Idiopathic Alopecia—A Retrospective Descriptive Study Integrated with the Current Literature
Abstract
1. Introduction
Evaluation of Hair Loss and Therapies for Hair Regeneration
2. Materials and Methods
3. Results
3.1. The Epidemiological Assessment
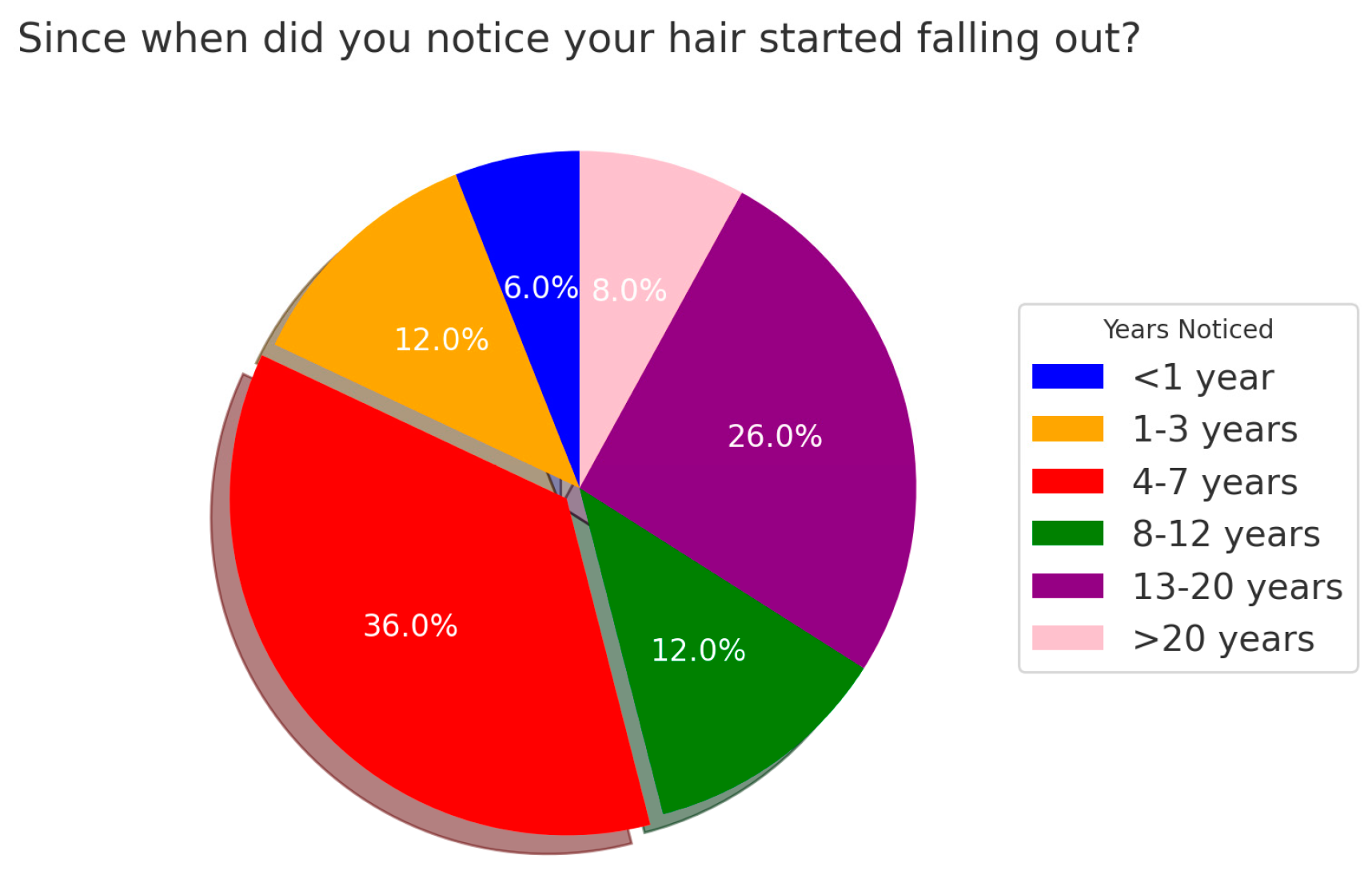
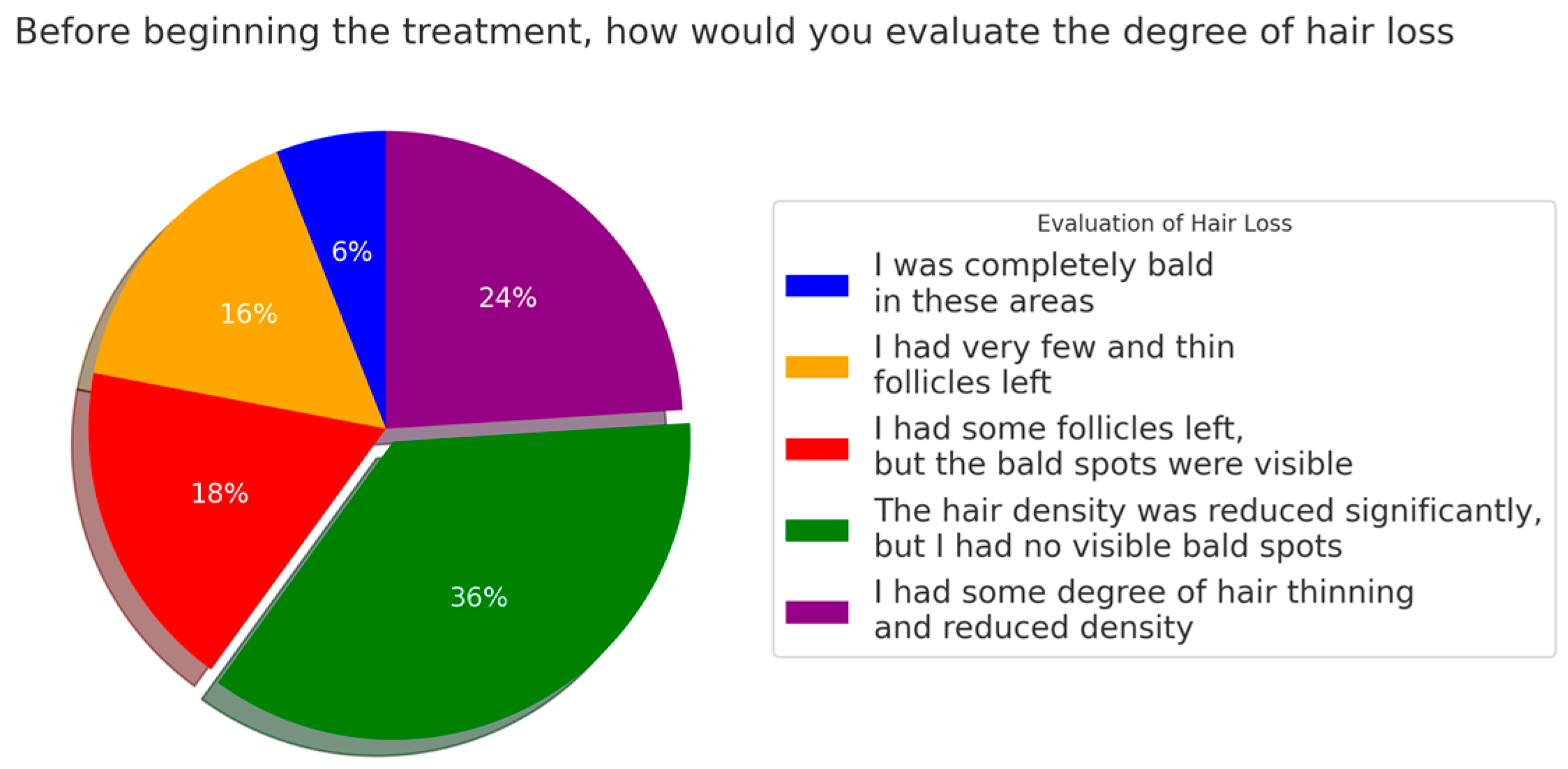
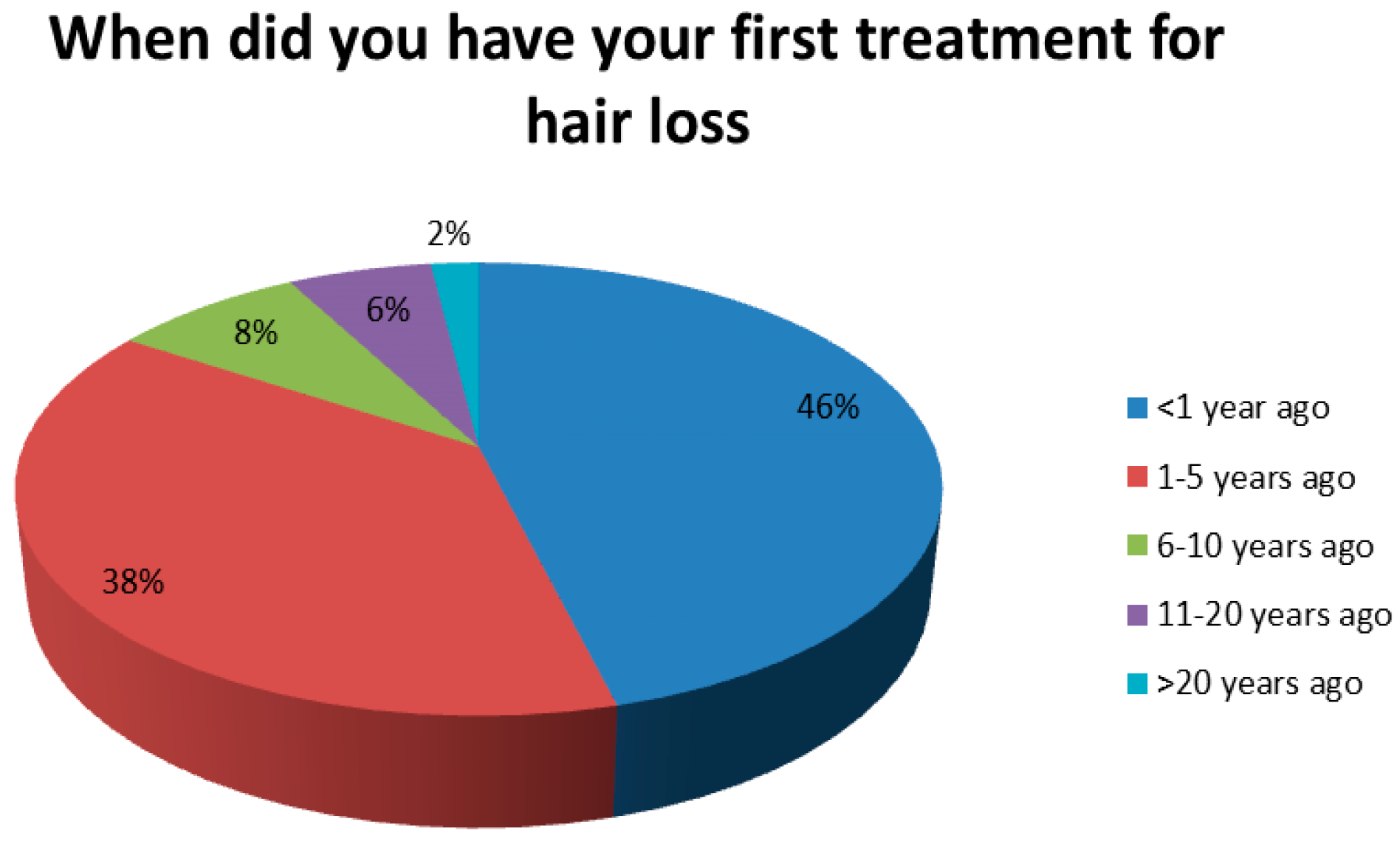
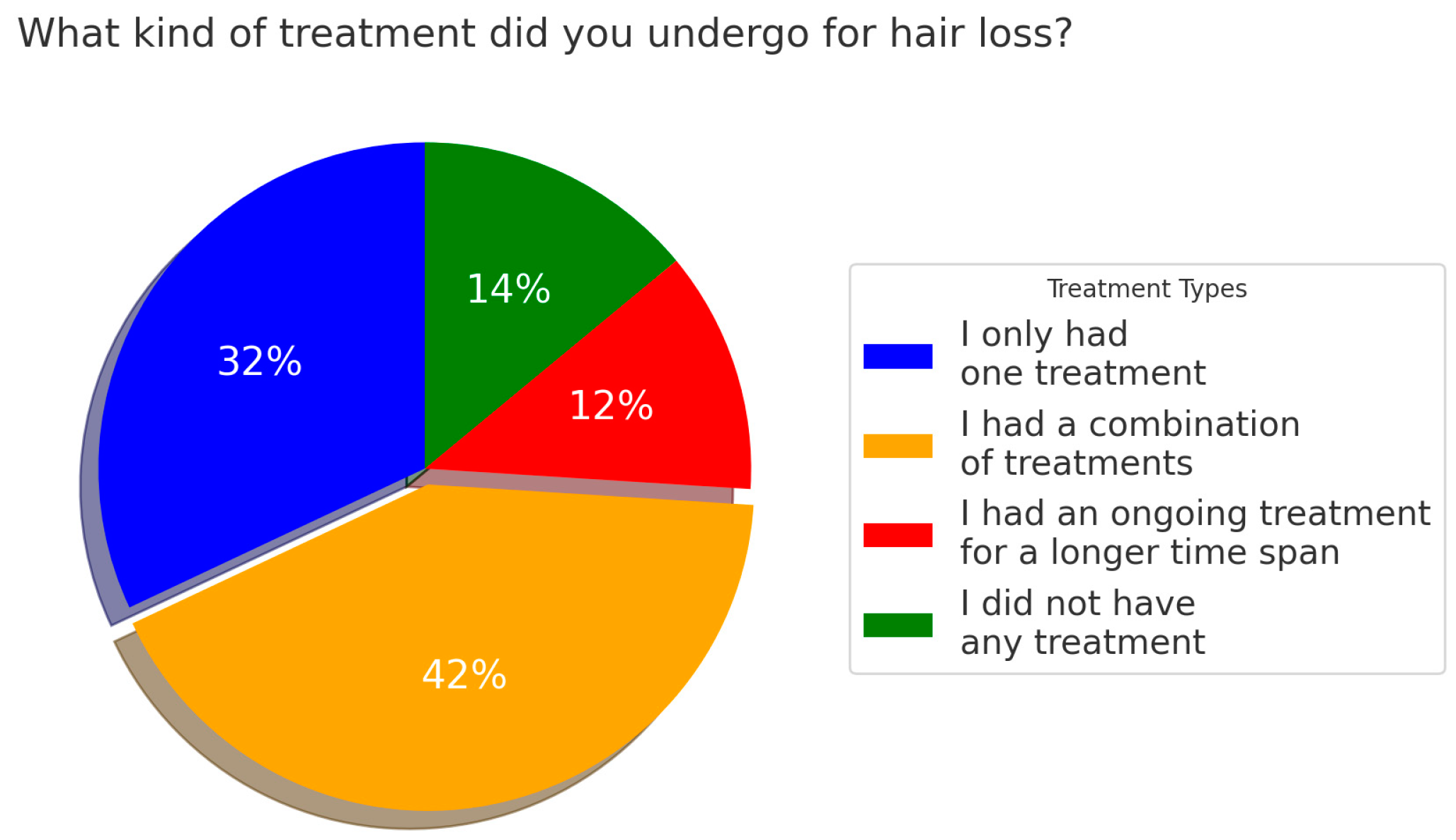
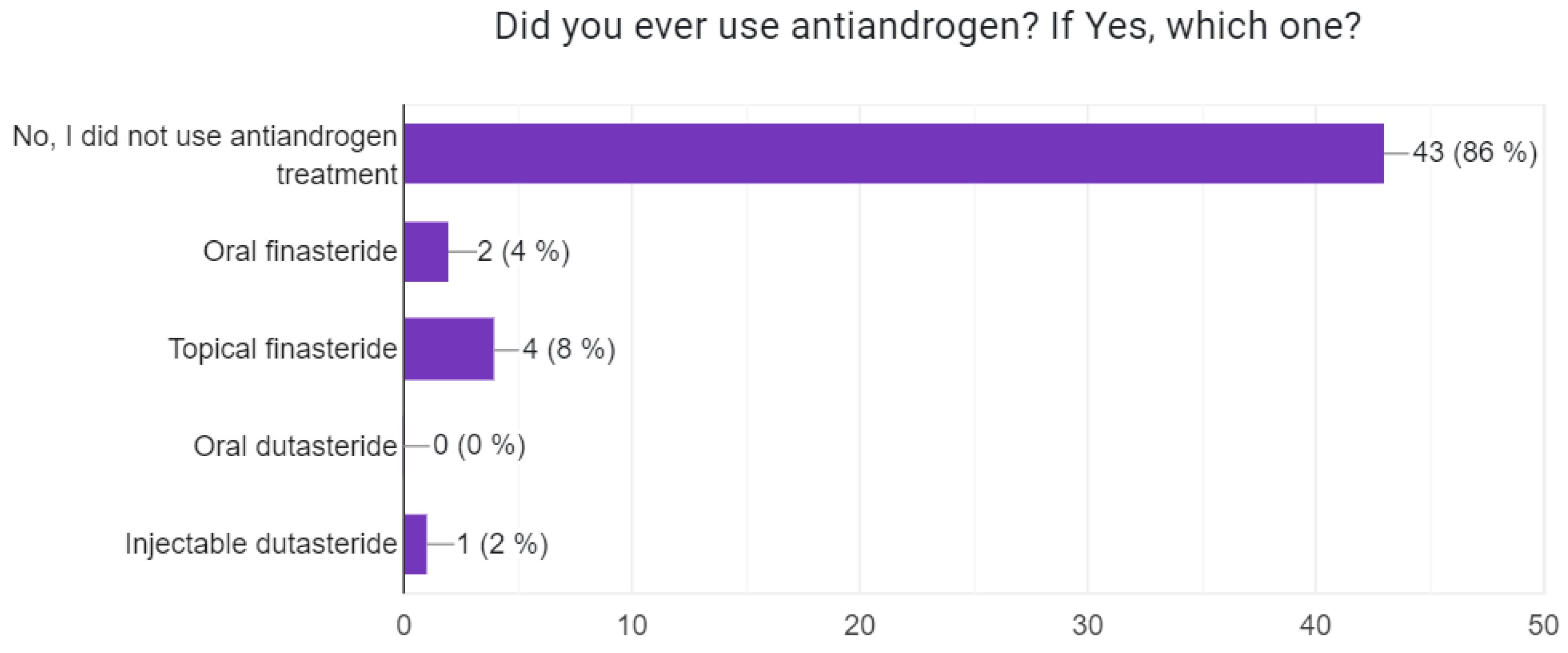
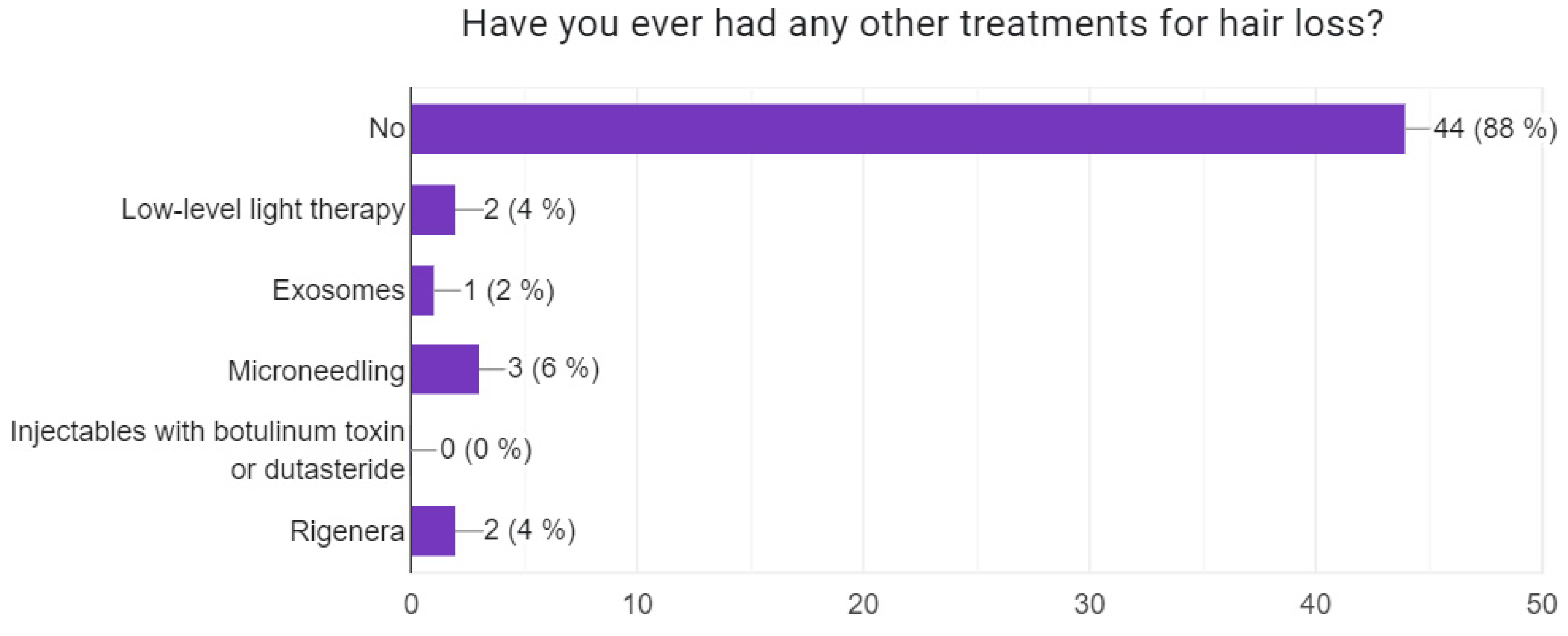
3.2. Information About the Patients’ Disease Status
- –
- Most patients (80%) did not undergo trichoscopy prior to their first hair treatment.
- –
- Most patients (66%) had not had any investigations performed prior to treatment, while only 26% had had a blood analysis and 8% dermoscopy.
- –
- Most patients (68%) did not have any other medical condition associated with their hair loss, followed by 12% of cases being associated with endocrine disorders, 8% gynecological ones, and 6% psychological ones; one patient reported autoimmune diseases and one nutrition deficiency.
- –
- A total of 56% of the patients had a family history of alopecia.
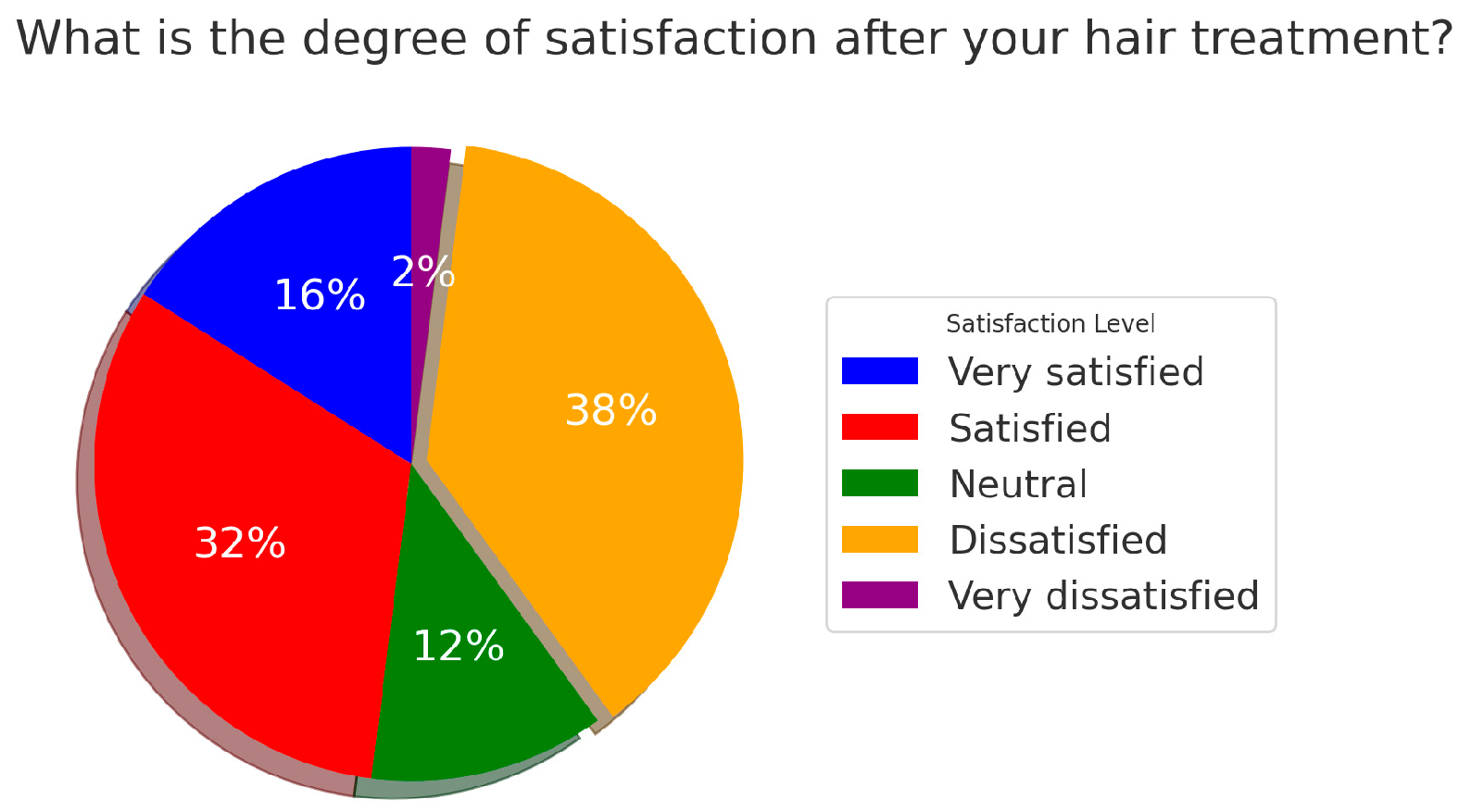
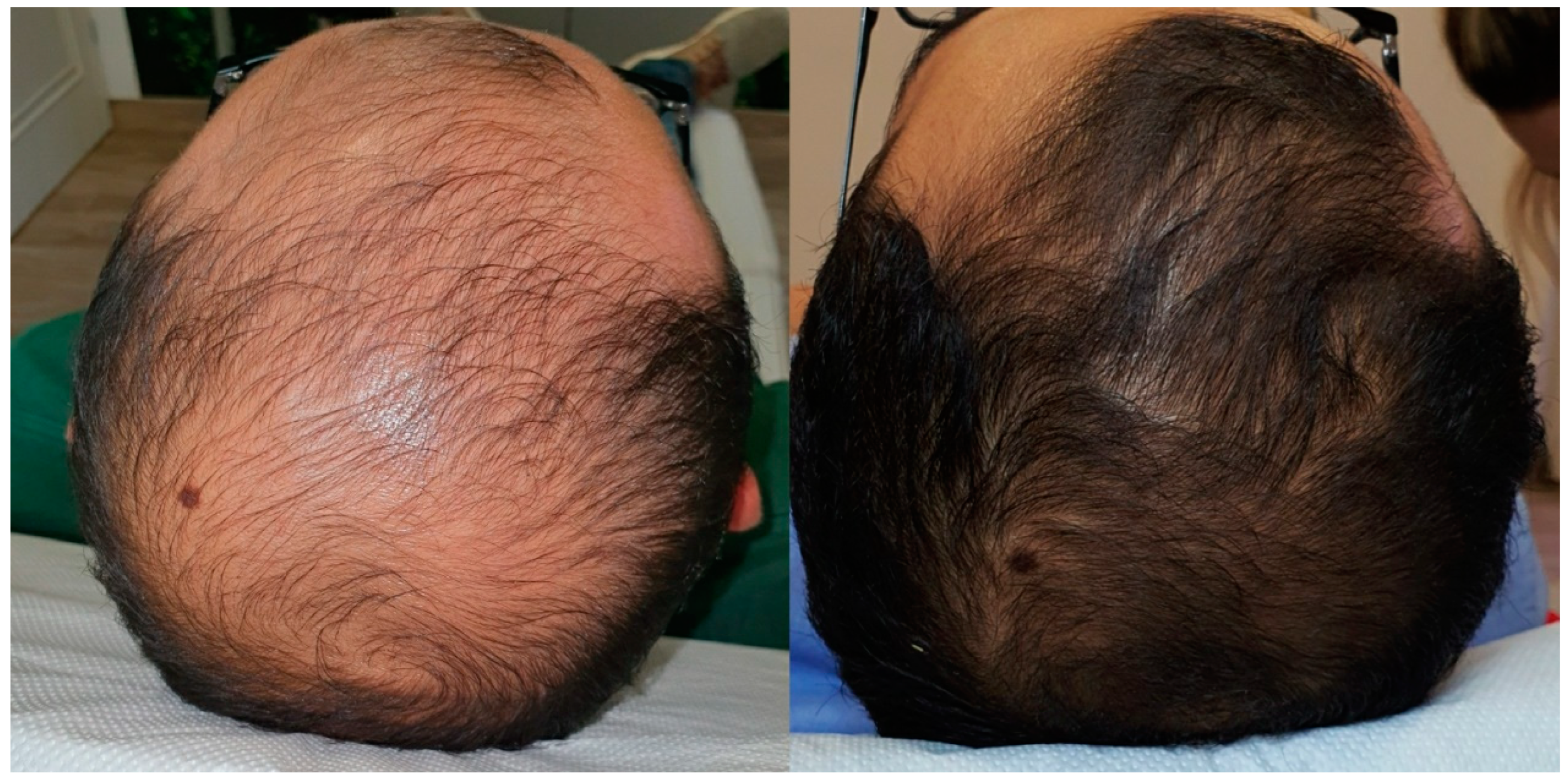
3.3. Clinical Outcomes
3.4. Statistical Findings
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, J.R. Techniques for Hair Restoration. Facial Plast. Surg. Clin. N. Am. 2020, 28, ix. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Băloi, S.-E.; Ion, D.; Andrei, C.-L.; Marinescu, S.A.; Dragomiroiu, G.T.A.B.; Giuglea, C. Clinical assessment of the therapeutic options for alopecia treatment. Farmacia 2021, 69, 6. [Google Scholar] [CrossRef]
- Shehan, J.N.; Spiegel, J.H. Hair Restoration Techniques. Facial Plast. Surg. 2023, 39, 512–516. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.M.; Miot, H.A. Female Pattern Hair Loss: A clinical and pathophysiological review. Bras. Dermatol. 2015, 90, 529–543. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thom, E. Stress and the Hair Growth Cycle: Cortisol-Induced Hair Growth Disruption. J. Drugs Dermatol. 2016, 15, 1001–1004. [Google Scholar] [PubMed]
- Stenn, K.S.; Paus, R. Controls of hair follicle cycling. Physiol. Rev. 2001, 81, 449–494. [Google Scholar] [CrossRef] [PubMed]
- Semalty, A.; Semalty, M.; Joshi, G.P.; Rawat, M.S. Techniques for the discovery and evaluation of drugs against alopecia. Expert Opin. Drug Discov. 2011, 6, 309–321. [Google Scholar] [CrossRef]
- Semalty, M.; Semalty, A.; Joshi, G.P.; Rawat, M.S. Hair growth and rejuvenation: An overview. J. Dermatol. Treat. 2011, 22, 123–132. [Google Scholar] [CrossRef]
- Sperling, L.C. Atlas of Hair Pathology; The Parthenon Publishing Group: Nashville, TN, USA, 2003; p. 1. [Google Scholar]
- Suchonwanit, P.; Thammarucha, S.; Leerunyakul, K. Minoxidil and its use in hair disorders: A review. Drug Des. Devel. Ther. 2019, 13, 2777–2786, Erratum in Drug Des. Devel. Ther. 2020, 14, 575. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devjani, S.; Ezemma, O.; Kelley, K.J.; Stratton, E.; Senna, M. Androgenetic Alopecia: Therapy Update. Drugs 2023, 83, 701–715. [Google Scholar] [CrossRef]
- Marin, A.; Savescu, M.; Marin, G.G.; Dricu, A.; Parasca, S.; Giuglea, C. Evaluation of muscle atrophy after sciatic nerve defect repair—Experimental model. Rom. J. Mil. Med. 2022, 125, 420–431. [Google Scholar] [CrossRef]
- Trambitas, C.; Pap, T.; Niculescu, R.; Popelea, M.C.; Cotoi, O.S.; Cordoș, B.; Domnariu, H.-P.; Marin, A.; Feier, A.M.; David, C.; et al. Biocompatible 3D-Printed Devices with Adipose Stem Cells in the Regenerative Process of Sciatic Nerve Lesions in Rodent Models: An Experimental Study. Cureus 2024, 16, e62412. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.S.; Smith, P.A. Clinical Update: Why PRP Should Be Your First Choice for Injection Therapy in Treating Osteoarthritis of the Knee. Curr. Rev. Musculoskelet. Med. 2018, 11, 583–592. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mlynarek, R.A.; Kuhn, A.W.; Bedi, A. Platelet-Rich Plasma (PRP) in Orthopedic Sports Medicine. Am. J. Orthop. 2016, 45, 290–326. [Google Scholar] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment options for androgenetic alopecia: Efficacy, side effects, compliance, financial considerations, and ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, H.; Jing, J.; Yu, L.; Wu, X.; Lu, Z. Regulation of hair follicle development by exosomes derived from dermal papilla cells. Biochem. Biophys. Res. Commun. 2018, 500, 325–332. [Google Scholar] [CrossRef]
- Dhurat, R.; Sukesh, M.; Avhad, G.; Dandale, A.; Pal, A.; Pund, P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: A pilot study. Int. J. Trichology 2013, 5, 6–11. [Google Scholar] [CrossRef]
- Marin, A.; Bălăceanu, L.-A.; Marinescu, S.-A.; Burcea-Dragomiroiu, G.-T.-A.; Dîdă, M.-A.; Mirică, A.; Giuglea, C. A double perspective on botulinum toxin in Romania used for aesthetic clinical applications. Farmacia 2024, 72, 2. [Google Scholar] [CrossRef]
- Ruiz, R.G.; Rosell, J.M.C.; Ceccarelli, G.; De Sio, C.; De Angelis, G.C.; Pinto, H.; Astarita, C.; Graziano, A. Progenitor-cell-enriched micrografts as a novel option for the management of androgenetic alopecia. J. Cell. Physiol. 2020, 235, 4587–4593. [Google Scholar] [CrossRef]
- Cho, Y.H.; Lee, S.Y.; Jeong, D.W.; Choi, E.J.; Kim, Y.J.; Lee, J.G.; Yi, Y.H.; Cha, H.S. Effect of pumpkin seed oil on hair growth in men with androgenetic alopecia: A randomized, double-blind, placebo-controlled trial. Evid. Based Complement. Altern. Med. 2014, 2014, 549721. [Google Scholar] [CrossRef]
- Rogers, N.E. Hair transplantation update. Semin. Cutan. Med. Surg. 2015, 34, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Ranjan, A. Follicular Unit Extraction (FUE) Hair Transplant: Curves Ahead. J. Maxillofac. Oral Surg. 2019, 18, 509–517. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohamed, N.E.; Soltan, M.R.; Galal, S.A.; El Sayed, H.S.; Hassan, H.M.; Khatery, B.H. Female Pattern Hair Loss and Negative Psychological Impact: Possible Role of Brain-derived Neurotrophic Factor (BDNF). Dermatol. Pract. Concept. 2023, 13, e2023139. [Google Scholar] [CrossRef] [PubMed]
- Rudnicka, L.; Olszewska, M.; Waśkiel, A.; Rakowska, A. Trichoscopy in Hair Shaft Disorders. Dermatol. Clin. 2018, 36, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Perera, E.; Yip, L.; Sinclair, R. Alopecia areata. Curr. Probl. Dermatol. 2015, 47, 67–75. [Google Scholar] [CrossRef]
- Zhou, C.; Li, X.; Wang, C.; Zhang, J. Alopecia Areata: An Update on Etiopathogenesis, Diagnosis, and Management. Clin. Rev. Allergy Immunol. 2021, 61, 403–423. [Google Scholar] [CrossRef]
- Sterkens, A.; Lambert, J.; Bervoets, A. Alopecia areata: A review on diagnosis, immunological etiopathogenesis and treatment options. Clin. Exp. Med. 2021, 21, 215–230. [Google Scholar] [CrossRef]
- Cranwell, W.C.; Lai, V.W.Y.; Photiou, L.; Meah, N.; Wall, D.; Rathnayake, D.; Joseph, S.; Chitreddy, V.; Gunatheesan, S.; Sindhu, K.; et al. Treatment of alopecia areata: An Australian expert consensus statement. Australas. J. Dermatol. 2019, 60, 163–170. [Google Scholar] [CrossRef]
- Alessandrini, A.; Starace, M.; Bruni, F.; Brandi, N.; Baraldi, C.; Misciali, C.; Fanti, P.A.; Piraccini, B.M. Alopecia areata incognita and diffuse alopecia areata: Clinical, trichoscopic, histopathological, and therapeutic features of a 5-year study. Dermatol. Pract. Concept. 2019, 9, 272–277. [Google Scholar] [CrossRef]
- Asz-Sigall, D.; González-De-Cossio-Hernández, A.C.; Rodríguez-Lobato, E.; Ortega-Springall, M.F.; Vega-Memije, M.E.; Guzmán, R.A. Differential diagnosis of female-pattern hair loss. Ski. Appendage Disord. 2016, 2, 18–21. [Google Scholar] [CrossRef]
- Ciongariu, A.M.; Țăpoi, D.A.; Dumitru, A.V.; Bejenariu, A.; Marin, A.; Costache, M. Pleomorphic Liposarcoma Unraveled: Investigating Histopathological and Immunohistochemical Markers for Tailored Diagnosis and Therapeutic Innovations. Medicina 2024, 60, 950. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.J.; Schwartz, R.A.; Janniger, C.K. Alopecia areata. Pathogenesis, diagnosis, and therapy. Am. J. Clin. Dermatol. 2000, 1, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.P.; Farrukh, S.N. Association between alopecia areata and thyroid dysfunction. Postgrad. Med. 2021, 133, 895–898. [Google Scholar] [CrossRef]
- Villasante Fricke, A.C.; Miteva, M. Epidemiology and burden of alopecia areata: A systematic review. Clin. Cosmet. Investig. Dermatol. 2015, 8, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Fechine, C.O.C.; Valente, N.Y.S.; Romiti, R. Lichen planopilaris and frontal fibrosing alopecia: Review and update of diagnostic and therapeutic features. Bras. Dermatol. 2022, 97, 348–357. [Google Scholar] [CrossRef]
- Kossard, S.; Lee, M.S.; Wilkinson, B. Postmenopausal frontal fibrosing alopecia: A frontal variant of lichen planopilaris. J. Am. Acad. Dermatol. 1997, 36, 59–66. [Google Scholar] [CrossRef]
- Giuglea, C.; Burlacu, E.C.; Dumitrache, S.; Tene, M.G.; Marin, A.; Jianu, D.M.; Marinescu, S.A. Negative Pressure Wound Therapy in Postbariatric Lower Body Lift—A Method of Decreasing Postoperative Complications. Aesthetic Plast. Surg. 2022, 46, 2882–2890. [Google Scholar] [CrossRef]
- Baibergenova, A.; Donovan, J. Lichen planopilaris: Update on pathogenesis and treatment. Skinmed 2013, 11, 161–165. [Google Scholar]
- Bertoli, M.J.; Sadoughifar, R.; Schwartz, R.A.; Lotti, T.M.; Janniger, C.K. Female pattern hair loss: A comprehensive review. Dermatol. Ther. 2020, 33, e14055. [Google Scholar] [CrossRef]
- Saini, K.; Mysore, V. Role of vitamin D in hair loss: A short review. J. Cosmet. Dermatol. 2021, 20, 3407–3414. [Google Scholar] [CrossRef] [PubMed]
- York, K.; Meah, N.; Bhoyrul, B.; Sinclair, R. A review of the treatment of male pattern hair loss. Expert Opin. Pharmacother. 2020, 21, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Love, R.P.; Harris, J.A. Old Friend or New Ally: A Comparison of Follicular Unit Transplantation and Follicular Unit Excision Methods in Hair Transplantation. Dermatol. Surg. 2020, 46, 1078–1083. [Google Scholar] [CrossRef] [PubMed]
- Maletic, A.; Dumic-Cule, I.; Zic, R.; Milosevic, M. Impact of Hair Transplantation on Quality of Life. Aesthetic Plast. Surg. 2024, 48, 1825–1830. [Google Scholar] [CrossRef] [PubMed]
- Varothai, S.; Bergfeld, W.F. Androgenetic alopecia: An evidence-based treatment update. Am. J. Clin. Dermatol. 2014, 15, 217–230. [Google Scholar] [CrossRef]
- Emer, J. Platelet-Rich Plasma (PRP): Current Applications in Dermatology. Ski. Ther. Lett. 2019, 24, 1–6. [Google Scholar]
- Paichitrojjana, A.; Paichitrojjana, A. Platelet Rich Plasma and Its Use in Hair Regrowth: A Review. Drug Des. Dev. Ther. 2022, 16, 635–645. [Google Scholar] [CrossRef]
- Jianu, D.M.; Marin, A. Invited Discussion on: Evaluation of Chlorhexidine Concentration on the Skin After Preoperative Surgical Site Preparation in Breast Surgery-A Randomized Controlled Trial. Aesthetic Plast. Surg. 2022, 46, 1523–1524. [Google Scholar] [CrossRef]
- Mysore, V.; Shashikumar, B.M. Guidelines on the use of finasteride in androgenetic alopecia. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 128–134. [Google Scholar] [CrossRef]
- Gentile, P.; Garcovich, S. Advances in Regenerative Stem Cell Therapy in Androgenic Alopecia and Hair Loss: Wnt pathway, Growth-Factor, and Mesenchymal Stem Cell Signaling Impact Analysis on Cell Growth and Hair Follicle Development. Cells 2019, 8, 466. [Google Scholar] [CrossRef]
- English, R.S., Jr.; Ruiz, S.; DoAmaral, P. Microneedling and Its Use in Hair Loss Disorders: A Systematic Review. Dermatol. Ther. 2022, 12, 41–60. [Google Scholar] [CrossRef] [PubMed]




| In Which Areas Do You Have Problems with Hair Falling? Sex Crosstabulation | |||||
|---|---|---|---|---|---|
| Sex | Total | ||||
| Female | Male | ||||
| In which areas do you have problems with hair falling? | Both the frontal and vertex areas | Count | 8 | 11 | 19 |
| % within “In which areas do you have problems with hair falling?” | 42.1% | 57.9% | 100.0% | ||
| Diffuse falling (including occipital and temporal regions) | Count | 11 | 1 | 12 | |
| % within “In which areas do you have problems with hair falling?” | 91.7% | 8.3% | 100.0% | ||
| Frontal area | Count | 6 | 9 | 15 | |
| % within “In which areas do you have problems with hair falling?” | 40.0% | 60.0% | 100.0% | ||
| Vertex area | Count | 2 | 2 | 4 | |
| % within “In which areas do you have problems with hair falling?” | 50.0% | 50.0% | 100.0% | ||
| Total | Count | 27 | 23 | 50 | |
| % within “In which areas do you have problems with hair falling” | 54.0% | 46.0% | 100.0% | ||
| Chi-Square Tests | |||
|---|---|---|---|
| Value | df | Asymptotic Significance (Two-Sided) | |
| Pearson Chi-Square | 9.146 a | 3 | 0.027 |
| Likelihood Ratio | 10.511 | 3 | 0.015 |
| N of Valid Cases | 50 | ||
| Have You Had a Hair Transplant? Satisfaction Degree_Colapsed Crosstabulation | ||||||
|---|---|---|---|---|---|---|
| Satisfaction Degree_Colapsed | Total | |||||
| Very Dissatisfied/Dissatisfied | Neutral | Satisfied/Very Satisfied | ||||
| Have you had a hair transplant? | No | Count | 7 | 19 | 18 | 44 |
| % within “Have you had a hair transplant?” | 15.9% | 43.2% | 40.9% | 100.0% | ||
| YES | Count | 0 | 0 | 6 | 6 | |
| % within “Have you had a hair transplant?” | 0.0% | 0.0% | 100.0% | 100.0% | ||
| Total | Count | 7 | 19 | 24 | 50 | |
| % within “Have you had a hair transplant?” | 14.0% | 38.0% | 48.0% | 100.0% | ||
| VAR00004 Satisfaction Degree_Colapsed Crosstabulation | ||||||
|---|---|---|---|---|---|---|
| Satisfaction Degree_Colapsed | Total | |||||
| Very Dissatisfied/Dissatisfied | Neutral | Satisfied/Very Satisfied | ||||
| VAR00004 | 0.00 | Count | 1 | 6 | 1 | 8 |
| % within VAR00004 | 12.5% | 75.0% | 12.5% | 100.0% | ||
| 1.00 | Count | 2 | 6 | 7 | 15 | |
| % within VAR00004 | 13.3% | 40.0% | 46.7% | 100.0% | ||
| 2.00 | Count | 2 | 4 | 5 | 11 | |
| % within VAR00004 | 18.2% | 36.4% | 45.5% | 100.0% | ||
| 3.00 | Count | 2 | 3 | 11 | 16 | |
| % within VAR00004 | 12.5% | 18.8% | 68.8% | 100.0% | ||
| Total | Count | 7 | 19 | 24 | 50 | |
| % within VAR00004 | 14.0% | 38.0% | 48.0% | 100.0% | ||
| Value | df | Asymptotic Significance (Two-Sided) | |
|---|---|---|---|
| Pearson Chi-Square | 8.203 a | 6 | 0.224 |
| Likelihood Ratio | 8.628 | 6 | 0.196 |
| N of Valid Cases | 50 |
| For How Long Have You Been Using Treatment for Hair Regeneration? vs. After the Undergone Treatment —How Did Hair Density Evolve? Crosstabulation | ||||||
|---|---|---|---|---|---|---|
| After the Undergone Treatment, How Did Hair Density Evolve? | Total | |||||
| Hair Density Improved Moderately | Hair Density Improved Significantly | There Was No Change | ||||
| For how long have you used treatment for hair regeneration? | I had treatment for a longer period of time and then I stopped | Count | 4 | 1 | 2 | 7 |
| % within “For how long have you been using treatment for hair regeneration?” | 57.1% | 14.3% | 28.6% | 100.0% | ||
| I have been on and off different hair treatments and I continue | Count | 12 | 1 | 2 | 15 | |
| % within “For how long have you been using treatment for hair regeneration?” | 80.0% | 6.7% | 13.3% | 100.0% | ||
| I have been on and off different hair treatments but I stopped | Count | 5 | 0 | 1 | 6 | |
| % within “For how long have you been using treatment for hair regeneration?” | 83.3% | 0.0% | 16.7% | 100.0% | ||
| I only had one treatment and then I stopped | Count | 4 | 1 | 17 | 22 | |
| % within “For how long have you been using treatment for hair regeneration?” | 18.2% | 4.5% | 77.3% | 100.0% | ||
| Total | Count | 25 | 3 | 22 | 50 | |
| % within For how long have you been using treatment for hair regeneration? | 50.0% | 6.0% | 44.0% | 100.0% | ||
| For How Long Have You Been Using Treatment for Hair Regeneration? After the Undergone Treatment —How Did the Hair Follicle Thickness Evolve? Crosstabulation | ||||||
|---|---|---|---|---|---|---|
| After the Undergone Treatment, How Did the Hair Follicle Thickness Evolve? | Total | |||||
| Hair Follicle Thickness Improved Moderately | Hair Follicle Thickness Improved Significantly | There Was No Change | ||||
| For how long have you been using treatment for hair regeneration? | I had treatment for a longer period of time and then I stopped | Count | 3 | 1 | 3 | 7 |
| % within “For how long have you been using treatment for hair regeneration?” | 42.9% | 14.3% | 42.9% | 100.0% | ||
| I have been on and off different hair treatments and I continue | Count | 13 | 1 | 1 | 15 | |
| % within “For how long have you been using treatment for hair regeneration?” | 86.7% | 6.7% | 6.7% | 100.0% | ||
| I have been on and off different hair treatments but I stopped | Count | 3 | 1 | 2 | 6 | |
| % within “For how long have you been using treatment for hair regeneration?” | 50.0% | 16.7% | 33.3% | 100.0% | ||
| I only had one treatment and then I stopped | Count | 4 | 1 | 17 | 22 | |
| % within “For how long have you been using treatment for hair regeneration?” | 18.2% | 4.5% | 77.3% | 100.0% | ||
| Total | Count | 23 | 4 | 23 | 50 | |
| % within “For how long have you been using treatment for hair regeneration?” | 46.0% | 8.0% | 46.0% | 100.0% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, A.; Băloi, S.-E.; Marinescu, S.A.; Dumitru, A.V.; Țăpoi, D.A.; Ciongariu, A.M.; Tampa, M.-Ș.; Caunic, M.-R.; Șerban, D.; Giuglea, C. Idiopathic Alopecia—A Retrospective Descriptive Study Integrated with the Current Literature. Cosmetics 2025, 12, 46. https://doi.org/10.3390/cosmetics12020046
Marin A, Băloi S-E, Marinescu SA, Dumitru AV, Țăpoi DA, Ciongariu AM, Tampa M-Ș, Caunic M-R, Șerban D, Giuglea C. Idiopathic Alopecia—A Retrospective Descriptive Study Integrated with the Current Literature. Cosmetics. 2025; 12(2):46. https://doi.org/10.3390/cosmetics12020046
Chicago/Turabian StyleMarin, Andrei, Sabina-Eliza Băloi, Silviu Adrian Marinescu, Adrian Vasile Dumitru, Dana Antonia Țăpoi, Ana Maria Ciongariu, Mircea-Ștefan Tampa, Maria-Roxana Caunic, Dragoș Șerban, and Carmen Giuglea. 2025. "Idiopathic Alopecia—A Retrospective Descriptive Study Integrated with the Current Literature" Cosmetics 12, no. 2: 46. https://doi.org/10.3390/cosmetics12020046
APA StyleMarin, A., Băloi, S.-E., Marinescu, S. A., Dumitru, A. V., Țăpoi, D. A., Ciongariu, A. M., Tampa, M.-Ș., Caunic, M.-R., Șerban, D., & Giuglea, C. (2025). Idiopathic Alopecia—A Retrospective Descriptive Study Integrated with the Current Literature. Cosmetics, 12(2), 46. https://doi.org/10.3390/cosmetics12020046








