Abstract
Nanofiber-based materials, due to their unique properties, are of remarkable interest across multiple fields of applications, including cosmetics. Collagen, a primary structural protein in human skin, is well-regarded for its critical role in maintaining skin health, elasticity, and enhancing skin regeneration. This study reports the characterization, safety, and efficacy evaluation of DermaLayr™, a novel collagen-based nanofiber platform, for skincare application. The collagen nanofibers were developed using a sonic electrospinning technique, and scanning electron microscopy (SEM) analyses indicated that the nanofibers were uniform with average fiber diameters ranging from 250 to 300 nm. The skin permeation studies on EpiDerm™ indicated that applying the test products resulted in around 5–25% higher collagen permeation into the epidermis, and 16–20% higher collagen permeation into the dermis when compared to the non-treated sample. Additionally, the safety of the developed nanofibers was assessed in vitro and in vivo and both the studies indicated their non-toxic and non-irritant properties. Moreover, clinical trials on human subjects further substantiated the clinical efficacy of DermaLayr™ by demonstrating significant improvement in several skin parameters such as hydration, elasticity, and overall skin health. In summary, the findings of this study emphasize the huge potential of DermaLayr™ nanofiber products for their safe application in cosmetics and skin therapeutics.
1. Introduction
When it comes to cosmetics and skin therapeutics, nanotechnology is widely researched as it is acknowledged as one of the revolutionary technologies across all the industries [1,2]. Innovations in cosmetic science brought about by the integration of nanotechnology have significantly raised the global consumer demand of cosmetics [3]. Through the application of nanotechnology, materials can be manipulated at the nano level, offering new opportunities for the cosmetics sector. Cosmetics and dermatological products based on nanomaterials are employed in cosmetics, skin disorders, and diagnostic applications.
Nanofibers (NFs) are a fascinating class of nanomaterial that have gained attention recently due to their extensive range of biomedical uses. In general, a fiber with a diameter of less than 1000 nm is referred to as NF [4]. A much higher specific surface area can result when the diameter of fibers decreases from micrometers (e.g., 10–100 μm) to sub micrometers or nanometers (e.g., 10–100 nm). Because of this inherent property, NFs are attractive for a wide range of applications where a high specific surface area is required [5]. NFs can be synthesized from natural polymers as well as synthetic polymers, or their combination, and they can be produced by several processing techniques, such as spraying, template synthesis, phase separation, self-assembly, electrospinning, and so on [6]. Electrospinning is the most popular method for preparing NFs for cosmetic applications [7]. Electrospun NFs have a wide range of uses in skincare, including the development of facial masks and cosmetic patches [8,9]. The ease with which active ingredients can be incorporated into electrospun fibers makes this method appealing for cosmetic applications.
NanoLayr Limited, based in Auckland, New Zealand, is large-scale manufacturer of advanced NF textiles with ISO9001 and cosmetic GMP certification [10]. NanoLayr specializes in producing NF-based products using its in-house developed needleless sonic electrospinning technology, which is used to convert the active-loaded polymeric solutions into NFs. It involves the deposition of NFs on a non-woven substrate layer and sandwiched between a protective layer. The developed NF products have bespoke applications in cosmetics, filtration, composite reinforcement, sound attenuation, and various other markets [10]. One such NF-based product developed by NanoLayr is a dermal delivery platform known as DermaLayr™. The DermaLayr™ Marine+ products are made from type I marine collagen, and they have the capability to encapsulate actives for their enhanced delivery into the dermal layers of the skin.
Collagen is the major structural protein that makes up a significant portion of the extracellular matrix of connective tissues, especially in skin, bones, tendons, and joints [11]. Because of its excellent cell accommodation capabilities made possible by its highly biocompatible and biodegradable properties, collagen is widely used in bioindustries such as cosmetics, food, and pharmaceuticals [12]. Additionally, compared to other types of biomaterials, collagen has relatively low immunogenicity. Collagen can originate from a variety of sources, including bovine, porcine, and marine sources [13]. There are approximately 29 different kinds of collagen discovered so far [14]. Type I collagen is the predominant type of collagen found in skin, and research has shown that marine collagen is rich in this type of collagen [15,16]. Therefore, marine collagen is the most desired collagen source in the cosmetic industry. Marine collagen can be obtained from a variety of sources, including fish and invertebrate marine organisms such as marine sponges and jellyfish [17]. Because of its excellent moisturizing, skin renewing, and film-forming capabilities, collagen is a key ingredient in cosmetic compositions [18,19]. According to research, collagen also promotes wound healing and aids in tissue regeneration [11]. However, native collagen cannot effectively cross the stratum corneum due to its highly hydrophilic nature and large molecular weight [20]. Commonly, dermal collagen fillers are injected to address cosmetic concerns such as wrinkles and folds. However, invasive intravascular injections are associated with various complications including risks of accidental injuries, hypersensitivity reactions, tissue necrosis, and infections [21]. To overcome these complications related to dermal delivery of collagen, NF-based products are an alternative non-invasive approach for its safe and effective skin delivery [22].
In this study, we have reported the in vitro and clinical evaluation of DermaLayr™ Marine+ NF products. The in vitro skin penetration potential of marine collagen from the NF products was evaluated using reconstructed human skin cells. The clinical efficacy was assessed to evaluate the potential of the NF product to treat women with wrinkles and facial skin sagging. The clinical trial also aimed to study the effectiveness of the NF product to improve the overall skin health in the study subjects. The safety profile of the NF products was also investigated both in vitro and in vivo.
2. Materials and Methods
2.1. Study Products
DermaLayr™ Marine+ products are collagen-based NF products developed by NanoLayr Ltd. (Auckland, New Zealand) using a proprietary needleless electrospinning technique. The 3 different NF test products used in this study are listed in Table 1. The ingredients used in this study are named as per the International Nomenclature Cosmetic Ingredient (INCI) system.

Table 1.
Description of the NF products used in this study.
2.2. Characterization of Nanofiber Products
The areal weights of the NFs were determined by weighing a sample 0.01m2 using an analytical balance (AUY220, Shimadzu, Kyoto, Japan) and calculated using Equation (1):
Grams per square meter (gsm) of sample = sample mass in grams/sample
area in square meters
area in square meters
The morphology of the NFs was analyzed using a scanning electron microscope (SEM) (JCM-5000, JEOL Ltd., Tokyo, Japan) and their average diameters and distribution were determined using MIPAR Image Analysis Software version 4.2.2 (MIPAR Software LLC, Worthington, OH, USA).
2.3. In Vitro Permeation of Type I Marine Collagen through Human-Derived Epidermal Keratinocytes (EpiDermTM)
The in vitro permeation of type I marine collagen from three different products (TP 1, TP 2, and TP 3) was analyzed using a reconstructed human epidermal (RHE) model, EpiDerm™ (EPI-200, MatTek, Ashland, MA, USA and Bratislava, Slovakia). The studies were conducted by following the standards set out by The Organisation for Economic Co-operation and Development (OECD) Test Guideline 428 [23,24] at MS Clinical Research Pvt. Ltd., Bengaluru, India.
EpiDerm™ is a highly differentiated multi-layered human epidermis model formed using normal human-derived epidermal keratinocytes. It consists of organized basal, spinous, and granular layers, as well as a multi-layered stratum corneum containing intercellular lamellar lipid layers arranged in patterns analogous to those found in vivo [25]. The EpiDerm™ tissues (surface 0.6 cm2) are cultured on specially prepared cell culture inserts that enables the tissue to act as a semipermeable membrane. To prepare the tissues, the EpiDerm™ tissue kit was conditioned in the EPI-100-LLMM-X culture media according to manufacturer guidelines. The schematic representation of EpiDerm™ is provided in Figure S1.
The test products, available in the form of sheets, were cut using a sterile biopsy punch of 8mm diameter at 4 g per cm2 and placed over the respective tissues in 6-well plates using dry sterile forceps and were allowed to incubate for 24 h. The degree of permeation of the actives into the epidermis and dermal layers of the skin was determined by analyzing the concentrations of actives in EpiDerm™ tissue lysate and the receiver solution, respectively, in comparison to the donor solution. Assessment was performed in triplicate for both the donor and receiver solutions, and their optical densities were determined according to the standard type I marine collagen ELISA kit protocol [26].
2.4. Clinical Evaluation of DermaLayr™ Marine+ Tighten
In this article, we report the clinical evaluation of DermaLayr™ Marine+ Tighten (TP 1). The clinical evaluation was performed at P&K Skin Research Center, Seoul, South Korea. All the recruited subjects were informed about the study and were required to sign a written consent form before participating in the study. The trials were conducted in accordance with the ethical principles founded in the Declaration of Helsinki. The study design has been presented in Table 2.

Table 2.
Study design for the clinical evaluation of DermaLayr™ Marine+ Tighten.
Study 1 was designed to evaluate the effect of DermaLayr™ Marine+ Tighten on various skin parameters. The devices used during this study are listed in Table 3. Study 2 was designed to evaluate the effectiveness of the test product to treat SLS-induced skin erythema. In total, 35 μL of 2% SLS was occluded on the test site of the subjects using the IQ Ultimate chamber and left on the test site for 24 h. The treated and the non-treated (control) sites were partitioned on the left forearm of the subject. The subjects were instructed to apply the test product on the left forearm, twice a day for 2 weeks. Skin redness and transepidermal water loss (TEWL) were measured before and after using the test product, as described in Table 3.

Table 3.
List of devices used for the clinical evaluation of DermaLayr™ Marine+ Tighten.
Skin absorbance of the test product was investigated in Study 3. In this study, ActivLayr® premium intensive collagen ampoule mist was used as a control. Two areas were partitioned on the forearm of the subject, and 4 µL/cm2 equivalent of the test product and control product was applied to the designated application areas, respectively. Skin absorbance was measured before and after 30 min of using the products using a 3D Raman microscopy system.
2.5. Safety Assessment of Nanofibers
2.5.1. In Vitro Skin Irritation Assessment
In this study, the in vitro skin irritation potential of the 3 test products (TP 1, TP 2, and TP 3) were evaluated. The test was conducted at MS Clinical Research Pvt Ltd., Bengaluru, India, based on OECD Test Guideline 439. RHE model, EpiDerm™ 24 tissue kit EPI-200-SIT was used for this study. The EpiDerm™ tissues were conditioned in the EPI-100-NMM culture media according to manufacturer guidelines to prepare the tissues and the assessment of the DermaLayr™ products was performed over a 24 h period in triplicates using an (3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide) MTT assay [27]. A positive control in the form of 5% sodium dodecyl sulphate (SDS) and a non-treated well was used as a negative control.
2.5.2. Clinical Safety Evaluation of DermaLayr™ Marine+ Tighten
DermaLayr™ Marine+ Tighten (TP 1) was also assessed for its skin irritation potential in humans. An open label study of 32 healthy subjects, aged between 20 and 50 years, without any acute or chronic physical disease, including infectious skin disease was conducted at P&K Skin Research Center. The test product was attached to IQ Ultimate chamber (Chemotechnique Diagnostics, Vellinge, Sweden), which was then applied to the back of the test subject for 24 h. Skin reactivity and irritation were evaluated based on the Frosch and Kligman, CTFA guideline, and Draize method, respectively, at 1 h and 24 h post patch removal.
2.6. Statistical Analysis
Statistical analysis was performed using SPSS 19.0 (IBM, Armonk, NY, USA). Significance was confirmed when p < 0.05. Continuous variables were summarized as mean ± standard deviation, and categorical variables were summarized as frequency and percentage. For comparison before and after use, a paired t-test was used after the normality test. The independent t-test and Mann–Whitney U test were used for normality testing.
3. Results and Discussion
3.1. Characterization of DermaLayr™ Test Products
The surface morphology of the NF products as analyzed via SEM are presented in Figure 1a–c. The NFs were dense and well-distributed with average diameters ranging from 255 to 266 nm. The characteristics exhibited by the NFs are consistent with fibrous non-woven materials such as fabrics which have a stacked layered morphology and are randomly oriented.
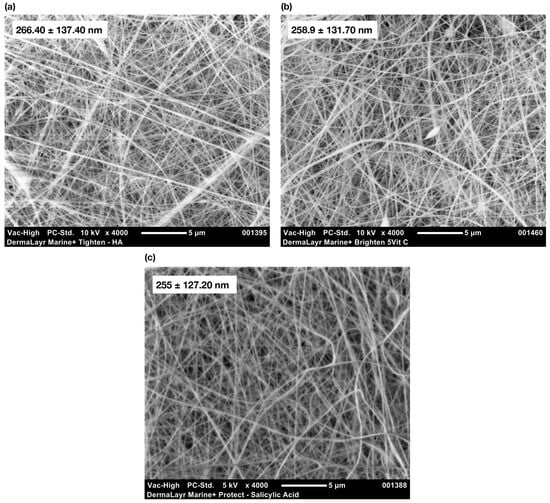
Figure 1.
In vitro evaluations of DermaLayr™ NF products: SEM characterizations of (a) DermaLayr™ Marine+ Tighten; (b) DermaLayr™ Marine⁺ Brighten 5Vit C; (c) DermaLayr™ Marine⁺ Protect 2SA.
3.2. In Vitro Permeation of DermaLayr™ Type I Marine Collagen through Human-Derived Epidermal Keratinocytes (EpiDermTM)
The concentration of type I marine collagen as measured by ELISA assay for each of the three test products and non-treated control in both the tissue lysate (dermis) and receiver solution (epidermis) are presented in Figure 2. All samples, including the non-treated control showed some level of type I marine collagen; however, all NF test products demonstrated higher concentrations of type I marine collagen in both the receiver solution (dermis) and tissue lysate (epidermis).
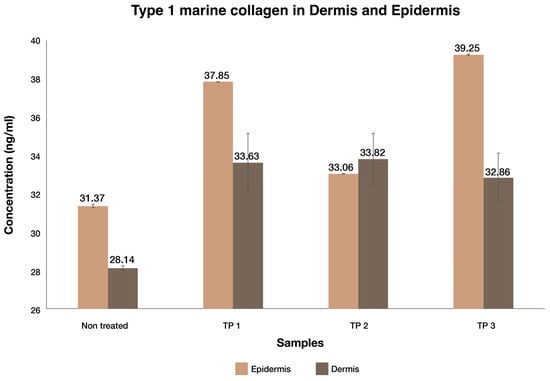
Figure 2.
Permeation of type I marine collagen from different test products across EpiDerm™.
To determine the degree of permeation, the non-treated control was used as the baseline against which the NF test product concentrations were compared. The percentage differences from baseline for each of the test products in both the tissue lysate and receiver solution are presented in Table 4.

Table 4.
Estimation of type 1 marine collagen in epidermis and dermis.
Based on the above results, it was determined that all three DermaLayr™ test products permeated into both the epidermal and dermal layers of the skin. The varying degree of percentage increase in type I marine collagen across the epidermis and dermis observed after one application of NF product is attributed to the different formulations of DermaLayr™ products tested. TP 1 showed 20.62% and 19.50% higher concentrations of type I marine collagen in the tissue lysate (epidermis) and receiver solution (dermis), respectively, compared to the non-treated control. Similarly, TP 3 permeated 25.11% higher concentrations of collagen into the epidermis and 16.75% higher concentrations into the dermis, compared to the non-treated sample. Additionally, compared to non-treated sample, TP 2 permeated only 5.38% and 20.16% higher concentrations of collagen in the epidermis and dermis, respectively. The significantly (p < 0.05) lower percentage of permeation of type I marine collagen into the epidermis for TP 2 may be explained by the presence of highly reactive and oxidizing active Vitamin C, in the form of sodium ascorbyl phosphate in the test product. Vitamin C may have caused the modification of collagen on application, inhibiting the ability to detect and quantify type I collagen using ELISA. Vitamin C is immediately mobilized when the test product is applied onto the skin and begins to permeate into the epidermis. However, due to its poor permeability through the epidermis [28], it does not reach the dermis while DermaLayr™ type I collagen continues to permeate through to the dermis (receiver solution). Hence, the modification of collagen occurs only in the epidermis resulting in lower levels of type I collagen detected in the epidermis compared to the dermis. The permeation of type I collagen for all DermaLayr™ products is further supported and strengthened by the clinical studies, which are discussed in the later sections of this article.
3.3. Clinical Evaluation of DermaLayr™ Marine+ Tighten
A total of 23 subjects were enrolled for this study and all the subjects completed the study. The measurements recorded for the tested parameters are presented in Table 5 and Table 6. Skin brightness, as measured using VISIA®, was expressed using the L* value (luminosity). The higher the L* value, the better the perceived skin brightness [29]. A significant increase of 0.68% (p < 0.01) and 1.51% in skin brightness (p < 0.01) was observed after 2 weeks and 4 weeks of using the test product, compared to the condition before use, as shown in Figure 3a,b. The lifting effect was determined by measuring the angle of the contour lines in the cheek or the eye regions, respectively. Eye and facial skin lifting were analyzed as described previously by Saito et al. [30]. Eye lifting measurements were performed by measuring the angle of the contour line from the captured contour image using the image analysis program. As the angle between the two lines increases, there is a lifting effect. Cheek lifting measurements were performed by drawing a straight line through the circle formed near the cheekbones and measuring the angle formed with the straight line drawn across the mouth. As the angle between the two straight lines decreases (decreased sagginess), it can be considered that there is an improvement effect on the facial (cheek) lifting [31]. Figure 3c shows that compared to baseline (before use of the test product), there was a significant increase (p < 0.05) in lifting around the eyes immediately after use of the test product. Also, there was a significant improvement (p < 0.01) in facial lifting after 2 weeks and 4 weeks of use when compared to the condition before use of the test product, as shown in Figure 3d. A significant decrease (p < 0.05) in deep eye, forehead, and nasolabial fold wrinkles were also observed after using the test product (Table 5) [32].

Table 5.
Estimation of improvement in skin brightness, eye and facial lifting, and wrinkle reduction before and after application of the test product.

Table 6.
Estimation of improvement in skin elasticity restoration, moisture, elasticity, and density before and after application of test product.
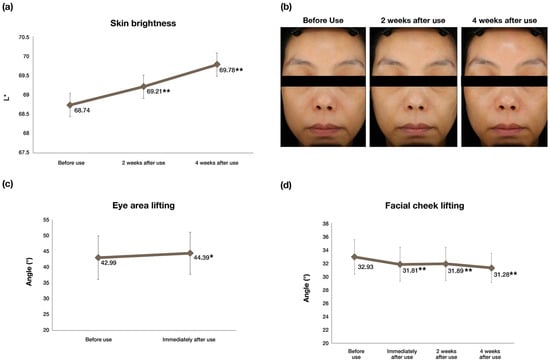
Figure 3.
Clinical efficacy evaluations of DermaLayr™ Marine+ Tighten: (a) Increase in skin brightness; (b) representative images demonstrating an increase in skin brightness in a study subject; (c) % improvement in eye area lifting and (d) improvement in facial lifting as demonstrated by decreased sagginess (n = 23; * p < 0.05, ** p < 0.01 indicate significant differences).
Skin elasticity denotes structural integrity and skin aging leads to evident decreases in skin elasticity restoration ability. The elastic restoring force of skin significantly increased (p < 0.01) after 2 weeks and 4 weeks of use of the test product when compared to before use, as demonstrated in Table 6. The data are also graphically represented in Figure 4a,b.
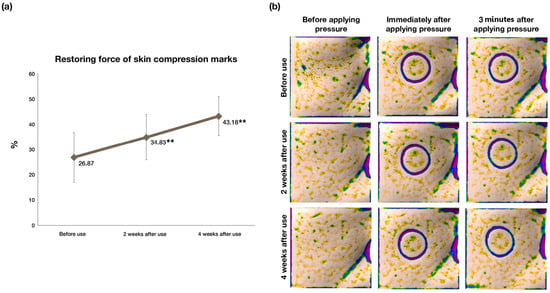
Figure 4.
Clinical efficacy evaluations of DermaLayr™ Marine+ Tighten: (a) % improvement in skin elasticity restoration; (b) representative Antera 3D images showing improvement in skin compression marks, thus demonstrating improvement in skin elasticity (n = 23; ** p < 0.01 indicate significant differences).
Skin moisture level was measured using corneometer, which specifies the hydration level of the stratum corneum by measuring skin dielectric properties. Greater values indicate higher amounts of skin hydration [33]. The results, as shown in Table 6 and Figure 5a, show a statistically significant increase (p < 0.01) of 17.41% in skin moisture levels after application of the test product for 4 weeks [32,34].
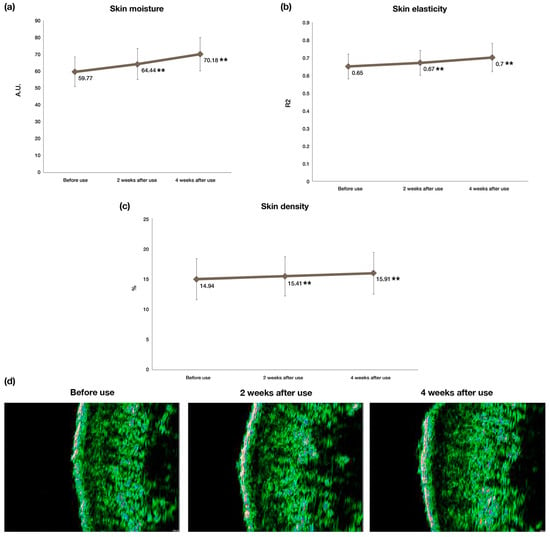
Figure 5.
Clinical efficacy evaluations of DermaLayr™ Marine+ Tighten: (a) increase in skin moisture level; (b) increase in skin elasticity; (c) increase in skin density and (d) representative DUBSkin Scanner images showing increase in skin density, as demonstrated by an increase in thickness of the fluorescent layer (n = 23; ** p < 0.01 indicate significant differences).
Skin elasticity is regarded as a sign of both age and general skin health. Therefore, determining the elasticity of skin and quantifying skin elasticity are crucial clinical results [35]. The cutometer assesses skin firmness and elasticity by measuring skin deformation when it is pulled by controlled vacuum into the circular aperture [35]. A significant improvement (p < 0.01) with a mean value increase of 7.69% was observed in skin elasticity (Figure 5b) after 4 weeks of application of the test product. Improved skin elasticity is commonly associated with increased skin moisture levels, as also demonstrated by our results [36].
Loss of skin density is associated with skin aging, leading to thinner skin and decreased skin elasticity [37]. In this study, the skin density (SD, %) was determined with a skin scanner. The dermis is thicker and has a brighter green color when the SD value is higher, which suggests that the skin has better density. A lower SD score, on the other hand, denotes poor skin density and as a result, the dermis is thinner. As demonstrated by Figure 5c, we can conclude that the skin density improved significantly (p < 0.01) with a mean increase of 6.5% after 4 weeks of product use. The skin scanner images shown in Figure 5d also show that after application of the test product, the thickness of the dermis increased.
Facial skin redness often decreases self-esteem despite not being a life-threatening condition [38]. Consequently, it is imperative to address this cosmetic concern. The effectiveness of the test product to alleviate SLS-induced skin erythema was investigated on 20 female subjects and the data are presented in Table 7. a* describes a colour ranging from green (negative value) to red (positive value) [29]. Significant (p < 0.05) signs of improvement in skin redness (Figure 6a) and reduction in TEWL (Figure 6b) were evident after 2 weeks of use of the test product, when compared to the condition before using the product. Representative images from the clinical trial showing reduction in skin redness are provided in Figure 6c. The amount of water that passively diffuses from the underlying skin layers to the skin surface is measured by TEWL. It is frequently considered a vital indicator of skin integrity [39]. It has been reported that TEWL for intact skin ranges between 4 and 9 g/m2/h, and exposure to irritants results in increases of 2–3× baseline values [40,41]. Therefore, it can be concluded that the test product helps in improving the skin barrier and soothe the skin damaged by external stimuli after 2 weeks of use. In addition, there was a significant difference (p < 0.05) in TEWL between the treated and the non-treated areas after 2 weeks. No adverse skin reactions were reported throughout the study period, and no abnormalities were observed on physical examination by the dermatologist.

Table 7.
Measurement of skin redness and TEWL.
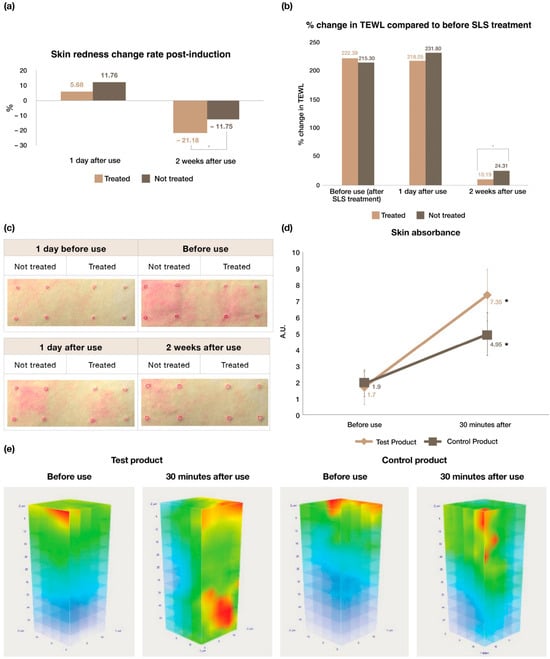
Figure 6.
Clinical efficacy evaluations of DermaLayr™ Marine+ Tighten: (a) % change in skin redness post-induction for the treated and non-treated areas; (b) % change in TEWL for the treated and non-treated areas; (c) representative images showing differences in reduction in skin redness for the treated and non-treated areas; (d) increase in skin absorbance of the test product compared to the control and (e) representative 3D Raman plots showing the increased skin absorbance of the test product compared to the control product (n = 20; * p < 0.05, indicate significant differences).
Not only was the test product effective in improving skin properties, but it also improved skin absorbance, as demonstrated by the clinical assessment of skin absorbance on 20 female subjects (Table 8). For example, skin absorbance was found to be significantly increased (p < 0.05) both for the test product group and the control group compared to before using the product (Figure 6d). Additionally, there was a significant difference (p < 0.05) in skin absorbance between the test product group and the control group. Compared to before use, the skin absorbance of the test product increased significantly (p < 0.05) by 449.667% after 30 min of product use. The control product group showed a significant increase (p < 0.05) of 198.333% when compared to before and 30 min after use. Representative 3D plots obtained using Raman microscopy are provided in Figure 6e.

Table 8.
Measurement of skin absorbance after application of the test product and control product.
3.4. In Vitro Skin Irritation Assessment
The RHE model, EpiDerm™ was used to assess the irritation potential of the test products in vitro. MTT assay was used to determine the viability of human epidermal cells, after being treated with three different NF products against positive and negative controls. Figure 7 shows the % viability of the Epiderm tissues, tested using the MTT assay.
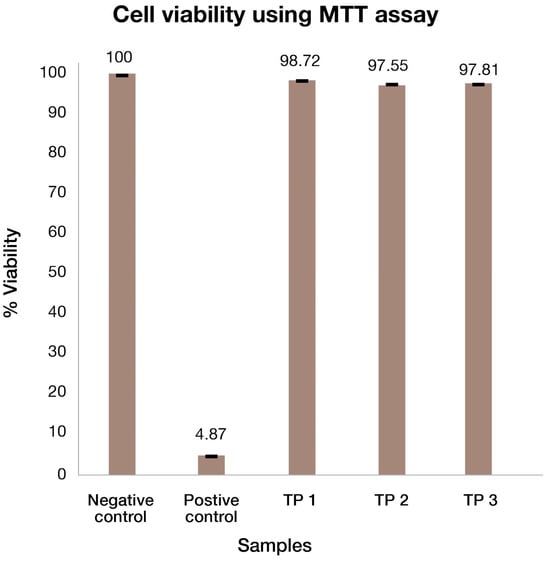
Figure 7.
MTT assay: cell viability is expressed as percentage (%) of viable RHE after being treated with different test products.
The percentage viability of positive and negative control was 4.87% and 100%, respectively. Overall cell viability of >95% is considered to show that there are no toxicity issues with skin tissue [42]. This test showed that all three NF products are not toxic to the cells and thus would not cause any toxicity to skin tissues. The studied DermaLayr™ NF products are, therefore, safe for use on human skin.
3.5. In Vivo Safety Evaluation
The chemical complexity of NF materials makes it possible for them to contain chemicals that could cause allergies or irritation. The evaluation of skin compatibility gives an indication of potential irritative and/or allergic processes, which are the primary concerns when evaluating the safety of new components for personal care products. The test product was found to be non-irritating when used on the test subjects. No adverse reactions were also reported during the trial period. Since no subject displayed any erythema, edema, or papules on their skin when exposed to the test products, the test products were deemed safe for use in topical applications.
4. Conclusions
In this research, novel NFs loaded with cosmetic ingredients were successfully developed and then investigated for their safety and efficacy. Skin permeation studies carried out using EpiDerm™ demonstrated the permeation of type I marine collagen into the dermis and epidermis layers of the skin, emphasizing the excellent skin absorptivity of the DermaLayr™ test products. Furthermore, the clinical assessment of the products on human subjects reinforced their excellent potential in cosmetic applications with a significant (p < 0.05) improvement in the appearance of fine lines, deep wrinkles, radiance, hydration, and increased firmness. Both in vitro and in vivo tests have demonstrated that the NF products are safe, they do not show cytotoxicity, and can be considered non-irritating. These results prove that DermaLayr™ effectively delivers collagen and actives into the skin for achieving enhanced dermatological benefits. This novel approach represents a safe, convenient, and effective way to address skin related concerns and improve appearance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cosmetics11010018/s1, Figure S1: Schematic representation of an EpiDerm™ tissue tested for skin permeation according to OECD 428 standards.
Author Contributions
Conceptualization, N.A.C.; methodology, N.A.C. and D.F.; validation, N.A.C.; investigation, N.A.C. and D.F.; resources, N.A.C. and D.F.; data curation, S.D.; writing—original draft preparation, S.D., N.A.C., and D.F.; writing—review and editing, S.D., N.A.C., and B.A.Y.-W.; visualization, S.D.; supervision, N.A.C. and B.A.Y.-W.; project administration, N.A.C. All authors have read and agreed to the published version of the manuscript.
Funding
Part of this research was funded by Cosmoreplus Co., Ltd. (191218).
Institutional Review Board Statement
The studies were conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of P&K Skin Research Center. Study 1: protocol code PNK-19D18-K1R and approved on 5 December 2019. Study 2: protocol code PNK-19D18-SB1R and approved on 5 December 2019. Study 3: protocol code PNK-19D18-RS1R and approved on 5 December 2019. Skin irritation study: protocol code PNK-20108-I7R and approved on 5 December 2019.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study, including the use of their photographs for publication.
Data Availability Statement
The data reported in this study are available on request.
Acknowledgments
The authors would like to acknowledge MS Clinical Research Pvt. Ltd. for successfully conducting the in vitro skin permeation and cytotoxicity studies. The authors would also like to thank PNK Skin Research Center for conducting the clinical studies. The authors would like to express their gratitude to New Zealand Trade and Enterprise (NZTE) and Callaghan Innovation for their support. The authors are also grateful to present and past staff at NanoLayr Ltd. for their contribution in the studies, and in the preparation of the manuscript. The authors would like to thank Kimberley Hassett and Mark Tongs for their kind assistance in graphical editing.
Conflicts of Interest
All the authors are employees of NanoLayr Limited, who has sponsored this study. The authors declare no other conflicts of interest.
References
- Kaul, S.; Gulati, N.; Verma, D.; Mukherjee, S.; Nagaich, U. Role of Nanotechnology in Cosmeceuticals: A Review of Recent Advances. J. Pharm. 2018, 2018, 3420204. [Google Scholar] [CrossRef]
- Raj, S.; Jose, S.; Sumod, U.; Sabitha, M. Nanotechnology in cosmetics: Opportunities and challenges. J. Pharm. Bioallied Sci. 2012, 4, 186–193. [Google Scholar] [CrossRef]
- Rodríguez, M.I.A.; Barroso, L.G.R.; Sánchez, M.L. Collagen: A review on its sources and potential cosmetic applications. J. Cosmet. Dermatol. 2017, 17, 20–26. [Google Scholar] [CrossRef]
- Gundloori, R.V.N.; Singam, A.; Killi, N. Nanobased Intravenous and Transdermal Drug Delivery Systems. In Applications of Targeted Nano Drugs and Delivery Systems: Nanoscience and Nanotechnology in Drug Delivery; Elsevier: Amsterdam, The Netherlands, 2018; pp. 551–594. [Google Scholar] [CrossRef]
- Zhang, Y.; Lim, C.T.; Ramakrishna, S.; Huang, Z.-M. Recent development of polymer nanofibers for biomedical and biotechnological applications. J. Mater. Sci. Mater. Med. 2005, 16, 933–946. [Google Scholar] [CrossRef]
- Kamble, P.; Sadarani, B.; Majumdar, A.; Bhullar, S. Nanofiber based drug delivery systems for skin: A promising therapeutic approach. J. Drug Deliv. Sci. Technol. 2017, 41, 124–133. [Google Scholar] [CrossRef]
- Reneker, D.H.; Chun, I. Nanometre diameter fibres of polymer, produced by electrospinning. Nanotechnology 1996, 7, 216–223. [Google Scholar] [CrossRef]
- Kim, C. Cosmetic Sheet Formed from Nanofiber with Controlled Dissolution Velocity and Method of Manufacturing the Same. U.S. Patent 14/437,234, 1 October 2015. [Google Scholar]
- Taepaiboon, P.; Rungsardthong, U.; Supaphol, P. Vitamin-Loaded Electrospun Cellulose Acetate Nanofiber Mats as Transdermal and Dermal Therapeutic Agents of Vitamin A Acid and Vitamin E. Eur. J. Pharm. Biopharm. 2007, 67, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, F.N.H.; Höfler, G.; Chand, N.A.; Beckermann, G.W. Electrospun Nanofibre Filtration Media to Protect against Biological or Nonbiological Airborne Particles. Polymers 2021, 13, 3257. [Google Scholar] [CrossRef]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-B.; Won, B.; Yang, S.-C.; Kim, D.-H. Asterias pectinifera derived collagen peptide-encapsulating elastic nanoliposomes for the cosmetic application. J. Ind. Eng. Chem. 2021, 98, 289–297. [Google Scholar] [CrossRef]
- Park, S.-H.; Song, T.; Bae, T.S.; Khang, G.; Choi, B.H.; Park, S.R.; Min, B.-H. Comparative analysis of collagens extracted from different animal sources for application of cartilage tissue engineering. Int. J. Precis. Eng. Manuf. 2012, 13, 2059–2066. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen—Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef]
- Epstein, E.; Munderloh, N. Human skin collagen. Presence of type I and type III at all levels of the dermis. J. Biol. Chem. 1978, 253, 1336–1337. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine Origin Collagens and Its Potential Applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef]
- Chung, J.H.; Seo, J.Y.; Choi, H.R.; Lee, M.K.; Youn, C.S.; Rhie, G.-E.; Cho, K.H.; Kim, K.H.; Park, K.C.; Eun, H.C. Modulation of Skin Collagen Metabolism in Aged and Photoaged Human Skin In Vivo. J. Investig. Dermatol. 2001, 117, 1218–1224. [Google Scholar] [CrossRef]
- Helfrich, Y.R.; Sachs, D.L.; Voorhees, J.J. Overview of Skin Aging and Photoaging. Dermatol. Nurs. 2008, 20, 177–183. [Google Scholar] [PubMed]
- Agustina, L.; Miatmoko, A.; Hariyadi, D.M. Challenges and strategies for collagen delivery for tissue regeneration. J. Public Health Afr. 2023, 14, 2505. [Google Scholar] [CrossRef]
- Sánchez-Carpintero, I.; Candelas, D.; Ruiz-Rodríguez, R. Dermal Fillers: Types, Indications, and Complications. Actas Dermo-Sifiliogr. 2010, 101, 381–393. [Google Scholar] [CrossRef]
- Ahmad, I.; Akhter, S.; Ahmad, M.Z.; Shamim, M.; Alam Rizvi, M.; Khar, R.K.; Ahmad, F.J. Collagen loaded nano-sized surfactant based dispersion for topical application: Formulation development, characterization and safety study. Pharm. Dev. Technol. 2013, 19, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Howes, D.; Guy, R.; Hadgraft, J.; Heylings, J.; Hoeck, U.; Kemper, F.; Maibach, H.; Marty, J.-P.; Merk, H.; Parra, J.; et al. Methods for Assessing Percutaneous Absorption. Altern. Lab. Anim. 1996, 24, 81–106. [Google Scholar] [CrossRef]
- OECD. Guidance Document for the Conduct of Skin Absorption Studies; OECD: Paris, France, 2004; ISBN 9789264078796. [Google Scholar]
- Neupane, R.; Boddu, S.H.; Renukuntla, J.; Babu, R.J.; Tiwari, A.K. Alternatives to Biological Skin in Permeation Studies: Current Trends and Possibilities. Pharmaceutics 2020, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Konstantinou, G.N. Enzyme-Linked Immunosorbent Assay (ELISA); StatPearls Publishing: Treasure Island, FL, USA, 2017; pp. 79–94. [Google Scholar]
- Van Merloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
- Gref, R.; Deloménie, C.; Maksimenko, A.; Gouadon, E.; Percoco, G.; Lati, E.; Desmaële, D.; Zouhiri, F.; Couvreur, P. Vitamin C–squalene bioconjugate promotes epidermal thickening and collagen production in human skin. Sci. Rep. 2020, 10, 16883. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Lee, M.R.; Kim, E.J.; Cho, J.C.; Lee, H.K. Comparison between Face Color Change and Its Recognition Difference on Asian: Korean, Indonesian and Vietnamian. J. Soc. Cosmet. Sci. Korea 2013, 39, 323–327. [Google Scholar]
- Saito, N.; Nishijima, T.; Fujimura, T.; Moriwaki, S.; Takema, Y. Development of a new evaluation method for cheek sagging using a Moire 3D analysis system. Ski. Res. Technol. 2008, 14, 287–292. [Google Scholar] [CrossRef]
- Borkow, G.; Elías, A.D.C. Facial Skin Lifting and Brightening Following Sleep on Copper Oxide Containing Pillowcases. Cosmetics 2016, 3, 24. [Google Scholar] [CrossRef]
- Aguirre-Cruz, G.; León-López, A.; Cruz-Gómez, V.; Jiménez-Alvarado, R.; Aguirre-Álvarez, G. Collagen Hydrolysates for Skin Protection: Oral Administration and Topical Formulation. Antioxidants 2020, 9, 181. [Google Scholar] [CrossRef]
- Berardesca, E.; Loden, M.; Serup, J.; Masson, P.; Rodrigues, L.M. The revised EEMCO guidance for the in vivo measurement of water in the skin. Ski. Res. Technol. 2018, 24, 351–358. [Google Scholar] [CrossRef]
- Banov, D.; Carvalho, M.; Schwartz, S.; Frumento, R. A randomized, double-blind, controlled study evaluating the effects of two facial serums on skin aging. Ski. Res. Technol. 2023, 29, e13522. [Google Scholar] [CrossRef]
- Woo, M.S.; Moon, K.J.; Jung, H.Y.; Park, S.R.; Moon, T.K.; Kim, N.S.; Lee, B.C. Comparison of skin elasticity test results from the Ballistometer® and Cutometer®. Ski. Res. Technol. 2014, 20, 422–428. [Google Scholar] [CrossRef]
- Yimam, M.; Lee, Y.-C.; Jiao, P.; Hong, M.; Brownell, L.; Jia, Q. A Randomized, Active Comparator-controlled Clinical Trial of a Topical Botanical Cream for Skin Hydration, Elasticity, Firmness, and Cellulite. J. Clin. Aesthetic Dermatol. 2018, 11, 51–57. [Google Scholar]
- Jasaitiene, D.; Valiukeviciene, S.; Linkeviciute, G.; Raisutis, R.; Jasiuniene, E.; Kazys, R. Principles of high-frequency ultrasonography for investigation of skin pathology. J. Eur. Acad. Dermatol. Venereol. 2010, 25, 375–382. [Google Scholar] [CrossRef]
- Huynh, T.T. Burden of Disease: The Psychosocial Impact of Rosacea on a Patient’s Quality of Life. Am. Health Drug Benefits 2013, 6, 348–354. [Google Scholar]
- Akdeniz, M.; Gabriel, S.; Lichterfeld-Kottner, A.; Blume-Peytavi, U.; Kottner, J. Transepidermal water loss in healthy adults: A systematic review and meta-analysis update. Br. J. Dermatol. 2018, 179, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Boer, M.; Duchnik, E.; Maleszka, R.; Marchlewicz, M. Structural and biophysical characteristics of human skin in maintaining proper epidermal barrier function. Postep. Dermatol. Alergol. 2016, 33, 1–5. [Google Scholar] [CrossRef]
- Kelchen, M.N.; Menon, G.; Eyck, P.T.; Prettypaul, D.; Brogden, N.K. A Pilot Study to Evaluate the Effects of Topically Applied Cosmetic Creams on Epidermal Responses. Ski. Pharmacol. Physiol. 2018, 31, 269–282. [Google Scholar] [CrossRef] [PubMed]
- López-García, J.; Lehocký, M.; Humpolíček, P.; Sáha, P. HaCaT Keratinocytes Response on Antimicrobial Atelocollagen Substrates: Extent of Cytotoxicity, Cell Viability and Proliferation. J. Funct. Biomater. 2014, 5, 43–57. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).