Abstract
The Amazon region’s rich biodiversity supports a bioindustry model that utilizes various biological assets from different plant species, and where it will add value to existing production chains, starting to supply bio industrialized products and not just primary products. Guarana (Paullinia cupana) is rich in bioactive compounds that interest the food and pharmaceutical industries. Thus, the main objective of this review is to present ways to add value to the guarana production chain by developing bioproducts using the residues generated in its processing. During processing, various residues are generated, as follows: peel (corresponding to 30% of the total mass of the fruit), and pulp (aryl), shell, and spent seeds, which have potential for application according to their characteristics. These residues were used to obtain bioactive compounds (catechins, theobromine, and caffeine) through different types of extraction (conventional, enzymatic, and pressurized liquid), and, subsequently, encapsulation. They were also applied in biodegradable and active packaging. Due to the high hemicellulose concentration, residual guarana seeds’ characteristics could potentially produce xylooligosaccharides (XOS). Therefore, the concept of biorefinery applied within the guarana production chain provides products that can be studied in the future to determine which processes are viable for expanding and valuing the productive chain of this fruit, in addition to strengthening sustainable development in the Amazon.
1. Introduction
The objective of this work is to present to the readers which residues are generated in the guarana production chain, what the composition of each residue is, and, based on this information, what the possibility of its application as a raw material is for a biorefinery seeking to obtain products with greater added value. In the end, it is expected to contribute to the presentation of another alternative for the sustainable development of the Amazon region through the valorization of the guarana productive chain. The need to develop regions with rich biodiversity sustainably increasingly demands research on improving processes and obtaining products based on biotechnology. The Amazon presents several species of interest to the chemical, pharmaceutical, and food industries; some natural species emerge, with enormous possibilities for economic exploitation, also promoting social and environmental development in these regions. However, the techniques that allow for the best use of these resources need to be evaluated and implemented, because, in this way, it is possible to apply an economic model that preserves the forest, using natural resources rationally and sustainably. The Amazon region has some of the most incredible biodiversity in the world, concentrating in a total area of approximately 6,300,000 km2, of which 5,000,000 km2 are in Brazilian territory, with more than 40,000 species of plants, with enormous potential for extracting bioproducts [1]. The guarana (Paullinia cupana) production chain, which is among those species that have high biotechnological potential, has a great deal of weight in the local economy, so the use of waste generated in its processing to produce compounds with higher added value emerges as an important scientific and technological challenge to be overcome. Transforming biological wealth into economic wealth and environmental conservation through sustainable development models is a great challenge for the Amazon [2].
Guarana (Paullinia cupana) is an Amazonian fruit widely used in the beverage industry in Brazil. About 70% of production is used to manufacture soft drinks; the rest is converted into byproducts, such as syrup, sticks, powder, and extract [3]. The fruit has beneficial health properties associated with bioactive compounds in the seed, such as methylxanthines (caffeine, theobromine, and theophylline) and polyphenols, determined by high-performance liquid chromatography coupled with a diode array detector (HPLC-DAD) [4,5]. Studies carried out by humans have detected antioxidants, anticarcinogenic, antitumor, and neuroprotective effects in guarana and improvements in cognitive function [6]. Due to these properties, guarana has been implemented in producing stimulant drinks, functional foods, pharmaceuticals, cosmetics, and others [7]. The beverage industry in Brazil uses the guarana extract obtained from the processing of the fruit seed, a process that generates depleted seeds as waste [8]. A study by Santana et al. [4] uses the guarana extract obtained from the processing of the fruit seed, a process that generates depleted seeds as waste. A study shows that, despite the seed being residual, it is possible to recover essential bioactive compounds, such as catechins and methylxanthines, from hydroalcoholic and enzymatic extraction, determined by HPLC-DAD. However, there is still little information in the literature regarding the advantages and applications of residual seeds. This makes it essential for studies to be undertaken that propose investigating and efficiently extracting relevant compounds from this substrate, consequently adding value to the guarana production chain.
Although the seed is the main product of the guarana fruit, a significant amount of waste is generated during processing, consisting of peel, pulp, and seed. Currently, these residues are underutilized and are mainly destined for organic fertilization. However, the many bioactive compounds and fibers in these residues transform these products into potential raw materials for producing compounds with high added value to generate income for producers, develop the local economy, and preserve the environment. The concept of biorefinery was followed, in which there is the integration of biomass conversion processes into various bioproducts, and the use of natural resources is carried out in such a way as to minimize effluents and maximize benefits and profit. This practice is also aligned with the principle of a circular economy, where the optimization of the use of natural resources and processes occurs through the use of different techniques to reuse waste previously generated in the linear model, so the circular economy presents itself as an advantageous model that should be applied in the most diverse production processes [9]. Within a biorefinery, there is an opportunity to add value to agro-industry residues that present the lignocellulosic portion as a potential substrate. The bioactive compounds can also be used for residues from the guarana production chain. These residues can be converted into fuels and value-added products, such as bioethanol, activated carbon, biochar, organic fertilizer, natural fiber compounds and nanocomposites, and other essential chemical inputs [10]. Recent research has shown that agro-industrial residues rich in fiber and polyphenols can be transformed into value-added products through physical, chemical, and biological pretreatments, opening up new possibilities in the food, pharmaceutical, and bioenergy sectors [11]. Studies have also demonstrated the potential of incorporating microbial biomass—such as yeasts—into circular economy strategies to generate biofuels, bioplastics, fertilizers, and nutraceuticals, thereby reducing waste [12].
Other promising applications include converting biowaste into biochar, organic acids, and bioplastics through thermochemical and biological pathways, contributing to environmental sustainability and economic benefits [13]. For instance, sugarcane bagasse has been successfully used to recover phytochemicals such as sterols, flavonoids, and terpenoids, as well as to produce biofuels and nutraceuticals, demonstrating the feasibility of applying biorefinery principles to agricultural residues [14]. Similarly, nutrient-rich residues from shrimp farming have been valorized through microbial fermentation and eco-friendly extraction techniques to recover chitin, proteins, carotenoids, and nanomaterials, highlighting the cross-sector potential of the biorefinery strategy.
This review addresses these gaps by providing a comprehensive and structured overview of Guarana processing residues. It compiles existing knowledge on the chemical composition of these byproducts, evaluates current extraction strategies, and contextualizes guarana residue valorization within the frameworks of biorefinery and a circular economy. This review was searched in the Scopus, Capes Periodics, Google, and PubMed databases. The search was performed using the following terms: “Paullinia cupana AND residues”, “Paullinia cupana AND waste”, “Paullinia cupana AND bioactive compounds”, “Paullinia cupana AND by-products”, “lignocellulosic residues AND cellulose”, “lignocellulosic residues AND hemicellulose”, “lignocellulosic residues AND lignin”, “lignocellulosic residues AND pretreatment”, and “biopackaging”, nanoparticles”, considering the title, abstract, and keywords. All articles from the searched selection were read in full, based on the criteria adopted for the theme.
The objective of this work is to present to the readers which residues are generated in the guarana production chain, what the composition of each residue is, and, based on this information, the possibility of its application as a raw material for a biorefinery seeking to obtain products with greater added value. In the end, it is expected to contribute to the presentation of another alternative for the sustainable development of the Amazon region through the valorization of the guarana productive chain.
2. Guarana Processing
Figure 1 presents a general summary of the topics covered in the review, from the processing of guarana to the production of byproducts/waste, the main bioactive compounds present in these byproducts, the main applications (encapsulation, application in food and packaging, potential in human health, and production of nanoparticles), and possible applications (extraction of hemicellulose).
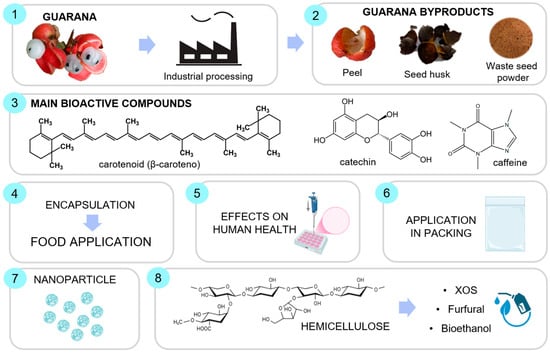
Figure 1.
A general summary of the topics presented in the review. Created with https://www.biorender.com (accessed on 13 May 2025).
2.1. Guarana Production in Brazil
Agribusiness is one of Brazil’s most important commercial activities, generating jobs and income, especially regarding fruits and their derivatives. The term guarana refers to the fruits extracted from Paullinia cupana H.B.K. Typica, which are mostly cultivated in the Venezuelan and Colombian Amazons, and the Paullinia cupana variety sorbilis (Martius) Ducke, mostly cultivated in the Brazilian Amazon, both belonging to the Sapindaceae family [15]. Brazil is practically the only country in the world that produces guarana, with small areas where the tree is planted in Venezuela and Peru. The guarana tree starts to produce fruit in its third or fourth year after planting, and around the fifth, it reaches the level of economic production, having a useful lifespan of up to twenty years [16].
The central producing state is Bahia, which represented 67.0% of national production in 2021, standing at 1.8 thousand tons, followed by the state of Amazonas, which represented 23.5% of national output in 2021, having produced 643 tons, with a reduction of 16.6% in comparison to the previous year. The decline in production in that state was due to cuts of 7.5% in the area destined for harvesting, and 9.6% in productivity. In the municipalities where guarana is produced, several family farmers benefit from its commercialization through cooperatives, which integrate companies and intermediaries that serve different buyers, such as the pharmaceutical and soft drink industries. The average price paid to the producer of guarana in Bahia in October 2022 was, on average, US$ 5.5/kg; in the state of Amazonas, for the same period, the guarana was off-season, but for 2021, the actual price paid to the producer was around US$ 3.90/kg. Among the factors that influence the difference in fees paid to the producer between the states are the product’s quality, the crop’s productivity, and the way of insertion in the production chain [17].
2.2. Waste Generated
The widely commercialized part of guarana is just the seed, with the other pieces of the fruit being discarded [6]. Processing steps include fermentation and pulping, washing, sieving, drying, roasting, selection, and grinding. At the end of the industrial processing of P. cupana, residues are generated, as follows: peel (corresponding to 30% of the total mass of the fruit), pulp (aryl), shell, and spent seeds (deprived of substances of commercial interest in significant quantities after processing) (Figure 2) [3,5].
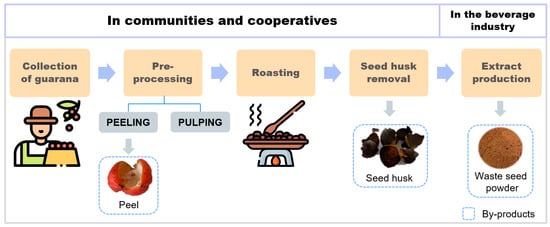
Figure 2.
Waste from guarana processing. Icons designed by Freepik from Flaticon. https://www.flaticon.com/br/ (accessed on 14 May 2023).
3. Biorefinery Based on Guarana Residues
Figure 3 presents the main applications found in the literature and the potential applications (da cellulose, hemicellulose, and lignin) of guarana residues. Below, we will explore these applications more fully.
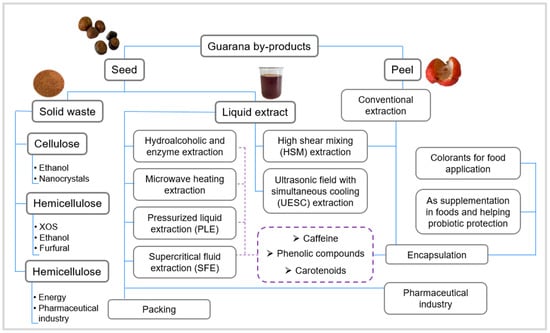
Figure 3.
Guarana byproducts in a biorefinery approach.
3.1. Bioactive Compounds
Guarana (Paullinia cupana) is the most widely consumed fruit, and it is typically fresh or processed [2]. Generating large amounts of waste during harvest can change from an environmental problem to an opportunity to create products with greater added value. Studies have indicated that plant byproducts contain high phenolic compounds, including guarana [5]. The few reports on using residues from the guarana processing chain indicate a vast field of study in its application for food products’ sensory and functional improvement.
Environmentally, replacing conventional petroleum-based packaging with biodegradable alternatives derived from agro-industrial residues reduces greenhouse gas emissions and post-consumer waste. The carbon footprint of biodegradable packaging is significantly lower than that of synthetic plastics, primarily when renewable residues like guarana waste are used [10]. Additionally, adopting these alternatives aligns with the life cycle assessment (LCA) goals for food packaging systems [13].
Guarana peels are byproducts that the food and pharmaceutical industries could use in many of their products. They are sources of macro- and micronutrients, caffeine, theobromine, phenolic compounds, and carotenoids (-carotene, a pigment converted into vitamin A by the oxidative breakdown of the central double bond and subsequent biochemical reduction of aldehyde and lutein) [5].
The recovery of bioactive compounds from agro-industrial waste is sustainable, providing antioxidants and increasing food probiotic viability [18]. Pinho et al. [5] identified high mineral and fiber content and the presence of carotenoids in guarana peels, indicating that this material can be used to produce supplements and nutraceuticals.
Therefore, the valorization of guarana peel to recover carotenoids combined with probiotics can strengthen food matrices as functional ingredients [19]. This combination may be beneficial due to the antioxidant potential of carotenoids to prolong the viability of probiotics during storage.
3.1.1. Bioactive Compounds Extraction
Aiming to recover bioactive compounds from guarana byproducts, some studies (Table 1) performed extraction using different sources and processes. Most of the studies extracted from the seed, while some also extracted the guarana peel. Figure 4 presents the main applications of guarana byproducts that will be given throughout the review.

Table 1.
Summary of guarana by-product applications and innovations; use of seeds.
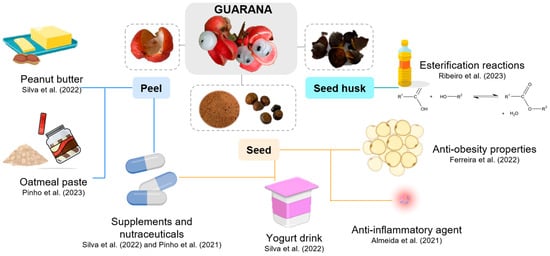
Figure 4.
Main applications of guarana byproducts. Created with https://www.biorender.com (accessed on 13 May 2025).
Caffeine is an important compound that can help fight several illnesses, such as cancer, immunomodulation, autoimmune diseases, and ocular, respiratory, neurodegenerative, and cardiovascular diseases [28]. Furthermore, caffeine also helps with resistance exercises [29]. Therefore, these compounds have potential both for pharmaceutical applications, treating different diseases, and as a supplement for sports.
Some studies also carried out the extraction of alkaloids (the group that caffeine belongs to) and methylxanthines (groups composed mainly of caffeine, theobromine, and theophylline). Furthermore, these works also carried out the extraction of phenolic compounds (Table 1). Among the results in the study by Santana et al. [20], the authors reported that extraction using the PLE (pressurized liquid extraction) method and enzymes showed greater catechin and caffeine extraction. In another study by Santana et al. [4], the authors showed better extraction using the hot hydroalcoholic maceration method. In the study by Santana et al. [8], the authors observed that 1-min using high-shear mixing was sufficient to increase phenolics and methylxanthines extraction. However, the previously mentioned PLE method showed better results. In addition to these methods, Marques et al. [30] used supercritical extraction to recover phenolic compounds. Interestingly, although some methods are used to eliminate bioactive compounds, several procedures, such as ultrasound and microwave-assisted extractions, have not yet been tested.
In addition to these compounds, guarana byproducts also presented an exciting number of carotenoids; as Pinho et al. [5] reported, an alternative use of these compounds may be in the food industry as antioxidant compounds.
3.1.2. Microencapsulation of Bioactive Compounds from Guarana Byproducts and Food Applications
Some studies microencapsulated bioactive compounds from guarana byproducts (Table 1). In the study by Silva et al. [22], the authors reported that encapsulation by spray-chilling showed promising results, and the phenolic compounds were practically completely released (99%) in the simulated gastrointestinal tract. In the study by Pinho et al. [5], the authors conducted the microencapsulation of carotenoids extracted from guarana seeds to be used as food colorants with antioxidant power. In the work of Silva et al. [26], the authors reported that the encapsulation of bioactive compounds from guarana seeds and peels helped protect probiotics, which have a potential for application in functional foods.
Two studies were developed to consider the potential use of functional foods. Silva et al. [19] microencapsulated guarana peel extracts with probiotics and added them to peanut butter, observing that the extracts helped protect during storage. In the work by Silva et al. [23], the authors verified storage protection and better sensory acceptance by adding guarana seed extract microencapsulated with probiotics to yogurt. In the study by Pinho et al. [25], the authors evaluated the addition of encapsulated guarana peel extract to oatmeal paste, resulting in a paste with a more intense color, a suitable particle size, and high carotenoid content.
In the study by Carvalho et al. (2019) [31], guarana seed extracts were used in lamb burgers. They reported a positive effect against color deterioration and lipid and protein oxidation without impairing sensorial characteristics. Thus, guarana seed extracts represent a promising alternative to synthetic antioxidants in lamb burgers.
Interesting studies have shown that residues from guarana processing have great potential for food application. Due to their content of bioactive compounds, these residues bring beneficial characteristics. Furthermore, these compounds can be used to prepare functional foods to help protect probiotics.
3.1.3. Guarana Byproducts on Human Health
As previously seen, guarana byproducts are rich in bioactive compounds, with the primary studies focusing on their encapsulation for better absorption of these compounds. However, some studies have reported beneficial effects on health from these compounds. In the work of Silva et al. (2019) [22], the authors noted that guarana seed extract has anti-obesity potential due to the high inhibitory activity of lipase.
In the study by Figueira et al. (2022) [32], the authors reported that digested guarana seed powder extract inhibits pancreatic lipase dose-dependently and decreases cholesterol micellar solubilization. In the study by Algarve et al. (2020) [33], the authors found that roasted guarana seeds have some neuroprotective effects against methylmercury-induced toxicity, evidenced by their anti-inflammatory effect.
Guarana byproducts generally have bioactive properties that have potential in several areas. These properties were initially addressed in relation to anti-obesity and anti-inflammatory properties. Future studies tend to elucidate these properties further.
3.1.4. Biodegradable and Active Packaging
Biodegradable packaging is one of the alternatives for replacing or reducing the use of conventional packaging with synthetic plastics. Active packaging is related to the conditions offered during packaging to guarantee an increase in the shelf life of the food, while its quality is maintained and its integrity is ensured. In countries like the USA, Japan, and Australia, due to consumer demands for less industrialized or minimally processed products and their potential to extend shelf life and maintain the sensory characteristics of products, active packaging is more sought after in the food sector [34].
Biodegradable packaging can be characterized as active due to the incorporation of natural compounds and phenolic compounds in its formulation, improving the barrier characteristics, antioxidant and antimicrobial activity, and mechanical properties observed for packaging with red bean flour and açaí seed extract [35] and jambolan bark extract [36], making it possible for this combination of bioactive compounds, biodegradable materials, and active functions relevant to food packaging.
Casting is the most opportune technique for forming the filmogenic solution of the packaging, and it is among the most known and used techniques due to its practicality and advantages in preparation time. Thus, the packaging is produced by mixing a filmogenic solution, traditionally starch and a solvent, and it is taken to a smooth surface for solvent evaporation and solidification of the mixture to form the film [37].
The result of the films obtained by the casting technique is subject to changes, such as changes in mechanical strength, barrier properties, thickness, and sensory properties, either due to the type of biopolymer used, environmental conditions, or packaging during the solution drying process, including filmogenic or plasticizing compounds [38].
Obtaining guarana residual seed extract is an alternative for recovering and reusing guarana bioactive compounds, such as phenolic compounds [20], that can be used to formulate active biodegradable packaging and that are associated with the phenolic compounds present in the chemical composition of the guarana seed. These packages can acquire active barrier and antioxidant characteristics, ensuring better storage conditions for food products [39].
The study by Bonilla et al. [40] used the guarana seed extract and other to elaborate packaging prepared using the casting technique. The packaging with the extract showed antibacterial and antioxidant activities and can be applied as active packaging. In this sense, the possibility of using the residual guarana seed extract to prepare active biodegradable packaging and guarantee an increase in the shelf life of foods is an alternative to add value to the residue and the guarana production chain. Additionally, it contributes to reducing the environmental impact and presents options for the food packaging sector.
3.1.5. Production of Metallic Nanoparticles
Given the potential for extracting valuable bioactive compounds from guarana bark and residual seeds, particularly phenolic compounds that have various applications in the food and pharmaceutical industries, this topic will explore the possibility of utilizing these plant extracts in the production of metallic nanoparticles. These nanoparticles are defined as structures with at least one dimension measuring less than 100 nanometers.
Metallic nanoparticles are of great interest and have received increasing attention due to their chemical and physical properties, which confer antimicrobial, magnetic, catalytic, optical, and electrical activities [41,42]. Among metallic nanoparticles, those most interesting are nanoparticles from noble metals (gold and silver) due to their promising contribution to plasmonic, renewable energy, photocatalysis, and biomedical applications [43,44,45]. Usually, metallic nanoparticles are synthesized by reducing metallic ions from a precursor through some reducing agent. Different synthesis methods can be used to produce nanoparticles, emphasizing chemical, physical, and biological methods.
In chemical methods, synthesis occurs through the use of chemical reagents, which are commonly harmful to the environment and human beings [46], and toxic compounds are used as a reducing agent, which is unsuitable for environmental sustainability, producing commonly harmful byproducts [47,48,49], and limiting the application of nanoparticles in areas that require high purity, such as medicine [50].
In physical methods, synthesis occurs through evaporation–condensation processes, amorphous crystallization, pyrolysis, high-energy ball milling, physical fragmentation, and other nanoscale processes. This method has a high production cost, as it requires much energy during the synthesis process [51], which may limit the use of nanoparticles due to the associated costs.
To reduce damage to the environment, physical and chemical methods have been replaced by green synthesis methods that use natural and more environmentally friendly materials, in addition to reducing the energy consumption of the process as a whole. Green methods use microorganisms (bacteria, fungi, microalgae) or extracts from different parts of a plant to produce a reducing medium, typically containing polyphenols and proteins, which aim to reduce the metallic ions of a precursor to its valence state zero [46]. Suppose the synthesis process is well-controlled (temperature, concentration, exposure to air, among others); in that case, green methods can produce nanoparticles of a superior quality to the physical and chemical processes, especially in nanoparticle size. Gokila et al. [52] obtained Fe3O4 nanoparticles from a green route in the 2–80 nm range, much lower than the same particles synthesized by the chemical method (87–400 nm).
For a plant matrix to be used as raw material to produce nanoparticles, it must have appreciable concentrations of bioactive compounds, such as polyphenols, carotenoids, vitamins, and lipids, which can be found in different parts of these plants. Such compounds are diversified by their polarity, solubility, molecular weight, bioavailability, and metabolic pathways, and this diversification plays a fundamental role in the synthesis process of the nanoparticles to be produced [53,54].
In the study by Almeida et al. [24], the authors carried out the synthesis of silver nanoparticles using Fusarium concolor with the addition of guarana seed extract and peels, verifying an antifungal action that can be used as an alternative to control plant diseases. In this sense, extracting guarana residues is an essential source of bioactive compounds of interest for producing metallic nanoparticles.
3.2. Hemicellulose
In a study by Santana et al. [4] on the effects of hydroalcoholic and enzymatic extraction processes on the recovery of catechins and methylxanthines from crude and residual guarana seeds (Table 1), a composition of guarana seed 10.82% in moisture was obtained, with 16.29% in protein, 1.6% in ash, 6.25% in lipids, and 65.03% in total carbohydrates. This same study obtained the composition of the residual seed generated by the cold hydroalcoholic maceration process, with 34.96% in moisture, 11.80% in proteins, 4.42% in lipids, 1.18% in ash, and 47.64% in total carbohydrates. The carbohydrate content in the residual guarana seed demonstrates that this substrate can potentially source essential polysaccharides. Polysaccharides are the main components of plant biomass and serve as storage for reserve foods, such as starch, or contribute to mechanical strength, such as cellulose and hemicelluloses [55]. Residual guarana seeds generated in the production process of extracts for the beverage industry, which generally occurs by hydroalcoholic extraction, present in their composition approximately 59% hemicellulose, 8% cellulose, and 13% lignin (values expressed on a dry basis), according to Pereira et al. [56]. In the work of de Oliveira Júnior et al. [21], the authors reported hemicellulose values of 32.8%, lignin of 6.1%, and 19.2% cellulose for processed guarana seeds; the composition was determined using the method proposed by Van Soest. Hemicellulose is the second most widespread polysaccharide in raw plant material, demonstrating its abundant availability [57]. Hemicelluloses are heteropolysaccharides formed by pentoses (xylose, arabinose), hexoses (mannose, glucose, and galactose), and uronic acid units. Xylan is the most abundant hemicellulosic polymer [58].
3.2.1. Hemicellulose Extraction Methods
The hemicellulose extraction methods involve subjecting the plant biomass to physical or chemical pretreatments, or a combination of these processes. Chemical methods such as alkaline and acid extractions present environmental pollution problems and low reaction efficiency. Therefore, new methods using solvents and green reagents, low energy consumption, and promoting a high yield and purity of hemicellulose are promising [59]. The physical methods of hydrothermal extraction (autohydrolysis) and steam explosion have the advantage of not using chemicals, which entails lower costs and environmental impacts, but require equipment that maintains high temperatures and pressure, which results in increased energy consumption [60]. The chemical method using ionic liquids (ILs) for dissolving hemicellulose can be implemented in biorefinery platforms [61]. The use of ILs is considered a green method because this reagent has the advantages of low vapor pressure, high thermal and chemical stability, and recyclability [62].
Although ionic liquids (ILs) are widely promoted as green solvents due to their negligible vapor pressure and high recyclability, recent studies have raised environmental concerns related to their toxicity and persistence. Some ILs are poorly biodegradable and can accumulate in aquatic environments if not properly recovered, representing a risk to ecosystems [63,64]. Additionally, physical pretreatment methods such as steam explosion and microwave-assisted extractions, although effective, often involve high energy consumption and infrastructure costs, which may offset the sustainability gains of the biorefinery approach [65,66,67]. Therefore, the adoption of greener, low-energy, and biodegradable alternatives is essential to ensure that the valorization of guarana residues is truly aligned with environmental sustainability goals.
Another green chemical treatment is high-pressure CO2/H2O, as they are renewable and non-flammable resources. Pressurized liquid CO2 favors the formation of carbonic acid, which favors the hydrolysis of the hemicellulose fraction of the biomass into sugars, generating an increase in enzymatic digestibility [68]. Combined methods have been employed to obtain better yields. The physical–chemical method of hemicellulose extraction assisted by ultrasonic technology has attracted attention since it has an essential sustainable contribution, high efficiency, low energy consumption, and mild conditions compared to other processes. In this method, ultrasonic waves generate high-frequency vibrations, which increase the effects of acoustic cavitation between the solute and solvent; tiny bubbles in the solution suddenly burst, thus destroying the cell wall structure and then separating the components [60]. Ultrasound-assisted alkaline extraction has been effective for hemicellulose extraction, obtaining good yields and shorter reaction times [69,70]. Microwave heating is another ecological hemicellulose extraction method that takes place using the action of electromagnetic radiation. The microwave-assisted biomass pretreatment has been widely applied, with the advantages of uniform heating, high thermal efficiency, strong penetrability, mild conditions, and a shorter reaction time [71].
3.2.2. Applications of Hemicellulose
Hemicellulose has applications in several segments, such as the food and pharmaceutical industries. Different substrates have been studied for using hemicellulose to produce bioproducts such as functional oligosaccharides, biofuels, and utility chemicals, such as furfural [72,73,74].
XOS Production
From hemicellulose, it is possible to obtain XOS (xylo-oligosaccharides). These unconventional sugars with prebiotic properties are obtained on an industrial scale, preferably by chemical and enzymatic hydrolysis of the hemicellulose substrate, whose main component is xylan [75]. XOS comprises xylose chains joined by β-(1,4) glycosidic bonds with a degree of polymerization from two to ten monosaccharides [76]. The methods for producing XOS from hemicellulose are feedstock-dependent; pretreatment is a common step to extract xylan from biomass, and it aims to provide a liquid fraction that is rich in depolymerized xylan as a substrate available for hydrolysis [77]. Production of XOS can be carried out by autohydrolysis, steam, microwave-assisted, and dilute acid or alkaline solutions. Applying chemical products (acids and bases) and autohydrolysis are commonly used for producing XOS. However, they are not considered biological and ecologically correct. These methods have disadvantages such as the generation of unwanted byproducts, including toxic ones, such as furfural and hydroxymethylfurfural (HMF); poor control of the degree of polymerization; and high costs, often requiring the use of harmful chemicals and robust equipment [78,79,80]. XOS is relatively more expensive to produce than other prebiotics. Currently, the industry is focused on developing efficient and more ecological processes for producing XOS, using renewable, cheap, and abundant lignocellulosic residues. Recent studies have employed green extraction technologies using enzyme application methods. The enzymatic process operating under mild conditions without harmful chemicals is more environmentally friendly and efficient in converting hemicellulose to oligosaccharides [81]. XOS can be considered emerging prebiotics. According to a study by Global Info Research, the worldwide XOS market is expected to grow from US$ 93 million in 2017 to US$ 130 million in 2023, with a compound annual growth rate of approximately 5.3% [82]. XOS, when consumed, has a prebiotic effect that enhances the growth of probiotic organisms in the gastrointestinal tract [83]. Studies report that dietary treatment with functional oligosaccharides has the effects of preventing obesity, diabetes, and related diseases, improving pancreas function, anti-inflammatory effects, and regulating the intestinal microbiota [84,85].
Furfural Production
From hemicellulose, it is possible to obtain furfural, a vital chemical product; the furan ring and the functional aldehyde group contained in molecules of this substance provide important reactive sites, making it a source for the manufacture of valuable chemical products for various industry segments [71,86]. Furfural is a component of herbicides, insecticides, pesticides, antiseptics, disinfectants, and rust removers, and it is involved in manufacturing pharmaceuticals, cosmetics, aromas, plastics, and resins [63]. Furfural is produced exclusively from the acid hydrolysis and dehydration of pentoses, mainly xylose. There are two furfural production processes, with one or two steps. Depolymerization of hemicellulose to xylose by acids and dehydration to furfural co-occur in the one-step process. In the two-step process, dissolution and depolymerization of hemicellulose occur under mild conditions, followed by dehydration of xylose to furfural [83]. In chemical process industries, homogeneous acid–base catalysis is widely used in organic conversion reactions. The production of furfural is one of the processes that involve using these catalysts, which can be toxic, dangerous, corrosive, and consequently damaging to the environment [84]. More sustainable furfural synthesis processes are currently sought without using these traditional catalysts. Organic acids such as formic acid, citric acid, and acetic acid are environmentally friendly, with low corrosivity, and can potentially replace mineral acids [85]. The use of metallic chlorides has become a research focus in recent years due to their low corrosivity and high catalytic activity [86]. Studies with metallic chlorides, such as SnCl4 (tin tetrachloride) and ZnCl2 (zinc chloride), have proven the efficient production of furfural from hemicellulose [87,88]. Using a two-phase system with DES (Deep Eutectic Solvent) as the catalytic phase is promising for sustainable furfural production. Biphasic systems can increase furfural yield, minimizing unwanted furfural degradation reactions. Organic solvents such as MIBK (methyl isobutyl ketone) and GVL (Gamma-valerolactone) are promising solvents that can be used as an extractive phase [89]. Another method considered green for furfural production is using ionic liquids, which have low volatility, a good dissolving capacity, and are recyclable, non-flammable, and toxic [63].
Bioethanol Production
The sustainable production of second-generation (2G) bioethanol from lignocellulosic biomass has emerged as a strategic alternative to reduce dependence on fossil fuels. In this context, guarana (Paullinia cupana) processing residues—primarily husks, discarded seeds, and pomace—represents a promising yet underutilized feedstock for integrated biorefinery processes. As the world’s largest producer of guarana, Brazil generates significant quantities of lignocellulosic waste during industrial processing, with biochemical composition analyses revealing favorable cellulose (30–50%) and hemicellulose (15–30%) content, comparable to sugarcane bagasse, the country’s primary 2G ethanol substrate [90]. This similarity suggests that technologies developed for other biomasses could be adapted for guarana residues, while their utilization offers notable strategic advantages by avoiding competition with food production chains and aligning with circular bioeconomy principles [91].
The efficient conversion of guarana residues faces multiple technical challenges, beginning with the recalcitrance of the lignocellulosic structure due to its lignin content (20–30%). Pretreatment methods, such as steam explosion and organosolv, have proven to be effective for other biomasses in enhancing enzymatic digestibility [92], though protocol optimization that is specific to guarana’s chemical particularities remains crucial. Subsequent enzymatic hydrolysis, while promising due to its specificity and mild reaction conditions, faces economic hurdles from high cellulase/hemicellulase costs at the industrial scale [93]. Potential solutions include in situ enzyme production by microorganisms (Trichoderma reesei, Aspergillus niger) [94], engineered enzymatic cocktails [95], and microbial consortia (Trichoderma, S. rolfsii, Aspergillus) [96].
Fermentation presents additional challenges in converting hexoses (C6) and pentoses (C5), with conventional Saccharomyces cerevisiae strains unable to process C5 sugars efficiently. Advances in modified yeast strains (Scheffersomyces stipitis), bacterial systems (E. coli, Zymomonas), and co-culture approaches [97,98,99] offer solutions, while integrated processing strategies—including separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF), and consolidated bioprocessing (CBP) [75]—show potential for cost reduction.
Despite this technical progress, scaling up faces the following substantial barriers: collection and storage logistics in Amazonian production regions, necessary adaptations at existing ethanol plants [100], and the absence of specific policy incentives for 2G ethanol in Brazil. Three key recommendations emerge for overcoming these challenges, which are as follows: (1) targeted investment in pretreatment optimization for guarana residues, (2) development of integrated biorefineries for high-value coproducts, and (3) academia-industry partnerships for pilot-scale implementation.
The lignocellulosic biomass from agricultural residues like guarana byproducts is widely recognized as the most viable option for sustainable biofuel production due to its low cost and non-competition with food [101]. However, despite scientific advances, large-scale lignocellulosic ethanol production remains hindered by persistent technical barriers in lignocellulose biodegradation [101,102]. The valorization of guarana residues presents a unique opportunity to combine regional development in the Amazon with energy sustainability, though realizing this potential will require coordinated research into biomass-specific process optimization, detailed techno-economic analyses, and policy frameworks to support this emerging bioenergy pathway.
3.3. Use of Lignin
Regarding the waste generated from guarana (Paullinia cupana), we can highlight the presence of lignin, an essential input for the natural cosmetics industry; in addition to presenting the potential for energy generation, its integration and use in a biorefinery is of great importance. Lignin is a natural aromatic polymer with a complex, heterogeneous, amorphous, three-dimensional structure. Due to these properties, it has several properties, such as biodegradability, excellent thermal stability, and antioxidant and antimicrobial properties [103,104]. It is an excellent natural preservative for use in the production of natural cosmetics.
It is also essential to emphasize the lignin’s ability to absorb ultraviolet radiation. Its aromatic structure is based on phenylpropane units linked through both carbon–carbon bonds, and it contains a wide variety of functional groups capable of absorbing radiation (carbon double bond, α-carbon, carbonyl, and other chromophores). Thus, lignin is suitable for broad-spectrum biological sunscreens [105].
Different delignification methods, such as thermal, chemical, or biochemical, extract lignin. More direct and ecologically correct methodologies are being studied to obtain lignin with a native structure [106]. The Kraft process is the most common method for producing lignin. According to the industrial process used, the most common types of lignin are sulfite, kraft, soda lignin, and organosolv lignin [107].
Lignin is a sustainable material that will be available in biorefineries and other applications in the future. However, the great potential of lignin needs to be satisfactorily exploited. Lignin can be used as a dispersant, adsorbent, carbon fiber precursor, and principal constituent of phenol-formaldehyde resins and thermoplastics. In addition, in cosmetics, lignin has other applications than those already described. It can also act as an emulsifier and stabilizer, components widely used in creams, lotions, and soaps for the face and body. Likewise, several other substances can be stored inside nanocapsules made with lignin, which would be helpful for the controlled release of active principles in cosmetic formulations [108].
Thus, this substance can be used as a raw material within an industry model based on the residual biomass generated in the guarana production chain without competition among other applications. Removing this fraction allows for better use of the others, such as cellulose and hemicellulose.
3.4. Cellulose Extraction and Technological Applications
The guarana (Paullinia cupana) supply chain generates considerable amounts of lignocellulosic residues that are rich in cellulose. Utilizing these residues as a source of cellulose presents a sustainable and economically viable opportunity, aligning with the principles of a circular bioeconomy and the valorization of agro-industrial waste.
Cellulose can be extracted from plant biomass through various methods, including acid hydrolysis, TEMPO-mediated oxidation, mechanical disintegration (e.g., high-pressure homogenization), or combinations of these techniques [109]. These processes yield different forms of cellulose—such as microcrystalline cellulose (MCC), cellulose nanofibrils (CNF), and cellulose nanocrystals (CNC)—each with distinct physicochemical properties like crystallinity, surface area, and chemical reactivity [110].
Nanocellulose, particularly CNF and CNC, stands out due to its superior structural properties. CNFs exhibit high aspect ratios and can form entangled networks, whereas CNCs are highly crystalline, rigid, and thermally stable [109]. These characteristics make nanocellulose an ideal candidate for various high-performance applications.
In the oil and gas industry, CNFs and CNCs are being explored as green additives in water-based drilling fluids (WBDFs), acting as rheology modifiers, fluid loss reducers, and shale stabilizers. Research has shown that CNFs significantly improve viscosity and cuttings transport efficiency, while CNCs and functionalized CNFs help minimize fluid loss by forming compact structures that seal the pore spaces of geological formations TEMPO-oxidized CNFs, in particular, exhibit excellent performance in high-temperature and high-salinity environments, maintaining rheological stability even after thermal aging [110].
Environmentally, nanocellulose is gaining momentum as a key material in water treatment systems. Hydrogels, aerogels, and membranes from CNF and CNC have demonstrated excellent adsorption capabilities for heavy metals (e.g., Pb2+, Cu2+, Hg2+), organic dyes, and pharmaceutical contaminants. Functionalizing cellulose surfaces with carboxyl, amino, or sulfonic groups enhances selectivity and binding affinity, enabling removal efficiencies above 90% in several cases [111].
Plant-derived cellulose is used in biomedical applications to develop biocompatible hydrogels for tissue engineering, drug delivery, and wound dressings. These materials mimic the extracellular matrix, offering high water retention, adjustable porosity, and nutrient and therapeutical permeability [112]. Their low cytotoxicity and ability to integrate with human cells make them excellent candidates for regenerative medicine.
Furthermore, cellulose-based materials are incorporated into sustainable composites, biodegradable films, and coatings with excellent gas and moisture barrier properties. These alternatives to conventional plastics expand the potential industrial applications of cellulose extracted from agricultural residues like guarana [109].
In summary, eco-friendly technologies can transform guarana processing residues—once considered low-value byproducts—into high-value cellulose. This approach enhances the sustainability of the guarana production chain while contributing to the development of advanced materials in accordance with green chemistry and circular economy principles.
3.5. Other Applications
Some initial studies were reported using the extract from guarana byproducts (Table 1). In the study by Júnior [21], the authors used processed seeds to produce lignocellulolytic enzymes through solid-state fermentation using Lentinus strigosus. The residue demonstrated the potential to be used in biotechnological processes.
Another biotechnological process in which the guarana waste, shell, and husk were utilized was that of Ribeiro et al. [27]. The authors used the residue as a catalyst for esterification, which can help with the accumulation of incorrect product disposal and is cheap, fast, and straightforward.
It is essential to highlight that waste guarana has the potential to be applied in several areas, as observed during our study. This material has great potential, and more studies must be conducted to verify the best alternatives.
3.6. Challenges and Future Research Directions
Despite the high biotechnological potential of the residues generated in the guarana (Paullinia cupana) production chain, several challenges still hinder their large-scale valorization. While the seeds are widely used, other fruit components—such as the peel, pulp, and exhausted seeds—remain underutilized, often being discarded, or employed in low-value applications such as organic fertilization [26].
One of the main limitations is the scarcity of studies providing an integrated and detailed characterization of these residues. There are limited data on chemical composition, compound stability, extraction efficiency, and real-world industrial applicability [4,8]. In addition, modern and sustainable extraction techniques, such as microwave-assisted extraction, ultrasound, ionic liquids, and deep eutectic solvents, remain largely unexplored in this context [7,25].
Another significant barrier is the lack of research addressing valorization processes’ techno-economic feasibility and scalability. Most studies remain confined to laboratory settings and lack cost–benefit analysis, environmental impact assessment, market integration potential, or alignment with local production chains [56].
Although the biorefinery concept has been proposed as a model for waste valorization, its practical implementation remains fragmented. Few studies adopt a truly integrated approach that combines bioactive compound recovery, energy production, biopolymers, and functional ingredients within a circular and sustainable model [9].
From an economic perspective, the valorization of guarana residues enables the creation of new revenue streams for local producers and cooperatives, especially in Amazonian communities, where guarana plays a key role in rural livelihoods. Studies emphasize that transforming agricultural waste into high-value products can significantly improve the profitability of small-scale agriculture and reduce reliance on primary product sales [9,56].
Cost considerations are also central to the viability of guarana residue valorization. Although biorefinery approaches offer long-term environmental and economic benefits, initial investment costs, infrastructure requirements, and technology transfer represent significant barriers to adoption in rural Amazonian contexts. Therefore, future research must integrate techno-economic assessments (TEA) and life cycle cost (LCC) analyses to ensure the scalability and adoption of sustainable innovations [95,101].
Therefore, valorizing guarana processing residues represents a concrete opportunity to strengthen the regional bioeconomy. A multidisciplinary scientific approach, aligned with technological innovation and sustainability principles, is essential to transform this environmental liability into economic assets. This strategy has the potential to foster local economies, reduce environmental burdens, and promote biodiversity conservation across the Amazon region.
4. Conclusions
Few studies have been found that focus on obtaining bioproducts from different parts of the guarana fruit. Investment into obtaining products with greater added value is an important alternative for developing regions that need to be conserved.
This review reveals the need to develop research to obtain bioproducts from waste, focusing on taking advantage of production chains established in regions that need environmental preservation. This statement is based on the necessity of obtaining greater economic returns sustainably from plant species with so much potential for biotechnology. In the case of guarana, the number of articles that mention obtaining bioproducts with an analysis of the technical and economic viability of the process is reduced. Some parts of the fruit have still been little studied, and few advanced techniques have been used for characterization, an essential step for better planning of utilization routes. Therefore, this literature search reveals that there are still many gaps that must be filled in the search for obtaining products with greater added value from waste generated in the guarana production chain.
The guarana production chain has the potential for the creation of a bioindustry where the use of different fractions of this fruit would be carried out, as well as where investment in research on the development of the proposed bioproducts and the expansion of the application of the biorefinery concept to other production chains in the Amazon can help to develop it sustainably.
Author Contributions
G.T.d.A., G.L.d.S., E.L., K.M.L., and G.H.B.d.S.: writing—original draft. F.R.M.B. and K.C.: writing—original draft and supervision. D.d.O., A.M.P., and L.d.S.S.S.: writing—original draft, supervision, conceptualization, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by FAPEAM (Fundação de Amparo à Pesquisa do Estado do Amazonas)—grant number: Edital N. 002/2021—Programa Amazônidas, Edital N. 010/2022—PDCA, PAIC/UFAM 2020–2021, PAIC/UFAM 2021–2022, Secretaria de Estado de Desenvolvimento Econômico, Ciência, Tecnologia e Inovação—SEDECTI, Governo do Estado do Amazonas, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—CAPES, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and PROPESP/UFAM (Federal University of Amazonas).
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank FAPEAM (Fundação de Amparo à Pesquisa do Estado do Amazonas). The author Eduardo Leonarski is grateful to the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico), Project number 150786/2024-7.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Santos, T.; Filho, V.A.; Rocha, V.M.; Menezes, J. Os impactos do desmatamento e queimadas de origem antrópica sobre o clima da amazônia brasileira: Um estudo de revisão. Rev. Geográfica Acadêmica 2017, 11, 157–181. [Google Scholar]
- Silva, L.M.R.; Sousa, P.H.M.; Sousa Sabino, L.B.; Prado, G.M.; Torres, L.B.V.; Maia, G.A.; de Figueiredo, R.W.; Ricardo, N.M.P.S. Brazilian (North and Northeast) Fruit By-Products. In Food Wastes and By-Products [Internet]; Wiley: Hoboken, NJ, USA, 2020; pp. 127–158. [Google Scholar] [CrossRef]
- Lopes, F.C.R.; Pereira, J.C.; Tannous, K. Thermal decomposition kinetics of guarana seed residue through thermogravimetric analysis under inert and oxidizing atmospheres. Bioresour. Technol. 2018, 270, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Santana, Á.L.; Macedo, G.A. Effects of hydroalcoholic and enzyme-assisted extraction processes on the recovery of catechins and methylxanthines from crude and waste seeds of guarana (Paullinia cupana). Food Chem. 2019, 281, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.S.; Silva, M.P.; Thomazini, M.; Cooperstone, J.L.; Campanella, O.H.; Costa Rodrigues, C.E.; Favaro-Trindade, C.S. Guaraná (Paullinia cupana) by-product as a source of bioactive compounds and as a natural antioxidant for food applications. J. Food Process. Preserv. 2021, 45, e15854. [Google Scholar] [CrossRef]
- Santana, Á.L.; Macedo, G.A. Health and technological aspects of methylxanthines and polyphenols from guarana: A review. J. Funct. Foods 2018, 47, 457–468. [Google Scholar] [CrossRef]
- Marques, L.L.M.; Ferreira, E.D.F.; de Paula, M.N.; Klein, T.; de Mello, J.C.P. Paullinia cupana: A multipurpose plant—A review. Rev. Bras. Farm. 2019, 29, 77–110. [Google Scholar] [CrossRef]
- Santana, Á.L.; Zanini, J.A.; Macedo, G.A. Dispersion-assisted extraction of guarana processing wastes to obtain polyphenols and alkaloids. J. Food Process. Eng. 2020, 43, e13381. [Google Scholar] [CrossRef]
- Embrapa. Bioeconomy [Internet]. 2020 [Cited 22 January 2023]. Available online: https://www.embrapa.br/en/tema-bioeconomia (accessed on 5 June 2023).
- Rathore, D.; Nizami, A.S.; Singh, A.; Pant, D. Key issues in estimating energy and greenhouse gas savings of biofuels: Challenges and perspectives. Biofuel Res. J. 2016, 3, 380–393. [Google Scholar] [CrossRef]
- Bala, S.; Garg, D.; Sridhar, K.; Inbaraj, B.S.; Singh, R.; Kamma, S.; Tripathi, M.; Sharma, M. Transformation of agro-waste into value-added bioproducts and bioactive compounds: Micro/nano formulations and application in the agri-food-pharma sector. Bioengineering 2023, 10, 152. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.K.; Rahayu, F.; Yustina, I.; Fatah, G.S.A.; Kariada, I.K.; Antarlina, S.S.; Jufri, A.; Pamungkas, D. Contribution of yeast and its biomass for the preparation of industrially essential materials: A boon to circular economy. Bioresour. Technol. Rep. 2023, 23, 101508. [Google Scholar] [CrossRef]
- Saravanan, A.; Karishma, S.; Senthil Kumar, P.; Rangasamy, G. A review on regeneration of biowaste into bio-products and bioenergy: Life cycle assessment and circular economy. Fuel 2023, 338, 127221. [Google Scholar] [CrossRef]
- Wani, A.K.; Rahayu, F.; Fauziah, L.; Suhara, C. Advances in safe processing of sugarcane and bagasse for the generation of biofuels and bioactive compounds. J. Agric. Food Res. 2023, 12, 100549. [Google Scholar] [CrossRef]
- Schimpl, F.C.; Da Silva, J.F.; Gonçalves, J.F.D.C.; Mazzafera, P. Guarana: Revisiting a highly caffeinated plant from the Amazon. J. Ethnopharmacol. 2013, 150, 14–31. [Google Scholar] [CrossRef]
- Weil, A.G.; Witkoski, A.C. A organização ancestral do trabalho na produção do guaraná em Maués/Amazonas. Acta Sci. Hum. Soc. Sci. 2023, 45, e69245. Available online: https://periodicos.uem.br/ojs/index.php/ActaSciHumanSocSci/article/view/69245 (accessed on 22 October 2023). [CrossRef]
- Conab. Histórico Mensal do Guaraná [Internet]. 2022 [Cited 11 January 2022]. Available online: https://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-mensal-de-Guarana (accessed on 6 March 2023).
- Akca, S.; Akpinar, A. The Effects of Grape, pomegranate, Sesame Seed Powder and Their Oils on Probiotic Ice Cream: Total phenolic contents, antioxidant activity and probiotic viability. Food Biosci. 2021, 42, 101203. [Google Scholar] [CrossRef]
- Silva, M.P.; Farsoni, E.G.; Gobato, C.F.; Thomazini, M.; Favaro-Trindade, C.S. Simultaneous encapsulation of probiotic and guaraná peel extract for development of functional peanut butter. Food Control. 2022, 138, 109050. [Google Scholar] [CrossRef]
- Santana, Á.L.; Queirós, L.D.; Martínez, J.; Macedo, G.A. Pressurized liquid- and supercritical fluid extraction of crude and waste seeds of guarana (Paullinia cupana): Obtaining of bioactive compounds and mathematical modeling. Food Bioprod. Process. 2019, 117, 194–202. [Google Scholar] [CrossRef]
- de Oliveira Júnior, S.D.; dos Santos Gouvêa, P.R.; de Aguiar, L.V.B.; Pessoa, V.A.; dos Santos Cruz Costa, C.L.; Chevreuil, L.R.; BritoNascimento, L.B.d.; dos Santos, E.S.; Sales-Campos, C. Production of Lignocellulolytic Enzymes and Phenolic Compounds by Lentinus strigosus from the Amazon Using Solid-State Fermentation (SSF) of Guarana (Paullinia cupana) Residue. Appl. Biochem. Biotechnol. 2022, 194, 2882–2900. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; Thomazini, M.; Holkem, A.T.; Pinho, L.S.; Genovese, M.I.; Fávaro-Trindade, C.S. Production and characterization of solid lipid microparticles loaded with guaraná (Paullinia cupana) seed extract. Food Res. Int. 2019, 123, 144–152. [Google Scholar] [CrossRef]
- Silva, M.P.; da SMesquita, M.; Fernanda, F.T.; Thomazini, M.; Favaro-Trindade, C.S. Fortification of yoghurt drink with microcapsules loaded with Lacticaseibacillus paracasei BGP-1 and guaraná seed extract. Int. Dairy J. 2022, 125, 105230. [Google Scholar] [CrossRef]
- de Almeida, A.S.F.; Corrêa Junior, A.; Bentes, J.L.d.S. Synthesis of silver nanoparticles (Agnps) by Fusarium concolor and inhibition of plant pathogens. Summa Phytopathol. 2021, 47, 9–15. [Google Scholar] [CrossRef]
- Pinho, L.S.; Patel, B.K.; Campanella, O.H.; Rodrigues, C.E.d.C.; Favaro-Trindade, C.S. Microencapsulation of Carotenoid-Rich Extract from Guaraná Peels and Study of Microparticle Functionality through Incorporation into an Oatmeal Paste. Foods 2023, 12, 1170. [Google Scholar] [CrossRef] [PubMed]
- Pinho, L.; Silva, M.P.; Martelli-Tosi, M.; Massarioli, A.P.; Melo, P.S.; Alencar, S.M.; Favaro-Trindade, C.S. Co-encapsulation of guaraná extracts and probiotics increases probiotic survivability and simultaneously delivers bioactive compounds in simulated gastrointestinal fluids. LWT 2022, 161, 113351. [Google Scholar] [CrossRef]
- Ribeiro, F.C.P.; Santos, V.O.; Araujo, R.O.; Santos, J.L.; Chaar, J.S.; Falcão, N.P.S.; Farias, M.A.S.; de Souza, L.K.C. Determination of the thermal stability of sulfonic groups in heterogeneous acid catalysts derived from residue of guarana amazon biomass. J. Therm. Anal. Calorim. 2023, 148, 23–35. [Google Scholar] [CrossRef]
- Saraiva, S.M.; Jacinto, T.A.; Gonçalves, A.C.; Gaspar, D.; Silva, L.R. Overview of Caffeine Effects on Human Health and Emerging Delivery Strategies. Pharmaceuticals 2023, 16, 1067. [Google Scholar] [CrossRef] [PubMed]
- Grgic, J. Effects of Caffeine on Resistance Exercise: A Review of Recent Research. Sports Med. 2021, 51, 2281–2298. [Google Scholar] [CrossRef]
- Marques, L.L.M.; Panizzon, G.P.; Aguiar, B.A.A.; Simionato, A.S.; Cardozo-Filho, L.; Andrade, G.; de Oliveira, A.G.; Guedes, T.A.; de Mello, J.C.P. Guaraná (Paullinia cupana) seeds: Selective supercritical extraction of phenolic compounds. Food Chem. 2016, 212, 703–711. [Google Scholar] [CrossRef]
- Carvalho, F.A.L.; Lorenzo, J.M.; Pateiro, M.; Bermúdez, R.; Purriños, L.; Trindade, M.A. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 °C. Food Res. Int. 2019, 125, 108554. [Google Scholar] [CrossRef]
- Figueira, M.S.; Soares, M.J.; Soares-Freitas, R.A.M.; Sampaio, G.R.; Pinaffi-Langley, A.C.; Santos, O.V.; De Camargo, A.C.; Rogero, M.M.; Torres, E.A.F.S. Effect of guarana seed powder on cholesterol absorption in vitro and in Caco-2 cells. Food Res. Int. 2022, 162, 111968. [Google Scholar] [CrossRef]
- Algarve, T.D.; Assmann, C.E.; Cadoná, F.C.; Machado, A.K.; Manica-Cattani, M.F.; Sato-Miyata, Y.; Asano, T.; Duarte, M.M.M.F.; Ribeiro, E.E.; Aigaki, T.; et al. Guarana Improves Behavior and Inflammatory Alterations Triggered by Methylmercury Exposure: An In Vivo Fruit Fly and In Vitro Neural Cells Study. Environmental Science and Pollution Research 2019, 26, 15069–15083. [Google Scholar] [CrossRef]
- Braga, L.R.; Silva, F.M. Embalagens ativas: Uma nova abordagem para embalagens alimentícias. Braz. J. Food Res. 2017, 8, 170. [Google Scholar] [CrossRef]
- Nogueira, D.; Marasca, N.S.; Latorres, J.M.; Costa, J.A.V.; Martins, V.G. Effect of an active biodegradable package made from bean flour and açaí seed extract on the quality of olive oil. Polym. Eng. Sci. 2022, 62, 1070–1080. [Google Scholar] [CrossRef]
- da Silva Filipini, G.; Romani, V.P.; Guimarães Martins, V. Biodegradable and active-intelligent films based on methylcellulose and jambolão (Syzygium cumini) skins extract for food packaging. Food Hydrocoll. 2020, 109, 106139. [Google Scholar] [CrossRef]
- Costa, E.K.D.C.; Rocha, I.S.; Silva, R.D.J.; Druzian, J.I. Estudo prospectivo relativo a depósitos de patentes relacionadas à produção de filmes elaborados pela técnica de casting. Cad. Prospecção 2016, 9, 280. [Google Scholar] [CrossRef][Green Version]
- Rocha, G.O.; Farias, M.G.; Carvalho CWPde Ascheri, J.L.R.; Galdeano, M.C. Filmes compostos biodegradáveis a base de amido de mandioca e proteína de soja. Polímeros 2014, 24, 587–595. [Google Scholar] [CrossRef]
- Mir, S.A.; Dar, B.N.; Wani, A.A.; Shah, M.A. Effect of plant extracts on the techno-functional properties of biodegradable packaging films. Trends Food Sci. Technol. 2018, 80, 141–154. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J.A. Investigation of the physicochemical, antimicrobial and antioxidant properties of gelatin-chitosan edible film mixed with plant ethanolic extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Noah, N. Green synthesis: Characterization and application of silver and gold nanoparticles. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Paniz, O.G.; Scheik, L.K.; da Silva, G.E.H.; Gonçalves, M.R.F.; de Oliveira, A.D.; Araújo, E.M.; Carreño, N.L.V. Cellulose acetate and silver particles composite synthesis to antimicrobial applications. Rev. Mater. 2018, 23, e12244. [Google Scholar]
- Bogireddy, N.K.R.; Pal, U.; Gomez, L.M.; Agarwal, V. Size controlled green synthesis of gold nanoparticles using Coffea arabica seed extract and their catalytic performance in 4-nitrophenol reduction. RSC Adv. 2018, 8, 24819–24826. [Google Scholar] [CrossRef]
- Chaicherd, S.; Killingsworth, M.C.; Pissuwan, D. Toxicity of gold nanoparticles in a commercial dietary supplement drink on connective tissue fibroblast cells. SN Appl. Sci. 2019, 1, 336. [Google Scholar] [CrossRef]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green synthesis of nanoparticles: Current developments and limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Solgi, M.; Taghizadeh, M. Biogenic synthesis of metal nanoparticles by plants. In Biogenic Nano-Particles and Their Use in Agro-ecosystems; Springer: Singapore, 2020. [Google Scholar]
- Dzimitrowicz, A.; Jamróz, P.; diCenzo, G.C.; Sergiel, I.; Kozlecki, T.; Pohl, P. Preparation and characterization of gold nanoparticles prepared with aqueous extracts of Lamiaceae plants and the effect of follow-up treatment with atmospheric pressure glow microdischarge. Arab. J. Chem. 2019, 12, 4118–4130. [Google Scholar] [CrossRef]
- Folorunso, A.; Akintelu, S.; Oyebamiji, A.K.; Ajayi, S.; Abiola, B.; Abdusalam, I.; Morakinyo, A. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J. Nanostructure Chem. 2019, 9, 111–117. [Google Scholar] [CrossRef]
- Islam, N.U.; Jalil, K.; Rauf, A.; Muhammad, N.; Khan, A.; Shah, M.R.; Khan, M.A. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab. J. Chem. 2019, 12, 2914–2925. [Google Scholar] [CrossRef]
- Kunoh, T.; Takeda, M.; Matsumoto, S.; Suzuki, I.; Takano, M.; Kunoh, H.; Takada, J. Green Synthesis of Gold Nanoparticles Coupled with Nucleic Acid Oxidation. ACS Sustain. Chem. Eng. 2018, 6, 364–373. [Google Scholar] [CrossRef]
- Wongyai, K.; Wintachai, P.; Maungchang, R.; Rattanakit, P. Exploration of the Antimicrobial and Catalytic Properties of Gold Nanoparticles Greenly Synthesized by Cryptolepis buchanani Roem. and Schult Extract. J. Nanomater. 2020, 2020, 1320274. [Google Scholar] [CrossRef]
- Gokila, V.; Perarasu, V.T.; Rufina, R.D.J. Qualitative comparison of chemical and green synthesized Fe3O4 nanoparticles. Adv. Nano Res. 2021, 10, 71–76. [Google Scholar]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from fruit processing wastes: Green approaches to valuable chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Linares-Pasten, J.A.; Aronsson, A.; Karlsson, E.N. Structural Considerations on the Use of Endo-Xylanases for the Production of prebiotic Xylooligosaccharides from Biomass. Curr. Protein Pept. Sci. 2018, 19, 48–67. [Google Scholar] [CrossRef]
- Pereira, J.C.; Lopes, F.C.R.; Tannous, K. Guarana Fruit: Traditional Applications and Sustainable Use of Residues; Todorov, S.D., Pieri, F.A., Eds.; Food Science and Technology Series; Nova Science Publishers: New York, NY, USA, 2018. [Google Scholar]
- Kaprelyants, L.; Zhurlova, O.; Shpyrko, T.; Pozhitkova, L. Xylooligosaccharides from agricultural by-products: Characterisation, production and physiological effects. Food Sci. Technol. 2017, 11, 25–34. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Cerqueira, M.A.; Silva, H.D.; Rodríguez-Jasso, R.M.; Vicente, A.A.; Teixeira, J.A. Biorefinery valorization of autohydrolysis wheat straw hemicellulose to be applied in a polymer-blend film. Carbohydr. Polym. 2013, 92, 2154–2162. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; He, Q.; Fan, G.; Cheng, Q.; Song, G. Extraction and modification of hemicellulose from lignocellulosic biomass: A review. Green Process. Synth. 2021, 10, 779–804. [Google Scholar] [CrossRef]
- Gallina, G.; Alfageme, E.R.; Biasi, P.; García-Serna, J. Hydrothermal extraction of hemicellulose: From lab to pilot scale. Bioresour. Technol. 2018, 247, 980–991. [Google Scholar] [CrossRef]
- Morais, E.S.; Da Costa Lopes, A.M.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of ionic liquids and deep eutectic solvents in polysaccharides dissolution and extraction processes towards sustainable biomass valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef]
- Hu, L.; Peng, H.; Xia, Q.; Zhang, Y.; Ruan, R.; Zhou, W. Effect of ionic liquid pretreatment on the physicochemical properties of hemicellulose from bamboo. J. Mol. Struct. 2020, 1210, 128067. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2020, 200, 905–913. [Google Scholar] [CrossRef]
- Foong, S.Y.; Liew, R.K.; Yang, Y.; Cheng, Y.W.; Yek, P.N.Y.; Mahari, W.A.W.; Lee, X.Y.; Han, C.S.; Vo, D.-V.N.; Van Le, Q.; et al. Valorization of biomass waste to engineered activated biochar by microwave pyrolysis: Progress, challenges, and future directions. Chem. Eng. J. 2020, 389, 124408. [Google Scholar] [CrossRef]
- Özbek, H.N.; Fockink, D.H.; Yanık, D.K.; Göğüş, F.; Lukasik, R. The green biorefinery concept for the valorisation of pistachio shell by high-pressure CO2/H2O system. J. Clean. Prod. 2018, 196, 842–851. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, X.; Ma, Z.; Gong, J.; Wang, H.; Lv, Y. Efficient extraction and structural characterization of hemicellulose from sugarcane bagasse pith. Polymers 2020, 12, 608. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Liu, Y. Ultrasonic-assisted extraction and purification of xylo-oligosaccharides from wheat bran. J. Food Process. Eng. 2022, 45, e14152. [Google Scholar] [CrossRef]
- Banerjee, S.; Patti, A.F.; Ranganathan, V.; Arora, A. Hemicellulose based biorefinery from pineapple peel waste: Xylan extraction and its conversion into xylooligosaccharides. Food Bioprod. Process. 2019, 117, 38–50. [Google Scholar] [CrossRef]
- Khaire, K.C.; Moholkar, V.S.; Goyal, A. Bioconversion of sugarcane tops to bioethanol and other value-added products: An overview. Mater. Sci. Energy Technol. 2021, 4, 54–68. [Google Scholar] [CrossRef]
- Delbecq, F.; Wang, Y.; Muralidhara, A.; El Ouardi, K.E.; Marlair, G.; Len, C. Hydrolysis of hemicellulose and derivatives—A review of recent advances in the production of furfural. Front. Chem. 2018, 6, 146. [Google Scholar] [CrossRef]
- Palaniappan, A.; Antony, U.; Emmambux, M.N. Current status of xylooligosaccharides: Production, characterization, health benefits and food application. Food Sci. Technol. 2021, 111, 506–519. [Google Scholar] [CrossRef]
- Belorkar, S.A.; Gupta, A.K. Oligosaccharides: A boon from nature’s desk. AMB Express 2016, 6, 82. [Google Scholar] [CrossRef]
- Poletto, P.; Pereira, G.N.; Monteiro, C.R.M.; Pereira, M.A.F.; Bordignon, S.E.; de Oliveira, D. Xylooligosaccharides: Transforming the lignocellulosic biomasses into valuable 5-carbon sugar prebiotics. Process. Biochem. 2020, 91, 352–363. [Google Scholar] [CrossRef]
- Gao, X.; Kumar, R.; Wyman, C.E. Fast hemicellulose quantification via a simple one-step acid hydrolysis. Biotechnol. Bioeng. 2014, 111, 1088–1096. [Google Scholar] [CrossRef]
- Chen, H.; Fu, X. Industrial technologies for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2016, 57, 468–478. [Google Scholar] [CrossRef]
- Bian, J.; Peng, P.; Peng, F.; Xiao, X.; Xu, F.; Sun, R.C. Microwave-assisted acid hydrolysis to produce xylooligosaccharides from sugarcane bagasse hemicelluloses. Food Chem. 2014, 156, 7–13. [Google Scholar] [CrossRef]
- Amorim, C.; Silvério, S.C.; Prather, K.L.J.; Rodrigues, L.R. From lignocellulosic residues to market: Production and commercial potential of xylooligosaccharides. Biotechnol. Adv. 2019, 37, 107397. [Google Scholar] [CrossRef]
- The Market Reports. Global Xylo-Oligosaccharide (XOS) Market by Manufacturers, Regions, Type and Application, Forecast to 2023 [Internet]. 2018 [Cited 1 January 2022]. Available online: https://www.themarketreports.com/report/global-xylo-oligosaccharide-xos-market-by-anufacturers-regions-type-and-application-forecast-to-2023 (accessed on 25 October 2023).
- Azelee, N.I.W.; Jahim, J.M.; Ismail, A.F.; Fuzi, S.F.Z.M.; Rahman, R.A.; Md Illias, R. High xylooligosaccharides (XOS) production from pretreated kenaf stem by enzyme mixture hydrolysis. Ind. Crops Prod. 2016, 81, 11–19. [Google Scholar] [CrossRef]
- Abdo, A.A.A.; Zhang, C.; Patil, P.; Teng, C.; Li, X.; Liang, X. Biological functions of nutraceutical xylan oligosaccharides as a natural solution for modulation of obesity, diabetes, and related diseases. Int. Food Res. J. 2022, 29, 236–247. [Google Scholar] [CrossRef]
- Zhu, D.; Yan, Q.; Liu, J.; Wu, X.; Jiang, Z. Can functional oligosaccharides reduce the risk of diabetes mellitus? FASEB J. 2019, 33, 11655–11667. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Peng, H.; Zhang, Y.; Fu, G.; Liu, Y.; Xiao, Z.; Huang, L.; Bi, H. Microwave-assisted furfural production from xylose and bamboo hemicellulose in a biphasic medium. Biomass Convers. Biorefin. 2021, 13, 7895–7907. [Google Scholar] [CrossRef]
- Machado, G.; Leon, S.; Santos, F.; Lourega, R.; Dullius, J.; Mollmann, M.E.; Eichler, P. Literature Review on Furfural Production from Lignocellulosic Biomass. Nat. Resour. 2016, 7, 115. [Google Scholar] [CrossRef]
- Dulie, N.W.; Woldeyes, B.; Demsash, H.D.; Jabasingh, A.S. An Insight into the Valorization of Hemicellulose Fraction of Biomass into Furfural: Catalytic Conversion and Product Separation. Waste Biomass Valorization 2020, 12, 531–552. [Google Scholar] [CrossRef]
- Yao, B.; Kang, Q.; Fu, J.; Liu, Y.; Ao, W.; Wang, L.; Jiang, Z.; Zhang, T.; Song, Y.; Deng, Z.; et al. Catalytic hydrolysis of corncob for production of furfural and cellulose-rich solids: Product characterization and analysis. Biomass Bioenergy 2023, 168, 106658. [Google Scholar] [CrossRef]
- Ye, L.; Han, Y.; Wang, X.; Lu, X.; Qi, X.; Yu, H. Recent progress in furfural production from hemicellulose and its derivatives: Conversion mechanism, catalytic system, solvent selection. Mol. Catal. 2021, 515, 111899. [Google Scholar] [CrossRef]
- Wang, W.; Ren, J.; Li, H.; Deng, A.; Sun, R. Direct transformation of xylan-type hemicelluloses to furfural via SnCl4 catalysts in aqueous and biphasic systems. Bioresour. Technol. 2015, 183, 188–194. [Google Scholar] [CrossRef]
- Hu, B.; Xie, W.L.; Wu, Y.T.; Liu, J.; Ma, S.W.; Wang, T.P.; Zheng, S.; Lu, Q. Mechanism study on the formation of furfural during zinc chloride-catalyzed pyrolysis of xylose. Fuel 2021, 295, 120656. [Google Scholar] [CrossRef]
- Lee, C.B.T.L.; Wu, T.Y. A review on solvent systems for furfural production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2021, 137, 110172. [Google Scholar] [CrossRef]
- Sánchez, Ó.J.; Cardona, C.A. Trends in biotechnological production of fuel ethanol from different feedstocks. Bioresour. Technol. 2008, 99, 5270–5295. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Gupta, R.; Singh, A. Microbial cellulases and their industrial applications. Enzym. Res. 2011, 2011, 280696. [Google Scholar] [CrossRef]
- Klein-Marcuschamer, D.; Oleskowicz-Popiel, P.; Simmons, B.A.; Blanch, H.W. The challenge of enzyme cost in the production of lignocellulosic biofuels. Biotechnol. Bioeng. 2012, 109, 1083–1087. [Google Scholar] [CrossRef]
- Andlar, M.; Rezić, T.; Marđetko, N.; Kracher, D.; Ludwig, R.; Šantek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef]
- Ko, J.K.; Um, Y.; Woo, H.M.; Kim, K.H.; Lee, S.M. Ethanol production from lignocellulosic hydrolysates using engineered Saccharomyces cerevisiae harboring xylose isomerase-based pathway. Bioresour. Technol. 2016, 209, 290–296. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, P.; Zhang, G.; Yang, Q.; Lu, J.; Xia, T.; Peng, L.; Wang, Y. Cascading of engineered bioenergy plants and fungi sustainable for low-cost bioethanol and high-value biomaterials under green-like biomass processing. Renew. Sustain. Energy Rev. 2021, 137, 110586. [Google Scholar] [CrossRef]
- Malik, K.; Sharma, P.; Yang, Y.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; et al. Lignocellulosic biomass for bioethanol: Insight into the advanced pretreatment and fermentation approaches. Ind. Crop. Prod. 2022, 188, 115569. [Google Scholar] [CrossRef]
- Lynd, L.R.; Laser, M.S.; Bransby, D.; E Dale, B.; Davison, B.; Hamilton, R.; Himmel, M.; Keller, M.; McMillan, J.D.; Sheehan, J.; et al. How biotech can transform biofuels. Nat. Biotechnol. 2008, 26, 169–172. [Google Scholar] [CrossRef]
- Taha, M.; Foda, M.; Shahsavari, E.; Aburto-Medina, A.; Adetutu, E.; Ball, A. Commercial feasibility of lignocellulose biodegradation: Possibilities and challenges. Curr. Opin. Biotechnol. 2016, 38, 190–197. [Google Scholar] [CrossRef]
- Avanthi, A.; Kumar, S.; Sherpa, K.C.; Banerjee, R. Bioconversion of hemicelluloses of lignocellulosic biomass to ethanol: An attempt to utilize pentose sugars. Biofuels 2016, 8, 431–444. [Google Scholar] [CrossRef]
- Patil, S.V.; Argyropoulos, D.S. Stable Organic Radicals in Lignin: A Review. ChemSusChem 2017, 10, 3284–3303. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Qian, Y.; Qiu, X.; Zhu, S. Lignin: A nature-inspired sun blocker for broadspectrum Sunscreens. Green Chem. 2015, 17, 320–324. [Google Scholar] [CrossRef]
- Poveda-Giraldo, J.A.; Solarte-Toro, J.C.; Cardona Alzate, C.A. The potential use of lignin as a platform product in biorefineries: A review. Renew. Sustain. Energy Rev. 2021, 138, 110688. [Google Scholar] [CrossRef]
- Chávez-Sifontes, M.; Domine, M.E. Lignin, Structure and Applications: Depolymerization Methods for Obtaining Aromatic Derivatives of Industrial Interest. Av. Cienc. Ingeniería 2013, 4, 15–46. [Google Scholar]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin valorization: Improving lignin processing in the biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-C.; Liu, X.; Lv, K.; Sun, J.; Dai, C.; Liao, B.; Liu, C.; Mei, C.; Wu, Q.; Hubbe, M. Cellulose nanomaterials in oil and gas industry: Current status and future perspectives. Prog. Mater. Sci. 2023, 139, 101187. [Google Scholar] [CrossRef]
- Khan, M.A.; Li, M.-C.; Lv, K.; Sun, J.; Liu, C.; Liu, X.; Shen, H.; Dai, L.; Lalji, S.M. Cellulose derivatives as environmentally-friendly additives in water-based drilling fluids: A review. Carbohydr. Polym. 2024, 342, 122355. [Google Scholar] [CrossRef] [PubMed]
- Azimi, B.; Sepahvand, S.; Ismaeilimoghadam, S.; Kargarzadeh, H.; Ashori, A.; Jonoobi, M.; Danti, S. Application of Cellulose-Based Materials as Water Purification Filters: A State-of-the-Art Review. J. Polym. Environ. 2023, 32, 345–366. [Google Scholar] [CrossRef]
- Selvaraj, S.; Chauhan, A.; Dutta, V.; Verma, R.; Rao, S.K.; Radhakrishnan, A.; Ghotekar, S. A state-of-the-art review on plant-derived cellulose-based green hydrogels and their multifunctional role in advanced biomedical applications. Int. J. Biol. Macromol. 2024, 265, 130991. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).