Abstract
Percutaneous Nephrolithotomy (PCNL) is a procedure used to treat kidney stones. In PCNL, a needle punctures the kidney through an incision in a patient’s back and thin tools are threaded through the incision to gain access to kidney stones for removal. Despite being one of the main endoscopic procedures for managing kidney stones, PCNL remains a difficult procedure to learn with a long and steep learning curve. Virtual reality simulation with haptic feedback is emerging as a new method for PCNL training. It offers benefits for both novices and experienced surgeons. In the first case, novices can practice and gain kidney access in a variety of simulation scenarios without offering any risk to patients. In the second case, surgeons can use the simulator for preoperative surgical rehearsal. This paper proposes the first preliminary study of PCNL surgical rehearsal using the Marion Surgical PCNL simulator. Preoperative CT scans of a patient scheduled to undergo PCNL are used in the simulator to create a 3D model of the renal system. An experienced surgeon then planned and practiced the procedure in the simulator before performing the surgery in the operating room. This is the first study involving survival rehearsal using a combination of VR and haptic feedback in PCNL before surgery. Preliminary results confirm that surgical rehearsal using a combination of virtual reality and haptic feedback strongly affects decision making during the procedure.
1. Introduction
Percutaneous Nephrolithotomy (PCNL) is a minimally invasive procedure for the treatment of nephrolithiasis (commonly known as kidney stones). It involves using a needle to puncture the kidney through a small incision in a patient’s back. A sheath is then placed through this entry path, and a nephroscope, shown in Figure 1, is passed through the sheath to gain access to kidney stones. Stones are then fragmented and removed through the nephroscope [1,2].
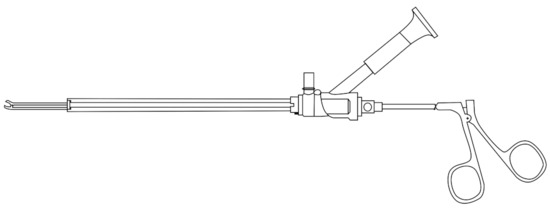
Figure 1.
A nephroscope: this tool is inserted into a patient’s back during a PCNL procedure. The eyepiece on the top of the nephroscope enables the surgeon to see inside the kidney. Tools are used through the nephroscope to breakup and remove kidney stones.
Even though more than 90% of kidney stones are passed without medical intervention or through the use of non-invasive procedures, PCNL is an integral treatment for more severe cases of large or irregularly shaped kidney stones, or where other treatment options have been unsuccessful [1,2]. Despite decades of clinical prevalence, it is challenging for novice surgeons to receive adequate training and gain experience in the procedure [3]. Such a lack of surgical proficiency may lead to poor treatment outcomes.
Despite being one of the main endoscopic procedures for managing kidney stones, PCNL remains a difficult procedure to learn with a long training period [4]. Traditional simulations such as cadavers are expensive and in short supply [5]. Other available PCNL training resources such as porcine training models require the use of fluoroscopy for tool guidance, which leads to unnecessary radiation exposure to trainees [6,7]. Virtual reality (VR) is emerging as a new method of delivering simulations for training in a variety of surgical procedures. It offers benefits for learners and educators through cost-effective, repeatable, and standardized clinical training on-demand [8,9]. Due to their versatility, simulators are becoming a new standard for effectively training novice surgeons in various surgical procedures such as general surgery [9], intracardiac interventions [10], cataract surgery [11], amongst others [8]. Gradually, this training option is being explored for PCNL as well [12]. Sommer et al. [9] found that surgical simulators improved a novice surgeon’s visual-spatial ability. Similarly, a cyber-physical teleoperative rehearsal framework is tested in [13], which found that novices benefit from haptic feedback during surgical training.
Once a novice surgeon has been trained, a variety of challenges still exist when performing this procedure in the real world. Arnold et al. [14] emphasized that health care is the only high-risk industry where rehearsals are not yet part of daily work. The development and growth of health care simulations can put an end to this model and provide an opportunity to rehearse high-risk, complex, and rare surgical procedures in a safe environment rather than on an actual patient. Yiasemidou et al. [15] conducted a meta-analysis of studies comparing preoperative rehearsals to standard treatment with two distinct groups of patients and demonstrated that real procedures were performed quicker if preoperative rehearsal took place. However, the immediate clinical outcome was similar for practiced and non-practiced operations. Current evidence suggests that patient-specific preoperative preparation is feasible, safe, and decreases operational time [15,16,17,18].
An example of surgical rehearsal is the SNAP VR 360 software (Surgical Theatre, Pepper Pike, OH, USA). It provides a neurosurgeon with a virtual walk-through and preplan of a keyhole surgery [19]. A 3D model used during the walk-through is generated from a patient’s computed tomography angiography and magnetic resonance imaging (MRI) [19]. While Surgical Theatre has gained FDA approval for their software to be used in cerebral and spine surgery rehearsal, it only provides a walk-through of the procedure and does not provide integrated real-time tactile feedback during the rehearsal. The implementation of haptic feedback has been proven to be beneficial to surgical rehearsal [13] and can be implemented to simulate surgical complications, including abnormal patient kidney anatomy, such as horseshoe kidneys, malrotated kidney, or duplex kidneys. Additionally, tactile feedback can imitate kidney movements from patient breathing, heart pumping, and general tissue resistance forces. Rehearsing with integrated real-time force feedback allows the surgeon to plan an appropriate path toward the kidney stones while receiving real-time feedback about the tissue displacement, which can ultimately reduce tissue damage during surgery.
Other surgical rehearsal approaches use patient-specific preoperative imaging to create a physical model of the relevant anatomy. The limitations of 3D printed models are that they are static, and thus, they lack the ability to simulate the dynamic conditions of real-world organs that result from pulsations of the heart or lung expansion and contraction. Therefore, incorporating accurate dynamic functionalities into the organ models is a key aspect to achieve more realistic surgical rehearsal [20].
Parkhomenko et al. [16] explored the effect of virtual reality models of a patient’s anatomy on preoperative planning for PCNL. Surgeons had the opportunity to interact with a 3D model (constructed from a patient’s CT scan) in a VR environment; 10 of the 25 surgeons altered their operative plan based on their interaction with the 3D model. Additionally, surgeons that used the rehearsal model inflicted less blood loss to patients, fewer incisions through the skin, and used fluoroscopy for shorter periods of time, while showing a higher stone clearance rate after the procedure when compared to surgeons that did not perform the rehearsal. The study thus provides significant evidence for the efficacy of virtual reality models based on patient-specific anatomy as beneficial rehearsal tools [16]. In [21], the surgeon could view and interact with a 3D model of a patient’s lung displayed next to other operative imaging, allowing them to have a better understanding of the patient’s anatomy.
While VR simulations provide a surgeon with a better understanding of patient anatomy, this paper takes the approach one step further and presents a PCNL rehearsal framework that includes 3D model generation from patient data, while including haptic feedback during the rehearsal training using a complete PCNL simulator. A patient agreed to have their preoperative full-body CT scan used in this study. First, a 3D model of the patient’s anatomy is constructed based on the preoperative imaging. The surgeon then rehearsed the procedure in the K181 simulator (Marion Surgical, Toronto, ON, Canada); this simulator provides haptic feedback to the user by mimicking tissue resistance forces. The PCNL surgery was then performed on the patient to remove their kidney stones. Questionnaires were provided to the surgeon pre/postoperatively to assess the benefit and quality of the simulated surgical rehearsal. The objective of this study is to assess the impact of both the 3D model and the real-time haptic feedback on the surgery.
A detailed description of the surgical simulator is provided in Section 2 and the process for generating 3D models from 2D imaging is discussed in Section 3. Preoperative and postoperative questionnaires were given to the surgeon to explore the viability of the simulator as surgical rehearsal, and its possible benefits. The nephrolithiasis case details, questionnaires, experimental procedure, surgical outcomes, and questionnaire results are described in Section 4. Finally, concluding remarks and a description of future work are given in Section 5.
2. Marion K181 PCNL Simulator
The Marion K181 PCNL simulator depicted in Figure 2 is a virtual reality PCNL surgical simulator. It provides users with real-time haptic feedback while they control a fluoroscopic arm and a needle for calyceal puncture. The user enters an immersive, 3D virtual operating room using a virtual reality headset from where they gain percutaneous renal access into virtual kidneys rendered from real patient anatomic data obtained from CT scan images [22]. The procedure is practiced/rehearsed in a virtual environment, which eliminates radioactivity exposure for the operator and allows the operator multiple attempts to perform the procedure. This will also enable a surgeon to explore the use of different entry points or directions if they are unsure which would be most appropriate.

Figure 2.
The virtual operating room of the Marion K181 PCNL simulator is shown with a user operating the haptic device, a virtual patient undergoing PCNL, virtual X-ray imaging, and the medical instruments and imaging device, which would be present in the real world operating room. The user is wearing a VR headset with the leap motion attachment for hand tracking. The TV screen behind the user shows a representation of what the user sees in VR, the X-ray view and the Virtual Reality Operating room. The user’s hands are holding the needle tool that is attached to the haptic robots that provide real-time force feedback.
While the headset provides the user with an immersive visual environment, a tool connected to a haptic device allows the user to experience real-time haptic feedback. Users control the tool connected to a haptic device while performing the virtual surgery. The haptic device is then able to generate resistive forces, which mimic tissue resistive forces while collecting accurate position data from the tool.
The 3D patient models are created by taking anonymized patient venous, delay, non-contrast, and full-body CT scans that are registered and segmented to generate 3D models of the abdominal organs, skin, and bone. These models are decimated and re-meshed into low-polygon versions while maintaining anatomical accuracy [23].
Preliminary Testing of Simulator
At the University of Toronto, a total of 18 participants with varying levels of PCNL experience benchmarked the Marion K181 against other commercially available surgical simulators, such as the PercMentor [6] and the porcine PCNL model by Cook [7]. Study participants concluded that the novel PCNL simulator was comparable to a high-fidelity porcine inanimate model and had adequate content validity evidence to support its use for beginner-level PCNL training. Participants felt it was a valuable teaching tool, equivalent to a high-fidelity porcine model, with the additional advantage of not requiring radiation exposure [7].
In another independent study conducted at the Department of Urology at Boston Medical Center, 20 participants with various levels of PCNL experience evaluated the efficacy of the K181 in the following categories: virtual reality experience, image control, and economy of motion of an immersive virtual reality simulator for percutaneous nephrostomy tract access [24]. This study concluded that the Immersive VR simulator for percutaneous collecting system access is a realistic and unique platform for surgical education and is highly recommended by participants. Almost all (95%) of the participants rated the VR simulation as a realistic experience.
The VR simulator has performed well in previous assessments of its quality and application for teaching novice surgeons. However, this paper explores its applicability to surgical rehearsal. The purpose of this study is to act as a pilot study for using Marion’s surgical simulator as a surgical rehearsal tool for PCNL. Specifically, this study aims to determine if a large study with more participants (patients and surgeons) is appropriate, and whether or not a haptic-assisted VR simulator is a suitable surgical rehearsal tool.
Once a patient has agreed to take part in this study, their CT scan data is used to construct a 3D model of the kidney anatomy and surrounding tissue. The surgeon can then rehearse the procedure in the simulator prior to performing the surgery on the actual patient.
3. Generating 3D Model Patient CT Scans
Patients considered for this study were anonymous and provided informed consent for their information to be used in these studies. Once a patient’s preoperative imaging was completed, the imaging was used to generate a 3D model for the simulator. The final 3D model consists of a finite element mesh containing all relevant structures from patient imaging.
3.1. Method
A combination of 3D Slicer, Maya, and Blender were used to generate the 3D models from CT scans. The algorithms utilized here are described in more detail by Wu et al. [23], although the general process is described below.
Converting the CT scans to 3D models first requires generating a voxel (3D pixel) representation from the various 2D image segments. Each image segment is stacked with the distance between them corresponding to the depth at which each segment is taken, see Figure 3. Pixel intensities are interpolated between image segments to generate voxels. Once this process has been completed, a basic 3D image of a patient’s anatomy exists. However, this 3-dimensional representation lacks clearly defined boundaries between anatomy and tool/tissue interaction and cannot be determined directly from it since this representation does not include specific tissue characteristics, shapes, or boundaries. Thus, it is necessary to create 3D meshes that represent anatomical structures.
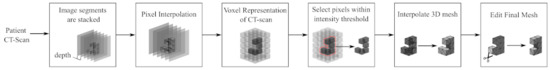
Figure 3.
Workflow process of converting CT scan data to 3D models to be displayed as virtual elements within the simulator. Image segments are layered so pixels can be interpolated between images. The pixels are interpolated into voxels (3D pixels), which can then be used to create approximate 3D meshes. The meshes are then completed through a final manual editing process.
These 3D models are constructed by considering voxels within a specified intensity threshold. Voxels within the determined threshold are used to determine the approximate geometric boundaries of an anatomical structure; these boundaries are used to construct a finite element mesh representation of the anatomical structure. The 3D model is then manually edited to create a final smooth, clean, and thoughtfully segmented model. Since the general 3D model is constructed from voxel intensity, some voxels may have been included or excluded incorrectly, leading to uneven mesh surfaces. Further, the editing process can ensure a particular mesh resolution (polygon count) is achieved, in addition to partitioning model components such as vascular components, different tissues, or different structures.
One of the most important components when considering PCNL is the specific size and location of each kidney stone. Incorrectly representing the size of kidney stones can lead to improper planning or practice for the procedure. Accurate 3D models are also integral to generating accurate haptic feedback within the simulator since haptic feedback is based on the mesh models.
3.2. Haptic Feedback Based on 3D Models
Haptic feedback is designed to mimic tissue resistance forces during PCNL. To generate these forces the system simulates how the virtual tool interacts with the tissue. The simulator tracks the motion of the surgical tool and the user’s motion through the virtual reality motion tracking cameras. The x-y-z positional data is then recorded at 100 Hz throughout the 2–10 min simulation. See Figure 4 for the interactions between various components used to generate an immersive simulation. The simulator’s physics engine is able to calculate the forces on the tissue, the total length of the path taken by the tool and the surgeon’s hand, and the direction of the surgeon’s gaze. The system uses three separate components in parallel at different frequencies:
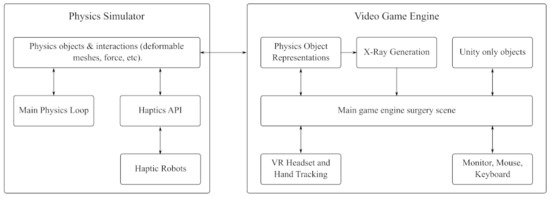
Figure 4.
Flow chart of the interactions between the physics simulator, the game engine, and the peripherals to generate an immersive VR experience for users.
Component 1: The dynamic model of the tool/tissue interaction that calculates tissue deformation and contact forces, and generates a virtual X-ray image. This component provides data for the subsequent two components.
Component 2: The graphical representation of the model displayed in the VR operating room takes the simulation information created in the first component and displays it to the user. The virtual operating room is based on the direction of the user’s eye line as well as their actions within the simulator.
Component 3: The haptic controller generates and applies force feedback to approximate real soft tissue interactions based on the virtual patient’s tissue model. This component takes the tool/tissue interaction that is determined in the first component to calculate the appropriate haptic forces and apply them through the haptic device.
4. Results from Surgical Rehearsal
Three anonymous patient’s agreed to have their imaging data used within the Marion K181 Simulator for use as a preoperative planning tool. Of these patients, one case has been selected to undergo a contrast-enhanced CT scan. A special contrast material was injected to help highlight the kidney duct system. The contrast material appears white on images, which emphasizes blood vessels, intestines, or other structures that are required to generate the 3D model. A contrast CT scan is necessary to create accurate 3D models of the patient’s anatomy. Some CT scan segments from the patient are shown in Figure 5 as well as the 3D model of the patient’s calyceal structure. The nephrolithiasis case being considered is one that qualifies for PCNL surgery, although it is a relatively simple case since the patient does not have anatomical abnormalities or a significantly large or severe case of nephrolithiasis. Thus, this case is an excellent way to demonstrate the effectiveness of haptic virtual reality simulation for preoperative planning.
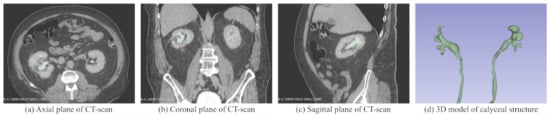
Figure 5.
3D model of the calyxial system within the kidney in (d) and overlayed onto the patient’s CT scans in (a–c), which are the axial, coronal, and sagittal planes of the CT scans, respectively.
4.1. Experimental Procedure
Once the patient was selected for the surgical rehearsal trial, their CT scans were used to construct 3D models. The 3D model constructed of the patient’s calyx structures within the kidney are shown in Figure 5d. This figure also depicts some of the CT scans taken of the patient that were used to construct the 3D model.
The surgeon then uses the simulator to run a virtual walk-through of his procedural plan. Once the surgeon is satisfied with his experience within the simulator, he fills out the preoperative questionnaire. The surgeon then performed the surgery at St. Joseph’s Hospital, Hamilton, Ontario, Canada. After the surgery has been completed, the surgeon fills out a postoperative questionnaire. These questionnaires aim to determine how beneficial the surgeon found the simulator for the use of preoperative planning. The pre and postoperative questionnaires contain questions aimed at identifying the surgeon’s skill level as well as aspects of the surgery performed. Several assessment questions are given both before and after the surgery to identify if the surgeon’s opinion about the rehearsal experience changed as a result of performing the surgery in the real world.
4.2. Results
After completing the surgical rehearsal in the simulator, the surgeon filled out the preoperative questionnaire. The first part of the questionnaire contained three questions aimed at identifying the surgeon’s skill level. These questions and their answers are provided in Table A1 in the Appendix A.
The results from part 1 of the questionnaire show that the surgeon is experienced when using a C-arm and has experienced some intraoperative errors as a result of technical errors (see Table A1 of the Appendix A). The second part of the questionnaire is also completed prior to surgery and focuses on the surgeon’s experience within the simulator and its realism. The results for the second part of the questionnaire are given in Table A1 in the Appendix A; most of these questions ask the surgeon to rate their experience with the simulator on a scale of 1 to 5, with 1 being poor or very unrealistic and 5 being very realistic. Part three of the preoperative survey concerns the construct validation of the simulator. Construct validation attempts to measure how well the simulator represents and measures the physical phenomena it is attempting to recreate. Finally, the surgeon gives an overall score for their experience with the simulator at the end of the preoperative survey.
The postoperative survey is more in-depth, asking the surgeon to reflect on the overall rehearsal experience with the simulator after performing the real-world surgery. Additionally, several of the same questions were repeated from the preoperative survey to determine whether the surgeon’s impression of the simulator was altered after performing the real-world procedure. The postoperative questionnaire results are separated into two tables in the Appendix A with Table A2 focusing on the surgeon’s impression of the simulator, while Table A3 contains questions specific to the surgeon’s surgical rehearsal experience within the simulator. This surgical rehearsal section specifically focuses on evaluating the simulator in terms of a surgical rehearsal tool. Within the rehearsal portion of the questionnaire, the participant is asked to evaluate how helpful the rehearsal was on a scale of 0 to 10 where 0 indicates that it was not helpful at all, while 10 indicates that it was very helpful.
The limitations of this study are largely due to its size, as only a single case is being considered. Even though the preliminary results indicate that the simulator improves kidney access during PCNL, a larger scale study with several patients and surgeons is required to fully determine the effectiveness of the simulator. Furthermore, quantitative rather than qualitative performance metrics are required to fully evaluate the performance of the simulator in a future study.
4.3. Discussion
The simulator was rated highly in most categories (see Table A1–Table A3 in Appendix A) before and after the surgery. The simulation appears to accurately depict the surgery performed in October 2021. It is suggested that the simulation is helpful in decision making on difficult cases to minimize fluoroscopy time (radiation exposure for clinician and patient). The rehearsal can result in less bleeding and can improve the success rate of the surgery.
The ability during the surgical rehearsal to determine the approach to take with the location of the ribs in relation to the targeted calyx in the kidney was rated as excellent and helped the surgeon get real-life access during the actual procedure. Pre-planning saves the surgeon time during the rehearsal phase since one can try different approaches to reach the kidney stones while getting familiar with the patient’s anatomy. The haptic feedback provided during the simulation was rated to be helpful to interpret the shape and texture of the skin and kidney using the needle. The force feedback that the surgeon experienced during rehearsal influenced the surgeon’s plan for the real surgery. The surgeon rated the construct validation higher in the postoperative survey, noting that the haptics had an influence on decisions in the actual surgery. The surgeon also noted that the simulator can potentially minimize fluoroscopy time and bleeding during the actual surgery, which could, in turn, improve the surgery success rate. This correlates with the surgeon having had time to reflect on the surgical rehearsal and having trained the procedure before going into the surgery.
5. Conclusions
Virtual reality simulators are becoming an essential tool in surgical training. Through virtual reality, novice surgeons can develop their surgical skills without posing any danger to the patient. Expert surgeons, on the other hand, can use a virtual reality simulator to plan a surgical intervention and practice it, before going into the actual surgery. The benefits of surgical rehearsal using virtual reality has been proven in several studies, including craniotomy [25], thoracic surgery [21] and PCNL [16].
Percutaneous renal surgery is a difficult procedure to learn and perform due to challenges with obtaining and/or maintaining percutaneous access [26]. The Marion Virtual Reality PCNL simulator with haptic feedback is a novel tool to allow the surgeon to rehearse and practice the difficult access part of the surgery without harming the patient. This paper describes the first pilot study using a combination of virtual reality and haptic feedback for kidney access rehearsal before PCNL surgery. An experienced surgeon used the simulator before the surgical procedure to plan and practice kidney access on a routine PCNL case before surgery. The survey data collected from the surgeon after the surgical rehearsal on the simulator and after performing the actual surgery on the patient indicates that the simulator improves confidence in the procedure, reduced the time taken by the surgeon to complete kidney access and reduced blood loss. To the best of the author’s knowledge, this is the first study combining virtual reality and haptic feedback in PCNL. It shows promising preliminary data for the efficacy of the simulator as a rehearsal tool.
In this paper, a pre-surgical rehearsal was conducted for a single patient case study. Even though the preliminary results indicate that the simulator does help improve kidney access during PCNL, a large-scale study with several patients and surgeons is required to fully determine the effectiveness of the simulator. Furthermore, quantitative rather than qualitative performance metrics are required in a future study. Further studies with a larger sample size of surgeons and residents at various levels of PCNL access experience are required to confirm the findings of this preliminary study. Such studies will run the rehearsals on more difficult cases to determine if the use of the simulated surgical rehearsal improves the outcomes of the actual surgery. Ultimately, a large clinical study to analyze and compare clinical outcomes of surgeries performed with and without the surgical rehearsal platform would confirm the suitability of simulation training in improving surgical outcomes for PCNL.
Author Contributions
B.S., J.R., M.G. and M.F. conceptualized the study and the methodology, B.S. carried out experimental validation, data collection and analyses, B.S. and O.W.; prepared the manuscript, C.R., B.S. and OW. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study is a case report. Ethical review and approval were waived.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The first author of this paper is affiliated with Marion Surgical.
Appendix A. Surgical Rehearsal Questionnaires and Results

Table A1.
Preoperative Assessments: Parts 1 Through 4.
Table A1.
Preoperative Assessments: Parts 1 Through 4.
| Questionnaire Part 1: User Demographics | ||
|---|---|---|
| 1. | How many PCNL access procedures have you performed in the last year | |
| with a C-arm? | 44 | |
| 2. | Have you experienced any intraoperative errors during PCNL procedures? | Yes |
| 3. | Was there error due to: | |
| Answer: Technical error (i.e., excessive force, tissue injury, etc.) | ||
| Questionnaire Part 2: Face Validation | ||
| How would you rate the virtual reality PCNL simulator with respect to: | ||
| 1. | Visual realism | 4/5 |
| 2. | Tactile feedback | 4/5 |
| 3. | Movement and instruments | 4/5 |
| 4. | Anatomical realism | 4/5 |
| 5. | How stable were the graphics and your sense of self inside the simulator? | 5/5 |
| 6. | Describe your experience/comment on any areas for improvement in realistic | |
| representation of the operating environment: | ||
| Answer: Graphics were very good. Graphics sometimes jumpy. | ||
| Questionnaire Part 3: Construct Validation | ||
| 1. | Were you able to interpret the shape and texture of the skin and kidney | |
| using the needle tool? | Yes | |
| 2. | Do you feel the tactile information was amplified in the simulation? | No |
| 3. | If applicable, did motion and force feedback influence your decision? | No |
| Questionnaire Part 4: Content Validation | ||
| 1. | Do you feel the tasks performed in the simulator reflected the real | |
| surgical procedure? | Yes | |
| 2. | Please provide comments of the overall simulation experience (VR) | |
| in content accuracy? | ||
| Answer: Location of rib impacting access was excellent. | ||
| Help me for real life access. | ||
| Overall Rating | ||
| 1. | Rate the experience training with the Marion Surgical PCNL simulator: | 4/5 |

Table A2.
Postoperative Assessments: Parts 1 through 3.
Table A2.
Postoperative Assessments: Parts 1 through 3.
| Questionnaire Part 1: Face Validation | ||
|---|---|---|
| How would you rate the virtual reality PCNL simulator with respect to: | ||
| 1. | Visual realism | 4/5 |
| 2. | Tactile feedback | 4/5 |
| 3. | Movement and instruments | 4/5 |
| 4. | Anatomical realism | 5/5 |
| 6. | How stable were the graphics and your sense of self inside the simulator? | 4/5 |
| 7. | Describe your experience/ comment on any areas for improvement in realistic | |
| representation of the operating environment: | ||
| Answer: The virtual rendering are an excellent simulation of actual | ||
| patient anatomy. Tactile feedback can always be improved | ||
| Questionnaire Part 2: Construct Validation | ||
| 1. | Were you able to interpret the shape and texture of the skin and kidney | |
| using the needle tool? | Yes | |
| 2. | Do you feel the tactile information was amplified in the simulation? | Yes |
| 3. | If applicable, did motion and force feedback influence your decision? | Yes |
| Questionnaire Part 3: Content Validation | ||
| 1. | Visual simulation is the most important factor in learning surgical motor skills: | 4/5 |
| 2. | Do you feel the tasks performed in the simulator reflected real | |
| surgical skills? | Yes | |
| 3. | You see the value in VR PNCL tool as a useful tool in Training PCNL skills: | 4/5 |
| 4. | You think this VR PCNL simulator is useful for Assessing/Testing PCNL skills: | 4/5 |
| 5. | What is the most difficult skill to learn during a full PCNL procedure? | |
| Answer: Actual renal access | ||
| 6. | Please provide comments of the overall simulation experience (VR) | |
| in having an educational role? | ||
| Answer: Being able to practice targeting of calyx/stone with needle | ||
| is valuable. Also without having excess radiation exposure | ||
| or harm to patient. | ||
| 7. | Please provide comments of the overall simulation experience (VR) | |
| in content accuracy? | ||
| Answer: It was help to rehearse access. Became aware of rib in the | ||
| way of calyx of interest. | ||
| Overall Rating | ||
| 1. | Rate the experience training with the Marion Surgical PCNL simulator: | 5/5 |

Table A3.
Postoperative Assessments: Rehearsal.
Table A3.
Postoperative Assessments: Rehearsal.
| Rehearsal | ||
|---|---|---|
| Did the rehearsal help you determine: | ||
| 1. | The location of the stone (with regard to the bulk of the stone)? | 9/10 |
| (a) Specifically, where is the bulk of the stone? | Lower Pole | |
| 2. | The size of the stone? | 10/10 |
| (a) What is the size of the largest stone in three dimensions? | 2 cm | |
| (b) What is the total volume of the largest stone? | 2 cm | |
| 3. | The shape and orientation of each stone-bearing calix? | 10/10 |
| 4. | The optimal calix of entry to perform the PCNL? | |
| (a) Into which calix (upper, mid, lower, and anterior or post- | ||
| erior) are you planning to place the nephrostomy track? | Lower Posterior | |
| 5. | How easily do you think you can navigate this patient’s pelvic | |
| caliceal system from your planned approach with a rigid | ||
| nephroscope? | 9/10 | |
| When you performed the actual surgery on this patient: | ||
| 6. | How close was the location of the stone relative to the rehearsal | |
| (specifically, with regard to the bulk of the stone)? | 9/10 | |
| (a) Specifically, where is the bulk of the stone: | Lower Posterior | |
| 7. | The size of the stone? | 10/10 |
| (a) What is the size of the largest stone in three dimensions? | 2 cm | |
| (b) What is the total volume of the largest stone? | 2 cm | |
| 8. | The shape and orientation of each stone-bearing calix? | 9/10 |
| 9. | The optimal calix of entry to perform the PCNL? | 9/10 |
| (a) Into which calix (upper, mid, lower, and anterior | ||
| or posterior) did you place the nephrostomy track? | Lower Posterior | |
| 10. | How easily were you able to navigate to this patient’s pelvic | |
| caliceal system from your planned rehearsal approach with a | ||
| rigid nephroscope? | 9/10 | |
References
- Aydın, A.; Al-Jabir, A.; Smith, B.; Ahmed, K. Training in Percutaneous Nephrolithotomy. In Percutaneous Nephrolithotomy; Zeng, G., Sarica, K., Eds.; Springer: Singapore, 2020; pp. 195–202. [Google Scholar] [CrossRef]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.G. Kidney stones. Nat. Rev. Dis. Prim. 2016, 2, 1–23. [Google Scholar]
- Bird, V.G.; Fallon, B.; Winfield, H.N. Practice patterns in the treatment of large renal stones. J. Endourol. 2003, 17, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.F. Training in percutaneous nephrolithotomy: The learning curve and options. Arab J. Urol. 2014, 12, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Bushey, C. Cadaver supply: The last industry to face big changes. CRAIN’s Chic. Bus. 2016, 15. [Google Scholar]
- Mishra, S.; Kurien, A.; Ganpule, A.; Muthu, V.; Sabnis, R.; Desai, M. Percutaneous renal access training: Content validation comparison between a live porcine and a virtual reality (VR) simulation model. BJU Int. 2010, 106, 1753–1756. [Google Scholar] [CrossRef]
- Farcas, M.; Reynolds, L.F.; Lee, J.Y. Simulation-Based Percutaneous Renal Access Training: Evaluating a Novel 3D Immersive Virtual Reality Platform. J. Endourol. 2021, 35, 695–699. [Google Scholar] [CrossRef]
- Pottle, J. Virtual reality and the transformation of medical education. Future Healthc. J. 2019, 6, 181. [Google Scholar] [CrossRef] [Green Version]
- Sommer, G.M.; Broschewitz, J.; Huppert, S.; Sommer, C.G.; Jahn, N.; Jansen-Winkeln, B.; Gockel, I.; Hau, H.M. The role of virtual reality simulation in surgical training in the light of COVID-19 pandemic: Visual spatial ability as a predictor for improved surgical performance: A randomized trial. Medicine 2021, 100, e27844. [Google Scholar] [CrossRef]
- Chiang, P.; Zheng, J.; Yu, Y.; Mak, K.H.; Chui, C.K.; Cai, Y. A VR simulator for intracardiac intervention. IEEE Comput. Graph. Appl. 2012, 33, 44–57. [Google Scholar] [CrossRef]
- Staropoli, P.C.; Gregori, N.Z.; Junk, A.K.; Galor, A.; Goldhardt, R.; Goldhagen, B.E.; Shi, W.; Feuer, W. Surgical simulation training reduces intraoperative cataract surgery complications among residents. Simul. Healthc. J. Soc. Simul. Healthc. 2018, 13, 11. [Google Scholar] [CrossRef]
- Badash, I.; Burtt, K.; Solorzano, C.A.; Carey, J.N. Innovations in surgery simulation: A review of past, current and future techniques. Ann. Transl. Med. 2016, 4, 453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilz, O.; Sainsbury, B.; Rossa, C. Constrained haptic-guided shared control for collaborative human–robot percutaneous nephrolithotomy training. Mechatronics 2021, 75, 102528. [Google Scholar] [CrossRef]
- Arnold, J.; Cashin, M.; Olutoye, O.O. Simulation-based clinical rehearsals as a method for improving patient safety. JAMA Surg. 2018, 153, 1143–1144. [Google Scholar] [CrossRef] [PubMed]
- Yiasemidou, M.; Glassman, D.; Jayne, D.; Miskovic, D. Is patient-specific pre-operative preparation feasible in a clinical environment? A systematic review and meta-analysis. Comput. Assist. Surg. 2018, 23, 57–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkhomenko, E.; O’Leary, M.; Safiullah, S.; Walia, S.; Owyong, M.; Lin, C.; James, R.; Okhunov, Z.; Patel, R.M.; Kaler, K.S.; et al. Pilot assessment of immersive virtual reality renal models as an educational and preoperative planning tool for percutaneous nephrolithotomy. J. Endourol. 2019, 33, 283–288. [Google Scholar] [CrossRef]
- Willaert, W.I.; Aggarwal, R.; Van Herzeele, I.; Cheshire, N.J.; Vermassen, F.E. Recent advancements in medical simulation: Patient-specific virtual reality simulation. World J. Surg. 2012, 36, 1703–1712. [Google Scholar] [CrossRef]
- Won, T.B.; Hwang, P.; Lim, J.H.; Cho, S.W.; Paek, S.H.; Losorelli, S.; Vaisbuch, Y.; Chan, S.; Salisbury, K.; Blevins, N.H. Early Experience with a Patient-Specific Virtual Surgical Simulation for Rehearsal of Endoscopic Skull-Base Surgery; Wiley Online Library: Hoboken, NJ, USA, 2018; Volume 8, pp. 54–63. [Google Scholar]
- Jean, W.C. Virtual reality surgical rehearsal and 2-dimensional operative video of a paramedian supracerebellar infratentorial approach endoscopic resection of pineocytoma: 2-dimensional operative video. Oper. Neurosurg. 2021, 20, E51–E52. [Google Scholar] [CrossRef]
- Qiu, K.; Haghiashtiani, G.; McAlpine, M.C. 3D printed organ models for surgical applications. Annu. Rev. Anal. Chem. 2018, 11, 287–306. [Google Scholar] [CrossRef]
- Guerrera, F.; Nicosia, S.; Costardi, L.; Lyberis, P.; Femia, F.; Filosso, P.L.; Arezzo, A.; Ruffini, E. Proctor-guided virtual reality–enhanced three-dimensional video-assisted thoracic surgery: An excellent tutoring model for lung segmentectomy. Tumori J. 2021, 107, NP1–NP4. [Google Scholar] [CrossRef]
- Sainsbury, B.; Łącki, M.; Shahait, M.; Goldenberg, M.; Baghdadi, A.; Cavuoto, L.; Ren, J.; Green, M.; Lee, J.; Averch, T.D.; et al. Evaluation of a virtual reality percutaneous nephrolithotomy (PCNL) surgical simulator. Front. Robot. AI 2020, 6, 145. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.O.; Sunderland, K.; Filippov, M.; Sainsbury, B.; Fichtinger, G.; Ungi, T. Workflow for creation and evaluation of virtual nephrolithotomy training models. In Proceedings of the Medical Imaging 2020: Image-Guided Procedures, Robotic Interventions, and Modeling, Houston, TX, USA, 15–20 February 2020; International Society for Optics and Photonics: Bellingham, WA, USA, 2020; Volume 11315, p. 1131524. [Google Scholar]
- Resad, S.; Parkhomenko, E.; Wang, D.S.; Wason, S.E. The Utility and Value of Immersive Virtual Reality Simulation for Percutaneous Nephrostomy Tract Access and Surgical Training; Boston University School of Medicine: Boston, MA, USA, 2019. [Google Scholar]
- Montemurro, N.; Condino, S.; Cattari, N.; D’Amato, R.; Ferrari, V.; Cutolo, F. Augmented reality-assisted craniotomy for parasagittal and convexity en plaque meningiomas and custom-made cranio-plasty: A preliminary laboratory report. Int. J. Environ. Res. Public Health 2021, 18, 9955. [Google Scholar] [CrossRef] [PubMed]
- Rais-Bahrami, S.; Friedlander, J.I.; Duty, B.D.; Okeke, Z.; Smith, A.D. Difficulties with access in percutaneous renal surgery. Ther. Adv. Urol. 2011, 3, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).