Efficacy and Skin Microbiome Modulation Effects of a Fixed-Concentration Combination of Benzoyl Peroxide 4% Plus Niacinamide 4% in a Film-Forming Cream in Subjects with Mild-to-Moderate Acne: A Non-Sponsored, Prospective, Assessor-Blinded, Pilot Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Study Design
2.2. Study Outcomes
2.3. Statistical Analysis
2.4. Ethical Issues
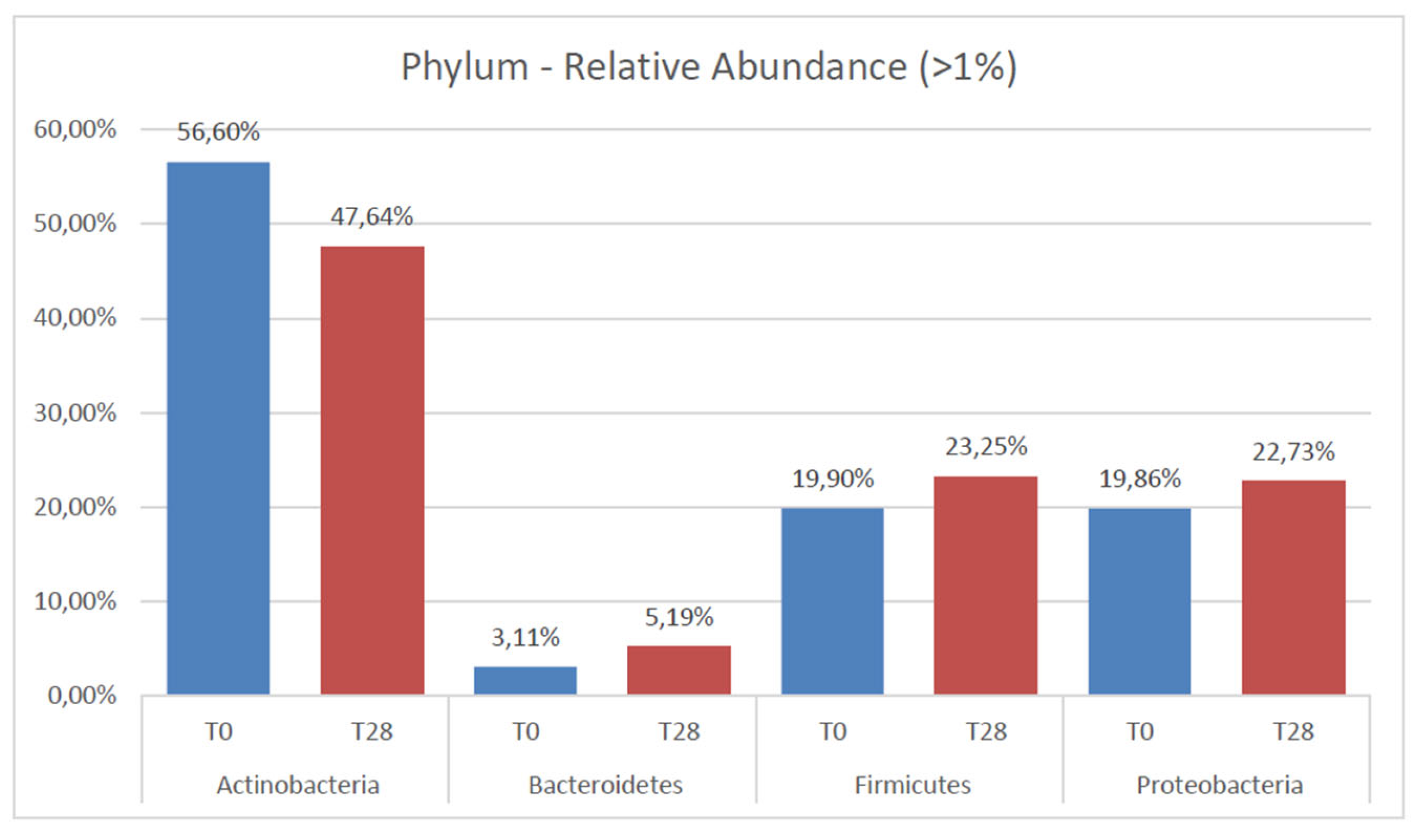
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kircik, L.H. Advances in the Understanding of the Pathogenesis of Inflammatory Acne. J. Drugs Dermatol. 2016, 15, s7–s10. [Google Scholar]
- Vasam, M.; Korutla, S.; Bohara, R.A. Acne vulgaris: A review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem. Biophys. Rep. 2023, 36, 101578. [Google Scholar] [CrossRef]
- Schachner, L.A.; Alexis, A.F.; Andriessen, A.; Berson, D.; Gold, M.; Goldberg, D.J.; Hu, S.; Keri, J.; Kircik, L.; Woolery-Lloyd, H. Insights into acne and the skin barrier: Optimizing treatment regimens with ceramide-containing skincare. J. Cosmet. Dermatol. 2023, 22, 2902–2909. [Google Scholar] [CrossRef]
- Rathi, S. Acne vulgaris treatment: The Current Scenario. Indian J. Dermatol. 2011, 56, 7. [Google Scholar] [CrossRef]
- Bhatia, A.C.; Jimenez, F. Rapid treatment of mild acne with a novel skin care system containing 1% salicylic acid, 10% buffered glycolic acid, and botanical ingredients. J. Drugs Dermatol. 2014, 13, 678–683. [Google Scholar]
- De Lucas, R.; Moreno-Arias, G.; Perez-López, M.; Vera-Casaño, Á.; Aladren, S.; Milani, M. Adherence to drug treatments and adjuvant barrier repair therapies are key factors for clinical improvement in mild to moderate acne: The ACTUO observational prospective multicenter cohort trial in 643 patients. BMC Dermatol. 2015, 15, 17. [Google Scholar] [CrossRef]
- Leyden, J.J.; Del Rosso, J.Q.; Webster, G.F. Clinical considerations in the treatment of acne vulgaris and other inflammatory skin disorders: Focus on antibiotic resistance. Cutis 2007, 79, 9–25. [Google Scholar]
- Araviiskaia, E.; Layton, A.M.; Estebaranz, J.L.L.; Ochsendorf, F.; Micali, G. The Synergy between Pharmacological Regimens and Dermocosmetics and Its Impact on Adherence in Acne Treatment. Dermatol. Res. Pract. 2022, 2022, 1–10. [Google Scholar] [CrossRef]
- Yamamoto, A.; Takenouchi, K.; Ito, M. Impaired water barrier function in acne vulgaris. Arch. Dermatol. Res. 1995, 287, 214–218. [Google Scholar] [CrossRef]
- Thiboutot, D.; Del Rosso, J.Q. Acne Vulgaris and the Epidermal Barrier: Is Acne Vulgaris Associated with Inherent Epidermal Abnormalities that Cause Impairment of Barrier Functions? Do Any Topical Acne Therapies Alter the Structural and/or Functional Integrity of the Epidermal Barrier? J. Clin. Aesthet. Dermatol. 2013, 6, 18–24. [Google Scholar]
- Walocko, F.M.; Eber, A.E.; Keri, J.E.; AL-Harbi, M.A.; Nouri, K. The role of nicotinamide in acne treatment. Dermatol. Ther. 2017, 30, e12481. [Google Scholar] [CrossRef]
- Forbat, E.; Al-Niaimi, F.; Ali, F.R. Use of nicotinamide in dermatology. Clin. Exp. Dermatol. 2017, 42, 137–144. [Google Scholar] [CrossRef]
- Kaewsanit, T.; Chakkavittumrong, P.; Waranuch, N. Clinical Comparison of Topical 2.5% Benzoyl Peroxide plus 5% Niacinamide to 2.5% Benzoyl Peroxide Alone in the Treatment of Mild to Moderate Facial Acne Vulgaris. J. Clin. Aesthet. Dermatol. 2021, 14, 35–41. [Google Scholar]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef]
- Muppalaneni, S.; Omidian, H. Polyvinyl Alcohol in Medicine and Pharmacy: A Perspective. J. Dev. Drugs 2013, 2, 1–5. [Google Scholar] [CrossRef]
- Rigano, L.; Savonelli, S.; Bencini, P.L. Use and properties of perfluoropolymethyl-isopropylethers in skin and hair cleaning; system stabilization and interference with sebum redistribution on skin and hair. Int. J. Cosmet. Sci. 1989, 11, 259–282. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Bai, Y.; Tai, X.; Wang, W.; Wang, G. Preparation and Property of Perfluoropolyether Emulsions. Polymers 2019, 11, 932. [Google Scholar] [CrossRef]
- Dutil, M. Benzoyl peroxide: Enhancing antibiotic efficacy in acne management. Skin. Therapy Lett. 2010, 15, 5–7. [Google Scholar]
- Worret, W.; Fluhr, J.W. Acne therapy with topical benzoyl peroxide, antibiotics and azelaic acid. JDDG J. Der Dtsch. Dermatol. Ges. 2006, 4, 293–300. [Google Scholar] [CrossRef]
- Draelos, Z.D.; Matsubara, A.; Smiles, K. The effect of 2% niacinamide on facial sebum production. J. Cosmet. Laser Ther. 2006, 8, 96–101. [Google Scholar] [CrossRef]
- Grange, P.A.; Raingeaud, J.; Calvez, V.; Dupin, N. Nicotinamide inhibits Propionibacterium acnes-induced IL-8 production in keratinocytes through the NF-κB and MAPK pathways. J. Dermatol. Sci. 2009, 56, 106–112. [Google Scholar] [CrossRef]
- Liu, C.; Takada, K.; Zhu, D. Targeting Wnt/β-Catenin Pathway for Drug Therapy. Med. Drug Discov. 2020, 8, 100066. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Choo, H.; Chen, Y.; Zhang, X.; Xu, R.-H.; Wang, X.; Wu, Y. Distinct bulge stem cell populations maintain the pilosebaceous unit in a β-catenin-dependent manner. IScience 2023, 26, 105805. [Google Scholar] [CrossRef]
- Cong, T.-X.; Hao, D.; Wen, X.; Li, X.-H.; He, G.; Jiang, X. From pathogenesis of acne vulgaris to anti-acne agents. Arch. Dermatol. Res. 2019, 311, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-H.; Shin, J.Y.; Kim, J.; Kang, N.-G.; Lee, S. Niacinamide Down-Regulates the Expression of DKK-1 and Protects Cells from Oxidative Stress in Cultured Human Dermal Papilla Cells. Clin. Cosmet. Investig. Dermatol. 2021, 14, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chang, I.-J.; Bolick, N.; Hsu, W.-T.; Su, C.-H.; Hsieh, T.-S.; Huang, I.-H.; Lee, C.-C. Comparative Efficacy of Pharmacological Treatments for Acne Vulgaris: A Network Meta-Analysis of 221 Randomized Controlled Trials. Ann. Fam. Med. 2023, 21, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.; Hu, A.; Fleming, P.; Lynde, C.W. The Impact of Acne Treatment on Skin Bacterial Microbiota: A Systematic Review. J. Cutan. Med. Surg. 2022, 26, 93–97. [Google Scholar] [CrossRef]
| Parameter | Time | Mean Score ± SD | % Variation vs. T0 | p-Value * |
|---|---|---|---|---|
| Non-inflammatory lesions (comedones + microcysts) | T0 | 23.18 ± 17.73 | −40.39% | <0.05 |
| T28 | 13.82 ± 12.71 | |||
| Inflammatory lesions (papules + pustules) | T0 | 10.68 ± 5.62 | −43.40% | <0.05 |
| T28 | 6.05 ± 3.12 | |||
| Total acne lesions | T0 | 33.86 ± 20.34 | −41.34% | <0.05 |
| T28 | 19.86 ± 14.24 |
| Parameter | Time | Mean Score ± SD | % Variation vs. T0 | p-Value * |
|---|---|---|---|---|
| Transepidermal water loss (g/m2/h) | T0 | 17.33 ± 4.95 | 6.48% | >0.05 |
| T28 | 18.45 ± 4.00 | |||
| Skin lipids (mg/cm2) | T0 | 137.05 ± 66.89 | −41.63% | <0.05 |
| T28 | 80.00 ± 31.34 | |||
| Skin redness (score) | T0 | 61.23 ± 15.28 | −11.14% | <0.05 |
| T28 | 54.41 ± 14.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puviani, M.; Eisendle, K. Efficacy and Skin Microbiome Modulation Effects of a Fixed-Concentration Combination of Benzoyl Peroxide 4% Plus Niacinamide 4% in a Film-Forming Cream in Subjects with Mild-to-Moderate Acne: A Non-Sponsored, Prospective, Assessor-Blinded, Pilot Trial. Cosmetics 2024, 11, 25. https://doi.org/10.3390/cosmetics11010025
Puviani M, Eisendle K. Efficacy and Skin Microbiome Modulation Effects of a Fixed-Concentration Combination of Benzoyl Peroxide 4% Plus Niacinamide 4% in a Film-Forming Cream in Subjects with Mild-to-Moderate Acne: A Non-Sponsored, Prospective, Assessor-Blinded, Pilot Trial. Cosmetics. 2024; 11(1):25. https://doi.org/10.3390/cosmetics11010025
Chicago/Turabian StylePuviani, Mario, and Klaus Eisendle. 2024. "Efficacy and Skin Microbiome Modulation Effects of a Fixed-Concentration Combination of Benzoyl Peroxide 4% Plus Niacinamide 4% in a Film-Forming Cream in Subjects with Mild-to-Moderate Acne: A Non-Sponsored, Prospective, Assessor-Blinded, Pilot Trial" Cosmetics 11, no. 1: 25. https://doi.org/10.3390/cosmetics11010025
APA StylePuviani, M., & Eisendle, K. (2024). Efficacy and Skin Microbiome Modulation Effects of a Fixed-Concentration Combination of Benzoyl Peroxide 4% Plus Niacinamide 4% in a Film-Forming Cream in Subjects with Mild-to-Moderate Acne: A Non-Sponsored, Prospective, Assessor-Blinded, Pilot Trial. Cosmetics, 11(1), 25. https://doi.org/10.3390/cosmetics11010025







