Abstract
Sensory systems allow the detection of external and internal cues essential for adaptive responses. Chemosensation exemplifies this integration, guiding feeding, mating, and toxin avoidance while also influencing physiological regulation. Across taxa, chemical detection relies on diverse receptor families, and emerging evidence reveals that transient receptor potential (TRP) channels—traditionally associated with phototransduction, thermosensation, and mechanotransduction—also mediate chemosensory functions. Studies in Drosophila melanogaster and vertebrates demonstrate that TRPs detect tastants, odorants, and internal chemical states, highlighting their evolutionary conservation and functional versatility. This review synthesizes current insights into the roles of TRP channels across four major domains: taste, smell, internal state, and central circuit modulation. Using D. melanogaster and mammalian systems as comparative frameworks, we highlight how TRP channels function as polymodal sensors, signal amplifiers, and modulators embedded within canonical receptor pathways rather than as standalone chemoreceptors. Recognizing these integrative functions not only expands our understanding of how organisms coordinate behavior with internal states but also points to TRP channels as potential targets for addressing chemosensory disorders and metabolic diseases. This framework highlights key directions for future research into TRP-mediated sensory and homeostatic regulation.
1. Introduction
How the five senses—vision, olfaction, audition, gustation, and somatosensation—interact with the central nervous system remains a fundamental enigma in neuroscience. Sensory perception is classically defined as the detection of external stimuli by specialized peripheral receptors followed by signal transmission to the brain, enabling organisms to experience their environment and thereby secure survival and reproduction. Increasing evidence demonstrates that sensory systems not only detect external cues but also integrate internal signals to regulate physiology and behavior in dynamic and adaptive ways.
Studies in Drosophila have been pivotal in advancing this paradigm [1,2]. For example, larval gustatory receptor neurons (GRNs) serve as nutrient sensors that modulate systemic growth by engaging central neuroendocrine circuits [3]. Similarly, in vertebrates, taste perception is not exclusively defined by peripheral input: activation or silencing of discrete cortical ensembles can elicit or abolish taste experiences independently of receptor activity [4]. Such findings highlight that sensory meaning is constructed centrally and that sensory modalities extend beyond external detection to include the monitoring of internal states, ultimately governing homeostasis [5,6].
Among all modalities, chemosensation most clearly exemplifies this integrative capacity. It provides a unique window into how organisms translate chemical cues into neural representations that shape physiology and behavior [7]. At its foundation, chemosensation relies on specialized receptors that transduce ligand binding into electrical and biochemical activity [8]. This occurs at the periphery, through external sensory organs that detect volatile or soluble compounds, and internally, where sensors continuously monitor chemical parameters such as pH, CO2, or osmolarity to sustain homeostasis [9]. The interplay of these systems underlies vital behaviors—including feeding, mating, and metabolic regulation—across diverse taxa [10,11,12,13,14].
Chemical perception is tightly linked to molecular properties. Volatile compounds disperse through the air and activate olfactory receptors, generating odors, whereas non-volatile or structurally incompatible molecules remain undetectable olfactorily. In contrast, water-soluble compounds activate gustatory receptors upon ingestion, providing critical information about the nutritive or toxic qualities of food [15,16,17]. Taste thus represents a fundamental chemosensory system that guides dietary decisions essential for survival, balancing nutrient acquisition against toxin avoidance. Despite pronounced anatomical divergence between insects and mammals, both taxa rely on shared principles of sensory transduction [18,19,20].
Among conserved molecular players, transient receptor potential (TRP) channels stand out. While originally recognized for their roles in thermosensation and mechanotransduction, TRP channels are now increasingly implicated in chemosensation across four domains: taste, smell, internal state sensing, and central circuit processing. Their evolutionary conservation and polymodal gating properties make them powerful models for understanding how chemical information is encoded and integrated across species.
In this review, we synthesize current knowledge on the role of TRP channels in chemosensation, with particular emphasis on D. melanogaster and comparisons to mammalian systems. By bridging molecular, cellular, and systems-level insights, we aim to highlight conserved principles, point to key divergences, and provide a framework for future research into how TRP channels contribute to the interface between chemical environments and organismal physiology.
2. TRP Channels as Integrators of Chemosensation
Chemosensation is not restricted to peripheral processes but instead operates as an organized system encompassing four interconnected domains: taste, smell, internal physiological state, and central neural circuits. Taste represents a core chemosensory modality that enables animals to evaluate the nutritional value and toxicity of ingested substances [21,22]. Classically defined by five conserved modalities—sweet, umami, salty, bitter, and sour—taste is closely aligned with ecological, physiological, and behavioral needs [23,24,25]. Yet this canonical framework has expanded. Drosophila, for instance, detects alkaline compounds via specialized gustatory circuits [26,27], and both insects and vertebrates sense fatty acids, calcium, and amino acids as distinct taste modalities [28,29,30,31,32]. These discoveries underscore that the taste system is evolutionarily dynamic, tuned to the nutritional pressures of distinct ecological niches [13,33].
In mammals, taste receptor cells are organized into taste buds distributed across the tongue and soft palate. On the tongue, taste buds are localized to three classes of papillae: fungiform papillae distributed across the anterior two-thirds of the tongue, foliate papillae along the posterior lateral edges, and a single circumvallate papilla positioned at the posterior midline. Taste buds on the tongue and soft palate are innervated by three major afferent nerves—the chorda tympani, greater superficial petrosal, and glossopharyngeal nerves—which convey gustatory information from taste receptor cells to the nucleus of the solitary tract (NST) in the brainstem. From the NST, taste responses are transmitted through the parabrachial nucleus and ventral posteromedial thalamus to the primary gustatory cortex in the insula, where gustatory information is integrated with other sensory modalities, including olfaction and texture, to shape feeding behavior and flavor perception [14,34,35,36] (Figure 1). Each basic taste modality is encoded by discrete receptor cell populations expressing defined receptor families, including T1R heterodimers for sweet and umami [37,38,39], T2R receptors for bitter compounds [40,41,42], epithelial sodium channels (ENaC) for amiloride-sensitive salt detection [43], PKD2L1-expressing cells for sour taste [44], and carbonic anhydrase IV–dependent mechanisms for carbonation sensing [45], which together project via the brainstem to cortical gustatory centers.
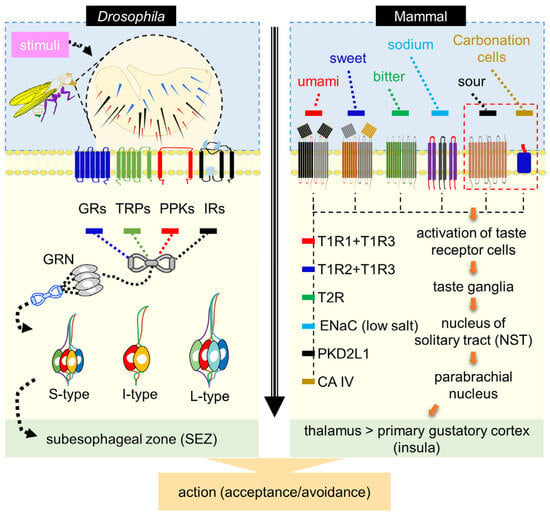
Figure 1.
Comparative taste transduction model in Drosophila and mammals. Left, in Drosophila, taste detection is mediated by gustatory receptor neurons (GRNs) distributed across multiple peripheral organs, including the proboscis (labellum), legs, wing margins, and ovipositor. Each labellar hemisphere contains 31 gustatory sensilla, classified as long (L), intermediate (I), or short (S) based on morphology and position. GRNs express diverse chemosensory receptors and ion channels, including gustatory receptors (GRs), ionotropic receptors (IRs), pickpocket channels (PPKs), and transient receptor potential (TRP) channels, enabling functional specialization for sugars, water, salts, and bitter compounds. Axons of these GRNs project to the subesophageal zone (SEZ), where peripheral taste information is integrated to drive feeding-related decisions. Right, in mammals, taste transduction occurs in epithelial taste receptor cells organized into taste buds on the tongue and soft palate. Distinct receptor cell populations encode specific taste modalities through defined receptor families (e.g., T1Rs, T2Rs, ENaC, PKD2L1, and carbonic anhydrase IV). Taste information is conveyed via dedicated afferent nerves to the nucleus of the solitary tract (NST) in the brainstem and subsequently relayed through parabrachial and thalamic nuclei to the primary gustatory cortex in the insula. Despite profound anatomical differences between insect and mammalian taste systems, both implement a shared organizational principle in which modality-specific receptor cells map chemical features onto labeled-line pathways that ultimately guide acceptance or avoidance behaviors.
By contrast, in D. melanogaster, GRNs are distributed across diverse tissues, including the labellum, ovipositor [46,47,48,49], internal pharynx [50], legs [51], and wings [52]. The labellum contains both hair-like sensilla and taste pegs. Labellar sensilla are classified into long (L), intermediate (I), and short (S) types, each housing distinct subsets of GRNs. S-type sensilla detect water, sugars, bitter compounds, and salts; I-type sensilla respond primarily to sugars and bitters; and L-type sensilla contain water- and sweet-sensitive neurons, with one neuron likely tuned to low sodium [47,49,53,54,55,56]. GRNs converge on the subesophageal zone (SEZ), the insect’s primary gustatory center. This distributed organization allows Drosophila to sample both external and internal chemical cues (Figure 1).
The molecular toolkit underlying insect chemosensation is equally diverse. GRs mediate responses to sugars [57,58,59,60] and bitters [61,62,63,64], IRs detect bitters [65], salts [66,67,68], acids [69,70,71] and alkalis [26,27], and members of the pickpocket (PPK) family contribute to pheromone and water sensing as well as mechanotransduction [72,73,74,75,76,77]. Together, these receptor families establish a robust sensory network that links food chemistry to behavior.
Olfaction mirrors taste in organizational logic but specializes in volatile detection. In mammals, odorant receptors (ORs) expressed by olfactory sensory neurons (OSNs) form a chemotopic map in the olfactory bulb, projecting to higher-order centers including the piriform cortex, amygdala, and hypothalamus [78,79,80]. These circuits not only mediate odor discrimination but also couple with emotional and physiological states. In insects, OSNs located in antennal and maxillary palp sensilla project to glomeruli in the antennal lobe, the analog of the mammalian olfactory bulb. Projection neurons then convey signals to the mushroom body and lateral horn [81,82,83]. Detection in insects relies on ORs, IRs, and in some cases GRs, reflecting evolutionary divergence in receptor repertoires despite conserved glomerular architecture [84,85,86].
While GRs, IRs, ORs, and PPKs define much of the classical chemosensory machinery, TRP channels have emerged as versatile and conserved players in chemical detection [87]. Originally identified in Drosophila phototransduction [88,89,90], TRPs are now known to encompass seven subfamilies—TRPA, TRPC, TRPM, TRPML, TRPN, TRPP, and TRPV—comprising 13 members in flies and 28 in mammals (Figure 2) [91,92,93,94].
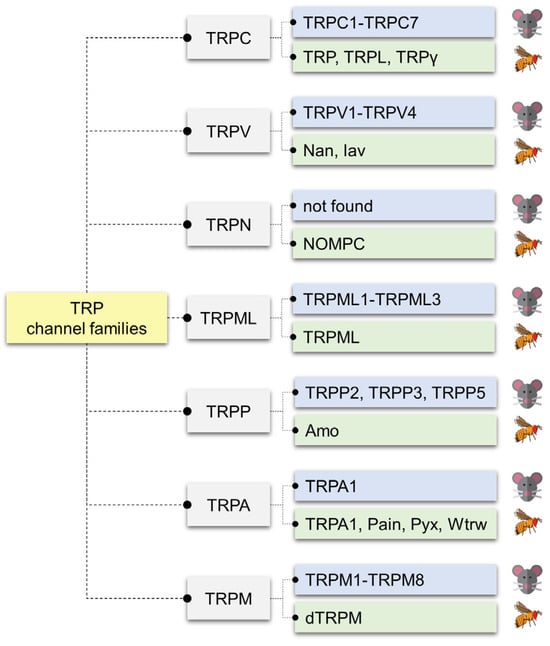
Figure 2.
Comparison of TRP channel families in mammals and Drosophila melanogaster. Transient receptor potential (TRP) channels are classified into seven major subfamilies: canonical (TRPC), vanilloid (TRPV), no mechanoreceptor potential C (TRPN), mucolipin (TRPML), polycystin (TRPP), ankyrin (TRPA), and melastatin (TRPM). Each family includes mammalian members (blue boxes) and their Drosophila homologs (green boxes). The TRPC subfamily comprises mammalian TRPC1–TRPC7 and Drosophila TRP, TRP-like (TRPL), and TRP-gamma (TRPγ). The TRPV family includes mammalian TRPV1–TRPV4 and Drosophila Nanchung (Nan) and Inactive (Iav). The TRPN family is not found in mammals but is represented in Drosophila by No mechanoreceptor potential C (NOMPC). The TRPML family includes mammalian TRPML1–TRPML3 and Drosophila TRP mucolipin (TRPML). The TRPP family consists of mammalian TRPP2, TRPP3, and TRPP5, and the Drosophila homolog Amo. The TRPA family includes mammalian TRPA1 and Drosophila TRPA1, Painless (Pain), Pyrexia (Pyx), and Water witch (Wtrw). The TRPM family includes mammalian TRPM1–TRPM8 and Drosophila TRPM (dTRPM).
From an evolutionary standpoint, TRPs epitomize both conservation and versatility. They participate in thermosensation, mechanotransduction, chemical detection, and intracellular signaling. Increasingly, they are appreciated as central integrators of chemosensory input in both insects and mammals, bridging canonical taste and olfactory pathways with broader physiological regulation. This positions TRP channels as a molecular nexus for chemosensory integration internally as well as externally and sets the stage for examining their modality-specific contributions.
3. Structural Importance and Chemosensory Function
The ability of TRP channels to detect diverse chemical cues arises from their highly adaptable structural design. All TRP channels share a conserved six-transmembrane (S1–S6) architecture that assembles into a tetrameric ion-conducting pore [95,96,97]. The pore is formed by the S5–S6 helices and the intervening loop, whereas the cytoplasmic N- and C-termini provide additional regulatory flexibility (Figure 3A).
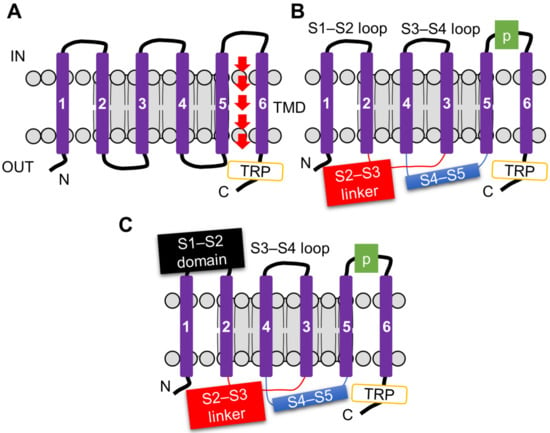
Figure 3.
Structural organization of transient receptor potential (TRP) channels. (A) Canonical TRP channel subunit composed of six transmembrane segments (S1–S6) with a pore loop between S5 and S6, and cytosolic N- and C-termini. The C-terminal region contains the conserved TRP helix adjacent to the membrane. (B) Representative membrane architecture of TRPV, TRPA1, TRPM, and TRPC channels, highlighting the voltage sensor–like domain (S1–S4), the S4–S5 linker, and the C-terminal TRP helix that couples conformational changes to gating. (C) Structural features of TRPML and TRPP channels, which lack the canonical TRP helix but possess an extended S1–S2 loop, reflecting subfamily-specific structural adaptations. These conserved and divergent elements define the gating and regulatory diversity of TRP channels across families.
A key feature of TRPs in chemosensation is that they couple a conserved structural core with evolutionary adaptations that broaden their functional repertoire. The S1–S4 region adopts a voltage-sensing-like fold; rather than serving as a classical voltage sensor, it communicates with the pore via the S4–S5 linker, enabling polymodal gating by chemicals, temperature, and mechanical stimuli [98] (Figure 3B). In many subfamilies, such as TRPV and TRPA1, a characteristic TRP helix functions as a molecular lever for gating, whereas TRPML and TRPP channels lack this helix and instead employ expanded S1–S2 loops, representing subfamily-specific strategies for stimulus detection [99] (Figure 3C). Equally critical are the cytoplasmic domains, which incorporate ankyrin repeats, coiled-coils, and specialized binding pockets for phosphoinositides, toxins, and metabolites. These domains act as integration hubs that align TRP activity with both internal metabolic status and external chemical challenges [100,101].
At higher resolution, different TRP subfamilies contain specialized ligand-binding or reactive motifs that directly couple chemical recognition to channel gating. For example, in TRPV1, a hydrophobic “vanilloid pocket” located between the S3–S4 helices, the S4–S5 linker, and the S6 helix binds capsaicin, resiniferatoxin, and related ligands. Structural and mutagenesis studies identify residues Y511, S512, M547, and T550 as key determinants of capsaicin binding and activation [95,96]. In electrophile-sensing TRPA1, reactive irritants modify a cluster of nucleophilic residues in the membrane-proximal N-terminus—most notably Cys621, Cys641, Cys665, and Lys710—forming an allosteric sensor that stabilizes the open state [97,102]. Together, these examples show how specific structural microdomains within the conserved TRP scaffold encode chemical specificity and link ligand binding directly to pore gating.
Thus, TRP channels embody a unifying principle: a conserved ion channel scaffold combined with modular extensions that confer chemosensory versatility. Their architecture is not simply structural—it provides the mechanistic basis by which a single channel family can mediate responses to sugars, irritants, toxins, pH, and a wide range of other chemical cues.
4. TRP Channels in Mammalian Taste and Trigeminal Chemesthesis
TRP channels form a large superfamily of cation-permeable ion channels that act as molecular sensors for a wide spectrum of physical and chemical stimuli, including temperature [103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121], metabolic state [122,123,124,125,126,127,128,129], mechanical forces [130,131,132,133,134,135,136,137,138,139], and diverse small molecules [140,141,142,143,144]. Mammals possess 28 TRP genes; in humans, 27 remain functional because TRPC2 has been pseudogenized in catarrhine primates (Old World monkeys and apes), but TRPC2 remains intact in many New World monkeys and prosimian species [145,146,147,148,149].
TRP channels are broadly expressed in neuronal [150,151,152,153,154], epithelial [155,156,157,158,159,160,161], and sensory tissues [162,163,164,165,166]. They regulate the movement of Ca2+, Na+, K+, and Mg2+ ions, thereby linking environmental and physiological inputs to excitability. While many family members operate at the plasma membrane, others function intracellularly—for example, TRPML1–3 in lysosomes [167,168] and TRPP2, TRPP3 and TRPP5 in cilia and endosomes [169].
All share a conserved six-transmembrane architecture that assembles as a tetramer, but their divergent N- and C-terminal domains generate distinct gating and regulatory properties. This structural modularity underlies the diverse sensory roles of TRPs, ex-tending from thermosensation and mechanotransduction to chemosensory functions in taste and chemesthesis (Table 1).

Table 1.
Transient Receptor Potential channels in mammalian chemosensation.
4.1. Taste
The best-established TRP in mammalian taste is TRPM5. In Type II taste cells, activation of T1R or T2R G-protein-coupled receptors (GPCRs) triggers a PLCβ2–IP3R3 signaling cascade, releasing Ca2+ from intracellular stores. Elevated cytosolic Ca2+ activates TRPM5 and TRPM4, leading to membrane depolarization and neurotransmitter release through CALHM1/3 channels. Knockout of TRPM5 or PLCβ2 abolishes sweet, bitter, and umami responses, confirming TRPM5 as essential for these modalities [171,208]. TRPM5 is also temperature-sensitive within the physiological range (~15–35 °C), which explains the enhancement of sweetness perception at warmer temperatures [116,209]. Dietary modulators such as steviol glycosides can potentiate TRPM5 activity, and human TRPM5 polymorphisms have been linked to variation in taste and metabolic phenotypes (Figure 4) [210,211,212].
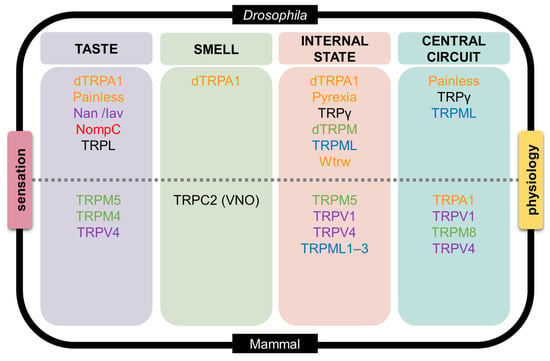
Figure 4.
An integrative model linking sensation and physiology through TRP channels in Drosophila and mammals. This schematic organizes representative TRP channels into four functional domains—taste, smell, internal physiological state, and central neural circuits—with Drosophila depicted in the upper half and mammals in the lower half. In Drosophila (top), taste-related processes include dTRPA1 and Painless, as well as mechanosensory TRP channels (Nanchung (Nan), Inactive (Iav), and NompC) that contribute to texture-dependent modulation of feeding behavior. TRPL is placed in the taste domain as a modulatory TRPC channel implicated in experience-dependent gustatory plasticity rather than primary tastant detection. Olfactory and irritant detection involves dTRPA1, while internal physiological state sensing engages dTRPA1, Pyrexia, TRPγ, dTRPM, TRPML, and Water witch (Wtrw), reflecting roles in thermosensation, hygrosensation, metabolic integration, and intracellular signaling. Central circuit modulation is mediated by Painless, TRPγ, and TRPML, which shape nociceptive processing, metabolic coupling, and glia–neuron interactions. In mammals (bottom), taste transduction is supported by TRPM5, TRPM4, and TRPV4 downstream of canonical taste receptors. Smell and chemesthesis involve TRPC2 in the vomeronasal organ and polymodal irritant receptors TRPV1, TRPA1, and TRPM8 in trigeminal pathways. Internal physiological sensing includes TRPM5, TRPV1, TRPV4, and TRPML1–3, which contribute to metabolic regulation, energy homeostasis, and lysosomal signaling. Central processing engages TRPA1, TRPV1, TRPM8, and TRPV4, where these channels regulate excitability, nociception, and sensory gain. Channel names are color-coded by TRP subfamily—TRPA (orange), TRPC (black), TRPN (red), TRPM (green), TRPV (purple), and TRPML (blue)—highlighting how conserved TRP families are deployed at different hierarchical levels to integrate external chemical cues with internal physiological state across taxa.
Additional TRPs contribute more indirectly. TRPV4 functions as an osmosensor [125,213,214] and mechanosensor [200,215,216,217], responding to hypotonicity, shear stress, and warm temperatures [108,218,219,220]. Recent evidence suggests that it may influence the differentiation of sour-sensing taste cells rather than act as a primary tastant transducer. Mice lacking TRPV4 show defects in the differentiation of Type III (sour-responsive) taste cells, indicating that TRPV4 contributes to epithelial patterning rather than direct tastant transduction [201].
4.2. Smell and Chemesthesis (Trigeminal Irritation)
Among mammalian TRPs, TRPC2 exemplifies a chemosensory specialization. In rodents, TRPC2 is expressed in the vomeronasal organ (VNO), where pheromone detection activates a phospholipase C–diacylglycerol pathway requiring TRPC2 function [221]. Genetic ablation of TRPC2 abolishes VNO responses and disrupts pheromone-dependent behaviors such as male–male aggression and mating [221,222].
Beyond pheromone signaling, several TRPs mediate chemesthesis in trigeminal pathways. TRPV1 detects capsaicin, noxious heat, acid, ethanol, and other pungent irritants through a hydrophobic “vanilloid pocket” formed by segments of the S3–S4 helices, the S4–S5 linker, and the S6 helices. Point mutations of residues such as Y511, S512, M547, and T550 in human TRPV1 strongly reduce capsaicin binding and gating, underscoring how this local pocket couples chemical occupancy to pore opening [95,96,104,117,183,223]. TRPA1 responds to reactive electrophiles such as isothiocyanates and cinnamaldehyde. In mammalian TRPA1, these electrophiles covalently modify a cluster of nucleophilic residues in the membrane-proximal N-terminus—notably Cys621, Cys641, Cys665, and, in some contexts, Lys710—within an allosteric nexus that couples chemical adduction to pore opening. This covalent gating mechanism allows TRPA1 to function as a broad-spectrum detector of pungent and irritant chemicals [97,102,184,224,225].
TRPM8 is activated by menthol, eucalyptol, linalool, icilin, and environmental cooling (<25 °C). Genetic knockout studies confirmed that TRPM8 is essential for neuronal and behavioral responses to cooling and menthol, making it the principal cold sensor in mammals [191,194,195]. Structural and mutagenesis studies localize the binding pocket for menthol and icilin to the voltage sensor–like domain (S1–S4) and the S4–S5 linker, where aromatic and hydrophobic residues create a cavity that stabilizes the open conformation in response to cooling compounds [226,227,228,229].
Together, these findings establish that TRP channels complement but do not replace canonical taste receptors. Beyond taste buds, TRPV1, TRPA1, and TRPM8 extend chemosensation into trigeminal irritation and thermal perception, shaping oral burning, pungent, and cooling sensations (Figure 4) [104,188,194].
4.3. Internal Sensing
Several TRP subfamilies monitor internal physiological state, contributing indirectly to chemosensory perception. TRPM5 links sweet and umami taste transduction to incretin signaling and glucose homeostasis. TRPV1 and TRPV4 influence energy expenditure and susceptibility to diet-induced obesity. TRPA1 and other TRPs contribute to thermogenesis and nutrient handling in adipose, hepatic, and neuronal tissues. TRPV1-null mice exhibit enhanced thermogenesis and resistance to high-fat–diet-induced obesity, directly demonstrating a role for TRPV1 in metabolic homeostasis [122,123,124,125,126,127,128,129]. TRPML1–3 function intracellularly in lysosomes to regulate Ca2+ and other cation release, lysosomal trafficking, autophagy, and organelle homeostasis [167,168]. These roles underscore the importance of mammalian TRP channels as metabolic sensors that shape chemosensory perception indirectly (Figure 4).
4.4. Central Processing
TRP channels also influence chemosensory processing at circuit levels. TRPA1 and TRPV1 are expressed in nociceptive and visceral sensory neurons, where they integrate thermal, mechanical, and chemical cues to regulate pain, irritation, and inflammatory responses. Genetic deletion of TRPA1 abolishes nocifensive responses to mustard oil, acrolein, and other electrophilic irritants, showing that TRPA1 is required for chemical nociception in vertebrates. TRPM8 contributes to central cold pathways by driving cooling-responsive trigeminal and spinal neurons, while TRPV4 modulates mechanotransduction and osmotic sensitivity in sensory ganglia [200,215,216,217]. In each case, TRP channels act as excitability regulators that shape the gain and quality of chemosensory inputs prior to their integration in higher-order circuits (Figure 4).
In mammals, TRP channels operate as polymodal sensors that integrate thermal, mechanical, and chemical information to shape chemosensory perception [230]. Although TRPC2 function remains debated, other TRPC members—TRPC1, TRPC3, and TRPC4—show expression in orofacial epithelia and trigeminal ganglia, but definitive roles in taste remain unresolved [231].
Notably, the mechanisms for CO2 detection differ strikingly between insects and mammals. In Drosophila, CO2 is sensed by a dedicated gustatory receptor pair, Gr63a/Gr21a, expressed in antennal neurons. These receptors are necessary and sufficient for CO2 sensitivity. When researchers knocked out plc21C, trp, or trpl, the CO2-sensitive neurons responded weakly, and flies showed reduced avoidance of CO2, indicating that while Gr63a/Gr21a serve as the primary detectors, the PLC–TRPC pathway boosts the signal to ensure strong behavioral responses [232,233]. This arrangement illustrates how, in insects, TRP channels act primarily as signal amplifiers downstream of dedicated chemoreceptors, rather than as the detectors themselves. In rodents, by contrast, CO2 detection does not rely on canonical odorant GPCRs. Instead, a distinct subset of olfactory sensory neurons expressing guanylyl cyclase-D (GC-D) responds selectively to CO2. These neurons detect bicarbonate, a metabolic product of CO2 formed by carbonic anhydrase activity, which directly stimulates GC-D to generate cGMP, thereby activating downstream cyclic nucleotide-gated channels and producing excitation [234,235]. These GC-D neurons form a parallel CO2-sensing pathway that is separate from the Golf–adenylyl cyclase III–cAMP–CNG cascade used by most odorant receptors in the olfactory epithelium. Thus, whereas flies rely on Gr63a/Gr21a GPCRs coupled to TRP/TRPL channels, mammals employ GC-D neurons with cGMP signaling, highlighting fundamentally different molecular logics for CO2 detection across phyla.
Finally, non-TRP channels complete the core taste repertoire: OTOP1 mediates sour detection, and ENaC underlies amiloride-sensitive salt responses, particularly in rodents [236,237]. Taken together, these findings establish that TRP channels function mainly as downstream excitability amplifiers in taste cells and as polymodal irritant receptors in trigeminal pathways, complementing canonical taste receptors such as T1Rs, T2Rs, OTOP1, and ENaC. This complementary role underscores the versatility of TRPs in shaping flavor perception through both taste-dependent and chemesthetic pathways.
5. TRP Channels Across Drosophila Chemosensors
Building on the mammalian framework presented above, this section examines how four analogous themes—taste, smell, internal sensing, and central processing—are implemented through distinct yet evolutionarily informative TRP mechanisms in Drosophila. The D. melanogaster genome encodes 13 TRP channels spanning all seven subfamilies—TRPC, TRPV, TRPA, TRPN, TRPM, TRPML, and TRPP [238] (Table 2). First identified through trp phototransduction mutants [88,89,90,239], these channels have become a central model for studying ion channel structure, gating, and multimodal sensory integration.

Table 2.
Transient Receptor Potential channels in Drosophila chemosensation.
5.1. Taste
In the gustatory system, TRP channels primarily act as aversive stimulus sensors rather than canonical taste receptors. Whereas mammalian taste relies on specialized taste buds with GPCR-driven transduction, Drosophila taste is organized through a distributed array of GRNs, where TRP channels act within fundamentally different receptor architectures to modulate gustatory coding. Expressed in gustatory receptor neurons (GRNs) of the labellum and antennae, dTRPA1 activation depolarizes these neurons, triggering aversive feeding for aristolochic acid [285,286,287].
Painless, another Drosophila TRPA channel, is expressed in class IV multidendritic neurons and in the proboscis, where it mediates aversive responses to wasabi (allyl isothiocyanate) and related isothiocyanates [243]. Painless is heat-activated and required for aversion to pungent isothiocyanates; heat responses are Ca2+-dependent, consistent with its role as a multimodal nociceptor in these circuits [118,243,244]. Its multimodal sensitivity allows Painless to integrate thermal and chemical harm cues, supporting larval nocifensive rolling and adult feeding avoidance behaviors. Beyond acute signal amplification, TRP channels—particularly the canonical TRPC member TRPL—contribute to experience-dependent modulation of gustatory sensitivity. Chronic exposure to the aversive but non-toxic compound camphor induces taste desensitization in Drosophila through TRPL-dependent mechanisms [272]. Rather than functioning as a primary tastant receptor, TRPL acts as a modulatory channel whose protein abundance is dynamically regulated via Ube3a-mediated ubiquitination and degradation. This reversible reduction in TRPL levels attenuates bitter-sensing GRN responsiveness and promotes adaptive changes in feeding behavior, identifying TRPL as a key molecular substrate for taste plasticity (Figure 4).
In addition to chemical detection, the taste organs also encode mechanical features of food (texture), which strongly shape feeding decisions. Mechanosensory neurons associated with labellar gustatory sensilla use TRP channels to detect physical properties during feeding. Nanchung (Nan) is required in labellar mechanosensory neurons for texture-dependent feeding choices and can suppress sweet-driven responses, whereas NompC localizes to neurons within gustatory sensilla and is essential for mechanosensory-evoked activity underlying texture detection; Nan acts together with its obligate TRPV partner Inactive (Iav) in TRPV-dependent mechanosensory signaling [275,288].
5.2. Smell
In contrast to mammalian olfaction—which depends on a vast GPCR repertoire and the olfactory bulb—Drosophila olfactory signaling operates through OR/IR-based ionotropic receptor systems, with TRP channels serving distinct modulatory roles within this invertebrate-specific framework. Among chemosensory TRPs, dTRPA1 is the most extensively characterized. It responds to electrophilic irritants, including isothiocyanates and acrolein, via covalent modification of nucleophilic cysteine residues in its N-terminal ankyrin repeat domain, in a membrane-proximal cluster analogous to the electrophile-sensing cysteine nexus defined in mammalian TRPA1, inducing conformational changes that open the pore and allow Na+ and Ca2+ influx [102,242]. In the antenna, dTRPA1 expression in olfactory circuits enables detection of volatile irritants: dTRPA1 activation depolarizes these neurons and triggers aversive positional responses for citronellal. Flies lacking dTRPA1 fail to avoid citronellal and other reactive volatiles, demonstrating that dTRPA1 is required for odor-evoked aversive behavior in vivo [285,286,287]. This multimodal gating makes dTRPA1 a sensor that integrates reactive volatile chemicals and temperature cues to drive odor-guided avoidance behaviors (Figure 4).
5.3. Internal Sensing
Unlike mammals, where TRPs often monitor visceral chemistry through epithelial and endocrine pathways, Drosophila internal sensing relies on neuroendocrine circuits and metabolic neurons in which TRP channels integrate nutritional, hormonal, and intracellular signals to regulate systemic physiology. Several TRP channels contribute to chemosensation by encoding internal thermal, ionic, metabolic, and hygrosensory states rather than directly detecting tastants or odorants. dTRPA1 contributes to temperature entrainment of the circadian clock neurons [289]. dTRPA1-expressing neurons form a thermo-sensitive circuit that regulates siesta duration and sleep timing in response to ambient temperature changes [290]. Additionally, dTRPA1 contributes to thermotaxis through intrinsic heat sensitivity arising from allosteric coupling among cytosolic ankyrin repeats, transmembrane helices (including the VSLD), and the pore [291,292]. Isoform-specific tuning further broadens its thermal responsiveness [240].
Beyond reproduction, the TRPP subfamily member Pkd2 is expressed in class III multidendritic neurons and contributes to larval nociception, particularly cold-evoked aversive behaviors [293]. Acid stimulation also elicits nociceptive rolling in larvae through multidendritic neurons [294], though a direct role of Pkd2 in acid sensing has not been established.
The sole Drosophila TRPM channel plays essential roles in physiology but is not a canonical chemosensory receptor. Early work established that dTRPM is required for zinc and magnesium homeostasis, with mutants displaying impaired viability, neuronal excitability, and male fertility [248,279]. More recently, dTRPM has been implicated in noxious cold sensing: it functions together with Pkd2 and NompC in class III multidendritic neurons to mediate aversive rolling and withdrawal behaviors in larvae exposed to low temperatures. Larvae lacking dTRPM show markedly reduced rolling and withdrawal responses when exposed to noxious cold, confirming its essential role in cold nociception [293]. By contrast, innocuous cool avoidance relies on ionotropic receptors such as IR21a, IR25a, and IR93a, rather than TRPM [295]. Thus, while dTRPM is indispensable for metal ion balance and noxious cold responses, there is no evidence that it directly contributes to gustatory or olfactory chemosensation.
Pyrexia (TRPA) is required for survival at high temperatures and for thermosensory signaling in the brain: it participates with TRPA1 in warm-sensing anterior-cell (AC) neurons and is necessary for temperature synchronization of circadian clock neurons (PERIOD) [109,255,256]. Water witch (TRPA) supports hygrosensation in antennal circuits (moist preference). Wtrw-null flies exhibit marked defects in moist-air attraction [254], confirming that Wtrw is required for hygrosensory-driven preference behavior. Meanwhile, Nanchung and Inactive (TRPV) form a heteromeric complex in chordotonal cilia that is essential for auditory transduction and proprioception [254,257,258,259,260]. These channels are also deployed in feeding-related mechanosensation at the labellum, where they contribute to texture-dependent modulation of taste-guided behavior [288]. IR-based dry/moist pathways also contribute to hygrosensation (Figure 4) [251]. In the CNS, TRPγ functions in neuroendocrine Dh44 neurons as a metabolic integrator rather than a classical chemoreceptor. In these cells, TRPγ-dependent Ca2+ influx is required for Dh44 neuropeptide release and for coupling post-ingestive nutrient status to systemic sugar and lipid handling: trpγ deficient mutants show altered crop physiology, reduced intracellular sugars and glycogen, impaired triacylglycerol homeostasis, and defective starvation-dependent switching from non-nutritive to nutritive sugar preference [262,264]. Consistent with a lipid-sensitive gating mechanism, TRPγ can be activated by polyunsaturated fatty acids and receptor signaling in heterologous systems [263], although the endogenous metabolite(s) that modulate Dh44 neurons remain to be fully defined [296]. Current evidence suggests that TRPγ does not directly bind lipid metabolites; instead, lipid metabolic state modulates upstream hormonal and phosphoinositide signaling pathways that converge on TRPγ to tune its activation. Consistently, trpγ mutants fail to switch from non-nutritive to nutritive sugar preference during starvation and display impaired crop contractions, demonstrating that TRPγ converts internal metabolic state into feeding decisions
Although TRPML1 is localized on lysosomal (endolysosomal) membranes rather than the plasma membrane, it nonetheless functions as a bona fide intracellular metabolic and ionic sensor. In both mammals and insects, TRPML channels mediate Ca2+ (and other cation) release from lysosomal stores into the cytosol in response to changes in lysosomal lipid composition, ionic environment, redox status, and phosphoinositide signaling such as PI(3,5)P2 [284,297,298,299,300]. Through this lysosomal Ca2+ signaling, TRPML channels regulate lysosomal biogenesis, autophagy, membrane trafficking, and organelle homeostasis, thereby shaping cellular metabolic state and global excitability [283,284,297,298,301,302]. In Drosophila, the single TRPML ortholog performs analogous lysosomal signaling functions but with added relevance to sensory physiology. Fly TRPML regulates microdomain Ca2+ transients in astrocytes and coordinates glia–neuron–trachea interactions that influence oxygen delivery and metabolic adaptation in the CNS [168,302]. These processes can modulate sensory gain and neuronal responsiveness, positioning Drosophila TRPML as an internal-state modulator that indirectly shapes chemosensory behavior by tuning neural circuit physiology rather than detecting tastants or odorants directly [283,303]. By modulating metabolic tone and neuron–glia signaling, TRPML indirectly contributes to sensory coding by altering the excitability landscape in which chemosensory inputs are interpreted.
Thus, across taxa, TRPML channels are best conceptualized as organelle-level internal-state sensors: they detect the ionic and metabolic status of the lysosome and convert these intracellular cues into Ca2+ signals that modulate neuronal and glial function. Within sensory systems, this modulatory capacity influences how external chemical stimuli are processed and integrated, complementing the roles of canonical plasma membrane TRP channels. Collectively, these roles show that fly TRPs broadly tune internal state and sensory gain—particularly in relation to energy balance, nutrient storage, and brain excitability—supporting chemosensory behaviors indirectly rather than serving as canonical taste/odor receptors.
5.4. Central Processing
While mammalian central processing integrates TRP activity across cortical and subcortical circuits, Drosophila relies on a simpler yet highly conserved architecture in which neuronal and glial TRP channels—such as TRPγ and TRPML—shape synaptic gain, metabolic coupling, and sensorimotor output. Painless, another Drosophila TRPA channel, was first identified for its role in nociception, with mutants showing deficits in responses to noxious heat and mechanical stimuli [118]. Its multimodal sensitivity allows Painless to integrate thermal and chemical harm cues, supporting larval nocifensive rolling and adult feeding avoidance behaviors. Larval nociceptive circuit work [304] and broader surveys of pain-related genes [305,306] show that Painless functions alongside other channels and modulators to integrate harmful stimuli in complex behavioral outputs (Figure 4).
TRPγ in chordotonal and associated support cells is required for fine motor coordination and gap crossing, highlighting a dual role in proprioception and metabolic control [261,262]. Beyond neurons, TRP channels operate in glia: TRPML in astrocytes mediates microdomain Ca2+ transients and interacts with CNS tracheal dynamics, and perineurial glia exhibit ER/GAP-junction–driven Ca2+ waves that modulate brain excitability [168,284,307].
In summary, Drosophila chemosensory TRPs—particularly dTRPA1, Painless, and Pkd2—mediate avoidance of harmful environmental cues through distinct gating mechanisms: covalent cysteine modification by electrophiles [242,285,291], thermal and pungent irritant sensitivity [118,243,244]. By contrast, TRPM contributes to ion homeostasis and noxious cold responses [248,250,279,293] but has no demonstrated role in direct chemosensory detection. Collectively, these channels illustrate the evolutionary versatility of TRPs, complementing canonical gustatory and olfactory receptors to ensure survival through robust detection and integration of environmental danger signals.
6. Future Perspectives
As the study on taste modalities continues to advance, the field is progressively shifting from peripheral to internal sensory systems. Recent discoveries, such as the identification of postprandial neurons mediating sodium sensation [308], exemplify this transition and highlight the growing complexity of chemosensory regulation. These findings collectively broaden our understanding of how sensory inputs are integrated across different domains. However, a central question that remains is when and how specific TRP channels operate as sensors, amplifiers, or modulators of sensory information across distinct physiological contexts. In addition to their sensory and homeostatic roles, several TRP channels represent emerging therapeutic targets. TRPA1 antagonists are under investigation for inflammatory pain and airway hypersensitivity (e.g., A-967079, HC-030031) [185,187]. TRPV1 modulators show promise in chronic pain and metabolic regulation, including diet-induced obesity [309,310]. TRPV4 inhibitors are being tested for edema, pulmonary barrier dysfunction, and diabetic neuropathy [311,312]. TRPM8 antagonists have entered clinical evaluation for chronic cough and neuropathic pain. Moreover, activation of the lysosomal channel TRPML1 with small-molecule agonists such as ML-SA1 is being explored as a therapeutic avenue for lysosomal storage disorders, including mucolipidosis type IV [313]. Together, these examples highlight the translational potential of TRP channels as modulators of sensory processing and systemic physiology.
Therefore, we proposed that the ongoing investigations into TRP function can be conceptualized across three interconnected levels: (I) the structural level, emphasizing channel function and gating dynamics; (II) the receptor level, focusing on interactions between TRPs and primary chemosensory receptors; and (III) the modulatory level, addressing how TRPs influence neural processing, physiological states, and behavior. This review positions TRP channels at the intersection of chemosensation and physiology. Additionally, several critical research directions remain. Identifying the endogenous ligands and modality-specific regulators that tune TRP activity in vivo is essential for understanding how these channels integrate external sensory cues with internal metabolic state. Advances in cryo-EM, in vivo imaging, and single-cell transcriptomics now provide the tools to study TRP channels across subcellular, cellular, and circuit scales. Interspecies comparisons between insects and vertebrates will be particularly valuable for revealing conserved principles of multisensory integration. Finally, ongoing development of isoform-selective pharmacological modulators will not only refine mechanistic studies but also accelerate the translation of TRP biology into therapeutic strategies for chemosensory disorders, chronic pain, metabolic diseases, and lysosomal dysfunction. Together, these avenues position TRP channels as both fundamental mechanistic nodes and promising intervention points across sensory and metabolic disorders.
To date, it has been established that TRPs act as complementary components that refine, extend, and contextualize sensory signaling. Their conserved capacity for polymodal gating across species positions them as key molecular regulators that align chemosensory meaning with internal physiological and environmental contexts. Moving forward, integrating peripheral TRP activity with central regulatory circuits will be critical for achieving a holistic understanding of sensory processing. Conceptualizing TRP channels as dynamic, context-dependent modulators embedded within established receptor architectures may ultimately enable a transition from descriptive to mechanistic models of chemosensory regulation across taxa.
Author Contributions
Both authors contributed to the study concept and design. Both authors wrote the manuscript. Y.L. supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants to Youngseok Lee from the National Research Foundation of Korea (NRF) funded by the Korea government (MIST) (RS-2021-NR058319).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
M.A. was supported by the Global Scholarship Program for Foreign Graduate Students at Kookmin University in Korea.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bellen, H.J.; Tong, C.; Tsuda, H. 100 Years of Drosophila Research and Its Impact on Vertebrate Neuroscience: A History Lesson for the Future. Nat. Rev. Neurosci. 2010, 11, 514–522. [Google Scholar] [CrossRef]
- Montell, C. Drosophila Sensory Receptors—A Set of Molecular Swiss Army Knives. Genetics 2021, 217, iyaa011. [Google Scholar] [CrossRef] [PubMed]
- Ohhara, Y.; Yamanaka, N. Internal Sensory Neurons Regulate Stage-Specific Growth in Drosophila. Development 2022, 149, dev200440. [Google Scholar] [CrossRef]
- Peng, Y.; Gillis-Smith, S.; Jin, H.; Tränkner, D.; Ryba, N.J.P.; Zuker, C.S. Sweet and Bitter Taste in the Brain of Awake Behaving Animals. Nature 2015, 527, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Grove, J.C.R.; Gray, L.A.; La Santa Medina, N.; Sivakumar, N.; Ahn, J.S.; Corpuz, T.V.; Berke, J.D.; Kreitzer, A.C.; Knight, Z.A. Dopamine Subsystems That Track Internal States. Nature 2022, 608, 374–380. [Google Scholar] [CrossRef]
- Mei, Y.; Zhou, B.-L.; Zhong, D.; Zheng, X.-J.; Deng, Y.-T.; Yu, L.; Jiang, B.-C. From Sensation to Regulation: The Diverse Functions of Peripheral Sensory Nervous System. Front. Immunol. 2025, 16, 1575917. [Google Scholar] [CrossRef]
- Yohe, L.R.; Brand, P. Evolutionary Ecology of Chemosensation and Its Role in Sensory Drive. Curr. Zool. 2018, 64, 525–533. [Google Scholar] [CrossRef]
- Wicher, D.; Marion-Poll, F. Editorial: Function and Regulation of Chemoreceptors. Front. Cell. Neurosci. 2018, 12, 496. [Google Scholar] [CrossRef]
- Huckstepp, R.T.R.; Dale, N. Redefining the Components of Central CO2 Chemosensitivity—Towards a Better Understanding of Mechanism. J. Physiol. 2011, 589, 5561–5579. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, C.I. Comparative Chemosensation from Receptors to Ecology. Nature 2006, 444, 295–301. [Google Scholar] [CrossRef]
- Crespo, J.G. A Review of Chemosensation and Related Behavior in Aquatic Insects. J. Insect Sci. 2011, 11, 62. [Google Scholar] [CrossRef]
- Gerber, B.; Stocker, R.F. The Drosophila Larva as a Model for Studying Chemosensation and Chemosensory Learning: A Review. Chem. Senses 2007, 32, 65–89. [Google Scholar] [CrossRef]
- Ruedenauer, F.A.; Parreño, M.A.; Grunwald Kadow, I.C.; Spaethe, J.; Leonhardt, S.D. The Ecology of Nutrient Sensation and Perception in Insects. Trends Ecol. Evol. 2023, 38, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J.P. Common Sense about Taste: From Mammals to Insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Chiera, F.; Costa, G.; Alcaro, S.; Artese, A. An Overview on Olfaction in the Biological, Analytical, Computational, and Machine Learning Fields. Arch. Pharm. 2025, 358, e2400414. [Google Scholar] [CrossRef] [PubMed]
- Fabela-Morón, M.F. Bioactive Compounds, Sensory Attributes, and Flavor Perceptions Involved in Taste-Active Molecules in Fruits and Vegetables. Front. Nutr. 2024, 11, 1427857. [Google Scholar] [CrossRef]
- Shrestha, B.; Lee, Y. Molecular Sensors in the Taste System of Drosophila. Genes Genom. 2023, 45, 693–707. [Google Scholar] [CrossRef]
- Engel, M.S. Insect Evolution. Curr. Biol. 2015, 25, R868–R872. [Google Scholar] [CrossRef]
- Hildebrand, J.G.; Shepherd, G.M. MECHANISMS OF OLFACTORY DISCRIMINATION: Converging Evidence for Common Principles Across Phyla. Annu. Rev. Neurosci. 1997, 20, 595–631. [Google Scholar] [CrossRef]
- WAAGE, J.K. The Evolution of Insect/Vertebrate Associations. Biol. J. Linn. Soc. 1979, 12, 187–224. [Google Scholar] [CrossRef]
- Huang, J.; Lee, Y. The Power of Drosophila Genetics in Studying Insect Toxicology and Chemical Ecology. Crop Health 2023, 1, 12. [Google Scholar] [CrossRef]
- Shim, J.; Lee, Y.; Jeong, Y.T.; Kim, Y.; Lee, M.G.; Montell, C.; Moon, S.J. The Full Repertoire of Drosophila Gustatory Receptors for Detecting an Aversive Compound. Nat. Commun. 2015, 6, 8867. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Hoon, M.A.; Ryba, N.J.P.; Zuker, C.S. The Receptors and Cells for Mammalian Taste. Nature 2006, 444, 288–294. [Google Scholar] [CrossRef]
- Lindemann, B. Chemoreception: Tasting the Sweet and the Bitter. Curr. Biol. 1996, 6, 1234–1237. [Google Scholar] [CrossRef]
- Roper, S.D. Taste Buds as Peripheral Chemosensory Processors. Semin. Cell Dev. Biol. 2013, 24, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Mi, T.; Mack, J.O.; Koolmees, W.; Lyon, Q.; Yochimowitz, L.; Teng, Z.-Q.; Jiang, P.; Montell, C.; Zhang, Y.V. Alkaline Taste Sensation through the Alkaliphile Chloride Channel in Drosophila. Nat. Metab. 2023, 5, 466–480. [Google Scholar] [CrossRef]
- Pandey, P.; Shrestha, B.; Lee, Y. Avoiding Alkaline Taste through Ionotropic Receptors. iScience 2024, 27, 110087. [Google Scholar] [CrossRef]
- Aryal, B.; Dhakal, S.; Shrestha, B.; Lee, Y. Molecular and Neuronal Mechanisms for Amino Acid Taste Perception in the Drosophila Labellum. Curr. Biol. 2022, 32, 1376–1386.e4. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Chandel, A.; Turner, H.; Wang, S.; Liman, E.R.; Montell, C. Requirement for an Otopetrin-like Protein for Acid Taste in Drosophila. Proc. Natl. Acad. Sci. USA 2021, 118, e2110641118. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Poudel, S.; Kim, Y.; Thakur, D.; Montell, C. Calcium Taste Avoidance in Drosophila. Neuron 2018, 97, 67–74.e4. [Google Scholar] [CrossRef]
- Liman, E.R.; Zhang, Y.V.; Montell, C. Peripheral Coding of Taste. Neuron 2014, 81, 984–1000. [Google Scholar] [CrossRef]
- Pradhan, R.N.; Shrestha, B.; Lee, Y. Molecular Basis of Hexanoic Acid Taste in Drosophila melanogaster. Mol. Cells 2023, 46, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Breslin, P.A.S. An Evolutionary Perspective on Food and Human Taste. Curr. Biol. 2013, 23, R409–R418. [Google Scholar] [CrossRef]
- Breslin, P.A.S.; Spector, A.C. Mammalian Taste Perception. Curr. Biol. 2008, 18, R148–R155. [Google Scholar] [CrossRef]
- Scott, K. Taste Recognition: Food for Thought. Neuron 2005, 48, 455–464. [Google Scholar] [CrossRef]
- Spector, A.C.; Travers, S.P. The Representation of Taste Quality in the Mammalian Nervous System. Behav. Cogn. Neurosci. Rev. 2005, 4, 143–191. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Hoon, M.A.; Chandrashekar, J.; Zhang, Y.; Ryba, N.J.P.; Zuker, C.S. Mammalian Sweet Taste Receptors. Cell 2001, 106, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Nelson, G.; Chandrashekar, J.; Hoon, M.A.; Feng, L.; Zhao, G.; Ryba, N.J.P.; Zuker, C.S. An Amino-Acid Taste Receptor. Nature 2002, 416, 199–202. [Google Scholar] [CrossRef]
- Li, X.; Staszewski, L.; Xu, H.; Durick, K.; Zoller, M.; Adler, E. Human Receptors for Sweet and Umami Taste. Proc. Natl. Acad. Sci. USA 2002, 99, 4692–4696. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Mueller, K.L.; Hoon, M.A.; Adler, E.; Feng, L.; Guo, W.; Zuker, C.S.; Ryba, N.J.P. T2Rs Function as Bitter Taste Receptors. Cell 2000, 100, 703–711. [Google Scholar] [CrossRef]
- Mueller, K.L.; Hoon, M.A.; Erlenbach, I.; Chandrashekar, J.; Zuker, C.S.; Ryba, N.J.P. The Receptors and Coding Logic for Bitter Taste. Nature 2005, 434, 225–229. [Google Scholar] [CrossRef]
- Meyerhof, W.; Behrens, M.; Brockhoff, A.; Bufe, B.; Kuhn, C. Human Bitter Taste Perception. Chem. Senses 2005, 30, i14–i15. [Google Scholar] [CrossRef] [PubMed]
- Heck, G.L.; Mierson, S.; DeSimone, J.A. Salt Taste Transduction Occurs Through an Amiloride-Sensitive Sodium Transport Pathway. Science 1984, 223, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.L.; Chen, X.; Hoon, M.A.; Chandrashekar, J.; Guo, W.; Tränkner, D.; Ryba, N.J.P.; Zuker, C.S. The Cells and Logic for Mammalian Sour Taste Detection. Nature 2006, 442, 934–938. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Yarmolinsky, D.; von Buchholtz, L.; Oka, Y.; Sly, W.; Ryba, N.J.P.; Zuker, C.S. The Taste of Carbonation. Science 2009, 326, 443–445. [Google Scholar] [CrossRef]
- Chen, Y.; Amrein, H. Ionotropic Receptors Mediate Drosophila Oviposition Preference through Sour Gustatory Receptor Neurons. Curr. Biol. 2017, 27, 2741–2750.e4. [Google Scholar] [CrossRef]
- Dunipace, L.; Meister, S.; McNealy, C.; Amrein, H. Spatially Restricted Expression of Candidate Taste Receptors in the Drosophila Gustatory System. Curr. Biol. 2001, 11, 822–835. [Google Scholar] [CrossRef]
- Joseph, R.M.; Devineni, A.V.; King, I.F.G.; Heberlein, U. Oviposition Preference for and Positional Avoidance of Acetic Acid Provide a Model for Competing Behavioral Drives in Drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 11352–11357. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Brady, R.; Cravchik, A.; Morozov, P.; Rzhetsky, A.; Zuker, C.; Axel, R. A Chemosensory Gene Family Encoding Candidate Gustatory and Olfactory Receptors in Drosophila. Cell 2001, 104, 661–673. [Google Scholar] [CrossRef]
- Chen, Y.-C.D.; Dahanukar, A. Molecular and Cellular Organization of Taste Neurons in Adult Drosophila Pharynx. Cell Rep. 2017, 21, 2978–2991. [Google Scholar] [CrossRef]
- Ling, F.; Dahanukar, A.; Weiss, L.A.; Kwon, J.Y.; Carlson, J.R. The Molecular and Cellular Basis of Taste Coding in the Legs of Drosophila. J. Neurosci. 2014, 34, 7148–7164. [Google Scholar] [CrossRef]
- Valmalette, J.C.; Raad, H.; Qiu, N.; Ohara, S.; Capovilla, M.; Robichon, A. Nano-Architecture of Gustatory Chemosensory Bristles and Trachea in Drosophila Wings. Sci. Rep. 2015, 5, 14198. [Google Scholar] [CrossRef]
- Hiroi, M.; Marion-Poll, F.; Tanimura, T. Differentiated Response to Sugars among Labellar chemosensilla in Drosophila. Zool. Sci. 2002, 19, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, M.; Meunier, N.; Marion-Poll, F.; Tanimura, T. Two Antagonistic Gustatory Receptor Neurons Responding to Sweet-Salty and Bitter Taste in Drosophila. J. Neurobiol. 2004, 61, 333–342. [Google Scholar] [CrossRef]
- Meunier, N.; Marion-Poll, F.; Rospars, J.-P.; Tanimura, T. Peripheral Coding of Bitter Taste in Drosophila. J. Neurobiol. 2003, 56, 139–152. [Google Scholar] [CrossRef]
- Wang, Z.; Singhvi, A.; Kong, P.; Scott, K. Taste Representations in the Drosophila Brain. Cell 2004, 117, 981–991. [Google Scholar] [CrossRef]
- Chyb, S.; Dahanukar, A.; Wickens, A.; Carlson, J.R. Drosophila Gr5a Encodes a Taste Receptor Tuned to Trehalose. Proc. Natl. Acad. Sci. USA 2003, 100, 14526–14530. [Google Scholar] [CrossRef]
- Fujii, S.; Yavuz, A.; Slone, J.; Jagge, C.; Song, X.; Amrein, H. Drosophila Sugar Receptors in Sweet Taste Perception, Olfaction, and Internal Nutrient Sensing. Curr. Biol. 2015, 25, 621–627. [Google Scholar] [CrossRef]
- Jiao, Y.; Moon, S.J.; Wang, X.; Ren, Q.; Montell, C. Gr64f Is Required in Combination with Other Gustatory Receptors for Sugar Detection in Drosophila. Curr. Biol. 2008, 18, 1797–1801. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Slone, J.; Song, X.; Amrein, H. A Fructose Receptor Functions as a Nutrient Sensor in the Drosophila Brain. Cell 2012, 151, 1113–1125. [Google Scholar] [CrossRef] [PubMed]
- Dweck, H.K.M.; Carlson, J.R. Molecular Logic and Evolution of Bitter Taste in Drosophila. Curr. Biol. 2020, 30, 17–30.e3. [Google Scholar] [CrossRef]
- Lee, Y.; Moon, S.J.; Montell, C. Multiple Gustatory Receptors Required for the Caffeine Response in Drosophila. Proc. Natl. Acad. Sci. USA 2009, 106, 4495–4500. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.J.; Lee, Y.; Jiao, Y.; Montell, C. A Drosophila Gustatory Receptor Essential for Aversive Taste and Inhibiting Male-to-Male Courtship. Curr. Biol. 2009, 19, 1623–1627. [Google Scholar] [CrossRef]
- Weiss, L.A.; Dahanukar, A.; Kwon, J.Y.; Banerjee, D.; Carlson, J.R. The Molecular and Cellular Basis of Bitter Taste in Drosophila. Neuron 2011, 69, 258–272. [Google Scholar] [CrossRef]
- Pradhan, R.N.; Shrestha, B.; Lee, Y. Avoiding Cantharidin through Ionotropic Receptors. J. Hazard. Mater. 2024, 466, 133497. [Google Scholar] [CrossRef]
- Jaeger, A.H.; Stanley, M.; Weiss, Z.F.; Musso, P.-Y.; Chan, R.C.; Zhang, H.; Feldman-Kiss, D.; Gordon, M.D. A Complex Peripheral Code for Salt Taste in Drosophila. eLife 2018, 7, e37167. [Google Scholar] [CrossRef] [PubMed]
- Sang, J.; Dhakal, S.; Shrestha, B.; Nath, D.K.; Kim, Y.; Ganguly, A.; Montell, C.; Lee, Y. A Single Pair of Pharyngeal Neurons Functions as a Commander to Reject High Salt in Drosophila melanogaster. eLife 2024, 12, RP93464. [Google Scholar] [CrossRef]
- Zhang, Y.V.; Ni, J.; Montell, C. The Molecular Basis for Attractive Salt-Taste Coding in Drosophila. Science 2013, 340, 1334–1338. [Google Scholar] [CrossRef] [PubMed]
- Mi, T.; Mack, J.O.; Lee, C.M.; Zhang, Y.V. Molecular and Cellular Basis of Acid Taste Sensation in Drosophila. Nat. Commun. 2021, 12, 3730. [Google Scholar] [CrossRef]
- Rimal, S.; Sang, J.; Poudel, S.; Thakur, D.; Montell, C.; Lee, Y. Mechanism of Acetic Acid Gustatory Repulsion in Drosophila. Cell Rep. 2019, 26, 1432–1442.e4. [Google Scholar] [CrossRef]
- Stanley, M.; Ghosh, B.; Weiss, Z.F.; Christiaanse, J.; Gordon, M.D. Mechanisms of Lactic Acid Gustatory Attraction in Drosophila. Curr. Biol. 2021, 31, 3525–3537.e6. [Google Scholar] [CrossRef] [PubMed]
- Cameron, P.; Hiroi, M.; Ngai, J.; Scott, K. The Molecular Basis for Water Taste in Drosophila. Nature 2010, 465, 91–95. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, Y.; Wang, Q.; Wang, Z. The Role of PPK26 in Drosophila Larval Mechanical Nociception. Cell Rep. 2014, 9, 1183–1190. [Google Scholar] [CrossRef]
- Jang, W.; Lim, J.Y.; Kang, S.; Kim, M.; Hwang, S.W.; Kim, C. Drosophila Ppk19 Encodes a Proton-Gated and Mechanosensitive Ion Channel. Sci. Rep. 2022, 12, 18346. [Google Scholar] [CrossRef]
- Thistle, R.; Cameron, P.; Ghorayshi, A.; Dennison, L.; Scott, K. Contact Chemoreceptors Mediate Male-Male Repulsion and Male-Female Attraction during Drosophila Courtship. Cell 2012, 149, 1140–1151. [Google Scholar] [CrossRef]
- Vijayan, V.; Thistle, R.; Liu, T.; Starostina, E.; Pikielny, C.W. Drosophila Pheromone-Sensing Neurons Expressing the Ppk25 Ion Channel Subunit Stimulate Male Courtship and Female Receptivity. PLoS Genet. 2014, 10, e1004238. [Google Scholar] [CrossRef]
- Zhong, L.; Hwang, R.Y.; Tracey, W.D. Pickpocket Is a DEG/ENaC Protein Required for Mechanical Nociception in Drosophila Larvae. Curr. Biol. 2010, 20, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Buck, L.; Axel, R. A Novel Multigene Family May Encode Odorant Receptors: A Molecular Basis for Odor Recognition. Cell 1991, 65, 175–187. [Google Scholar] [CrossRef]
- Mombaerts, P. Odorant Receptor Gene Choice in Olfactory Sensory Neurons: The One Receptor-One Neuron Hypothesis Revisited. Curr. Opin. Neurobiol. 2004, 14, 31–36. [Google Scholar] [CrossRef]
- Mori, K.; Sakano, H. How Is the Olfactory Map Formed and Interpreted in the Mammalian Brain? Annu. Rev. Neurosci. 2011, 34, 467–499. [Google Scholar] [CrossRef] [PubMed]
- de Bruyne, M.; Clyne, P.J.; Carlson, J.R. Odor Coding in a Model Olfactory Organ: The Drosophila Maxillary Palp. J. Neurosci. 1999, 19, 4520–4532. [Google Scholar] [CrossRef] [PubMed]
- Jefferis, G.S.X.E.; Potter, C.J.; Chan, A.M.; Marin, E.C.; Rohlfing, T.; Maurer, C.R.; Luo, L. Comprehensive Maps of Drosophila Higher Olfactory Centers: Spatially Segregated Fruit and Pheromone Representation. Cell 2007, 128, 1187–1203. [Google Scholar] [CrossRef]
- Masse, N.Y.; Turner, G.C.; Jefferis, G.S.X.E. Olfactory Information Processing in Drosophila. Curr. Biol. 2009, 19, R700–R713. [Google Scholar] [CrossRef] [PubMed]
- Anton, S.; Rössler, W. Plasticity and Modulation of Olfactory Circuits in Insects. Cell Tissue Res. 2021, 383, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Benton, R. On the ORigin of Smell: Odorant Receptors in Insects. Cell. Mol. Life Sci. 2006, 63, 1579–1585. [Google Scholar] [CrossRef]
- del Mármol, J.; Yedlin, M.A.; Ruta, V. The Structural Basis of Odorant Recognition in Insect Olfactory Receptors. Nature 2021, 597, 126–131. [Google Scholar] [CrossRef]
- Boonen, B.; Startek, J.B.; Talavera, K. Chemical Activation of Sensory TRP Channels. In Taste and Smell; Krautwurst, D., Ed.; Topics in Medicinal Chemistry; Springer International Publishing: Cham, Switzerland, 2017; pp. 73–113. ISBN 978-3-319-48927-8. [Google Scholar]
- Cosens, D.J.; Manning, A. Abnormal Electroretinogram from a Drosophila Mutant. Nature 1969, 224, 285–287. [Google Scholar] [CrossRef]
- Minke, B. Drosophila Mutant with a Transducer Defect. Biophys. Struct. Mech. 1977, 3, 59–64. [Google Scholar] [CrossRef]
- Montell, C.; Rubin, G.M. Molecular Characterization of the Drosophila Trp Locus: A Putative Integral Membrane Protein Required for Phototransduction. Neuron 1989, 2, 1313–1323. [Google Scholar] [CrossRef]
- Bloomquist, B.T.; Shortridge, R.D.; Schneuwly, S.; Perdew, M.; Montell, C.; Steller, H.; Rubin, G.; Pak, W.L. Isolation of a Putative Phospholipase c Gene of Drosophila, norpA, and Its Role in Phototransduction. Cell 1988, 54, 723–733. [Google Scholar] [CrossRef]
- Hardie, R.C. A Brief History of Trp: Commentary and Personal Perspective. Pflug. Arch. Eur. J. Physiol. 2011, 461, 493–498. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Montell, C. TRP Channels. Annu. Rev. Biochem. 2007, 76, 387–417. [Google Scholar] [CrossRef]
- Wes, P.D.; Chevesich, J.; Jeromin, A.; Rosenberg, C.; Stetten, G.; Montell, C. TRPC1, a Human Homolog of a Drosophila Store-Operated Channel. Proc. Natl. Acad. Sci. USA 1995, 92, 9652–9656. [Google Scholar] [CrossRef] [PubMed]
- Cao, E.; Liao, M.; Cheng, Y.; Julius, D. TRPV1 Structures in Distinct Conformations Reveal Activation Mechanisms. Nature 2013, 504, 113–118. [Google Scholar] [CrossRef]
- Liao, M.; Cao, E.; Julius, D.; Cheng, Y. Structure of the TRPV1 Ion Channel Determined by Electron Cryo-Microscopy. Nature 2013, 504, 107–112. [Google Scholar] [CrossRef]
- Paulsen, C.E.; Armache, J.-P.; Gao, Y.; Cheng, Y.; Julius, D. Structure of the TRPA1 Ion Channel Suggests Regulatory Mechanisms. Nature 2015, 520, 511–517. [Google Scholar] [CrossRef]
- Hellmich, U.A.; Gaudet, R. Structural Biology of TRP Channels. Handb. Exp. Pharmacol. 2014, 223, 963–990. [Google Scholar] [CrossRef]
- Madej, M.G.; Ziegler, C.M. Dawning of a New Era in TRP Channel Structural Biology by Cryo-Electron Microscopy. Pflug. Arch. Eur. J. Physiol. 2018, 470, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Owsianik, G.; Voets, T. Transient Receptor Potential Channels Meet Phosphoinositides. EMBO J. 2008, 27, 2809–2816. [Google Scholar] [CrossRef]
- Siemens, J.; Zhou, S.; Piskorowski, R.; Nikai, T.; Lumpkin, E.A.; Basbaum, A.I.; King, D.; Julius, D. Spider Toxins Activate the Capsaicin Receptor to Produce Inflammatory Pain. Nature 2006, 444, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, L.J.; Dubin, A.E.; Evans, M.J.; Marr, F.; Schultz, P.G.; Cravatt, B.F.; Patapoutian, A. Noxious Compounds Activate TRPA1 Ion Channels through Covalent Modification of Cysteines. Nature 2007, 445, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.; Rang, H.; Nagy, I. The Putative Role of Vanilloid Receptor-like Protein-1 in Mediating High Threshold Noxious Heat-Sensitivity in Rat Cultured Primary Sensory Neurons. Eur. J. Neurosci. 2002, 16, 1483–1489. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The Capsaicin Receptor: A Heat-Activated Ion Channel in the Pain Pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef]
- Caterina, M.J.; Rosen, T.A.; Tominaga, M.; Brake, A.J.; Julius, D. A Capsaicin-Receptor Homologue with a High Threshold for Noxious Heat. Nature 1999, 398, 436–441. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired Nociception and Pain Sensation in Mice Lacking the Capsaicin Receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid Receptor-1 Is Essential for Inflammatory Thermal Hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef]
- Güler, A.D.; Lee, H.; Iida, T.; Shimizu, I.; Tominaga, M.; Caterina, M. Heat-Evoked Activation of the Ion Channel, TRPV4. J. Neurosci. 2002, 22, 6408–6414. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, Y.; Lee, J.; Bang, S.; Hyun, S.; Kang, J.; Hong, S.-T.; Bae, E.; Kaang, B.-K.; Kim, J. Pyrexia Is a New Thermal Transient Receptor Potential Channel Endowing Tolerance to High Temperatures in Drosophila melanogaster. Nat. Genet. 2005, 37, 305–310. [Google Scholar] [CrossRef]
- Lewinter, R.D.; Skinner, K.; Julius, D.; Basbaum, A.I. Immunoreactive TRPV-2 (VRL-1), a Capsaicin Receptor Homolog, in the Spinal Cord of the Rat. J. Comp. Neurol. 2004, 470, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Moqrich, A.; Hwang, S.W.; Earley, T.J.; Petrus, M.J.; Murray, A.N.; Spencer, K.S.R.; Andahazy, M.; Story, G.M.; Patapoutian, A. Impaired Thermosensation in Mice Lacking TRPV3, a Heat and Camphor Sensor in the Skin. Science 2005, 307, 1468–1472. [Google Scholar] [CrossRef] [PubMed]
- Obata, K.; Katsura, H.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Tominaga, M.; Noguchi, K. TRPA1 Induced in Sensory Neurons Contributes to Cold Hyperalgesia after Inflammation and Nerve Injury. J. Clin. Investig. 2005, 115, 2393–2401. [Google Scholar] [CrossRef]
- Peier, A.M.; Reeve, A.J.; Andersson, D.A.; Moqrich, A.; Earley, T.J.; Hergarden, A.C.; Story, G.M.; Colley, S.; Hogenesch, J.B.; McIntyre, P.; et al. A Heat-Sensitive TRP Channel Expressed in Keratinocytes. Science 2002, 296, 2046–2049. [Google Scholar] [CrossRef]
- Smith, G.D.; Gunthorpe, M.J.; Kelsell, R.E.; Hayes, P.D.; Reilly, P.; Facer, P.; Wright, J.E.; Jerman, J.C.; Walhin, J.-P.; Ooi, L.; et al. TRPV3 Is a Temperature-Sensitive Vanilloid Receptor-like Protein. Nature 2002, 418, 186–190. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like Channel Expressed in Nociceptive Neurons, Is Activated by Cold Temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Talavera, K.; Yasumatsu, K.; Voets, T.; Droogmans, G.; Shigemura, N.; Ninomiya, Y.; Margolskee, R.F.; Nilius, B. Heat Activation of TRPM5 Underlies Thermal Sensitivity of Sweet Taste. Nature 2005, 438, 1022–1025. [Google Scholar] [CrossRef]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Tracey, W.D.; Wilson, R.I.; Laurent, G.; Benzer, S. Painless, a Drosophila Gene Essential for Nociception. Cell 2003, 113, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Vlachová, V.; Teisinger, J.; Susánková, K.; Lyfenko, A.; Ettrich, R.; Vyklický, L. Functional Role of C-Terminal Cytoplasmic Tail of Rat Vanilloid Receptor 1. J. Neurosci. 2003, 23, 1340–1350. [Google Scholar] [CrossRef]
- Voets, T.; Droogmans, G.; Wissenbach, U.; Janssens, A.; Flockerzi, V.; Nilius, B. The Principle of Temperature-Dependent Gating in Cold- and Heat-Sensitive TRP Channels. Nature 2004, 430, 748–754. [Google Scholar] [CrossRef]
- Xu, H.; Ramsey, I.S.; Kotecha, S.A.; Moran, M.M.; Chong, J.A.; Lawson, D.; Ge, P.; Lilly, J.; Silos-Santiago, I.; Xie, Y.; et al. TRPV3 Is a Calcium-Permeable Temperature-Sensitive Cation Channel. Nature 2002, 418, 181–186. [Google Scholar] [CrossRef]
- Dhakal, S.; Lee, Y. Transient Receptor Potential Channels and Metabolism. Mol. Cells 2019, 42, 569–578. [Google Scholar] [CrossRef]
- Kusudo, T.; Wang, Z.; Mizuno, A.; Suzuki, M.; Yamashita, H. TRPV4 Deficiency Increases Skeletal Muscle Metabolic Capacity and Resistance against Diet-Induced Obesity. J. Appl. Physiol. 2012, 112, 1223–1232. [Google Scholar] [CrossRef]
- Lee, J.-E.; Kim, Y.; Kim, K.H.; Lee, D.Y.; Lee, Y. Contribution of Drosophila TRPA1 to Metabolism. PLoS ONE 2016, 11, e0152935. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Choe, Y.; Martí-Renom, M.A.; Bell, A.M.; Denis, C.S.; Sali, A.; Hudspeth, A.J.; Friedman, J.M.; Heller, S. Vanilloid Receptor-Related Osmotically Activated Channel (VR-OAC), a Candidate Vertebrate Osmoreceptor. Cell 2000, 103, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Motter, A.L.; Ahern, G.P. TRPV1-Null Mice Are Protected from Diet-Induced Obesity. FEBS Lett. 2008, 582, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Nijenhuis, T.; Hoenderop, J.G.J.; Bindels, R.J.M. TRPV5 and TRPV6 in Ca2+ (Re)Absorption: Regulating Ca2+ Entry at the Gate. Pflug. Arch. Eur. J. Physiol. 2005, 451, 181–192. [Google Scholar] [CrossRef]
- Takezawa, R.; Cheng, H.; Beck, A.; Ishikawa, J.; Launay, P.; Kubota, H.; Kinet, J.-P.; Fleig, A.; Yamada, T.; Penner, R. A Pyrazole Derivative Potently Inhibits Lymphocyte Ca2+ Influx and Cytokine Production by Facilitating Transient Receptor Potential Melastatin 4 Channel Activity. Mol. Pharmacol. 2006, 69, 1413–1420. [Google Scholar] [CrossRef]
- Ye, L.; Kleiner, S.; Wu, J.; Sah, R.; Gupta, R.K.; Banks, A.S.; Cohen, P.; Khandekar, M.J.; Boström, P.; Mepani, R.J.; et al. TRPV4 Is a Regulator of Adipose Oxidative Metabolism, Inflammation, and Energy Homeostasis. Cell 2012, 151, 96–110. [Google Scholar] [CrossRef]
- Agarwal, P.; Lee, H.; Smeriglio, P.; Grandi, F.; Goodman, S.; Chaudhuri, O.; Bhutani, N. A Dysfunctional TRPV4–GSK3β Pathway Prevents Osteoarthritic Chondrocytes from Sensing Changes in Extracellular Matrix Viscoelasticity. Nat. Biomed. Eng. 2021, 5, 1472–1484. [Google Scholar] [CrossRef]
- Cox, C.D.; Poole, K.; Martinac, B. Re-Evaluating TRP Channel Mechanosensitivity. Trends Biochem. Sci. 2024, 49, 693–702. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Z.-Y.; Wu, J.; Gong, H.; Kesteven, S.; Iismaa, S.E.; Chan, A.Y.; Holman, S.; Pinto, S.; Pironet, A.; et al. The Ca2+-Activated Cation Channel TRPM4 Is a Positive Regulator of Pressure Overload-Induced Cardiac Hypertrophy. eLife 2021, 10, e66582. [Google Scholar] [CrossRef]
- Jo, A.O.; Lakk, M.; Rudzitis, C.N.; Križaj, D. TRPV4 and TRPC1 Channels Mediate the Response to Tensile Strain in Mouse Müller Cells. Cell Calcium 2022, 104, 102588. [Google Scholar] [CrossRef]
- Katoh, T.A.; Omori, T.; Mizuno, K.; Sai, X.; Minegishi, K.; Ikawa, Y.; Nishimura, H.; Itabashi, T.; Kajikawa, E.; Hiver, S.; et al. Immotile Cilia Mechanically Sense the Direction of Fluid Flow for Left-Right Determination. Science 2023, 379, 66–71. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Corey, D.P. TRP Channels in Mechanosensation. Curr. Opin. Neurobiol. 2005, 15, 350–357. [Google Scholar] [CrossRef]
- Liu, C.; Montell, C. Forcing Open TRP Channels: Mechanical Gating as a Unifying Activation Mechanism. Biochem. Biophys. Res. Commun. 2015, 460, 22–25. [Google Scholar] [CrossRef]
- Sternberg, J.R.; Prendergast, A.E.; Brosse, L.; Cantaut-Belarif, Y.; Thouvenin, O.; Orts-Del’Immagine, A.; Castillo, L.; Djenoune, L.; Kurisu, S.; McDearmid, J.R.; et al. Pkd2l1 Is Required for Mechanoception in Cerebrospinal Fluid-Contacting Neurons and Maintenance of Spine Curvature. Nat. Commun. 2018, 9, 3804. [Google Scholar] [CrossRef]
- Yin, J.; Kuebler, W.M. Mechanotransduction by TRP Channels: General Concepts and Specific Role in the Vasculature. Cell Biochem. Biophys. 2010, 56, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-Y.; Gong, H.; Kesteven, S.; Guo, Y.; Wu, J.; Li, J.V.; Cheng, D.; Zhou, Z.; Iismaa, S.E.; Kaidonis, X.; et al. Piezo1 Is the Cardiac Mechanosensor That Initiates the Cardiomyocyte Hypertrophic Response to Pressure Overload in Adult Mice. Nat. Cardiovasc. Res. 2022, 1, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Häfner, S.; Urban, N.; Schaefer, M. Discovery and Characterization of a Positive Allosteric Modulator of Transient Receptor Potential Canonical 6 (TRPC6) Channels. Cell Calcium 2019, 78, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, W.; Zhao, S.; Chen, S.; Chen, H.; Wang, S.; Li, Z.; Qian, H.; Tian, X. Discovery of First-in-Class Highly Selective TRPV1 Antagonists with Dual Analgesic and Hypoglycemic Effects. Bioorg. Med. Chem. 2024, 107, 117750. [Google Scholar] [CrossRef]
- Tang, Q.; Guo, W.; Zheng, L.; Wu, J.-X.; Liu, M.; Zhou, X.; Zhang, X.; Chen, L. Structure of the Receptor-Activated Human TRPC6 and TRPC3 Ion Channels. Cell Res. 2018, 28, 746–755. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, X.; Tian, J.; Xiao, Y.; Tian, T.; Xu, F.; Hong, X.; Zhu, M.X. TRPC Channels: Structure, Function, Regulation and Recent Advances in Small Molecular Probes. Pharmacol. Ther. 2020, 209, 107497. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, X.; Leung, L.K. Zeranol Induces COX-2 Expression through TRPC-3 Activation in the Placental Cells JEG-3. Toxicol. In Vitr. 2016, 35, 17–23. [Google Scholar] [CrossRef]
- Oh, H.J.; Choi, D.; Goh, C.J.; Hahn, Y. Loss of Gene Function and Evolution of Human Phenotypes. BMB Rep. 2015, 48, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Yohe, L.R.; Abubakar, R.; Giordano, C.; Dumont, E.; Sears, K.E.; Rossiter, S.J.; Dávalos, L.M. Trpc2 Pseudogenization Dynamics in Bats Reveal Ancestral Vomeronasal Signaling, Then Pervasive Loss. Evolution 2017, 71, 923–935. [Google Scholar] [CrossRef]
- Liman, E.R.; Innan, H. Relaxed Selective Pressure on an Essential Component of Pheromone Transduction in Primate Evolution. Proc. Natl. Acad. Sci. USA 2003, 100, 3328–3332. [Google Scholar] [CrossRef]
- Zhang, J.; Webb, D.M. Evolutionary Deterioration of the Vomeronasal Pheromone Transduction Pathway in Catarrhine Primates. Proc. Natl. Acad. Sci. USA 2003, 100, 8337–8341. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Antunes, A. Vomeronasal Receptors in Vertebrates and the Evolution of Pheromone Detection. Annu. Rev. Anim. Biosci. 2017, 5, 353–370. [Google Scholar] [CrossRef]
- Dalmazzo, S.; Antoniotti, S.; Ariano, P.; Gilardino, A.; Lovisolo, D. Expression and Localisation of TRPC Channels in Immortalised GnRH Neurons. Brain Res. 2008, 1230, 27–36. [Google Scholar] [CrossRef]
- Nilius, B. Transient Receptor Potential (TRP) Channels in the Brain: The Good and the Ugly. Eur. Rev. 2012, 20, 343–355. [Google Scholar] [CrossRef]
- Silverman, H.A.; Chen, A.; Kravatz, N.L.; Chavan, S.S.; Chang, E.H. Involvement of Neural Transient Receptor Potential Channels in Peripheral Inflammation. Front. Immunol. 2020, 11, 590261. [Google Scholar] [CrossRef] [PubMed]
- Stotz, S.C.; Vriens, J.; Martyn, D.; Clardy, J.; Clapham, D.E. Citral Sensing by Transient Receptor Potential Channels in Dorsal Root Ganglion Neurons. PLoS ONE 2008, 3, e2082. [Google Scholar] [CrossRef]
- Vandewauw, I.; Owsianik, G.; Voets, T. Systematic and Quantitative mRNA Expression Analysis of TRP Channel Genes at the Single Trigeminal and Dorsal Root Ganglion Level in Mouse. BMC Neurosci. 2013, 14, 21. [Google Scholar] [CrossRef]
- De Clercq, K.; Van den Eynde, C.; Hennes, A.; Van Bree, R.; Voets, T.; Vriens, J. The Functional Expression of Transient Receptor Potential Channels in the Mouse Endometrium. Hum. Reprod. 2017, 32, 615–630. [Google Scholar] [CrossRef]
- Hoenderop, J.G.J.; Voets, T.; Hoefs, S.; Weidema, F.; Prenen, J.; Nilius, B.; Bindels, R.J.M. Homo- and Heterotetrameric Architecture of the Epithelial Ca2+ Channels TRPV5 and TRPV6. EMBO J. 2003, 22, 776–785. [Google Scholar] [CrossRef]
- Kärki, T.; Rajakylä, E.K.; Acheva, A.; Tojkander, S. TRPV6 Calcium Channel Directs Homeostasis of the Mammary Epithelial Sheets and Controls Epithelial Mesenchymal Transition. Sci. Rep. 2020, 10, 14683. [Google Scholar] [CrossRef]
- Kunert-Keil, C.; Bisping, F.; Krüger, J.; Brinkmeier, H. Tissue-Specific Expression of TRP Channel Genes in the Mouse and Its Variation in Three Different Mouse Strains. BMC Genom. 2006, 7, 159. [Google Scholar] [CrossRef]
- Moayedi, Y.; Michlig, S.; Park, M.; Koch, A.; Lumpkin, E.A. Localization of TRP Channels in Healthy Oral Mucosa from Human Donors. eNeuro 2022, 9, ENEURO.0328-21.2022. [Google Scholar] [CrossRef]
- van de Graaf, S.F.J.; Hoenderop, J.G.J.; Gkika, D.; Lamers, D.; Prenen, J.; Rescher, U.; Gerke, V.; Staub, O.; Nilius, B.; Bindels, R.J.M. Functional Expression of the Epithelial Ca2+ Channels (TRPV5 and TRPV6) Requires Association of the S100A10–Annexin 2 Complex. EMBO J. 2003, 22, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, C.; De Clercq, K.; Vriens, J. Transient Receptor Potential Channels in the Epithelial-to-Mesenchymal Transition. Int. J. Mol. Sci. 2021, 22, 8188. [Google Scholar] [CrossRef] [PubMed]
- Kaske, S.; Krasteva, G.; König, P.; Kummer, W.; Hofmann, T.; Gudermann, T.; Chubanov, V. TRPM5, a Taste-Signaling Transient Receptor Potential Ion-Channel, Is a Ubiquitous Signaling Component in Chemosensory Cells. BMC Neurosci. 2007, 8, 49. [Google Scholar] [CrossRef]
- Koltzenburg, M. The Role of TRP Channels in Sensory Neurons. Novartis Found. Symp. 2004, 260, 206–213, discussion 213–220, 277–279. [Google Scholar]
- Liman, E.R.; Corey, D.P.; Dulac, C. TRP2: A Candidate Transduction Channel for Mammalian Pheromone Sensory Signaling. Proc. Natl. Acad. Sci. USA 1999, 96, 5791–5796. [Google Scholar] [CrossRef]
- Lübbert, M.; Kyereme, J.; Schöbel, N.; Beltrán, L.; Wetzel, C.H.; Hatt, H. Transient Receptor Potential Channels Encode Volatile Chemicals Sensed by Rat Trigeminal Ganglion Neurons. PLoS ONE 2013, 8, e77998. [Google Scholar] [CrossRef]
- Nakashimo, Y.; Takumida, M.; Fukuiri, T.; Anniko, M.; Hirakawa, K. Expression of Transient Receptor Potential Channel Vanilloid (TRPV) 1–4, Melastin (TRPM) 5 and 8, and Ankyrin (TRPA1) in the Normal and Methimazole-Treated Mouse Olfactory Epithelium. Acta Otolaryngol. 2010, 130, 1278–1286. [Google Scholar] [CrossRef]
- Micsenyi, M.C.; Dobrenis, K.; Stephney, G.; Pickel, J.; Vanier, M.T.; Slaugenhaupt, S.A.; Walkley, S.U. Neuropathology of the Mcoln1−/− Knockout Mouse Model of Mucolipidosis Type IV. J. Neuropathol. Exp. Neurol. 2009, 68, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, K.; Long, A.A.; Elsaesser, R.; Nikolaeva, D.; Broadie, K.; Montell, C. Motor Deficit in a Drosophila Model of Mucolipidosis Type IV Due to Defective Clearance of Apoptotic Cells. Cell 2008, 135, 838–851. [Google Scholar] [CrossRef]
- Scarinci, N.; Perez, P.L.; Cantiello, H.F.; Cantero, M.d.R. Polycystin-2 (TRPP2) Regulates Primary Cilium Length in LLC-PK1 Renal Epithelial Cells. Front. Physiol. 2022, 13, 995473. [Google Scholar] [CrossRef]
- Ádám, D.; Arany, J.; Tóth, K.F.; Pető, O.; Nyitrai, T.; Tóth, B.I.; Póliska, S.; Zouboulis, C.C.; Oláh, A. The TRPM5 Antagonist Triphenylphosphine Oxide Increases Sebaceous Lipogenesis and Modulates Immune Phenotype of Human Sebocytes in a TRPM5-Independent Manner. Exp. Dermatol. 2025, 34, e70115. [Google Scholar] [CrossRef]
- Damak, S.; Rong, M.; Yasumatsu, K.; Kokrashvili, Z.; Pérez, C.A.; Shigemura, N.; Yoshida, R.; Mosinger, B.; Glendinning, J.I.; Ninomiya, Y.; et al. Trpm5 Null Mice Respond to Bitter, Sweet, and Umami Compounds. Chem. Senses 2006, 31, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.K.; Atwal, K.; Bakaj, I.; Carlucci-Derbyshire, S.; Buber, M.T.; Cerne, R.; Cortés, R.Y.; Devantier, H.R.; Jorgensen, V.; Pawlyk, A.; et al. Triphenylphosphine Oxide Is a Potent and Selective Inhibitor of the Transient Receptor Potential Melastatin-5 Ion Channel. Assay Drug Dev. Technol. 2010, 8, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Talavera, K.; Yasumatsu, K.; Yoshida, R.; Margolskee, R.F.; Voets, T.; Ninomiya, Y.; Nilius, B. The Taste Transduction Channel TRPM5 Is a Locus for Bitter-Sweet Taste Interactions. FASEB J. 2008, 22, 1343–1355. [Google Scholar] [CrossRef]
- Zhang, Y.; Hoon, M.A.; Chandrashekar, J.; Mueller, K.L.; Cook, B.; Wu, D.; Zuker, C.S.; Ryba, N.J.P. Coding of Sweet, Bitter, and Umami Tastes: Different Receptor Cells Sharing Similar Signaling Pathways. Cell 2003, 112, 293–301. [Google Scholar] [CrossRef]
- Arullampalam, P.; Preti, B.; Ross-Kaschitza, D.; Lochner, M.; Rougier, J.-S.; Abriel, H. Species-Specific Effects of Cation Channel TRPM4 Small-Molecule Inhibitors. Front. Pharmacol. 2021, 12, 712354. [Google Scholar] [CrossRef]
- Dutta Banik, D.; Medler, K.F. Defining the Role of TRPM4 in Broadly Responsive Taste Receptor Cells. Front. Cell. Neurosci. 2023, 17, 1148995. [Google Scholar] [CrossRef]
- Fearey, B.C.; Binkle, L.; Mensching, D.; Schulze, C.; Lohr, C.; Friese, M.A.; Oertner, T.G.; Gee, C.E. A Glibenclamide-Sensitive TRPM4-Mediated Component of CA1 Excitatory Postsynaptic Potentials Appears in Experimental Autoimmune Encephalomyelitis. Sci. Rep. 2022, 12, 6000. [Google Scholar] [CrossRef] [PubMed]
- Guinamard, R.; Hof, T.; Del Negro, C.A. The TRPM4 Channel Inhibitor 9-Phenanthrol. Br. J. Pharmacol. 2014, 171, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B.; Mahieu, F.; Prenen, J.; Janssens, A.; Owsianik, G.; Vennekens, R.; Voets, T. The Ca2+-activated Cation Channel TRPM4 Is Regulated by Phosphatidylinositol 4,5-biphosphate. EMBO J. 2006, 25, 467–478. [Google Scholar] [CrossRef]
- Richter, P.; Andersen, G.; Kahlenberg, K.; Mueller, A.U.; Pirkwieser, P.; Boger, V.; Somoza, V. Sodium-Permeable Ion Channels TRPM4 and TRPM5 Are Functional in Human Gastric Parietal Cells in Culture and Modulate the Cellular Response to Bitter-Tasting Food Constituents. J. Agric. Food Chem. 2024, 72, 4906–4917. [Google Scholar] [CrossRef]
- Wang, C.; Naruse, K.; Takahashi, K. Role of the TRPM4 Channel in Cardiovascular Physiology and Pathophysiology. Cells 2018, 7, 62. [Google Scholar] [CrossRef]
- Hara, T.; Chiba, T.; Abe, K.; Makabe, A.; Ikeno, S.; Kawakami, K.; Utsunomiya, I.; Hama, T.; Taguchi, K. Effect of Paclitaxel on Transient Receptor Potential Vanilloid 1 in Rat Dorsal Root Ganglion. PAIN® 2013, 154, 882–889. [Google Scholar] [CrossRef]
- Trevisani, M.; Smart, D.; Gunthorpe, M.J.; Tognetto, M.; Barbieri, M.; Campi, B.; Amadesi, S.; Gray, J.; Jerman, J.C.; Brough, S.J.; et al. Ethanol Elicits and Potentiates Nociceptor Responses via the Vanilloid Receptor-1. Nat. Neurosci. 2002, 5, 546–551. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious Cold Ion Channel TRPA1 Is Activated by Pungent Compounds and Bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Banzawa, N.; Saito, S.; Imagawa, T.; Kashio, M.; Takahashi, K.; Tominaga, M.; Ohta, T. Molecular Basis Determining Inhibition/Activation of Nociceptive Receptor TRPA1 Protein. J. Biol. Chem. 2014, 289, 31927–31939. [Google Scholar] [CrossRef]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.-E.; Zygmunt, P.M. Pungent Products from Garlic Activate the Sensory Ion Channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef] [PubMed]
- Eid, S.R.; Crown, E.D.; Moore, E.L.; Liang, H.A.; Choong, K.-C.; Dima, S.; Henze, D.A.; Kane, S.A.; Urban, M.O. HC-030031, a TRPA1 Selective Antagonist, Attenuates Inflammatory- and Neuropathy-Induced Mechanical Hypersensitivity. Mol. Pain. 2008, 4, 48. [Google Scholar] [CrossRef]
- Jordt, S.-E.; Bautista, D.M.; Chuang, H.-H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard Oils and Cannabinoids Excite Sensory Nerve Fibres through the TRP Channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Macpherson, L.J.; Geierstanger, B.H.; Viswanath, V.; Bandell, M.; Eid, S.R.; Hwang, S.; Patapoutian, A. The Pungency of Garlic: Activation of TRPA1 and TRPV1 in Response to Allicin. Curr. Biol. 2005, 15, 929–934. [Google Scholar] [CrossRef]
- Sallomy, C.; Awolade, P.; Rahnasto-Rilla, M.; Hämäläinen, M.; Nousiainen, L.P.; Johansson, N.G.; Hiltunen, S.; Turhanen, P.; Moilanen, E.; Lahtela-Kakkonen, M.; et al. TRPA1 Inhibition Effects by 3-Phenylcoumarin Derivatives. ACS Med. Chem. Lett. 2024, 15, 1221–1226. [Google Scholar] [CrossRef]
- Dhaka, A.; Murray, A.N.; Mathur, J.; Earley, T.J.; Petrus, M.J.; Patapoutian, A. TRPM8 Is Required for Cold Sensation in Mice. Neuron 2007, 54, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-S.; Su, H.-H.; Chien, S.-Y.; Chung, H.-Y.; Luo, S.-T.; Chu, Y.-T.; Wang, Y.-H.; MacDonald, I.J.; Lee, H.-H.; Chen, Y.-H. Activation of Peripheral TRPM8 Mitigates Ischemic Stroke by Topically Applied Menthol. J. Neuroinflamm. 2022, 19, 192. [Google Scholar] [CrossRef]
- Lewis, C.M.; Griffith, T.N. Ion Channels of Cold Transduction and Transmission. J. Gen. Physiol. 2024, 156, e202313529. [Google Scholar] [CrossRef]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a Cold Receptor Reveals a General Role for TRP Channels in Thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef]
- Peier, A.M.; Moqrich, A.; Hergarden, A.C.; Reeve, A.J.; Andersson, D.A.; Story, G.M.; Earley, T.J.; Dragoni, I.; McIntyre, P.; Bevan, S.; et al. A TRP Channel That Senses Cold Stimuli and Menthol. Cell 2002, 108, 705–715. [Google Scholar] [CrossRef]
- Thiel, G.; Rössler, O.G. Signal Transduction of Transient Receptor Potential TRPM8 Channels: Role of PIP5K, Gq-Proteins, and c-Jun. Molecules 2024, 29, 2602. [Google Scholar] [CrossRef] [PubMed]
- Foote, A.G.; Tibbetts, J.; Bartley, S.M.; Thibeault, S.L. Localization of TRPV3/4 and PIEZO1/2 Sensory Receptors in Murine and Human Larynges. Laryngoscope Investig. Otolaryngol. 2022, 7, 1963–1972. [Google Scholar] [CrossRef]
- Gao, X.; Wu, L.; O’Neil, R.G. Temperature-Modulated Diversity of TRPV4 Channel Gating: Activation by Physical Stresses and Phorbol Ester Derivatives through Protein Kinase C-Dependent and -Independent Pathways. J. Biol. Chem. 2003, 278, 27129–27137. [Google Scholar] [CrossRef]
- Hossain, M.Z.; Ando, H.; Unno, S.; Roy, R.R.; Kitagawa, J. Pharmacological Activation of Transient Receptor Potential Vanilloid 4 Promotes Triggering of the Swallowing Reflex in Rats. Front. Cell. Neurosci. 2023, 17, 1149793. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W. TRPV4 Plays an Evolutionary Conserved Role in the Transduction of Osmotic and Mechanical Stimuli in Live Animals. J. Physiol. 2005, 567, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ohishi, A.; Iwatsuki, K.; Yamazaki, K.; Takayanagi, S.; Tsuji, M.; Aihara, E.; Utsumi, D.; Tsukahara, T.; Tominaga, M.; et al. Transient Receptor Potential Vanilloid 4 Mediates Sour Taste Sensing via Type III Taste Cell Differentiation. Sci. Rep. 2019, 9, 6686. [Google Scholar] [CrossRef]
- Mizuno, A.; Matsumoto, N.; Imai, M.; Suzuki, M. Impaired Osmotic Sensation in Mice Lacking TRPV4. Am. J. Physiol. -Cell Physiol. 2003, 285, C96–C101. [Google Scholar] [CrossRef]
- Kim, S.; Ma, L.; Yu, C.R. Requirement of Calcium-Activated Chloride Channels in the Activation of Mouse Vomeronasal Neurons. Nat. Commun. 2011, 2, 365. [Google Scholar] [CrossRef]
- Leypold, B.G.; Yu, C.R.; Leinders-Zufall, T.; Kim, M.M.; Zufall, F.; Axel, R. Altered Sexual and Social Behaviors in Trp2 Mutant Mice. Proc. Natl. Acad. Sci. USA 2002, 99, 6376–6381. [Google Scholar] [CrossRef]
- Murata, K.; Itakura, T.; Touhara, K. Neural Basis for Pheromone Signal Transduction in Mice. Front. Neural Circuits 2024, 18, 1409994. [Google Scholar] [CrossRef]
- Pfau, D.R.; Baribeau, S.; Brown, F.; Khetarpal, N.; Marc Breedlove, S.; Jordan, C.L. Loss of TRPC2 Function in Mice Alters Sex Differences in Brain Regions Regulating Social Behaviors. J. Comp. Neurol. 2023, 531, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Stowers, L.; Holy, T.E.; Meister, M.; Dulac, C.; Koentges, G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science 2002, 295, 1493–1500. [Google Scholar] [CrossRef]
- Dutta Banik, D.; Martin, L.E.; Freichel, M.; Torregrossa, A.-M.; Medler, K.F. TRPM4 and TRPM5 Are Both Required for Normal Signaling in Taste Receptor Cells. Proc. Natl. Acad. Sci. USA 2018, 115, E772–E781. [Google Scholar] [CrossRef]
- Uchida, K. TRPM3, TRPM4, and TRPM5 as Thermo-Sensitive Channels. J. Physiol. Sci. 2024, 74, 43. [Google Scholar] [CrossRef] [PubMed]
- Karuppan, S.; Schrag, L.G.; Pastrano, C.M.; Jara-Oseguera, A.; Zubcevic, L. Structural Dynamics at Cytosolic Interprotomer Interfaces Control Gating of a Mammalian TRPM5 Channel. Proc. Natl. Acad. Sci. USA 2024, 121, e2403333121. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Taruno, A.; Ohmoto, M.; Jyotaki, M.; Lim, J.C.; Miyazaki, H.; Niisato, N.; Marunaka, Y.; Lee, R.J.; Hoff, H.; et al. CALHM3 Is Essential for Rapid Ion Channel-Mediated Purinergic Neurotransmission of GPCR-Mediated Tastes. Neuron 2018, 98, 547–561.e10. [Google Scholar] [CrossRef]
- Philippaert, K.; Pironet, A.; Mesuere, M.; Sones, W.; Vermeiren, L.; Kerselaers, S.; Pinto, S.; Segal, A.; Antoine, N.; Gysemans, C.; et al. Steviol Glycosides Enhance Pancreatic Beta-Cell Function and Taste Sensation by Potentiation of TRPM5 Channel Activity. Nat. Commun. 2017, 8, 14733. [Google Scholar] [CrossRef]
- Liedtke, W. TRPV4 as Osmosensor: A Transgenic Approach. Pflug. Arch. Eur. J. Physiol. 2005, 451, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Friedman, J.M. Abnormal Osmotic Regulation in Trpv4−/− Mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef]
- Fernandes, J.; Lorenzo, I.M.; Andrade, Y.N.; Garcia-Elias, A.; Serra, S.A.; Fernández-Fernández, J.M.; Valverde, M.A. IP3 Sensitizes TRPV4 Channel to the Mechano- and Osmotransducing Messenger 5′-6′-Epoxyeicosatrienoic Acid. J. Cell Biol. 2008, 181, 143–155. [Google Scholar] [CrossRef]
- Fu, S.; Meng, H.; Inamdar, S.; Das, B.; Gupta, H.; Wang, W.; Thompson, C.L.; Knight, M.M. Activation of TRPV4 by Mechanical, Osmotic or Pharmaceutical Stimulation Is Anti-Inflammatory Blocking IL-1β Mediated Articular Cartilage Matrix Destruction. Osteoarthr. Cartil. 2021, 29, 89–99. [Google Scholar] [CrossRef]
- Goswami, R.; Arya, R.K.; Sharma, S.; Dutta, B.; Stamov, D.R.; Zhu, X.; Rahaman, S.O. Mechanosensing by TRPV4 Mediates Stiffness-Induced Foreign Body Response and Giant Cell Formation. Sci. Signal 2021, 14, eabd4077. [Google Scholar] [CrossRef]
- Becker, D.; Bereiter-Hahn, J.; Jendrach, M. Functional Interaction of the Cation Channel Transient Receptor Potential Vanilloid 4 (TRPV4) and Actin in Volume Regulation. Eur. J. Cell Biol. 2009, 88, 141–152. [Google Scholar] [CrossRef]
- Benfenati, V.; Caprini, M.; Dovizio, M.; Mylonakou, M.N.; Ferroni, S.; Ottersen, O.P.; Amiry-Moghaddam, M. An Aquaporin-4/Transient Receptor Potential Vanilloid 4 (AQP4/TRPV4) Complex Is Essential for Cell-Volume Control in Astrocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 2563–2568. [Google Scholar] [CrossRef]
- Shibasaki, K. TRPV4 Activation by Thermal and Mechanical Stimuli in Disease Progression. Lab. Investig. 2020, 100, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Zufall, F. The TRPC2 Ion Channel and Pheromone Sensing in the Accessory Olfactory System. Naunyn Schmiedebergs Arch. Pharmacol. 2005, 371, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.; Ukhanov, K.; Leinders-Zufall, T.; Zufall, F. A Diacylglycerol-Gated Cation Channel in Vomeronasal Neuron Dendrites Is Impaired in TRPC2 Mutant Mice. Neuron 2003, 40, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Szallasi, A.; Sheta, M. Targeting TRPV1 for Pain Relief: Limits, Losers and Laurels. Expert. Opin. Investig. Drugs 2012, 21, 1351–1369. [Google Scholar] [CrossRef]
- Bautista, D.M.; Jordt, S.-E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 Mediates the Inflammatory Actions of Environmental Irritants and Proalgesic Agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef]
- Habgood, M.; Seiferth, D.; Zaki, A.-M.; Alibay, I.; Biggin, P.C. Atomistic Mechanisms of Human TRPA1 Activation by Electrophile Irritants through Molecular Dynamics Simulation and Mutual Information Analysis. Sci. Rep. 2022, 12, 4929. [Google Scholar] [CrossRef]
- Lu, X.; Yao, Z.; Wang, Y.; Yin, C.; Li, J.; Chai, L.; Dong, W.; Yuan, L.; Lai, R.; Yang, S. The Acquisition of Cold Sensitivity during TRPM8 Ion Channel Evolution. Proc. Natl. Acad. Sci. USA 2022, 119, e2201349119. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Le, S.C.; Hsu, A.L.; Borgnia, M.J.; Yang, H.; Lee, S.-Y. Structural Basis of Cooling Agent and Lipid Sensing by the Cold-Activated TRPM8 Channel. Science 2019, 363, eaav9334. [Google Scholar] [CrossRef]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.-E.; Julius, D. The Menthol Receptor TRPM8 Is the Principal Detector of Environmental Cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef]
- Bandell, M.; Dubin, A.E.; Petrus, M.J.; Orth, A.; Mathur, J.; Hwang, S.W.; Patapoutian, A. High-Throughput Random Mutagenesis Screen Reveals TRPM8 Residues Specifically Required for Activation by Menthol. Nat. Neurosci. 2006, 9, 493–500. [Google Scholar] [CrossRef]
- Clapham, D.E. TRP Channels as Cellular Sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef]
- Obukhov, A.G.; Nowycky, M.C. TRPC4 Can Be Activated by G-Protein-Coupled Receptors and Provides Sufficient Ca2+ to Trigger Exocytosis in Neuroendocrine Cells. J. Biol. Chem. 2002, 277, 16172–16178. [Google Scholar] [CrossRef] [PubMed]
- Jones, W.D.; Cayirlioglu, P.; Kadow, I.G.; Vosshall, L.B. Two Chemosensory Receptors Together Mediate Carbon Dioxide Detection in Drosophila. Nature 2007, 445, 86–90. [Google Scholar] [CrossRef]
- Suh, G.S.B.; Wong, A.M.; Hergarden, A.C.; Wang, J.W.; Simon, A.F.; Benzer, S.; Axel, R.; Anderson, D.J. A Single Population of Olfactory Sensory Neurons Mediates an Innate Avoidance Behaviour in Drosophila. Nature 2004, 431, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhong, C.; Ding, C.; Chi, Q.; Walz, A.; Mombaerts, P.; Matsunami, H.; Luo, M. Detection of Near-Atmospheric Concentrations of CO2 by an Olfactory Subsystem in the Mouse. Science 2007, 317, 953–957. [Google Scholar] [CrossRef]
- Leinders-Zufall, T.; Cockerham, R.E.; Michalakis, S.; Biel, M.; Garbers, D.L.; Reed, R.R.; Zufall, F.; Munger, S.D. Contribution of the Receptor Guanylyl Cyclase GC-D to Chemosensory Function in the Olfactory Epithelium. Proc. Natl. Acad. Sci. USA 2007, 104, 14507–14512. [Google Scholar] [CrossRef]
- Chandrashekar, J.; Kuhn, C.; Oka, Y.; Yarmolinsky, D.A.; Hummler, E.; Ryba, N.J.P.; Zuker, C.S. The Cells and Peripheral Representation of Sodium Taste in Mice. Nature 2010, 464, 297–301. [Google Scholar] [CrossRef]
- Teng, B.; Wilson, C.E.; Tu, Y.-H.; Joshi, N.R.; Kinnamon, S.C.; Liman, E.R. Cellular and Neural Responses to Sour Stimuli Require the Proton Channel Otop1. Curr. Biol. 2019, 29, 3647–3656.e5. [Google Scholar] [CrossRef]
- Montell, C. The TRP Superfamily of Cation Channels. Sci. STKE 2005, 2005, re3. [Google Scholar] [CrossRef]
- Minke, B.; Wu, C.-F.; Pak, W.L. Isolation of Light-Induced Response of the Central Retinula Cells from the Electroretinogram of Drosophila. J. Comp. Physiol. 1975, 98, 345–355. [Google Scholar] [CrossRef]
- Du, E.J.; Ahn, T.J.; Wen, X.; Seo, D.-W.; Na, D.L.; Kwon, J.Y.; Choi, M.; Kim, H.-W.; Cho, H.; Kang, K. Nucleophile Sensitivity of Drosophila TRPA1 Underlies Light-Induced Feeding Deterrence. eLife 2016, 5, e18425. [Google Scholar] [CrossRef]
- Hamada, F.N.; Rosenzweig, M.; Kang, K.; Pulver, S.R.; Ghezzi, A.; Jegla, T.J.; Garrity, P.A. An Internal Thermal Sensor Controlling Temperature Preference in Drosophila. Nature 2008, 454, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Pulver, S.R.; Panzano, V.C.; Chang, E.C.; Griffith, L.C.; Theobald, D.L.; Garrity, P.A. Analysis of Drosophila TRPA1 Reveals an Ancient Origin for Human Chemical Nociception. Nature 2010, 464, 597–600. [Google Scholar] [CrossRef]
- Al-Anzi, B.; Tracey, W.D.; Benzer, S. Response of Drosophila to Wasabi Is Mediated by Painless, the Fly Homolog of Mammalian TRPA1/ANKTM1. Curr. Biol. 2006, 16, 1034–1040. [Google Scholar] [CrossRef]
- Sokabe, T.; Tsujiuchi, S.; Kadowaki, T.; Tominaga, M. Drosophila Painless Is a Ca2+-Requiring Channel Activated by Noxious Heat. J. Neurosci. 2008, 28, 9929–9938. [Google Scholar] [CrossRef]
- Köttgen, M.; Hofherr, A.; Li, W.; Chu, K.; Cook, S.; Montell, C.; Watnick, T. Drosophila Sperm Swim Backwards in the Female Reproductive Tract and Are Activated via TRPP2 Ion Channels. PLoS ONE 2011, 6, e20031. [Google Scholar] [CrossRef]
- Li, W.; Liang, J.; Outeda, P.; Turner, S.; Wakimoto, B.T.; Watnick, T. A Genetic Screen in Drosophila Reveals an Unexpected Role for the KIP1 Ubiquitination-Promoting Complex in Male Fertility. PLoS Genet. 2020, 16, e1009217. [Google Scholar] [CrossRef]
- Watnick, T.J.; Jin, Y.; Matunis, E.; Kernan, M.J.; Montell, C. A Flagellar Polycystin-2 Homolog Required for Male Fertility in Drosophila. Curr. Biol. 2003, 13, 2179–2184. [Google Scholar] [CrossRef]
- Georgiev, P.; Okkenhaug, H.; Drews, A.; Wright, D.; Lambert, S.; Flick, M.; Carta, V.; Martel, C.; Oberwinkler, J.; Raghu, P. TRPM Channels Mediate Zinc Homeostasis and Cellular Growth during Drosophila Larval Development. Cell Metab. 2010, 12, 386–397. [Google Scholar] [CrossRef]
- Hofmann, T.; Chubanov, V.; Chen, X.; Dietz, A.S.; Gudermann, T.; Montell, C. Drosophila TRPM Channel Is Essential for the Control of Extracellular Magnesium Levels. PLoS ONE 2010, 5, e10519. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wolfner, M.F. The Drosophila Trpm Channel Mediates Calcium Influx during Egg Activation. Proc. Natl. Acad. Sci. USA 2019, 116, 18994–19000. [Google Scholar] [CrossRef] [PubMed]
- Enjin, A.; Zaharieva, E.E.; Frank, D.D.; Mansourian, S.; Suh, G.S.B.; Gallio, M.; Stensmyr, M.C. Humidity Sensing in Drosophila. Curr. Biol. 2016, 26, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, R.; Costea, P.I.; Stam, L.; Nesterov, A. TRPV Channel Nanchung and TRPA Channel Water Witch Form Insecticide-Activated Complexes. Insect Biochem. Mol. Biol. 2022, 149, 103835. [Google Scholar] [CrossRef]
- Knecht, Z.A.; Silbering, A.F.; Ni, L.; Klein, M.; Budelli, G.; Bell, R.; Abuin, L.; Ferrer, A.J.; Samuel, A.D.; Benton, R.; et al. Distinct Combinations of Variant Ionotropic Glutamate Receptors Mediate Thermosensation and Hygrosensation in Drosophila. eLife 2016, 5, e17879. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Wang, R.; Yin, C.; Dong, Q.; Hing, H.; Kim, C.; Welsh, M.J. Drosophila Hygrosensation Requires the TRP Channels Water Witch and Nanchung. Nature 2007, 450, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Roessingh, S.; Rosing, M.; Marunova, M.; Ogueta, M.; George, R.; Lamaze, A.; Stanewsky, R. Temperature Synchronization of the Drosophila Circadian Clock Protein PERIOD Is Controlled by the TRPA Channel PYREXIA. Commun. Biol. 2019, 2, 246. [Google Scholar] [CrossRef] [PubMed]
- Wolfgang, W.; Simoni, A.; Gentile, C.; Stanewsky, R. The Pyrexia Transient Receptor Potential Channel Mediates Circadian Clock Synchronization to Low Temperature Cycles in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 2013, 280, 20130959. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Son, W.; Chung, Y.D.; Kim, J.; Shin, D.W.; McClung, C.A.; Lee, Y.; Lee, H.W.; Chang, D.-J.; Kaang, B.-K.; et al. Two Interdependent TRPV Channel Subunits, Inactive and Nanchung, Mediate Hearing in Drosophila. J. Neurosci. 2004, 24, 9059–9066. [Google Scholar] [CrossRef]
- Kim, J.; Chung, Y.D.; Park, D.; Choi, S.; Shin, D.W.; Soh, H.; Lee, H.W.; Son, W.; Yim, J.; Park, C.-S.; et al. A TRPV Family Ion Channel Required for Hearing in Drosophila. Nature 2003, 424, 81–84. [Google Scholar] [CrossRef]
- Lehnert, B.P.; Baker, A.E.; Gaudry, Q.; Chiang, A.-S.; Wilson, R.I. Distinct Roles of TRP Channels in Auditory Transduction and Amplification in Drosophila. Neuron 2013, 77, 115–128. [Google Scholar] [CrossRef]
- Li, B.; Li, S.; Zheng, H.; Yan, Z. Nanchung and Inactive Define Pore Properties of the Native Auditory Transduction Channel in Drosophila. Proc. Natl. Acad. Sci. USA 2021, 118, e2106459118. [Google Scholar] [CrossRef]
- Akitake, B.; Ren, Q.; Boiko, N.; Ni, J.; Sokabe, T.; Stockand, J.D.; Eaton, B.A.; Montell, C. Coordination and Fine Motor Control Depend on Drosophila TRPγ. Nat. Commun. 2015, 6, 7288. [Google Scholar] [CrossRef]
- Dhakal, S.; Ren, Q.; Liu, J.; Akitake, B.; Tekin, I.; Montell, C.; Lee, Y. Drosophila TRPγ Is Required in Neuroendocrine Cells for Post-Ingestive Food Selection. eLife 2022, 11, e56726. [Google Scholar] [CrossRef]
- Jörs, S.; Kazanski, V.; Foik, A.; Krautwurst, D.; Harteneck, C. Receptor-Induced Activation of Drosophila TRPγ by Polyunsaturated Fatty Acids. J. Biol. Chem. 2006, 281, 29693–29702. [Google Scholar] [CrossRef]
- Nath, D.K.; Dhakal, S.; Lee, Y. TRPγ Regulates Lipid Metabolism through Dh44 Neuroendocrine Cells. eLife 2025, 13, RP99258. [Google Scholar] [CrossRef]
- Xu, X.Z.; Chien, F.; Butler, A.; Salkoff, L.; Montell, C. TRPγ, a Drosophila TRP-Related Subunit, Forms a Regulated Cation Channel with TRPL. Neuron 2000, 26, 647–657. [Google Scholar] [CrossRef]
- Delgado, R.; Muñoz, Y.; Peña-Cortés, H.; Giavalisco, P.; Bacigalupo, J. Diacylglycerol Activates the Light-Dependent Channel TRP in the Photosensitive Microvilli of Drosophila melanogaster Photoreceptors. J. Neurosci. 2014, 34, 6679–6686. [Google Scholar] [CrossRef]
- Hardie, R.C.; Minke, B. The Trp Gene Is Essential for a Light-Activated Ca2+ Channel in Drosophila Photoreceptors. Neuron 1992, 8, 643–651. [Google Scholar] [CrossRef]
- Niemeyer, B.A.; Suzuki, E.; Scott, K.; Jalink, K.; Zuker, C.S. The Drosophila Light-Activated Conductance Is Composed of the Two Channels TRP and TRPL. Cell 1996, 85, 651–659. [Google Scholar] [CrossRef]
- Rosenzweig, M.; Kang, K.; Garrity, P.A. Distinct TRP Channels Are Required for Warm and Cool Avoidance in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 2008, 105, 14668–14673. [Google Scholar] [CrossRef]
- Meyer, N.E.; Joel-Almagor, T.; Frechter, S.; Minke, B.; Huber, A. Subcellular Translocation of the eGFP-Tagged TRPL Channel in Drosophila Photoreceptors Requires Activation of the Phototransduction Cascade. J. Cell Sci. 2006, 119, 2592–2603. [Google Scholar] [CrossRef]
- Parnas, M.; Katz, B.; Minke, B. Open Channel Block by Ca2+ Underlies the Voltage Dependence of Drosophila TRPL Channel. J. Gen. Physiol. 2007, 129, 17–28. [Google Scholar] [CrossRef]
- Zhang, Y.V.; Raghuwanshi, R.P.; Shen, W.L.; Montell, C. Food Experience-Induced Taste Desensitization Modulated by the Drosophila TRPL Channel. Nat. Neurosci. 2013, 16, 1468–1476. [Google Scholar] [CrossRef]
- Hehlert, P.; Effertz, T.; Gu, R.-X.; Nadrowski, B.; Geurten, B.R.H.; Beutner, D.; de Groot, B.L.; Göpfert, M.C. NOMPC Ion Channel Hinge Forms a Gating Spring That Initiates Mechanosensation. Nat. Neurosci. 2025, 28, 259–267. [Google Scholar] [CrossRef]
- Lee, J.; Moon, S.; Cha, Y.; Chung, Y.D. Drosophila TRPN(= NOMPC) Channel Localizes to the Distal End of Mechanosensory Cilia. PLoS ONE 2010, 5, e11012. [Google Scholar] [CrossRef]
- Sánchez-Alcañiz, J.A.; Zappia, G.; Marion-Poll, F.; Benton, R. A Mechanosensory Receptor Required for Food Texture Detection in Drosophila. Nat. Commun. 2017, 8, 14192. [Google Scholar] [CrossRef]
- Walker, R.G.; Willingham, A.T.; Zuker, C.S. A Drosophila Mechanosensory Transduction Channel. Science 2000, 287, 2229–2234. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, Y.; Li, G.; Liu, C.; Wang, L.; Zhang, A.; Yan, Z.; Song, C. The Push-to-Open Mechanism of the Tethered Mechanosensitive Ion Channel NompC. eLife 2021, 10, e58388. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, W.; He, Y.; Gorczyca, D.; Xiang, Y.; Cheng, L.E.; Meltzer, S.; Jan, L.Y.; Jan, Y.N. Drosophila NOMPC Is a Mechanotransduction Channel Subunit for Gentle-Touch Sensation. Nature 2013, 493, 221–225. [Google Scholar] [CrossRef]
- Zhang, W.; Yan, Z.; Jan, L.Y.; Jan, Y.N. Sound Response Mediated by the TRP Channels NOMPC, NANCHUNG, and INACTIVE in Chordotonal Organs of Drosophila Larvae. Proc. Natl. Acad. Sci. USA 2013, 110, 13612–13617. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, L.E.; Kittelmann, M.; Li, J.; Petkovic, M.; Cheng, T.; Jin, P.; Guo, Z.; Göpfert, M.C.; Jan, L.Y.; et al. Ankyrin Repeats Convey Force to Gate the NOMPC Mechanotransduction Channel. Cell 2015, 162, 1391–1403. [Google Scholar] [CrossRef]
- Edwards-Jorquera, S.S.; Bosveld, F.; Bellaïche, Y.A.; Lennon-Duménil, A.-M.; Glavic, Á. Trpml Controls Actomyosin Contractility and Couples Migration to Phagocytosis in Fly Macrophages. J. Cell Biol. 2020, 219, e201905228. [Google Scholar] [CrossRef]
- Feng, X.; Huang, Y.; Lu, Y.; Xiong, J.; Wong, C.-O.; Yang, P.; Xia, J.; Chen, D.; Du, G.; Venkatachalam, K.; et al. Drosophila TRPML Forms PI(3,5)P2-Activated Cation Channels in Both Endolysosomes and Plasma Membrane. J. Biol. Chem. 2014, 289, 4262–4272. [Google Scholar] [CrossRef]
- Ma, Z.; Freeman, M.R. TrpML-Mediated Astrocyte Microdomain Ca2+ Transients Regulate Astrocyte–Tracheal Interactions. eLife 2020, 9, e58952. [Google Scholar] [CrossRef]
- Venkatachalam, K.; Wong, C.-O.; Zhu, M.X. The Role of TRPMLs in Endolysosomal Trafficking and Function. Cell Calcium 2015, 58, 48–56. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Montell, C. Drosophila TRPA1 Channel Mediates Chemical Avoidance in Gustatory Receptor Neurons. Proc. Natl. Acad. Sci. USA 2010, 107, 8440–8445. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, S.H.; Ronderos, D.S.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Smith, D.P.; Montell, C. Drosophila TRPA1 Channel Is Required to Avoid the Naturally Occurring Insect Repellent Citronellal. Curr. Biol. 2010, 20, 1672–1678. [Google Scholar] [CrossRef]
- Thakur, D.; Hunt, S.; Tsou, T.; Petty, M.; Rodriguez, J.M.; Montell, C. Control of Odor Sensation by Light and Cryptochrome in the Drosophila Antenna. iScience 2025, 28, 112443. [Google Scholar] [CrossRef]
- Jeong, Y.T.; Oh, S.M.; Shim, J.; Seo, J.T.; Kwon, J.Y.; Moon, S.J. Mechanosensory Neurons Control Sweet Sensing in Drosophila. Nat. Commun. 2016, 7, 12872. [Google Scholar] [CrossRef]
- Lee, Y.; Montell, C. Drosophila TRPA1 Functions in Temperature Control of Circadian Rhythm in Pacemaker Neurons. J. Neurosci. 2013, 33, 6716–6725. [Google Scholar] [CrossRef]
- Lamaze, A.; Öztürk-Çolak, A.; Fischer, R.; Peschel, N.; Koh, K.; Jepson, J.E.C. Regulation of Sleep Plasticity by a Thermo-Sensitive Circuit in Drosophila. Sci. Rep. 2017, 7, 40304. [Google Scholar] [CrossRef]
- Moparthi, L.; Sinica, V.; Moparthi, V.K.; Kreir, M.; Vignane, T.; Filipovic, M.R.; Vlachova, V.; Zygmunt, P.M. The Human TRPA1 Intrinsic Cold and Heat Sensitivity Involves Separate Channel Structures beyond the N-ARD Domain. Nat. Commun. 2022, 13, 6113. [Google Scholar] [CrossRef]
- SINICA, V.; VLACHOVÁ, V. Transient Receptor Potential Ankyrin 1 Channel: An Evolutionarily Tuned Thermosensor. Physiol. Res. 2021, 70, 363–381. [Google Scholar] [CrossRef]
- Turner, H.N.; Armengol, K.; Patel, A.A.; Himmel, N.J.; Sullivan, L.; Iyer, S.C.; Bhattacharya, S.; Iyer, E.P.R.; Landry, C.; Galko, M.J.; et al. The TRP Channels Pkd2, NompC, and Trpm Act in Cold-Sensing Neurons to Mediate Unique Aversive Behaviors to Noxious Cold in Drosophila. Curr. Biol. 2016, 26, 3116–3128. [Google Scholar] [CrossRef]
- Lopez-Bellido, R.; Himmel, N.J.; Gutstein, H.B.; Cox, D.N.; Galko, M.J. An Assay for Chemical Nociception in Drosophila Larvae. Philos. Trans. R. Soc. B 2019, 374, 20190282. [Google Scholar] [CrossRef]
- Ni, L.; Bronk, P.; Chang, E.C.; Lowell, A.M.; Flam, J.O.; Panzano, V.C.; Theobald, D.L.; Griffith, L.C.; Garrity, P.A. A Gustatory Receptor Paralogue Controls Rapid Warmth Avoidance in Drosophila. Nature 2013, 500, 580–584. [Google Scholar] [CrossRef]
- Dus, M.; Lai, J.S.-Y.; Gunapala, K.M.; Min, S.; Tayler, T.D.; Hergarden, A.C.; Geraud, E.; Joseph, C.M.; Suh, G.S.B. Nutrient Sensor in the Brain Directs the Action of the Brain-Gut Axis in Drosophila. Neuron 2015, 87, 139–151. [Google Scholar] [CrossRef]
- Dong, X.; Shen, D.; Wang, X.; Dawson, T.; Li, X.; Zhang, Q.; Cheng, X.; Zhang, Y.; Weisman, L.S.; Delling, M.; et al. PI(3,5)P2 Controls Membrane Trafficking by Direct Activation of Mucolipin Ca2+ Release Channels in the Endolysosome. Nat. Commun. 2010, 1, 38. [Google Scholar] [CrossRef]
- Xu, H.; Ren, D. Lysosomal Physiology. Annu. Rev. Physiol. 2015, 77, 57–80. [Google Scholar] [CrossRef]
- Brunetti, V.; Berra-Romani, R.; Conca, F.; Soda, T.; Biella, G.R.; Gerbino, A.; Moccia, F.; Scarpellino, G. Lysosomal TRPML1 Triggers Global Ca2+ Signals and Nitric Oxide Release in Human Cerebrovascular Endothelial Cells. Front. Physiol. 2024, 15, 1426783. [Google Scholar] [CrossRef] [PubMed]
- Yim, W.W.-Y.; Mizushima, N. Lysosome Biology in Autophagy. Cell Discov. 2020, 6, 6. [Google Scholar] [CrossRef]
- Medina, D.L.; Ballabio, A. Lysosomal Calcium Regulates Autophagy. Autophagy 2015, 11, 970–971. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, V.; Petrozziello, T.; Secondo, A. Calcium Dyshomeostasis and Lysosomal Ca2+ Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2019, 8, 1216. [Google Scholar] [CrossRef] [PubMed]
- De Backer, J.-F.; Grunwald Kadow, I.C. A Role for Glia in Cellular and Systemic Metabolism: Insights from the Fly. Curr. Opin. Insect Sci. 2022, 53, 100947. [Google Scholar] [CrossRef] [PubMed]
- Boivin, J.-C.; Zhu, J.; Ohyama, T. Nociception in Fruit Fly Larvae. Front. Pain Res. 2023, 4. [Google Scholar] [CrossRef]
- Gibbons, M.; Crump, A.; Barrett, M.; Sarlak, S.; Birch, J.; Chittka, L. Chapter Three—Can Insects Feel Pain? A Review of the Neural and Behavioural Evidence. In Advances in Insect Physiology; Jurenka, R., Ed.; Academic Press: Cambridge, MA, USA, 2022; Volume 63, pp. 155–229. [Google Scholar]
- He, J.; Li, B.; Han, S.; Zhang, Y.; Liu, K.; Yi, S.; Liu, Y.; Xiu, M. Drosophila as a Model to Study the Mechanism of Nociception. Front. Physiol. 2022, 13, 854124. [Google Scholar] [CrossRef]
- Weiss, S.; Clamon, L.C.; Manoim, J.E.; Ormerod, K.G.; Parnas, M.; Littleton, J.T. Glial ER and GAP Junction Mediated Ca2+ Waves Are Crucial to Maintain Normal Brain Excitability. Glia 2022, 70, 123–144. [Google Scholar] [CrossRef]
- Kim, B.; Hwang, G.; Yoon, S.-E.; Kuang, M.C.; Wang, J.W.; Kim, Y.-J.; Suh, G.S.B. Postprandial Sodium Sensing by Enteric Neurons in Drosophila. Nat. Metab. 2024, 6, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, M.J.; Schillers, F.; Eberhardt, E.M.; Risser, L.; de la Roche, J.; Herzog, C.; Echtermeyer, F.; Leffler, A. Reactive Metabolites of Acetaminophen Activate and Sensitize the Capsaicin Receptor TRPV1. Sci. Rep. 2017, 7, 12775. [Google Scholar] [CrossRef]
- Rahman, M.M.; Jo, Y.-Y.; Kim, Y.H.; Park, C.-K. Current Insights and Therapeutic Strategies for Targeting TRPV1 in Neuropathic Pain Management. Life Sci. 2024, 355, 122954. [Google Scholar] [CrossRef]
- Kumar, M.; Zaman, M.K.; Das, S.; Goyary, D.; Pathak, M.P.; Chattopadhyay, P. Transient Receptor Potential Vanilloid (TRPV4) Channel Inhibition: A Novel Promising Approach for the Treatment of Lung Diseases. Biomed. Pharmacother. 2023, 163, 114861. [Google Scholar] [CrossRef]
- Thorneloe, K.S.; Cheung, M.; Bao, W.; Alsaid, H.; Lenhard, S.; Jian, M.-Y.; Costell, M.; Maniscalco-Hauk, K.; Krawiec, J.A.; Olzinski, A.; et al. An Orally Active TRPV4 Channel Blocker Prevents and Resolves Pulmonary Edema Induced by Heart Failure. Sci. Transl. Med. 2012, 4, 159ra148. [Google Scholar] [CrossRef]
- Millqvist, E. TRPV1 and TRPM8 in Treatment of Chronic Cough. Pharmaceuticals 2016, 9, 45. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.