Prognostic Factors Associated with Breast Cancer-Specific Survival from 1995 to 2022: A Systematic Review and Meta-Analysis of 1,386,663 Cases from 30 Countries
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
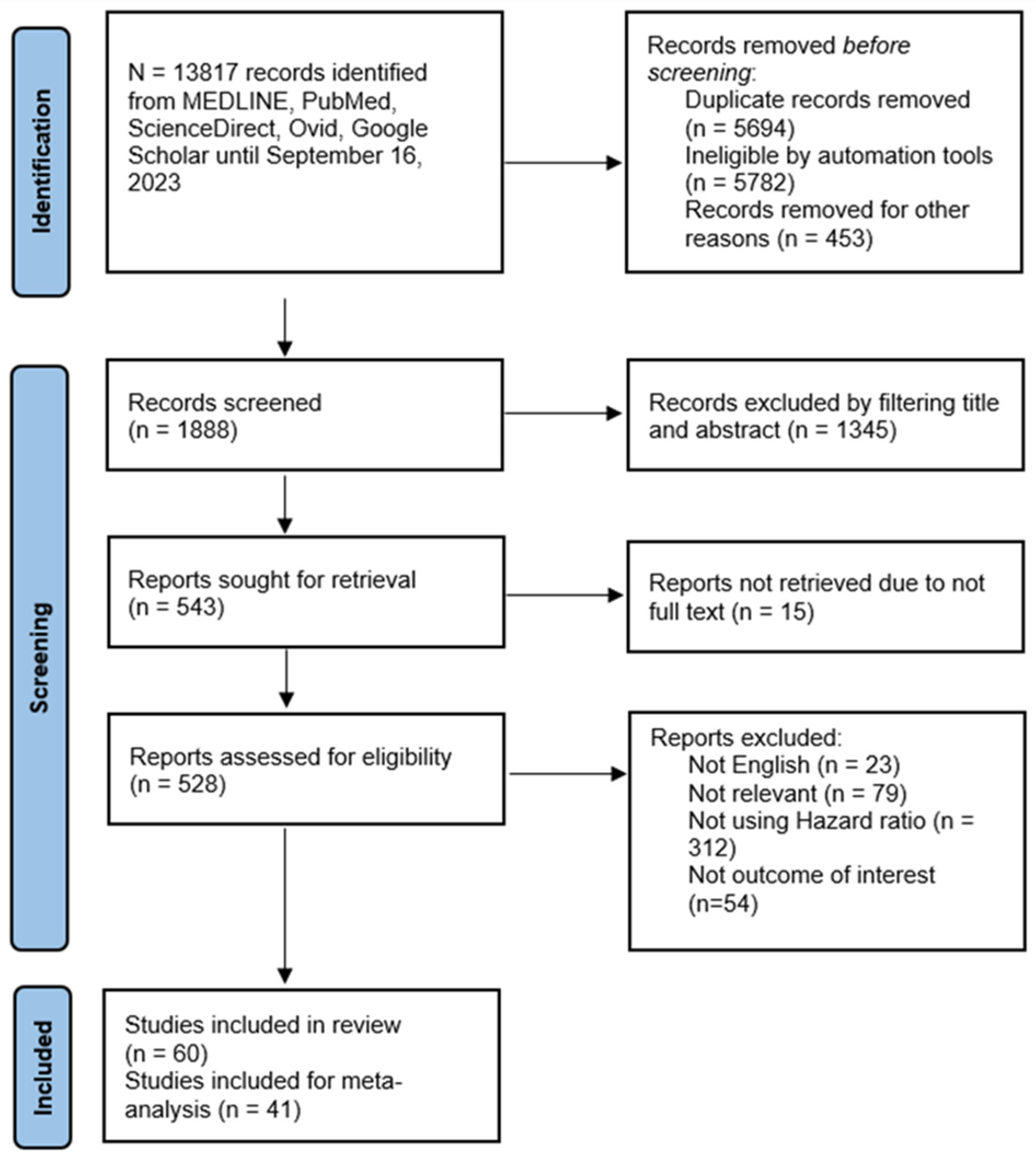
2.3. Study Selection
2.4. Quality Assessment
2.5. Data Extraction
2.6. Statistical Analyses
3. Results
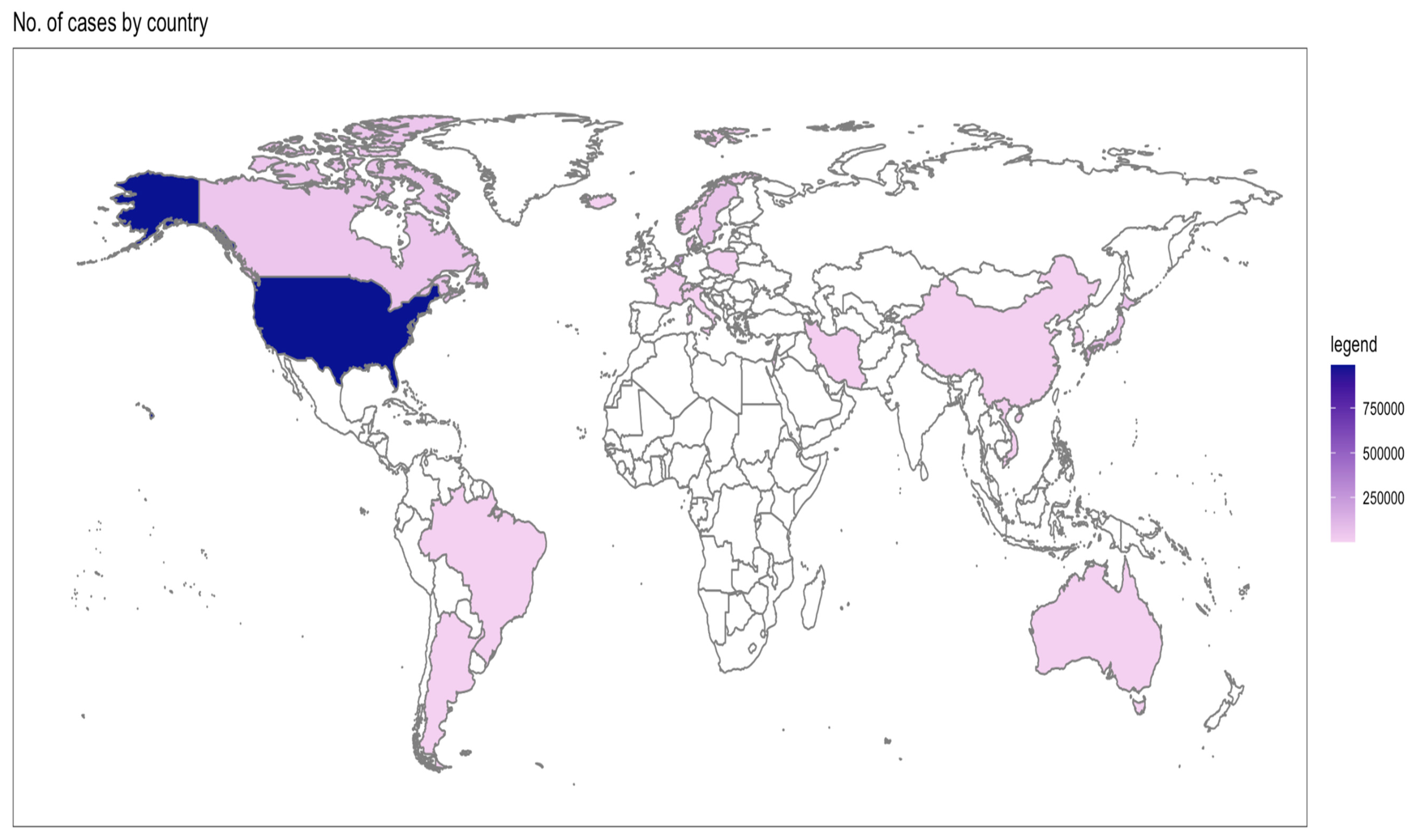
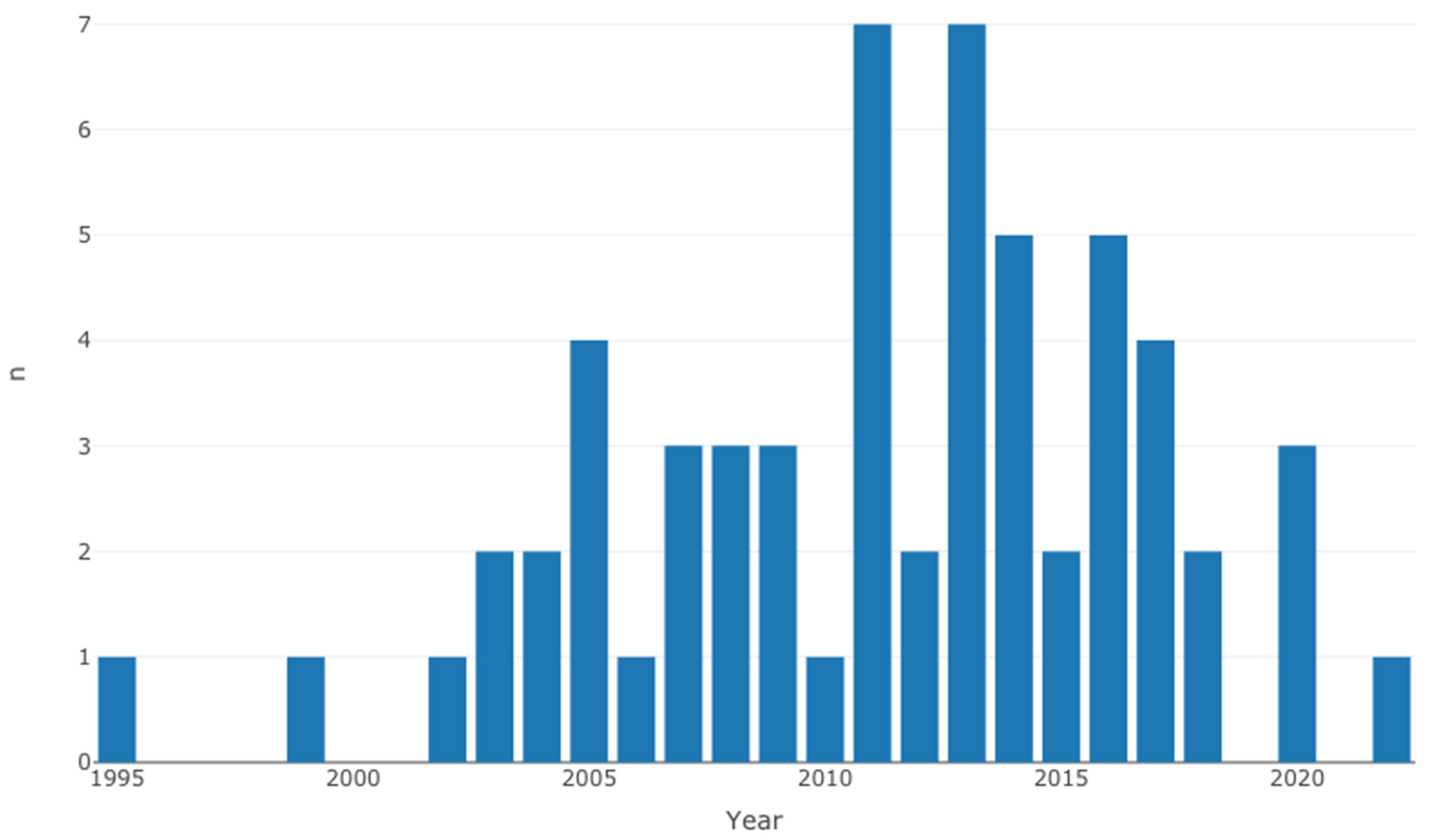
3.1. Study Characteristics
3.2. Pooled Effects of Breast Cancer-Specific Survival
3.3. Heterogeneity
3.4. Publication Bias
3.5. Meta-Regression Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; Dandona, L.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Naghavi, M.; Allen, C.; Barber, R.M.; Carter, A.; Casey, D.C. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1459–1544. [Google Scholar] [CrossRef]
- Azamjah, N.; Soltan-Zadeh, Y.; Zayeri, F. Global Trend of Breast Cancer Mortality Rate: A 25-Year Study. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 2015–2020. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.S.M.; Vieira, A.R.; Aune, D.; Bandera, E.V.; Greenwood, D.C.; McTiernan, A.; Rosenblatt, D.N.; Thune, I.; Vieira, R.; Norat, T. Body mass index and survival in women with breast cancer—Systematic literature review and meta-analysis of 82 follow-up studies. Ann. Oncol. 2014, 25, 1901–1914. [Google Scholar] [CrossRef]
- Maajani, K.; Jalali, A.; Alipour, S.; Khodadost, M.; Tohidinik, H.R.; Yazdani, K. The Global and Regional Survival Rate of Women with Breast Cancer: A Systematic Review and Meta-analysis. Clin. Breast Cancer 2019, 19, 165–177. [Google Scholar] [CrossRef]
- Salehi, M.; Gohari, M.R.; Vahabi, N.; Zayeri, F.; Yahyazadeh, S.H.; Kafashian, M.R. Comparison of artificial neural network and cox regression models in survival prediction of breast cancer patients. J. Ilam Univ. Med. Sci. 2013, 21, 120–128. [Google Scholar]
- National Cancer Institute. Measures of Cancer Survival. 2021. Available online: https://surveillance.cancer.gov/survival/measures.html (accessed on 2 October 2023).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, G. meta: An R package for meta-analysis. R News 2007, 7, 40–45. [Google Scholar]
- Viechtbauer, W. Conducting meta-analyses in R with the metafor package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- E Rohan, T.; Fu, W.; Hiller, J.E. Physical activity and survival from breast cancer. Eur. J. Cancer Prev. 1995, 4, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Homewood, J.; Haviland, J.; Bliss, J.M. Influence of psychological response on breast cancer survival: 10-Year follow-up of a population-based cohort. Eur. J. Cancer 2005, 41, 1710–1714. [Google Scholar] [CrossRef] [PubMed]
- Vinh-Hung, V.; Burzykowski, T.; Van de Steene, J.; Storme, G.; Soete, G. Post-surgery radiation in early breast cancer: Survival analysis of registry data. Radiother. Oncol. 2002, 64, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Kaffashian, F.; Godward, S.; Davies, T.; Solomon, L.; McCann, J.; Duffy, S.W. Socioeconomic effects on breast cancer survival: Proportion attributable to stage and morphology. Br. J. Cancer 2003, 89, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.; Lynch, H.T.; Ghadirian, P.; Tung, N.; Olivotto, I.; Warner, E.; Olopade, O.I.; Eisen, A.; Weber, B.; McLennan, J.; et al. Contralateral breast cancer in BRCA1 and BRCA2 mutation carriers. J. Clin. Oncol. 2004, 22, 2328–2335. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, S.M.; Shu, X.-O.; Ruan, Z.X.; Dai, Q.; Cai, Q.; Gao, Y.-T.; Zheng, W. Soyfood intake and breast cancer survival: A followup of the Shanghai Breast Cancer Study. Breast Cancer Res. Treat. 2005, 92, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Rapiti, E.; Fioretta, G.; Verkooijen, H.M.; Vlastos, G.; Schäfer, P.; Sappino, A.-P.; Kurtz, J.; Neyroud-Caspar, I.; Bouchardy, C. Survival of young and older breast cancer patients in Geneva from 1990 to 2001. Eur. J. Cancer 2005, 41, 1446–1452. [Google Scholar] [CrossRef] [PubMed]
- Le, G.M.; O’Malley, C.D.; Glaser, S.L.; Lynch, C.F.; Stanford, J.L.; Keegan, T.H.; West, D.W. Breast implants following mastectomy in women with early-stage breast cancer: Prevalence and impact on survival. Breast Cancer Res. BCR 2005, 7, 184–193. [Google Scholar] [CrossRef]
- Field, T.S.; Buist, D.S.M.; Doubeni, C.; Enger, S.; Fouayzi, H.; Hart, G.; Korner, E.J.; Lamerato, L.; Bachman, D.J.; Ellis, J.; et al. Disparities and survival among breast cancer patients. J. Natl. Cancer Institute. Monogr. 2005, 01605, 88–95. [Google Scholar] [CrossRef]
- de Bock, G.H.; van der Hage, J.A.; Putter, H.; Bonnema, J.; Bartelink, H.; van de Velde, C.J. Isolated loco-regional recurrence of breast cancer is more common in young patients and following breast conserving therapy: Long-term results of European Organisation for Research and Treatment of Cancer studies. Eur. J. Cancer 2006, 42, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.N.; Steck, S.E.; Wolff, M.S.; Britton, J.A.; Kabat, G.C.; Gaudet, M.M.; Abrahamson, P.E.; Bell, P.; Schroeder, J.C.; Teitelbaum, S.L.; et al. Dietary flavonoid intake and breast cancer survival among women on long island. Cancer Epidemiol. Biomark. Prev. 2007, 16, 2285–2292. [Google Scholar] [CrossRef] [PubMed]
- Sohn, V.Y.; Arthurs, Z.M.; Sebesta, J.A.; Brown, T.A. Primary tumor location impacts breast cancer survival. Am. J. Surg. 2008, 195, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.K.; Altieri, A.; Sundquist, J.; Hemminki, K. Influence of education level on breast cancer risk and survival in Sweden between 1990 and 2004. Int. J. Cancer 2008, 122, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Dal Maso, L.; Zucchetto, A.; Talamini, R.; Serraino, D.; Stocco, C.F.; Vercelli, M.; Falcini, F.; Franceschi, S. Prospective Analysis of Case-Control Studies on Environmental Factors and Health (PACE) Study Group. Effect of obesity and other lifestyle factors on mortality in women with breast cancer. Int. J. Cancer 2008, 123, 2188–2194. [Google Scholar] [CrossRef] [PubMed]
- Nichols, H.B.; Trentham-Dietz, A.; Egan, K.M.; Titus-Ernstoff, L.; Holmes, M.D.; Bersch, A.J.; Holick, C.N.; Hampton, J.M.; Stampfer, M.J.; Willett, W.C. Body mass index before and after breast cancer diagnosis: Associations with all-cause, breast cancer, and cardiovascular disease mortality. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Gnerlich, J.L.; Deshpande, A.D.; Jeffe, D.B.; Sweet, A.; White, N.; Margenthaler, J.A. Elevated Breast Cancer Mortality in Women Younger than Age 40 Years Compared with Older Women Is Attributed to Poorer Survival in Early-Stage Disease. J. Am. Coll. Surg. 2009, 208, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Peel, J.B.; Sui, X.; Adams, S.A.; Hébert, J.R.; Hardin, J.W.; Blair, S.N. A prospective study of cardiorespiratory fitness and breast cancer mortality. Med. Sci. Sports Exerc. 2009, 41, 742–748. [Google Scholar] [CrossRef]
- Powe, D.G.; Voss, M.J.; Zänker, K.S.; Habashy, H.O.; Green, A.R.; Ellis, I.O.; Entschladen, F. Beta-blocker drug therapy reduces secondary cancer formation in breast cancer and improves cancer specific survival. Oncotarget 2010, 1, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.A.C.; Stefanick, M.L.; Saquib, N.; Natarajan, L.; Patterson, R.E.; Bardwell, W.; Flatt, S.W.; Newman, V.A.; Rock, C.L.; Thomson, C.A.; et al. Physical activity, additional breast cancer events, and mortality among early-stage breast cancer survivors: Findings from the WHEL Study. Cancer Causes Control 2011, 22, 427–435. [Google Scholar] [CrossRef]
- Ewertz, M.; Jensen, M.-B.; Gunnarsdóttir, K.Á.; Højris, I.; Jakobsen, E.H.; Nielsen, D.; Stenbygaard, L.E.; Tange, U.B.; Cold, S. Effect of obesity on prognosis after early-stage breast cancer. J. Clin. Oncol. 2011, 29, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Conroy, S.M.; Maskarinec, G.; Wilkens, L.R.; White, K.K.; Henderson, B.E.; Kolonel, L.N. Obesity and breast cancer survival in ethnically diverse postmenopausal women: The Multiethnic Cohort Study. Breast Cancer Res. Treat. 2011, 129, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Noh, W.C.; Han, W.; Noh, D. Prognostic significance of young age (<35 Years) by subtype based on ER, PR, and HER2 status in breast cancer: A nationwide registry-based study. World J. Surg. 2011, 35, 1244–1253. [Google Scholar] [CrossRef] [PubMed]
- Colzani, E.; Liljegren, A.; Johansson, A.L.; Adolfsson, J.; Hellborg, H.; Hall, P.F.; Czene, K. Prognosis of patients with breast cancer: Causes of death and effects of time since diagnosis, age, and tumor characteristics. J. Clin. Oncol. 2011, 29, 4014–4021. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, P.J.; Phillips, K.-A.; West, D.W.; Ennis, M.; Hopper, J.L.; John, E.M.; O’Malley, F.P.; Milne, R.L.; Andrulis, I.L.; Friedlander, M.L.; et al. Breast cancer prognosis in BRCA1 and BRCA2 mutation carriers: An international prospective breast cancer family registry population-based cohort study. J. Clin. Oncol. 2012, 30, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.J.; Alexander, B.; Schnitt, S.J.; Comander, A.; Gallagher, B.; Garber, J.E.; Tung, N. Clinical outcome of triple negative breast cancer in BRCA1 mutation carriers and noncarriers. Cancer 2011, 117, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Vostakolaei, F.A.; Broeders, M.J.M.; Rostami, N.; van Dijck, J.A.A.M.; Feuth, T.; Kiemeney, L.A.L.M.; Verbeek, A.L.M. Age at Diagnosis and Breast Cancer Survival in Iran. Int. J. Breast Cancer 2012, 2012, 517976. [Google Scholar] [CrossRef] [PubMed]
- Movahedi, M.; Haghighat, S.; Khayamzadeh, M.; Moradi, A.; Ghanbari-Motlagh, A.; Mirzaei, H.; Esmail-Akbari, M. Survival rate of breast cancer based on geographical variation in Iran: A national study. Iran. Red Crescent Med. J. 2012, 14, 798–804. [Google Scholar] [CrossRef]
- Lan, N.; Laohasiriwong, W.; Stewart, J. Survival probability and prognostic factors for breast cancer patients in Vietnam. Glob. Health Action 2013, 6, 18860. [Google Scholar] [CrossRef]
- Keegan, T.H.M.; Press, D.J.; Tao, L.; DeRouen, M.C.; Kurian, A.W.; A Clarke, C.; Gomez, S.L. Impact of breast cancer subtypes on 3-year survival among adolescent and young adult women. Breast Cancer Res. 2013, 15, R95. [Google Scholar] [CrossRef]
- Hwang, E.S.; Lichtensztajn, D.Y.; Gomez, S.L.; Fowble, B.; Clarke, C.A. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: The effect of age and hormone receptor status. Cancer 2013, 119, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Nechuta, S.; Lu, W.; Zheng, Y.; Cai, H.; Bao, P.-P.; Gu, K.; Zheng, W.; Shu, X.O. Comorbidities and breast cancer survival: A report from the Shanghai Breast Cancer Survival Study. Breast Cancer Res. Treat. 2013, 139, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Valentini, A.; Lubinski, J.; Byrski, T.; Ghadirian, P.; Moller, P.; Lynch, H.T.; Ainsworth, P.; Neuhausen, S.L.; Weitzel, J.; The Hereditary Breast Cancer Clinical Study Group; et al. The impact of pregnancy on breast cancer survival in women who carry a BRCA1 or BRCA2 mutation. Breast Cancer Res. Treat. 2013, 142, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wray, C.J.; Phatak, U.R.; Robinson, E.K.; Wiatek, R.L.; Rieber, A.G.; Gonzalez, A.; Ko, T.C.; Kao, L.S. The effect of age on race-related breast cancer survival disparities. Ann. Surg. Oncol. 2013, 20, 2541–2547. [Google Scholar] [CrossRef]
- Huzarski, T.; Byrski, T.; Gronwald, J.; Górski, B.; Domagała, P.; Cybulski, C.; Oszurek, O.; Szwiec, M.; Gugała, K.; Stawicka, M.; et al. Ten-year survival in patients with BRCA1-negative and BRCA1-positive breast cancer. J. Clin. Oncol. 2013, 31, 3191–3196. [Google Scholar] [CrossRef]
- Ali, H.R.; Provenzano, E.; Dawson, S.-J.; Blows, F.M.; Liu, B.; Shah, M.; Earl, H.M.; Poole, C.J.; Hiller, L.; Dunn, J.A.; et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12,439 patients. Ann. Oncol. 2014, 25, 1536–1543. [Google Scholar] [CrossRef] [PubMed]
- de Glas, N.A.; de Craen, A.J.M.; Bastiaannet, E.; Op’t Land, E.G.; Kiderlen, M.; van de Water, W.; Siesling, S.; Portielje, J.E.A.; Schuttevaer, H.M.; de Bock, G.T.H. Effect of implementation of the mass breast cancer screening programme in older women in the Netherlands: Population based study. BMJ 2014, 349. [Google Scholar] [CrossRef]
- Abadi, A.; Yavari, P.; Dehghani-Arani, M.; Alavi-Majd, H.; Ghasemi, E.; Amanpour, F.; Bajdik, C. Cox models survival analysis based on breast cancer treatments. Iran. J. Cancer Prev. 2014, 7, 124–129. [Google Scholar]
- Vollebergh, M.A.; Lips, E.H.; Nederlof, P.M.; Wessels, L.F.A.; Wesseling, J.; Vd Vijver, M.J.; de Vries, E.G.E.; Van Tinteren, H.; Jonkers, J.; Hauptmann, M.; et al. Genomic patterns resembling BRCA1- and BRCA2-mutated breast cancers predict benefit of intensified carboplatin-based chemotherapy. Breast Cancer Res. 2014, 16, R47. [Google Scholar] [CrossRef] [PubMed]
- Kriege, M.; Hollestelle, A.; Jager, A.; E A Huijts, P.; Berns, E.M.; Sieuwerts, A.M.; Gelder, M.E.M.-V.; Collée, J.M.; Devilee, P.; Hooning, M.J.; et al. Survival and contralateral breast cancer in CHEK2 1100delC breast cancer patients: Impact of adjuvant chemotherapy. Br. J. Cancer 2014, 111, 1004–1013. [Google Scholar] [CrossRef]
- Saadatmand, S.; Bretveld, R.; Siesling, S.; A Tilanus-Linthorst, M.M. Influence of tumour stage at breast cancer detection on survival in modern times: Population based study in 173,797 patients. BMJ 2015, 351, h4901. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, H.G.; Malmgren, J.A.; Atwood, M.K.; Calip, G.S. Effect of treatment and mammography detection on breast cancer survival over time: 1990-2007. Cancer 2015, 121, 2553–2561. [Google Scholar] [CrossRef]
- Eng, L.G.; Dawood, S.; Sopik, V.; Haaland, B.; Tan, P.S.; Bhoo-Pathy, N.; Warner, E.; Iqbal, J.; Narod, S.A.; Dent, R. Ten-year survival in women with primary stage IV breast cancer. Breast Cancer Res. Treat. 2016, 160, 145–152. [Google Scholar] [CrossRef]
- Kim, J.; Mersereau, J.E.; Khankari, N.; Bradshaw, P.T.; McCullough, L.E.; Cleveland, R.; Shantakumar, S.; Teitelbuam, S.L.; Neugut, A.I.; Senie, R.T. Polycystic ovarian syndrome (PCOS), related symptoms/sequelae, and breast cancer risk in a population-based case–control study. Cancer Causes Control. 2016, 27, 403–414. [Google Scholar] [CrossRef]
- Kataoka, A.; Iwamoto, T.; Tokunaga, E.; Tomotaki, A.; Kumamaru, H.; Miyata, H.; Niikura, N.; Kawai, M.; Anan, K.; Hayashi, N.; et al. Young adult breast cancer patients have a poor prognosis independent of prognostic clinicopathological factors: A study from the Japanese Breast Cancer Registry. Breast Cancer Res. Treat. 2016, 160, 163–172. [Google Scholar] [CrossRef]
- Partridge, A.H.; Hughes, M.E.; Warner, E.T.; Ottesen, R.A.; Wong, Y.-N.; Edge, S.B.; Theriault, R.L.; Blayney, D.W.; Niland, J.C.; Winer, E.P.; et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J. Clin. Oncol. 2016, 34, 3308–3314. [Google Scholar] [CrossRef]
- Odén, L.; Akbari, M.; Zaman, T.; Singer, C.F.; Sun, P.; Narod, S.A.; Salmena, L.; Kotsopoulos, J. Plasma osteoprotegerin and breast cancer risk in BRCA1 and BRCA2 mutation carriers. Oncotarget 2016, 7, 86687–86694. [Google Scholar] [CrossRef]
- Chagpar, A.B.; Hatzis, C.; Pusztai, L.; DiGiovanna, M.P.; Moran, M.; Mougalian, S.; Sanft, T.; Evans, S.; Hofstatter, E.; Wilson, L.D.; et al. Association of LN Evaluation with Survival in Women Aged 70 Years or Older with Clinically Node-Negative Hormone Receptor Positive Breast Cancer. Ann. Surg. Oncol. 2017, 24, 3073–3081. [Google Scholar] [CrossRef] [PubMed]
- Johnsson, A.; Broberg, P.; Krüger, U.; Johnsson, A.; Tornberg, B.; Olsson, H. Physical activity and survival following breast cancer. Eur. J. Cancer Care 2019, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fallahpour, S.; Navaneelan, T.; De, P.; Borgo, A. Breast cancer survival by molecular subtype: A population-based analysis of cancer registry data. CMAJ Open 2017, 5, E734–E739. [Google Scholar] [CrossRef]
- Schmidt, M.K.; Van Den Broek, A.J.; Tollenaar, R.A.; Smit, V.T.; Westenend, P.; Brinkhuis, M.; Oosterhuis, W.J.W.; Wesseling, J.; Janssen-Heijnen, M.L.; Jobsen, J.J.; et al. Breast Cancer Survival of BRCA1/BRCA2 Mutation Carriers in a Hospital-Based Cohort of Young Women. J. Natl. Cancer Inst. 2017, 109, djw329. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.Y.; Tham, W.Y.; Nei, W.L.; Lim, C.; Miao, H. Age exerts a continuous effect in the outcomes of Asian breast cancer patients treated with breast-conserving therapy. Cancer Commun. 2018, 38, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Copson, E.R.; Maishman, T.C.; Tapper, W.J.; I Cutress, R.; Greville-Heygate, S.; Altman, D.G.; Eccles, B.; Gerty, S.; Durcan, L.T.; Jones, L.; et al. Germline BRCA mutation and outcome in young-onset breast cancer (POSH): A prospective cohort study. Lancet Oncol. 2018, 19, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Lambertini, M.; Ameye, L.; Hamy, A.-S.; Zingarello, A.; Poorvu, P.D.; Carrasco, E.; Grinshpun, A.; Han, S.; Rousset-Jablonski, C.; Ferrari, A.; et al. Pregnancy after breast cancer in patients with germline BRCA mutations. J. Clin. Oncol. 2020, 38, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Olafsdottir, E.J.; Borg, A.; Jensen, M.-B.; Gerdes, A.-M.; Johansson, A.L.V.; Barkardottir, R.B.; Johannsson, O.T.; Ejlertsen, B.; Sonderstrup, I.M.H.; Hovig, E.; et al. Breast cancer survival in Nordic BRCA2 mutation carriers—Unconventional association with oestrogen receptor status. Br. J. Cancer 2020, 123, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- De Talhouet, S.; Peron, J.; Vuilleumier, A.; Friedlaender, A.; Viassolo, V.; Ayme, A.; Bodmer, A.; Treilleux, I.; Lang, N.; Tille, J.C.; et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci. Rep. 2020, 10, 7073. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, S.; Freedman, R.A.; Partridge, A.H. The impact of young age at diagnosis (age <40 years) on prognosis varies by breast cancer subtype: A U.S. SEER database analysis. Breast 2022, 61, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Headon, H.; Wazir, U.; Kasem, A.; Mokbel, K. Surgical treatment of the primary tumour improves the overall survival in patients with metastatic breast cancer: A systematic review and meta-analysis. Mol Clin Oncol 2016, 4, 863–867. [Google Scholar] [CrossRef]
- Lundqvist, A.; Andersson, E.; Ahlberg, I.; Nilbert, M.; Gerdtham, U. Socioeconomic inequalities in breast cancer incidence and mortality in Europe—A systematic review and meta-analysis. Eur. J. Public Health 2016, 26, 804–813. [Google Scholar] [CrossRef]
- de Lemos, L.L.P.; de Souza, M.C.; Moreira, D.P.; Almeida, P.H.R.F.; Godman, B.; Verguet, S.; Guerra, A.A.; Cherchiglia, M.L. Stage at diagnosis and stage-specific survival of breast cancer in Latin America and the Caribbean: A systematic review and meta-analysis. PLoS ONE 2019, 14, e0224012. [Google Scholar] [CrossRef]
- Salas, M.; Henderson, M.; Sundararajan, M.; Tu, N.; Islam, Z.; Ebeid, M.; Horne, L. Use of comorbidity indices in patients with any cancer, breast cancer, and human epidermal growth factor receptor-2-positive breast cancer: A systematic review. PLoS ONE 2021, 16, e0252925. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Rumble, R.B.; Carey, L.A.; Come, S.E.; Davidson, N.E.; Di Leo, A.; Gralow, J.; Hortobagyi, G.N.; Moy, B.; Yee, D.; et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2–negative (or unknown) advanced breast cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 2014, 32, 3307. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Henley, J.; Thun, M. All-cause and cause-specific death rates by educational status for two million people in two American Cancer Society cohorts, 1959–1996. Am. J. Epidemiol. 2002, 156, 11–21. [Google Scholar] [CrossRef] [PubMed]
| Study | Study Participation | Study Attrition | Prognostic Factor Measurement | Outcome Measurement | Study Confounding | Statistical Analysis and Reporting | Overall Quality |
|---|---|---|---|---|---|---|---|
| Rohan et al. [12] | x | x | x | x | Moderate | ||
| Watson et al. [13] | x | x | x | x | Moderate | ||
| Vinh-Hung et al. [14] | x | x | x | x | x | High | |
| Kaffashian et al. [15] | x | x | x | Moderate | |||
| Robson et al. [16] | x | x | x | x | x | High | |
| Metcalfe et al. [17] | x | x | x | x | Moderate | ||
| Boyapati [18] | x | x | x | x | x | High | |
| Rapiti et al. [19] | x | x | x | x | Moderate | ||
| Le et al. [20] | x | x | x | x | Moderate | ||
| Field et al. [21] | x | x | x | x | x | x | High |
| de Bock et al. [22] | x | x | x | x | Moderate | ||
| Fink et al. [23]. | x | x | x | x | Moderate | ||
| Sohn et al. [24] | x | x | x | x | x | High | |
| Hussain et al. [25] | x | x | x | x | x | High | |
| Maso et al. [26] | x | x | x | x | x | High | |
| Nichols et al. [27] | x | x | x | x | Moderate | ||
| Gnerlich et al. [28] | x | x | x | x | Moderate | ||
| Peel et al. [29] | x | x | x | x | x | High | |
| Powe et al. [30] | x | x | x | x | x | High | |
| Bertram [31] | x | x | x | x | x | High | |
| Ewertz et al. [32] | x | x | x | x | x | High | |
| Conroy et al. [33] | x | x | x | x | x | High | |
| Kim EK et al. [34] | x | x | x | x | Moderate | ||
| Colzani et al. [35] | x | x | x | x | Moderate | ||
| Goodwin et al. [36] | x | x | x | x | x | High | |
| Lee et al. [37] | x | x | x | x | Moderate | ||
| Vostakolaei et al. [38] | x | x | x | x | x | High | |
| Movahedi et al. [39] | x | x | x | x | Moderate | ||
| Lan et al. [40] | x | x | x | x | x | High | |
| Keegan et al. [41] | x | x | x | x | Moderate | ||
| Hwang et al. [42] | x | x | x | x | Moderate | ||
| Nechuta et al. [43] | x | x | x | x | Moderate | ||
| Valentini et al. [44] | x | x | x | x | Moderate | ||
| Wray et al. [45] | x | x | x | x | x | High | |
| Huzarski et al. [46] | x | x | x | x | x | High | |
| Ali et al. [47] | x | x | x | x | x | x | High |
| de Glas et al. [48] | x | x | x | x | Moderate | ||
| Abadi et al. [49] | x | x | x | x | Moderate | ||
| Vollebergh et al. [50] | x | x | x | x | Moderate | ||
| Kriege et al. [51] | x | x | x | x | x | High | |
| Saadatmand et al. [52] | x | x | x | x | Moderate | ||
| Kaplan et al. [53] | x | x | x | x | x | High | |
| Eng et al. [54] | x | x | x | x | Moderate | ||
| Kim et al. [55] | x | x | x | x | x | x | High |
| Kataoka et al. [56] | x | x | x | x | Moderate | ||
| Partridge et al. [57] | x | x | x | x | Moderate | ||
| Odén et al. [58] | x | x | x | x | x | High | |
| Chagpar et al. [59] | x | x | x | x | Moderate | ||
| Johnsson et al. [60] | x | x | x | x | Moderate | ||
| Fallahpour et al. [61] | x | x | x | x | Moderate | ||
| Schmidt et al. [62] | x | x | x | x | Moderate | ||
| Wong et al. [63] | x | x | x | x | x | High | |
| Copson et al. [64] | x | x | x | x | x | x | High |
| Lambertini et al. [65] | x | x | x | x | x | High | |
| Olafsdottir et al. [66] | x | x | x | x | Moderate | ||
| Talhouet et al. [67] | x | x | x | x | Moderate | ||
| Kim et al. [68] | x | x | x | x | Moderate |
| Author | Year | Country | Study Design/Database | Sample Size | Sample | Prognostic Factor(s) Included |
|---|---|---|---|---|---|---|
| Rohan et al. [12] | 1995 | Australia | Population-based cohort study | 412 | Women with breast cancer | Physical activity level |
| Watson et al. [13] | 1999 | United Kingdom | Prospective survival study | 578 | Women with early-stage breast cancer | Mental Adjustment to Cancer (MAC) scale-predominant responses |
| Vinh-Hung et al. [14] | 2002 | USA | SEER 9-registries database | 186,549 | Women with partial or total mastectomy Breast cancer | Race, marital status, histology, differentiation, ER status, PR status, and treatment |
| Kaffashian et al. [15] | 2003 | United Kingdom | East Anglia Cancer Registry database | 10,865 | Women with breast cancer | Stage, grade, morphology, and social class |
| Robson et al. [16] | 2003 | USA | Retrospective cohort study | 496 | Women with breast cancer | BRCA1 mutation, tumour size, axillary node, and age of diagnosis |
| Metcalfe et al. [17] | 2004 | Canada | Retrospective cohort study | 491 | Women with breast cancer with BRCA1/2 mutation | BRCA |
| Boyapati [18] | 2005 | China | Retrospective cohort study | 1459 | Women with breast cancer | Total Isoflavone |
| Rapiti et al. [19] | 2005 | Switzerland | Retrospective data analysis | 2997 | Women with breast cancer | Age at diagnosis, method of discovery, socio-economic status, stage, histology, differentiation, ER status, surgery, radiotherapy, chemotherapy, and hormonal therapy |
| Le et al. [20] | 2005 | USA | Surveillance, Epidemiology and End Results Breast Implant Surveillance Study | 4968 | Women < 65 years with breast cancer | Implant status, age at diagnosis, race, grade, morphology, and radiation therapy |
| Field et al. [21] | 2005 | USA | Retrospective cohort study; Cancer research network | 21,155 | Women with breast cancer | Race, stage, grade, estrogen receptor, progesterone receptor, and tumour size |
| de Bock et al. [22] | 2006 | Netherlands | Retrospective data analysis | 1073 | Women with breast cancer | Age at diagnosis, tumour size, nodal state, surgical therapy, chemotherapy, adjuvant chemotherapy, adjuvant radiotherapy, and tamoxifen |
| Fink et al. [23] | 2007 | USA | Population-based case–control; Long Island Breast Cancer Study | 1383 | Women with breast cancer | Total Isoflavone |
| Sohn et al. [24] | 2008 | USA | Retrospective cohort study | 13,984 | Women with breast cancer | Age at diagnosis, race, grade, stage, and location |
| Hussain et al. [25] | 2008 | Sweden | Swedish Family Cancer Database | 43,222 | Women with invasive breast cancer | Histology, age at diagnosis, and education |
| Maso et al. [26] | 2008 | Italy | Multicentre case–control study | 1453 | Women with breast cancer | Age at diagnosis, tumour size, lymph node-positive, stage, ER status, PR status, BMI, work physical activity, leisure time, vegetable and fruit intake, total protein intake, total fat intake, and glycaemic load |
| Nichols et al. [27] | 2009 | USA | Population-based case–control | 3993 | Women with invasive non-metastatic breast cancers | BMI |
| Gnerlich et al. [28] | 2009 | USA | SEER 9-registries database | 243,012 | Women with breast cancer | Stage |
| Peel et al. [29] | 2009 | USA | Prospective study; Aerobics Center Longitudinal study | 14,811 | Women with breast cancer | Age at diagnosis, BMI, oral contraceptive use, and estrogen use |
| Powe et al. [30] | 2010 | United Kingdom | Prospective study | 466 | Women with breast cancer | Tumour size, grade, stage, and beta-blocker treatment |
| Bertram [31] | 2011 | USA | Randomized controlled trial | 2361 | Women with post-treatment breast cancer survivor | Total PA |
| Ewertz et al. [32] | 2011 | Denmark | Retrospective cohort study | 5868 | Women with early-stage breast cancer | BMI |
| Conroy et al. [33] | 2011 | USA | Prospective study (Multi-ethnic cohort study) | 3842 | Women with breast cancer aged 50 and above | Age at diagnosis, ethnicity, BMI, cardiovascular comorbidity, surgery, chemotherapy, and radiotherapy |
| Kim EK et al. [34] | 2011 | Korea | Nationwide registry from Seoul National University Hospital Breast Cancer Center (SNUHBCC) and Korean Breast Cancer Registry (KBCR) | 2474 | Women with breast cancer | Age at diagnosis, tumour size, LN positive, histology grade, hormone receptor, and HER2 status |
| Colzani et al. [35] | 2011 | Sweden | Stockholm Breast Cancer Registry | 12,850 | Women with breast cancer | Age at diagnosis, treatment, nodes, Estrogen-receptor status, and tumour size |
| Goodwin et al. [36] | 2011 | Canada, USA, Australia | International population-based cohort study | 3220 | Women with breast cancer | BRCA, chemotherapy, and hormone therapy |
| Lee et al. [37] | 2011 | USA | Retrospective data analysis; Clinical database and annotated Specialized Program of Research Excellence (SPORE) | 117 | Women with breast cancer (BRCA1 and noncarriers) | BRCA, Age, AJCC stage, lymph node, and tumour size |
| Vostakolaei et al. [38] | 2012 | Iran | Retrospective data analysis | 1500 | Women with breast cancer | Age at diagnosis, stage, grade, estrogen receptor, progesterone receptor, and HER2 |
| Movahedi et al. [39] | 2012 | Iran | Retrospective data analysis | 6147 | Women with breast cancer | Age at diagnosis |
| Lan et al. [40] | 2013 | Vietnam | Retrospective data analysis | 948 | Women with breast cancer | Marital status, hormone therapy, education level, and stage |
| Keegan et al. [41] | 2013 | USA | California cancer registry | 5331 | Adolescent and young adult breast cancer | Her2, race, marital status, lymph nodes, and tumour grade |
| Hwang et al. [42] | 2013 | USA | Retrospective data analysis | 112,514 | Women with early-stage breast cancer | Surgery, grade, nodes, race, socio-economic status, tumour size, age at diagnosis, and hormone receptor status |
| Nechuta et al. [43] | 2013 | China | Population-based prospective study; Shanghai Breast Cancer Survival study | 4664 | Women with breast cancer | Comorbidity |
| Valentini et al. [44] | 2013 | Canada, USA, Asia, Europe | Multicentre, historical cohort study | 397 | Women with breast cancer (at least one mutation in the BRCA1 or BRCA2 gene) | Birth after diagnosis, age at diagnosis, chemotherapy, surgery, tumour size, lymph node, and receptor status |
| Wray et al. [45] | 2013 | USA | Retrospective data analysis; Harris Country Hospital District and Memorial Hermann Healthcare System | 9249 | Women with breast cancer | Age at diagnosis, race, stage, receptor, and hospital system |
| Huzarski et al. [46] | 2013 | Poland | Retrospective data analysis | 3345 | Women with breast cancer | Age at diagnosis, ER status, PR status, HER2 status, tumour size, nodes, oophorectomy, tamoxifen, chemotherapy, and BRCA |
| Ali et al. [47] | 2014 | Canada | Retrospective observational studies | 8775 | Women with breast cancer (estrogen-receptor positive) | Age at diagnosis, No. positive nodes, tumour size, grade, hormone therapy, chemotherapy, morphology, PR status, HER2 status, and molecular subtype |
| de Glas et al. [48] | 2014 | Netherlands | Randomised controlled trial | 300 | Women with breast cancer, aged < 65 at diagnosis, postmenopausal, hormone receptor-positive | Physical activity (MET-hrs/week) |
| Abadi et al. [49] | 2014 | Canada | Population-based British Columbia Cancer Registry | 15,830 | Women with breast cancer (Stage I ≤ 50 years) | Surgery |
| Vollebergh et al. [50] | 2014 | Netherlands | Retrospective multicentre RCT study | 249 | Women with breast cancer | Stage, grade, BRCA |
| Kriege et al. [51] | 2014 | Netherlands | Retrospective cohort study | 4722 | Women with breast cancer | Chemotherapy |
| Saadatmand et al. [52] | 2015 | Netherlands | Prospective nationwide population-based study | 173,797 | Women with breast cancer | Age at diagnosis, tumour category, pathological node category, morphology, Estrogen receptor status, Progesterone receptor status, HER2 status, breast surgery, chemotherapy, hormone therapy, and radiotherapy |
| Kaplan et al. [53] | 2015 | USA | Institutional Breast Cancer Clinical Database Registry | 2998 | Women with breast cancer aged 50 to 69 | Detection method, radiation therapy, hormone treatment, and chemotherapy |
| Eng et al. [54] | 2016 | USA | SEER 18-registries database | 25,323 | Women with breast cancer (Stage IV) | Income status, age at diagnosis, tumour size, node status, estrogen receptor status, progesterone receptor status, and race |
| Kim et al. [55] | 2016 | USA | Population-based Long Islan Breast Cancer Study | 1413 | Women with breast cancer | Chemotherapy, hormone therapy, and radiation therapy |
| Kataoka et al. [56] | 2016 | Japan | Japanese Breast Cancer Registry | 53,670 | Women with breast cancer | Age at diagnosis, grade, node, subtype, and adjuvant therapy |
| Partridge et al. [57] | 2016 | USA | Longitudinal cohort study; National Comprehensive Cancer Network Breast Cancer Outcome Project database | 17,575 | Women with breast cancer | Age at diagnosis |
| Odén et al. [58] | 2016 | Canada | Prospective randomised trial | 206 | Women with breast cancer (BRCA1 or 2 mutation carriers) | BRCA, oophorectomy, and oral contraceptive use |
| Chagpar et al. [59] | 2017 | USA | Retrospective data analysis | 157,584 | Women aged ≥ 70 years diagnosed with cLN- HR+ breast cancer | Age at diagnosis, tumour size, race, tumour grade, and radiation therapy |
| Johnsson et al. [60] | 2017 | Sweden | Prospective population-based cohort; Swedish National Cancer Registry | 847 | Women with breast cancer | Stage, physical activity, oral contraception use, age at first childbirth, family history of breast cancer, education, BMI, smoking status, and alcohol |
| Fallahpour et al. [61] | 2017 | Canada | Population-based study; Ontario, Cancer Registry | 17,598 | Women with breast cancer (Luminal A) | Age at diagnosis, residence, histology, stage, and Charlson comorbidity index |
| Schmidt et al. [62] | 2017 | Netherlands | Retrospective cohort study | 6478 | Women with breast cancer (less than 50 years old) | BRCA, age at diagnosis, grade, tumour size, nodes, ER status, chemotherapy, and surgery |
| Wong et al. [63] | 2018 | Singapore | Retrospective data analysis; National Cancer Centre Singapore | 2492 | Women with breast cancer | Age at diagnosis |
| Copson et al. [64] | 2018 | United Kingdom | Prospective cohort study | 2733 | Women with breast cancer (40 years old or younger) | BRCA, BMI, grade, HER2 status, ER status, Race, and chemotherapy |
| Lambertini et al. [65] | 2020 | Europe, North America, Latin America, Israel | International, multicentre, retrospective cohort study | 1252 | Women with breast cancer (with germline deleterious BRCA mutations) | BRCA and hormone receptor status |
| Olafsdottir et al. [66] | 2020 | Nordics—Denmark, Iceland, Norway, Sweden | Retrospective data analysis | 608 | Women with breast cancer | Tumour size, lymph node, grade, ER status, surgery, chemotherapy, and radiation |
| Talhouet et al. [67] | 2020 | France, Switzerland | Retrospective cohort study | 677 (French), 248 (Swiss) | Women with breast cancer (BRCA 1/2 or noncarriers) | BRCA, grade, age at diagnosis, and nodal status |
| Kim et al. [68] | 2022 | USA | SEER 18-registries database | 158,253 | Women with breast cancer (hormone receptor+, lower grade) | Age at diagnosis |
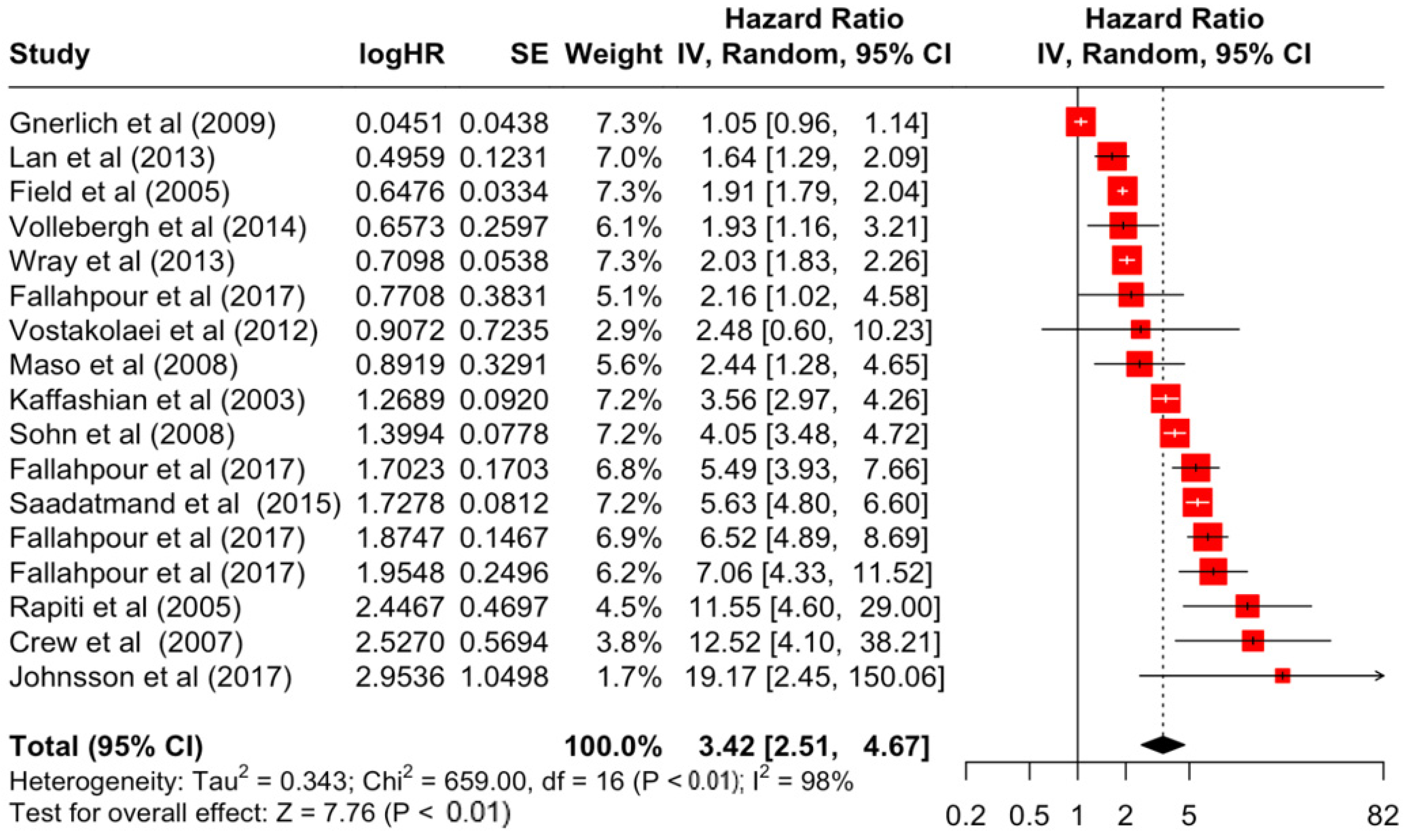
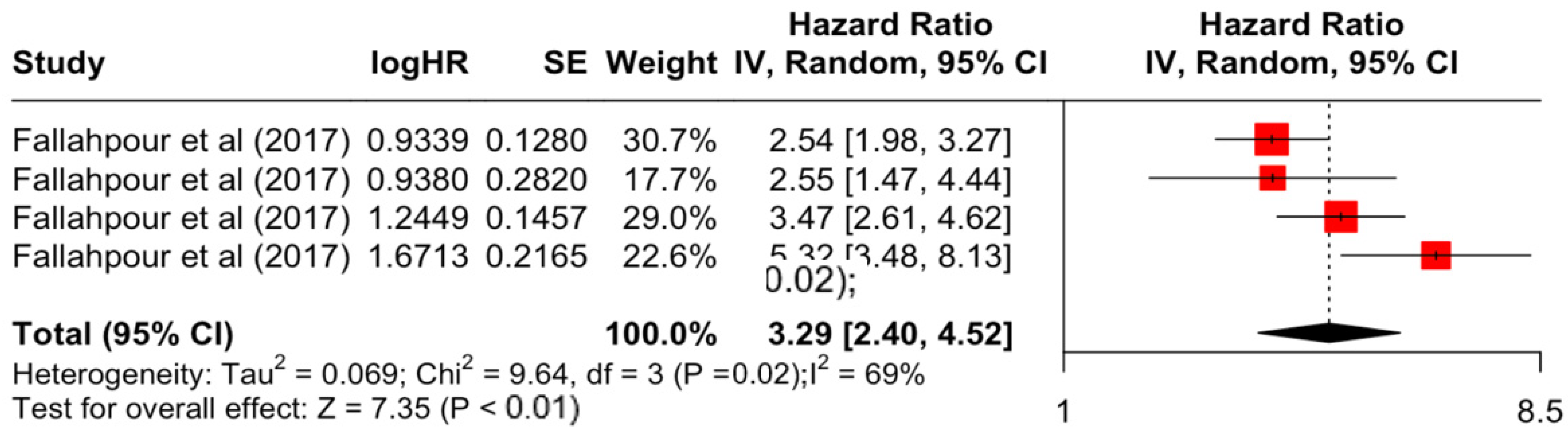
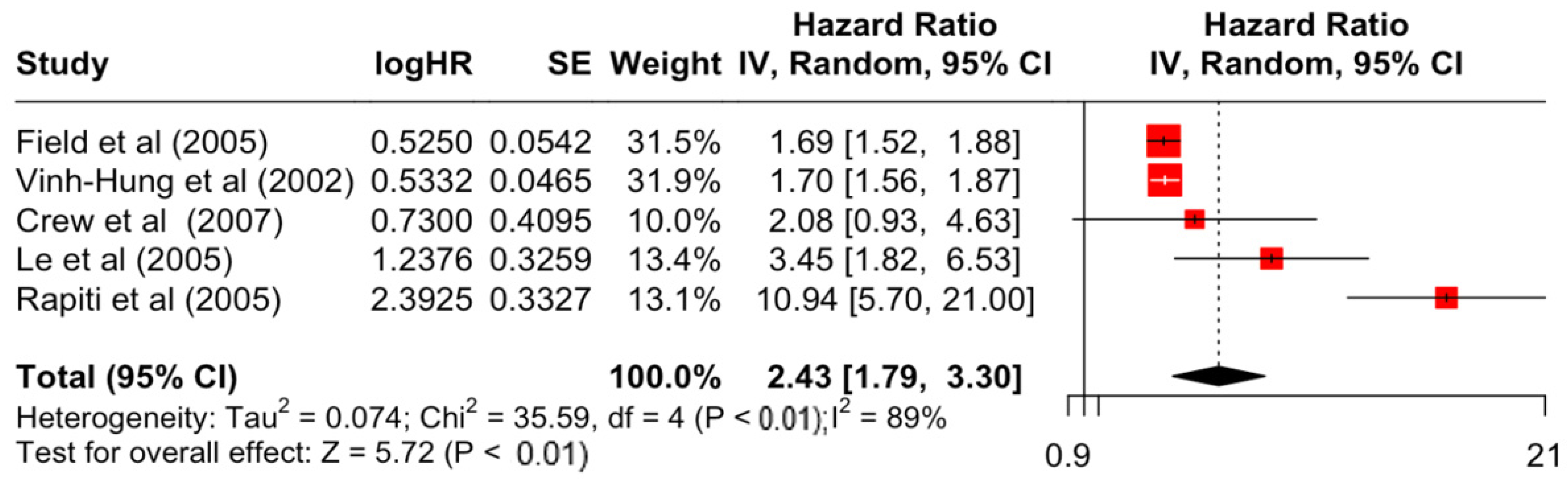
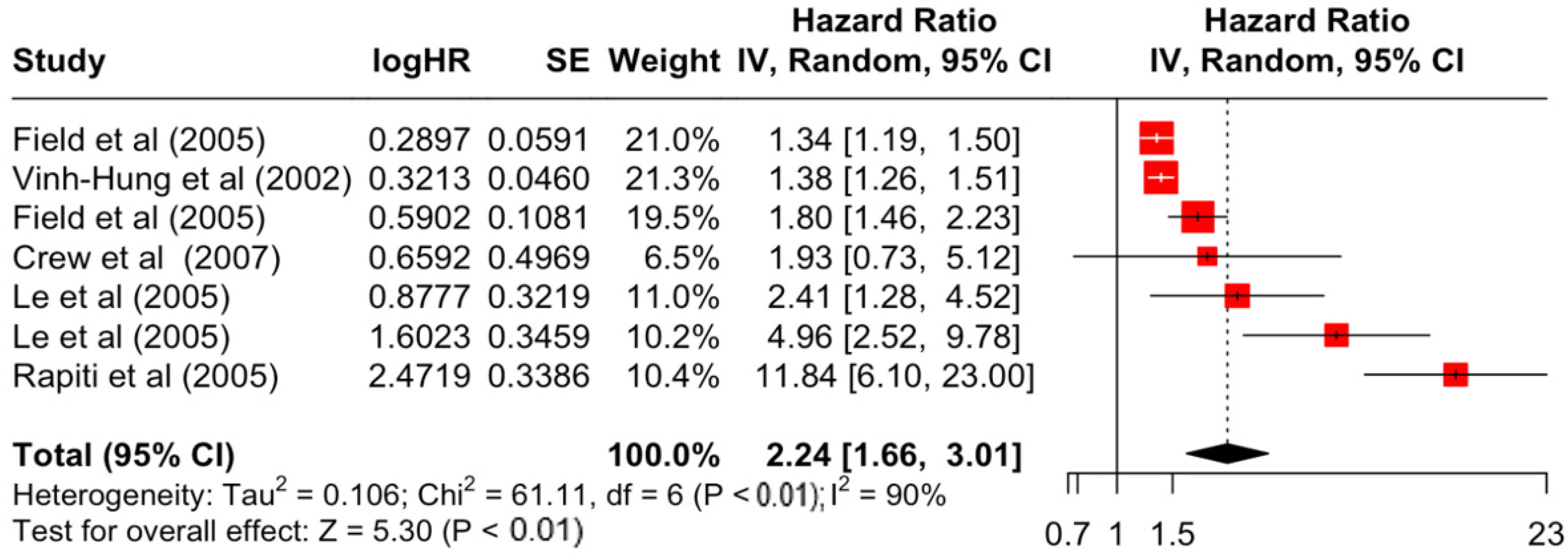
| Factor | Group | HR | Lower | Upper | z-Stats | p-Value |
|---|---|---|---|---|---|---|
| Age | Below 35 | 1.53 | 1.26 | 1.87 | 4.19 | <0.001 |
| 35 to 60 | 1.11 | 1.01 | 1.21 | 2.27 | 0.023 | |
| Above 60 | 1.45 | 1.21 | 1.72 | 4.11 | <0.001 | |
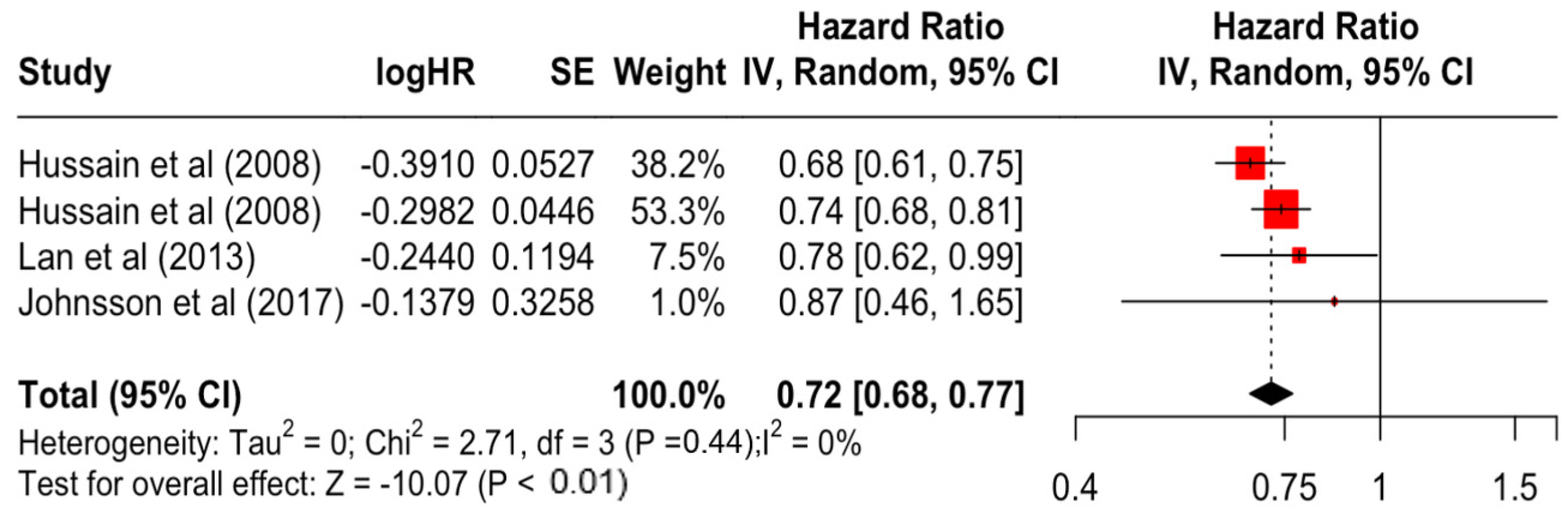
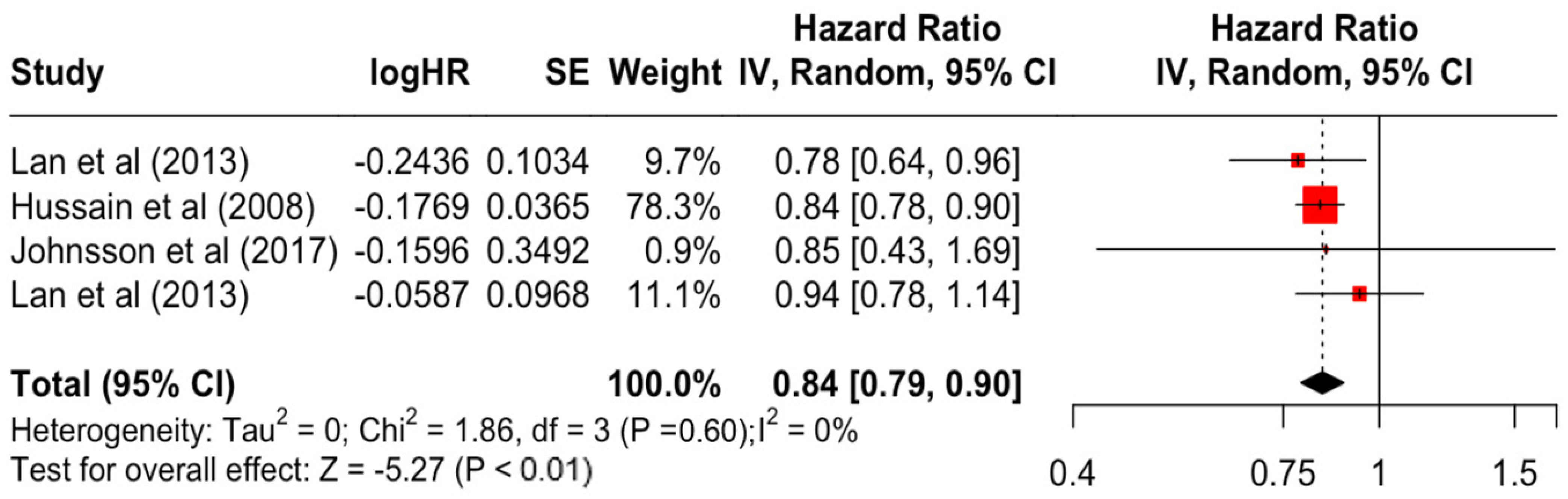
| Education | Secondary | 0.84 | 0.79 | 0.90 | −5.27 | <0.001 |
| Higher | 0.72 | 0.68 | 0.77 | −10.07 | <0.001 | |
| Race | Black | 1.39 | 1.33 | 1.45 | 14.45 | <0.001 |
| Asian | 0.84 | 0.76 | 0.93 | −3.23 | <0.001 | |
| Hispanic | 1.16 | 0.98 | 1.36 | 1.74 | 0.082 | |
| Grade | 2 | 1.54 | 1.27 | 1.87 | 4.44 | <0.001 |
| 3 | 1.92 | 1.33 | 2.76 | 3.47 | <0.001 | |
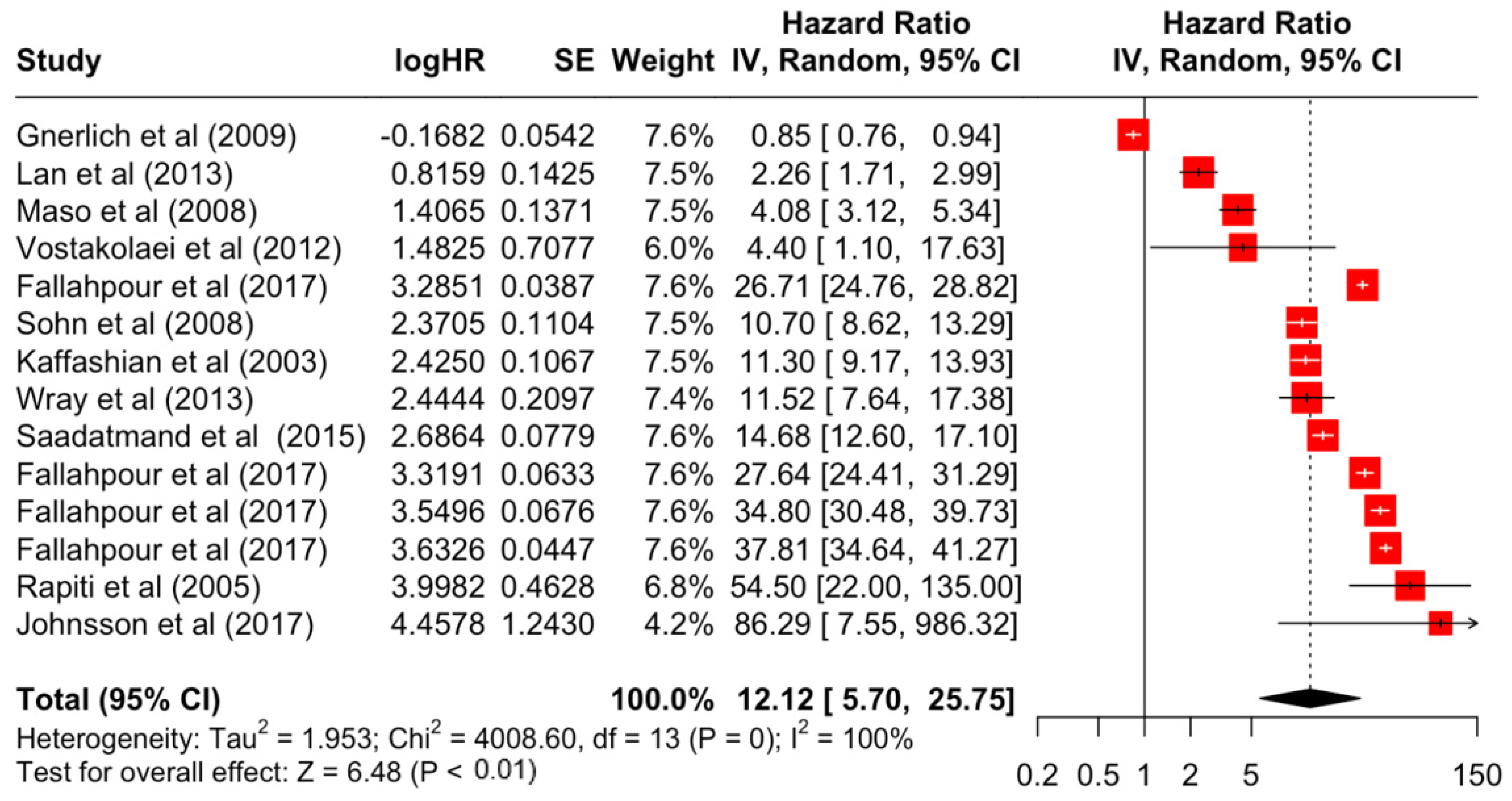
| Stage | 2 | 1.93 | 1.48 | 2.51 | 4.86 | <0.001 |
| 3 | 3.42 | 2.51 | 4.67 | 7.76 | <0.001 | |
| 4 | 12.12 | 5.70 | 25.76 | 6.48 | <0.001 | |
| Differentiation | Moderate | 1.49 | 1.15 | 1.93 | 3.02 | 0.003 |
| Poor | 2.43 | 1.79 | 3.30 | 5.72 | <0.001 | |
| Undifferentiated | 2.24 | 1.66 | 3.01 | 5.3 | <0.001 | |
| Nodes | Positive | 1.71 | 1.42 | 2.05 | 5.77 | <0.001 |
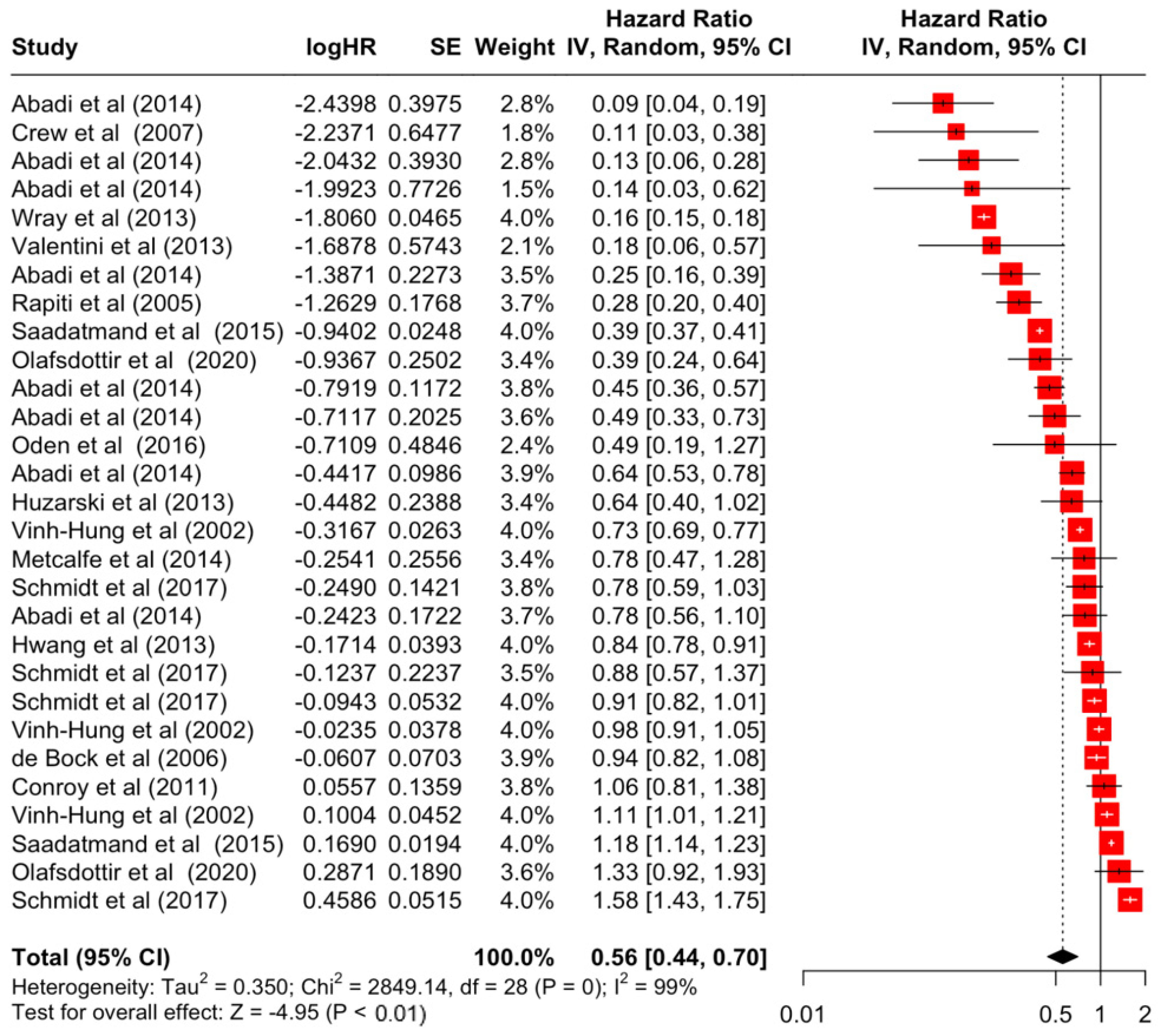
| Surgery | Yes | 0.56 | 0.44 | 0.70 | −4.95 | <0.001 |
| Tumour size | ≥2 cm | 1.39 | 1.35 | 1.42 | 24.75 | <0.001 |
| Histology | Lobular | 1.09 | 0.88 | 1.34 | 0.81 | 0.420 |
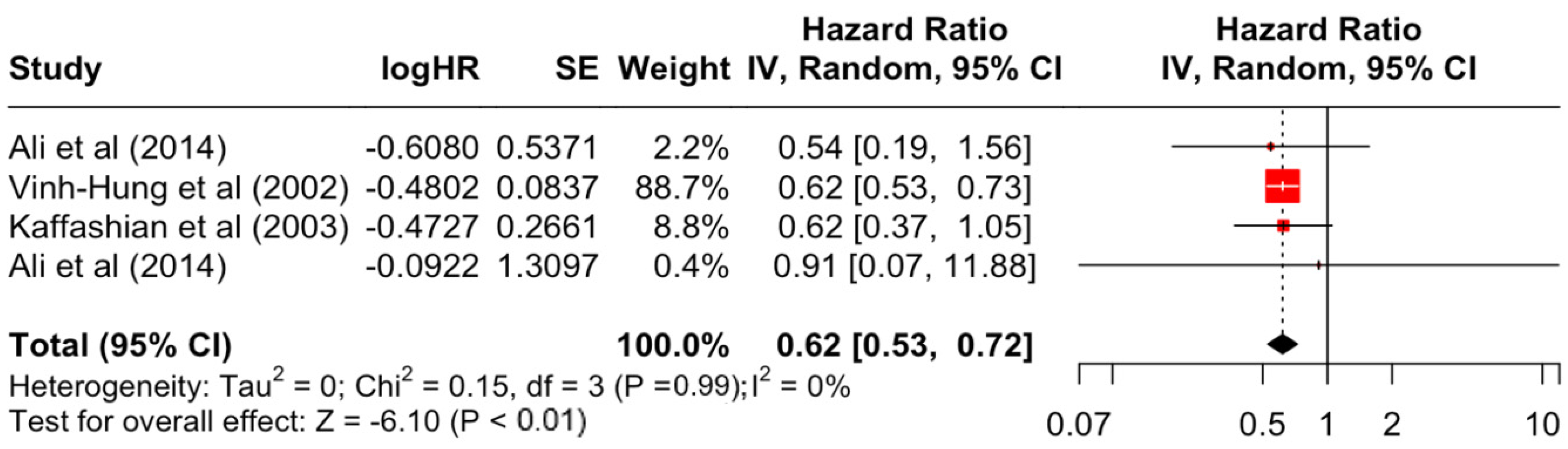
| Medullary | 0.62 | 0.53 | 0.72 | −6.1 | <0.001 | |
| Others | 0.90 | 0.71 | 1.15 | −0.81 | 0.420 | |
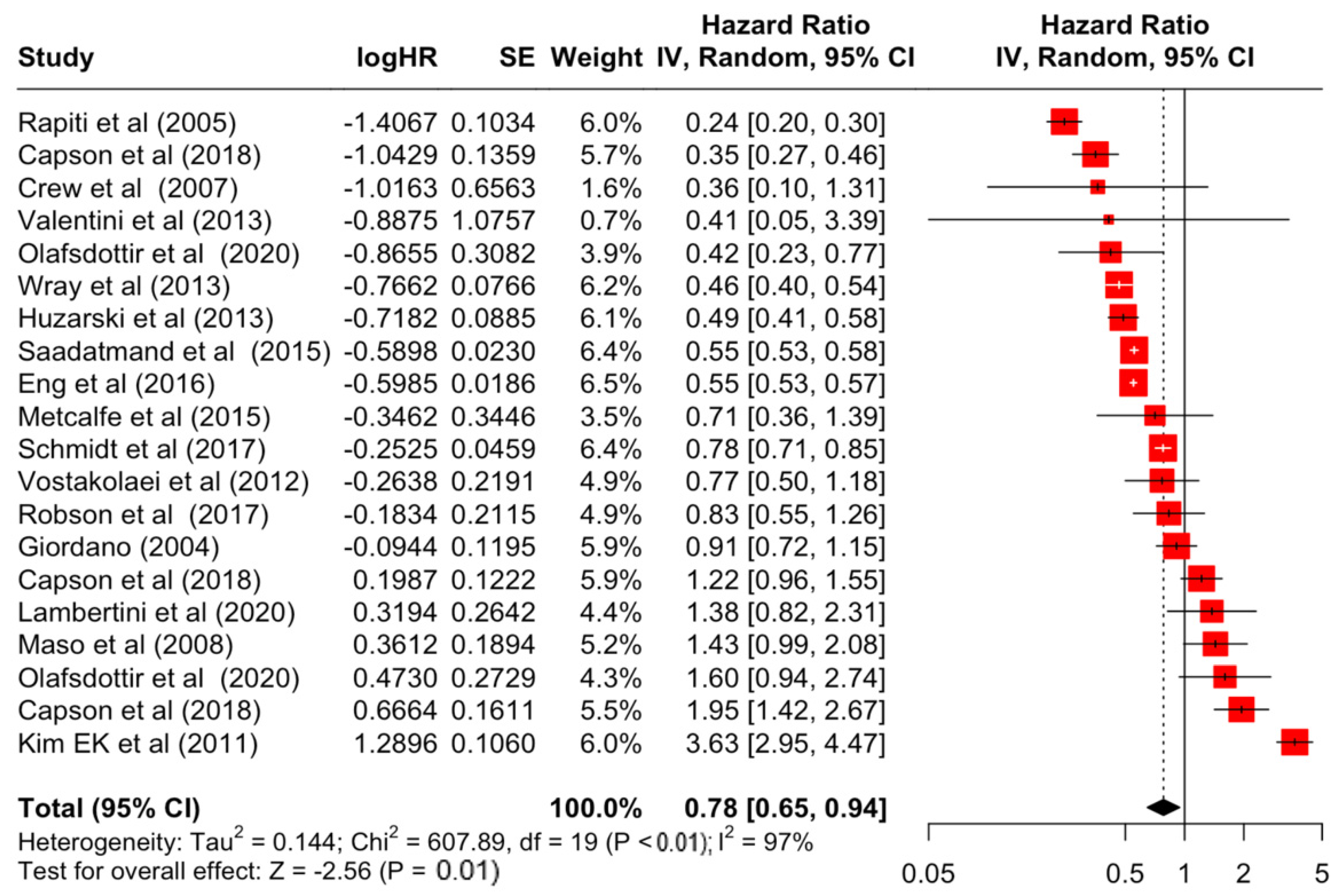
| Estrogen receptor | Positive | 0.78 | 0.65 | 0.94 | −2.56 | 0.011 |
| Negative | 1.55 | 1.13 | 2.13 | 2.69 | 0.007 | |
| HER2 receptor | Positive | 1.29 | 0.93 | 1.79 | 1.53 | 0.125 |
| Negative | 1.16 | 0.79 | 1.70 | 0.76 | 0.448 | |
| Body Mass Index | Overweight/Obese | 1.20 | 1.09 | 1.33 | 3.69 | <0.001 |
| Comorbidity Index | 1 to 2 | 1.87 | 1.35 | 2.61 | 3.72 | <0.001 |
| ≥3 | 3.29 | 2.40 | 4.52 | 7.35 | <0.001 | |
| BRCA | 1 | 0.86 | 0.61 | 1.19 | −0.91 | 0.363 |
| 2 | 1.02 | 0.78 | 1.33 | 0.13 | 0.898 | |
| Oral contraceptive use | Yes | 0.97 | 0.72 | 1.31 | −0.19 | 0.852 |
| Physical activity | Light/Moderate | 0.98 | 0.80 | 1.20 | −0.21 | 0.833 |
| High/Vigorous | 0.99 | 0.68 | 1.44 | −0.06 | 0.950 | |
| Progesterone receptor | Positive | 0.87 | 0.70 | 1.07 | −1.3 | 0.193 |
| Negative | 1.21 | 0.78 | 1.88 | 0.86 | 0.391 | |
| Hormone therapy | Yes | 0.98 | 0.74 | 1.29 | −0.16 | 0.872 |
| Chemotherapy | Yes | 1.02 | 0.81 | 1.28 | 0.16 | 0.869 |
| Radiotherapy | Yes | 1.14 | 0.83 | 1.57 | 0.8 | 0.423 |
| Tamoxifen | Yes | 0.74 | 0.48 | 1.16 | −1.32 | 0.187 |
| Bold values indicate statistical significance at 0.05 | ||||||
| Factor | Group | Q-Statistics | df | p-Value | I-Square | Classification |
|---|---|---|---|---|---|---|
| Age | Below 35 | 8.81 | 4 | 0.066 | 55% | moderate |
| 35 to 60 | 629.27 | 41 | <0.001 | 93% | very high | |
| Above 60 | 731.92 | 30 | <0.001 | 96% | very high | |
| Education | Secondary | 1.86 | 3 | 0.601 | 0% | low |
| Higher | 2.71 | 3 | 0.439 | 0% | low | |
| Race | Black | 17.21 | 11 | 0.102 | 36% | moderate |
| Asian | 9.78 | 6 | 0.134 | 39% | moderate | |
| Hispanic | 22.9 | 5 | <0.001 | 78% | very high | |
| Grade | 2 | 164.76 | 11 | <0.001 | 93% | very high |
| 3 | 1077.9 | 12 | <0.001 | 99% | very high | |
| Stage | 2 | 352.18 | 13 | <0.001 | 96% | very high |
| 3 | 659 | 16 | <0.001 | 98% | very high | |
| 4 | 4008.6 | 13 | <0.001 | 100% | very high | |
| Differentiation | Moderate | 21.59 | 3 | <0.001 | 86% | very high |
| Poor | 35.59 | 4 | <0.001 | 89% | very high | |
| Undifferentiated | 61.11 | 6 | <0.001 | 90% | very high | |
| Nodes | Positive | 2295.3 | 32 | <0.001 | 99% | very high |
| Surgery | Yes | 2849.14 | 28 | <0.001 | 99% | very high |
| Tumour size | ≥2 cm | 2409.95 | 24 | <0.001 | 99% | very high |
| Histology | Lobular | 170.94 | 11 | <0.001 | 94% | very high |
| Medullary | 0.15 | 3 | 0.986 | 0% | low | |
| Others | 403.92 | 12 | <0.001 | 97% | very high | |
| Estrogen receptor | Positive | 607.89 | 19 | <0.001 | 97% | very high |
| Negative | 244.5 | 6 | <0.001 | 98% | very high | |
| HER2 receptor | Positive | 470.86 | 10 | <0.001 | 98% | very high |
| Negative | 23.44 | 2 | <0.001 | 91% | very high | |
| Body Mass Index | Overweight/Obese | 36.79 | 11 | <0.001 | 70% | high |
| Comorbidity Index | 1 to 2 | 6.36 | 5 | 0.273 | 21% | low |
| ≥3 | 9.64 | 3 | 0.022 | 69% | high | |
| BRCA | 1 | 45.2 | 8 | <0.001 | 82% | very high |
| 2 | 27.12 | 9 | <0.001 | 67% | high | |
| Oral contraceptive use | Yes | 1.83 | 2 | 0.400 | 0% | low |
| Physical activity | Light/Moderate | 3.63 | 4 | 0.459 | 0% | low |
| High/Vigorous | 6.1 | 5 | 0.296 | 18% | low | |
| Progesterone receptor | Positive | 468.94 | 12 | <0.001 | 97% | very high |
| Negative | 536.84 | 6 | <0.001 | 99% | very high | |
| Hormone therapy | Yes | 180.85 | 10 | <0.001 | 94% | very high |
| Chemotherapy | Yes | 935.86 | 27 | <0.001 | 97% | very high |
| Radiotherapy | Yes | 1329.58 | 16 | <0.001 | 99% | very high |
| Tamoxifen | Yes | 13.95 | 2 | <0.001 | 86% | very high |
| Bold values indicate statistical significance at 0.05 | ||||||
| Factor | Group | t-Statistics | df | p-Value |
|---|---|---|---|---|
| Age | Below 35 | −1.14 | 3 | 0.336 |
| 35 to 60 | 2.39 | 40 | 0.022 | |
| Above 60 | −1.51 | 29 | 0.141 | |
| Education | Secondary | 0.26 | 2 | 0.822 |
| Higher | 0.72 | 2 | 0.546 | |
| Race | Black | −0.16 | 10 | 0.873 |
| Asian | −0.07 | 5 | 0.943 | |
| Hispanic | 1.33 | 4 | 0.256 | |
| Grade | 2 | −0.27 | 10 | 0.793 |
| 3 | −0.41 | 11 | 0.692 | |
| Stage | 2 | 1.62 | 12 | 0.132 |
| 3 | 2.12 | 15 | 0.052 | |
| 4 | −0.57 | 12 | 0.577 | |
| Differentiation | Moderate | 2.62 | 2 | 0.120 |
| Poor | 2.13 | 3 | 0.123 | |
| Undifferentiated | 3.09 | 5 | 0.027 | |
| Nodes | Positive | 0.81 | 31 | 0.427 |
| Surgery | Yes | −0.89 | 27 | 0.383 |
| Tumour size | ≥2 cm | 5.96 | 23 | <0.001 |
| Histology | Lobular | 0.03 | 10 | 0.978 |
| Medullary | 0.27 | 2 | 0.812 | |
| Others | −1.32 | 11 | 0.215 | |
| Estrogen receptor | Positive | 1.58 | 18 | 0.132 |
| Negative | −0.76 | 5 | 0.479 | |
| HER2 receptor | Positive | −0.03 | 9 | 0.978 |
| Negative | 0.49 | 1 | 0.713 | |
| Body Mass Index | Overweight/Obese | 3.38 | 10 | 0.007 |
| Comorbidity Index | 1 to 2 | 0.45 | 4 | 0.678 |
| ≥3 | 0.57 | 2 | 0.628 | |
| BRCA | 1 | −1.07 | 7 | 0.321 |
| 2 | −0.29 | 8 | 0.778 | |
| Oral contraceptive use | Yes | 0.96 | 1 | 0.513 |
| Physical activity | Light/Moderate | −1.22 | 3 | 0.309 |
| High/Vigorous | −1.1 | 4 | 0.335 | |
| Progesterone receptor | Positive | 1.67 | 11 | 0.123 |
| Negative | −0.83 | 5 | 0.447 | |
| Hormone therapy | Yes | −0.95 | 9 | 0.368 |
| Chemotherapy | Yes | 2.79 | 26 | 0.010 |
| Radiotherapy | Yes | 3.56 | 15 | 0.003 |
| Tamoxifen | Yes | −0.04 | 1 | 0.972 |
| Bold values indicate statistical significance at 0.05 | ||||
| Year of Study | ||||
|---|---|---|---|---|
| Estimate | SE | p-Value | ||
| Age | Below 35 | −0.01 | 0.02 | 0.598 |
| 35 to 60 | 0.01 | 0.01 | 0.199 | |
| Above 60 | 0.05 | 0.01 | 0.002 | |
| Education | Secondary | 0.01 | 0.02 | 0.699 |
| Higher | 0.02 | 0.02 | 0.344 | |
| Race | Black | 0.00 | 0.00 | 0.706 |
| Asian | 0.01 | 0.02 | 0.578 | |
| Hispanic | 0.00 | 0.03 | 0.853 | |
| Grade | 2 | 0.00 | 0.02 | 0.961 |
| 3 | −0.02 | 0.03 | 0.661 | |
| Stage | 2 | −0.06 | 0.03 | 0.061 |
| 3 | 0.02 | 0.04 | 0.533 | |
| 4 | 0.09 | 0.06 | 0.108 | |
| Differentiation | Moderate | 0.19 | 0.29 | 0.519 |
| Poor | 0.09 | 0.22 | 0.677 | |
| Undifferentiated | 0.14 | 0.16 | 0.394 | |
| Nodes | Positive | −0.01 | 0.03 | 0.646 |
| Surgery | Yes | 0.00 | 0.03 | 0.911 |
| Tumour size | ≥2 cm | 0.02 | 0.01 | 0.145 |
| Histology | Lobular | −0.02 | 0.11 | 0.872 |
| Medullary | 0.00 | 0.04 | 0.983 | |
| Others | −0.01 | 0.04 | 0.898 | |
| Estrogen receptor | Positive | −0.03 | 0.08 | 0.747 |
| Negative | 0.00 | 0.02 | 0.944 | |
| HER2 receptor | Positive | 0.00 | 0.04 | 0.918 |
| Negative | −0.05 | 0.03 | 0.036 | |
| Body Mass Index | Overweight/Obese | −0.01 | 0.03 | 0.583 |
| Comorbidity Index | 1 to 2 | −0.02 | 0.05 | 0.682 |
| ≥3 | 0.02 | 0.02 | 0.314 | |
| BRCA | 1 | −0.02 | 0.02 | 0.386 |
| 2 | 0.00 | 0.09 | 0.977 | |
| Oral contraceptive use | Yes | −0.34 | 0.19 | 0.073 |
| Physical activity | Light/Moderate | 0.01 | 0.02 | 0.453 |
| High/Vigorous | −0.05 | 0.03 | 0.088 | |
| Progesterone receptor | Positive | 0.01 | 0.02 | 0.531 |
| Negative | −0.06 | 0.03 | 0.052 | |
| Hormone therapy | Yes | 0.00 | 0.03 | 0.968 |
| Chemotherapy | Yes | 0.06 | 0.04 | 0.181 |
| Radiotherapy | Yes | −0.04 | 0.04 | 0.365 |
| Tamoxifen | Yes | - | - | - |
| Bold values indicate statistical significance at 0.05 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdul Rahman, H.; Zaim, S.N.N.; Suhaimei, U.S.; Jamain, A.A. Prognostic Factors Associated with Breast Cancer-Specific Survival from 1995 to 2022: A Systematic Review and Meta-Analysis of 1,386,663 Cases from 30 Countries. Diseases 2024, 12, 111. https://doi.org/10.3390/diseases12060111
Abdul Rahman H, Zaim SNN, Suhaimei US, Jamain AA. Prognostic Factors Associated with Breast Cancer-Specific Survival from 1995 to 2022: A Systematic Review and Meta-Analysis of 1,386,663 Cases from 30 Countries. Diseases. 2024; 12(6):111. https://doi.org/10.3390/diseases12060111
Chicago/Turabian StyleAbdul Rahman, Hanif, Siti Nurzaimah Nazhirah Zaim, Ummi Salwa Suhaimei, and Al Amin Jamain. 2024. "Prognostic Factors Associated with Breast Cancer-Specific Survival from 1995 to 2022: A Systematic Review and Meta-Analysis of 1,386,663 Cases from 30 Countries" Diseases 12, no. 6: 111. https://doi.org/10.3390/diseases12060111
APA StyleAbdul Rahman, H., Zaim, S. N. N., Suhaimei, U. S., & Jamain, A. A. (2024). Prognostic Factors Associated with Breast Cancer-Specific Survival from 1995 to 2022: A Systematic Review and Meta-Analysis of 1,386,663 Cases from 30 Countries. Diseases, 12(6), 111. https://doi.org/10.3390/diseases12060111






