Abstract
This study introduces a novel oxygenating facial mask enriched with hemp seed extract, which uniquely combines advanced bubble-generating technology with botanically derived antioxidants for enhanced skin care. The innovative mask forms microbubbles that simulate targeted oxygen delivery, accelerating cell renewal and improving active ingredient absorption. In a randomized, controlled trial, forty participants used either the hemp seed extract mask (F1) or a placebo (F2) over eight weeks. Both formulations demonstrated excellent physical stability for 60 days, maintaining consistent pH, color, fragrance, viscosity, and foaming properties. Notably, F1 demonstrated superior foam persistence and product stability. Clinically, the hemp mask significantly increased skin hydration (up to 65.7%, p < 0.05), reduced sebum levels (32.9%), and lowered erythema (up to 46.9 AU or 12.9%, p < 0.01), without altering skin color or causing adverse effects. Consumer satisfaction with F1 exceeded F2 by 10.7%. The novelty of this work lies in the integration of oxygenating bubble technology and hemp seed extract—demonstrating synergistic effects on skin barrier function, hydration, sebum control, and erythema reduction. These findings highlight the mask’s potential as a next-generation cosmeceutical with meaningful clinical and commercial value.
1. Introduction
Skin hydration primarily depends on the architecture of the stratum corneum and the distribution of water-binding natural moisturizing factors (NMF) within corneocytes, together with the organization of intercellular lipids that create an effective barrier to transepidermal water loss (TEWL). Humectants such as glycerol, alongside ceramides, contribute to water retention and optimal barrier function. Disruption in these mechanisms—through lipid deficits or impaired NMF—leads to reduced hydration and visible dryness [1]. Sebum is produced in sebaceous glands located within the dermis, made up of a complex mix of triglycerides, wax esters, free fatty acids, and squalene. Sebum traverses the hair follicle canals to the skin surface, sealing in moisture and defending against microbial threats. Androgenic stimulation and dysregulation of 5α-reductase activity commonly lead to excessive sebum production, contributing to oily skin and acne. Imbalanced sebum synthesis not only affects the skin’s hydration but also modulates immune responses and increases inflammation in the skin layers [2].
Facial masks are a crucial element of contemporary skincare treatments, functioning as effective carriers for bioactive ingredients that enhance skin health, rejuvenate radiance, and maintain optimal hydration levels [3]. These topical treatments facilitate the penetration of essential nutrients into the dermal layers, addressing many skin issues, including aging, environmental damage, and inflammatory conditions. Advanced skincare treatments are crucial due to the adverse impacts of aging, environmental pollutants, and UV radiation on skin health. Recent advancements in cosmetic science have recognized oxygenating masks as an innovative method for facial treatment. These formulations provide visible bubbling effects upon application, stimulating oxygen delivery to the skin while facilitating cellular renewal and detoxification processes. Oxygenating masks significantly enhance the skin’s brightness, promote the removal of pollutants and dead skin cells, reduce the appearance of visible pores, prevent the development of acne, and maintain skin hydration [4]. It facilitates greater oxygen delivery from the microcirculation to skin cells. This enhanced oxygenation promotes cellular repair, accelerates skin renewal and exfoliation, boosts tissue hydration, and visibly reduces wrinkles and pore size [5]. Additionally, increased oxygen availability stimulates collagen synthesis and neocollagenesis, contributing to improved skin elasticity and a more youthful appearance.
The addition of botanical extracts and bioactive substances, combined with oxygenating technology, enhances the therapeutic effectiveness of these formulations [6]. The findings indicate that the hemp seed extract oxygenating mask exhibits established efficacy, substantial stability, and advanced skin-rejuvenating technology, making it a promising candidate for future development as a premium skincare product.
The regulatory endorsement of cannabis-derived compounds for cosmetic applications in the skincare industry has unveiled novel opportunities for product development. Extracts of Cannabis sativa L., cannabidiol (CBD), and hemp seed oil provide considerable therapeutic advantages for dermatological purposes [7]. CBD exhibits significant anti-inflammatory and antioxidant effects [8,9], effectively mitigating photodamage, promoting wound healing, and reducing the appearance of hyperpigmentation and erythema. The effects mediated by interactions with the cannabinoid will preserve skin health. Hemp seed oil, derived from the seeds of Cannabis sativa L., contains very high concentrations of essential fatty acids for skin health [10,11]. Linoleic acid (omega-6 fatty acid) contributes 54–60% of the oil’s composition, providing significant benefits, including enhanced skin barrier function, diminished transepidermal water loss, improved cellular moisture retention, and powerful antioxidant properties that prevent cellular damage and skin aging. Alpha-linolenic acid (omega-3 fatty acid), comprising 15–20%, improves skin smoothness, diminishes wrinkles, enhances firmness and elasticity, increases hydration, prevents acne, and offers protection against UV damage and environmental stressors [12].
Additionally, the regulatory framework regulating cannabis-derived cosmetic ingredients defines the limits for tetrahydrocannabinol (THC) across various application categories, thereby ensuring product safety and compliance [13]. Current evidence suggests that CBD possesses anti-inflammatory, antioxidant, moisturizing, anti-acne, wound-healing, and anti-aging properties, making it a desirable ingredient for cosmetic formulations [14].
The transparent, stable gel formulation of the hemp seed oil-based oxygen face mask was chosen as the optimal delivery platform because such masks ensure maximal localized contact, prolonged exposure time, and efficient transfer of bioactives compared to conventional leave-on or rinse-off preparations. This reasoning supports the creation and clinical testing of a hemp seed extract-enriched oxygenating facial mask as a next-generation option for better skin hydration, oil control, and soothing effects in modern skincare.
Although oxygenating facial masks and hemp-derived ingredients are both emerging trends in modern skincare, current research predominantly focuses on their separate benefits. Currently, no clinical investigations have evaluated the synergistic efficacy of integrating oxygenating bubble technology with hemp seed extract in a single topical formulation. Furthermore, there is a deficiency of objective, instrument-based evaluations of skin barrier function, erythema, and sebum, as well as a structured assessment of consumer satisfaction for these advanced cosmeceutical products. This study uniquely addresses these gaps by clinically evaluating a novel facial mask that combines these two technologies, providing the first comprehensive evidence of their combined dermatological effects in humans.
2. Materials and Methods
2.1. Materials
Hemp seed oil was purchased from JKKN Supply and Services Co., Ltd. (Phitsanulok, Thailand). According to the certificate of analysis, the oil contained 55.93% linolenic acid (omega-3), 15.42% linoleic acid (omega-6), and 71.84% polyunsaturated fatty acids, with total THC content lower than 0.2% w/w. Other ingredients included distilled water, sodium EDTA, Carbomer Ultrez 21, 1,3-propanediol, Plantacare 810, Polyglyceryl-6 Caprylate, Cocamidopropyl Betaine, cationic hyaluronic acid (HyaCoat™), Reservoir-Tech, niacinamide, phenoxyethanol, fragrance, triethanolamine (TEA), and Gransil SiW-7100 as a bubble generator. All chemicals were of cosmetic grade.
2.2. Development of Hemp Oxygenating Facial Masks
Two transparent self-foaming gel mask formulations were created: F1, an oxygenating mask containing hemp seed, and F2, a placebo oxygenating mask devoid of hemp seed oil. Both formulations possessed similar base compositions and differed only in the presence or absence of hempseed oil in the active phase. The detailed qualitative and quantitative composition of each formulation is enclosed in Table 1, expressed as a percentage by weight (g per 100 g of finished product).

Table 1.
Summarizes the composition, percentage by weight (% w/w), and functional role of each ingredient in F1 and F2.
2.2.1. Equipment and Processing Conditions
All batches were prepared in a stainless steel main vessel equipped with a homogenizer (IKA T25 D, IKA-Werke GmbH & Co. KG, Staufen im Breisgau, Germany). Processing was performed at ambient temperature (25 ± 2 °C) under atmospheric pressure. The mixing speeds were controlled within the ranges of 300–500 rpm for low-shear stirring and 1500–3000 rpm for high-shear homogenization, as specified in each step below. The pH of the formulations was monitored using a calibrated bench-top pH meter (ST3000, OHAUS, Parsippany, NJ, USA).
2.2.2. Preparation Protocol
The hemp oxygenating gel masks were prepared using a six-phase process (Phases A–F), as described below. The same procedure was applied to both F1 and F2; hemp seed oil was included only in F1.
- Phase A: Gel base preparation
Distilled water was weighed into the main vessel, and sodium EDTA and Carbomer Ultrez 21 were slowly sprinkled onto the vortex under overhead stirring at 400 rpm for 10 min to avoid clumping. The mixture was further homogenized with a high-shear mixer at 2500 rpm for 15 min until the carbomer was completely hydrated, resulting in a clear, homogenous gel base [15]. Subsequently, 1,3-propanediol was added under gentle stirring at 300 rpm for 5 min.
- Phase B: Surfactant blend incorporation
In a separate beaker, Plantacare 810, polyglyceryl-6 caprylate, and cocamidopropyl betaine [16] were premixed using a propeller stirrer at 500 rpm for 10 min to obtain a homogeneous surfactant blend. The premix (Phase B) was gradually mixed into Phase A for a period of 10 min while maintaining continuous stirring at 400 rpm to minimize air entrapment, followed by an additional 10 min of mixing at the same speed to ensure thorough dispersion.
- Phase C: Active ingredients and hemp seed oil (F1 only)
HyaCoat (cationic hyaluronic acid), Reservoir-Tech, and niacinamide were dispersed into the bulk gel (Phases A + B) under overhead stirring at 400 rpm for 15 min [17]. In F1, cold-pressed cosmetic-grade hemp seed oil was gradually added dropwise to the same vessel over a period of 10 min while stirring at 500 rpm, followed by homogenization at 2000 rpm for 5 min to achieve a delicate dispersion of oil droplets within the gel matrix. The concentration range of 0.80–1.20% hemp seed oil in F1 was selected according to industry standards and contemporary research, which consistently indicate this range as effective and safe for topical cosmetic applications. For F2, this step was performed without hemp seed oil, keeping all other conditions identical.
- Phase D: Preservative and fragrance addition
Phenoxyethanol and fragrance were premixed in a small beaker and then added to the main batch with gentle stirring at 300 rpm. The fragrance level was adjusted “quantum satis” to achieve the desired olfactory profile without affecting the physical stability of the gel [18].
- Phase E: Neutralization and pH adjustment
Triethanolamine (Phase E) was added dropwise to the bulk formulation under continuous stirring at 300 rpm [19]. The pH was monitored after each addition until it reached the range of 5.5–6.0, which is suitable for facial skin compatibility. After reaching the target pH, the batch was mixed for an additional 15 min at 400 rpm to allow complete carbomer neutralization and uniform viscosity development.
- Phase F: Incorporation of the oxygenating silicone elastomer gel
Gransil SiW-7100 was slowly incorporated as the final phase. The silicone elastomer gel was gradually mixed into the neutralized gel base while stirring at 300 rpm for 20 min, followed by a brief high-shear homogenization at 1500 rpm for 5 min to achieve a smooth, self-foaming gel consistency. This step enables the formation of an oxygenating bubble network that appears upon application to moist skin, as recommended by the supplier’s formulation guidelines for oxygenating gel masks.
The finished formulation is removed of any remaining air, sealed in vacuum-sealed glass bottles, and stored at 25 ± 2 °C for further stability and efficacy assessments.
2.3. Physical Property Characterization and Stability Assessment
The physicochemical properties of both formulations were thoroughly assessed over a 60-day accelerated stability period under controlled storage conditions (25 ± 2 °C, 60 ± 5% relative humidity) in accordance with ICH guidelines [20]. pH measurements were obtained every 48 h using a calibrated digital pH meter (ST3000, OHAUS, Parsippany, NJ, USA) with an accuracy of ±0.01 pH units, following standardization with buffer solutions at pH 4.00 and 7.00 [21]. The target pH range was maintained between 5.5 and 6.0 to ensure compatibility with skin physiological conditions [22].
Foam characterization analysis was performed utilizing a standardized protocol derived from recognized cosmetic testing methodologies. Precisely 1.5 g of each formulation was uniformly distributed on a 10 × 10 cm2 artificial leather substrate (treated with synthetic sebum). The foam formation time (T1) was defined as the period necessary for achieving maximum foam volume, whereas the foam disappearance time (T2) indicated the duration until the foam vanishes.
2.4. Clinical Test of the Oxygenating Mask’s Efficiency
2.4.1. Ethical Approval and Subject Recruitment
The study received ethical approval and recruited participants. The study was conducted in accordance with the Declaration of Helsinki and received approval from the Institutional Review Board of Phranakhon Rajabhat University (Approval Protocol No. 02.018/67). Forty-three healthy male and female volunteers aged 18 years or older were enrolled after providing written informed consent. All study participants in this work were Thai nationals of Thai ethnicity.
The study design and randomization were executed. A randomized, single-blind, placebo-controlled, parallel study was conducted over eight weeks [23]. Participants were categorized by age and skin type, then randomized (1:1) into a treatment group that received the hemp-seed-oil oxygen mask (Formula 1) and a control group that received the placebo base (Formula 2).
- Inclusion criteria: Forty-three healthy male and female volunteers, each aged 18 years or older, did not have skin diseases or abnormalities such as rashes, erythema, or eczema, were not pregnant, and still had to breastfeed. All volunteers were informed of the test objectives and procedures; for example, during the test, volunteers must avoid the risk of using other facial products, avoid working outdoors for a long time, and be aware of the potential side effects. The volunteers must pass allergy and irritation tests as mentioned in Section 2.4.2 before participating in the treatment with the developed products for two months. All subjects were required to provide informed consent before participating in the study. The investigators adhered to all criteria and procedures for submitting research applications on human subjects.
- Exclusion criteria: Participants had a history of corticosteroid or antihistamine usage within two weeks before the beginning of the study.
- Withdrawal criteria of the study: Subjects experienced an allergic reaction to the product during the test, became pregnant, or did not have time for the test.
- Termination of study criteria: subjects had an allergic reaction to the product.
The protocol for applying the product was followed: Volunteers applied the assigned formulation twice weekly under investigator supervision. Each application was left on the face for 15 min and rinsed with warm water.
2.4.2. Patch Test
The irritation and allergic reactions were evaluated using patch testing. The researchers recruited the volunteers and conducted the following. All volunteers were informed of the objectives, possible adverse effects, and test procedures, and were rewarded for their participation. The approved study code from the Ethics Committee of Phranakhon Rajabhat University, Bangkok, Thailand, Approval Protocol No. 02.018/67. All volunteers provided consent before entering the study. Volunteers had the right to withdraw from the test at any time without consequence or penalty.
Stepwise protocol:
- Ten drops of product were applied to a 5.5 × 6.5 cm2 area on the inner forearm.
- Covered with an occlusive patch for 30 min.
- Patch removed, and site observed for erythema or edema within 30 min post-exposure.
- Grading documented; negative replies addressed in accordance with ethical guidelines, including no irritation, redness, or edema.
2.4.3. Efficacy Evaluation Through Modern Non-Invasive Measurements of the Skin
The instrumentation for biophysical measurements and objective skin assessments was a Multi Probe Adapter MPA 580 system (Courage + Khazaka Electronic GmbH, Cologne, Germany). The Corneometer® CM 825, Skin-Glossy Meter® GL 200, and Mexameter® MX 18 probes (all from Courage + Khazaka Electronic GmbH, Cologne, Germany) were used for biophysical assessments. The Corneometer® CM 825 probe measured stratum corneum hydration via the electrical capacitance method, with readings expressed in arbitrary units (AU) ranging from 0 to 120. The Skin-Glossy Meter® GL 200 probe assessed skin surface gloss and optical properties by measuring specular reflection at a 60° angle. The Mexameter® MX 18 probe quantified melanin and erythema indices through narrow-band reflectance spectroscopy at wavelengths of 568 nm (erythema) and 660 nm (melanin) [24]. All measurements were conducted in triplicate at standardized face sites under controlled conditions (temperature 20–22 °C, relative humidity 50 ± 5%) after a 30 min acclimatization period.
Stepwise protocol:
- The volunteer acclimated in a controlled room (20–22 °C, 50 ± 5% RH) for 30 min.
- Skin was cleansed with a pH-neutral cleanser; no other skincare products were applied for 12 h.
- Each measurement (hydration, gloss/sebum, melanin/erythema) was performed in triplicate on both the right and left cheeks using:
- Corneometer CM 825
- Skin-Glossy Meter (GL 200)
- Mexameter MX 18 (MPA 580)
- Mean ± SD calculated for each parameter.
- Statistical significance was assessed with a paired t-test (SPSS Version 26).
Triplicate readings were obtained from the right cheek region using a non-invasive bioengineering approach at predetermined time intervals: baseline (Week 0, pre-treatment) and post-treatment assessments at Weeks 5, 6, 7, and 8. The same trained investigator performed all measurements.
2.5. Preference Test
A randomized, double-blind, controlled evaluation of consumer satisfaction was performed with 40 healthy volunteers, who evaluated two formulations: F1 (containing hemp extract) and F2 (control). Consumer satisfaction was evaluated utilizing a validated structured questionnaire rooted in recognized cosmetic evaluation methodologies [25], employing a 5-point Likert scale (1 = very dissatisfied, 5 = very satisfied) across six parameters: foaming sensation, skin cleansing efficacy, skin penetration capability, post-application moisturization, product fragrance, and overall satisfaction. Participants evaluated both products under controlled conditions using the same application protocols. The data were analyzed using paired t-tests to compare the formulations (p < 0.05). The formula for calculating the superiority percentage is Superiority = [(Mean F1 − Mean F2)/Mean F2] × 100. Positive values mean that the hemp extract formulation worked better than the control, and mean differences greater than 10% are considered commercially significant improvements in consumer perception, according to established satisfaction measurements. The study protocol obtained ethical approval, with informed consent from all participants, safety confirmed through preliminary compatibility testing, and adverse event monitoring conducted during the evaluation period, according to Good Clinical Practice guidelines for consumer research studies.
- Stepwise protocol:
- Conducted a double-blind study with n = 40 healthy volunteers.
- Each subject used both F1 and F2 formulas.
- Evaluation via a validated 5-point Likert scale questionnaire covering six aspects (e.g., foaming, cleansing, moisturization, satisfaction).
- Data analyzed for superiority and significance (p < 0.05).
2.6. Statistical Analysis
Statistical analyses were conducted utilizing SPSS software version 26 (IBM, Chicago, IL, USA). The data were presented as means ± standard deviation (SD). Statistical significance for comparisons between time points and groups was evaluated using the two-tailed paired samples t-test.
3. Results
3.1. Development of Hemp Oxygenating Facial Masks
Two formulations of oxygenating gel masks were successfully created: F1, which includes hemp seed extract, and F2, a placebo base devoid of hemp. As summarized in Table 1, F1 and F2 share an identical gel-mask base and differ only by the inclusion of 0.80–1.20% w/w hemp seed oil in F1. The concentration range for F1 (hemp extract mask) was selected based on industry standards and contemporary research, which reliably identifies effective and safe concentrations of hemp seed oil for topical application within this range [26,27]. Both masks exhibited transparent to slightly opalescent gel textures with similar color, aroma, and sensory characteristics, producing fine, uniform bubbles upon application to the skin. Figure 1 shows the visual appearance of the two formulations.
Figure 1.
The appearance of Hemp mask (a) and Hemp-Free mask (b).
The finished formulations had several essential characteristics of gel-based oxygenating face masks, including suitable viscosity for facial application, physical stability during the study period, and foam production. These optimized compositions were used for all stability, clinical, and consumer studies reported in the following sections.
3.2. Physical Property Characterization and Stability Assessment
As described in Section 3.2, the physical property evaluation revealed distinct performance characteristics between the two formulations over the 30-measurement times (60-day study); the pH of the hemp seed oxygen mask ranged from pH 5.5 to pH 6.0. Table 2 illustrates the results of testing the physical properties of the oxygenating mask.

Table 2.
Results of testing the physical properties of the oxygenating mask.
In this study, the formulation was designed as a self-foaming, oxygenating gel mask, where rapid oxygen bubble generation and foam formation cause continuous, non-linear changes in rheological behavior during application. Under these highly dynamic conditions, conventional single-point viscosity and spreadability measurements did not yield reproducible or meaningful values and were therefore not selected as primary stability endpoints. Instead, physical stability and operational performance were characterized using parameters appropriate for bubble-based systems, including the onset time of bubble formation, foam expansion, bubble stability, and collapse time on the skin, in addition to pH and visual/phase stability under accelerated storage. This approach is consistent with recent reports highlighting bubble-based hydrogels and oxygen-generating gel masks, where foam behavior and dynamic rheology are considered more representative indicators of product performance than static viscosity values [28,29].
- Foam Formation Time Analysis
Both formulations demonstrated relatively consistent foam formation times throughout the evaluation period. The hemp extract formulation exhibited an average foam formation time of 15.50 ± 0.51 s, while the control formulation (without hemp extract) showed an average of 15.43 ± 0.78 s. The maximum percentage deviation from initial measurements was 11.76% for the hemp extract formulation and 6.67% for the control formulation.
3.3. Physical Property Characterization and Stability Assessment
As described in Section 3.2, the physical property evaluation revealed distinct performance characteristics between the two formulations over the 30-measurement times (60-day study); the pH of the hemp seed oxygen mask ranged from pH 5.5 to pH 6.0. Table 2 illustrates the results of testing the physical properties of the oxygenating mask.
- Foam Formation Time Analysis
Both formulations demonstrated relatively consistent foam formation times throughout the evaluation period. The hemp extract formulation exhibited an average foam formation time of 15.50 ± 0.51 s, while the control formulation (without hemp extract) showed an average of 15.43 ± 0.78 s. The maximum percentage deviation from initial measurements was 11.76% for the hemp extract formulation and 6.67% for the control formulation.
- Foam Disappearance Time Analysis
The hemp extract formulation exhibited enhanced foam stability, with an average disappearance time of 785.80 ± 50.12 s, in contrast to 758.00 ± 67.45 s for the control formulation. Maximum percentage deviations were 16.98% and 24.69%, respectively.
All measurements maintained coefficients of variation below 9% (CV = 3.29–8.95%), demonstrating excellent reproducibility and manufacturing consistency that meets pharmaceutical quality standards. The enhanced foam stability provides an extended contact time for active ingredient delivery while maintaining an equivalent user application experience, supporting the clinical advantages of incorporating hemp seed extract without compromising formation kinetics. It confirms the formulation’s exceptional performance and its appropriateness for regulatory approval and commercial use.
Figure 2 illustrates the maximum variance in foam disappearance time. When compared to the freshly made (first) product, both masks exhibited a difference of less than 25%. The maximum difference in foam disappearance time between the two groups is 3.67% as indicated. The dashed lines indicate the mean time between foam disappearance for each group. Figure 2 presents the duration of foam disappearance for the hemp extract formulation (F1) and the placebo formulation (F2) throughout 30 repeated assessments, along with comprehensive mean ± SD and variability data in Table 2.
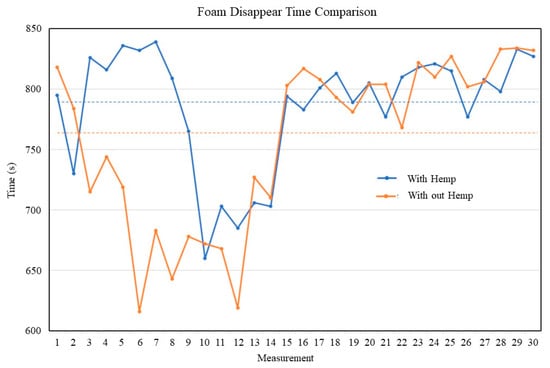
Figure 2.
Foam disappearance time comparison between formulations with and without hemp extract, recorded over 30 repeated measurements. The blue and red lines represent foam disappearance times (seconds) for formulations containing hemp and without hemp, respectively.
The hemp extract formulation demonstrated enhanced foam stability with longer persistence times and reduced variability in foam disappearance measurements. While foam formation times remained statistically equivalent between formulations, the hemp extract contributed to improved foam longevity and better overall stability, as evidenced by the lower maximum percentage deviation in foam disappearance time.
These results suggest that hemp extract incorporation provides beneficial effects on product performance characteristics, particularly in maintaining foam structure over extended periods.
3.4. Clinical Test of the Oxygenating Masks’ Efficiency
Among the group of 43 volunteers, three volunteers experienced slight itching and redness and withdrew before testing the mask’s effectiveness. The remaining 40 volunteers, who had no allergic reactions or skin irritations, were tested continuously. Skin examination of moisture, melanin, erythema, and oil levels was conducted over a duration of eight weeks, as detailed in Section 2. The statistical significance levels are as follows: p < 0.05, * p < 0.01, and *** p < 0.001, indicating differences compared to baseline and between groups.
3.4.1. Coreneometer
This eight-week clinical study demonstrated that oxygenating facial masks containing hemp seed extract (F1) exhibited significantly superior moisturizing efficacy compared to control formulations without hemp extract (F2), as measured by a Corneometer CM 825 assessment on 40 volunteers who completed the trial without experiencing any adverse reactions [23]. The hemp-enhanced formulation achieved a remarkable 65.65% improvement in skin hydration, significantly outperforming the control group’s 38.25% improvement (Table 3). Statistical significance was shown as early as week 5 for F1 (p < 0.05) compared to week 6 for F2, indicating the enhanced and prolonged moisturizing efficacy of formulations including hemp seed extract. This higher performance aligns with established research demonstrating that hemp seed oil’s high concentration of essential fatty acids, particularly gamma-linolenic acid and linoleic acid, promotes superior skin barrier function and cellular regeneration [30,31,32]. The symmetrical consistency found in both formulations validates the dependability of Corneometer measurements for hydration assessment, with the device demonstrating coefficients of variation under 10% in controlled conditions. The swift onset and enduring effectiveness of masks enriched with hemp extract encourage their incorporation into advanced cosmetic formulations to enhance moisturizing properties, consistent with clinical evidence that illustrates hemp oil’s quick skin absorption and moisture retention capacities [33,34]. These findings represent clinically meaningful improvements that translate to measurable therapeutic benefits, with the 1.5-fold greater efficacy of hemp extract formulations providing compelling evidence for its utility in professional skincare applications targeting enhanced facial hydration and restoring barrier function.

Table 3.
Changes in skin hydration measured by Corneometer CM 825 over eight weeks in volunteers treated with oxygenating facial masks containing hemp seed extract (F1) and control formulation (F2).
3.4.2. Skin-Glossy Meter
This thorough eight-week clinical trial utilizing dual-mode Skin-glossy meter assessments (standard and Glossy DSC modes) revealed the enhanced sebum control effectiveness of hemp seed extract-infused oxygenating face masks (HSE) in comparison to placebo formulations. The hemp-infused formulation exhibited significant sebum regulation on the right side of the face in both measurement modalities, resulting in a 32.9% reduction in standard glossiness measurements (2.96 ± 2.11 AU, p < 0.001) and a 21.6% decrease in DSC mode (2.28 ± 1.13 AU) at week 8. Conversely, placebo formulations elevated sebum levels by 22.4% and 30.0%, respectively, on the right side. Measurements on the left side revealed modest but consistent improvements with HSE treatment, showing an 11.5% reduction in standard mode and a 21.3% decrease in DSC mode, compared to the placebo group, which exhibited a 17.1% increase in glossiness in standard measurements, despite a 7.5% decline in DSC mode, as described in Table 4. This asymmetric response pattern, marked by increased efficacy on the right side, aligns with dermatological studies that indicate natural symmetrical differences in the distribution and function of facial sebaceous glands, possibly influenced by variations in androgen receptor expression and hormonal sensitivity [35]. The exceptional sebum-regulating attributes of hemp extract are due to its elevated levels of gamma-linolenic acid (GLA) and linoleic acid, which influence sebaceous gland function via the regulation of the prostaglandin E1 pathway, inhibition of 5α-reductase, and cannabinoid receptor-mediated signaling, thereby normalizing sebum production and enhancing skin barrier integrity [36]. The enhancement noted on the right side signifies clinically meaningful progress in sebum regulation, with the dual-measurement method offering an extensive evaluation of both surface shine and deeper sebaceous function, indicating possible uses in professional acne treatment strategies where precise sebum control is therapeutically advantageous.

Table 4.
Comparative effects of hemp seed extract versus placebo facial masks on skin gloss and sebum levels assessed by standard and Glossy DSC modes during an eight-week clinical trial.
3.4.3. Mexameter
This eight-week randomized clinical study utilizing Mexameter MX-18 assessments (Table 5) demonstrates strong evidence that oxygenating facial masks containing hemp seed extract (F1) exhibit enhanced anti-inflammatory efficiency compared to control formulations (F2), especially in terms of diminishing cutaneous erythema. Statistically significant erythema reduction was observed in the F1 group bilaterally, attaining a reduction of 12.9% on the right side (315.97 ± 71.27 AU) and 8.7% on the left side from baseline after eight weeks (p < 0.01), with significance emerging as early as week 5 (p < 0.05), with higher significance (p < 0.01) observed from week 6 onwards, whereas the control group yielded only a 7.7% reduction without statistical significance on either facial side during the study period. This consistent bilateral improvement affirms the reliability of objective erythema quantification via dual-sided Mexameter measurements. Furthermore, both formulations maintained pigmentation homeostasis, with melanin indices showing no significant changes from baseline throughout the eight weeks (p > 0.05), thus confirming no risk of undesirable hyperpigmentation or depigmentation. These findings address safety concerns by demonstrating that potent erythema reduction is achievable without pigmentary disruption—a critical consideration for long-term topical interventions in sensitive and inflamed skin conditions. The enhanced anti-inflammatory response to the hemp extract formulation is supported by mechanistic data demonstrating that phytocannabinoids such as cannabidiol modulate cutaneous inflammatory pathways, notably by downregulating pro-inflammatory cytokines (e.g., IL-1β and TNF-α) via CB2 receptor activation in human keratinocytes [37,38]. The observed greater erythema reduction with F1 is clinically meaningful, suggesting potential for hemp-based formulations as adjuncts in sensitive skin management, post-inflammatory hyperpigmentation prevention, and therapeutic regimens for chronic inflammatory dermatoses such as rosacea, where targeted erythema reduction without pigmentation alteration is especially desirable [39].

Table 5.
Changes in melanin and erythema levels (arbitrary units, AU) assessed bilaterally by Mexameter MX-18 following 8-week treatment with oxygenating facial masks containing hemp seed extract (F1) or control formulation (F2). Values are mean ± SD (n = 20 per group).
3.5. Preference Test
The double-blind consumer satisfaction study involving forty volunteers demonstrated superior acceptability and perceived efficacy of the hemp extract-containing oxygenating facial mask (F1) compared with the control formulation (F2) across all assessed sensory and functional parameters, as shown in Table 6. The superiority percentage was determined using the formula:
Superiority (%) = [(Mean F1 − Mean F2)/Mean F2] × 100

Table 6.
Average product satisfaction in each aspect.
Positive values signify the superior performance of F1 over the controls. The hemp-enhanced formulation achieved consistently higher satisfaction scores in all six evaluation criteria, with the most pronounced advantages observed in product fragrance (13.8% superiority; 4.55 ± 0.83 vs. 4.00 ± 0.97), foaming sensation (12.2% improvement; 4.60 ± 0.68 vs. 4.10 ± 0.79), and skin cleansing effectiveness (12.0% enhancement; 4.65 ± 0.67 vs. 4.15 ± 0.75). Moreover, F1 maintained excellent performance in functional attributes, including skin penetration ability (4.85 ± 0.37 vs. 4.40 ± 0.68) and post-application moisturization (4.95 ± 0.22 vs. 4.65 ± 0.67).
This method of calculating relative percentages presents a standard way to measure F1′s performance advantages over F2. All positive values show that the hemp extract formulation is consistently higher than F2 across all parameters. The overall product satisfaction score of 4.95 ± 0.22 for the hemp extract formulation reflects exceptionally high consumer acceptance, outperforming the control formulation by 10.0% (4.50 ± 0.83). The aggregate satisfaction advantage across all parameters averaged 10.7%, signifying that consumers rated the hemp extract formulation 10.7% higher than the control, representing a clinically and commercially meaningful difference that surpasses the commonly recognized 5–10% threshold for perceptible consumer-level improvements.
The results align with prior studies demonstrating that the inclusion of natural, hemp-derived components improves perceived product quality, sensory appearance, and overall satisfaction through enhancements in texture, absorption, and scent qualities [33]. The superior consumer satisfaction observed in this study can be attributed to the multifunctional properties of hemp extract, including its essential fatty acid composition, excellent skin compatibility, contribution to formulation stability, and distinctive sensory attributes that reinforce a perception of premium product quality [40].
The consistently positive superiority values across all functional and hedonic parameters (ranging from 6.5% to 13.8%) provide quantitative confirmation that F1 systematically outperformed F2 in every evaluated aspect. The finding indicates the enhanced benefits of incorporating hemp extract into oxygenating facial mask formulations, besides supporting its potential for greater market acceptance in the premium skincare sector, where product satisfaction is integral to consumer preference, commercial success, and brand loyalty.
4. Discussion
This study provides the first robust clinical evidence supporting the multi-functional dermatological benefits of oxygenating facial masks infused with hemp seed extract (HSE) using a double-blinded, placebo-controlled randomized design. Unlike previous reports that primarily relied on short-term or preclinical models, our rigorous eight-week human trial offers compelling data applicable to global skincare markets, directly reflecting the need for safe and innovative cosmetics.
4.1. Moisturization and Skin Barrier Effects
Eight-week Corneometer CM 825 assessments revealed that the hemp-infused mask resulted in significantly greater enhancements in skin moisture (approximately 65.65%) compared to the control (approximately 38.25%), with statistical significance observed as early as week five. The superior moisturizing effect is due to the high levels of essential fatty acids in hemp seed oil, especially gamma-linolenic and linoleic acids. These acids enhance barrier function, prevent transepidermal water loss, and facilitate cellular regeneration [32,33,41]. These findings align with recent clinical studies, such as Bennardo et al., which reported that topical hemp seed extract improved stratum corneum hydration by over 44% and exhibited favorable barrier protection in volunteers [4]. Similarly, Žugić et al. found that hemp seed creams rapidly improved water retention and barrier recovery in irritated skin. Comprehensive reviews substantiate the penetrative ability and hydrating characteristics of hemp oil, thereby reinforcing its application in sophisticated cosmetic formulations [33].
4.2. Sebum Regulation and Gloss Reduction
Eight-week Mexameter and Sebumeter measurements revealed significant reductions in sebum levels following application of the hemp seed extract oxygenating mask (mean reduction 32.9%), outperforming the placebo control. This effect is attributable to bioactive compounds in hemp seed oil, such as polyunsaturated fatty acids and phytosterols, which are known to control sebaceous gland activity and exhibit anti-inflammatory properties [35]. These findings align with recent clinical trials assessing hemp-based formulations: Žugić et al. found that hemp oil creams reduced sebum production and enhanced skin oiliness in participants with combination skin [33]. Additionally, recent reviews confirm that both hemp seed oil and cannabidiol (CBD) suppress lipid synthesis in sebocytes and normalize sebum excretion. The clinically relevant sebum-regulating effect of hemp seed extract is associated with its ability to modulate sebaceous activity through prostaglandin E1 pathways, 5α-reductase inhibition, and cannabinoid receptor-mediated mechanisms [42]. The present results further validate the sebum-regulating potential of hemp-based cosmetic products for oily and acne-prone skin.
4.3. Erythema Reduction and Anti-Inflammatory Activity
Eight-week Mexameter evaluations demonstrated that the hemp seed extract oxygenating mask significantly reduced skin erythema (up to 46.9 AU, or 12.9%) compared to the control, with statistical significance established at the end of the trial. When compared to the control group, the potent anti-inflammatory qualities of hemp extract result in a 1.6-fold reduction in erythema. Hemp seed oil contains phytocannabinoids, polyphenols, and omega-3 and omega-6 fatty acids, which are known to affect epidermal inflammatory pathways and reduce skin redness. Recent clinical studies support these findings, which Oláh et al. corroborated by showing that cannabidiol (CBD) and hemp extracts downregulate pro-inflammatory cytokines and normalize skin response in various dermatological conditions. This work aligns with preclinical work that demonstrates phytocannabinoids (cannabidiol) downregulate pro-inflammatory cytokines in keratinocytes via CB2 receptors [36]. Research studies confirm that topical hemp formulations are effective for managing erythema and mild inflammation, contributing to skin soothing and recovery.
4.4. Consumer Perception and Acceptance
Results from structured consumer satisfaction surveys provided further validation, with the HSE mask achieving a 10.7% aggregate satisfaction advantage over F2 (controlled formulation) for overall parameters. Improvements in foaming sensation, cleansing efficiency, product fragrance, and moisturizing after-feel highlight not only the functional but also the experiential benefits of hemp actives. This considerable consumer approval enhances commercial viability and market placement within the premium skincare sector.
4.5. Repetitiveness, Safety, and Legal Consequences
All objective measurements demonstrated high repeatability (CV < 10%) and no adverse responses during the eight weeks, satisfying regulatory and scientific standards for reproducibility and dermal safety. The complete absence of clinically significant pigmentary alteration underscores the suitability of these actives for sensitive skin and prolonged care environments.
4.6. Constraints and Prospective Pathways
Despite the rigorous design, the study was limited; involving a broader diversity of phototypes, larger sample sizes, and longer-term follow-up is warranted. Additionally, biomarker-based measurements of anti-inflammatory activity and clinical endpoints (e.g., in post-procedural erythema) could provide added mechanistic insights. This double-blind, placebo-controlled study yielded significant information that oxygenating facial masks containing hemp seed extract (HSE) deliver comprehensive dermatological benefits, including clinically meaningful enhancements in skin hydration, sebum control, erythema reduction, and user satisfaction, regardless of damaging pigmentary changes or irritation. These findings emphasize the varied usefulness and safety of cannabis-derived substances in advanced skincare.
In conclusion, this study demonstrates that an oxygenating facial mask enriched with hemp seed extract provided significant dermatological benefits, including enhanced skin hydration, improved barrier function, decreased sebum production, and reduced erythema. The clinical data demonstrate that the hemp-derived product surpasses the placebo in all outcomes, with measurable improvements corroborated by non-invasive biophysical evaluations. Recent research confirms the findings and exhibits the synergistic effects of essential fatty acids, polyphenols, and phytocannabinoids in enhancing skin health. These findings support the development of hemp seed extract oxygenating masks as advanced cosmeceuticals for both moisturizing and soothing applications and establish their potential for use in premium skin care products targeting hydration, oil control, and skin sensitivity.
Author Contributions
Conceptualization, O.A.; methodology, O.A. and S.J.; software, O.A.; validation, O.A. and S.J.; formal analysis, O.A.; investigation, O.A.; resources, S.J.; data curation, O.A.; writing—original draft preparation, O.A.; writing—review and editing, O.A. and S.J.; funding acquisition, O.A. and S.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Thailand Science Research and Innovation, Grant Number 194624.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Phranakhon Rajabhat University (Approval Protocol No. 02.018/67).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The proposed experiments were carried out in the Department of Cosmetic Science, Phranakhon Rajabhat University.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verdier-Sévrain, S.; Bonté, F. Skin hydration: A review on its molecular mechanisms. J. Cosmet. Dermatol. 2007, 6, 75–82. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. The primary role of sebum in the pathophysiology of acne. J. Dermatol. Treat. 2024, 35, 247–253. [Google Scholar] [CrossRef]
- Perugini, P.; Bleve, M.; Redondi, R.; Cortinovis, F.; Colpani, A. In vivo evaluation of the effectiveness of biocellulose facial masks as active delivery systems to skin. J. Cosmet. Dermatol. 2019, 18, 1606–1615. [Google Scholar] [CrossRef]
- Bennardo, L.; Del Duca, E.; Dastoli, S.; Schipani, G.; Scali, E.; Silvestri, M.; Nisticò, S.P. Potential Applications of Topical Oxygen Therapy in Dermatology. Dermatol. Pract. Concept. 2018, 8, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Draelos, Z.D.; Shamban, A. A pilot study evaluating the anti-aging benefits of a CO2-emitting facial mask. J. Cosmet. Dermatol. 2023, 22, 2198–2204. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.S.; Magalhães, M.C.; Sousa-Lobo, J.M.; Cruz, M.T.; Almeida, I.F. Trends in the use of botanicals in anti-aging cosmetics. Molecules 2021, 26, 3584. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.M.; Gomes, A.L.; Vilas Boas, I.; Marto, J.; Ribeiro, H.M. Cannabis-Based Products for the Treatment of Skin Inflammatory Diseases: A Timely Review. Pharmaceuticals 2022, 15, 210. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and anti-inflammatory properties of cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- Liu, C.; Li, H.; Xu, F.; Jiang, X.; Ma, H.; Seeram, N.P. Cannabidiol protects human skin keratinocytes from hydrogen-peroxide-induced oxidative stress via modulation of the Caspase-1–IL-1β axis. J. Nat. Prod. 2021, 84, 1563–1572. [Google Scholar] [CrossRef]
- Tănase Apetroaei, V.; Pricop, E.M.; Istrati, D.I.; Vizireanu, C. Hemp seeds (Cannabis sativa L.) as a valuable source of natural ingredients for functional foods—A review. Molecules 2024, 29, 2097. [Google Scholar] [CrossRef]
- Şeker, M.; Esen, Ö. The Effect of Hemp Seed Oil on Skin and Soap Performance. Int. J. Life Sci. Biotechnol. 2021, 4, 420–438. [Google Scholar] [CrossRef]
- Mikulcová, V.; Kašpárková, V.; Humpolíček, P.; Buňková, L. Formulation, characterization, and properties of hemp seed oil macroemulsions. Molecules 2017, 22, 700. [Google Scholar] [CrossRef]
- Jairoun, A.A.; Al-Hemyari, S.S.; Shahwan, M.; Ibrahim, B.; Hassali, M.A.; Zyoud, S.H. Risk assessment of over-the-counter cannabinoid-based cosmetics: Legal and regulatory issues governing the safety of cannabinoid-based cosmetics in the UAE. Cosmetics 2021, 8, 57. [Google Scholar] [CrossRef]
- Tassaneesuwan, N.; Khongkow, M.; Jansrinual, S.; Khongkow, P. Discovering the potential of cannabidiol for cosmeceutical development at the cellular level. Pharmaceuticals 2025, 18, 202. [Google Scholar] [CrossRef]
- Parente, M.E.; Ochoa Andrade, A.; Ares, G.; Russo, F.; Jiménez-Kairuz, Á. Bioadhesive hydrogels for cosmetic applications. Int. J. Cosmet. Sci. 2015, 37, 511–518. [Google Scholar] [CrossRef]
- Kim, D.; Seok, J.K.; Kim, M.; Choi, S.; Hong, J.; Yoon, Y.A.; Chung, H.; Bae, O.N.; Kwack, S.J.; Kim, K.B.; et al. Safety assessment of cocamidopropyl betaine, a cosmetic ingredient. Toxicol. Res. 2024, 40, 361–375. [Google Scholar] [CrossRef]
- Boo, Y.C. Mechanistic basis and clinical evidence for the applications of nicotinamide (niacinamide) to control skin aging and pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Dréno, B.; Zuberbier, T.; Gelmetti, C.; Gontijo, G.; Marinovich, M. Safety review of phenoxyethanol when used as a preservative in cosmetics. J. Eur. Acad. Dermatol. Venereol. 2019, 33 (Suppl. S7), 15–24. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef]
- ICH. Stability Testing of New Drug Substances and Products Q1A(R2); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2003. Available online: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf (accessed on 2 November 2025).
- Sanjeevini, M.; Harini Priya, P.; Rakshitha, L.; Murthy, P.N.; Pragathi, S.G.; Matadh, A.V.; Murthy, S.N.; Srinath, R.; Shivakumar, H.N.; Maibach, H.; et al. Skin can modulate the pH of topical creams and gels. AAPS PharmSciTech 2025, 26, 166. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Li, S.; He, X.; Zhang, Z.; Zhang, X.; Niu, Y.; Steel, A.; Wang, H. Efficacy and safety of a facial serum and a mask containing salicylic acid and lipohydroxy acid in acne management: A randomized controlled trial. J. Cosmet. Dermatol. 2023, 22, 2502–2511. [Google Scholar] [CrossRef]
- Hua, W.; Fan, L.M.; Dai, R.; Luan, M.; Xie, H.; Li, A.Q.; Li, L. Comparison of two series of non-invasive instruments used for the skin physiological properties measurements: The DermaLab® from Cortex Technology vs. the series of detectors from Courage & Khazaka. Skin Res. Technol. 2017, 23, 70–78. [Google Scholar] [CrossRef]
- Ayob, A.; Awadh, A.I.; Hadi, H.; Jaffri, J.; Jamshed, S.; Ahmad, H.M.A. Malaysian consumers’ awareness, perception, and attitude toward cosmetic products: Questionnaire development and pilot testing. J. Pharm. Bioallied Sci. 2016, 8, 203–209. [Google Scholar] [CrossRef]
- Palmieri, B.; Laurino, C.; Vadalà, M. A Therapeutic Effect of CBD-Enriched Ointment in Inflammatory Skin Diseases and Cutaneous Scars. Clin. Ter. 2019, 170, e93–e99. [Google Scholar] [CrossRef] [PubMed]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic potential of cannabidiol (CBD) for skin health and disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Messerschmidt, V.; Tsipursky, M.; Irudayaraj, J. Oxygen Nanobubbles-Embedded Hydrogel as Wound Dressing to Accelerate Healing. ACS Appl. Nano Mater. 2023, 6, 13116–13126. [Google Scholar] [CrossRef] [PubMed]
- Brummer, R.; Godersky, S. Rheological Studies to Objectify Sensations Occurring When Cosmetic Emulsions Are Applied to the Skin. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 89–94. [Google Scholar] [CrossRef]
- Imokawa, G.; Yada, Y.; Higuchi, K.; Okuda, M.; Ohashi, Y.; Kawamata, A. Pseudo-acylceramide with linoleic acid produces selective recovery of diminished cutaneous barrier function in essential fatty acid-deficient rats and has an inhibitory effect on epidermal hyperplasia. J. Clin. Investig. 1994, 94, 89–96. [Google Scholar] [CrossRef]
- Mao-Qiang, M.; Elias, P.M.; Feingold, K.R. Fatty acids are required for epidermal permeability barrier function. J. Clin. Investig. 1993, 92, 791–798. [Google Scholar] [CrossRef]
- Kawamura, A.; Ooyama, K.; Kojima, K.; Kachi, H.; Abe, T.; Amano, K.; Aoyama, T. Dietary supplementation of gamma-linolenic acid improves skin parameters in subjects with dry skin and mild atopic dermatitis. J. Oleo Sci. 2011, 60, 597–607. [Google Scholar] [CrossRef]
- Žugić, A.; Martinović, M.; Tadić, V.; Rajković, M.; Racić, G.; Nešić, I.; Koren, A. Comprehensive insight into cutaneous application of hemp. Pharmaceutics 2024, 16, 748. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.S.; Zomer, R.; Mantri, N. Nanoformulations as a strategy to overcome the delivery limitations of cannabinoids. Phytother. Res. 2023, 37, 1526–1538. [Google Scholar] [CrossRef]
- Seo, Y.J.; Li, Z.J.; Choi, D.K.; Sohn, K.C.; Kim, H.R.; Lee, Y.; Kim, C.D.; Lee, Y.H.; Shi, G.; Lee, J.H.; et al. Regional difference in sebum production by androgen susceptibility in human facial skin. Exp. Dermatol. 2014, 23, 70–72. [Google Scholar] [CrossRef]
- Dobrosi, N.; Tóth, B.I.; Nagy, G.; Dózsa, A.; Géczy, T.; Nagy, L.; Zouboulis, C.C.; Paus, R.; Kovács, L.; Bíró, T. Endocannabinoids enhance lipid synthesis and apoptosis of human sebocytes via cannabinoid receptor-2-mediated signaling. FASEB J. 2008, 22, 3685–3695. [Google Scholar] [CrossRef]
- Anil, S.M.; Peeri, H.; Koltai, H. Medical cannabis activity against inflammation: Active compounds and modes of action. Front. Pharmacol. 2022, 13, 908198. [Google Scholar] [CrossRef]
- Sermet, S.; Li, J.; Bach, A.; Crawford, R.B.; Kaminski, N.E. Cannabidiol selectively modulates interleukin (IL)-1β and IL-6 production in toll-like receptor activated human peripheral blood monocytes. Toxicology 2021, 464, 153016. [Google Scholar] [CrossRef]
- Shrestha, C.; Yoo, E.H.; Deshar, B.; Hwang, M.; Kang, S.; Bin, B.H.; Lee, J.H.; Kim, J. Cannabidiol as a therapeutic agent for rosacea through simultaneous inhibition of multiple inflammatory pathways. BMB Rep. 2025, 58, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Ziomek, M.; Żbikowska, A. Stability of cosmetic emulsion containing different amount of hemp oil. Int. J. Cosmet. Sci. 2015, 37, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Hartop, P.J.; Prottey, C. Changes in transepidermal water loss and the composition of epidermal lecithin after applications of pure fatty acid triglycerides to skin of essential fatty acid-deficient rats. Br. J. Dermatol. 1976, 95, 255–264. [Google Scholar]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllősi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).