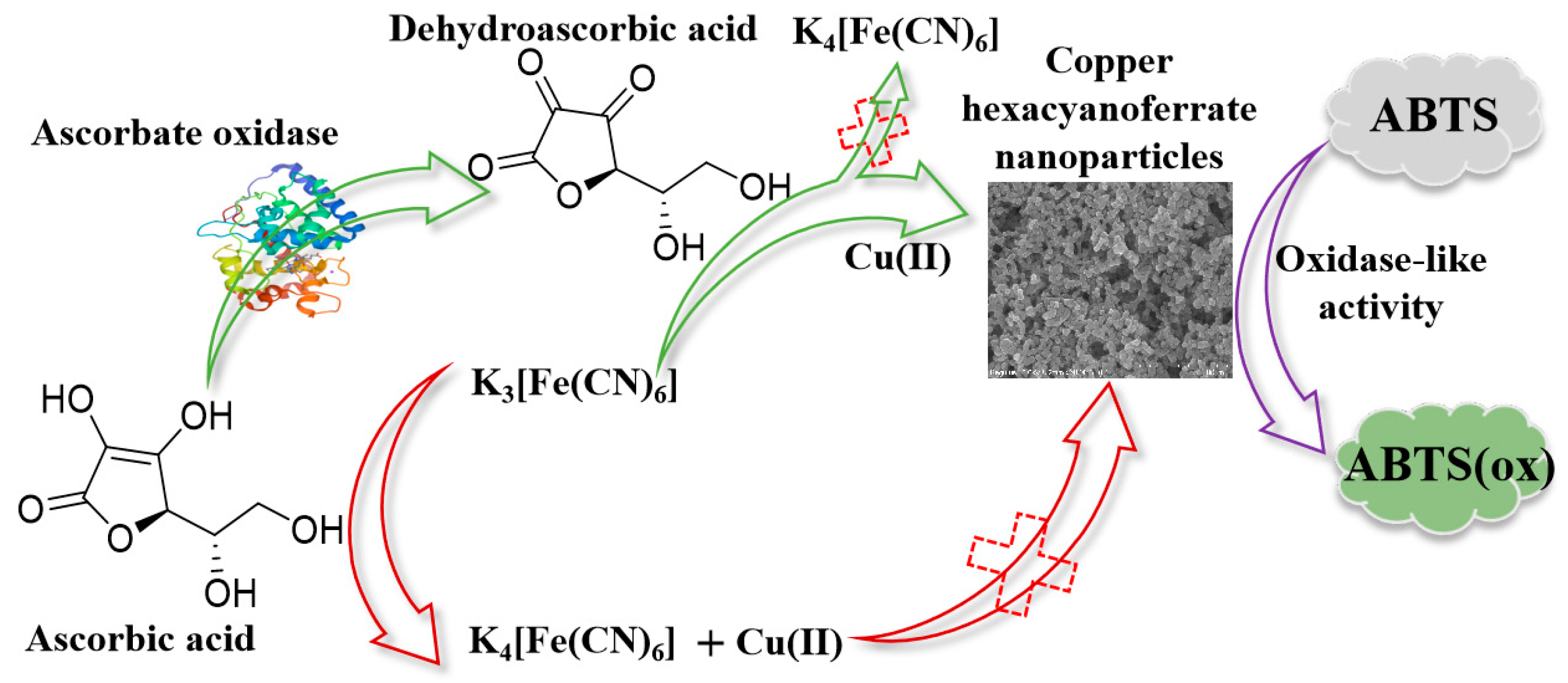
Enzyme-Regulated In Situ Formation of Copper Hexacyanoferrate Nanoparticles with Oxidase-Mimetic Behaviour for Colorimetric Detection of Ascorbate Oxidase
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Instrumentation
2.3. Preparation of Sample Solutions
2.4. Oxidase-Mimetic Behaviour of Copper Hexacyanoferrate Nanoparticles
2.5. Catalytic Mechanism of Copper Hexacyanoferrate Nanoparticles
2.6. Kinetic Analysis
2.7. Determination of AAO Activity Using the Colorimetric Sensing Assay
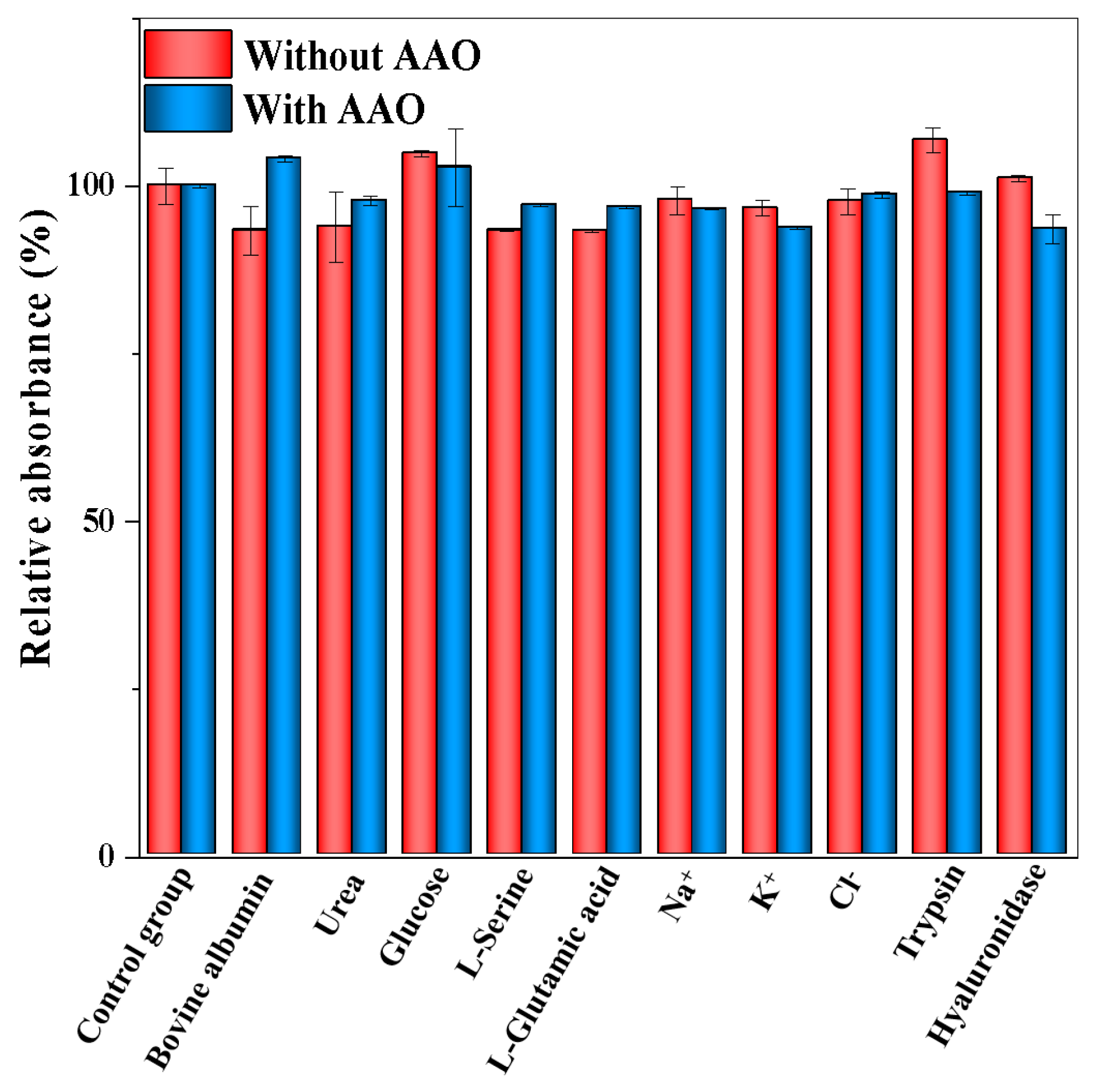
2.8. Selectivity and Interference Study
3. Results and Discussion
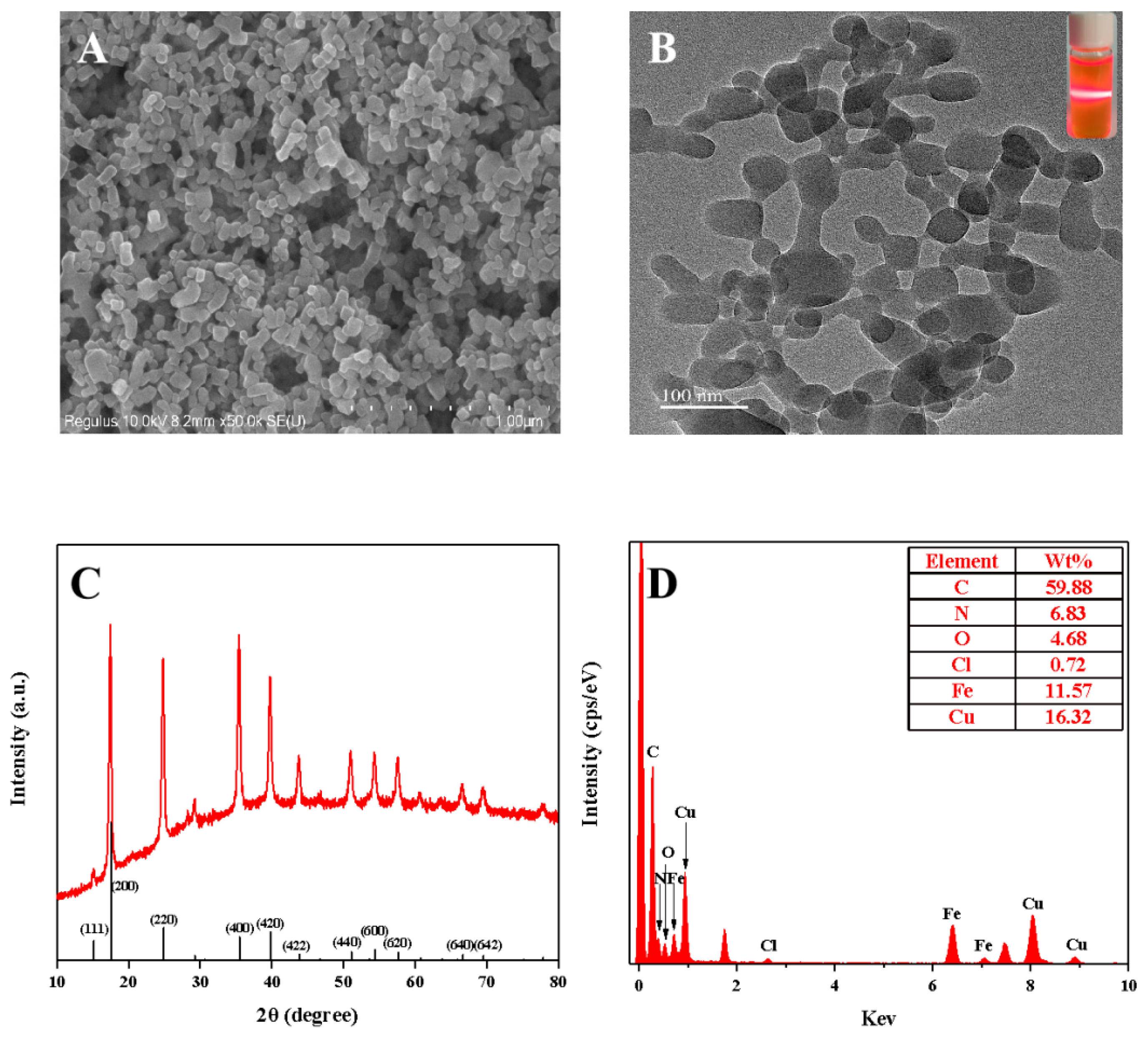
3.1. Characterization of the Copper Hexacyanoferrate Nanoparticles
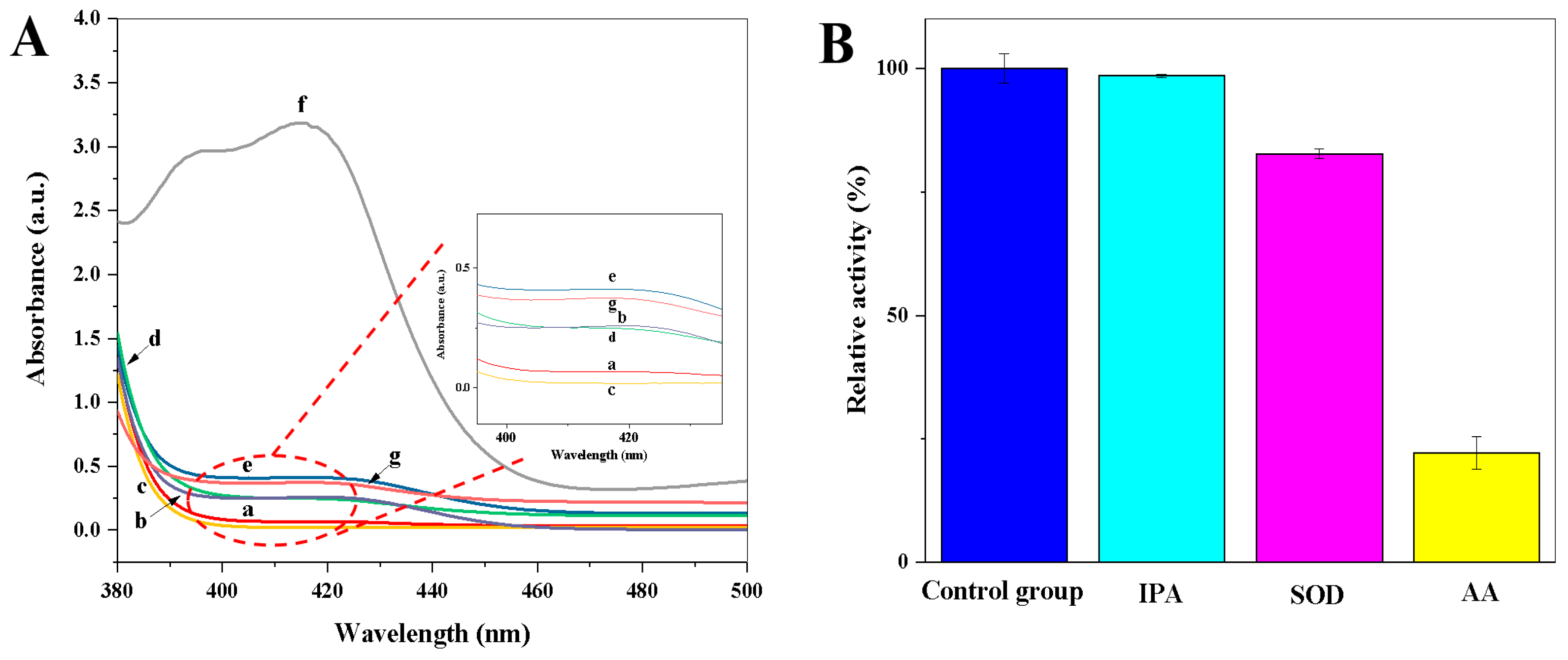
3.2. Oxidase-Mimetic Behaviour of Copper Hexacyanoferrate Nanoparticles
3.3. Catalytic Mechanism of Copper Hexacyanoferrate Nanoparticles
3.4. Kinetic Analysis
3.5. Feasibility of the Colorimetric Detection of AAO Activity
3.6. Optimization of the Colorimetric Detection Method
3.7. Feasibility of the Colorimetric Detection of AAO Activity
3.8. Real Samples Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Liu, Y.; Li, H.; Zhang, Y.; Yao, S. Non-oxidation reduction strategy for highly selective detection of ascorbic acid with dualratio fluorescence and colorimetric signals. Sens. Actuator B-Chem. 2019, 281, 983–988. [Google Scholar] [CrossRef]
- Wu, Z.; Nan, D.; Yang, H.; Pan, S.; Liu, H.; Hu, X. A ratiometric fluorescence-scattered light strategy based on MoS2 quantum dots/CoOOH nanoflakes system for ascorbic acid detection. Anal. Chim. Acta 2019, 1091, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Li, H.; Liu, S.; Wang, W.; Chen, H.; Xiao, L.; Ren, C.; Chen, X. Carbon dots as fluorescent/colorimetric probes for real-time detection of hypochlorite and ascorbic acid in cells and body fluid. Anal. Chem. 2019, 91, 15477–15483. [Google Scholar] [CrossRef]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001. [Google Scholar] [CrossRef]
- Gan, L.L.; Su, Q.; Chen, Z.B.; Yang, X.M. Exploration of pH-responsive carbon dots for detecting nitrite and ascorbic acid. Appl. Surf. Sci. 2020, 530, 147269. [Google Scholar] [CrossRef]
- Murugan, N.; Jerome, R.; Preethika, M.; Sundaramurthy, A.; Sundramoorthy, A.K. 2D-titanium carbide (MXene) based selective electrochemical sensor for simultaneous detection of ascorbic acid, dopamine and uric acid. J. Mater. Sci. Technol. 2021, 72, 122–131. [Google Scholar] [CrossRef]
- Paixão, T.R.L.C.; Bertotti, M. FIA determination of ascorbic acid at low potential using a ruthenium oxide hexacyanoferrate modified carbon electrode. J. Pharm. Biomed. Anal. 2008, 46, 528–533. [Google Scholar] [CrossRef]
- Bohndiek, S.E.; Kettunen, M.I.; Hu, D.-E.; Kennedy, B.W.C.; Boren, J.; Gallagher, F.A.; Brindle, K.M. Hyperpolarized [1-13C]-ascorbic and dehydroascorbic acid: Vitamin C as a probe for imaging redox status in vivo. J. Am. Chem. Soc. 2011, 133, 11795–11801. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Ciraci, S.; Liso, R.; Arrigoni, O. Ascorbic acid oxidase is dynamically regulated by light and oxygen. A tool for oxygen management in plants? J. Plant Physiol. 2007, 164, 39–46. [Google Scholar] [CrossRef]
- Li, N.; Zhang, F.; Sun, W.; Zhang, L.; Su, X. Redox reaction-modulated fluorescence biosensor for ascorbic acid oxidase assay by using MoS2 quantum dots as fluorescence probe. Talanta 2021, 222, 121522. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Jiang, S.; Wang, M.; Xie, X.; Su, X. Self-assembled dual-emissive nanoprobe with metal-organic frameworks as scaffolds for enhanced ascorbic acid and ascorbate oxidase sensing. Sens. Actuator B-Chem. 2021, 339, 129910. [Google Scholar] [CrossRef]
- Liu, S.; Pang, S. A dual-model strategy for fluorometric determination of ascorbic acid and of ascorbic acid oxidase activity by using DNA-templated gold-silver nanoclusters. Microchim. Acta 2018, 185, 426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Y.; Liu, W.; Ding, F.; Zou, P.; Wang, X.; Zhao, Q.; Rao, H. A carbon dot-based ratiometric fluorometric and colorimetric method for determination of ascorbic acid and of the activity of ascorbic acid oxidase. Microchim. Acta 2019, 186, 246. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.Z.; Zhuang, J.; Nie, L.; Zhang, J.B.; Zhang, Y.; Gu, N.; Wang, T.H.; Feng, J.; Yang, D.L.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E.K. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Lai, W.Q.; Guo, J.Q.; Wang, Y.Q.; Lin, Y.X.; Ye, S.; Zhuang, J.Y.; Tang, D.P. Enzyme-controllable just-in-time production system of copper hexacyanoferrate nanoparticles with oxidase-mimicking activity for highly sensitive colorimetric immunoassay. Talanta 2022, 247, 123546. [Google Scholar] [CrossRef] [PubMed]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically synthesized prussian blue nanoparticles defeating natural enzyme peroxidase. J. Am. Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Y.; Zhang, H.; Yang, F.Q. Enhanced peroxidase-like activity of copper phosphate modified by hydrophilic phytic-acid and its application in colorimetric detection of hydrogen peroxide. Microchem. J. 2021, 168, 106489. [Google Scholar] [CrossRef]
- Peng, L.J.; Zhang, C.Y.; Zhang, W.Y.; Zhou, H.Y.; Yang, F.Q. The peroxidase-like catalytic activity of in situ prepared cobalt carbonate and its applications in colorimetric detection of hydrogen peroxide, glucose and ascorbic acid. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129744. [Google Scholar] [CrossRef]
- Goud, B.S.; Shin, G.; Vattikuti, S.V.P.; Mameda, N.; Kim, H.; Koyyada, G.; Kim, J. Enzyme-integrated biomimetic cobalt metal-organic framework nanozyme for one-step cascade glucose biosensing via tandem catalysis. Biochem. Eng. J. 2022, 188, 108669. [Google Scholar] [CrossRef]
- Zhan, X.Q.; Tang, Y.; Liu, Y.Y.; Tao, H.; Wu, Y.G. A novel colorimetric strategy for rapid detection of dimethoate residue in vegetables based on enhancing oxidase-mimicking catalytic activity of cube-shape Ag2O particles. Sens. Actuator B-Chem. 2020, 361, 131720. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, J.J.; Zou, L.; Ye, B.X.; Li, G.P. Ratiometric fluorescence sensing of glutathione by using the oxidase-mimicking activity of MnO2 nanosheet. Anal. Chim. Acta 2021, 1145, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.W.; Ni, D.L.; Rosenkrans, Z.T.; Huang, P.; Yan, X.Y.; Cai, W.B. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Rao, H.H.; Huang, H.Y.; Zhang, K.H.; Wei, M.M.; Luo, M.Y.; Xue, X.; Xue, Z.H.; Lu, X.Q. A sensitive photothermometric biosensor based on redox reaction-controlled nanoprobe conversion from Prussian blue to Prussian white. Anal. Bioanal. Chem. 2021, 413, 6627–6637. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, G.Q.; Guan, Y.; Yang, T.; Hu, R.; Yang, Y.H. Modulation of inner filter effect between persistent luminescent particles and 2, 3-diaminophenazine for ratiometric fluorescent assay of ascorbic acid and ascorbate oxidase activity. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2022, 280, 121564. [Google Scholar] [CrossRef]
- Han, X.Y.; Chen, Z.H.; Fan, Q.X.; Li, K.N.; Mu, F.Y.; Luo, Q.Y.; Jin, Z.W.; Shi, G.Y.; Zhang, M. Manganese(II)-doped zinc/germanium oxide nanoparticles as a viable fluorescent probe for visual and time-resolved fluorometric determination of ascorbic acid and its oxidase. Microchim. Acta 2019, 186, 466. [Google Scholar] [CrossRef]
- Wang, M.J.; Wang, M.K.; Wang, G.N.; Su, X.G. A fluorescence “off–on–off” sensing platform based on bimetallic gold/silver nanoclusters for ascorbate oxidase activity monitoring. Analyst 2020, 145, 1001–1007. [Google Scholar] [CrossRef]
- Peng, L.J.; Yin, S.J.; Chen, L.; Tian, T.; Zhang, W.Y.; Zhou, H.Y.; Yang, F.Q. Investigating the oxidase-like activity of a Co–Fe Prussian blue analogue nanocube prepared in situ and its applications in the colorimetric detection of ascorbic acid, alkaline phosphatase, α-glucosidase, and ascorbic acid oxidase. New J. Chem. 2023, 47, 1156–1164. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, X.M.; Liu, S.J.; Zheng, L.L.; Bu, Y.M.; Deng, H.H.; Chen, R.T.; Peng, H.P.; Lin, X.H.; Chen, W. Colorimetric acid phosphatase sensor based on MoO3 nanozyme. Anal. Chim. Acta 2020, 1105, 162–168. [Google Scholar] [CrossRef]
- Wang, N.; Shi, J.; Liu, Y.; Sun, W.; Su, X. Constructing bifunctional metaleorganic framework based nanozymes with fluorescence and oxidase activity for the dualchannel detection of butyrylcholinesterase. Anal. Chim. Acta 2022, 1205, 339717. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Gao, Y.C.; Li, H.W.; Wu, Y.Q. Gold-platinum bimetallic nanoclusters for oxidase-like catalysis. ACS Appl. Nano Mater. 2020, 3, 9318–9328. [Google Scholar] [CrossRef]
- Qin, W.J.; Su, L.; Yang, C.; Ma, Y.H.; Zhang, H.J.; Chen, X.G. Colorimetric detection of sulfite in foods by a TMB-O2-Co3O4 nanoparticles detection system. J. Agric. Food Chem. 2014, 62, 5827–5834. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.X.; Li, H.; Deng, Y.Q.; He, Y. Blue light-gated reversible silver nanozyme reaction networks that achieve life-like adaptivity. ACS Sustain. Chem. Eng. 2020, 8, 5076–5081. [Google Scholar] [CrossRef]


| Materials | Detection Methods | Linear Range (U/L) | LOD (U/L) | Ref. |
|---|---|---|---|---|
| Copper hexacyanoferrate NPs a | Colorimetry | 1.1–35.7 | 0.52 | This study |
| MoS2 QDs b | Fluorimetry | 2.0–40.0 | 0.8 | [10] |
| ZIF-8 c @QDs and CDs d | Fluorimetry | 0.05–4.0 | 0.02 | [11] |
| Prussian blue NPs | Photothermometry | 0.25–14 | 0.21 | [24] |
| CoOOH nanosheets | Fluorimetry | 1.0–20.0 | 0.25 | [25] |
| Manganese(II)-doped zinc/germanium oxide NPs | Fluorimetry/Scanometric analysis | 1250−2500/1000–4000 | 728/850 | [26] |
| DNA-templated gold-silver nanoclusters | Fluorimetry/Colorimetry | 10.0−200.0 | 4.8/6.8 | [12] |
| Papain-protected bimetallic gold/silver nanoclusters | Fluorimetry | 5.0−80.0 | 1.72 | [27] |
| CDs | Fluorimetry/Colorimetry | 0.04–5.0/0.04–8.0 | 0.017/0.012 | [13] |
| Co-Fe Prussian blue analog nanocube | Colorimetry | 0.25–5.0 | 0.16 | [28] |
| Sample | Added (U/L) | Found (SD) (U/L) | Recovery (%) a |
|---|---|---|---|
| Human serum | 0 | 0 b | - |
| 2.2 | 2.4 (0.7) | 108.8 | |
| 8.9 | 7.8 (1.0) | 87.4 | |
| 35.7 | 34.6 (0.6) | 96.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Yang, D.-N.; Li, Y.; Yang, F.-Q. Enzyme-Regulated In Situ Formation of Copper Hexacyanoferrate Nanoparticles with Oxidase-Mimetic Behaviour for Colorimetric Detection of Ascorbate Oxidase. Biosensors 2023, 13, 344. https://doi.org/10.3390/bios13030344
Zhang H, Yang D-N, Li Y, Yang F-Q. Enzyme-Regulated In Situ Formation of Copper Hexacyanoferrate Nanoparticles with Oxidase-Mimetic Behaviour for Colorimetric Detection of Ascorbate Oxidase. Biosensors. 2023; 13(3):344. https://doi.org/10.3390/bios13030344
Chicago/Turabian StyleZhang, Hao, Dan-Ni Yang, Yan Li, and Feng-Qing Yang. 2023. "Enzyme-Regulated In Situ Formation of Copper Hexacyanoferrate Nanoparticles with Oxidase-Mimetic Behaviour for Colorimetric Detection of Ascorbate Oxidase" Biosensors 13, no. 3: 344. https://doi.org/10.3390/bios13030344
APA StyleZhang, H., Yang, D.-N., Li, Y., & Yang, F.-Q. (2023). Enzyme-Regulated In Situ Formation of Copper Hexacyanoferrate Nanoparticles with Oxidase-Mimetic Behaviour for Colorimetric Detection of Ascorbate Oxidase. Biosensors, 13(3), 344. https://doi.org/10.3390/bios13030344






