Abstract
Gamma−aminobutyric acid (GABA) presents several potential skin benefits, but its water−soluble nature poses challenges for effectively penetrating the skin to produce its effects. This research aimed to improve GABA’s skin penetration and availability by employing a nanoemulsion formulation, both with and without the aid of the penetration enhancer 1,8−cineole. Using a high−pressure homogenizer, an oil−in−water nanoemulsion was created, and its size and distribution were analyzed. The stability of these nanoemulsions was tested under various conditions, revealing their resilience over six months, even at elevated temperatures of 40 °C. In vitro tests on pig skin showed that GABA−loaded nanoemulsions, particularly those without the enhancer, demonstrated a nearly 2.89−fold increase in skin permeation compared to the solution form. Moreover, the addition of the enhancer amplified this effect, resulting in over a 3.37−fold increase in skin permeation compared to the solution. These results emphasize the potential of nanoemulsion formulations as effective tools for enhancing GABA’s skin permeation and availability, potentially expanding its use in dermatological applications. Further exploration and research are necessary to fully exploit GABA’s capabilities in supporting skin health and wellness.
1. Introduction
GABA, or gamma−aminobutyric acid (Figure 1), has emerged as a promising cosmeceutical active compound with multiple potential benefits for the skin. It enhances the skin’s barrier function by increasing the expression of key genes involved in the synthesis of hyaluronic acid and filaggrin protein, such as human beta−defensin−2 (HBD−2), human hyaluronan synthase (HAS1), and filaggrin [1]. To improve skin elasticity, GABA regulates the expression of genes related to type I collagen and inhibits matrix metalloproteinase−1 (MMP−1), as shown in human dermal fibroblasts [2]. Additionally, it could stimulate elastin synthesis, promote the formation of elastic fibers, and enhance the production of hyaluronic acid, thereby supporting the survival of dermal fibroblasts even under conditions of oxidative stress [3]. Notably, GABA has demonstrated its potential in wound healing by reducing inflammation, promoting the proliferation of fibroblast cells, and stimulating the expression of growth factors [4] and has shown potential in improving hyperpigmentation disorders [5]. Recently, GABA was found to be able to enhance filaggrin and aquaporin 3 in keratinocytes, showing that GABA can effectively improve skin moisturization [6]. These findings highlight the significant benefits of GABA for skincare applications.
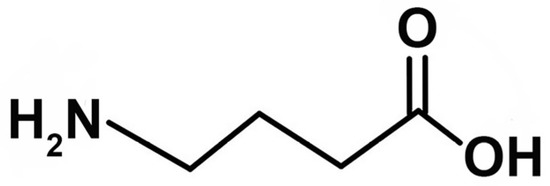
Figure 1.
Chemical structure of gamma−aminobutyric acid (GABA).
The efficacy of active compounds found in cosmetics or pharmaceuticals affecting skin function greatly relies on the molecules’ ability to penetrate through the skin barrier. GABA is an amino acid that contains four carbon atoms (Figure 1). GABA is a white powder and a hydrophilic molecule with a molecular weight of 103.12 g/moL, a partition coefficient (Log PO/W) of −3.17, and a water solubility of 1.3 g/mL. GABA is stable in aqueous solution at high temperatures up to 90 °C [7]. According to the skin permeability theory, GABA encounters difficulties in crossing the stratum corneum (SC), the primary obstacle to the percutaneous absorption of this external substance. The permeability of hydrophilic GABA through intact skin remains consistently low, thereby hindering the attainment of desired therapeutic benefits. Several approaches, including the utilization of nanotechnology, have been documented to enhance the transportation of water−soluble compounds to the skin. Nanoemulsions (NEs), characterized by their nano−sized droplets ranging from 20 to 200 nm, have gained great interest as an efficient means of drug delivery and as a premium option for personal care and cosmetic products. The oil−in−water (O/W) nanoemulsions, incorporating 5−aminolevulinic acid (5−ALA), an amino acid utilized in photodynamic therapy, demonstrated a significant 2.6−fold enhancement in 5−ALA flux through porcine skin compared to the aqueous solution [8]. Notably, O/W nanoemulsions showed larger particle sizes (216.2 to 256.3 nm) compared to water−in−oil (W/O) nanoemulsions (18.1 to 33.6 nm), but O/W nanoemulsions outperformed W/O nanoemulsions and aqueous solutions in terms of skin permeation. In this case, the O/W nanoemulsion not only demonstrated a clear advantage but also provided a pleasant skin sensation.
Soybean oil is chosen as the exclusive oil phase in our nanoemulsion formulations, with a concentration of 10% w/w. Lin et al. [9], in a review of the topical application of plant oils, highlighted that soybean oil has been extensively researched due to its favorable safety profile. In forearm skin applications, soybean oil extracts have demonstrated a reduction in transepidermal water loss (TEWL) and contributed to the restoration of the skin barrier [9]. As emulsifiers, a combination of nonionic surfactants, specifically polyoxyethylene sorbitan esters (Tween®) and sorbitan esters (Span®), has been chosen. These surfactants are widely employed in pharmaceutical emulsion formulations because of their comparatively low toxicity and minimal irritation potential [10].
Permeation enhancers have effectively been utilized to reversibly facilitate the passage of the active ingredient. Specifically, 1,8−cineole (eucalyptol) has demonstrated its ability to enhance the permeation of hydrophilic compounds in various studies [11,12,13]. For instance, caffeine nanoemulsions incorporating eucalyptol notably boosted caffeine permeation through both open hair follicles (49−fold) and untreated skin (42−fold) when compared to an aqueous solution [12]. This indicated that nanoemulsions with penetration enhancers not only increased permeation through the SC but also into and through the hair follicles. Similar findings were observed in a study conducted by the same research group [13], where eucalyptol nanoemulsions demonstrated enhanced minoxidil retention in both the SC and deeper layers of the skin. These studies demonstrate that the utilization of nanotechnology combined with penetration enhancement could elevate percutaneous permeation. This may be achieved by enhancing drug solubility within the stratum corneum (SC), primarily through the surfactants used in nanoemulsions. Additionally, it may involve disrupting the organization of SC lipids and augmenting their fluidity through a penetration enhancer.
While W/O nanoemulsions of 5−ALA were found to yield smaller nanoparticles compared to O/W nanoemulsions in the study mentioned by Zhang et al. [8], it was noted that the permeation of O/W nanoemulsions was more effective. This could be attributed to the drug’s hydrophilic nature, as it tends to remain in the aqueous phase rather than the oil phase, which it must pass through before entering the skin. Nevertheless, W/O nanoemulsions have also demonstrated success in delivering water−soluble substances, as reported by Abd et al. [12], possibly due to their oil−water partition coefficient. GABA, an amino acid like 5−ALA and possessing a Log Po/w value of −3.17, would anticipate experiencing the same behavior. Therefore, in this study, O/W nanoemulsion was chosen as the preferred formulation.
The primary objective of this study was to enhance the skin permeation capabilities of GABA, aiming for enhanced skin benefits. To achieve this goal, GABA−loaded nanoemulsions were formulated both with and without a penetration enhancer, namely 1,8 cineole, to attain physically and chemically stable nanoemulsions. Subsequently, these GABA−loaded nanoemulsions were subjected to physical and chemical characterization, stability assessment, and an evaluation of how the enhancer influenced its permeation property through newborn pig skin, using Franz diffusion cells.
2. Materials and Methods
2.1. Materials
4−Aminobutyric acid (GABA), 3−mercaptopropionic acid (MPA), and o−phthalaldehyde (OPA) were purchased from Merck (Darmstadt, Germany). 2−hydroxynaphthaldehyde (HN) was purchased from Sigma-Aldrich, Corporation (St. Louis, MO, USA). Tween® 80 (polyoxyethylene (20) sorbitan monooleate) was purchased from Croda (Thailand) Corporation, Limited (Bangkok, Thailand). Span® 80 (sorbitan oleate) was purchased from NOF Corporation (Tokyo, Japan). 1,8−cineole was purchased from beScents® (Chanjao Longevity Corporation, Limited. Bangkok, Thailand). All chromatography reagents were analytical reagent grade. Deionized water and ultrapure water were used in this study.
2.2. Methods
2.2.1. Nanoemulsions Preparation
Compositions of GABA−loaded nanoemulsions are presented in Table 1. The oil phase was composed of 10% w/w soybean oil and a 5% w/w surfactant mixture consisting of Tween® 80 and Span® 80 resulting in an HLB value of 8. When the formulation contained 1,8−cineole (5% w/w), it was blended with the oil phase, while GABA (10% w/w) was dissolved in the water phase. Coarse emulsions were prepared by combining the oil phase and water phase and subjecting them to high−speed homogenization (Ultra−Turrax® X1020, IKA Works (Thailand) Corporation, Limited, Bangkok, Thailand) at 5000 rpm for 5 min. Subsequently, this coarse emulsion was subjected to four passes through a high−pressure homogenizer (Microfluidizer® LM20, Microfluidics International Corporation, Westwood, MA, USA) at a pressure of 100 MPa, and an outlet cooler was employed to rapidly cool down the nanoemulsions to 25 °C [14]. The resulting nanoemulsions were allowed to equilibrate overnight at room temperature while being protected from light before undergoing characterization.

Table 1.
Compositions of GABA−loaded nanoemulsions.
2.2.2. Nanoemulsion Characterization
Size, Size Distribution, and Zeta−Potential
The mean droplet sizes, size distribution (polydispersity index, PDI), and zeta−potential were determined using dynamic light scattering (DLS) (Zetasizer Nano ZS™ instrument, Malvern Instruments, Malvern WR14 1XZ, UK). Each data point represents the average of 10 consecutive runs, each lasting 60 s. Prior to measurement, the nanoemulsions were diluted 1:100 with ultrapure water. The measurements were conducted in triplicate for each sample.
Viscosity Measurement
Approximately 30 mL of sample was measured in triplicate with a viscometer (Brookfield LVDV−II+, AMETEK (Thailand) Corporation, Limited, Bangkok, Thailand) using a S00 spindle at 25 ± 2 °C. The measurements were conducted in triplicate for each sample.
pH Measurement
The pH value of the nanoemulsion systems was measured by using a pH meter (SevenCompact™ pH/ion meter S220, Mettler Toledo, Greifensee, Switzerland) at 25 ± 2 °C. The measurements were conducted in triplicate for each sample.
% Loading Efficiency
The GABA−loaded nanoemulsions, both with and without a penetration enhancer, underwent a 500−fold dilution with methanol. Subsequently, the drug content was assessed through high−performance liquid chromatography (HPLC). The percentages of loading and labeled amount can be determined using the following equations:
2.2.3. HPLC Determination of GABA
GABA Derivatization
GABA was subjected to derivatization using either 2−hydroxynaphthaldehyde (HN) or o−phthalaldehyde (OPA) in conjunction with 3−mercaptopropionic acid (MPA) as previously described [15]. The specifics are outlined below.
- GABA−HN derivatization
A diluted sample for the assay was mixed with 0.6 mL of borate buffer (pH 8) and 1 mL of 2−hydroxynaphthaldehyde solution (0.3% w/v in methanol) at 80 °C for 10 min, then cooled down and protected from light for 10 min. The final volume was adjusted to 5 mL with methanol before HPLC analysis.
- GABA−OPA/MPA derivatization (for the samples from receptor compartment in in vitro skin permeation study)
A 500 µL volume of sample was mixed with 100 µL of methanolic OPA solution (25 µg/mL), 375 µL of borate buffer (pH 9.9), and 25 µL of MPA. The resulting solution was vortexed and then stored in the dark at room temperature for 1 min before analysis.
HPLC Analytical Method
Subsequently, the analysis and validation were conducted utilizing two distinct HPLC methods (Shimadzu Model SIL−20A, Shimadzu Corporation, Tokyo, Japan) as previously described [15]. The HPLC analysis method underwent validation following standard criteria for specificity, linearity, accuracy, and precision. The method was modified from ICH guideline Q2 (R1) [16].
- GABA−HN derivative
The following HPLC conditions were used to quantify the GABA−HN derivative and then to calculate the GABA amount. The HPLC conditions were described as follows.
| Column | Phenomenex Luna C18 (4.6 × 250 mm) |
| Mobile phase | methanol:water (66:34, v/v) |
| Injection volume | 5 µL |
| Flow rate | 0.8 mL/min |
| Detector | UV detector at 330 nm |
| Temperature | 25 °C |
| Duration | 20 min |
| Concentration range | 40–600 μg/mL |
- GABA−OPA/MPA derivative
The following HPLC conditions were used to quantify the GABA−OPA/MPA derivative. The HPLC conditions were described as follows.
| Column | Agilent Zorbax 300SB−C18 (4.6 × 150 mm) |
| Mobile phase | 0.05 M sodium acetate:tetrahydrofuran:methanol (50:1:49, v/v) pH 4.0 |
| Injection volume | 10 µL |
| Flow rate | 1.0 mL/min |
| Detector | Fluorescence detector |
| Excitation wavelength | 337 nm |
| Emission wavelength | 454 nm |
| Temperature | 25 °C |
| Duration | 10 min |
| Concentration range | 0.2–0.9 μg/mL |
2.2.4. Stability Testing
Physical Stability
All nanoemulsion formulations underwent centrifugation at 3500 rpm and 25 °C for 30 min to detect any phase separation. Only those formulations that exhibited no phase separation were subjected to further examination. To assess physical stability, a heating−cooling cycle was implemented by storing the nanoemulsions in a refrigerator at 4 °C for 48 h, followed by exposure to a hot air oven at 45 °C for 48 h, constituting one cycle. The average droplet size and PDI of all samples were determined at the conclusion of each cycle, with a total of six cycles being performed.
Chemical Stability
For chemical stability assessment, GABA−loaded nanoemulsions, both with and without the penetration enhancer, were placed in sealed glass vials and stored at 40 °C, shielded from light, for varying durations of 0, 1, 2, 3, and 6 months. The remaining percentage of GABA was quantified using the previously described high−performance liquid chromatography (HPLC) method.
2.2.5. In Vitro Skin Permeation Study
In this study, abdominal porcine skin from newborn pigs was utilized. The carcasses of newborn piglets that had perished at birth were acquired from a local pig slaughterhouse. Surgical scissors were employed to separate the epidermis and dermis layers, resulting in full−thickness skin samples. These skin samples were then wrapped in aluminum foil and stored at −20 °C until needed. When required, the frozen skin was thawed at room temperature, cut into 4 cm × 4 cm pieces, and hydrated in ultrapure water for 1 h prior to the experiment.
The permeation of GABA was assessed using Franz diffusion cells (FDC−6 Transdermal Diffusion Cell Drive System, Logan Instruments Corporation, Somerset, NJ, USA). The receptor chambers were maintained at a constant temperature of 37 °C using a circulating water jacket. The various test formulations, including a GABA solution (the control group), GABA−loaded nanoemulsions, and GABA−loaded nanoemulsions with a penetration enhancer, were applied to the skin surface. This study was performed in two conditions: non−occlusive application or occlusion using a parafilm plug lined with aluminum foil.
The receptor medium used was ultrapure water. At specified intervals, 1 mL of receptor medium was collected and replaced with an equal volume of fresh medium. After 24 h, the skin was removed, cleansed with methanol, cut into small pieces, and soaked in methanol to extract GABA from the skin. The cumulative amounts of GABA remaining on the skin after 24 h (Q24), in the receptor medium, and extracted from the skin (Qskin) were quantified using HPLC. This procedure was conducted with four replicates (n = 4).
The steady−state flux, (Jss, µg/cm2·h) was determined from the slope of the linear portion of the permeation profile, plotting the cumulative amount permeated per unit area (Q, µg/cm2) against time (h). The permeability coefficient (P) was calculated with the following equation:
The Jss, P, Q24, and Qskin of GABA−loaded nanoemulsions with and without a penetration enhancer were compared to the control group.
2.2.6. Statistical Analysis
Each experiment was conducted in triplicate (n = 3), and the results are presented as the mean ± standard deviation (SD). Mean values were subjected to a one−way analysis of variance (ANOVA) followed by the least significant difference (LSD) test using IBM SPSS Statistics for Windows, Version 28.0. (IBM Corp SPSS software, Armonk, NY, USA). Statistical significance was determined when p < 0.05.
3. Results
3.1. Nanoemulsion Preparation and Characterization
3.1.1. Size, Size Distribution, and Zeta−Potential
The high−pressure homogenizer effectively prepared nanoemulsions, which, following the preparation process and overnight equilibration, appeared as white liquids with a bluish tint and droplets smaller than 200 nm. The GABA nanoemulsion demonstrated Newtonian flow and a viscosity value of 4.12 ± 0.04 centipoises, and this viscosity remained consistent across all formulated variations. All blank formulations exhibited a narrow size distribution of droplets, as shown in Table 2. This indicated that the high−energy process parameters, such as the pressure and number of cycles, efficiently produced soybean oil nanoemulsions with a uniform dispersion of droplet sizes. Table 2 reveals that the droplet sizes of GABA−loaded nanoemulsions, both with and without the penetration enhancer, were smaller than those of the blank nanoemulsion (130.7 ± 0.6 nm and 131.1 ± 1.4 nm versus 169.4 ± 1.1 nm, respectively, p < 0.05). This reduction in droplet size may be attributed to the emulsifying properties of amino acids like GABA. However, there was no significant difference in droplet sizes between GABA−loaded nanoemulsions with and without the penetration enhancer (p > 0.05). The zeta−potential of blank and GABA−loaded nanoemulsions was about −40 mV (Table 2) and pH was neutral (i.e., pH 7.33 ± 0.01).

Table 2.
The droplet size, size distribution (PDI), and zeta−potential of blank nanoemulsion and the GABA−loaded nanoemulsions presented as mean ± SD (n = 3).
3.1.2. % Loading Efficiency
In comparison to 10.04 ± 0.08% for the solution, the percentage of GABA loading was 9.11 ± 0.07% and 9.11 ± 0.09% with and without penetration enhancer nanoemulsions, respectively. The GABA solution, which was not processed through the Microfluidizer®, attained a labeled amount of 100.03 ± 0.70%. In contrast, the GABA−loaded nanoemulsions, both with and without the enhancer, that underwent Microfluidizer® treatment only reached 91.04 ± 0.69% and 90.88 ± 0.75%, respectively. The rigorous conditions involving high pressure and heat in the preparation process suggested potential partial loss of amino acids like GABA. An additional quantity might be necessary to achieve 100% of the labeled amount.
3.2. Nanoemulsion Stability Studies
3.2.1. Physical Stability
After centrifugation, none of the formulations showed signs of phase separation and all successfully passed the initial screening. The nanoemulsions underwent testing through multiple heating−cooling cycles, and the results showed that the average droplet sizes and PDI values for all formulations remained constant, as depicted in Figure 2.
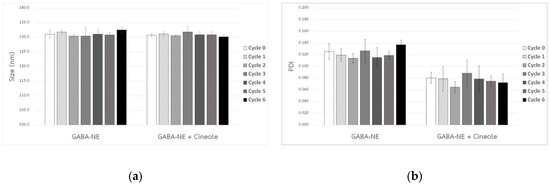
Figure 2.
Droplet size (a) and PDI value (b) of GABA−loaded nanoemulsions, both with and without the penetration enhancer, following multiple heating–−cooling cycles (mean ± SD, n = 3).
The Ostwald ripening rates (ω) for GABA−loaded nanoemulsions, with and without the penetration enhancer, were virtually negligible when stored at 40 °C, as indicated by the nearly zero slopes (0.0001 and −0.0002 nm³/h) in Figure 3. When compared to their initial sizes, the droplet sizes of these GABA−loaded nanoemulsions did not alter, either with or without the penetration enhancer (Figure 4a). Furthermore, as shown in Figure 4b, both variations of the GABA−loaded nanoemulsions showed a narrow size distribution of droplets (PDI). These findings confirm that all GABA−loaded nanoemulsions exhibited exceptional physical stability when stored for a minimum of six months at 40 °C.
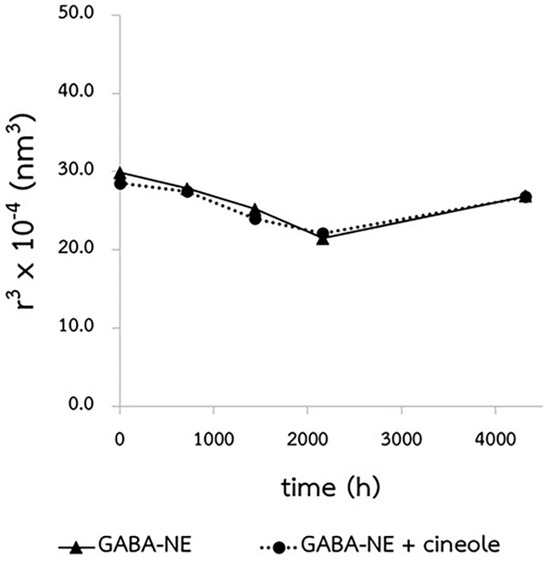
Figure 3.
Ostwald ripening plot of the GABA−loaded nanoemulsions with and without the penetration enhancer, conducted at 40 °C.
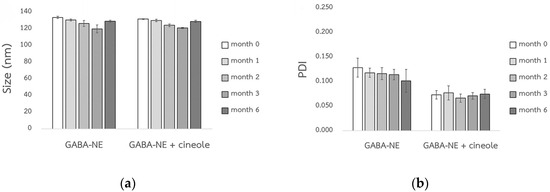
Figure 4.
Droplet size (a) and PDI value (b) of the GABA−loaded nanoemulsions, both with and without the penetration enhancer, following storage at 40 °C (mean ± SD, n = 3).
3.2.2. Chemical Stability
Under accelerated conditions (40 °C), the formulations showed a gradual decline in the remaining percentage of GABA, as depicted in Figure 5. After 6 months, the remaining GABA percentages in the solution and the GABA−loaded nanoemulsions with and without the enhancer dropped to 90.38 ± 2.06%, 91.65 ± 2.93%, and 86.54 ± 1.69%, respectively. However, the high percentage of retained drug labels suggested acceptable chemical stability. This reduction might be linked to potential GABA degradation induced by long−term heat exposure, a phenomenon previously documented [17].
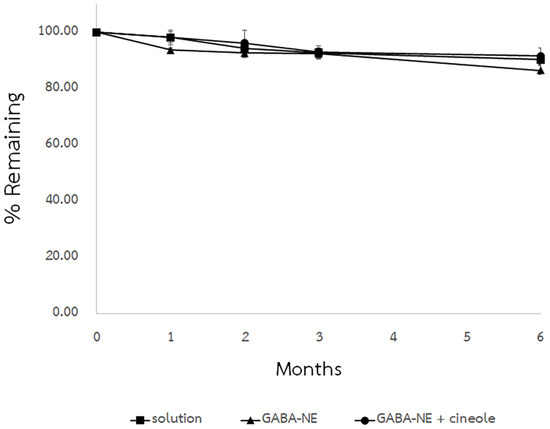
Figure 5.
Chemical stabilities of GABA solution and GABA−loaded nanoemulsions with and without the penetration enhancer, conducted at 40 °C (mean ± SD, n = 3).
3.3. In Vitro Skin Permeation Study
The skin permeation of GABA solution, GABA−loaded nanoemulsions, and GABA−loaded nanoemulsions with the penetration enhancer was assessed using Franz diffusion cells with full−thickness abdominal porcine skin. Recovery percentages for all formulations ranged from 91.32% to 96.78%, indicating the suitability of the method. GABA amounts remaining on the skin surface (donor part) and extracted from the skin were determined via HPLC using the GABA−HN derivative method. The GABA quantities are displayed as % GABA concerning the applied amount on the skin surface in Table 3 and Figure 6. Across all tested formulations, the % GABA remaining in the donor compartment exhibited no notable differences (p > 0.05). Nonetheless, a lower % GABA was noted in the donor chamber for all GABA−loaded nanoemulsions in both non−occlusive and occlusive applications compared to the solution.

Table 3.
In vitro skin permeation of GABA at 24 h (mean ± SD, n = 4).
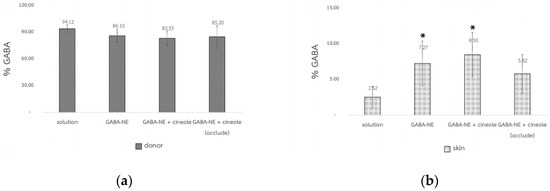
Figure 6.
The GABA percentage in the donor compartment (Q24) (a) and in the skin (Qskin) at 24 h (b) (mean ± SD, n = 4), * p < 0.05 significantly different from the solution.
The GABA content that permeated into the receptor chamber was analyzed by HPLC using the GABA−OPA/MPA derivative method, chosen for its higher sensitivity compared to the GABA−HN derivative method as noted by [15]. GABA was detectable in the receptors for all tested formulations (Figure 7), indicating its ability to permeate the skin. However, the amounts of detected GABA were below the LOQ (0.02 µg/mL), rendering accurate calculations for the reported values of permeability coefficient (P) and flux (Jss) unfeasible. All formulations had similar permeability characteristics (Figure 7), highlighting that GABA−loaded nanoemulsions with 1,8−cineole exhibited the highest skin permeation under occlusive conditions.
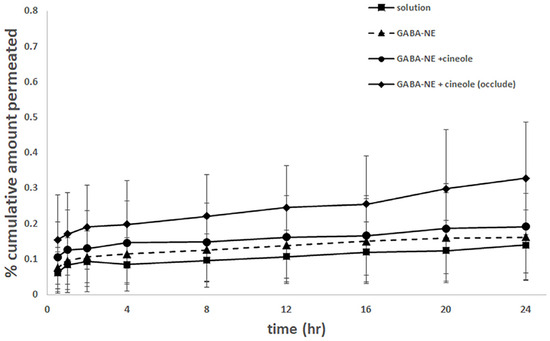
Figure 7.
The permeation profiles of GABA solution, GABA−loaded nanoemulsions, GABA−loaded nanoemulsions with the penetration enhancer, and GABA−loaded nanoemulsions with the penetration enhancer under occlusion (mean ± SD, n = 4).
4. Discussion
Stable nanoemulsions were formed by blending hydrophilic (Tween®) and lipophilic (Span®) surfactants to achieve an optimal HLB of 8. In comparison to the blank nanoemulsion, both GABA−loaded nanoemulsions, with or without the penetration enhancer, displayed smaller droplet sizes. Yiase [18] demonstrated the potential of amino acids as emulsifiers in oil/water emulsions, enhancing emulsion stability by prolonging phase separation time. This aligns with observations by Thaiphanit and Anprung [19], who found that edible protein concentrate from coconut, rich in various amino acids, exhibited similar emulsifying effects by notably reducing surface and interfacial tension in natural oil−water blends.
The % GABA content in the skin indicated that GABA−loaded nanoemulsions, both without and with the penetration enhancer (in non−occlusive and occlusive applications), enhanced GABA penetration into the skin by 2.88, 3.37, and 2.31 times, respectively, compared to the solution. This might be attributed to their smaller droplet sizes and significantly larger surface area, potentially facilitating enhanced absorption into the stratum corneum compared to larger droplets [20,21]. Additionally, soybean oil, a component used in the oil phase, contains fatty acids like linoleic acid and oleic acid, known for their penetration−enhancing properties [22,23], suggesting that soybean oil could contribute as a penetration enhancer. An additional potential mechanism might involve GABA interacting within the interfacial layer of the nanoemulsions by forming hydrogen bonds with the poly(oxyethylene) units present in the nonionic surfactants investigated (Figure 8). This interaction could potentially aid in the concurrent penetration of both oil droplets and GABA through the skin [24].
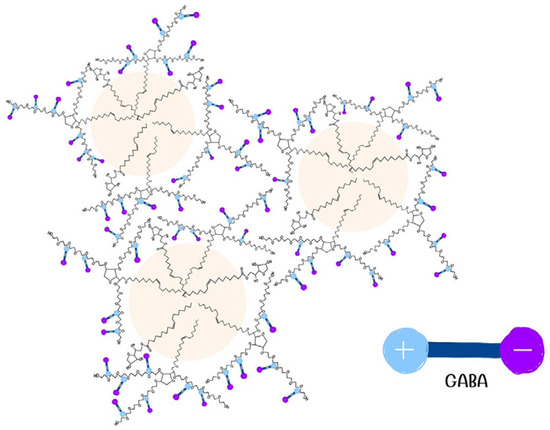
Figure 8.
Electrostatic interaction of GABA with nonionic surfactants. The negative end of GABA (−COO−) is represented by a purple circle with a minus sign, and the positive end (−NH3+) by a blue circle with a plus sign.
Dash et al. [25] investigated the interaction between glycine, an amino acid, and two nonionic surfactants featuring a nonylphenyl hydrocarbon chain along with hydrophilic heads containing 15 and 20 oxyethylene units, respectively. The introduction of glycine to these nonionic surfactants resulted in a more negatively charged zeta potential. The researchers hypothesized that the positive end of glycine (−NH3+) could potentially interact with the hydrophilic section of the nonionic surfactant (oxyethylene chain), causing the exposure of its negative charge (−COO−) to the external side. This interaction led to a more negative zeta potential value. Given that GABA−loaded nanoemulsions exhibited a zeta potential of −40 mV (Table 2), a similar reaction could be inferred as shown in Figure 8. This interaction may enhance skin permeation when the nanoemulsion comes into contact with the skin, providing one plausible explanation.
The % GABA extracted from the skin of the GABA−loaded nanoemulsion with the penetration enhancer in non−occlusive application (8.50 ± 3.13%) showed a significant increase compared to the solution (2.52 ± 1.53%) (p < 0.05), though it was not significantly higher than that of the GABA−loaded nanoemulsion without the enhancer (7.27 ± 3.15%). The compound 1,8−cineole, an oxide terpene, has been noted to potentially disrupt the lipid structure of the stratum corneum, thereby reducing the skin barrier [26]. The skin permeation of GABA from the nanoemulsion may be marginally improved by the permeation enhancer, though. In this case, using nanoemulsions was the primary method to improve skin penetration of amino acids such as GABA. This finding aligns with Zhang et al. [8], where 5−ALA loaded in O/W nanoemulsions utilizing α−terpineol as an enhancer did not show significant enhancement in skin permeation. This lack of enhancement might be due to the enhancer being in the inner phase, unable to directly interact with the lipid present in the stratum corneum.
Regarding the occlusive experiments, a thin occlusive film of GABA−loaded nanoemulsions and a parafilm plug lined with aluminum foil protected water and the enhancer from evaporation. Water could potentially hydrate the skin, while 1,8−cineole might disrupt the skin structure, resulting in higher GABA amounts detected in the receptor compared to other conditions. Additionally, the occlusive effect might reduce the skin’s barrier function [27], facilitating GABA permeation. However, the % GABA extracted from the skin with GABA−loaded nanoemulsions with the penetration enhancer in both non−occlusive and occlusive applications did not exhibit significant differences (p > 0.05).
5. Conclusions
Both the GABA−loaded nanoemulsions, with and without the penetration enhancer, maintained their physical stability after undergoing heating−cooling cycles and showed satisfactory chemical stability during storage at 40 °C for a duration of 6 months. In the in vitro skin permeation study, the GABA−loaded nanoemulsions along with the enhancer displayed the most significant enhancement compared to the solution. Nanoemulsions, owing to their nano−sized structure, were primarily intended to efficiently facilitate the movement of active compounds into the skin. This was attributed to their small droplet sizes and notably large surface area, allowing for enhanced absorption into the stratum corneum. Meanwhile, the addition of 1,8−cineole as a penetration enhancer exhibited slight improvements in both non−occlusive and occlusive applications. In the future, developing GABA nanoemulsions with or without a permeation enhancer should be further investigated in clinical studies for their safety and effectiveness.
6. Patents
This research endeavor has been officially patented under the Thai Pretty Patent number 21982, granted on 30 June 2023.
Author Contributions
Conceptualization, methodology, funding acquisition, supervision, writing—original draft preparation, D.C.; methodology; investigation, formal analysis, visualization, K.S.; conceptualization, methodology, formal analysis, resources, funding acquisition, writing—review and editing, supervision, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
The research has been funded by a scholarship from Chulalongkorn University’s Cosmetic Science Graduate Program.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The corresponding author can provide the data from this study upon request. Because of an intellectual property dispute, the data are not publicly accessible.
Acknowledgments
The authors acknowledge the Department of Pharmaceutics and Industrial Pharmacy, Faculty of Pharmaceutical Sciences, Chulalongkorn University, for its administrative support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; De Angelis, M.; Giuliani, G.; Meloni, M.; De Servi, B.; Gobbetti, M. Synthesis of Gamma−Aminobutyric Acid (GABA) by Lactobacillus plantarum DSM19463: Functional Grape must Beverage and Dermatological Applications. Appl. Microbiol. Biotechnol. 2010, 86, 731–741. [Google Scholar] [CrossRef]
- Uehara, E.; Hokazono, H.; Sasaki, T.; Yoshioka, H.; Matsuo, N. Effects of GABA on the Expression of Type I Collagen Gene in Normal Human Dermal Fibroblasts. Biosci. Biotechnol. Biochem. 2017, 81, 376–379. [Google Scholar] [CrossRef]
- Ito, K.; Tanaka, K.; Nishibe, Y.; Hasegawa, J.; Ueno, H. GABA−Synthesizing Enzyme, GAD67, from Dermal Fibroblasts: Evidence for a New Skin Function. Biochim. Biophys. Acta (BBA)−Gen. Subj. 2007, 1770, 291–296. [Google Scholar] [CrossRef]
- Han, D.; Kim, H.-Y.; Lee, H.-J.; Shim, I.; Hahm, D.-H. Wound Healing Activities of Gamma−Aminobutyric acid (GABA) in Rats. J. Microbiol. Biotechnol. 2007, 17, 1661–1669. [Google Scholar]
- Molagoda, I.M.N.; Kavinda, M.H.D.; Ryu, H.W.; Choi, Y.H.; Jeong, J.-W.; Kang, S.; Kim, G.-Y. Gamma−Aminobutyric Acid (GABA) Inhibits α−Melanocyte−Stimulating Hormone−Induced Melanogenesis through GABAA and GABAB Receptors. Int. J. Mol. Sci. 2021, 22, 8257. [Google Scholar] [CrossRef]
- Zhao, H.; Park, B.; Kim, M.J.; Hwang, S.H.; Kim, T.J.; Kim, S.U.; Kwon, I.; Hwang, J.S. The Effect of γ−Aminobutyric Acid Intake on UVB− Induced Skin Damage in Hairless Mice. Biomol. Ther. 2023, 31, 640–647. [Google Scholar] [CrossRef]
- Le, P.H.; Le, T.T.; Raes, K. Effects of pH and Heat Treatment on the Stability of γ−Aminobutyric Acid (GABA) in Germinated Soymilk. J. Food Process. Preserv. 2020, 44, e14301. [Google Scholar] [CrossRef]
- Zhang, L.-W.; Al-Suwayeh, S.A.; Hung, C.-F.; Chen, C.-C.; Fang, J.-Y. Oil Components Modulate the Skin Delivery of 5−Aminolevulinic Acid and Its Ester Prodrug from Oil−in−Water and Water−in−Oil Nanoemulsions. Int. J. Nanomed. 2011, 6, 693–704. [Google Scholar] [CrossRef]
- Lin, T.K.; Zhong, L.; Santiago, J.L. Anti−Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, J.; Singh, H. Microemulsions: A Potential Delivery System for Bioactives in Food. Crit. Rev. Food Sci. Nutr. 2006, 46, 221–237. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, B.S.M.; Hasani, M. The Effect of Chemical and Physical Enhancers on Trolamine Salicylate Permeation through Rat Skin. Trop. J. Pharm. Res. 2011, 9, 541–548. [Google Scholar] [CrossRef]
- Abd, E.; Benson, H.A.E.; Roberts, M.S.; Grice, J.E. Follicular Penetration of Caffeine from Topically Applied Nanoemulsion Formulations Containing Penetration Enhancers: In Vitro Human Skin Studies. Skin Pharmacol. Physiol. 2018, 31, 252–260. [Google Scholar] [CrossRef]
- Abd, E.; Benson, H.A.E.; Roberts, M.S.; Grice, J.E. Minoxidil Skin Delivery from Nanoemulsion Formulations Containing Eucalyptol or Oleic acid: Enhanced Diffusivity and Follicular Targeting. Pharmaceutics 2018, 10, 19. [Google Scholar] [CrossRef]
- Singpanna, K.; Charnvanich, D.; Panapisal, V. Effect of the Hydrophilic−Lipophilic Balance Values of Non−Ionic Surfactants on Size and Size Distribution and Stability of Oil/Water Soybean Oil Nanoemulsions. Thai J. Pharm. Sci. 2021, 45, 487–491. [Google Scholar]
- Panrod, K.; Tansirikongkol, A.; Panapisal, V. Comparison of Validated High Performance Liquid Chromatography Methods using Two Derivatizing Agents for Gamma−Aminobutyric Acid Quantification. Thai J. Pharm. Sci. 2016, 40, 203–208. [Google Scholar]
- Borman, P.; Elder, D. Q2(R1) Validation of Analytical Procedures. In ICH Quality Guidelines; The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, 2017; pp. 127–166. [Google Scholar]
- Khan, W.; Bhatt, P.C.; Panda, B. Degradation Kinetics of Gamma Amino Butyric Acid in Monascus−Fermented Rice. J. Food Qual. 2015, 38, 123–129. [Google Scholar] [CrossRef]
- Yiase, S.G. Amino Acids as Potential Emulsifiers in Stabilizing Oil/Water Emulsions. Int. J. Innov. Sci. Res. 2015, 15, 409–414. [Google Scholar]
- Thaiphanit, S.; Anprung, P. Physicochemical and Emulsion Properties of Edible Protein Concentrate from Coconut (Cocos nucifera L.) Processing By−Products and the Influence of Heat Treatment. Food Hydrocoll. 2016, 52, 756–765. [Google Scholar] [CrossRef]
- Kotyla, T.; Kuo, F.; Moolchandani, V.; Wilson, T.; Nicolosi, R. Increased Bioavailability of a Transdermal Application of a Nano−Sized Emulsion Preparation. Int. J. Pharm. 2008, 347, 144–148. [Google Scholar] [CrossRef]
- Sutradhar, K.B.; Amin, M.L. Nanoemulsions: Increasing Possibilities in Drug Delivery. Eur. J. Nanomed. 2013, 5, 97–110. [Google Scholar] [CrossRef]
- Baughman, W.F.; Jamieson, G.S. The Chemical Composition of Soya Bean Oil. J. Am. Chem. Soc. 1922, 44, 2947–2952. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Del. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Shaker, D.S.; Ishak, R.A.H.; Ghoneim, A.; Elhuoni, M.A. Nanoemulsion: A Review on Mechanisms for the Transdermal Delivery of Hydrophobic and Hydrophilic Drugs. Sci. Pharm. 2019, 87, 17. [Google Scholar] [CrossRef]
- Dash, U.; Meher, J.; Misra, P.K. Organization of Amphiphiles, Part XII: Studies on the Interaction of Glycine with Aqueous Micelles of Polyoxyethylated Nonyl Phenols. J. Mol. Liq. 2013, 177, 317–324. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.D.; Chai, Y.P.; Zhang, H.; Peng, P.; Yang, X.X. Natural Terpenes as Penetration Enhancers for Transdermal Drug Delivery. Molecules 2016, 21, 1709. [Google Scholar] [CrossRef]
- Agner, T.; Serup, J. Time Course of Occlusive Effects on Skin Evaluated by Measurement of Transepidermal Water Loss (TEWL) Including Patch Tests with Sodium lauryl sulphate and Water. Contact Dermat. 1993, 28, 6–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).