Abstract
Exposure to ultraviolet (UV) radiation from the sun significantly damages the skin, leading to premature aging, hyperpigmentation, and oxidative stress that disrupts skin homeostasis. UV radiation increases the production of reactive oxygen species (ROS), accelerating skin deterioration. Although sunscreens remain the primary method for UV protection, chemical-based formulations are often associated with side effects, such as allergic reactions and acne. To address these concerns, the inclusion of natural ingredients in sunscreen formulations has gained attention. Curcumin, an active compound found in turmeric (Curcuma longa) and Java turmeric (Curcuma xanthorrhiza), is well-known for its antioxidant and anti-inflammatory properties. This review explores the potential of curcumin as a natural ingredient for enhancing the Sun Protection Factor (SPF) of sunscreen products. A systematic literature review was conducted, analyzing 200 articles sourced from Google Scholar and PubMed using keywords such as “Curcumin”, “Curcuma”, “Antioxidant”, “Anti-Inflammatory”, and “Sun Protection Factor”. Studies unrelated to UV protection were excluded. The findings, presented in tabular form, indicate that curcumin and Curcuma exhibit significant potential to enhance SPF values due to their antioxidant, anti-inflammatory, and UV-absorbing properties. Additionally, curcumin may aid in skin repair following UV-induced damage. However, the specific concentration of curcumin in various Curcuma species remains unknown, and further research is necessary to determine its optimal use. Consideration of additional excipients in sunscreen formulations is also required to maximize efficacy. In conclusion, curcumin demonstrates considerable promise as a sustainable and effective natural ingredient for protecting the skin from UV radiation, offering a safer alternative to conventional chemical-based sunscreens.
1. Introduction
The exposure of human skin to solar ultraviolet radiation (UVR) significantly increases the production of reactive oxygen species (ROS), disrupting the natural redox balance and promoting a pro-oxidative state, which ultimately leads to oxidative stress [1,2,3]. The detrimental effects of oxidative stress manifest through various mechanisms, including alterations in proteins and lipids, inflammation, immune suppression, DNA damage, and the activation of signaling pathways that regulate gene transcription, cell cycle control, proliferation, and apoptosis [1]. Consequently, maintaining ROS levels within a physiological range is critical for preserving normal skin homeostasis [1,2,3,4,5,6,7].
To mitigate the detrimental effects of oxidative stress, reactive oxygen species (ROS) can be regulated by various compounds, including antioxidants such as vitamin C, vitamin E, and glutathione, as well as enzymatic antioxidants like superoxide dismutase (SOD), catalase, and glutathione peroxidase, which play crucial roles in reducing ROS levels and maintaining cellular redox homeostasis [8,9,10]. In addition to these compounds, natural substances such as curcumin, kaempferol, ellagic acid, capsaicin, and anthocyanin have been shown to contribute significantly to ROS regulation [11,12,13,14,15,16,17]. Among these, curcumin, a bioactive compound derived from turmeric (Curcuma longa) and Java turmeric (Curcuma xanthorrhiza), stands out for its potent antioxidant properties [11,12,13]. Curcumin not only neutralizes free radicals but also modulates ROS-metabolizing enzymes, thereby offering protection against oxidative stress-induced skin damage [18,19].
Recent studies have highlighted the potential of curcumin in the cosmetic industry, particularly for maintaining skin homeostasis and regulating ROS levels. Curcumin has been shown to enhance cellular antioxidant defense mechanisms through the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway, which plays a pivotal role in mitigating oxidative stress [20,21,22,23]. As a natural active ingredient, curcumin not only aids in repairing UV-damaged skin but also acts as an anti-aging agent due to its potent antioxidant and anti-inflammatory properties [24,25,26,27]. Its potential as a natural UV protector has garnered significant attention, as it can absorb UV radiation and prevent skin damage, including erythema, hyperpigmentation, and wrinkles [28,29,30,31,32,33,34]. Furthermore, curcumin has been found to inhibit UVB-induced tumor necrosis factor (TNF) mRNA expression and reduce matrix metalloproteinase-1 (MMP-1) expression in keratinocytes and fibroblasts, contributing to its protective effects against photoaging [35,36].
Given this potential, curcumin is increasingly being explored as an alternative ingredient in UV protection products. Its efficacy as a UV protectant lies in its ability to absorb UV rays and mitigate oxidative stress induced by sun exposure [37,38,39,40]. While studies have shown that curcumin can enhance Sun Protection Factor (SPF) values, further research is required to fully evaluate its potential as a standalone agent in effective sunscreen formulations [41,42,43].
Sunscreen has long been recognized as one of the primary methods for protecting the skin from ultraviolet (UV) radiation [44,45]. These products reduce the harmful effects of UV rays by absorbing, reflecting, or scattering radiation and are specifically formulated for topical application [46,47]. The effectiveness of sunscreen is often enhanced by incorporating antioxidants, which boost photoprotective properties [48,49]. The SPF value is a key metric for determining a sunscreen’s efficacy in preventing sunburn; higher SPF values indicate greater protection [50,51]. However, traditional sunscreens frequently rely on chemical filters, which are a leading cause of photoallergic reactions and can trigger both acute and chronic allergic symptoms [52,53,54]. Additionally, inorganic filters may interfere with percutaneous absorption, disrupt endocrine function, clog pores, and contribute to acne. In response to these concerns, the pharmaceutical industry is increasingly focusing on the development of sunscreens made from safer and more cost-effective natural ingredients, such as curcumin [55,56].
Curcumin, a bioactive compound derived from plants of the Curcuma genus in the Zingiberaceae family, has been extensively utilized as a natural active ingredient in herbal cosmetics, including as a photoprotective agent [57,58,59]. Recent studies demonstrate that curcumin protects skin cells from UV radiation and prevents sunburn through its potent antioxidant and anti-inflammatory properties [60,61,62]. Moreover, curcumin enhances cellular antioxidant defenses by activating the Nrf2 pathway, which plays a pivotal role in mitigating oxidative stress [63,64]. As a result, antioxidants such as curcumin are highly valuable in preventing various UV-induced skin conditions, including premature aging [49,65,66,67]. In sunscreen formulations, UV-absorbing ingredients are designed to absorb UV rays within the 290–400 nm wavelength range, effectively preventing skin damage such as erythema, hyperpigmentation, and premature aging [68,69].
Several studies on plants of the Curcuma genus have demonstrated their potential to enhance the SPF values of sunscreen formulations. Despite these promising findings, a comprehensive evaluation of curcumin as a standalone sunscreen agent remains unexplored. This review aims to summarize and analyze the potential of curcumin as a natural and effective ingredient for sunscreen products.
2. Materials and Methods
2.1. Focal Question
This systematic review is conducted to answer the following question: “Can curcumin be used as sun protector agent in sunscreen formulation?”.
2.2. Literature Search
This systematic review was conducted using both national and international databases, specifically Google Scholar and PubMed. The search utilized keywords such as “curcumin”, “Curcuma”, “antioxidant”, “anti-inflammation”, and “sun protection factor”.
2.3. Inclusion and Exclusion Criteria
The inclusion criteria for this systematic review encompassed in vitro and in vivo studies that reported the antioxidant or anti-inflammatory activities of curcumin and Curcuma and included their Sun Protection Factor (SPF) values. It is acknowledged that SPF values in formulations can be influenced by excipients. The exclusion criteria consisted of studies focusing on the activities of curcumin and Curcuma in relation to specific diseases.
2.4. Study Selection
This systematic review adhered to the guidelines of PRISMA (The Preferred Reporting Items for a Systematic Review and Meta-Analysis), with the corresponding flow diagram presented in Figure 1. The primary outcome considered in this review was the SPF value of curcumin.
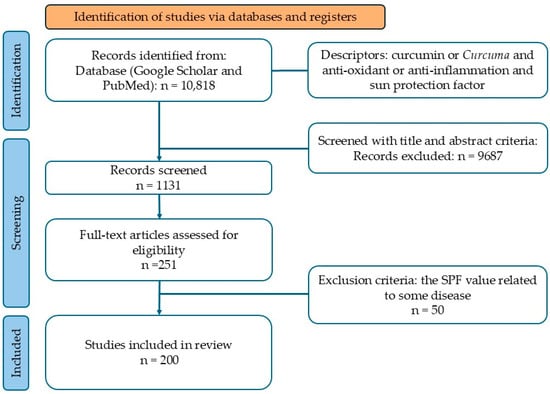
Figure 1.
Flow diagram according to PRISMA guidelines.
2.5. Data Analysis
A qualitative assessment of the outcomes from the included studies is provided in this systematic review. No meta-analysis was performed. The results are presented descriptively and supplemented with tables that summarize the evidence.
3. Results
Based on the defined search terms, a total of 10,818 studies were identified. Following a full-text screening aligned with the inclusion criteria, 251 studies were selected. Among these, 50 were excluded as their SPF values were associated with specific diseases. Consequently, 200 studies were included in this review. The study selection process adhered to the PRISMA guidelines and is illustrated in Figure 1.
3.1. Physicochemical Characteristics of Curcumin
Curcumin is recognized as an effective natural sunscreen, largely attributable to its active components, particularly flavonoids. The chemical structure of curcumin is presented in Figure 2.
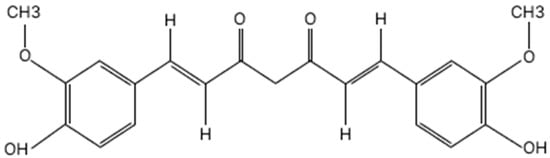
Figure 2.
Chemical structure of curcumin.
Curcumin is a yellow–orange pigment derived from plants of the Curcuma genus, including Curcuma longa, Curcuma xanthorrhiza, Curcuma zedoaria, Curcuma mangga, and other species [70,71,72,73,74]. Curcumin extracted from different species exhibits unique physicochemical characteristics, as summarized in Table 1. These distinct properties are essential for the formulation and therapeutic applications of curcumin in sunscreen products [75,76].

Table 1.
Physicochemical characteristics of curcumin.
3.2. Antioxidant Activity of Curcumin
Antioxidant activity can be evaluated using various methods, including the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay, oxygen radical absorption capacity (ORAC), reducing power assay (RPA), 2-deoxyribose degradation assay (2-DR), ferric reducing antioxidant power (FRAP), 2,2-azinobis(3-ethylbenzothiazoline)-6-sulfonic acid (ABTS), and cupric reducing antioxidant capacity (CUPRAC) [77,78,79,80,81,82,83,84,85,86]. The DPPH and 2-DR methods allow for the determination of free radical inhibition percentages and the inhibition concentration (IC50) value, where a lower IC50 indicates higher antioxidant activity [77]. In several studies, antioxidant activity measured using the ORAC, ABTS, and CUPRAC methods is expressed as Trolox equivalents in micromoles per gram of extract (μmol TE/g). In contrast, the antioxidant activity in the RPA method is reported as absorbance [77,78,79,80,81,82,83,84,85,86]. Higher ORAC, ABTS, and CUPRAC values indicate greater antioxidant activity, while higher absorbance values in the RPA method reflect stronger reducing power and antioxidant activity [77]. Antioxidant activity in the FRAP assay is expressed as micromoles of ferrous equivalent (μM Fe[II] per 100 g), with higher FRAP values indicating stronger antioxidant potential [85]. Several studies have highlighted the antioxidant activity of curcumin, as summarized in Table 2.

Table 2.
Antioxidant activity of curcumin.
Research has shown that the flavonoids present in curcumin exhibit strong antioxidant properties, effectively preventing the formation of reactive oxygen species (ROS) and lipid peroxidation induced by UV exposure [79,100]. These processes are closely associated with conditions such as photoaging and skin cancer [101].
3.3. Anti-Inflammatory Properties of Curcumin
The anti-inflammatory activities of curcumin have been extensively investigated in numerous studies, both in vivo and in vitro, as summarized in Table 3.

Table 3.
Anti-inflammatory properties of curcumin.
Recent research indicates that curcumin, a flavonoid compound, exhibits anti-inflammatory effects by suppressing the release of pro-inflammatory cytokines and scavenging free radicals, thereby protecting tissues and cells from damage [133,134].
3.4. Potential of Curcumin (Curcuma Spesies) as SPF Agent
Several studies have demonstrated that curcumin improves SPF values, as outlined in Table 4. Its ability to enhance the skin’s defense against ultraviolet (UV) radiation is attributed to its antioxidant and UV-absorbing properties [60,61,62]. By neutralizing free radicals and reducing oxidative stress caused by UV exposure, curcumin enhances the photoprotective capacity of sunscreen formulations [48,49]. Due to these properties, curcumin holds potential as a valuable addition to sun care products, not only as an SPF-boosting agent but also for its broader skin-protective benefits, including preventing photoaging and reducing inflammation [55,56,101].

Table 4.
Potential of curcumin (Curcuma species) as SPF agent.
The SPF values of different Curcuma species vary due to the differing curcumin con-centrations among them. Even within the same species, variations in SPF values can occur due to differences in the material’s origin and the processing methods employed.
3.5. Mechanism of Curcumin as a Sun Protector Agent
Curcumin has several mechanisms of action that make it an ideal ingredient for cosmetic formulations, particularly as an SPF booster [101]. These mechanisms include its anti-inflammatory, antioxidant, and sun-protective properties, as summarized in Table 5.

Table 5.
Mechanism of curcumin as a sun protector agent.
The anti-inflammatory and antioxidant properties of curcumin are strongly correlated with its potential as a sun-protective agent [167,170]. Evidence from research highlights curcumin’s ability to reduce inflammatory markers and alleviate oxidative damage, both of which are critical factors in skin damage induced by UV radiation [28,29,30,31,32,33,34]. Through its capacity to mitigate inflammation and oxidative stress, curcumin plays a pivotal role in shielding the skin from UV-related damage, thus serving as a multifunctional component in sun care formulations [55,56].
4. Discussion
Curcumin, the primary bioactive compound derived from Curcuma longa, has attracted considerable interest due to its broad spectrum of bioactive properties, notably its antioxidant, anti-inflammatory, and UV-protective effects [101,167,170]. The flavonoid components of curcumin demonstrate robust antioxidant capabilities, efficiently inhibiting the generation of oxygen free radicals and lipid peroxidation triggered by UV radiation [79,100,172]. These protective effects are crucial in mitigating conditions such as photoaging and skin cancer [24,25,26,27,101]. Additionally, curcumin alleviates UV-induced inflammatory responses by suppressing the activation of NF-κB and other pro-inflammatory transcription factors, ultimately reducing inflammation and safeguarding the skin against UV-induced damage [87].
As a UV protector, curcumin holds promise for inclusion in natural sunscreen formulations, offering a range of opportunities, advantages, and challenges [173,174,175]. Natural sunscreens present an opportunity to leverage plant-based compounds such as flavonoids, polyphenols, and other secondary metabolites, known for their strong antioxidant and UV-absorbing properties [176,177]. These compounds can shield the skin from oxidative damage caused by UV radiation while reducing inflammation and mitigating aging effects [176,178,179,180]. The integration of natural ingredients with nanotechnology, such as nanoparticles and liposomes, further expands the potential of natural sunscreens. These advanced delivery systems enhance the stability, bioavailability, and efficacy of the active compounds, improving protection against UV rays [181]. Research indicates that natural ingredients can significantly enhance the photoprotective capabilities of sunscreens, providing antioxidant benefits and addressing skin inflammation, barrier damage, and UV-induced aging [182,183,184]. However, natural sunscreens face notable challenges, including their relatively lower SPF and photostability compared with chemical-based sunscreens [185,186,187]. Stabilization techniques, such as incorporating natural compounds into nanoparticles, are often required to improve their performance and resilience under sun exposure [181]. For instance, a study by Sari and Susiloningrum (2022) demonstrated that combining Curcuma mangga with TiO2 in sunscreen formulations resulted in higher SPF values compared with TiO2-only formulations [152]. This suggests that natural ingredients can act as SPF boosters, potentially reducing the reliance on synthetic UV filters. The composition of natural sunscreens, which typically involves fewer synthetic UV filters, offers additional benefits, including safety, non-toxicity, and a lower risk of irritation or side effects compared with formulations with chemical or synthetic ingredients [188,189]. This makes natural sunscreens an appealing choice for consumers seeking effective and safer alternatives for sun protection [188,189].
The physicochemical properties of curcumin, summarized in Table 1, play a significant role in its applicability within cosmetic formulations. This compound is recognized for its yellowish-orange hue, mild earthy aroma, and bitter taste, yet these characteristics pose challenges in product development [70,73]. Curcumin’s limited solubility in water significantly restricts its bioavailability, though it is readily soluble in organic solvents such as ethanol, acetone, and dimethyl sulfoxide (DMSO) [71,73]. This poor solubility necessitates advanced formulation strategies to enhance both its stability and integration into cosmetic products. Furthermore, curcumin exhibits instability under specific conditions, degrading when exposed to sunlight and becoming unstable at pH levels exceeding 6.5 [70]. These issues must be meticulously managed during formulation to preserve its efficacy [75,76]. On the other hand, curcumin demonstrates favorable heat resistance, tolerating temperatures up to 140 °C for short durations, an attribute beneficial for certain manufacturing processes [70]. Additionally, the ash and water content of curcumin extracts, typically ranging between 4 and 6% and 8 and 9%, respectively, influence both its stability and shelf life [72,74]. Addressing these factors is crucial for optimizing its use in sustainable and effective cosmetic formulations.
Curcumin’s antioxidant activity, as detailed in Table 2, has been evaluated using a range of assays, including DPPH radical scavenging, ORAC, FRAP, ABTS, and others. These studies highlight variations in antioxidant potential among different Curcuma species, primarily influenced by differences in curcumin content [77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99]. Furthermore, environmental factors such as geographic region, solvent selection, and extraction methodologies significantly impact the antioxidant properties of curcumin, as summarized in Table 2 [89,92,95,97]. The antioxidant activity of curcumin across various Curcuma species demonstrates notable variability, with values ranging from 291.3 ± 3.1 μg/mL to 1.08 μg/mL for Curcuma longa, 19.0 ± 1.7 μg/mL to 1973.38 ± 219.93 μg/mL for Curcuma xanthorrhiza, 20.2 ± 2.0 μg/mL to 956.16 ± 20.27 μg/mL for Curcuma zedoaria, 37.75 μg/mL to 500 μg/mL for Curcuma heyneana, 6.0313 μg/mL to 1724 μg/mL for Curcuma aeruginosa, and 37.338 ± 1.851 μg/mL to 268.802 ± 43.573 μg/mL for Curcuma mangga [77,79,87,89,92,95,97,99]. Notably, curcumin in its extract form demonstrates superior antioxidant activity compared with formulated products, likely due to the reduced bioavailability of the active compound in complex formulations [90,151]. The enhanced antioxidant activity of curcumin is closely linked to its photoprotective properties, including reductions in sunburn cell formation, erythema, and UV-induced immunosuppression [182,190,191]. Consequently, optimizing the concentration of curcumin in formulations is essential to ensure adequate antioxidant and photoprotective effects.
Curcumin’s anti-inflammatory properties have been widely studied through both in vivo and in vitro approaches, as summarized in Table 3. In vivo experiments, such as those utilizing TPA-induced and xylene-induced ear edema models in mice, have demonstrated curcumin’s efficacy in reducing inflammation by assessing changes in edema volume in the ears of the test subjects [108,129]. Additionally, the application of curcumin, topically, has been shown to accelerate wound contraction in streptozotocin-induced models, with higher concentrations of curcumin extracts correlating with improved wound healing outcomes [125]. In vitro studies, employing spectrophotometric methods and high-performance liquid chromatography (HPLC), further support curcumin’s anti-inflammatory potential. These studies have demonstrated reductions in pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, as detailed in Table 3. Variations in these effects are influenced by curcumin concentration and the extraction methods used [89,92,95,97]. For instance, research by Indriani et al. (2018) highlighted that curcumin extract exhibited anti-inflammatory efficacy comparable to the positive control [113]. Similarly, Arisonya et al. (2018) reported a significant reduction in ulcers on the labial mucosa diameter in male white rats treated with curcumin extract topically, further validating its potent anti-inflammatory effects [116]. The anti-inflammatory properties of curcumin are closely associated with its photoprotective benefits. These include the inhibition of edema and erythema, as well as reductions in hyperplastic responses, inflammatory leukocyte infiltration, skin aging, and the risk of skin cancer formation [192,193,194]. These findings highlight curcumin’s potential as a multifunctional agent in both therapeutic and cosmetic applications.
As a photoprotective agent, curcumin demonstrates considerable variability in SPF values, as outlined in Table 4. In vitro testing using UV–Vis spectroscopy, with ethanol as a blank, evaluated curcumin’s absorbance within the 290–320 nm wavelength range [107]. These analyses revealed that curcumin’s SPF values span from minimal protection (SPF 1–4) to ultra protection (SPF > 15) [135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155]. Table 4 highlights that SPF values of curcumin in Curcuma extracts are generally higher than those observed in formulated products, emphasizing the need to optimize curcumin concentrations in sunscreen formulations to enhance photoprotective efficacy [46,47]. Additionally, as shown in Table 4, Curcuma extracts at concentrations ranging from 0.1 to 15% exhibited SPF values between 1 and 27.40. Extracts with concentrations of 200–1500 ppm demonstrated SPF values ranging from approximately 0.33 to 37.46 [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86]. These findings indicate a positive correlation between extract concentration and SPF value, suggesting that higher extract concentrations result in enhanced UV absorption and greater protection against UVB radiation [172,195]. This relationship highlights the potential for higher curcumin content to provide extended and more effective sun protection [196]. However, a notable limitation is the lack of detailed information regarding curcumin concentrations in different Curcuma species. This gap underscores the importance of further standardization and the quantification of curcumin content to maximize its potential as a photoprotective agent.
Several factors influence the SPF value generated by Curcuma extracts and curcumin, including the choice of solvent, extraction temperature, and formulation ingredients [89,92,95,97]. Research by Kanani et al. (2017) demonstrated that using ethyl acetate as a solvent at 30 °C resulted in higher SPF values compared with methanol at elevated temperatures [173]. This is attributed to ethyl acetate’s ability to extract more potent antioxidant compounds, which are strongly associated with increased SPF values [173,197,198]. Antioxidant compounds extracted by ethyl acetate contain chromophores and aliphatic CH groups capable of absorbing UV rays, thereby enhancing photoprotective efficacy [199,200]. These findings underscore the importance of selecting an appropriate solvent and optimizing extraction temperature during the formulation process to maximize the efficacy of sunscreens derived from Curcuma extracts containing curcumin [89,92,95,97,173]. Such considerations are critical to ensuring the development of effective and stable photoprotective products.
Curcumin enhances the effectiveness of sunscreen formulations by complementing other SPF-active ingredients, as evidenced by its multifunctional properties [152]. Acting as a skin protector, curcumin’s antioxidant and anti-inflammatory activities provide substantial benefits [60,61,62]. Specifically, its anti-inflammatory effects include the ability to reduce redness and tissue damage caused by inflammation following sunlight exposure [133,134]. Curcumin achieves this by inhibiting the production of pro-inflammatory cytokines, such as IL-β, IL-6, and TNF-α, while reducing arachidonic acid release through the suppression of phospholipase A2 and phospholipase C g1 activity [37,156]. Additionally, curcumin inhibits the synthesis of nitric oxide (NO), COX-2, and lipoxygenase, further mitigating inflammation [37,133]. As detailed in Table 5, plants containing curcumin protect skin cells from oxidative damage via their antioxidant properties, preserving cell membrane integrity and preventing oxidative degradation. The phenolic group in curcumin enables the scavenging of free radicals, providing additional photoprotection by absorbing UV light, reducing UV-induced oxidative damage, and decreasing TNF-α expression [162]. In summary, curcumin’s combined antioxidant, anti-inflammatory, and UV-protective effects make it a valuable ingredient in sunscreen formulations, as demonstrated in Figure 3. These attributes highlight its potential to improve the overall efficacy and multifunctionality of sun care products.
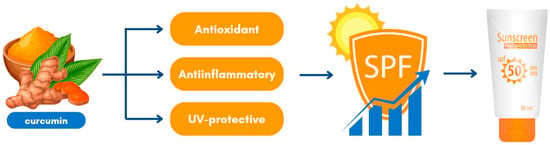
Figure 3.
Curcumin could be used in sunscreen formulations.
Studies, such as those conducted by Arizona and Zulkarnain (2018), have shown that increasing curcumin concentrations in lotions significantly enhances SPF values [151]. However, the impact of other excipients included in the formulation must also be considered, as these components can influence SPF values and alter the overall sun protection efficacy. Consequently, optimizing both the concentration of curcumin and the selection of suitable excipients is crucial to maximizing its photoprotective potential in sunscreen formulations. This strategic approach ensures the development of effective and stable sunscreen products that fully leverage curcumin’s properties.
5. Conclusions
Based on the reviewed literature, curcumin demonstrates significant potential as a sunscreen agent, primarily due to its ability to enhance SPF values through its synergistic antioxidant, anti-inflammatory, and UV-protective properties. These activities collectively strengthen curcumin’s effectiveness as an SPF booster, providing dual protection against UV-induced damage. Increasing curcumin concentrations in sunscreen formulations has been shown to result in higher SPF values, further highlighting its potential utility. However, the inclusion of various excipients must be carefully considered, as they can influence SPF values and potentially affect the overall efficacy of the formulation. Therefore, further research is necessary to identify the optimal concentration of curcumin and to evaluate its interaction with excipients, ensuring the development of effective and stable sunscreen products.
Author Contributions
Conceptualization, A.M.S., R.S.S.A., S.S. and I.M.; and methodology, I.N.P., H.P.D., R.A.S. and R.N.A.; resources, S.S., I.M. and R.N.A.; writing—original draft preparation, A.M.S., R.S.S.A., I.N.P. and S.R.M.; writing—review and editing, A.M.S., H.P.D., R.A.S., I.M., E.A. and N.A.P.; Supervision, S.S. and N.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are contained within the article.
Acknowledgments
The authors would like to extend their gratitude to Sriwidodo, Soraya Ratnawulan Mita, Eri Amalia, and Norisca Aliza Putriana for their invaluable assistance in editing the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dunaway, S.; Odin, R.; Zhou, L.; Ji, L.; Zhang, Y.; Kadekaro, A.L. Natural antioxidants: Multiple mechanism to protect skin from solar radiation. Front. Pharmacol. 2018, 9, 392. [Google Scholar] [CrossRef] [PubMed]
- Schuch, A.P.; Moreno, N.C.; Schuch, N.J.; Menck, C.F.M.; Garcia, C.C.M. Sunlight damage to cellular DNA: Focus on oxidatively generated lesions. Free Radic. Biol. Medic. 2017, 107, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; He, X.; Liu, N.; Deng, H. Role of reactive oxygen species in ultraviolet-induced photodamage of the skin. Cell Div. 2024, 19, 1. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Boiti, A.; Vallone, D.; Foulkes, N.S. Reactive oxygen species signaling and oxidative stress: Transcriptional regulation and evolution. Antioxidants 2024, 13, 312. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.M.; Cheng, M.Y.; Xun, M.H.; Zhao, Z.W.; Zhang, Y.; Tang, W.; Cheng, J.; Ni, J.; Wang, W. Possible mechanisms of oxidative stress-induced skin cellular senescence, inflammation, and cancer and the therapeutic potential of plant polyphenols. Int. J. Mol. Sci. 2023, 24, 3755. [Google Scholar] [CrossRef]
- Martins, S.G.; Zilhão, R.; Thorsteinsdóttir, S.; Carlos, A.R. Linking oxidative stress and DNA damage to changes in the expression of extracellular matrix components. Front. Genet. 2021, 12, 673002. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Chen, M.; Qian, C.; Jin, B.; Hu, C.; Zhang, L.; Wang, M.; Zhou, B.; Zuo, W.; Huang, L.; Wang, Y. Curcumin analog WZ26 Induces ROS and Cell Death via Inhibition of STAT3 in cholangiocarcinoma. Cancer Biol. Ther. 2023, 24, 2162807. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, B.H.; Qiu, X.; Wang, H.S.; Zhang, F.; Fang, R.; Wang, X.F.; Cai, S.H.; Du, J.; Bu, X.Z. A New 4-Arylidene Curcumin Analogue, Induces Cell Cycle Arrest and Apoptosis Through Activation of the Reactive Oxygen Species-FOXO3 A Pathway in Lung Cancer Cells. Free Radic. Biol. Med. 2012, 53, 2204–2217. [Google Scholar] [CrossRef] [PubMed]
- Gersey, Z.C.; Rodriguez, G.A.; Barbarite, E.; Sanchez, A.; Walters, W.M.; Ohaeto, K.C.; Komotar, R.J.; Graham, R.M. Curcumin Decreases Malignant Characteristics of Glioblastoma Stem Cells via Induction of Reactive Exygen Species. BMC Cancer 2017, 17, 99. [Google Scholar] [CrossRef]
- Choi, J.K.; Kwon, O.Y.; Lee, S.H. Kaempferide Prevents Photoaging of Ultraviolet-B Irradiated NIH-3T3 Cell and Mouse Skin via Regulating the Reactive Oxygen SPecies-Mediated Signalings. Antioxidants 2022, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Aslan, A.; Gok, O.; Beyaz, S.; Uslu, H.; Erman, F.; Erman, O.; Baspinar, S. Ellagic acid inhibits proinflammatory intermediary manufacture by suppressing nf-kb/akt, vegf and activating nrf-2/caspase-3 signaling pathways in rat testicular damage: A new way for testicular damage cure and in silico approach. Toxicol. Mech. Methods. 2022, 32, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.R.; Bort, A.; Morrel, C.; Henche, N.R.; Laviada, I.D. The Pepper’s Natural Ingredient Capsaicin Induced Autophagy Blockage in Prostate Cancer Cells. Oncotarget 2015, 7, 1569–1583. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Anthocyanins Induce the Activation of Phase II Enzymes Through the Antioxidant Response Element Pathway Against Oxidative Stresss-Induced Apoptosis. J. Argic. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef]
- Rahmat, E.; Lee, J.; Kang, Y. Javanese turmeric (Curcuma xanthorrhiza Roxb.): Ethnobotany, phytochemistry, biotechnology, and pharmacological activities. Evid. Based Complement. Alternat. Med. 2021, 2021, 9960813. [Google Scholar] [CrossRef] [PubMed]
- Fuloria, S.; Mehta, J.; Chandel, A.; Sekar, M.; Rani, N.N.I.M.; Begum, M.Y.; Subramaniyan, V.; Chidambaram, K.; Thangavelu, L.; Nordin, R.; et al. A Comprehensive Review on the Therapeutic Potential of Curcuma longa Linn. in Relation to its Major Active Constituent Curcumin. Front. Pharmacol. 2022, 13, 820806. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Raina, N.; Wahi, A.; Goh, K.W.; Sharma, P.; Nagpal, R.; Jain, A.; Ming, L.C.; Gupta, M. Wound-Healing Effects of Curcumin and Its Nanoformulations: A Comprehensive Review. Pharmaceutics 2022, 14, 2288. [Google Scholar] [CrossRef] [PubMed]
- Rahban, M.; Rezaei, M.H.; Mazaheri, M.; Saso, L.; Movahedi, A.A.M. Anti-Viral Potential and Modulation of Nrf2 by Curcumin: Pharmacological Implications. Antioxidants 2020, 9, 1228. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Mendes, N.M.; de Lima, E.P.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Bueno, M.S.; Araujo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16, 2721. [Google Scholar] [CrossRef]
- Singh, S.; Aggarwal, B.B. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) (*). J. Biol. Chem. 1995, 270, 24995–25000. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Deng, L.; Yin, L.; Mao, Z.; Gao, X. Curcumin promotes skin wound healing by activating Nrf2 signaling pathways and inducing apoptosis in mice. Turk. J. Med. Sci. 2023, 53, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Vollono, L.; Falconi, M.; Gaziano, R.; Lacovelli, F.; Dika, E.; Terracciano, C.; Bianchi, L.; Campione, E. Potential of Curcumin in Skin Disorders. Nutrients 2019, 11, 2169. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.R.; Prasad, S.; Kannappan, R.; Ravindran, J.; Chaturvedi, M.M.; Vaahtera, L.; Parkkinen, J.; Aggarwal, B.B. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem. Pharmacol. 2010, 80, 1021–1032. [Google Scholar] [CrossRef]
- Abe, Y.; Hashimoto, S.; Horie, T. Curcumin inhibition of inflammatory cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacol. Res. 1999, 39, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- D’Orazio, J.; Jarrett, S.; Ortiz, A.A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C. Antioxidant activity of curcumin protects against the radiation-induced micronuclei formation in cultured human peripheral blood lymphocytes exposed to various doses of γ-Radiation. Int. J. Radiat. Biol. 2021, 97, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, A.R.; Branum, A.; Sivamani, R.K. Effects of Turmeric (Curcuma longa) on Skin Health: A Systematic Review of the Clinical Evidence. Phyther. Res. 2016, 30, 1243–1264. [Google Scholar] [CrossRef]
- Mohanty, C.; Sahoo, S.K. Curcumin and its topical formulations for wound healing applications. Drug Discov. 2017, 22, 1582–1592. [Google Scholar] [CrossRef]
- Benameur, T.; Soleti, R.; Panaro, M.A.; Torre, M.E.L.; Monda, V.; Messina, G.; Porro, C. Curcumin as Prospective Anti-Aging Natural Compound: Focus on Brain. Molecules 2021, 26, 4794. [Google Scholar] [CrossRef] [PubMed]
- Drozd, K.K.; Nizinski, P.; Hawryt, A.; Gancarz, M.; Hawryt, D.; Oliwa, W.; Patkaa, M.; Markowska, J.; Oniszczuk, A. Potential of Curcumin in the Management of Skin Disease. Int. J. Mol. Sci. 2024, 25, 3617. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Hu, D.N.; Roberts, J.; Shen, X.; Lee, C.Y.; Nien, C.W.; Lin, H.Y. Inhibition Effect of Curcumin on UVB-Induced Secretion of Pro-Inflammatory Cytokines from Corneal Limbus Epithelial Cells. Int. J. Ophthalmol. 2017, 10, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Hwang, B.M.; Noh, E.M.; Kim, J.S.; Kim, J.M.; You, Y.O.; Hwang, J.K.; Kwon, K.B.; Lee, Y.R. Curcumin Inhibits UVB-Induced Matrix Metalloproteinase-⅓ Expression by Suppressing the MAPK-p38/JNK Pathways in Human Dermal Fibroblasts. Exp. Dermatol. 2013, 22, 358–379. [Google Scholar] [CrossRef]
- Deng, H.; Wan, M.; Li, H.; Liang, B.; Zhu, H. Curcumin protection against ultraviolet-induced photo-damage in Hacat cells by regulating nuclear factor erythroid 2-related factor 2. Bioengineered 2021, 12, 9993–10006. [Google Scholar] [CrossRef]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2015, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Sandur, S.K.; Pandey, M.K.; Sung, B.; Ahn, K.S.; Murakami, A.; Sethi, G.; Limtrakul, P.; Badmaev, V.; Aggarwal, B.B. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis 2007, 28, 1765–1773. [Google Scholar] [CrossRef]
- Kitture, R.; Ghosh, S.; More, P.A.; Date, K.; Gaware, S.; Datar, S.; Chopade, B.A.; Kale, S.N. Curcumin-Loaded, Self-Assembled Aloevera Template for Superior Antioxidant Activity and Trans-Membrane Drug Release. J. Nanosci. Nanotechnol. 2015, 15, 4039–4045. [Google Scholar] [CrossRef]
- Hoang, H.T.; Moon, J.Y.; Lee, Y.C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
- Takshak, S.; Agrawal, S. Defense potential of secondary metabolites in medicinal plants under UV-B stress. J. Photochem. Photobiol. B 2019, 193, 51–88. [Google Scholar] [CrossRef] [PubMed]
- Coradini, K.; Lima, F.O.; Oliveira, C.M.; Chaves, P.S.; Athayde, M.L.; Carvalho, L.M.; Beck, R.C.R. Co-encapsulation of resveratrol and curcumin in lipid-core nanocapsules improves their in vitro antioxidant effects. Eur. J. Pharm. Biopharm. 2014, 88, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Geoffrey, K.; Mwangi, A.N.; Maru, S.M. Sunscreen products: Rationale for use, formulation development and regulatory considerations. Saudi Pharm. J. 2019, 27, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Bahashwan, E. Awareness and knowledge of sun exposure and use of sunscreen among adults in Aseer region, Saudi Arabia. Saudi Pharm. J. 2024, 32, 102019. [Google Scholar] [CrossRef] [PubMed]
- Lowe, N.J. An overview of ultraviolet radiation, sunscreens, and photo-induced dermatoses. Dermatol. Clin. 2006, 24, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Colantonio, S.; Dawson, A.; Lin, X.; Beecker, J. Sunscreen application, safety, and sun protection: The evidence. J. Cutan. Med. Surg. 2019, 23, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.; Lupuliasa, D.; Dima, I.A.; Nitulescu, G.M. Ultraviolet Filters for Cosmetic Applications. Cosmetics 2023, 10, 101. [Google Scholar] [CrossRef]
- Budiati, A.; Rahmat, D.; Alwiyah, Z. Antioxidant and Sunscreen Activity from Nanoparticles Extract of Temulawak Rhizome (Curcuma xanthorrhiza Roxb.) and Formulation in The Form of A Cream. J. Jamu Indones. 2021, 6, 72–83. [Google Scholar] [CrossRef]
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The efficacy and safety of sunscreen use for the prevention of skin cancer. CMAJ 2020, 192, 1802–1808. [Google Scholar] [CrossRef]
- Hughes, M.C.B.; Williams, G.M.; Baker, P.; Green, C.A. Sunscreen and prevention of skin aging: A randomized trial. Ann. Intern. Med. 2013, 158, 781–790. [Google Scholar] [CrossRef]
- Iannacone, M.R.; Hughes, M.C.B.; Green, A.C. Effects of sunscreen on skin cancer and photoaging. Photodermatol. Photoimmunol. Photomed. 2014, 30, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kohli, I.; Nicholson, C.L.; Williams, J.D.; Lyons, A.B.; Seo, I.S.; Maitra, P.; Tian, X.; Atillasoy, E.; Lim, H.W.; Hamzavi, I.H. Greater efficacy of SPF 100+ sunscreen compared with SPF 50+ in sunburn prevention during 5 consecutive days of sunlight exposure: A randomized, double-blind clinical trial. J. Am. Acad. Dermatol. 2020, 82, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D.; Maitra, P.; Atillasoy, E.; Wu, M.M.; Farberg, A.S.; Rigel, D.S. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: Results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J. Am. Acad. Dermatol. 2018, 78, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Pham, C.; Smith, J.; Mesinkovska, N.A. The banned sunscreen ingredients and their impact on human health: A systematic review. Int. J. Dermatol. 2020, 59, 1033–1042. [Google Scholar] [CrossRef]
- Sumule, A.; Pamudji, G.; Ikasari, E.D. Optimasi Aristoflex® AVC dan Propilen Glikol Gel Tabir Surya Rimpang Kunyit dengan Metode Desain Faktorial. J. Farm. Dan Ilmu Kefarmasian Indones. 2021, 8, 168. [Google Scholar] [CrossRef]
- Setyani, D.A.; Rahayu, D.U.C.; Handayani, S.; Sugita, P. Phytochemical and Anti Acne investigation of Indonesian White Turmeric (Curcuma zedoaria) Rhizomes. IOP Conf. Ser. Mater. Sci Eng 2020, 902, 012066. [Google Scholar] [CrossRef]
- Li, H.; Gao, A.; Jiang, N.; Liu, Q.; Liang, B.; Li, R.; Zhang, E.; Li, Z.; Zhu, H. Protective Effect of Curcumin Against Acute Ultraviolet B Irradiation-Induced Photo-Damage. Photochem. Photobiol. 2016, 92, 808–815. [Google Scholar] [CrossRef]
- Djawad, K.; Yusuf, I.; Miskad, U.A.; Patellongi, I.J.; Massi, M.N. Topical Curcumin as Chemoprotector Against Photoproducts Production: The Role of Cyclobutyl Pyrimidine Dimers, 8-Hydroxy2′Deoxyguanosine Expression and Epidermal Hyperplasia in Acute and Chronic UVB-Induced Mice. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Amalraj, A.; Pius, A.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2016, 7, 205–233. [Google Scholar] [CrossRef]
- Rahaman, M.M.; Rakib, A.; Mitra, S.; Tareq, A.M.; Emran, T.B.; Shahid-Ud-Daula, A.F.M.; Amin, M.N.; Simal-Gandara, J. The Genus Curcuma and Inflammation: Overview of the Pharmacological Perspectives. Plants 2021, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar] [PubMed]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [PubMed]
- Zhang, Y.; Sun, B.; Wang, L.; Shen, W.; Shen, S.; Cheng, X.; Liu, X.; Xia, H. Curcumin-Loaded Liposomes in Gel Protect the Skin of Mice against Oxidative Stress from Photodamage Induced by UV Irradiation. Gels 2024, 10, 596. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.B.S.; Daré, R.G.; Alves, B.L.; Hoshino, L.V.C.; Baesso, M.L.; Laustenschlager, S.O.S.; Bruschi, M.L. Development, in vitro and ex vivo evaluation of bioadhesive colloidal systems for curcumin skin delivery aiming the antioxidant and photoprotective activities. J. Drug Deliv. Sci. Technol. 2024, 91, 105195. [Google Scholar] [CrossRef]
- Djawad, K.; Patellongi, I.J.; Miskad, U.A.; Massi, M.N.; Yusuf, I.; Faruk, M. Single or Daily Application of Topical Curcumin Prevents Ultraviolet B-Induced Apoptosis in Mice. Molecules 2023, 28, 371. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.; Sola, Y.; Gilaberte, Y.; Trullas, C. Outdoor Testing of the Photoprotection Provided by a New Water-Based Broad-Spectrum SPF50+ Sunscreen Product: Two Double-Blind, Split-Face, Randomized Controlled Studies in Healthy Adults. Clin. Cosmet. Investig. Dermatol. 2019, 12, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Lestari, U.; Muhaimin, M.; Chaerunisaa, A.Y.; Sujarwo, W. Anti-Aging Potential of Plants of the Anak Dalam Tribe, Jambi, Indonesia. Pharmaceuticals 2023, 16, 1300. [Google Scholar] [CrossRef] [PubMed]
- Handayani, D.; Halimatushadyah, E.; Krismayadi, K. Standarisasi Mutu Simplisia Rimpang Kunyit Dan Ekstrak Etanol Rimpang Kunyit (Curcuma longa Linn). Pharm. Genius 2023, 2, 43–59. [Google Scholar] [CrossRef]
- Malahayati, N.; Widowati, T.W.; Febrianti, A. Karakterisasi Ekstrak Kurkumin dari Kunyit Putih (Kaemferia rotunda L.) dan Kunyit Kuning (Curcuma domestica Val.). AgriTech 2020, 41, 134–144. [Google Scholar] [CrossRef]
- Rezki, R.S.; Anggori, D.; Siswarni, E. Multi Tahap Kurkumin DariI Kunyit (Curcuma domestica Valet) Menggunakan Pelarut Etanol. J. Tek. Kim. USU 2015, 4, 29–34. [Google Scholar] [CrossRef]
- Kementerian Kesehatan Republik Indonesia. Farmakope Herbal Indonesia, 2nd ed.; Kementerian Kesehatan Republik Indonesia: Jakarta, Indonesia, 2017; pp. 272–273. [Google Scholar]
- Aziz, Y.S.; Ardyanto, M.; Ikhza, M. Standarisasi Parameter Non Spesifik Simplisia Rimpang Kunyit (Curcumae Domestica Rizhoma) Dan Temulawak (Curcuma xanthorrhiza Roxb.) Di Kabupaten Ponorogo. J. Delima Harapan 2019, 6, 89–94. [Google Scholar] [CrossRef]
- Zheng, B.; McClements, D.J. Formulation of More Efficacious Curcumin Delivery Systems Using Colloid Science: Enhanced Solubility, Stability, and Bioavailability. Molecules 2020, 25, 2791. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Kather, F.S.; Morsy, M.A.; Boddu, S.H.S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A.B. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 4, 672. [Google Scholar] [CrossRef] [PubMed]
- Akter, J.; Hossain, M.A.; Takara, K.; Islam, M.Z.; Hou, D.X. Antioxidant Activity of Different Species and Varieties of Turmeric (Curcuma spp.): Isolation of active compounds. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 215, 9–17. [Google Scholar] [CrossRef]
- Triyono, T.; Chaerunisaa, A.Y.; Subarnas, A. Antioxidant Activity of Combination Ethanol Extract of Turmeric Rhizome (Curcuma Domestica Val.) and Ethanol Extract of Trengguli Bark (Cassia fistula L.) with DPPH Method. Indones. J. Pharm. Sci. Technol. 2018, 5, 43–48. [Google Scholar] [CrossRef]
- Tanvir, E.M.; Hossen, M.S.; Hossain, M.F.; Afroz, R.; Gan, S.H.; Khalil, I.; Karim, N. Antioxidant Properties of Popular Turmeric (Curcuma longa) Varieties from Bangladesh. J. Food Qual. 2017, 2017, 8471785. [Google Scholar] [CrossRef]
- Rahul, R.; Arrivukkarasan, S.; Anhuradha, S. In Vitro Antioxidant and Free Radical Scavenging Activity of Curcuma longa, Acorus calamus, and Camellia sinensis. Food Nutr. Sci. 2020, 13, 750–760. [Google Scholar]
- Kusumaningrum, M.; Ardhiansyah, H.; Putranto, A.W.; Trihardini, A.; Kinanti, P.A.; Maslahah, D.N.; Harianingsih, H. Turmeric Extraction (Curcuma longa L.) Using the Reflux Method and Characterization. J. Bahan Alam Terbarukan 2022, 11, 85–91. [Google Scholar] [CrossRef]
- Rakhmayanti, R.D.; Rusita, Y.D. Aktivitas Antioksidan Masker Peel-Off Kopi (Coffea arabica) dan Kunyit (Curcuma longa) Menggunakan Metode DPPH. J. Ilmu Kefarmasian Indones. 2022, 20, 87–92. [Google Scholar] [CrossRef]
- Pratiwi, D.; Sidoretno, W.M.; Aisha, N. The Combination of Turmeric (Curcuma domestica) Rhizome Extract and Collagen in a Serum Formulation as an Antioxidant. Borneo J. Pharm. 2021, 4, 36–42. [Google Scholar] [CrossRef]
- Partio, E.K.U.; Tedjakusuma, F.; Surya, R. Analysis of Antioxidant and Hedonic Acceptance of Turmeric Extract-Enriched Milk. IOP Conference Series. Earth Environ. Sci. 2023, 1169, 012091. [Google Scholar] [CrossRef]
- Asyhar, R.; Minarni, M.; Arista, R.A.; Nurcholis, W. Total Phenolic and Flavonoid Contents and Their Antioxidant Capacity of Curcuma xanthorrhiza Accessions from Jambi. Biodiversitas 2023, 24. [Google Scholar] [CrossRef]
- Yodi, G.; Artika, I.M.; Nurcholis, W. Effect of Varieties of Curcuma xanthorrhiza and Extraction Solvent on Total Phenolic, Total Flavonoid Content, and Antioxidant Capacity. Biodiversitas 2023, 24. [Google Scholar] [CrossRef]
- Malau, R.C.; Nasution, S.W.; Nasution, A.N.; Widowati, W.; Nindya, F.S.; Kusuma, H.S.W. Antioxidant Potential of Temulawak (Curcuma xanthorrhiza) Extract Gel as a Candidate for Wound Healing. J. Vocat. Health Stud. 2024, 7. [Google Scholar] [CrossRef]
- Suryani, S.; Al Anshory, A.C.; Marlin, M.; Artika, I.M.; Ambasari, L.; Nurcholis, W. Variability Total Phenolic Content and Antioxidant Activity of Curcuma zanthorrhiza and C. aeruginosa Cultivated in Three Different Locations in West Java, Indonesia. Biodiversitas J. Biol. Divers. 2022, 23, 434. [Google Scholar] [CrossRef]
- Marliani, L.; Budiana, W.; Anandari, Y. The Effect of Extraction Condition on The Polyphenol Content and Antioxifant Activity of Curcuma zedoria (Christm.) Roschoe Rhizome. Indones. J. Pharm. Sci. Technol. 2017, 4, 57. [Google Scholar] [CrossRef]
- Desmiaty, Y.; Winarti, W.; Lindawati, L.; Fahleni, F. Formulasi Curcuma zedoaria sebagai Emulgel Antioksidan. J. Ilmu Kefarmasian Indones. 2020, 18, 34–40. [Google Scholar]
- Budiansyah, A.; Haroen, U.; Syafwan, S.; Kurniawan, K. Antioxidant and Antibacterial Activities of the Rhizome Extract of Curcuma zedoaria Extracted Using Some Organic Solvents. J. Adv. Vet. Anim. Res. 2023, 10, 347. [Google Scholar] [CrossRef]
- Kusumawati, I.; Kurniawan, K.O.; Rullyansyah, S.; Prijo, T.A.; Widyowati, R.; Ekowati, J.; Hestianah, E.P.; Maat, S.; Matsunami, K. Anti-aging Properties of Curcuma heyneana Valeton & Zipj: A Scientific Approach to Its Use in Javanese Tradition. J. Ethopharmacol. 2018, 225, 64–70. [Google Scholar] [CrossRef]
- Manuhara, Y.S.W.; Sugiharto, S.; Kristanti, A.N.; Aminah, N.S.; Wibowo, A.T.; Wardana, A.P.; Putro, Y.K.; Sugiarso, D. Antioxidant Activities, Total Phenol, Flavonoid, and Mineral Content in the Rhizome of Various Indonesian Herbal Plants. Rasayan J. Chem. 2022, 15, 2724–2730. [Google Scholar] [CrossRef]
- Marianne, M.; Patilaya, P.; Barus, B.T. Uji Aktivitas Antioksidan Kombinasi Ekstrak Etanol Rimpang Temu Giring (Curcuma heyneana) dan Daun Pugun Tanoh (Curanga Fel-Terrae) Menggunakan Metode Diphenyl Picrylhydrazil (DPPH). Talent. Conf. Ser. 2018, 2, 398–404. [Google Scholar] [CrossRef]
- Sukandiarsyah, F.; Purwaningsih, I.; Ratnawaty, G.J. Aktivitas Antioksidan Ekstrak Metanol dan n-Heksana Rimpang Temu Ireng (Curcuma aeruginosa Roxb.) Metode DPPH. J. Mandala Pharmacon. Indones. 2023, 9, 62–70. [Google Scholar] [CrossRef]
- Dewi, I.P.; Verawaty, V.; Taslim, T.; Sinabutar, P.P. Aktivitas Antioksidan Masker Gel Peel-off Ekstrak Rimpang Temu Ireng (Curcuma aeruginosa Roxb.). J. Kesehat. Perintis 2024, 11, 19–27. [Google Scholar] [CrossRef]
- Sari, R.P. Identifikasi Senyawa Flavonoid dan Uji Antioksidan Ekstrak Etanol Rimpang Temu Hitam (Curcuma aeruginosa Roxb) dengan Metode DPPH. Biol. Educ. Sci. Technol. 2024, 7, 2032–2038. [Google Scholar]
- Susiloningrum, D.; Sari, D.E.M. Uji Aktivitas Antioksidan dan Penetapan Kadar Flavonoid Total Ekstrak Temu Mangga (Curcuma mangga Valeton & Zipj) dengan Variasi Konsentrasi Pelarut. Cendekia J. Pharm. 2021, 5, 117–127. [Google Scholar] [CrossRef]
- Hartono, Y.I.; Widyastuti, I.; Luthfiah, H.Z.; Islamadina, R.; Can, A.T.; Rohman, A. Total Flavonoid Content and Antioxidant Activity of Temu Mangga (Curcuma mangga Val. & Zijp) and its Classification with Chemometrics. J. Food Pharm. Sci. 2020, 8, 202–214. [Google Scholar] [CrossRef]
- Alabdali, A.; Kzar, M.; Chinnappan, S.; Mogana, R.; Khalivula, S.I.; Rahman, H.; Razik, B.M.A. Antioxidant Activity of Curcumin. Res. J. Pharm. Technol. 2021, 14, 12. [Google Scholar] [CrossRef]
- Dalla, E.; Koumentakou, I.; Bikiaris, N.; Balla, E.; Lykidou, S.; Nikolaidis, N. Formulation, Characterization and Evaluation of Innovative O/W Emulsions Containing Curcumin Derivatives with Enhanced Antioxidant Properties. Antioxidants 2022, 11, 2271. [Google Scholar] [CrossRef] [PubMed]
- Milasari, M.; Jamaluddin, A.W.; Adikurniawan, Y.M. Pengaruh Pemberian Salep Ekstrak Kunyit Kuning (Curcuma longa Linn) terhadap Penyembuhan Luka Sayat pada Tikus Putih (Rattus norvegicus). J. Ilm. Ibnu Sina 2019, 4, 186–202. [Google Scholar] [CrossRef]
- Kaban, V.E.; Nasri; Rani, Z.; Suci, N.; Sekali., E.S.K.; Segala, H.U.B. The Effect of Turmeric Parent Extract Gel (Curcuma longa Linn) on Incision Wound Healing in Male White Rats (Rattus norvegicus). J. Pharm. Sci. 2024, 7, 616–627. [Google Scholar] [CrossRef]
- Adeliana; Usman, A.N.; Ahmad, M.; Arifuddin, S.; Yulianty, R.; Prihantono. Effectiveness of Turmeric (Curcuma longa Linn) Gel Extract (GE) on wound healing: Pre-Clinical Test. Gac. Sanit. 2021, 35, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Susanto, Y.; Solehah, F.A.; Fadya, A.; Khaerati, K. Potensi Kombinasi Ekstrak Rimpang Kunyit (Curcuma longa L.) dan Kapur Sirih Sebagai Anti Inflamasi dan Penyembuh Luka Sayat. J. Pharm. Sci. 2023, 8, 32–45. [Google Scholar] [CrossRef]
- Qabaha, K.; Abu-Lafi, S.; Al-Rimawi, F. Anti-inflammatory Activities of Ethanolic Extracts of curcuma Longa (Turmeric) and cinnamon (Cinnamomum verum). JFNR 2017, 5, 668–673. [Google Scholar]
- Kim, K.W.; Lee, Y.S.; Yoon, D.; Kim, G.S.; Lee, D.Y. The ethanolic extract of Curcuma longa grown in Korea exhibits anti-neuroinflammatory effects by activating of nuclear transcription factor erythroid-2-related factor 2/heme oxygenase-1 signaling pathway. BMC Complement. Med. Ther. 2022, 22, 343–346. [Google Scholar] [CrossRef]
- Shi, Y.; Liang, X.; Chi, L.; Chen, Y.; Liang, L.; Zhao, J.; Luo, Y.; Zhang, W.; Cai, Q.; Wu, X.; et al. Ethanol extracts from twelve Curcuma species rhizomes in China: Antimicrobial, antioxidative and anti-inflammatory activities. S. Afr. J. Bot. 2021, 140, 167–172. [Google Scholar] [CrossRef]
- Khatun, M.; Nur, M.A.; Biswas, S.; Khan, M.; Amin, M.Z. Assessment of the anti-oxidant, anti-inflammatory and anti-bacterial activities of different types of turmeric (Curcuma longa) powder in Bangladesh. J. Agric. Food Res. 2021, 6, 100201. [Google Scholar] [CrossRef]
- Muhammed, A.I.; Nasiry, B.S.A.N.A.; Rudha, A.M.H.A.A.; Dakheel, M.M.; Ali, S. Applications of Curcumin Extract Formulation for the Healing Efficacy on Mice Wounds. Int. J. Appl. Sci. Technol. 2022, 4, 17–184. [Google Scholar] [CrossRef]
- Amin, I.; Rashid, S.M.; Shubeena, S.; Hussain, I.; Ahmas, S.B.; Mir, M.U.R.; Alshehri, S.; Bukhari, S.I.; Mir, T.M.; Rehman, M.U. TLR4/NFκB-Mediated Anti-Inflammatory and Antioxidative Effect of Hexanic and Ethanolic Extracts of Curcuma longa L. in Buffalo Mammary Epithelial Cells. Separations 2022, 9, 414. [Google Scholar] [CrossRef]
- Pawar, R.; Toppo, F.; Mandloi, A.; Shaikh, S. Exploring the role of curcumin containing ethanolic extract obtained from Curcuma longa (rhizomes) against retardation of wound healing process by aspirin. Indian J. Pharmacol. 2015, 47, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Indriani, U.; Idiawati, N.; Wibowo, M.A. Uji Aktivitas Antiinflamasi dan Toksisitas Infus Kunyit (Curcuma domestica val.), Asam Jawa (Tamarindus indica L.) dan Sirih (Piper betle L.). J. Kim. Khatulistiwa 2018, 7, 107–112. [Google Scholar]
- Rahman, A.A.R.; Maryam, S.; Razak, R. Aktivitas Antiinflamasi Ekstrak Etanol Rimpang Kunyit (Curcuma domestica Val.) secara In Vitro. Makassar Pharm. Sci. J. 2024, 2, 336–343. [Google Scholar]
- Sukmawati, K.; Wati, A.; Muflihunna, A. Uji Aktivitas Ekstrak Kombinasi Rimpang Kunyit (Curcuma domestica Val.) dan Kurma (Phoenix dactylifera L.) sebagai Antiinflamasi Secara In Vitro. Window Health 2022, 5, 735–744. [Google Scholar] [CrossRef]
- Arisonya, S.; Wibisono, G.; Aditya, G. Efektivitas Ekstrak Kunyit (Curcuma domestica) terhadap Jumlah Sel Makrofag dan Diameter pada Lesi Ulkus Traumatikus (Suatu Penelitian In Vivo pada Tikus Putih Jantan (Rattus norvegiccus)). B-Dent J. Kedokt. Gigi Univ. Baiturrahmah 2018, 1, 118–125. [Google Scholar] [CrossRef][Green Version]
- Setyono, J.; Harini, I.M.; Sarmoko, S.; Rujito, L. Supplementation of curcuma domestica extract reduces cox-2 and inos expression on raw 264.7 cells. J. Phys. Conf. Ser. 2019, 1246, 12059. [Google Scholar] [CrossRef]
- Maulidi, R.R.; Girsang, E.; Nasution, A.N.; Ginting, S.F. Effectiveness of Ethanol Extract of Turmeric (Curcuma Domestic Valet) Gel Against Grade Iia Burn in White Rats (Rattus norvegicus). Int. J. Health Pharm. 2022, 2, 566–574. [Google Scholar] [CrossRef]
- Farida, Y.; Rahmat, D.; Amanda, A.W. Anti-Inflammation Activity Test of Nanoparticles Ethanol Extract of Temulawak Rhizome (Curcuma xanthorrhiza Roxb.) with Protein Denaturation Inhibition Method. J. Ilmu Kefarmasian Indones. 2018, 16, 225–230. [Google Scholar] [CrossRef]
- Ariyani, F.; Handharyani, E.; Sutardi, L.N. Wound Healing Using White Turmeric (Curcuma zedoaria) Extract Nanoparticles: Macroscopic and Microscopic Observation. J. Vet. 2022, 23, 441–447. [Google Scholar] [CrossRef]
- Andrina, S.; Churiyah, C.; Nuralih, N. Anti-Inflammatory Effect of Ethanolic Extract of Curcuma aeruginosa Roxb Rhizome, Morinda Citrifolia Fruit and Apium graveolens Leaf on Lipopplysaccharide-induce RAW 264.7 Cell Lines. Indones J. Cancer Chemoprevent. 2017, 6, 84–88. [Google Scholar] [CrossRef][Green Version]
- Srirod, S.; Tewtrakul, S. Anti-Inflammatory and Wound Healing Effects of Cream Containing Curcuma mangga Extract. J. Ethnopharmacol. 2019, 238, 111828. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Strong Anti-Inflammatory Effects of Curcumin. J. Nutr. Health Sci. 2016, 3, 205. [Google Scholar]
- Yuan, M.; Niu, J.; Li, F.; Ya, H.; Liu, X.; Li, K.; Fan, Y.; Zhang, Q. Dipeptide-1 modified nanostructured lipid carrier-based hydrogel with enhanced skin retention and topical efficacy of curcumin. RSC Adv. 2023, 13, 29152–29162. [Google Scholar] [CrossRef] [PubMed]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, S.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Li, J.; Xu, Y.; Wang, X.; Chen, H. In situ injectable nano-composite hydrogel composed of curcumin, N,O-carboxymethyl chitosan and oxidized alginate for wound healing application. Int. J. Pharm. 2012, 437, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Hamam, F.; Nasr, A. Curcumin-loaded mesoporous silica particles as wound-healing agent: An in vivo study. Saudi J. Med. Med. Sci. 2020, 8, 17–24. [Google Scholar] [CrossRef] [PubMed]
- González-Ortega, L.A.; Acosta-Osorio, A.A.; Grube-Pagola, P.; Palmeros-Exsome, C.; Cano-Sarmiento, C.; García-Varela, R.; García, H.S. Anti-inflammatory Activity of Curcumin in Gel Carriers on Mice with Atrial Edema. J. Oleo Sci. 2020, 69, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.Z.; Akter, J.; Hossain, M.A.; Islam, M.S.; Islam, P.; Goswami, C.; Nguyen, H.T.T.; Miyamoto, A. Anti-Inflammatory, Wound Healing, and Anti-Diabetic Effects of Pure Active Compounds Present in the Ryudai Gold Variety of Curcuma longa. Molecules 2024, 29, 2795. [Google Scholar] [CrossRef]
- Mohammad, C.A.; Ali, K.M.; Sha, A.M.; Gul, S.S. Effect of Curcumin gel on Inflammatory and Anti-Inflammatory Biomarkers in Experimental Induced Periodontitis in Rats: A Biochemical and Immunological Study. Front. Microbiol. 2023, 14, 1274189. [Google Scholar] [CrossRef]
- Yen, Y.H.; Pu, C.M.; Liu, C.W.; Chen, Y.C.; Chen, Y.C.; Liang, C.J.; Hsieh, J.H.; Huang, H.F.; Chen, Y.L. Curcumin Accelerates Cutaneous Wound Healing via Multiple Biological Actions: The Involvement of TNF-α, MMP-9, α-SMA, and Collagen. Intternational Wound J. 2018, 15, 605–617. [Google Scholar] [CrossRef]
- Frei, G.; Haimhoffer, A.; Csapo, E.; Bodnar, K.; Vasvari, G.; Nemes, D.; Lekli, I.; Gyongyosi, A.; Bacskay, I.; Feher, P.; et al. In Vitro and In Vivo Efficacy of Topical Dosage Forms Containing Sel-Nanoemulsifying Drug Delivery System Loaded with Curcumin. Pharmaceutics 2023, 15, 2054. [Google Scholar] [CrossRef]
- Herawati, H.; Oktanella, Y.; Anisa, A.K.; Wuragil, D.K.; Aulanni’am, A. In silico Analysis of Active Compounds from Ethanol Extract of Curcuma xanthorrhiza on COX-2 Receptors as Anti-Inflammation Candidate. In AIP Conference Proceedings; IOP Proceedings: Bristol, UK, 2021; pp. 1–8. [Google Scholar] [CrossRef]
- Fretes, F.D.; Rayanti, R.E.; Gintu, A.R. The Antioxidant Activity, Antibacterial Assay, and the Application of Turmeric (Curcuma domestica) Crude Extract with Various Solvents. Nusant. Sci. Tech. Proc. 2023, 2023, 80–93. [Google Scholar] [CrossRef]
- Bambal, V.; Mishra, M. Evaluation of in Vitro Sunscreen Activity of Herbal Cream Containing Extract of Curcuma longa and Butea monosperma. World J. Pharm. Res. 2014, 3, 3026–3035. [Google Scholar]
- Alfian, M.; Hasanudin, M.N.; Maulana, M.L.; Mustainin, M. Uji Aktivitas Tabir Surya Ekstrak dan Lotion Kunyit (Curcuma domestica Val.) secara In Vitro Menggunakan Spektrofotometri UV-Vis. J. Ilm. Ibnu Sina 2024, 9, 78–88. [Google Scholar]
- Narwaria, A.; Chakrabarty, A.K.; Bishayee, S.; Mohanty, S.; Banerjee, D.; Sharma, S.; Katiyar, C.K.; Dubey, S.K. Preparation, evaluation, and in vitro studies of sustained-release topical hydrogel of Curcuma longa L. targeting skin disorders. Int. J. Ayurveda Res. 2024, 5, 94–107. [Google Scholar] [CrossRef]
- Tiwari, R.; Singh, I.; Gupta, M.; Singh, L.P.; Tiwari, G. Formulation and Evaluation of Herbal Sunscreens: An Assessment Towards Skin Protection from Ultraviolet Radiation. Pharmacophore 2022, 13, 41–49. [Google Scholar] [CrossRef]
- Indarto, I.; Ikhsan, H.; Kuswannto, E. Aktifitas Tabir Surya dari Kombinasi Ekstrak Kunyit (Curcuma longa) dan Ganggang Hijau (Haematococus pluviaris) Secara In Vitro. Organisms 2021, 1, 119–124. [Google Scholar] [CrossRef]
- Nurhasnawati, H.; Sundu, R.; Sukmawati, A. Study of Curcuma Diversity from Central Java, Indonesia for Sunscreen and Antioxidant Activity based on Quantitative Phytochemical Analysis. Biodeiversitas 2023, 24, 6880–6887. [Google Scholar] [CrossRef]
- Khelker, T.; Haque, N.; Agrawal, A. Ultraviolet Protection potential of Curcuma longa L. and Citrus sinensis (L.) Osbeck. Res. J. Pharm. Tech. 2017, 10, 4282–4284. [Google Scholar] [CrossRef]
- Son, D.; Jun, J.S.; Hong, K. Photoprotection effect of Pu’er tea and Curcuma longa L. extracts against UV and blue lights. J. Appl. Biol. Chem. 2023, 66, 106–113. [Google Scholar] [CrossRef]
- Wilapangga, A.; Rahmat, D.; Rachmaniar, R. Formulasi dan Evaluasi Sediaan Gel Nanopartikel Ekstrak Temulawak (Curcuma xanthorrhiza) sebagai Tabir Surya. Indones. J. Pharm. 2023, 3, 26–32. [Google Scholar] [CrossRef]
- Pratiwi, P.D.; Sani, F.K.; Lestari, U. Determination of radical scavenging and sun protection factor of cream preparation contain ethanolic extract of Curcuma longa and Curcuma zedoaria. J. Pharm. Sci. 2021, 1, 240–245. [Google Scholar]
- Syarifah, A.L.; Andini, A.; Alfad, H.; Alfurida, A. Pengaruh Variasi Konsentrasi Ekstrak Temugiring (Curcuma heyneana) dalam Sediaan Krim terhadap Nilai SPF. J. Islam. Pharm. 2021, 6, 63–67. [Google Scholar] [CrossRef]
- Andriani, I.; Mardianingrum, R.; Susanti, S. Sunserum Wajah Sari Rimpang Temu Giring (Curcuma heyneana) Terfermentasi Lactobacillus bulgaricus. J. Ilm. Farm. Imelda 2023, 7, 20–33. [Google Scholar] [CrossRef]
- Maulida, A.N.; Supartono, S. Uji Efektivitas Krim Ekstrak Temu Giring (Curcuma heyneana Val) sebagai Tabir Surya. Indones. J. Chem. Sci. 2016, 5, 98–102. [Google Scholar]
- Saputra, A.; Purpratama, A.C.; Febriani, H.; Aznam, N. Uji Aktivitas Sediaan Gel Rimpang Temu Giring (Curcuma heyneana) sebagai Tabir Surya secara In Vitro. J. Ilm. Penal. Dan Penelit. Mhs. 2019, 3, 83–90. [Google Scholar]
- Ermawati, D.E.; Budiasih, D.Y. The Effect of Zinc Oxide and Curcuma heyneana Val, Combination on Stability and Sun Protection Factor (SPF) of Lotion. Pharmaciana 2022, 12, 327–334. [Google Scholar] [CrossRef]
- Nurwaini, S.; Mawarni, V. Formulasi Krim Tabir Surya Kombinasi Ekstrak Etanol Temu Mangga (Curcuma mangga) dan Seng Oksida. Camellia 2023, 2, 132–141. [Google Scholar] [CrossRef]
- Arizona, M.; Zulkarnain, A.K. Optimasi Formula dan Uji Aktivitas Secara In Vitro Lotion O/W Ekstrak Etanolik Rimpang Temu Mangga (Curcuma mangga Val. dan van Zijp) sebagai Tabir Surya. Maj. Farm. 2018, 14, 29. [Google Scholar] [CrossRef]
- Sari, D.E.M.; Susiloningrum, D. Penentuan Nilai SPF Krim Tabir Surya yang Mengandung Ekstrak Temu Mangga (Curcuma mangga Valeton & Zijp) dan Titanium Dioksida. Cendekia J. Pharm. 2022, 6, 102–111. [Google Scholar]
- Singh, B.G.; Bagora, N.; Nayak, M.; Ajish, J.K.; Gupta, N.; Kunwar, A. The Preparation of Curcumin-Loaded Pickering Emulsion Using Gelatin-Chitosan Colloidal Particles as Emulsifier for Possible Application as a Bio-Inspired Cosmetic Formulation. Pharmaceutics 2024, 16, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Sawant, M.; Chandrakant, P.; Hingane, L.D. Formulation and Evaluation of Herbal Sunscreen. IJCRT 2022, 10, 2320–2882. [Google Scholar] [CrossRef]
- Donglikar, M.M.; Deore, S.L. Development and Evaluation of Herbal Sunscreen. Pharmacog. J. 2017, 9, 83–97. [Google Scholar] [CrossRef]
- Shan, C.Y.; Iskandar, Y. Studi Kandungan Kimia dan Aktivitas Farmakologi Tanaman Kunyit (Curcuma longa L.). Farmaka 2018, 16, 547–555. [Google Scholar]
- Rana, M.; Maury, P.; Reddy, S.S.; Singh, V.; Ahmad, H.; Dwivedi, A.K.; Dikshit, M.; Barthwal, M. A Standardized Chemically Modified Curcuma longa Extract Modulates IRAK-MAPK Signaling in Inflammation and Potentiates Cytotoxicity. Front. Pharmacol. 2016, 7, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Suprihatin, T.; Rahayu, S.; Rifa’i, M.; Widyarti, S. Senyawa pada serbuk rimpang kunyit (Curcuma longa L.) yang berpotensi sebagai antioksidan. Bul. Anat. Dan Fisiol. 2020, 5, 35–42. [Google Scholar] [CrossRef]
- Threskeia, A.; Sandhika, W.; Rahayu, R.P. Effect of Turmeric (Curcuma longa) Extract Administration on Tumor Necrosis Fac-tor-Alpha and Type 1 Collagen Expression in UVB-Light Radiated BALB/c mice. J. Appl. Pharm. Sci. 2023, 13, 121–125. [Google Scholar] [CrossRef]
- Suryani, S.; Benny, F.; Wahyuni, W. Uji Efek Antiinflamasi secara In Vivo Nanopartikel Kurkumin yang Diformulasikan menggunakan Metode Reinforcement Gelasi Ionik. Pharmauho 2015, 1, 20–24. [Google Scholar]
- Rahmayunita, G.; Jacob, T.N.A.; Novianto, E.; Indriatami, W.; Rihatmadja, R.; Pusponegoro, E.H.D. A double-blind randomized controlled trial of topical Curcuma xanthorrhiza Roxb. on mild psoriasis: Clinical manifestations, histopathological features, and K6 expressions. Med. J. Indones. 2018, 27, 178–184. [Google Scholar] [CrossRef]
- Yunarto, N.; Aini, N.; Oktoberia, I.S.; Sulistyowati, I.; Kurniatri, A.A. Aktivitas Antioksidan serta Penghambatan HMG CoA dan Lipase dari Kombinasi Ekstrak Daun Binahong-Rimpang Temu Lawak. J. Kefarmasian Indones. 2019, 30, 89–96. [Google Scholar] [CrossRef]
- Sagita, N.D.; Sopyan, I.; Hadisaputri, Y.E. Kunir Putih (Curcuma zedoaria Rocs.): Formulasi, Kandungan Kimia dan Aktivitas Biologi. Maj. Farmasetika 2022, 7, 189–205. [Google Scholar] [CrossRef]
- Wulandari, R.; Puspitasari, P. Pengaruh Infusa Rimpang Temu Putih (Curcuma zedoaria (Berg.) Roscoe) terhadap Jumlah Leukosit dan Differential Counting (Diffcount) pada Kesembuhan Luka Laparatomi Pasca Bedah. J. Med. Lab. Sci. Technol. 2019, 2, 1689. [Google Scholar] [CrossRef]
- Ha, S.J.; Song, K.M.; Lee, J.; Kim, Y.H.; Lee, N.Y.; Kim, Y.E.; Lee, S.; Jung, S.K. Preventive Effect of Curcuma zedoaria Extract on UVB-Induced Skin In-flammation and Photoaging. J. Food Biochem. 2018, 42, 12598. [Google Scholar] [CrossRef]
- Paramita, S.; Moerad, E.B.; Ismail, S.; Marliana, E. Tracheospasmolytic and anti-inflammatory activity of indigenous Curcuma species as traditional antiasthmatic medicines. Nusant. Biosci. 2018, 10, 105–110. [Google Scholar] [CrossRef]
- Salman, S.; Indriana, M. Activity Ethanol Extract of Mangga (Curcuma mangga Val.) Feet Udem Rat White. J. Pharm. Sci. 2019, 2, 41–46. [Google Scholar] [CrossRef]
- Muchtaromah, B.; Mutmainah, F.N.; Prahardika, B.A.; Ahmad, M. Antioxidant and Antifungal Activities of Temu mangga (Curcuma mangga Val.) Extract in Some Solvents: Antioxidant and Antifungal Activities of C. mangga. Iran. J. Pharm. Sci. 2020, 16, 1–18. [Google Scholar]
- Yulianti, E.; Adelsa, A.; Putri, A. Penentuan nilai SPF (Sun Protection Factor) Ekstrak Etanol 70% Temu Mangga (Curcuma mangga) dan Krim Ekstrak Etanol 70% Temu Mangga (Curcuma mangga) secara In Vitro Menggunakan Metode Spektro-fotometri. Maj. Kesehat. Maj. Kesehat. 2015, 2, 41–50. [Google Scholar]
- Liu, X.; Zhang, R.; Shi, H.; Li, X.; Li, Y.; Taha, A.; Xu, C. Protective effect of curcumin against ultraviolet A irradiation induced photoaging in human dermal fibroblasts. Mol. Med. Rep. 2018, 20, 7227–7237. [Google Scholar] [CrossRef]
- Mohamad, E.A.; Rageh, M.; Ahmed, H. Curcumin provides skin Protection against UV radiation. Egypt. J. Chem. 2022, 65, 1341–1343. [Google Scholar] [CrossRef]
- Ismail, I.; Handayany, G.N.; Wahyuni, D.; Juliandri, J. Formulasi dan Penentuan Nilai SPF (Sun Protecting Factor) Sediaan Krim Tabir Surya Ekstrak Etanol dan Kemangi (Ocimum sanctum L.). J. Farm. Fak. Ilmu Kesehat. Univ. Islam 2014, 2, 6–11. [Google Scholar]
- Kanani, N. Pengaruh Temperatur terhadap Nilai Sun Protecting Factor (SPF) pada Ekstrak Kunyit Putih sebagai Bahan Pembuat Tabir Surya Menggunakan Pelarut Etil Asetat dan Metanol. J. Integr. Proses 2017, 6, 143–147. [Google Scholar] [CrossRef]
- Raymond-Lezman, J.R.; Riskin, S.I. Sunscreen Safety and Efficacy for the Prevention of Cutaneous Neoplasm. Cureus 2024, 16, e56369. [Google Scholar] [CrossRef]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural Components in Sunscreens: Topical Formulations with Sun Protection Factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural products and extracts from plants as natural UV filters for sunscreens: A review. Anim. Model Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-Based Active Photoprotectants for Sunscreen. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Salvo, E.D.; Gangemi, S.; Genovese, C.; Cicero, N.; Casciaro, M. Polyphenols from Mediterranean Plants: Biological Activities for Skin Photoprotection in Atopic Dermatitis, Psoriasis, and Chronic Urticaria. Plants 2023, 12, 3579. [Google Scholar] [CrossRef] [PubMed]
- Nisa, R.U.; Nisa, A.N.; Tantray, A.Y.; Shah, A.H.; Jan, A.T.; Shah, A.A.; Wani, I.A. Plant Phenolics with Promising Therapeutic Applications Against Skin Disorders: A Mechanistic Review. J. Agric. Food Res. 2024, 16, 101090. [Google Scholar] [CrossRef]
- Hedge, A.R.; Kunder, M.U.; Narayanaswamy, M.; Murugesan, S.; Furtado, S.C.; Veerabhadraiah, B.B.; Srinivasan, B. Advancements in Sunscreen Formulations: Integrating Polyphenolic Nanocarrirers and Nanotechnology for Enhanced UV Protection. Environ. Sci. Pollut. Res. 2024, 31, 38061–38082. [Google Scholar]
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in Sunscreens: Which and What For? Antioxidants 2023, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Resende, D.I.S.P.; Jesus, A.; Sousa, L.J.M.; Sousa, E.; Cruz, M.T.; Cidade, H.; Almeida, I.F. Up-to-Date Overview of the Use of Natural Ingredients in Sunscreens. Pharmaceuticals 2022, 15, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Contreras, A.M.T.; Baeza, A.G.; Limon, H.R.V.; Renteria, I.B.; Cabrera, M.A.R.; Estrada, K.R. Plant Secondary Metabolites Against Skin Photodamage: Mexican Plants, a Potential Source of UV-Radiation Protectant Molecules. Plants 2022, 11, 220–225. [Google Scholar] [CrossRef] [PubMed]
- Egambaram, O.P.; Pillai, S.K.; Ray, S.S. Materials Science Challenges in Skin UV Protection: A Review. Photochem. Photobiol. 2019, 96, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Lorquin, F.; Lorquin, J.; Bruno, M.C.; Rollet, M.; Robin, M.; Giorgio, C.; Piccerelle, P. Lignosulfonate is an efficient SPF booster: Application to eco-friendly sunscreen formulations. Sustain. Chem. Pharm. 2021, 24, 100539. [Google Scholar] [CrossRef]
- Chaabana, H.; Ioannoua, I.; Paris, C.; Charbonnel, C.; Ghoula, M. The photostability of flavanones, flavonols and flavones and evolution of their antioxidant activity. J. Photochem. Photobiol. Chem. 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Mansuri, R.; Diwan, A.; Kumar, H.; Dangwal, K.; Yadav, D. Potential of Natural Compounds as Sunscreen Agents. Pharmacogn. Rev. 2021, 15, 47–56. [Google Scholar] [CrossRef]
- Darmawan, M.A.; Ramadhani, N.H.; Hubeis, N.A.; Ramadhan, M.Y.A.; Sahlan, M.; Aziz, S.A.; Gozan, M. Natural sunscreen formulation with a high sun protection factor (SPF) from tengkawang butter and lignin. Ind. Crops Prod. 2022, 177, 114466. [Google Scholar] [CrossRef]
- Chen, L.; Hu, J.Y.; Wang, S.Q. The role of antioxidants in photoprotection: A critical review. J. Am. Acad. Dermatol. 2012, 67, 1013–1024. [Google Scholar] [CrossRef]
- Kockler, J.; Oelgemöller, M.; Robertson, S.; Glass, B.D. Photostability of sunscreens. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 91–110. [Google Scholar] [CrossRef]
- Kolbe, L.; Pissavini, M.; Tricaud, C.; Trullás, C.C.; Dietrich, E.; Matts, P.J. Anti-inflammatory/anti-oxidant activity of ingredients of sunscreen products? Implications for SPF. Int. J. Cosmet. Sci. 2019, 41, 320–324. [Google Scholar] [CrossRef]
- Meeran, S.M.; Akhtar, S.; Katiyar, S.K. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. J. Investig. Dermatol. 2009, 129, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef]
- Wiraningtyas, A.; Ruslan, R.; Agustina, S.; Hasanah, U. Penentuan Nilai Sun Protection Factor (SPF) dari Ekstrak Kulit Bawang Merah. J. Redoks J. Pendidik. Kim. Dan Ilmu Kim. 2019, 2, 34–43. [Google Scholar] [CrossRef]
- Saputri, M.; Razali, M.; Sari, N.; Nadia, S.; Anggreini, D. Mengenali Lebih Dekat Nilai SPF (Sun Protecting Factor) dalam Kosmetik. J. Pengabdi. Masy. Tjut Nyak Dhien 2024, 3, 33–38. [Google Scholar] [CrossRef]
- Afsar, T.; Razak, S.; Shabbir, M.; Khan, M.R. Antioxidant activity of polyphenolic compounds isolated from ethyl-acetate fraction of Acacia hydaspica R. Parker. Chem. Cent. J. 2018, 12, 5. [Google Scholar] [CrossRef]
- Sugihartini, N.; Firsty, G.R.; Laila, W.K.; Mulyaningsih, S.; Rais, I.R. Antioxidant, Tyrosinase Inhibition Activity, and In Vitro SPF Evaluation of Pepino Fruit Extract (Solanum muricatum Aiton) in Different Solvent Types and Concentrations. Pharm. Sci. Res. 2024, 11, 27–33. [Google Scholar]
- Sisa, M.; Bonnet, S.L.; Ferreira, D.; Van, W.J.H. Photochemistry of flavonoids. Molecules 2010, 15, 5196–5245. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.C.; Orellana, P.J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric Methods for Measurement of Antioxidant Activity in Food and Pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).