Abstract
Increasing evidence suggests that environmental stress, such as UV radiation, generates reactive oxygen and nitrogen species in skin cells, leading to histochemical changes including skin disorders and aging, hyper pigmentation, and increased formation of wrinkles. Besides the defensive system in skin composed of vitamins and intrinsic antioxidant enzymes, topical and skin conditioning products have been used commonly to eradicate or eliminate these skin ailments. Among various ingredients providing nourishing and moisturizing effect in skin, antioxidants have been reported to be a key ingredient to counteract skin aging processes and skin disorders. Derived from a patented extraction process, a polyphenol rich sugarcane concentrate (Officinol™) becomes the focus of this study due to its rich content of polyphenols known to be strong antioxidants. In this work, we carried out a series of cell-based in vitro studies to examine the use of Officinol™ in anti-aging and skin care functions. Our studies show that Officinol™ activated telomerase, a major biomarker that have been reported to be associated with slowed cellular aging process. When skin cells were under environmental stress such as UV radiation, Officinol™ inhibited MMP-1, an interstitial collagenase in skin cells, and deterred the breakdown of collagen that provides supple texture in skin. Officinol™ also inhibited cellular expression of melanin pigmentation and tyrosinase activity, two major biomarkers causing skin pigmentation and aging spots, and inhibited elastase, an enzyme that facilities the reduction of skin elasticity. At the end of the investigation, we carried out a 10-person, pilot study to examine the effect of Officinol™ on skin lightening and fine line and wrinkle reduction in human skin. The combination of the in vitro and the human pre-study indicates that Officinol™ could provide significant preventative and protective functions including antioxidant, anti-aging, wrinkle reduction, and skin brightening for human skin suffering from aging and other stress. These findings are to be confirmed with a larger scale clinical study at a later stage.
Keywords:
anti-aging; UV protection; MMP-1; melanin pigmentation; wrinkle reduction; tyrosinase; elastase 1. Introduction
As humans age, the accumulation of damages caused by environmental factors such as reactive oxygen radicals, sun exposure, high stress levels, environmental pollution, cigarette smoking, alcohol, and drug abuse attribute to the effect of skin disease and skin aging. At the molecular level, environmental and chronic stress causes DNA damage, increases pro-inflammatory cytokine levels (e.g., interleukins) that causes skin inflammation. The skin becomes thinner, sweat- and oil- secreting glands in the skin decrease, leaving the skin dry and thin. Production of the proteins collagen and elastin in the skin also declines, leading to decreased firmness and formation of wrinkles. Melanin, the pigment that gives skin its color, becomes unevenly distributed causing freckles and age spots.
In order to understand the skin aging mechanism, active components and phytochemicals for skin care have been investigated extensively aiming to intervene with the exogenous and endogenous aging agents to slow down aging and other skin deterioration effects. One category of the notable phytochemicals that have been reported to provide such therapeutic skin care functions is polyphenols. Polyphenols (polyphenolic compounds), are known to be a major group of naturally occurring phytochemicals containing conjugated phenol ring(s) as a common molecular structure. A key feature of these polyphenolic compounds is their antioxidant function expressed by neutralizing reactive oxygen and nitrogen species (ROS/RNS, referred as ROS in this paper) facilitated by the conjugated phenol ring(s) [1]. As an example, Resveratrol, a polyphenol from red wine, has demonstrated to be a front performer in prevention of photo-aging due to its strong antioxidant properties [2]. Based on a flaky skin mouse model, researchers also demonstrated that green tea polyphenol reduced the symptoms of epidermal pathology in mouse skin [3].
Sugarcane (Saccharum officinarum), a perennial grass indigenous to tropical South Asia, Southeast Asia, and New Guinea, has mainly been used as a food source up until the recent discovery of polyphenols and other bioactives in the plant. Pharmacological studies revealed several polyphenolic compounds such as phenolic acids, flavonoids, and different glycosides in sugarcane juices and its unrefined products (e.g., molasses), as well as fatty acids in its stems and leaves [4]. Since then, sugarcane has attracted much research attention in its potential therapeutic usage in a number of areas including diabetic management and neurological therapy [5]. However, the use of sugarcane phytochemicals in skin care has not been fully explored.
We recently reported that a polyphenol rich sugarcane extract (PRSE) obtained from sugarcane molasses using a patented extraction process contains a high level of phenolic compounds including tricin, apigenin, and luteolin, and displays a full spectrum of antioxidant property that effectively neutralizes six different species of ROS [6]. In another study, we also examined the anti-inflammatory effect of Officinol™ via its inhibitive effect on pro-inflammatory cytokine tumor necrosis factor (TNF-α) and activation effect on nuclear factor erythroid 2-related factor 2 (Nrf2), a redox-sensitive transcription factor deployed by a cellular system to counter oxidative stress and inflammation [7].
Based on the unique therapeutic functions we observed in PRSE, we theorized that the high level of polyphenols and its anti-oxidation and anti-inflammatory effect may render it a candidate as an active ingredient for skin care. In this study, we prepared a polyphenol-rich sugarcane concentrate (Officinol™) specifically for skin care application. We first examined the general functions of Officinol™ as an anti-aging agent, using cellular telomerase activity as biomarkers for aging. Telomerase is a ribonucleoprotein complex that contributes to the maintenance of telomeric structure and length that are associated with life span [8]. We then undertook a range of preclinical studies to examine the specific effect of Officinol™ on the biology and function of skin related enzymes and cells. These include the effect of Officinol™ on: (1) UV protection and tissue integrity with matrix metalloproteinase-1 (MMP-1) as an indicator; (2) melanin modulation in melanocytes, and skin brightness based on activity of tyrosinase, the key enzyme that catalyzes the first step of melanin biosynthesis; (3) wrinkle prevention using elastase, an enzyme that facilities the reduction of skin elasticity, as the indicator.
At the conclusion of this investigation, we carried out a 10-person, clinical pre-study to examine the effect of Officinol™ on skin lightening and fine line and wrinkle reduction in human skin. The combination of the in vitro and clinical studies demonstrated that Officinol™ could provide significant preventative and protective functions including anti-aging, UV protection, wrinkle reduction, and skin brightening for human skin suffering from aging and other chronicle and environmental stresses. The findings are to be further verified via a larger scale clinical study at a later stage.
2. Materials and Methods
2.1. Reagents
Unless otherwise specified, all chemicals were obtained from Sigma-Aldrich, St. Louis, MO, USA. The cell culture, cell media, and other biological reagents were obtained from sources indicated in the method description below.
2.2. Study Material
A proprietary, polyphenol rich sugarcane plant concentrate (Officinol™) obtained from sugarcane molasses was used in this study. The material was developed and prepared by The Product Makers Pty Ltd. (Melbourne, Australia). In short, sugarcane molasses is diluted to 50 Brix in deionized water, mixed well in room temperature until homogeneous. To this feedstock, food grade 95% ethanol is added slowly while stirring until the final ethanol level is 75%. The clear yellow supernatant is aspirated and collected and then centrifuged at 2500× g for 15 min to remove any remaining particulate matter. The supernatant is collected and then filtered through a Whatman grade GF/A filter under vacuum and the filtrate collected. Extract is concentrated to 70 Brix in a Buchi Rotary Evaporator. Quantification of general antioxidant capacity with both chemical (6-radical Oxygen Radical Absorption Capacity assay, ORAC 6.0) and biological (Cellular antioxidant assay, CAA) quantification methods were also carried out as previously described (6).
For the following cell-based studies, Officinol™ was suspended in 50% EtOH to yield a 1g/mL mixture. The mixture was then thoroughly vortexed for 30 s. Subsequently the solution was ultrasonicated in an ice bath for 15 min and vortexed occasionally during this time. Following ultrasonication, the solution was vortexed again for 30 s and centrifuged at 1780× g (or 3900 rpm @105mm radius) under 4 °C for 15 min to separate out any residue in the mix. The supernatant was then collected as the stock extract (1 g/mL) and stored under 4 °C until use. For each in vitro study, a series of dilutions of the stock extract were used according to specific study requirements. A minimum 1:33 dilution of the stock extract in cellular media was used when adding the sample into cell cultures, making the final concentration of ethanol in cell cultures less than 1.5%.
2.3. Cellular Telomerase Activity Assay
Cellular telomerase activity assay was carried out basing on an established study model with modification [9]. Human embryonic kidney cell line 293 T (HEK 293 T) from American Type Culture Collection (ATCC, Manassas, VA, USA) were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 0.5% fetal calf serum (FCS), 1 unit/mL of penicillin/streptomycin, and 2 mM glutamine. in a humidified atmosphere of 5% CO2. After 4–5 passages, the cells were counted and seeded at the density of 2.5 × 105 cells per 60-mm gelatin-coated well. Cells were then further cultivated, and assays were carried out when cells reached approximately 80–90% confluence in the wells. Unless otherwise indicated, cells were seeded with similar density and cell cultures reaching 80–90% confluence in culture wells were used for the rest of the cellular assays in this paper.
On the day of assay, the cells were treated with treated various concentrations of Officinol™, or cycloastragenol (used as the assay positive control), or phosphate-buffered saline (PBS) at pH 7.4 (negative control). After 24 h of treatment, a Telomerase Repeated Amplification Protocol (TRAP) assay [10] was performed using the TRAPEZE XL telomerase detection kit (Millipore, Burlington, MA) according to the manufacturer’s instructions and measured in a fluorescence plate reader after transfer of the polymerase chain reaction (PCR) products into black 96-well plates. Briefly, cells were lysed with CHAPS lysis buffer containing 100–200 units/mL of RNase inhibitor. The cell pellet was washed once with PBS at pH 7.4, and re-suspended in 800 µL of CHAPS Lysis Buffer. The suspension was incubated on ice for 30 min, and spun in a microcentrifuge at 12,000× g for 20 min at 4 °C. 500 µL of the supernatant was transferred into a fresh tube and the protein concentration determined. PCR amplification was then performed to determine the telomerase products. In short, aliquots of 48 µL of a TRAP reaction mix including telomerase substrate (TS) primer, a TS oligo (sequence: 5′-AAT CCG TCG AGC AGA GTT-3), ACX reverse primer (sequence: 5′-GCGCGGCTTACCCTTACCCTTACCCTAACC-3′) modified with proprietary fluorescent energy transfer dyes, and Taq polymerase were added into a RNase-free PCR tube. 2 µL of cell lysis extracts or controls were added into each tube. The tubes were placed in the thermocycler block, and incubated at 30 °C for 30 min. Then, in a thermocycler, a 4-step PCR (94 °C/30 s, 59 °C/30 s, 72 °C/1 min) was performed for 36 cycles followed by a 72 °C/3 min extension step and then at 55 °C/25 min, concluding with a 4 °C incubation. The yield of the PCR reaction was then determined by measuring the fluorescence in a spectrofluorometer. In each PCR reaction, a proprietary internal standard (K2) was also included which produced a detectable 56 bp sulforhodamine amplification product that served as a control for PCR amplification efficiency. The telomerase products of cells treated with Officinol™ or positive control were normalized against cells without treatments. Two biological replicates with a minimum two technical measurements per replicate were carried out for each assay in this paper.
2.4. UV Protection Study: Cellular Matrix Metalloproteinase-1 (MMP-1) Assay
HaCaT cells, an immortalized human keratinocyte cell line (ATCC, Manassas, VA, USA), were cultured in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% heat-inactivated FBS and 1% antibiotic-antimycotic (GIBCO-BRL, Grand Island, NY, USA) at 37 °C under a humidified atmosphere of 95% air and 5% CO2.
On the day of assay, the cells were treated by various concentrations of Officinol™ or avobenzone (used as the assay positive control), or PBS at pH 7.4 (negative control) for 24 h. Then, the cells were exposed to UV irradiation of 50 mJ/cm2 generated by an UVB lamp. After UV irradiation, the cells were washed with PBS at pH 7.4, and incubated with serum-free DMEM for 24 h. Mock-irradiated controls followed the same schedule of medium changes without UVB irradiation.
Quantitative real time PCR (qRT-PCR) was then performed to determine the MMP-1 expression level in each treatment group. Extraction of total RNA was performed by using the MagMAX™-96 Total RNA Isolation Kit. cDNA was synthesized from total RNA by using High Capacity RNA-to-cDNA Kits and a Veriti thermal cycler. The primer pair used was: TCTGACGTTGATCCCAGAGAGCAG (forward) and CAGGGTGACACCAGTGACTGCAC (reverse). Peptidyl-prolyl cis-trans isomerasa (Ppia) was used as the house keeping gene. qRT-PCR was performed in Applied Biosystems Real-Time Quantitative PCR system. MMP-1 expression levels in human skin cells treated with Officinol™ and positive controls were normalized against cells without treatments.
2.5. Cellular Melanin Pigmentation Inhibition
Cellular melanin pigmentation inhibition study was carried out according to a previously published procedure with minor adjustment [11]. Briefly, human melanoma cells MNT-1 (Thermo Fisher Scientific, Waltham, MA, USA) were maintained in DMEM with 10% heat-inactivated FCS and a 1% antibiotic-antimycotic at 37 °C in a humidified 5% CO2 atmosphere before use.
On the day of assay, the cells were treated by various concentrations of Officinol™ or chloroquine (used as the assay positive control), or PBS at pH 7.4 (negative control) for 72 h. The cells were then washed with PBS at pH 7.4, and lysed in 200 µL of 1M NaOH and boiled for 5 min to solubilize the melanin. 100 µL of each lysate was added into in a 96-well microplate, and the absorbance at 490 nm was measured with a microplate reader. The level of melanin pigmentation formation in human skin cells treated with Officinol™ and positive controls were normalized against that in the cells without treatments.
2.6. Enzymatic Tyrosinase Inhibition Assay
The inhibition of tyrosinase by Officinol™ was investigated using an enzymatic tyrosinase inhibitor screening assay kit (Abcam, Cambridge, UK) according to the manufacturer’s instruction, following an assay design tailored towards inhibition activity study. In principle, tyrosinase catalyzes the oxidation of tyrosine, producing a chromophore that can be detected at OD 510 nm. A series of concentration of Officinol™, or Kojic Acid (a reversible inhibitor of tyrosinase that is used as the assay control) were allowed to react with a mixture of tyrosinase and tyrosinase substrate for 10 min in room temperature (25 °C). The optical signal of each reaction mix was then recorded and normalized against that of the assay background. Inhibition curves and IC50s were then generated by Prism 8.0 (GraphPad Software, San Diego, CA, USA).
2.7. Enzymatic Elastase Inhibition Assay
The inhibition of elastase by Officinol™ was investigated using an enzymatic elastase assay kit (EnzChek®, Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instruction, with a modified assay design tailored towards inhibition activity study. In brief, a soluble bovine neck ligament elastin that has been labeled with BODIPY® FL (Thermo Fisher Scientific, Waltham, MA, USA), a fluorescent dye, such that the conjugate’s fluorescence is quenched. When elastase is introduced, this non-fluorescent substrate can be digested by elastase to yield a highly fluorescent product.
A series of concentration of Officinol™, or N-Methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone (a known inhibitor of elastase that is used as the assay control) were allowed to react with a mixture of the above non-fluorescent substrate and elastase for 30 min under room temperature (25 °C) in dark. Tej fluorescence signal of each reaction mix is then measured using a plate reader (Synergy HT, BioTek, Winooski, VT, USA) and normalized again the assay background control. The degree of the fluorescent signal change indicates the activity of elastase present in the reaction mix. Inhibition curves and IC50s were then generated by Prism 8.0 (GraphPad Software, San Diego, CA, USA).
2.8. Clinical Study
A clinical study was carried out to evaluate the in-use performance of Officinol™ after its application to the face. To facilitate the clinical study, a study product was prepared by adding Officinol™ at 1:20 (w/w) ratio into an inert carrier that possessed no active properties. The biophysical effects of the topically applied product were evaluated pre and post product application when used by the same test panelists. 11 subjects were recruited for the study, with results tabulated for all 10 who completed the study period of 12 weeks. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the guideline of Australian/New Zealand Standard (AS/NAS) 2604:2012, ISO 24444, the Declaration of Helsinki and the relevant Australian National Health and Medical Research Council (NHMRC) Act 1992. Wrinkle reduction was determined using silicone impressions and Profilometry and skin blemishes using a Minolta spectrophotometer. A photographic record was also taken of each test participant.
Healthy adult volunteers between the ages of 40–65 years were recruited for this study. The choice of the age criteria results from a combination consideration of the biological effect to be investigated, selection of a representative sample, and minimization of comorbidity. Inclusion criteria included the following: not taking medication or under the care of a physician for a period of one month prior to commencement and throughout the entire test period; free of any dermatological or systemic disorder that would interfere with the results, at the discretion of the Investigator; with self-described dry skin.
Exclusion criteria include: individuals who are under doctor’s care or are taking medication which in the opinion of the investigator would mask or interfere with the results; with any history of sensitivity to cosmetics in general and moisturizers in particular; with any form of skin cancer, or any disease that would interfere with the test results; diagnosed with chronic skin allergies; Female volunteers who indicate that they are pregnant or nursing an infant; Individuals with excessive hair on the test sites; Individuals with known hypersensitivity to cosmetic products.
Eleven participants had been recruited for the study, of which ten participants completed 12 weeks of study. The results were tabulated for all 10 who completed the full study period.
2.8.1. Study Protocol
After completing the informed consent forms, all participants were required to abstain from use of moisturizers and any other skin treatments on the test area for a period of 10 days prior to study commencement. At the completion of the 10 days ‘washout’ period, panelists were required to apply the study product (Officinol™) evenly applied to the face twice per day (Morning & Evening) for consecutive 12 weeks. During the 12 weeks test period, the participants were required to visit the clinic site for three times: week 0, week 8, week 12. At each site visit, following skin evaluation tests were conducted:
Instrumental Description Profilometry (Wrinkles and Roughness)
At each visit, a single silicone replica was made of the target area and a photographic record was kept of this target for subsequent relocation. The samples were stored in controlled conditions for comparative measurement. Comparative analysis of skin profilometry was conducted, using surface roughness and wrinkle depth analysis. Concurrent use of other moisturizer or skin care products did not occur in the skin area under study. The height of the replicated wrinkles were measured using Miyomoto Surftest profilometer. Ry (depth) and Ra (mean roughness) were recorded at each time of measuring operation. The area scanned from each sample was clearly mapped so as to determine the same area in respective two months sampling.
Instrumental Description L*a*b* Color Measurement
A Minolta Chromometer hand held spectrophotometer was utilized. This tri-stimulus instrument was in an unpigmented adjoining area. Specular component included (SCI) values are documented.
Digital Photography
At each time point, a series of high-resolution digital photographs were collected. The subject was presented with a clean face, with hair pulled off face, with no jewelry (unless permanent) and with a black drape used to standardize clothing. Subject positioning was reproduced upon return visit. A light booth was used so as to provide controlled reproducible light conditions. The booth consists of an array of eight equally spaced fluorescent tubes in a semicircular configuration. The software driven system allows the position and expression of the test subjects to be aligned to a high degree. Lux values were calibrated and documented.
2.9. Statistical Analysis
For the clinical study, a statistical analysis was performed on the data of roughness, wrinkle, and pigment color by analysis of variance (ANOVA). The analysis was conducted with time as the fixed effect and subject as the random effect, followed by the contrast test to compare the values obtained at weeks 0, 8, and 12 if the time effect was statistically significant. The statistical significance of the change of the data is determined by the probability value (p-value), i.e., probability that the difference of the data obtained at different time points being zero. When p-value was <0.05, the difference between the two sets of data was considered significant. The analysis was performed using JMP IN version 3 (SAS, Cary, NC).
3. Results
3.1. Total Phenols, Antioxidant Capacity, and Other General Properties of Officinol™
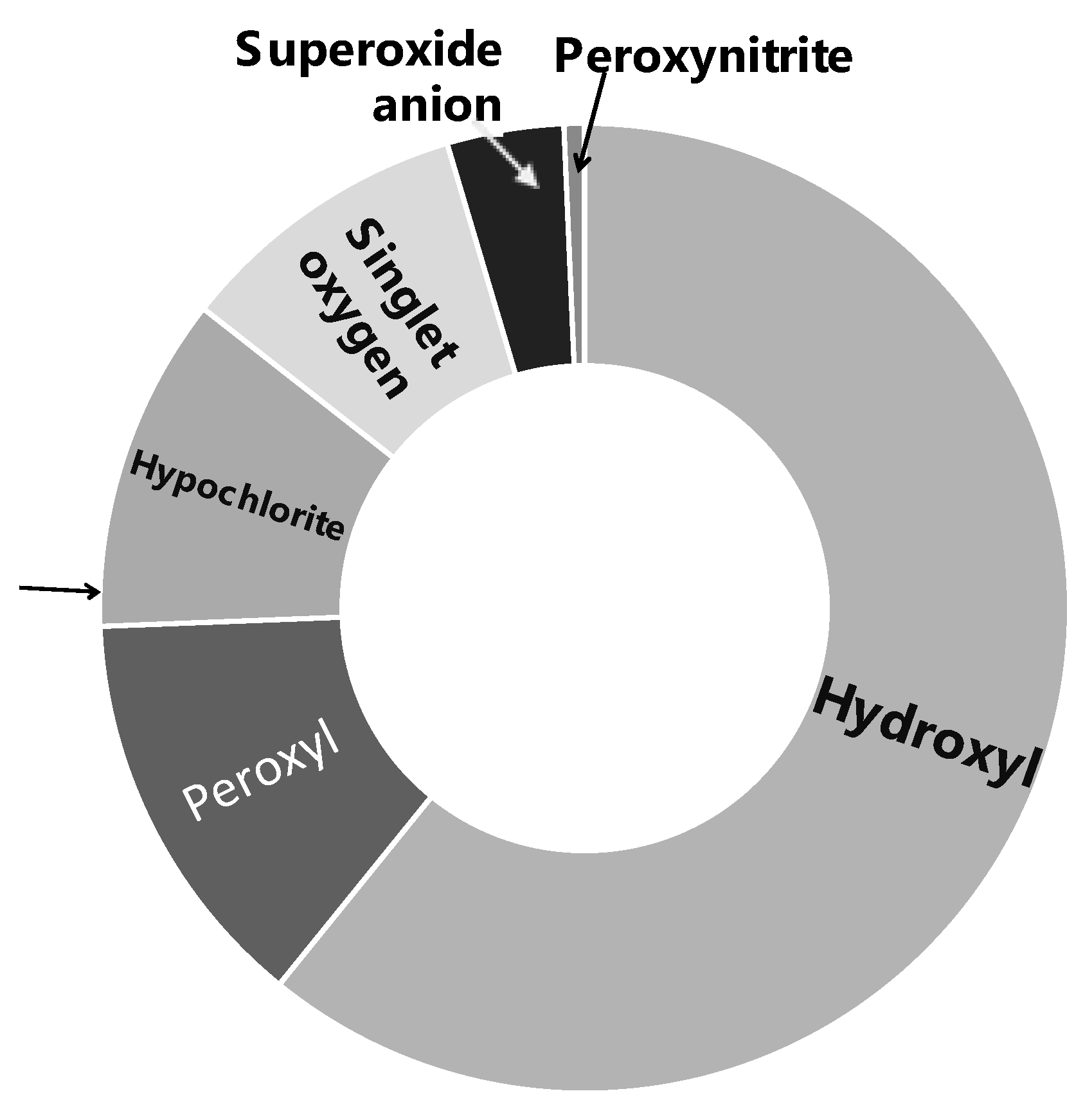
A wide range of characterization studies of the study material Officinol™ indicated that it contains 26 mg/g gallic acid equivalency (GAE) of total phenol, with a total antioxidant capacity (ORAC 6.0) of 1668.27 μmole trolox equivalency (TE)/g. General chemical and physical properties of Officinol™ are summarized in Table 1 and Figure 1. Phenolics profile of the study material Officinol™ has also been investigated via liquid chromatography–mass spectrometry (LCMS) technique as described previously [12], and summary of the findings is exhibited in Table 2.

Table 1.
Physical Properties of Officinol™.
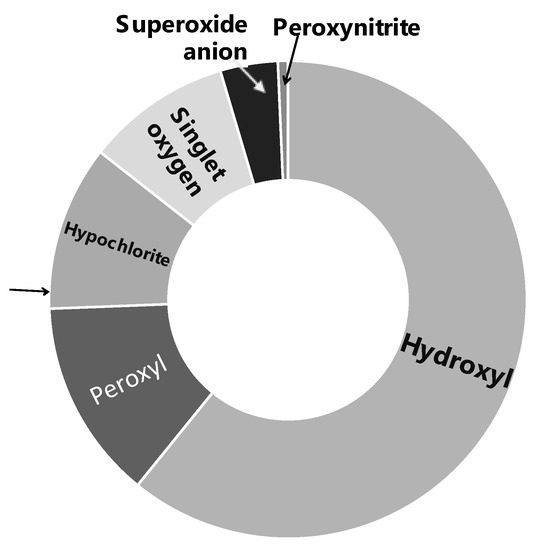
Figure 1.
Full spectrum antioxidant capacity of.

Table 2.
Polyphenol composition of Officinol™ by LCMS analysis *.
3.2. Activation of Cellular Telomerase Activity
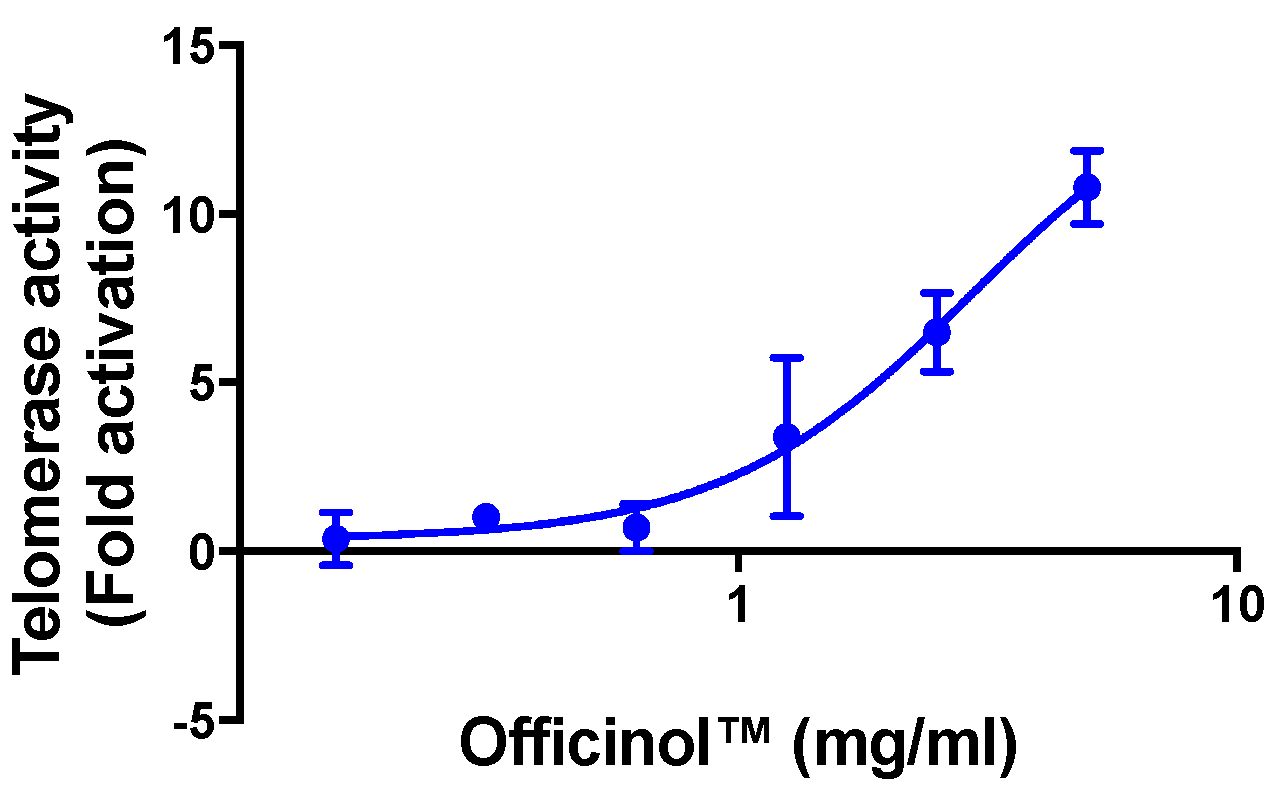
Treatment of HEK 293T cells with Officinol™ effectively activated cellular telomerase in a dose-dependent manner, with the half maximal activation concentration (EC50) of 2.94 mg/mL (Figure 2). Cycloastragenol, a known telomerase activator used as a reference control in the assay, enhanced telomerase activity with the EC50 of 0.39 μg/mL (0.79 μM, Table 2).
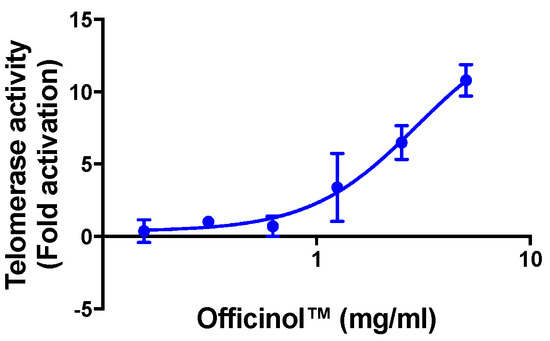
Figure 2.
Concentration-dependent activation effect of Officinol™ on telomerase activity in HEK 293T cells. Graph on a logarithmic scale.
3.3. Officinol™ Protects Skin Cells from UV Irradiation, with MMP-1 Inhibition as a Possible Pathway
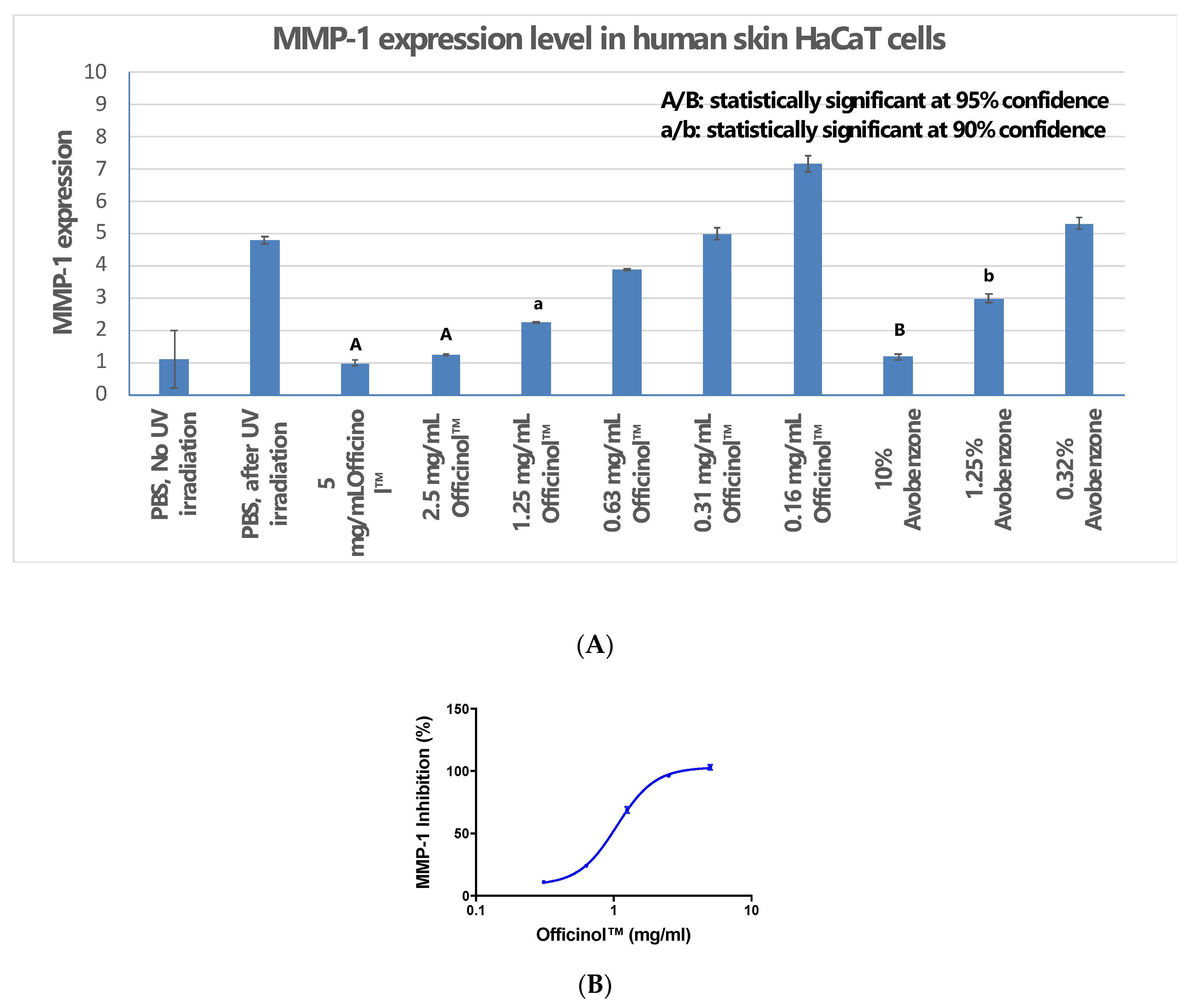
UV irradiation is one of the major environmental factors that induce skin disorders. Our study indicated that when human skin cells were under UV irradiation, Officinol™ effectively inhibits expression of MMP-1. Figure 3A shows that a pathogenically high level of UV irradiation at 50 mJ/cm2 significantly induced MMP-1 expression in human skin HaCaT cells. Treatment of HaCaT cells with Officinol™ inhibited MMP-1 expression in a dose-dependent manner with an IC50 of 1.05 mg/mL (Figure 3B). In a parallel study, avobenzone, a commercially available UV blocker, also exhibited strong MMP-1 inhibitive effect in a concentration-dependent manner. Treating the skin cells with 1.23 μg/mL of avobenzone inhibited the MMP-1 by 50%, comparable to the effect reached by 1.05 mg/mL of Officinol™.
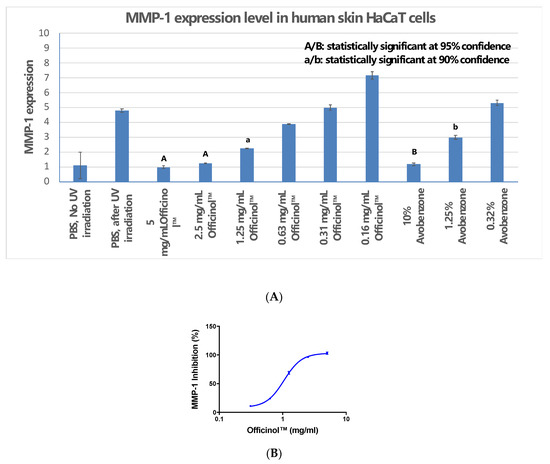
Figure 3.
Officinol™ protects human skin HaCaT cells from UV irradiation damage, possibly through MMP-1 inhibition as one of the function pathways. (A) Effect of UV irradiation on MMP-1 level in HaCaT cells, and the inhibition of MMP-1 by treatments of Officinol™ and avobenzone; (B) Dose-dependent MMP-1 inhibition effect by treatment of Officinol™ in HaCaT cells under UV irradiation. Graph on a logarithmic scale.
3.4. Mitigation of Skin Cell Melanin Biosynthesis and Tyrosinase Activity
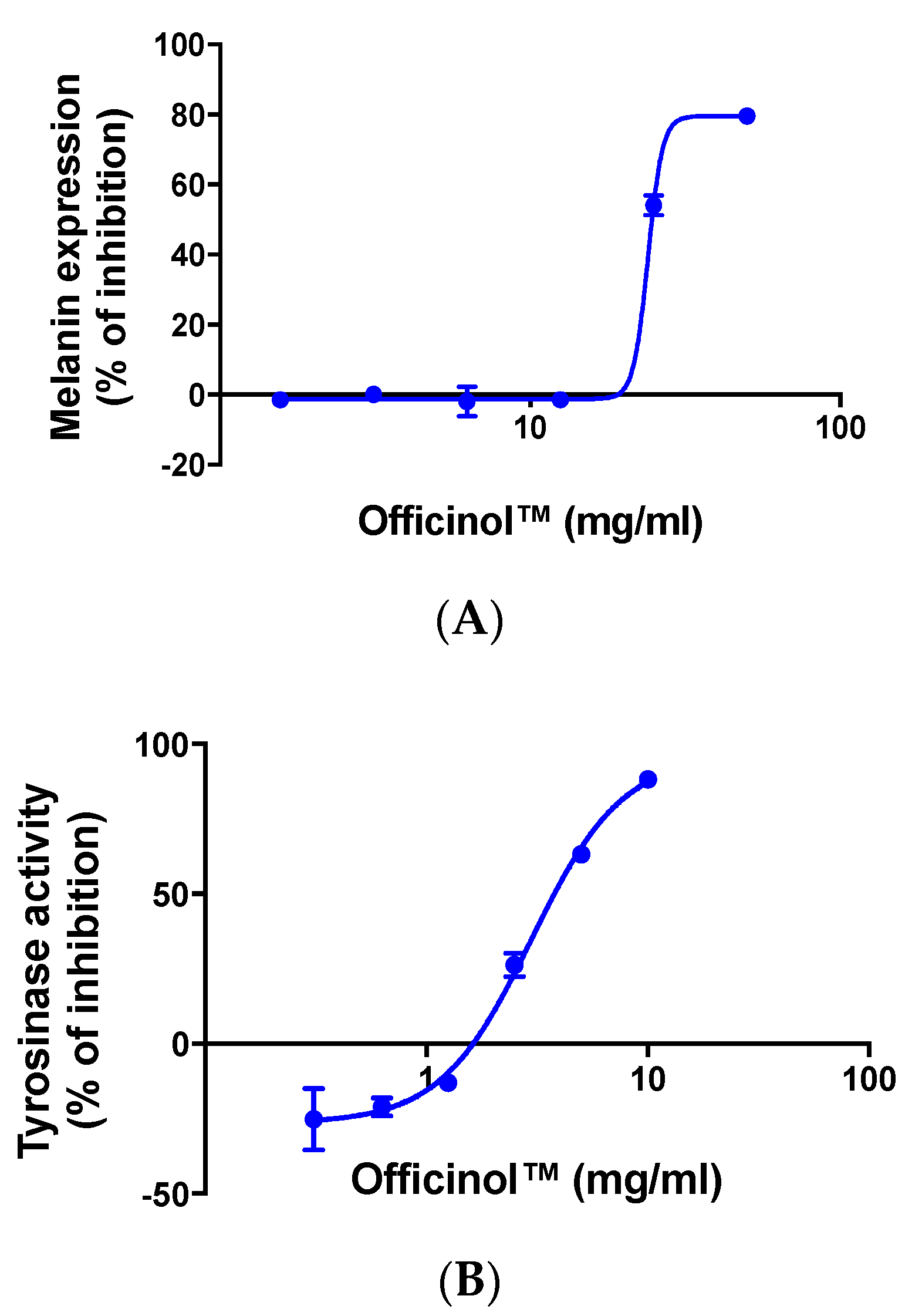
We monitored the effect of Officinol™ on melanin production in human melanocytes MNT-1. Results in Figure 4A show that treating the cells with Officinol™ effectively inhibited melanin pigmentation in human melanocytes in a concentration-dependent manner with an IC50 of 23.98 mg/mL.
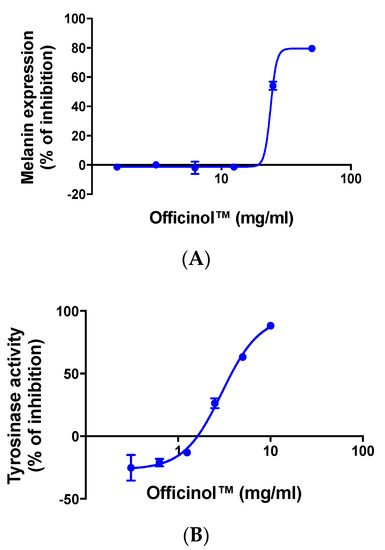
Figure 4.
Officinol™ mitigates melanin pigmentation and tyrosinase activity. (A) Concentration-dependent inhibition effect of Officinol™ on melanin pigmentation in human melanocytes MNT-1 cells. Graph on a logarithmic scale. (B) Concentration-dependent inhibition effect of Officinol™ on enzymatic tyrosinase activity. Graph on a logarithmic scale.
In the melanin biosynthesis process, tyrosinase is the key enzyme that catalyzes the first step of melanogenesis. Inhibition of tyrosinase effectively deters melanin pigmentation. We further evaluated the effect of Officinol™ on tyrosinase activity, and observed a dose-dependent inhibition effect with an IC50 of 3.62 mg/mL (Figure 4B).
3.5. Officinol™ Inhibits Elastase, a Cosmetic Target for Wrinkle Reduction
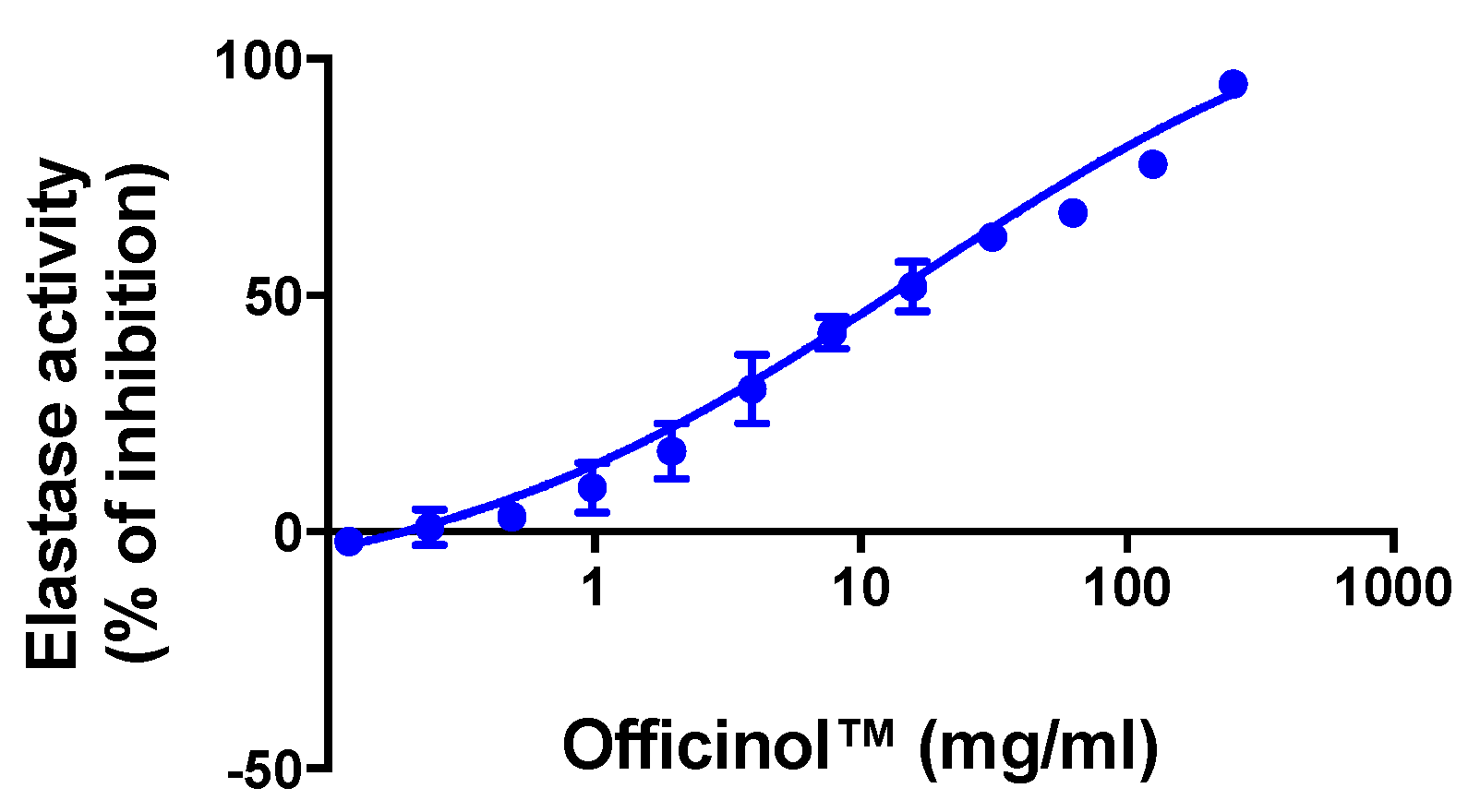
Wrinkle formation in the skin is accompanied by decrease in skin elasticity and the curling of elastic fibers such as elastin in the dermis. Elastase inhibitors suppress elastase activity and prevent the damage of dermal elastin, thus helping mitigate wrinkle formation. Our study indicates that Officinol™ effectively inhibited elastase activity with an IC50 of 14.38 mg/mL (Figure 5).
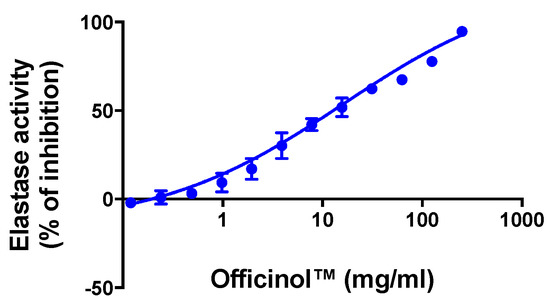
Figure 5.
Concentration-dependent inhibition effect of Officinol™ on enzymatic elastase activity, a cosmetic target for wrinkle reduction. Graph on a logarithmic scale.
Results from all above in vitro studies are summarized in Table 3 below:

Table 3.
Effect of Officinol™ vs. performance standards on biomarkers of aging and skin condition.
3.6. Clinical Study
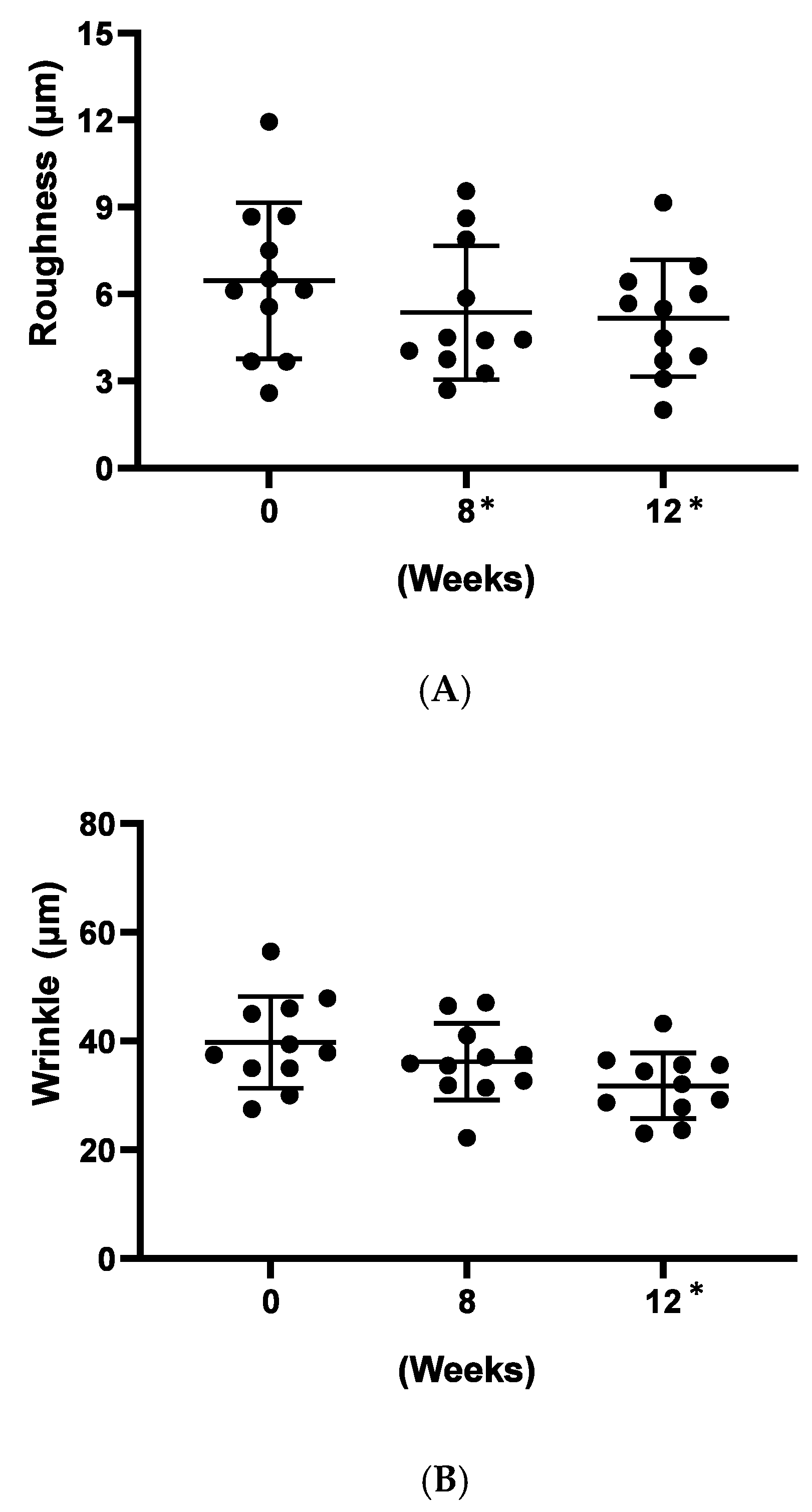
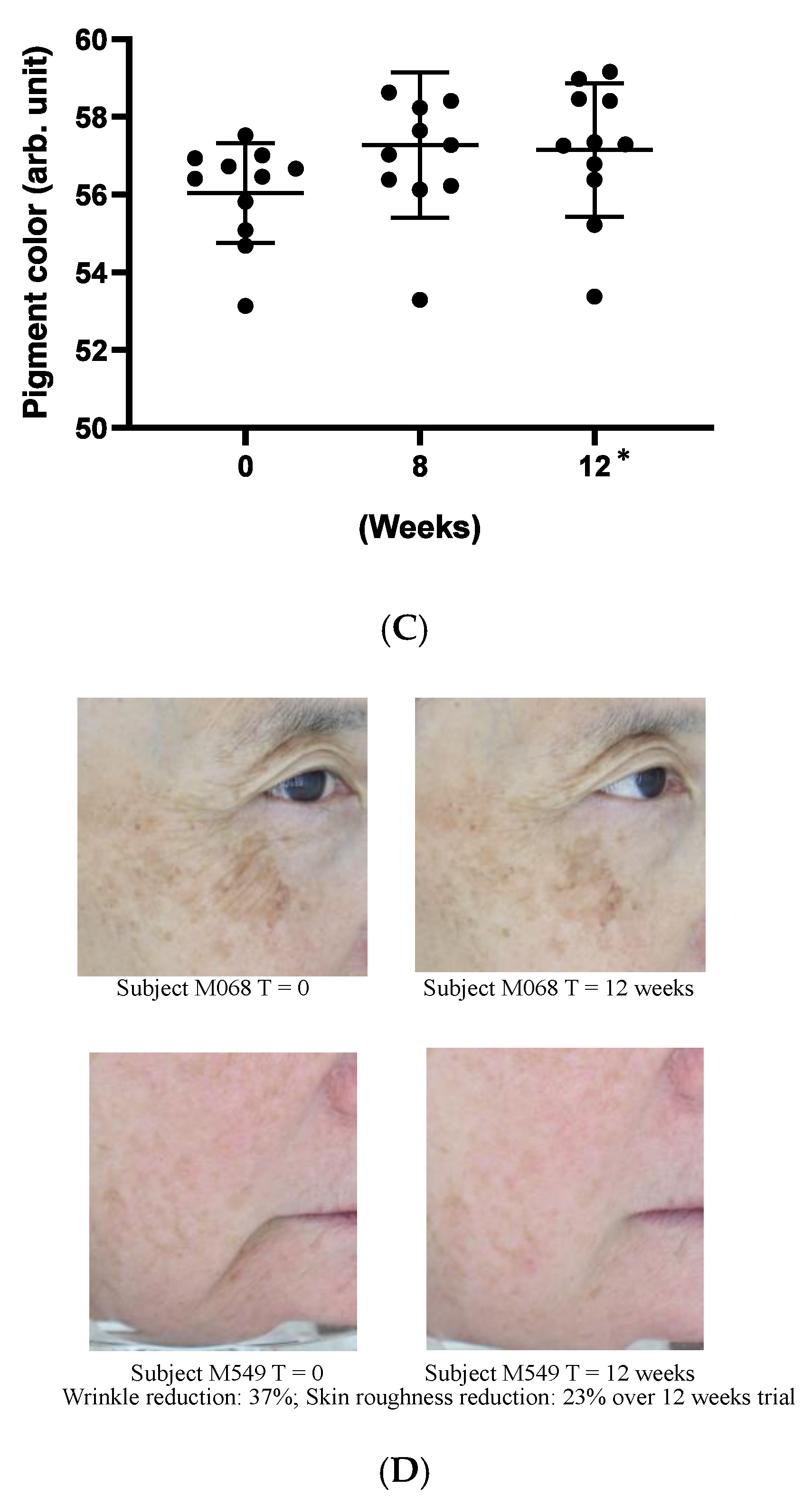
As a preliminary confirmation of these functions on clinic level, we carried out a small, pilot human trial where study subjects applied a cosmetic gel formulated with 5% (w/w) Officinol™ on facial skins for over 12 weeks. Biophysical instrumental measurements focused on the parameters normally considered for anti-aging effects were carried out to monitor the function of the Officinol™ cosmetic gel. 10 test subjects completed the study. The values of the skin measurements for each participant at Time 0 were subtracted from the values measured at various time points to reveal the true effect of the extract. The study results indicated that skin roughness, measured as Ra was reduced by 17% at 8 weeks (p < 0.05) and 20% at 12 weeks (p < 0.05). Wrinkle depth, measured as Ry, was reduced 20% as 12 weeks (p < 0.05). The pigmented area of sun spots measured on the L*a*b* color space, was reduced by 2% at 12 weeks (p < 0.05) of the use of Officinol™ cosmetic gel. Examples of the improvements in skin care conditions captured by digital photographs are shown in Figure 6D. A clinical study with a larger group is to be performed to confirm these preliminary findings.
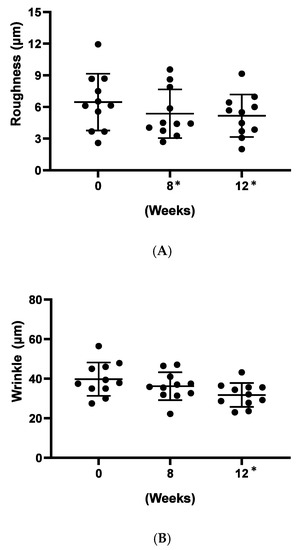
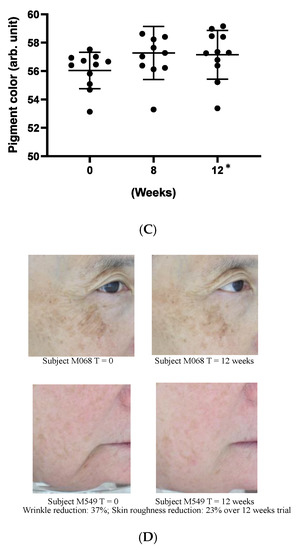
Figure 6.
Effect of Officinol™ cosmetic gel on human skins after up to 12 weeks of topical application. Top and bottom bars on each graph represent the standard deviation of the skin condition measurements of all the subjects studied at a specific time point. (A) skin roughness (Ra) was reduced by 17% at 8 weeks (p < 0.05) and 20% at 12 weeks (p < 0.05). (B) wrinkle depth (Ry) was reduced 20% at 12 weeks (p < 0.05). (C) Pigment color scale: white = 100, black = 0. The pigmented area of sun spots (L*a*b* color space) was reduced by 2% at 12 weeks (p < 0.05). (D) Examples of the improvements in skin care conditions captured by digital photographs. * Denotes the weeks when the data collected had statistically significant variance from those obtained at time point 0.
4. Discussion
4.1. Officinol™ Provides Anti-Aging Function, Possibly through Activation of Telomerase Pathways
Aging is complex biological process, affected by both heritage and environment, that serves as one of the major causes for skin deterioration such as wrinkles, skin pigmentation (aging spots), and other skin disorders. Volumes of studies reported the use of dietary polyphenols to mitigate age-associated cellular damage induced by metabolic production of ROS [13], and its use as active ingredients in cosmetics [14]. Although specific functioning mechanisms are not clear, some studies attributed the anti-aging and skin care functions of polyphenols to its aromatic, conjugated phenol ring structure which prevents and scavenges the formation of ROS. Some studies had also explored the potential impact of polyphenols in aging biomarkers such as telomere length and SIRT1 (Sirtuin 1: silent mating type information regulation 2 homolog) [15,16].
Discovery of rich phytochemicals in sugarcane plants has triggered the exploration of sugarcane plants as a therapeutic agent in a number of health concern fields including diabetic management, inflammation modulation, and neurological health. To collect the non-sugar phytochemicals effectively from sugarcane, we applied a patented hydrophobic extraction process to obtain Officinol™, a polyphenol-rich concentrate containing 26 mg/g gallic acid equivalency (GAE) of total phenol (Table 1), and explored its anti-aging properties from telomerase activation pathways. Telomerase is a ribonucleoprotein complex that adds a telomere repeating sequence to the 3’ end of telomeres and contributes to the maintenance of telomeric structure and length. The relationship between activation of telomerase, telomere length stabilization, and the extension of the life span of the human cell has been reported, making telomerase activity a biomarker for anti-aging. Our studies show that Officinol™ significantly enhanced cellular telomerase activity. A five-fold telomerase activation was obtained by treating the human HEK 293T cells with 2.94 mg/mL of Officinol™ (Figure 1A), comparable to the activation achieved by treating the cells with 0.39 μg/mL (0.79 μM) of cycloastragen, a known potent telomerase activator (Table 2).
One consideration in comparing the activation effect of above substances is that Officinol™ is a complex botanic extract, unlike its comparison material cycloastragen that is in a pure form (>90% purity). As a botanic plant extract, Officinol™ by nature contains various polyphenol components that function independently or synergistically or contradictorily. Besides these potential bioactives, Officinol™ contains other components such as metals and minerals that may not contribute to the specific activation effect being studied. Presumably, pure compounds such as cycloastragen are more concentrated and therefor more potent. This explains the ~10-fold difference in the activation potency between Officinol™ and cycloastragen observed in the above study. This consideration is also applicable when interpreting the rest of the study findings of this work.
The high level of polyphenols in the concentrate correlated with the strong antioxidant capacity of Officinol™, which exhibited a full spectrum oxidant absorption capacity against six primary radicals (peroxyl radical, hydroxyl radical, superoxide anion, singlet oxygen, peroxynitrite, and hypochlorite), with a total antioxidant capacity (ORAC 6.0) assessed at 1668.27 μmole trolox equivalency (TE)/g (shown in Table 1) using methods described previously (6). As exhibited in Table 4, the antioxidant capacity of Officinol™ is at an order of 2–10 magnitudes higher than that of raw fruits or juices, even dry fruits and cocoa powder that are known to contain high levels of antioxidants.

Table 4.
Polyphenol content and antioxidant activity of Officinol™ vs. other natural fruits and nuts *.
Based on established in vitro models for aging biomarkers, our findings demonstrated the potential of Officinol™ in age-deterring function. These findings substantiated the following studies on the therapeutical effect of Officinol™ in aging-associated skin disorders such as wrinkle production and age-spots.
4.2. Officinol™ Protects Human Skin Cells from UV Irradiation, with MMP-1 Inhibition One of the Potential Pathways
Collagen, the most abundant protein in skin which makes up 75–80% of the organ, is the key protein that gives skin its fullness and plumpness. As humans age, collagen synthesis in human tissue reduces, while collagen breakdown catalyzed by collagenase, a key collagen-degrading enzyme also called matrix metalloprotease-1 (MMP-1), speeds up and overwhelms the collagen synthesis process. Environmental stress such as UV exposure stimulates the production of collagenase, further exasperates the collagen degradation. Direct inhibition of collagenase by plant compounds has been discovered an effective approach to mitigate collagen breakdown and skin aging [17].
We monitored the protective effect of Officinol™ on human skin cells that were exposed to a pathogenically high level of UV irradiation. Our findings indicated that at 50 mJ/cm2 UV irradiation, MMP-1 production in human skin cells increased significantly, to a level of nearly 4-fold higher under our assay system (Figure 2A). This finding supported previous studies demonstrated that UV-irradiated epidermal keratinocytes released cytokine, which indirectly induce MMP-1 production [18]. Treatment with Officinol™ effectively inhibits MMP-1 in a dose-dependent manner with an IC50 of 1.05 mg/mL, comparing to IC50 of 1.23 μg/mL achieved by avobenzone, a commercially available UV blocker in skin care (Figure 2B). This finding resonates with a previous study based on mouse models where Katyer et al. reported the use of a (–)-epigallocatechin-3-gallate, a polyphenol extracted from green tea, to inhibit UV radiation-induced oxidative stress in skin, a known trigger of the activation of transcription factor NFκB that regulates cytokine production [19].
4.3. Officinol™ Inhibits Melanin Pigmentation, Possibly through Tyrosinase Inhibition as a Pathway
Melanin production by melanocytes is one of the key photoprotective functions of human skin to mitigate ultraviolet radiation. However, over production of melanin is also a major consequence of UV damage and aging process that induces pigmentation disorders such as freckles and senile lentigo (i.e., age spots), more severely, skin disease such as melanoma. Excessive melanin has also been viewed as melanoma precursor. Melanin inhibition is a desirable effect sought in various fronts of cosmetic industry not only to achieve skin whitening and improved aging appearance, but also to prevent melanoma. We monitored the effect of Officinol™ on melanin production in human melanocytes. Treating the cells with Officinol™ effectively inhibited melanin pigmentation in human melanocytes in a concentration-dependent manner with an IC50 of 23.98 mg/mL (Figure 3A), comparing to IC50 of 0.46 μg/mL achieved by chloroquine, a commercial drug for melanoma treatment.
To further understand the possible function pathway of Officinol™’s melanin inhibtion effect, we studied the effect of Officinol™ on tyrosinase, the key enzyme that catalyzes the first step also a rate-limiting step of melanogenesis, and observed a dose-dependent inhibition effect with an IC50 of 3.62 mg/mL (Figure 3B). Kojic acid, a widely used skin whitening agent [20], also exhibited a strong concentration-dependent inhibition effect with an IC50 at 32.19 μg/mL (226.50 μM, Table 2). Substantial studies have shown that melanin reduction and skin-whitening can be achieved, at least partially, by deactivating of tyrosinase. Tyrosinase inhibitors have become increasingly important in cosmetic and medicinal products used in the prevention of hyperpigmentation [21]. Various synthetic compounds, natural extracts and active ingredients from plants, bacteria, and fungi have been reported to be effective tyrosinase inhibitors. A major group of these tyrosinase inhibitors are simple phenols such as hydroquinone [22] and plant polyphenols. More than 100 natural plants have been reported to contain bioactives for tyrosinase inhibition [23], however, the use of sugarcane plants in tyrosinase inhibition has not been studied extensively. In 2011, via preliminary in vitro studies, Chung Y.M. et al. reported the findings of antioxidant and tyrosinase inhibitory constituents from a desugared sugar cane extract [24]. Our results agreed with previous findings, suggesting the use of Officinol™ to control melanin pigmentation presumably via tyrosinase inhibition.
4.4. Officinol™ Inhibits Elastase, a Biomarker for Wrinkle Formation
Aging, along with habitual and environmental factors such as smoking and sun exposure, contributes to the damage and loss of elastin that supplies skin elasticity and rebound. As a result, skin becomes thinner, wrinkles, and roughness appear. Inhibition of elastase, a key enzyme that catalyzes the degradation of elastin, is an approach widely used in skin care to mitigate the loss of elastin and the formation of wrinkles [25]. In a 2018 study, 100 plant extracts were screened via an enzymatic assay to search for natural elastase inhibitors. The study identified 17 extracts inhibited elastase and/or tyrosinase, and the enzyme inhibition capability of the extracts positively correlates with their phenolic and flavonoid content [26].
Based on a similar enzymatic assay, we demonstrated that Officinol™ was able to inhibit elastase activity in a concentration-dependent manner with an IC50 of 14.38 mg/mL (Figure 4). N-methoxysuccinyl-Ala-Ala-Pro-Val-chloromethyl ketone, a cell permeable inhibitor of human leukocyte elastase, showed strong inhibition effect in the same assay system with an IC50 of 0.42 μg/mL (0.83 μM, Table 2). We attribute the elastase inhibition effect of Officinol™ to its rich phenolic content, as discussed in previous reports that the elastase and/or tyrosinase inhibition capability of herbal extracts positively correlates with their phenolic content (26).
4.5. Preliminary Validation of Officinol™ Skin Care Function through a Clinical Pre-Study
Results from all above in vitro studies are summarized in Table 2. These cellular investigations indicated that on cellular level Officinol™ provides anti-aging and protective functions for human skins against from UV irradiation, skin pigmentation/age-spot, and wrinkle formation. As a preliminary confirmation of these functions on clinic level, we carried out a small, pilot human trial where 10 study subjects applied a cosmetic gel formulated with 5% Officinol™ on facial skins for 12 weeks. The study results in Figure 5 indicated that both skin roughness (measured as Ra) and wrinkle depth (measured as Ry) showed significant reduction of 20% at 12 weeks (p < 0.05). The pigmented area of sun spots measured on the L*a*b* color space, was reduced by 2% at 8 weeks of the use of Officinol™ cosmetic gel (p < 0.05). This is in line with other actives tested under the same conditions—typical values being around 1% per month. Preliminary clinical studies supported the findings from in vitro studies that Officinol™ cosmetic gel has potential to improve skin roughness, and reduce wrinkle depth and age spots. A clinical study with a larger group is to be performed to confirm these preliminary findings.
5. Conclusions
The sugarcane concentrate, Officinol™, exhibits significant anti-aging properties, potentially through telomerase activation pathway. Further studies in human keratinocytes indicated that Officinol™ protects human keratinocytes from UV irradiation by inhibiting MMP-1 as a possible pathway, inhibits melanin pigmentation in the skin cells, possibly through tyrosinase inhibition, and inhibits elastase, a key biomarker for wrinkle production. A 10-person clinical study has also been carried out to investigate the effect of Officinol™ on human skins. The human trial demonstrated that Officinol™ formulated cosmetic gel provided significant improvements in skin roughness, wrinkle depth, and skin pigmentation of the test subjects.
Our findings are also in accordance with previous reports on the use of polyphenols extracted from plants to improve skin appearance including skin pigmentation and wrinkle reduction [27]. One consideration in applying natural plant extracts is the possible synergistic or competing effects from various components in the extract. In a review article, Scheepens et al. discussed in depth designed synergies to improve oral bioavailability as well as efficacy of beneficial polyphenols. In this current study, we reported the combined effect from Officinol™, a mixture plant extract concentrate. While we have completed preliminary phytochemical profiling work to support the possible bioactives, we are carrying out further composition profiling and characterization studies to provide more insight on the key bioactive(s) in Officinol™.
We recognize that current study findings are based on human cells and a small human pre-study, which is still a distance away towards the translation into clinical success. Regardless, our work encourages further in vitro and in vivo studies to verify these potential therapeutic effects, and explore signal pathway in more depth. In our laboratory, a clinical study of minimum 20 subjects are being planned to further verify the study findings above. The success of these follow-up studies would provide an opportunity of discovering the skin care benefits of the bioactives extracted from sugar production, resulting in a new active skin care component based on a globally abundant crop.
Author Contributions
Conceptualization, B.K. and J.J.; Data Curation, X.Y., Z.P.S., and B.K.; Writing—Original Draft Preparation, J.J. and Z.P.S.; Writing—Review and Editing, M.F., J.N., M.-L.B., and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by The Product Makers, Pty Ltd., Melbourne, Australia.
Acknowledgments
The authors thank Brunswick Laboratories for conducting in vitro studies and Dermatest Pty Ltd./Eurofins Scientific for conducting clinical study.
Conflicts of Interest
Xin Yang, Matthew Flavel, Julian Neoh, Mae-Ling Bowen, and Barry Kitchen are employees of The Product Maker Pty Ltd., Melbourne, Australia, the producer of Officinol™.
References
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R.; Farzei, M.H. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Baxter, R. Anti-aging properties of resveratrol: Review and report of a potent new antioxidant skin care formulation. J. Cosmet. Dermatol. 2008, 7, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.; Dickinson, D.; Borke, J.; Walsh, U.S.; Wood, J.; Qin, H.; Winger, J.; Pearl, H.; Schuster, G.; Bollag, W.B. Green tea polyphenol induces caspase 14 in epidermal keratinocytes via MAPK pathways and reduces psoriasiform lesions in the flaky skin mouse model. Exp. Dermatol. 2007, 16, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Lal, U.; Mukhtar, H.M.; Singh, P.S.; Shah, G.; Dhawan, R.K. Phytochemical profile of sugarcane and its potential health aspects. Pharmacogn. Rev. 2015, 9, 45–54. [Google Scholar] [CrossRef]
- Duarte-Almeida, J.; Novoa, A.V.; Linares, A.F.; Lajolo, F.M.; Genovese, M.I. Antioxidant Activity of Phenolics Compounds From Sugar Cane (Saccharum officinarum L.) Juice. Plant Foods Hum. Nutr. 2006, 61, 187–192. [Google Scholar] [CrossRef]
- Ji, J.; Yang, X.; Flavel, M.; Shields, Z.P.-I.; Kitchen, B. Antioxidant and Anti-Diabetic Functions of a Polyphenol-Rich Sugarcane Extract. J. Am. Coll. Nutr. 2019, 38, 670–680. [Google Scholar] [CrossRef]
- Ji, J.; Flavel, M.; Yang, X.; Chen, O.C.; Downey, L.; Stough, C.; Kitchen, B. A polyphenol rich sugarcane extract as a modulator for inflammation and neurological disorders. PharmaNutrition 2020, 12, 100187. [Google Scholar] [CrossRef]
- Polychronopoulou, S.; Koutroumba, P. Telomere Length and Telomerase Activity: Variations With Advancing Age and Potential Role in Childhood Malignancies. J. Pediatric Hematol. 2004, 26, 342–350. [Google Scholar] [CrossRef]
- Fajkus, J. Detection of telomerase activity by the TRAP assay and its variants and alternatives. Clin. Chim. Acta 2006, 371, 25–31. [Google Scholar] [CrossRef]
- Mender, I.; Shay, J.W. Telomerase Repeated Amplification Protocol (TRAP). Bio Protoc. 2015, 5, e1657. [Google Scholar] [CrossRef]
- Fujii, T.; Saito, M. Inhibitory effect of quercetin isolated from rose hip (Rosa canina L.) against melanogenesis by mouse melanoma cells. Biosci. Biotechnol. Biochem. 2009, 73, 1989–1993. [Google Scholar] [CrossRef] [PubMed]
- Deseo, M.A.; Elkins, A.; Rochfort, S.; Kitchen, B. Antioxidant Activity and Polyphenol Composition of Sugarcane Molasses Extract. Food Chem. 2020, 314, 126180. [Google Scholar] [CrossRef]
- Queen, B.L.; Tollefsbol, T.O. Polyphenols and aging. Curr. Aging Sci. 2010, 3, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Eisner, P.; Kerscher, M.; Schweiggert-Weisz, U. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, V.; Paolisso, G.; Mecocci, P. Nutrition and lifestyle in healthy aging: The telomerase challenge. Aging 2016, 8, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Allard, J.S.; Perez, E.; Zou, S.; De Cabo, R. Dietary activators of Sirt1. Mol. Cell. Endocrinol. 2008, 299, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Wang, X.; Bi, Z.; Chu, W.; Wan, Y. IL-1 receptor antagonist attenuates MAP kinase/AP-1 activation and MMP1 expression in UVA-irradiated human fibroblasts induced by culture medium from UVB-irradiated human skin keratinocytes. Int. J. Mol. Med. 2005, 16, 1117–1124. [Google Scholar] [CrossRef]
- Katiyar, S.K.; Afaq, F.; Perez, A.; Mukhtar, H. Green tea polyphenol (–)-epigallocatechin-3-gallate treatment of human skin inhibits ultraviolet radiation-induced oxidative stress. Carcinogenesis 2001, 22, 287–294. [Google Scholar] [CrossRef]
- Saeedi, M.; Eslamifar, M.; Khezri, K. Kojic acid applications in cosmetic and pharmaceutical preparations. Biomed. Pharmacother. 2019, 110, 582–593. [Google Scholar] [CrossRef]
- Chang, T.-S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, K.; Ogawa, M.; Sugibayashi, K.; Yamada, K.-I.; Yamamoto, K. Relationship between tyrosinase inhibitory action and oxidation-reduction potential of cosmetic whitening ingredients and phenol derivatives. Arch. Pharmacal Res. 1999, 22, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Khan, M.T.H.; Muñoz-Muñoz, J.L.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-M.; Wang, H.; El-Shazly, M.; Leu, Y.-L.; Cheng, M.-C.; Lee, C.-L.; Chang, F.-R.; Wu, Y.-C. Antioxidant and Tyrosinase Inhibitory Constituents from a Desugared Sugar Cane Extract, a Byproduct of Sugar Production. J. Agric. Food Chem. 2011, 59, 9219–9225. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The Role of Elastases Secreted by Fibroblasts in Wrinkle Formation: Implication Through Selective Inhibition of Elastase Activity. Photochem. Photobiol. 2001, 74, 283–290. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crop. Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Saraf, S.; Kaur, C.D. Phytoconstituents as photoprotective novel cosmetic formulations. Pharmacogn. Rev. 2010, 4, 1–11. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).