1.99 ± 0.16 Bq·kg−1, compared to the nominal value of 2.27 ± 0.40 Bq·kg−1. Similarly, for Sample 05, the measured activity concentration for was 4.81 ± 0.54 Bq·kg−1, close to the certified value of 4.12 ± 0.28 Bq·kg−1.
The research focused on overcoming the analytical challenges related to the accurate measurement of anthropogenic radionuclides in environmental matrices. These isotopes, originating from nuclear weapons testing and accidents, persist in the environment due to their significant half-lives and pose a major threat to health and the environment. One of the key innovations of this study is the good chemical separation achieved through ion chromatography. However, certain technical limitations encountered during the AMS analysis may have affected signal quality. Identified issues, including those related to data acquisition and spectrum monitoring, are currently under review and will be addressed in future work to ensure improved system performance. Although progress has been made, it is acknowledged that further optimization is necessary to improve the separation efficiency and reduce interferences in complex environmental samples. Nevertheless, this method has proven capable of delivering results within nominal value ranges. For example, the / ratio measured in the Am standard was 0.158 at·at−1 (3%), in excellent agreement with the nominal value of 0.154 at·at−1. Additionally, the / ratio in the ColPuS standard was determined to be 0.029 at·at−1 (7%). These results validate not only the accuracy of the method but also its potential for effective environmental monitoring and radionuclide analysis.
Author Contributions
Formal analysis, E.M., I.S. and D.P.; investigation, E.M., D.P., A.D., O.G. and D.V.M.; methodology, I.S. and D.P.; supervision, I.S.; writing—original draft, E.M. and I.S.; writing—review and editing, D.P., A.D., O.G. and D.V.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Research Programme Partnership in Priority Areas PNII MEN-UEFISCDI, contract PN 23210201. The experiments carried out using the 1 MV Tandetron accelerator from IFIN-HH were supported by the Romanian Government Programme through the National Programme for Infrastructure of National Interest (IOSIN).
Data Availability Statement
All relevant data supporting the findings of this study are included in the paper.
Acknowledgments
We would like to thank Elena Chamizzo (Centro Nacional de Aceleradores (CNA), Universidad de Sevilla, Spain) for valuable discussions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- National Nuclear Data Center. National Nuclear Data Center (NNDC) Wallet Cards; National Nuclear Data Center: Upton, NY, USA, 2025. [Google Scholar]
- Kelley, J.; Bond, L.; Beasley, T. Global distribution of Pu isotopes and 237Np. Sci. Total Environ. 1999, 237, 483–500. [Google Scholar] [CrossRef] [PubMed]
- Hirose, K.; Povinec, P.P. Sources of plutonium in the atmosphere and stratosphere-troposphere mixing. Sci. Rep. 2015, 5, 15707. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Oh, J.; Lee, J.; Lee, K.; Park, T.; Lujaniene, G.; Valiulis, D.; Šakalys, J. Distribution characteristics of 137Cs, Pu isotopes and 241Am in soil in Korea. Appl. Radiat. Isot. 2013, 81, 315–320. [Google Scholar] [CrossRef]
- Molero, J.; Sanchez-Cabeza, J.; Merino, J.; Batlle, J.V.; Mitchell, P.; Vidal-Quadras, A. Particulate distribution of plutonium and americium in surface waters from the Spanish Mediterranean coast. J. Environ. Radioact. 1995, 28, 271–283. [Google Scholar] [CrossRef]
- Wang, H.; Ni, Y.; Men, W.; Wang, Z.; Liu, M.; Xiao, D.; Zheng, J. Distributions of fallout 137Cs, 239+240Pu and 241Am in a soil core from South Central China. J. Environ. Radioact. 2022, 251, 106971. [Google Scholar] [CrossRef]
- Tinsley, T.; Sarsfield, M. Recycle of Am-241 Obtained from Long Term Stored Plutonium for Use in Radioisotope Power Systems-15035; WM Symposia, Inc.: Tempe, AZ, USA, 2015. [Google Scholar]
- Steier, P.; Hrnecek, E.; Priller, A.; Quinto, F.; Srncik, M.; Wallner, A.; Wallner, G.; Winkler, S. AMS of the minor plutonium isotopes. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2013, 294, 160–164. [Google Scholar] [CrossRef]
- Pacesila, D. Accelerator Mass Spectrometry in the Actinides Field. Ph.D. Thesis, Polytechnic University of Bucharest, Bucharest, Romania, 2019. [Google Scholar]
- Burducea, I.; Ghiţă, D.; Sava, T.; Straticiuc, M. Tandem accelerators in Romania: Multi-tools for science, education and technology. AIP Conf. Proc. 2017, 1852, 060001. [Google Scholar]
- International Atomic Energy Agency. Certification of Massic Activities of Radionuclides in IAEA-410 Bikini Atoll Sediment; International Atomic Energy Agency: Vienna, Austria, 2018. [Google Scholar]
- Chamizo, E.; López-Lora, M.; Villa, M.; Casacuberta, N.; López-Gutiérrez, J.; Pham, M. Plutonium isotope ratio measurements in marine samples using AMS at the CNA (Seville, Spain). Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2015, 361, 535–540. [Google Scholar] [CrossRef]
- Mauring, A.; Patterson, S.; Seslak, B.; Tarjan, S.; Trinkl, A. IAEA-TEL-2021-03 World Wide Open Proficiency Test Exercise, Pie-Charts, S-Shapes and Reported Results with Scores; International Atomic Energy Agency: Vienna, Austria, 2021. [Google Scholar]
- Horwitz, E.; Dietz, M.; Chiarizia, R.; Diamond, H.; Maxwell, S.; Nelson, D. Separation and Preconcentration of Actinides from Acidic Media by Extraction Chromatography. Anal. Chim. Acta 1993, 281, 361–372. [Google Scholar] [CrossRef]
- Horwitz, E.; McAlister, D.; Bond, A.; Barrans, R. Separation and Preconcentration of Actinides by Extraction Chromatography using a Supported Liquid Anion Exchanger: Application to the Characterization of High-Level Nuclear Waste Solutions. Anal. Chim. Acta 1995, 310, 63–78. [Google Scholar] [CrossRef]
- Horwitz, E.; Kalina, D.; Kaplan, L.; Mason, G. Novel Extraction Chromatographic Resins Based on Tetraalkyldiglycolamides: Characterization and Potential Applications. Solvent Extr. Ion Exch. 2005, 23, 219–236. [Google Scholar] [CrossRef]
- Maxwell, S.; Culligan, B.; Nichols, S.; Noyes, G. Rapid Analysis of Emergency Urine and Water Samples. J. Radioanal. Nucl. Chem. 2008, 275, 497–502. [Google Scholar] [CrossRef]
- Enachescu, M.; Stan-Sion, C.; Petre, A.R.; Postolache, C.; Fugaru, V. 3H and 14C measurements of the irradiated graphite from the decommissioned VVR-S reactor in NIPNE Bucharest. J. Anal. At. Spectrom. 2018, 33, 431–436. [Google Scholar] [CrossRef]
- Enachescu, M.; Stan-Sion, C.; Petre, A. The Bucharest 1 MV HVEE Accelerator Mass Spectrometer extended for measurements of hydrogen isotopes. Nucl. Instruments Methods Phys. Res. Sect. B 2019, 461, 149–153. [Google Scholar] [CrossRef]
- Pacesila, D.; Bishop, S.; Stanciu, I.; Enachescu, M.; Petre, A.R.; Virgolici, M.; Iancu, D.; Tugulan, L.; Done, L.; Petraglia, A.; et al. Ultrasensitive detection of 244Pu in environmental samples by accelerator mass spectrometry. J. Anal. At. Spectrom. 2022, 37, 2581–2588. [Google Scholar] [CrossRef]
- Pacesila, D.; Stanciu, I.; Simion, C.; Galin, S.; Mirea, M.; Lechintan, M.; Mereuta, P.; Mosu, V. Quantification of 239Pu radioactivity in swipe samples by accelerator mass spectrometry. Nucl. Instruments Methods Phys. Res. Sect. B 2023, 539, 43–46. [Google Scholar] [CrossRef]
- López-Lora, M.; Chamizo, E. Accelerator mass spectrometry of 237Np, 239Pu and 240Pu for environmental studies at the Centro Nacional de Aceleradores. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2019, 455, 39–51. [Google Scholar] [CrossRef]
- Scognamiglio, G.; Chamizo, E.; López-Gutiérrez, J.M.; Müller, A.M.; Padilla, S.; Santos, F.J.; López-Lora, M.; Vivo-Vilches, C.; García-León, M. Recent developments of the 1 MV AMS facility at the Centro Nacional de Aceleradores. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2016, 375, 17–25. [Google Scholar] [CrossRef][Green Version]
- Calvo, E.C.; Santos, F.J.; López-Gutiérrez, J.M.; Padilla, S.; García-León, M.; Heinemeier, J.; Schnabel, C.; Scognamiglio, G. Status report of the 1 MV AMS facility at the Centro Nacional de Aceleradores. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2015, 361, 13–19. [Google Scholar] [CrossRef]
- Stanciu, I.; Pacesila, D.; Bishop, S.; Enachescu, M.; Petre, A.; Virgolici, M.; Serban, A.; Albota, F.; Erhan, I.; Fugaru, V.; et al. Status report on AMS measurements of plutonium isotopes using the 1MV Tandetron Accelerator at IFIN-HH. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2022, 529, 1–6. [Google Scholar] [CrossRef]
- Dai, X.; Christl, M.; Kramer-Tremblay, S.; Synal, H.A. Ultra-trace determination of plutonium in urine samples using a compact accelerator mass spectrometry system operating at 300 kV. J. Anal. At. Spectrom. 2012, 27, 126–130. [Google Scholar] [CrossRef]
- Christl, M.; Dai, X.; Lachner, J.; Kramer-Tremblay, S.; Synal, H.A. Low energy AMS of americium and curium. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2014, 331, 225–232. [Google Scholar] [CrossRef]
- Kazi, Z.H.; Cornett, R.J.; Zhao, X.; Kieser, W.E. Comparison of the measurement of Pu and Am isotopes by AMS using fluoride and oxide anion beams. J. Anal. At. Spectrom. 2015, 30, 2235–2240. [Google Scholar] [CrossRef]
- Hotchkis, M.; Child, D.; Froehlich, M.; Wallner, A.; Wilcken, K.; Williams, M. Actinides AMS on the VEGA accelerator. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2019, 438, 70–76. [Google Scholar] [CrossRef]
- Dittmann, B.A.; Buompane, R.; Chamizo, E.; Christl, M.; Dewald, A.; Dunai, T.; Feuerstein, C.; Fifield, K.; Fröhlich, M.; Heinze, S.; et al. ColPuS, a new multi-isotope plutonium standard for Accelerator Mass Spectrometry. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2019, 438, 189–192. [Google Scholar] [CrossRef]
- Dittmann, B.A.; Dunai, T.J.; Dewald, A.; Heinze, S.; Feuerstein, C.; Strub, E.; Fifield, L.K.; Froehlich, M.; Tims, S.; Wallner, A.; et al. Preparation of a multi-isotope plutonium AMS standard and preliminary results of a first inter-lab comparison. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2015, 361, 327–331. [Google Scholar] [CrossRef]
- Preparation and Certification of IRMM-081a and IRMM-086 Spike Isotopic Reference Materials. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC40700 (accessed on 25 October 2023).
- Certification of 240Pu Spike Reference Material: IRMM-083. Available online: https://crm.jrc.ec.europa.eu/p/40454/40475/By-application-field/Nuclear/IRMM-083-PLUTONIUM-240-SPIKE-NITRATE-SOLUTION/IRMM-083 (accessed on 25 October 2023).
- Certification of 244Pu Spike Reference Material: IRMM-042a: Certified Reference Material for the Amount Content of 244Pu and Pu Isotope Amount Ratios. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC108889 (accessed on 25 October 2023).
- López-Lora, M.; Chamizo, E.; Levy, I.; Olszewski, G.; Victoria, L.; López Fuentes, A. Sequential Extraction of U, Np, Pu and Am from Sediment Samples for AMS Studies at the Centro Nacional de Aceleradores (CNA, Spain). In Proceedings of the AMS16 Conference, Guilin, China, 20–26 October 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).