Abstract
Phytosphingosine-based 1-O-acylceramide (CerENP) from the human stratum corneum has not been reported on. High-resolution mass spectrometry was used to identify CerENP from skin samples. A vehicle-controlled human study was performed to investigate the physiological interaction between ceramide NP (CerNP) and CerENP with respect to skin hydration, cohesion, and TEWL, all of which were measured. Twenty volunteers were treated with test creams containing CerENP together with CerNP, which significantly improved skin barrier parameters after four weeks of application: 1. Skin hydration was increased by 26% compared to when CerNP was used alone, and moisture retention was better than it was with the CerNP control. 2. Stratum corneum (SC) cohesion was strengthened significantly only when using the test cream formulated with CerENP. The results suggested the boosting effects of CerENP on the skin barrier functions exerted by CerNP since only a small amount is required, as low as one-tenth of CerNP. This is the first report on the identification of CerENP in the human SC and its skin barrier activities in human skin. In conclusion, the combinatorial use of CerENP and CerNP at an appropriate relative ratio could be a new normal in developing an ideal moisturizer for dry and atopic skin.
1. Introduction
Ceramides form an essential permeability barrier in the human stratum corneum in combination with cholesterol and free fatty acids (FFAs) [1,2]. A plethora of information on ceramides’ biological and skin physiological functions has been published [3,4]. Among the three skin physiological lipids, the molecular diversity of ceramides is the most complicated. The permeability of the epidermal barrier varies depending on the amount of ceramides and compositional changes among different ceramide classes [5,6,7,8]. The classes of ceramides in the human stratum corneum are categorized depending on sphingoids and fatty acids binding to the amino group of sphingoids. Sphingosine (S), dihydrosphingosine (DS), phytosphingosine (P), and 6-hydroxysphingosine (H) are four major sphingoids. Three different types of fatty acids, including nonhydroxy fatty acid (N), α-hydroxy fatty acid (A), and ω-esterified fatty acid (EO), bind to the sphingoid to make a ceramide. Ceramide NP is formed by conjugating a nonhydroxy fatty acid to phytosphingosine, and α-hydroxy fatty acid is attached to make ceramide AP. The number of ceramide subclasses has increased with the advent of analytical methods [9,10,11]. Most recently, Suzuki M. et al. reported that the total number of ceramide classes is 25 based on the newly identified sphingoid 1,14-sphinganine and a β-hydroxy fatty acid [12]. In addition to ceramide classes, the chain length of ceramides is also an essential factor that determines the lipid lamellar organization’s physical and chemical characteristics [13,14,15,16,17]. Since Man et al. first showed that topical applications with ceramides could normalize abnormal skin barrier conditions, ceramide NP has been dominantly utilized over the last 30 years [18,19,20,21,22]. Recently, ceramide NS (CerNS), ceramide NDS (CerNDS), ceramide AP (CerAP), and ceramide EOP (CerEOP) have become available. Accordingly, the number of investigations on the effects of CerNS, CerNP, CerAP, and CerEOP, individually or in combination, on barrier permeability, lipid organization, and interactions between specific ceramide classes has increased [23,24,25,26,27].
1-O-acylceramide (1-OAC) was first identified in human and mouse epidermis in 2013 and named ceramide CER 1-O-ENS (CerENS) based on the CerNS backbone and 1-O-EAS (CerEAS) based on CerAS [28]. In 2017, the complexity of 1-O-acylceramide isolated from mouse epidermis was unveiled to find ceramide ENS with C18-sphingosine as the major 1-O-acylceramide. As a minor class, ceramide CER 1-O-ENDS (CerENDS) was also identified [29]. Another group also reported the presence of ENS in fetal vernix caseosa and speculated about the waterproof function of 1-O-acylceramide [30]. In addition, a new 1-O-acylceramide having an esterified ω-hydroxy acyl group was identified in the reconstructed human epidermis obtained from the culture of human-derived epidermal keratinocytes [31]. However, the existence of 1-O-acylceramide having phytosphingosine as a sphingoid backbone has not been reported yet, though CER 1-O-EAP (CerEAP) was found in cultured human keratinocytes [31].
The biological function of 1-O-acylceramide remains unknown. However, it is speculated that 1-O-acylceramide might play an essential role in the formation of lipid multilamellar organization although it consists of only 2~3% of the total ceramides. One report showed that 1-O-acylceramide affected skin permeability barrier function in mice [32]. Identifying ENS in fetal vernix caseosa would suggest the importance of 1-O-acylceramide in the epidermal permeability barrier’s function. The finding of a more than 2-fold increase in 1-O-acylceramide when the biosynthetic process of ω-esterified ceramides such as CerEOS and CerEOP was blocked in a mouse model further supports this notion [30]. This result suggested that 1-O-acylceramide could compensate for the deficiency of ω-esterified ceramides by forming an extended structure that has C24 lignoceric acid esterified at the 1-O-position that can still support the long periodicity phase (LPP) in lamellar organization [32]. However, the precise role of this new ceramide in lipid lamellar organization needs further investigation.
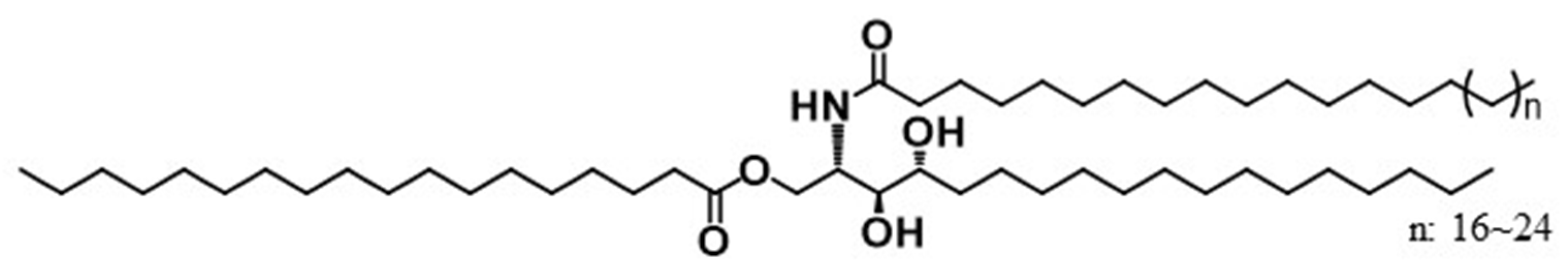
Previously, we reported on the synthesis of 1-O-stearoyl ceramide NP [33]. The chemical structure of CerENP resembles an ‘anchor bolt’ having a hydrophilic head group in the center while also having bidirectionally splayed hydrophobic acyl groups. Based on this characteristic structure, we proposed a ‘Bidirectional Anchoring Model’ for CerENP’s mode of action, which plays a role as a linker ceramide. To obtain a shred of evidence for this model, we performed physicochemical analyses and molecular dynamic simulations. Stratum corneum-mimetic nanovesicles were prepared using CerNP/cholesterol/fatty acid (model SC lipids) in combination with CerENP. The results strongly suggested that CerENP tightened the multilamellar structure of SCNV, improving long-term stability [33]. All-atom molecular dynamic simulation with the sandwich model framework of the LPP was conducted to gain more insight into the role of ENP in lamellar organization and skin barrier permeability. The results showed that the presence of ENP induces a compact lipid matrix in the lateral dimension of the skin barrier model. In addition, the data demonstrated that ENP retarded the penetration of ethanol through a lipid matrix [34]. When a reconstructed epidermis (RHE) was treated with ENP, the integrity of the stratum corneum was well maintained, while the untreated control was significantly disturbed [35]. This result again suggested that ENP could strengthen the lipid lamellar structure.
Having these outcomes from in vitro characterizations and molecular dynamic simulations, we wanted to identify the presence of ENP in the human skin before investigating its influence on the human skin barrier’s functions in combination with CerNP. Identifying CerENP in the human epidermis should make it more physiologically meaningful when proven to positively affect skin barrier function.
2. Materials and Methods
2.1. Identification of 1-O-Stearoyl-Ceramide NP (18:0-t18:0/18:1) in Human Stratum Corneum
Human stratum corneum samples were obtained by five tape strippings using D-squame standard tape (Cuderm, Dallas, TX, USA, diameter, 2.2 mm). Skin ceramides were extracted using methanol, and the solvent extracts were dried under a nitrogen stream at 30 °C. To analyze ceramides, the extracts were dissolved in 100 μL of chloroform/methanol (1/9, v/v). Aliquots were subjected to liquid chromatography/high-resolution mass spectrometry (LC-HRMS) to determine 1-O-stearoyl-ceramide NP (18:0-t18:0/18:1) in the human stratum corneum samples. A Vanquish LC system coupled with a Q Exactive Focus Orbitrap mass spectrometer was used, and chromatograms and mass spectra were analyzed using the Thermo Xcalibur 4.5 software (Thermo Fisher Scientific Inc., Waltham, MA, USA). A Kinetex C18 column (100 × 2.1 mm, 2.6 μm particle size, Phenomenex, Torrance, CA, USA) was used to separate samples. The mobile phase consisted of 10 mM ammonium acetate in 90% methanol (A) and 10 mM ammonium acetate in isopropyl alcohol/methanol (1/1, v/v) mixtures (B) and was set as 30% B (0 min), 95% B (15–20 min), and 30% B (20.1–25 min). The flow rate was set to 0.2 mL/min. A full scan in positive mode was performed at 70,000 FWMH resolution (full width at half maximum), and MS/MS spectra were acquired at m/z 200–900 at a resolution of 17,500 in the daughter ion scan mode. Parallel reaction monitoring (PRM) was also employed, and the PRM transition m/z 848.8066 (MH+) was used for the detection of 1-O-stearoyl-ceramide NP (18:0-t18:0/18:1).
2.2. Ceramides and Test Creams
Ceramide NP (HP-EcoCeramide LCSTM) was produced using a process previously reported with some modifications to adjust the fatty acid compositions [36]. The product was a mixture of ceramide NPs with different fatty acids that originated from 4 different natural oils, Shea butter, Moringa, Meadowfoam, and Macadamia. The percentile profiles of N-acyl moieties in ceramide NP are C16(4.0), C16:1(0.9), C18:0(35.8), C18:1(44.1), C18:2(4.6), C20:0(1.9), C20:1(4.0), C22:0(0.9), C22:1(1.1), and C24(0.3). N-stearoyl phytosphingosine and N-oleoyl phytosphingosine are the two most abundant subclasses of this ceramide NP mixture. Ceramide ENP (Figure 1) was synthesized by conjugating stearic acid to the first hydroxyl group of CerNP as described before. Test creams were prepared by mixing each ceramide with the base cream formulation to produce a targeted ceramide content. The base cream avoids glycerin and emollients as much as possible to minimize the effects on SC hydration measurement. Then, 0.02%, 0.05%, 0.2%, and 0.5% of ENP were formulated together with a vehicle cream containing 0.2% of CerNP as a control (Table 1).
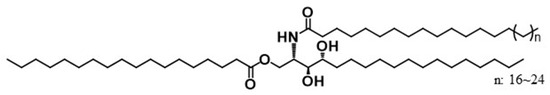
Figure 1.
1-O-stearoyl ceramide NP (CER 1-O-ENP).

Table 1.
Ingredients for vehicle cream and test creams.
2.3. Human Study
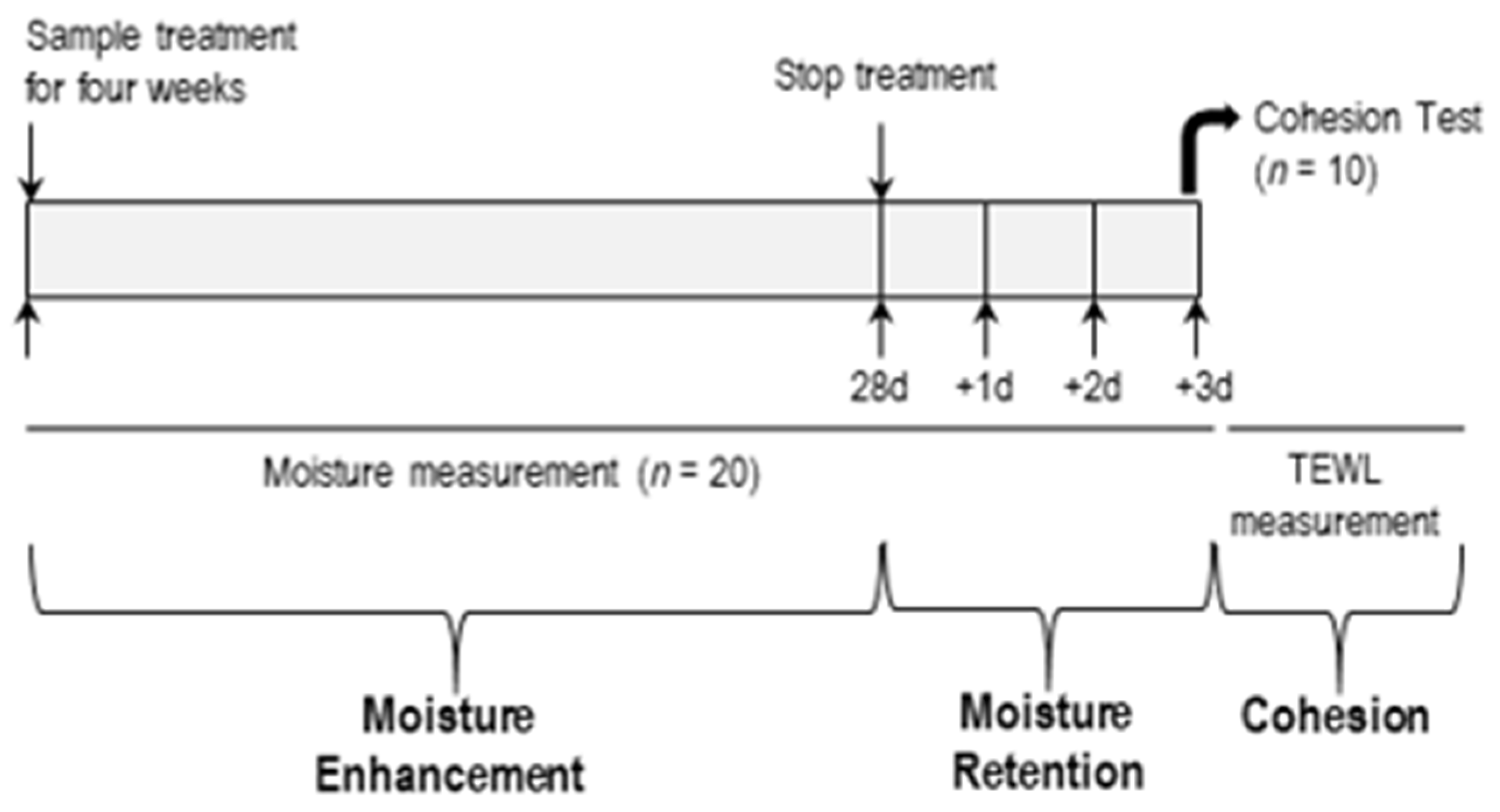
A double-blind, intra-subject, vehicle-controlled human study was performed. The human study aimed to investigate the effects of CerENP on skin barrier functions in combination with CerNP. Twenty women aged 20–29 (3), 30–39 (13), and 40–49 (4) who volunteered for this study and had no history of previous abnormal skin conditions were recruited with written consent. The study scheme is outlined in Figure 2, and skin barrier parameters include SC hydration, the retention of hydration, and SC cohesion. All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki. This study was approved by the Institutional Review Board of Dongguk University Committee on Human Research (Approval no.: DUIRB-2022051-01; Approval date: 30 May 2022). Written consent for participation from each volunteer was documented according to the ethical standards.
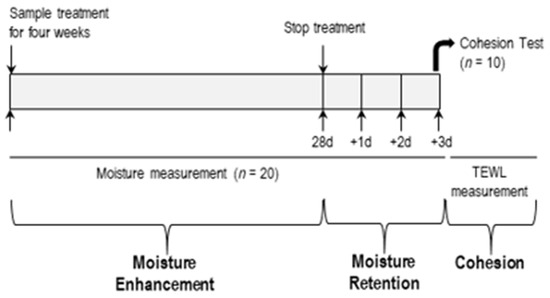
Figure 2.
Scheme of human study.
2.4. Measurement of Skin Hydration, Retention of Hydration, and SC Cohesion
2.4.1. Assessment of SC Hydration and Retention of Hydration
The assessment of SC hydration was also performed according to a previously described procedure [32]. To measure hydration, a 2.5 cm2 area of the forearms of all volunteers was treated twice a day, morning and evening, with 25 µL (10 µL/cm2) of each test cream for four weeks. Skin hydration measurements were conducted in week 2 and week 4 using a Corneometer CM820 device (Courage & Khazaka Electronic GmbH, Koln, Germany). Moisture retention was analyzed for three days after stopping the application of the respective test creams. Volunteers were asked not to apply any other cosmetic moisturizer during this period. All test sites were acclimated for 30 min in a controlled room (temperature of 24.1 ± 1 °C and relative humidity of 42.5 ± 1%). Before measurements were taken, any materials on the skin sites were gently wiped off using soft tissue paper. The changed value of arbitrary units was calculated from each baseline and expressed as the mean ± standard error of the mean (SEM).
2.4.2. SC Cohesion
SC cohesion was expressed as Δ TEWL between baseline and after four weeks of applying the test cream on the application sites. Volunteers were asked to apply the designated test creams to a 2.5 cm2 area of the forearms twice a day. TEWL was measured to calculate SC cohesion immediately after tape stripping 15 times with D-Squame tape. A D500-D-Squame Pressure Instrument was used for tape stripping in order to maintain experimental consistency. The pressure applied was 225 g/cm2, and tape stripping was conducted in an air-conditioned room at 20.4 ± 0.2 °C and 40.5 ± 1.0% relative humidity [37]. D-Squame tape and the D500-D-Squame Pressure Instrument were purchased from CuDerm Corporation, Dallas, TX, USA.
2.5. Microscopic Observations of Maltese Cross Appearance
Microscope images were observed for test creams formulated with CerENP and Cer NP at two time points: immediately after preparation and after 6 months of RT storage. Optical anisotropy was observed under a cross-polarized light microscope (Nikon Corporation, Tokyo, Japan).
2.6. Statistical Analysis
All data are represented as the mean ± standard deviation (SD), with differences between means assessed with IBM SPSS Statistics for Windows version 23 (IBM Corp., Armonk, NY, USA). Data analysis was performed using an ANOVA (one-way analysis of variance) followed by Tukey’s honest significant difference. Significant was defined as a p-value less than 0.05.
3. Results
3.1. Identification of 1-O-Stearoyl-Ceramide NP (18:0-t18:0/18:1) in Human Stratum Corneum
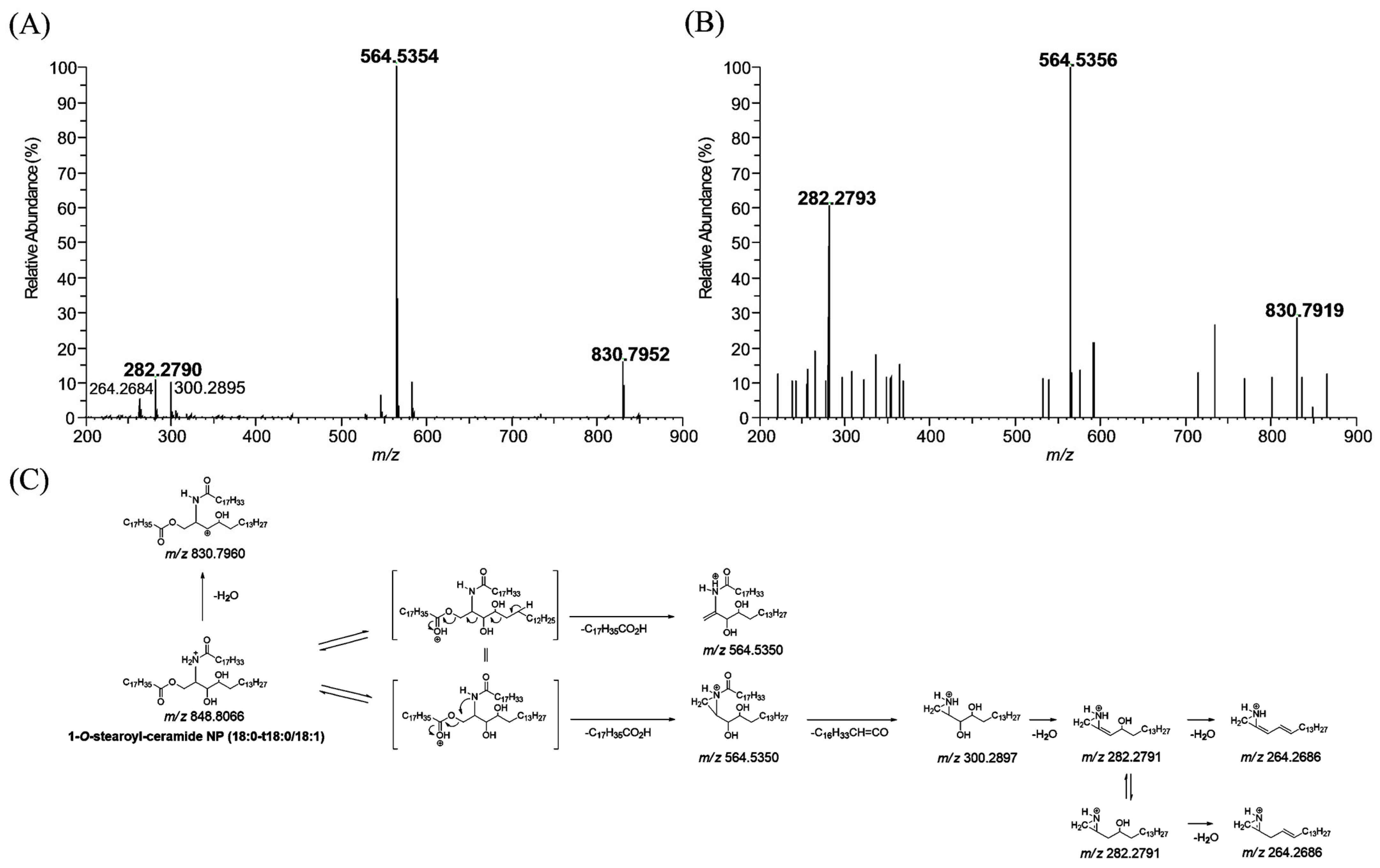
The MS/MS spectrum of 1-O-stearoyl-ceramide NP (18:0-t18:0/18:1) standard, which has a protonated molecular ion [M + H]+ at m/z 848.8066, yielded m/z 564.5354 (base peak) and m/z 282.2790, arising from the elimination of the 1-O-stearoyl group and further cleavage of the N-(9Z)-octadecenoyl substituent with water loss (Figure 3A), respectively. 1-O-stearoyl-ceramide NP can also lose water, resulting in a fragment ion at m/z 830.7952. The part of the proposed fragmentation pathway of 1-O-stearoyl-ceramide NP (18:0-t18:0/18:1) standard is illustrated. 1-O-stearoyl ceramide NP (18:0-t18:0/18:1) was also observed in human stratum corneum extracts. The MS/MS spectrum of the m/z 848.8066 ion which was found in human stratum corneum extracts gave characteristic fragment ions at m/z 830.7919, 564.5356, and 282.2793 (mass error < 5 ppm), which were also observed in 1-O-stearoyl-ceramide NP (18:0-t18:1/18:0) standard (Figure 3B).
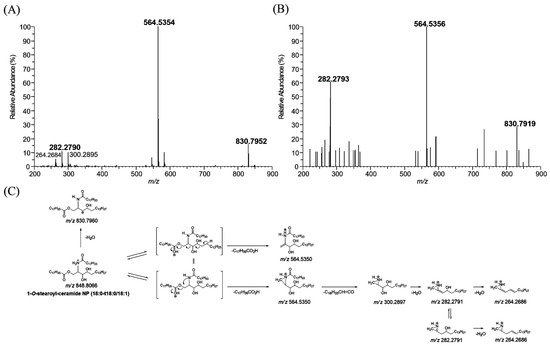
Figure 3.
Production scan mass spectra of 1-O-stearoyl ceramide NP (18:0-t18:0/18:1, m/z 848.8066) obtained by liquid chromatography/high-resolution mass spectrometric analysis of 1-O-stearoyl ceramide NP (18:0-t18:0/18:1, m/z 848.8066) standard (A) and human stratum corneum extracts (B), and their proposed fragmentation scheme (C).
3.2. Profiling of 1-O-Acylceramide NP (CerENP) in Human Stratum Corneum
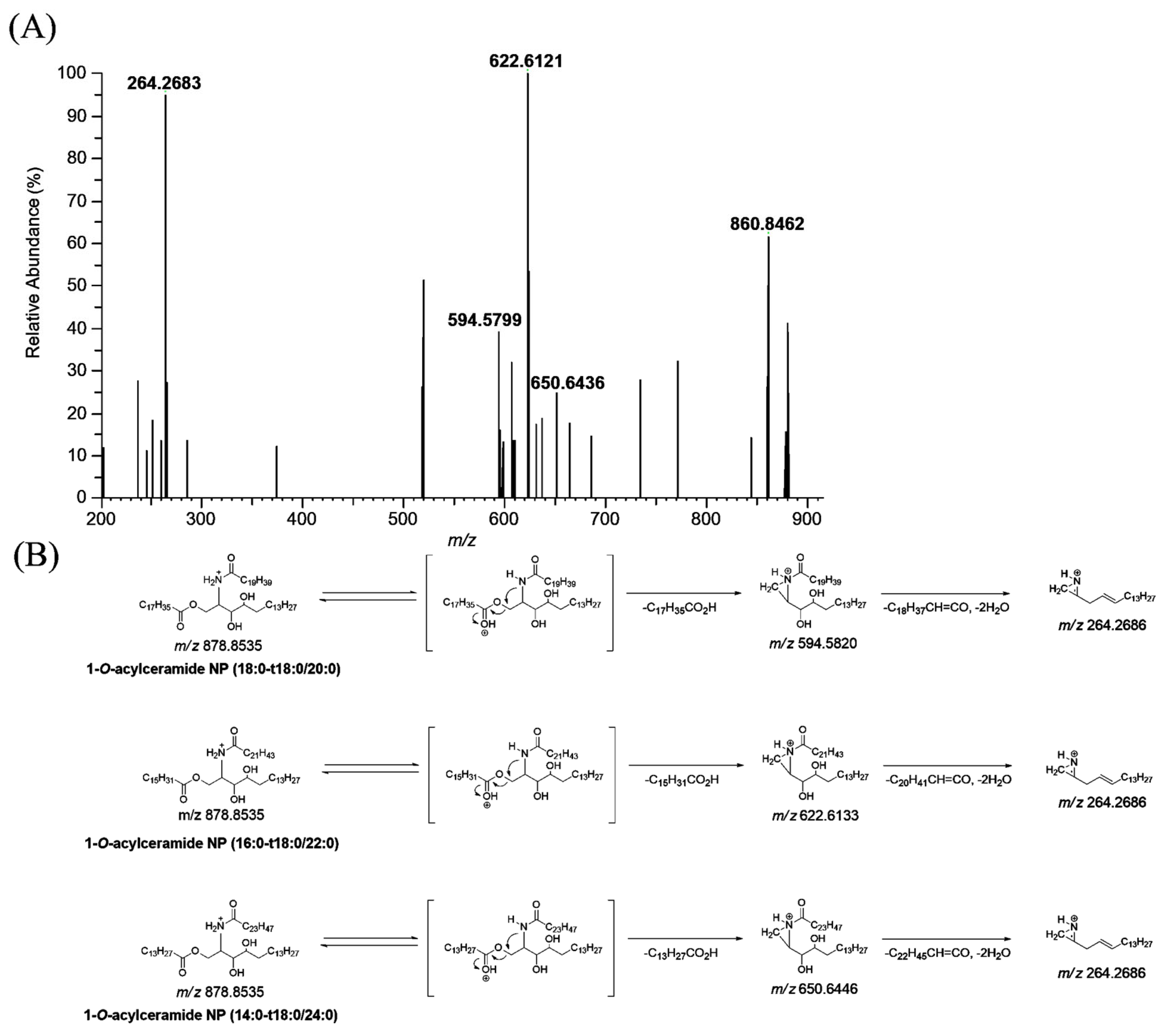
CerENP in the human stratum corneum was profiled using LC-HRMS. The MS/MS spectra of ENP were obtained from the parallel reaction monitoring (PRM) of the protonated adduct ion ([M + H]+) (Figure 4). The extracted ion chromatograms (EICs) for the detected CerENP are shown in Figure S1. Based on the established mass fragmentation patterns of ENP (18:0-t18:0/18:1), nine species of CerENP were identified in the human stratum corneum (Table 2). Interestingly, CerENPs with an elemental composition of CnH2nO5N1 (n = 52–66), suggesting that N-acyl substituents consist of saturated forms, were mainly observed in the human SC. Analyzing the fragments revealed CerENP molecules that differ in ester- and amide-linked fatty acid chain lengths. Multiple isobaric isomers were observed for most CerENP species, which existed as a mixture of two or seven species in the MS/MS spectra. For example, the MS/MS spectra of the ions of m/z 878.8535 (Figure 4A) showed that they included three ceramide NP moieties (fragment ions at m/z 594.5799, 622.6121, and 650.6436 corresponding to dehydrated NP (t18:0/20:0), NP (t18:0/22:0), and NP (t18:0/24:0), respectively, from the cleavage of the 1-O-acyl substituent) (Figure 4B). In addition, the observation of the fragment ion at m/z 264.2683, corresponding to the d18:1 long chain base, reflected the presence of phytosphingosine resulting from the cleavage of the N-acyl substituent and two water molecules from ceramide NP moieties. The confirmation of the suggested structures is further supported by the elemental composition extracted by high-resolution mass spectrometry (deviation within 5 ppm). Therefore, the CerENP species at m/z 878.8535 was identified as the mixture of CerENP (18:0-t18:0/20:0), (16:0-t18:0/22:0), and (14:0-t18:0/24:0). Based on these kinds of fragmentation patterns, the structures of other CerENPs were additionally identified (Table 2).
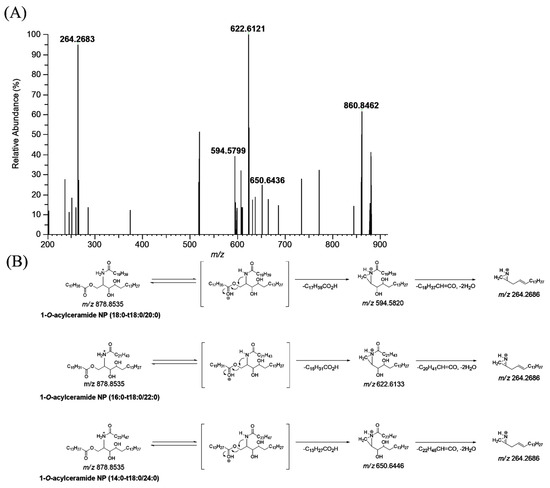
Figure 4.
Production scan mass spectra of 1-O-acylceramide NP (m/z 878.8535) obtained by liquid chromatography/high-resolution mass spectrometric analysis of human stratum corneum extracts (A), and their proposed fragmentation scheme (B).

Table 2.
Summary of 1-O-acylceramide NP identified in the human stratum corneum.
3.3. Effects of CER 1-O-ENP on Multilamellar Formation and Its Stability
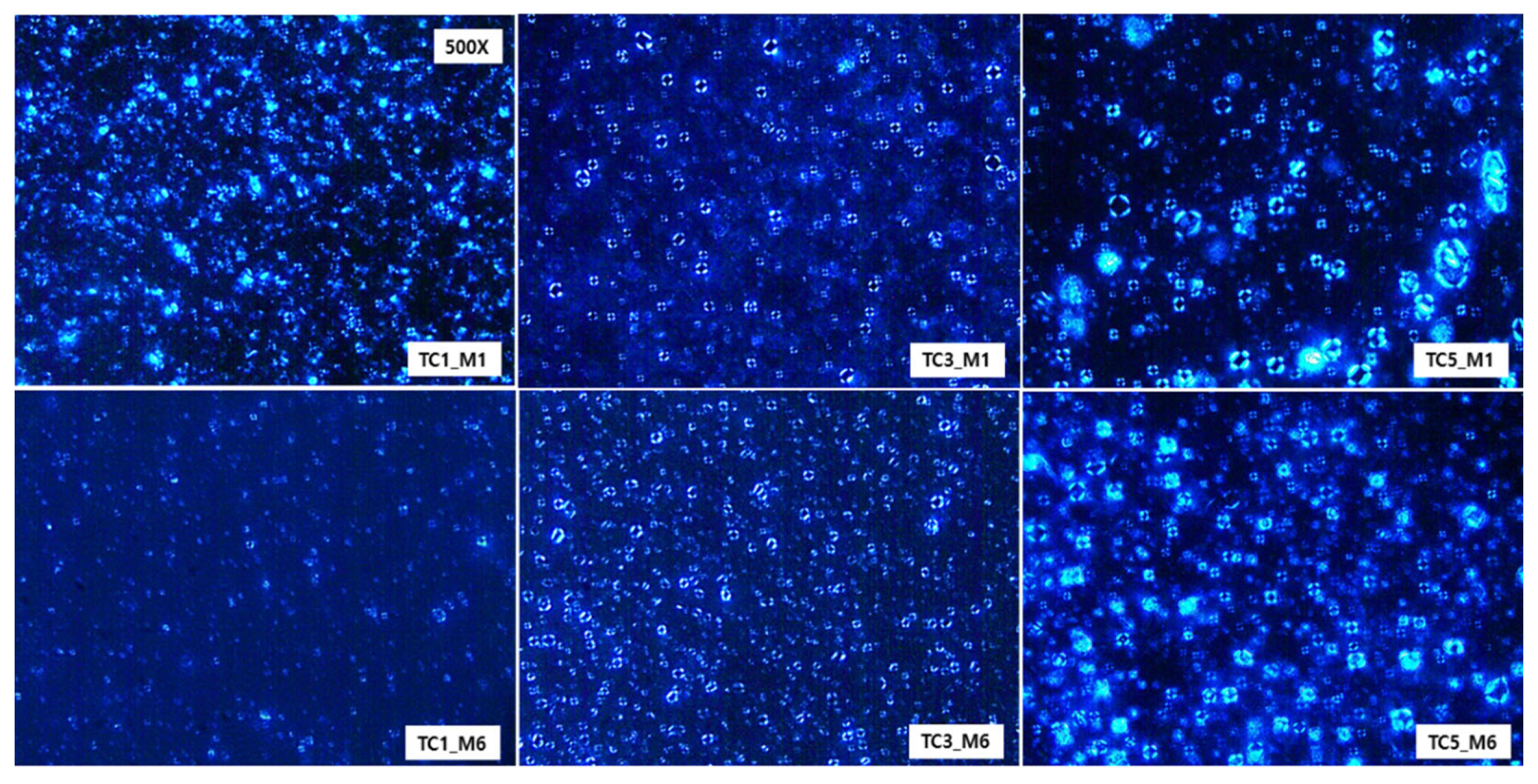
Once test creams were prepared, we assessed the effects of CerENP on forming a multilayer lamellar structure in combination with CerNP and evaluated the long-term stability of the multilamellar structure. The ingredients of test creams are listed in Table 1. A typical concentric lamellar structure could be observed as a Maltese cross appearance under a cross-polarized microscope when ceramide is formulated with cholesterol and free fatty acid. This Maltese cross appearance, optical anisotropy, indicates appropriate lipid lamellar formation. It is generally accepted that a moisturizer with a more typical cross pattern is better for repairing the skin barrier. A month after the preparation of test creams, TC1 containing 0.2% CerNP alone showed almost no Maltese cross formation; even if a few crosses remained, their size became smaller. Test cream TC3, prepared with 0.2% CerNP and 0.05% CerENP, presented numerous typical Maltese crosses. With increasing CerENP (TC5) concentration, the Maltese cross became more prominent in size and of a higher order in the multilamellar organization. This indicated that CerENP enhanced the formation of the multilamellar structure. (Figure 5). After six months of storage at RT, the Maltese cross of TC3 was maintained well but with minor deformation, while TC5 retained its Maltese cross fairly well at almost the same time as the preparation time. TC1 showed a few Maltese crosses.
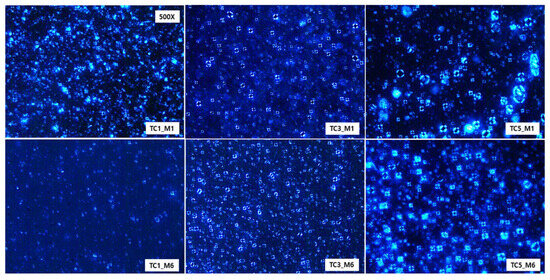
Figure 5.
A cross-polarized microscopic observation (500×) of test creams containing CerENP in combination with CerNP was performed one month (M1) and six months (M6) after the preparation to analyze the formation of Maltese crosses. Samples were kept at room temperature. Test creams were TC1 (0.2% CerNP), TC3 (0.2% CerNP plus 0.05% CerENP), and TC5 (0.2% CerNP plus 0.5% CerENP).
3.4. The Influence of CER 1-O-ENP on Skin Hydration
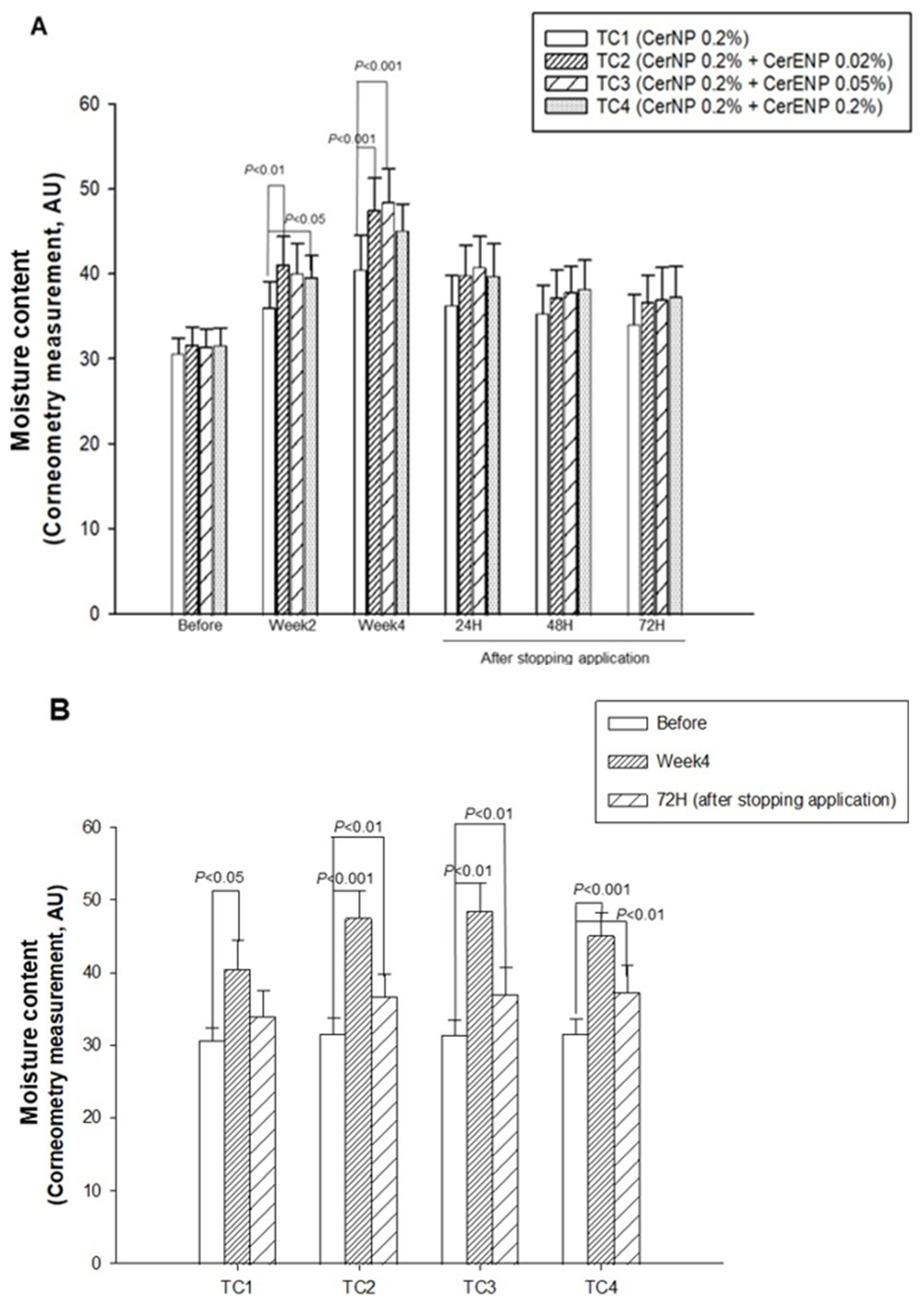
With these results, we investigated the effects of CerENP in combination with CerNP on the human skin barrier’s function. The content of CerNP in test creams was set to be 0.2%. The concentration of CerENP in the test creams was adjusted to be 10 or 25% of the CerNP used in the test creams. It is known that the concentration of 1-O-acylceramide was estimated to be 2~3% of the total ceramides in the human SC. Meanwhile, ceramide NP’s concentration in the human skin barrier was around 22%, indicating that the relative CerENP concentration to CerNP is in the range of 9~13%. The relative content ratio of CerNP to CerENP in the test creams was adjusted within the range that reflects the content ratio of the human skin. As shown in Figure 6A, after four weeks of application, significant enhancements in SC hydration by 32%, 50%, 54%, and 43% were observed on all the skin sites where the test creams TC1, TC2, TC3, and TC4 were applied, respectively. The hydration level of the skin on which TC1 was applied, which was formulated by using only 0.2% CerNP, was considerably increased, as expected. The addition of 0.02% or 0.05% of CerENP to TC1 brought about a dramatic enhancement in SC hydration. More than 20% enhancement, which is statistically significant, was induced by adding CerENP compared to the level of SC hydration enhanced by the application of TC1. The long-lasting moisturizing effects, or retention of hydration, were found to be even greater when ENP was combined with CerNP (Figure 6B). The moisture content of all three test creams formulated with both ENP and CerNP—TC2, TC3, and TC4—remained significantly higher for three days after application ceased compared to the baseline. This was not the case for TC1, which contained only CerNP. This result clearly showed that CerENP in the test cream confers a long-lasting moisturizing effect when applied to the human skin.
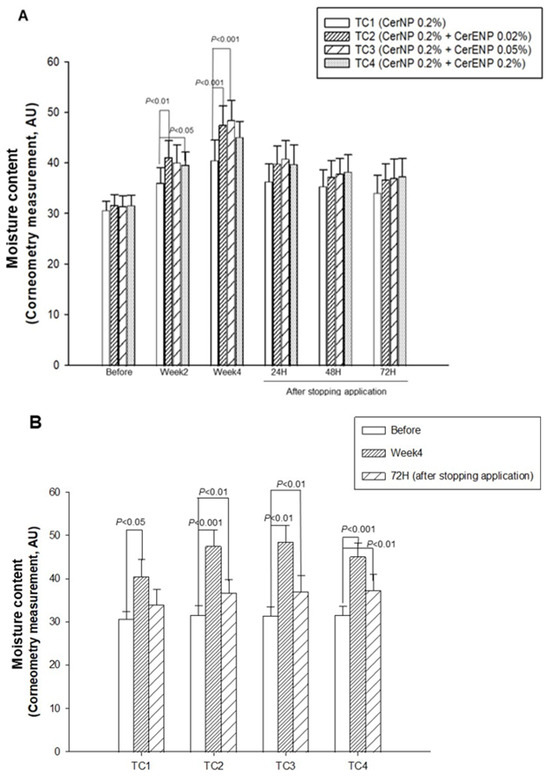
Figure 6.
The effects of CerENP on the moisture level in human skin were measured. The skin hydration level after four weeks of the application of test creams (A) and moisture retention for three days after stopping application (B) were measured. The hydration level is presented in arbitrary units for the capacitance measure by the use of a Corneometer. The data are expressed as the mean ± SD (n = 20).
3.5. CER 1-O-ENP Fostered SC Cohesion
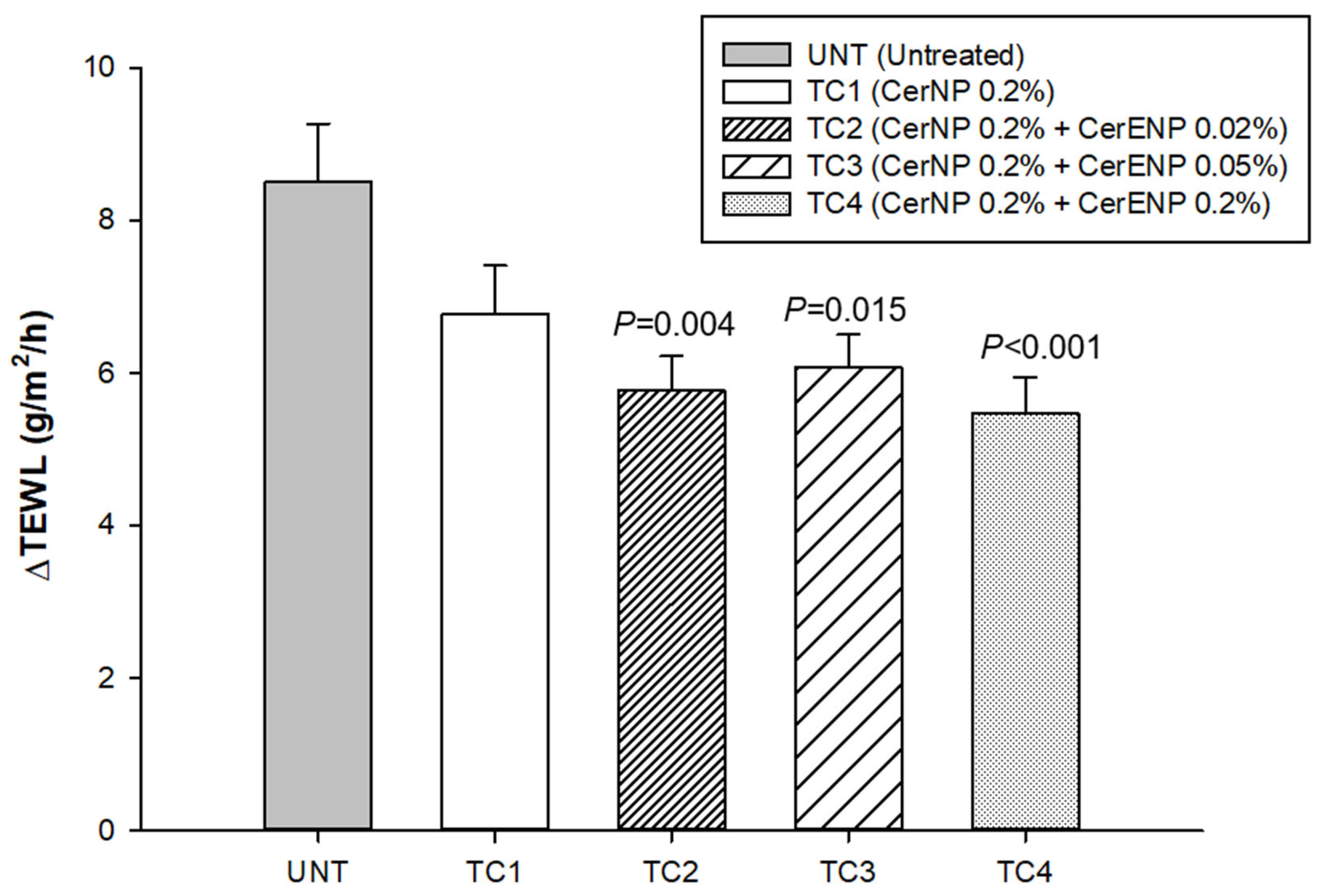
TEWL was measured to calculate ΔTEWL before and immediately after tape stripping 15 times with Desquame disk tapes (CuDerm, Dallas, TX, USA). All the ΔTEWL values obtained from the skin sites where test creams were applied containing ENP (TC2, TC3, TC4) were significantly lower than those of the control cream (TC1) containing only ceramide NP (Figure 7). The results indicated that the stratum corneum became more resistant against the physical force of tape stripping. However, no additional improvement in SC cohesion was observed from a concentration of CerENP higher than 0.02% (TC3 and TC4). The CerENP-to-CerNP ratio seems appropriate at 10 to 25%, similar to the ratio found in human skin. In conclusion, CerENP significantly boosted SC cohesion in combination with CerNP and other ceramides.
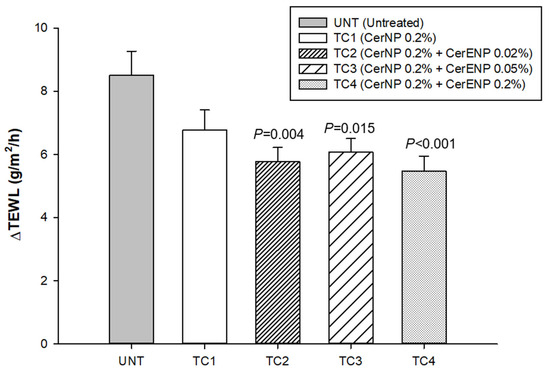
Figure 7.
CerENP fostered SC cohesion. ΔTEWL was calculated between baseline TEWL and TEWL measured after 15 tape strippings after four weeks of application of test cream containing CerENP compared to vehicle cream. Data represent mean ± SD (n = 20).
4. Discussion
This study aimed to investigate the physiological role of CerENP in combination with CerNP in several skin barrier parameters, for example, hydration, moisture retention, and SC cohesion. Although CerENS was identified as the primary class of 1-O-acylceramide, we produced CerENP since phytosphingosine is only affordable on a large scale. So far, no reports on the identification of CerENP have been published. We report for the first time the presence of CerENP in the human stratum corneum, specifically focusing on a sub-species known as 1-O-stearoyl ceramide NP (18:0-t18:0/18:1). This sub-species is the primary component of the CerENP examined in this study. According to a separate ongoing study by Liu with another group, CerENP was estimated to be about one-quarter of CerENS (un-published data). It was also reported that about 30% of sphingosine ceramides converted into CerENS and CerEAS [28]. Identifying CerENP in the human epidermis should make it more physiologically meaningful when proven to positively affect skin barrier function. With this finding, CerENP could be classified as a genuine human skin ceramide.
Multilamellar lipid organization as a permeability barrier is a typical feature of the intercellular space of the skin barrier. It is manifested as a Maltese cross under a polarized light microscope [38]. As shown in Figure 5, CerENP stimulated the formation of a typical Maltese cross more compared to the base cream and the CerNP cream. With the increasing concentrations of CerENP, the Maltese cross became thicker and more prominent. This may indicate that the number of lamellar layers in the Maltese cross increased. More importantly, a striking enhancement in the stability of the Maltese cross after six months of storage at RT was observed from the test cream containing CerENP. This observation aligned with previous findings from different multilamellar vesicle experiments; CerENP demonstrated that increasing CerENP application could make the multilamellar vesicle (SCNV model) more stable [33]. This finding was further supported by the results from a different multilamellar nanovesicle model, where the addition of CerENP significantly promoted the formation of multilamellar nanovesicles and enhanced their stability during repeated freeze/thaw cycles. [39]. Our study, which utilized the RHE skin model, provides further confirmation that CerENP exerts a stabilizing effect on the lipid multilamellar matrix of the reconstructed human epidermal stratum corneum [34]. This additional evidence underscores the significant role of CerENP in enhancing skin barrier properties.
In this human study, the concentration of CerNP was fixed at 0.2% for every test cream with different CerENP contents because 0.2% CerNP was the lowest concentration to present a marginal yet marked enhancement in skin barrier function. Therefore, we could expect that any CerENP was responsible for any changes from the topical application of test creams with varying concentrations. The results demonstrated that this is the case. Figure 6A shows that when 0.02% and 0.05% CerENPs were added to a control cream containing 0.2% CerNP, a significantly greater skin moisturizing effect was observed compared to the control. However, no further improvement in skin barrier parameters was observed from the skin site treated with the test creams containing more than 0.05% CerENP. The results indicated that 0.02% or 0.05% CerENP seemed appropriate in this formulation context, representing 1/10~1/4 of CerNP. This ratio is similar to that of the actual skin because 1-O-acylceramide only comprises less than 5% of the total ceramides in the skin barrier. A healthy skin barrier functions effectively due to its composition of ceramides and maintains proper moisture balance, primarily because of the high level of NMF found within the corneocytes. It is understood that the long-lasting moisture content in the skin barrier largely depends on the NMF levels in the corneocytes. Thus, the enhanced skin hydration observed with the application of CerENP and its long-lasting moisturizing effects can be attributed to the increased NMF, which enhances the water-holding capacity of the corneocytes. Further studies will be required to reveal whether the NMF level is enhanced by CerENP treatment.
Corneodesmosome is a primary factor responsible for SC cohesion. However, the lipid multilamellar organization interdigitated with cornified lipid envelopes may also contribute to SC cohesion as a minor determinant. In this study, we tried to measure SC cohesion as a whole, not to measure the amount of corneodesmosome. A significant decrease in ΔTEWL (Figure 7) was observed only from test creams. This result suggested that ENP fostered SC cohesion, most probably by increasing corneodesmosome. However, SC cohesion could also be strengthened by fostering lipid multilamellar organization via the anchoring action of CerENP. Further studies are required to elucidate whether CerENP can enhance the formation of corneodesmosome. Data from human studies were not well correlated with those from the experiment on the effects of Maltese cross formation, which exhibited the dose-dependent effects of CerENP. With the increasing concentration of CerENP, Maltese cross formation was significantly increased with enhanced stability. This difference could be attributed to the difference between in vivo human ceramide complexity and a simple and defined in vitro experimental condition where only limited classes of ceramides were involved. There are more than 20 classes of ceramides with several hundreds of different ceramide species.
Changes in ceramide composition concerning ceramide classes and chain lengths, commonly observed in abnormal skin, are also known to be the primary causes of impaired skin barrier function. However, more investigations are needed to elucidate molecular interactions among different ceramides in the stratum corneum. Recently, Kono et al. conducted a systematic review of the literature describing the effects of ceramide on the human skin from clinical viewpoints and concluded that no report provided details of the composition of ceramides and the concentrations of formulations [19,21,22]. In this study, we used defined dosages of ceramides with complete descriptions of their chemical nature. Some studies provided insights into the interactions between the individual ceramide classes, such as CerNS, CerAP, CerNP, and CerEOS, in forming lipid lamellar organization [25,26,27]. For example, CerNS was shown to have low miscibility with other lipids, and on the other hand, CerNP formed a strong in-plane H-bonding network [27]. Others reported that the ratio between CerNP and CerAP was critical concerning maintaining lamellar stability, and they found that a 2:1 ratio was optimal [25]. It is generally accepted that the presence of EO-class ceramides such as CerEOS and CerEOP is required to establish an LPP organization [23,24]. In this study, we showed a novel interaction between two individual ceramide classes, CerNP and CerENP, showing that a tiny amount of CerENP could significantly promote key skin barrier parameters by boosting the CerNP activity that is otherwise marginal. The boosting effects of CerENP can be explained by findings from previous molecular dynamic simulations [38]. These simulations indicated that splayed CerENP induces notable alterations in the organization of the lipid matrix. Such changes include modifications in surface morphology and lateral packing density, which contribute to a more compact lipid matrix within the stratum corneum lipid lamellar structure, thereby strengthening the permeability barrier. In this regard, our result, the boosting effects of CerENP on the skin barrier activity of CerNP, provided new insight into the relative role of each specific ceramide class. It would be interesting to find whether CerENP has similar boosting activity to other classes of ceramides, such as CerNS or CerNDS.
5. Conclusions
We identified 1-O-stearoyl ceramide NP (18:0/t18:0/18:1), a species of CerENP, along with other sub-species of diverse O-acyl and N-acyl chain length in the human stratum corneum. The results from the human study using a test cream co-formulated with the human skin-identical CerENP, and CerNP suggest there is a boosting effect of CerENP on the improvement in skin barrier functions by CerNP since a surprisingly small amount of CerENP is required. Our findings are the first that report on the physiological role of CerENP in the human skin barrier.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/cosmetics12020047/s1, Figure S1: Representative extracted ion chromatograms of 1-O-acylceramide NP obtained from liquid chromatography/high-resolution mass spectrometric analysis of human stratum corneum extracts.
Author Contributions
B.-G.K. performed liquid chromatography/high-resolution mass spectrometry (LC-HRMS); K.-H.L. conducted data analysis and the interpretation of LC-HRMS data; H.K.C. conducted the human study and data processing; S.K.H. supervised the whole process of the human study; J.W.K. prepared test creams and observed Maltese cross formation; E.O.L. produced CER 1-O-ENP; and C.S.P. supervised this project, designed the human study, gave an interpretation of data, and prepared the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (HP20C0018 and HP23C0129), and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (RS-2024-00411329), Republic of Korea (grant number: HP20C0018).
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Dongguk University, Seoul Korea (Approval date: DUIRB-202205-91; Approval date: 30 May 2022).
Informed Consent Statement
Written consent for participation from each volunteer was documented according to the ethical standards.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
The authors also thank SY Lee from Dongguk University for supporting the Chemdrawing of the CerENP structure.
Conflicts of Interest
The authors EO Lee, JW KIM, and CS Park are employed by the company LCS Biotech and the remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Lampe, M.A.; Burlingame, A.L.; Whitney, J.; Williams, M.L.; Brown, B.E.; Roitman, E.; Elias, P.M. Human stratum corneum lipids: Characterization and regional variations. J. Lipid Res. 1983, 24, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Abe, A.; Jin, K.; Higaki, Y.; Kawashima, M.; Hidano, A. Decreased level of ceramides in stratum corneum of atopic dermatitis: An etiologic factor in atopic dry skin? J. Investig. Dermatol. 1991, 96, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta 2006, 1758, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Janssens, M.; Gooris, G.S.; Bouwstra, J.A. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta 2014, 1841, 295–313. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Hoppel, L.; van der Heijden, R.; Hankemeier, T.; Vreeken, R.J.; Bouwstra, J.A. LC/MS analysis of stratum corneum lipids: Ceramide profiling and discovery. J. Lipid Res. 2011, 52, 1211–1221. [Google Scholar] [CrossRef] [PubMed]
- Zettersten, E.M.; Ghadially, R.; Feingold, K.R.; Crumrine, D.; Elias, P.M. Optimal ratios of topical stratum corneum lipids improve barrier recovery in chronologically aged skin. J. Am. Acad. Dermatol. 1997, 37, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kircik, L.H. Effect of skin barrier emulsion cream vs. a conventional moisturizer on transepidermal water loss and corneometry in atopic dermatitis: A pilot study. J. Drugs Dermatol. 2014, 13, 1482–1484. [Google Scholar] [PubMed]
- Danby, S.G.; Andrew, P.V.; Brown, K.; Chittock, J.; Kay, L.J.; Cork, M.J. An Investigation of the Skin Barrier Restoring Effects of a Cream and Lotion Containing Ceramides in a Multi-vesicular Emulsion in People with Dry, Eczema-Prone, Skin: The RESTORE Study Phase 1. Dermatol. Ther. 2020, 10, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Masukawa, Y.; Narita, H.; Sato, H.; Naoe, A.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; et al. Comprehensive quantification of ceramide species in human stratum corneum. J. Lipid Res. 2009, 50, 1708–1719. [Google Scholar] [CrossRef] [PubMed]
- Masukawa, Y.; Narita, H.; Shimizu, E.; Kondo, N.; Sugai, Y.; Oba, T.; Homma, R.; Ishikawa, J.; Takagi, Y.; Kitahara, T.; et al. Characterization of overall ceramide species in human stratum corneum. J. Lipid Res. 2008, 49, 1466–1476. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Boiten, W.A.; Hankemeier, T.; Rissmann, R.; Bouwstra, J.A.; Vreeken, R.J. Combined LC/MS-platform for analysis of all major stratum corneum lipids, and the profiling of skin substitutes. Biochim. Biophys. Acta 2014, 1841, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Ohno, Y.; Kihara, A. Whole picture of human stratum corneum ceramides, including the chain-length diversity of long-chain bases. J. Lipid Res. 2022, 63, 100235. [Google Scholar] [CrossRef] [PubMed]
- Janssens, M.; van Smeden, J.; Gooris, G.S.; Bras, W.; Portale, G.; Caspers, P.J.; Vreeken, R.J.; Hankemeier, T.; Kezic, S.; Wolterbeek, R.; et al. Increase in short-chain ceramides correlates with an altered lipid organization and decreased barrier function in atopic eczema patients. J. Lipid Res. 2012, 53, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Oguri, M.; Gooris, G.S.; Bito, K.; Bouwstra, J.A. The effect of the chain length distribution of free fatty acids on the mixing properties of stratum corneum model membranes. Biochim. Biophys. Acta 2014, 1838, 1851–1861. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.C.; Hartkamp, R.; Iacovella, C.R.; Bunge, A.L.; McCabe, C. Effect of Ceramide Tail Length on the Structure of Model Stratum Corneum Lipid Bilayers. Biophys. J. 2018, 114, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Pullmannová, P.; Pavlíková, L.; Kováčik, A.; Sochorová, M.; Školová, B.; Slepička, P.; Maixner, J.; Zbytovská, J.; Vávrová, K. Permeability and microstructure of model stratum corneum lipid membranes containing ceramides with long (C16) and very long (C24) acyl chains. Biophys. Chem. 2017, 224, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Uche, L.E.; Gooris, G.S.; Bouwstra, J.A.; Beddoes, C.M. Increased Levels of Short-Chain Ceramides Modify the Lipid Organization and Reduce the Lipid Barrier of Skin Model Membranes. Langmuir ACS J. Surf. Colloids 2021, 37, 9478–9489. [Google Scholar] [CrossRef] [PubMed]
- Man, M.-Q.; Brown, B.E.; Wu-Pong, S.; Feingold, K.R.; Elias, P.M. Exogenous nonphysiologic vs. physiologic lipids. Divergent mechanisms for correction of permeability barrier dysfunction. Arch. Dermatol. 1995, 131, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Miyachi, Y.; Kawashima, M. Clinical significance of the water retention and barrier function-improving capabilities of ceramide-containing formulations: A qualitative review. J. Dermatol. 2021, 8, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Lane, M.E. Letter to the Editor Regarding ‘An Investigation of the Skin Barrier Restoring Effects of a Cream and Lotion Containing Ceramides in a Multi-Vesicular Emulsion in People with Dry, Eczema-Prone Skin: The RESTORE Study Phase 1’. Dermatol. Ther. 2021, 11, 2245–2248. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.V.; Lane, M.E. Comment on “Clinical significance of the water retention and barrier function-improving capabilities of ceramide-containing formulations: A qualitative review”. J. Dermatol. 2022, 49, e121–e123. [Google Scholar] [CrossRef] [PubMed]
- van Smeden, J.; Bouwstra, J.A. Stratum Corneum Lipids: Their Role for the Skin Barrier Function in Healthy Subjects and Atopic Dermatitis Patients. Curr. Probl. Dermatol. 2016, 49, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Opálka, L.; Kováčik, A.; Maixner, J.; Vávrová, K. Omega-O-Acylceramides in Skin Lipid Membranes: Effects of Concentration, Sphingoid Base, and Model Complexity on Microstructure and Permeability. Langmuir ACS J. Surf. Colloids 2016, 32, 12894–12904. [Google Scholar] [CrossRef] [PubMed]
- Nakaune-Iijima, A.; Sugishima, A.; Omura, G.; Kitaoka, H.; Tashiro, T.; Kageyama, S.; Hatta, I. Topical treatments with acylceramide dispersions restored stratum corneum lipid lamellar structures in a reconstructed human epidermis model. Chem. Phys. Lipids 2018, 215, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, T.; Lange, S.; Dobner, B.; Sonnenberger, S.; Hauß, T.; Neubert, R.H.H. Investigation of a CER[NP]- and [AP]-Based Stratum Corneum Modeling Membrane System: Using Specifically Deuterated CER Together with a Neutron Diffraction Approach. Langmuir ACS J. Surf. Colloids 2018, 34, 1742–1749. [Google Scholar] [CrossRef] [PubMed]
- Uche, L.E.; Gooris, G.S.; Beddoes, C.M.; Bouwstra, J.A. New insight into phase behavior and permeability of skin lipid models based on sphingosine and phytosphingosine ceramides. Biochim. Biophys. Acta Biomembr. 2019, 1861, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Yokose, U.; Ishikawa, J.; Morokuma, Y.; Naoe, Y.; Inoue, Y.; Yasuda, Y.; Tsujimura, H.; Fujimura, T.; Murase, T.; Hatamochi, A. The ceramide [NP]/[NS] ratio in the stratum corneum is a potential marker for skin properties and epidermal differentiation. BMC Dermatol. 2020, 20, 6. [Google Scholar] [CrossRef] [PubMed]
- Rabionet, M.; Bayerle, A.; Marsching, C.; Jennemann, R.; Gröne, H.J.; Yildiz, Y.; Wachten, D.; Shaw, W.; Shayman, J.A.; Sandhoff, R. 1-O-acylceramides are natural components of human and mouse epidermis. J. Lipid Res. 2013, 54, 3312–3321. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Miner, J.H.; Turk, J.; Hsu, F.-F. Linear ion-trap MSn with high-resolution MS reveals structural diversity of 1-O-acylceramide family in mouse epidermis. J. Lipid Res. 2017, 58, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Harazim, E.; Vrkoslav, V.; Buděšínský, M.; Harazim, P.; Svoboda, M.; Plavka, R.; Bosáková, Z.; Cvačka, J. Nonhydroxylated 1-O-acylceramides in vernix caseosa. J. Lipid Res. 2018, 59, 2164–2173. [Google Scholar] [CrossRef] [PubMed]
- Assi, A.; Bakar, J.; Libong, D.; Sarkees, E.; Solgadi, A.; Baillet-Guffroy, A.; Michael-Jubeli, R.; Tfayli, A. Comprehensive characterization and simultaneous analysis of overall lipids in reconstructed human epidermis using NPLC/HR-MSn:1-O-E (EO) Cer, a new ceramide subclass. Anal. Bioanal. Chem. 2020, 412, 777–793. [Google Scholar] [CrossRef] [PubMed]
- Rabionet, M.; Bernard, P.; Pichery, M.; Marsching, C.; Bayerle, A.; Dworski, S.; Kamani, M.A.; Chitraju, C.; Gluchowski, N.L.; Gabriel, K.R.; et al. Epidermal 1-O-acylceramides appear with the establishment of the water permeability barrier in mice and are produced by maturating keratinocytes. Lipids 2022, 57, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.O.; Kim, J.W.; Liu, K.H.; Shin, K.; Nam, Y.S.; Kim, J.-W.; Park, C.S. A Novel Phytosphingosine Based 1-O-Acylceramide: Synthesis, Physicochemical Characterization, and Role in the Lipid Lamellar Organization. In Proceedings of the 32nd IFSCC Congress 2022, London, UK, 19–22 September 2022. [Google Scholar]
- Yang, M.Y.; Lee, E.; Park, C.S.; and Nam, Y.S. Molecular Dynamics Investigation into CerENP’s Effect on the Lipid Matrix of Stratum Corneum. J. Phys. Chem. B 2024, 128, 5378–5386. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Lee, E.O.; Kim, J.W.; Liu, K.H.; Lee, S.; Lim, K.M. Synthesis and Characterization of a Novel Phytosphingosine-Based 1-O-Acylceramide. IFSCC Mag. 2022, 25, 359–362. [Google Scholar]
- Oh, M.J.; Cho, Y.H.; Cha, S.Y.; Lee, E.O.; Kim, J.W.; Kim, S.K.; Park, C.S. Novel phytoceramides containing fatty acids of diverse chain lengths are better than a single C18-ceramide N-stearoyl phytosphingosine to improve the physiological properties of human stratum corneum. Clin. Cosmet. Investig. Dermatol. 2017, 10, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Kim, E.J.; Lee, C.H.; Park, G.H.; Yoo, K.M.; Nam, S.J.; Shin, K.-O.; Park, K.; Choi, E.H. A Lipid Mixture Enriched by Ceramide NP with Fatty Acids of Diverse Chain Lengths Contributes to Restore the Skin Barrier Function Impaired by Topical Corticosteroid. Ski. Pharmacol. Physiol. 2022, 35, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Park, B.D.; Youm, J.K.; Jeong, S.K.; Choi, E.H.; Ahn, S.K.; Lee, S.H. The characterization of molecular organization of multilamellar emulsions containing pseudoceramide and type III synthetic ceramide. J. Invest. Dermatol. 2003, 121, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.I.; Lee, D.J.; Rahman, R.T.; and Nam, Y.S. Biomimetic Multilayered Lipid Nanovesicles for Potent Protein Vaccination. Adv. Healthc. Mater. 2024, 13, 2304109. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).