Abstract
Breast cancer is the fifth-ranked cancer globally. Despite early diagnosis and advances in treatment, breast cancer mortality is increasing. This meta-analysis aims to examine all possible prognostic factors that improve/deteriorate breast cancer-specific survival. MEDLINE, PubMed, ScienceDirect, Ovid, and Google Scholar were systematically searched until September 16, 2023. The retrieved studies from 1995 to 2022 accumulated 1,386,663 cases from 30 countries. A total of 13 out of 22 prognostic factors were significantly associated with breast cancer-specific survival. A random-effects model provided a pooled estimate of the top five poorest prognostic factors, including Stage 4 (HR = 12.12; 95% CI: 5.70, 25.76), followed by Stage 3 (HR = 3.42, 95% CI: 2.51, 4.67), a comorbidity index ≥ 3 (HR = 3.29; 95% CI: 4.52, 7.35), the poor differentiation of cancer cell histology (HR = 2.43; 95% CI: 1.79, 3.30), and undifferentiated cancer cell histology (HR = 2.24; 95% CI: 1.66, 3.01). Other survival-reducing factors include positive nodes, age, race, HER2-receptor positivity, and overweight/obesity. The top five best prognostic factors include different types of mastectomies and breast-conserving therapies (HR = 0.56; 95% CI: 0.44, 0.70), medullary histology (HR = 0.62; 95% CI: 0.53, 0.72), higher education (HR = 0.72; 95% CI: 0.68, 0.77), and a positive estrogen receptor status (HR = 0.78; 95% CI: 0.65, 0.94). Heterogeneity was observed in most studies. Data from developing countries are still scarce.
1. Introduction
According to the Global Burden of Disease Cancer study, breast cancer remains the fifth-ranked cancer globally, with an increase from 2005 to 2015 of 17.2% (95% CI: 9.3%, 24.3%) in absolute years of life lost (A-YLLs) [1] and a significant increase in total YLLs [2]. The number of breast cancer cases has generally increased in recent years due to population growth, ageing populations, and age-specific cases [3]. Despite increases in the number of breast cancer survivors due to early diagnosis and advances in treatment [4] breast cancer mortality rate has increased by 21.3% (95% CI: 14.9%, 27.2%) [2].
Survival rate is one of the main outcome measures to predict the effectiveness of treatment or intervention for a specific period after diagnosis [5,6]. The accuracy of breast cancer survival prediction models requires the identification of the most salient factors that establish risk factors, such as age, race, stage at diagnosis, tumour size, hormonal receptor status, type of treatment, and family history of breast cancer, which could provide some evidence [5]. However, over-simplified or parsimonious models are often poor when applied to future trends and, thus, a comprehensive review of other biological and non-biological factors providing a more realistic interplay of this complex relationship needs to be considered. Furthermore, the availability of various cancer survival statistics, such as all-cause mortality, cancer-specific mortality, crude probability, and relative survival rate, adds to the difficulty of having a constant unit of measurement across studies [7].
Based on the above and a lack of systematic review and meta-analysis that comprehensively covers major factors related to breast cancer-specific survival, we conducted a systematic review and meta-analysis of longitudinal observational studies that report breast cancer-specific survival using a Hazard Ratio and a 95% confidence interval according to the preferred items for reporting systematic review and meta-analysis (PRISMA) guidelines [8].
2. Materials and Methods
2.1. Eligibility Criteria
Studies with the following eligibility characteristics were included: (1) they employed a longitudinal (retrospective or prospective) design, (2) they examined the survival of breast cancer patients using breast cancer-specific mortality, (3) a minimum sample size of 100, and (4) they provided a measure of survival statistics, particularly a Hazard Ratio (HR) and the corresponding 95% confidence interval (CI) for the estimation. Whenever necessary, the authors were contacted to provide more information to calculate these statistics. Studies that report survival statistics other than breast cancer-specific survival were excluded.
2.2. Search Strategy
A systematic search was performed using electronic databases, including (1) MEDLINE, (2) PubMed, (3) ScienceDirect, (4) Ovid, and (5) Google Scholar, spanning from 1990 to 2023. The search strategy was a combination of keywords that consisted of (“breast cancer” OR “breast carcinoma” OR “breast neoplasm” OR “tumor breast”) AND (“mortality” OR “survival”) AND (“factor” OR “prognostic factor”) AND (“Hazard ratio” OR “Cox model” OR “proportional hazard model”). The reference list of selected studies in the present review was also hand-searched in order to retrieve any additional relevant articles. Other non-primary sources such as editorials, conference proceedings, and reviews were excluded from the search.
2.3. Study Selection
The results of the systematic search were entered into a reference manager software (Mendeley v2.115.0), and two reviewers (HAR and SNNZ) independently screened the study titles, abstracts, and full texts based on the eligibility criteria. If there was disagreement, it was resolved through consensus with a third party.
2.4. Quality Assessment
The Quality in Prognosis Studies (QUIPS) tool was used to assess the risk of bias in the selected studies and consisted of the following items: (1) study participation, (2) study attrition, (3) prognostic factor measurement, (4) outcome measurement, (5) study confounding, and (6) statistical analysis and reporting. Two reviewers (HAR and SNNZ) assessed the quality of the retrieved articles independently. The inter-rater agreement using weighted kappa was used to obtain proper agreement between the two reviewers where values were tiered into 40–59% (low quality), 60–79% (moderate quality), and more than 90% (high quality). Only studies that were rated moderate and high were included in the review and meta-analysis.
2.5. Data Extraction
The data were extracted by two reviewers (HAR and SNNZ) independently using standard pre-defined features and formatting, including the first author’s name, the year of publication, the country of study, the study design/database used, the sample size/number of study participants, study population/sample characteristics, and prognostic factors studies in association with breast cancer-specific survival.
2.6. Statistical Analyses
To estimate the pooled effects of breast cancer-specific survival in association with each prognostic factor, the Hazard Ratios and corresponding 95% confidence intervals were combined and reported as fixed-effects models or random-effects models when heterogeneity is present. The pooled effect is considered statistically significant if the p-value is less than 0.05. The weight of each study calculated based on the inverse of the standard error is reported in percentages where a higher percentage indicates a higher weight. A forest plot is used to visualise the output of individual studies and pooled estimates where the diamond in the bottom represents the pooled effect size.
To explore between-study heterogeneity, the I-square (I2) statistics based on Cochran’s Q (following a Chi-square distribution), were used. I2 statistics above 50% are considered substantial [9]. Publication bias was assessed visually using funnel plots and Egger’s test to investigate the asymmetry among the study estimates. Microsoft Excel was used to input and prepare the data for analysis. All statistical analyses were performed in RStudio (v1.4.1717) using the meta [10] and metafor [11] packages.
3. Results
3.1. Study Characteristics
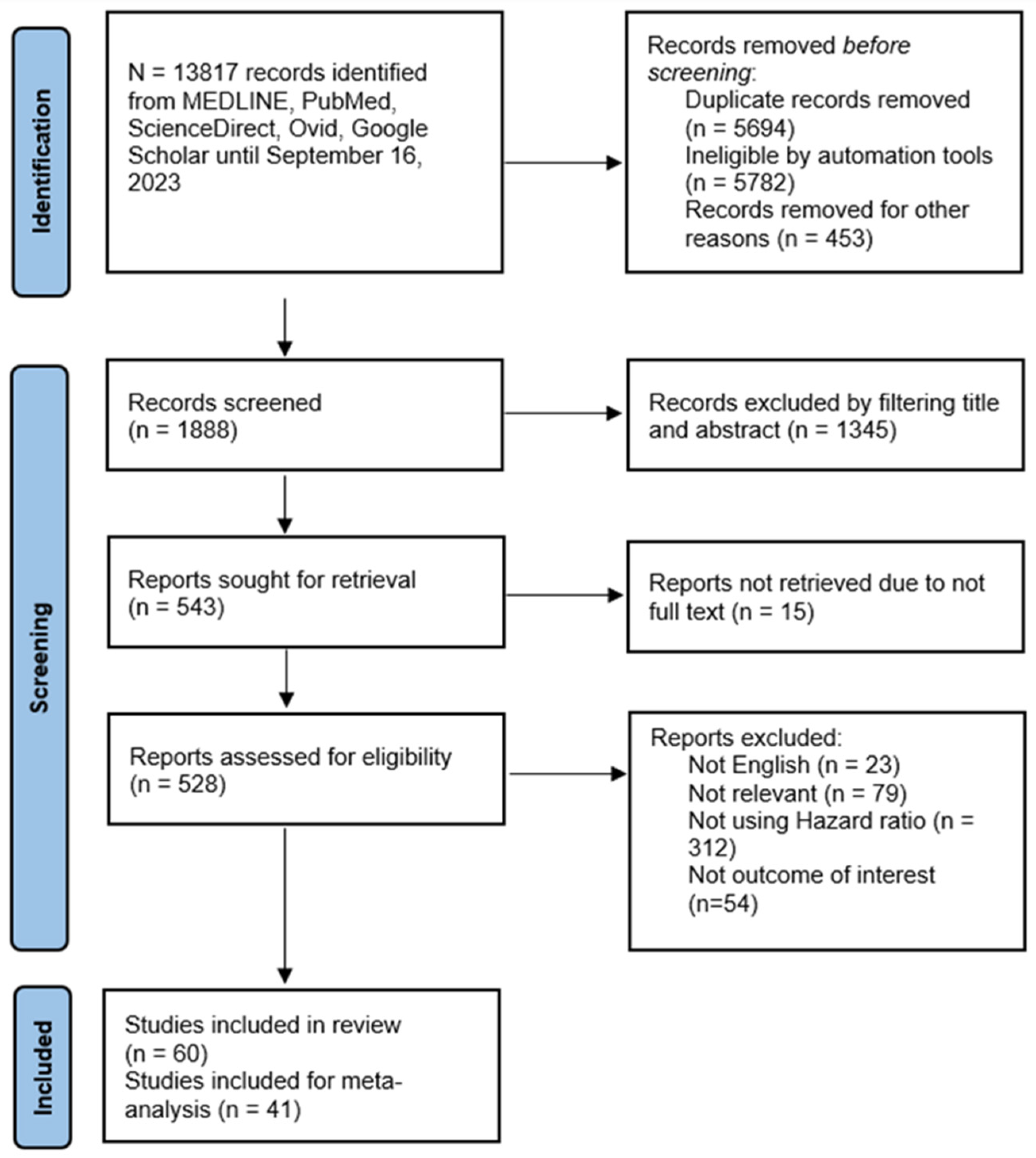
Figure 1 depicts the flow diagram of the systematic search process and study selection results. The initial search yielded 13,817 studies. After the exclusion of irrelevant studies, duplication, and other reasons, 1888 were screened based on title and abstract. Further exclusion based on eligibility criteria resulted in 60 studies included in this review, of which 33 (55%) were of moderate quality and 27 (45%) were of high quality (Table 1). Forty-one studies were eligible for meta-analysis.
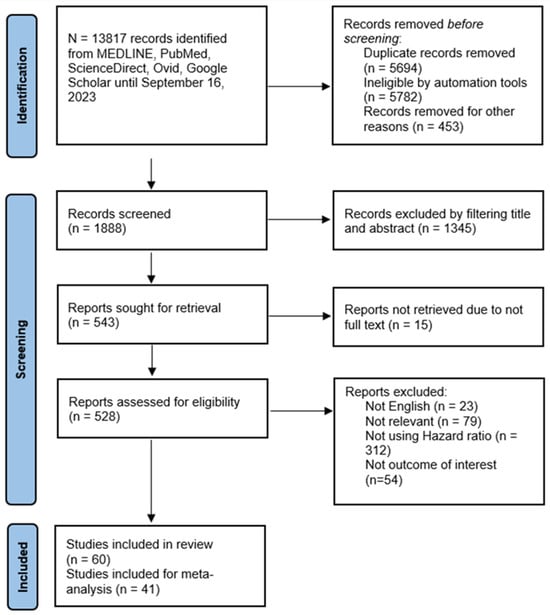
Figure 1.
PRISMA flowchart of systematic search and study selection outcome.

Table 1.
Quality assessment according to a 6-component checklist from the Quality in Prognosis Studies (QUIPS) tool.
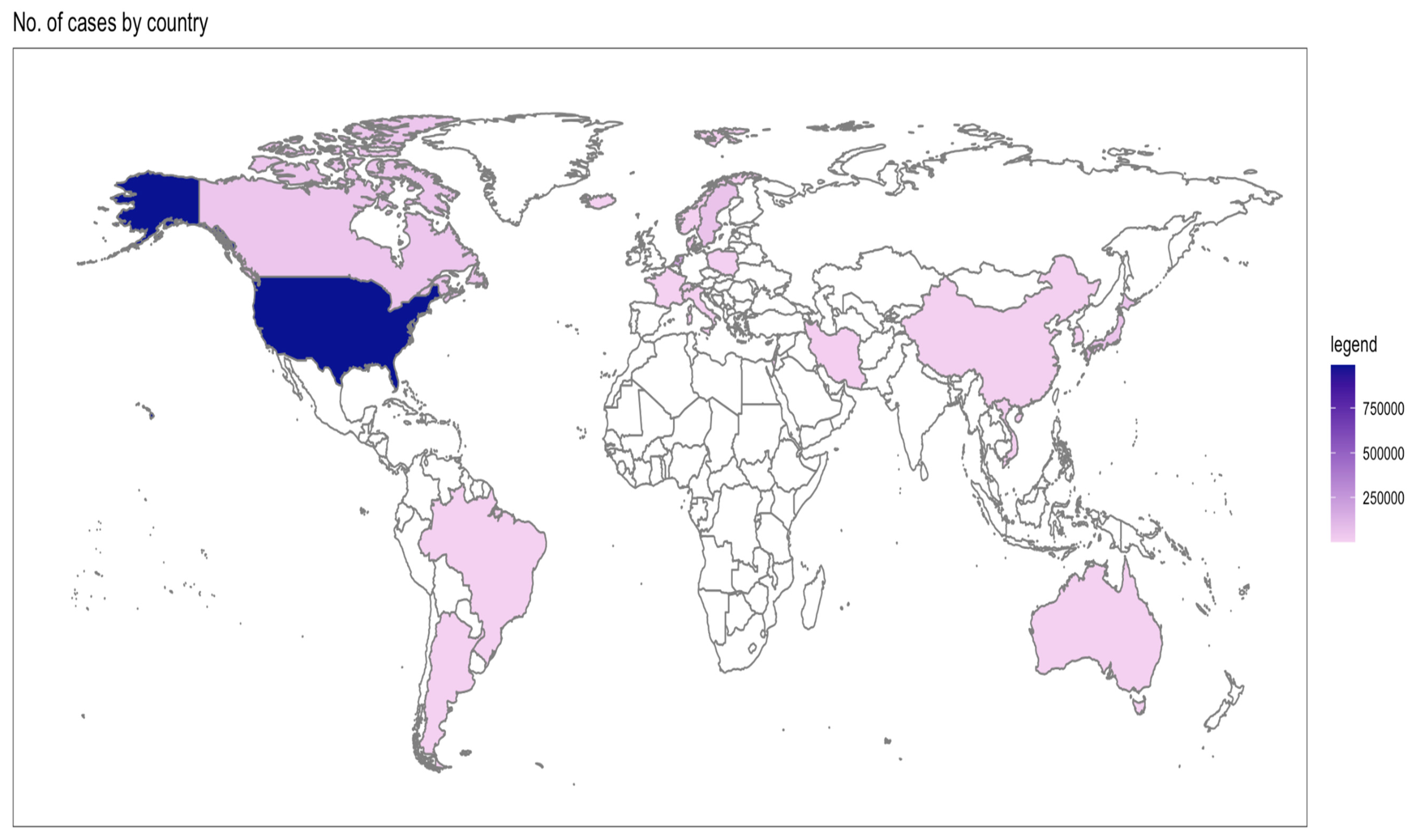
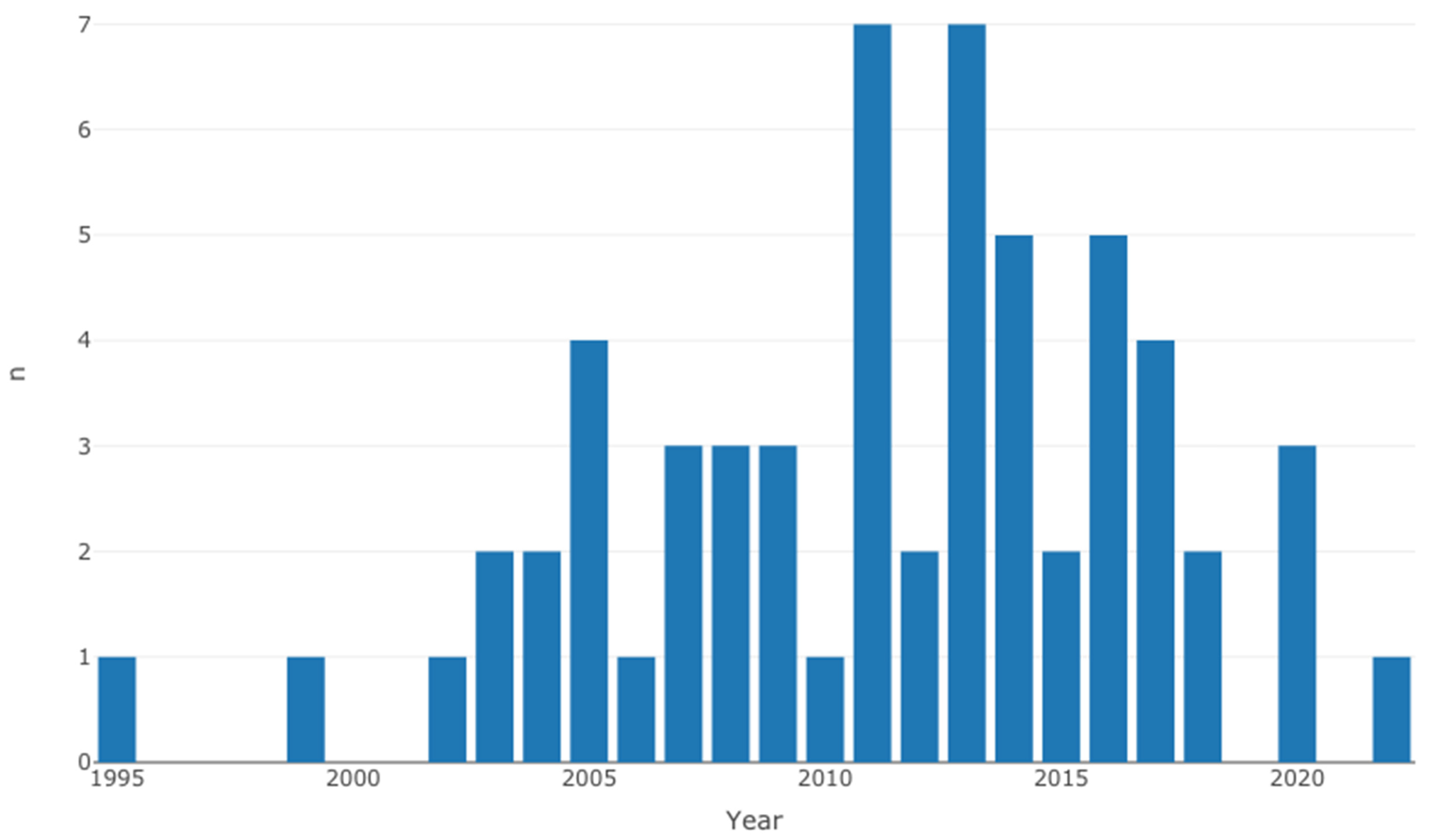
Table 2 shows the individual characteristics of selected studies [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68]. Overall, the selected studies originated from 30 countries, where the majority of the cases were from North America (Figure 2). Even though the search strategy was inclusive of 1990 to 2023, the eligible studies range between 1995 to 2022 (Figure 3).

Table 2.
Characteristics of individual selected studies.
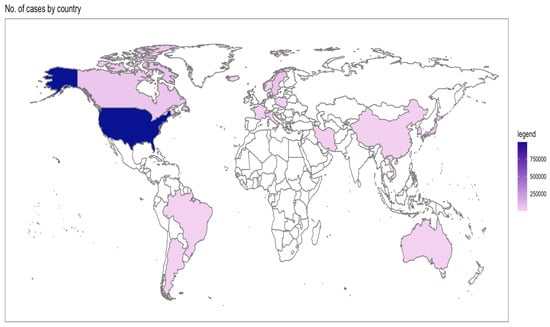
Figure 2.
Distribution of selected studies by number of cases and country.
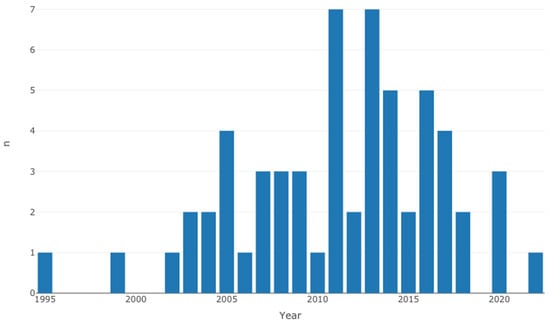
Figure 3.
Distribution of selected studies by year of publication.
3.2. Pooled Effects of Breast Cancer-Specific Survival
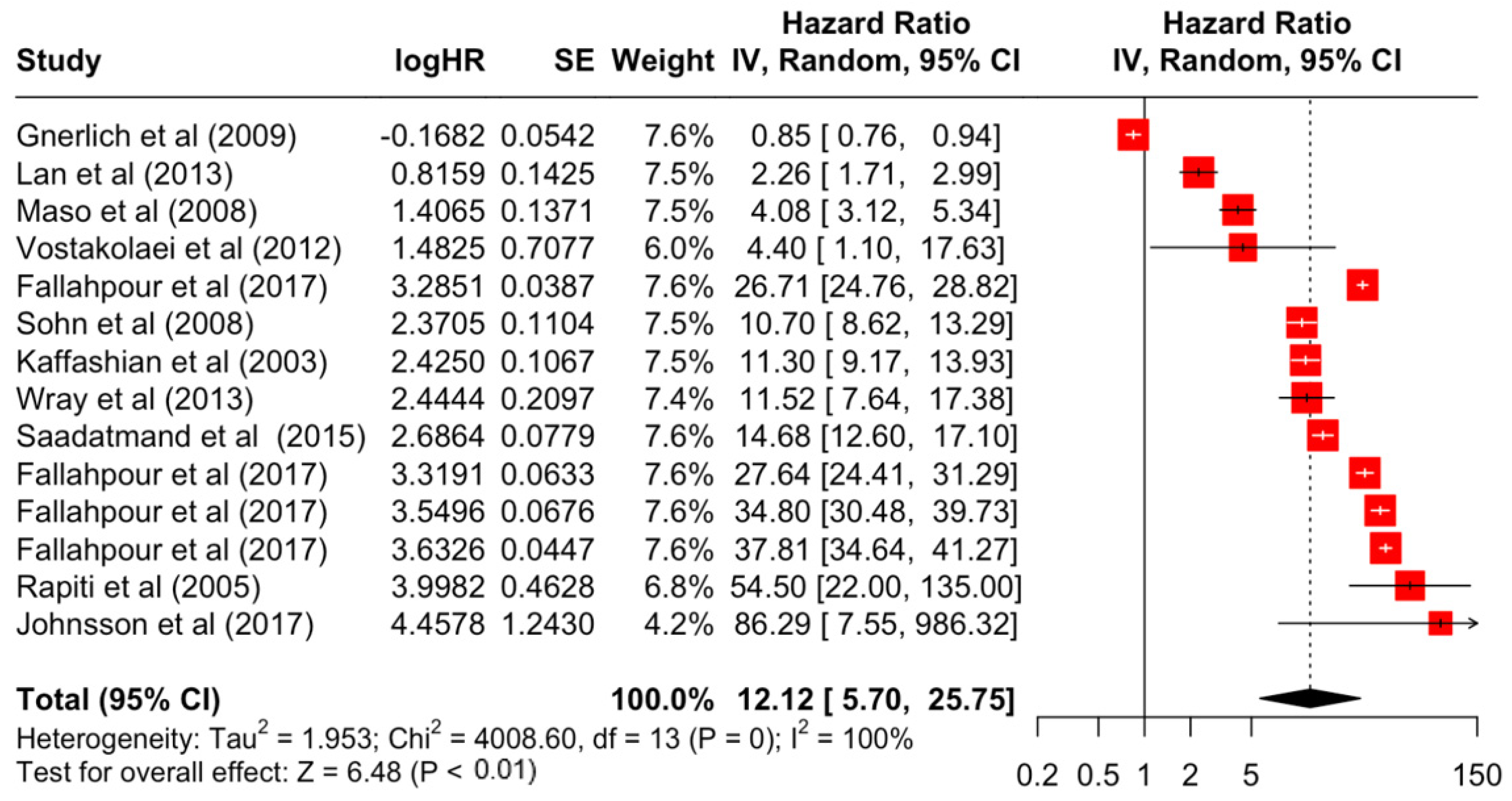
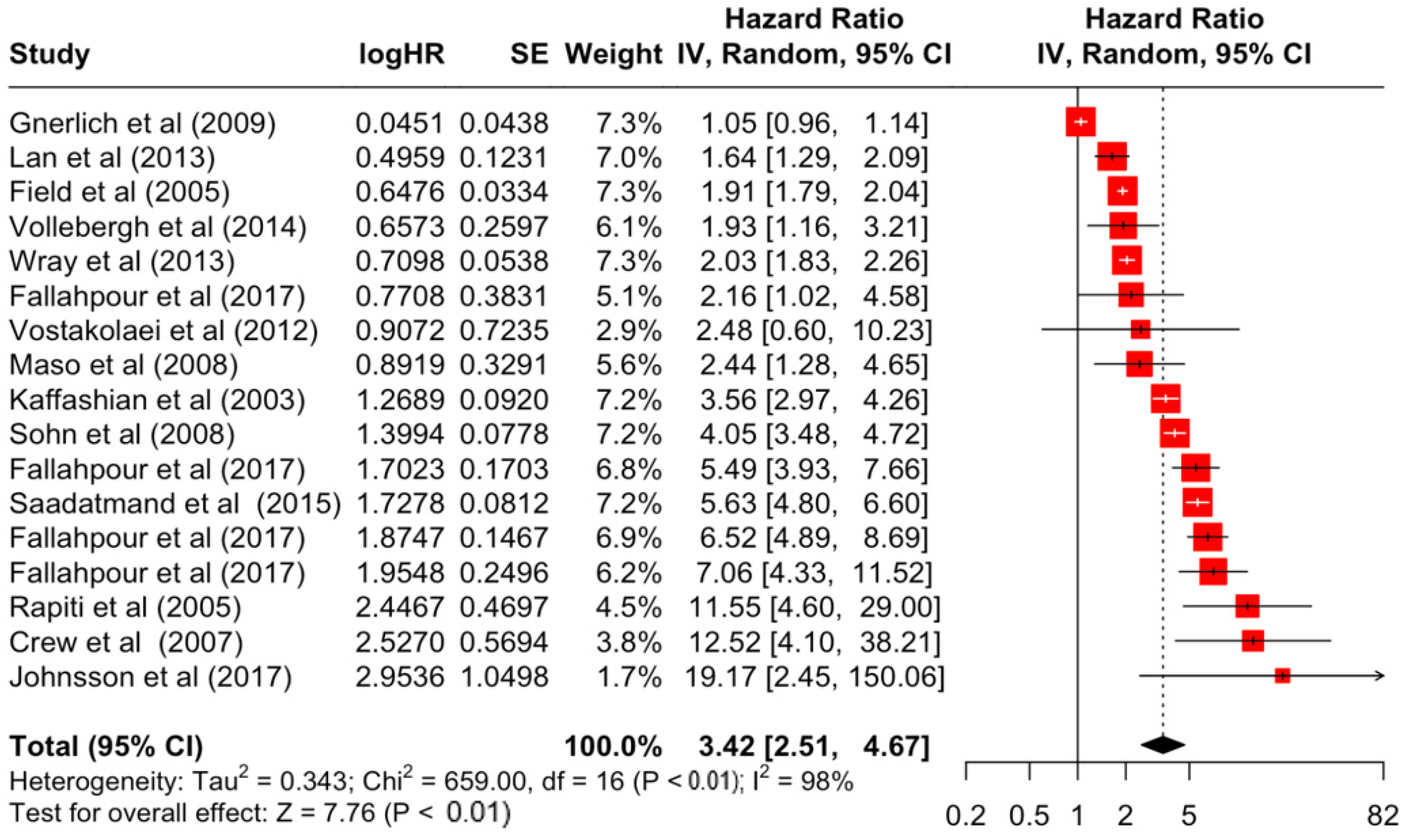
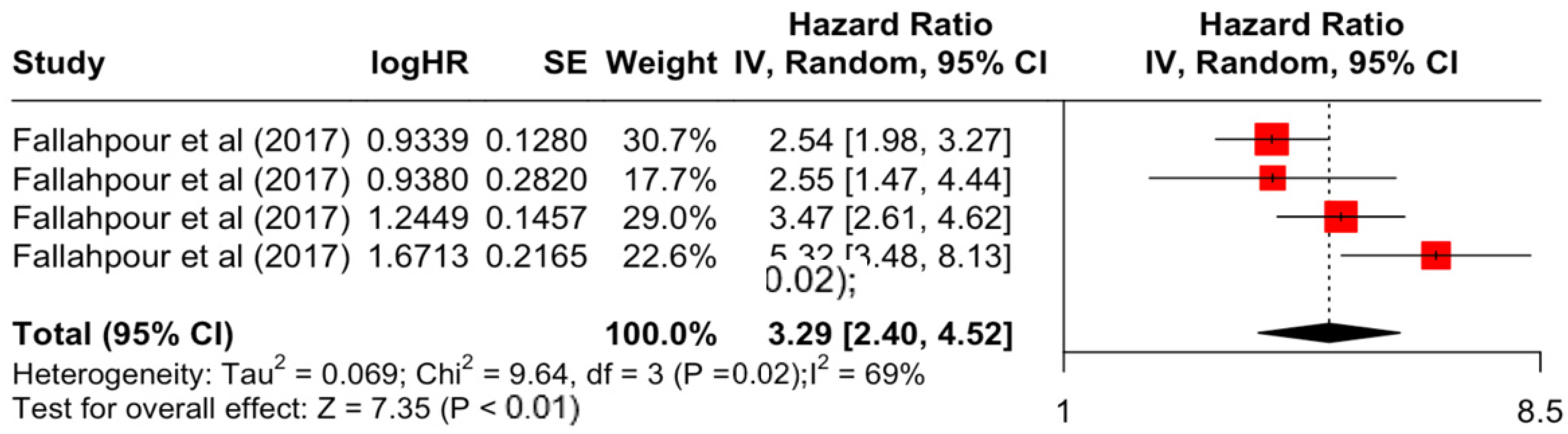
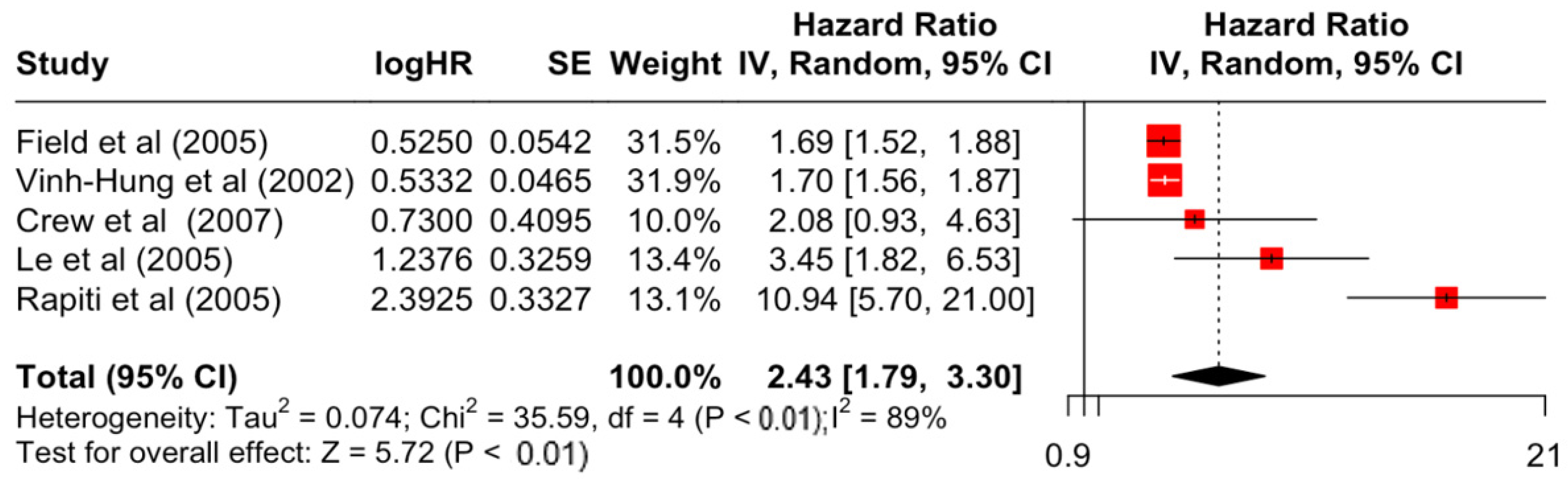
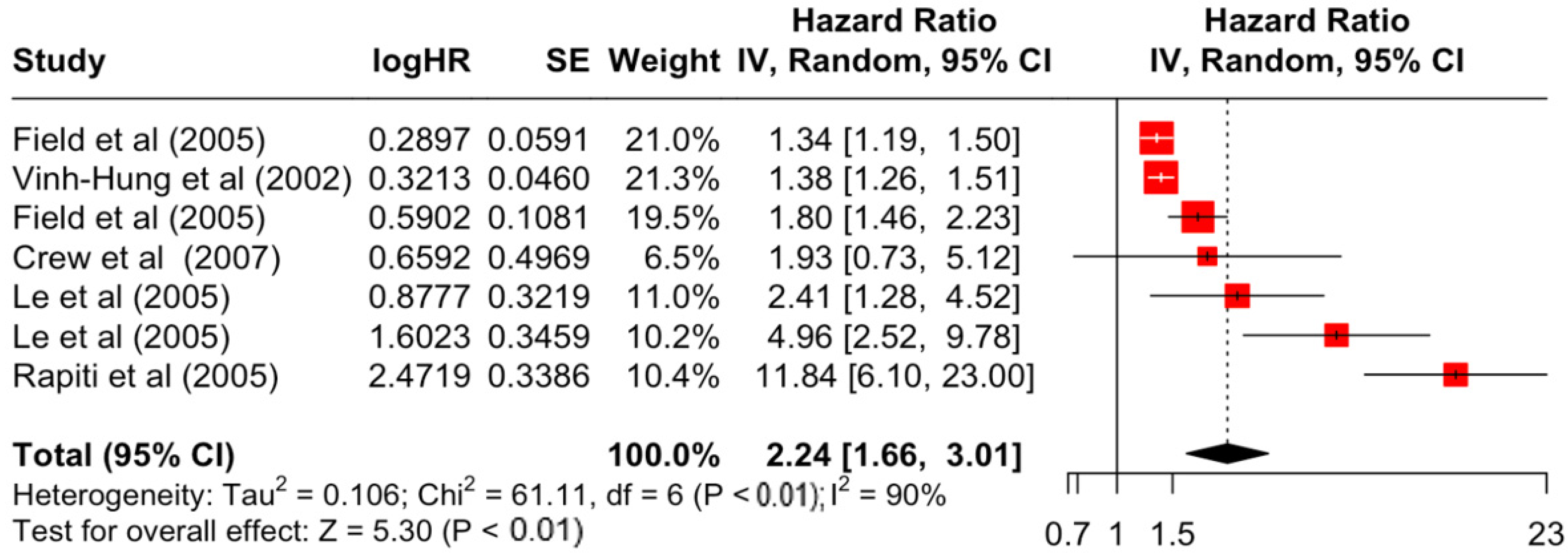
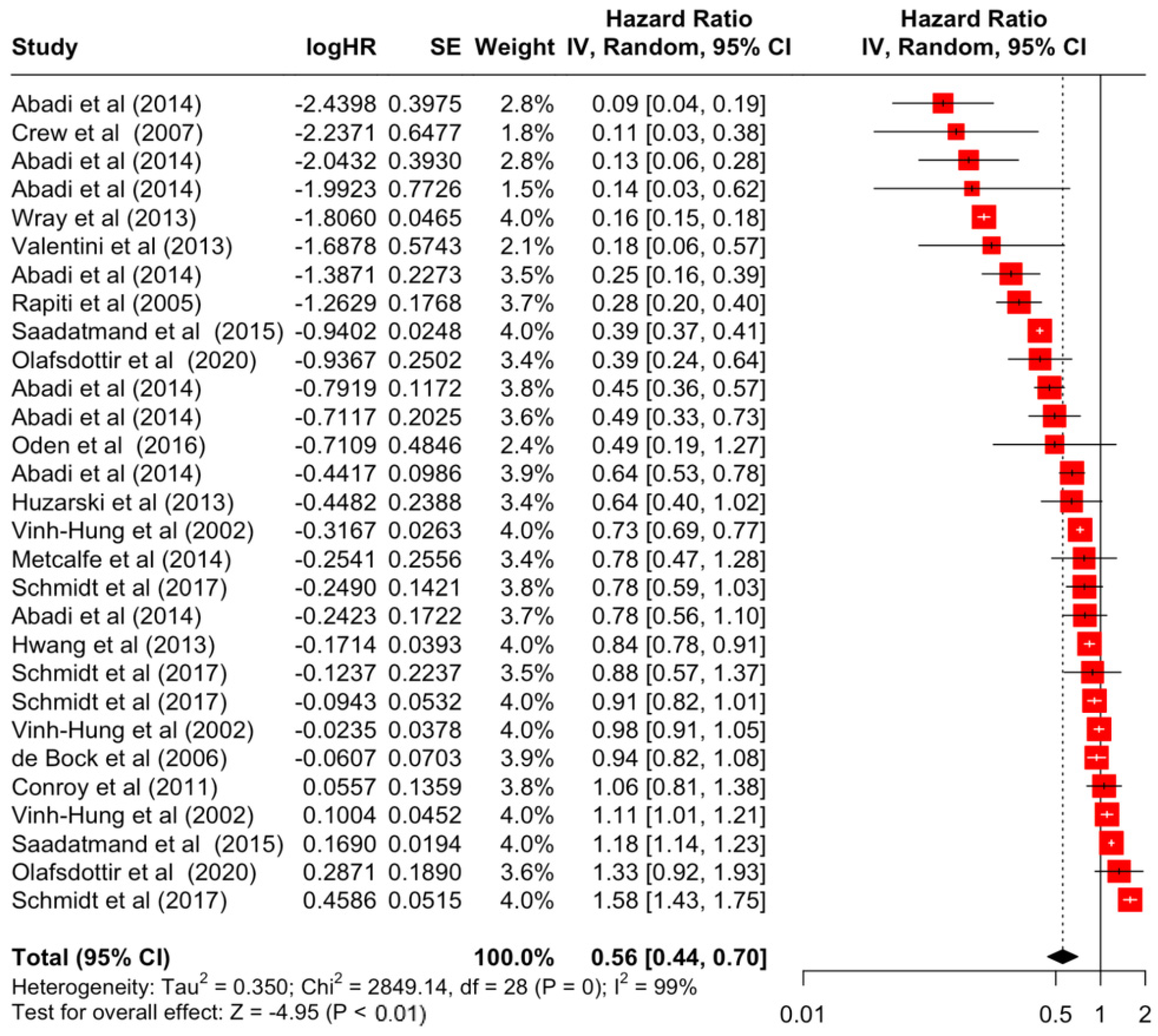
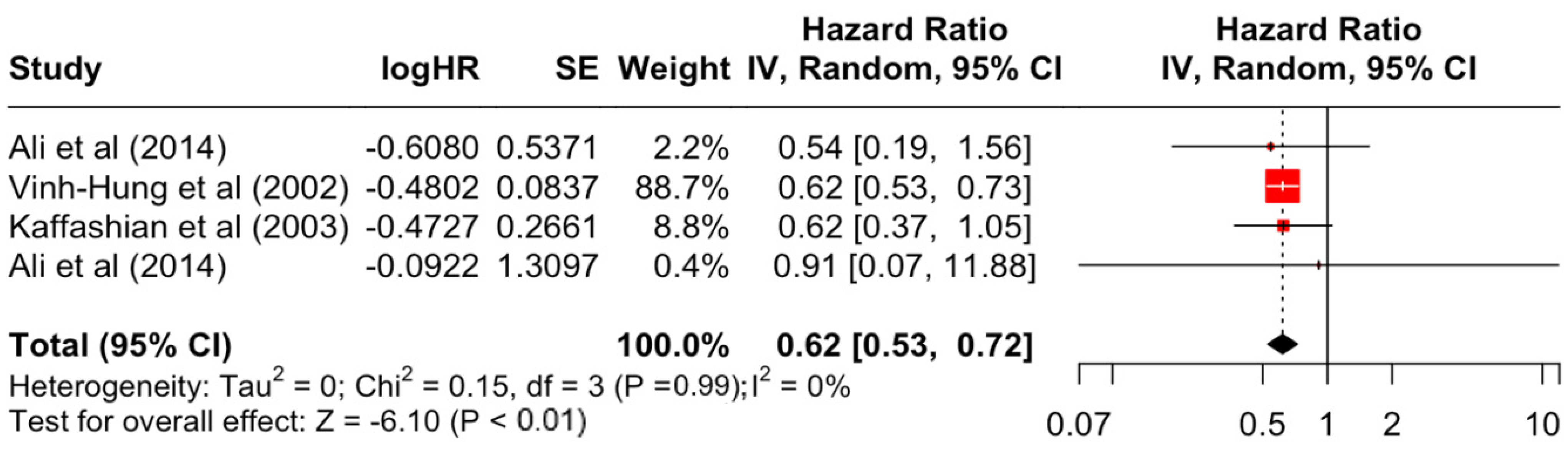
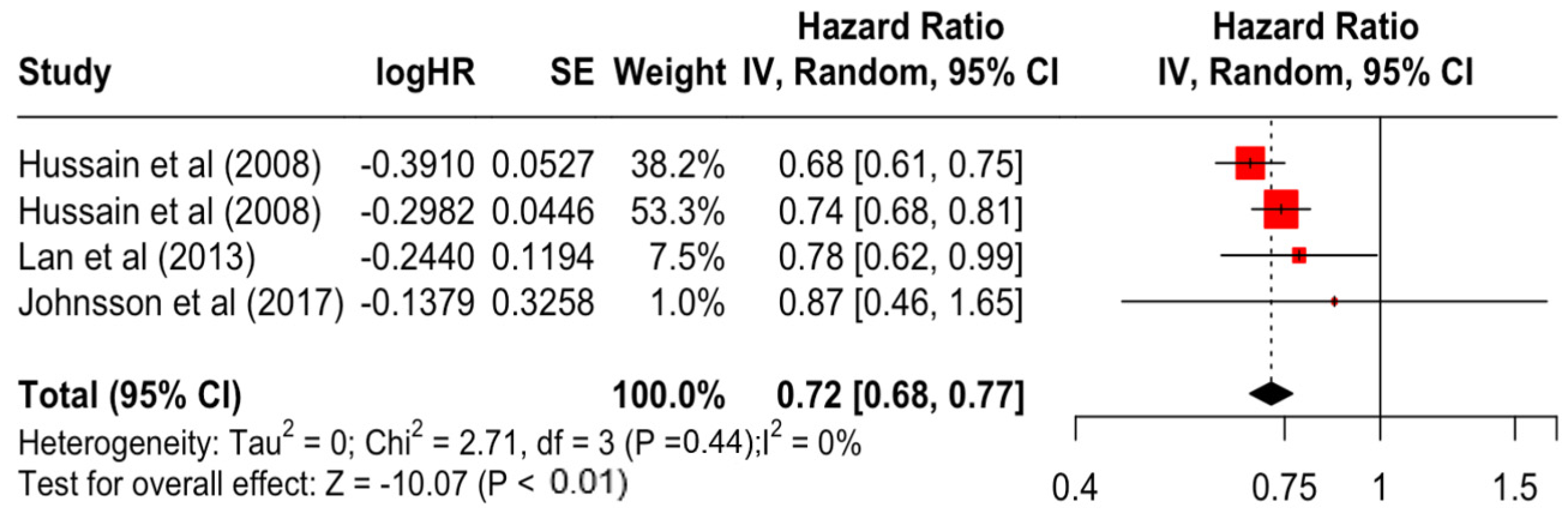
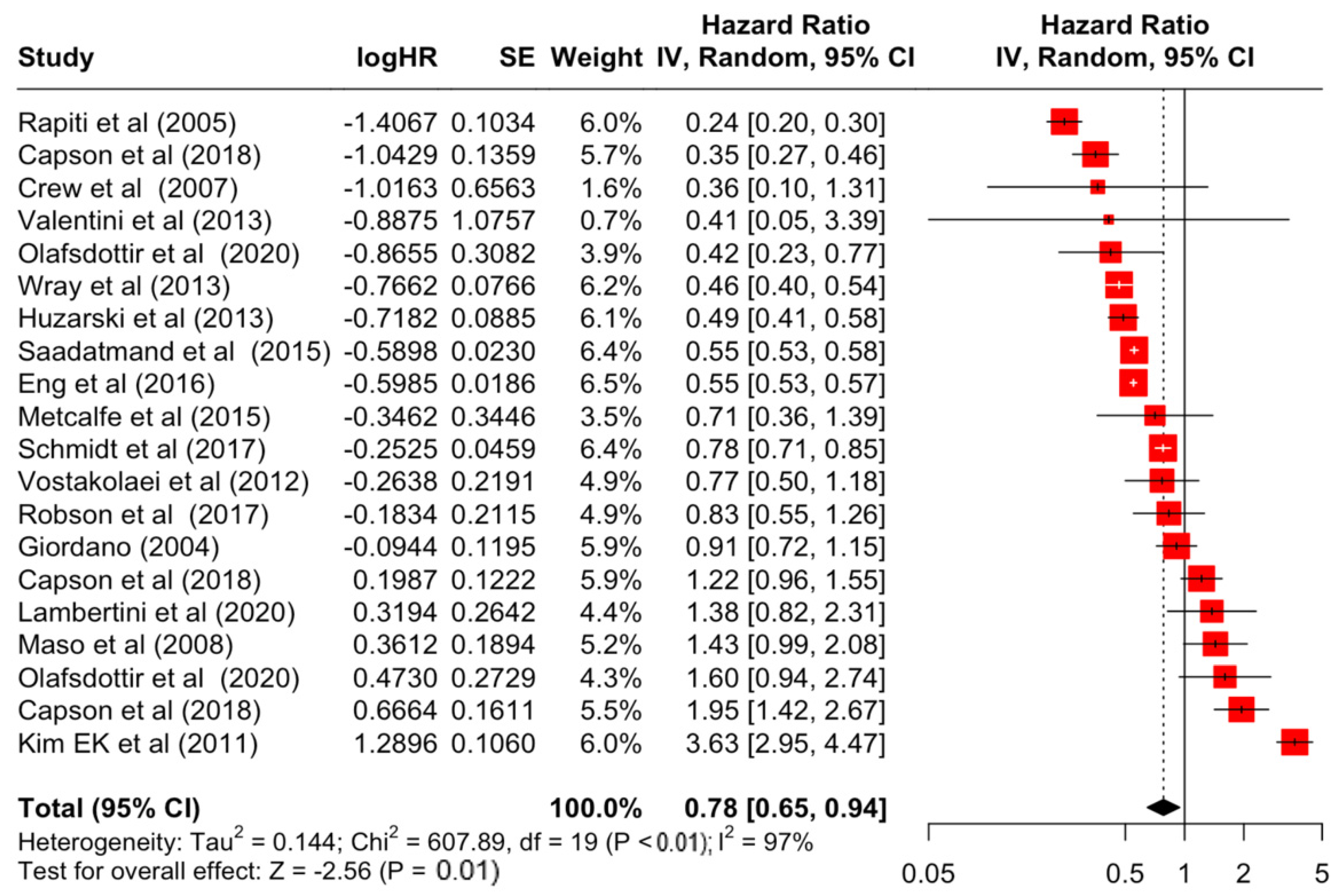
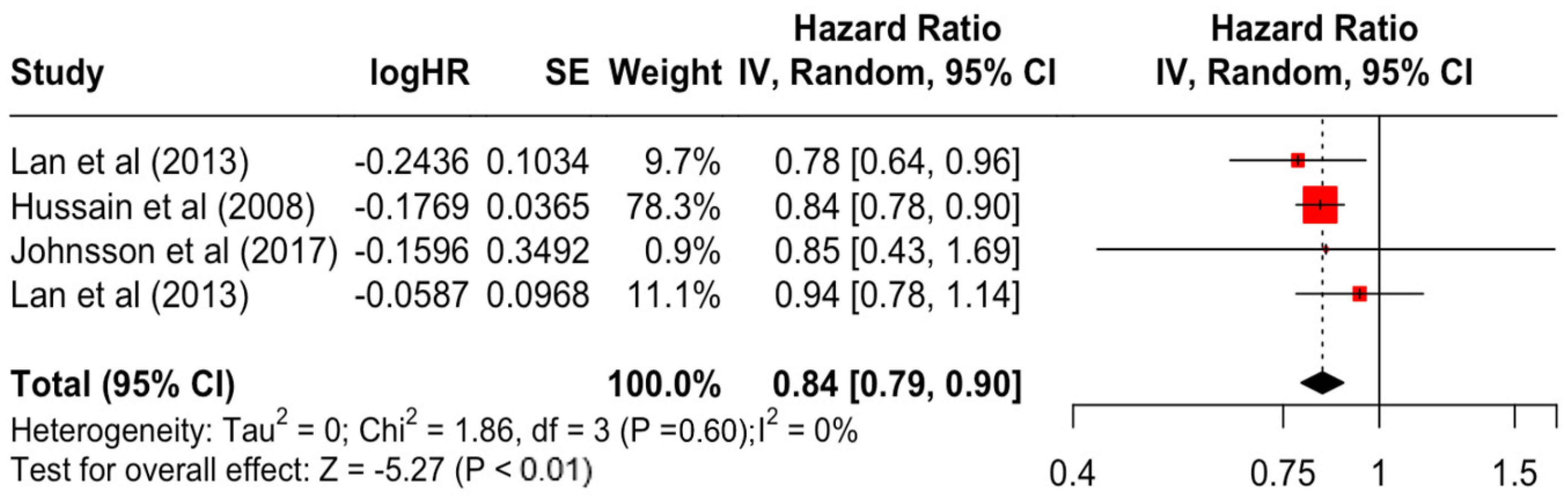
Table 3 presents the pooled estimate of breast cancer-specific survival for each prognostic factor. Following the meta-analysis procedure, we discovered several prognostic factors that are significantly associated with increased or decreased breast cancer survival. The top five poorest prognostic factors were Stage 4 cancer (HR = 12.12; 95% CI: 5.70, 25.76), followed by Stage 3 cancer (HR = 3.42, 95% CI: 2.51, 4.67), comorbidity index ≥ 3 (HR = 3.29; 95% CI: 4.52, 7.35), poor differentiation of cancer cell histology (HR = 2.43; 95% CI: 1.79, 3.30), and undifferentiated cancer cell histology (HR = 2.24; 95% CI: 1.66, 3.01). Other survival-reducing factors include positive nodes, age, race, Human Epidermal Growth factor receptor-2 (HER2) positivity, and body mass index. On the other hand, the top five prognostic factors that improve survival were surgery, including different types of mastectomies and breast-conserving therapies (HR = 0.56; 95% CI: 0.44, 0.70); medullary histology (HR = 0.62; 95% CI: 0.53, 0.72); higher education (HR = 0.72; 95% CI: 0.68, 0.77); positive estrogen receptor status (HR = 0.78; 95% CI: 0.65, 0.94); and secondary-level education (HR = 0.84; 95% CI: 0.79, 0.90). Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11, Figure 12 and Figure 13 illustrate the forest plots of breast cancer-specific survival for the top five survival-reducing and survival-improving factors. The forest plots of the remaining factors can be viewed in Supplementary File S1.

Table 3.
Pooled effects of breast cancer-specific survival by each prognostic factor.
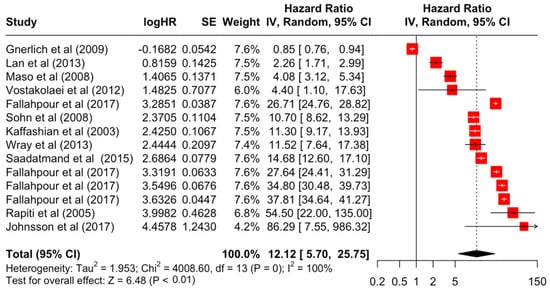
Figure 4.
Forest Plot for Random-effects Hazard Ratio Model of Stage 4.
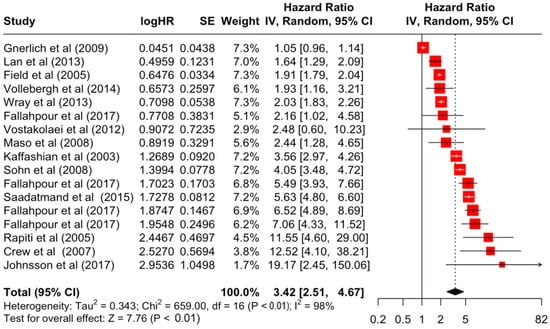
Figure 5.
Forest Plot for Random-effects Hazard Ratio Model of Stage 3.
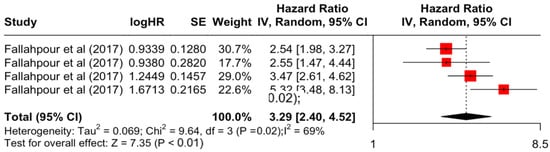
Figure 6.
Forest Plot for Random-effects Hazard Ratio Model of Comorbidity Index (≥3).
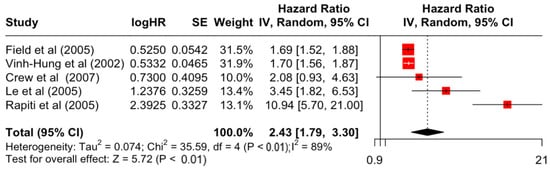
Figure 7.
Forest Plot for Random-effects Hazard Ratio Model of Differentiation (Poor).
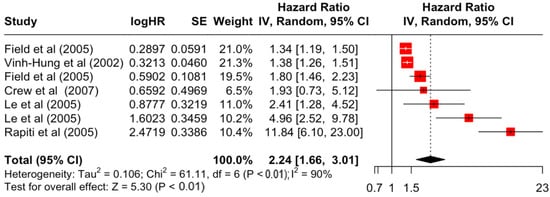
Figure 8.
Forest Plot for Random-effects Hazard Ratio Model of Differentiation (Undifferentiated).
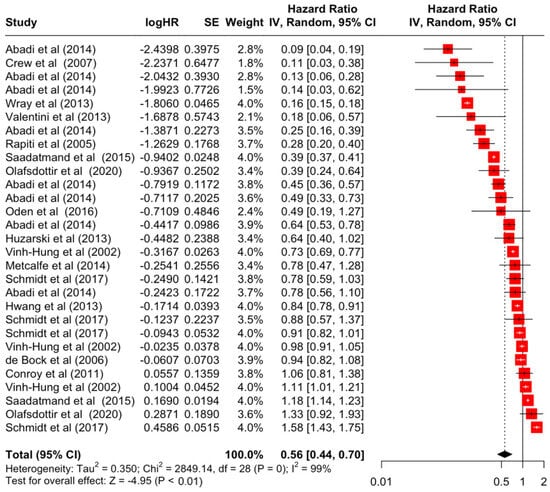
Figure 9.
Forest Plot for Random-effects Hazard Ratio Model of Surgery (Yes).
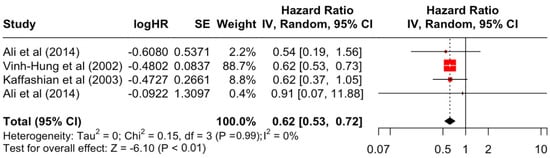
Figure 10.
Forest Plot for Random-effects Hazard Ratio Model of Histology (Medullary).
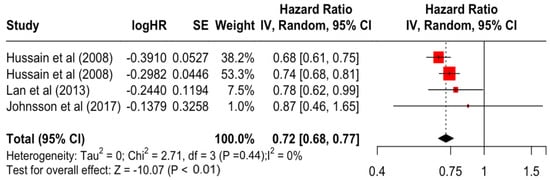
Figure 11.
Forest Plot for Random-effects Hazard Ratio Model of Education (Higher).
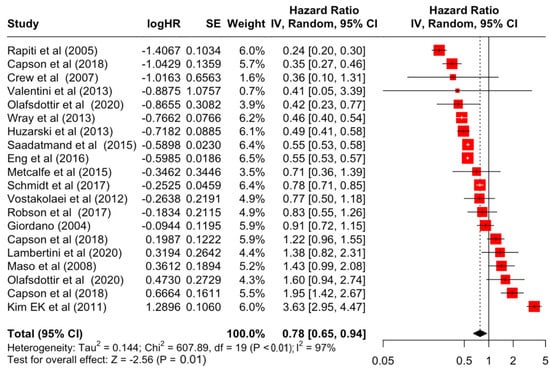
Figure 12.
Forest Plot for Random-effects Hazard Ratio Model of Estrogen Receptor (Positive).
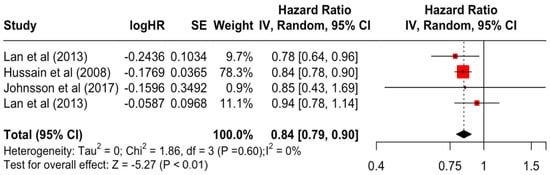
Figure 13.
Forest Plot for Random-effects Hazard Ratio Model of Education (Secondary).
3.3. Heterogeneity
The result from the Q and I2 statistics indicated considerable between-study heterogeneity in all prognostic factors except for age above 60 (I2 = 0%), higher education (I2 = 0%), medullary histology (I2 = 0%), comorbidity index 1 to 2 (I2 = 21%), oral contraceptive use (I2 = 0%), light to moderate physical activity (I2 = 0%), and high to vigorous physical activity (I2 = 18%) (Table 4).

Table 4.
Between-study heterogeneity statistics of each prognostic factor.
3.4. Publication Bias
Funnel plots of each prognostic factor are presented in Supplementary File S2. Egger’s test indicated that publication bias is present in ages 35 to 60, Stage 3, undifferentiated cancer cells, tumour size, overweight/obese, chemotherapy, and radiotherapy (Table 5).

Table 5.
Egger’s test results for publication bias.
3.5. Meta-Regression Analysis
Further exploring the source of heterogeneity, the univariable meta-regression analysis presented in Table 6, showed that there was a significant increase in studies of the above-60 age group (ß = 0.05, p = 0.002) and a significant decrease in studies of the HER2 receptor-negative group (ß = −0.05, p = 0.036) in increasing years of study. No significant change was observed in the sample size or study design.

Table 6.
Meta-regression univariable analysis for the effect of each prognostic factor association with the year of study and sample size.
4. Discussion
In the present systematic review and meta-analysis, we summarised the evidence for breast cancer-specific survival and discovered 13 out of 22 significant prognostic factors including surgery, estrogen receptor status, education, histology, body mass index, HER2 receptor status, race, tumour size, tumour differentiation, age, grade, node affected, comorbidity index, and cancer staging. The results showed that the top factor for improving breast cancer-specific survival was the surgical resection of the primary tumour using different types of mastectomies and breast-conserving therapies, with an increased survival rate of 44%. This reiterated the benefits of surgery by reducing the number of circulating tumour cells and improving disease outcomes, not just for breast cancer, but also potentially for other cancer types [69]. Nevertheless, the studies included in this meta-analysis of surgery were mostly from developed countries such as Canada, the USA, and the Nordic countries, and a clear evidence gap still exists for developing countries. In this meta-analysis, education status is also highlighted, where an increase in one’s level of education leads to an increase in breast cancer-specific survival by 28%. Women with a higher level of education generally have better uptake in breast cancer screening and, therefore, a better likelihood of early detection that results in higher breast cancer incidence and better survival outcome [70].
At the other extreme, breast cancer-specific survival was poorest in those with Stages 3 and 4 advanced cancer, which decreases survival rates by 3 and 12 times, respectively. This result further emphasises the importance of the early detection and diagnosis of breast cancer, and the implementation of screening programmes to provide timely treatment [71]. In this meta-analysis, the results also highlighted the important role of the comorbidity index. A higher score on the comorbidity index is significantly associated with higher mortality by more than three times. The current treatment guidelines for breast cancer have limited recommendations for comorbidities in decision-making, and no guidance to tailor treatments based on comorbidities [72]. This is mainly due to the challenges of obtaining evidence of drug efficacy for patients with comorbidities in clinical trials, which often exclude patients with comorbidities [73].
Other prognostic factors had significant associations but were not as strong as above. In addition, evidence of heterogeneity was observed in most studies and publication bias is present in certain factors including ages 35 to 60, Stage 3 cancer, undifferentiated cancer cells, tumour size, overweight/obese, chemotherapy, and radiotherapy. The source of heterogeneity was explored and did not yield significance. We postulated that due to the large sample size of observational studies selected in this meta-analysis, this would result in higher-power-to-detect, even clinically unimportant, heterogeneity [74].
Limitations of this study need to be considered when interpreting the results. The primary aim of this meta-analysis was to estimate all possible prognostic factors on breast cancer-specific survival; however, due to the restricted number of studies on certain factors, only 22 factors were analysed. A large number of studies originated from developed countries, and caution is warranted when applying the results to developing countries, where evidence is still lacking. Some prognostic factors have a relatively small number of studies. The survival rates of this study are as reported in each study and not distinguished, so they may be 1-, 3-, 5-, or more than 10-year survivals. Regardless of these limitations, this meta-analysis generated conclusive evidence for estimating the top five poorest and best prognostic factors for breast cancer-specific survival.
In conclusion, this meta-analysis estimated the pooled effects of breast cancer-specific survival in a large sample originating from 30 countries. The results highlight the beneficial effects of surgery, higher education, early detection, and the consideration of comorbidities in the treatment of breast cancer patients.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diseases12060111/s1: Supplementary File S1: Forest plots for other factors; Supplementary File S2: contains visualisations of Funnel plots generated for each factor to illustrate the distribution of publication bias, corresponding to the Egger’s test results, which indicated that publication bias is present in ages 35 to 60, Stage 3 cancer, undifferentiated cancer cells, tumour size, overweight/obese, chemotherapy, and radiotherapy.
Author Contributions
H.A.R.; S.N.N.Z.; U.S.S. and A.A.J. contributed to the conception or design of the paper. H.A.R. conducted the data analysis. All authors contributed to the data interpretation and the drafting/editing of the manuscript. All authors were involved in revising the manuscript, provided critical comments, and agreed to be accountable for all aspects of the work and any issues related to the accuracy or integrity of any part of the work. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received in the undertaking of this study.
Data Availability Statement
The data that support the findings of this study are not openly available due to institutional permission but are available from the corresponding author upon reasonable request.
Conflicts of Interest
No authors have conflicts of interest to declare.
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Carter, A.; Casey, D.C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Maajani, K.; Jalali, A.; Alipour, S.; Khodadost, M.; Tohidinik, H.R.; Yazdani, K. The Global and Regional Survival Rate of Women with Breast Cancer: A Systematic Review and Meta-analysis. Clin. Breast Cancer 2019, 19, 165–177. [Google Scholar] [CrossRef]
- Salehi, M.; Gohari, M.R.; Vahabi, N.; Zayeri, F.; Yahyazadeh, S.H.; Kafashian, M.R. Comparison of artificial neural network and cox regression models in survival prediction of breast cancer patients. J. Ilam Univ. Med. Sci. 2013, 21, 120–128. [Google Scholar]
- National Cancer Institute. Measures of Cancer Survival. 2021. Available online: https://surveillance.cancer.gov/survival/measures.html (accessed on 2 October 2023).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, G. meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- E Rohan, T.; Fu, W.; Hiller, J.E. Physical activity and survival from breast cancer. Eur. J. Cancer Prev. 1995, 4, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Homewood, J.; Haviland, J.; Bliss, J.M. Influence of psychological response on breast cancer survival: 10-Year follow-up of a population-based cohort. Eur. J. Cancer 2005, 41, 1710–1714. [Google Scholar] [CrossRef] [PubMed]
- Vinh-Hung, V.; Burzykowski, T.; Van de Steene, J.; Storme, G.; Soete, G. Post-surgery radiation in early breast cancer: Survival analysis of registry data. Radiother. Oncol. 2002, 64, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Kaffashian, F.; Godward, S.; Davies, T.; Solomon, L.; McCann, J.; Duffy, S.W. Socioeconomic effects on breast cancer survival: Proportion attributable to stage and morphology. Br. J. Cancer 2003, 89, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.; Lynch, H.T.; Ghadirian, P.; Tung, N.; Olivotto, I.; Warner, E.; Olopade, O.I.; Eisen, A.; Weber, B.; McLennan, J.; et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 2004, 22, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, S.M.; Shu, X.-O.; Ruan, Z.X.; Dai, Q.; Cai, Q.; Gao, Y.-T.; Zheng, W. Soyfood intake and breast cancer survival: A followup of the Shanghai Breast Cancer Study. Breast Cancer Res. Treat. 2005, 92, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Rapiti, E.; Fioretta, G.; Verkooijen, H.M.; Vlastos, G.; Schäfer, P.; Sappino, A.-P.; Kurtz, J.; Neyroud-Caspar, I.; Bouchardy, C. Survival of young and older breast cancer patients in Geneva from 1990 to 2001. Eur. J. Cancer 2005, 41, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Le, G.M.; O’Malley, C.D.; Glaser, S.L.; Lynch, C.F.; Stanford, J.L.; Keegan, T.H.; West, D.W. Breast implants following mastectomy in women with early-stage breast cancer: Prevalence and impact on survival. Breast Cancer Res. BCR 2005, 7, 184–193. [Google Scholar] [CrossRef]
- Field, T.S.; Buist, D.S.M.; Doubeni, C.; Enger, S.; Fouayzi, H.; Hart, G.; Korner, E.J.; Lamerato, L.; Bachman, D.J.; Ellis, J.; et al. Disparities and survival among breast cancer patients. J. Natl. Cancer Institute. Monogr. 2005, 01605, 88–95. [Google Scholar] [CrossRef]
- de Bock, G.H.; van der Hage, J.A.; Putter, H.; Bonnema, J.; Bartelink, H.; van de Velde, C.J. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: Long-term results of European Organisation for Research and Treatment of Cancer studies. Eur. J. Cancer 2006, 42, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.N.; Steck, S.E.; Wolff, M.S.; Britton, J.A.; Kabat, G.C.; Gaudet, M.M.; Abrahamson, P.E.; Bell, P.; Schroeder, J.C.; Teitelbaum, S.L.; et al. Dietary flavonoid intake and breast cancer survival among women on long island. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Sohn, V.Y.; Arthurs, Z.M.; Sebesta, J.A.; Brown, T.A. Primary tumor location impacts breast cancer survival. Am. J. Surg. 2008, 195, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.K.; Altieri, A.; Sundquist, J.; Hemminki, K. Influence of education level on breast cancer risk and survival in Sweden between 1990 and 2004. Int. J. Cancer 2008, 122, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Dal Maso, L.; Zucchetto, A.; Talamini, R.; Serraino, D.; Stocco, C.F.; Vercelli, M.; Falcini, F.; Franceschi, S. Prospective Analysis of Case-Control Studies on Environmental Factors and Health (PACE) Study Group. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int. J. Cancer 2008, 123, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.B.; Trentham-Dietz, A.; Egan, K.M.; Titus-Ernstoff, L.; Holmes, M.D.; Bersch, A.J.; Holick, C.N.; Hampton, J.M.; Stampfer, M.J.; Willett, W.C. Body mass index before and after breast cancer diagnosis: Associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Gnerlich, J.L.; Deshpande, A.D.; Jeffe, D.B.; Sweet, A.; White, N.; Margenthaler, J.A. Elevated Breast Cancer Mortality in Women Younger than Age 40 Years Compared with Older Women Is Attributed to Poorer Survival in Early-Stage Disease. J. Am. Coll. Surg. 2009, 208, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Peel, J.B.; Sui, X.; Adams, S.A.; Hébert, J.R.; Hardin, J.W.; Blair, S.N. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med. Sci. Sports Exerc. 2009, 41, 742–748. [Google Scholar] [CrossRef]
- Powe, D.G.; Voss, M.J.; Zänker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.A.C.; Stefanick, M.L.; Saquib, N.; Natarajan, L.; Patterson, R.E.; Bardwell, W.; Flatt, S.W.; Newman, V.A.; Rock, C.L.; Thomson, C.A.; et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: Findings from the WHEL Study. Cancer Causes Control 2011, 22, 427–435. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, M.-B.; Gunnarsdóttir, K.Á.; Højris, I.; Jakobsen, E.H.; Nielsen, D.; Stenbygaard, L.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.M.; Maskarinec, G.; Wilkens, L.R.; White, K.K.; Henderson, B.E.; Kolonel, L.N. Obesity and breast cancer survival in ethnically diverse postmenopausal women: The Multiethnic Cohort Study. Breast Cancer Res. Treat. 2011, 129, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Noh, W.C.; Han, W.; Noh, D. Prognostic significance of young age (<35 Years) by subtype based on ER, PR, and HER2 status in breast cancer: A nationwide registry-based study. World J. Surg. 2011, 35, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Colzani, E.; Liljegren, A.; Johansson, A.L.; Adolfsson, J.; Hellborg, H.; Hall, P.F.; Czene, K. Prognosis of patients with breast cancer: Causes of death and effects of time since diagnosis, age, and tumor characteristics. J. Clin. Oncol. 2011, 29, 4014–4021. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Phillips, K.-A.; West, D.W.; Ennis, M.; Hopper, J.L.; John, E.M.; O’Malley, F.P.; Milne, R.L.; Andrulis, I.L.; Friedlander, M.L.; et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: An international prospective breast cancer family registry population-based cohort study. J. Clin. Oncol. 2012, 30, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Alexander, B.; Schnitt, S.J.; Comander, A.; Gallagher, B.; Garber, J.E.; Tung, N. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer 2011, 117, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Vostakolaei, F.A.; Broeders, M.J.M.; Rostami, N.; van Dijck, J.A.A.M.; Feuth, T.; Kiemeney, L.A.L.M.; Verbeek, A.L.M. Age at Diagnosis and Breast Cancer Survival in Iran. Int. J. Breast Cancer 2012, 2012, 517976. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Haghighat, S.; Khayamzadeh, M.; Moradi, A.; Ghanbari-Motlagh, A.; Mirzaei, H.; Esmail-Akbari, M. Survival rate of breast cancer based on geographical variation in Iran: A national study. Iran. Red Crescent Med. J. 2012, 14, 798–804. [Google Scholar] [CrossRef]
- Lan, N.; Laohasiriwong, W.; Stewart, J. Survival probability and prognostic factors for breast cancer patients in Vietnam. Glob. Health Action 2013, 6, 18860. [Google Scholar] [CrossRef]
- Keegan, T.H.M.; Press, D.J.; Tao, L.; DeRouen, M.C.; Kurian, A.W.; A Clarke, C.; Gomez, S.L. Impact of breast cancer subtypes on 3-year survival among adolescent and young adult women. Breast Cancer Res. 2013, 15, R95. [Google Scholar] [CrossRef]
- Hwang, E.S.; Lichtensztajn, D.Y.; Gomez, S.L.; Fowble, B.; Clarke, C.A. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: The effect of age and hormone receptor status. Cancer 2013, 119, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.; Lu, W.; Zheng, Y.; Cai, H.; Bao, P.-P.; Gu, K.; Zheng, W.; Shu, X.O. Comorbidities and breast cancer survival: A report from the Shanghai Breast Cancer Survival Study. Breast Cancer Res. Treat. 2013, 139, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Lubinski, J.; Byrski, T.; Ghadirian, P.; Moller, P.; Lynch, H.T.; Ainsworth, P.; Neuhausen, S.L.; Weitzel, J.; The Hereditary Breast Cancer Clinical Study Group; et al. The impact of pregnancy on breast cancer survival in women who carry a BRCA1 or BRCA2 mutation. Breast Cancer Res. Treat. 2013, 142, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.J.; Phatak, U.R.; Robinson, E.K.; Wiatek, R.L.; Rieber, A.G.; Gonzalez, A.; Ko, T.C.; Kao, L.S. The effect of age on race-related breast cancer survival disparities. Ann. Surg. Oncol. 2013, 20, 2541–2547. [Google Scholar] [CrossRef]
- Huzarski, T.; Byrski, T.; Gronwald, J.; Górski, B.; Domagała, P.; Cybulski, C.; Oszurek, O.; Szwiec, M.; Gugała, K.; Stawicka, M.; et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J. Clin. Oncol. 2013, 31, 3191–3196. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.-J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- de Glas, N.A.; de Craen, A.J.M.; Bastiaannet, E.; Op’t Land, E.G.; Kiderlen, M.; van de Water, W.; Siesling, S.; Portielje, J.E.A.; Schuttevaer, H.M.; de Bock, G.T.H. Effect of implementation of the mass breast cancer screening programme in older women in the Netherlands: Population based study. BMJ 2014, 349. [Google Scholar] [CrossRef]
- Abadi, A.; Yavari, P.; Dehghani-Arani, M.; Alavi-Majd, H.; Ghasemi, E.; Amanpour, F.; Bajdik, C. Cox models survival analysis based on breast cancer treatments. Iran. J. Cancer Prev. 2014, 7, 124–129. [Google Scholar]
- Vollebergh, M.A.; Lips, E.H.; Nederlof, P.M.; Wessels, L.F.A.; Wesseling, J.; Vd Vijver, M.J.; de Vries, E.G.E.; Van Tinteren, H.; Jonkers, J.; Hauptmann, M.; et al. Genomic patterns resembling BRCA1- and BRCA2-mutated breast cancers predict benefit of intensified carboplatin-based chemotherapy. Breast Cancer Res. 2014, 16, R47. [Google Scholar] [CrossRef] [PubMed]
- Kriege, M.; Hollestelle, A.; Jager, A.; E A Huijts, P.; Berns, E.M.; Sieuwerts, A.M.; Gelder, M.E.M.-V.; Collée, J.M.; Devilee, P.; Hooning, M.J.; et al. Survival and contralateral breast cancer in CHEK2 1100delC breast cancer patients: Impact of adjuvant chemotherapy. Br. J. Cancer 2014, 111, 1004–1013. [Google Scholar] [CrossRef]
- Saadatmand, S.; Bretveld, R.; Siesling, S.; A Tilanus-Linthorst, M.M. Influence of tumour stage at breast cancer detection on survival in modern times: Population based study in 173,797 patients. BMJ 2015, 351, h4901. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.G.; Malmgren, J.A.; Atwood, M.K.; Calip, G.S. Effect of treatment and mammography detection on breast cancer survival over time: 1990-2007. Cancer 2015, 121, 2553–2561. [Google Scholar] [CrossRef]
- Eng, L.G.; Dawood, S.; Sopik, V.; Haaland, B.; Tan, P.S.; Bhoo-Pathy, N.; Warner, E.; Iqbal, J.; Narod, S.A.; Dent, R. Ten-year survival in women with primary stage IV breast cancer. Breast Cancer Res. Treat. 2016, 160, 145–152. [Google Scholar] [CrossRef]
- Kim, J.; Mersereau, J.E.; Khankari, N.; Bradshaw, P.T.; McCullough, L.E.; Cleveland, R.; Shantakumar, S.; Teitelbuam, S.L.; Neugut, A.I.; Senie, R.T. Polycystic ovarian syndrome (PCOS), related symptoms/sequelae, and breast cancer risk in a population-based case–control study. Cancer Causes Control. 2016, 27, 403–414. [Google Scholar] [CrossRef]
- Kataoka, A.; Iwamoto, T.; Tokunaga, E.; Tomotaki, A.; Kumamaru, H.; Miyata, H.; Niikura, N.; Kawai, M.; Anan, K.; Hayashi, N.; et al. Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: A study from the Japanese Breast Cancer Registry. Breast Cancer Res. Treat. 2016, 160, 163–172. [Google Scholar] [CrossRef]
- Partridge, A.H.; Hughes, M.E.; Warner, E.T.; Ottesen, R.A.; Wong, Y.-N.; Edge, S.B.; Theriault, R.L.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J. Clin. Oncol. 2016, 34, 3308–3314. [Google Scholar] [CrossRef]
- Odén, L.; Akbari, M.; Zaman, T.; Singer, C.F.; Sun, P.; Narod, S.A.; Salmena, L.; Kotsopoulos, J. Plasma osteoprotegerin and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Oncotarget 2016, 7, 86687–86694. [Google Scholar] [CrossRef]
- Chagpar, A.B.; Hatzis, C.; Pusztai, L.; DiGiovanna, M.P.; Moran, M.; Mougalian, S.; Sanft, T.; Evans, S.; Hofstatter, E.; Wilson, L.D.; et al. Association of LN Evaluation with Survival in Women Aged 70 Years or Older with Clinically Node-Negative Hormone Receptor Positive Breast Cancer. Ann. Surg. Oncol. 2017, 24, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, A.; Broberg, P.; Krüger, U.; Johnsson, A.; Tornberg, B.; Olsson, H. Physical activity and survival following breast cancer. Eur. J. Cancer Care 2019, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fallahpour, S.; Navaneelan, T.; De, P.; Borgo, A. Breast cancer survival by molecular subtype: A population-based analysis of cancer registry data. CMAJ Open 2017, 5, E734–E739. [Google Scholar] [CrossRef]
- Schmidt, M.K.; Van Den Broek, A.J.; Tollenaar, R.A.; Smit, V.T.; Westenend, P.; Brinkhuis, M.; Oosterhuis, W.J.W.; Wesseling, J.; Janssen-Heijnen, M.L.; Jobsen, J.J.; et al. Breast Cancer Survival of BRCA1/BRCA2 Mutation Carriers in a Hospital-Based Cohort of Young Women. J. Natl. Cancer Inst. 2017, 109, djw329. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.Y.; Tham, W.Y.; Nei, W.L.; Lim, C.; Miao, H. Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy. Cancer Commun. 2018, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; I Cutress, R.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Ameye, L.; Hamy, A.-S.; Zingarello, A.; Poorvu, P.D.; Carrasco, E.; Grinshpun, A.; Han, S.; Rousset-Jablonski, C.; Ferrari, A.; et al. Pregnancy after breast cancer in patients with germline BRCA mutations. J. Clin. Oncol. 2020, 38, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, E.J.; Borg, A.; Jensen, M.-B.; Gerdes, A.-M.; Johansson, A.L.V.; Barkardottir, R.B.; Johannsson, O.T.; Ejlertsen, B.; Sonderstrup, I.M.H.; Hovig, E.; et al. Breast cancer survival in Nordic BRCA2 mutation carriers—Unconventional association with oestrogen receptor status. Br. J. Cancer 2020, 123, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- De Talhouet, S.; Peron, J.; Vuilleumier, A.; Friedlaender, A.; Viassolo, V.; Ayme, A.; Bodmer, A.; Treilleux, I.; Lang, N.; Tille, J.C.; et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci. Rep. 2020, 10, 7073. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.; Freedman, R.A.; Partridge, A.H. The impact of young age at diagnosis (age <40 years) on prognosis varies by breast cancer subtype: A U.S. SEER database analysis. Breast 2022, 61, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Headon, H.; Wazir, U.; Kasem, A.; Mokbel, K. Surgical treatment of the primary tumour improves the overall survival in patients with metastatic breast cancer: A systematic review and meta-analysis. Mol Clin Oncol 2016, 4, 863–867. [Google Scholar] [CrossRef]
- Lundqvist, A.; Andersson, E.; Ahlberg, I.; Nilbert, M.; Gerdtham, U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe—A systematic review and meta-analysis. Eur. J. Public Health 2016, 26, 804–813. [Google Scholar] [CrossRef]
- de Lemos, L.L.P.; de Souza, M.C.; Moreira, D.P.; Almeida, P.H.R.F.; Godman, B.; Verguet, S.; Guerra, A.A.; Cherchiglia, M.L. Stage at diagnosis and stage-specific survival of breast cancer in Latin America and the Caribbean: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0224012. [Google Scholar] [CrossRef]
- Salas, M.; Henderson, M.; Sundararajan, M.; Tu, N.; Islam, Z.; Ebeid, M.; Horne, L. Use of comorbidity indices in patients with any cancer, breast cancer, and human epidermal growth factor receptor-2-positive breast cancer: A systematic review. PLoS ONE 2021, 16, e0252925. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Rumble, R.B.; Carey, L.A.; Come, S.E.; Davidson, N.E.; Di Leo, A.; Gralow, J.; Hortobagyi, G.N.; Moy, B.; Yee, D.; et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2–negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 3307. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Henley, J.; Thun, M. All-cause and cause-specific death rates by educational status for two million people in two American Cancer Society cohorts, 1959–1996. Am. J. Epidemiol. 2002, 156, 11–21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).