Radiomics Applications in Head and Neck Tumor Imaging: A Narrative Review
Abstract
:Simple Summary
Abstract
1. Introduction
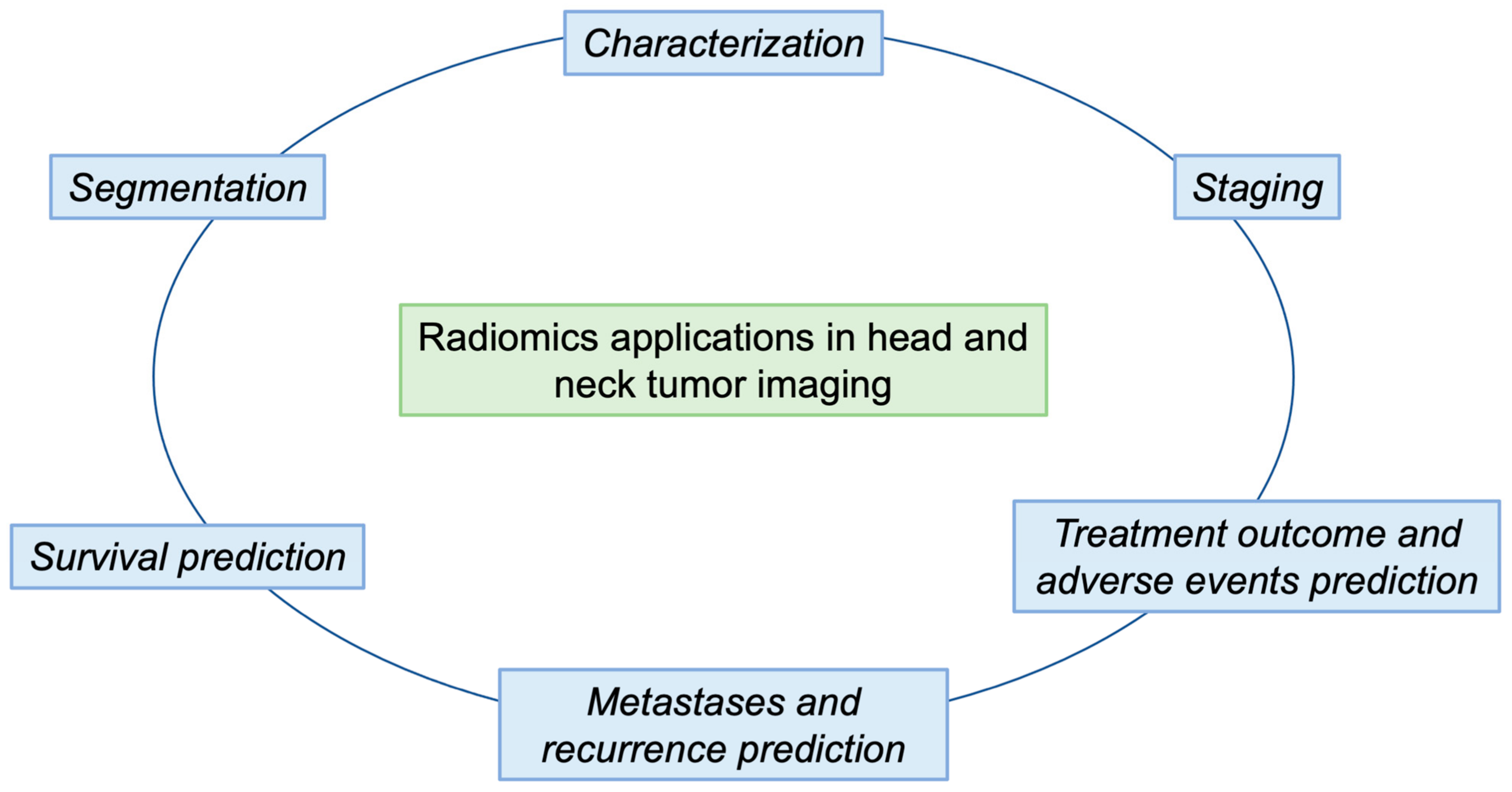
2. Segmentation
3. Characterization
4. Staging
5. Treatment
5.1. Pre- and Intra-Treatment Imaging
5.2. Short-Term Outcome and Adverse Events
5.3. Long-Term Outcome and Adverse Events
6. Metastases and Recurrence
7. Survival
8. Limitations of Radiomics
8.1. Current Issues
8.2. Potential Solutions
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Cramer, J.D.; Burtness, B.; Le, Q.T.; Ferris, R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019, 16, 669–683. [Google Scholar] [CrossRef]
- Hedberg, M.L.; Grandis, J.R. The Molecular Pathogenesis of Head and Neck Cancer. In The Molecular Basis of Cancer; Elsevier: Amsterdam, The Netherlands, 2015; pp. 491–498.e2. [Google Scholar]
- Maier, H.; Dietz, A.; Gewelke, U.; Heller, W.D.; Weidauer, H. Tobacco and alcohol and the risk of head and neck cancer. Clin. Investig. 1992, 70, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.K.; Engels, E.A.; Pfeiffer, R.M.; Hernandez, B.Y.; Xiao, W.; Kim, E.; Jiang, B.; Goodman, M.T.; Sibug-Saber, M.; Cozen, W.; et al. Human Papillomavirus and Rising Oropharyngeal Cancer Incidence in the United States. J. Clin. Oncol. 2011, 29, 4294–4301. [Google Scholar] [CrossRef] [PubMed]
- Sturgis, E.M.; Cinciripini, P.M. Trends in head and neck cancer incidence in relation to smoking prevalence. Cancer 2007, 110, 1429–1435. [Google Scholar] [CrossRef]
- Yu, M.C.; Yuan, J.-M. Epidemiology of nasopharyngeal carcinoma. Semin. Cancer Biol. 2002, 12, 421–429. [Google Scholar] [CrossRef]
- Jicman (Stan), D.; Niculet, E.; Lungu, M.; Onisor, C.; Rebegea, L.; Vesa, D.; Bezman, L.; Bujoreanu, F.; Sarbu, M.; Mihailov, R.; et al. Nasopharyngeal carcinoma: A new synthesis of literature data (Review). Exp. Ther. Med. 2021, 23, 136. [Google Scholar] [CrossRef]
- Stelow, E.B.; Wenig, B.M. Update From The 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Nasopharynx. Head Neck Pathol. 2017, 11, 16–22. [Google Scholar] [CrossRef]
- Badoual, C. Update from the 5th Edition of the World Health Organization Classification of Head and Neck Tumors: Oropharynx and Nasopharynx. Head Neck Pathol. 2022, 16, 19–30. [Google Scholar] [CrossRef]
- Tortora, M.; Gemini, L.; D’Iglio, I.; Ugga, L.; Spadarella, G.; Cuocolo, R. Spectral Photon-Counting Computed Tomography: A Review on Technical Principles and Clinical Applications. J. Imaging 2022, 8, 112. [Google Scholar] [CrossRef]
- van Timmeren, J.E.; Cester, D.; Tanadini-Lang, S.; Alkadhi, H.; Baessler, B. Radiomics in medical imaging—“how-to” guide and critical reflection. Insights Imaging 2020, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Sanduleanu, S.; Woodruff, H.C.; de Jong, E.E.C.; van Timmeren, J.E.; Jochems, A.; Dubois, L.; Lambin, P. Tracking tumor biology with radiomics: A systematic review utilizing a radiomics quality score. Radiother. Oncol. 2018, 127, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Avanzo, M.; Wei, L.; Stancanello, J.; Vallières, M.; Rao, A.; Morin, O.; Mattonen, S.A.; El Naqa, I. Machine and deep learning methods for radiomics. Med. Phys. 2020, 47. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Rios Velazquez, E.; Leijenaar, R.; Jermoumi, M.; Carvalho, S.; Mak, R.H.; Mitra, S.; Shankar, B.U.; Kikinis, R.; Haibe-Kains, B.; et al. Robust Radiomics Feature Quantification Using Semiautomatic Volumetric Segmentation. PLoS ONE 2014, 9, e102107. [Google Scholar] [CrossRef]
- Kuhl, C.K.; Truhn, D. The Long Route to Standardized Radiomics: Unraveling the Knot from the End. Radiology 2020, 295, 339–341. [Google Scholar] [CrossRef]
- Gitto, S.; Cuocolo, R.; Emili, I.; Tofanelli, L.; Chianca, V.; Albano, D.; Messina, C.; Imbriaco, M.; Sconfienza, L.M. Effects of Interobserver Variability on 2D and 3D CT- and MRI-Based Texture Feature Reproducibility of Cartilaginous Bone Tumors. J. Digit. Imaging 2021, 34, 820–832. [Google Scholar] [CrossRef]
- Huan, Y.; Caldwell, C.; Mah, K.; Mozeg, D. Coregistered FDG PET/CT-Based Textural Characterization of Head and Neck Cancer for Radiation Treatment Planning. IEEE Trans. Med. Imaging 2009, 28, 374–383. [Google Scholar] [CrossRef]
- Yu, H.; Caldwell, C.; Mah, K.; Poon, I.; Balogh, J.; MacKenzie, R.; Khaouam, N.; Tirona, R. Automated Radiation Targeting in Head-and-Neck Cancer Using Region-Based Texture Analysis of PET and CT Images. Int. J. Radiat. Oncol. 2009, 75, 618–625. [Google Scholar] [CrossRef]
- Buch, K.; Fujita, A.; Li, B.; Kawashima, Y.; Qureshi, M.M.; Sakai, O. Using Texture Analysis to Determine Human Papillomavirus Status of Oropharyngeal Squamous Cell Carcinomas on CT. Am. J. Neuroradiol. 2015, 36, 1343–1348. [Google Scholar] [CrossRef]
- Fujita, A.; Buch, K.; Li, B.; Kawashima, Y.; Qureshi, M.M.; Sakai, O. Difference Between HPV-Positive and HPV-Negative Non-Oropharyngeal Head and Neck Cancer. J. Comput. Assist. Tomogr. 2016, 40, 43–47. [Google Scholar] [CrossRef]
- Vallieres, M.; Kumar, A.; Sultanem, K.; El Naqa, I. FDG-PET Image-Derived Features Can Determine HPV Status in Head-and-Neck Cancer. Int. J. Radiat. Oncol. 2013, 87, S467. [Google Scholar] [CrossRef]
- Payabvash, S.; Brackett, A.; Forghani, R.; Malhotra, A. Differentiation of lymphomatous, metastatic, and non-malignant lymphadenopathy in the neck with quantitative diffusion-weighted imaging: Systematic review and meta-analysis. Neuroradiology 2019, 61, 897–910. [Google Scholar] [CrossRef]
- Payabvash, S. Quantitative diffusion magnetic resonance imaging in head and neck tumors. Quant. Imaging Med. Surg. 2018, 8, 1052–1065. [Google Scholar] [CrossRef]
- Marzi, S.; Piludu, F.; Avanzolini, I.; Muneroni, V.; Sanguineti, G.; Farneti, A.; D’Urso, P.; Benevolo, M.; Rollo, F.; Covello, R.; et al. Multifactorial Model Based on DWI-Radiomics to Determine HPV Status in Oropharyngeal Squamous Cell Carcinoma. Appl. Sci. 2022, 12, 7244. [Google Scholar] [CrossRef]
- Suh, C.H.; Lee, K.H.; Choi, Y.J.; Chung, S.R.; Baek, J.H.; Lee, J.H.; Yun, J.; Ham, S.; Kim, N. Oropharyngeal squamous cell carcinoma: Radiomic machine-learning classifiers from multiparametric MR images for determination of HPV infection status. Sci. Rep. 2020, 10, 17525. [Google Scholar] [CrossRef]
- Sohn, B.; Choi, Y.S.; Ahn, S.S.; Kim, H.; Han, K.; Lee, S.; Kim, J. Machine Learning Based Radiomic HPV Phenotyping of Oropharyngeal SCC: A Feasibility Study Using MRI. Laryngoscope 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Aerts, H.J.W.L.; Velazquez, E.R.; Leijenaar, R.T.H.; Parmar, C.; Grossmann, P.; Carvalho, S.; Bussink, J.; Monshouwer, R.; Haibe-Kains, B.; Rietveld, D.; et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat. Commun. 2014, 5, 4006. [Google Scholar] [CrossRef] [PubMed]
- Zwirner, K.; Hilke, F.J.; Demidov, G.; Fernandez, J.S.; Ossowski, S.; Gani, C.; Thorwarth, D.; Riess, O.; Zips, D.; Schroeder, C.; et al. Radiogenomics in head and neck cancer: Correlation of radiomic heterogeneity and somatic mutations in TP53, FAT1 and KMT2D. Strahlentherapie und Onkol. 2019, 195, 771–779. [Google Scholar] [CrossRef]
- Huang, C.; Cintra, M.; Brennan, K.; Zhou, M.; Colevas, A.D.; Fischbein, N.; Zhu, S.; Gevaert, O. Development and validation of radiomic signatures of head and neck squamous cell carcinoma molecular features and subtypes. EBioMedicine 2019, 45, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Mohamed, A.S.R.; Lai, S.Y.; Yang, S.; Kanwar, A.; Wei, L.; Kamal, M.; Sengupta, S.; Elhalawani, H.; Skinner, H.; et al. Imaging-Genomic Study of Head and Neck Squamous Cell Carcinoma: Associations Between Radiomic Phenotypes and Genomic Mechanisms via Integration of The Cancer Genome Atlas and The Cancer Imaging Archive. JCO Clin. Cancer Inform. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-Y.; Lin, Y.-C.; Shen, W.-C.; Hsieh, T.-C.; Yen, K.-Y.; Chen, S.-W.; Kao, C.-H. Associations of Tumor PD-1 Ligands, Immunohistochemical Studies, and Textural Features in 18F-FDG PET in Squamous Cell Carcinoma of the Head and Neck. Sci. Rep. 2018, 8, 105. [Google Scholar] [CrossRef]
- Brown, A.M.; Nagala, S.; McLean, M.A.; Lu, Y.; Scoffings, D.; Apte, A.; Gonen, M.; Stambuk, H.E.; Shaha, A.R.; Tuttle, R.M.; et al. Multi-institutional validation of a novel textural analysis tool for preoperative stratification of suspected thyroid tumors on diffusion-weighted MRI. Magn. Reson. Med. 2016, 75, 1708–1716. [Google Scholar] [CrossRef]
- Jansen, J.F. Texture analysis on parametric maps derived from dynamic contrast-enhanced magnetic resonance imaging in head and neck cancer. World J. Radiol. 2016, 8, 90. [Google Scholar] [CrossRef]
- Kim, S.; Loevner, L.A.; Quon, H.; Kilger, A.; Sherman, E.; Weinstein, G.; Chalian, A.; Poptani, H. Prediction of Response to Chemoradiation Therapy in Squamous Cell Carcinomas of the Head and Neck Using Dynamic Contrast-Enhanced MR Imaging. Am. J. Neuroradiol. 2010, 31, 262–268. [Google Scholar] [CrossRef]
- Shukla-Dave, A.; Lee, N.Y.; Jansen, J.F.A.; Thaler, H.T.; Stambuk, H.E.; Fury, M.G.; Patel, S.G.; Moreira, A.L.; Sherman, E.; Karimi, S.; et al. Dynamic Contrast-Enhanced Magnetic Resonance Imaging as a Predictor of Outcome in Head-and-Neck Squamous Cell Carcinoma Patients With Nodal Metastases. Int. J. Radiat. Oncol. 2012, 82, 1837–1844. [Google Scholar] [CrossRef]
- Dang, M.; Lysack, J.T.; Wu, T.; Matthews, T.W.; Chandarana, S.P.; Brockton, N.T.; Bose, P.; Bansal, G.; Cheng, H.; Mitchell, J.R.; et al. MRI Texture Analysis Predicts p53 Status in Head and Neck Squamous Cell Carcinoma. Am. J. Neuroradiol. 2015, 36, 166–170. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, B.; Wu, X.; Liu, L.; Fang, J.; Chen, Q.; Li, M.; Chen, Z.; Li, Y.; Dong, D.; et al. Radiomic Nomogram Improves Preoperative T Category Accuracy in Locally Advanced Laryngeal Carcinoma. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Ren, J.; Tian, J.; Yuan, Y.; Dong, D.; Li, X.; Shi, Y.; Tao, X. Magnetic resonance imaging based radiomics signature for the preoperative discrimination of stage I-II and III-IV head and neck squamous cell carcinoma. Eur. J. Radiol. 2018, 106, 1–6. [Google Scholar] [CrossRef]
- Romeo, V.; Cuocolo, R.; Ricciardi, C.; Ugga, L.; Cocozza, S.; Verde, F.; Stanzione, A.; Napolitano, V.; Russo, D.; Improta, G.; et al. Prediction of Tumor Grade and Nodal Status in Oropharyngeal and Oral Cavity Squamous-cell Carcinoma Using a Radiomic Approach. Anticancer Res. 2020, 40, 271–280. [Google Scholar] [CrossRef]
- Wang, H.; Song, B.; Ye, N.; Ren, J.; Sun, X.; Dai, Z.; Zhang, Y.; Chen, B.T. Machine learning-based multiparametric MRI radiomics for predicting the aggressiveness of papillary thyroid carcinoma. Eur. J. Radiol. 2020, 122, 108755. [Google Scholar] [CrossRef]
- Fave, X.; Zhang, L.; Yang, J.; Mackin, D.; Balter, P.; Gomez, D.; Followill, D.; Jones, A.K.; Stingo, F.; Liao, Z.; et al. Delta-radiomics features for the prediction of patient outcomes in non–small cell lung cancer. Sci. Rep. 2017, 7, 588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tian, J.; Dong, D.; Gu, D.; Dong, Y.; Zhang, L.; Lian, Z.; Liu, J.; Luo, X.; Pei, S.; et al. Radiomics Features of Multiparametric MRI as Novel Prognostic Factors in Advanced Nasopharyngeal Carcinoma. Clin. Cancer Res. 2017, 23, 4259–4269. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, L.; Yuan, C.; Huang, Y.; Liu, Z.; Liang, C. Pretreatment MR imaging radiomics signatures for response prediction to induction chemotherapy in patients with nasopharyngeal carcinoma. Eur. J. Radiol. 2018, 98, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, Y.; Li, Z.; Zhang, D.; Zhang, Z.; Hao, S.; Li, B. Use of texture analysis based on contrast-enhanced MRI to predict treatment response to chemoradiotherapy in nasopharyngeal carcinoma. J. Magn. Reson. Imaging 2016, 44, 445–455. [Google Scholar] [CrossRef]
- Hawkins, P.G.; Lee, J.Y.; Mao, Y.; Li, P.; Green, M.; Worden, F.P.; Swiecicki, P.L.; Mierzwa, M.L.; Spector, M.E.; Schipper, M.J.; et al. Sparing all salivary glands with IMRT for head and neck cancer: Longitudinal study of patient-reported xerostomia and head-and-neck quality of life. Radiother. Oncol. 2018, 126, 68–74. [Google Scholar] [CrossRef]
- Sheikh, K.; Lee, S.H.; Cheng, Z.; Lakshminarayanan, P.; Peng, L.; Han, P.; McNutt, T.R.; Quon, H.; Lee, J. Predicting acute radiation induced xerostomia in head and neck Cancer using MR and CT Radiomics of parotid and submandibular glands. Radiat. Oncol. 2019, 14, 131. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, H.; Huang, S.; Chen, X.; Zhou, H.; Chang, H.; Xia, Y.; Wang, G.; Yang, X. Early prediction of acute xerostomia during radiation therapy for nasopharyngeal cancer based on delta radiomics from CT images. Quant. Imaging Med. Surg. 2019, 9, 1288–1302. [Google Scholar] [CrossRef]
- van Dijk, L.V.; Thor, M.; Steenbakkers, R.J.H.M.; Apte, A.; Zhai, T.-T.; Borra, R.; Noordzij, W.; Estilo, C.; Lee, N.; Langendijk, J.A.; et al. Parotid gland fat related Magnetic Resonance image biomarkers improve prediction of late radiation-induced xerostomia. Radiother. Oncol. 2018, 128, 459–466. [Google Scholar] [CrossRef]
- van Dijk, L.V.; Brouwer, C.L.; van der Schaaf, A.; Burgerhof, J.G.M.; Beukinga, R.J.; Langendijk, J.A.; Sijtsema, N.M.; Steenbakkers, R.J.H.M. CT image biomarkers to improve patient-specific prediction of radiation-induced xerostomia and sticky saliva. Radiother. Oncol. 2017, 122, 185–191. [Google Scholar] [CrossRef]
- Thor, M.; Tyagi, N.; Hatzoglou, V.; Apte, A.; Saleh, Z.; Riaz, N.; Lee, N.Y.; Deasy, J.O. A magnetic resonance imaging-based approach to quantify radiation-induced normal tissue injuries applied to trismus in head and neck cancer. Phys. Imaging Radiat. Oncol. 2017, 1, 34–40. [Google Scholar] [CrossRef]
- Abdollahi, H.; Mostafaei, S.; Cheraghi, S.; Shiri, I.; Mahdavi, S.R.; Kazemnejad, A. Cochlea CT radiomics predicts chemoradiotherapy induced sensorineural hearing loss in head and neck cancer patients: A machine learning and multi-variable modelling study. Phys. Medica 2018, 45, 192–197. [Google Scholar] [CrossRef]
- Kann, B.H.; Aneja, S.; Loganadane, G.V.; Kelly, J.R.; Smith, S.M.; Decker, R.H.; Yu, J.B.; Park, H.S.; Yarbrough, W.G.; Malhotra, A.; et al. Pretreatment Identification of Head and Neck Cancer Nodal Metastasis and Extranodal Extension Using Deep Learning Neural Networks. Sci. Rep. 2018, 8, 14036. [Google Scholar] [CrossRef]
- Kann, B.H.; Hicks, D.F.; Payabvash, S.; Mahajan, A.; Du, J.; Gupta, V.; Park, H.S.; Yu, J.B.; Yarbrough, W.G.; Burtness, B.A.; et al. Multi-Institutional Validation of Deep Learning for Pretreatment Identification of Extranodal Extension in Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2020, 38, 1304–1311. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, D.; Li, H.; Tian, J.; Ouyang, F.; Mo, X.; Zhang, B.; Luo, X.; Lian, Z.; Pei, S.; et al. Development and validation of a magnetic resonance imaging-based model for the prediction of distant metastasis before initial treatment of nasopharyngeal carcinoma: A retrospective cohort study. EBioMedicine 2019, 40, 327–335. [Google Scholar] [CrossRef]
- Bogowicz, M.; Riesterer, O.; Ikenberg, K.; Stieb, S.; Moch, H.; Studer, G.; Guckenberger, M.; Tanadini-Lang, S. Computed Tomography Radiomics Predicts HPV Status and Local Tumor Control After Definitive Radiochemotherapy in Head and Neck Squamous Cell Carcinoma. Int. J. Radiat. Oncol. 2017, 99, 921–928. [Google Scholar] [CrossRef]
- Li, S.; Wang, K.; Hou, Z.; Yang, J.; Ren, W.; Gao, S.; Meng, F.; Wu, P.; Liu, B.; Liu, J.; et al. Use of Radiomics Combined With Machine Learning Method in the Recurrence Patterns After Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma: A Preliminary Study. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Kuno, H.; Qureshi, M.M.; Chapman, M.N.; Li, B.; Andreu-Arasa, V.C.; Onoue, K.; Truong, M.T.; Sakai, O. CT Texture Analysis Potentially Predicts Local Failure in Head and Neck Squamous Cell Carcinoma Treated with Chemoradiotherapy. Am. J. Neuroradiol. 2017, 38, 2334–2340. [Google Scholar] [CrossRef]
- Investigation of radiomic signatures for local recurrence using primary tumor texture analysis in oropharyngeal head and neck cancer patients. Sci. Rep. 2018, 8, 1524. [CrossRef]
- Zhang, L.; Zhou, H.; Gu, D.; Tian, J.; Zhang, B.; Dong, D.; Mo, X.; Liu, J.; Luo, X.; Pei, S.; et al. Radiomic Nomogram: Pretreatment Evaluation of Local Recurrence in Nasopharyngeal Carcinoma based on MR Imaging. J. Cancer 2019, 10, 4217–4225. [Google Scholar] [CrossRef]
- Shen, H.; Wang, Y.; Liu, D.; Lv, R.; Huang, Y.; Peng, C.; Jiang, S.; Wang, Y.; He, Y.; Lan, X.; et al. Predicting Progression-Free Survival Using MRI-Based Radiomics for Patients With Nonmetastatic Nasopharyngeal Carcinoma. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Yuan, Y.; Ren, J.; Shi, Y.; Tao, X. MRI-based radiomic signature as predictive marker for patients with head and neck squamous cell carcinoma. Eur. J. Radiol. 2019, 117, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Parmar, C.; Grossmann, P.; Rietveld, D.; Rietbergen, M.M.; Lambin, P.; Aerts, H.J.W.L. Radiomic Machine-Learning Classifiers for Prognostic Biomarkers of Head and Neck Cancer. Front. Oncol. 2015, 5. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, J.P.; Sinha, S.; Goda, J.S.; Joshi, K.; Mhatre, R.; Kannan, S.; Laskar, S.G.; Gupta, T.; Murthy, V.; Budrukkar, A.; et al. Tumor radiomic features complement clinico-radiological factors in predicting long-term local control and laryngectomy free survival in locally advanced laryngo-pharyngeal cancers. Br. J. Radiol. 2020, 93, 20190857. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Cao, Y.; Diao, W.; Cheng, Y.; Jia, Z.; Peng, X. Radiomics-based prediction of survival in patients with head and neck squamous cell carcinoma based on pre- and post-treatment 18F-PET/CT. Aging 2020, 12, 14593–14619. [Google Scholar] [CrossRef] [PubMed]
- Zhai, T.-T.; Langendijk, J.A.; van Dijk, L.V.; Halmos, G.B.; Witjes, M.J.H.; Oosting, S.F.; Noordzij, W.; Sijtsema, N.M.; Steenbakkers, R.J.H.M. The prognostic value of CT-based image-biomarkers for head and neck cancer patients treated with definitive (chemo-)radiation. Oral Oncol. 2019, 95, 178–186. [Google Scholar] [CrossRef]
- Leijenaar, R.T.H.; Carvalho, S.; Hoebers, F.J.P.; Aerts, H.J.W.L.; van Elmpt, W.J.C.; Huang, S.H.; Chan, B.; Waldron, J.N.; O’sullivan, B.; Lambin, P. External validation of a prognostic CT-based radiomic signature in oropharyngeal squamous cell carcinoma. Acta Oncol. 2015, 54, 1423–1429. [Google Scholar] [CrossRef]
- Bogowicz, M.; Tanadini-Lang, S.; Veit-Haibach, P.; Pruschy, M.; Bender, S.; Sharma, A.; Hüllner, M.; Studer, G.; Stieb, S.; Hemmatazad, H.; et al. Perfusion CT radiomics as potential prognostic biomarker in head and neck squamous cell carcinoma. Acta Oncol. 2019, 58, 1514–1518. [Google Scholar] [CrossRef]
- Zhang, H.; Graham, C.M.; Elci, O.; Griswold, M.E.; Zhang, X.; Khan, M.A.; Pitman, K.; Caudell, J.J.; Hamilton, R.D.; Ganeshan, B.; et al. Locally Advanced Squamous Cell Carcinoma of the Head and Neck: CT Texture and Histogram Analysis Allow Independent Prediction of Overall Survival in Patients Treated with Induction Chemotherapy. Radiology 2013, 269, 801–809. [Google Scholar] [CrossRef]
- Mao, J.; Fang, J.; Duan, X.; Yang, Z.; Cao, M.; Zhang, F.; Lu, L.; Zhang, X.; Wu, X.; Ding, Y.; et al. Predictive value of pretreatment MRI texture analysis in patients with primary nasopharyngeal carcinoma. Eur. Radiol. 2019, 29, 4105–4113. [Google Scholar] [CrossRef]
- Cheng, N.-M.; Fang, Y.-H.D.; Chang, J.T.-C.; Huang, C.-G.; Tsan, D.-L.; Ng, S.-H.; Wang, H.-M.; Lin, C.-Y.; Liao, C.-T.; Yen, T.-C. Textural Features of Pretreatment 18 F-FDG PET/CT Images: Prognostic Significance in Patients with Advanced T-Stage Oropharyngeal Squamous Cell Carcinoma. J. Nucl. Med. 2013, 54, 1703–1709. [Google Scholar] [CrossRef]
- Park, V.Y.; Han, K.; Lee, E.; Kim, E.-K.; Moon, H.J.; Yoon, J.H.; Kwak, J.Y. Association Between Radiomics Signature and Disease-Free Survival in Conventional Papillary Thyroid Carcinoma. Sci. Rep. 2019, 9, 4501. [Google Scholar] [CrossRef]
- Zdilar, L.; Vock, D.M.; Marai, G.E.; Fuller, C.D.; Mohamed, A.S.R.; Elhalawani, H.; Elgohari, B.A.; Tiras, C.; Miller, A.; Canahuate, G. Evaluating the Effect of Right-Censored End Point Transformation for Radiomic Feature Selection of Data From Patients With Oropharyngeal Cancer. JCO Clin. Cancer Inform. 2018, 1–19. [Google Scholar] [CrossRef]
- Zhuo, E.-H.; Zhang, W.-J.; Li, H.-J.; Zhang, G.-Y.; Jing, B.-Z.; Zhou, J.; Cui, C.-Y.; Chen, M.-Y.; Sun, Y.; Liu, L.-Z.; et al. Radiomics on multi-modalities MR sequences can subtype patients with non-metastatic nasopharyngeal carcinoma (NPC) into distinct survival subgroups. Eur. Radiol. 2019, 29, 5590–5599. [Google Scholar] [CrossRef]
- Haider, S.P.; Zeevi, T.; Baumeister, P.; Reichel, C.; Sharaf, K.; Forghani, R.; Kann, B.H.; Judson, B.L.; Prasad, M.L.; Burtness, B.; et al. Potential Added Value of PET/CT Radiomics for Survival Prognostication beyond AJCC 8th Edition Staging in Oropharyngeal Squamous Cell Carcinoma. Cancers 2020, 12, 1778. [Google Scholar] [CrossRef]
- Leijenaar, R.T.; Bogowicz, M.; Jochems, A.; Hoebers, F.J.; Wesseling, F.W.; Huang, S.H.; Chan, B.; Waldron, J.N.; O’Sullivan, B.; Rietveld, D.; et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: A multicenter study. Br. J. Radiol. 2018, 20170498. [Google Scholar] [CrossRef]
- Stanzione, A.; Cuocolo, R.; Ugga, L.; Verde, F.; Romeo, V.; Brunetti, A.; Maurea, S. Oncologic Imaging and Radiomics: A Walkthrough Review of Methodological Challenges. Cancers 2022, 14, 4871. [Google Scholar] [CrossRef]
- Zhao, B. Understanding Sources of Variation to Improve the Reproducibility of Radiomics. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Rizzo, S.; Botta, F.; Raimondi, S.; Origgi, D.; Fanciullo, C.; Morganti, A.G.; Bellomi, M. Radiomics: The facts and the challenges of image analysis. Eur. Radiol. Exp. 2018, 2, 36. [Google Scholar] [CrossRef]
- Prezioso, E.; Izzo, S.; Giampaolo, F.; Piccialli, F.; Orabona, G.D.; Cuocolo, R.; Abbate, V.; Ugga, L.; Califano, L. Predictive Medicine for Salivary Gland Tumours Identification Through Deep Learning. IEEE J. Biomed. Heal. Inform. 2022, 26, 4869–4879. [Google Scholar] [CrossRef]
- Lewis, J.S.; Beadle, B.; Bishop, J.A.; Chernock, R.D.; Colasacco, C.; Lacchetti, C.; Moncur, J.T.; Rocco, J.W.; Schwartz, M.R.; Seethala, R.R.; et al. Human Papillomavirus Testing in Head and Neck Carcinomas: Guideline From the College of American Pathologists. Arch. Pathol. Lab. Med. 2018, 142, 559–597. [Google Scholar] [CrossRef]
- Payabvash, S.; Chan, A.; Jabehdar Maralani, P.; Malhotra, A. Quantitative diffusion magnetic resonance imaging for prediction of human papillomavirus status in head and neck squamous-cell carcinoma: A systematic review and meta-analysis. Neuroradiol. J. 2019, 32, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.J.; Ferris, R.L.; Blumenschein, G.; Colevas, A.D.; Fayette, J.; Licitra, L.; Kasper, S.; Even, C.; Vokes, E.E.; Worden, F.; et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): Health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 2017, 18, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.M.; Orosco, R.K.; Shen, J.P.; Egloff, A.M.; Carter, H.; Hofree, M.; Choueiri, M.; Coffey, C.S.; Lippman, S.M.; Hayes, D.N.; et al. Multi-tiered genomic analysis of head and neck cancer ties TP53 mutation to 3p loss. Nat. Genet. 2014, 46, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Yasui, T.; Ishikawa-Fujiwara, T.; Morii, E.; Yamamoto, Y.; Yoshii, T.; Takenaka, Y.; Nakahara, S.; Todo, T.; Hongyo, T.; et al. Human papillomavirus and p53 mutations in head and neck squamous cell carcinoma among Japanese population. Cancer Sci. 2014, 105, 409–417. [Google Scholar] [CrossRef]
- Wichmann, G.; Rosolowski, M.; Krohn, K.; Kreuz, M.; Boehm, A.; Reiche, A.; Scharrer, U.; Halama, D.; Bertolini, J.; Bauer, U.; et al. The role of HPV RNA transcription, immune response-related gene expression and disruptive TP53 mutations in diagnostic and prognostic profiling of head and neck cancer. Int. J. Cancer 2015, 137, 2846–2857. [Google Scholar] [CrossRef]
- Yokota, T.; Hamauchi, S.; Shirasu, H.; Onozawa, Y.; Ogawa, H.; Onoe, T.; Onitsuka, T. How Should We Approach Locally Advanced Squamous Cell Carcinoma of Head and Neck Cancer Patients Ineligible for Standard Non-surgical Treatment? Curr. Oncol. Rep. 2020, 22, 118. [Google Scholar] [CrossRef]
- Dionisi, F.; Fiorica, F.; D’Angelo, E.; Maddalo, M.; Giacomelli, I.; Tornari, E.; Rosca, A.; Vigo, F.; Romanello, D.; Cianchetti, M.; et al. Organs at risk’s tolerance and dose limits for head and neck cancer re-irradiation: A literature review. Oral Oncol. 2019, 98, 35–47. [Google Scholar] [CrossRef]
- Seeburg, D.P.; Baer, A.H.; Aygun, N. Imaging of Patients with Head and Neck Cancer. Oral Maxillofac. Surg. Clin. N. Am. 2018, 30, 421–433. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Wang, Y.; Jiang, S.; Fan, R.; Zhang, H.; Jiang, W. Application of radiomics and machine learning in head and neck cancers. Int. J. Biol. Sci. 2021, 17, 475–486. [Google Scholar] [CrossRef]
- Oosting, S.F.; Haddad, R.I. Best Practice in Systemic Therapy for Head and Neck Squamous Cell Carcinoma. Front. Oncol. 2019, 9. [Google Scholar] [CrossRef]
- Fave, X.; Mackin, D.; Yang, J.; Zhang, J.; Fried, D.; Balter, P.; Followill, D.; Gomez, D.; Kyle Jones, A.; Stingo, F.; et al. Can radiomics features be reproducibly measured from CBCT images for patients with non-small cell lung cancer? Med. Phys. 2015, 42, 6784–6797. [Google Scholar] [CrossRef]
- Scalco, E.; Moriconi, S.; Rizzo, G. Texture analysis to assess structural modifications induced by radiotherapy. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; 2015; pp. 5219–5222. [Google Scholar]
- Dirix, P.; Nuyts, S. Evidence-based organ-sparing radiotherapy in head and neck cancer. Lancet Oncol. 2010, 11, 85–91. [Google Scholar] [CrossRef]
- Epstein, J.B.; Thariat, J.; Bensadoun, R.-J.; Barasch, A.; Murphy, B.A.; Kolnick, L.; Popplewell, L.; Maghami, E. Oral complications of cancer and cancer therapy. CA. Cancer J. Clin. 2012, 62, 400–422. [Google Scholar] [CrossRef]
- Concha-Benavente, F.; Kansy, B.; Moskovitz, J.; Moy, J.; Chandran, U.; Ferris, R.L. PD-L1 Mediates Dysfunction in Activated PD-1+ NK Cells in Head and Neck Cancer Patients. Cancer Immunol. Res. 2018, 6, 1548–1560. [Google Scholar] [CrossRef]
- Ang, K.K.; Zhang, Q.; Rosenthal, D.I.; Nguyen-Tan, P.F.; Sherman, E.J.; Weber, R.S.; Galvin, J.M.; Bonner, J.A.; Harris, J.; El-Naggar, A.K.; et al. Randomized Phase III Trial of Concurrent Accelerated Radiation Plus Cisplatin With or Without Cetuximab for Stage III to IV Head and Neck Carcinoma: RTOG 0522. J. Clin. Oncol. 2014, 32, 2940–2950. [Google Scholar] [CrossRef]
- Colevas, A.D.; Bahleda, R.; Braiteh, F.; Balmanoukian, A.; Brana, I.; Chau, N.G.; Sarkar, I.; Molinero, L.; Grossman, W.; Kabbinavar, F.; et al. Safety and clinical activity of atezolizumab in head and neck cancer: Results from a phase I trial. Ann. Oncol. 2018, 29, 2247–2253. [Google Scholar] [CrossRef]
- Ulrich, E.J.; Menda, Y.; Ponto, L.L.B.; Anderson, C.M.; Smith, B.J.; Sunderland, J.J.; Graham, M.M.; Buatti, J.M.; Beichel, R.R. FLT PET Radiomics for Response Prediction to Chemoradiation Therapy in Head and Neck Squamous Cell Cancer. Tomography 2019, 5, 161–169. [Google Scholar] [CrossRef]
- El Naqa, I.; Grigsby, P.W.; Apte, A.; Kidd, E.; Donnelly, E.; Khullar, D.; Chaudhari, S.; Yang, D.; Schmitt, M.; Laforest, R.; et al. Exploring feature-based approaches in PET images for predicting cancer treatment outcomes. Pattern Recognit. 2009, 42, 1162–1171. [Google Scholar] [CrossRef]
- Moskowitz, C.S.; Welch, M.L.; Jacobs, M.A.; Kurland, B.F.; Simpson, A.L. Radiomic Analysis: Study Design, Statistical Analysis, and Other Bias Mitigation Strategies. Radiology 2022, 304, 265–273. [Google Scholar] [CrossRef]
- Ugga, L.; Spadarella, G.; Pinto, L.; Cuocolo, R.; Brunetti, A. Meningioma Radiomics: At the Nexus of Imaging, Pathology and Biomolecular Characterization. Cancers 2022, 14, 2605. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer analysis project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Khosravi, B.; Vahdati, S.; Faghani, S.; Nugen, F.; Rassoulinejad-Mousavi, S.M.; Moassefi, M.; Jagtap, J.M.M.; Singh, Y.; Rouzrokh, P.; et al. Mitigating Bias in Radiology Machine Learning: 2. Model Development. Radiol. Artif. Intell. 2022, 4. [Google Scholar] [CrossRef] [PubMed]
- Faghani, S.; Khosravi, B.; Zhang, K.; Moassefi, M.; Jagtap, J.M.; Nugen, F.; Vahdati, S.; Kuanar, S.P.; Rassoulinejad-Mousavi, S.M.; Singh, Y.; et al. Mitigating Bias in Radiology Machine Learning: 3. Performance Metrics. Radiol. Artif. Intell. 2022, 4. [Google Scholar] [CrossRef] [PubMed]
- Rouzrokh, P.; Khosravi, B.; Faghani, S.; Moassefi, M.; Vera Garcia, D.V.; Singh, Y.; Zhang, K.; Conte, G.M.; Erickson, B.J. Mitigating Bias in Radiology Machine Learning: 1. Data Handling. Radiol. Artif. Intell. 2022, 4. [Google Scholar] [CrossRef]
- Willemink, M.J.; Koszek, W.A.; Hardell, C.; Wu, J.; Fleischmann, D.; Harvey, H.; Folio, L.R.; Summers, R.M.; Rubin, D.L.; Lungren, M.P. Preparing Medical Imaging Data for Machine Learning. Radiology 2020, 295, 4–15. [Google Scholar] [CrossRef]
- Eng, J. Sample Size Estimation: How Many Individuals Should Be Studied? Radiology 2003, 227, 309–313. [Google Scholar] [CrossRef]
- Huang, S.-C.; Pareek, A.; Seyyedi, S.; Banerjee, I.; Lungren, M.P. Fusion of medical imaging and electronic health records using deep learning: A systematic review and implementation guidelines. npj Digit. Med. 2020, 3, 136. [Google Scholar] [CrossRef]
- Ying, X. An Overview of Overfitting and its Solutions. J. Phys. Conf. Ser. 2019, 1168, 022022. [Google Scholar] [CrossRef]
- Ayinde, B.O.; Inanc, T.; Zurada, J.M. Regularizing Deep Neural Networks by Enhancing Diversity in Feature Extraction. IEEE Trans. Neural Netw. Learn. Syst. 2019, 30, 2650–2661. [Google Scholar] [CrossRef]
- Akbar, S.; Peikari, M.; Salama, S.; Nofech-Mozes, S.; Martel, A.L. The transition module: A method for preventing overfitting in convolutional neural networks. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2019, 7, 260–265. [Google Scholar] [CrossRef]
- Buda, M.; Maki, A.; Mazurowski, M.A. A systematic study of the class imbalance problem in convolutional neural networks. Neural Netw. 2018, 106, 249–259. [Google Scholar] [CrossRef]
- He, Q.; Li, X.; Kim, D.W.N.; Jia, X.; Gu, X.; Zhen, X.; Zhou, L. Feasibility study of a multi-criteria decision-making based hierarchical model for multi-modality feature and multi-classifier fusion: Applications in medical prognosis prediction. Inf. Fusion 2020, 55, 207–219. [Google Scholar] [CrossRef]
- Erickson, B.J.; Kitamura, F. Magician’s Corner: 9. Performance Metrics for Machine Learning Models. Radiol. Artif. Intell. 2021, 3, e200126. [Google Scholar] [CrossRef]
- Common Limitations of Image Processing Metrics: A Picture Story. Available online: https://arxiv.org/abs/2104.05642 (accessed on 20 December 2022).
- Metrics Reloaded: Pitfalls and Recommendations for Image Analysis Validation. Available online: https://arxiv.org/abs/2206.01653 (accessed on 20 December 2022).
- Sokolova, M.; Japkowicz, N.; Szpakowicz, S. Beyond Accuracy, F-Score and ROC: A Family of Discriminant Measures for Performance Evaluation. In AI 2006: Advances in Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 2006; pp. 1015–1021. [Google Scholar]
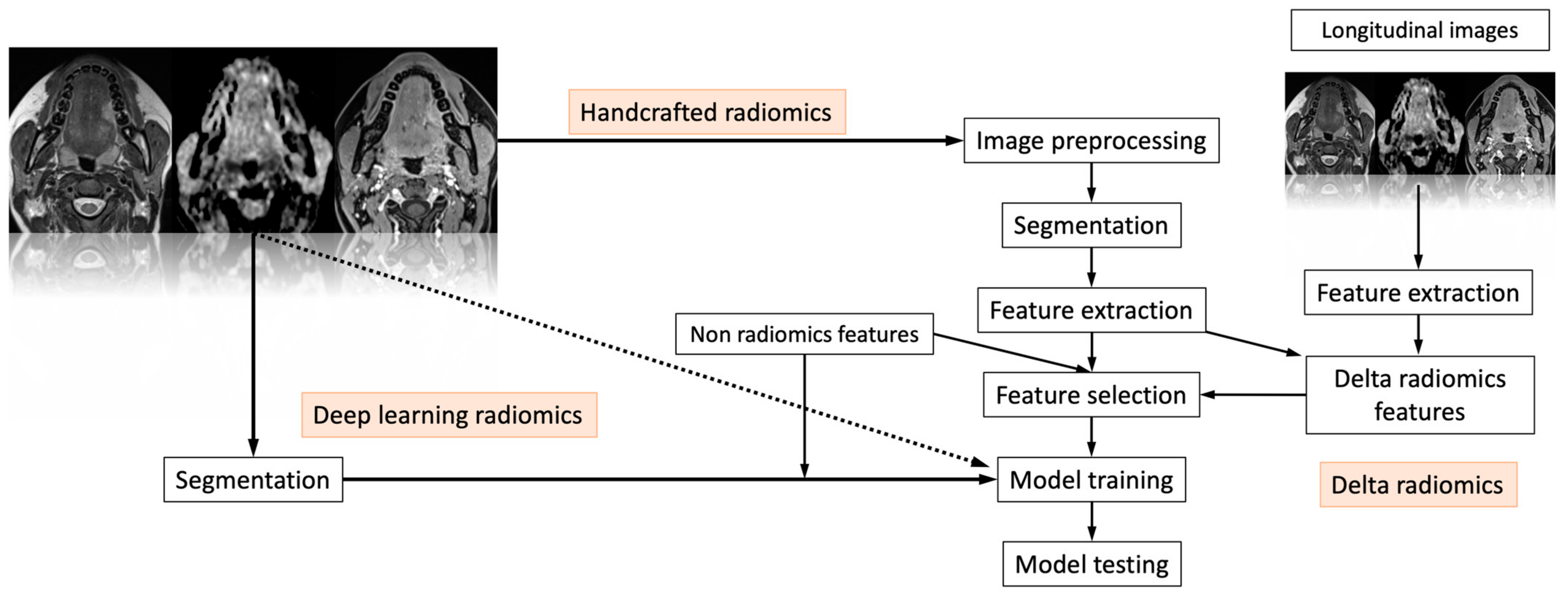
| Article | Number of Patients | Subsite | Imaging | Analyzed Endpoint | Statistical Findings | Conclusion |
|---|---|---|---|---|---|---|
| Segmentation | ||||||
| C. Parmar et al. Robust Radiomics Feature Quantification Using Semiautomatic Volumetric Segmentation [15] | 20 | Lung NSCLC | CT | Segmentation | 56 3D radiomic features, quantifying phenotypic differences based on tumor intensity, shape and texture | Radiomic features extracted from 3D slicer segmentations had significantly higher reproducibility, were more robust and overlapping with the feature ranges extracted from manual contouring. |
| Kuhl, C.K.; Truhn, D. The Long Route to Standardized Radiomics: Unraveling the Knot from the End [16] | 51 | Soft-tissue sarcoma | CT, MRI and PET | Segmentation | 169 preselected features | 167 features demonstrated good to excellent reproducibility and 71 were reproducible after a comprehensive inter- and intra-CT image acquisition analysis. |
| Gitto, S. et al. Effects of Interobserver Variability on 2D and 3D CT- and MRI-Based Texture Feature Reproducibility of Cartilaginous Bone Tumors [17] | 30 | Bone tumors | CT and MRI | Segmentation | 783 and 1132 features were extracted | The features extracted were reproducible. 3D and 2D MRI-based texture analyses provided similar rates of stable features. |
| Huan Yu et al. Coregistered FDG PET/CT-Based Textural Characterization of Head and Neck Cancer for Radiation Treatment Planning [18] | 40 | Head and neck cancer and lung cancer | F-FDG PET and CT | Segmentation | Texture features | Gray-tone difference matrices (NGTDM) (PET coarseness, PET contrast and CT coarseness) provided good discrimination performance. |
| Yu, H. et al. Automated Radiation Targeting in Head-and-Neck Cancer Using Region-Based Texture Analysis of PET and CT Images [19] | 10 | Head and neck cancer | F-FDG PET and CT | Segmentation | Co-registered multimodality pattern analysis segmentation system (COMPASS) | Tumor delineation was similar to those of the radiation oncologists. |
| Characterization | ||||||
| Buch, K. et al. Using Texture Analysis to Determine Human Papillomavirus Status of Oropharyngeal Squamous Cell Carcinomas on CT [20] | 40 | Oropharyngeal carcinoma | CT | Characterization | A t-test evaluated differences in 42 texture features between HPV-positive and -negative carcinoma | There are statistically significant differences in some texture features between human-papillomavirus-positive and human-papillomavirus-negative oropharyngeal tumors. |
| Fujita, A et al. Difference Between HPV-Positive and HPV-Negative Non-Oropharyngeal Head and Neck Cancer [21] | 46 | Oral cavity, larynx and hypopharynx cancer | CT | Characterization | Texture analysis program extracted 42 texture features | 16 texture parameters showed significant differences in relation to HPV status. |
| Vallieres, M. et al. FDG-PET Image-Derived Features Can Determine HPV Status in Head-and-Neck Cancer [22] | 67 | Hypopharynx | FDG-PET | Characterization | Six texture features, two SUV measures and three shape features were extracted, and logistic regression and support vector machine were performed | It is possible to predict HPV status and treatment failure in HNSCC using a combination of FDG-PET texture and morphological features. |
| Payabvash, S. et al. Differentiation of lymphomatous, metastatic, and non-malignant lymphadenopathy in the neck with quantitative diffusion-weighted imaging: Systematic review and meta-analysis [23] | Review (27 studies and 1165 patients) | Neck lymph nodes | MRI (Diffusion Weighted Imaging, DWI) | Characterization | Random-effects models, pooled diagnostic odds ratio (DOR), summary receiver operating characteristics (sROC), area under the curve (AUC) were determined | Quantitative valuation of ADC can help with differentiation of cervical lymph nodes. Lower ADC values are linked to malignancy and HPV positive status. |
| Payabvash, S. et al. Quantitative diffusion magnetic resonance imaging for prediction of human papillomavirus status in head and neck squamous-cell carcinoma: A systematic review and meta-analysis [24] | Review (5 studies and 264 patients) | HNSCC | MRI (DWI) | Characterization | Meta-analysis | HPV-positive HNSCC primary lesions have lower ADC. |
| Marzi, S.et al. Multifactorial Model Based on DWI-Radiomics to Determine HPV Status in Oropharyngeal Squamous Cell Carcinoma [25] | 144 | Oropharyngeal carcinoma | MRI (DWI) | Characterization | Different families of machine-learning (ML) algorithms and five-fold cross-validation | DWI-based radiomics can help in differentiating HPV-positive from HPV-negative patients. |
| Suh, C.H. et al. Oropharyngeal squamous cell carcinoma: Radiomic machine-learning classifiers from multiparametric MR images for determination of HPV infection status [26] | 60 | Oropharyngeal carcinoma | MRI | Characterization | 1618 quantitative features extraction, features selection, three machine-learning classifiers (logistic regression, random forest and XG boost) | The highest diagnostic accuracies were achieved when using all sequences, and the difference was significant only when the combination did not include the ADC map. |
| Sohn, B. et al. Machine Learning Based Radiomic HPV Phenotyping of Oropharyngeal SCC: A Feasibility Study Using MRI [27] | 62 | Oropharyngeal carcinoma | MRI | Characterization | 170 radiomic features | Six radiomic features with strong association with HPV status of SCC were selected using least absolute shrinkage and selection operator (LASSO). |
| Aerts, H.J.W.L. et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach [28] | 1019 | Lung or head-and-neck cancer | CT | Characterization | 440 features | Some radiomic features had prognostic power associated with underlying gene expression patterns. |
| Zwirner, K. et al. Radiogenomics in head and neck cancer: Correlation of radiomic heterogeneity and somatic mutations in TP53, FAT1 and KMT2D [29] | 20 | HNSCC | CT | Characterization | Radiomic features and genetic analysis | Somatic mutations in FAT1 and smaller primary tumor volumes were associated with reduced radiomic intra-tumor heterogeneity. |
| Huang, C. et al. Development and validation of radiomic signatures of head and neck squamous cell carcinoma molecular features and subtypes [30] | 113 | HNSCC | CT | Characterization | 540 features, logistic regression, AUC | Quantitative image features can distinguish several molecular phenotypes. |
| Zhu, Y. et al. Imaging-Genomic Study of Head and Neck Squamous Cell Carcinoma: Associations Between Radiomic Phenotypes and Genomic Mechanisms via Integration of The Cancer Genome Atlas and The Cancer Imaging Archive [31] | 126 | HNSCC | CT | Characterization | Linear regression and gene set enrichment analysis | Associations between genomic features and radiomic features |
| Chen, R.-Y. et al.; Associations of Tumor PD-1 Ligands, Immunohistochemical Studies, and Textural Features in 18F-FDG PET in Squamous Cell Carcinoma of the Head and Neck [32] | 53 | HNSCC | 18F-FDG PET | Characterization | Associations of tumor PD-1 ligands, immunohistochemical studies and textural features | PD-L1 expressions were positively correlated with Ki-67 c-Met and p16. |
| Brown, A.M. et al.; Multi-institutional validation of a novel textural analysis tool for preoperative stratification of suspected thyroid tumors on diffusion-weighted MRI [33] | Thyroid tumors | MRI (DWI) | Characterization | 21 textural features | Textural analysis (TA) could characterize thyroid nodules using diffusion-weighted MRI (DW-MRI). | |
| Jansen, J.F. Texture analysis on parametric maps derived from dynamic contrast-enhanced magnetic resonance imaging in head and neck cancer [34] | 19 | HNSCC | Dynamic contrast enhanced (DCE)-MRI | Characterization | Image texture analysis was employed on maps of Ktrans and ve, generating two texture measures | Chemoradiation treatment in HNSCC significantly reduced the heterogeneity of tumors. |
| Kim, S. et al. Prediction of Response to Chemoradiation Therapy in Squamous Cell Carcinomas of the Head and Neck Using Dynamic Contrast-Enhanced MR Imaging [35] | 33 | HNSCC | DCE-MRI | Characterization | The data were analyzed by using SSM for estimation of Ktrans, ve and τi | Pretreatment DCE-MR imaging can potentially be used for prediction of response to chemoradiation therapy. |
| Shukla-Dave et al. Dynamic Contrast-Enhanced Magnetic Resonance Imaging as a Predictor of Outcome in Head-and-Neck Squamous Cell Carcinoma Patients with Nodal Metastases [36] | 64 | HNSCC | DCE-MRI | Characterization | DCE-MRI data were analyzed using the Tofts model | Important role of pretreatment DCE-MRI parameter K{sup trans} as a predictor of outcome |
| Dang, M. et al.; MRI Texture Analysis Predicts p53 Status in Head and Neck Squamous Cell Carcinoma [37] | 16 | HNSCC | MRI | Characterization | Texture analysis | MR imaging texture analysis predicted p53 status. |
| Staging | ||||||
| Wang, F. et al. Radiomic Nomogram Improves Preoperative T Category Accuracy in Locally Advanced Laryngeal Carcinoma [38] | 211 | Laryngeal carcinoma | CT | Staging | 1390 radiomic features extracted and analyzed | Eight features were found associated with preoperative T category. |
| Ren, J. et al.; Magnetic resonance imaging based radiomics signature for the preoperative discrimination of stage I-II and III-IV head and neck squamous cell carcinoma [39] | 127 | HNSCC | MRI | Staging | Radiomics signatures were constructed with least absolute shrinkage and selection operator (LASSO) logistic regression and analyzed | Radiomics signature based on MRI could discriminate stage I–II from stage III–IV HNSCC. |
| Romeo, V. et al. Prediction of Tumor Grade and Nodal Status in Oropharyngeal and Oral Cavity Squamous-cell Carcinoma Using a Radiomic Approach [40] | 40 | Oropharyngeal oral cavity carcinoma | CT | Staging | TA features | Tumor grade (TG) and nodal status (NS) could be predicted. |
| Wang, H. et al.; Machine learning-based multiparametric MRI radiomics for predicting the aggressiveness of papillary thyroid carcinoma [41] | 120 | Papillary thyroid carcinoma | MRI | Staging | 1393 features | Aggressive and non-aggressive PTC could be distinguished preoperatively through machine-learning-based multiparametric MR imaging radiomics. |
| Article | Number of Patients | Subsite | Imaging | Analyzed Endpoint | Statistical Findings | Conclusion |
|---|---|---|---|---|---|---|
| Treatment | ||||||
| Fave X et al. Delta-radiomics features for the prediction of patient outcomes in non-small cell lung cancer [42] | 107 | NSCC (lung) | CT | Overall survival, distant metastases and local recurrence | Multivariate models were built for overall survival, distant metastases and local recurrence using only clinical factors, clinical factors combined with pretreatment radiomics features, and a combination of clinical factors, pretreatment radiomics features and delta radiomics features | For overall survival and distant metastases, pretreatment compactness improved the c-index. For local recurrence, pretreatment imaging features were not prognostic, while texture strength measured at the end of treatment significantly stratified high- and low-risk patients. |
| Jansen JF et al. Texture analysis on parametric maps derived from dynamic contrast-enhanced magnetic resonance imaging in head and neck cancer [34] | 19 | HNSCC | CT and MRI | Prediction of treatment response | Image texture analysis was employed on maps of Ktrans and Ve, generating two texture measures: energy (E) and homogeneity | Chemoradiation treatment in HNSCC significantly reduced the heterogeneity of tumors. |
| Brown AM et al. Multi-institutional validation of a novel textural analysis tool for preoperative stratification of suspected thyroid tumors on diffusion-weighted MRI [33] | 44 | Thyroid cancer | MRI | Preoperative stratification | Apparent diffusion coefficients (ADCs) were obtained from regions of interest (ROIs) defined on thyroid nodules. TA, linear discriminant analysis (LDA) and feature reduction were also performed using the 21 MaZda-generated texture parameters that best distinguished benign and malignant ROIs | TA classified thyroid nodules with high sensitivity and specificity. |
| Zhang B et al. Radiomics Features of Multiparametric MRI as Novel Prognostic Factors in Advanced Nasopharyngeal Carcinoma [43] | 118 | Nasopharynx carcinoma | MRI | Progression-free survival (PFS) | A total of 970 radiomics features were extracted from T2-weighted (T2-w) and contrast-enhanced T1-weighted (CET1-w) MRI. Least absolute shrinkage and selection operator (LASSO) regression was applied to select features for progression-free survival (PFS) nomograms | Multiparametric MRI-based radiomics nomograms provided improved prognostic ability in advanced NPC. |
| Wang, G et al. Pretreatment MR imaging radiomics signatures for response prediction to induction chemotherapy in patients with nasopharyngeal carcinoma [44] | 120 | Nasopharynx carcinoma | MRI | Pretreatment prediction of early response to induction chemotherapy | Radiomics signatures were obtained with the least absolute shrinkage and selection operator method (LASSO) logistic regression model | Pretreatment morphological MR imaging radiomics signatures can predict early response to induction chemotherapy in patients with NPC. |
| Liu, J et al. Use of texture analysis based on contrast-enhanced MRI to predict treatment response to chemoradiotherapy in nasopharyngeal carcinoma [45] | 53 | Nasopharynx carcinoma | MRI | Pretreatment prediction of response to chemotherapy | Quantitative image parameters were extracted and statistically filtered to identify a subset of reproducible and non-redundant parameters, which were used to construct the predictive model. Internal validation was performed using stratified 10-fold cross-validation in the training set, and external validation was performed in the testing set. McNemar’s test was used to test the statistical difference between the performances of the extracted parameters in predicting the treatment response | Texture analysis based on T1 W, T2 W and DWI could act as imaging biomarkers of tumor response to chemoradiotherapy in NPC patients. |
| Romeo, V. et al. Prediction of Tumor Grade and Nodal Status in Oropharyngeal and Oral Cavity Squamous-cell Carcinoma Using a Radiomic Approach [40] | 40 | Oropharyngeal (OP) and oral cavity (OC) squamous-cell carcinoma (SCC) | CT | Prediction of tumor grade (TG) and nodal status (NS) | CT images were post-processed to extract TA features from primary tumor lesions. A feature selection method and different ML algorithms were applied to find the most accurate subset of features to predict TG and NS | A radiomic ML approach applied to PTLs was able to predict TG and NS in patients with OC and OP SCC. |
| Hawkins, P.G. et al. Sparing all salivary glands with IMRT for head and neck cancer: Longitudinal study of patient-reported xerostomia and head-and-neck quality of life [46] | 252 | HNSCC | Radiation Therapy | Prediction of xerostomia | Longitudinal regression was used to assess the relationship between questionnaire scores and mean bilateral parotid gland (bPG), contralateral submandibular gland (cSMG) and oral cavity (OC) doses. Marginal R2 and Akaike information criterion (AIC) were used for model evaluation | Reducing doses to all salivary glands maximized PROMs. A cSMG dose constraint of ≤39Gy did not increase failure risk. |
| Sheikh, K. et al. Predicting acute radiation induced xerostomia in head and neck Cancer using MR and CT Radiomics of parotid and submandibular glands [47] | 266 | HNSCC | CT and MRI | Prediction of xerostomia | CT and MR images were registered, on which glands were contoured. Image features were extracted for glands relative to the location of the primary tumor. Dose-volume-histogram (DVH) parameters were also acquired. Features were preselected based on Spearman correlation | Baseline CT and MR image features may reflect baseline salivary gland function and potential risk of radiation injury. |
| Liu, Y. et al. Early prediction of acute xerostomia during radiation therapy for nasopharyngeal cancer based on delta radiomics from CT images [48] | 35 | Nasopharynx cancer | CT | Prediction of xerostomia | RidgeCV and recursive feature elimination (RFE) were used for feature selection, while linear regression was used for predicting SA30F | Investigating radiation-induced changes of computed tomography (CT) radiomics in parotid glands (PGs) and saliva amount (SA) can predict acute xerostomia during the RT for nasopharyngeal cancer (NPC). |
| van Dijk, L.V. et al. Parotid gland fat related Magnetic Resonance image biomarkers improve prediction of late radiation-induced xerostomia [49] | 68 | HNSCC | MRI | Prediction of xerostomia | The performance of the resulting multivariable logistic regression models after bootstrapped forward selection was compared with that of the logistic regression reference model | Pretreatment MR-imaging biomarkers were associated with radiation-induced xerostomia, which supported the hypothesis that the amount of predisposed fat within the parotid glands is associated with Xer12m. In addition, xerostomia prediction was improved with MR-IBMs compared to the reference model. |
| van Dijk, L.V. et al. CT image biomarkers to improve patient-specific prediction of radiation-induced xerostomia and sticky saliva [50] | 249 | HNSCC | CT | Prediction of xerostomia | The potential IBMs represent geometric, CT intensity and textural characteristics of the parotid and submandibular glands. LASSO regularization was used to create multivariable logistic regression models, which were internally validated by bootstrapping | Prediction of XER12m and STIC12m was improved by including IBMs representing heterogeneity and density of the salivary glands, respectively. These IBMs could guide additional research into the patient-specific response of healthy tissue to radiation dose. |
| Thor, M. et al. A magnetic resonance imaging-based approach to quantify radiation-induced normal tissue injuries applied to trismus in head and neck cancer [51] | 10 | HNSCC | MRI | Prediction of trismus | Univariate logistic regression with bootstrapping (1000 populations) was applied to compare the muscle mean dose and textures between cases and controls (ipsilateral muscles); candidate predictors were suggested with an average p ≤ 0.20 across all bootstrap populations | TA identified the critical muscle(s) for radiation-induced trismus. |
| Abdollahi, H. et al. Cochlea CT radiomics predicts chemoradiotherapy induced sensorineural hearing loss in head and neck cancer patients: A machine learning and multi-variable modelling study [52] | 47 | HNSCC | CT | Prediction of sensorineural hearing loss | Different ML algorithms and LASSO logistic regression were implemented on radiomic features for feature selection, classification and prediction | A combination of radiomic features with clinical and dosimetric variables can model radiotherapy outcome, such as sensorineural hearing loss. |
| Metastases and Recurrence | ||||||
| Kann, B.H. et al. Pretreatment Identification of Head and Neck Cancer Nodal Metastasis and Extranodal Extension Using Deep Learning Neural Networks [53] | 270 | HNSCC | CT | Identification of metastasis (nodal metastasis and tumor extranodal extension) | Three-dimensional convolutional neural network using a dataset of 2,875 CT-segmented lymph node samples with correlating pathology labels, cross-validated and tested on a blinded test set | The model has the potential for clinical decision making. |
| Kann, B.H. et al. Multi-Institutional Validation of Deep Learning for Pretreatment Identification of Extranodal Extension in Head and Neck Squamous Cell Carcinoma [54] | 200 lymph nodes | HNSCC | CT | Identification of metastasis (extranodal extension ENE) | Deep-learning algorithm performance | Deep learning successfully identified ENE in pretreatment imaging. |
| Zhang, L. et al. Development and validation of a magnetic resonance imaging-based model for the prediction of distant metastasis before initial treatment of nasopharyngeal carcinoma: A retrospective cohort study [55] | 176 | Nasopharyngeal carcinoma | MRI | Identification of metastasis | Features of primary tumors were extracted; then, minimum redundancy–maximum relevance, LASSO and selection operator algorithms were performed. To select the strongest features, a logistic model for DM prediction was built | The model could be used as a prognostic model and can improve treatment decisions. |
| Bogowicz, M. et al. Computed Tomography Radiomics Predicts HPV Status and Local Tumor Control After Definitive Radiochemotherapy in Head and Neck Squamous Cell Carcinoma [56] | 149 | HNSCC | CT | Prediction of local tumor control (LC) after radiochemotherapy and HPV status | 317 CT radiomic features were calculated. Cox and logistic regression models were built. The quality of the models was assessed using the concordance index (CI) for modeling of LC and receiver operating characteristics area under the curve (AUC) | Heterogeneity of HNSCC tumor density is associated with LC after radiochemotherapy and HPV status. |
| Li, S. et al. Use of Radiomics Combined With Machine Learning Method in the Recurrence Patterns After Intensity-Modulated Radiotherapy for Nasopharyngeal Carcinoma: A Preliminary Study [57] | 306 | Nasopharyngeal carcinoma | MRI, PET | Prediction of recurrence and radio resistance | 1117 radiomic features were quantified from the tumor region intraclass correlation coefficients (ICC), and Pearson correlation coefficient (PCC) was calculated to identify the influential feature subset. Kruskal–Wallis test and receiver operating characteristic (ROC) analysis were employed to assess the ability of each feature in NPC-in-field recurrences prediction. Artificial neural network (ANN), k-nearest neighbor (KNN) and support vector machine (SVM) models were trained and validated by using stratified 10-fold cross-validation | In-field and high-dose region relapses were the main recurrence patterns, which may be due to the radioresistance. After integration with the clinical workflow, radiomic analyses can serve as imaging biomarkers to facilitate early salvage for NPC patients who are at risk of in-field recurrence. |
| Kuno, H. et al. CT Texture Analysis Potentially Predicts Local Failure in Head and Neck Squamous Cell Carcinoma Treated with Chemoradiotherapy [58] | 62 | HNSCC | CT | Prediction of local failure | Texture analysis | Independent primary tumor CT texture analysis features are linked to local failure after chemoradiotherapy in patients with HNSCC. |
| MDACC Head. Investigation of radiomic signatures for local recurrence using primary tumor texture analysis in oropharyngeal head and neck cancer patients [59] | 465 | Oropharyngeal cancer | CT, MRI, PET | Prediction of local recurrence | Two texture analysis features from pre-therapy imaging were extracted, and the resultant groups were analyzed | There is robust discrimination of recurrence probability and local control rate (LCR) differences between “favorable” and “unfavorable” clusters. |
| Zhang, L. et al. Radiomic Nomogram: Pretreatment Evaluation of Local Recurrence in Nasopharyngeal Carcinoma based on MR Imaging [60] | 140 | Nasopharyngeal carcinoma | MRI | Prediction of local recurrence | 970 radiomic features were extracted. Univariate and multivariate analyses were used. Eight CET1-w image features and seven T2-w image features were selected to build a Cox proportional hazard model in the training cohort | This study demonstrates that MR-imaging-based radiomics can be used to categorize patients into low- and high-risk groups. |
| Survival | ||||||
| Shen, H. et al. Predicting Progression-Free Survival Using MRI-Based Radiomics for patients with nonmetastatic Naso-pharyngeal Carcinoma [61] | 327 | Nasopharynx carcinoma | MRI | Prediction of progression-free survival (PFS) | The clinical and MRI data were collected. The least absolute shrinkage selection operator (LASSO) and recursive feature elimination (RFE) were used to select radiomic features. Five models were constructed. The prognostic performances of these models were evaluated by Harrell’s concordance index (C-index). The Kaplan–Meier method was applied for the survival analysis | The model incorporating radiomics, overall stage and Epstein–Barr virus DNA showed better performance in predicting PFS in non-metastatic NPC patients. |
| Yuan, Y. et al. MRI-based radiomic signature as predictive marker for patients with head and neck squamous cell carcinoma [62] | 85 | HNSCC | MRI | Prediction of prognosis | LASSO Cox regression model was used to select the most useful prognostic features with their coefficients, upon which a radiomic signature was generated | MRI-based radiomic signature is an independent prognostic factor for HNSCC patients. |
| Parmar, C. et al. Radiomic Machine-Learning Classifiers for Prognostic Biomarkers of Head and Neck Cancer [63] | 196 | HNSCC | CT | Prediction of overall survival | A total of 440 radiomic features were extracted from the segmented tumor regions in CT images. Feature selection and classification methods were compared using an unbiased evaluation framework | The study identified prognostic and reliable machine-learning methods for the prediction of overall survival of head and neck cancer patients. |
| Agarwal, J.P. et al. Tumor radiomic features complement clinico-radiological factors in predicting long-term local control and laryngectomy free survival in locally advanced laryngo-pharyngeal cancers [64] | 60 | Laryngopharynx cancer | CT | Prediction of long-term local control and laryngectomy-free survival (LFS) | The ability of texture analysis to predict LFS or local control was determined using Kaplan–Meier analysis and multivariate Cox model | Medium texture entropy is a predictor for inferior local control and laryngectomy-free survival in locally advanced laryngo-pharyngeal cancer, and this can complement clinico-radiological factors in predicting the prognosis of these tumors. |
| Liu, Z. et al. Radiomics-based prediction of survival in patients with head and neck squamous cell carcinoma based on pre- and post-treatment 18F-PET/CT [65] | 171 | HNSCC | PET-CT | Prediction of survival | Receiver operating characteristic (ROC) curves and decision curves were used to compare the predictions of ML models with those of a model incorporating only clinicopathological features | Combining clinicopathological characteristics with radiomics features of pre-treatment PET/CT or post-treatment PET/CT assessment of primary tumor sites as positive or negative may substantially improve the prediction of overall survival and disease-free survival of HNSCC patients. |
| Zhai, T.-T. et al. The prognostic value of CT-based image-biomarkers for head and neck cancer patients treated with definitive (chemo-)radiation [66] | 444 | HNSCC | CT | Prediction of local control (LC), regional control (RC), distant-metastasis-free survival (DMFS) and disease-free survival (DFS) | Models were created from multivariable Cox proportional hazard analyses based on clinical features and IBMs for LC, RC, DMFS and DFS | For prediction of HNC treatment outcomes, image biomarkers performed as well or slightly better than clinical variables. |
| Leijenaar, R.T.H. et al. External validation of a prognostic CT-based radiomic signature in oropharyngeal squamous cell carcinoma [67] | 542 | Oropharyngeal carcinoma | CT | Prognosis prediction | Signature model was tested and fit in a Cox regression and assessed model discrimination with Harrell’s c-index. Kaplan–Meier survival curves between high and low signature predictions were compared with a log-rank test | Signature had significant prognostic power, regardless of whether patients with CT artifacts were included. |
| Liu, J. et al. Use of texture analysis based on contrast-enhanced MRI to predict treatment response to chemoradiotherapy in nasopharyngeal carcinoma [45] | 53 | Nasopharyngeal carcinoma | MRI | Treatment prediction | Quantitative image parameters were extracted and statistically filtered to identify a subset of reproducible and non-redundant parameters, which were used to construct the predictive model. McNemar’s test was used to test the statistical difference in predicting the treatment response | Texture analyses based on T1 W, T2 W and DWI could act as imaging biomarkers of tumor response to chemoradiotherapy in NPC patients and serve as a new radiological analysis tool for treatment prediction. |
| Bogowicz, M. et al. Perfusion CT radiomics as potential prognostic biomarker in head and neck squamous cell carcinoma [68] | 45 | HNSCC | CT perfusion (CTP) | Prediction of local tumor control | Each feature was assigned to a principal component group based on feature–principal component correlation. Univariate Cox regression analysis was used to define the best prognostic feature in each group | CTP radiomics is a prognostic factor for local tumor control after definitive radiochemotherapy. |
| Zhang, H. et al. Locally Advanced Squamous Cell Carcinoma of the Head and Neck: CT Texture and Histogram Analysis Allow Independent Prediction of Overall Survival in Patients Treated with Induction Chemotherapy [69] | 72 | HNSCC | CT | Prediction of overall survival | CT texture and histogram analyses of primary mass on pretherapy CT images were performed by using TexRAD software before and after application of spatial filters at different anatomic scales, ranging from fine detail to coarse features. Cox proportional hazards models were used to examine the association between overall survival and the baseline CT imaging measurements and clinical variables | Independent of tumor size, N stage and other clinical variables, primary mass CT texture and histogram analysis parameters were associated with overall survival in patients with locally advanced squamous cell carcinoma of the head and neck who were treated with induction TPF. |
| Mao, J. et al. Predictive value of pretreatment MRI texture analysis in patients with primary nasopharyngeal carcinoma [70] | 79 | Nasopharyngeal carcinoma | MRI | Prediction of progression-free survival (PFS) | The Cox proportional hazards model was used to determine the association of texture features, tumor volume and the tumor node metastasis (TNM) stage with PFS. Survival curves were plotted using the Kaplan–Meier method. The prognostic performance was evaluated with the receiver operating characteristic (ROC) analyses and C-index | A texture parameter of pretreatment CE-T1WI-based uniformity improved the prediction of PFS in NPC patients. |
| Cheng, N.-M. et al. Textural Features of Pretreatment 18 F-FDG PET/CT Images: Prognostic Significance in Patients with Advanced T-Stage Oropharyngeal Squamous Cell Carcinoma [71] | 70 | Oropharyngeal carcinoma | PET-CT | Prediction of prognosis | Uniformity extracted from the normalized gray-level co-occurrence matrix represents an independent prognostic predictor in patients with advanced T-stage OPSCC | Uniformity extracted from the normalized gray-level co-occurrence matrix represents an independent prognostic predictor in patients with advanced T-stage OPSCC. |
| Park, V.Y. et al. Association Between Radiomics Signature and Disease-Free Survival in Conventional Papillary Thyroid Carcinoma [72] | 768 | Thyroid carcinoma | Ultrasound | Identification of biomarkers for risk stratification | A radiomics signature (Rad-score) was generated by using the least absolute shrinkage and selection operator (LASSO) method in Cox regression | Radiomics features from pretreatment US may be potential imaging biomarkers for risk stratification in patients with conventional papillary carcinoma. |
| Zdilar, L. et al. Evaluating the Effect of Right-Censored End Point Transformation for Radiomic Feature Selection of Data From Patients With Oropharyngeal Cancer [73] | 529 | Oropharyngeal carcinoma | - | Prediction of overall survival (OS) and relapse-free survival (RFS) | Radiomic signatures combined with clinical variables were used for risk prediction. Three metrics for accuracy and calibration were used to evaluate eight feature selectors and six predictive models | Random regression forest and random survival forest performed best for OS and RFS, respectively. |
| Zhuo, E.-H. et al. Radiomics on multi-modalities MR sequences can subtype patients with non-metastatic nasopharyngeal carcinoma (NPC) into distinct survival subgroups [74] | 658 | Nasopharyngeal carcinoma | MRI | Revelation of distinct survival subtypes | Each patient in the validation cohort was assigned to the risk model using the trained classifier. Harrell’s concordance index (C-index) was used to measure the prognosis performance, and differences between subgroups were compared using the log-rank test | Quantitative multi-modalities MRI image phenotypes revealed distinct survival subtypes. |
| Haider, S.P. et al. Potential Added Value of PET/CT Radiomics for Survival Prognostication beyond AJCC 8th Edition Staging in Oropharyngeal Squamous Cell Carcinoma [75] | 311 | Oropharyngeal carcinoma | CT/ PET | Definition of staging scheme for survival prognostication and risk stratification | Harrell’s C-index quantified survival model performance; risk stratification was evaluated in Kaplan–Meier analysis | Radiomics imaging features extracted from pretreatment PET/CT may provide complementary information to the current American Joint Committee on Cancer staging scheme for survival prognostication and risk stratification of HPV-associated OPSCC. |
| Leijenaar, R.T. et al. Development and validation of a radiomic signature to predict HPV (p16) status from standard CT imaging: A multicenter study [76] | 778 | Oropharyngeal carcinoma | CT | Identification of the HPV status (p16) of OPSCC and prognosis | Multivariable modeling was performed using least absolute shrinkage and selection operator. Kaplan–Meier survival analysis was performed to compare HPV status based on p16 and radiomic model predictions | Radiomics has the potential to identify clinically relevant molecular phenotypes influencing the prognosis. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortora, M.; Gemini, L.; Scaravilli, A.; Ugga, L.; Ponsiglione, A.; Stanzione, A.; D’Arco, F.; D’Anna, G.; Cuocolo, R. Radiomics Applications in Head and Neck Tumor Imaging: A Narrative Review. Cancers 2023, 15, 1174. https://doi.org/10.3390/cancers15041174
Tortora M, Gemini L, Scaravilli A, Ugga L, Ponsiglione A, Stanzione A, D’Arco F, D’Anna G, Cuocolo R. Radiomics Applications in Head and Neck Tumor Imaging: A Narrative Review. Cancers. 2023; 15(4):1174. https://doi.org/10.3390/cancers15041174
Chicago/Turabian StyleTortora, Mario, Laura Gemini, Alessandra Scaravilli, Lorenzo Ugga, Andrea Ponsiglione, Arnaldo Stanzione, Felice D’Arco, Gennaro D’Anna, and Renato Cuocolo. 2023. "Radiomics Applications in Head and Neck Tumor Imaging: A Narrative Review" Cancers 15, no. 4: 1174. https://doi.org/10.3390/cancers15041174
APA StyleTortora, M., Gemini, L., Scaravilli, A., Ugga, L., Ponsiglione, A., Stanzione, A., D’Arco, F., D’Anna, G., & Cuocolo, R. (2023). Radiomics Applications in Head and Neck Tumor Imaging: A Narrative Review. Cancers, 15(4), 1174. https://doi.org/10.3390/cancers15041174









