Simple Summary
Head and neck tumors (HNTs) are associated with a high mortality due to their commonly insidious and asymptomatic development. Regarding risk stratification and long-term patient outcome prediction, routine clinical evaluation by radiologists has several limitations. Numerous researchers have assessed the usefulness of radiomics and artificial intelligence in the context of head and neck tumor imaging given the exponential development of these technologies in medical imaging. These were geared at the creation of reliable and reproducible models based on quantitative data. Even if there are still a few obstacles to their widespread usage in clinical practice, it is clear that they have the potential to be revolutionary. In this paper, we provide a thorough overview of radiomics and artificial intelligence applications in head and neck tumor imaging.
Abstract
Recent advances in machine learning and artificial intelligence technology have ensured automated evaluation of medical images. As a result, quantifiable diagnostic and prognostic biomarkers have been created. We discuss radiomics applications for the head and neck region in this paper. Molecular characterization, categorization, prognosis and therapy recommendation are given special consideration. In a narrative manner, we outline the fundamental technological principles, the overall idea and usual workflow of radiomic analysis and what seem to be the present and potential challenges in normal clinical practice. Clinical oncology intends for all of this to ensure informed decision support for personalized and useful cancer treatment. Head and neck cancers present a unique set of diagnostic and therapeutic challenges. These challenges are brought on by the complicated anatomy and heterogeneity of the area under investigation. Radiomics has the potential to address these barriers. Future research must be interdisciplinary and focus on the study of certain oncologic functions and outcomes, with external validation and multi-institutional cooperation in order to achieve this.
1. Introduction
Head and neck tumors (HNTs) account for over 830,000 cases annually worldwide [1] and are associated with a high mortality due to their commonly insidious and asymptomatic development. This often leads to a late diagnosis of disease, in more advanced stages. HNTs comprise a large spectrum of tumors and tumor-like conditions [2], which can arise from different tissues included in this anatomical region, such as paranasal sinuses, pharynx, oral cavity, larynx, thyroid, lymph nodes and associated soft tissues and bones. Squamous cell carcinomas (SCCs) represent the most common histological type, accounting for 90% of all HNTs [3]. Risk factors include tobacco and alcohol use [4] as well as, more recently identified, human papillomavirus (HPV) infection [5]. The latter represents the main cause of an increasing incidence of head and neck squamous cell carcinoma (HNSCC) in the USA [6], mainly consisting of oropharyngeal squamous cell carcinoma (OPSCC) and nasopharyngeal carcinoma (NPC). Among HNSCCs, NPC represents a peculiar entity with a distinct epidemiology. It is a rare malignant epithelial tumor [7] arising from the superior region of the pharynx’s mucosa, usually from the lateral pharyngeal recess (i.e., Rosenmüller’s fossa) [8], with evidence of squamous differentiation. An interplay of different causes is involved in the etiology of this disease, including genetic, viral and environmental risk factors, such as nitrosamine-containing food consumption and smoking. Its strong association with the Epstein–Barr virus infection makes the incidence of NPC significantly higher in some endemic regions, including southeast Asia and China [9]. According to the 2017 World Health Organization (WHO) Classification of HNTs, NPCs are classified into three subtypes, non-keratinizing (NK-NPC), keratinizing (K-NPC) and basaloid [10], as also confirmed in the recent 5th edition of the WHO Classification. NK-NPC represents the most common histologic subtype. Magnetic resonance imaging (MRI) is the modality of choice for diagnosis, staging and in the evaluation of response to treatment in HNTs, as its high soft tissue contrast allows a more accurate delineation of tumor margins compared to computed tomography (CT) and spectral photon-counting CT [11]. Furthermore, the emerging field of radiomics has shown that a large amount of additional quantitative information, otherwise undetectable, can be extracted from medical images of these patients.
Radiomics is a quantitative method of approaching medical imaging, which seeks to augment the data already available to doctors through sophisticated mathematical analysis. Using analytical techniques, radiomics quantifies textural information by mathematically extracting the spatial distribution of signal intensities and pixel inter-relationships [12]. Numerous imaging studies from different fields have already been published using this approach. The use of radiomics in the study of the head and neck region, particularly for neoplastic pathology, is one of the newer use areas. This is achievable through the characterization of pixel gray level distribution patterns, which can then be analyzed by machine-learning (ML) algorithms, potentially providing information on tumor physiology, which could have an important impact on the management and improve prognosis of these tumors in the near future. Figure 1 shows a classic “radiomic workflow” involving a series of steps for reproducible and consistent extraction of imaging data. These steps include image acquisition, feature extraction and feature selection. This may be possible through deep-learning (DL) radiomics, handcrafted radiomics and delta radiomics.
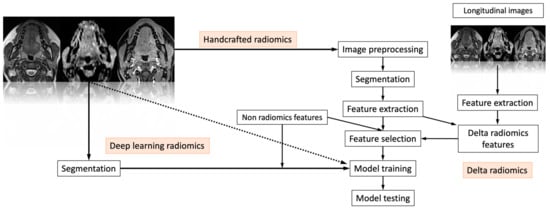
Figure 1.
The “radiomic workflow” involves a series of iterative steps for reproducible and consistent extraction of imaging data. These steps include image acquisition, feature extraction and feature selection. This may be possible through deep-learning radiomics, handcrafted radiomics and delta radiomics. Finally, the selected features are used to test the final model.
Based on how images are converted into data that can be mined, radiomics has two primary branches: “deep-learning” and “handcrafted radiomics”. In contrast to deep learning, which uses complex networks to “extract and analyze” its own features, handcrafted features are obtained by formulae that are mostly based on intensity histograms, shape attributes and texture matrices, which can be used to identify phenotypical properties of the radiological image [13]. Delta radiomics is the study of characteristics throughout time and how they change in order to predict a patient’s response to treatments [14]. Finally, the selected features are used to test the final model.
In this review, we will provide an overview of radiomics and machine-learning studies, focusing on the different research areas in which these techniques can be implemented in relation to HNTs, such as lesion segmentation, grading, differential diagnosis, prediction of prognosis, evaluation of treatment response and prediction (Figure 2). Table 1 and Table 2 present a summary of the studies discussed in the text, arranged by subtopic.
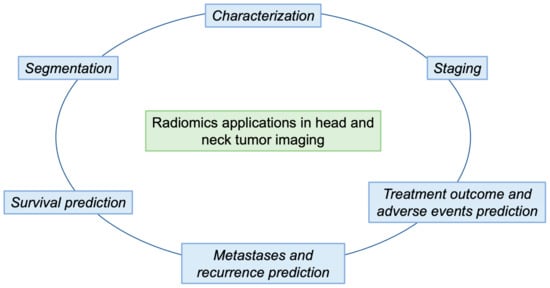
Figure 2.
The outline of our paper is shown in Figure 2. In particular, we describe the main steps of a radiomic workflow (segmentation and characterization) in order to obtain predictions of survival, metastasis and recurrence, and treatment responses.

Table 1.
Overview of study characteristics, divided by topic.

Table 2.
Overview of study characteristics, divided by topic.
2. Segmentation
Grouping portions of a picture that belong to the same class of objects is known as segmentation. Because it establishes a tumor’s region of interest (ROI), from which imaging data are collected and processed into machine-readable quantitative attributes, segmentation is essential to the creation of a radiomic workflow. Depending on the approach used, the tumor lesion may be delineated as a two-dimensional or three-dimensional ROI or volume of interest (VOI), respectively [77]. Segmentation can be performed with different methods: manual, semi-automatic and automatic. There are advantages and disadvantages of each method [78]. Precision definitions of ROIs and/or VOIs are possible with manual segmentation using a mouse or a graphic tablet, especially when trained radiologists apply it to small datasets. However, this method involves time-consuming procedures and may be subject to high intra- and inter-observer variability, resulting in bias in radiomic pipeline results. Applying algorithms that make use of various image delineation strategies, such as region-growing, level set, graph cut and active contour (snake) techniques, is the process of semi-automatic segmentation [79]. Despite the fact that this technique reduces labor tasks and improves radiomic feature robustness [15], the stability of radiomic models remains susceptible to subjective bias, especially in cases of intensive user correction. Medical image segmentation has recently used a completely automated technique. In the identification and segmentation of lesions, it has shown excellent results, and it has also eliminated potential intra- and inter-observer differences [78]. Large data requirements and the generalizability of the taught algorithms are the key drawbacks [79]. On the one hand, these approaches could help reduce the workload and increase reproducibility in craft radiomic research. On the other hand, they do not need picture segmentation for classification. Given the absence of standardized segmentation techniques, which might result in inconsistently replicable models, tumor segmentation presents a significant barrier to the robustness of radiomic characteristics, particularly for manual and semi-automated approaches [15]. Indeed, emerging exploratory work has been aimed at assessing the extent to which the stability of radiomic features may be affected by segmentation variability [17]. Texture analysis has also been applied to create automated segmentation models. Using radiomics to distinguish between normal and pathologic tissue in HNTs, Yu et al. developed a multivariate model, which could identify pathological pixels using a combination of positron emission tomography (PET) and CT gray-tone difference features [18]. Using this approach, a co-registered multimodality pattern analysis segmentation system (COMPASS) has been developed to identify the radiation therapy target using PET and CT images. This is able to identify the tumoral area with results comparable to manual segmentation by expert radiation oncologists [19], possibly reducing inter-observer variability and improving treatment planning accuracy. In a recently published paper, Prezioso et al. provide another example of automatic segmentation of head and neck lesions using a DL-based framework for automatic segmentation of salivary gland tumors [80].
3. Characterization
Recent radiomics research works have demonstrated the correlation between bio-imaging traits (human papilloma virus (HPV) status, somatic mutations, methylation, subtypes of gene expression and PD-L1 protein expression levels) in HNSCC. The subject that has generated the most attention among them is HPV status. Younger patient age at presentation, unique tumor morphology (smaller original tumors, significant cervical adenopathy) and a better response to radiation treatment are all related to the virus’ existence [81]. If supported by sufficient evidence, radiomics-based biomarkers could be used in the future as a viable alternative to confirm HPV status after positive p16 immunohistochemical tests [81]. Several studies have been conducted to define HPV status in HNSCC using texture analysis. To date, the majority of these have investigated the value of CT-based radiomics. For example, both Buch et al. and Fujita et al. examined the association of individual texture features with HPV status. The first research group identified three features (histogram-derived median and entropy and gray-level co-occurrence matrix (GLCM) entropy) on contrast-enhanced CT images of primary oropharyngeal squamous cell carcinoma, which showed statistically significant differences in relation to patient HPV status [20]. Similarly, Fujita et al. were able to identify 16 texture parameters with the potential to distinguish HPV status in non-oropharyngeal carcinoma [21]. Regarding other imaging modalities, Vallieres et al. reported on the value of Fludeoxyglucose (FDG)-PET features as HPV status biomarkers when used in combination with different machine-learning algorithms [22]. With regard to MRI-based biomarkers, some studies assessed that MRI radiomics models could be used in future as an effective imaging biomarker to confirm HPV status after positive p16 immunohistochemical tests [81]. Differences in the apparent diffusion coefficient (ADC) value have been reported in some previous MRI-based studies [23,24,82]. Moreover, Marzi et al. obtained good results in differentiating HPV status in OPSCC using a multifactorial model incorporating diffusion-weighted imaging (DWI) and clinical features. They extracted first- and second-order radiomic features from ADC maps and trained different machine-learning algorithms from that dataset [25]. Chong Hyun Suh et al. conducted a retrospective study in 60 patients with new histological diagnosis of OPSCC. They manually delineated the tumor area in four sequences (axial T1 weighted images (WI), fat-suppressed T2 WI, axial fat-suppressed contrast-enhanced T1 WI and ADC maps from DWI) and then demonstrated that three machine-learning classifiers (logistic regression, random forest and XGboost) trained with quantitative radiomics features extracted from those variously combined sequences were accurate in predicting HPV status [26]. Additionally, Sohn et al. developed a model for diagnosing HPV status in patients with oropharyngeal cancer using six MRI radiomic features (post-contrast 3D T1WI and T2 WI sequences) [27]. Beyond HPV status, the use of radiomics biomarkers has also been proposed to identify HNSCC molecular subtypes in several studies. Aerts et al. [28] associated three radiomics features with the presence of mutations in as many driver genes (TP53, FAT1 and KMT2D) and found that FAT1 had a significant association with all of them [29]. Huang et al. [30] studied several molecular HNSCC “phenotypes” (five DNA methylation subtypes, four previously identified HNSCC gene expression subtypes and five common somatic gene mutations) and considered 540 CT radiomics features extracted from pretreatment scans of 113 patients. Zhu et al. [31] reported a correlation between radiomic features extracted from contrast-enhanced CT images and genome data in a public cohort of 126 HNSCC patients, identifying over 5000 statistically significant associations. Interestingly, Chen et al. [32] reported a significant association between FDG PET textural features and expression of PD-L1, which correlate with response to PD-1 blockers, such as nivolumab or pembrolizumab [83], in patients with oropharyngeal and hypopharyngeal SCC. In the field of preoperative stratification of thyroid tumors, an algorithm that used linear discriminant analysis focused on DWI and ADC data was proposed. The authors report a greater performance of textural features in differentiating between benign and malign lesions (area under the curve (AUC) = 0.97, sensitivity = 92%, specificity = 96%) compared to ADC alone (AUC = 0.73, sensitivity = 70%, specificity = 63%) [33]. Jansen et al. analyzed the parametric maps of Ktrans and Ve, which are indices of tumor vascularity, in HNSCC patients before and during the treatment, obtained with dynamic contrast-enhanced perfusion imaging. They showed a significantly higher energy feature in the scans performed during the treatment, suggesting that texture analysis could be used together with standard MRI perfusion maps to provide additional information in head and neck oncological patients [34,35,36]. In addition, some studies aim to predict p53 status, as a positive status is associated with poor prognosis [84,85,86]. Dang et al. showed that MRI texture analysis could predict p53 status in oropharyngeal squamous cell carcinoma with an accuracy of 81.3% (p < 0.05). The variables that stood out significantly were those thought to be due to differences in vascularity between p53(+) and p53(−) status [37].
4. Staging
Pre-treatment staging is an important point in diagnosis and therapeutic planning, as well as a factor closely related to tumor prognosis. The main treatment is surgery, but there are also several treatment options, including induction chemotherapy, concomitant chemoradiotherapy, targeted therapy or immunotherapy [87,88]. Studies have shown that the T-stage of head and neck tumors and lymph node status greatly influence the treatment choice, and thus, the prognosis of cancer patients [89,90]. Prior to therapy, a reliable radiomics evaluation of the tumor’s stage can assist direct treatment decisions, ensuring lower risk of adverse effects and recurrence. Radiomics could be used to successfully establish a T-staging model of locally progressed laryngeal cancer [88]. In particular, MRI radiomic signature was shown to be a supplemental tool for preoperative staging, differentiating stage III–IV from stage I–II squamous cell carcinoma [89]. Romeo et al. [40] predicted tumor grade and lymph node status (N) in squamous cell carcinoma of the oropharynx and oral cavity using a radiomic approach based on contrast-enhanced CT images. The determination of extra-nodal extension (ENE) of the tumor is important, since it represents an unfavorable prognostic factor and is associated with a higher risk of developing recurrent disease [91], as will be discussed in the next sections. Finally, a prospective study of a cohort of 96 patients with papillary thyroid carcinoma (PTC) enrolled patients who underwent neck MRI and subsequent thyroidectomy during the study interval. Aggressive and non-aggressive cancers can be distinguished using machine-learning MRI-based prediction algorithms. This is crucial before surgery, since it makes it easier to create individualized treatment strategies [91].
5. Treatment
Leaving out the prediction of surgical treatment outcomes (which can be predicted by the surgeon based on planned resection according to current guidelines), it would be appropriate to focus on the predictive ability of radiomics models regarding radiotherapy and chemotherapy outcomes [42].
5.1. Pre- and Intra-Treatment Imaging
Oncologists can devise individualized treatment plans and implement preventative measures to enhance therapy outcome and patient’s tolerance to therapy.
During radiation therapy (RT) for NSCLC, features computed from pre-treatment and weekly intra-treatment CT alter dramatically [42].
Cone beam CT (CBCT) systems may be able to conduct delta radiomics for image-guided radiation, enabling extensive research on tumor response to total dose, fractionation and fraction dosage. It has been demonstrated that repeatable characteristics from CBCT are just as effective in predicting overall survival in NSCLC patients as features from CT [92]. However, studies on CBCT delta radiomics are still only capable of evaluating repeatability and feasibility.
During pre- and intra-treatment evaluations, the preferred MRI sequences differed across investigations. The sequences selected vary, though; for instance, some authors utilize DCE-MRI to integrate pharmacokinetic modeling [34], while others employ DWI to increase the precision of lesion stratification [33]. The repeatability of pictures and, by extension, the textural qualities obtained from them may vary between MRI modalities in addition to sequence selection due to differences in scanner features. The possibility of bias from characteristics taken from a single sequence can be decreased using multiparametric techniques [43].
5.2. Short-Term Outcome and Adverse Events
A few studies aiming to estimate early response to induction chemotherapy and chemoradiotherapy (CRT) [44,45] in nasopharyngeal carcinoma have been conducted. In addition, it might also be useful to predict outcomes in adjacent non-cancerous tissues, such as glandular tissues (parotid and major salivary glands). A general decrease in parotid tissue complexity and heterogeneity has been observed in the literature [90]. The change in mean volume and intensity was found to be correlated with pre-treatment dosimetric parameters, suggesting a relationship between the dose schedule and estimated structural change after radiotherapy [93].
5.3. Long-Term Outcome and Adverse Events
CRT represents a usual treatment regimen [91]; however, adverse symptoms are occasionally seen, even in the long term. These include hearing loss, trismus and xerostomia. Radiation xerostomia is a common side effect and poses a challenge in the long-term management of patients [46,94]. A number of studies with heterogeneous endpoints have been performed in this regard: Sheikh et al. [47] predicted a binary endpoint of xerostomia at 3 months after radiation therapy; Liu et al. [48] applied regression analysis for the prediction of acute xerostomia; van Dijk et al. [49,50] used three different imaging modalities (CT, MRI, FDG-PET) for the classification of the binary outcome of long-term xerostomia. Although these results appear promising, their clinical application is limited due to lack of external validation, heterogeneity in image processing, statistical analysis and treatment outcome measures. Trismus may result from involvement of masticatory muscles in radiation therapy treatment fields, surgery or tumor invasion into mastication structures, or neural innervation of masticatory muscles [95]. Thor et al. [51] compared 24 imaging features extracted from post-contrast T1 WI sequences of four masticatory muscles in 10 patients with radiation-induced trismus after treatment with 10 control subjects. The medial pterygoid muscle was shown to have the greatest radiomic predictor discriminative capacity. The outcome is not statistically significant, but it may be a hint of how well radiomic biomarkers can predict post-radiation trismus. Cochlear radiomics may be used to anticipate hearing loss brought on by chemotherapy and radiation therapy, according to Abdollahi et al. They showed that the combination of radiomic features with clinical and dosimetric variables can predict radiotherapy-induced neural sensory hearing loss.
In the context of long-term outcome, we reserve the right to address “metastases and recurrence” and “survival” separately in the following sections.
6. Metastases and Recurrence
ENE of cervical lymph node metastases is an adverse prognostic feature linked to a higher probability of recurrent illness. This supports the use of chemotherapy in combination with adjuvant radiotherapy [40]. In individuals who are likely to need adjuvant chemoradiation, the detection of ENE might assist direct treatment decisions, lower morbidity and prevent surgery. Quantitative imaging methods were created and validated by Kann et al. for the identification of ENE prior to surgery [51,95]. On contrast-enhanced CT images, they segmented more than 600 lymph nodes and extracted 99 radiomic characteristics. These provided AUC values for ENE identification and lymph node metastases detection close to 0.9 by training ML classifiers. These results highlight the potential for quantitative imaging to enhance radiologist’s performance and guide the treatment of HNSCC. Zhang et al. [55] developed a model for pre-treatment risk assessment of distant metastasis in patients with nasopharyngeal carcinoma using MRI. They extracted 2780 radiomic features, among which 7 were selected to build a logistic regression model to classify patients at low or high risk of distant metastasis. They trained the model using a retrospective cohort of 123 untreated patients with non-metastatic status (AUC 5.827) and validated the trained model using an independent retrospective cohort of 53 patients (AUC 5.792). Other studies suggest the use of MRI, CT and/or PET imaging radiomics and ML to predict tumor recurrence after radiotherapy and/or chemotherapy for several HNTs [56,57,58]. Through the study of a large dataset of pre-treatment contrast-enhanced CT scans (465 cases of oropharyngeal squamous cell cancer), a model capable of significantly discriminating between high and low probability of recurrence groups was analyzed by the M.D. Anderson Cancer Center’s Quantitative Head and Neck Imaging Working Group [59]. Finally, in relation to recurrence, it was possible, through the selection of eleven imaging features, to construct a radiomic score (Rad-score) capable of predicting local recurrence-free survival (LRFS). Rad-scores were generated using Cox’s proportional hazards regression model and can reliably predict LRFS in patients with non-metastatic T4 stage [60].
7. Survival
With the development of medical diagnosis and treatment technology, great progress has been made in the treatment of HNTs [96,97,98]. However, at the time of first diagnosis, many patients are already in an advanced stage of disease. With five-year survival rates ranging from 25% for hypopharyngeal carcinoma (HPC) to 80% for nasopharyngeal carcinoma (NPC), the prognosis is still poor [61,62]. In order to create even more correct treatment programs, it is necessary to predict patients’ survival rates more precisely.
In research reports on the use of radiomics in HNC, radiomic models related to survival prediction are the most numerous. Shen et al. [61] sought to explore the predictive value of the radiomic model based on MRI features. Out of 327 patients, they established five models. The prognostic performance of these models was evaluated by Harrell’s concordance index (C-index). It was found that the best model was the one incorporating radiomics, global health stage and DNA in non-metastatic tumors. Yuan et al. [62] found that radiomic signature based on MRI is an independent prognostic factor for patients with HNSCC, as also highlighted by another study [63]. In addition, others have used pre- and post-operative PET/CT radiomic features for HNSCC and found that combining clinico-pathological features with pre- and post-treatment PET/CT radiomic features can substantially improve the prediction of overall survival (OS) of HNSCC patients [64,65]. Zhai et al. [66], using 240 contrast-enhanced CT data, reported significantly better prognostic performance with a combined model than a model based on clinical variables alone for disease-free survival in HNSCC. Using 542 cases of oropharyngeal SCC from Canada, Leijenaar et al. [67] validated a radiomics model previously devised by Aerts et al. [28] on 422 cases, which showed significant prognostic differentiation in Kaplan–Meier analysis of OS in all sub-cohorts. Radiomics-based outcome prediction used CT with and without contrast, T1WI and T2WI MRI sequences (with contrast) and FDG-PET, as well as DWI [45], 18F-fluorothymidine PET [99] and perfusion CT [68]. Most of the studies applied multivariate Cox proportional hazard models. The performance, expressed by the hazard ratio of the Cox model, and the accuracy, expressed by the C-index, of the radiomic or combined models were superior to the clinical models with respect to the prediction of various outcomes. An investigation by Parmar et al. [63], analyzing features extracted from pre-treatment CT images of four independent cohorts of HNTs (878 patients in total), showed that radiomic clusters are significantly associated with patient survival and tumor stage. Parmar et al. analyzed 13 feature selection methods and 11 machine-learning classification methods chosen for simplicity, efficiency and popularity in the literature. Specifically, they identified three classifiers and feature selection methods that demonstrated high performance and stability in predicting 3-year OS in head and neck cancer, suggesting that these machine-learning methods should be the starting point for radiomics-based prognostic analyses due to their consistency. El Naqa et al. [100] examined the characteristics of pre-treatment PET images of nine HNT patients. Using the most predictive features, they were able to construct a two-metric model predicting OS with an AUC of 1. In a retrospective study of 72 patients using 2D CT texture analysis, textural features were found to be associated with OS in patients with locally advanced HNSCC treated with induction chemotherapy [69]. In addition, texture analysis of CT, PET or MR images before treatment has been used to predict progression-free survival or OS in several HNTs of the mucosa or thyroid [70,71,72,73,74,75]. Leijenaar et al., using contrast-enhanced CT radiomic features, assessed that p16 and the radiomics-based classifier had the same potential to differentiate the risk of patients in the survival curve using Kaplan–Maier analysis [76].
8. Limitations of Radiomics
8.1. Current Issues
Radiomic analyses are very promising in assessing several characteristics of head and neck malignancies, but there are a number of limitations that must also be considered [77]. The majority of recent radiomics research works have a retrospective, monocentric design, which advocates for caution in interpreting the reported findings. In particular, the small sample size frequently characterizing these works may lead to a patient selection bias, not accurately reflecting the overall population [101]. Furthermore, few device manufacturers and data acquisition techniques are often employed, and this could also result in random patterns that add biases into the models. Unfortunately, these are difficult to identify without access to larger and more varied datasets. In general, these issues may lead to models that cannot duplicate their performance in new research trials. The low level of uniformity of radiological imaging protocols, which may also have an impact on the generalizability of the models and, consequently, their clinical application, represents another issue somewhat related to the latter [102]. Another problem is the extremely frequent lack of external validation, which may cause the predictive model to overfit. Extreme variability in the segmentation, feature extraction and selection, as well as the adopted modeling procedures still represent further drawbacks. In general, rather than the underlying biological lesion features, all the aforementioned issues constitute a possible source of unwelcome heterogeneity brought on by the traits of the patient cohort and imaging data. Instead of identifying this heterogeneity as the noise it actually is and dismissing it, radiomics pipelines run the risk of seeing it as a source of information and including it in the model’s classification process. Future works should therefore focus on radiomics pipeline standardization and prospective multicenter trial designs. Despite being in its early stages, efforts are being made to increase awareness of the methodological problems affecting current radiomics research, to encourage editors and reviewers to focus more on the technical details and clinical applicability of this work, to educate potential customers about commercial solutions based on radiomics and to gather curated, open medical images [103].
8.2. Potential Solutions
Briefly, the main limitations of radiomics are due to bias in three main steps: data collection and handling; model development; performance metrics. To lessen such bias, it is essential to be aware of it [104,105,106].
Careful data collection is critical to ML model development. Estimating the types, features and sizes of the data required is essential for locating and gathering the right datasets. First, a comprehensive study of the literature that incorporates advice from medical experts aids in the task [107]. The minimal dataset size required to demonstrate an effect and guarantee the brilliance of the trained model may be determined using statistical power estimate approaches and knowledge of similarly created ML models [108].
Training a heterogeneous model can help machine-learning systems perform better [109]. To this goal, data collection from several institutions with various patient compositions is beneficial. This issue can be improved thanks to the development of data de-identification technologies, federated learning and cloud data storage. The second strategy is to obtain information from many suppliers (such as imaging equipment or electronic medical records) while staying within the same organization. It might be beneficial to amass many brands and models, even older ones. Using open datasets is the third strategy [106].
Regarding model development, frequent bias is caused by overfitting. Early stopping, which tracks the model’s performance on the validation set and halts training when the validation measure drops or its validation loss rises over a few steps, is one method to lessen overfitting [110]. Model capacity reduction is another strategy for reducing overfitting. Fewer parameters limit the network’s ability to learn erroneous characteristics, pushing it to focus on learning the most crucial ones [111]. Regularization is another strategy to lessen overfitting. Regularization techniques include dropout layers and regularizations. Ensemble modeling might also help address overfitting [112]. The risk of overfitting is reduced by oversampling and undersampling, which prevent the model from seeing significantly more instances of one class than others during training [113].
Finally, attention must be paid to performance metrics to minimize bias [105]. Predictive models for head and neck cancer have been developed using twelve distinct classifiers [63]. A multi-classifier model that makes the most of the data gleaned from various classifiers can be used to lessen bias. This technique states that if one classifier is regarded as “expert”, aggregating the judgments of numerous “experts” will result in a more trustworthy outcome [114].
The three types of classification tasks that can be performed are binary, multiclass and multilabel.
A confusion matrix can be created by adding the outcomes of a binary classifier [115]. Metrics are typically produced based on combinations of values in the confusion matrix to reduce bias because each number represents a different facet of performance, and focusing on just one of them can introduce bias [116,117]. Additionally, the clinical context of the condition has a significant impact on the metrics of choice. High sensitivity is crucial, for instance, if a model is intended to screen for cancer; however, if the goal is to confirm cancer, a more specific model would be preferable. Setting up a relevant threshold for metrics is therefore crucial. If there is a significant imbalance in the data, relying on the “accuracy” statistic to assess model performance may result in bias. The ROC curve may more effectively illustrate model performance on uneven data than accuracy [118].
9. Conclusions
HNTs represent real challenges for clinicians and radiologists due to the complex regional anatomy, their often small sizes, the oncologic pathology variability, as well as the modifications of the anatomical site after treatment. Numerous promising studies have focused on radiomics and machine-learning applications for HNTs. While these techniques have the potential to overcome the current limitations of imaging in the head and neck area, future efforts must be directed toward robust external validation within multi-institutional collaborative efforts to standardize, refine and finally implement radiomics and machine-learning software in clinical practice.
Author Contributions
Conceptualization, L.U. and R.C.; methodology, L.U. and R.C.; writing—original draft preparation, M.T., L.G. and A.S. (Alessandra Scaravilli); writing—review and editing, M.T., L.U., A.P., A.S. (Arnaldo Stanzione), F.D., G.D. and R.C. All authors have read and agreed to the published version of the manuscript.
Funding
The authors state that this work has not received any funding.
Conflicts of Interest
The authors have nothing to disclose.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Hedberg, M.L.; Grandis, J.R. The Molecular Pathogenesis of Head and Neck Cancer. In The Molecular Basis of Cancer; Elsevier: Amsterdam, The Netherlands, 2015; pp. 491–498.e2. [Google Scholar]
- Maier, H.; Dietz, A.; Gewelke, U.; Heller, W.D.; Weidauer, H. Tobacco and alcohol and the risk of head and neck cancer. Clin. Investig. 1992, 70, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Sturgis, E.M.; Cinciripini, P.M. Trends in head and neck cancer incidence in relation to smoking prevalence. Cancer 2007, 110, 1429–1435. [Google Scholar] [CrossRef]
- Yu, M.C.; Yuan, J.-M. Epidemiology of nasopharyngeal carcinoma. Semin. Cancer Biol. 2002, 12, 421–429. [Google Scholar] [CrossRef]
- Jicman (Stan), D.; Niculet, E.; Lungu, M.; Onisor, C.; Rebegea, L.; Vesa, D.; Bezman, L.; Bujoreanu, F.; Sarbu, M.; Mihailov, R.; et al. Nasopharyngeal carcinoma: A new synthesis of literature data (Review). Exp. Ther. Med. 2021, 23, 136. [Google Scholar] [CrossRef]
- Stelow, E.B.; Wenig, B.M. Update From The 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Nasopharynx. Head Neck Pathol. 2017, 11, 16–22. [Google Scholar] [CrossRef]
- Badoual, C. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Oropharynx and Nasopharynx. Head Neck Pathol. 2022, 16, 19–30. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112. [Google Scholar] [CrossRef]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Sanduleanu, S.; Woodruff, H.C.; de Jong, E.E.C.; van Timmeren, J.E.; Jochems, A.; Dubois, L.; Lambin, P. Tracking tumor biology with radiomics: A systematic review utilizing a radiomics quality score. Radiother. Oncol. 2018, 127, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, M.; Wei, L.; Stancanello, J.; Vallières, M.; Rao, A.; Morin, O.; Mattonen, S.A.; El Naqa, I. Machine and deep learning methods for radiomics. Med. Phys. 2020, 47. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Rios Velazquez, E.; Leijenaar, R.; Jermoumi, M.; Carvalho, S.; Mak, R.H.; Mitra, S.; Shankar, B.U.; Kikinis, R.; Haibe-Kains, B.; et al. Robust Radiomics Feature Quantification Using Semiautomatic Volumetric Segmentation. PLoS ONE 2014, 9, e102107. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Truhn, D. The Long Route to Standardized Radiomics: Unraveling the Knot from the End. Radiology 2020, 295, 339–341. [Google Scholar] [CrossRef]
- Gitto, S.; Cuocolo, R.; Emili, I.; Tofanelli, L.; Chianca, V.; Albano, D.; Messina, C.; Imbriaco, M.; Sconfienza, L.M. Effects of Interobserver Variability on 2D and 3D CT- and MRI-Based Texture Feature Reproducibility of Cartilaginous Bone Tumors. J. Digit. Imaging 2021, 34, 820–832. [Google Scholar] [CrossRef]
- Huan, Y.; Caldwell, C.; Mah, K.; Mozeg, D. Coregistered FDG PET/CT-Based Textural Characterization of Head and Neck Cancer for Radiation Treatment Planning. IEEE Trans. Med. Imaging 2009, 28, 374–383. [Google Scholar] [CrossRef]
- Yu, H.; Caldwell, C.; Mah, K.; Poon, I.; Balogh, J.; MacKenzie, R.; Khaouam, N.; Tirona, R. Automated Radiation Targeting in Head-and-Neck Cancer Using Region-Based Texture Analysis of PET and CT Images. Int. J. Radiat. Oncol. 2009, 75, 618–625. [Google Scholar] [CrossRef]
- Buch, K.; Fujita, A.; Li, B.; Kawashima, Y.; Qureshi, M.M.; Sakai, O. Using Texture Analysis to Determine Human Papillomavirus Status of Oropharyngeal Squamous Cell Carcinomas on CT. Am. J. Neuroradiol. 2015, 36, 1343–1348. [Google Scholar] [CrossRef]
- Fujita, A.; Buch, K.; Li, B.; Kawashima, Y.; Qureshi, M.M.; Sakai, O. Difference Between HPV-Positive and HPV-Negative Non-Oropharyngeal Head and Neck Cancer. J. Comput. Assist. Tomogr. 2016, 40, 43–47. [Google Scholar] [CrossRef]
- Vallieres, M.; Kumar, A.; Sultanem, K.; El Naqa, I. FDG-PET Image-Derived Features Can Determine HPV Status in Head-and-Neck Cancer. Int. J. Radiat. Oncol. 2013, 87, S467. [Google Scholar] [CrossRef]
- Payabvash, S.; Brackett, A.; Forghani, R.; Malhotra, A. Differentiation of lymphomatous, metastatic, and non-malignant lymphadenopathy in the neck with quantitative diffusion-weighted imaging: Systematic review and meta-analysis. Neuroradiology 2019, 61, 897–910. [Google Scholar] [CrossRef]
- Payabvash, S. Quantitative diffusion magnetic resonance imaging in head and neck tumors. Quant. Imaging Med. Surg. 2018, 8, 1052–1065. [Google Scholar] [CrossRef]
- Marzi, S.; Piludu, F.; Avanzolini, I.; Muneroni, V.; Sanguineti, G.; Farneti, A.; D’Urso, P.; Benevolo, M.; Rollo, F.; Covello, R.; et al. Multifactorial Model Based on DWI-Radiomics to Determine HPV Status in Oropharyngeal Squamous Cell Carcinoma. Appl. Sci. 2022, 12, 7244. [Google Scholar] [CrossRef]
- Suh, C.H.; Lee, K.H.; Choi, Y.J.; Chung, S.R.; Baek, J.H.; Lee, J.H.; Yun, J.; Ham, S.; Kim, N. Oropharyngeal squamous cell carcinoma: Radiomic machine-learning classifiers from multiparametric MR images for determination of HPV infection status. Sci. Rep. 2020, 10, 17525. [Google Scholar] [CrossRef]
- Sohn, B.; Choi, Y.S.; Ahn, S.S.; Kim, H.; Han, K.; Lee, S.; Kim, J. Machine Learning Based Radiomic HPV Phenotyping of Oropharyngeal SCC: A Feasibility Study Using MRI. Laryngoscope 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, K.; Hilke, F.J.; Demidov, G.; Fernandez, J.S.; Ossowski, S.; Gani, C.; Thorwarth, D.; Riess, O.; Zips, D.; Schroeder, C.; et al. Radiogenomics in head and neck cancer: Correlation of radiomic heterogeneity and somatic mutations in TP53, FAT1 and KMT2D. Strahlentherapie und Onkol. 2019, 195, 771–779. [Google Scholar] [CrossRef]
- Huang, C.; Cintra, M.; Brennan, K.; Zhou, M.; Colevas, A.D.; Fischbein, N.; Zhu, S.; Gevaert, O. Development and validation of radiomic signatures of head and neck squamous cell carcinoma molecular features and subtypes. EBioMedicine 2019, 45, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mohamed, A.S.R.; Lai, S.Y.; Yang, S.; Kanwar, A.; Wei, L.; Kamal, M.; Sengupta, S.; Elhalawani, H.; Skinner, H.; et al. Imaging-Genomic Study of Head and Neck Squamous Cell Carcinoma: Associations Between Radiomic Phenotypes and Genomic Mechanisms via Integration of The Cancer Genome Atlas and The Cancer Imaging Archive. JCO Clin. Cancer Inform. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Y.; Lin, Y.-C.; Shen, W.-C.; Hsieh, T.-C.; Yen, K.-Y.; Chen, S.-W.; Kao, C.-H. Associations of Tumor PD-1 Ligands, Immunohistochemical Studies, and Textural Features in 18F-FDG PET in Squamous Cell Carcinoma of the Head and Neck. Sci. Rep. 2018, 8, 105. [Google Scholar] [CrossRef]
- Brown, A.M.; Nagala, S.; McLean, M.A.; Lu, Y.; Scoffings, D.; Apte, A.; Gonen, M.; Stambuk, H.E.; Shaha, A.R.; Tuttle, R.M.; et al. Multi-institutional validation of a novel textural analysis tool for preoperative stratification of suspected thyroid tumors on diffusion-weighted MRI. Magn. Reson. Med. 2016, 75, 1708–1716. [Google Scholar] [CrossRef]
- Jansen, J.F. Texture analysis on parametric maps derived from dynamic contrast-enhanced magnetic resonance imaging in head and neck cancer. World J. Radiol. 2016, 8, 90. [Google Scholar] [CrossRef]
- Kim, S.; Loevner, L.A.; Quon, H.; Kilger, A.; Sherman, E.; Weinstein, G.; Chalian, A.; Poptani, H. Prediction of Response to Chemoradiation Therapy in Squamous Cell Carcinomas of the Head and Neck Using Dynamic Contrast-Enhanced MR Imaging. Am. J. Neuroradiol. 2010, 31, 262–268. [Google Scholar] [CrossRef]
- Shukla-Dave, A.; Lee, N.Y.; Jansen, J.F.A.; Thaler, H.T.; Stambuk, H.E.; Fury, M.G.; Patel, S.G.; Moreira, A.L.; Sherman, E.; Karimi, S.; et al. Dynamic Contrast-Enhanced Magnetic Resonance Imaging as a Predictor of Outcome in Head-and-Neck Squamous Cell Carcinoma Patients With Nodal Metastases. Int. J. Radiat. Oncol. 2012, 82, 1837–1844. [Google Scholar] [CrossRef]
- Dang, M.; Lysack, J.T.; Wu, T.; Matthews, T.W.; Chandarana, S.P.; Brockton, N.T.; Bose, P.; Bansal, G.; Cheng, H.; Mitchell, J.R.; et al. MRI Texture Analysis Predicts p53 Status in Head and Neck Squamous Cell Carcinoma. Am. J. Neuroradiol. 2015, 36, 166–170. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, B.; Wu, X.; Liu, L.; Fang, J.; Chen, Q.; Li, M.; Chen, Z.; Li, Y.; Dong, D.; et al. Radiomic Nomogram Improves Preoperative T Category Accuracy in Locally Advanced Laryngeal Carcinoma. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Ren, J.; Tian, J.; Yuan, Y.; Dong, D.; Li, X.; Shi, Y.; Tao, X. Magnetic resonance imaging based radiomics signature for the preoperative discrimination of stage I-II and III-IV head and neck squamous cell carcinoma. Eur. J. Radiol. 2018, 106, 1–6. [Google Scholar] [CrossRef]
- Romeo, V.; Cuocolo, R.; Ricciardi, C.; Ugga, L.; Cocozza, S.; Verde, F.; Stanzione, A.; Napolitano, V.; Russo, D.; Improta, G.; et al. Prediction of Tumor Grade and Nodal Status in Oropharyngeal and Oral Cavity Squamous-cell Carcinoma Using a Radiomic Approach. Anticancer Res. 2020, 40, 271–280. [Google Scholar] [CrossRef]
- Wang, H.; Song, B.; Ye, N.; Ren, J.; Sun, X.; Dai, Z.; Zhang, Y.; Chen, B.T. Machine learning-based multiparametric MRI radiomics for predicting the aggressiveness of papillary thyroid carcinoma. Eur. J. Radiol. 2020, 122, 108755. [Google Scholar] [CrossRef]
- Fave, X.; Zhang, L.; Yang, J.; Mackin, D.; Balter, P.; Gomez, D.; Followill, D.; Jones, A.K.; Stingo, F.; Liao, Z.; et al. Delta-radiomics features for the prediction of patient outcomes in non–small cell lung cancer. Sci. Rep. 2017, 7, 588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, J.; Dong, D.; Gu, D.; Dong, Y.; Zhang, L.; Lian, Z.; Liu, J.; Luo, X.; Pei, S.; et al. Radiomics Features of Multiparametric MRI as Novel Prognostic Factors in Advanced Nasopharyngeal Carcinoma. Clin. Cancer Res. 2017, 23, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, L.; Yuan, C.; Huang, Y.; Liu, Z.; Liang, C. Pretreatment MR imaging radiomics signatures for response prediction to induction chemotherapy in patients with nasopharyngeal carcinoma. Eur. J. Radiol. 2018, 98, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, Y.; Li, Z.; Zhang, D.; Zhang, Z.; Hao, S.; Li, B. Use of texture analysis based on contrast-enhanced MRI to predict treatment response to chemoradiotherapy in nasopharyngeal carcinoma. J. Magn. Reson. Imaging 2016, 44, 445–455. [Google Scholar] [CrossRef]
- Hawkins, P.G.; Lee, J.Y.; Mao, Y.; Li, P.; Green, M.; Worden, F.P.; Swiecicki, P.L.; Mierzwa, M.L.; Spector, M.E.; Schipper, M.J.; et al. Sparing all salivary glands with IMRT for head and neck cancer: Longitudinal study of patient-reported xerostomia and head-and-neck quality of life. Radiother. Oncol. 2018, 126, 68–74. [Google Scholar] [CrossRef]
- Sheikh, K.; Lee, S.H.; Cheng, Z.; Lakshminarayanan, P.; Peng, L.; Han, P.; McNutt, T.R.; Quon, H.; Lee, J. Predicting acute radiation induced xerostomia in head and neck Cancer using MR and CT Radiomics of parotid and submandibular glands. Radiat. Oncol. 2019, 14, 131. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, H.; Huang, S.; Chen, X.; Zhou, H.; Chang, H.; Xia, Y.; Wang, G.; Yang, X. Early prediction of acute xerostomia during radiation therapy for nasopharyngeal cancer based on delta radiomics from CT images. Quant. Imaging Med. Surg. 2019, 9, 1288–1302. [Google Scholar] [CrossRef]
- van Dijk, L.V.; Thor, M.; Steenbakkers, R.J.H.M.; Apte, A.; Zhai, T.-T.; Borra, R.; Noordzij, W.; Estilo, C.; Lee, N.; Langendijk, J.A.; et al. Parotid gland fat related Magnetic Resonance image biomarkers improve prediction of late radiation-induced xerostomia. Radiother. Oncol. 2018, 128, 459–466. [Google Scholar] [CrossRef]
- van Dijk, L.V.; Brouwer, C.L.; van der Schaaf, A.; Burgerhof, J.G.M.; Beukinga, R.J.; Langendijk, J.A.; Sijtsema, N.M.; Steenbakkers, R.J.H.M. CT image biomarkers to improve patient-specific prediction of radiation-induced xerostomia and sticky saliva. Radiother. Oncol. 2017, 122, 185–191. [Google Scholar] [CrossRef]
- Thor, M.; Tyagi, N.; Hatzoglou, V.; Apte, A.; Saleh, Z.; Riaz, N.; Lee, N.Y.; Deasy, J.O. A magnetic resonance imaging-based approach to quantify radiation-induced normal tissue injuries applied to trismus in head and neck cancer. Phys. Imaging Radiat. Oncol. 2017, 1, 34–40. [Google Scholar] [CrossRef]
- Abdollahi, H.; Mostafaei, S.; Cheraghi, S.; Shiri, I.; Mahdavi, S.R.; Kazemnejad, A. Cochlea CT radiomics predicts chemoradiotherapy induced sensorineural hearing loss in head and neck cancer patients: A machine learning and multi-variable modelling study. Phys. Medica 2018, 45, 192–197. [Google Scholar] [CrossRef]
- Kann, B.H.; Aneja, S.; Loganadane, G.V.; Kelly, J.R.; Smith, S.M.; Decker, R.H.; Yu, J.B.; Park, H.S.; Yarbrough, W.G.; Malhotra, A.; et al. Pretreatment Identification of Head and Neck Cancer Nodal Metastasis and Extranodal Extension Using Deep Learning Neural Networks. Sci. Rep. 2018, 8, 14036. [Google Scholar] [CrossRef]
- Kann, B.H.; Hicks, D.F.; Payabvash, S.; Mahajan, A.; Du, J.; Gupta, V.; Park, H.S.; Yu, J.B.; Yarbrough, W.G.; Burtness, B.A.; et al. Multi-Institutional Validation of Deep Learning for Pretreatment Identification of Extranodal Extension in Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2020, 38, 1304–1311. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, D.; Li, H.; Tian, J.; Ouyang, F.; Mo, X.; Zhang, B.; Luo, X.; Lian, Z.; Pei, S.; et al. Development and validation of a magnetic resonance imaging-based model for the prediction of distant metastasis before initial treatment of nasopharyngeal carcinoma: A retrospective cohort study. EBioMedicine 2019, 40, 327–335. [Google Scholar] [CrossRef]
- Bogowicz, M.; Riesterer, O.; Ikenberg, K.; Stieb, S.; Moch, H.; Studer, G.; Guckenberger, M.; Tanadini-Lang, S. Computed Tomography Radiomics Predicts HPV Status and Local Tumor Control After Definitive Radiochemotherapy in Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2017, 99, 921–928. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Hou, Z.; Yang, J.; Ren, W.; Gao, S.; Meng, F.; Wu, P.; Liu, B.; Liu, J.; et al. Use of Radiomics Combined With Machine Learning Method in the Recurrence Patterns After Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma: A Preliminary Study. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Kuno, H.; Qureshi, M.M.; Chapman, M.N.; Li, B.; Andreu-Arasa, V.C.; Onoue, K.; Truong, M.T.; Sakai, O. CT Texture Analysis Potentially Predicts Local Failure in Head and Neck Squamous Cell Carcinoma Treated with Chemoradiotherapy. Am. J. Neuroradiol. 2017, 38, 2334–2340. [Google Scholar] [CrossRef]
- Investigation of radiomic signatures for local recurrence using primary tumor texture analysis in oropharyngeal head and neck cancer patients. Sci. Rep. 2018, 8, 1524. [CrossRef]
- Zhang, L.; Zhou, H.; Gu, D.; Tian, J.; Zhang, B.; Dong, D.; Mo, X.; Liu, J.; Luo, X.; Pei, S.; et al. Radiomic Nomogram: Pretreatment Evaluation of Local Recurrence in Nasopharyngeal Carcinoma based on MR Imaging. J. Cancer 2019, 10, 4217–4225. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Y.; Liu, D.; Lv, R.; Huang, Y.; Peng, C.; Jiang, S.; Wang, Y.; He, Y.; Lan, X.; et al. Predicting Progression-Free Survival Using MRI-Based Radiomics for Patients With Nonmetastatic Nasopharyngeal Carcinoma. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Yuan, Y.; Ren, J.; Shi, Y.; Tao, X. MRI-based radiomic signature as predictive marker for patients with head and neck squamous cell carcinoma. Eur. J. Radiol. 2019, 117, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Grossmann, P.; Rietveld, D.; Rietbergen, M.M.; Lambin, P.; Aerts, H.J.W.L. Radiomic Machine-Learning Classifiers for Prognostic Biomarkers of Head and Neck Cancer. Front. Oncol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.P.; Sinha, S.; Goda, J.S.; Joshi, K.; Mhatre, R.; Kannan, S.; Laskar, S.G.; Gupta, T.; Murthy, V.; Budrukkar, A.; et al. Tumor radiomic features complement clinico-radiological factors in predicting long-term local control and laryngectomy free survival in locally advanced laryngo-pharyngeal cancers. Br. J. Radiol. 2020, 93, 20190857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cao, Y.; Diao, W.; Cheng, Y.; Jia, Z.; Peng, X. Radiomics-based prediction of survival in patients with head and neck squamous cell carcinoma based on pre- and post-treatment 18F-PET/CT. Aging 2020, 12, 14593–14619. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.-T.; Langendijk, J.A.; van Dijk, L.V.; Halmos, G.B.; Witjes, M.J.H.; Oosting, S.F.; Noordzij, W.; Sijtsema, N.M.; Steenbakkers, R.J.H.M. The prognostic value of CT-based image-biomarkers for head and neck cancer patients treated with definitive (chemo-)radiation. Oral Oncol. 2019, 95, 178–186. [Google Scholar] [CrossRef]
- Leijenaar, R.T.H.; Carvalho, S.; Hoebers, F.J.P.; Aerts, H.J.W.L.; van Elmpt, W.J.C.; Huang, S.H.; Chan, B.; Waldron, J.N.; O’sullivan, B.; Lambin, P. External validation of a prognostic CT-based radiomic signature in oropharyngeal squamous cell carcinoma. Acta Oncol. 2015, 54, 1423–1429. [Google Scholar] [CrossRef]
- Bogowicz, M.; Tanadini-Lang, S.; Veit-Haibach, P.; Pruschy, M.; Bender, S.; Sharma, A.; Hüllner, M.; Studer, G.; Stieb, S.; Hemmatazad, H.; et al. Perfusion CT radiomics as potential prognostic biomarker in head and neck squamous cell carcinoma. Acta Oncol. 2019, 58, 1514–1518. [Google Scholar] [CrossRef]
- Zhang, H.; Graham, C.M.; Elci, O.; Griswold, M.E.; Zhang, X.; Khan, M.A.; Pitman, K.; Caudell, J.J.; Hamilton, R.D.; Ganeshan, B.; et al. Locally Advanced Squamous Cell Carcinoma of the Head and Neck: CT Texture and Histogram Analysis Allow Independent Prediction of Overall Survival in Patients Treated with Induction Chemotherapy. Radiology 2013, 269, 801–809. [Google Scholar] [CrossRef]
- Mao, J.; Fang, J.; Duan, X.; Yang, Z.; Cao, M.; Zhang, F.; Lu, L.; Zhang, X.; Wu, X.; Ding, Y.; et al. Predictive value of pretreatment MRI texture analysis in patients with primary nasopharyngeal carcinoma. Eur. Radiol. 2019, 29, 4105–4113. [Google Scholar] [CrossRef]
- Cheng, N.-M.; Fang, Y.-H.D.; Chang, J.T.-C.; Huang, C.-G.; Tsan, D.-L.; Ng, S.-H.; Wang, H.-M.; Lin, C.-Y.; Liao, C.-T.; Yen, T.-C. Textural Features of Pretreatment 18 F-FDG PET/CT Images: Prognostic Significance in Patients with Advanced T-Stage Oropharyngeal Squamous Cell Carcinoma. J. Nucl. Med. 2013, 54, 1703–1709. [Google Scholar] [CrossRef]
- Park, V.Y.; Han, K.; Lee, E.; Kim, E.-K.; Moon, H.J.; Yoon, J.H.; Kwak, J.Y. Association Between Radiomics Signature and Disease-Free Survival in Conventional Papillary Thyroid Carcinoma. Sci. Rep. 2019, 9, 4501. [Google Scholar] [CrossRef]
- Zdilar, L.; Vock, D.M.; Marai, G.E.; Fuller, C.D.; Mohamed, A.S.R.; Elhalawani, H.; Elgohari, B.A.; Tiras, C.; Miller, A.; Canahuate, G. Evaluating the Effect of Right-Censored End Point Transformation for Radiomic Feature Selection of Data From Patients With Oropharyngeal Cancer. JCO Clin. Cancer Inform. 2018, 1–19. [Google Scholar] [CrossRef]
- Zhuo, E.-H.; Zhang, W.-J.; Li, H.-J.; Zhang, G.-Y.; Jing, B.-Z.; Zhou, J.; Cui, C.-Y.; Chen, M.-Y.; Sun, Y.; Liu, L.-Z.; et al. Radiomics on multi-modalities MR sequences can subtype patients with non-metastatic nasopharyngeal carcinoma (NPC) into distinct survival subgroups. Eur. Radiol. 2019, 29, 5590–5599. [Google Scholar] [CrossRef]
- Haider, S.P.; Zeevi, T.; Baumeister, P.; Reichel, C.; Sharaf, K.; Forghani, R.; Kann, B.H.; Judson, B.L.; Prasad, M.L.; Burtness, B.; et al. Potential Added Value of PET/CT Radiomics for Survival Prognostication beyond AJCC 8th Edition Staging in Oropharyngeal Squamous Cell Carcinoma. Cancers 2020, 12, 1778. [Google Scholar] [CrossRef]
- Leijenaar, R.T.; Bogowicz, M.; Jochems, A.; Hoebers, F.J.; Wesseling, F.W.; Huang, S.H.; Chan, B.; Waldron, J.N.; O’Sullivan, B.; Rietveld, D.; et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: A multicenter study. Br. J. Radiol. 2018, 20170498. [Google Scholar] [CrossRef]
- Stanzione, A.; Cuocolo, R.; Ugga, L.; Verde, F.; Romeo, V.; Brunetti, A.; Maurea, S. Oncologic Imaging and Radiomics: A Walkthrough Review of Methodological Challenges. Cancers 2022, 14, 4871. [Google Scholar] [CrossRef]
- Zhao, B. Understanding Sources of Variation to Improve the Reproducibility of Radiomics. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Prezioso, E.; Izzo, S.; Giampaolo, F.; Piccialli, F.; Orabona, G.D.; Cuocolo, R.; Abbate, V.; Ugga, L.; Califano, L. Predictive Medicine for Salivary Gland Tumours Identification Through Deep Learning. IEEE J. Biomed. Heal. Inform. 2022, 26, 4869–4879. [Google Scholar] [CrossRef]
- Lewis, J.S.; Beadle, B.; Bishop, J.A.; Chernock, R.D.; Colasacco, C.; Lacchetti, C.; Moncur, J.T.; Rocco, J.W.; Schwartz, M.R.; Seethala, R.R.; et al. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch. Pathol. Lab. Med. 2018, 142, 559–597. [Google Scholar] [CrossRef]
- Payabvash, S.; Chan, A.; Jabehdar Maralani, P.; Malhotra, A. Quantitative diffusion magnetic resonance imaging for prediction of human papillomavirus status in head and neck squamous-cell carcinoma: A systematic review and meta-analysis. Neuroradiol. J. 2019, 32, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.M.; Orosco, R.K.; Shen, J.P.; Egloff, A.M.; Carter, H.; Hofree, M.; Choueiri, M.; Coffey, C.S.; Lippman, S.M.; Hayes, D.N.; et al. Multi-tiered genomic analysis of head and neck cancer ties TP53 mutation to 3p loss. Nat. Genet. 2014, 46, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Yasui, T.; Ishikawa-Fujiwara, T.; Morii, E.; Yamamoto, Y.; Yoshii, T.; Takenaka, Y.; Nakahara, S.; Todo, T.; Hongyo, T.; et al. Human papillomavirus and p53 mutations in head and neck squamous cell carcinoma among Japanese population. Cancer Sci. 2014, 105, 409–417. [Google Scholar] [CrossRef]
- Wichmann, G.; Rosolowski, M.; Krohn, K.; Kreuz, M.; Boehm, A.; Reiche, A.; Scharrer, U.; Halama, D.; Bertolini, J.; Bauer, U.; et al. The role of HPV RNA transcription, immune response-related gene expression and disruptive TP53 mutations in diagnostic and prognostic profiling of head and neck cancer. Int. J. Cancer 2015, 137, 2846–2857. [Google Scholar] [CrossRef]
- Yokota, T.; Hamauchi, S.; Shirasu, H.; Onozawa, Y.; Ogawa, H.; Onoe, T.; Onitsuka, T. How Should We Approach Locally Advanced Squamous Cell Carcinoma of Head and Neck Cancer Patients Ineligible for Standard Non-surgical Treatment? Curr. Oncol. Rep. 2020, 22, 118. [Google Scholar] [CrossRef]
- Dionisi, F.; Fiorica, F.; D’Angelo, E.; Maddalo, M.; Giacomelli, I.; Tornari, E.; Rosca, A.; Vigo, F.; Romanello, D.; Cianchetti, M.; et al. Organs at risk’s tolerance and dose limits for head and neck cancer re-irradiation: A literature review. Oral Oncol. 2019, 98, 35–47. [Google Scholar] [CrossRef]
- Seeburg, D.P.; Baer, A.H.; Aygun, N. Imaging of Patients with Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 421–433. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Wang, Y.; Jiang, S.; Fan, R.; Zhang, H.; Jiang, W. Application of radiomics and machine learning in head and neck cancers. Int. J. Biol. Sci. 2021, 17, 475–486. [Google Scholar] [CrossRef]
- Oosting, S.F.; Haddad, R.I. Best Practice in Systemic Therapy for Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Fave, X.; Mackin, D.; Yang, J.; Zhang, J.; Fried, D.; Balter, P.; Followill, D.; Gomez, D.; Kyle Jones, A.; Stingo, F.; et al. Can radiomics features be reproducibly measured from CBCT images for patients with non-small cell lung cancer? Med. Phys. 2015, 42, 6784–6797. [Google Scholar] [CrossRef]
- Scalco, E.; Moriconi, S.; Rizzo, G. Texture analysis to assess structural modifications induced by radiotherapy. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; 2015; pp. 5219–5222. [Google Scholar]
- Dirix, P.; Nuyts, S. Evidence-based organ-sparing radiotherapy in head and neck cancer. Lancet Oncol. 2010, 11, 85–91. [Google Scholar] [CrossRef]
- Epstein, J.B.; Thariat, J.; Bensadoun, R.-J.; Barasch, A.; Murphy, B.A.; Kolnick, L.; Popplewell, L.; Maghami, E. Oral complications of cancer and cancer therapy. CA. Cancer J. Clin. 2012, 62, 400–422. [Google Scholar] [CrossRef]
- Concha-Benavente, F.; Kansy, B.; Moskovitz, J.; Moy, J.; Chandran, U.; Ferris, R.L. PD-L1 Mediates Dysfunction in Activated PD-1+ NK Cells in Head and Neck Cancer Patients. Cancer Immunol. Res. 2018, 6, 1548–1560. [Google Scholar] [CrossRef]
- Ang, K.K.; Zhang, Q.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef]
- Colevas, A.D.; Bahleda, R.; Braiteh, F.; Balmanoukian, A.; Brana, I.; Chau, N.G.; Sarkar, I.; Molinero, L.; Grossman, W.; Kabbinavar, F.; et al. Safety and clinical activity of atezolizumab in head and neck cancer: Results from a phase I trial. Ann. Oncol. 2018, 29, 2247–2253. [Google Scholar] [CrossRef]
- Ulrich, E.J.; Menda, Y.; Ponto, L.L.B.; Anderson, C.M.; Smith, B.J.; Sunderland, J.J.; Graham, M.M.; Buatti, J.M.; Beichel, R.R. FLT PET Radiomics for Response Prediction to Chemoradiation Therapy in Head and Neck Squamous Cell Cancer. Tomography 2019, 5, 161–169. [Google Scholar] [CrossRef]
- El Naqa, I.; Grigsby, P.W.; Apte, A.; Kidd, E.; Donnelly, E.; Khullar, D.; Chaudhari, S.; Yang, D.; Schmitt, M.; Laforest, R.; et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009, 42, 1162–1171. [Google Scholar] [CrossRef]
- Moskowitz, C.S.; Welch, M.L.; Jacobs, M.A.; Kurland, B.F.; Simpson, A.L. Radiomic Analysis: Study Design, Statistical Analysis, and Other Bias Mitigation Strategies. Radiology 2022, 304, 265–273. [Google Scholar] [CrossRef]
- Ugga, L.; Spadarella, G.; Pinto, L.; Cuocolo, R.; Brunetti, A. Meningioma Radiomics: At the Nexus of Imaging, Pathology and Biomolecular Characterization. Cancers 2022, 14, 2605. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Khosravi, B.; Vahdati, S.; Faghani, S.; Nugen, F.; Rassoulinejad-Mousavi, S.M.; Moassefi, M.; Jagtap, J.M.M.; Singh, Y.; Rouzrokh, P.; et al. Mitigating Bias in Radiology Machine Learning: 2. Model Development. Radiol. Artif. Intell. 2022, 4. [Google Scholar] [CrossRef] [PubMed]
- Faghani, S.; Khosravi, B.; Zhang, K.; Moassefi, M.; Jagtap, J.M.; Nugen, F.; Vahdati, S.; Kuanar, S.P.; Rassoulinejad-Mousavi, S.M.; Singh, Y.; et al. Mitigating Bias in Radiology Machine Learning: 3. Performance Metrics. Radiol. Artif. Intell. 2022, 4. [Google Scholar] [CrossRef] [PubMed]
- Rouzrokh, P.; Khosravi, B.; Faghani, S.; Moassefi, M.; Vera Garcia, D.V.; Singh, Y.; Zhang, K.; Conte, G.M.; Erickson, B.J. Mitigating Bias in Radiology Machine Learning: 1. Data Handling. Radiol. Artif. Intell. 2022, 4. [Google Scholar] [CrossRef]
- Willemink, M.J.; Koszek, W.A.; Hardell, C.; Wu, J.; Fleischmann, D.; Harvey, H.; Folio, L.R.; Summers, R.M.; Rubin, D.L.; Lungren, M.P. Preparing Medical Imaging Data for Machine Learning. Radiology 2020, 295, 4–15. [Google Scholar] [CrossRef]
- Eng, J. Sample Size Estimation: How Many Individuals Should Be Studied? Radiology 2003, 227, 309–313. [Google Scholar] [CrossRef]
- Huang, S.-C.; Pareek, A.; Seyyedi, S.; Banerjee, I.; Lungren, M.P. Fusion of medical imaging and electronic health records using deep learning: A systematic review and implementation guidelines. npj Digit. Med. 2020, 3, 136. [Google Scholar] [CrossRef]
- Ying, X. An Overview of Overfitting and its Solutions. J. Phys. Conf. Ser. 2019, 1168, 022022. [Google Scholar] [CrossRef]
- Ayinde, B.O.; Inanc, T.; Zurada, J.M. Regularizing Deep Neural Networks by Enhancing Diversity in Feature Extraction. IEEE Trans. Neural Netw. Learn. Syst. 2019, 30, 2650–2661. [Google Scholar] [CrossRef]
- Akbar, S.; Peikari, M.; Salama, S.; Nofech-Mozes, S.; Martel, A.L. The transition module: A method for preventing overfitting in convolutional neural networks. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2019, 7, 260–265. [Google Scholar] [CrossRef]
- Buda, M.; Maki, A.; Mazurowski, M.A. A systematic study of the class imbalance problem in convolutional neural networks. Neural Netw. 2018, 106, 249–259. [Google Scholar] [CrossRef]
- He, Q.; Li, X.; Kim, D.W.N.; Jia, X.; Gu, X.; Zhen, X.; Zhou, L. Feasibility study of a multi-criteria decision-making based hierarchical model for multi-modality feature and multi-classifier fusion: Applications in medical prognosis prediction. Inf. Fusion 2020, 55, 207–219. [Google Scholar] [CrossRef]
- Erickson, B.J.; Kitamura, F. Magician’s Corner: 9. Performance Metrics for Machine Learning Models. Radiol. Artif. Intell. 2021, 3, e200126. [Google Scholar] [CrossRef]
- Common Limitations of Image Processing Metrics: A Picture Story. Available online: https://arxiv.org/abs/2104.05642 (accessed on 20 December 2022).
- Metrics Reloaded: Pitfalls and Recommendations for Image Analysis Validation. Available online: https://arxiv.org/abs/2206.01653 (accessed on 20 December 2022).
- Sokolova, M.; Japkowicz, N.; Szpakowicz, S. Beyond Accuracy, F-Score and ROC: A Family of Discriminant Measures for Performance Evaluation. In AI 2006: Advances in Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1015–1021. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).