Photodynamic Therapy by Diaryl-Porphyrins to Control the Growth of Candida albicans
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Culture Conditions
2.2. Photosensitizers
2.3. Light Source
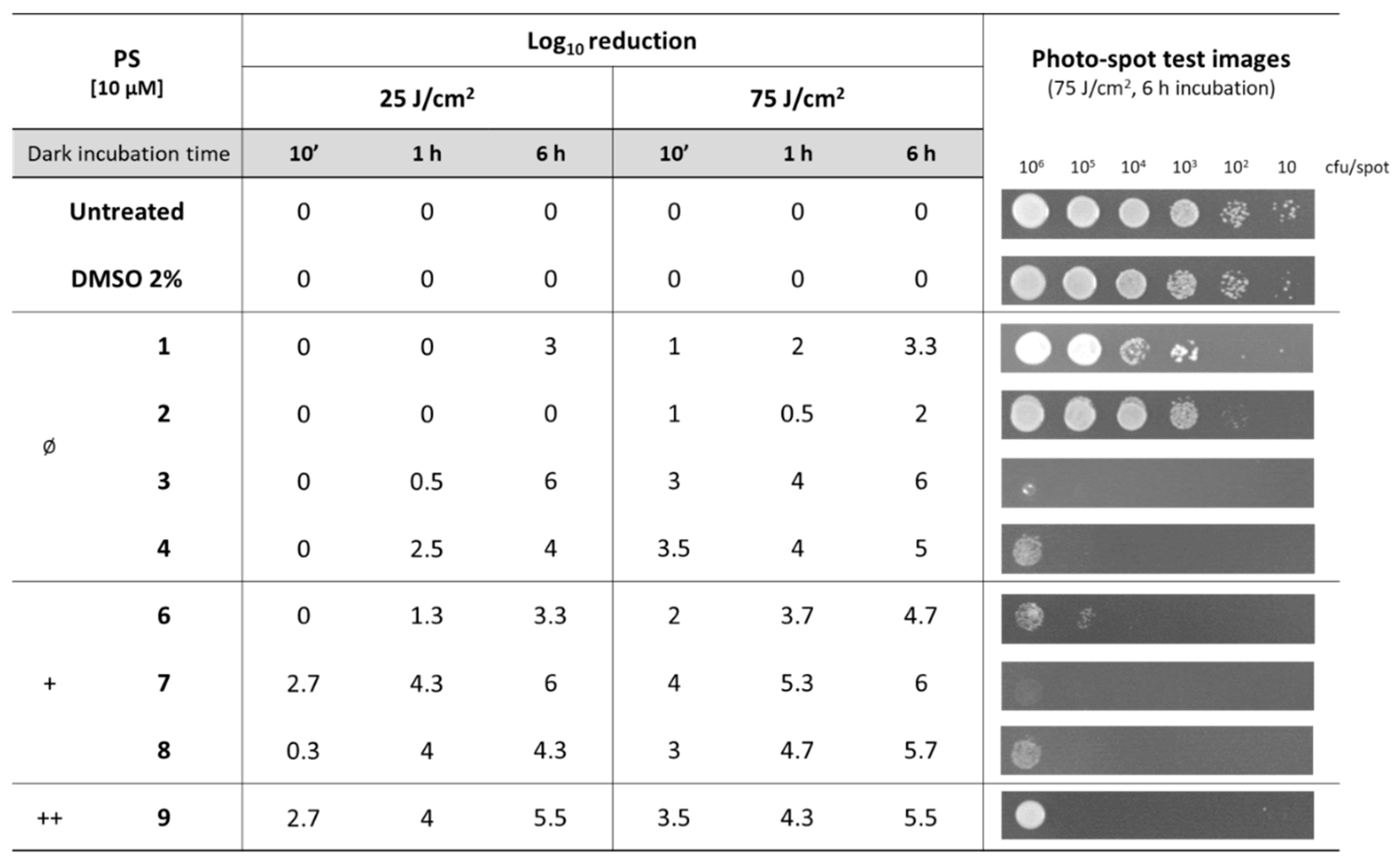
2.4. Photo-Spot Test
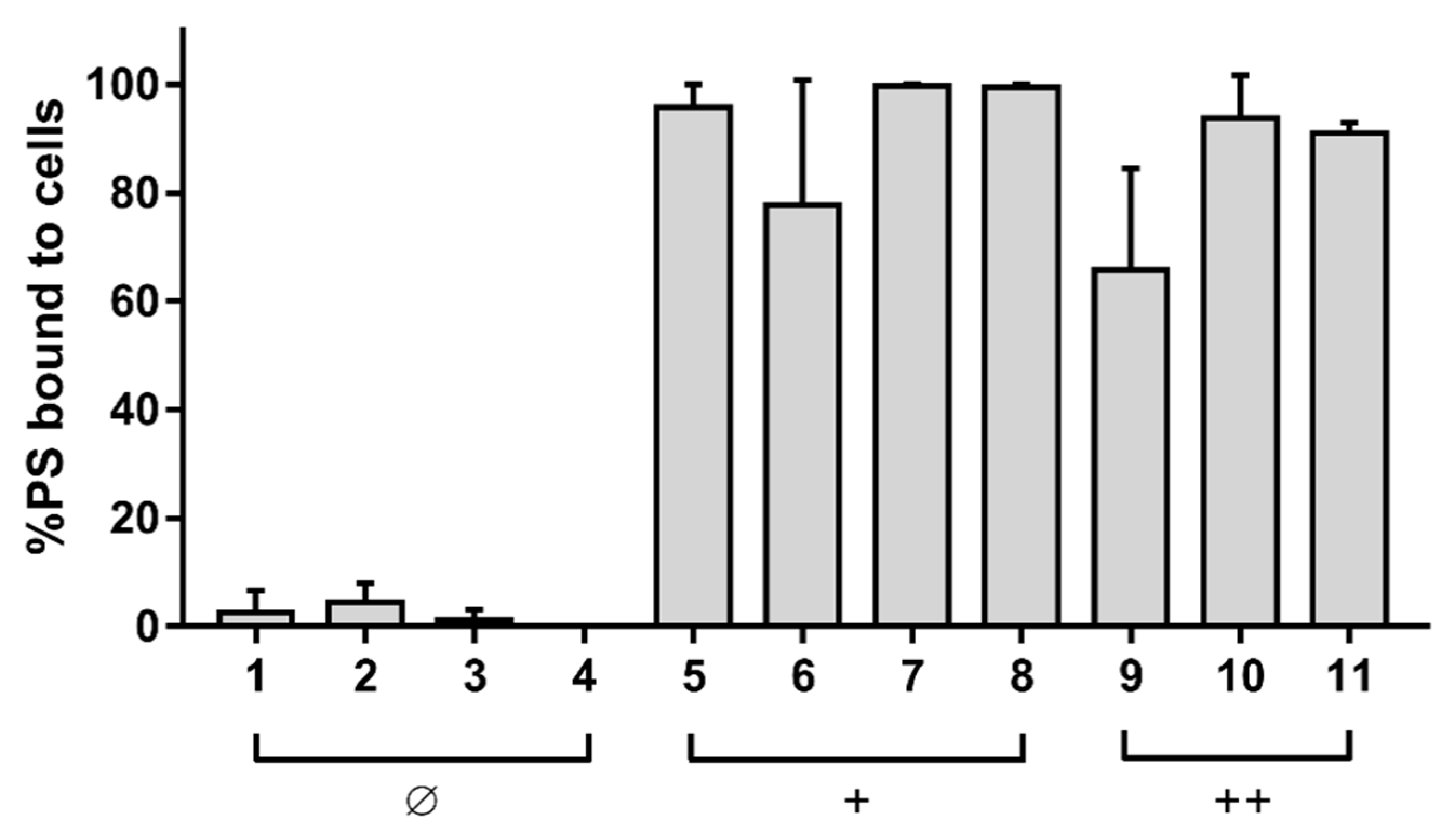
2.5. Binding Assay
2.6. Photoinactivation of Suspended Cells
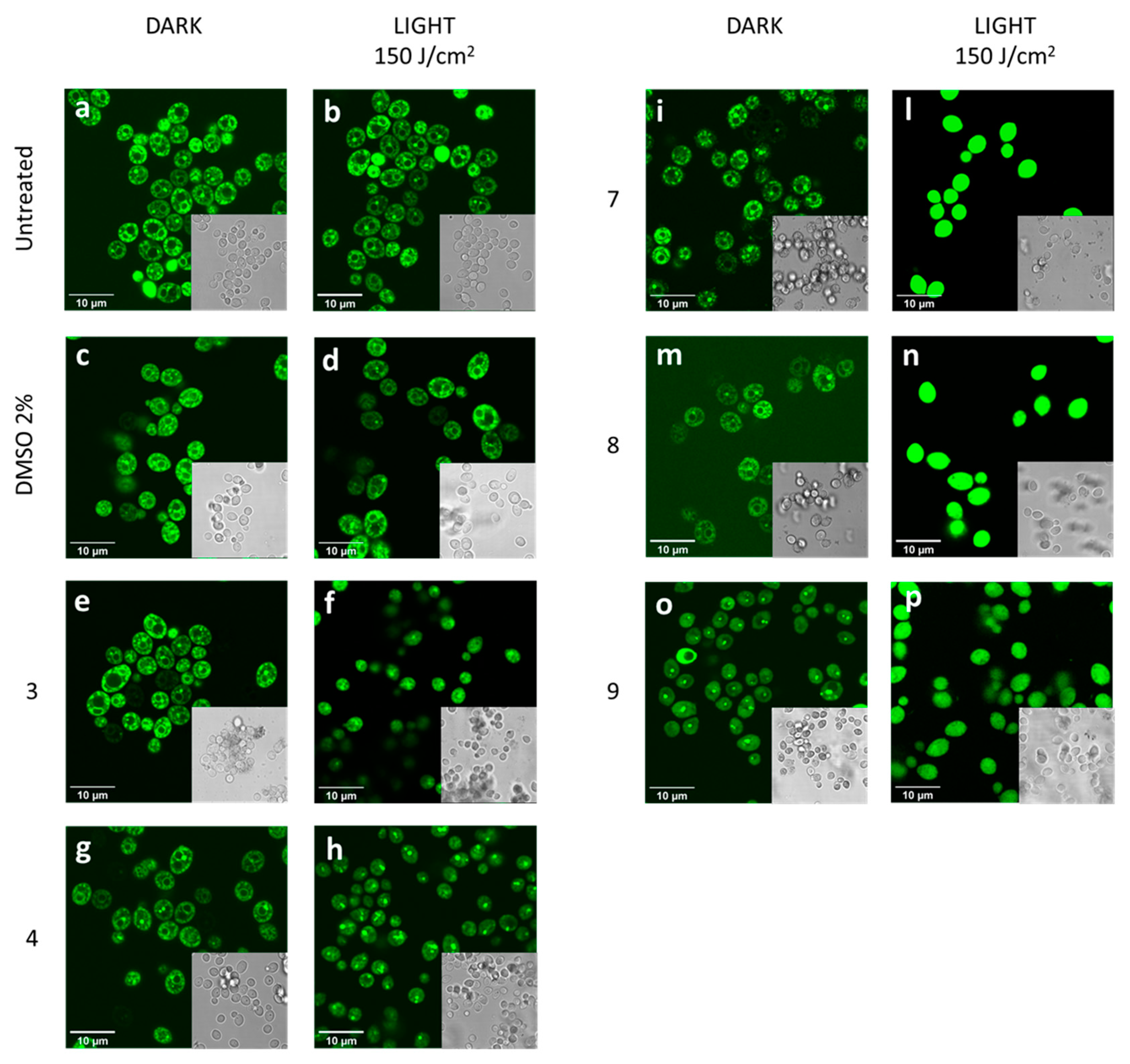
2.7. Confocal Microscopy Analysis
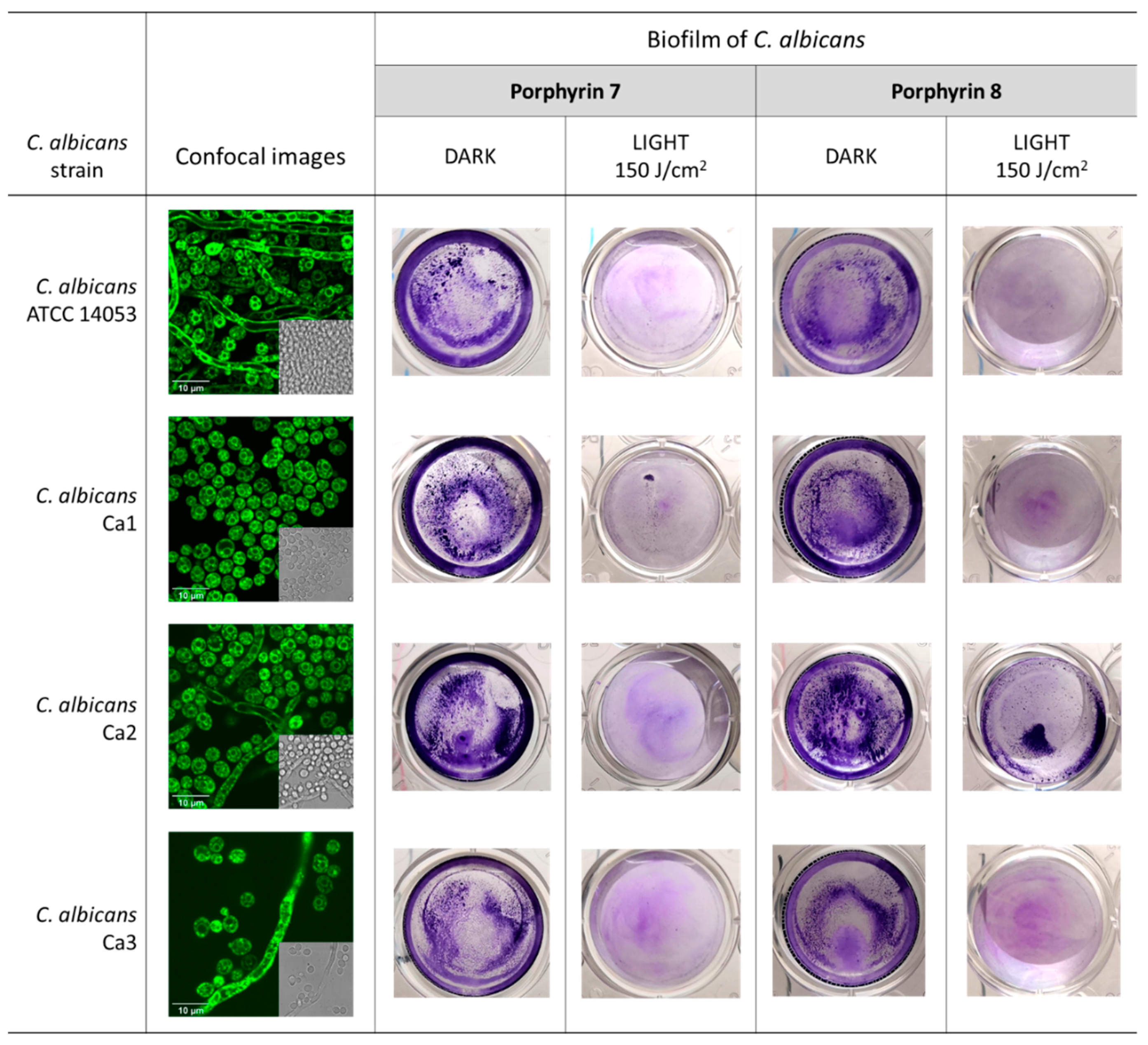
2.8. Biofilm Photoinactivation Assay
2.9. Statistical Analyses
3. Results
3.1. Effect of Diaryl-Porphyrins on Candida albicans Viability
3.2. Photodynamic Activity of Diaryl-Porphyrins on Candida albicans
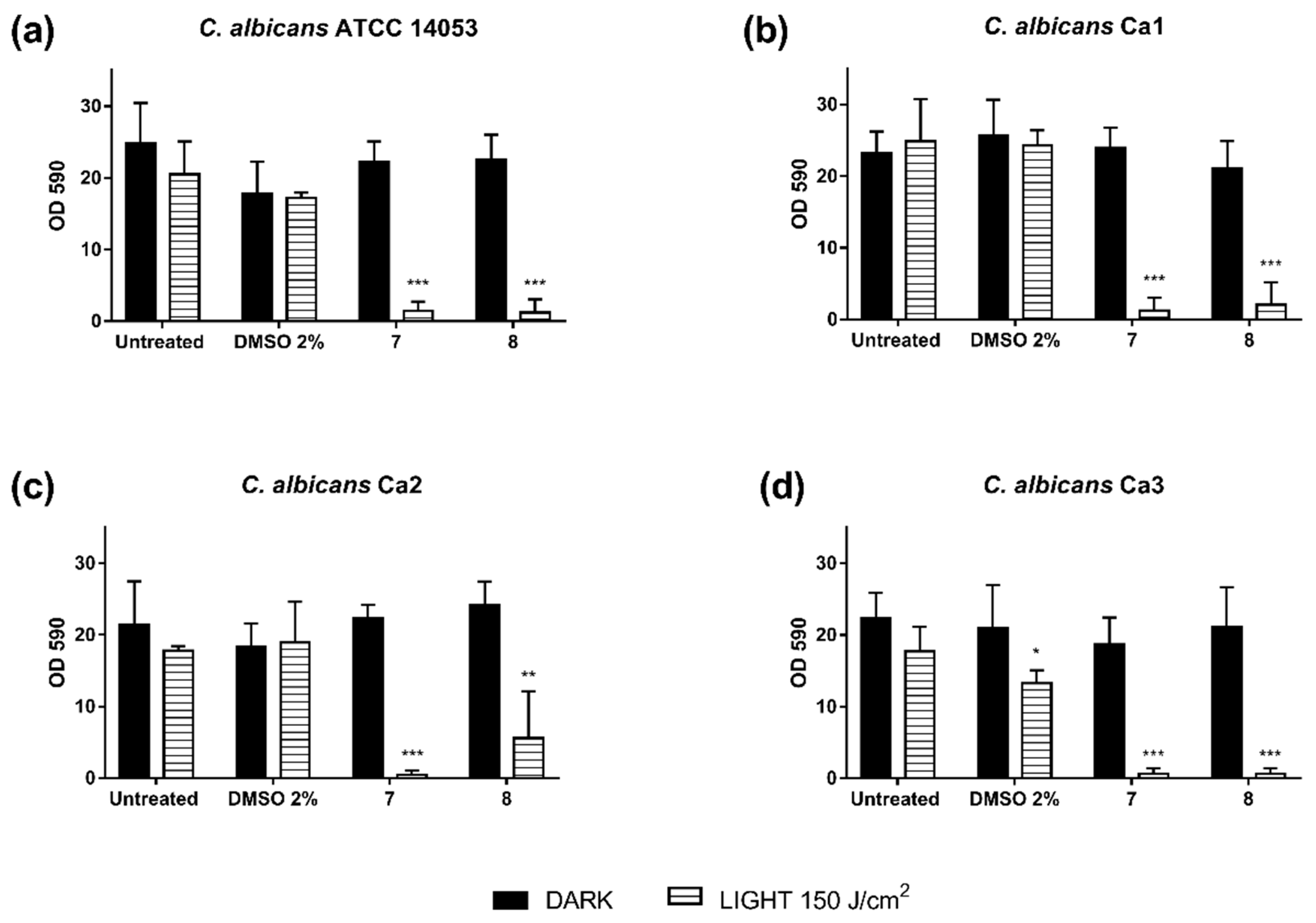
3.3. Photodynamic Activity of Diaryl-Porphyrins on Clinical Isolates of Candida albicans
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.; Woo, E.R.; Lee, D.G. Synergistic Antifungal Activity of Isoquercitrin: Apoptosis and Membrane Permeabilization Related to Reactive Oxygen Species in Candida albicans. IUBMB Life 2019, 71, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, Z.; Peng, Y.; Guo, Y.; Yao, M.; Dong, J. Application of 460 nm visible light for the elimination of Candida albicans in vitro and in vivo. Mol. Med. Rep. 2018, 18, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Shenderovich, J.; Al-Quntar, A.A.A.; Friedman, M.; Steinberg, D. Sustained release of a novel anti-quorum-sensing agent against oral fungal biofilms. Antimicrob. Agents Chemother. 2015, 59, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Bil De Arce, V.J.; Tegos, G.P.; Hamblin, M.R. Blue dye and red light, a dynamic combination for prophylaxis and treatment of cutaneous Candida albicans infections in mice. Antimicrob. Agents Chemother. 2011, 55, 5710–5717. [Google Scholar] [CrossRef] [PubMed]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy–what we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.C.B.P.; Campos Rasteiro, V.M.; Da Silva Hashimoto, E.S.H.; Araújo, C.F.; Pereira, C.A.; Junqueira, J.C.; Jorge, A.O.C. Effect of erythrosine- and LED-mediated photodynamic therapy on buccal candidiasis infection of immunosuppressed mice and Candida albicans adherence to buccal epithelial cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 67–74. [Google Scholar] [CrossRef]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, challenges, and promising strategies. Front. Med. 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Abe, M.; Nakamura, S.; Kinjo, Y.; Masuyama, Y.; Mitsuyama, J.; Kaku, M.; Miyazaki, Y. Efficacy of T-2307, a novel arylamidine, against ocular complications of disseminated candidiasis in mice. J. Antimicrob. Chemother. 2019, 74, 1327–1332. [Google Scholar] [CrossRef]
- Machado-de-Sena, R.M.; Corrêa, L.; Kato, I.T.; Prates, R.A.; Senna, A.M.; Santos, C.C.; Picanço, D.A.; Ribeiro, M.S. Photodynamic therapy has antifungal effect and reduces inflammatory signals in Candida albicans-induced murine vaginitis. Photodiagnosis Photodyn. Ther. 2014, 11, 275–282. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Passarelli, P.C.; De Leonardis, M.; Piccirillo, G.B.; Desantis, V.; Papa, R.; Rella, E.; Bonaviri, G.N.M.; Papi, P.; Pompa, G.; Pasquantonio, G.; et al. The effectiveness of chlorhexidine and air polishing system in the treatment of candida albicans infected dental implants: An experimental in vitro study. Antibiotics 2020, 9, 179. [Google Scholar] [CrossRef]
- Benzaid, C.; Belmadani, A.; Djeribi, R.; Rouabhia, M. The effects of mentha × piperita essential oil on C. albicans growth, transition, biofilm formation, and the expression of secreted aspartyl proteinases genes. Antibiotics 2019, 8, 10. [Google Scholar] [CrossRef]
- Rosa, L.P.; da Silva, F.C.; Viana, M.S.; Meira, G.A. In vitro effectiveness of 455-nm blue LED to reduce the load of Staphylococcus aureus and Candida albicans biofilms in compact bone tissue. Lasers Med. Sci. 2016, 31, 27–32. [Google Scholar] [CrossRef]
- Huang, M.C.; Shen, M.; Huang, Y.J.; Lin, H.C.; Chen, C.T. Photodynamic inactivation potentiates the susceptibility of antifungal agents against the planktonic and biofilm cells of Candida albicans. Int. J. Mol. Sci. 2018, 19, 434. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef]
- Rodrigues, M.E.; Silva, S.; Azeredo, J.; Henriques, M. Novel strategies to fight Candida species infection. Crit. Rev. Microbiol. 2016, 42, 594–606. [Google Scholar]
- Gupta, A.K.; Ahmad, I.; Summerbell, R.C. Fungicidal activities of commonly used disinfectants and antifungal pharmaceutical spray preparations against clinical strains of Aspergillus and Candida species. Med. Mycol. 2002, 40, 201–208. [Google Scholar] [CrossRef][Green Version]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic therapy: A new concept in medical treatment. Brazilian J. Med. Biol. Res. 2000, 33, 869–880. [Google Scholar] [CrossRef]
- Kato, I.T.; Prates, R.A.; Sabino, C.P.; Fuchs, B.B.; Tegos, G.P.; Mylonakis, E.; Hamblin, M.R.; Ribeiro, M.S. Antimicrobial photodynamic inactivation inhibits Candida albicans virulence factors and reduces in vivo pathogenicity. Antimicrob. Agents Chemother. 2013, 57, 445–451. [Google Scholar] [CrossRef]
- Lin, C.H.; Chien, H.F.; Lin, M.H.; Chen, C.P.; Shen, M.; Chen, C.T. Chitosan inhibits the rehabilitation of damaged microbes induced by photodynamic inactivation. Int. J. Mol. Sci. 2018, 19, 19. [Google Scholar]
- Queiroga, A.S.; Trajano, V.N.; Lima, E.O.; Ferreira, A.F.M.; Queiroga, A.S.; Limeira, F.A. In vitro photodynamic inactivation of Candida spp. by different doses of low power laser light. Photodiagnosis Photodyn. Ther. 2011, 8, 332–336. [Google Scholar] [CrossRef]
- Costa, A.C.B.P.; Rasteiro, V.M.C.; Pereira, C.A.; Rossoni, R.D.; Junqueira, J.C.; Jorge, A.O.C. The effects of rose bengal- and erythrosine-mediated photodynamic therapy on Candida albicans. Mycoses 2012, 55, 56–63. [Google Scholar] [CrossRef]
- Pupo, Y.M.; Gomes, G.M.; Santos, E.B.; Chaves, L.; Michel, M.D.; Kozlowski, V.A.; Gomes, O.M.M.; Gomes, J.C. Susceptibility of Candida albicans to photodynamic therapy using methylene blue and toluidine blue as photosensitizing dyes. Acta Odontol. Latinoam. 2011, 24, 188–192. [Google Scholar]
- Munin, E.; Giroldo, L.M.; Alves, L.P.; Costa, M.S. Study of germ tube formation by Candida albicans after photodynamic antimicrobial chemotherapy (PACT). J. Photochem. Photobiol. B Biol. 2007, 88, 16–20. [Google Scholar] [CrossRef]
- Teichert, M.C.; Jones, J.W.; Usacheva, M.N.; Biel, M.A. Treatment of oral candidiasis with methylene blue-mediated photodynamic therapy in an immunodeficient murine model. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 155–160. [Google Scholar] [CrossRef]
- Calzavara-Pinton, P.G.; Venturini, M.; Sala, R. A comprehensive overview of photodynamic therapy in the treatment of superficial fungal infections of the skin. J. Photochem. Photobiol. B Biol. 2005, 78, 1–6. [Google Scholar] [CrossRef]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal photodynamic therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef]
- Peloi, L.S.; Soares, R.R.S.; Biondo, C.E.G.; Souza, V.R.; Hioka, N.; Kimura, E. Photodynamic effect of light emitting diode light on cell growth. J. Biosci. 2008, 33, 231–237. [Google Scholar] [CrossRef]
- Wainwright, M.; Giddens, R.M. Phenothiazinium photosensitisers: Choices in synthesis and application. Dye. Pigment. 2003, 57, 245–257. [Google Scholar] [CrossRef]
- Ma, J.; Shi, H.; Sun, H.; Li, J.; Bai, Y. Antifungal effect of photodynamic therapy mediated by curcumin on Candida albicans biofilms in vitro. Photodiagnosis Photodyn. Ther. 2019, 27, 280–287. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Zhang, J.H.; Chuang, W.C.; Yu, K.H.; Huang, X.B.; Lee, Y.C.; Lee, C.I. An in vitro study on the effect of combined treatment with photodynamic and chemical therapies on Candida albicans. Int. J. Mol. Sci. 2018, 19, 337. [Google Scholar] [CrossRef]
- Janeth Rimachi Hidalgo, K.; Cabrini Carmello, J.; Carolina Jordão, C.; Aboud Barbugli, P.; de Sousa Costa, C.A.; Garcia de Oliveira Mima, E.; Pavarina, A.C. Antimicrobial photodynamic therapy in combination with nystatin in the treatment of experimental oral candidiasis induced by candida albicans resistant to fluconazole. Pharmaceuticals 2019, 12, 140. [Google Scholar] [CrossRef]
- Hsieh, Y.H.; Chuang, W.C.; Yu, K.H.; Jheng, C.P.; Lee, C.I. Sequential photodynamic therapy with phthalocyanine encapsulated chitosan-tripolyphosphate nanoparticles and flucytosine treatment against candida tropicalis. Pharmaceutics 2019, 11, 16. [Google Scholar] [CrossRef]
- Diogo, P.; Faustino, M.F.A.; Neves, G.M.P.M.S.; Palma, P.J.; Baptista, I.P.; Gonçalves, T.; Santos, J.M. An insight into advanced approaches for photosensitizer optimization in endodontics—a critical review. J. Funct. Biomater. 2019, 10, 44. [Google Scholar] [CrossRef]
- Caruso, E.; Malacarne, M.C.; Banfi, S.; Gariboldi, M.B.; Orlandi, V.T. Cationic diarylporphyrins: In vitro versatile anticancer and antibacterial photosensitizers. J. Photochem. Photobiol. B Biol. 2019, 197, 111548. [Google Scholar] [CrossRef]
- Gunasegar, S.; Himratul-Aznita, W.H. Nicotine enhances the thickness of biofilm and adherence of Candida albicans ATCC 14053 and Candida parapsilosis ATCC 22019. FEMS Yeast Res. 2019, 19, 1–11. [Google Scholar] [CrossRef]
- Campana, R.; Ciandrini, E.; Baffone, W. Experimental approach for a possible integrated protocol to determine sanitizer activity against both planktonic bacteria and related biofilms. Food Res. Int. 2018, 111, 472–479. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Caruso, E.; Tettamanti, G.; Banfi, S.; Barbieri, P. Photoinduced antibacterial activity of two dicationic 5,15-diarylporphyrins. J. Photochem. Photobiol. B Biol. 2013, 127, 123–132. [Google Scholar] [CrossRef]
- Caruso, E.; Cerbara, M.; Malacarne, M.C.; Marras, E.; Monti, E.; Gariboldi, M.B. Synthesis and photodynamic activity of novel non-symmetrical diaryl porphyrins against cancer cell lines. J. Photochem. Photobiol. B Biol. 2019, 195, 39–50. [Google Scholar] [CrossRef]
- Martegani, E.; Bolognese, F.; Trivellin, N.; Orlandi, V.T. Effect of blue light at 410 and 455 nm on Pseudomonas aeruginosa biofilm. J. Photochem. Photobiol. B Biol. 2020, 204, 111790. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Martegani, E.; Bolognese, F. Catalase A is involved in the response to photooxidative stress in Pseudomonas aeruginosa. Photodiagnosis Photodyn. Ther. 2018, 22, 233–240. [Google Scholar] [CrossRef]
- Orlandi, V.T.; Caruso, E.; Banfi, S.; Barbieri, P. Effect of organic matter on the in Vitro Photoeradication of Pseudomonas aeruginosa by means of a cationic tetraaryl-porphyrin. Photochem. Photobiol. 2012, 88, 557–564. [Google Scholar] [CrossRef]
- Sunahara, H.; Urano, Y.; Kojima, H.; Nagano, T. Design and synthesis of a library of BODIPY-based environmental polarity sensors utilizing photoinduced electron-transfer-controlled fluorescence ON/OFF switching. J. Am. Chem. Soc. 2007, 129, 5597–5604. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Y.; Murray, C.K.; Hamblin, M.R.; Hooper, D.C.; Dai, T. Antimicrobial blue light inactivation of pathogenic microbes: State of the art. Drug Resist. Updat. 2017, 33–35, 1–22. [Google Scholar] [CrossRef]
- Lyon, J.P.; Rezende, R.R.; Rabelo, M.P.; de Lima, C.J.; Moreira, L.M. Synergic Effect of Photodynamic Therapy with Methylene Blue and Surfactants in the Inhibition of Candida albicans. Mycopathologia 2013, 175, 159–164. [Google Scholar] [CrossRef]
- Sousa, V.; Gomes, A.T.P.C.; Freitas, A.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Almeida, A. Photodynamic inactivation of candida albicans in blood plasma and whole blood. Antibiotics 2019, 8, 221. [Google Scholar] [CrossRef]
- Scanone, A.C.; Gsponer, N.S.; Alvarez, M.G.; Durantini, E.N. Porphyrins containing basic aliphatic amino groups as potential broad-spectrum antimicrobial agents. Photodiagnosis Photodyn. Ther. 2018, 24, 220–227. [Google Scholar] [CrossRef]
- Martinez De Pinillos Bayona, A.; Mroz, P.; Thunshelle, C.; Hamblin, M.R. Design features for optimization of tetrapyrrole macrocycles as antimicrobial and anticancer photosensitizers. Chem. Biol. Drug Des. 2017, 89, 192–206. [Google Scholar] [CrossRef]
- Kapteyn, J.C.; Montijn, R.C.; Dijkgraaf, G.J.P.; Van den Ende, H.; Klis, F.M. Covalent association of β-1,3-glucan with β-1,6-glucosylated mannoproteins in cell walls of Candida albicans. J. Bacteriol. 1995, 177, 3788–3792. [Google Scholar] [CrossRef]
- Hall, R.A.; Gow, N.A.R. Mannosylation in candida albicans: Role in cell wall function and immune recognition. Mol. Microbiol. 2013, 90, 1147–1161. [Google Scholar] [CrossRef]
- Cormick, M.P.; Alvarez, M.G.; Rovera, M.; Durantini, E.N. Photodynamic inactivation of Candida albicans sensitized by tri- and tetra-cationic porphyrin derivatives. Eur. J. Med. Chem. 2009, 44, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Nobile, C.J. Candida albicans biofilms: Development, regulation, and molecular mechanisms. Microbes Infect. 2016, 18, 310–321. [Google Scholar] [CrossRef]
- Grinholc, M.; Szramka, B.; Kurlenda, J.; Graczyk, A.; Bielawski, K.P. Bactericidal effect of photodynamic inactivation against methicillin-resistant and methicillin-susceptible Staphylococcus aureus is strain-dependent. J. Photochem. Photobiol. B Biol. 2008, 90, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Pereira Gonzales, F.; Maisch, T. Photodynamic inactivation for controlling Candida albicans infections. Fungal Biol. 2012, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, C.; Sotgiu, G.; Sagnella, A.; Varchi, G.; Guerrini, A.; Giuri, D.; Polo, E.; Orlandi, V.T.; Marras, E.; Gariboldi, M.; et al. Wool Keratin 3D Scaffolds with Light-Triggered Antimicrobial Activity. Biomacromolecules 2016, 17, 2882–2890. [Google Scholar] [CrossRef] [PubMed]



| PS | Chemical Structure | Chemical Denomination | Ref | |
|---|---|---|---|---|
| Neutral (Ø) | 1 |  | 5-Pentafluorophenyl-15-[4-(4-Bromobutoxy)Phenyl]-21H,23H-porphyrin | [39] |
| 2 |  | 5- Pentafluorophenyl-15-[4-(8-Bromooctaoxy)Phenyl]-21H,23H-porphyrin | [39] | |
| 3 |  | 5-Phenyl-15-[4-(4-bromobutoxy)phenyl]-21H,23H-porphyrin | [35] | |
| 4 |  | 5,15-Di[4-(4-bromobutoxy)phenyl]-21H,23H-porphyrin | [35] | |
| Monocationic (+) | 5 |  | 5-Phenyl-15-[4-(4-pyridinobutoxy)phenyl]-21H,23H-porphyrin | [35] |
| 6 |  | 5-Phenyl-15-[4-(4-pyridinooctaoxy)phenyl]-21H,23H-porphyrin | [35] | |
| 7 |  | 5-Pentafluorophenyl-15-[4-(4-Pyridinobutoxy)Phenyl]-21H,23H-porphyrin | [39] | |
| 8 |  | 5-Pentafluorophenyl-15-[4-(4-Pyridinooctaoxy)Phenyl]-21H,23H-porphyrin | [39] | |
| Dicationic (++) | 9 |  | 5,15-di(N-benzyl-4-pyridyl)porphyrin | [38] |
| 10 |  | 5,15-Di[4-(4-pyridinobutoxy)phenyl]-21H,23H-porphyrin | [35] | |
| 11 |  | 5,15-Di[4-(4-pyridinooctaoxy)phenyl]-21H,23H-porphyrin | [35] |
| Adherent Biomass (cfu/well) | Planktonic Biomass (cfu/mL) | ||||
|---|---|---|---|---|---|
| C. albicans Strain | DARK | LIGHT 150 J/cm2 | DARK | LIGHT 150 J/cm2 | |
| C. albicans ATCC 14053 | Untreated | 6.1 ± 0.85 × 107 | 6.63 ± 1.3 × 107 | 3.85 ± 3.9 × 106 | 3.77 ± 2.6 × 106 |
| DMSO 4% | 6.1 ± 1.2 × 107 | 5.55 ± 1.48 × 107 | 1.32 ± 1.7 × 106 | 1.98 ± 2.4 × 106 | |
| 7 | 5.87 ± 1.7 × 107 | 9.67 ± 1.6 × 104 * | 2.85 ± 2.7 × 106 | 2.53 ± 4.2 × 102 ** | |
| 8 | 6.45 ± 0.07 × 107 | 5.5 ± 6.3 × 10 *** | 2.3 ± 2.4 × 106 | 10 ± 0.0 *** | |
| C. albicans Ca1 | Untreated | 4.93 ± 0.7 × 107 | 4.9 ± 1.6 × 107 | 3.87 ± 2.7 × 106 | 6.17 ± 3.5 × 106 |
| DMSO 4% | 5.35 ± 0.2 × 107 | 6.15 ± 0.07 × 107 | 2.41 ± 3.1 × 106 | 2.83 ± 3.1 × 106 | |
| 7 | 5.3 ± 0.5 × 107 | 1.1 ± 1.9 × 105 * | 2.73 ± 1.1 × 106 | 5 ± 8.6 × 104 * | |
| 8 | 6.25 ± 0.07 × 107 | 10 ± 0.0 *** | 2.65 ± 3.6 × 106 | 10 ± 0.0 *** | |
| C. albicans Ca2 | Untreated | 4.75 ± 0.07 × 107 | 4.6 ± 0.1 × 107 | 6.6 ± 3.3 × 106 | 2.3 ± 0.1 × 106 |
| DMSO 4% | 5 ± 0.4 × 107 | 4.35 ± 0.9 × 107 | 3.5 ± 2.1 × 106 | 2.4 ± 1.2 × 106 | |
| 7 | 3.95 ± 0.6 × 107 | 10 ± 0.0 *** | 1.65 ± 0.2 × 106 | 10 ± 0.0 *** | |
| 8 | 4.7 ± 0.0 × 107 | 10 ± 0.0 *** | 3.4 ± 0.1 × 106 | 10 ± 0.0 *** | |
| C. albicans Ca3 | Untreated | 6.67 ± 3.1 × 107 | 3.33 ± 2.3 × 107 | 1.57 ± 1.5 × 107 | 3.57 ± 1 × 106 |
| DMSO 4% | 5.6 ± 0.2 × 107 | 5.9 ± 0.4 × 107 | 2.7 ± 2.2 × 106 | 4.05 ± 0.9 × 106 | |
| 7 | 3.27 ± 1.4 × 107 | 2.26 ± 3.8 × 105 | 4.57 ± 3.6 × 106 | 9.01 ± 10 × 103 * | |
| 8 | 4 ± 2.6 × 107 | 3.36 ± 5.7 × 103 ** | 2.9 ± 0.7 × 106 | 16.7 ± 11 *** | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlandi, V.T.; Martegani, E.; Bolognese, F.; Trivellin, N.; Maťátková, O.; Paldrychová, M.; Baj, A.; Caruso, E. Photodynamic Therapy by Diaryl-Porphyrins to Control the Growth of Candida albicans. Cosmetics 2020, 7, 31. https://doi.org/10.3390/cosmetics7020031
Orlandi VT, Martegani E, Bolognese F, Trivellin N, Maťátková O, Paldrychová M, Baj A, Caruso E. Photodynamic Therapy by Diaryl-Porphyrins to Control the Growth of Candida albicans. Cosmetics. 2020; 7(2):31. https://doi.org/10.3390/cosmetics7020031
Chicago/Turabian StyleOrlandi, Viviana Teresa, Eleonora Martegani, Fabrizio Bolognese, Nicola Trivellin, Olga Maťátková, Martina Paldrychová, Andreina Baj, and Enrico Caruso. 2020. "Photodynamic Therapy by Diaryl-Porphyrins to Control the Growth of Candida albicans" Cosmetics 7, no. 2: 31. https://doi.org/10.3390/cosmetics7020031
APA StyleOrlandi, V. T., Martegani, E., Bolognese, F., Trivellin, N., Maťátková, O., Paldrychová, M., Baj, A., & Caruso, E. (2020). Photodynamic Therapy by Diaryl-Porphyrins to Control the Growth of Candida albicans. Cosmetics, 7(2), 31. https://doi.org/10.3390/cosmetics7020031








