Abstract
Etifoxine is an anxiolytic drug with a dual mechanism of action. In contrast to conventional benzodiazepine anxiolytics, which induce cognitive dysfunction and myorelaxation, no memory impairment nor a decrease in motor activity is observed with etifoxine. This study aims to evaluate the effects of etifoxine on locomotor activity and passive learning in rats with diazepam-induced memory deficit. Male Wistar rats were treated intraperitoneally for 7 days with: (1) saline; (2) diazepam 2.5 mg/kg bw or (3) diazepam 2.5 mg/kg bw and etifoxine in a dose of 50 mg/kg bw. Activity cage test was used for evaluation of locomotor activity, and step-through and step-down tests were performed to study the passive learning. Etifoxine increased the number of horizontal movements on the 7th and 14th days of the experiment. The drug exhibits anti-amnesic effect in a model of diazepam-induced anterograde amnesia by enhancing long-term memory in passive learning tests. The data obtained suggest that etifoxine can reduce the benzodiazepine-induced cognitive deficit. Moreover, such a combination can alleviate the negative influence of benzodiazepines on locomotor activity. However, additional studies are necessary to translate these results into clinical practice.
Keywords:
etifoxine; passive learning; diazepam; anterograde amnesia; step-through test; step-down test; cognition; rats 1. Introduction
Anxiety and adjustment disorders (phobias, separation anxiety disorder, social anxiety disorder, etc.) have a high prevalence in society. The treatment of this type of disorders includes SSRI, SRNI, benzodiazepines, buspirone, hydroxyzine, etc. However, some of these drugs have disadvantages. For example, antidepressants have delayed onset of action, and benzodiazepines induce adverse drug reactions, such as dependency, anterograde amnesia, sedation, etc. Recently, the interest in new therapeutic agents with better safety is increasing [1,2].
Etifoxine is an anxiolytic drug with a non-benzodiazepine structure, which also has anticonvulsant properties. Etifoxine has a unique mechanism of action: (1) a direct positive allosteric effect on GABA-A receptors, and (2) an indirect mechanism involving the stimulation of translocator protein (TSPO) with subsequent production of neurosteroids. Allopregnanolone is an endogenous neurosteroid, which also acts as a positive modulator of GABA-A receptors. The dual potentiation of GABAergic neurotransmission leads to prolonged anxiolytic effects evaluated in animal studies [2,3,4].
Etifoxine is used for treatment of anxiety disorders and its efficacy has been proven in clinical trials. Etifoxine has shown a similar anxiolytic effect to alprazolam [5], lorazepam [6,7], clonazepam [1], and buspirone [8] in patients with adjustment disorder with anxiety (ADWA). However, conventional benzodiazepine anxiolytics induce side effects, including cognitive dysfunction and myorelaxation. In contrast, etifoxine does not induce memory impairment and sedation at anxiolytic concentrations nor is associated with dependence and adverse psychomotor effects [5,7]. Benzodiazepines bind to α5 subunits of the GABA-A receptor, which induce a decrease in the cognitive function [9]. Etifoxine interacts with β2 or β3 subunits of the same receptor complex. Based on the similarity of the target structure for the two drugs, we choose the diazepam-induced model of anterograde amnesia for our studies.
Previously, we reported that etifoxine does not impair the muscle tone and the locomotor activity of rats after a single intraperitoneal application [10]. In another study, we found no statistical difference in the tests for active learning performed on rats treated with etifoxine in doses of 50 and 100 mg/kg in comparison to control rats. In this case, the evaluation of the effects on cognition was performed after multiple applications of etifoxine (pretreatment duration: 7 days) [11]. Rats, which received etifoxine for one week, showed increased latency in the passive avoidance tests. These results reveal the potential of etifoxine to improve short- and long-term memory in rats, subjected to passive avoidance tests. Interestingly, the locomotor activity of the rats was increased after prolonged treatment [12].
In another study, we also evaluated the effect of etifoxine on active learning in rats with diazepam-induced cognitive deficit. The comparison between rats treated with 2.5 mg/kg bw diazepam, and rats treated simultaneously with diazepam (2.5 mg/kg bw) and etifoxine (50 mg/kg bw) showed an increased number of avoidances in the etifoxine-treated group. The difference was significant on the 2nd, 4th, 5th, and 12th day of the test, revealing the potential of etifoxine to restore the normal cognition in rats with diazepam-induced amnesia [13]. Based on these data, we choose the dose of 50 mg/kg bw for our current research.
The aim of this study is to determine the effects of etifoxine on locomotor activity and passive learning in rats with diazepam-induced memory deficit. Here, we hypothesize that etifoxine decreases the negative effects of diazepam on the spontaneous exploratory activity in rats and ameliorates the anterograde amnesia induced by diazepam.
2. Materials and Methods
2.1. Chemicals
Etifoxine, 2-ethylamino-6-chloro-4-methyl-4-phenyl-4H-3,1benzoxazine hydrochloride (Stresam®, Biocodex, Gentilly, France), and diazepam (Diazepam Sopharma®.sol. inj. 5 mg/mL, 2 mL, Sopharma, Sofia, Bulgaria) were purchased from a pharmacy store. Etifoxine was dissolved in saline before the intraperitoneal application and 0.1% Tween 20 was added to increase the solubility of the substance.
2.2. Animals
Thirty male Wistar rats with an average weight of 180–215 g were used. Animals were housed under standard laboratory conditions: temperature 22 ± 1 °C, humidity 45%, 12:12 h light/dark cycle, food, and water ad libitum.
The animals were divided into three groups (n = 10) and treated intraperitoneally (i.p.) as follows:
- Group 1 (Control) with an equivalent volume (0.1 mL/100 g bw) of vehicle (0.1% Tween 20 in 0.9% NaCl solution);
- Group 2 with diazepam 2.5 mg/kg bw;
- Group 3 with diazepam 2.5 mg/kg bw and etifoxine in a dose of 50 mg/kg bw.
Group 3 rats received a diazepam injection 30 min after the injection of etifoxine.
2.3. Activity Cage Test
After one week of pretreatment of the rats, their locomotor activity was evaluated using the Activity cage apparatus (Ugo Basile, Gemonio, Italy), as described previously [10]. Thirty minutes after the intraperitoneal application of the substances, the animals were placed in the apparatus and they were allowed to explore the new environment for 5 min. The number of horizontal and vertical movements of each rat was recorded.
2.4. Model of Diazepam-Induced Amnesia
The cognitive deficit was induced as described by Georgieva-Kotetarova and Kostadinova (2013) [14]. Briefly, after one week of pretreatment of the rats, the effects of etifoxine and diazepam on cognition were evaluated with passive avoidance tests. The tests (step-through and step-down) were performed one hour after the application of the substances. On the days when both tests were held, each animal was tested first on the step-through test and immediately after that on the step-down test.
2.4.1. Step-Through Test
This test was performed using an automatic device for passive avoidance with negative reinforcement (step-through) (Ugo Basile, Gemonio, Italy). The test was performed in a cage that consists of two compartments—one of them darkened and the other brightened. The chambers are connected by a door.
The test starts with training with a duration of 2 days. Every day the rats are subjected to a standard program consisting of 3 training sessions, performed at an interval of 60 min. Each session has the following parameters: 7 s delay before the door between the compartments is opened and 12 s during which the door remains open. The rat is placed in the bright chamber. Moving to the darkened chamber triggered a closure of the door and following electrical stimulation on the floor of the cage for 9 s with an intensity of 0.4 mA. If the rat remains in the brightened chamber, a timer counts the time (in seconds) spent by the animal in this chamber. The maximal remaining time for each animal is 3 min (180 ± 2 s).
Twenty-four hours after the training session (3rd day of the experiment) a test for short-term memory is performed. The test for long-term memory is performed on the 11th day of the experiment. Both tests use the same parameters, as described above, with one difference—the intensity of the electrical stimulation is 0.3 mA.
Latency time over 178 s in two consecutive sessions is considered a criterion for cognition.
2.4.2. Step-Down Test
A passive avoidance test was also carried out using another device for passive avoidance with negative reinforcement (step-down) (Ugo Basile, Gemonio, Italy). The apparatus consists of a standard chamber with a plastic platform on the floor.
Initial training was performed for 2 consecutive days. Each day the rats were subjected to a standard program: 2 training sessions at 60 min intervals. The animal was placed on the plastic platform of the apparatus. The device is switched on and the platform starts to vibrate vertically. A timer counts the latency (in seconds) during which the rat remains on the platform. Stepping down off the platform on 3 or 4 paws trigger a 10 s electrical stimulation (0.4 mA) delivered by the metallic mesh floor of the cage outside the platform. Again, two tests were performed to evaluate the cognitive functions: (1) a test for short-term memory on day 3 of the experiment, and (2) a test for long-term memory on day 7 after the start of the training session. The parameters of the tests were similar to the training program, however, no electrical stimulation was used. Latency time over 60 s in two consecutive sessions is considered a criterion for cognition.
2.5. Statistical Analysis
Statistical analysis was performed using SPSS 17.0. The normal distribution was evaluated with One-sample Kolmogorov–Smirnov test. One-way ANOVA and Tukey’s post hoc test were employed for intergroup comparison. For comparison of results obtained in the same group on different days, Paired samples t-test was used. Non-parametric Two independent samples test (Mann–Whitney U test) and Two related samples test were applied to analyze results with a non-homogenous distribution. The number of tested animals is given as n. The results are presented as mean ± SEM and are considered significant at p < 0.05.
3. Results
3.1. Activity Cage
Application of a single dose of diazepam induced a significant decrease in the number of horizontal movements in comparison to controls (336.3 ± 28.5 vs. 464.3 ± 32.9; p < 0.05), as shown in Figure 1. Similar results were obtained for the number of the vertical movements on the same day, however, the difference did not reach statistical significance. The rats treated with etifoxine (397.9 ± 22.7) showed an increased number of horizontal movements in comparison to the diazepam-only group (336.3 ± 28.5) with no statistical significance. On the 7th day of the experiment, the application of etifoxine significantly elevated the number of horizontal movements (308.9 ± 36.2) in comparison to the diazepam-only group (143.8 ± 29.1; p < 0.01) and control group (193.7 ± 28.0; p < 0.05). This tendency was also present on the 14th day of treatment when compared to diazepam (268.5 ± 13.4 vs. 153.5 ± 13.2; p < 0.01) and controls (268.5 ± 13.4 vs. 135.1 ± 34.2; p < 0.01).
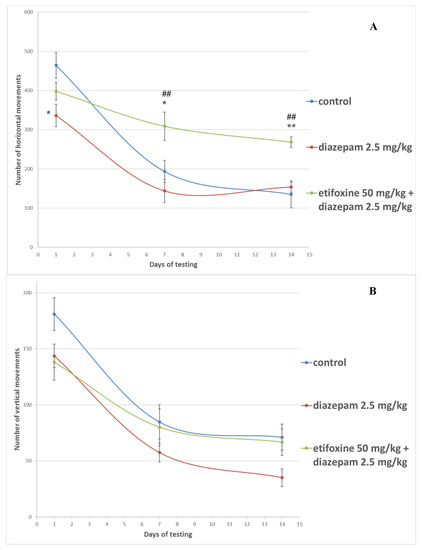
Figure 1.
Changes in the horizontal (panel (A)) and vertical (panel (B)) exploration movements in rats with diazepam-induced amnesia. * p < 0.05 vs. control group on the same day; ** p < 0.01 vs. control group on the same day; ## p < 0.01 vs. diazepam-only treated rats on the same day.
3.2. Step-Through Test
Comparing the latencies of the control group during the step-through test, we registered a significant increase in the latency on the 2nd day of the training session (133.32 ± 19.41 vs. 73.36 ± 16.42, p ≤ 0.01), as well as in the short-term memory test on day 3 (141.1 ± 13.34 vs. 73.36 ± 16.42, p ≤ 0.01), and in the long-term memory test on day 11 (148.09 ± 13.51 vs. 73.36 ± 16.42, p ≤ 0.01) compared to day 1, as shown in Figure 2. Treatment with diazepam resulted in shortened latency time during the two-day training session compared to controls, and the difference was significant in the short-term and long-term memory tests (91.73 ± 6.19 vs. 141.1 ± 13.34, p ≤0.05 and 94.93 ± 9.25 vs. 148.09 ± 13.51, p ≤ 0.05, respectively) when compared to control rats on the same day. Animals receiving etifoxine and diazepam performed similarly to controls during the whole experiment. During the training session, the experimental group treated with both substances demonstrated a tendency to increase the latency time compared to the group treated with diazepam alone. The difference was statistically significant in the two memory tests (short-term memory test: 138.54 ± 17.19 vs. 91.73 ± 6.19, p ≤ 0.05, and long-term memory test: 144.89 ± 13 vs. 94.93 ± 9.25, p ≤ 0.05).

Figure 2.
Effect of etifoxine on latency in rats with diazepam-induced amnesia evaluated with step-through test for passive learning. °° p ≤ 0.01 vs. controls on day 1; * p ≤ 0.05 vs. controls on the same day; # p ≤ 0.05 vs. diazepam-only treated rats on the same day.
3.3. Step-Down Test
In the step-down passive learning test, the control group demonstrated significantly prolonged time spent on the platform on the 2nd day of the training session (41.25 ± 5.69 vs. 30.92 ± 3.78, p ≤ 0.05), in the short-term memory test (54.7 ± 2.98 vs. 30.92 ± 3.78, p ≤ 0.01), and in the long-term memory test (49.54 ± 4.17 vs. 30.92 ± 3.78, p ≤ 0.01) compared to the first day of training. As shown in Figure 3, the group treated only with diazepam showed a tendency of decreased latency in comparison to control animals during the whole experiment. However, the difference did not reach statistical significance. No significant difference was found between the controls and the group treated with diazepam + etifoxine during the memory tests. Rats receiving both substances spent longer time on the platform compared to the diazepam group during the training session. On the 3rd day (of the short-term memory test), the two experimental groups (diazepam and diazepam + etifoxine) showed similar results. Treatment with etifoxine resulted in significantly prolonged latency time on the 7th day (of the long-term memory test) in comparison to the diazepam-only treated group (55.75 ± 2.14 vs. 38.07 ± 5.67, p ≤ 0.05).

Figure 3.
Effect of etifoxine on latency in rats with diazepam-induced amnesia evaluated with step-down test for passive learning. ° p ≤ 0.05 vs. controls on day 1; °° p ≤ 0.01 vs. controls on day 1; # p ≤ 0.05 vs. diazepam-only treated rats on the same day.
4. Discussion
Etifoxine as an anxiolytic may potentiate the effect of other CNS depressant drugs (e.g., benzodiazepines). The binding sites of etifoxine and benzodiazepines to GABA-A receptors are different, which provides the background for etifoxine to be combined with benzodiazepines to enhance their effect without competing with them for their GABA-A receptor binding site [6,15]. Apparently, such combination can alleviate the negative influence of benzodiazepines on the locomotor activity. Benzodiazepines decreased the number of horizontal and vertical movements of the rats, while etifoxine + diazepam increased the number of horizontal movements on the 7th and 14th day (Figure 1). The number of vertical movements was similar to the control group, however, their count was higher than the diazepam-only treated rats. In our previous research, we also registered increased locomotor activity in native rats after treatment with etifoxine [12]. Our results are in accordance with Girard et al. (2009) and Shehadeh et al. (2019), who also reported enhanced locomotor function in rats with traumatic brain injury and brain edema, respectively [16,17].
In our experiments, the administration of diazepam led to a significant impairment of short-term and long-term memory in the step-through passive learning test (Figure 2). In the step-down test, the application of diazepam worsened the cognition, without reaching a significant difference to the control group. The results show that etifoxine at a dose of 50 mg/kg (i.p.) exhibits an anti-amnesic effect in a model of diazepam-induced anterograde amnesia. Administration of this drug diminished the amnestic effect of diazepam and significantly improved long-term memory in passive learning tests (Figure 2 and Figure 3).
Girard et al. (2009) reported that etifoxine (25 and 50 mg/kg, two times a day, p.o.) treatment of animals with brain edema (from the 4th day of triethyltin application) for 5 consecutive days improved locomotor activity, reduced brain edema, electrolyte Na+, and Cl− content, neurological changes, and mortality [16]. A recent study by Palzur et al. (2021) demonstrated a significant improvement in cognitive functions and faster recovery of rats treated intraperitoneally with etifoxine (50 mg/kg bw) in a model of traumatic brain injury. The authors also reported restoration of mitochondrial oxidative phosphorylation and a possible relation to the improved behavioral and cognitive function [18]. Simon-O’Brien et al. (2016) reported improved functional recovery and a significant positive influence on sensorimotor functions in rats treated with etifoxine at a dose of 50 mg/kg, i.p. in a model of traumatic brain injury [19]. The article by Shehadeh et al. (2019) also reported enhanced motor and behavioral activity by etifoxine in doses of 25 and 50 mg/kg [17]. Some clinical trials are also available. Deplanque et al. (2018) compared the effects of lorazepam and etifoxine in healthy elderly and found no deterioration in cognitive function and alertness in comparison to the placebo group. However, lorazepam induced such deterioration in comparison to the controls [20].
A recent research by Tian et al. (2022) showed the beneficial effects of etifoxine in a model of neurodegenerative disease, induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Etifoxine significantly reduced MPTP-induced neurotoxicity and neuroinflammation, motor function disturbances, diminished the production of inflammatory mediators, and infiltration of leukocytes in the brain after MPTP exposure in mice [21]. Our results are also in accordance with the report of Zhang et al. (2020). The authors revealed the effect of etifoxine pretreatment in mice with LPS-induced neuroinflammation and cognitive dysfunction. The results showed alleviated hippocampal inflammation, increased brain levels of progesterone and allopregnanolone, and attenuated cognitive dysfunction in LPS-injected mice treated with etifoxine [22].
As mentioned before, etifoxine has a dual mechanism of action: (1) binding to β2 or β3 subunits of the GABA-A receptor (the anxiolytic effects of etifoxine are mainly related to this activity), and (2) interaction with TSPO, which induce increased neurosteroid synthesis in the brain [20]. The anti-amnesic effects of etifoxine may be related to both mechanisms: the increased direct and indirect GABAergic neurotransmission and the increased synthesis of neurosteroids. Thanapreedawat et al. (2013) reported improved long-term object recognition memory and working memory after GABA administration [23]. One of the strategies to achieve neuroprotection is based on increased GABA concentration in the brain [24,25]. However, some agents that increase GABAergic mediation such as clomethiazole, muscimol, tiagabine, and vigabatrin, have shown such an effect in various animal models, whereas, with other potent GABA-A receptor agonists, such as barbiturates and benzodiazepines, neuroprotection has not been demonstrated. The reasons for these differences are not fully understood and may include the complex interplay between GABAergic transmission and glutamatergic activity in the brain, and the different types of receptor subunits, to which these ligands bind [20]. Green et al. (2000) suggest that increasing GABA function reduces glutamatergic mediation in the brain and may provide neuroprotection in cerebral ischemia [24]. Improved GABA transmission decreases the excitotoxicity due to the excessive release of glutamate and acetylcholine in a model of traumatic CNS injury [19].
TSPO is a transmembrane protein located in the mitochondrial membrane and is involved in many cellular functions, such as steroid hormone synthesis, cholesterol transport, apoptosis, inflammation, etc. [26]. In the CNS, TSPO has high expression in glial cells (microglia and astrocytes) and elevated levels of this protein are associated with neuroinflammation. It can be assumed that the anti-amnesic effects of etifoxine are also related to the stimulation of neurosteroid synthesis. Neurosteroids are steroids, which modulate the activity of the CNS. Their main effects are related to interaction with the GABA-A receptors, however, neurosteroids can also modulate other receptors (e.g., AMPA, NMDA, kainate, and serotonin receptors) [27]. For example, progesterone and allopregnanolone have been shown to modulate GABA-A and NMDA receptors and induce rapid neuronal growth and neuroprotection [28,29]. Evidence of the neuroprotective effects of progesterone and the improvement of cognitive functions following its administration in a mouse model of Alzheimer’s disease has been reported by Frye and Walf (2008) [30]. Progesterone reduces the levels of inflammatory cytokines and cerebral edema in a rat model of traumatic brain injury [31] and restores neurological functions in a model of focal cerebral ischemia in rats [32]. Post-traumatic progesterone treatment of rats with frontal cortex damage has been reported to reduce cerebral edema and may improve cognitive recovery [33,34,35]. In the nervous system, the neurosteroids allopregnanolone, dehydroepiandrosterone, pregnenolone, and progesterone, exhibit neuroprotective properties by improving myelination and synaptic function [36,37]. Administration of etifoxine to rats evokes a dose-dependent increase in concentrations of pregnenolone, progesterone, 5α-dihydroprogesterone, and allopregnanolone in plasma and cerebrospinal fluid [38]. Moreover, another TSPO ligand (PK11195) was shown to significantly ameliorate cognitive deficits in mice with a model of Alzheimer’s disease [39], which discloses the involvement of this mechanism in the anti-amnesic properties of etifoxine.
Summarizing the literature overview, the anti-amnesic properties of etifoxine on memory may be due to increased GABAergic neurotransmission and/or increased neurosteroids.
To our knowledge, this is the first study on the influence of etifoxine on passive learning in rats with diazepam-induced cognitive deficit. A limitation of the research is related to the study objects. Results obtained in rats do not always correlate to humans and a negative outcome could not be excluded if this combination is applied to other species.
5. Conclusions
Etifoxine exhibits anti-amnesic effect in a model of diazepam-induced anterograde amnesia by enhancing long-term memory in passive learning tests. The data obtained suggest that etifoxine can reduce the benzodiazepine-induced cognitive deficit and provide a background for a possible co-administration of these drugs. Moreover, such a combination can alleviate the negative influence of benzodiazepines on locomotor activity. However, additional studies are necessary to translate these results into clinical practice.
Author Contributions
Conceptualization, methodology, investigation, V.K. and E.A.; writing—original draft preparation, V.K.; writing—review and editing, E.A.; statistical analysis, V.K.; visualization, E.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Before the experiments, approvals were received from the Bulgarian Food Safety Agency (permit number: 87/9.01.2014) and the Ethics Committee of the Medical University, Plovdiv, Bulgaria (protocol number: 5/29.09.2016). The study was conducted in accordance with the following guidelines: ARRIVE, the EU Directive 2010/63/EU for animal experiments, and the relevant national and institutional rules and regulations.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vicente, B.; Saldivia, S.; Hormazabal, N.; Bustos, C.; Rubí, P. Etifoxine is non-inferior than clonazepam for reduction of anxiety symptoms in the treatment of anxiety disorders: A randomized, double blind, non-inferiority trial. Psychopharmacology 2020, 237, 3357–3367. [Google Scholar] [CrossRef] [PubMed]
- Poisbeau, P.; Gazzo, G.; Calvel, L. Anxiolytics targeting GABAA receptors: Insights on etifoxine. World J. Biol. Psychiatry 2018, 19, S36–S45. [Google Scholar] [CrossRef] [PubMed]
- Verleye, M.; Dumas, S.; Heulard, I.; Krafft, N.; Gillardin, J.-M. Differential effects of etifoxine on anxiety-like behaviour and convulsions in BALB/cByJ and C57BL/6J mice: Any relation to overexpression of central GABAA receptor beta2 subunits? Eur. Neuropsychopharmacol. 2011, 21, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Verleye, M.; Heulard, I.; Gillardin, J.-M. The anxiolytic etifoxine protects against convulsant and anxiogenic aspects of the alcohol withdrawal syndrome in mice. Alcohol 2009, 43, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J. Etifoxine versus alprazolam for the treatment of adjustment disorder with anxiety: A randomized controlled trial. Adv. Ther. 2015, 32, 57–68. [Google Scholar] [CrossRef]
- Nguyen, N.; Fakra, E.; Pradel, V.; Jouve, E.; Alquier, C.; Le Guern, M.-E.; Micallef, J.; Blin, O. Efficacy of etifoxine compared to lorazepam monotherapy in the treatment of patients with adjustment disorders with anxiety: A double-blind controlled study in general practice. Hum. Psychopharmacol. Clin. Exp. 2006, 21, 139–149. [Google Scholar] [CrossRef]
- Micallef, J.; Soubrouillard, C.; Guet, F.; Le Guern, M.E.; Alquier, C.; Bruguerolle, B.; Blin, O. A double blind parallel group placebo controlled comparison of sedative and amnesic effects of etifoxine and lorazepam in healthy subjects. Fundam. Clin. Pharmacol. 2001, 15, 209–216. [Google Scholar] [CrossRef]
- Servant, D.; Graziani, P.L.; Moyse, D.; Parquet, P.J. Treatment of adjustment disorder with anxiety: Efficacy and tolerance of eti-foxine in a double blind controlled study. Encephale 1998, 24, 569–574. [Google Scholar]
- Dubrovina, N.I. GABA Receptors in the Modulation of Fear Memory Extinction. Neurosci. Behav. Physiol. 2017, 47, 573–584. [Google Scholar] [CrossRef]
- Kokova, V.Y.; Zagorchev, P.I.; Apostolova, E.G.; Peychev, L.P. Etifoxine does not impair muscle tone and motor function in rats as assessed by in vivo and in vitro methods. Gen. Physiol. Biophys. 2020, 39, 179–186. [Google Scholar] [CrossRef]
- Kokova, V.; Apostolova, E.; Peychev, L. Effects of etifoxine on learning and memory of intact rats. Trakia J. Sci. 2015, 13, 40–44. [Google Scholar] [CrossRef]
- Kokova, V.; Apostolova, E.; Peychev, L. P.1.j.004 Effects of etifoxine on passive avoidance tests and locomotor activity in rats. Eur. Neuropsychopharmacol. 2015, 25 (Suppl. S1), S334–S335. [Google Scholar] [CrossRef]
- Kokova, V.; Apostolova, E. Effect of etifoxine on learning and memory processes in rats with diazepam-induced amnesia. Sci. Rep. Compet. Sess. Sci. Youth 2016, 1, 399–403. [Google Scholar]
- Georgieva-Kotetarova, M.T.; Kostadinova, I. Effect of Atorvastatin and Rosuvastatin on Learning and Memory in Rats with Diazepam-Induced Amnesia. Folia Med. 2013, 55, 58–65. [Google Scholar] [CrossRef]
- Khan, Z.; Ghosh, A. Possible modulation of neurobehavioural patterns by anxiolytics drugs. Int. J. Pharm. Sci. 2010, 1, 457–464. [Google Scholar]
- Girard, P.H.; Pansart, Y.; Gillardin, J.M. Preventive and curative effects of etifoxine in a rat model of brain oedema. Clin. Exp. Pharmacol. 2009, 36, 655–661. [Google Scholar] [CrossRef]
- Shehadeh, M.; Palzur, E.; Apel, L.; Soustiel, J.F. Reduction of Traumatic Brain Damage by Tspo Ligand Etifoxine. Int. J. Mol. Sci. 2019, 20, 2639. [Google Scholar] [CrossRef]
- Palzur, E.; Edelman, D.; Sakas, R.; Soustiel, J.F. Etifoxine Restores Mitochondrial Oxidative Phosphorylation and Improves Cognitive Recovery Following Traumatic Brain Injury. Int. J. Mol. Sci. 2021, 22, 12881. [Google Scholar] [CrossRef]
- Simon-O’brien, E.; Gauthier, D.; Riban, V.; Verleye, M. Etifoxine improves sensorimotor deficits and reduces glial activation, neuronal degeneration, and neuroinflammation in a rat model of traumatic brain injury. J. Neuroinflamm. 2016, 13, 203. [Google Scholar] [CrossRef]
- Deplanque, D.; Machuron, F.; Waucquier, N.; Jozefowicz, E.; Duhem, S.; Somers, S.; Colin, O.; Duhamel, A.; Bordet, R. Etifoxine impairs neither alertness nor cognitive functions of the elderly: A randomized, double-blind, placebo-controlled crossover study. Eur. Neuropsychopharmacol. 2018, 28, 925–932. [Google Scholar] [CrossRef]
- Tian, Q.; Yang, X.; Du, J.; Huang, H.; Liu, W.; Zhao, P. Translocator Protein Ligand Etifoxine Attenuates MPTP-Induced Neurotoxicity. Front. Mol. Neurosci. 2022, 15, 850904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, L.; Guo, W.-Z.; Jiao, L.-B.; Zhao, H.-Y.; Ma, Y.-Q.; Hao, X.-M. TSPO ligand etifoxine attenuates LPS-induced cognitive dysfunction in mice. Brain Res. Bull. 2020, 165, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Thanapreedawat, P.; Kobayashi, H.; Inui, N.; Sakamoto, K.; Kim, M.; Yoto, A.; Yokogoshi, H. GABA Affects Novel Object Recognition Memory and Working Memory in Rats. J. Nutr. Sci. Vitaminol. 2013, 59, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Green, A.; Hainsworth, A.H.; Jackson, D.M. GABA potentiation: A logical pharmacological approach for the treatment of acute ischaemic stroke. Neuropharmacology 2000, 39, 1483–1494. [Google Scholar] [CrossRef]
- Costa, C.; Leone, G.; Saulle, E.; Pisani, F.; Bernardi, G.; Calabresi, P. Coactivation of GABA A and GABA B Receptor Results in Neuroprotection During In Vitro Ischemia. Stroke 2004, 35, 596–600. [Google Scholar] [CrossRef]
- Lee, Y.; Park, Y.; Nam, H.; Lee, J.-W.; Yu, S.-W. Translocator protein (TSPO): The new story of the old protein in neuroinflammation. BMB Rep. 2020, 53, 20–27. [Google Scholar] [CrossRef]
- Longone, P.; di Michele, F.; D’Agati, E.; Romeo, E.; Pasini, A.; Rupprecht, R. Neurosteroids as neuromodulators in the treatment of anxiety disorders. Front. Endocrinol. 2011, 2, 55. [Google Scholar] [CrossRef]
- Stoffel-Wagner, B. Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 2001, 145, 669–679. [Google Scholar] [CrossRef]
- Wojtal, K.; Trojnar, M.K.; Czuczwar, S.J. Endogenous neuroprotective factors: Neurosteroids. Pharmacol. Rep. 2006, 58, 335–340. [Google Scholar]
- Frye, C.A.; Walf, A.A. Effects of progesterone administration and APPswe+PSEN1Δe9 mutation for cognitive performance of mid-aged mice. Neurobiol. Learn. Mem. 2008, 89, 17–26. [Google Scholar] [CrossRef]
- VanLandingham, J.W.; Cutler, S.M.; Virmani, S.; Hoffman, S.W.; Covey, D.F.; Krishnan, K.; Hammes, S.R.; Jamnongjit, M.; Stein, D.G. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology 2006, 51, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Chopp, M.; Stein, D.; Feit, H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996, 735, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Roof, R.L.; Duvdevani, R.; Heyburn, J.W.; Stein, D.G. Progesterone Rapidly Decreases Brain Edema: Treatment Delayed up to 24 Hours Is Still Effective. Exp. Neurol. 1996, 138, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.G.; Hoffman, S.W. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr. Rehabil. 2003, 6, 13–22. [Google Scholar] [CrossRef]
- Wright, D.W.; Kellermann, A.L.; Hertzberg, V.S.; Clark, P.L.; Frankel, M.; Goldstein, F.C.; Salomone, J.P.; Dent, L.L.; Harris, O.A.; Ander, D.S.; et al. ProTECT: A Randomized Clinical Trial of Progesterone for Acute Traumatic Brain Injury. Ann. Emerg. Med. 2007, 49, 391–402.e2. [Google Scholar] [CrossRef]
- Schumacher, M.; Akwa, Y.; Guennoun, R.; Robert, F.; Labombarda, F.; Désarnaud, F.; Robel, P.; De Nicola, A.F.; Baulieu, E.-E. Steroid synthesis and metabolism in the nervous system: Trophic and protective effects. J. Neurocytol. 2000, 29, 307–326. [Google Scholar] [CrossRef]
- Schumacher, M.; Guennoun, R.; Mercier, G.; Désarnaud, F.; Lacor, P.; Bénavides, J.; Ferzaz, B.; Robert, F.; Baulieu, E.E. Progesterone synthesis and myelin formation in peripheral nerves. Brain Res. Rev. 2001, 37, 343–359. [Google Scholar] [CrossRef]
- Verleye, M.; Akwa, Y.; Liere, P.; Ladurelle, N.; Pianos, A.; Eychenne, B.; Schumacher, M.; Gillardin, J.-M. The anxiolytic etifoxine activates the peripheral benzodiazepine receptor and increases the neurosteroid levels in rat brain. Pharmacol. Biochem. Behav. 2005, 82, 712–720. [Google Scholar] [CrossRef]
- Christensen, A.; Pike, C.J. TSPO ligand PK11195 improves Alzheimer-related outcomes in aged female 3xTg-AD mice. Neurosci. Lett. 2018, 683, 7–12. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).