Natural Deep Eutectic Solvents as a Novel Bio-Based Matrix for Ready-to-Use Natural Antioxidants-Enriched Ingredients: Extraction and Formulation Optimization
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Biomass
2.3. NaDES Preparation
2.4. Calendula Extraction
2.4.1. NaDES Screening
2.4.2. Process Optimization Using the Design of Experiment Approach
2.4.3. Extraction Scale-Up
2.5. Extract Analysis
2.5.1. Total Carotenoid Content (TCC)
2.5.2. Total Flavonoid Content (TFC)
2.5.3. High-Performance Liquid Chromatography (HPLC)
2.6. Cream Formulation
2.7. Static Multiple Light Scattering-Based Stability Analysis
2.8. Rheology
2.9. Statistical Analysis
3. Results and Discussions
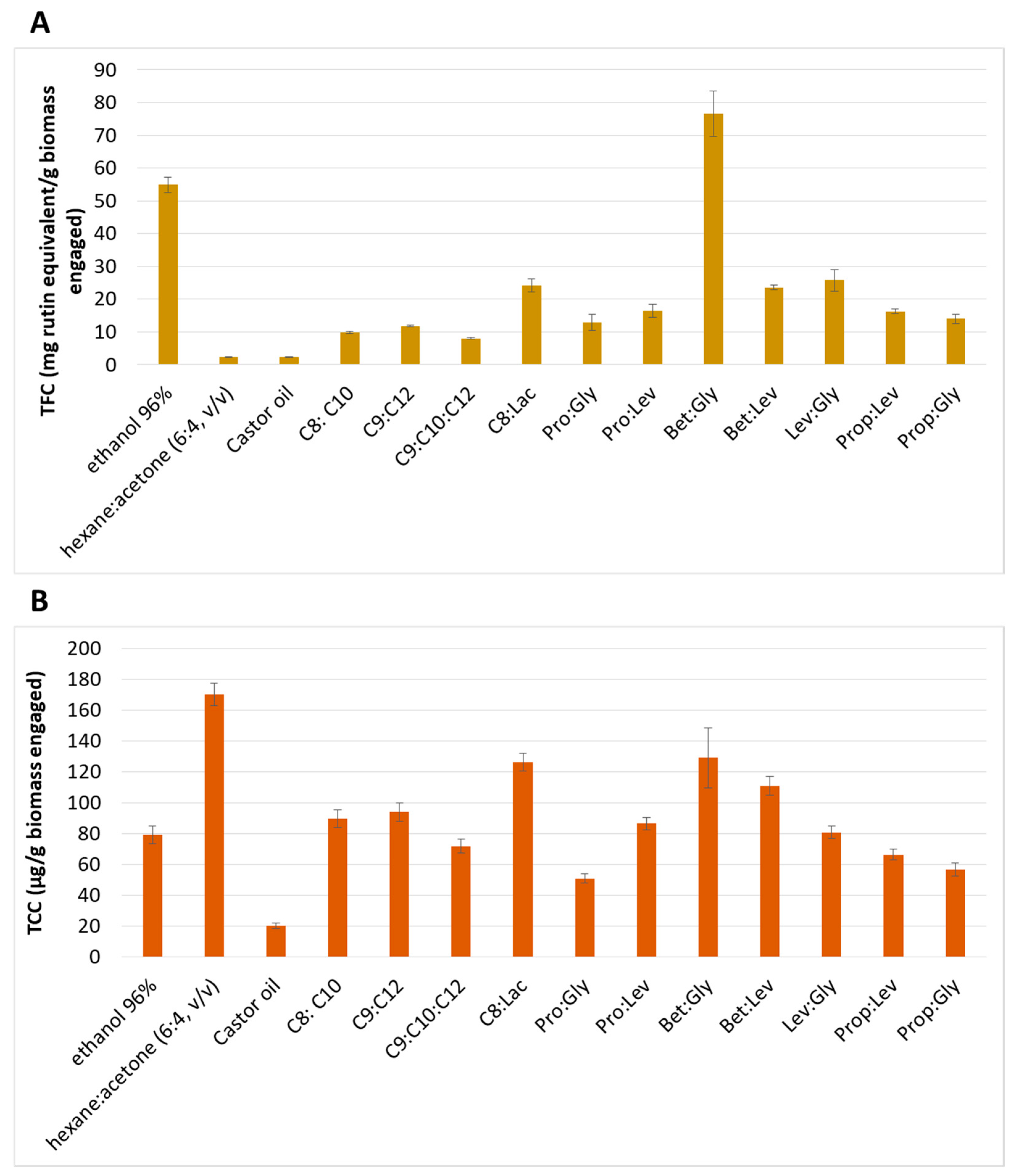
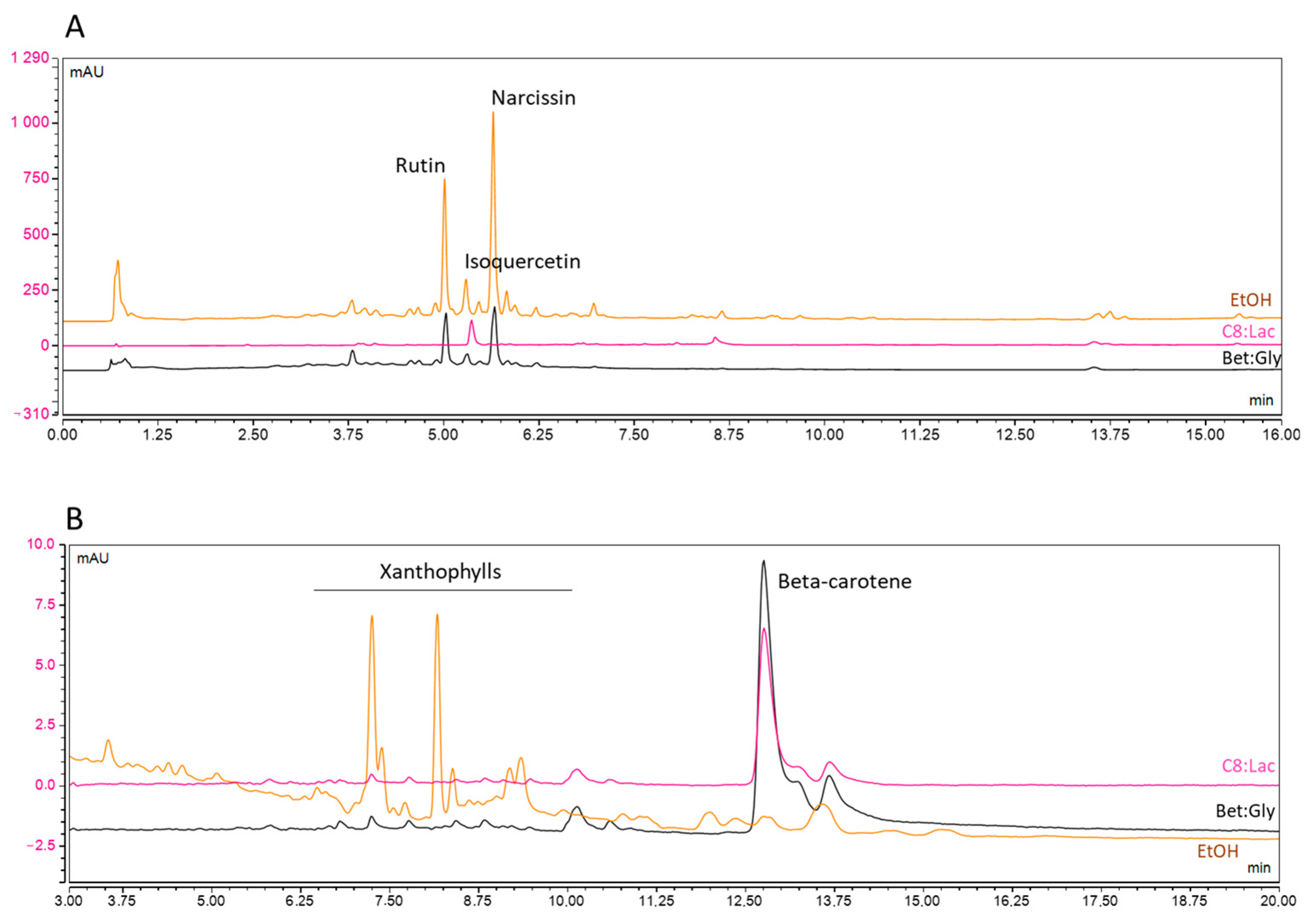
3.1. NaDES Screening
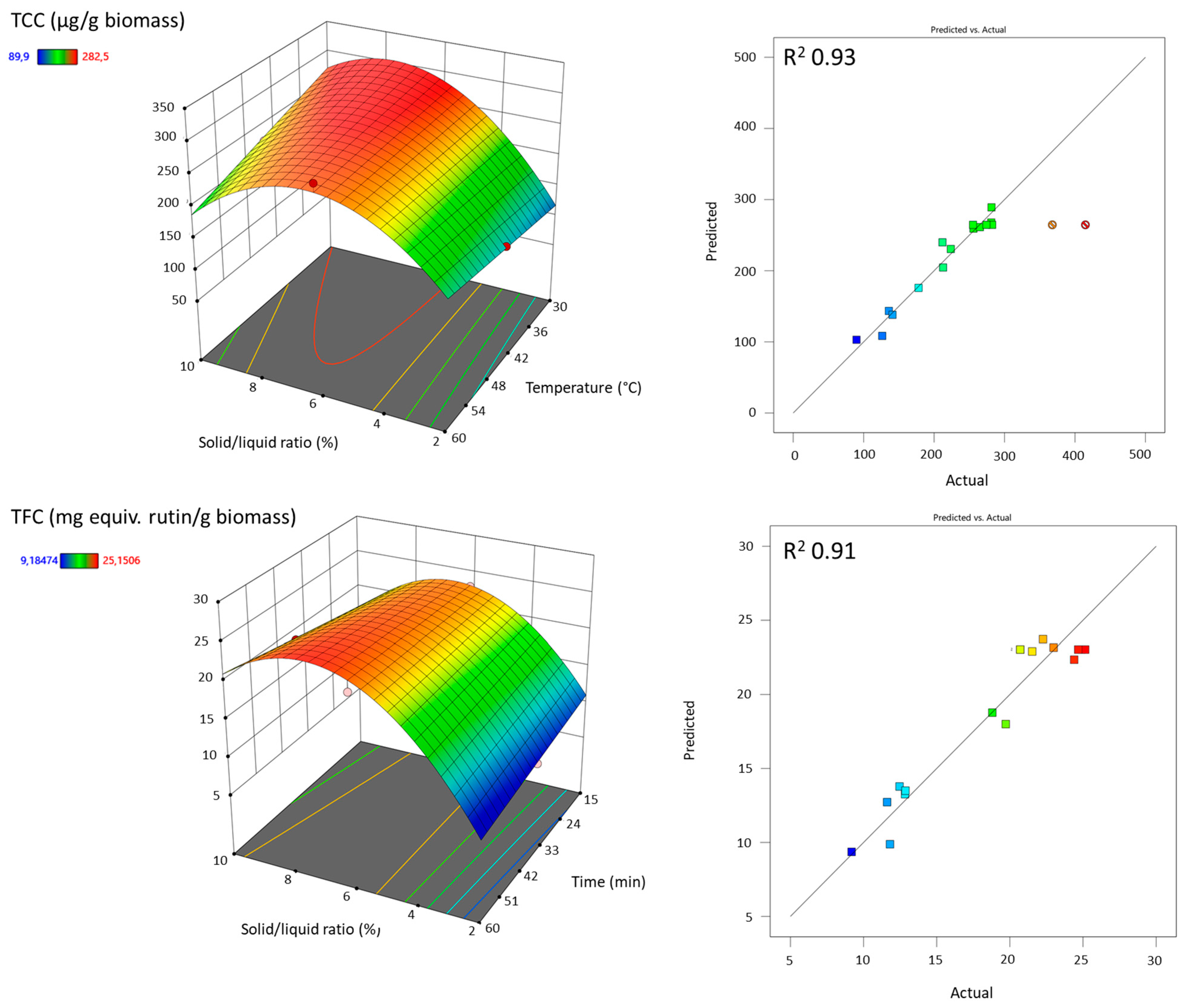
3.2. Extraction Optimization Using the Design of Experiment Approach
3.2.1. Ultrasound-Assisted Extraction (UAE)
Temperature − 0.680000 Time − 0.516241 solid/liquid ratio × Temperature +
0.010278 solid/liquid ratio × Time − 5.88146 solid/liquid ratio2
0.234605 Temperature − 0.118983 Time − 0.043623 Solid/liquid ratio ×
Temperature + 0.021898 Solid/liquid ratio × Time − 0.586024 Solid/liquid
ratio2
3.2.2. Dual Asymmetric Centrifugation (DAC)
+ 0.050855 − 0.003535 Speed solid/liquid ratio × Speed − 0.000865 Speed ×
Time − 7.81179 solid/liquid ratio2
0.524081 Time + 0.034061 Solid/liquid ratio × Time − 0.734255 Solid/liquid
ratio2 − 0.011086 Time2
3.3. Cosmetic Formulation of Bet:Gly Calendula Extract
3.3.1. Cream Preparation
3.3.2. Cream pH Measurements
3.3.3. Static Multiple Light Scattering-Based Stability Analysis
3.3.4. Rheology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M. Plant Extracts as Skin Care and Therapeutic Agents. Int. J. Mol. Sci. 2023, 24, 15444. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kucharska, E.; Kowalczyk, T.; Zajdel, K.; Cegliński, T.; Zajdel, R. Antioxidant Properties of Plant-Derived Phenolic Compounds and Their Effect on Skin Fibroblast Cells. Antioxidants 2021, 10, 726. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Escriche, I. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res. Int. 2015, 74, 10–36. [Google Scholar] [CrossRef] [PubMed]
- Shahane, K.; Kshirsagar, M.; Tambe, S.; Jain, D.; Rout, S.; Ferreira, M.K.M.; Mali, S.; Amin, P.; Srivastav, P.P.; Cruz, J.; et al. An Updated Review on the Multifaceted Therapeutic Potential of Calendula officinalis L. Pharmaceuticals 2023, 16, 611. [Google Scholar] [CrossRef] [PubMed]
- Balázs, V.L.; Gulyás-Fekete, G.; Nagy, V.; Zubay, P.; Szabó, K.; Sándor, V.; Agócs, A.; Deli, J. Carotenoid Composition of Calendula officinalis Flowers with Identification of the Configuration of 5,8-Epoxy-carotenoids. ACS Agric. Sci. Technol. 2023, 3, 1092–1102. [Google Scholar] [CrossRef]
- Selvamuthukumaran, M.; Shi, J. Recent advances in extraction of antioxidants from plant by-products processing industries. Food Qual. Saf. 2017, 1, 61–81. [Google Scholar] [CrossRef]
- Jeong, K.M.; Zhao, J.; Ko, J.; Yoo, Y.; Jin, D.E.; Han, S.Y.; Lee, J. Multi-functioning deep eutectic solvents as extraction and storage media for bioactive natural products that are readily applicable to cosmetic products. J. Clean. Prod. 2017, 151, 87–95. [Google Scholar] [CrossRef]
- Dai, Y.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as a New Extraction Media for Phenolic Metabolites in Carthamus tinctorius L. Anal. Chem. 2013, 85, 6272–6278. [Google Scholar] [CrossRef]
- Afonso, J.; Mezzetta, A.; Marrucho, I.M.; Guazzelli, L. History repeats itself again: Will the mistakes of the past for ILs be repeated for DESs? From being considered ionic liquids to becoming their alternative: The unbalanced turn of deep eutectic solvents. Green Chem. 2023, 25, 59–105. [Google Scholar] [CrossRef]
- Choi, H.; van Spronsen, J.; Dai, Y.; Verberne, M.; Hollmann, F.; Arends, I.W.C.E.; Witkamp, G.-J.; Ver-Poorte, R. Are Natural Deep Eutectic Solvents the Missing Link in Understanding Cellular Metabolism and Physiology? Plant Physiol. 2011, 156, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; van Spronsen, J.; Witkamp, G.-J.; Verpoorte, R.; Choi, Y.H. Natural deep eutectic solvents as new potential media for green technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Verpoorte, R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019, 26, 87–93. [Google Scholar] [CrossRef]
- Liu, Y.; Friesen, J.B.; McAlpine, J.B.; Lankin, D.C.; Chen, S.-N.; Pauli, G.F. Natural Deep Eutectic Solvents: Properties, Applications, and Perspectives. J. Nat. Prod. 2018, 81, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Wils, L.; Hilali, S.; Boudesocque-Delaye, L. Biomass Valorization Using Natural Deep Eutectic Solvents: What’s New in France? Molecules 2021, 26, 6556. [Google Scholar] [CrossRef] [PubMed]
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; De Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Colombo Dugoni, G.; Ferro, M.; et al. Are natural deep eutectic solvents always a sustainable option? A bioassay-based study. Environ. Sci. Pollut. Res. 2023, 30, 17268–17279. [Google Scholar] [CrossRef] [PubMed]
- Thiery, E.; Delaye, P.-O.; Thibonnet, J.; Boudesocque-Delaye, L. Mechanochemical Suzuki-Miyaura Cross-Coupling with Natural Deep Eutectic Solvent as Liquid-Assisted Grinding Additive: Merging Two Fields for a Greener Strategy. Eur. J. Org. Chem. 2023, 26, e202300727. [Google Scholar] [CrossRef]
- Delaye, P.O.; Pénichon, M.; Boudesocque-Delaye, L.; Enguehard-Gueiffier, C.; Gueiffier, A. Natural Deep Eutectic Solvents as sustainable solvent for Suzuki-Miyaura cross-coupling reactions applied to imidazo-fused heterocycles. SynOpen 2018, 2, 306–311. [Google Scholar]
- Alonso, D.A.; Burlingham, S.J.; Chinchilla, R.; Guillena, G.; Ramón, D.J.; Tiecco, M. Asymmetric Organocatalysis in Deep Eutectic Solvents. Eur. J. Org. Chem. 2021, 2021, 4065. [Google Scholar] [CrossRef]
- Nolan, M.D.; Mezzetta, A.; Guazzelli, L.; Scanlan, E.M. Radical-mediated thiol–ene ‘click’ reactions in deep eutectic solvents for bioconjugation. Green Chem. 2022, 24, 1456–1462. [Google Scholar] [CrossRef]
- Hadi Nematollahi, M.; Carvalho, P.J. Green solvents for CO2 capture. Curr. Opin. Green Sustain. Chem. 2019, 18, 25–30. [Google Scholar] [CrossRef]
- He, W.; Zhan, T.; Han, H.; Xu, Y. Optimization of Deep Eutectic Solvents Enables Green and Efficient Cryopreservation. Langmuir 2024, 40, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Caprin, B.; Charton, V.; Vogelgesang, B. The use of NADES to support innovation in the cosmetic industry. In Advances in Botanical Research, 1st ed.; Verpoorte, R., Witkamp, G.-J., Choi, Y.H., Eds.; Elsevier: London, UK, 2021; Volume 97, pp. 309–332. [Google Scholar] [CrossRef]
- Le Bras, C.; Maillet, G.O.; Lubrano, C. Base de Formulation Cosmétique D’origine Naturelle ou Végétale et Composition Cosmétique. Patent FR 3 017 292-A1, 14 August 2015. [Google Scholar]
- Lubrano, C.; Maillet, G.O.; Laperdrix, C. Utilisation Cosmétique d’un Solvant Eutectique pour Améliorer L’aspect de la Peau. Patent FR 3 046 352-A1, 7 July 2017. [Google Scholar]
- Laperdrix, C.; Sennelier, B. Cosmetic Use of Vegetable Ivory Extract (Phytelephas sp.). Patent WO 2019/081859-A1, 15 July 2021. [Google Scholar]
- Lubrano, C.; Sennelier, B.; Mervoyer, C. Cosmetic Use of Syringa vulgaris L. Meristematic Cells for a Soothing and/or Softening Action on the Skin. Patent WO 2019/063929-A1, 29 March 2019. [Google Scholar]
- Sennelier, B.; Laugier-Cassin, F.; Mervoyer, C. Cosmetic Use of Syringa vulgaris L. Meristematic Cells for Anti-Ageing Action on the Skin. Patent WO 2019/063927-A1, 4 April 2019. [Google Scholar]
- Laperdrix, C.; Lubrano, C.; Portet, B. Utilisation d’un Extrait de Réglisee (Glycyrrhiza glabra L.) pour une Action D’hydratation de la Peau, de ses Annexes ou des Muqueuses. Patent FR 3 042 415-A1, 21 April 2017. [Google Scholar]
- Caprin, B.; Charton, V.; Demargne, F. Extraits Végétaux Destinés à la Cosmétique, Solvants et Procédés pour les Obtenir. Patent FR 3036618-A1, 2 December 2016. [Google Scholar]
- Caprin, B.; Charton, V.; Demargne, F. Solvants Eutectiques pour la Dissolution de Stilbenoides ou leurs Dérivés. Patent FR 3068352-A1, 4 January 2019. [Google Scholar]
- Lavaud, A.; Laguerre, M.; Birtic, S.; Fabiano-Tixier, A.S.; Roller, M.; Chemat, F.; Bily, A.C. Solvant Eutectique D’extraction, Procédé D’extraction par Eutectigénèse Utilisant Ledit Solvant, et Extrait issu dudit Procédé. Patent FR 3034625-A1, 14 October 2016. [Google Scholar]
- Laguerre, M.; Harris, R.; Lavaud, A.; Tenon, M.; Birtic, S.; Bily, A.C.; Abbott, A.P. Eutectic Extract Formation and Purification. Patent WO 2019/219774-A2, 30 September 2021. [Google Scholar]
- Wils, L.; Leman-Loubière, C.; Bellin, N.; Clément-Larosiére, B.; Pinault, M.; Chevalier, S.; Enguehard-Gueiffier, C.; Bodet, C.; Boudesocque-Delaye, L. Natural deep eutectic solvent formulations for spirulina: Preparation, intensification, and skin impact. Algal Res. 2021, 56, 102317. [Google Scholar] [CrossRef]
- Hilali, S.; Van Gheluwe, L.; Yagmur, M.; Wils, L.; Phelippe, M.; Clément-Larosière, B.; Montigny, B.; Jacquemin, J.; Thiery, T.; Boudesocque-Delaye, L. NaDES-based biorefinery of Spirulina (Arthrospira platensis): A new path for sustainable high value-added metabolites. Sep. Purif. Technol. 2024, 329, 125123. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, C.; Xu, C.; Zhang, P.; Chen, C.; Tang, L.; Zhang, J.; Zhang, H.; Jiang, S. Simultaneous extraction of crocin and geniposide from gardenia fruits (Gardenia jasminoides Ellis) by probe-type ultrasound-assisted natural deep eutectic solvents and their inhibition effects on low density lipoprotein oxidation. Ultrason. Sonochem. 2023, 101, 106658. [Google Scholar] [CrossRef] [PubMed]
- Obluchinskaya, E.D.; Pozharitskaya, O.N.; Zakharova, L.V.; Daurtseva, A.V.; Flisyuk, E.V.; Shikov, A.N. Efficacy of Natural Deep Eutectic Solvents for Extraction of Hydrophilic and Lipophilic Compounds from Fucus vesiculosus. Molecules 2021, 26, 4198. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, S.; Zhou, H.; Wu, H.; Wang, S.; Yong, X.; Zhou, J. Separation and purification of glabridin from a deep eutectic solvent extract of Glycyrrhiza glabra residue by macroporous resin and its mechanism. Sep. Purif. Technol. 2023, 315, 123731. [Google Scholar] [CrossRef]
- Rente, D.; Cvjetko Bubalo, M.; Panić, M.; Paiva, A.; Caprin, B.; Radojčić Redovniković, I.; Duarte, A.R.C. Review of deep eutectic systems from laboratory to industry, taking the application in the cosmetics industry as an example. J. Clean. Prod. 2022, 380, 135147. [Google Scholar] [CrossRef]
- Jamaleddine, A.; Urrutigoïty, M.; Bouajila, J.; Merah, O.; Evon, P.; de Caro, P. Ecodesigned Formulations with Tomato Pomace Extracts. Cosmetics 2023, 10, 7. [Google Scholar] [CrossRef]
- Rocha, D.; Freitas, D.S.; Magalhães, J.; Fernandes, M.; Silva, S.; Noro, J.; Ribeiro, A.; Cavaco-Paulo, A.; Martins, M.; Silva, C. NADES-Based Cork Extractives as Green Ingredients for Cosmetics and Textiles. Processes 2023, 11, 309. [Google Scholar] [CrossRef]
- Barros Santos, M.C.; Barouh, N.; Baréa, B.; Villeneuve, P.; Bourlieu-Lacanal, C.; Simões Larraz Ferreira, M.; Durand, E. Sequential one-pot NaDES assisted extraction and biotransformation of rice bran: A new strategy to boost antioxidant activity of natural extracts. Process Biochem. 2022, 117, 110–116. [Google Scholar] [CrossRef]
- Wils, L.; Yagmur, M.; Phelippe, M.; Montigny, B.; Clément-Larosière, B.; Jacquemin, J.; Boudesocque-Delaye, L. Alternative Solvents for the Biorefinery of Spirulina: Impact of Pretreatment on Free Fatty Acids with High Added Value. Mar. Drugs 2022, 20, 600. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ying, T.; Bao, B.; Huang, X. Characteristics of fruit ripening in tomato mutant epi. J. Zhejiang Univ. Sci. B 2005, 6, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Yang, M.-H.; Wen, H.-M.; Chern, J.-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J. Food Drug Anal. 2002, 10, 3. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Morais, E.S.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Extraction of High Value Triterpenic Acids from Eucalyptus globulus Biomass Using Hydrophobic Deep Eutectic Solvents. Molecules 2020, 25, 210. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Czaikoski, A.; Aparecida Almeida, N.; Fraga, S.; de Oliveira Rocha, L.; Lopes Cunha, R.; Maximo, G.J.; Caldas Batista, E.A. Extraction optimization, biological activities, and application in O/W emulsion of deep eutectic solvents-based phenolic extracts from olive pomace. Food Res. 2022, 161, 111753. [Google Scholar] [CrossRef] [PubMed]
- Tenambergen, F.; Maruiama, C.H.; Mäder, K. Dual asymmetric centrifugation as an alternative preparation method for parenteral fat emulsions in preformulation development. Int. J. Pharm. 2013, 447, 31–37. [Google Scholar] [CrossRef]
- Gharehbeglou, P.; Jafari, S.M.; Homayouni, A.; Hamishekar, H.; Mirzaei, H. Fabrication of double W1/O/W2 nano-emulsions loaded with oleuropein in the internal phase (W1) and evaluation of their release rate. Food Hydrocoll. 2019, 89, 44–55. [Google Scholar] [CrossRef]
- Kusmita, L.; Mutmainah, N.; Sabdono, A.; Trianto, A.; Radjasa, O.K.; Pangestuti, R. Characteristic Evaluation of Various Formulations of Anti-Aging Cream from Carotenoid Extract of Bacterial Symbiont Virgibacillus salarius Strain 19.PP.Sc1.6. Cosmetics 2021, 8, 120. [Google Scholar] [CrossRef]
- Franyoto, Y.D.; Kusmita, L.; Mutmainah, N.; Angrena, R.D. Total flavonoid content and formulation antioxidant cream stem of jatropha multifida. J. Phys. Conf. Ser. 2017, 1025, 012130. [Google Scholar] [CrossRef]
- Baldassarre, F.; Schiavi, D.; Di Lorenzo, V.; Biondo, F.; Vergaro, V.; Colangelo, G.; Balestra, G.M.; Ciccarella, G. Cellulose Nanocrystal-Based Emulsion of Thyme Essential Oil: Preparation and Characterisation as Sustainable Crop Protection Tool. Molecules 2023, 28, 7884. [Google Scholar] [CrossRef]
- Moravkova, T.; Stern, P. Rheological and textural properties of cosmetic emulsions. Appl. Rheol. 2011, 21, 35200. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, Y.; Zhu, L.; Liu, X.; Zhao, G.; Wang, S.; Yang, L.; Liu, H. Stability and rheological properties of water-in-oil (W/O) emulsions prepared with a soyasaponin-PGPR system. Future Food 2021, 4, 100096. [Google Scholar] [CrossRef]
- Wang, W.; Jia, R.; Hui, Y.; Liu, Y.; Song, Y.; Wang, B. Utilization of two plant polysaccharides to improve fresh goat milk cheese: Texture, rheological properties, and microstructure characterization. J. Dairy Sci. 2023, 106, 3900–3917. [Google Scholar] [CrossRef]
- de la Guardia, C.; Virno, A.; Musumeci, M.; Bernardin, A.; Silberberg, M.B. Rheologic and Physicochemical Characteristics of Hyaluronic Acid Fillers: Overview and Relationship to Product Performance. Facial Plast. Surg. 2022, 38, 116–123. [Google Scholar] [CrossRef]

| NaDES | Component 1 | Component 2 | Component 3 | Molar Ratio | Water Amount (% w/w) | |
|---|---|---|---|---|---|---|
| Nonpolar | C8:C10 | Octanoic acid | Decanoic acid | 3:1 | ||
| C9:C12 | Nonanoic acid | Lauric acid | 3:1 | |||
| C9:C10:C12 | Nonanoic acid | Decanoic acid | Lauric acid | 3:2:1 | ||
| C8:Lac | Octanoic acid | Lactic acid | 5:1 | 1 | ||
| Polar | Pro:Gly | Proline | Glycerol | 1:3 | ||
| Pro:Lev | Proline | Levulinic acid | 2:1: | 20 | ||
| Bet:Gly | Betaine | Glycerol | 1:8 | 20 | ||
| Bet:Lev | Betaine | Levulinic acid | 1:1 | |||
| Lev:Gly | Levulinic acid | Glycerol | 1:1 | |||
| Prop:Lev | 1,3-propanediol | Levulinic acid | 1:1 | |||
| Prop:Gly | 1,3-propanediol | Glycerol | 1:1 |
| %wt in the Analyzed Samples | |||
|---|---|---|---|
| Ingredients | Function | Control | Calendula Creams |
| BGCE (%wt) | Active | 0 | 1 or 10 |
| Xanthan gum (%wt) | Thickener | 0.25 | 0.25 |
| Sclerotium gum (%wt) | Thickener | 0.75 | 0.75 |
| Glycerol (%wt) | Humectant | 10 | 9 or 0 |
| Hydrogenated lecithin (%wt) | Emulsifier | 3 | 3 |
| Caprylic/capric triglycerides (%wt) | Emollient | 25 | 25 |
| Dehydroacetic acid/ benzyl alcohol mix (%wt) | Antimicrobial agent | 1 | 1 |
| Water (%wt) | Solvent | q.s 100 | |
| Run | Solid/Liquid Ratio (%) | Temperature (°C) | Time (min) | TCC (µg/g) | TFC (mg eq Rutin/g) |
|---|---|---|---|---|---|
| 1 | 6 | 60 | 60 | 211.9 | 21.5 |
| 2 | 6 | 45 | 37.5 | 255.3 | 20.7 |
| 3 | 6 | 30 | 60 | 265.0 | 22.3 |
| 4 | 6 | 30 | 15 | 281.5 | 23.0 |
| 5 | 10 | 45 | 15 | 223.7 | 12.9 |
| 6 | 6 | 60 | 15 | 281.2 | 24.4 |
| 7 | 6 | 45 | 37.5 | 282.5 | 20.7 |
| 8 | 2 | 60 | 37.5 | 135.8 | 12.5 |
| 9 | 10 | 30 | 37.5 | 255.9 | 18.8 |
| 10 | 2 | 45 | 15 | 141.0 | 12.8 |
| 11 | 2 | 30 | 37.5 | 89.9 | 9.2 |
| 12 | 10 | 60 | 37.5 | 177.9 | 11.6 |
| 13 | 6 | 45 | 37.5 | 274.3 | 24.7 |
| 14 | 2 | 45 | 60 | 126.4 | 11.8 |
| 15 | 6 | 45 | 37.5 | 414.8 | 25.1 |
| 16 | 6 | 45 | 37.5 | 367.9 | 24.9 |
| 17 | 10 | 45 | 60 | 212.8 | 19.7 |
| Run | Solid/Liquid Ratio (%) | Time (min) | Speed (rpm) | TCC (µg/g) | TFC (mg eq rutin/g) |
|---|---|---|---|---|---|
| 1 | 2 | 37.5 | 3500 | 238.6 | 18.6 |
| 2 | 6 | 37.5 | 2000 | 340.3 | 30.2 |
| 3 | 6 | 60 | 500 | 384.7 | 29.0 |
| 4 | 10 | 37.5 | 500 | 167.1 | 34.9 |
| 5 | 2 | 60 | 2000 | 240.5 | 24.9 |
| 6 | 10 | 60 | 2000 | 173.3 | 32.7 |
| 7 | 2 | 15 | 2000 | 240.3 | 18.8 |
| 8 | 10 | 15 | 2000 | 169.1 | 30.9 |
| 9 | 6 | 37.5 | 2000 | 387.3 | 38.5 |
| 10 | 6 | 37.5 | 2000 | 301.4 | 38.8 |
| 11 | 2 | 37.5 | 500 | 188.8 | 20.5 |
| 12 | 6 | 15 | 3500 | 316.3 | 37.7 |
| 13 | 6 | 37.5 | 2000 | 318.9 | 41.7 |
| 14 | 10 | 37.5 | 3500 | 132.1 | 34.7 |
| 15 | 6 | 37.5 | 2000 | 303.9 | 41.1 |
| 16 | 6 | 60 | 3500 | 302.1 | 32.6 |
| 17 | 6 | 15 | 500 | 192.0 | 33.9 |
| D1 | D90 | |
|---|---|---|
| Control | 5.4 ± 0.2 | 5.4 ± 0.1 |
| 1% BGCE Cream | 5.4 ± 0.1 | 5.0 ± 0.3 |
| 10% BGCE Cream | 5.3 ± 0.1 | 5.8 ± 0.1 |
| TSI | Droplet Diameter (µm) | |||
|---|---|---|---|---|
| D1 | D90 | D1 | D90 | |
| Control | 0.83 ± 0.21 | 0.90 ± 0.10 | 9.3 ± 0.2 | 9.7 ± 0.2 |
| 1% BGCE Cream | 1.23 ± 0.23 | 0.83 ± 0.15 | 9.2 ± 0.2 | 10.2 ± 0.2 |
| 10% BGCE Cream | 0.97 ± 0.35 | 1.00 ± 0.44 | 7.3 ± 0.3 | 8.6 ± 0.2 |
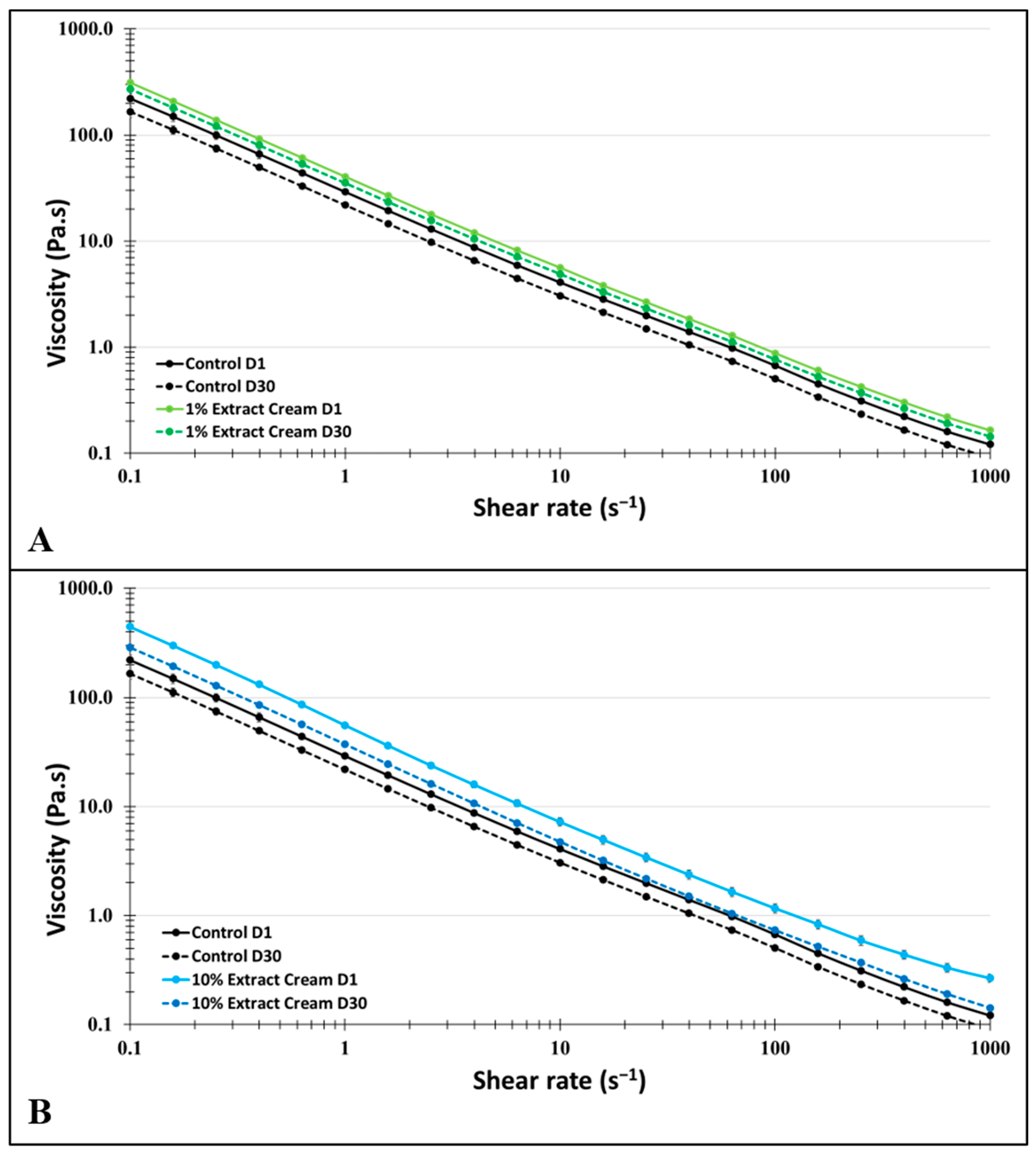
| Creams | η (0.01) | η (0.1) | η (1) | η (10) | η (100) | η (1000) |
|---|---|---|---|---|---|---|
| Control D1 | 1430.0 ± 37.8 | 221.1 ± 23.0 | 29.1 ± 0.7 | 4.1 ± 0.0 | 0.7 ± 0.0 | 0.1 ± 0.0 |
| Control D30 | 1072.5 ± 124.2 | 165.8 ± 19.8 | 21.9 ± 0.6 | 3.1 ± 0.1 | 0.5 ± 0.0 | 0.1 ± 0.0 |
| 1% BGCE D1 | 2236.3 ± 61.2 | 311.6 ± 7.3 | 40.6 ± 1.2 | 5.6 ± 0.1 | 0.9 ± 0.0 | 0.2 ± 0.0 |
| 1% BGCE D30 | 1945.6 ± 53.3 | 271.1 ± 6.3 | 35.3 ± 1.1 | 4.9 ± 0.1 | 0.8 ± 0.0 | 0.1 ± 0.0 |
| 10% BGCE D1 | 3084.0 ± 49.5 | 446.5 ± 19.9 | 55.8 ± 2.7 | 7.2 ± 0.6 | 1.2 ± 0.1 | 0.3 ± 0.0 |
| 10% BGCE D30 | 1694.7 ± 87.8 | 287.9 ± 10.8 | 37.2 ± 1.0 | 4.8 ± 0.1 | 0.7 ± 0.0 | 0.1 ± 0.0 |
| D1 | D30 | |||
|---|---|---|---|---|
| G′ (Pa) | G″ (Pa) | G′ (Pa) | G″ (Pa) | |
| Control | 188.1 ± 3.1 | 36.0 ± 3.2 | 215.7 ± 7.0 | 40.6 ± 5.6 |
| 1% BGCE Cream | 260.2 ± 7.9 | 47.1 ± 8.0 | 226.3 ± 4.3 | 41.0 ± 3.0 |
| 10% BGCE Cream | 389.6 ± 10.4 | 68.2 ± 3.8 | 228.7 ± 7.3 | 50.6 ± 4.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boudesocque-Delaye, L.; Ardeza, I.M.; Verger, A.; Grard, R.; Théry-Koné, I.; Perse, X.; Munnier, E. Natural Deep Eutectic Solvents as a Novel Bio-Based Matrix for Ready-to-Use Natural Antioxidants-Enriched Ingredients: Extraction and Formulation Optimization. Cosmetics 2024, 11, 17. https://doi.org/10.3390/cosmetics11010017
Boudesocque-Delaye L, Ardeza IM, Verger A, Grard R, Théry-Koné I, Perse X, Munnier E. Natural Deep Eutectic Solvents as a Novel Bio-Based Matrix for Ready-to-Use Natural Antioxidants-Enriched Ingredients: Extraction and Formulation Optimization. Cosmetics. 2024; 11(1):17. https://doi.org/10.3390/cosmetics11010017
Chicago/Turabian StyleBoudesocque-Delaye, Leslie, Iron Mike Ardeza, Alexis Verger, Roxane Grard, Isabelle Théry-Koné, Xavier Perse, and Emilie Munnier. 2024. "Natural Deep Eutectic Solvents as a Novel Bio-Based Matrix for Ready-to-Use Natural Antioxidants-Enriched Ingredients: Extraction and Formulation Optimization" Cosmetics 11, no. 1: 17. https://doi.org/10.3390/cosmetics11010017
APA StyleBoudesocque-Delaye, L., Ardeza, I. M., Verger, A., Grard, R., Théry-Koné, I., Perse, X., & Munnier, E. (2024). Natural Deep Eutectic Solvents as a Novel Bio-Based Matrix for Ready-to-Use Natural Antioxidants-Enriched Ingredients: Extraction and Formulation Optimization. Cosmetics, 11(1), 17. https://doi.org/10.3390/cosmetics11010017