Abstract
In this study, a copper hexacyanoferrate nanoparticle with excellent oxidase-mimetic behaviour has been synthesized through a simple precipitation method. The synthesized copper hexacyanoferrate nanoparticle has intrinsic oxidase-like activity, which can catalyze the chromogenic reaction of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) through an O2•− reactive oxygen-species-participated process. On the other hand, K3[Fe(CN)6] can be reduced by ascorbic acid (AA) to produce K4[Fe(CN)6], thereby inhibiting the formation of the copper hexacyanoferrate nanoparticles. Furthermore, ascorbate oxidase (AAO) can catalyze the oxidation of AA to produce dehydroascorbic acid, which cannot reduce K3[Fe(CN)6]. Thus, a system for an AAO-regulated in situ formation of copper hexacyanoferrate nanoparticles was constructed by coupling a prepared copper hexacyanoferrate nanozyme with AA for the detection of AAO activity. This colorimetric sensing assay shows high sensitivity and selectivity for the detection of AAO activity (the limit of detection is 0.52 U/L) with a linear range of 1.1–35.7 U/L. Finally, the developed method was applied to detect the activity of AAO in normal human serum with a satisfactory sample spiked recovery (87.4–108.8%). In short, this study provides a good strategy for the construction of nanozyme-based multi-enzyme cascade-signal amplification assay.
1. Introduction
Ascorbic acid (AA, namely, vitamin C), which is an important nutrient common in humans and animals, plays a vital role in the biochemical metabolism of organisms, through processes such as antioxidation, free radical scavenging, and immune enhancement [1,2,3]. The daily intake level of AA is about 2000 mg according to the Food and Nutrition Committee of the Institute of Medicine of the National Academy of Sciences [4]. However, elevated intake of AA can cause some negative effects, such as urinary stones, taste degradation, and diarrhea [5,6]. On the other hand, insufficient intake of AA is commonly correlated with the incidence of a number of diseases, such as skin diseases and cancer [7]. Ascorbate oxidase (AAO), a versatile copper-containing enzyme, can catalyze the oxidation of AA to dehydroascorbic acid (DHA) to balance the levels of AA in the human body [8]. AAO plays an important role in delaying aging and metabolism by acting as a terminal oxidative enzyme in conjunction with other redox reactions in an organism. In addition, AAO may also be involved in the dynamic homeostasis system of plant oxygen management [9]. Therefore, sensitive methods are highly meaningful to trace and analyze AAO activity.
To date, colorimetry and fluorometry [10,11,12,13] have been reported for the detection of AAO activity. Among them, fluorometry for AAO activity detection has been reported more frequently due to its high sensitivity. Nevertheless, the colorimetry methods associated with the AAO activity detection have rarely been reported. Liu et al. [12] developed a fluorometric and colorimetric detection of AAO activity method through AA-induced growth and aggregation of DNA-templated gold/silver nanoclusters. The limits of detection (LODs) for fluorometric and colorimetric detection of AAO activity are 4.8 U/L and 6.8 U/L, respectively. Wang et al. [13] combined the advantages of ratiometric fluorometry and colorimetry to develop a dual-model strategy for fluorometric and colorimetric detection of AAO activity using carbon dots and o-phenylenediamine. The LODs for fluorometric and colorimetric detection of AAO activity are 0.017 U/L and 0.012 U/L, respectively. However, these reported methods usually require expensive fluorescent materials, tedious synthesis processes, and specialized operators.
Nanomaterials with enzyme-like activities (nanozymes) have attracted attention continually since Gao et al. [14] first reported that Fe3O4 nanoparticles have intrinsic peroxidase-like characteristics. Nanozymes with high catalytic activity are comparable to natural enzymes and have the benefits of high stability, controllable catalytic activity, and flexible functionality over natural enzymes [15,16,17]. Thus far, several nanozymes with peroxidase-like activity have been reported [18,19,20], but they require the addition of H2O2 as an electron acceptor to oxidize the substrate. It is worth noting that H2O2 is not only easily decomposed and loses its oxidation ability, but its strong oxidation ability may also destroy the analytes in the constructed sensing system. Alternatively, oxidase-like nanozymes can directly catalyze the oxidation of substrates without H2O2. For example, Zhan et al. [21] developed a colorimetric method for the detection of dimethoate residue by improving the oxidase-like catalytic activity of cube-shape Ag2O, which can directly oxidize the chromogenic substrate of 3,3′,5,5′-tetramethylbenzidine (TMB) to oxidized TMB. Cao et al. [22] developed a ratiometric fluorescence sensor for the detection of glutathione using the oxidase-like activity of a MnO2 nanosheet. However, some disadvantages of these nanozymes are nonnegligible, such as the tedious design and synthesis processes. The use of heavy and precious metal materials will pollute the environment and waste resources.
In this study, a system for an AAO-regulated in situ formation of copper hexacyanoferrate nanoparticles was constructed to detect AAO activity. Considering the development of cost-efficient enzyme mimics with an environmentally friendly and simple synthesis process, the choice was made to synthesize copper hexacyanoferrate nanoparticles. The principle is shown in Figure 1: (1) Unlike the traditional tedious process of nanozyme design and synthesis, copper hexacyanoferrate nanoparticles were synthesized by simply mixing a solution of CuCl2·2H2O and K3[Fe(CN)6]; (2) K3[Fe(CN)6] can be reduced by AA to produce K4[Fe(CN)6], which can inhibit the formation of the copper hexacyanoferrate nanoparticles; (3) AAO can catalyze the oxidation of AA to generate DHA, decreasing the reduction of K3[Fe(CN)6] to K4[Fe(CN)6]. When CuCl2·2H2O is added, the yield of copper hexacyanoferrate nanoparticles will be increased because of the coordination reaction between Cu(II) and K3[Fe(CN)6], leading to an increase in the absorption value of oxidized 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS). The oxidase-mimetic behaviour and the catalytic mechanism of copper hexacyanoferrate nanoparticles were investigated by using ABTS as a substrate. A series of experimental parameters, including the enzymatic reaction time between AAO and AA, AA concentration, K3[Fe(CN)6] concentration, and the enzymatic reaction time between copper hexacyanoferrate nanoparticles and ABTS, were systematically studied. Finally, the developed colorimetric detection method was used to determine the AAO activity in normal human serum.
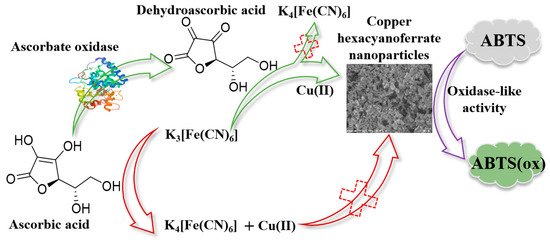
Figure 1.
Schematic illustration of enzyme-regulated in situ formation of copper hexacyanoferrate nanoparticles with oxidase-mimetic behaviour for colorimetric detection of ascorbate oxidase.
2. Materials and Methods
2.1. Chemicals and Materials
Ascorbate oxidase, superoxide dismutase (SOD), L-glutamate sodium, trypsin, and hyaluronidase were obtained from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). ABTS, isopropyl alcohol (IPA), K4[Fe(CN)6], and urea were purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Copper(II) chloride dihydrate (CuCl2·2H2O) was obtained from Shanghai Titan Scientific Co., Ltd. (Shanghai, China). L-Serine was purchased from Tianjin Guangfu Fine Chemical Research Institute (General Partnership) (Tianjin, China). L(+)-Ascorbic acid, hydrochloric acid (HCl), D-glucose monohydrate, sodium chloride (NaCl), and potassium chloride (KCl) were purchased from Chengdu Chron Chemicals Co., Ltd. (Chengdu, China). Normal human serum was purchased from Beijing Solarbio Science and Technology Co., Ltd. (Beijing, China). Sodium dihydrogen phosphate (NaH2PO4), K3[Fe(CN)6], and bovine albumin were obtained from Shanghai Adamas Reagent Co., Ltd. (Shanghai, China).
2.2. Instrumentation
An FE 28 pH meter (Mettler-Toledo Instruments, Shanghai, China) was used to measure the pH of the solution. A UC-2H ultrasonic cleaner (Shanghai Titan Scientific Co., Ltd., Shanghai, China) was used to prepare the solution. UV-Vis absorption spectra were recorded with a UV-2600 spectrophotometer (Shimadzu Instruments (Suzhou) Co., Ltd., Suzhou, China). A DHG-9035A drying oven (Shanghai Yiheng Technology Instrument Co., Ltd., Shanghai, China) was used to control the temperature. The crystal structure of the copper hexacyanoferrate nanoparticles was studied by X’Pert Powder X-ray diffraction (XRD) (Empyrean, PANalytical, the Netherlands). Transmission electron microscopy (TEM) images were collected by a JEM-2100F (JEOL, Tokyo, Japan). Scanning electronic microscopy (SEM) images were collected with an SU8100 (Hitachi, Tokyo, Japan).
2.3. Preparation of Sample Solutions
K3[Fe(CN)6] (2.0 mM) and CuCl2·2H2O (4.0 mM) were prepared by dissolving them in deionized water. The HCl solution (1:50, v/v) was prepared by diluting concentrated HCl with deionized water. The 0.5 mM of ABTS was prepared in deionized water. AA (0.2 mM) was prepared by dissolving it in phosphate buffer (10.0 mM, pH = 6.0). Different concentrations of AAO were prepared by dissolving AAO in phosphate buffer (10.0 mM, pH 6.0). The interfering substrates of Na+, K+, Ag+, Cl−, glucose, L-serine, L-glutamic acid, urea, bovine albumin, trypsin, and hyaluronidase were prepared in deionized water. In addition, 0.2 mg/mL of AA and 2.0 mg/mL of SOD were prepared in deionized water.
2.4. Oxidase-Mimetic Behaviour of Copper Hexacyanoferrate Nanoparticles
The oxidase-mimetic behaviour of copper hexacyanoferrate nanoparticles was investigated using ABTS as the substrate. Experiments were performed in the presence of 0.5 mM of K3[Fe(CN)6], 2.0 μM of CuCl2·2H2O, and 5.0 mM of ABTS, with a total reaction volume of 225.0 μL. After being incubated at room temperature for 3.0 min, the UV-Vis absorbance spectra of the reaction mixture solution were recorded in the range of 380–500 nm.
2.5. Catalytic Mechanism of Copper Hexacyanoferrate Nanoparticles
The catalytic mechanism of the oxidase-mimetic behaviour of copper hexacyanoferrate nanoparticles was investigated. To explore which kind of reactive oxygen species (ROS) was involved in the catalytic process of copper hexacyanoferrate nanoparticles toward ABTS, different radical scavengers, including AA (scavenges •OH and O2•−), SOD (scavenges O2•−), and IPA (scavenges •OH), were introduced. A total volume of 260.0 μL of reaction mixture solution containing 100.0 μL of Cu2+ solution (1.0 mM), 100.0 μL of K3[Fe(CN)6] solution (2.0 mM), 10.0 μL of ABTS solution (1.0 mM), and 50.0 μL of varied amounts of radical scavengers were incubated at room temperature for 3.0 min, respectively. Absorbance values at 412 nm were measured.
2.6. Kinetic Analysis
The kinetic experiments were performed using 1.0 mM of K3[Fe(CN)6] and 2.0 mM of CuCl2·2H2O with different concentrations of ABTS, and absorbance at 412 nm was recorded. The kinetic parameter of the Michaelis–Menten constant (Km) was obtained according to the Lineweaver-Burk double reciprocal plot: 1/v = Km/(vmax[S]) + 1/vmax, where v and vmax represent the initial reaction velocity and the maximum reaction velocity of the enzymatic reaction, respectively, and [S] is the ABTS concentration.
2.7. Determination of AAO Activity Using the Colorimetric Sensing Assay
First, 10.0 μL of deionized water, 10.0 μL of AAO solution, and 50.0 μL of AA solution (0.2 mM) were mixed and reacted at 37 °C for 30.0 min. Then, 10.0 μL of HCl solution, 10.0 μL of K3[Fe(CN)6] solution (2.0 mM), and 10.0 μL of CuCl2·2H2O solution (4.0 mM) were added to the above reaction mixture and incubated for 1.0 min at room temperature. Finally, 10.0 μL of ABTS solution (0.5 mM) was added to the above reaction mixture and reacted for 3.0 min at room temperature. The absorbance of the solution at 412 nm was collected using a UV-2600 spectrophotometer. The change in the absorbance value ΔA (ΔA = A − A0, where A and A0 are the absorbance values of mixture solutions in the presence and absence of AAO, respectively) was calculated, and a calibration curve between AAO activity and ΔA was plotted.
2.8. Selectivity and Interference Study
The selectivity of the colorimetric method for AAO activity detection was investigated, and the following interfering substrates that may exist in the serum sample were analyzed under the optimal conditions: Na+, K+, Ag+, Cl−, glucose, L-serine, L-glutamic acid, urea, bovine albumin, trypsin, and hyaluronidase. In the interference study, these interfering substances were, respectively, mixed with AAO and then analyzed by the UV-Vis spectrophotometer. The initial concentrations of AAO activity and the interfering substances are 0.1 U/mL and 0.5 mg/mL, respectively.
3. Results and Discussion
3.1. Characterization of the Copper Hexacyanoferrate Nanoparticles
The copper hexacyanoferrate nanoparticles were synthesized through the coordination reaction between Cu(II) (4.0 mM) and K3[Fe(CN)6] (2.0 mM) (Figure 1). Figure 2A displays the morphology of the copper hexacyanoferrate nanoparticles investigated through the SEM. Spherical nanoparticles can be observed, indicating the successful formation of copper hexacyanoferrate nanoparticles through the coordination reaction between Cu(II) and K3[Fe(CN)6]. The synthesized copper hexacyanoate nanoparticles were further characterized using TEM. As shown in Figure 2B, the morphology of nanoparticles is spherical with an average diameter of about 100 nm. When the generated orange-yellow colloid solution was irradiated with a red laser, a clear and straight laser beam passing through the colloid sample can be observed due to the Tyndall effect (insert in Figure 2B), indicating that the nanoparticles are well-dispersed in the aqueous solution. Powder X-ray diffraction (XRD) for nanoparticles was conducted to determine the phase and crystalline pattern. As shown in Figure 2C, eleven main characteristic diffraction peaks can be observed at XRD patterns, which correspond to (111), (200), (220), (400), (420), (422), (440), (600), (620), (640), and (642) lattice planes of Cu3[Fe(CN)6]2, respectively. These main diffraction peaks are well-consistent with the standard JCPDS card No. 70–2702. These results revealed the successful synthesis of copper hexacyanoferrate nanoparticles. Moreover, the surface characteristics of the copper hexacyanoferrate nanoparticles were further characterized by EDX analysis (Figure 2D), and the results indicate the presence of C, N, Fe, and Cu elements on the surface of the copper hexacyanoferrate nanoparticles.
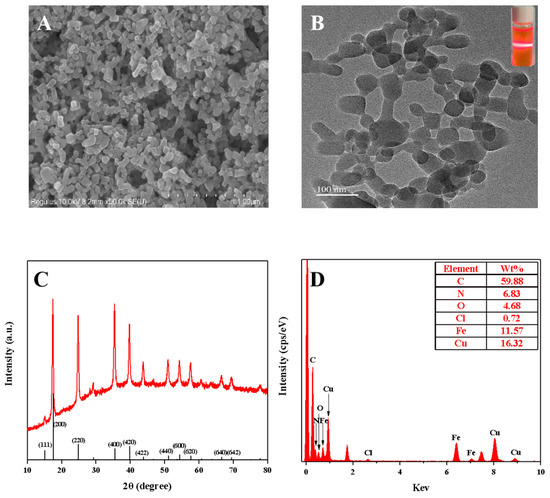
Figure 2.
(A) SEM and TEM; (B) images of the copper hexacyanoferrate nanoparticles (inset: the image of the copper hexacyanoferrate nanoparticles under the irradiation of a red laser); (C) XRD plot of the copper hexacyanoferrate nanoparticles; (D) EDS plot of the copper hexacyanoferrate nanoparticles.
3.2. Oxidase-Mimetic Behaviour of Copper Hexacyanoferrate Nanoparticles
The oxidase-mimetic behaviour of copper hexacyanoferrate nanoparticles was investigated using ABTS as the chromogenic substrate. As shown in Figure 3A, there is no obvious color observed for the CuCl2·2H2O solution (Figure 3A, a curve); the K3[Fe(CN)6] solution (Figure 3A, b curve); the ABTS solution (Figure 3A, c curve); and the mixture solutions of CuCl2·2H2O and ABTS (Figure 3A, d curve) or K3[Fe(CN)6] and ABTS (Figure 3A, e curve). However, a characteristic absorption peak of oxidized ABTS at 412 nm was observed in the coexistence of CuCl2·2H2O, K3[Fe(CN)6], and ABTS (Figure 3A, f curve), indicating that copper hexacyanoferrate nanoparticles have good oxidase-like activity. In addition, the oxidase-mimetic behaviour of the CuCl2·2H2O, K4[Fe(CN)6], and ABTS mixture solution was investigated. The CuCl2·2H2O, K4[Fe(CN)6], and ABTS mixture solution cannot cause the oxidation of ABTS (Figure 3A, g curve). Thus, the synthesized copper hexacyanoferrate nanoparticles through the coordination reaction between Cu(II) and K3[Fe(CN)6] in situ can be used as an oxidase mimic to catalyze the oxidation of ABTS.
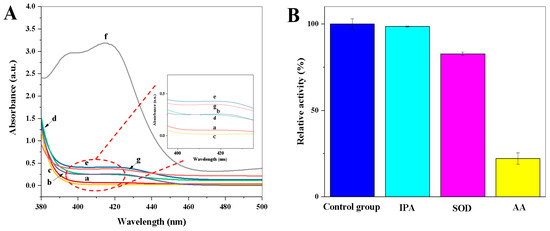
Figure 3.
(A) UV–Vis absorption spectra of (a) CuCl2·2H2O solution, (b) K3[Fe(CN)6] solution, (c) ABTS solution, (d) CuCl2·2H2O + ABTS mixture solution, (e) K3[Fe(CN)6] + ABTS mixture solution, (f) CuCl2·2H2O + K3[Fe(CN)6] + ABTS mixture solution, (g) CuCl2·2H2O + K4[Fe(CN)6] + ABTS mixture solution; (B) The relative activity (%) of copper hexacyanoferrate nanoparticles in the presence of different radical scavengers of IPA (scavenges •OH), AA (scavenges •OH and O2•−), and SOD (scavenges O2•−) (n = 3). The relative activity (%) = the absorbance value in the presence of radical scavengers/the absorbance value in the absence of radical scavengers × 100.
3.3. Catalytic Mechanism of Copper Hexacyanoferrate Nanoparticles
To explore the role of ROS in the catalytic process of copper hexacyanoferrate nanoparticles toward ABTS, different radical scavengers of IPA (scavenges •OH), AA (scavenges •OH and O2•−), and SOD (scavenges O2•−) were introduced [23]. As shown in Figure 3B, the change in relative activity (%) is almost negligible after the introduction of IPA, while the relative activity (%) is significantly decreased after the introduction of SOD and AA, respectively. These results indicate that O2•– is the main ROS active species involved in the copper hexacyanoferrate nanoparticles-ABTS system.
3.4. Kinetic Analysis
The oxidase-mimetic behaviour of copper hexacyanoferrate nanoparticles was investigated by detecting the steady-state kinetic parameter using ABTS as the chromogenic substrate. As shown in Figure S1 (in Supplementary Material), the obtained Lineweaver–Burk double reciprocal plot shows a satisfactory linear correlation (Y = 7.0954X + 0.3182, R2 = 0.9841). The Km value of copper hexacyanoferrate nanoparticles to the substrate (ABTS) is 22.30 μM. In general, a smaller Km value represents a better affinity of the enzyme for the substrate. Compared with previously reported oxidase-like nanozymes (Table S1), the Km value of the copper hexacyanoferrate nanoparticles with ABTS as the chromogenic substrate is much lower than other oxidase-like nanozymes, indicating a much higher affinity between the nanoparticles and ABTS.
3.5. Feasibility of the Colorimetric Detection of AAO Activity
The feasibility of the colorimetric detection of AAO activity was investigated by several control experiments. As shown in Figure S2, an obvious color (insert in Figure S2) and a characteristic absorption peak of oxidized ABTS at 412 nm was observed in the coexistence of AAO, AA, CuCl2·2H2O, K3[Fe(CN)6], and ABTS (Figure S2, a curve), as well as in the coexistence of AAO, CuCl2·2H2O, K3[Fe(CN)6], and ABTS (Figure S2, b curve), indicating that AAO can oxidize the AA to produce DHA that cannot reduce the K3[Fe(CN)6]. In addition, there were no obvious colors observed in the other mixture solutions (Figure S2, c–f curve). Thus, the synthesized copper hexacyanoferrate nanoparticles with oxidase-like activity can be used for the colorimetric detection of AAO activity.
3.6. Optimization of the Colorimetric Detection Method
To obtain a good performance of the assay, several experimental parameters were optimized, including the enzymatic reaction time between AAO and AA (5.0–40.0 min), AA concentration (0.07–0.70 mM), K3[Fe(CN)6] concentration (0.11–0.77 mM), and the enzymatic reaction time between copper hexacyanoferrate nanoparticles and ABTS (0–7.0 min). According to the information provided by the manufacturer, AAO has good activity at pH = 6.0. In addition, the normal human body temperature of 37 °C was selected for the optimization of the colorimetric reaction.
The effect of enzymatic reaction time between AAO and AA (5.0–40.0 min) on the absorbance value was investigated. As shown in Figure 4A, the absorbance value at 412 nm is increased with the increase in enzymatic reaction time from 5.0 to 30.0 min. However, the absorbance values at 412 nm are almost constant when the enzymatic reaction time exceeded 30.0 min. Therefore, the enzymatic reaction time of 30.0 min was chosen for the next study. Then, the effect of AA concentration (0.07–0.70 mM) on the absorbance value was explored. As shown in Figure 4B, there is a maximum decrement of the absorbance value when the final concentration of the AA solution reaches 0.14 mM, while the absorbance value is almost constant when the AA concentration exceeds 0.14 mM. Therefore, the optimal final concentration of AA is 0.14 mM. Moreover, the variation in absorbance value with the concentration of K3[Fe(CN)6] (0.1–0.70 mM) and the corresponding spectrogram are shown in Figure 4C,D, respectively. The absorbance value is increased with the increase in the concentration of K3[Fe(CN)6] from 0.11 to 0.77 mM, and there is a maximum increment of the absorbance value between 0.22 mM and 0.33 mM. To obtain a good sensitivity for AAO detection (Figure 4D), 0.22 mM of K3[Fe(CN)6] was chosen. Finally, the reaction time between copper hexacyanoferrate nanoparticles and ABTS (0–7.0 min) was investigated. As shown in Figure S3, when the reaction time exceeds 1.0 min, the absorbance value is almost constant. Therefore, 1.0 min of reaction time between copper hexacyanoferrate nanoparticles and ABTS was selected in the following experiments.
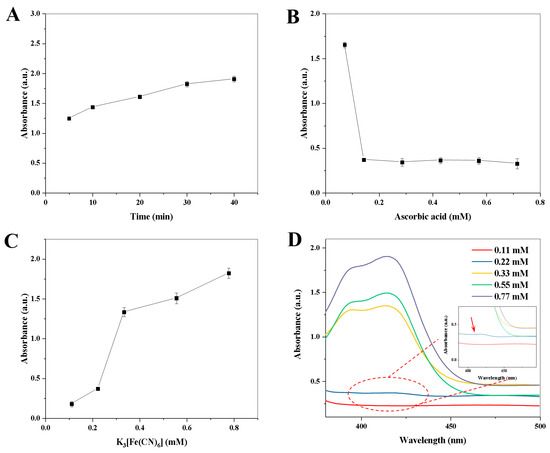
Figure 4.
Effects of enzymatic reaction time (A) and ascorbic acid concentration (B) on the absorbance value at 412 nm.; Effect of K3[Fe(CN)6] concentration on the absorbance value at 412 nm (C), and the corresponding UV–Vis absorption spectra (D). Error bars represent standard deviations (n = 3).
3.7. Feasibility of the Colorimetric Detection of AAO Activity
As shown in Figure 5A, the absorbance value of ABTS at 412 nm is gradually increased with the increase in the activity of AAO ranging from 0 to 35.7 U/L. The corresponding calibration curve of the change in the absorbance value (ΔA) versus AAO activity is shown in Figure 5B. Moreover, different colors appeared with the different activities of AAO (inset in Figure 5B). The linear calibration equation is ΔA = 0.0305X + 0.2289 (where X is the AAO activity, ΔA is the increment of absorbance value, R2 = 0.9913), and the linear range for AAO activity is from 1.1 to 35.7 U/L. The LOD is calculated to be 0.52 U/L according to the 3 σ/S (where σ is the standard deviation, S is the slope of the calibration curve). Compared with other AAO detection methods (Table 1), the developed method has a satisfactory linear range and a low LOD value. The selectivity and interference study of the developed method was performed, and the following interfering substrates that may exist in serum samples were analyzed under the optimal conditions: Na+, K+, Ag+, Cl−, glucose, L-glutamic acid, L-serine, urea, bovine albumin, trypsin, and hyaluronidase. As shown in Figure 6, no obvious changes in the relative absorbance (%) are observed in the presence or absence of AAO. These results indicate that the colorimetric detection for AAO activity has a satisfactory selectivity.
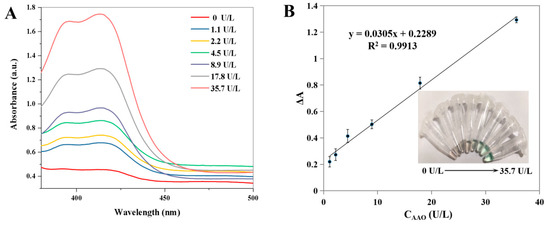
Figure 5.
UV–Vis absorption spectra of ABTS system after the addition of different activities of AAO (A). The corresponding calibration plots for AAO (B) (Insets: the corresponding images). Error bars represent standard deviations (n = 3).

Table 1.
Comparisons of the reported methods for the detection of AAO activity.
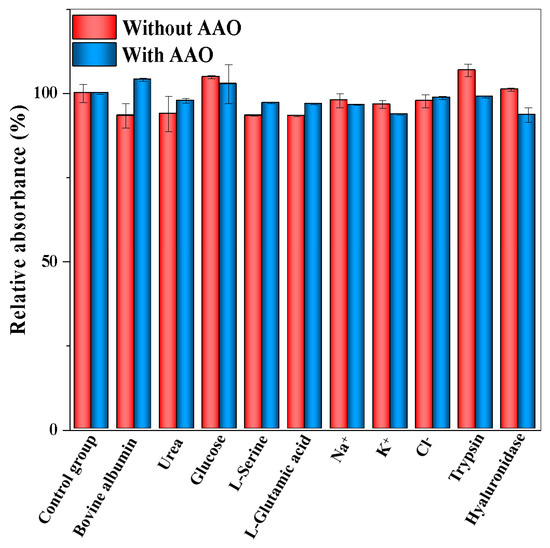
Figure 6.
Selectivity and interference study of the colorimetric assay for AAO activity detection. Error bars represent standard deviations (n = 3).
3.8. Real Samples Analysis
The practical application of the nanozyme-based colorimetric method was investigated by sample spiked recovery to detect AAO activity in normal human serum. Before the analysis, normal human serum was diluted 500 times using deionized water. The serum samples were spiked with three different activities of AAO (2.2, 8.9, and 35.7 U/L) and analyzed through the nanozyme-based colorimetric method. In brief, 10.0 μL of the human serum sample, 10.0 μL of AAO solution, and 50.0 μL of AA solution (0.2 mM) were mixed and reacted at 37 °C for 30.0 min. Then, 10.0 μL of HCl solution, 10.0 μL of K3[Fe(CN)6] solution (2.0 mM), and 10.0 μL of CuCl2·2H2O solution (4.0 mM) were added to the above reaction mixture and incubated for 1.0 min at room temperature. Finally, 10.0 μL of ABTS solution (0.5 mM) was added to the above reaction mixture and reacted for 3.0 min at room temperature. The absorbance of the solution at 412 nm was collected using a UV-2600 spectrophotometer. The recoveries (Table 2) of the AAO in the human serum sample spiked with three different activities of AAO are in the range of 87.4–108.8%. The results show that the developed method has good reliability in the detection of AAO activity in real samples.

Table 2.
Detection of AAO activity in normal human serum using the nanozyme-based colorimetric method (n = 3).
4. Conclusions
In summary, a colorimetric detection method for AAO activity was established for the first time through an enzyme-regulated in situ formation of copper hexacyanoferrate nanoparticles-based nanozyme through a simple precipitation method. The synthesized copper hexacyanoferrate nanozyme was coupled with AA to construct an AAO-regulated in situ formation of copper hexacyanoferrate nanoparticles system for the detection of AAO activity with an LOD as low as 0.52 U/L. In short, the colorimetric method proposed in this study has the following merits: (1) it avoids the tedious design and synthesis processes of other nanozymes; (2) the detection process is simple and does not require large equipment; (3) it provides a simple bioanalytical method using a bioenzyme–nanozyme cascade reaction, which has great potential in constructing sensitive cascade-reaction assaying methods.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bios13030344/s1, Figure S1: The double-reciprocal plot of copper hexacyanoferrate nanoparticles. Error bars represent standard deviations (n = 3); Figure S2: UV–vis absorption spectra of (a) AAO + AA + K3[Fe(CN)6] + CuCl2·2H2O + ABTS mixture solution, (b) AAO + K3[Fe(CN)6] + CuCl2·2H2O + ABTS mixture solution, (c) AA + K3[Fe(CN)6] + CuCl2·2H2O + ABTS mixture solution, (d) AAO + AA + CuCl2·2H2O + ABTS mixture solution, (e) AAO + AA + K3[Fe(CN)6] + ABTS mixture solution, (f) AAO + AA + K3[Fe(CN)6] + CuCl2·2H2O mixture solution (Insets: the corresponding images); Figure S3: The effect of reaction time between copper hexacyanoferrate nanoparticles and ABTS on the absorbance value at 412 nm. Error bars represent standard deviations (n = 3); Table S1: Comparison of apparent Michaelis−Menten Constant (Km) of copper hexacyanoferrate nanoparticles for the oxidation of ABTS with other reported oxidase-like nanozymes. References [29,30,31,32,33] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, H.Z.; methodology, H.Z.; investigation, H.Z. and D.-N.Y.; software, D.-N.Y. and Y.L.; data curation, D.-N.Y. and Y.L., writing—original draft preparation, H.Z.; writing—review and editing, H.Z. and F.-Q.Y.; supervision, F.-Q.Y. and H.Z.; project administration, H.Z.; funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Chongqing Postdoctoral Science Foundation, China, grant number CSTB2022NSCQ-BHX0687, Chongqing Medical Scientific Research Project (Joint project of Chongqing Health Commission and Science and Technology Bureau), China, grant number 2023MSXM006, and the Chongqing Medical and Pharmaceutical College, China, grant number YGZ2021133.
Institutional Review Board Statement
The normal human serum used in this study was purchased from Beijing Solarbio Science and Technology Co., Ltd., China, and is a biological product. Thus, it is not applicable for an ethics statement.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, H.; Liu, Y.; Li, H.; Zhang, Y.; Yao, S. Non-oxidation reduction strategy for highly selective detection of ascorbic acid with dualratio fluorescence and colorimetric signals. Sens. Actuator B-Chem. 2019, 281, 983–988. [Google Scholar] [CrossRef]
- Wu, Z.; Nan, D.; Yang, H.; Pan, S.; Liu, H.; Hu, X. A ratiometric fluorescence-scattered light strategy based on MoS2 quantum dots/CoOOH nanoflakes system for ascorbic acid detection. Anal. Chim. Acta 2019, 1091, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, H.; Liu, S.; Wang, W.; Chen, H.; Xiao, L.; Ren, C.; Chen, X. Carbon dots as fluorescent/colorimetric probes for real-time detection of hypochlorite and ascorbic acid in cells and body fluid. Anal. Chem. 2019, 91, 15477–15483. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Gan, L.L.; Su, Q.; Chen, Z.B.; Yang, X.M. Exploration of pH-responsive carbon dots for detecting nitrite and ascorbic acid. Appl. Surf. Sci. 2020, 530, 147269. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Paixão, T.R.L.C.; Bertotti, M. FIA determination of ascorbic acid at low potential using a ruthenium oxide hexacyanoferrate modified carbon electrode. J. Pharm. Biomed. Anal. 2008, 46, 528–533. [Google Scholar] [CrossRef]
- Bohndiek, S.E.; Kettunen, M.I.; Hu, D.-E.; Kennedy, B.W.C.; Boren, J.; Gallagher, F.A.; Brindle, K.M. Hyperpolarized [1-13C]-ascorbic and dehydroascorbic acid: Vitamin C as a probe for imaging redox status in vivo. J. Am. Chem. Soc. 2011, 133, 11795–11801. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Ciraci, S.; Liso, R.; Arrigoni, O. Ascorbic acid oxidase is dynamically regulated by light and oxygen. A tool for oxygen management in plants? J. Plant Physiol. 2007, 164, 39–46. [Google Scholar] [CrossRef]
- Li, N.; Zhang, F.; Sun, W.; Zhang, L.; Su, X. Redox reaction-modulated fluorescence biosensor for ascorbic acid oxidase assay by using MoS2 quantum dots as fluorescence probe. Talanta 2021, 222, 121522. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, S.; Wang, M.; Xie, X.; Su, X. Self-assembled dual-emissive nanoprobe with metal-organic frameworks as scaffolds for enhanced ascorbic acid and ascorbate oxidase sensing. Sens. Actuator B-Chem. 2021, 339, 129910. [Google Scholar] [CrossRef]
- Liu, S.; Pang, S. A dual-model strategy for fluorometric determination of ascorbic acid and of ascorbic acid oxidase activity by using DNA-templated gold-silver nanoclusters. Microchim. Acta 2018, 185, 426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Liu, W.; Ding, F.; Zou, P.; Wang, X.; Zhao, Q.; Rao, H. A carbon dot-based ratiometric fluorometric and colorimetric method for determination of ascorbic acid and of the activity of ascorbic acid oxidase. Microchim. Acta 2019, 186, 246. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.Z.; Zhuang, J.; Nie, L.; Zhang, J.B.; Zhang, Y.; Gu, N.; Wang, T.H.; Feng, J.; Yang, D.L.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E.K. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Lai, W.Q.; Guo, J.Q.; Wang, Y.Q.; Lin, Y.X.; Ye, S.; Zhuang, J.Y.; Tang, D.P. Enzyme-controllable just-in-time production system of copper hexacyanoferrate nanoparticles with oxidase-mimicking activity for highly sensitive colorimetric immunoassay. Talanta 2022, 247, 123546. [Google Scholar] [CrossRef] [PubMed]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically synthesized prussian blue nanoparticles defeating natural enzyme peroxidase. J. Am. Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Zhang, H.; Yang, F.Q. Enhanced peroxidase-like activity of copper phosphate modified by hydrophilic phytic-acid and its application in colorimetric detection of hydrogen peroxide. Microchem. J. 2021, 168, 106489. [Google Scholar] [CrossRef]
- Peng, L.J.; Zhang, C.Y.; Zhang, W.Y.; Zhou, H.Y.; Yang, F.Q. The peroxidase-like catalytic activity of in situ prepared cobalt carbonate and its applications in colorimetric detection of hydrogen peroxide, glucose and ascorbic acid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129744. [Google Scholar] [CrossRef]
- Goud, B.S.; Shin, G.; Vattikuti, S.V.P.; Mameda, N.; Kim, H.; Koyyada, G.; Kim, J. Enzyme-integrated biomimetic cobalt metal-organic framework nanozyme for one-step cascade glucose biosensing via tandem catalysis. Biochem. Eng. J. 2022, 188, 108669. [Google Scholar] [CrossRef]
- Zhan, X.Q.; Tang, Y.; Liu, Y.Y.; Tao, H.; Wu, Y.G. A novel colorimetric strategy for rapid detection of dimethoate residue in vegetables based on enhancing oxidase-mimicking catalytic activity of cube-shape Ag2O particles. Sens. Actuator B-Chem. 2020, 361, 131720. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, J.J.; Zou, L.; Ye, B.X.; Li, G.P. Ratiometric fluorescence sensing of glutathione by using the oxidase-mimicking activity of MnO2 nanosheet. Anal. Chim. Acta 2021, 1145, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.W.; Ni, D.L.; Rosenkrans, Z.T.; Huang, P.; Yan, X.Y.; Cai, W.B. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Rao, H.H.; Huang, H.Y.; Zhang, K.H.; Wei, M.M.; Luo, M.Y.; Xue, X.; Xue, Z.H.; Lu, X.Q. A sensitive photothermometric biosensor based on redox reaction-controlled nanoprobe conversion from Prussian blue to Prussian white. Anal. Bioanal. Chem. 2021, 413, 6627–6637. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, G.Q.; Guan, Y.; Yang, T.; Hu, R.; Yang, Y.H. Modulation of inner filter effect between persistent luminescent particles and 2, 3-diaminophenazine for ratiometric fluorescent assay of ascorbic acid and ascorbate oxidase activity. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 280, 121564. [Google Scholar] [CrossRef]
- Han, X.Y.; Chen, Z.H.; Fan, Q.X.; Li, K.N.; Mu, F.Y.; Luo, Q.Y.; Jin, Z.W.; Shi, G.Y.; Zhang, M. Manganese(II)-doped zinc/germanium oxide nanoparticles as a viable fluorescent probe for visual and time-resolved fluorometric determination of ascorbic acid and its oxidase. Microchim. Acta 2019, 186, 466. [Google Scholar] [CrossRef]
- Wang, M.J.; Wang, M.K.; Wang, G.N.; Su, X.G. A fluorescence “off–on–off” sensing platform based on bimetallic gold/silver nanoclusters for ascorbate oxidase activity monitoring. Analyst 2020, 145, 1001–1007. [Google Scholar] [CrossRef]
- Peng, L.J.; Yin, S.J.; Chen, L.; Tian, T.; Zhang, W.Y.; Zhou, H.Y.; Yang, F.Q. Investigating the oxidase-like activity of a Co–Fe Prussian blue analogue nanocube prepared in situ and its applications in the colorimetric detection of ascorbic acid, alkaline phosphatase, α-glucosidase, and ascorbic acid oxidase. New J. Chem. 2023, 47, 1156–1164. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.M.; Liu, S.J.; Zheng, L.L.; Bu, Y.M.; Deng, H.H.; Chen, R.T.; Peng, H.P.; Lin, X.H.; Chen, W. Colorimetric acid phosphatase sensor based on MoO3 nanozyme. Anal. Chim. Acta 2020, 1105, 162–168. [Google Scholar] [CrossRef]
- Wang, N.; Shi, J.; Liu, Y.; Sun, W.; Su, X. Constructing bifunctional metaleorganic framework based nanozymes with fluorescence and oxidase activity for the dualchannel detection of butyrylcholinesterase. Anal. Chim. Acta 2022, 1205, 339717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Gao, Y.C.; Li, H.W.; Wu, Y.Q. Gold-platinum bimetallic nanoclusters for oxidase-like catalysis. ACS Appl. Nano Mater. 2020, 3, 9318–9328. [Google Scholar] [CrossRef]
- Qin, W.J.; Su, L.; Yang, C.; Ma, Y.H.; Zhang, H.J.; Chen, X.G. Colorimetric detection of sulfite in foods by a TMB-O2-Co3O4 nanoparticles detection system. J. Agric. Food Chem. 2014, 62, 5827–5834. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.X.; Li, H.; Deng, Y.Q.; He, Y. Blue light-gated reversible silver nanozyme reaction networks that achieve life-like adaptivity. ACS Sustain. Chem. Eng. 2020, 8, 5076–5081. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).