Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
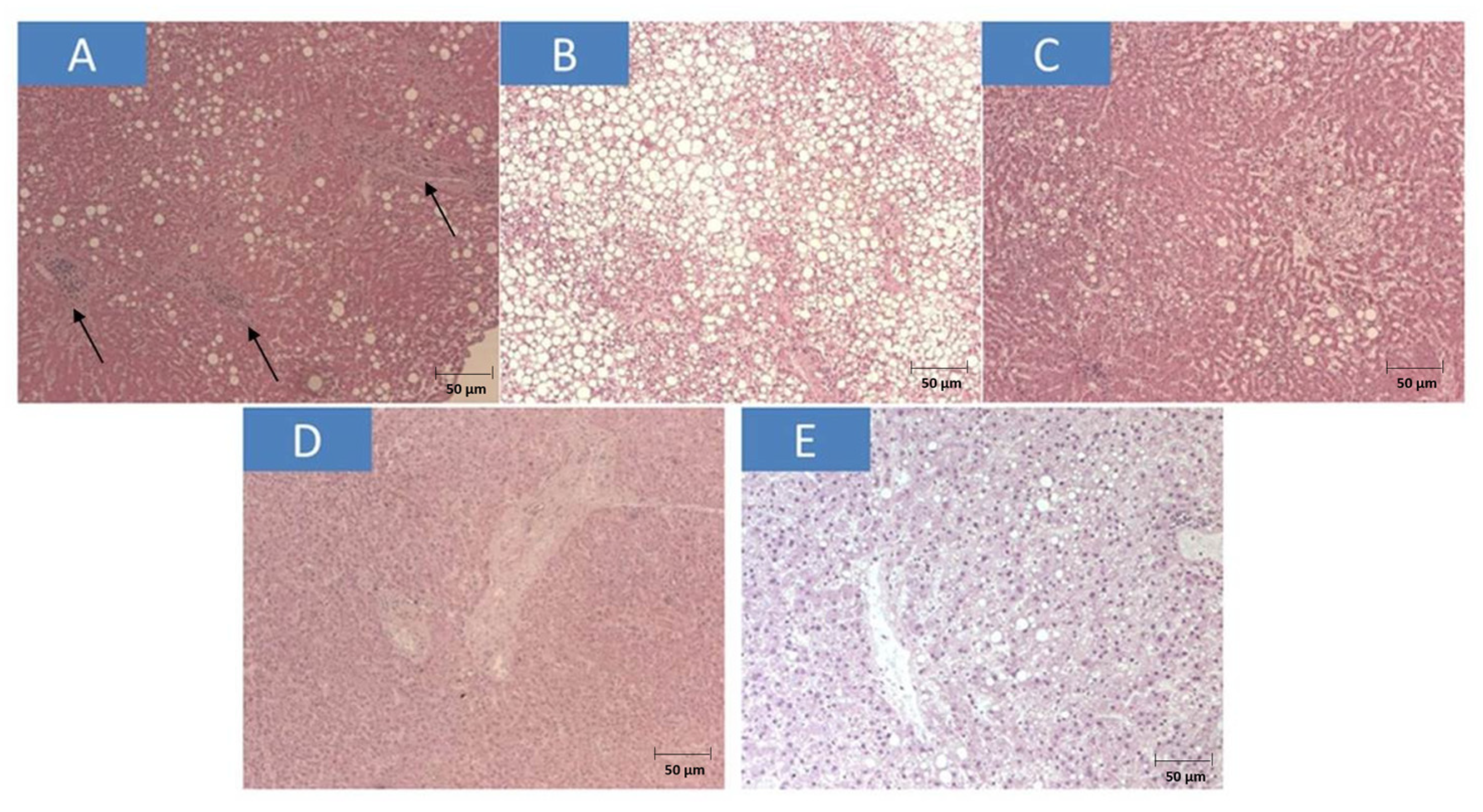
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Borczuk, A.C.; Yantiss, R.K. The pathogenesis of coronavirus-19 disease. J. Biomed. Sci. 2022, 29, 87. [Google Scholar] [CrossRef] [PubMed]
- Montori, M.; Baroni, G.S.; Santori, P.; Di Giampaolo, C.; Ponziani, F.; Abenavoli, L.; Scarpellini, E. Liver Damage and COVID-19: At Least a “Two-Hit” Story in Systematic Review. Curr. Issues Mol. Biol. 2023, 45, 3035–3047. [Google Scholar] [CrossRef]
- Zanza, C.; Racca, F.; Longhitano, Y.; Piccioni, A.; Franceschi, F.; Artico, M.; Abenavoli, L.; Maiese, A.; Passaro, G.; Volonnino, G.; et al. Risk Management and Treatment of Coagulation Disorders Related to COVID-19 Infection. Int. J. Environ. Res. Public Health 2021, 18, 1268. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Cinaglia, P.; Procopio, A.C.; Serra, R.; Aquila, I.; Zanza, C.; Longhitano, Y.; Artico, M.; Larussa, T.; Boccuto, L.; et al. SARS-CoV-2 Spread Dynamics in Italy: The Calabria Experience. Rev. Recent Clin. Trials 2021, 16, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Mousavizadeh, L.; Ghasemi, S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J. Microbiol. Immunol. Infect. 2021, 54, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Redondo, N.; Zaldívar-López, S.; Garrido, J.J.; Montoya, M. SARS-CoV-2 Accessory Proteins in Viral Pathogenesis: Knowns and Unknowns. Front. Immunol. 2021, 12, 708264. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- V’kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef]
- Roberts, M.C.; Levi, M.; McKee, M.; Schilling, R.; Lim, W.S.; Grocott, M.P.W. COVID-19: A complex multisystem disorder. Br. J. Anaesth. 2020, 125, 238–242. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The signal pathways and treatment of cytokine storm in COVID-19. Signal. Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef] [PubMed]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Kulanthaivel, S.; Kaliberdenko, V.B.; Balasundaram, K.; Shterenshis, M.V.; Scarpellini, E.; Abenavoli, L. Tocilizumab in SARS-CoV-2 Patients with the Syndrome of Cytokine Storm: A Narrative Review. Rev. Recent Clin. Trials 2021, 16, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Zanza, C.; Tassi, M.F.; Romenskaya, T.; Piccolella, F.; Abenavoli, L.; Franceschi, F.; Piccioni, A.; Ojetti, V.; Saviano, A.; Canonico, B.; et al. Lock, Stock and Barrel: Role of Renin-Angiotensin-Aldosterone System in Coronavirus Disease 2019. Cells 2021, 10, 1752. [Google Scholar] [CrossRef] [PubMed]
- Yüce, M.; Filiztekin, E.; Özkaya, K.G. COVID-19 diagnosis—A review of current methods. Biosens. Bioelectron. 2021, 172, 112752. [Google Scholar] [CrossRef] [PubMed]
- Dell’Aquila, M.; Cattani, P.; Fantoni, M.; Marchetti, S.; Aquila, I.; Stigliano, E.; Carbone, A.; Oliva, A.; Arena, V. Postmortem Swabs in the Severe Acute Respiratory Syndrome Coronavirus 2 Pandemic: Report on 12 Complete Clinical Autopsy Cases. Arch. Pathol. Lab. Med. 2020, 144, 1298–1302. [Google Scholar] [CrossRef] [PubMed]
- Hanley, B.; Lucas, S.B.; Youd, E.; Swift, B.; Osborn, M. Autopsy in suspected COVID-19 cases. J. Clin. Pathol. 2020, 73, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Sánchez, N.; Valencia-Rodríguez, A.; Qi, X.; Yoshida, E.M.; Romero-Gómez, M.; George, J.; Eslam, M.; Abenavoli, L.; Xie, W.; Teschke, R.; et al. What Has the COVID-19 Pandemic Taught Us so Far? Addressing the Problem from a Hepatologist’s Perspective. J. Clin. Transl. Hepatol. 2020, 8, 0024. [Google Scholar] [CrossRef]
- Testino, G.; Pellicano, R. Alcohol consumption in the COVID-19 era. Minerva Gastroenterol. Dietol. 2020, 66, 90–92. [Google Scholar] [CrossRef]
- Spearman, C.W.; Aghemo, A.; Valenti, L.; Sonderup, M.W. COVID-19 and the liver: A 2021 update. Liver Int. 2021, 41, 1988–1998. [Google Scholar] [CrossRef]
- Actis, G.C.; Ribaldone, D.G.; Fagoonee, S.; Pellicano, R. COVID-19: A user’s guide, status of the art and an original proposal to terminate viral recurrence. Minerva Med. 2021, 112, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Chinese Digestion Association; Chinese Medical Doctor Association; Chinese Society of Hepatology; Chinese Medical Association. The protocol for prevention, diagnosis and treatment of liver injury in coronavirus disease 2019. Zhonghua Gan Zang Bing Za Zhi 2020, 28, 218–221. [Google Scholar] [CrossRef]
- Testino, G.; Pellicano, R. Acute-on-chronic liver failure by SARS-CoV-2 in active alcohol use disorder cirrhotic patient. Minerva Gastroenterol. 2021, 67, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Saito, M.; Nagai, H.; Yamamoto, S.; Ikeuchi, K.; Lim, L.A.; Adachi, E.; Koga, M.; Okushin, K.; Akai, H.; et al. Association of coagulopathy with liver dysfunction in patients with COVID-19. Hepatol. Res. 2021, 51, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Sonzogni, A.; Previtali, G.; Seghezzi, M.; Grazia Alessio, M.; Gianatti, A.; Licini, L.; Morotti, D.; Zerbi, P.; Carsana, L.; Rossi, R.; et al. Liver histopathology in severe COVID 19 respiratory failure is suggestive of vascular alterations. Liver Int. 2020, 40, 2110–2116. [Google Scholar] [CrossRef] [PubMed]
- D’Ardes, D.; Boccatonda, A.; Cocco, G.; Fabiani, S.; Rossi, I.; Bucci, M.; Guagnano, M.T.; Schiavone, C.; Cipollone, F. Impaired coagulation, liver dysfunction and COVID-19: Discovering an intriguing relationship. World J. Gastroenterol. 2022, 28, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Rapporto ISS COVID-19 n. 6/2020—Procedura per L’esecuzione di Riscontri Diagnostici in Pazienti Deceduti Con Infezione da SARS-CoV-2. Versione del 23 Marzo 2020. Available online: https://www.iss.it/documents/20126/0/Rapporto+COVID-19+n.+6_2020+Autopsie+v27+marzo.pdf/c4b363a1-a246-c36c-d007-ae24ed7e648b?t=1585307031219 (accessed on 10 July 2023).
- Mussini, C.; Falcone, M.; Nozza, S.; Sagnelli, C.; Parrella, R.; Meschiari, M.; Petrosillo, N.; Mastroianni, C.; Cascio, A.; Iaria, C.; et al. Therapeutic strategies for severe COVID-19: A position paper from the Italian Society of Infectious and Tropical Diseases (SIMIT). Clin. Microbiol. Infect. 2021, 27, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Aquila, I.; Sacco, M.A.; Procopio, A.C.; Cinaglia, P.; Zanza, C.; Longhitano, Y.; Arena, V.; Fagoonee, S.; Ricci, P.; et al. Liver injury associated with high value of D-dimer plasmatic level in COVID-19 patients. Minerva Gastroenterol. 2023, 69, 141–148. [Google Scholar] [CrossRef]
- Bryce, C.; Grimes, Z.; Pujadas, E.; Ahuja, S.; Beasley, M.B.; Albrecht, R.; Hernandez, T.; Stock, A.; Zhao, Z.; Al Rasheed, M.R.; et al. Pathophysiology of SARS-CoV-2: The Mount Sinai COVID-19 autopsy experience. Mod. Pathol. 2021, 34, 1456–1467. [Google Scholar] [CrossRef]
- Miranda, C.; Garlatti, E.; Da Porto, A.; Rinaldo, E.; Grazioli, S.; Zanette, G.; Tonizzo, M. Liver injury in COVID-19 patients with non-alcoholic fatty liver disease: An update. Arch. Med. Sci. Atheroscler. Dis. 2023, 8, e1–e10. [Google Scholar] [CrossRef]
- Dietrich, C.G.; Geier, A.; Merle, U. Non-alcoholic fatty liver disease and COVID-19: Harmless companions or disease intensifier? World J. Gastroenterol. 2023, 29, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Ciavarella, A.; Gnocchi, D.; Custodero, C.; Lenato, G.M.; Fiore, G.; Sabbà, C.; Mazzocca, A. Translational insight into prothrombotic state and hypercoagulation in nonalcoholic fatty liver disease. Thromb. Res. 2021, 198, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Louie, C.Y.; Pham, M.X.; Daugherty, T.J.; Kambham, N.; Higgins, J.P. The liver in heart failure: A biopsy and explant series of the histopathologic and laboratory findings with a particular focus on pre-cardiac transplant evaluation. Mod. Pathol. 2015, 28, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Masarone, M.; Rosato, V.; Aglitti, A.; Bucci, T.; Caruso, R.; Salvatore, T.; Sasso, F.C.; Tripodi, M.F.; Persico, M. Liver biopsy in type 2 diabetes mellitus: Steatohepatitis represents the sole feature of liver damage. PLoS ONE 2017, 12, e0178473. [Google Scholar] [CrossRef] [PubMed]
- Spiezia, L.; Boscolo, A.; Poletto, F.; Cerruti, L.; Tiberio, I.; Campello, E.; Navalesi, P.; Simioni, P. COVID-19-Related Severe Hypercoagulability in Patients Admitted to Intensive Care Unit for Acute Respiratory Failure. Thromb. Haemost. 2020, 120, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Rampotas, A.; Pavord, S. Platelet aggregates, a marker of severe COVID-19 disease. J. Clin. Pathol. 2021, 74, 750–751. [Google Scholar] [CrossRef] [PubMed]
- Fanni, D.; Cerrone, G.; Saba, L.; Demontis, R.; Congiu, T.; Piras, M.; Gerosa, C.; Suri, J.S.; Coni, P.; Caddori, A.; et al. Thrombotic sinusoiditis and local diffuse intrasinusoidal coagulation in the liver of subjects affected by COVID-19: The evidence from histology and scanning electron microscopy. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Esmaeel, H.M.; Ahmed, H.A.; Elbadry, M.I.; Khalaf, A.R.; Mohammed, N.A.; Mahmoud, H.A.; Taha, E.M. Coagulation parameters abnormalities and their relation to clinical outcomes in hospitalized and severe COVID-19 patients: Prospective study. Sci. Rep. 2022, 12, 13155. [Google Scholar] [CrossRef]
- Grobler, C.; Maphumulo, S.C.; Grobbelaar, L.M.; Bredenkamp, J.C.; Laubscher, G.J.; Lourens, P.J.; Steenkamp, J.; Kell, D.B.; Pretorius, E. COVID-19: The Rollercoaster of Fi-brin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets, and Erythrocytes. Int. J. Mol. Sci. 2020, 21, 5168. [Google Scholar] [CrossRef]
- Medeiros, A.K.; Barbisan, C.C.; Cruz, I.R.; Araújo, E.M.; Libânio, B.B.; Albuquerque, K.S.; Torres, U.S. Higher frequency of hepatic steatosis at CT among COVID-19-positive patients. Abdom. Radiol. 2020, 45, 2748–2754. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Prichett, L.; Tao, X.; Alqahtani, S.A.; Hamilton, J.P.; Mezey, E.; Strauss, A.T.; Kim, A.; Potter, J.J.; Chen, P.H.; et al. Abnormal liver chemistries as a predictor of COVID-19 severity and clinical outcomes in hospitalized patients. World J. Gastroenterol. 2022, 28, 570–587. [Google Scholar] [CrossRef] [PubMed]
- Da, B.L.; Suchman, K.; Roth, N.; Rizvi, A.; Vincent, M.; Trindade, A.J.; Bernstein, D.; Satapathy, S.K. Northwell COVID-19 Research Consortium. Cholestatic liver injury in COVID-19 is a rare and distinct entity and is associated with increased mortality. J. Intern. Med. 2021, 290, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, H.; Wang, J.; Li, X.; Xue, C.; Niu, C.; Liao, P. Serum Albumin Levels are a Predictor of COVID-19 Patient Prognosis: Evidence from a Single Cohort in Chongqing, China. Int. J. Gen. Med. 2021, 14, 2785–2797. [Google Scholar] [CrossRef] [PubMed]
- Turcato, G.; Zaboli, A.; Kostic, I.; Melchioretto, B.; Ciccariello, L.; Zaccaria, E.; Olivato, A.; Maccagnani, A.; Pfeifer, N.; Bonora, A. Severity of SARS-CoV-2 infection and albumin levels recorded at the first emergency department evaluation: A multicentre retrospective observational study. Emerg. Med. J. 2022, 39, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Wiedermann, C.J. Hypoalbuminemia as Surrogate and Culprit of Infections. Int. J. Mol. Sci. 2021, 22, 4496. [Google Scholar] [CrossRef]
| Patients | #1 | #2 | #3 | #4 | #5 | #6 | #7 | #8 | #9 | #10 |
|---|---|---|---|---|---|---|---|---|---|---|
| Age | 51 | 52 | 85 | 74 | 77 | 62 | 64 | 71 | 83 | 21 |
| Sex | M | M | M | M | F | M | F | M | F | M |
| Hospital stay (days) | 9 | 18 | 27 | 12 | 10 | 26 | 7 | 2 | 1 | 1 |
| Comorbidities | - | - | HF | PF | - | AH | DM2 | AH | Stroke | - |
| Cause of death | PE | PNA | PNA | PE | CVA | PE | DIC | PE | DIC | DIC |
| INR | ||||||||||
| T0 | 1.06 | 0.96 | 0.89 | 1.22 | 3.57 | 0.91 | 1 | 1.13 | 1.3 | 1.42 |
| T1 | 1.03 | 1.17 | 3.03 | 1.33 | 1.28 | 1.77 | 1.13 | 1.2 | 1.37 | 1.45 |
| aPTT (s) | ||||||||||
| T0 | 31 | 15 | 35 | 39 | 37 | 33 | 35 | 58 | 39 | 31 |
| T1 | 115 | 40 | 67 | 39 | 40 | 121 | 40 | 67 | 40 | 35 |
| Fibrinogen (mg/dL) | ||||||||||
| T0 | 546 | 164 | 368 | 303 | 618 | 603 | 636 | 542 | 448 | 604 |
| T1 | 960 | 500 | 220 | 182 | 812 | 585 | 708 | 563 | 453 | 608 |
| AT-III (%) | ||||||||||
| T0 | 80 | 120 | 85 | 92 | 97 | 106 | 100 | 84 | 95 | 100 |
| T1 | 71 | 118 | 110 | 106 | 82 | 73 | 80 | 88 | 97 | 111 |
| D-Dimers (μg/L) | ||||||||||
| T0 | 0.77 | 15.38 | 0.75 | 0.44 | 5.89 | 0.38 | 0.95 | 1.13 | 1.89 | 0.79 |
| T1 | 6.83 | 15.38 | 0.75 | 0.38 | 5.89 | 4.44 | 3.67 | 1.13 | 1.86 | 0.81 |
| AST (UI/L) | ||||||||||
| T0 | 45 | 27 | 25 | 18 | 15 | 24 | 27 | 27 | 38 | 19 |
| T1 | 92 | 63 | 1886 | 87 | 641 | 24 | 30 | 23 | 56 | 13 |
| ALT (UI/L) | ||||||||||
| T0 | 62 | 62 | 12 | 19 | 19 | 19 | 38 | 51 | 54 | 12 |
| T1 | 52 | 36 | 162 | 23 | 469 | 18 | 40 | 53 | 50 | 25 |
| GGT (UI/L) | ||||||||||
| T0 | 21 | 68 | 21 | 28 | 69 | 38 | 53 | 91 | 34 | 30 |
| T1 | 39 | 271 | 96 | 14 | 151 | 13 | 58 | 73 | 47 | 45 |
| ALP (UI/L) | ||||||||||
| T0 | 46 | 74 | 80 | 84 | 62 | 63 | 67 | 42 | 81 | 75 |
| T1 | 180 | 261 | 103 | 55 | 128 | 72 | 105 | 30 | 101 | 100 |
| Albumin (g/dL) | ||||||||||
| T0 | 3.2 | 3.3 | 3.7 | 3.2 | 3.0 | 3.9 | 3.5 | 3.4 | 3.8 | 3.1 |
| T1 | 2 | 2.6 | 3.5 | 3.1 | 3.2 | 2.9 | 3.2 | 2.8 | 2.4 | 2.4 |
| T0 | T1 | p-Value | |
|---|---|---|---|
| INR | 1.35 ± 0.8 | 1.48 ± 0.58 | 0.114 |
| aPTT (s) | 35.3 ± 10.54 | 60.4 ± 32.5 | 0.009 |
| Fibrinogen (mg/dL) | 438.2 ± 158.72 | 559.1 ± 240.8 | 0.226 |
| AT-III (%) | 95.88 ± 11.76 | 85.27 ± 32.01 | 0.689 |
| D-Dimers (μg/L) | 2.84 ± 4.7 | 4.13 ± 4.57 | 0.052 |
| AST (UI/L) | 26.5 ± 9.1 | 291.5 ± 590.1 | 0.17 |
| ALT (UI/L) | 34.8 ± 20.9 | 92.8 ± 138.4 | 0.20 |
| GGT (UI/L) | 45.7 ± 23.7 | 80.7 ± 78.3 | 0.19 |
| ALP (UI/L) | 67.5 ± 14.5 | 113.5 ± 65.9 | 0.044 |
| Albumin (g/dL) | 3.41 ± 0.3 | 2.71 ± 0.5 | 0.0009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abenavoli, L.; Aquila, I.; Sacco, M.A.; Scarlata, G.G.M.; Procopio, A.C.; Boccuto, L.; Scarpellini, E.; Greco, M.; Foti, D.P.; Ricci, P.; et al. Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series. Diseases 2023, 11, 141. https://doi.org/10.3390/diseases11040141
Abenavoli L, Aquila I, Sacco MA, Scarlata GGM, Procopio AC, Boccuto L, Scarpellini E, Greco M, Foti DP, Ricci P, et al. Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series. Diseases. 2023; 11(4):141. https://doi.org/10.3390/diseases11040141
Chicago/Turabian StyleAbenavoli, Ludovico, Isabella Aquila, Matteo Antonio Sacco, Giuseppe Guido Maria Scarlata, Anna Caterina Procopio, Luigi Boccuto, Emidio Scarpellini, Marta Greco, Daniela Patrizia Foti, Pietrantonio Ricci, and et al. 2023. "Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series" Diseases 11, no. 4: 141. https://doi.org/10.3390/diseases11040141
APA StyleAbenavoli, L., Aquila, I., Sacco, M. A., Scarlata, G. G. M., Procopio, A. C., Boccuto, L., Scarpellini, E., Greco, M., Foti, D. P., Ricci, P., & Luzza, F. (2023). Liver Damage and Impaired Coagulation in COVID-19 Patients: A Case Series. Diseases, 11(4), 141. https://doi.org/10.3390/diseases11040141














