1. Introduction
Skin whitening and depigmentation practices are prevalent in specific ethnic groups, notably in Asia, Africa, and the Middle East. This widespread phenomenon can be attributed to the intricate interplay of cultural, social, political, and psychological factors [
1]. Among Asian women, the popularity of skin-lightening products has significantly increased, driven largely by their desire to counter color-based discrimination [
2]. Globally, there is a rapid demand for combination skin-lightening products. It has been estimated that their market value will reach USD 7.68 billion by 2028, with a projected growth rate of 6.23% from 2021 to 2028 [
3].
Whitening is the process of reducing the amount of melanin, or pigment, in the skin to make it appear lighter. Melanin is part of a group of natural pigments that is ubiquitous in nearly all living organisms, playing an essential role in epidermal homeostasis and serving as a defense against environmental stressors, such as ultraviolet radiation from the sun [
4]. In contrast, abnormal melanin synthesis can be associated with various dermatological conditions, including the formation of freckles, solar lentigo, melasma, vitiligo, melanoma, and other hyperpigmented skin disorders [
5]. Hyperpigmentation-related skin disorders can be treated with depigmentation agents, including conditions such as melasma, post-inflammatory hyperpigmentation, congenital melanocytic naevi, lentigo, erythromelanosis follicularis faciei et colli, and erythema dyschromicum perstans [
6].
The overproduction of melanin by the melanosomes leads to hyperpigmentation, a result of melanogenesis. These processes begin when
l-tyrosine is converted into three major types of pigments, including eumelanin, pheomelanin, and mixed melanin [
7]. Tyrosinase (TYR) plays a crucial role in melanogenesis, catalyzing the initial two stages involved in the conversion of
l-tyrosine into
l-DOPA and the subsequent conversion of
l-DOPA into dopaquinone. Additionally, tyrosinase-related protein-1 (TYRP-1) and tyrosinase-related protein-2 (TRP-2), also known as dopachrome tautomerase (DCT), are the enzymes specifically involved in eumelanin biosynthesis. TYRP-2 catalyzes the tautomerization of dopachrome to 5,6-dihydroxyindole-2-carboxylic acid (DHICA). This compound is subsequently converted into eumelanin by TYRP-1 [
8]. Therefore, the identification of tyrosinase inhibitors has been the most frequently used approach for suppressing or reducing melanogenesis, or for skin whitening [
7].
Numerous studies have documented the presence of TYR inhibitors in natural sources, with the majority being identified in plants. Skin-whitening agents sourced from nature hamper melanin biosynthesis by directly suppressing TYR activity. They also disturb the melanin synthesis cascade, affecting pathways such as the microphthalmia-associated transcription factor (MITF) pathway [
9]. MITF, a pivotal basic helix-loop-helix leucine zipper transcription factor, plays a crucial role in the regulation of genes within the tyrosinase and tyrosinase-related proteins family in melanocytes. Various reports indicate that natural products deregulate the MITF pathway during stimulated melanin synthesis in melanocytes. They also suppress melanosome’s uptake and distribution in keratinocytes [
9]. Commonly utilized in cosmetics and dermatology, whitening agents include arbutin, azelaic acid, hydroquinone, kojic acid, and resveratrol. Despite their efficacy, each of these agents raises safety concerns [
10]. Many skin-whitening products contain ingredients that are toxic when used cosmetically for extended periods without medical guidance. These ingredients not only have the potential to harm the skin but also to cause life-threatening illnesses [
10]. For instance, hydroquinone, a natural phenolic compound considered the gold standard for skin whitening, has been used for decades. However, its long-term use has been associated with various adverse effects, including contact dermatitis, conjunctival melanosis, corneal degeneration, exogenous ochronosis, nail discoloration, and skin irritation. Moreover, it has been shown to be toxic to the kidneys, bone marrow, and the immune system [
1]. Furthermore, in the European Union (EU), hydroquinone has been regulated, with restrictions imposed on its use in cosmetic products due to concerns about the carcinogenicity of its metabolite. Another natural whitening agent, kojic acid, is known for its storage stability issues and carcinogenic activity [
1]. Several natural tyrosinase activity inhibitors have proven ineffective in human applications due to their low bioavailability. Consequently, researchers in academia and in industry are actively exploring novel potent and safe tyrosinase inhibitors from both natural and synthetic sources.
Patchouli essential oil (PEO) is a volatile extract obtained from the dried leaves of
Pogostemon cablin (Blanco) Benth. (Lamiaceae) through steam distillation or hydrodistillation methods. Renowned for its distinctive woody aroma, it stands as a key ingredient in perfumery, cosmetics, toiletries, detergents, and the pharmaceutical industry [
11]. Indeed, it has been emphasized that “patchouli oil is one of the most crucial materials available to the perfumer”. Significant research has been conducted on the chemical constituents and bioactivities of patchouli oil [
11]. Patchouli alcohol (syn. patchoulol), a tricyclic sesquiterpene, has emerged as a significant bioactive component in the oil extracted from the aerial parts of
P. cablin. Prior investigations have documented the broad spectrum of bioactivities associated with patchouli alcohol, including anti-influenza virus, anti-depressive, anti-nociceptive, vasorelaxation, lung protection, brain protection, anti-ulcerogenic, anti-colitis, prebiotic-like, anti-inflammatory, anti-cancer, and protective activities against metabolic diseases [
12]. Up to this point, there have been no reported findings concerning the potential skin-whitening effects of either patchouli oil or its primary bioactive compound, patchouli alcohol. The present study aims to investigate the anti-melanogenic properties of patchouli essential oil and patchouli alcohol utilizing a murine melanoma cell model.
2. Materials and Methods
2.1. Chemicals and Reagents
Patchouli essential oil (PEO) was provided by Bio-Jourdeness International Groups Co., Ltd. (Taichung, Taiwan). Patchouli alcohol was obtained from Biosynth International, Inc. (San Diego, CA, USA). The compound’s purity was determined to be above 99%, as confirmed by both gas chromatography (GC) and proton nuclear magnetic resonance (1H-NMR) analyses. Fetal bovine serum (FBS), Roswell park memorial institute (RPMI) 1640 medium, penicillin, and streptomycin were procured from Life Technologies (Grand Island, NY, USA). 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), tyrosinase (EC 1.14.18.1, activity of 6680 units/mg), melanin, and kojic acid (KA) were purchased from Sigma-Aldrich (St. Louis, CA, USA). Forskolin (FRK) was acquired from Selleckchem (Houston, TX, USA). An antibody against tyrosinase was obtained from Genetex, Irvin, CA, USA. Antibodies against GAPDH, tyrosinase-related protein-1, and tyrosinase-related protein-2 were obtained from Santa-Cruz Biotechnology (Dallas, TX, USA). Horseradish peroxidase (HRP)-linked anti-mouse IgG and anti-rabbit IgG antibodies were sourced from Cell Signaling Technology (Danvers, MA, USA). All other chemicals used were of reagent grade or HPLC grade and were provided by either Merck (Darmstadt, Germany) or Sigma-Aldrich.
2.2. Cell Culture and Cell Viability Assay
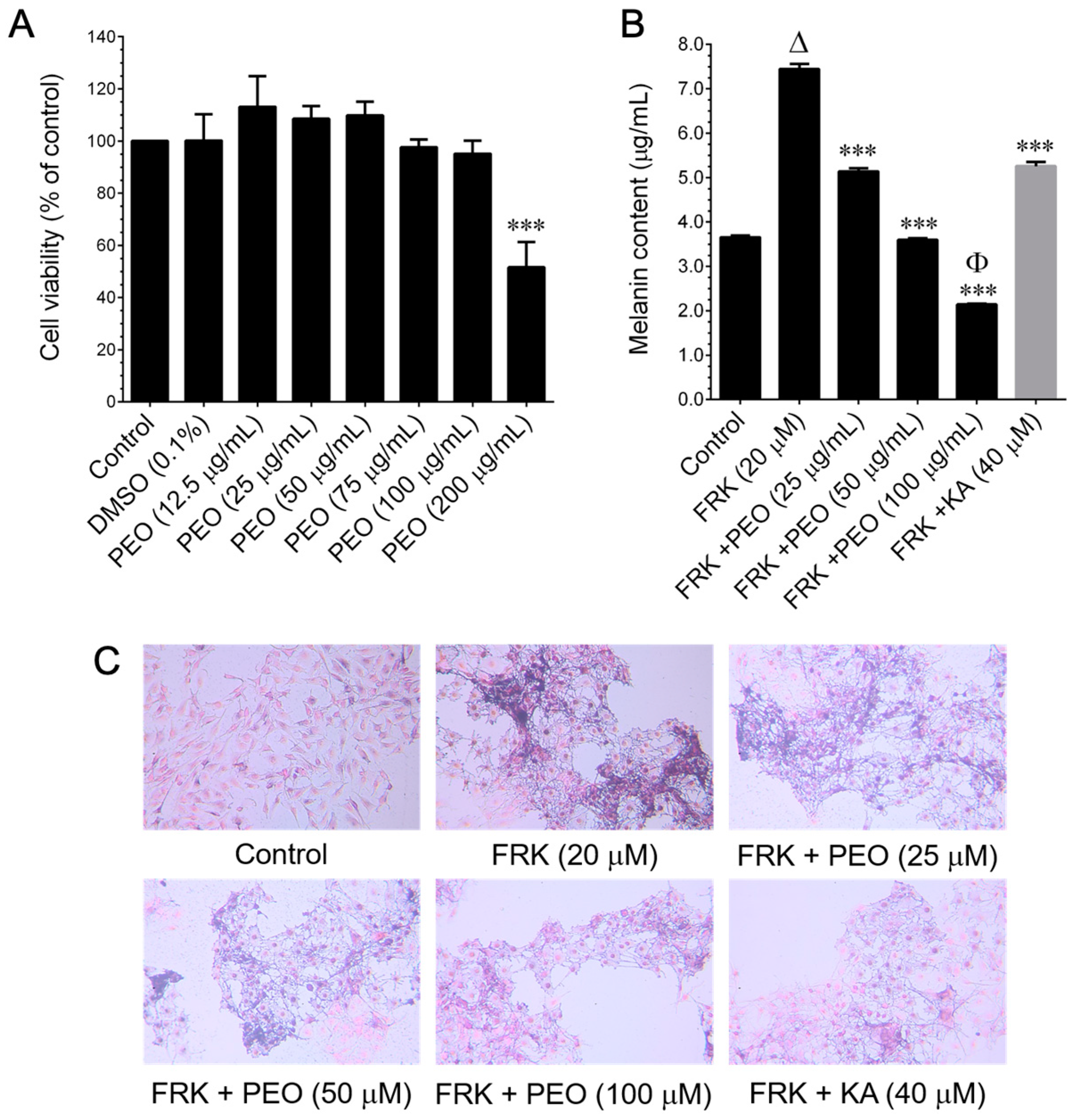
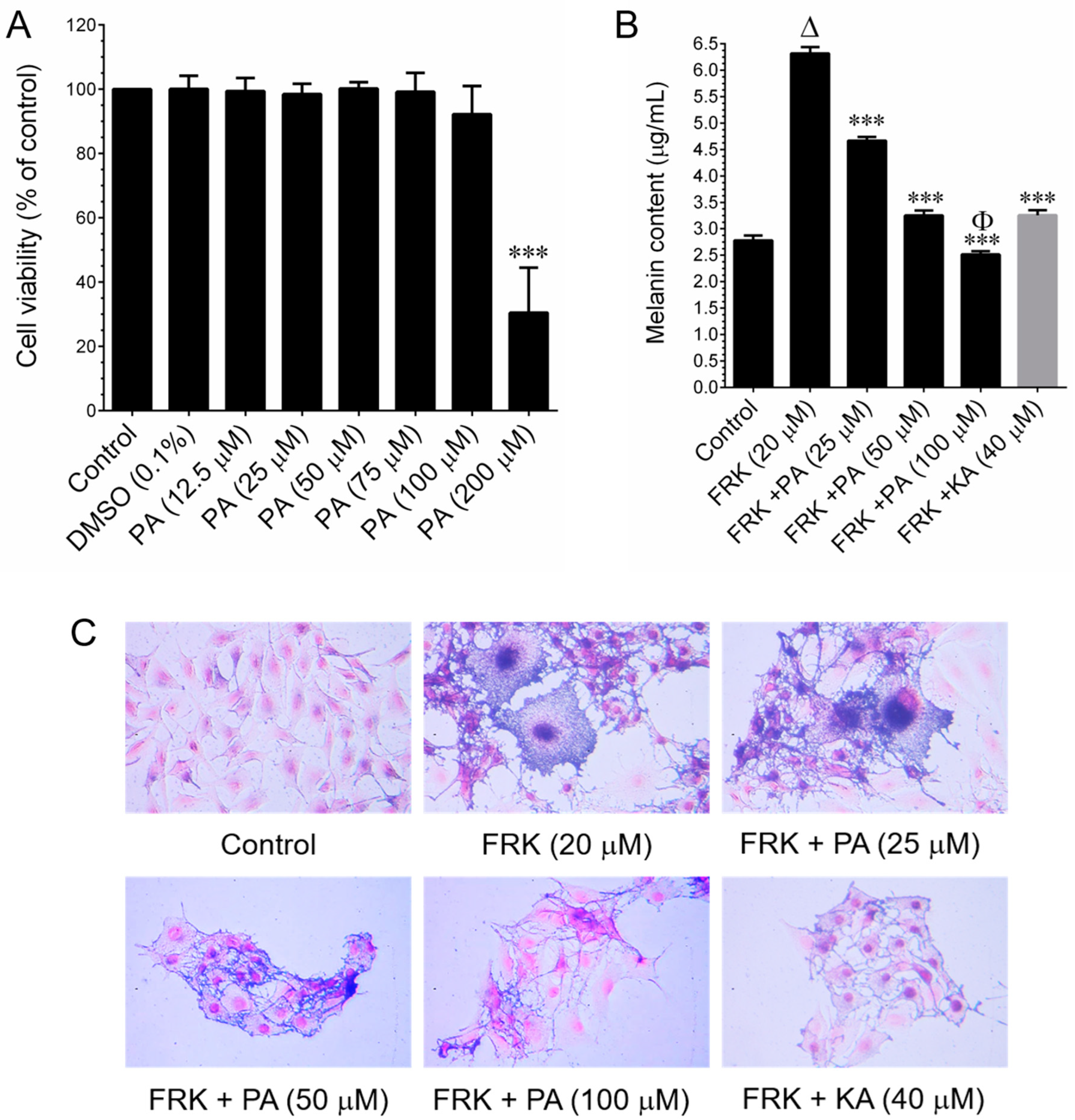
The murine melanoma (B16–F10) cell line was procured from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI-1640 medium supplemented with glucose, penicillin, and streptomycin, and 10% FBS. They were grown in 10 cm culture dishes and incubated in a humidified atmosphere containing 5% CO2 at 37 °C. The sub-culturing of cells was performed at three-day intervals. Cell viability was assessed using the MTT colorimetric assay. B16–F10 cells were seeded in a 96-well plate at a density of 1 × 104 cells/well. After 24 h of incubation, cells were treated with various concentrations (25, 50, 100, 150, and 200 μg/mL) of PEO or 25–100 μM PA or 20 μM FRK for an additional 48 h. Control cells were treated with 0.1% DMSO/RPMI for 48 h. After removing the cell culture supernatant, 1 mg/mL of MTT in 200 μL of fresh culture medium was added. The resulting MTT formazan crystals were dissolved in 200 μL of DMSO. Subsequently, the samples were measured at 570 nm (A570) using an ELISA microplate reader (Bio-Tek Instruments, Winooski, VT, USA). The percentage of cell viability was determined using the following formula: (A570 of treated cells/A570 of untreated cells) × 100.
2.3. Determination of Melanin Content and Cellular Tyrosinase Activity
Melanin content and cellular tyrosinase activity were assessed following previously established procedures [
13]. In brief, B16–F10 cells were seeded in 6 cm cell culture dishes at a density of 1 × 10
5 cells/dish. When the cell confluence reached 50%, cells were treated with FRK at a concentration of 20 μM, either in the presence or absence of PEO at concentrations ranging from 25 to 100 μg/mL, or PA (25–100 μM), or KA (20 μM), for a duration of 48 h. Subsequently, the cells were harvested, washed twice with PBS, and the intracellular melanin was solubilized in 1N NaOH, and then incubated at 68 °C for 20 min. The melanin content was quantified by measuring the absorbance at 475 nm using an ELISA microplate reader. In another set of experiments, cells were subjected to similar conditions for 48 h. Cultured cells were lysed with a lysis buffer and subsequently clarified by centrifugation at 16,000×
g for 10 min. A total of 90 μL from each lysate, containing an equal amount of protein (100 µg), was dispensed into a 96-well plate. Subsequently, 10 µL of 15 mM
l-DOPA was introduced to each well. Following an incubation period, at 37 °C, of 20 min, the formation of dopachrome was quantified at 475 nm using an ELISA microplate reader.
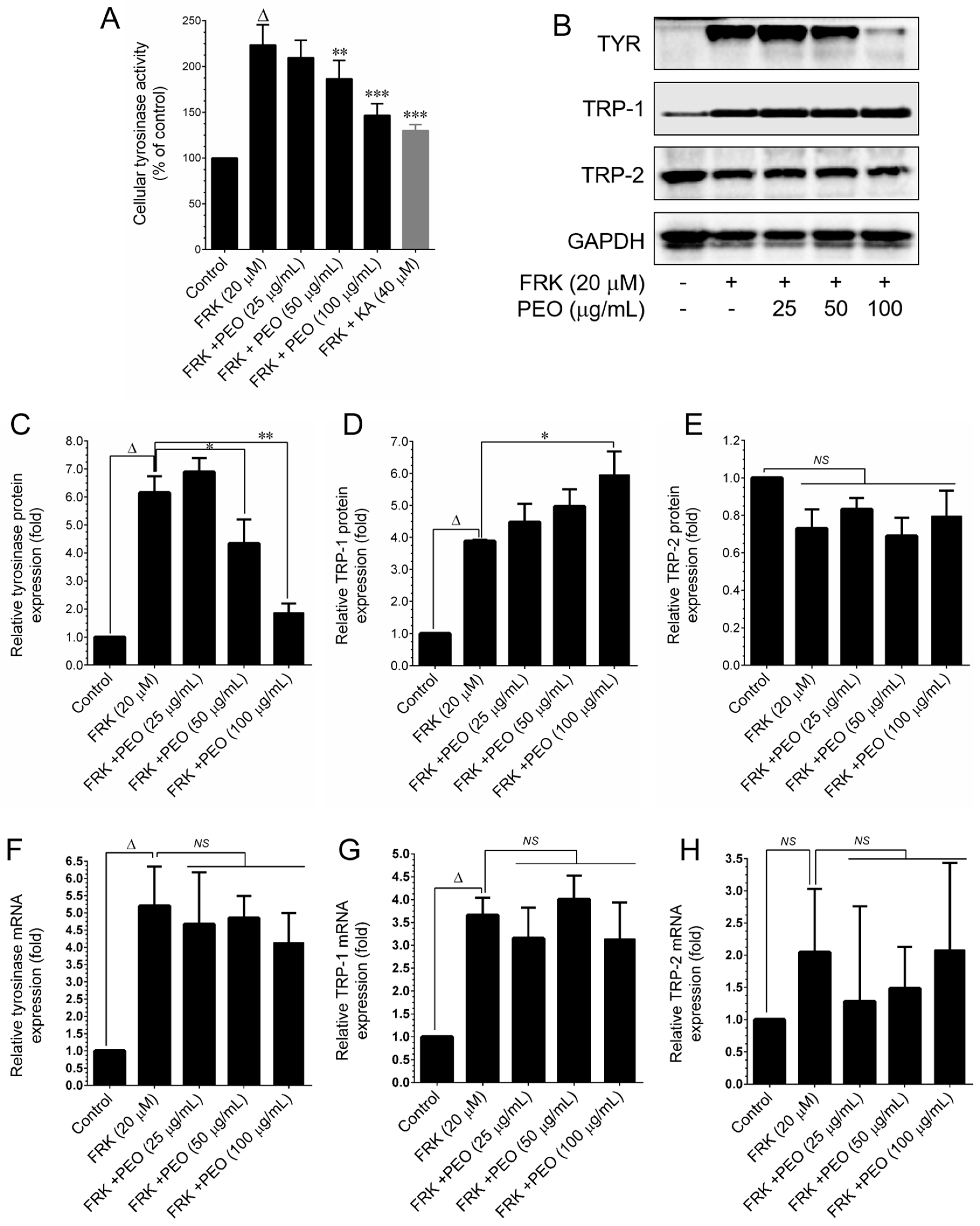
2.4. Protein Extraction and Western Blot Analysis
Cell lysis was performed using radioimmunoprecipitation assay buffer (RIPA buffer, Pierce Biotechnology, Rockford, IL, USA). Protein concentrations were determined using the Bio-Rad protein assay reagent (Bio-Rad Laboratories, Hercules, CA, USA), which is based on the Bradford dye-binding method. Subsequently, equal amounts of the protein samples (100 µg) were subjected to separation by 8–12% SDS-PAGE, and the separated proteins were transferred onto a polyvinylidene fluoride (PVDF) membrane. After transfer, the protein membranes were blocked with 5% non-fat skim milk for 30 min, followed by an overnight incubation with specific primary antibodies. Subsequently, the membranes were probed with HRP-conjugated anti-rabbit or anti-mouse antibodies for 2 h. Immunoblots were visualized using enhanced chemiluminescence (ECL) reagents (Advansta Inc., San Jose, CA, USA), and the ChemiDoc XRS+ docking system was employed to capture images. Quantitative analysis of the protein bands was conducted using Imagelab software version 6.0.1 from Bio-Rad Laboratories.
2.5. Immunofluorescence and Fluorescence Microscopy
B16–F10 cells (2 × 104 cells/well) were cultured on Nunc Lab-Tek® cell culture slides (ThermoFisher Scientific, Waltham, MA, USA) and subjected to treatment with FRK, with or without PA (100 µM) or KA (50 µM), for 24 h. Following treatment, the culture media were aspirated, and the cells were fixed in 2% paraformaldehyde for 15 min. Following fixation, the cells were permeabilized with 0.1% Triton X-100 for 10 min, washed, and then blocked with 10% FBS in PBS. Following this, the cells were incubated for 2 h with anti-tyrosinase antibody in 1.5% FBS. The cells were subsequently incubated with the fluorescein isothiocyanate (FITC)-conjugated secondary antibody for an additional 1 h in 6% bovine serum albumin (BSA). Following that, the cells were stained with 1 µg/mL of DAPI for 5 min, washed with PBS, and visualized using a fluorescence microscope (Olympus Corp., Tokyo, Japan) at 20× magnification.
2.6. RNA Extraction and q-PCR Analyses
Total RNA extraction was performed using the GeneMark Total RNA Purification Kit (GeneMark, New Taipei City, Taiwan), following two washes of the B16–F10 cells with cold PBS. The SuperScript™ IV First-Strand Synthesis Kit (Invitrogen, Waltham, MA, USA) was utilized to convert 2 μg of extracted RNA into cDNA. Subsequently, mRNA expression levels were quantified using the Applied Biosystems Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) and Power SYBR Green Master Mix (Applied Biosystems). The amplification process was conducted under the following conditions: the qPCR reaction involved an initial denaturation step at 96 °C for 3 min, followed by 40 cycles of denaturation at 96 °C for 1 min, annealing at 50 °C for 30 s, and extension at 72 °C for 90 s. The primer sequences for each gene in the qPCR were as follows: TYR—forward primer (F), 5′-TATTGAGCCTTACTTGGAAC-3′; reverse primer (R), 5′-AAATAGGTCGAGTGAGGTAA-3′, TRP-1—forward primer (F), 5′-TGCAGGAGCCTTCTTTCTC-3′; reverse primer (R), 5′-AAGACGCTGCACTGCTGGTCT-3′, TRP-2—forward primer (F), 5′-GGATGACCGTGAGCAATGGCC-3′; reverse primer (R), 5′-CGGTTGTGACCAATGGGTGCC-3′, and GAPDH—forward primer (F), 5′-TCAACGGCACAGTCAAGG-3′; reverse primer (R), 5′-ACTCCACGACATACTCAGC-3′. The copy number for each transcript was determined by calculating the relative copy number, which was normalized to the GAPDH copy number. The relative abundance of the target mRNA in each sample was calculated based on the ΔCt values of the target and the endogenous reference gene GAPDH, employing the 2ΔCt cycle threshold method.
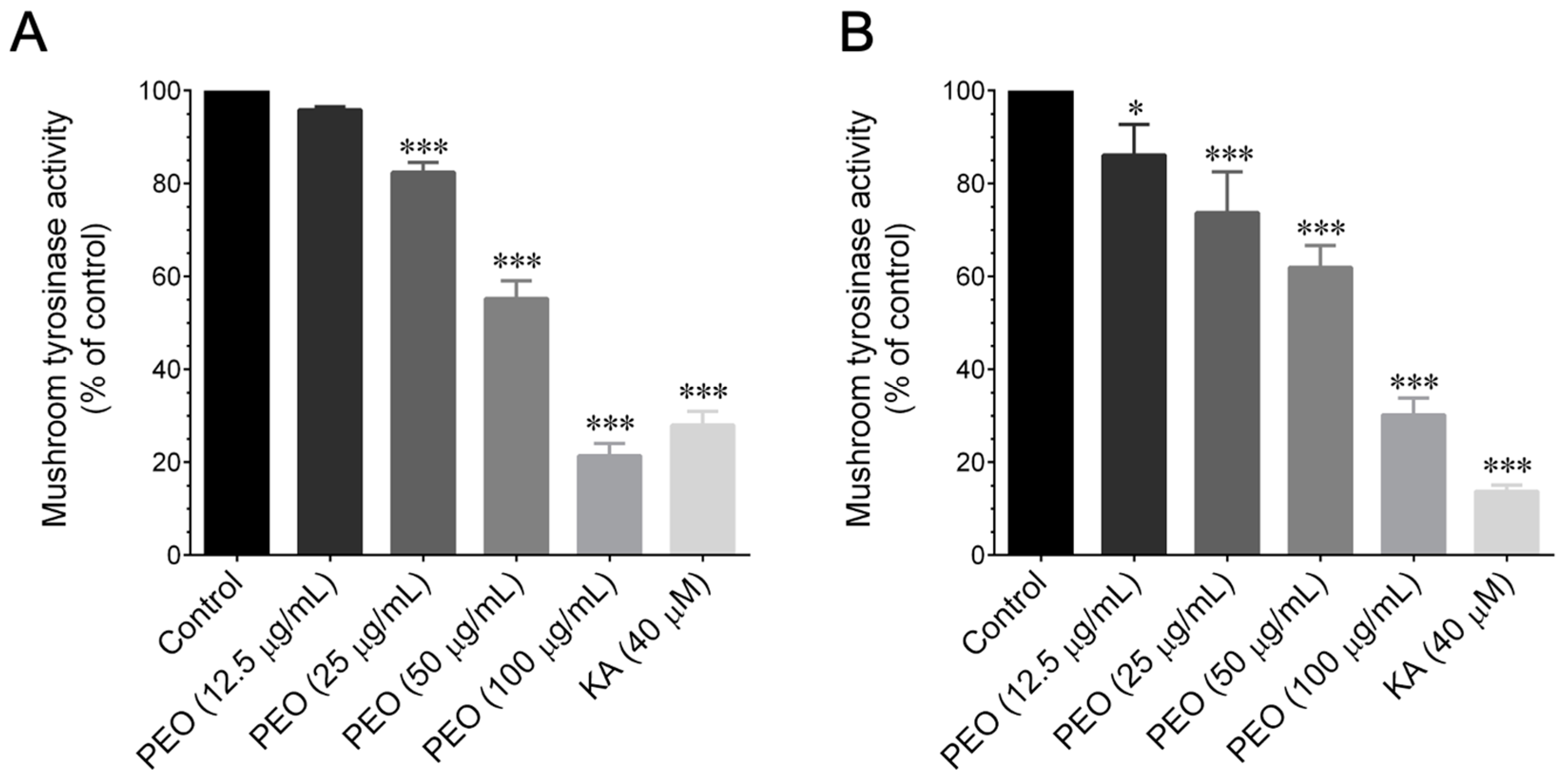
2.7. Mushroom Tyrosinase Activity Inhibition Assay
The ex vivo mushroom tyrosinase assay utilized
l-tyrosine and
l-DOPA as substrates for tyrosinase activity. The inhibitory activity of PEO and PA against the tyrosinase-catalyzed oxidation of
l-tyrosine was determined following the method outlined by Chang [
14]. In brief, a 40 μL volume of 1.5 mM substrate (
l-tyrosine) dissolved in 0.1 M phosphate buffer (pH 6.8) was combined with 120 μL of 0.1 M phosphate buffer. To this mixture, 20 μL of different concentrations of PEO (12.5–100 μg/mL) or PA (12.5–100 μM) were added. Following this, 20 μL of mushroom tyrosinase (2000 U/mL in phosphate buffer) was introduced to initiate the reaction. The assay mixture was then incubated at 37 °C for 15 min. A microplate reader (μQuant, BioTek Instruments, Winooski, VA, USA) was employed to monitor the increase in absorbance at 475 nm, indicative of dopachrome formation. The inhibitory effect of PEO and PA on mushroom tyrosinase in
l-DOPA oxidation was assessed. A mixture of 100 μL of 0.1 M phosphate buffer and 20 μL of different concentrations of POE (5–100 μg/mL) or PA (12.5–100 μM) was prepared. Following that, 20 μL of mushroom tyrosinase (2000 U/mL in phosphate buffer) was added to initiate the reaction. The mixture was then incubated at 37 °C for 5 min before introducing 40 μL of
l-DOPA (4 mM in 0.1 M of phosphate buffer). Subsequently, the mixture underwent incubation for an additional 10 min at 37 °C, and the absorbance, at 475 nm, of the reaction mixture was recorded. As a positive control, kojic acid (40 μM) was employed for the assay. The percentage inhibition of
l-tyrosine or
l-DOPA oxidation was calculated using the following formula: % inhibition = 100 − (B/A × 100), where A =
ΔOD
475 over 10 min without the sample, and B =
ΔOD
475 over 10 min with the tested sample.
2.8. In Silico Molecular Docking Study
2.8.1. Ligand and Receptor Selection
In this study, a total of 9 aromatic organic compounds were selected as ligands for in silico molecular docking study. Three-dimensional structures of all the selected ligands were downloaded from
https://pubchem.ncbi.nlm.nih.gov accessed on 19 October 2023. The list of ligands is α-bulnesene, α-guaiene, azulene, β-patchoulene, δ-guaiene, δ-patchoulene, patchouli alcohol, and seychellene. The predicted 3D structure of the human tyrosinase receptor, generated by alpha-Fold, was obtained from
https://www.uniprot.org with the molecular ID AF-L8B082-F1 was accessed on 2 September 2023. During processing, residues with a confidence score below 70 were excluded, and the core structure was utilized for the docking study.
2.8.2. Prediction of Ligand and Protein Interaction
We used Schrodinger 2023-3 software to prepare and optimize the structure of the protein, using the OPLS_2005 force field to remove heteroatoms (Schrodinger, LLC, New York, NY, USA). The binding site of the protein structure was identified using SitMap (Sitemap, Version 4.3), available in Maestro, and some other physical characteristics such as hydrogen bonding, hydrophobicity, size, and linking point [
15]. A grid created at 12 Å × 12 Å × 12 Å was virtually screened and run in VSW (virtual screening workflow module). Then, the virtual screening workflow was performed in QikProp (ligand filter), LigPrep, and glide docking (HTVS, SP, and XP).
2.9. Statistical Analysis
The data are presented as mean ± SD. Statistical analysis was conducted using GraphPad Prism version 6.0 for Windows (GraphPad Software, La Jolla, CA, USA). A one-way ANOVA followed by Dunnett’s test for multiple comparisons was employed for statistical evaluation. p values of less than 0.05 *, 0.01 **, and 0.001 *** were considered statistically significant for the FRK treatment vs. the PEO or PA or KA treatment groups. Additionally, p values of less than 0.01Δ were considered statistically significant for the FRK treatment vs. the control group.
4. Discussion
For over a millennium, essential oils have been utilized in pharmaceutical and cosmetic formulations, due to their diverse health benefits and preservative effect. Additionally, essential oils are widely recognized for their aromatic qualities, and, beyond fragrance, they have a spectrum of therapeutic properties [
17]. This multifaceted nature proves to be a boon for the cosmetic industry when essential oils are integrated into their formulations. In recent years, essential oils (EOs) and essential oil components (EOCs) have gained significant popularity as ingredients in skincare products [
18]. The increasing interest in utilizing these molecules in skincare formulations seeks to leverage their diverse biological properties, such as their antimicrobial, anti-inflammatory, and antioxidant effects [
18]. This trend aims to contribute to maintaining youthful, healthy, and fresh skin while providing protection against environmental damage. The pharmacological potential of essential oils (EOs) derived from plants has been extensively studied in relation to their capacity to block melanin formation, making them a subject of significant interest as skin-lightening agents [
19].
Pogostemon cablin has been extensively utilized in traditional Chinese medicine to address various ailments, particularly skin disorders. Notably,
P. cablin holds a prominent position among the ten most commonly utilized traditional Chinese medicines within the context of skin beauty and care regimens [
20]. Upon hydrodistillation, the dry leaves of
P. cablin yield an essential oil known as patchouli essential oil (PEO). Cahyono et al. identified nine compounds in patchouli oil. The major compounds they reported included α-guaiene, α-patchoulene, δ-guaiene, and patchouli alcohol [
16]. Fensia et al. [
21] successfully isolated 13 compounds from patchouli oil, with patchouli alcohol identified as the major component. Furthermore, a comparative analysis of the chemical fingerprints of two
Pogostemon species unveiled the presence of 26 compounds in
P. heyneanus and 32 compounds in
P. cablin. Notably, the primary compound in
P. cablin was identified as being patchouli alcohol, constituting 38.3% of its compounds. Conversely, acetophenone dominated in
P. heyneanus, comprising 51% of its compounds, with patchouli alcohol as the second major component at 14% [
22]. Collectively, these studies suggest that patchouli alcohol, identified as one of the major chemical constituents of patchouli oil, plays a significant role in imparting its intense aromatic odor. Currently, PEO stands as a pivotal ingredient in cosmetic products, valued for its herbaceous notes and fixative properties. Pharmacological studies have revealed that PEO has diverse bioactive components, which demonstrate anti-allergic and anti-acne properties, and antibacterial effects on the skin, as well as anti-oxidative and anti-inflammatory benefits [
23,
24,
25]. A previous study by Lin et al. [
20] reported that the topical application of patchouli oil prevents the cutaneous photoaging induced by UV radiation in mice by enhancing the skin’s antioxidant defense mechanism. However, the impact of PEO on cutaneous melanin biosynthesis was not explored. Likewise, patchouli alcohol (PA) is a tricyclic sesquiterpene widely utilized in the fragrance industry, in soaps, and in other cosmetic products [
12,
26]. Recent scientific investigations have documented with a broad spectrum of PA’s bio-activities, including anti-influenza virus, anti-depressive, anti-nociceptive, vasorelaxation, lung and brain protection, anti-ulcerogenic, anti-colitis, prebiotic-like, anti-inflammatory, anti-cancer, and protective effects against metabolic diseases [
24,
27]. PA has been shown to offer potential skin health benefits. Kim et al. [
28] demonstrated its ability to promote wound healing in obese mice. Additionally, another study by Feng et al. [
29] illustrated that the topical application of PA protects mice skin from UV-induced premature skin aging. However, the skin-whitening/lightening effect of PA remains unexplored.
Melanin biosynthesis involves a series of sequential steps, encompassing receptor activation, intracellular cAMP production, the transcriptional activation of MITF, and the transcription of genes within the tyrosinase family [
30]. In this study, we induced melanin synthesis in vitro using forskolin (FRK), a cAMP agonist known to trigger melanogenesis [
30]. Subsequently, we investigated the inhibitory effects of PEO on melanin synthesis under these conditions. Our study revealed that treatment with PEO significantly inhibited melanin synthesis in B16–F10 cells. This finding aligns well with others’ observations that essential oils from various sources, including
Alpinia nantoensis,
Alpinia zerumbet,
Cinnamomum cassia,
Eucalyptus camaldulensis,
Melaleuca quinquenervia,
Calocedrus formosana, and
Origanum ehrenbergii [
13,
31,
32,
33,
34,
35], demonstrated robust melanin synthesis inhibition under conditions similar to those our in vitro experiments. Furthermore, essential oils exhibit the ability to inhibit melanin biosynthesis through two mechanisms [
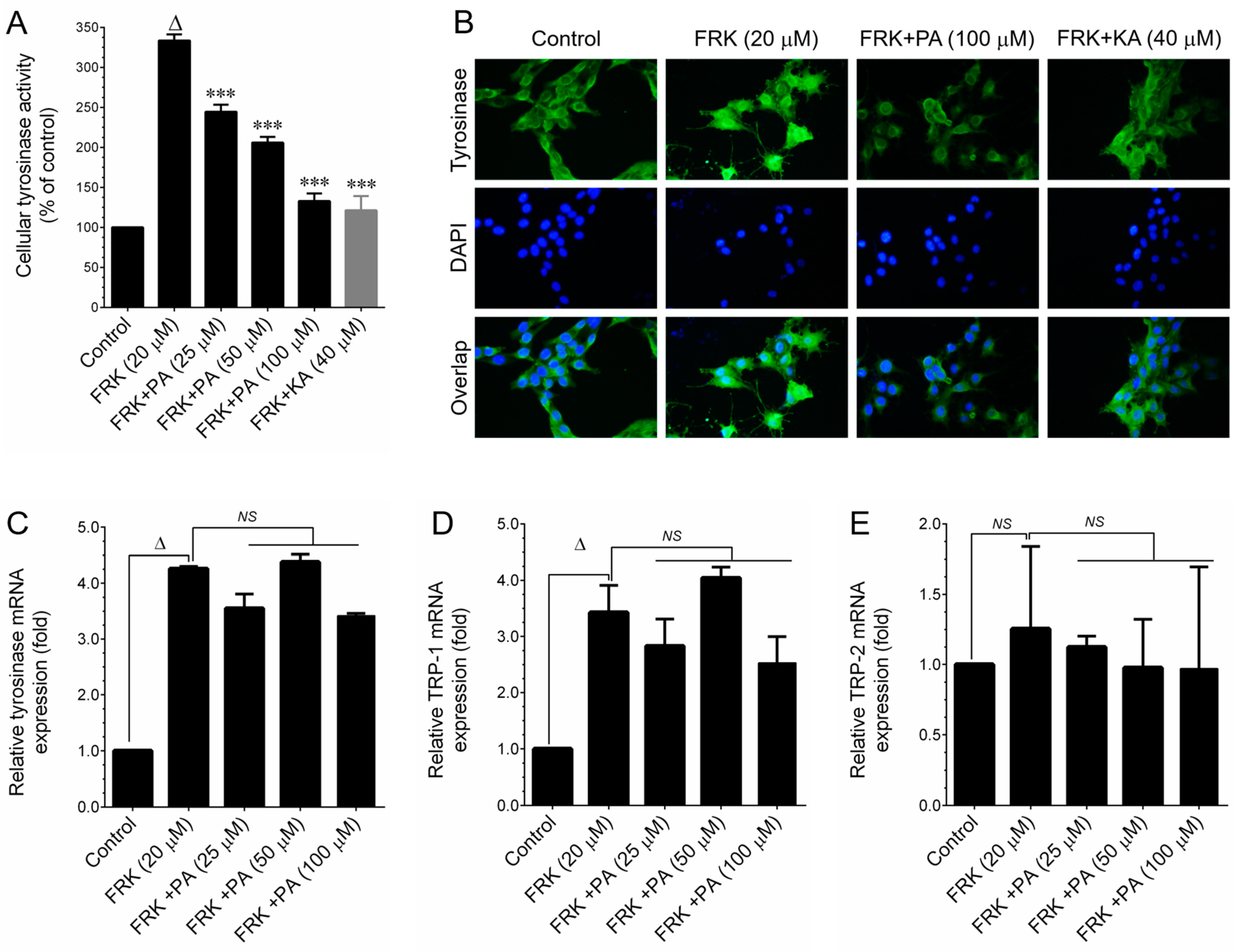
36]. This includes the direct inhibition of tyrosinase enzyme activity and the downregulation of the melanin biosynthesis pathway by modulating cellular signaling cascades. Tyrosinase family proteins, such as TYR, TRP-1, and TRP-2, play pivotal roles in melanin biosynthesis. Our study revealed that both PEO and PA significantly inhibited FRK-induced cellular tyrosinase activity. However, a noticeable reduction in TYR protein expression was observed only at a higher dose of both PEO and PA, while the levels of TRP-1 and TRP-2 remained unaffected. Furthermore, the FRK-induced elevation of TYR, TRP-1, and TRP-2 mRNA expression levels was unaltered by either PEO or PA. This observation suggests that PEO and PA inhibit melanin biosynthesis not by altering signaling cascades, but possibly through the direct inhibition of tyrosinase activity.
Numerous essential oils have undergone extensive study with regard to their direct tyrosinase inhibition properties [
19]. Momtaz et al. [
37] stated that plant oils are abundant in compounds that include hydrophobic components, which can function as competitive inhibitors for the enzyme tyrosinase, thereby influencing melanin synthesis. This distinctive characteristic positions them as crucial ingredients in the development of skin-lightening agents, showcasing their potential to address and alleviate skin pigmentation issues. Similarly, numerous photo compounds have been investigated and demonstrated to possess direct tyrosinase inhibitory effects [
38,
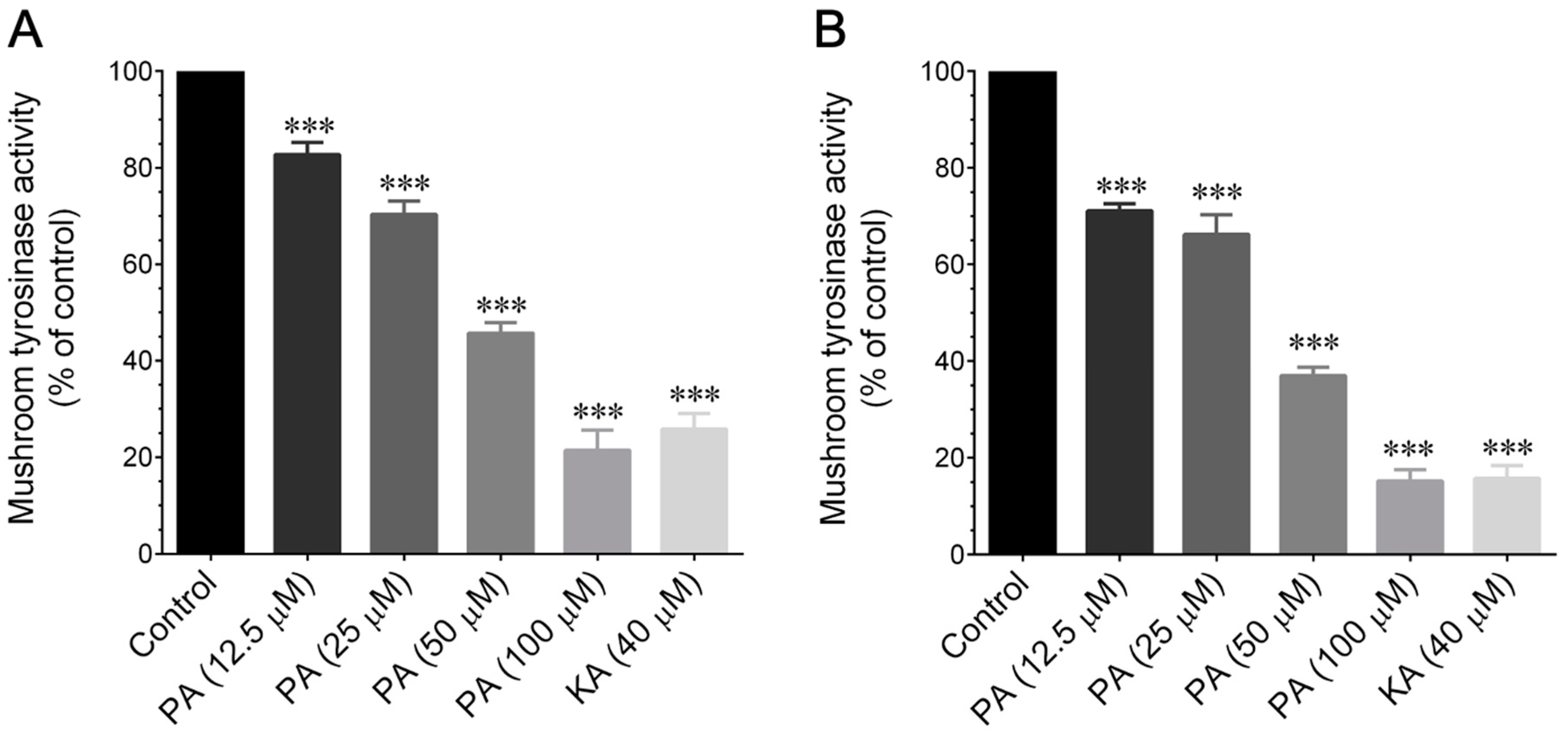
39]. Hence, we aimed to investigate whether PEO or PA could modulate tyrosinase enzyme activity. A mushroom tyrosinase inhibitory assay was employed, as it is a widely used method for assessing the skin-whitening effects of candidate agents in a cell-free system. This is based on the rationale that tyrosinase serves as the limiting enzyme in melanin formation in the skin. Using this assay, we assessed the tyrosinase inhibitory effects of PEO and PA, utilizing
l-tyrosine and
l-DOPA as substrates. Our findings revealed that both PEO and PA inhibit mushroom tyrosinase activity in a cell-free system at both the monophenolase and diphenolase phases. This observation correlates with other studies that have suggested that several herbal extracts and phytocompounds can inhibit tyrosinase activity without affecting the upstream signaling cascades of tyrosinase production [
14].
To further explore the interactions between enzymes and ligands, we conducted a molecular docking analysis. Typically, natural products and protein peptides with tyrosinase inhibitory activity contain high levels of hydrophobic (Trp, Phe, Gly, Val, Leu, Ile, Ala, Pro, and Met) and aromatic (Tyr, Trp, and Phe) amino acids [
40]. Our docking analysis revealed that patchouli alcohol forms six hydrogen bonds with the tyrosinase enzyme at residues Leu382, Val392, His215, 377, 381, and Phe362. Previous studies have demonstrated that potent tyrosinase inhibitors bind to these residues [
41]. Specifically, the amino acids His, Val, Thr, Met, and Leu are crucial interaction sites for tyrosinase inhibitors [
42]. Additionally, various tyrosinase inhibitors, such as carvacrol derivatives, tyrosol derivatives, ketones, hesperetin, oxoethyl derivatives, and certain food peptides, have been found to inactivate the enzyme by interacting with these critical residues [
43].