Abstract
We previously found that fermented extract of Dendrobium officinale using Lactobacillus plantarum GT-17F has a stronger antioxidant effect, especially in free radical scavenging. The result provided a basis for further studies to evaluate the effectiveness of fermented D. officinale in preventing UV-mediated damage and photoaging in humans. Therefore, in this study, we aimed to assess the anti-aging efficacy of D. officinale fermented with GT-17F strain in a clinical trial, which was conducted as a double-blind, placebo-controlled, randomized parallel-group comparative study with 99 volunteers with visible wrinkles. During the study, subjects were instructed to apply one pump of each essence, which contains fermented, unfermented, or no D. officinale extract, evenly to their face, both in the morning and evening, following their cleansing routine. At 28 days post-treatment, compared to baseline, subjects in the fermented extract group demonstrated significant improvements in stratum corneum water content, skin elasticity, skin glossiness, wrinkle area and ratio, erythema area, and erythema area ratio. In contrast, the unfermented extract group showed a significant difference only in improving erythema index levels in the skin. This comprehensive study has rigorously investigated the anti-aging effects of D. officinale and its fermented version on human skin, highlighting a notable contribution to dermatological research.
1. Introduction
Aging refers to the progressive decline in the function of organs and tissues over time. This decline is a multifaceted manifestation of various biochemical reactions within the body and is influenced by a plethora of internal and external factors including genetics, mental stress, and environmental pollutants [1,2]. The skin, being the most expansive organ in the human body, prominently displays signs of aging [3].
Factors contributing to skin aging can be bifurcated into endogenous and exogenous contributors [4]. Endogenous factors encompass the body’s natural aging process, leading to manifestations such as fine lines on dry skin [5]. Conversely, exogenous factors entail elements like increased reactive oxygen species, hormonal fluctuations, mitochondrial DNA damage, and telomere attrition [6]. Beyond intrinsic aging, various environmental elements, including but not limited to smoking, ultraviolet (UV) radiation, unhealthy lifestyle choices, and bacterial infections, can expedite skin aging [7]. Ultraviolet radiation is a pivotal exogenous determinant in skin aging. UVA, with its profound penetrative ability, can infiltrate the skin’s dermis, compromising the integrity of collagen and elastin structures. It also instigates melanin oxidation in the epidermis, thereby elevating melanin levels and causing insidious damage that accumulates over time [8]. UVB, on the other hand, can denature nucleic acids or proteins in the epidermal cells, leading to sunburn, skin peeling, erythema, and related symptoms [9]. UVC, though possessing weaker penetrative power and being largely filtered by the ozone layer, is gradually becoming a concern due to escalating environmental pollution and its potential detrimental effects on the skin [10].
The quest for beauty has spanned millennia and is reflected in the global contemporary trend of pursuing radiant and youthful skin. Cosmetics are ubiquitously utilized to enhance physical appearance and conceal imperfections. In response to burgeoning consumer demand, numerous companies have introduced compounds with purported anti-aging properties, many of which are derived from natural botanical sources, resonating with consumers globally [11,12].
Dendrobium officinale, a prized orchid with considerable medicinal attributes, is revered as one of the nine elixirs in traditional Chinese folklore [13]. This plant boasts a plethora of active compounds, including polysaccharides, alkaloids, flavonoids, phenanthrenes, benzyl compounds, sesquiterpenes, and steroids. Contemporary pharmacological research underscores the antioxidative and potential anti-aging benefits of D. officinale [14,15]. There was a study showing the repair effect of D. officinale extract on oxidative damage caused by UV radiation. The results showed that compared with the model group (UV irradiation), the positive control group and the treatment with 10, 25 and 50 mg/mL of D. officinale extract. By increasing the expression levels of catalase, superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and reducing thiobarbituric acid reactive substances (TBARS) and matrix metalloproteinases (MMPs), it can reduce erythema and protect the skin from dryness. By enhancing the antioxidant system, it can protect the skin from dryness and effectively reduce erythema [16]. In addition, one study reported that D. officinale extract (DOE) significantly increased SOD production and inhibited malondialdehyde (MDA) activity, indicating that the myocardial toxicity caused by diabetes was alleviated by inhibiting oxidative stress [17]. In D-galactose-induced (0.125 g/kg) aging mice, oral administration of a high dose of 1 g/kg extract of D. officinale showed significant increases in serum SOD, GSH-Px and total antioxidant capacity (T-AOC). SOD levels in the kidney and brain were significantly increased [18].
This study explored the anti-aging properties of fermented D. officinale compared to its unfermented counterpart. The polysaccharides from fermented D. officinale were found to intervene in human splenic fibroblast (HSF) cells damaged by UVA, resulting in an enhanced antioxidant capacity [19,20]. This intervention consequently reduced the decomposition of skin-essential molecules, such as collagen, elastin, and hyaluronic acid, offering substantial protection to skin tissue. While in vitro laboratory results have indicated the efficacy of Lactobacillus plantarum GT-17F against UV-mediated skin photoaging, clinical data supporting this claim remain sparse. Various metrics and instruments are employed to study the external signs of skin aging. Melanin, a pivotal pigment, plays a significant role in determining skin appearance [21]. The Individual Typology Angle (ITA°), grounded in the standardized Commission Internationale de l’Eclairage (CIE) L*a*b* color system, serves as a recognized standard for gauging skin radiance [22]. A higher ITA° value correlates to lighter skin tones, whereas a lower value indicates darker hues [23].
With these findings in mind, the central aim of our clinical study is to discern whether D. officinale fermented with L. plantarum GT-17F presents a superior alternative to its unfermented version as an anti-aging cosmetic ingredient.
2. Materials and Methods
2.1. Settings and Locations
The study was conducted from July 2022 to December 2022 at Huiwen Testing Co., Ltd. (Shanghai, China), which was validated by a ‘Quality Management System Certificate’ (CMA; certificate no. 210910341970). Huiwen Testing operates as an independent, third-party laboratory specializing in the in vitro, in vivo, chemical, microbiological, and clinical testing of products ranging from cosmetics and medical devices to nutraceuticals. The study protocol and the informed consent form were reviewed and approved by the institutional review board at Huiwen Skin Research Center, with the designated Protocol No. HUIWEN BIO_20220601.
2.2. Test Products
In this experiment, three distinct groups were studied: FDO (stem of D. officinale was squeezed and prepared as juice, fermented with L. plantarum GT-17F, lyophilized into powder), DO (stem of D. officinale was squeezed and prepared as juice, lyophilized into powder), and placebo. As shown in Table 1, each test sample was comprised of glycerin, water, and preservatives. In addition to the basic composition, the FDO group contained D. officinale extract fermented with L. plantarum GT-17F at a concentration of 0.5%. In contrast, the DO group had the base composition with the addition of unfermented D. officinale extract at a concentration of 0.5%. The placebo sample was made using the base composition with water, replacing the D. officinale extract.

Table 1.
Test-product formulas.
All essence components were homogenized at 30 °C and 5500 rpm using a PRIMIX homogenizer (PRIMIX Corporation, Hyogo, Japan) for 4 min and subsequently mixed with a mechanical stirrer (EUROSTAR 60, IKA-Werke Gmbh&CO.KG, Staufen, Germany). The freshly prepared essence had a pH value of 6.1. Further details are provided in Table 1.
2.3. Subjects
All subjects were from Shanghai, China. The study sought healthy women aged 30–60 years with an average age distribution. Dermatologists evaluated each subject, confirming a crow’s feet grade of 3–5 according to the method for assessment of cosmetics anti-wrinkle efficacy, which is the Chinese Standard No. T/ZHCA 006-2019 [24]. These women were able to cooperate effectively with the experimenters and maintain regular lifestyles throughout the study. Subjects needed to read, understand, and voluntarily sign the informed consent form. They also agreed not to use cosmetics, drugs, or health products during the trial that could affect the results. All subjects were given verbal and written informed consent statements per regulations. This statement explained the study’s nature, purpose, and potential risks, emphasizing voluntary participation. Participants could exit the study whenever they wished. Informed consent signatures were obtained before the study commenced. Exclusion criteria for subjects: (1) Used antihistamines within the past week or immunosuppressants within the past month; (2) Applied any anti-inflammatory drugs to the test site in the last two months; (3) History of skin diseases such as psoriasis, eczema, or skin cancer; (4) Insulin-dependent diabetes mellitus; (5) Undergoing treatment for asthma or other chronic respiratory diseases; (6) Underwent anticancer chemotherapy within the last six months; (7) Diagnosed with immunodeficiency or autoimmune diseases; (8) Lactating or pregnant women; (9) Had a bilateral mastectomy and bilateral axillary lymph node resection; (10) Presence of scars, pigment disparities, atrophy, port wine nevus, uneven skin tone, folliculitis, or other skin defects at the test site; and (11) Any other significant health issues or chronic diseases.
2.4. Study Design
The study followed a single-center, randomized, double-blind trial design. Over a period of 56 days, volunteers used the product continuously. The skin data between different groups of subjects before and after using the product were analyzed using specific instruments. Eligible subjects were randomly allocated to one of three groups. The randomization process was overseen by personnel not involved in the clinical trial or its data analysis. Crucially, both the subjects and the data analysts remained blinded to the group assignments and specific sample details.
During the study, subjects were instructed to apply one pump (0.5 g) of the essence evenly to their face, both in the morning and evening, following their cleansing routine. For the entire 56-day period, no other skincare products were to be used. After each application, the subjects were required by the clinical trial personnel to record photographic evidence of the application and upload it to the company’s server. This allowed for effective monitoring of each participant’s adherence to the regimen. Follow-up evaluations for subjects were set for the baseline and at 28 and 56 days. Before each test, the face of the subject needs to be cleaned with a light touch of water at constant temperature for 1 min and then cleaned by wiping with a uniform dry paper towel for 1 min. Participants then rested in a controlled environment maintained at 20 ± 1 °C and a relative humidity of 50 ± 10% for 30 min. Following this, the clinical trial personnel utilized the C+K multiprobe skin testing system (Courage+Khazaka Electronic GmbH, Cologne, Germany) and standard digital images (VISIA-CR, Canfield Scientific, Inc., Parsippany-Troy Hills, NJ, USA.) to measure parameters including wrinkle depth (VISIA-CR), transepidermal water loss (TEWL), skin hydration, skin elasticity, skin erythema index levels, skin melanin concentration, skin radiance, skin profilometry, and skin erythema.
2.5. Skin Moisturization
Skin hydration was assessed using the Corneometer® CM 825 (Courage+Khazaka electronic GmbH, Cologne, Germany) [25]. Measurements were taken at three distinct points on the right cheek. These measurement points were marked on a photograph of the participant’s face, ensuring accuracy during subsequent visits.
TEWL was evaluated using the Tewameter® TM 300 (Courage+Khazaka electronic GmbH, Cologne, Germany). This measurement was centralized on the right cheek, and the selected measurement site was documented three times to ensure precision. Similarly, this point was marked on a photograph of the participant’s face for consistent measurements during follow-up sessions.
2.6. Skin Elasticity
Skin elasticity was assessed using the Cutometer® MPA580 (Courage+Khazaka electronic GmbH, Cologne, Germany) [26]. First, the skin surface of the right cheek was positioned within the 3 mm aperture of the probe. Then, a negative pressure of 450 mbar was applied for 3 s. Finally, the pressure was released, and the skin was allowed to revert for another 3 s. This process evaluates the skin’s resistance to mechanical force and its ability to return to its original state, often referred to as R2 or gross elasticity. We selected the right half of the face of all subjects as the research area, and the specific measurement site was documented three times for precision. This point was consistently marked on a photograph of the participant’s face to ensure accuracy during subsequent evaluations. After 28 and 56 days of using the product, in order to obtain accurate data, the test must be conducted again in the same area and location.”
2.7. Skin Whitening
The Skin Colorimeter® CL 400 (Courage+Khazaka electronic GmbH, Cologne, Germany) uses white LED light to illuminate the skin evenly in a ring-shaped pattern [27]. Reflected light from the skin is captured by the probe and quantified. Measurements for skin radiance are initially provided as XYZ values corresponding to the three primary colors. These XYZ readings can subsequently be converted to L*a*b* color values. Importantly, the L* and b* parameters derived from the colorimeter readings are integrated into the Individual Typology Angle (ITA°) formula: ITA° = Arctan [(L* − 50)/b*] × 180/π). The Mexameter® MX 18 (Courage+Khazaka electronic GmbH, Cologne, Germany) probe emits light at three specific wavelengths. The instrument measures the light that is diffusely reflected from the skin. Because the initial amount of emitted light is known, we can calculate the light absorbed by the skin. Melanin levels in the skin are ascertained using two of these wavelengths, chosen specifically for their differential melanin-absorption efficiencies.
For both instruments, measurements were centralized on the right cheek. The exact measurement location was marked three times for precision. These measurement points were consistently indicated on a photograph of each participant’s face to ensure uniformity in subsequent evaluations.
2.8. Eye Wrinkle
Standard facial images were captured using the VISIA-CR 2.3 system equipped with Mirror Photo Tools image management software 7.5.6 (Canfield Scientific, Inc., Parsippany, NJ, USA) [28]. Images of the right side of the face were taken following a consistent protocol. For each participant, at every visit, the target areas on these images were manually adjusted by a trained image-analysis technician to ensure accuracy. To evaluate the efficacy of wrinkle reduction, we quantified the volume of the skin in the eye-corner regions, specifically focusing on “crow’s feet.” This was done by measuring the total skin volume and subtracting the volume of the wrinkles. The resulting data provided insights into wrinkle improvement over time.
2.9. Skin Soothing
Researchers use the VISIA-CR 2.3 system to take standard facial images of subjects at different stages of using the product and select pictures of cross-polarized light in five light sources. The R channel of cross-polarized light can separate the red zone image showing the status of blood vessels. To export the image, the image to be analyzed was exported and imported into the Image-Pro Plus analysis software 7.0. The macro menu was selected to pull down and open the skin comprehensive management software to analyze the selected area. The evaluation aimed to measure the area and proportion of erythema on the skin’s surface. The derived data assisted in gauging the effects of soothing improvements [29].
For a precise erythema measurement, the Mexameter® MX 18 (Courage+Khazaka electronic GmbH, Cologne, Germany) probe was employed [30]. This probe emits light at three distinct wavelengths. The integrated receiver gauges the light reflected off the skin. The specific positioning of the transmitter and receiver ensures the capture of only diffused and scattered reflected light. Since the quantity of emitted light is predetermined, the quantity absorbed by the skin can be computed. Two specific wavelengths are crucial in this measurement. The first corresponds to the spectral absorption peak of the erythema index, while the second is carefully chosen to circumvent interference from other colors, such as bilirubin. The resultant values are displayed on the screen, represented as values ranging between 0 and 999.
2.10. Self-Assessment Questionnaire
Upon the study’s conclusion, all participants who successfully completed the test received a self-assessment questionnaire. This questionnaire assessed their satisfaction with the product, their tolerance to the product, and the product’s perceived efficacy. The experimenter explained the questions and the 5-point scale to the subjects as follows: “5—Strongly agree”, “4—Agree”, “3—Neutral”, “2—Disagree”, and “1—Strongly disagree”. The percentage of positive responses (those indicating agreement) was calculated for the participant cohort. It was postulated that perceived efficacy was confirmed if at least 80% of the participants provided positive feedback.
2.11. Valid Cases for Subjects
Over the 56-day testing period, subjects who adhered to all experimental protocols and used more than half of the provided samples were deemed to have successfully completed the trial. Data from these subjects were included in the analysis. Exclusions from the data set included (1) Termination: Subjects who withdrew due to adverse events; (2) Dropouts: Subjects who left the trial for personal reasons or became untraceable; and (3) Non-compliance: Subjects who did not use the test samples as instructed or violated usage guidelines.
2.12. Adverse Events
Adverse events encompass any negative health occurrences noted after using the test sample, irrespective of a direct causal link to the sample. This includes new diseases or exacerbation of preexisting conditions. However, common symptoms presented before sample use, such as pain, are not considered adverse events unless they worsened after sample use. All adverse skin reactions that arose during product use, including redness, tingling, itching, and burning, were to be documented. Severe adverse reactions would have been reported promptly.
2.13. Statistical Analysis
For this study, specific facial or periocular areas of subjects were chosen. Following the evaluation of relevant metrics, data from all subjects who met the criteria were analyzed and supplemented with visual evidence from photographs. GraphPad Prism 9 was the statistical software utilized for data analysis. Descriptive statistics were expressed as the mean ± standard deviation. A normality test was executed on the data. If the data conformed to a normal distribution, a two-way ANOVA was applied, considering both the type of sample used and the test time. Sidak’s multiple comparisons test was employed for intragroup comparisons, analyzing the same sample at different times. Conversely, if the data did not adhere to a normal distribution, the rank sum test was utilized for paired samples, comparing rank data before and after the application. For intergroup comparisons, either the independent sample t-test or rank sum test was chosen based on data distribution. All statistical procedures were two-tailed, with a significance threshold set at α = 0.05.
3. Results
3.1. Test Procedures and Participant Information
Prior to the initiation of the study, a thorough screening was conducted on potential participants from the subject database to ascertain their suitability for the project. Once appropriate candidates were identified, the recruitment process was set in motion. Of the 124 volunteers who expressed interest, 20 decided to withdraw upon receiving a detailed briefing about the study’s instructions and requirements. Subsequently, 104 individuals arrived at the test location. Five individuals were deemed ineligible after comprehensive evaluations by a dermatologist and in-depth discussions about the study. The final cohort consisted of 99 participants. They were randomized by the study coordinator into three groups, with each group consisting of 33 participants designated to use different products (Figure 1). Pertinent details and baseline skin data for all participants were meticulously recorded (Table 2). It is crucial to note that the individuals responsible for this participant allocation were not to be involved in any subsequent stages of skin assessment or data analysis.
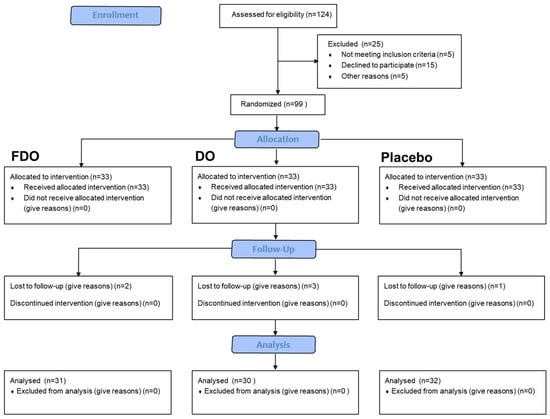
Figure 1.
Subject flow diagram.

Table 2.
Subject information and skin baseline parameters.
3.2. Skin Moisturization
Following 28 and 56 days of product usage, the following respective results were observed: In the FDO group, the stratum corneum water content was increased by 7.20% (p = 0.002) and 12.60% (p < 0.001), and TEWL decreased by 8.41% (p = 0.006) and 16.41% (p < 0.001). In the DO group, water content was increased by 4.44% (p = 0.010) and 6.16% (p = 0.001), and TEWL was reduced by 2.77% (p = 0.803) and 9.14% (p = 0.007). The placebo group had no significant changes in water content or TEWL. When statistically comparing stratum corneum water content increase among the groups, the FDO group’s increase was notably distinct from the placebo group (p < 0.01, p < 0.001). However, the DO group only showed significant differences after 56 days (p < 0.05). Importantly, the FDO group’s increase was notably different from that of the DO group after 56 days (p < 0.05). Detailed results are presented in Figure 2A. For TEWL, only the FDO group displayed a significant decrease when compared with the placebo group after 56 days (p < 0.01). This is further detailed in Figure 2B.
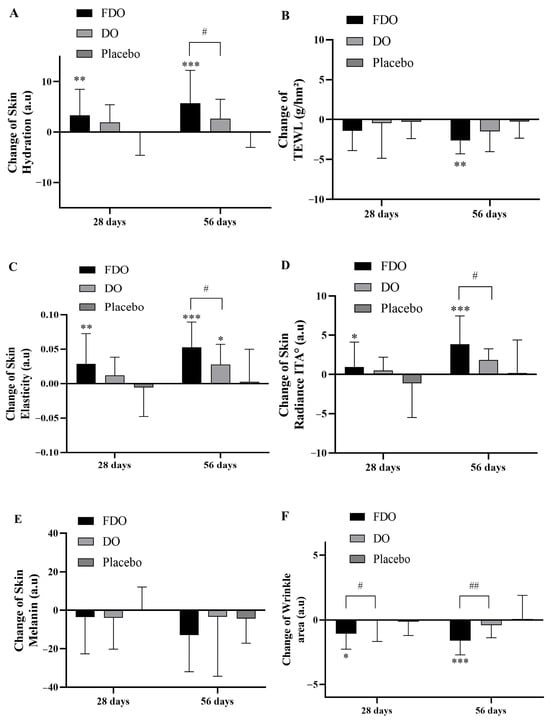
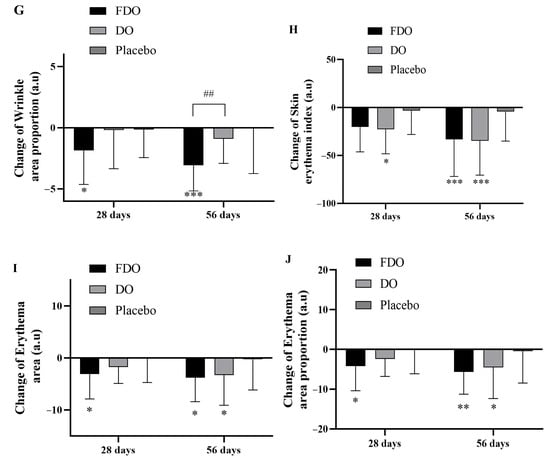
Figure 2.
Changes in clinical skin parameters with FDO, DO, and placebo administration for 56 consecutive days. (A) Changes in skin hydration. (B) Changes in TEWL. (C) Changes in skin elasticity. (D) Changes in skin radiance ITA°. (E) Changes in skin melanin. (F) Changes in wrinkle area. (G) Changes in wrinkle area proportion. (H) Changes in skin erythema index. (I) Changes in skin erythema area. (J) Changes in skin erythema area proportion. * Indicates comparison within the placebo; p-value (* p < 0.05, ** p < 0.01, and *** p < 0.001). # Indicates a comparison between the FDO group and the DO group (# p < 0.05 and ## p < 0.01, respectively).
3.3. Skin Elasticity
Skin elasticity is one of the indicators of human aging. The more obvious the degree of aging, the less skin elasticity. In the experiment results, after subjects used the product for 28 and 56 days, compared with the baseline value, the FDO group’s skin elasticity increased by 5.18% (p = 0.002) and 9.27% (p < 0.001), respectively; the DO group’s skin elasticity increased by 2.05% (p = 0.035) and 4.73% (p < 0.001), respectively; and the placebo group did not have significant changes. The increase in skin elasticity of each group was statistically calculated. Compared with the placebo group, the increase in the FDO group was significantly different (p < 0.01 and p < 0.001 after 28 and 56 days, respectively), while the DO group only had significant differences after 56 days (p < 0.05). Most importantly, after 56 days, the increase in the FDO group’s subjects was significantly different from that of the DO group (p < 0.05) (Figure 2C).
3.4. Skin Whitening
The experiment results show that following 28 and 56 days of product application, the ITA° values of the FDO group increased by 1.94% (p = 0.227) and 8.35% (p < 0.001), respectively; the ITA° values of the DO group showed an elevation of 1.20% (p = 0.160) and 4.21% (p < 0.001), respectively; and the placebo group had no marked changes in ITA°. Statistical evaluation highlighted that the FDO group’s rise in ITA°, when compared to the placebo group, was significant (p < 0.05 and p < 0.001 after 28 and 56 days, respectively). The DO group, however, did not show any significant variations. Notably, after 56 days, the increase observed in the FDO group contrasted significantly with that of the DO group (p < 0.05). See Figure 2D for a detailed analysis. Regarding melanin levels, the FDO group had a decrease of 2.14% (p = 0.496) and 6.59% (p = 0.001), while the DO group’s melanin decreased by 2.15% (p = 0.336) and 1.84% (p = 0.777) after 28 and 56 days, respectively; the placebo group had no significant alterations in melanin. Statistical assessment of the melanin levels across all groups revealed no substantial differences between them. Refer to Figure 2E for further insights.
3.5. Eye Wrinkle
After 28 and 56 days of product usage, compared to baseline values, the FDO group experienced a wrinkle-area reduction of 15.51% (p < 0.001) and 22.86% (p < 0.001), respectively; the DO group observed a decrease of 0.98% (p = 0.951) and 6.32% (p = 0.188), respectively; and the placebo group showed no significant changes. When statistically evaluating the wrinkle-area reduction across the groups, the FDO group showed a significant decrease compared to the placebo group (p < 0.05 and p < 0.001 after 28 and 56 days, respectively). The DO group, however, did not exhibit notable differences. The decline in the FDO group’s wrinkle area was significantly more pronounced than in the DO group at 28 and 56 days (p < 0.01). Detailed results are provided in Figure 2F. Regarding the proportion of wrinkle area, the FDO group showed a decrease of 15.12% (p = 0.001) and 24.82% (p < 0.001), while the DO group’s melanin decreased by 2.15% (p = 0.336) and 1.84% (p = 0.777) after 28 and 56 days, respectively; the placebo group had no significant alterations in melanin.
3.6. Skin Soothing
The results of the experiment show that, after using the product for 28 and 56 days in comparison to baseline values, the FDO group’s erythema index decreased by 8.59% (p < 0.001) and 13.59% (p < 0.001), respectively. The DO group’s erythema index decreased by 9.20% (p < 0.001) and 14.03% (p < 0.001), respectively; no significant changes were observed in the placebo group. Compared to the placebo group, the decrease in the DO group was significant (p < 0.05 and p < 0.001 after 28 and 56 days, respectively), while the FDO group showed significance after 56 days (p < 0.001) (Figure 2H). For erythema area measurements, the FDO group’s proportion decreased by 10.75% (p = 0.002) and 12.39% (p < 0.001), while the DO group’s proportion decreased by 6.88% (p = 0.010) and 12.97% (p = 0.008) after 28 and 56 days, respectively. Again, the placebo group showed no significant changes. When compared to the placebo group, the reduction in erythema area in the FDO group was significant (p < 0.05 at both 28 and 56 days), and the DO group showed significance after 56 days (p < 0.05) (Figure 2I). The findings for the proportion of erythema area mirrored these results. The FDO group’s proportion decreased by 11.06% (p = 0.001) and 14.67% (p < 0.001), while the DO group’s proportion decreased by 7.17% (p = 0.012) and 13.67% (p = 0.007) after 28 and 56 days, respectively; the placebo group remained unchanged. The trends in the proportion of erythema area for each group were consistent with the erythema-area results (Figure 2J and Figure 3).
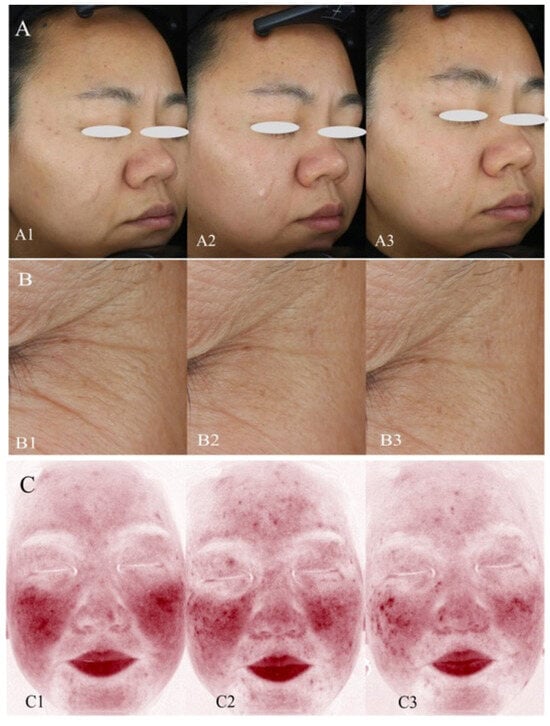
Figure 3.
VISIA analysis of skin wrinkles and erythema area in a subject who used FDO. Standard photographic (upper row) and cross-polarized (lower row) images captured with the VISIA-CR 2.3. (A) Subject’s overall wrinkles. (A1) Baseline. (A2) After using FDO for 28 days. (A3) After using FDO for 56 days. (B) Subjects’ crow’s feet. (B1) Baseline. (B2) After using FDO for 28 days. (B3) After using FDO for 56 days. (C) Subject’s erythema area. (C1) Baseline. (C2) After using FDO for 28 days. (C3) After using FDO for 56 days.
3.7. Self-Assessment Questionnaire
The Self-Assessment Questionnaire will serve as the secondary efficacy endpoint. Subjects are asked to give a rating response based on a scoring system from 5 (completely agree) to 1 (completely disagree). The most favorable outcome is a “completely agree” response to the provided statements/questions. Nearly all subjects reported positive feedback regarding the safety of the products. Regarding the seven product performance-related concerns, the FDO group expressed satisfaction. In contrast, the DO group indicated dissatisfaction with two aspects: wrinkles and elasticity. This feedback aligned with the findings from objective, instrument-based tests. The results of the questionnaire are presented in Figure 4.
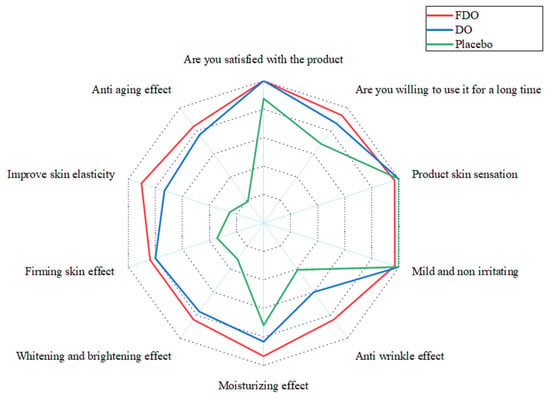
Figure 4.
Subject flow diagram of self-assessment questionnaire results. The red line indicates the statistical results of the questionnaire for subjects in the FDO group. The blue line indicates the statistical results of the questionnaire for subjects in the DO group. The green line indicates the statistical results of the questionnaire for subjects in the placebo group.
4. Discussion
Aging is an intricate and irreversible biological process. Skin aging directly reflects systemic aging, primarily evident as increased wrinkles, skin dryness and atrophy, redness, inflammation, pigmentation, and loss of elasticity. Beyond aesthetic concerns, skin aging can lead to psychological issues like depression and feelings of inadequacy and is linked to various diseases [31]. Although skin aging is inevitable, interventions can potentially delay it. Identifying potent anti-aging agents and incorporating them into cosmetics to enhance individuals’ well-being is a pivotal focus in current research. The use of plant-derived ingredients in anti-aging cosmetics is increasingly recognized, and developing efficient, safe, and distinctive plant-based materials holds considerable market potential [32]. Plant resources offer a multitude of active ingredients, conferring unique anti-inflammatory, antioxidant, moisturizing, and anti-aging benefits [33]. Advanced fermentation engineering can amplify the concentration of plant-derived active ingredients, augment their effectiveness, minimize toxic side effects, and bolster the potency and safety of plant-based cosmetic ingredients [34].
In recent years, numerous reports have been on fermenting probiotics and plants to enhance the potential anti-aging properties. Shin et al. reported that when Pogostemon cablin (patchouli) was fermented using probiotics, it led to a downregulation in MMP-9 expression in HaCaT keratinocytes influenced by UVB and reactive oxygen species (ROS), simultaneously enhancing the secretion of antioxidant components glutathione and SOD to bolster the skin’s resistance to photoaging [35]. In a study by Lee et al., red ginseng (RG) fermented with Lactobacillus brevis revealed that the overall saponin content remained largely unchanged post-fermentation. Upon fermenting RG, the fermented RG (FRG) showed increased concentrations of ginsenoside metabolites, such as Rg3, Rg5, Rk1, compound K, Rh1, F2, and Rg2, along with heightened flavonoid content. These modified saponins exhibited enhanced anti-wrinkle and whitening properties with reduced toxicity [36]. Ha et al. investigated the protective impact of Lavandula angustifolia (English lavender) extract, once fermented with Pediococcus pentosaceus DK1, against UVB-induced damage. This protective effect was noticeable even at fermented extract concentrations below those of their unfermented counterparts. Fibroblasts treated with the fermented extract showed a 20% smaller decrease in collagen production upon UVB exposure compared to those treated with unfermented extracts. Moreover, UVB-irradiated fibroblasts exposed to the fermented extract showed a 50% increase in ROS inhibition relative to cells treated with the unfermented version [37]. D. officinale fermented with L. plantarum GT-17F (referred to as FDO) has demonstrated potential in mitigating the effects of UV-mediated photoaging [19]. The study aimed to evaluate the impact of UV damage on skin fibroblasts. Additionally, the effects of FDO were assessed on in vitro reconstituted epidermis and full-thickness skin models. The findings indicated that FDO effectively mitigated UV-induced oxidative and collagen damage in fibroblasts and demonstrated a positive impact on barrier repair.
In the current study on the clinical anti-aging of D. officinale, a study of 22 Thai volunteers showed that Dendrobium orchid polysaccharide extract was not irritating to the skin but had a better moisturizing effect than untreated skin and baseline skin, indicating that the extract is a safe and effective treatment of dry skin special ingredients [38]. Some studies have also shown that D. officinale polysaccharide multilayer emulsion can improve the stability and skin absorption to improve the skin aging of photoaging mice [39]. There are also many reports on the clinical improvement of anti-aging of microbial fermentation plants; for example, Lee et al. assessed the anti-aging effects of blackberry fermented with L. plantarum JBMI F5 as a randomized, double-blind, and placebo-controlled clinical trial showing that following a 12-week oral regimen of BB-1000 (800 mg/day), participants exhibited a notable reduction in wrinkles [40]. However, there are no reports on the clinical anti-aging of microbial fermentation of D. officinale. We prepared some fresh D. officinale juice, fermented with L. plantarum GT-17F, which was freeze-dried to preserve its composition post-fermentation. Concurrent clinical trials evaluated the comparative anti-aging effects of fermented and unfermented D. officinale. These findings lay the foundation for its potential as an ideal anti-aging ingredient in future cosmetic applications [41,42]. The current research results show that at 28 days, compared with the baseline, the subjects using FDO had significantly improved stratum corneum water content, skin elasticity, glossiness (ITA°), wrinkle area, wrinkle area ratio, erythema area, and erythema area ratio. At 28 days post-treatment, subjects using FDO showed notable enhancements in various skin metrics, including stratum corneum hydration, elasticity, and glossiness (ITA°) and reductions in wrinkle and erythema areas.
In contrast, the DO group mainly significantly improved erythema index levels. Comparisons revealed that FDO had a more pronounced effect on wrinkle reduction than did DO. By the 56-day mark, FDO positively influenced almost all assessed parameters, while DO’s benefits were more limited. Crucially, FDO outperformed its unfermented counterpart in enhancing skin elasticity and reducing wrinkles and other metrics. In addition to the detection of skin indicators by instruments and equipment, we also refer to the methods of other randomized, double-blind studies and collect questionnaires from the subjects in order to verify the subjective effects of the products [43,44], which further verified the excellent anti-aging effect of FDO. In essence, this study not only illuminates the potential of D. officinale and its fermented version for anti-aging skin care products but also lays the foundation for more in-depth and inclusive research in the future.
5. Conclusions
This comprehensive study rigorously investigated the anti-aging effects of D. officinale and its fermented version on human skin, providing a significant contribution to skin disease research. This study used a rigorous, randomized, double-blind, placebo-controlled approach with encouraging results. In the sample group, compared with placebo, after 56 days of using skin care products containing FDO ingredients, the skin hydration, TEWL, elasticity, skin brightening, melanin content and erythema index measured by corresponding instruments were all improved, and the differences were statistically significant (p < 0.05). According to the VISIA-CR skin pictures, it can be observed that the erythema area and the proportion of erythema area gradually decrease over time in the cheek area, and the wrinkle area and the proportion of wrinkle area in the crow’s feet area also begin to decrease, indicating that the skin care products containing FDO ingredients have the effect of moisturizing, brightening, soothing, refining wrinkles and tightening the skin. However, when the sample group containing FDO ingredients was compared with the sample group containing DO ingredients after 56 days of using the skin care product containing FDO ingredients, the cuticle moisture content, skin elasticity, skin gloss, skin wrinkles and other parameters were significantly improved (p < 0.05). It shows that skin care products containing FDO ingredients have better moisturizing, brightening, anti-wrinkle and tightening skin effects. Importantly, these findings were supported by both objective instrument test results and subjective participant feedback, enhancing confidence in the results. In addition, none of the subjects who participated in this study reported any adverse effects. While providing compelling evidence for the potential of D. officinale for anti-aging skin care, this study also highlights the need for more extensive studies across different populations and regions to further validate these findings.
Author Contributions
Conceptualization, W.F. and M.S.; methodology, W.F.; formal analysis, W.F.; investigation, W.F.; resources, W.F. and M.S.; data curation, W.F., M.N. and M.S.; writing—original draft preparation, W.F.; writing—review and editing, W.F., M.N., N.D. and M.S.; visualization, W.F.; supervision, M.N. and M.S.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board for Huiwen Skin Research Center (protocol code HUIWEN BIO_20220601 on 1 June 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in the study are available in the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Csekes, E.; Račková, L. Skin aging, cellular senescence and natural polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef] [PubMed]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 9, 759. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.; Khan, A.; Gupta, M. Skin ageing: Pathophysiology and current market treatment approaches. Curr. Aging Sci. 2020, 13, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Addor, F.A.S. Beyond photoaging: Additional factors involved in the process of skin aging. Clin. Cosmet. Investig. Dermatol. 2018, 11, 437–443. [Google Scholar] [CrossRef]
- Wong, Q.Y.A.; Chew, F.T. Defining skin aging and its risk factors: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 22075. [Google Scholar] [CrossRef]
- Khmaladze, I.; Leonardi, M.; Fabre, S.; Messaraa, C.; Mavon, A. The skin interactome: A holistic “genome-microbiome-exposome” approach to understand and modulate skin health and aging. Clin. Cosmet. Investig. Dermatol. 2020, 13, 1021–1040. [Google Scholar] [CrossRef]
- Amaro-Ortiz, A.; Yan, B.; D’Orazio, J.A. Ultraviolet radiation, aging and the skin: Prevention of damage by topical cAMP manipulation. Molecules 2014, 19, 6202–6219. [Google Scholar] [CrossRef]
- Iwasaki, K.; Izawa, M.; Mihara, M. UV-induced apoptosis in rat skin. J. Dermatol. Sci. 1996, 12, 31–35. [Google Scholar] [CrossRef]
- Ploydaeng, M.; Rajatanavin, N.; Rattanakaemakorn, P. UV-C light: A powerful technique for inactivating microorganisms and the related side effects to the skin. Photodermatol. Photoimmunol. Photomed. 2021, 37, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Goyal, N.; Jerold, F. Biocosmetics: Technological advances and future outlook. Environ. Sci. Pollut. Res. Int. 2023, 30, 25148–25169. [Google Scholar] [CrossRef] [PubMed]
- Salvioni, L.; Morelli, L.; Ochoa, E.; Labra, M.; Fiandra, L.; Palugan, L.; Prosperi, D.; Colombo, M. The emerging role of nanotechnology in skincare. Adv. Colloid Interface Sci. 2021, 293, 102437. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.S.; Jia, M.; Chen, L.; Zhu, B.; Dong, H.X.; Si, J.P.; Peng, W.; Han, T. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules 2015, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Lu, J.; Zhang, J.; Wu, J.; Yu, L.; Qin, L.; Zhu, B. Traditional uses, phytochemistry, pharmacology, and quality control of Dendrobium officinale Kimura et. Migo. Front. Pharmacol. 2021, 12, 726528. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Qi, J.; Du, D.; Liu, Y.; Jiang, X. Current advances of Dendrobium officinale polysaccharides in dermatology: A literature review. Pharm. Biol. 2020, 58, 664–673. [Google Scholar] [CrossRef]
- Mai, Y.; Niu, Z.; He, W.; Lai, X.; Huang, S.; Zheng, X. The reparative effect of Dendrobium officinale protocorms against photodamage caused by UV-irradiation in hairless mice. Biol. Pharm. Bull. 2019, 42, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, D.; Dou, M.; Li, M.; Zhang, J.; Zhao, X. Dendrobium officinale Kimura et Migo attenuates diabetic cardiomyopathy through inhibiting oxidative stress, inflammation and fibrosis in streptozotocin-induced mice. Biomed. Pharmacother. 2016, 84, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.Y.; Liang, Y.M.; Liu, H.Z.; Zhu, D.M.; Hou, S.Z.; Wu, Y.Y.; Huang, S.; Lai, X.P. Effect of Dendrobium officinale on D-galactose-induced aging mice. Chin. J. Integr. Med. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Fei, W.; Noda, M.; Danshiitsoodol, N.; Sugiyama, M. Dendrobium officinale extract fermented with Lactobacillus plantarum GT-17F enhances the protection of UV-mediated photoaging. Biol. Pharm. Bull. 2023, 10, 1451–1460. [Google Scholar] [CrossRef]
- Zhang, Y.; You, S.; Wang, D.; Zhao, D.; Zhang, J.; An, Q.; Li, M.; Wang, C. Fermented Dendrobium officinale polysaccharides protect UVA-induced photoaging of human skin fibroblasts. Food Sci. Nutr. 2022, 10, 1275–1288. [Google Scholar] [CrossRef]
- Alaluf, S.; Heath, A.; Carter, N.; Atkins, D.; Mahalingam, H.; Barrett, K.; Kolb, R.; Smit, N. Variation in melanin content and composition in type V and VI photoexposed and photoprotected human skin: The dominant role of DHI. Pigment Cell Res. 2001, 14, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, X.X.; Yang, R.Y.; Yi, F. Study of the characteristics of facial skin tone status in 1092 young Chinese females according to the ITA°. J. Cosmet. Dermatol. 2022, 21, 2073–2081. [Google Scholar] [CrossRef] [PubMed]
- Chardon, A.; Cretois, I.; Hourseau, C. Skin colour typology and suntanning pathways. Int. J. Cosmet. Sci. 1991, 13, 191–208. [Google Scholar] [CrossRef] [PubMed]
- T/ZHCA 006-2019; Zhejiang Health Products and Cosmetic Industry Association. Method for Assessment of Cosmetics Anti-wrinkle Efficacy. Standards Press of China: Beijing, China, 2019.
- Ye, L.; Wang, Z.; Li, Z.; Lv, C.; Man, M.Q. Validation of GPSkin Barrier® for assessing epidermal permeability barrier function and stratum corneum hydration in humans. Skin Res. Technol. 2019, 25, 25–29. [Google Scholar] [CrossRef]
- Ohshima, H.; Kinoshita, S.; Oyobikawa, M.; Futagawa, M.; Takiwaki, H.; Ishiko, A.; Kanto, H. Use of Cutometer area parameters in evaluating age-related changes in the skin elasticity of the cheek. Skin Res. Technol. 2013, 19, 238–242. [Google Scholar] [CrossRef]
- Matias, A.R.; Ferreira, M.; Costa, P.; Neto, P. Skin colour, skin redness and melanin biometric measurements: Comparison study between Antera (®) 3D, Mexameter (®) and Colorimeter (®). Skin Res. Technol. 2015, 21, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Holcomb, J.D.; Doolabh, V.; Lin, M.; Zimmerman, E. High energy, double pass helium plasma dermal resurfacing: A prospective, multicenter, single-arm clinical study. Lasers Surg. Med. 2022, 54, 648–662. [Google Scholar] [CrossRef]
- Xu, D.T.; Yan, J.N.; Cui, Y.; Liu, W. Quantifying facial skin erythema more precisely by analyzing color channels of The VISIA Red images. J. Cosmet. Laser Ther. 2016, 18, 296–300. [Google Scholar] [CrossRef]
- Nobile, V.; Zanoletti, V.; Manzoni, V.; Romagnoli, S.; Cestone, E. Soothing effect of a cosmetic product on skin discomforts induced by a chemical irritant (capsaicin) and UV-radiation, and after mosquito bites and sunburn in a real-world setting. Cosmetics 2022, 9, 130. [Google Scholar] [CrossRef]
- Swift, A.; Liew, S.; Weinkle, S.; Garcia, J.K.; Silberberg, M.B. The facial aging process from the “inside out”. Aesthet. Surg. J. 2021, 41, 1107–1119. [Google Scholar] [CrossRef]
- Costa, E.F.; Magalhães, W.V.; Di Stasi, L.C. Recent advances in herbal-derived products with skin anti-aging properties and 586cosmetic applications. Molecules 2022, 27, 7518. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential health benefits of plant food-derived bioactive components: An overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Li, L.; Wang, L.; Fan, W.; Jiang, Y.; Zhang, C.; Li, J.; Peng, W.; Wu, C. The application of fermentation technology in traditional Chinese medicine: A review. Am. J. Chin. Med. 2020, 48, 899–921. [Google Scholar] [CrossRef]
- Shin, D.; Lee, Y.; Huang, Y.H.; Lim, H.W.; Jang, K.; Kim, D.D.; Lim, C.J. Probiotic fermentation augments the skin anti-photoaging properties of Agastache rugosa through up-regulating antioxidant components in UV-B-irradiated HaCaT keratinocytes. BMC Complement. Altern. Med. 2018, 18, 196. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, M.R.; Park, Y.; Park, H.J.; Chang, U.J.; Kim, S.Y.; Suh, H.J. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: in vitro and animal study. J. Med. Food. 2012, 15, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.H.; Kim, A.R.; Lee, K.S.; Xuan, S.H.; Kang, H.C.; Lee, D.H.; Cha, M.Y.; Kim, H.J.; An, M.; Park, S.N. Anti-aging activity of Lavandula angustifolia extract fermented with Pediococcus pentosaceus DK1 isolated from Diospyros kaki fruit in UVB-irradiated human skin fibroblasts and analysis of principal components. J. Microbiol. Biotechnol. 2019, 29, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Pawakongbun, T.; Lourith, N. Dendrobium orchid polysaccharide extract: Preparation, characterization and in vivo skin hydrating efficacy. Chin. Herb. Med. 2019, 11, 400–405. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Y.; Pu, Y.; Mao, S.; Nie, Y.; Liu, Y.; Jiang, X. Dendrobium officinale Kimura & Migo polysaccharide and its multilayer emulsion protect skin photoaging. J. Ethnopharmacol. 2024, 318, 116974. [Google Scholar]
- Lee, S.W.; Sin, H.S.; Hurh, J.; Kim, S.Y. Anti-wrinkle effect of BB-1000: a double-blind, randomized controlled study. Cosmetics 2022, 9, 50. [Google Scholar] [CrossRef]
- Chen, H.; Shi, X.; Cen, L.; Zhang, L.; Dai, Y.; Qiu, S.; Zeng, X.; Wei, C. Effect of yeast fermentation on the physicochemical properties and bioactivities of polysaccharides of Dendrobium officinale. Foods 2022, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Dai, L.; Lu, S.; Luo, Z.; Qiu, Z.; Li, J.; Li, P.; Du, B. Effect of Bacillus sp. DU-106 fermentation on Dendrobium officinale polysaccharide: structure and immunoregulatory activities. Int. J. Biol. Macromol. 2019, 135, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, F.; Roveda, G.; Michelotti, A.; Ruggeri, F.; Tursi, F. Anti-skin-aging effect of a treatment with a cosmetic product and a food supplement based on a new hyaluronan: A randomized clinical study in healthy women. Cosmetics 2022, 9, 54. [Google Scholar] [CrossRef]
- Nobile, V.; Schiano, I.; Germani, L.; Cestone, E.; Navarro, P.; Jones, J.; Caturla, N. Skin anti-aging efficacy of a four-botanical blend dietary ingredient: A randomized, double blind, clinical study. Cosmetics 2023, 10, 16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).