Pilot Clinical Safety and Efficacy Evaluation of a Topical 3% Tranexamic Acid Cream and Serum Protocol for Managing Facial Hyperpigmentation in Caucasian Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. In-Use Test Design
2.2. In-Use Test Demographics
2.3. Investigational Test Items
2.4. Protocol for Test Item Application
2.5. Endpoints and Clinical Assessments
2.5.1. Clinical Assessments
2.5.2. Photographic Recording
2.5.3. Colorimetric Evaluation of Dark Spots
2.5.4. Participant Questionnaires
2.5.5. Cutaneous Acceptability of Test Items
2.6. Statistical Analyses
3. Results
3.1. Test Item Formulation Analysis
3.2. Cutaneous Acceptability
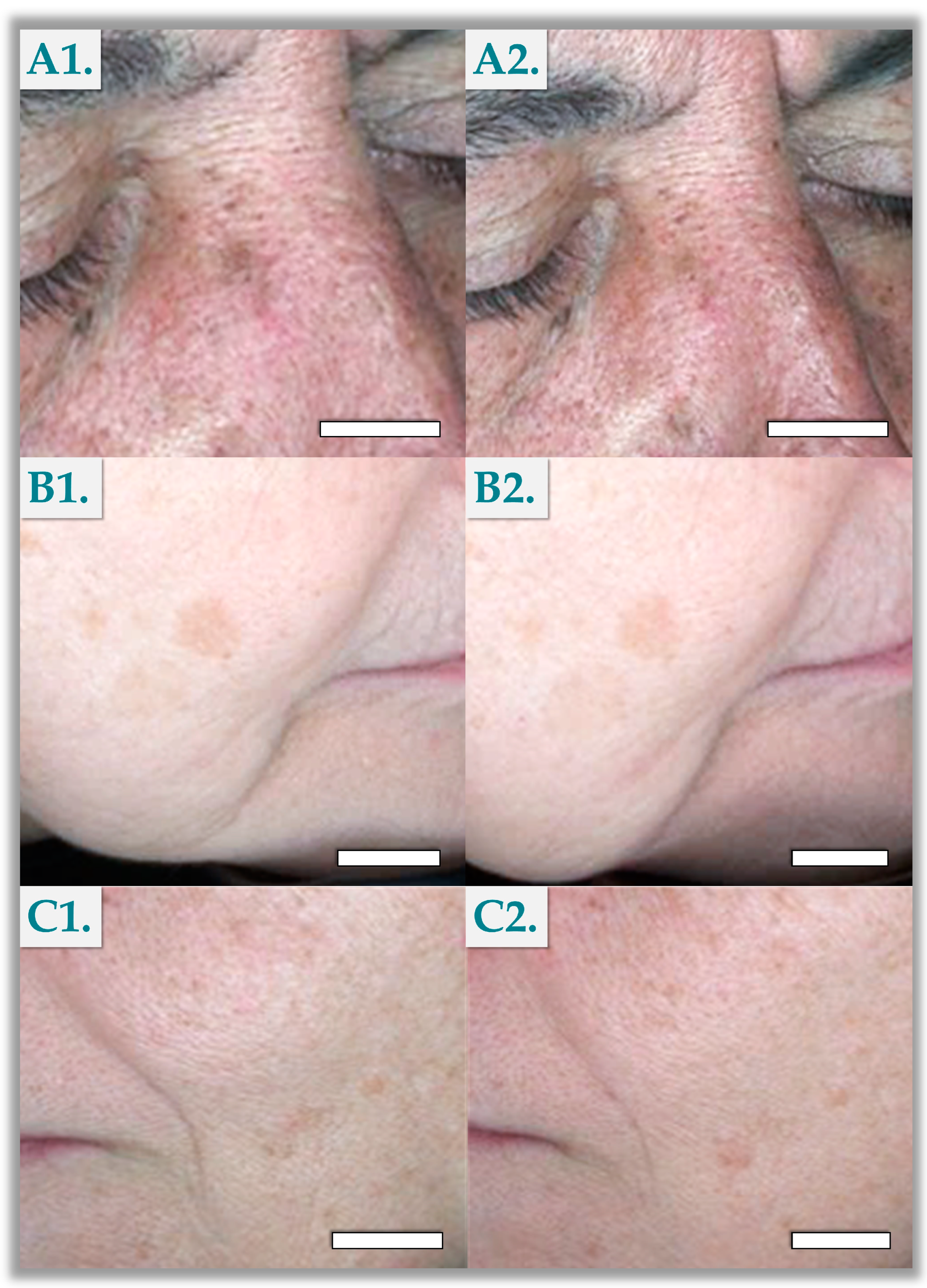
3.3. Standardized Photography Results
3.4. Colorimetric Evaluations
3.5. Self-Assessment Questionnaires
3.6. Clinical Scoring Results
4. Discussion
4.1. Combining Functional Ingredients for Topical Management of Hyperpigmentation
4.2. Assessing Tolerability and Efficacy of TXA-Based Facial Cream and Serum
4.3. Study Limitations and Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Abbreviations
| AGE | advanced glycation end-products |
| CHUV | Lausanne University Hospital |
| CRO | contract research organization |
| DNA | deoxyribonucleic acid |
| ECHA | European Chemicals Agency |
| FDA | US Food and Drug Administration |
| HA | hyaluronic acid |
| kDA | kiloDalton |
| MDa | megaDalton |
| MMP | matrix metalloproteinase |
| mRNA | messenger ribonucleic acid |
| NAD | nicotinamide adenine dinucleotide |
| NADP | nicotinamide adenine dinucleotide phosphate |
| NIA | niacinamide |
| Ph. Eur. | European Pharmacopoeia |
| PIH | post-inflammatory hyperpigmentation |
| ROS | reactive oxygen species |
| TXA | tranexamic acid |
| USA | United States of America |
| UV | ultraviolet |
| VEGFR | vascular endothelial growth factor receptor |
References
- Thawabteh, A.M.; Jibreen, A.; Karaman, D.; Thawabteh, A.; Karaman, R. Skin pigmentation types, causes and treatment-A review. Molecules 2023, 28, 4839. [Google Scholar] [CrossRef] [PubMed]
- Plensdorf, S.; Livieratos, M.; Dada, N. Pigmentation disorders: Diagnosis and management. Am. Fam. Physician 2017, 96, 797–804. [Google Scholar] [PubMed]
- Dalgard, F.J.; Gieler, U.; Tomas-Aragones, L.; Lien, L.; Poot, F.; Jemec, G.B.E.; Misery, L.; Szabo, C.; Linder, D.; Sampogna, F.; et al. The psychological burden of skin diseases: A cross-sectional multicenter study among dermatological out-patients in 13 European countries. J. Investig. Dermatol. 2015, 135, 984–991. [Google Scholar] [CrossRef]
- Dabas, G.; Vinay, K.; Parsad, D.; Kumar, A.; Kumaran, M.S. Psychological disturbances in patients with pigmentary disorders: A cross-sectional study. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 392–399. [Google Scholar] [CrossRef]
- Moolla, S.; Miller-Monthrope, Y. Dermatology: How to manage facial hyperpigmentation in skin of colour. Drugs Context 2022, 11, 2021-11-2. [Google Scholar] [CrossRef]
- Sarkar, R.; Arora, P.; Garg, K.V. Cosmeceuticals for hyperpigmentation: What is available? J. Cutan. Aesthet. Surg. 2013, 6, 4–11. [Google Scholar] [CrossRef]
- Clark, A.; Sivamani, R. Phytochemicals in the treatment of hyperpigmentation. Bot. Targets Ther. 2016, 6, 89–96. [Google Scholar] [CrossRef]
- González-Molina, V.; Martí-Pineda, A.; González, N. Topical treatments for melasma and their mechanism of action. J. Clin. Aesthet. Dermatol. 2022, 15, 19–28. [Google Scholar] [PubMed]
- Gaćina, K.; Krstanović Ćosić, A. The use of tranexamic acid in dermatology. Acta Clin. Croat. 2023, 62, 368–372. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, J.Y.; Shibata, T.; Fujiwara, R.; Kang, H.Y. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin. Exp. Dermatol. 2016, 41, 480–485. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Naeini, F.F. Topical tranexamic acid as a promising treatment for melasma. J. Res. Med. Sci. 2014, 19, 753–757. [Google Scholar] [PubMed]
- Atefi, N.; Dalvand, B.; Ghassemi, M.; Mehran, G.; Heydarian, A. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol. Ther. 2017, 7, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Lim, H.W. The uses of tranexamic acid in dermatology: A review. Int. J. Dermatol. 2023, 62, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Otte, N.; Borelli, C.; Korting, H.C. Nicotinamide-Biologic actions of an emerging cosmetic ingredient. Int. J. Cos. Sci. 2005, 27, 255–261. [Google Scholar] [CrossRef]
- Bissett, D.L.; Miyamoto, K.; Sun, P.; Li, J.; Berge, C.A. Topical niacinamide reduces yellowing, wrinkling, red blotchiness, and hyperpigmented spots in aging facial skin. Int. J. Cos. Sci. 2004, 26, 231–238. [Google Scholar] [CrossRef]
- Hakozaki, T.; Minwalla, L.; Zhuang, J.; Chhoa, M.; Matsubara, A.; Miyamoto, K.; Greatens, A.; Hillebrand, G.G.; Bissett, D.L.; Boissy, R.E. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br. J. Dermatol. 2002, 147, 20–31. [Google Scholar] [CrossRef]
- Couteau, C.; Alvarez Rueda, N.; Breton, B.; Coiffard, L. A review of homemade cosmetics based on a study of 150 blogs and their authors. Int. J. Cos. Sci. 2023, 45, 539–547. [Google Scholar] [CrossRef]
- Wu, Y.; Tanaka, T.; Akimoto, M. Utilization of individual typology angle (ITA) and hue angle in the measurement of skin color on images. Bioimages 2020, 28, 1–8. [Google Scholar]
- Alaluf, S.; Atkins, D.; Barrett, K.; Blount, M.; Carter, N.; Heath, A. The impact of epidermal melanin on objective measurements of human skin colour. Pigment Cell Res. 2002, 15, 119–126. [Google Scholar] [CrossRef]
- Jdid, R.; Pedrazzani, M.; Lejeune, F.; Fischman, S.; Cazorla, G.; Forestier, S.; Khalifa, Y.B. Skin dark spot mapping and evaluation of brightening product efficacy using Line-field Confocal Optical Coherence Tomography (LC-OCT). Ski. Res. Technol. 2024, 30, e13623. [Google Scholar] [CrossRef]
- Makino, E.T.; Huang, P.; Cheng, T.; Acevedo, S.F.; de Oliveira, C.; Mehta, R.C. 12-Week, single-center study of a targeted pigment-correcting dark spot treatment for post-inflammatory hyperpigmentation and solar lentigines. Clin. Cosmet. Investig. Dermatol. 2023, 16, 2677–2686. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Hadjab, F.; Porcello, A.; Lourenço, K.; Scaletta, C.; Abdel-Sayed, P.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Mechanistic insights into the multiple functions of niacinamide: Therapeutic implications and cosmeceutical applications in functional skincare products. Antioxidants 2024, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Jatana, G.K.; Sonthalia, S. Cosmeceuticals. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Ali, A.; Skedung, L.; Burleigh, S.; Lavant, E.; Ringstad, L.; Anderson, C.D.; Wahlgren, M.; Engblom, J. Relationship between sensorial and physical characteristics of topical creams: A comparative study on effects of excipients. Int. J. Pharm. 2022, 613, 121370. [Google Scholar] [CrossRef]
- Nebogina, N.A.; Prozorova, I.V.; Yudina, N.V. The influence of the temperature of formation of water-oil emulsions on their dispersion. AIP Conf. Proc. 2020, 2310, 020221. [Google Scholar] [CrossRef]
- Kim, K.M.; Oh, H.M.; Lee, J.H. Controlling the emulsion stability of cosmetics through shear mixing process. Korea-Aust. Rheol. J. 2020, 32, 243–249. [Google Scholar] [CrossRef]
- Maeda, K.; Tomita, Y. Mechanism of the inhibitory effect of tranexamic acid on melanogenesis in cultured human melanocytes in the presence of keratinocyte-conditioned medium. J. Health Sci. 2007, 53, 389–396. [Google Scholar] [CrossRef]
- Zhu, J.W.; Ni, Y.J.; Tong, X.Y.; Guo, X.; Wu, X.P. Activation of VEGF receptors in response to UVB promotes cell proliferation and melanogenesis of normal human melanocytes. Exp. Cell Res. 2020, 387, 111798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.W.; Ni, Y.J.; Tong, X.Y.; Guo, X.; Wu, X.P.; Lu, Z.F. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int. J. Med. Sci. 2020, 17, 903–911. [Google Scholar] [CrossRef]
- Kim, H.J.; Moon, S.H.; Cho, S.H.; Lee, J.D.; Kim, H.S. Efficacy and safety of tranexamic acid in melasma: A meta-analysis and systematic review. Acta Derm. Venereol. 2017, 97, 776–781. [Google Scholar] [CrossRef]
- Walocko, F.M.; Eber, A.E.; Keri, J.E.; Al-Harbi, M.A.; Nouri, K. The role of nicotinamide in acne treatment. Dermatol. Ther. 2017, 30, e12481. [Google Scholar] [CrossRef]
- Cho, Y.H.; Park, J.E.; Lim, D.S.; Lee, J.S. Tranexamic acid inhibits melanogenesis by activating the autophagy system in cultured melanoma cells. J. Dermatol. Sci. 2017, 88, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Bang, S.H.; Kim, J.H.; Shin, H.J.; Choi, J.H.; Chang, S.E. Tranexamic acid diminishes laser-induced melanogenesis. Ann. Dermatol. 2015, 27, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kimball, A.B.; Kaczvinsky, J.R.; Li, J.; Robinson, L.R.; Matts, P.J.; Berge, C.A.; Miyamoto, K.; Bissett, D.L. Reduction in the appearance of facial hyperpigmentation after use of moisturizers with a combination of topical niacinamide and N-acetyl glucosamine: Results of a randomized, double-blind, vehicle-controlled trial. Br. J. Dermatol. 2010, 162, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Mechanistic basis and clinical evidence for the applications of nicotinamide (niacinamide) to control skin aging and pigmentation. Antioxidants 2021, 10, 1315. [Google Scholar] [CrossRef]
- Yanez, M.; Jhanji, M.; Murphy, K.; Gower, R.M.; Sajish, M.; Jabbarzadeh, E. Nicotinamide augments the anti-inflammatory properties of resveratrol through PARP1 activation. Sci. Rep. 2019, 9, 10219. [Google Scholar] [CrossRef]
- Biedroń, R.; Ciszek, M.; Tokarczyk, M.; Bobek, M.; Kurnyta, M.; Słominska, E.M.; Smoleński, R.T.; Marcinkiewicz, J. 1-Methylnicotinamide and nicotinamide: Two related anti-inflammatory agents that differentially affect the functions of activated macrophages. Arch. Immunol. Ther. Exp. 2008, 56, 127–134. [Google Scholar] [CrossRef]
- Ong, R.R.; Goh, C.F. Niacinamide: A review on dermal delivery strategies and clinical evidence. Drug Del. Transl. Res. 2024, 1–37. [Google Scholar] [CrossRef]
- Minoretti, P.; Emanuele, E. Clinically actionable topical strategies for addressing the hallmarks of skin aging: A primer for aesthetic medicine practitioners. Cureus 2024, 16, e52548. [Google Scholar] [CrossRef]
- Huber, R.; Wong, A. Nicotinamide: An update and review of safety & differences from niacin. Ski. Ther. Let. 2020, 25, 7–11. [Google Scholar]
- Schalka, S. New data on hyperpigmentation disorders. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 18–21. [Google Scholar] [CrossRef]
- Qian, H.; Shan, Y.; Gong, R.; Lin, D.; Zhang, M.; Wang, C.; Wang, L. Mechanism of action and therapeutic effects of oxidative stress and stem cell-based materials in skin aging: Current evidence and future perspectives. Front. Bioeng. Biotechnol. 2023, 10, 1082403. [Google Scholar] [CrossRef] [PubMed]
- Porcello, A.; Chemali, M.; Marques, C.; Scaletta, C.; Lourenço, K.; Abdel-Sayed, P.; Raffoul, W.; Hirt-Burri, N.; Applegate, L.A.; Laurent, A. Dual functionalization of hyaluronan dermal fillers with vitamin B3: Efficient combination of bio-stimulation properties with hydrogel system resilience enhancement. Gels 2024, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, Y.; Kim, J.; Lee, S.; Park, J. The synergistic effect of hyaluronic acid and vitamin C in hydrating and brightening the skin. Int. J. Cosmet. Sci. 2018, 40, 325–331. [Google Scholar]
- Boo, Y.C. Ascorbic acid (vitamin C) as a cosmeceutical to increase dermal collagen for skin antiaging purposes: Emerging combination therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Al-Niaimi, F.; Chiang, N.Y.Z. Topical vitamin C and the skin: Mechanisms of action and clinical applications. J. Clin. Aesthet. Dermatol. 2017, 10, 14–17. [Google Scholar]
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The roles of vitamin C in skin health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Machado, B.H.B.; Frame, J.; Zhang, J.; Najlah, M. Comparative study on the outcome of periorbital wrinkles treated with laser-assisted delivery of vitamin C or vitamin C plus growth factors: A randomized, double-blind, clinical trial. Aesthet. Plast. Surg. 2021, 45, 1020–1032. [Google Scholar] [CrossRef]
- Mainville, L.; Smilga, A.S.; Fortin, P.R. Effect of nicotinamide in skin cancer and actinic keratoses chemoprophylaxis, and adverse effects related to nicotinamide: A systematic review and meta-analysis. J. Cutan. Med. Surg. 2022, 26, 297–308. [Google Scholar] [CrossRef]
- Lee, D.H.; Oh, I.Y.; Koo, K.T.; Suk, J.M.; Jung, S.W.; Park, J.O.; Kim, B.J.; Choi, Y.M. Reduction in facial hyperpigmentation after treatment with a combination of topical niacinamide and tranexamic acid: A randomized, double-blind, vehicle-controlled trial. Ski. Res. Technol. 2014, 20, 208–212. [Google Scholar] [CrossRef]
- Telang, P.S. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef] [PubMed]
- Farris, P.K. Topical vitamin C: A useful agent for treating photoaging and other dermatologic conditions. Dermatol. Surg. 2005, 31, 814–818. [Google Scholar] [CrossRef]
- Jacques, C.; Genies, C.; Bacqueville, D.; Tourette, A.; Borotra, N.; Chaves, F.; Sanches, F.; Gaudry, A.L.; Bessou-Touya, S.; Duplan, H. Ascorbic acid 2-glucoside: An ascorbic acid pro-drug with longer-term antioxidant efficacy in skin. Int. J. Cosmet. Sci. 2021, 43, 691–702. [Google Scholar] [CrossRef]
- Stamford, N.P. Stability, transdermal penetration, and cutaneous effects of ascorbic acid and its derivatives. J. Cosmet. Dermatol. 2012, 11, 310–317. [Google Scholar] [CrossRef]
- Starr, N.J.; Abdul Hamid, K.; Wibawa, J.; Marlow, I.; Bell, M.; Pérez-García, L.; Barrett, D.A.; Scurr, D.J. Enhanced vitamin C skin permeation from supramolecular hydrogels, illustrated using in situ ToF-SIMS 3D chemical profiling. Int. J. Pharm. 2019, 563, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Lee, P.C.; Huang, L.K.; Lu, L.P.; Liao, W.C. Stability studies of ascorbic acid 2-glucoside in cosmetic lotion using surface response methodology. Bioorgan. Med. Chem. Let. 2013, 23, 1583–1587. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E. pH in nature, humans and skin. J. Dermatol. 2018, 45, 1044–1052. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and anti-inflammatory activity of ascorbic acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- Bravo, B.; Correia, P.; Gonçalves Junior, J.E.; Sant’Anna, B.; Kerob, D. Benefits of topical hyaluronic acid for skin quality and signs of skin aging: From literature review to clinical evidence. Dermatol. Ther. 2022, 35, e15903. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, E.S.; Nam, S.M.; Choi, C.Y. Comparison of effectiveness and safety of a botulinum toxin monotherapy and a combination therapy with hyaluronic acid filler for improving glabellar frown. Aesthet. Plast. Surg. 2022, 46, 1872–1880. [Google Scholar] [CrossRef]
- Juncan, A.M.; Moisă, D.G.; Santini, A.; Morgovan, C.; Rus, L.L.; Vonica-Țincu, A.L.; Loghin, F. Advantages of hyaluronic acid and its combination with other bioactive ingredients in cosmeceuticals. Molecules 2021, 26, 4429. [Google Scholar] [CrossRef] [PubMed]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O.J.S.R. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Ski. Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Beygi, M.; Mohammad, T.F.; Alijanianzadeh, M.; Pillaiyar, T.; Garcia-Molina, P.; Garcia-Canovas, F.; Munoz-Munoz, J.; Saboury, A.A. Targeting tyrosinase in hyperpigmentation: Current status, limitations and future promises. Biochem. Pharmacol. 2023, 212, 115574. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enz. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Wang, R.F.; Ozog, D.; Lim, H.W.; Mohammad, T.F. Disorders of hyperpigmentation. Part II. Review of management and treatment options for hyperpigmentation. J. Am. Acad. Dermatol. 2023, 88, 291–320. [Google Scholar] [CrossRef]
- Nautiyal, A.; Wairkar, S. Management of hyperpigmentation: Current treatments and emerging therapies. Pigment Cell Melan. Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, J.; Jiang, L.; Mu, Y. The application of skin care product in melasma treatment. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1165–1171. [Google Scholar] [CrossRef]
- Steiner, M.; Peppelman, M.; Houben, E.; van Erp, P.; van de Kerkhof, P.; Luttge, R. The effectiveness of tranexamic acid in combination with laser treatments for melasma. Lasers Med. Sci. 2019, 34, 1237–1243. [Google Scholar]
- Maeda, K. New method of measurement of epidermal turnover in humans. Cosmetics 2017, 4, 47. [Google Scholar] [CrossRef]
- Collier, A.E.; Wek, R.C.; Spandau, D.F. Human keratinocyte differentiation requires translational control by the eIF2α kinase GCN2. J. Invest. Dermatol. 2017, 137, 1924–1934. [Google Scholar] [CrossRef]
- Feng, X.; Su, H.; Xie, J. Efficacy and safety of tranexamic acid in the treatment of adult melasma: An updated meta-analysis of randomized controlled trials. J. Clin. Pharm. Therap. 2021, 46, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Michael, S.; Reimers, K.; Strauß, S.; Vogt, P.M.; Chichkov, B. Bioprinting for skin. In 3D Bioprinting and Nanotechnology in Tissue Engineering and Regenerative Medicine; Academic Press: Cambridge, MA, USA, 2022; pp. 397–425. [Google Scholar]
- Austin, E.; Nguyen, J.K.; Jagdeo, J. Topical treatments for melasma: A systematic review of randomized controlled trials. J. Drugs Dermatol. 2019, 18, S1545961619P1156X. [Google Scholar] [PubMed]
- Desai, S.; Ayres, E.; Bak, H.; Manco, M.; Lynch, S.; Raab, S.; Du, A.; Green, D.; Skobowiat, C.; Wangari-Talbot, J.; et al. Effect of a tranexamic acid, kojic acid, and niacinamide containing serum on facial dyschromia: A clinical evaluation. J. Drugs Dermatol. 2019, 18, 454–459. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
| Study Phase/Activities | Day 0 | Day 55 | Day 56 |
|---|---|---|---|
| Clinical examination | Yes | / | Yes |
| Participant inclusion | Yes | / | / |
| Clinical scoring by the dermatologist investigator | Yes | / | Yes |
| Color measurements of dark spots with colorimeter | Yes | / | Yes |
| Photographs of the face | Yes | / | Yes |
| Application of the products under normal conditions of use | Daily application | / | |
| Subjective assessment of the cosmetic qualities and efficacy of the tested products | / | / | Yes |
| Origin/Ethnicity | Caucasian: 22 (100%) |
|---|---|
| Sex | Female: 18 (82%) Male: 4 (18%) |
| Age | 45 to 67 years old |
| Skin Nature | Normal: 3 (14%) Dry: 10 (45%) Oily: 1 (5%) Mixed Oily: 8 (36%) |
| Skin Sensitivity | 1 (5%) |
| Atopic Tendency | 1 (5%) |
| Facial Spots | 22 (100%) |
| Key Ingredient | Role | Percentage in Finished Product (%) | Temperature of Incorporation (°C) | Supplier |
|---|---|---|---|---|
| Aqua/Water | Solvent | 54.31% | 70 °C | Internal |
| Niacinamide | Anti-inflammatory and brightening agent | 5.00% | 20 °C | DSM |
| Tranexamic Acid | Skin-lightening agent | 3.00% | 20 °C | Ami Lifesciences |
| Hyaluronic Acid | Moisturizing agent | 0.25% | 20 °C | Givaudan |
| Key Ingredient | Role | Percentage in Finished Product (%) | Temperature of Incorporation (°C) | Supplier |
|---|---|---|---|---|
| Aqua/Water | Solvent | 62.80% | 70 °C | Internal |
| Niacinamide | Antioxidant and brightening agent | 5.00% | 20 °C | DSM |
| Tranexamic Acid | Brightening agent | 3.00% | 20 °C | Ami Lifesciences |
| Ascorbyl Glucoside | Antioxidant and brightening agent | 2.00% | 20 °C | Hayashibara |
| Low-Molecular-Weight Hyaluronic Acid | Moisturizing agent | 0.50% | 20 °C | Givaudan |
| Medium-Molecular-Weight Hyaluronic Acid | Moisturizing agent | 0.25% | 20 °C | Givaudan |
| High-Molecular-Weight Hyaluronic acid | Moisturizing agent | 0.25% | 20 °C | Givaudan |
| Parameter | n | Means and Standard Deviations 1 | Paired t-Test p-Value | Percentage of Variation (%) | Delta | |
|---|---|---|---|---|---|---|
| D0 Initial Values | D56 Final Values | |||||
| L* | 21 | 57.55 ± 0.56 | 58.20 ± 0.54 | 0.0326 | +1% | +0.66 |
| b* | 17.54 ± 0.34 | 17.51 ± 0.32 | 0.9359 | +0% | −0.03 | |
| ITA° | 23.06 ± 1.61 | 24.98 ± 1.57 | 0.0162 | +8% | +1.92 | |
| Parameter | n | Means and Standard Deviations | Wilcoxon Test p-Value | Percentage of Variation (%) | |
|---|---|---|---|---|---|
| D0 Initial Values | D56 Final Values | ||||
| Color Intensity of the Spot | 22 | 5.86 ± 0.21 | 6.55 ± 0.16 | < 0.0001 | +13% |
| Size of the Spot (cm) | 22 | 1.02 ± 0.14 | 0.95 ± 0.13 | 0.0020 | −6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsin, S.; Lourenço, K.; Porcello, A.; Marques, C.; Rodriguez, C.; Raffoul, W.; Scaletta, C.; Abdel-Sayed, P.; Hadjab, B.; Applegate, L.A.; et al. Pilot Clinical Safety and Efficacy Evaluation of a Topical 3% Tranexamic Acid Cream and Serum Protocol for Managing Facial Hyperpigmentation in Caucasian Patients. Cosmetics 2024, 11, 168. https://doi.org/10.3390/cosmetics11050168
Hsin S, Lourenço K, Porcello A, Marques C, Rodriguez C, Raffoul W, Scaletta C, Abdel-Sayed P, Hadjab B, Applegate LA, et al. Pilot Clinical Safety and Efficacy Evaluation of a Topical 3% Tranexamic Acid Cream and Serum Protocol for Managing Facial Hyperpigmentation in Caucasian Patients. Cosmetics. 2024; 11(5):168. https://doi.org/10.3390/cosmetics11050168
Chicago/Turabian StyleHsin, Sarah, Kelly Lourenço, Alexandre Porcello, Cíntia Marques, Clara Rodriguez, Wassim Raffoul, Corinne Scaletta, Philippe Abdel-Sayed, Basste Hadjab, Lee Ann Applegate, and et al. 2024. "Pilot Clinical Safety and Efficacy Evaluation of a Topical 3% Tranexamic Acid Cream and Serum Protocol for Managing Facial Hyperpigmentation in Caucasian Patients" Cosmetics 11, no. 5: 168. https://doi.org/10.3390/cosmetics11050168
APA StyleHsin, S., Lourenço, K., Porcello, A., Marques, C., Rodriguez, C., Raffoul, W., Scaletta, C., Abdel-Sayed, P., Hadjab, B., Applegate, L. A., & Laurent, A. (2024). Pilot Clinical Safety and Efficacy Evaluation of a Topical 3% Tranexamic Acid Cream and Serum Protocol for Managing Facial Hyperpigmentation in Caucasian Patients. Cosmetics, 11(5), 168. https://doi.org/10.3390/cosmetics11050168











