Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/iBRA-NET, NCT 05559411)
Abstract
Simple Summary
Abstract
1. Introduction
2. Current Evidence of Different Localization Techniques
2.1. Wire-Guided Localization (WGL)
2.2. Radioactive Localization
2.3. Magnetic and Paramagnetic Localization
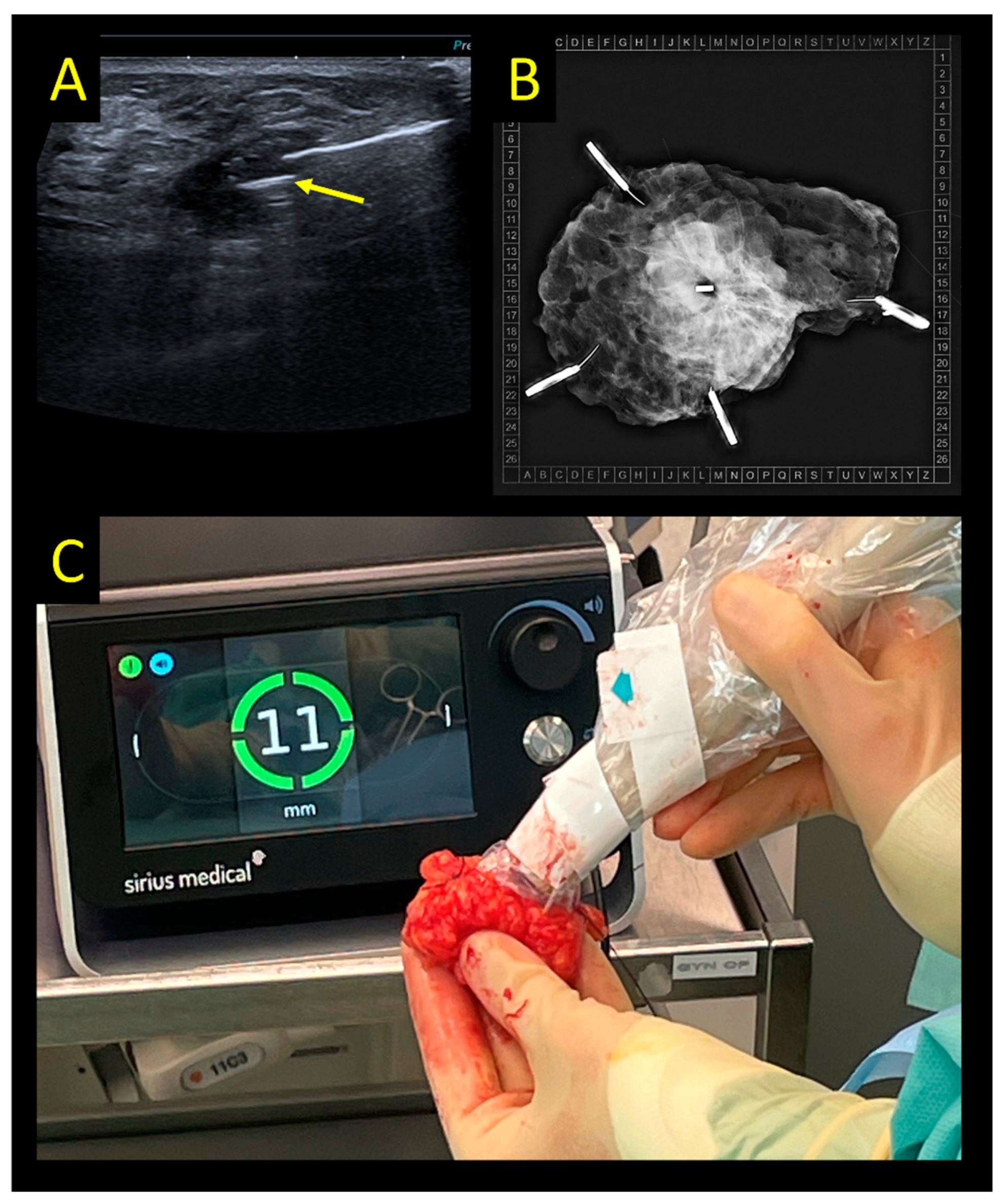
2.4. Sirius Pintuition
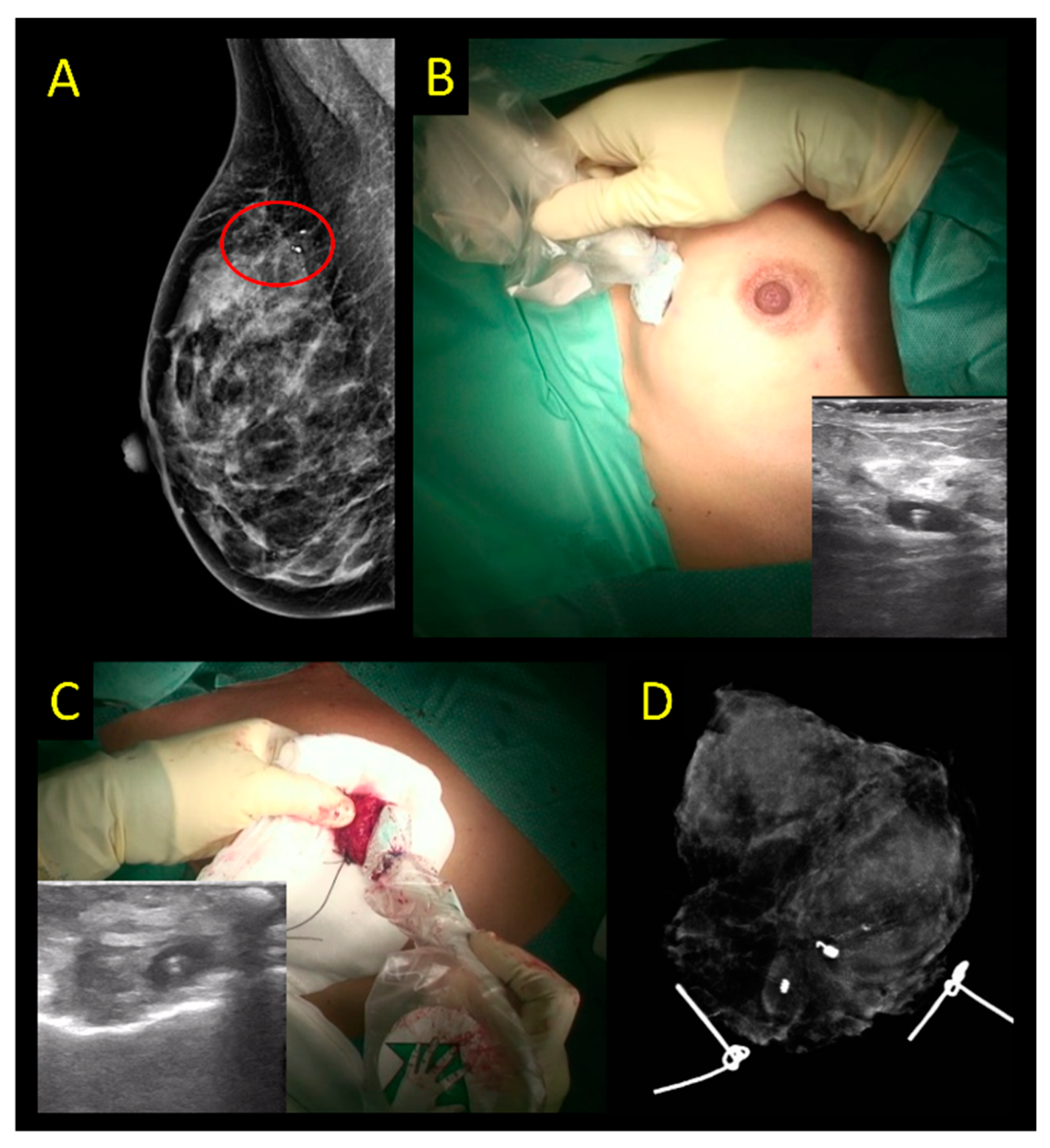
2.5. Radar Reflector Localization
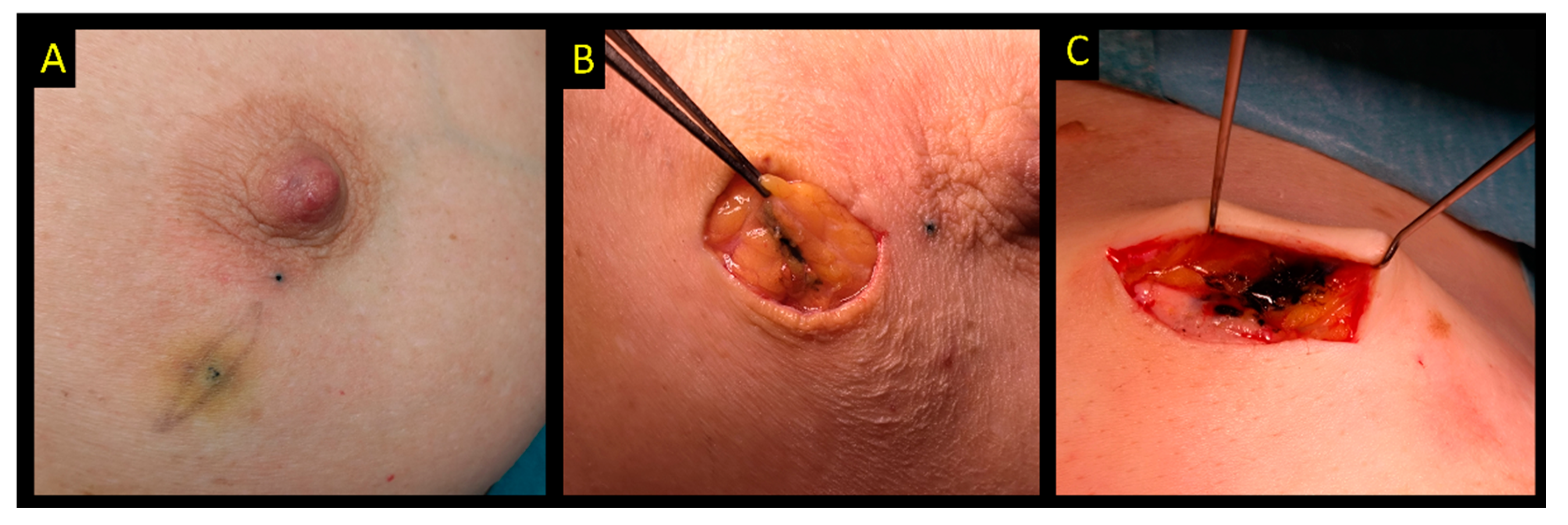
2.6. Radiofrequency Identification Tags
2.7. Intraoperative Ultrasound
2.8. Carbon Suspension
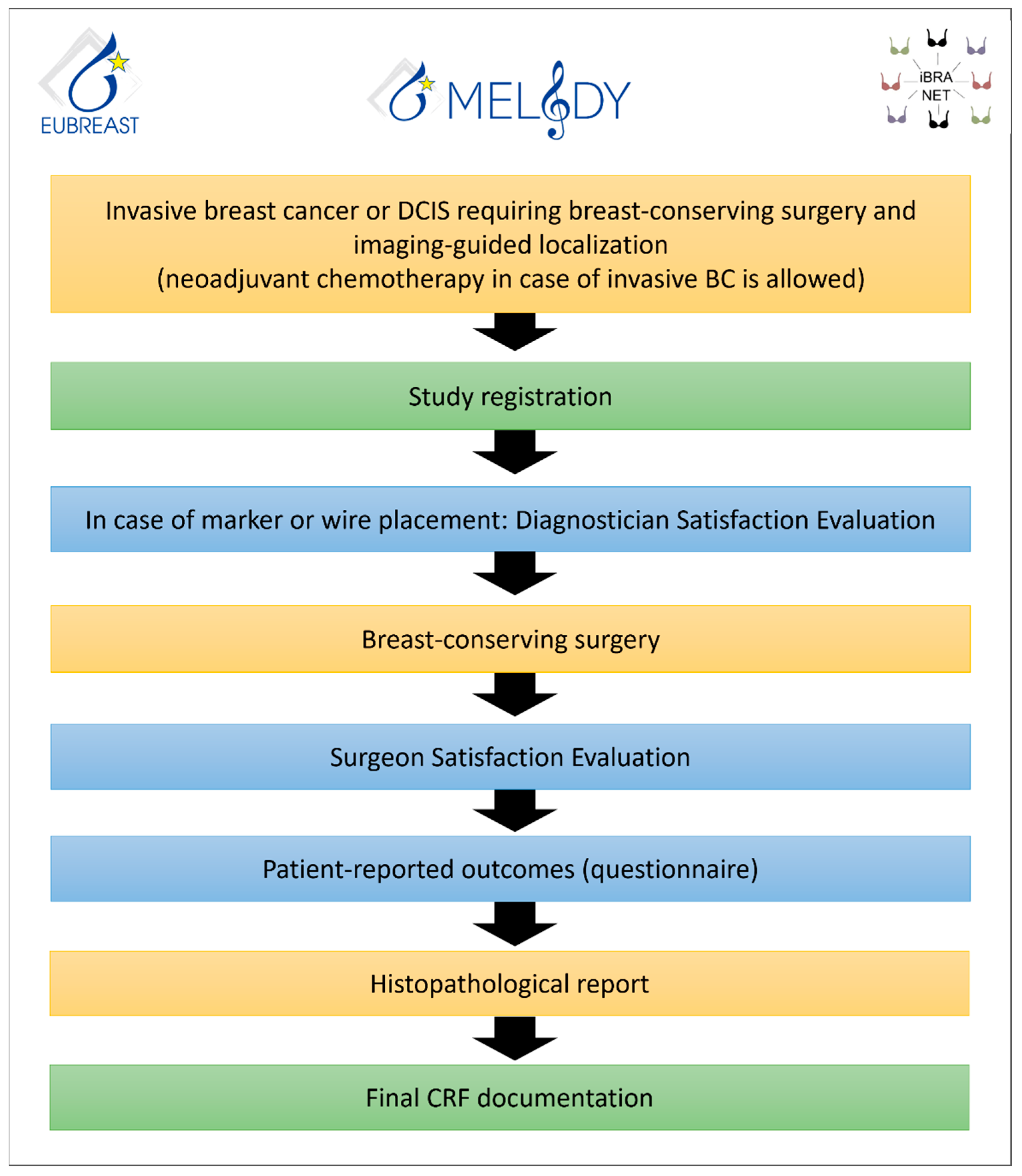
3. The MELODY Study
- Intended target lesion and/or marker removal, independent of margin status on final histopathology;
- Negative resection margin rates (defined as lesion removal with no invasive or non-invasive carcinoma on ink) at first surgery.
- 3.
- Rates of second surgery;
- 4.
- Rates of secondary mastectomy;
- 5.
- Resection ratio, defined as actual resection volume divided by the calculated optimum specimen volume;
- 6.
- Duration of surgery in BC patients, defined as time between first incision and end of skin closure (patients receiving simultaneous reconstructive, oncoplastic or contralateral surgery will be excluded from this analysis);
- 7.
- Marker dislocation rates;
- 8.
- Rates of marker placement failure, i.e., marker dislocation requiring a placement of a second marker;
- 9.
- Rates of localization failure, i.e., failed removal of marker or lesion, or necessity to switch to another intraoperative localization method;
- 10.
- Patient-reported outcomes (e.g., patient discomfort, pain level, and impairment of breathing);
- 11.
- Diagnostician/radiologist satisfaction with marking technique;
- 12.
- Surgeon satisfaction with localization technique;
- 13.
- Rates of “lost markers” (defined as markers placed prior to surgery and not retrieved at surgery);
- 14.
- Volume and weight of resected tissue;
- 15.
- Impact of experience of study sites on other outcome measures, depending on the localization technique used;
- 16.
- Impact of self-reported ethnicity on outcome measures;
- 17.
- Evaluation of surgical standards of care in different countries;
- 18.
- Evaluation of economic resources required for different localization techniques (material costs, operative time etc.);
- 19.
- Evaluation of MRI artifacts;
- 20.
- Evaluation of complication rates related to marker placement;
- 21.
- Evaluation of peri- and postoperative complication rates.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schermers, B.; van Riet, Y.E.; Schipper, R.J.; Vrancken Peeters, M.J.; Voogd, A.C.; Nieuwenhuijzen, G.A.P.; Ten Haken, B.; Ruers, T.J.M. Nationwide registry study on trends in localization techniques and reoperation rates in non-palpable ductal carcinoma in situ and invasive breast cancer. Br. J. Surg. 2021, 109, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.K.; Potter, S.; Elgammal, S.; Maxwell, A.J.; Sami, A.S.; Down, S.K.; Dave, R.V.; Harvey, J. Impalpable breast lesion localisation, a logistical challenge: Results of the UK iBRA-NET national practice questionnaire. Breast Cancer Res. Treat. 2021, 185, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Hirst, A.; Agha, R.A.; Rosin, D.; McCulloch, P. How can we improve surgical research and innovation?: The IDEAL framework for action. Int. J. Surg. 2013, 11, 1038–1042. [Google Scholar] [CrossRef] [PubMed]
- Kasem, I.; Mokbel, K. Savi Scout(R) Radar Localisation of Non-palpable Breast Lesions: Systematic Review and Pooled Analysis of 842 Cases. Anticancer Res. 2020, 40, 3633–3643. [Google Scholar] [CrossRef]
- Lowes, S.; Bell, A.; Milligan, R.; Amonkar, S.; Leaver, A. Use of Hologic LOCalizer radiofrequency identification (RFID) tags to localise impalpable breast lesions and axillary nodes: Experience of the first 150 cases in a UK breast unit. Clin. Radiol. 2020, 75, 942–949. [Google Scholar] [CrossRef]
- Ergina, P.L.; Barkun, J.S.; McCulloch, P.; Cook, J.A.; Altman, D.G.; Group, I. IDEAL framework for surgical innovation 2: Observational studies in the exploration and assessment stages. BMJ 2013, 346, f3011. [Google Scholar] [CrossRef]
- Ditsch, N.; Woeckel, A.; Untch, M.; Jackisch, C.; Albert, U.S.; Banys-Paluchowski, M.; Bauerfeind, I.; Blohmer, J. AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer (EBC): Update 2022. Breast Care 2022, 17, 403–420. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Rubio, I.T.; Karadeniz Cakmak, G.; Esgueva, A.; Krawczyk, N.; Paluchowski, P.; Gruber, I.; Marx, M.; Brucker, S.Y.; Bundgen, N.; et al. Intraoperative Ultrasound-Guided Excision of Non-Palpable and Palpable Breast Cancer: Systematic Review and Meta-Analysis. Ultraschall Med. 2022, 43, 367–379. [Google Scholar] [CrossRef]
- Chan, B.K.; Wiseberg-Firtell, J.A.; Jois, R.H.; Jensen, K.; Audisio, R.A. Localization techniques for guided surgical excision of non-palpable breast lesions. Cochrane Database Syst. Rev. 2015, 12, CD009206. [Google Scholar] [CrossRef]
- Davey, M.G.; O’Donnell, J.P.M.; Boland, M.R.; Ryan, E.J.; Walsh, S.R.; Kerin, M.J.; Lowery, A.J. Optimal localization strategies for non-palpable breast cancers—A network meta-analysis of randomized controlled trials. Breast 2022, 62, 103–113. [Google Scholar] [CrossRef]
- Athanasiou, C.; Mallidis, E.; Tuffaha, H. Comparative effectiveness of different localization techniques for non-palpable breast cancer. A systematic review and network meta-analysis. Eur. J. Surg. Oncol. 2022, 48, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.V.; Barrett, E.; Morgan, J.; Chandarana, M.; Elgammal, S.; Barnes, N.; Sami, A.; Masudi, T.; Down, S.; Holcombe, C.; et al. Wire- and magnetic-seed-guided localization of impalpable breast lesions: iBRA-NET localisation study. Br. J. Surg. 2022, 109, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.S.; Kaptanis, S.; Haddow, J.B.; Mondani, G.; Elsberger, B.; Tasoulis, M.K.; Obondo, C.; Johns, N.; Ismail, W.; Syed, A.; et al. Current margin practice and effect on re-excision rates following the publication of the SSO-ASTRO consensus and ABS consensus guidelines: A national prospective study of 2858 women undergoing breast-conserving therapy in the UK and Ireland. Eur. J. Cancer 2017, 84, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Langhans, L.; Jensen, M.B.; Talman, M.M.; Vejborg, I.; Kroman, N.; Tvedskov, T.F. Reoperation Rates in Ductal Carcinoma In Situ vs Invasive Breast Cancer After Wire-Guided Breast-Conserving Surgery. JAMA Surg. 2017, 152, 378–384. [Google Scholar] [CrossRef]
- Struik, G.M.; Schermers, B.; Mares, I.; Lont, H.E.; Bradshaw, J.W.; Ten Haken, B.; Ruers, T.J.M.; Mourik, J.E.M.; Birnie, E.; Klem, T. Randomized controlled trial comparing magnetic marker localization (MaMaLoc) with wire-guided localization in the treatment of early-stage breast cancer. Breast J. 2021, 27, 638–650. [Google Scholar] [CrossRef]
- Look Hong, N.; Wright, F.C.; Semple, M.; Nicolae, A.M.; Ravi, A. Results of a phase I, non-randomized study evaluating a Magnetic Occult Lesion Localization Instrument (MOLLI) for excision of non-palpable breast lesions. Breast Cancer Res. Treat. 2020, 179, 671–676. [Google Scholar] [CrossRef]
- Kurita, T.; Taruno, K.; Nakamura, S.; Takei, H.; Enokido, K.; Kuwayama, T.; Kanada, Y.; Akashi-Tanaka, S.; Matsuyanagi, M.; Hankyo, M.; et al. Magnetically Guided Localization Using a Guiding-Marker System((R)) and a Handheld Magnetic Probe for Nonpalpable Breast Lesions: A Multicenter Feasibility Study in Japan. Cancers 2021, 13, 2923. [Google Scholar] [CrossRef]
- Tayeh, S.; Wazir, U.; Mokbel, K. The Evolving Role of Radiofrequency Guided Localisation in Breast Surgery: A Systematic Review. Cancers 2021, 13, 4996. [Google Scholar] [CrossRef]
- Shaughnessy, E.; Vijapura, C.; Reyna, C.; Lewis, J.; Lewis, K.; Lee, S.J.; Sobel, L.; Wahab, R.; Rosen, L.; Brown, A. Exploiting the advantages of a wireless seed localization system that differentiates between the seeds: Breast cancer resection following neoadjuvant chemotherapy. Cancer Rep. 2022, 6, e1690. [Google Scholar] [CrossRef]
- Arman, A.; Kilicoglu, G.; Guner, H.H.; Celik, L. Marking of nonpalpable breast lesions using a custom carbon suspension. Acta Radiol. 2001, 42, 599–601. [Google Scholar] [CrossRef]
- Moss, H.A.; Barter, S.J.; Nayagam, M.; Lawrence, D.; Pittam, M. The use of carbon suspension as an adjunct to wire localisation of impalpable breast lesions. Clin. Radiol. 2002, 57, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Rose, A.; Collins, J.P.; Neerhut, P.; Bishop, C.V.; Mann, G.B. Carbon localisation of impalpable breast lesions. Breast 2003, 12, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Mazy, S.; Galant, C.; Berliere, M.; Mazy, G. Localization of non-palpable breast lesions with black carbon powder (experience of the Catholic University of Louvain). J. Radiol. 2001, 82, 161–164. [Google Scholar] [PubMed]
- Riedl, C.C.; Pfarl, G.; Helbich, T.H.; Memarsadeghi, M.; Wagner, T.; Rudas, M.; Fuchsjager, M. Comparison of wire versus carbon localization of non-palpable breast lesions. Rofo 2002, 174, 1126–1131. [Google Scholar] [CrossRef]
- Svane, G. A stereotaxic technique for preoperative marking of non-palpable breast lesions. Acta Radiol. Diagn. 1983, 24, 145–151. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Gruber, I.V.; Hartkopf, A.; Paluchowski, P.; Krawczyk, N.; Marx, M.; Brucker, S.; Hahn, M. Axillary ultrasound for prediction of response to neoadjuvant therapy in the context of surgical strategies to axillary dissection in primary breast cancer: A systematic review of the current literature. Arch. Gynecol. Obstet. 2020, 301, 341–353. [Google Scholar] [CrossRef]
- Dauway, E.L.; Sanders, R.; Freidland, J. Innovative diagnostics for breast cancer: New frontiers for the new millennium using radioactive seed localization. Presented at the 85th Annual American College of Surgeons Clinical Congress, Chicago, IL, USA; 1999. [Google Scholar]
- Simons, J.M.; van Nijnatten, T.J.; Koppert, L.B.; Van der Pol, C.C.; Van Diest, P.J.; Jager, A.; Van Klaveren, D.; Kam, B.L.R.; Lobbes, M.B.I.; De Boer, M.; et al. Radioactive Iodine Seed placement in the Axilla with Sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer: Results of the prospective multicenter RISAS trial. In Proceedings of the San Antonio Breast Cancer Symposium 2020, Abstract GS1-10, Virtual, 8–11 December 2020. [Google Scholar]
- Donker, M.; Straver, M.E.; Wesseling, J.; Loo, C.E.; Schot, M.; Drukker, C.A.; van Tinteren, H.; Sonke, G.S.; Rutgers, E.J.; Vrancken Peeters, M.J. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: The MARI procedure. Ann. Surg. 2015, 261, 378–382. [Google Scholar] [CrossRef]
- Langhans, L.; Tvedskov, T.F.; Klausen, T.L.; Jensen, M.B.; Talman, M.L.; Vejborg, I.; Benian, C.; Roslind, A.; Hermansen, J.; Oturai, P.S.; et al. Radioactive Seed Localization or Wire-guided Localization of Nonpalpable Invasive and In Situ Breast Cancer: A Randomized, Multicenter, Open-label Trial. Ann. Surg. 2017, 266, 29–35. [Google Scholar] [CrossRef]
- Bloomquist, E.V.; Ajkay, N.; Patil, S.; Collett, A.E.; Frazier, T.G.; Barrio, A.V. A Randomized Prospective Comparison of Patient-Assessed Satisfaction and Clinical Outcomes with Radioactive Seed Localization versus Wire Localization. Breast J. 2016, 22, 151–157. [Google Scholar] [CrossRef]
- Taylor, D.B.; Bourke, A.G.; Westcott, E.J.; Marinovich, M.L.; Chong, C.Y.L.; Liang, R.; Hughes, R.L.; Elder, E.; Saunders, C.M. Surgical outcomes after radioactive 125I seed versus hookwire localization of non-palpable breast cancer: A multicentre randomized clinical trial. Br. J. Surg. 2021, 108, 40–48. [Google Scholar] [CrossRef]
- Lovrics, P.J.; Goldsmith, C.H.; Hodgson, N.; McCready, D.; Gohla, G.; Boylan, C.; Cornacchi, S.; Reedijk, M. A multicentered, randomized, controlled trial comparing radioguided seed localization to standard wire localization for nonpalpable, invasive and in situ breast carcinomas. Ann. Surg. Oncol. 2011, 18, 3407–3414. [Google Scholar] [CrossRef]
- Gray, R.J.; Salud, C.; Nguyen, K.; Dauway, E.; Friedland, J.; Berman, C.; Peltz, E.; Whitehead, G.; Cox, C.E. Randomized prospective evaluation of a novel technique for biopsy or lumpectomy of nonpalpable breast lesions: Radioactive seed versus wire localization. Ann. Surg. Oncol. 2001, 8, 711–715. [Google Scholar] [CrossRef] [PubMed]
- Parvez, E.; Cornacchi, S.D.; Hodgson, N.; Thoma, A.; Kong, I.; Foster, G.; Cheng, J.; Goldsmith, C.H.; Dao, D.; Lovrics, P.J. A cosmesis outcome substudy in a prospective, randomized trial comparing radioguided seed localization with standard wire localization for nonpalpable, invasive, and in situ breast carcinomas. Am. J. Surg. 2014, 208, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Tsikouras, P.; Zuo, H.Q.; Huang, M.Q.; Peng, L.; Bothou, A.; Zervoudis, S.; Tobias Teichmann, A. Radioactive seed localization and wire guided localization in breast cancer: A systematic review and meta-analysis. J. BUON 2019, 24, 48–60. [Google Scholar]
- Pouw, B.; de Wit-van der Veen, L.J.; Stokkel, M.P.; Loo, C.E.; Vrancken Peeters, M.J.; Valdes Olmos, R.A. Heading toward radioactive seed localization in non-palpable breast cancer surgery? A meta-analysis. J. Surg. Oncol. 2015, 111, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Bhatt, A.; Felmlee, J.P.; Trester, P.; Lanners, D.; Paulsen, A.; Brunette, J. How to Safely Perform Magnetic Resonance Imaging-guided Radioactive Seed Localizations in the Breast. J. Clin. Imaging Sci. 2020, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Lindenberg, M.; van Beek, A.; Retel, V.; van Duijnhoven, F.; van Harten, W. Early budget impact analysis on magnetic seed localization for non-palpable breast cancer surgery. PLoS ONE 2020, 15, e0232690. [Google Scholar] [CrossRef]
- Zhang, Y.; Seely, J.; Cordeiro, E.; Hefler, J.; Thavorn, K.; Mahajan, M.; Domina, S.; Aro, J.; Ibrahim, A.M.; Arnaout, A.; et al. Radioactive Seed Localization Versus Wire-Guided Localization for Nonpalpable Breast Cancer: A Cost and Operating Room Efficiency Analysis. Ann. Surg. Oncol. 2017, 24, 3567–3573. [Google Scholar] [CrossRef]
- Loving, V.A.; Edwards, D.B.; Roche, K.T.; Steele, J.R.; Sapareto, S.A.; Byrum, S.C.; Schomer, D.F. Monte Carlo simulation to analyze the cost-benefit of radioactive seed localization versus wire localization for breast-conserving surgery in fee-for-service health care systems compared with accountable care organizations. AJR Am. J. Roentgenol. 2014, 202, 1383–1388. [Google Scholar] [CrossRef]
- Alderliesten, T.; Loo, C.E.; Pengel, K.E.; Rutgers, E.J.T.; Gilhuijs, K.G.A.; Vrancken Peeters, M.J. Radioactive Seed Localization of Breast Lesions: An Adequate Localization Method without Seed Migration. Breast J. 2011, 17, 594–601. [Google Scholar] [CrossRef]
- Luini, A.; Zurrida, S.; Paganelli, G.; Galimberti, V.; Sacchini, V.; Monti, S.; Veronesi, P.; Viale, G.; Veronesi, U. Comparison of radioguided excision with wire localization of occult breast lesions. Br. J. Surg. 1999, 86, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.; Galimberti, V.; Trifiro, G.; De Cicco, C.; Peradze, N.; Brenelli, F.; Fernandez-Rodriguez, J.; Rotmensz, N.; Latronico, A.; Berrettini, A.; et al. Occult breast lesion localization plus sentinel node biopsy (SNOLL): Experience with 959 patients at the European Institute of Oncology. Ann. Surg. Oncol. 2007, 14, 2928–2931. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; Wiltgen, J.E.; Bodanese, B.; Schmitt, R.L.; Gutfilen, B.; da Fonseca, L.M. Radioguided breast surgery for occult lesion localization—Correlation between two methods. J. Exp. Clin. Cancer Res. 2008, 27, 29. [Google Scholar] [CrossRef] [PubMed]
- Medina-Franco, H.; Abarca-Perez, L.; Ulloa-Gomez, J.L.; Romero, C. Radioguided localization of clinically occult breast lesions (ROLL): A pilot study. Breast J. 2007, 13, 401–405. [Google Scholar] [CrossRef]
- Rampaul, R.S.; Bagnall, M.; Burrell, H.; Pinder, S.E.; Evans, A.J.; Macmillan, R.D. Randomized clinical trial comparing radioisotope occult lesion localization and wire-guided excision for biopsy of occult breast lesions. Br. J. Surg. 2004, 91, 1575–1577. [Google Scholar] [CrossRef]
- Duarte, C.; Bastidas, F.; de los Reyes, A.; Martinez, M.C.; Hurtado, G.; Gomez, M.C.; Sanchez, R.; Manrique, J. Randomized controlled clinical trial comparing radioguided occult lesion localization with wire-guided lesion localization to evaluate their efficacy and accuracy in the localization of nonpalpable breast lesions. Surgery 2016, 159, 1140–1145. [Google Scholar] [CrossRef]
- Ocal, K.; Dag, A.; Turkmenoglu, O.; Gunay, E.C.; Yucel, E.; Duce, M.N. Radioguided occult lesion localization versus wire-guided localization for non-palpable breast lesions: Randomized controlled trial. Clinics 2011, 66, 1003–1007. [Google Scholar] [CrossRef]
- Kanat, N.B.; Tuncel, M.; Aksoy, T.; Firat, A.; Demirkazik, F.; Onat, D.; Caglar Tuncali, M.; Caner, B.E. Comparison of wire-guided localization and radio-guided occult lesionlocalization in preoperative localization of nonpalpable breast lesions. Turk. J. Med. Sci. 2016, 46, 1829–1837. [Google Scholar] [CrossRef]
- Postma, E.L.; Verkooijen, H.M.; van Esser, S.; Hobbelink, M.G.; van der Schelling, G.P.; Koelemij, R.; Witkamp, A.J.; Contant, C.; van Diest, P.J.; Willems, S.M.; et al. Efficacy of ‘radioguided occult lesion localisation’ (ROLL) versus ‘wire-guided localisation’ (WGL) in breast conserving surgery for non-palpable breast cancer: A randomised controlled multicentre trial. Breast Cancer Res. Treat. 2012, 136, 469–478. [Google Scholar] [CrossRef]
- Alikhassi, A.; Saeed, F.; Abbasi, M.; Omranipour, R.; Mahmoodzadeh, H.; Najafi, M.; Gity, M.; Kheradmand, A. Applicability of Radioguided Occult Lesion Localization for NonPalpable Benign Breast Lesions, Comparison with Wire Localization, a Clinical Trial. Asian Pac. J. Cancer Prev. 2016, 17, 3185–3190. [Google Scholar]
- Tang, J.; Xie, X.M.; Wang, X.; Xie, Z.M.; He, J.H.; Wu, Y.P.; Fan, W.; Fu, J.H.; Yang, M.T. Radiocolloid in combination with methylene dye localization, rather than wire localization, is a preferred procedure for excisional biopsy of nonpalpable breast lesions. Ann. Surg. Oncol. 2011, 18, 109–113. [Google Scholar] [CrossRef]
- Mariscal Martinez, A.; Sola, M.; de Tudela, A.P.; Julian, J.F.; Fraile, M.; Vizcaya, S.; Fernandez, J. Radioguided localization of nonpalpable breast cancer lesions: Randomized comparison with wire localization in patients undergoing conservative surgery and sentinel node biopsy. AJR Am. J. Roentgenol. 2009, 193, 1001–1009. [Google Scholar] [CrossRef]
- Philadelpho Arantes Pereira, F.; Martins, G.; Gregorio Calas, M.J.; Fonseca Torres de Oliveira, M.V.; Gasparetto, E.L.; Barbosa da Fonseca, L.M. Magnetic resonance imaging-radioguided occult lesion localization (ROLL) in breast cancer using Tc-99m macro-aggregated albumin and distilled water control. BMC Med. Imaging 2013, 13, 33. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.K. Update on Preoperative Breast Localization. Radiol. Clin. North Am. 2017, 55, 591–603. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, A.; Trombadori, C.M.L.; Caprini, F.; Lo Cicero, S.; Longo, V.; Ferrara, F.; Palma, S.; Conti, M.; Franco, A.; Scardina, L.; et al. Efficacy and Accuracy of Using Magnetic Seed for Preoperative Non-Palpable Breast Lesions Localization: Our Experience with Magseed. Curr. Oncol. 2022, 29, 8468–8474. [Google Scholar] [CrossRef] [PubMed]
- Zacharioudakis, K.; Down, S.; Bholah, Z.; Lee, S.; Khan, T.; Maxwell, A.J.; Howe, M.; Harvey, J. Is the future magnetic? Magseed localisation for non palpable breast cancer. A multi-centre non randomised control study. Eur. J. Surg. Oncol. 2019, 45, 2016–2021. [Google Scholar] [CrossRef]
- Harvey, J.R.; Lim, Y.; Murphy, J.; Howe, M.; Morris, J.; Goyal, A.; Maxwell, A.J. Safety and feasibility of breast lesion localization using magnetic seeds (Magseed): A multi-centre, open-label cohort study. Breast Cancer Res. Treat. 2018, 169, 531–536. [Google Scholar] [CrossRef]
- Micha, A.E.; Sinnett, V.; Downey, K.; Allen, S.; Bishop, B.; Hector, L.R.; Patrick, E.P.; Edmonds, R.; Barry, P.A.; Krupa, K.D.C.; et al. Patient and clinician satisfaction and clinical outcomes of Magseed compared with wire-guided localisation for impalpable breast lesions. Breast Cancer 2021, 28, 196–205. [Google Scholar] [CrossRef]
- Morgan, J.L.; Bromley, H.L.; Dave, R.V.; Masannat, Y.; Masudi, T.; Mylvaganam, S.; Elgammal, S.; Barnes, N.; Down, S.; Holcombe, C.; et al. Results of shared learning of a new magnetic seed localisation device–A UK iBRA-NET breast cancer localisation study. Eur. J. Surg. Oncol. 2022, 48, 2408–2413. [Google Scholar] [CrossRef]
- Powell, M.; Gate, T.; Kalake, O.; Ranjith, C.; Pennick, M.O. Magnetic Seed Localization (Magseed) for excision of impalpable breast lesions-The North Wales experience. Breast J. 2021, 27, 529–536. [Google Scholar] [CrossRef]
- Bessems, M.; van Breest Smallenburg, V.; van Bebber, I.; van Dijk, E.; van der Giessen, A.; Schermers, B.; Malloni, M. Safety and performance of Sirius Pintuition—A novel wire-free and non-radioactive localization system for breast cancer surgery. Eur. J. Surg. Oncol. 2022, 47, E1. [Google Scholar] [CrossRef]
- Clement, C.; Heeren, A.; den Hoed, I.; Jansen, P.; Venmans, A. First experience with Sirius Pintuition®—A novel magnetic localization system for breast cancer surgery. Eur. J. Surg. Oncol. 2022, 48, E70. [Google Scholar] [CrossRef]
- Bromley, H.L.; Dave, R.; Holcombe, C.; Potter, S.; Maxwell, A.J.; Kirwan, C.; Mylvaganam, S.; Elgammal, S.; Morgan, J.; Down, S.; et al. A Novel Mixed-Methods Platform Study Protocol for Investigating New Surgical Devices, with Embedded Shared Learning: ibra-net Breast Lesion Localisation Study. Int. J. Surg. Protoc. 2021, 25, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Banys-Paluchowski, M.; Thill, M.; Kuhn, T.; Ditsch, N.; Heil, J.; Wockel, A.; Fallenberg, E.; Friedrich, M.; Kummel, S.; Muller, V.; et al. AGO Recommendations for the Surgical Therapy of Breast Cancer: Update 2022. Geburtshilfe Frauenheilkd 2022, 82, 1031–1043. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.E.; Russell, S.; Prowler, V.; Carter, E.; Beard, A.; Mehindru, A.; Blumencranz, P.; Allen, K.; Portillo, M.; Whitworth, P.; et al. A Prospective, Single Arm, Multi-site, Clinical Evaluation of a Nonradioactive Surgical Guidance Technology for the Location of Nonpalpable Breast Lesions during Excision. Ann. Surg. Oncol. 2016, 23, 3168–3174. [Google Scholar] [CrossRef] [PubMed]
- Tayeh, S.; Muktar, S.; Heeney, J.; Michell, M.J.; Perry, N.; Suaris, T.; Evans, D.; Malhotra, A.; Mokbel, K. Reflector-guided Localization of Non-palpable Breast Lesions: The First Reported European Evaluation of the SAVI SCOUT(R) System. Anticancer Res. 2020, 40, 3915–3924. [Google Scholar] [CrossRef]
- Falcon, S.; Weinfurtner, R.J.; Mooney, B.; Niell, B.L. SAVI SCOUT(R) localization of breast lesions as a practical alternative to wires: Outcomes and suggestions for trouble-shooting. Clin. Imaging 2018, 52, 280–286. [Google Scholar] [CrossRef]
- Cox, C.E.; Garcia-Henriquez, N.; Glancy, M.J.; Whitworth, P.; Cox, J.M.; Themar-Geck, M.; Prati, R.; Jung, M.; Russell, S.; Appleton, K.; et al. Pilot Study of a New Nonradioactive Surgical Guidance Technology for Locating Nonpalpable Breast Lesions. Ann. Surg. Oncol. 2016, 23, 1824–1830. [Google Scholar] [CrossRef]
- Hayes, M.K.; Bloomquist, E.V.; Wright, H.R.; Long Term SCOUT® Placement in Breast and Axillary Node Prior to Neoadjuvant Chemotherapy. Clinical Case Review. 2018. Available online: https://www.merit.com/wp-content/uploads/2019/12/Case-Review-Long-Term-Placement-in-Breast-and-Node-Prior-to-NAC.pdf (accessed on 28 December 2022).
- Benoy, I.H.; Elst, H.; Van der Auwera, I.; Van Laere, S.; van Dam, P.; Van Marck, E.; Scharpe, S.; Vermeulen, P.B.; Dirix, L.Y. Real-time RT-PCR correlates with immunocytochemistry for the detection of disseminated epithelial cells in bone marrow aspirates of patients with breast cancer. Br. J. Cancer 2004, 91, 1813–1820. [Google Scholar] [CrossRef]
- Lamb, L.R.; Gilman, L.; Specht, M.; D’Alessandro, H.A.; Miles, R.C.; Lehman, C.D. Retrospective Review of Preoperative Radiofrequency Tag Localization of Breast Lesions in 848 Patients. AJR Am. J. Roentgenol. 2021, 217, 605–612. [Google Scholar] [CrossRef]
- Dauphine, C.; Reicher, J.J.; Reicher, M.A.; Gondusky, C.; Khalkhali, I.; Kim, M. A prospective clinical study to evaluate the safety and performance of wireless localization of nonpalpable breast lesions using radiofrequency identification technology. AJR Am. J. Roentgenol. 2015, 204, W720–W723. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Juette, A. Radio-Frequency Identifier Devices (RFIDs): Our Experience With Wireless Localisation in Non-palpable Breast Masses at a UK Tertiary Breast Imaging Unit. Cureus 2022, 14, e22402. [Google Scholar] [CrossRef] [PubMed]
- DiNome, M.L.; Kusske, A.M.; Attai, D.J.; Fischer, C.P.; Hoyt, A.C. Microchipping the breast: An effective new technology for localizing non-palpable breast lesions for surgery. Breast Cancer Res. Treat. 2019, 175, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Wazir, U.; Tayeh, S.; Perry, N.; Michell, M.; Malhotra, A.; Mokbel, K. Wireless Breast Localization Using Radio-frequency Identification Tags: The First Reported European Experience in Breast Cancer. Vivo 2020, 34, 233–238. [Google Scholar] [CrossRef]
- Heindl, F.; Schulz-Wendtland, R.; Jud, S.; Erber, R.; Hack, C.C.; Preuss, C.; Behrens, A.; Poschke, P.; Emons, J. Evaluation of a Wireless Localization System for Nonpalpable Breast Lesions—Feasibility and Cost-effectiveness in Everyday Clinical Routine. Vivo 2022, 36, 2342–2349. [Google Scholar] [CrossRef]
- Schwartz, G.F.; Goldberg, B.B.; Rifkin, M.D.; D’Orazio, S.E. Ultrasonography: An alternative to x-ray-guided needle localization of nonpalpable breast masses. Surgery 1988, 104, 870–873. [Google Scholar]
- Rubio, I.T.; Henry-Tillman, R.; Klimberg, V.S. Surgical use of breast ultrasound. Surg. Clin. North Am. 2003, 83, 771–788. [Google Scholar] [CrossRef]
- Karadeniz Cakmak, G.; Emre, A.U.; Tascilar, O.; Bahadir, B.; Ozkan, S. Surgeon performed continuous intraoperative ultrasound guidance decreases re-excisions and mastectomy rates in breast cancer. Breast 2017, 33, 23–28. [Google Scholar] [CrossRef]
- Kaufman, C.S.; Jacobson, L.; Bachman, B.; Kaufman, L. Intraoperative ultrasound facilitates surgery for early breast cancer. Ann. Surg. Oncol. 2002, 9, 988–993. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Paluchowski, P.; Krawczyk, N. Twinkle artifact in sonographic breast clip visualization. Arch. Gynecol. Obstet. 2022. [Google Scholar] [CrossRef]
- Pan, H.; Wu, N.; Ding, H.; Ding, Q.; Dai, J.; Ling, L.; Chen, L.; Zha, X.; Liu, X.; Zhou, W.; et al. Intraoperative ultrasound guidance is associated with clear lumpectomy margins for breast cancer: A systematic review and meta-analysis. PLoS ONE 2013, 8, e74028. [Google Scholar] [CrossRef] [PubMed]
- Banys-Paluchowski, M.; Thill, M.; Kühn, T.; Ditsch, N.; Heil, J.; Wöckel, A.; Fallenberg, E.; Friedrich, M.; Kümmel, S.; Müller, V.; et al. AGO Breast Committee Recommendations: Surgical Therapy Update 2022. AGO Empfehlungen zur Operativen Therapie des Mammakarzinoms: Update 2022; GebFra: Bielefeld, Germany, 2022. [Google Scholar]
- Esgueva, A.; Rodriguez-Revuelto, R.; Espinosa-Bravo, M.; Salazar, J.P.; Rubio, I.T. Learning curves in intraoperative ultrasound guided surgery in breast cancer based on complete breast cancer excision and no need for second surgeries. Eur. J. Surg. Oncol. 2019, 45, 578–583. [Google Scholar] [CrossRef]
- Konen, J.; Murphy, S.; Berkman, A.; Ahern, T.P.; Sowden, M. Intraoperative Ultrasound Guidance With an Ultrasound-Visible Clip: A Practical and Cost-effective Option for Breast Cancer Localization. J. Ultrasound Med. 2020, 39, 911–917. [Google Scholar] [CrossRef]
- Volders, J.H.; Haloua, M.H.; Krekel, N.M.; Negenborn, V.L.; Kolk, R.H.; Lopes Cardozo, A.M.; Bosch, A.M.; de Widt-Levert, L.M.; van der Veen, H.; Rijna, H.; et al. Intraoperative ultrasound guidance in breast-conserving surgery shows superiority in oncological outcome, long-term cosmetic and patient-reported outcomes: Final outcomes of a randomized controlled trial (COBALT). Eur. J. Surg. Oncol. 2017, 43, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.; Diaz, J.C.; Ramos, T.; Ruano, R.; Aparicio, M.; Sancho, M.; Gonzalez-Orus, J.M. Ultrasound-guided excision combined with intraoperative assessment of gross macroscopic margins decreases the rate of reoperations for non-palpable invasive breast cancer. Breast 2013, 22, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.T.; Esgueva-Colmenarejo, A.; Espinosa-Bravo, M.; Salazar, J.P.; Miranda, I.; Peg, V. Intraoperative Ultrasound-Guided Lumpectomy Versus Mammographic Wire Localization for Breast Cancer Patients After Neoadjuvant Treatment. Ann. Surg. Oncol. 2016, 23, 38–43. [Google Scholar] [CrossRef]
- Krekel, N.M.; Lopes Cardozo, A.M.; Muller, S.; Bergers, E.; Meijer, S.; van den Tol, M.P. Optimising surgical accuracy in palpable breast cancer with intra-operative breast ultrasound—Feasibility and surgeons’ learning curve. Eur. J. Surg. Oncol. 2011, 37, 1044–1050. [Google Scholar] [CrossRef]
- Canavese, G.; Catturich, A.; Vecchio, C.; Tomei, D.; Estienne, M.; Moresco, L.; Imperiale, A.; Parodi, G.C.; Massa, T.; Badellino, F. Pre-operative localization of non-palpable lesions in breast cancer by charcoal suspension. Eur. J. Surg. Oncol. 1995, 21, 47–49. [Google Scholar] [CrossRef]
- Ko, K.; Han, B.K.; Jang, K.M.; Choe, Y.H.; Shin, J.H.; Yang, J.H.; Nam, S.J. The value of ultrasound-guided tattooing localization of nonpalpable breast lesions. Korean J. Radiol. 2007, 8, 295–301. [Google Scholar] [CrossRef]
- Mathieu, M.C.; Bonhomme-Faivre, L.; Rouzier, R.; Seiller, M.; Barreau-Pouhaer, L.; Travagli, J.P. Tattooing breast cancers treated with neoadjuvant chemotherapy. Ann. Surg. Oncol. 2007, 14, 2233–2238. [Google Scholar] [CrossRef]
- Porpiglia, M.; Borella, F.; Chieppa, P.; Brino, C.; Ala, A.; Marra, V.; Castellano, I.; Benedetto, C. Carbon tattooing of axillary lymph nodes in breast cancer patients before neoadjuvant chemotherapy: A retrospective analysis. Tumori 2022. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.M.; van Nijnatten, T.J.A.; van der Pol, C.C.; van Diest, P.J.; Jager, A.; van Klaveren, D.; Kam, B.L.R.; Lobbes, M.B.I.; de Boer, M.; Verhoef, C.; et al. Diagnostic Accuracy of Radioactive Iodine Seed Placement in the Axilla with Sentinel Lymph Node Biopsy After Neoadjuvant Chemotherapy in Node-Positive Breast Cancer. JAMA Surg. 2022, 157, 991–999. [Google Scholar] [CrossRef] [PubMed]
- de Boniface, J.; Frisell, J.; Kuhn, T.; Wiklander-Brakenhielm, I.; Dembrower, K.; Nyman, P.; Zouzos, A.; Gerber, B.; Reimer, T.; Hartmann, S. False-negative rate in the extended prospective TATTOO trial evaluating targeted axillary dissection by carbon tattooing in clinically node-positive breast cancer patients receiving neoadjuvant systemic therapy. Breast Cancer Res. Treat. 2022, 193, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Kuhn, T.; de Boniface, J.; Stachs, A.; Winckelmann, A.; Frisell, J.; Wiklander-Brakenhielm, I.; Stubert, J.; Gerber, B.; Reimer, T. Carbon tattooing for targeted lymph node biopsy after primary systemic therapy in breast cancer: Prospective multicentre TATTOO trial. Br. J. Surg. 2021, 108, 302–307. [Google Scholar] [CrossRef]
- Goyal, A.; Puri, S.; Marshall, A.; Valassiadou, K.; Hoosein, M.M.; Carmichael, A.R.; Erdelyi, G.; Sharma, N.; Dunn, J.; York, J. A multicentre prospective feasibility study of carbon dye tattooing of biopsied axillary node and surgical localisation in breast cancer patients. Breast Cancer Res. Treat. 2021, 185, 433–440. [Google Scholar] [CrossRef]
- Banys-Paluchowski, M.; Untch, M.; Krawczyk, N.; Thurmann, M.; Kuhn, T.; Sehouli, J.; Gasparri, M.L.; de Boniface, J.; Gentilini, O.D.; Stickeler, E.; et al. Current trends in diagnostic and therapeutic management of the axilla in breast cancer patients receiving neoadjuvant therapy: Results of the German-wide NOGGO MONITOR 24 survey. Arch. Gynecol. Obstet. 2022. [Google Scholar] [CrossRef]





| Successful Excision | Positive Margins 1 | Re-Operation Rate | Data Quality | |
|---|---|---|---|---|
| Wire-guided localization (WGL) | 99% [9,12] | 15–21% [9,10,12,14] | 14–19% [9,10] | High; Meta-analyses of RCTs available (LoE 1a) |
| Radioactive seed localization (RSL) | 100% [9] | 12–13% [9,10] | 10–15% [9,10] | High; Meta-analyses of RCTs available (LoE 1a) |
| Radio-guided Occult Lesion Localization (ROLL) | 99.5% [9] | 12–17% [9,10] | 9–10% [9,10] | High; Meta-analyses of RCTs available (LoE 1a) |
| Magseed | 99.8% [12] | 13.3% [12] | 12% [12] | Large cohort studies [12], no RCTs (LoE 2b) |
| Sirius Pintuition | 100% [15] | 8% [15] | 4% [15] | Small cohort studies, one small RCT 3 [15] (LoE 2b) |
| MOLLI | 100% [16] | 0% [16] | 0% [16] | Small phase I cohort study (LoE 4) |
| TAKUMI | 100% [17] | 7.3% [17] | 4.9% [17] | Small cohort study (LoE 4) |
| SAVI SCOUT | 99.64% [4] | n.d. | 12.8% [4] | Systemic review and pooled analysis [4] (LoE 2b) |
| LOCalizer | 99.9% [18] | n.d. | 13.9% [18] | Systemic review and pooled analysis [18] (LoE 2b) |
| EnVisio | n.d. | n.d. | n.d. | Case report [19] (LoE 5) |
| Intraoperative ultrasound (IOUS) | 100% [8] 2 | 5% [8,10,11] 2 | 5–7% [8,10] 2 | High; Meta-analyses of RCTs available (LoE 1a) 2 |
| Carbon | 79.0–99.1% [20,21,22,23,24] | 75.0–96.4% [21,22,25] | 7.1% [25] | Cohort studies, no RCTs (LoE 4) |
| Advantages | Disadvantages | |
|---|---|---|
| Wire-guided localization (WGL) |
|
|
| Radioactive seed localization (RSL) |
|
|
| Radio-guided Occult Lesion Localization (ROLL) |
|
|
| Magnetic and paramagnetic localization Commercially available systems:
|
|
|
| Radar reflector-based localization Commercially available systems:
|
|
|
| Radiofrequency identification tags (RFID) Commercially available systems:
|
|
|
| Intraoperative ultrasound (IOUS) |
|
|
| Carbon |
|
|
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banys-Paluchowski, M.; Kühn, T.; Masannat, Y.; Rubio, I.; de Boniface, J.; Ditsch, N.; Karadeniz Cakmak, G.; Karakatsanis, A.; Dave, R.; Hahn, M.; et al. Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/iBRA-NET, NCT 05559411). Cancers 2023, 15, 1173. https://doi.org/10.3390/cancers15041173
Banys-Paluchowski M, Kühn T, Masannat Y, Rubio I, de Boniface J, Ditsch N, Karadeniz Cakmak G, Karakatsanis A, Dave R, Hahn M, et al. Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/iBRA-NET, NCT 05559411). Cancers. 2023; 15(4):1173. https://doi.org/10.3390/cancers15041173
Chicago/Turabian StyleBanys-Paluchowski, Maggie, Thorsten Kühn, Yazan Masannat, Isabel Rubio, Jana de Boniface, Nina Ditsch, Güldeniz Karadeniz Cakmak, Andreas Karakatsanis, Rajiv Dave, Markus Hahn, and et al. 2023. "Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/iBRA-NET, NCT 05559411)" Cancers 15, no. 4: 1173. https://doi.org/10.3390/cancers15041173
APA StyleBanys-Paluchowski, M., Kühn, T., Masannat, Y., Rubio, I., de Boniface, J., Ditsch, N., Karadeniz Cakmak, G., Karakatsanis, A., Dave, R., Hahn, M., Potter, S., Kothari, A., Gentilini, O. D., Gulluoglu, B. M., Lux, M. P., Smidt, M., Weber, W. P., Aktas Sezen, B., Krawczyk, N., ... Harvey, J. (2023). Localization Techniques for Non-Palpable Breast Lesions: Current Status, Knowledge Gaps, and Rationale for the MELODY Study (EUBREAST-4/iBRA-NET, NCT 05559411). Cancers, 15(4), 1173. https://doi.org/10.3390/cancers15041173












