Simple Summary
All living species are the result of countless generations of “survival of the fittest”. Therefore, one may suppose that all living species, small and large, have attained the same high level of fitness, at least approximately. Indeed, according to the recently proposed “equal fitness paradigm” (EFP), this fitness universally approximates 22.4 kJ/g, which is quantified as the lifetime energetic production of surviving offspring per parental body mass. However, as I argue, the EFP has several problems. They include a questionable measure of fitness and flawed methods used to quantify and compare it among species. Other measures of fitness, which include the timing of reproduction, a critical factor affecting fitness not considered by the EFP, vary considerably with body size and habitat. The EFP also ignores the profound effects of population abundance and geographical range size on the survival and multiplication of species. In addition, if the EFP were true, natural selection at the species level, which depends on fitness variation, would be impossible. Therefore, I advocate a “variable fitness paradigm” (VFP) originally proposed by Charles Darwin and Alfred Wallace. According to the VFP, fitness varies significantly at multiple levels of biological organization, thus permitting selection at all these levels.
Abstract
My article criticizes the view held by many ecologists that species have evolved essentially equivalent levels of fitness, thus permitting their coexistence. I show that a recently proposed version of this view called the “equal fitness paradigm” (EFP) has multiple problems, empirically and conceptually. Some of these problems are (1) an energetic fitness measure (OPG = lifetime production of surviving offspring per parental body mass) that ignores the critical effect of the timing of reproduction; (2) flawed methods and data used to calculate and interpret the body-size scaling invariance of OPG upon which the EFP is based; (3) omission of the profound effects of population size and geographical range size on species-level fitness; and (4) lack of recognition that if the EFP were true, species-level selection would not be able to operate. By contrast, the “variable fitness paradigm” (VFP), which is a mainstay of modern evolutionary biology, is supported by numerous lines of evidence at multiple levels of biological organization. Extensive fitness variation allows natural selection to operate at all these levels. Distinguishing fitness and adaptiveness as reproductive power and efficiency of resource acquisition, respectively, helps explain species coexistence within the conceptual framework of the VFP. No EFP is needed.
1. Introduction
In a democracy, it is said that all people are created equal because they have equal rights under the law. However, in nature, is it also true that all organisms (species) are created equal in some sense? According to some religions, the answer is “yes”, because they were created to fit into a perfectly orchestrated divine design. Furthermore, even if one believes that all species evolved naturally rather than being the result of divine creation, one could consider all living species to be equal because they are “equally evolved” ([1], p. 3). Given that living species are the result of countless generations of natural selection rejecting poorly adapted organisms, one might suppose that they have attained equally high adaptive fits to specific environments, or nearly so. Indeed, several ecologists have argued that species coexistence in ecological communities is the result of the evolution of “equalized fitness” achieved in diverse ways by demographic or ecological trade-offs between major components of fitness, including survival versus growth/reproduction or competitive versus colonizing abilities (e.g., [2,3,4,5,6,7,8,9]). This outlook permits one to embrace two apparently contradictory perspectives at the same time: living species are essentially “equal” in terms of their present survival and thus evolutionary success after millions of years of “struggling for existence”, while also being very different in terms of their form, function, life history, and ecology.
This equal fitness perspective has been developed further from an energetic point of view. According to some ecologists, all species, small and large, have evolved an approximately equal energetic fitness, as defined by surviving offspring production during a lifetime [10,11,12,13]. According to this “equal fitness paradigm” (EFP), although species have evolved in diverse ways along a life-history continuum from high rates of reproduction and mortality (and thus short lives) to low rates of reproduction and mortality (and thus long lives), they have all attained equal or nearly equal lifetime offspring production. Although the EFP has been recently promoted by ecologists with little critical discussion so far (but see [14]), it appears to contradict a fundamental assumption of the Darwinian principle of natural selection, i.e., that evolutionary fitness varies substantially among individuals, populations, species, or other units of selection (see [15,16,17] and Section 3). Indeed, selection (sorting) at any hierarchical level cannot occur without variable fitness. Thus, Darwinian evolution, as commonly accepted, is based on a “variable fitness paradigm” (VFP) in contrast to the recently proposed EFP. Is one of these paradigms incorrect or can they both be accommodated within a larger synthetic framework?
The purpose of my commentary is to address this important question, and thereby show that the EFP, as currently developed, has multiple problems conceptually and empirically. I describe these problems in some detail, and in the process provide vindication for the VFP as originated by Charles Darwin [15] and Alfred Wallace [18] and embraced by mainstream evolutionary biology. Although this vindication may seem obvious to many evolutionary biologists, I chiefly direct my analysis toward ecologists and other scientists who may be contemplating using the EFP in their research program. Note that fitness has been defined and estimated in many ways, and the purpose of my article is not to advocate the universal application of any one fitness measure, but to consider multiple measures when evaluating the relative merits of the EFP and VFP (see Appendix A). However, I do advocate that the concept of fitness should be used in operational, non-tautological ways that allow quantitative comparisons among individuals, populations, species and clades. I also argue that at the population/species level the concept of “fitness” should be distinguished from that of “adaptiveness”, as I operationalize quantitatively in Section 6 and Appendix A.3.
2. The “Equal Fitness Paradigm”
The recently proposed “equal fitness paradigm” (EFP) is based on comparing the interspecific body-mass scaling of the mass-specific production rate of surviving offspring (OP) and generation time (G) or lifetime [10,11,12,13]. From these analyses it is claimed that OP and G scale inversely and thus OP x G (or OPG) is “invariant” because it scales with body mass zerometrically (i.e., with a slope of 0), or nearly so. Since OPG is regarded as a useful energy- and time-based measure of fitness, it is claimed that all species have equal fitness, at least approximately, regardless of their body size.
However, the EFP and the analyses used to support it have several problems, which are explained in the next few sections. They include (1) insufficient justification of the fitness measure OPG used to support the EFP, (2) assuming steady-state populations, though most populations and their energy supplies fluctuate, often greatly and to various degrees, (3) assuming the validity of the “rate of living theory”, though it is not generally applicable, (4) assuming that biological time represents an independent 4th dimension in 3/4-power biological scaling, though it scales allometrically (disproportionately) with other spatial dimensions (e.g., body length), (5) ignoring substantial effects of population size and geographical range size on fitness at the population or species level, (6) providing insufficient support for the EFP by using allometric scaling analyses that have multiple conceptual and empirical problems, including that OPG does not scale zerometrically with body mass in birds and mammals (see Section 2.6), contrary to the EFP, (7) not appreciating that a supposed scaling invariance of OPG is not sufficient to support the EFP, (8) underappreciating and not sufficiently explaining the extensive species variation around the body-mass scaling relationships of OP, G and OPG used to support the EFP, (9) not acknowledging that if the EFP were true, species selection, which depends on variable fitness, would not be possible, and (10) insufficient justification for a claimed biophysical basis of the EFP.
2.1. EFP Fitness Measure Has Not Been Sufficiently Justified
Proponents of the EFP have not adequately justified why their measure of fitness should be preferred over others that have been proposed, though they acknowledge that there are other fitness indicators [10,11]. Fitness has been defined in multiple ways and has been applied at multiple hierarchical levels of biological organization (see Appendix A). Even if we restrict our definitions to those that are energy- or time-based, as have the proponents of the EFP, there are multiple possibilities. For energy-based fitness, why not use the concepts of “expansive energy” [19] or “reproductive power” [20,21] (see also Appendix A.2)? For time-based fitness, why not use the concept of “stability” or “persistence” (i.e., relative survival time), as applied to various units of selection from genes to clades and ecological communities (see, e.g., [22,23,24,25,26,27] and Appendix A.1)? One might suggest that OPG (total offspring production during a generation time or lifetime) is a preferred measure of fitness because it is both energy- and time-based, though this has not been adequately justified/explained by EFP proponents (cf. [11,28]). If so, why not consider alternative fitness measures that are also energy- and time-based, such as OP/G (offspring production rate divided by generation time), OP/L (offspring production rate divided by lifetime) or OPL/G (offspring production over a lifetime divided by generation time)? One may consider these measures better ways to compare fitness among species with different body sizes and life-history durations than OPG. They are analogous to how evolutionary rate has been scaled by dividing evolutionary change (or rate) by generation time [29,30]. OPL/G also resembles the intrinsic rate of increase (r), an oft-used measure of fitness (see below). Using OPL/G, r, or other fitness indicators reveals that fitness scales strongly with body size, and clearly is not invariant, as further explained in Section 2.6. Note the consistency of the results for each parameter when compared between birds and mammals. Indeed, they are not significantly different from one another (see 95% confidence intervals in Figure 1 and Figure 2 legends in Section 2.6). In addition, the scaling exponents for OPL/G (−0.308 ± 0.066 and −0.314 ± 0.046) have 95% CI that overlap or nearly overlap that for rmax (−0.262 for 44 species of mammals [31]; and −0.26 for 42 species of organisms from viruses to mammals [32].
Consider that the quantity OPG resembles the demographic concept of net reproductive rate (R0 = “the mean number of female offspring produced per female over her lifetime” [33], p. 56), which has been frequently used as an indicator of fitness [11,23,34,35,36,37,38,39,40,41,42,43]. For a population to persist R0 must be ≥1. Furthermore, across species of insects, fishes, lizards, birds and mammals, R0 appears to vary independently of body size or nearly so [44,45,46,47], though this may not be generally true across the tree of life [45]. Similarly, across eukaryotic species, OPG has been calculated to be an average 1 g/g or 22.4 kJ/g over a lifetime regardless of body size [12,13], which are simply biomass and energetic expressions, respectively, of the demographic necessity that R0 = 1 in steady-state populations. This observation has two implications: (a) biophysical processes or constraints are not needed to explain (purportedly invariant) values of OPG, contrary to claims made by proponents of the EFP [12] (see also Section 2.9); and (b) using OPG or R0 as a fitness indicator does not adequately account for the effects of reproductive timing on the rate of reproduction or population growth [37,48], i.e., it is not properly scaled to time [41,49]. For example, a deer and deer mouse may have a similar R0 (or OPG) but since deer mice mature at younger ages and thus have more generations per unit time than deer, their populations can grow significantly faster. According to Roff [36], r is a better fitness measure when populations are growing or unstable, whereas R0 is a better measure when populations are stationary (see also [50]). Hence, the intrinsic rate of increase (r = ln R0/G) is often used as an alternative measure of fitness or population growth capacity (see [16,23,33,34,37,38,39,40,41,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]). Using G in this way results in a fitness index that is not invariant but rather scales strongly with body size (see, e.g., [31,32,44,55,63,64,65,66,67], Section 2.6, and Appendix A.3).
2.2. The EFP’s Questionable Assumption of Steady-State Populations
The OPG fitness concept of the EFP assumes steady-state populations and constant energy supply. However, most populations fluctuate, often considerably, and generally in a body-size dependent way [55,68,69,70,71]. Energy supply (and thus population carrying capacity) may also vary spatially and temporally [14]. Therefore, during population growth spurts that frequently and episodically occur in small bodied species and in variable environments, birth rates exceed death rates. This leads to selection for higher reproductive power (thus enabling rapid exploitation of temporarily abundant resources and habitats) than observed in large bodied species or in relatively stable environments where birth and death rates are more consistently balanced (following r- and K-selection theory: ([55,68,72,73,74]; also see [21,75], Section 6, and Appendix A.3).
2.3. The Rate of Living Theory Assumed by the EFP Is Not Generally Applicable
Proponents of the EFP [12] assume that metabolic rate drives the pace of life and death (and thus lifetime), following the rate of living theory [76,77] and the metabolic theory of ecology [78], but this assumption is not generally applicable [79,80,81,82]. More evidence supports the view that the rate of mortality, as mediated by various environmental factors, drives the pace of life, including growth, developmental and reproductive rates, and in some cases, by association, metabolic rate, as well [83].
2.4. Biological Time Is Not an Independent 4th Dimension in Biological Scaling
Proponents of the EFP have assumed that biological time represents an independent 4th dimension in quarter-power biological scaling [11,12]. The EFP claims that fitness (OPG) is invariant with respect to body size because its components (OP and G) scale inversely with −1/4 and 1/4 powers, respectively. This quarter-power scaling is assumed to result from biological time (e.g., generation time, G) being an independent 4th dimension commensurate with the three spatial dimensions of biological volumes (see also [45,84,85,86,87,88,89]). However, this view has three problems. First, as described in Section 2.6, the OP and G of birds and mammals do not show quarter-power scaling. Second, G is not an independent 4th dimension, but rather scales allometrically (disproportionately) with the dimension of body length (L) in a variety of organisms, i.e., log–log slopes are typically <1, rather than ≈ 1, as assumed [90]. Third, even if G scaled as L1, dimensional scaling analysis [91] indicates that G should scale as M1/3, not as M1/4 (where M = body mass) as has been assumed [11,12,89]. This claim follows logically from two basic geometric scaling relationships, where G is assumed to scale as L1, and M scales as L3, assuming that M is proportional to body volume, and body shape is constant.
2.5. The Species-Level Fitness Measure of the EFP Ignores Effects of Population and Geographical Range Sizes
The OPG fitness definition of the EFP ignores differences in population size or geographical range size that strongly influence the persistence of populations and species (a major indicator of fitness at these levels: see [92,93,94] and other references cited in Section 3). This is a major omission. Abundant, widespread species can be considered more fit than rare, geographically restricted species. The former often have higher reproductive power (e.g., [21,95]), faster rates of growth and resource acquisition [96], and suffer less extinction relative to allied restricted species (e.g., [97,98,99,100,101,102]), thus being selected for at the species level. They may also have higher speciation rates ([18,92,101,103,104,105,106,107]; but see [108,109,110]), another important driver of species selection [93,111].
In addition, conspecific populations can vary greatly in fitness, as supported by source-sink metapopulation theory [112,113,114]. Small “sink” populations (mortality > natality) are highly vulnerable to extirpation and often can persist only by immigration of individuals from other populations (the so-called “rescue effect”: [115,116]). By contrast, large “source” populations (natality > mortality) that support sink populations with immigrants are more resistant to extirpation and thus are more fit. The relative rates of reproduction and mortality of conspecific populations can vary greatly (see also Section 3); and moreover, these rates do not necessarily exactly balance as assumed by the EFP, because population sizes are also influenced by rates of immigration and emigration not considered by the EFP. Furthermore, immigration can affect the genetic diversity and fitness of individuals and populations [117,118].
2.6. Problems with Data and Scaling Analyses Used to Support the EFP
The data and scaling analyses used to support the EFP have several problems (see also [21]). They include (a) conflating generation time and lifetime (they are not the same, contrary to the definition used by [11,12]), (b) the data used for generation time by [10,12] are in many cases unreliable (e.g., many small birds and mammals were reported to have generation times >10 years, which often exceeds their expected lifetimes in nature), (c) the fecundity schedule, an important component of fitness [48,49,119], is ignored, (d) the inverse quarter-power scaling of offspring production rate and generation time across various eukaryotic species reported by [10,12] is based on analyses of datasets that do not contain the same groups of species and higher taxa and thus are not strictly comparable, and (e) analyses of birds and mammals based on the same species do not show inversely related quarter-power scaling, thus invalidating the EFP for these two major taxa [21].
Indeed, the OPG of birds and mammals scales allometrically with body mass (Figure 1A and Figure 2B: based on data collected in [10]), as do alternative fitness measures OP/G, OP/L, and OPL/G (Figure 1B–D and Figure 2B–D; based on data from [10], as well as alternative data assembled in [120] on female age at maturity, an approximate indicator of G, following [49,121], though this measure does not include the effects of iteroparous reproduction). Note that OPG resembles the net reproductive rate (R0), whereas OPL/G resembles the intrinsic rate of increase (r). Ordinary least-squares regression analyses were used because I wanted to determine how variation in each fitness measure is predicted by variation in body mass [122,123]. This method is also appropriate when the Y variable is determined with more error than the X variable [124,125], as is probably the case for my analyses. In addition, I did not use phylogenetically informed analyses because I merely wanted to know whether the slope of a relationship was significantly different from zero. As seen by the obviously negatively trending scatter of points in each graph of Figure 1 and Figure 2, phylogenetic adjustments may alter the exact value of each slope but unlikely its negativity (significantly < 0). Further statistical analyses based on other fitness measures and other taxa are needed to test the generality of the patterns that I have documented.
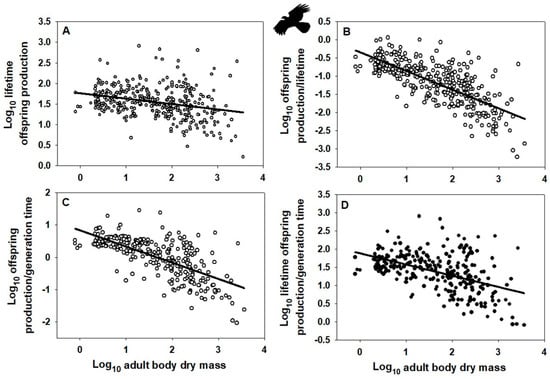
Figure 1.
Log–log scaling relationships of various “fitness” indices with adult body dry mass (g) across species of birds (data from [10,120]). (A) Mass-specific surviving offspring production (g/g) over a female’s lifetime: Y = 1.757 − 0.131 ± 0.046 (X); r = 0.292; n = 361; p < 0.001. (B) Mass-specific surviving offspring production rate (g/g/y) divided by a female’s lifetime (yrs): Y = −0.343 − 0.514 ± 0.048 (X); r = 0.744; n = 361; p < 0.001. (C) Mass-specific surviving offspring production (g/g/y) divided by generation time (approximated by female age at maturity, yrs): Y = 0.833 − 0.501 ± 0.064 (X); r = 0.692; n = 276; p < 0.001. (D) Mass-specific surviving offspring production (g/g) over a female’s lifetime divided by generation time (female age at maturity, yrs): Y = 1.833 − 0.308 ± 0.066 (X); r = 0.496; n = 276; p < 0.001. Error terms for scaling exponents are 95% confidence intervals. Note that the fitness indices in A and D resemble the demographic parameters “net reproductive rate” (R0) and “intrinsic rate of increase” (r). Bird silhouette from https://www.phylopic.org/images/, (accessed on 24 December 2025).
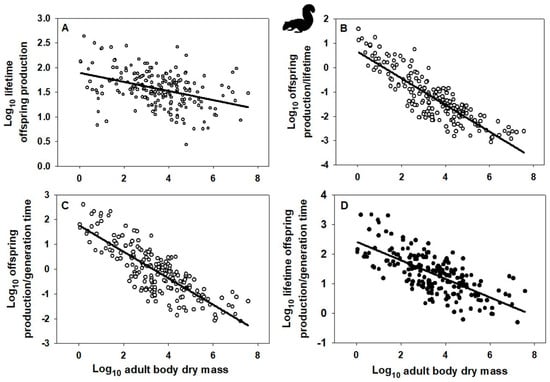
Figure 2.
Log–log scaling relationships of various “fitness” indices with adult body dry mass (g) across species of mammals (data from [10,120]). (A) Mass-specific surviving offspring production (g/g) over a female’s lifetime: Y = 1.891 − 0.092 ± 0.030 (X); r = 0.395; n = 209; p < 0.001. (B) Mass-specific surviving offspring production rate (g/g/y) divided by a female’s lifetime (yrs): Y = 0.657 − 0.548 ± 0.046 (X); r = 0.860; n = 209; p < 0.001. (C) Mass-specific surviving offspring production rate (g/g/y) divided by generation time (approximated by female age at maturity, yrs): Y = 1.771 − 0.535 ± 0.054 (X); r = 0.665; n = 195; p < 0.001. (D) Mass-specific surviving offspring production (g/g) over a female’s lifetime divided by generation time (female age at maturity, yrs): Y = 2.416 − 0.314 ± 0.046 (X); r = 0.698; n = 195; p < 0.001. Error terms for scaling exponents are 95% confidence intervals. Note that the fitness indices in A and D resemble the demographic parameters “net reproductive rate” (R0) and “intrinsic rate of increase” (r). Mammal silhouette from https://www.phylopic.org/images/, (accessed on 24 December 2025).
2.7. Problems with Basing the EFP on a Supposed Body-Mass Scaling Invariance
Proponents of the EFP focus on body-mass scaling relationships while largely ignoring the substantial influence of other factors. This leads to two major problems as described below.
2.7.1. Body-Mass Independent Variation in Fitness
Allometry-based invariance analyses are inadequate because they ignore variation independent of body size. Even if an allometric invariance is found (scaling slope = 0), much residual variation remains. Indeed, the residual variation for the fitness measure OPG is about two orders of magnitude (100-fold) or more for birds and mammals (Figure 1A and Figure 2A). EFP proponents acknowledge the existence of body-size independent residual variation but regard it as being due to methodological error or other factors of secondary importance [12]. Furthermore, direct relationships between the parameters (e.g., OP and G) comprising a so-called invariant ratio or multiplied quantity (e.g., OPG) may not show a proportional positive/negative 1:1 relationship as assumed [21,90]. In addition, when each parameter is scaled against body size, they may not show exactly inverse relationships as expected for an invariance (including offspring production rate and lifetime or generation time, as assumed by the EFP: see scaling analyses for birds and mammals reported in [21,32]). As a result, the fitness measure (OPG) upon which the EFP is based scales allometrically in relation to body mass (Figure 1A,B) and therefore cannot be considered invariant.
Consider how reproduction and survival often show trade-offs within and among individuals in a population (as reviewed by [36,37,119]; see also data analysis by [126]; and more recent studies by [127,128,129]). The existence of individuals showing such trade-offs implies that reproductive output over a lifetime is less variable than each component parameter examined by itself. However, this pattern does not mean that all individuals have equal or nearly equal fitness. Yet, this appears to be the reasoning analogously used at the species level by proponents of the EFP. Trade-offs between offspring production rate (OP) and survival (lifetime or generation time) occur among species but given that OP over a lifetime may be less variable than each component parameter by itself, does not mean that all species have attained similar fitness. Lifetime reproduction varies considerably among individuals and species (see Section 3). For example, individual variation in growth rate can cause large variation in lifetime reproduction [130]. As noted in Section 2.1, using lifetime reproduction as a fitness indicator also ignores major effects of the rate/timing of reproduction on fitness [37,48]. Species with earlier reproduction may have higher fitness [129,131].
2.7.2. There Are Many Kinds of Scaling Invariances That by Themselves Do Not Provide Sufficient Support for the EFP
The energetic fitness measure of the EFP represents one of several supposedly invariant quantities that have been reported based on allometric analyses (see e.g., [45,82,89,132,133,134,135]). If they are valid (but see Section 2.7.1 and below), one could argue that other invariant quantities could qualify just as well as evidence for equal fitness in species, small and large. For example, the number of breaths or heart beats per lifetime has been reported to be relatively constant across small to large mammals (e.g., [45,136]) and animals generally [82]. If so, does this indicate equal fitness across animal species? I have yet to see a good argument supporting this view. Of course, one may regard these invariants as inappropriate examples because they do not involve reproductive fitness. Wieser [137] showed that the rate of energy expenditure for reproduction scales isometrically or nearly so (slope ~ 1) with litter or clutch mass across animal species, thus resulting in an approximately invariant energy cost of reproduction per unit offspring mass (~250 kJ/kg/d), but this is not surprising given the similar biochemistry underlying biosynthesis in all animals. Moreover, lifetime reproductive effort (a dimensionless quantity analogous to OPG) does not necessarily show zerometric scaling (scaling slope = 0), as predicted by the EFP. Although the scaling slope is apparently not significantly different from 0 in squamates and birds, it is significantly negative in amphibians and mammals [135]. Similarly, Peters [134] examined the scaling of various measures of reproductive effort in mammals, most of which showed a negative relationship with body size (see also the negatively allometric scaling analyses of mass-specific reproductive rates reported by [138]. As another example, across various animal populations, production efficiency (production/assimilation) has been shown to scale with a slope ~ 0 [139,140]. Given that energetic efficiency is often thought to be maximized by natural selection (in fact, Slobodkin [141] proposed this specifically for population production efficiency; see also Appendix A.2), one could conclude that a scaling invariance for population production efficiency is evidence for equal population-level fitness across animals with different body sizes. If so, we have a quandary because some EFP proponents have argued that natural selection does not maximize efficiency (see e.g., [13,20,28]). However, this quandary is only apparent because none of the so-called invariant quantities that have been reported show that fitness is truly invariant. They merely show zerometric scaling with body mass (slope ≈ 0), while ignoring extensive variation around each scaling line. Furthermore, these and other so-called invariances (e.g., the apparent zerometric scaling of total population energy expenditure with body mass, which was originally reported in mammals and other terrestrial animals and called the “energy equivalence principle” [142,143] and later also observed in some autotrophic organisms ([144]; but see deviant comparative results reported for other taxa: e.g., [145,146,147,148]; and in a controlled experimental system [149]), merely show that multiple traits exhibit either parallel or inverse relationships with body size, or nearly so (see [45,134]). Inferring that zerometrically scaling ratios or multiples of these traits represent equal fitness across body-size classes is problematic because no single preferred fitness measure has been conclusively identified. In addition, correlations between life-history traits (e.g., trade-offs between offspring production and longevity, whether related to body size or not) are “inevitable” because “in an isolated constant-sized population, natality must be balanced by mortality” [150]. Demography of persistent populations prevents rates of growth, reproduction and mortality from varying independently [151]. However, demographic constraints need not entail the attainment of equal fitness by all species. Indeed, differences in population size can have major effects on population or species fitness (see Section 3).
2.8. If the EFP Were True, Species-Level Selection Would Not Be Possible
If equal fitness occurred among individuals, populations and species, selection (or sorting) would not be possible at these levels (see also Appendix A.1). Accordingly, in this sense the EFP is fundamentally anti-Darwinian, even though it was derived based on an energetic definition of Darwinian fitness. If the EFP were true, a lack of species fitness variation would paralyze species selection, thus creating a fundamental problem for current evolutionary theory.
Although proponents of the EFP clearly declare that “at steady state, species are equally fit because they allocate an equal quantity per gram of energy and biomass to surviving offspring” [13], one might loosen this claim by allowing fitness to vary somewhat around a specified central tendency or approximate “invariant” quantity. Indeed, I have carefully used the word “approximately” and similar qualifying words throughout my article to refer to the “nearly” equal fitness claim as presented by [11]. Nevertheless, even in this case, selection would be severely limited by an alledgedly narrow canalization of fitness variation around a reputedly constant mean value (effectively constituting a uniformly restricted “fitness zone”) across species with different body sizes (see also Section 7 and Section 8). Furthermore, EFP proponents claim that their energetic measure of fitness should be regarded as “distinct” from other fitness indicators that are subject to natural selection [10,11]. Indeed, they regard the equalization of their fitness measure across species as being applicable only under steady-state conditions where natural selection is not operating. Thus, they use the term “fitness” in a way that is essentially non-Darwinian and outside mainstream evolutionary thought.
2.9. The Biophysical Basis of the EFP Is Questionable
In my opinion, the proposed EFP embodies another problematic attempt to explain evolution simply in terms of universal physical principles or processes (see also [152]). Proponents claim a biophysical basis for the EFP, when none is needed because it is simply an expression of a fundamental demographic constraint (birth and death rates must balance in stable persistent populations; see also Section 2.1) that has been recognized in evolutionary and life-history studies since the 1800s (e.g., [18,45,73,92,150,151,153,154,155]). Other failed attempts to explain evolution in terms of simple physical laws or internal forces include Haacke’s and Nägeli’s orthogenesis, Haeckel’s recapitulation theory, and Kleiber’s 3/4-power law [156,157,158,159,160]. The application of physics to evolutionary theory and biological scaling continues to be a controversial subject demanding more clarity (see e.g., [152,159,161,162,163,164,165,166,167] and Section 4). In any case, the EFP represents another questionable endeavor to impose order on a contingent world, a recurring theme in the history of biology (see e.g., [168,169,170]).
However, I want to make clear that I am not against attempts to find/explain major laws or general trends concerning evolution, which I have acknowledged approvingly or even proposed myself (e.g., the evolution of general allometric and habitat-specific trends in energetic power and efficiency) (see Section 4 and Section 6 and Appendix A.2 and Appendix A.3). Rather I am against developing allegedly universal, over-simplistic, physical laws of evolution that ignore the multi-mechanistic basis of evolution and its diverse, opportunistic, and contingent pathways that depend on specific biological and ecological contexts.
3. The “Variable Fitness Paradigm”
An alternative view to the EFP is the variable fitness paradigm (VFP) originated by Darwin [15,92,171] and Wallace [18], which continues to be a major conceptual framework used by mainstream evolutionary theory (neo-Darwinism). The VFP recognizes extensive variation in fitness (or its components) at the individual, population and species levels. Note that although EFP proponents claim that fitness equality exists at the species level, they acknowledge that fitness varies among individuals in a population [11]. However, fitness variation at one level may affect fitness variation at other levels. Hence, in this section, I will briefly discuss fitness variation at various hierarchical levels, with a special emphasis on their cross-level interactive effects.
Individual variation (both genotypic and phenotypic) in vital rates, including survival (mortality), growth, and reproductive rates, as well as lifetime reproduction (a commonly used measure of fitness) within populations has been well documented (e.g., [35,42,52,53,92,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190,191]). Fitness variation in populations is expected to be promoted by sexual reproduction (recombination), which according to theory and experimental evidence facilitates rapid evolutionary adaptation to new or changing environments [192,193,194,195,196,197,198] and furthers population persistence [199]. In addition, fitness and its components (e.g., survival and reproductive success) vary considerably across populations occupying different parts of a species geographical range. Geographical fitness variation in animals and plants may relate to a variety of demographic and abiotic/biotic environmental factors (e.g., [200,201,202,203,204,205,206,207,208]). Fitness and associated traits (e.g., aerobic scope, scope for growth, lifetime reproductive success, population size, and population growth rate) usually decrease in stressful environments (e.g., [209,210,211,212,213,214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229]). Population fluctuations, which can affect extinction risk (and thus population-level fitness), may also vary considerably across species geographical ranges [230].
Furthermore, extensive variation in persistence (extinction vulnerability) and speciation rate occurs among species, both across the tree of life and within clades of related species [93,109,231,232,233,234,235,236,237,238,239]. Emergent traits at the species level can affect this variation, including population abundance/dynamics and geographical range size ([92,93,94,98,99,100,106,110,233,234,235,239,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259,260,261]; but see [262]). The wide variation in these species-level traits alone indicates that fitness must vary greatly among species, thus further supporting the VFP. Indeed, the geographical range sizes of species can vary by as much as 12 orders of magnitude [263], and related species-level fitness traits (e.g., rates of speciation and extinction) should thus vary considerably as well.
Individual traits and their variability may also affect emergent species-level fitness because populations and species with more individual variability may persist longer, especially during stressful periods, than those with lower variability ([97,178,264,265,266,267]; but see [100]). Therefore, variable fitness at the individual level can promote selection both within and among populations or species. Abundant, widespread species often (but not always) exhibit higher genetic variation than rarer, more restricted, related species (see e.g., [92,268,269,270,271,272,273,274,275,276,277,278,279]), a trend also documented by general reviews or meta-analyses [277,280,281,282,283]. Furthermore, the population size of both animals and plants is significantly correlated with both genetic variation [277,283,284,285] and reproductive fitness (defined as adult progeny production or population growth rate [286], or as female reproductive output gauged as number of flowers, seeds or fruits [283]). The effectiveness of sexual reproduction in promoting adaptation to changing environments (and thus greater evolutionary persistence) is also higher in larger populations [195,287]. In addition, individual traits such as body size, fecundity, parental care, torpor ability, morphological complexity and niche specialization can affect extinction risk (e.g., [72,231,232,233,234,237,243,247,249,250,251,288,289,290,291,292,293,294,295,296,297,298,299]. Various individual physiological and life-history traits may also affect the growth rate and thus fitness of populations (e.g., [66,300,301,302,303]). Furthermore, the genetic variability of animal populations and thus their prospects for evolution and survival are generally negatively related to body size and longevity [304]. The above analyses connect fitness indicators estimated at the individual and population levels.
Population size or its variability is often correlated with geographical range size, thus connecting these fitness indicators at the population and species (metapopulation) levels [20,69,70,108,240,305,306,307,308,309,310,311,312], as well. Connections between fitness at the individual and species levels are also supported by positive correlations observed in some taxa between geographical range size and reproductive output (e.g., [21,95,307], and references therein) or individual/population growth rate (e.g., [308,313]). Fitness measures may not only be estimated at several hierarchical levels, a “hierarchical expansion” of the theory of natural selection [266,314,315,316], but they may also be linked in multiple ways (see also [25,317]).
Other observations attest to the importance of population size, geographical range size and body size in affecting extinction rates. For example, geographically restricted species tend to occupy relatively stable, predictable, sheltered, or non-seasonal habitats, where extinction risk is reduced, thus revealing species-level selection for population stability (see e.g., [95,242,307,318,319,320,321,322,323,324]). In addition, the maximum body size of animals is negatively correlated with habitat area, because population sizes of large animals are too small for extended persistence in small habitat areas (see [289,325,326,327]). Similarly on continents, large fishes, birds and mammals have relatively large geographical ranges, presumably because those with smaller ranges (and related low population abundances) went extinct [20,108,309]. In addition, theoretical analyses show that time to extinction decreases with increasing population carrying capacity (K) and with increasing population growth rate (r) under many demographic conditions [328].
Further research is needed to characterize fitness variation taxonomically, allometrically, and ecologically in both space and time. For example, the “fitness landscape” concept [329,330,331,332,333,334] may be used to characterize individual or taxonomic variation in fitness or its components. As a case in point, Beausoleil et al. [335] showed a multi-species pattern of fitness peaks and valleys (based on relative survival) for phenotypic variation in beak morphology among species of Darwin’s finches. Allometric variation can be represented by the body-size scaling of fitness measures such as OP/G (offspring production per generation time) OP/L (offspring production per life time) or OPL/G (offspring production over a lifetime per generation time) (Figure 1B–D and Figure 2B–D) or the intrinsic rate of increase (see Section 2.1, as predicted by [21,68]). One way to consider ecological variation of fitness is to plot a fitness measure along an environmental gradient in space or time [334]. For example, relative reproductive and mortality rates should be expected to vary with resource availability, habitat stability or duration, and stage of ecological succession. Species that occur in relatively unstable, disturbed or seasonal habitats with episodically high resource supplies tend to have higher mass-specific reproductive outputs than related species that occupy relatively stable crowded habitats where resource competition is intense [55,72,73,95,307], see also Appendix A.3). Shifts in relative allocation of resources to reproduction versus competitive survival (r- to K-selection) also occur during the ecological or seasonal succession of various kinds of aquatic and terrestrial communities [336,337,338] and with the evolution of increasing body size [339]. All in all, fitness and its components may vary in response to a variety of physical, biological and ecological factors (constraints). Adopting a VFP is not only important for evolutionary theory but also ecological theory (see e.g., [178,265,340,341,342,343,344]). Ecologists who have promoted the view that species coexistence is facilitated by the evolution of equalized species fitness would do well to contemplate the extensive evidence supporting the VFP.
4. Equal or Variable Fitness: A Question of Universal Determinism Versus Contextual Contingency
One might argue that the EFP is to be expected because how else does one explain why our biosphere contains a diversity of species rather than only one dominant maximally fit species. Since all living species are the descendants of lineages that have been subjected to countless generations of selection, it might seem reasonable to suppose that they have all attained equal (maximal or nearly maximal) levels of fitness, or nearly so, in various ways (including by demographic trade-offs), thus allowing their present coexistence (see also Section 1). However, coexistence (non-replacement) is not a sufficient indicator of equal fitness, because heterogenous environmental conditions at various spatial and temporal scales (local to global and hours to millennia), the continual occurrence of mutations, sexual recombination and other kinds of “accidents” (random events), and the complexity of organisms, which creates conflicting fitness advantages for various traits, can cause individuals in populations and species in clades to have different abilities to survive and multiply, thus continually generating variable fitness. Stochastic genotypic and phenotypic variation in variable, heterogeneous environments not only fuels selection but also prevents the attainment of equal fitness, which is not as paradoxical as it may seem. Equal (maximal) fitness can only be attained in a constant, homogenous, completely deterministic world (cf. [14]). Indeed, some population geneticists and mathematical biologists have claimed that maximal fitness may often not be attainable, as it strongly depends on contingent conditions (see the varied discussions of [345,346,347]). The stochasticity, variability and heterogeneity of the world continually create diversity, a trend that is so ubiquitous that it has been considered a universal law of evolution [348]. This is ironic because the EFP itself represents an attempt to find a universal law of life based on deterministic physical principles or processes. However, like the biogenetic and 3/4-power laws, the EFP does not represent a universal law (see also Section 2.9). Recapitulation of phylogeny by ontogeny (as dictated by the biogenetic law) is not universal (as evidenced by diverse patterns of heterochrony [157,349,350], nor is the 3/4-power law (as evidenced by diverse biological scaling relationships [83,160,351,352,353,354]). The EFP is not universal either, because of ubiquitous variation in fitness at multiple levels of biological organization (see also Section 3 and Section 6 and Appendix A.3).
The VFP may even apply to the structure and function of ecological systems, which may vary in terms of their long-term stability, thus subjecting them to persistence selection [24,25,27,355,356]. Examples include various food-web patterns [357], biochemical cycles [26,358,359,360] and other functional networks of multi-species communities and ecosystems [361,362]. The VFP coupled with persistence (stability) selection may also have played an important role in the origin of life itself [22,363,364,365,366,367]. This makes sense because stability selection undoubtedly has played an important role in the evolution of chemical systems. As Calow ([368], p. 5) remarked: “Only stable chemicals persist”.
Accepting the widespread applicability of the VFP not only represents a vindication of the view of life originated by Darwin and Wallace but also represents an important step toward rejecting overly simplistic, deterministic, biophysical, “Newtonian” approaches to the study of evolution in favor of multi-faceted, contextual, ecological “Darwinian” approaches (as has also occurred in the field of biological scaling [160]). However, this does not mean that life does not obey fundamental physical principles. As recognized by Darwin [92] and other evolutionary biologists, life has evolved in a physical world and is thus constrained by physical principles, though the nature and extent of influence of these constraints is not wholly clear or commonly accepted (see e.g., [21,159,160,166,167,369,370,371,372,373,374,375,376,377,378,379,380]). Physical (energetic) constraints are manifested in part by the covariation between energetic power and efficiency observed in living systems, as discussed in [21], Section 6, and Appendix A.3. However, despite these inescapable constraints, life has evolved a remarkable diversity of form, function and fitness, which has been made possible by the coordinated acquisition and use of both energy (resources) and information, the subject of Section 5.
5. Toward Evolutionary Theory That Integrates the Acquisition and Use of Both Energy and Information
I contend that fully understanding evolutionary fitness and how it varies among individuals, populations, species and clades requires that we view fitness not only in terms of “energy” or “genes”, but both. Organisms have evolved to be “well-informed resource users” [159,381]. Their fitness and adaptiveness are not simply passive results of energetic/physical constraints but are flexible, well-informed (actively regulated) responses to diverse environments. Genes and various biological regulatory systems play important roles in controlling the expression of fitness in various ecological contexts. We need to unite the energy and information paradigms discussed by Van Valen [382]. I believe that this will be important not only for evolutionary biology specifically, but also biology generally. Eldredge [383] similarly suggested that genetic and economic (energetic) views of life should be synthesized to obtain a comprehensive understanding of evolution. However, I argue that we should go beyond Eldredge’s view that “Genetic information acts as a ledger book, a record of the status of biological systems up to a given moment” (p. 352, see also [25]) to consider how genetic information and other information-based regulatory systems determine how and to what extent organisms acquire and use energy (resources) in the face of various environmental challenges [80,381]. Interactions between the acquisition/use of energy and information are important causes of fitness variation and in turn the operation of natural selection in the living world (see also [62,384,385,386,387,388]). The evolution of fitness is not constrained merely in a “passive” way by biophysical (energetic) factors (as assumed by EFP proponents), but rather is mediated by “active”, highly flexible biological regulation based on information acquired about the external world (cf. [389]). Studies of how regulatory networks determine the effects of environmental stress on the growth and fitness of organisms and populations illustrate this fact very well (e.g., [224,225,390,391,392,393,394,395,396,397]). A related viewpoint is that “agency” (i.e., goal-directed power) can significantly alter the availability of energy for fitness-related activities [14].
An analogous situation exists for many ontogenetic growth models, which assume that growth (an important component of fitness) is merely the passive result of energy acquisition exceeding energy costs for maintenance, such that when they become equal growth stops (e.g., [398,399,400]). These models ignore the influence of information-based regulatory systems, which can cause significant variation in growth rates and the energetic processes supporting them ([80,393,396]), as revealed by responses of prey growth (and metabolic) rates to different predation regimes (e.g., [401,402]).
Given the importance of both energy (resources) and information (about resources and environmental hazards) for evolutionary fitness, I recommend future studies that examine the spatial and temporal variation of fitness in relation to energy, nutrient, and fear landscapes and the abilities of organisms to gain useful information about them. Energy landscapes concern variable energy costs of movement across landscapes [403,404,405] that can impact foraging for survival, growth, reproduction, and ultimately fitness [406]. Nutrient landscapes concern variable nutrient availability across landscapes [407,408,409] that influence the ability to survive, grow and reproduce, and ultimately fitness [410,411]. Landscapes of fear concern variability of predation risk across landscapes [405,412,413] that can affect survival and foraging needed for growth and reproduction, and ultimately fitness [405,414,415]. Temporal variation in energy costs, nutrient availability and predation risk can also cause variation in fitness over time [14,407,416,417,418].
6. Species Diversity and Coexistence Are Enabled by Size and Habitat Spectra of Fitness (Power) and Adaptiveness (Efficiency)
How do so many living species coexist, if they have not attained a similar level of fitness and thus evolutionary competitiveness, as proposed by the EFP and other ecological species coexistence models? If fitness (as defined by “reproductive power” or the rate of offspring production or population growth rate: see Section 2.1 and Appendix A.2 and Appendix A.3) is highest in the smallest organisms and lowest in the largest organisms, how were large organisms able to evolve and persist without eventually being replaced by smaller organisms. Why isn’t the modern world dominated only by high-fitness microbes with high reproductive power? This may be because large organisms have compensated for their lower reproductive power by having higher survival ability that is associated with higher adaptiveness and efficiency of resource acquisition. Many large organisms eat smaller organisms or outcompete them for shared resources because of their higher efficiency of resource acquisition. The living world can be envisioned as a size-spectrum from high fitness and reproductive power at the small end to high survival, adaptiveness, and efficiency of resource acquisition at the large end (Figure 3; see [21,339] for supporting details).
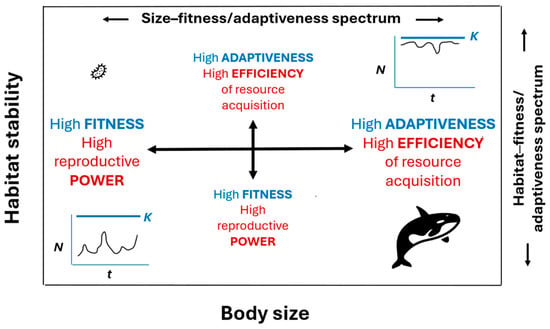
Figure 3.
Schematic representation of the hypothesis that fitness (as indicated by reproductive power) decreases, whereas adaptiveness (as indicated by efficiency of resource acquisition) increases with increasing body size (from microbes to whales) and habitat stability. Both trends are linked to decreasing mortality rates and increasing intraspecific competition (and thus decreasing resource availability per capita) that relate to more stable populations whose size (N) over time (t) is closer to their carrying capacity (K). For more explanation, see the text (Section 6 and Appendix A.3) and [21,83,339]).
According to Glazier [21] and r- and K- selection theory (as interpreted by [55,68]), species follow a similar trend from high fitness (power) to high adaptiveness (efficiency) along a habitat-spectrum from low to high stability (Figure 3). These hypothetical spectra depend on defining fitness and adaptiveness energetically (see Appendix A.2) and as reproductive power versus efficiency of resource acquisition, respectively (see Appendix A.3 for details). Although Glazier [21,83,339] provides empirical support for both spectra, they require further testing. Furthermore, these indicators of fitness and adaptiveness are proposed as useful comparative tools at the population and species levels, and not as universally applicable measures at all levels of biological organization.
Intriguingly, my conceptual scheme partially parallels the EFP and other ecological models that explain the coexistence of species as the result of their having evolved different life-history strategies along a continuum involving a fundamental trade-off between reproduction and survival. However, unlike the EFP and some ecological species coexistence models that are based on species fitness equalization, my scheme is based on a fundamental trade-off between fitness (power) and adaptiveness (efficiency). My view has the advantage of helping to explain the diversity and coexistence of species, while working within the VFP, which is required for natural selection to operate, as commonly accepted by evolutionary biologists. It also makes sense in terms of a recently proposed “mortality theory of ecology” that helps explain diverse biological scaling patterns [83,90,339,419]. Small organisms, or those living in relatively unstable habitats, suffer relatively high rates of mortality that favor high rates (power) of compensatory reproduction and thus fitness, as mediated by natural selection and relatively high resource availability per capita (due to populations being frequently below their carrying capacity: see Figure 3). By contrast, large organisms, or those living in relatively stable habitats, experience lower rates of mortality that enable relatively high survival (longevity) and the evolution of relatively high adaptiveness (as indicated by higher efficiency of resource acquisition), which is advantageous because of relatively high intraspecific competition and low resource availability per capita (due to populations being frequently at or near their carrying capacity; see Figure 3, Appendix A.3, and [83,339]).
7. Prospectus for Assessing the Equality/Variability of Species Fitness and Adaptiveness
In this section, I outline some ways that the equality/variability of species fitness and adaptiveness should be assessed, thus further addressing the major question asked in my commentary: “Are all species created equal?”. In doing so, the EFP may serve as a useful null hypothesis [14]. (1) Statistical tests should use a variety of operational (quantifiable) measures of species fitness and adaptiveness to compare among species, not merely the one limited measure used to support the EFP (cf. [14]). (2) Tests should be performed at various spatial and temporal scales, from local communities to the biosphere and over various geological time periods (cf. [14]). The EFP has been formulated based only on existing species that have been sampled indiscriminately from across the globe and thus ignores how species fitness or adaptiveness may change across different kinds of habitats and geographical regions and over evolutionary time. The EFP also posits that species have equal fitness “at all scales, from local populations and communities to the global biota” ([11], p. 1272), a bold claim that should be tested. (3) Comparisons of species fitness and adaptiveness should be examined among habitats with different levels of stability (e.g., seasonal versus non-seasonal; and disturbed versus undisturbed) to determine whether species fitness equalization more likely occurs in stable habitats, thus following the assumption of the EFP that species have steady-state populations. (4) Levels of species fitness and adaptiveness should be assessed across landscapes varying in energy/nutrient availability and predatory risk all of which can affect survival, reproduction, and rates/efficiencies of energy/nutrient uptake/use that contribute to fitness and adaptiveness (see also Section 5). (5) Variability of species fitness and adaptiveness should be compared among different phylogenetic groups both with respect to body size (as I have done for birds and mammals: see Section 2.6) and independently of body size. Species fitness may vary greatly, independently of body size, and thus estimates of species fitness equality should not be based merely on whether a species fitness measure is “invariant” in an allometric analysis (i.e., shows a log–log slope ≈ 0) (see also Section 2.7). Related studies may include assessing how narrow the residual variation of species fitness around an allometric regression line should be to provide support for the EFP versus VFP, and whether the components of a fitness measure (e.g., offspring production rate and lifetime) covary with negative isometry (log–log slope ≈ −1), as predicted by the EFP. (6) Following recommendation 5, it may also be useful to consider whether an intermediate view between the EFP and VFP best applies in some cases. Perhaps fitness varies but only within specific constraints or boundaries: if so, this could be called the constrained fitness paradigm (CFP). Methods are needed to distinguish between the EFP, CFP and VFP.
8. Conclusions
I have contrasted the recently proposed EFP with the VFP originated by Darwin and Wallace. The EFP claims that since individuals of all species produce about the same amount of offspring biomass (or energy equivalent) per adult mass per generation (lifetime), they all have the same fitness (defined as OPG), at least approximately. However, the EFP has several conceptual and empirical problems, the most important of which are provided here as conclusions (see Section 2 for details). First, the measure of fitness (OPG) proposed is not justified. Why it should be preferred over other possible fitness measures (indicators) is not explained adequately (see also Appendix A.1). Second, the EFP assumes that species populations are generally stable, or nearly so, such that rates of reproduction and mortality balance (which underlies the claimed constancy of OPG among species), but most natural populations fluctuate greatly, especially for relatively small organisms, which according to life-history theory can have great consequences for the evolution of fitness (reproductive success). Third, despite being based on interspecific analyses, the EFP ignores the profound effects of population abundance and geographical range size on fitness at the population and species levels. Fourth, the EFP is based on comparing broad interspecific scaling analyses of eukaryotic offspring production and generation time, which is problematic because these analyses are based on misleading and frequently unrealistic estimates of generation time, as well as comparisons of scaling relationships that contain many non-overlapping species and taxa. Therefore, these scaling analyses are unreliable and non-comparable. Fifth, scaling relationships for mass-specific offspring production rate and generation time (using data collected by proponents of the EFP) analyzed for the same sets of species in two major taxa of animals (birds and mammals) are not exactly inverse, thus invalidating the EFP in these taxa. Sixth, the EFP is based on a purported “scaling invariance” that not only does not exist in birds and mammals but also ignores extensive variation unrelated to body size. Seventh, mass-specific offspring-production rate and generation time in birds and mammals are not related in a negatively isometric way (slope ≠ −1), as predicted by the EFP. Thus, OPG is not constant in either birds or mammals, but varies significantly with body size, as do alternative fitness measures (e.g., the intrinsic rate of increase, r, OP/G, OP/L, and OPL/G: see Figure 1 and Figure 2; and Section 2.1 and Section 2.6).
By contrast, the VFP recognizes extensive fitness variation at the individual, population and species levels. This view is vindicated by multiple energetic, allometric, comparative, and hierarchical analyses, as discussed in Section 3. First, I review numerous studies that show that fitness, defined as differential multiplication and/or persistence, varies enormously at the individual, population, species and clade levels. At the population and species levels, important fitness correlates include population abundance and geographical range size. In general, abundant, widespread species tend to persist longer than do rare, geographically restricted species. Second, fitness variation is essential for continuing multilevel selection at the individual, population, species and clade levels, which would not be possible at the species level if the EFP were true. The existence of equal fitness at specific hierarchical levels would cause evolutionary change via natural selection to be at a standstill at those levels. Third, fitness variation at the individual level may affect fitness variation and resulting selection at higher levels. For example, populations or species with relatively high genetic variation among individuals may persist longer, on average, than those with low variation. In addition, clades containing abundant widespread species may persist longer, on average, than those with rare restricted species. Fourth, individual traits such as body size and reproductive output (which relate to population abundance and geographical range size in various taxa) may affect the persistence (and thus fitness) of populations and species. Often species with smaller body sizes and higher reproductive rates persist longer than related species with larger body sizes and lower reproductive rates. Fifth, I recommend that “fitness” and “adaptiveness” at the levels of populations and species be distinguished as productive “power” versus “efficiency” of resource (energy) acquisition for production (see also [21], Section 6, and Appendix A.3). This distinction reconciles two largely alternative approaches that have long been used to characterize evolutionary fitness and adaptation (often regarded as equivalent) as either energetic power or efficiency (see Appendix A.2). Distinguishing them in this energetic way allows one to use the concepts of fitness and adaptiveness operationally and non-tautologically so that they can be compared among species with different biological and ecological characteristics. Sixth, following the above distinction, it appears that fitness (productive power) and adaptiveness (efficiency of energy acquisition for production) decrease and increase, respectively, in species with larger body size and/or that use resources and habitats that are more stable (see Figure 3, Section 6, Appendix A.3, and [21]). This distinction also helps explain the coexistence of so many species, small and large, in the living world, within the conceptual framework of the VFP. In Section 7, I describe some ways of further assessing the relative variability of species fitness and adaptiveness, and thus the relative validity of the EFP, VFP and a possible intermediate view that I have called the “constrained fitness paradigm” (CFP).
Logic and currently available evidence strongly favor the VFP over the EFP. Consider that the EFP uses selected aspects of the life-histories of currently existing species to formulate a measure of species-level fitness (OPG) and thus represents a time-limited snapshot view of species survival. An implication of the EFP, which is not articulated by its proponents, is that it regards the successful current survival of existing species as being due to their equally maximized OPG attained because of countless past episodes of selection. As such, the EFP represents a “present existence” view of species fitness. In this sense, it is an “equal existence paradigm” that is a truism with little value for predicting future survival or reproductive success. By contrast, the VFP takes a more time-expansive (prospective) view of species fitness as a propensity for “future persistence” (see also [14,23,420,421]). By focusing on how currently living species trade off offspring production with mortality rate (as indicated by generation time or lifetime), a demographic necessity for relatively stable populations, the EFP gives incomplete information about the likely future survival and reproductive success of a species. For example, compare two species with purportedly equal fitness (as defined by OPG) but one is abundant and widespread whereas the other is rare and restricted. The EFP gives us no clue about the likely greater future persistence of the widespread versus restricted species, even though they may have equal OPG. The large amount of variation in fitness (and fitness-related traits), as related to likely future persistence and multiplication, that has been observed at multiple levels of biological organization firmly supports the VFP over the EFP (see Section 3 and Section 4). Therefore, the viewpoint of Darwin and Wallace regarding the importance of fitness variation in evolution is vindicated, thus upholding a continuing Darwinian Revolution in scientific thought.
Funding
This research received no external funding.
Data Availability Statement
All data used in this article can be found in the cited references.
Acknowledgments
I thank Max Reuter, Geerat Vermeij, and an anonymous reviewer for their helpful comments on previous versions of my commentary.
Conflicts of Interest
The author declares no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| EFP | Equal fitness paradigm |
| VFP | Variable fitness paradigm |
| OP | Offspring production |
| G | Generation time (not equal to lifetime) |
| OPG | Offspring production per generation time |
| OPL | Offspring production per lifetime |
| OP/G | Offspring production/generation time |
| OP/L | Offspring production/lifetime |
| OPG/L | Offspring production per lifetime/generation time |
Appendix A
Appendix A.1. Controversy About Defining and Estimating Evolutionary Fitness
The concept of fitness is central to studies of evolution by natural selection, but precisely defining and applying it has been a source of continual controversy among biologists and philosophers alike (see, e.g., [23,36,37,38,40,48,50,75,130,382,420,421,422,423,424,425,426,427,428,429,430,431,432] and references therein). Many kinds of fitness estimates exist, all of which have their uses in specific cases. The purpose of my article has not been to advocate one universally applicable measure of fitness, but to discuss various measures in the context of evaluating the relative merits of the EFP and VFP.
According to Darwin [92] and Wallace [18], individuals with higher fitness (i.e., greater reproductive success) in a population are selected over those with lower fitness. Reproductive success entails the number of offspring that survive to reproduce themselves, and ultimately the number of genes passed onto future generations [22,423]. Thus, natural selection is more than just “survival of the fittest” (a slogan originally used by Spencer [433]), because it also importantly involves differential reproduction (including mating success), as especially occurs during sexual selection [434]. Indeed, Spencer ([433], p. 444) wrote that “survival of the fittest, implies multiplication of the fittest”. In short, Darwinian evolution fundamentally depends upon individual fitness variation in populations [16,17,36,37,168,169,427,435,436,437,438,439]. Indeed, according to the “fundamental theorem of natural selection”, the effectiveness of natural selection in increasing fitness is positively correlated with the fitness variation in a population [16].
Darwin [92] and Wallace [18] also viewed selection as operating at the level of species, with more fit (abundant and widespread) species dominating and eventually replacing less fit (rare and geographically restricted) species, a view further promoted by [103,269,299,440,441,442]. The idea that selection occurs at the population, species and even clade levels, which has been advocated by several biologists, paleontologists, and philosophers during the past several decades (e.g., [17,93,111,153,231,238,253,266,314,315,316,360,361,382,443,444,445,446]), requires that populations, species or clades vary in fitness (i.e., their ability to persist, grow, spread, and/or speciate). Otherwise, selection could not occur at these levels of biological organization.
Appendix A.2. Energetic Views of Fitness and Adaptation
Ever since Darwin and Wallace proposed their theory of natural selection, many scientists have suggested that evolutionary fitness should be defined and studied energetically. Doing so, it is claimed, may help (1) give the fitness concept more operational value (i.e., make it more measurable and comparable among populations and species [19,447]) and (2) eliminate the belief that natural selection of/for the most fit is a tautology based on circular reasoning (e.g., [161,448,449,450]), though this has been considered a problematic criticism because it misrepresents the complex multi-mechanistic process of natural selection with the oversimplistic slogan “survival of the fittest” (see also [23,169,266,421,451,452,453]).
Notably, Boltzmann [454] remarked that the “struggle for existence” is primarily about organisms obtaining enough free energy to support their ordered (negentropic) state. Subsequently, Lotka [370,455] proposed that within certain constraints natural selection favors those organisms that can obtain and use energy at the fastest rate: i.e., high fitness entails high energetic power. Selection for maximal power of energy (resource) uptake and use, especially for growth and reproduction (i.e., “maximal power theory”), has been further discussed and elaborated by many others (e.g., [14,19,20,21,161,456,457,458,459,460,461,462]). Some scientists have also suggested that natural selection for increased power has occurred at the expense of decreased efficiency (e.g., [20,450,457,463,464]).
Although little cited, experimental studies have shown that the energetic “scope for growth” (i.e., net energy gain for growth and reproduction) in Daphnia magna is strongly correlated with the intrinsic rate of increase (r) and net reproductive rate (R0), which are commonly used measures of fitness ([60,217]; see also [61,209,465]; Section 2.1 and Appendix A.1). In addition, Glazier [214] showed that two small populations of the amphipod crustacean Gammarus minus in freshwater springs with relatively low pH and ionic conductivity had relatively low energetic scopes for growth compared to those of two large populations in relatively alkaline springs. Smaller populations can be considered less fit, because of their higher vulnerability to extirpation (see also Section 3); in fact, the two small populations that Glazier [214] studied no longer exist (D. S. Glazier, personal observations). Furthermore, plant fitness has been linked to photosynthetic activity [466].
Various intrinsic and extrinsic constraints can prevent the maximization of energy fluxes or power in living systems (reviewed by [21]). For example, low food availability, diet specialization, and high resource competition can limit the power of growth and reproduction, while favoring high efficiency of food energy acquisition (a possibility recognized by [370,455,458,467], and supported by many examples reviewed in [21]). Glazier [21] further applied a “maximum efficiency principle” derived from physical systems to argue that efficiency may be favored at the expense of power in some living systems.
What then does natural selection favor? Is it energetic power, as advocated by some of the authors cited above, or is it energetic efficiency, “economy”, or other measures of functional efficacy, as has been advocated by many other biologists (e.g., [92,141,218,384,443,468,469,470,471,472,473,474,475,476,477]). Glazier [21] proposed a resolution to this challenging fundamental question by (1) suggesting that either or both power or efficiency may be favored depending on the biology and ecology of the organisms involved (see also [161,368,463,478,479]), and (2) distinguishing the concepts of “fitness” and “adaptation” as indicators of productive “power” versus “efficiency” of resource acquisition (see Section 6 and Appendix A.3).
Appendix A.3. Distinguishing Fitness and Adaptiveness as Power Versus Efficiency
Since Darwin [15] and Spencer [433], many evolutionary biologists have regarded fitness and adaptation as essentially synonymous. However, some biologists and philosophers have argued for distinguishing fitness and adaptiveness (see e.g., [355,421,480,481]) “because the one does not necessarily imply or give rise to the other” ([23], p. 43). For example, within populations sexual selection may favor reproductive success and fitness, while also increasing vulnerability to environmental hazards, thus decreasing survival and adaptation to the environment [434,435]. Consequently, increasing reproductive success (fitness) may not increase the degree of “fit” (adaptiveness) of an organism with its environment (see also [38,426,482]). In addition, a population or species may be currently well adapted to a specialized niche, but its prospects for future survival and reproductive success (i.e., its fitness) may be bleak because it is geographically restricted (e.g., the Californian redwood tree [23,480]). Other ways to distinguish fitness and adaptiveness (adaptedness) are discussed by [23,421,483].
I have recently suggested that it may be advantageous, at least at the population and species levels, to distinguish “fitness” as the energetic “power” (rate of energy uptake or use) of (re)production versus “adaptiveness” as the energetic “efficiency” (ratio of resource uptake to resource supply) of acquiring resources for (re)production [21] (see also Section 6). Employing this dual energetic perspective allows one to compare levels of fitness and adaptiveness among populations and species, thus increasing the comparative operationality of the concepts of fitness and adaptation, while avoiding a possible criticism of tautology. Indeed, I have argued that fitness (power) and adaptiveness (efficiency) decrease and increase, respectively, with increasing body size and habitat stability [21] (see also Figure 3).
When viewing the size-spectrum of all life, one sees that species of small organisms (e.g., microbes) are highly vulnerable to unpredictable environmental hazards and thus subject to high mortality rates, whereas species of large organisms (e.g., trees and whales) are more protected and thus experience relatively low mortality rates (as reviewed in [83,90,339]). One can also infer from this size-mortality spectrum that population stability and the intensity of per capita intraspecific competition for resources should increase with increasing body size, which is supported by observations reviewed by [83]), though more tests are needed. As a result, productive power (fitness) tends to be highest in the smallest, most vulnerable organisms (as supported by Figure 1 and Figure 2), whereas resource-acquisition efficiency (adaptiveness) tends to be highest in the largest, most protected organisms [21]. This size-spectrum of power (productivity) versus efficiency (Figure 3) parallels a scheme based on r- and K-selection, as envisioned by Pianka [68] (see also [55,73,484,485]).
An alternative (complementary) way to distinguish fitness and adaptiveness is to regard them as relative reproductive and survival success, respectively. Accordingly, across the size-spectrum of life, fitness (as estimated by reproductive success) is highest in the smallest organisms, whereas adaptiveness (as estimated by survival success) is highest in the largest organisms (cf. [339]). In broad terms, as body size increases, one sees a shift from “reproduction of the most fit” to “survival of the most adapted”. This scheme corresponds nicely with the previously proposed size-related transition from power to efficiency, which is driven by gradients of (1) decreasing mortality rate and per capita resource availability and (2) increasing resource competition and functional effectiveness (economy) made possible by increasingly sophisticated regulatory systems [21]. These size-related transitions may also be driven by an increase in the selectivity of mortality (see also [83,419]). Small organisms are especially vulnerable to catastrophic mortality events that destroy all or most genotypes indiscriminately. In response to such high levels of random, largely non-selective mortality, generalized evolutionary responses involving rapid growth, maturation and reproduction should be favored to compensate for the large number of individuals that are rapidly/frequently lost (see e.g., [153,154,486,487]). By contrast, mortality may be more selective in large, well-protected organisms, which in turn, should favor more specialized adaptive responses involving enhanced survival [21,153], a hypothesis requiring testing.
Transitions in the emphasis of selection on high fitness versus high adaptiveness can also be seen along environmental gradients in space and time. Gradients from low to high habitat stability tend to be associated with increased resource competition and thus organismal switches from high (re)productive power to high efficiency of resource acquisition [21] (see also Figure 3), a pattern predicted by r- to K-selection theory, as envisioned by Pianka [68] and others (e.g., [55,73,74,484]). Dobzhansky [486] and Williams [154] also claimed that higher population stability promotes greater adaptation and efficiency of resource acquisition. Parsons [218] further argued that selection for adaptive energetic efficiency occurs in stable and resource-poor environments.
In addition, over ecological and evolutionary time, increased species richness and associated resource competition should favor a greater focus on adaptive survival and energetic efficiency [368,463,486,488,489]. During ecological succession and geological history, these trends are often accompanied by increases in maximal body size [336,472,490,491], which are also associated with a switch from high power to high efficiency [21,336,464,486,492]. “Taxon cycles”, involving expansion and restriction of species ranges occurring on either islands [493,494,495,496,497,498,499] or continents [95,500,501], may also show shifts from high fitness to high adaptiveness. Species with broad niches and/or high dispersal ability and reproductive power can colonize many kinds of habitats [95,239,299,502,503,504,505,506,507], thus exhibiting high fitness. After a species colonizes a specific locality, isolate selection [242,320], increasing specialization, K-selection in relatively stable crowded habitats [95,242], and/or more intense counteradaptation from interacting species [495,497] may drive the taxon cycle through later ecological stages of increasing endemism and adaptiveness. According to the concepts of counteradaptation and zero-sum energy, the fitness and expansive energy of a species may be reduced by fitness maximization in other exploitative species [14,19,456], especially in species-rich habitats. More intense species interactions in species-rich areas may also reduce the niche (habitat) breadth of species [508,509]. Therefore, shifts in the relative maximization of fitness versus adaptiveness may occur in both space (“fitness landscapes”) and time (“fitness timescapes”) in association with changes in resource availability, habitat stability, and species richness.
Tradeoffs between power and efficiency can help explain how both abundant widespread and rare restricted species may coexist in the modern biota, contrary to Darwin’s expectation that abundant species should competitively dominate and ultimately replace restricted species. Widespread species may opportunistically exploit ephemeral, disturbed or seasonally variable habitats, whereas related geographically restricted and ecologically specialized species are often competitively dominant in relatively stable habitats (see e.g., [21,95,244,307,509]). Accordingly, widespread colonizing species may be largely limited by “time” (temporary availability of abundant resources: see also Southwood [484]), whereas related localized and specialized species in relatively stable habitats are more limited by “resources” whose availability per capita is continuously low due to high intraspecific competition.
References
- Margulis, L. Symbiotic Planet: A New Look at Evolution; Basic Books: New York, NY, USA, 1998. [Google Scholar]
- Chesson, P. Mechanisms of maintenance of species diversity. Annu. Rev. Ecol. Syst. 2000, 31, 343–366. [Google Scholar] [CrossRef]
- Hubbell, S.P. The Unified Neutral Theory of Biodiversity and Biogeography; Princeton University Press: Princeton, NJ, USA, 2001. [Google Scholar]
- Ostling, A. Do fitness-equalizing tradeoffs lead to neutral communities? Theor. Ecol. 2012, 5, 181–194. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Zhang, B.Y.; Lin, K.; Jiang, X.; Tao, Y.; Hubbell, S.; He, F.; Ostling, A. Demographic trade-offs determine species abundance and diversity. J. Plant Ecol. 2012, 5, 82–88. [Google Scholar] [CrossRef]
- DeLong, J.P.; Hanley, T.C. The rate-size trade-off structures intraspecific variation in Daphnia ambigua life history parameters. PLoS ONE 2013, 8, e81024. [Google Scholar] [CrossRef]
- Jops, K.; O’Dwyer, J.P. Life history complementarity and the maintenance of biodiversity. Nature 2023, 618, 986–991. [Google Scholar] [CrossRef] [PubMed]
- Ontiveros, V.J.; Capitán, J.A.; Casamayor, E.O.; Alonso, D. Colonization–persistence trade-offs in natural bacterial communities. Proc. R. Soc. B Biol. Sci. 2023, 290, 20230709. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, A.B.; Otto, S.P.; Nelson, W.A.; Day, T. Variety is the spice of life: Nongenetic variation in life histories influences population growth and evolvability. J. Evol. Biol. 2024, 37, 1244–1263. [Google Scholar] [CrossRef]
- Brown, J.H.; Hall, C.A.S.; Sibly, R.M. Equal fitness paradigm explained by a trade-off between generation time and energy production rate. Nat. Ecol. Evol. 2018, 2, 262–268. [Google Scholar] [CrossRef]
- Burger, J.R.; Hou, C.; Brown, J.H. Universal rules of life: Metabolic rates, biological times and the equal fitness paradigm. Ecol. Lett. 2021, 24, 1262–1281. [Google Scholar] [CrossRef]
- Brown, J.H.; Burger, J.R.; Hou, C.; Hall, C.A.S. The pace of life: Metabolic energy, biological time, and life history. Integr. Comp. Biol. 2022, 62, 1479–1491. [Google Scholar] [CrossRef]
- Brown, J.H.; Hou, C.; Hall, C.A.S.; Burger, J.R. Life, death and energy: What does nature select? Ecol. Lett. 2024, 27, e14517. [Google Scholar] [CrossRef]
- Vermeij, G.J.; Grosberg, R.K.; Roopnarine, P.D. Energetics and evolutionary fitness. Proc. Natl. Acad. Sci. USA 2025, 122, e2423684122. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. On the Origin of Species by Means of Natural Selection or the Preservation of Favoured Races in the Struggle for Life; John Murray: London, UK, 1859. [Google Scholar]
- Fisher, R.A. The Genetical Theory of Natural Selection; Dover: New York, NY, USA, 1958. [Google Scholar]
- Lewontin, R.C. The units of selection. Annu. Rev. Ecol. Syst. 1970, 1, 1–18. [Google Scholar] [CrossRef]
- Wallace, A.R. Darwinism: An Exposition of the Theory of Natural Selection, with Some of Its Applications; MacMillan: New York, NY, USA, 1889. [Google Scholar]
- Van Valen, L.M. Energy and evolution. Evol. Theor. 1976, 1, 179–229. [Google Scholar]
- Brown, J.H. Macroecology; University of Chicago Press: Chicago, IL, USA, 1995. [Google Scholar]
- Glazier, D.S. Power and efficiency in living systems. Sci 2024, 6, 28. [Google Scholar] [CrossRef]
- Dawkins, R. The Selfish Gene; Oxford University Press: Oxford, UK, 1976. [Google Scholar]
- Endler, J.A. Natural Selection in the Wild; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Toman, J.; Flegr, J. Stability-based sorting: The forgotten process behind (not only) biological evolution. J. Theor. Biol. 2017, 435, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Lenton, T.M.; Kohler, T.A.; Marquet, P.A.; Boyle, R.A.; Crucifix, M.; Wilkinson, D.M.; Scheffer, M. Survival of the systems. Trends Ecol. Evol. 2021, 36, 333–344. [Google Scholar] [CrossRef]
- Boyle, R.A.; Moody, E.R.; Babcock, G.; McShea, D.W.; Álvarez-Carretero, S.; Lenton, T.M.; Donoghue, P.C. Persistence selection between simulated biogeochemical cycle variants for their distinct effects on the Earth system. Proc. Natl. Acad. Sci. USA 2025, 122, e2406344122. [Google Scholar] [CrossRef]
- Damuth, J.; Ginzburg, L.R. Nonadaptive Selection: An Evolutionary Source of Ecological Laws; University of Chicago Press: Chicago, IL, USA, 2025. [Google Scholar]
- Burger, J.R.; Brown, J.H. Does nature select to maximize power? BioScience 2025, 75, 502–504. [Google Scholar] [CrossRef]
- Gingerich, P.D. Rates of evolution. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 657–675. [Google Scholar] [CrossRef]
- Okie, J.G.; Boyer, A.G.; Brown, J.H.; Costa, D.P.; Ernest, S.M.; Evans, A.R.; Fortelius, M.; Gittleman, J.L.; Hamilton, M.J.; Harding, L.E.; et al. Effects of allometry, productivity and lifestyle on rates and limits of body size evolution. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131007. [Google Scholar] [CrossRef]
- Hennemann, W.W., III. Relationship among body mass, metabolic rate and the intrinsic rate of natural increase in mammals. Oecologia 1983, 56, 104–108. [Google Scholar] [CrossRef]
- Blueweiss, L.; Fox, H.; Kudzma, V.; Nakashima, D.; Peters, R.; Sams, S. Relationships between body size and some life history parameters. Oecologia 1978, 37, 257–272. [Google Scholar] [CrossRef]
- Gotelli, N.J. A Primer of Ecology, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2008. [Google Scholar]
- Charlesworth, B. Evolution in Age-Structured Populations; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Clutton-Brock, T.H. (Ed.) Reproductive Success: Studies of Individual Variation in Contrasting Breeding Systems; University of Chicago Press: Chicago, IL, USA, 1988. [Google Scholar]
- Roff, D.A. The Evolution of Life Histories: Theory and Analysis; Chapman and Hall: New York, NY, USA, 1992. [Google Scholar]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- De Jong, G. The fitness of fitness concepts and the description of natural selection. Q. Rev. Biol. 1994, 69, 3–29. [Google Scholar] [CrossRef]
- Kozłowski, J. Adaptation: A life history perspective. Oikos 1999, 86, 185–194. [Google Scholar] [CrossRef]
- Brommer, J.E. The evolution of fitness in life-history theory. Biol. Rev. 2000, 75, 377–404. [Google Scholar] [CrossRef] [PubMed]
- Huey, R.B.; Berrigan, D. Temperature, demography, and ectotherm fitness. Am. Nat. 2001, 158, 204–210. [Google Scholar] [CrossRef]
- van Daalen, S.F.; Caswell, H. Lifetime reproductive output: Individual stochasticity, variance, and sensitivity analysis. Theor. Ecol. 2017, 10, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Kindsvater, H.K.; Juan-Jordá, M.J.; Dulvy, N.K.; Horswill, C.; Matthiopoulos, J.; Mangel, M. Size-dependence of food intake and mortality interact with temperature and seasonality to drive diversity in fish life histories. Evol. Appl. 2024, 17, e13646. [Google Scholar] [CrossRef]
- Gaston, K.J. The intrinsic rates of increase of insects of different sizes. Ecol. Entomol. 1988, 13, 399–409. [Google Scholar] [CrossRef]
- Calder, W.A. Size, Function, and Life History; Dover: Mineola, NY, USA, 1996. [Google Scholar]
- Charnov, E.L.; Warne, R.; Moses, M. Lifetime reproductive effort. Am. Nat. 2007, 170, E129–E142. [Google Scholar] [CrossRef]
- Ginzburg, L.R.; Burger, O.; Damuth, J. The May threshold and life-history allometry. Biol. Lett. 2010, 6, 850–853. [Google Scholar] [CrossRef] [PubMed]
- Brommer, J.E.; Merilä, J.; Kokko, H. Reproductive timing and individual fitness. Ecol. Lett. 2002, 5, 802–810. [Google Scholar] [CrossRef]
- Steiner, U.K.; Tuljapurkar, S.; Coulson, T. Generation time, net reproductive rate, and growth in stage-age-structured populations. Am. Nat. 2014, 183, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.J.; Reeve, J.P. Natural Selection. In Evolutionary Ecology: Concepts and Case Studies; Fox, C.W., Roff, D.A., Fairbairn, D.J., Eds.; Oxford University Press: Oxford, UK, 2001; pp. 29–43. [Google Scholar]
- Hairston, N.G.; Tinkle, D.W.; Wilbur, H.M. Natural selection and the parameters of population growth. J. Wildl. Manag. 1970, 34, 681–690. [Google Scholar] [CrossRef]
- Doyle, R.W.; Hunte, W. Demography of an estuarine amphipod (Gammarus lawrencianus) experimentally selected for high “r”: A model of the genetic effects of environmental change. Can. J. Fish. Aquat. Sci. 1981, 38, 1120–1127. [Google Scholar] [CrossRef]
- Doyle, R.W.; Hunte, W. Genetic changes in “fitness” and yield of a crustacean population in a controlled environment. J. Exp. Mar. Biol. Ecol. 1981, 52, 147–156. [Google Scholar] [CrossRef]
- Kirkwood, T.B.L. Repair and Its Evolution: Survival Versus Reproduction. In Physiological Ecology: An Evolutionary Approach to Resource Use; Townsend, C.R., Calow, P., Eds.; Sinauer Associates: Sunderland, MA, USA, 1981; pp. 165–189. [Google Scholar]
- Southwood, T.R.E. Bionomic Strategies and Population Parameters. In Theoretical Ecology: Principles and Applications; May, R.M., Ed.; Sinauer Associates: Sunderland, MA, USA, 1981; pp. 30–52. [Google Scholar]
- Southwood, T.R.E. Tactics, strategies and templets. Oikos 1988, 52, 3–18. [Google Scholar] [CrossRef]
- Lande, R. A quantitative genetic theory of life history evolution. Ecology 1982, 63, 607–615. [Google Scholar] [CrossRef]
- Sibly, R.M.; Calow, P. Physiological Ecology of Animals: An Evolutionary Approach; Blackwell: Oxford, UK, 1986. [Google Scholar]
- Sibly, R.M. The life-history approach to physiological ecology. Funct. Ecol. 1991, 5, 184–191. [Google Scholar] [CrossRef]
- Baillieul, M.; Selens, M.; Blust, R. Scope for growth and fitness of Daphnia magna in salinity-stressed conditions. Funct. Ecol. 1996, 10, 227–233. [Google Scholar] [CrossRef]
- Lampert, W.; Trubetskova, I. Juvenile growth rate as a measure of fitness in Daphnia. Funct. Ecol. 1996, 10, 631–635. [Google Scholar] [CrossRef]
- Usinowicz, J.; O’Connor, M.I. The fitness value of ecological information in a variable world. Ecol. Lett. 2023, 26, 621–639. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, T. Intrinsic rate of natural increase: The relationship with body size. Oecologia 1974, 14, 317–326. [Google Scholar] [CrossRef]
- Thompson, S.D. Body size, duration of parental care, and the intrinsic rate of natural increase in eutherian and metatherian mammals. Oecologia 1987, 71, 201–209. [Google Scholar] [CrossRef]
- Ross, C. The intrinsic rate of natural increase and reproductive effort in primates. J. Zool. 1988, 214, 199–219. [Google Scholar] [CrossRef]
- Denney, N.H.; Jennings, S.; Reynolds, J.D. Life–history correlates of maximum population growth rates in marine fishes. Proc. R. Soc. B Biol. Sci. 2002, 269, 2229–2237. [Google Scholar] [CrossRef] [PubMed]
- Hatton, I.A.; Dobson, A.P.; Storch, D.; Galbraith, E.D.; Loreau, M. Linking scaling laws across eukaryotes. Proc. Natl. Acad. Sci. USA 2019, 116, 21616–21622. [Google Scholar] [CrossRef]
- Pianka, E.R. On r- and K-selection. Am. Nat. 1970, 104, 592–597. [Google Scholar] [CrossRef]
- Gaston, K.J. Patterns in the local and regional dynamics of moth populations. Oikos 1988, 53, 49–57. [Google Scholar] [CrossRef]
- Gaston, K.J.; Lawton, J.H. Patterns in body size, population dynamics, and regional distribution of bracken herbivores. Am. Nat. 1988, 132, 662–680. [Google Scholar] [CrossRef]
- Anderson, D.M.; Gillooly, J.F. Allometric scaling of Lyapunov exponents in chaotic populations. Popul. Ecol. 2020, 62, 364–369. [Google Scholar] [CrossRef]
- MacArthur, R.H.; Wilson, E.O. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967. [Google Scholar]
- Southwood, T.R.E.; May, R.M.; Hassell, M.P.; Conway, G.R. Ecological strategies and population parameters. Am. Nat. 1974, 108, 791–804. [Google Scholar] [CrossRef]
- Spitzer, K.; Rejmánek, M.; Soldán, T. The fecundity and long-term variability in abundance of noctuid moths (Lepidoptera, Noctuidae). Oecologia 1984, 62, 91–93. [Google Scholar] [CrossRef]
- Sæther, B.E.; Engen, S. The concept of fitness in fluctuating environments. Trends Ecol. Evol. 2015, 30, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Rubner, M. Über den Einfluss der Körpergrösse auf Stoff- und Kraftwechsel. Z. Biol. 1883, 19, 535–562. [Google Scholar]
- Pearl, R. The Rate of Living; University of London Press: London, UK, 1928. [Google Scholar]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Economos, A.C. Beyond rate of living. Gerontology 1981, 27, 258–265. [Google Scholar] [CrossRef]
- Glazier, D.S. Is metabolic rate a universal ‘pacemaker’ for biological processes? Biol. Rev. 2015, 90, 377–407. [Google Scholar] [CrossRef]
- Stark, G.; Pincheira-Donoso, D.; Meiri, S. No evidence for the “rate-of-living” theory across the tetrapod tree of life. Glob. Ecol. Biogeogr. 2020, 29, 857–884. [Google Scholar] [CrossRef]
- Escala, A. Universal relation for life-span energy consumption in living organisms: Insights for the origin of aging. Sci. Rep. 2022, 12, 2407. [Google Scholar] [CrossRef]
- Glazier, D.S. Does death drive the scaling of life? Biol. Rev. 2025, 100, 586–619. [Google Scholar] [CrossRef]
- Günther, B. Dimensional analysis and theory of biological similarity. Physiol. Rev. 1975, 55, 659–699. [Google Scholar] [CrossRef]
- Hainsworth, F.R. Animal Physiology: Adaptations in Function; Addison-Wesley: Reading, MA, USA, 1981. [Google Scholar]
- Lindstedt, S.L.; Calder, W.A. Body size, physiological time, and longevity of homeothermic animals. Q. Rev. Biol. 1981, 56, 1–16. [Google Scholar] [CrossRef]
- Günther, B.; Morgado, E. Allometric scaling of biological rhythms in mammals. Biol. Res. 2005, 38, 207–212. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.K.L.; Garcia, G.J.; Barbosa, L.A. Allometric scaling laws of metabolism. Phys. Life Rev. 2006, 3, 229–261. [Google Scholar] [CrossRef]
- Ginzburg, L.; Damuth, J. The space-lifetime hypothesis: Viewing organisms in four dimensions, literally. Am. Nat. 2008, 171, 125–131. [Google Scholar] [CrossRef]
- Glazier, D.S. The relevance of time in biological scaling. Biology 2023, 12, 1084. [Google Scholar] [CrossRef]
- Lambert, R.; Teissier, G. Théorie de la similitude biologique. Ann. Physiol. Physicochim. Biol. 1927, 13, 212–246. [Google Scholar]
- Darwin, C. On the Origin of Species by Means of Natural Selection or the Preservation of Favoured Races in the Struggle for Life, 6th ed.; John Murray: London, UK, 1872. [Google Scholar]
- Stanley, S.M. Macroevolution: Pattern and Process; Freeman: San Francisco, CA, USA, 1979. [Google Scholar]
- Fowler, C.W.; MacMahon, J.A. Selective extinction and speciation: Their influence on the structure and functioning of communities and ecosystems. Am. Nat. 1982, 119, 480–498. [Google Scholar] [CrossRef]
- Glazier, D.S. Ecological shifts and the evolution of geographically restricted species of North American Peromyscus (mice). J. Biogeogr. 1980, 7, 63–83. [Google Scholar] [CrossRef]
- Blumenthal, D.M.; Diez, J.; Pearse, I.; Sofaer, H.R.; Sorte, C.J.B.; Barnett, D.; Beaury, E.M.; Bradley, B.A.; Corbin, J.D.; Dukes, J.S.; et al. Why are non-native plants successful? Consistently fast economic traits and novel origin jointly explain abundance across US ecoregions. New Phytol. 2025, 248, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Liow, L.H. Does versatility as measured by geographic range, bathymetric range and morphological variability contribute to taxon longevity? Glob. Ecol. Biogeogr. 2007, 16, 117–128. [Google Scholar] [CrossRef]
- Payne, J.L.; Finnegan, S. The effect of geographic range on extinction risk during background and mass extinction. Proc. Natl. Acad. Sci. USA 2007, 104, 10506–10511. [Google Scholar] [CrossRef] [PubMed]
- Harris, G.; Pimm, S.L. Range size and extinction risk in forest birds. Conserv. Biol. 2008, 22, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, M.J. How species longevity, intraspecific morphological variation, and geographic range size are related: A comparison using Late Cambrian trilobites. Evolution 2011, 65, 3253–3273. [Google Scholar] [CrossRef]
- Castiglione, S.; Mondanaro, A.; Melchionna, M.; Serio, C.; Di Febbraro, M.; Carotenuto, F.; Raia, P. Diversification rates and the evolution of species range size frequency distribution. Front. Ecol. Evol. 2017, 5, 147. [Google Scholar] [CrossRef]
- Newsome, T.M.; Wolf, C.; Nimmo, D.G.; Kopf, R.K.; Ritchie, E.G.; Smith, F.A.; Ripple, W.J. Constraints on vertebrate range size predict extinction risk. Glob. Ecol. Biogeogr. 2020, 29, 76–86. [Google Scholar] [CrossRef]
- Brown, W.L., Jr. Centrifugal speciation. Q. Rev. Biol. 1957, 32, 247–277. [Google Scholar] [CrossRef]
- Rosenzweig, M.L. Species Diversity in Space and Time; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Li, P.; Wiens, J.J. What drives diversification? Range expansion tops climate, life history, habitat and size in lizards and snakes. J. Biogeogr. 2022, 49, 237–247. [Google Scholar] [CrossRef]
- Hay, E.M.; McGee, M.D.; Chown, S.L. Geographic range size and speciation in honeyeaters. BMC Ecol. Evol. 2022, 22, 86. [Google Scholar] [CrossRef]
- Smyčka, J.; Toszogyova, A.; Storch, D. The relationship between geographic range size and rates of species diversification. Nat. Comm. 2023, 14, 5559. [Google Scholar] [CrossRef]
- Gaston, K.J.; Blackburn, T.M. Pattern and Process in Macroecology; Blackwell: Malden, MA, USA, 2000. [Google Scholar]
- Jablonski, D.; Roy, K. Geographical range and speciation in fossil and living molluscs. Proc. R. Soc. B Biol. Sci. 2003, 270, 401–406. [Google Scholar] [CrossRef]
- Jablonski, D.; Roy, K.; Valentine, J.W. Evolutionary Macroecology and the Fossil Record. In Macroecology—Concepts and Consequences; Blackburn, T.M., Gaston, K.J., Eds.; Cambridge University Press: Cambridge, UK, 2003; pp. 368–390. [Google Scholar]
- Jablonski, D. Species selection: Theory and data. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 501–524. [Google Scholar] [CrossRef]
- Roughgarden, J.; Iwasa, Y. Dynamics of a metapopulation with space-limited subpopulations. Theor. Pop. Biol. 1986, 29, 235–261. [Google Scholar] [CrossRef]
- Pulliam, H.R. Sources, sinks, and population regulation. Am. Nat. 1988, 132, 652–661. [Google Scholar] [CrossRef]
- Dias, P.C. Sources and sinks in population biology. Trends Ecol. Evol. 1996, 11, 326–330. [Google Scholar] [CrossRef]
- Brown, J.H.; Kodric-Brown, A. Turnover rates in insular biogeography: Effect of immigration on extinction. Ecology 1977, 58, 445–449. [Google Scholar] [CrossRef]
- Gotelli, N.J. Metapopulation models: The rescue effect, the propagule rain, and the core-satellite hypothesis. Am. Nat. 1991, 138, 768–776. [Google Scholar] [CrossRef]
- Goedert, D.; Jensen, H.; Dickel, L.; Reid, J.M. Multi-generational fitness legacies of natural immigration: Theoretical and empirical perspectives and opportunities. Biol. Rev. 2025, 100, 1250–1271. [Google Scholar] [CrossRef] [PubMed]
- Saatoglu, D.; Niskanen, A.K.; Froy, H.; Ranke, P.S.; Goedert, D.; Reid, J.; Ringsby, T.H.; Sæther, B.E.; Araya-Ajoy, Y.G.; Jensen, H. Metapopulation-level analyses reveal positive fitness consequences of immigration in a small bird. J. Anim. Ecol. 2025, 94, 1180–1192. [Google Scholar] [CrossRef]
- Roff, D.A. Life History Evolution; Sinauer Associates: Sunderland, MA, USA, 2002. [Google Scholar]
- Myhrvold, N.P.; Baldridge, E.; Chan, B.; Sivam, D.; Freeman, D.L.; Ernest, S.M. An amniote life-history database to perform comparative analyses with birds, mammals, and reptiles: Ecological Archives E096–269. Ecology 2015, 96, 3109. [Google Scholar] [CrossRef]
- Bonner, J.T. Size and Cycle: An Essay on the Structure of Biology; Princeton University Press: Princeton, NJ, USA, 1965. [Google Scholar]
- Sokal, R.R.; Rohlf, F.J. Biometry; Freeman: New York, NY, USA, 1995. [Google Scholar]
- Smith, R.J. Use and misuse of the reduced major axis for line-fitting. Am. J. Phys. Anthropol. 2009, 140, 476–486. [Google Scholar] [CrossRef]
- White, C.R. Allometric estimation of metabolic rates in animals. Comp. Biochem. Physiol. A 2011, 158, 346–357. [Google Scholar] [CrossRef]
- Kilmer, J.T.; Rodríguez, R.L. Ordinary least squares regression is indicated for studies of allometry. J. Evol. Biol. 2017, 30, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S. Trade-offs between reproductive and somatic (storage) investments in animals: A comparative test of the Van Noordwijk and De Jong model. Evol. Ecol. 1999, 13, 539–555. [Google Scholar] [CrossRef]
- Barbraud, C.; Weimerskirch, H. Environmental conditions and breeding experience affect costs of reproduction in blue petrels. Ecology 2005, 86, 682–692. [Google Scholar] [CrossRef]
- Travers, L.M.; Garcia-Gonzalez, F.; Simmons, L.W. Live fast die young life history in females: Evolutionary trade-off between early life mating and lifespan in female Drosophila melanogaster. Sci. Rep. 2015, 5, 15469. [Google Scholar] [CrossRef] [PubMed]
- Wadgymar, S.M.; Sheth, S.; Josephs, E.; DeMarche, M.; Anderson, J. Defining fitness in evolutionary ecology. Int. J. Plant Sci. 2024, 185, 218–227. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Keevil, M.G.; Rollinson, N.; Brooks, R.J. Subtle individual variation in indeterminate growth leads to major variation in survival and lifetime reproductive output in a long-lived reptile. Funct. Ecol. 2018, 32, 752–761. [Google Scholar] [CrossRef]
- Sibly, R.M.; Calow, P. Why breeding earlier is always worthwhile. J. Theor. Biol. 1986, 123, 311–319. [Google Scholar] [CrossRef]
- Stahl, W.R. Similarity and dimensional methods in biology: They promise to show quantitative similarities between biological organisms and models of biological systems. Science 1962, 137, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Charnov, E.L. Life History Invariants; Oxford University Press: Oxford, UK, 1993. [Google Scholar]
- Peters, R.H. The Ecological Implications of Body Size; Cambridge University Press: Cambridge, UK, 1983. [Google Scholar]
- Babich Morrow, C.; Ernest, S.M.; Kerkhoff, A.J. Macroevolution of dimensionless life-history metrics in tetrapods. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210200. [Google Scholar] [CrossRef]
- Levine, H.J. Rest heart rate and life expectancy. J. Am. Coll. Cardiol. 1997, 30, 1104–1106. [Google Scholar]
- Wieser, W. A new look at energy conversion in ectothermic and endothermic animals. Oecologia 1985, 66, 506–510. [Google Scholar] [CrossRef]
- Hendriks, A.J.; Mulder, C. Scaling of offspring number and mass to plant and animal size: Model and meta-analysis. Oecologia 2008, 155, 705–716. [Google Scholar] [CrossRef]
- Humphreys, W.F. Production and respiration in animal populations. J. Anim. Ecol. 1979, 48, 427–453. [Google Scholar] [CrossRef]
- Lavigne, D.M. Similarity in energy budgets of animal populations. J. Anim. Ecol. 1982, 51, 195–206. [Google Scholar] [CrossRef]
- Slobodkin, L.B. On the inconstancy of ecological efficiency and the form of ecological theories. Trans. Conn. Acad. Arts Sci. 1972, 44, 293–305. [Google Scholar]
- Damuth, J. Population density and body size in mammals. Nature 1981, 290, 699–700. [Google Scholar] [CrossRef]
- Damuth, J. Interspecific allometry of population density in mammals and other animals: The independence of body mass and population energy-use. Biol. J. Linn. Soc. 1987, 31, 193–246. [Google Scholar] [CrossRef]
- Perkins, D.M.; Perna, A.; Adrian, R.; Cermeño, P.; Gaedke, U.; Huete-Ortega, M.; White, E.P.; Yvon-Durocher, G. Energetic equivalence underpins the size structure of tree and phytoplankton communities. Nat. Comm. 2019, 10, 255. [Google Scholar] [CrossRef]
- Hayward, A.; Khalid, M.; Kolasa, J. Population energy use scales positively with body size in natural aquatic microcosms. Glob. Ecol. Biogeogr. 2009, 18, 553–562. [Google Scholar] [CrossRef]
- DeLong, J.P. Energetic inequivalence in eusocial insect colonies. Biol. Lett. 2011, 7, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Munn, A.J.; Dunne, C.; Müller, D.W.; Clauss, M. Energy in-equivalence in Australian marsupials: Evidence for disruption of the continent’s mammal assemblage, or are rules meant to be broken? PLoS ONE 2013, 8, e57449. [Google Scholar] [CrossRef] [PubMed]
- Ehnes, R.B.; Pollierer, M.M.; Erdmann, G.; Klarner, B.; Eitzinger, B.; Digel, C.; Ott, D.; Maraun, M.; Scheu, S.; Brose, U. Lack of energetic equivalence in forest soil invertebrates. Ecology 2014, 95, 527–537. [Google Scholar] [CrossRef]
- Malerba, M.E.; Marshall, D.J. Size-abundance rules? Evolution changes scaling relationships between size, metabolism and demography. Ecol. Lett. 2019, 22, 1407–1416. [Google Scholar] [CrossRef]
- Sutherland, W.J.; Grafen, A.; Harvey, P.H. Life history correlations and demography. Nature 1986, 320, 88. [Google Scholar] [CrossRef]
- Sibly, R.M.; Calow, P. Ecological compensation—A complication for testing life-history theory. J. Theor. Biol. 1987, 125, 177–186. [Google Scholar] [CrossRef]
- Lynch, M. Complexity myths and the misappropriation of evolutionary theory. Proc. Natl. Acad. Sci. USA 2025, 122, e2425772122. [Google Scholar] [CrossRef]
- Schmalhausen, I.I. Factors of Evolution: The Theory of Stabilizing Selection; Blakiston: Toronto, ON, Canada, 1949. [Google Scholar]
- Williams, G.C. Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought; Princeton University Press: Princeton, NJ, USA, 1966. [Google Scholar]
- Promislow, D.E.; Harvey, P.H. Living fast and dying young: A comparative analysis of life-history variation among mammals. J. Zool. 1990, 220, 417–437. [Google Scholar] [CrossRef]
- Jepsen, G.L. Selection, “orthogenesis,” and the fossil record. Proc. Am. Philos. Soc. 1949, 93, 479–500. [Google Scholar] [PubMed]
- Gould, S.J. Ontogeny and Phylogeny; Harvard University Press: Cambridge, MA, USA, 1977. [Google Scholar]
- Levit, G.S.; Olsson, L. ‘Evolution on rails’: Mechanisms and levels of orthogenesis. Ann. Hist. Philos. Biol. 2006, 11, 99–138. [Google Scholar]
- Glazier, D.S. Metabolic scaling in complex living systems. Systems 2014, 2, 451–540. [Google Scholar] [CrossRef]
- Glazier, D.S. Variable metabolic scaling breaks the law: From ‘Newtonian’ to ‘Darwinian’ approaches. Proc. R. Soc. B Biol. Sci. 2022, 289, 20221605. [Google Scholar] [CrossRef] [PubMed]
- Wicken, J.S. A thermodynamic theory of evolution. J. Theor. Biol. 1980, 87, 9–23. [Google Scholar] [CrossRef]
- Wicken, J.S. On the Increase in Complexity in Evolution. In Beyond Neo-Darwinism: An Introduction to the New Evolutionary Paradigm; Ho, M.-W., Saunders, P.T., Eds.; Academic Press: Orlando, FL, USA, 1984; pp. 89–112. [Google Scholar]
- Annila, A.; Salthe, S. Physical foundations of evolutionary theory. J. Non-Equilibr. Thermodyn. 2010, 35, 301–321. [Google Scholar] [CrossRef]
- Bejan, A.; Lorente, S. The constructal law and the evolution of design in nature. Phys. Life Rev. 2011, 8, 209–240. [Google Scholar] [CrossRef]
- Glazier, D.S. Scaling of metabolic scaling within physical limits. Systems 2014, 2, 425–450. [Google Scholar] [CrossRef]
- Kempes, C.P.; Koehl, M.A.R.; West, G.B. The scales that limit: The physical boundaries of evolution. Front. Ecol. Evol. 2019, 7, 242. [Google Scholar] [CrossRef]
- Glazier, D.S.; Avilés, L.; Okie, J.G. Size and Metabolism from Cells to Social Groups: Universal Geometric Constraints and Adaptive Responses. In Scaling in Biology: A New Synthesis; Enquist, B.J., O’Connor, M.I., Kempes, C.P., Eds.; Santa Fe Institute Press: Sante Fe, NM, USA, 2026; in press. [Google Scholar]
- Jacob, F. The Logic of Life: A History of Heredity; Random House: New York, NY, USA, 1973. [Google Scholar]
- Mayr, E. The Growth of Biological Thought: Diversity, Evolution, and Inheritance; Harvard University Press: Cambridge, MA, USA, 1982. [Google Scholar]
- Blount, Z.D.; Lenski, R.E.; Losos, J.B. Contingency and determinism in evolution: Replaying life’s tape. Science 2018, 362, eaam5979. [Google Scholar] [CrossRef]
- Darwin, C. The Variation of Animals and Plants Under Domestication; Appleton: New York, NY, USA, 1896. [Google Scholar]
- Mousseau, T.A.; Roff, D.A. Natural selection and the heritability of fitness components. Heredity 1987, 69, 181–197. [Google Scholar] [CrossRef]
- Newton, I. Lifetime Reproduction in Birds; Academic Press: London, UK, 1989. [Google Scholar]
- Albon, S.D.; Clutton-Brock, T.H.; Langvatn, R. Cohort Variation in Reproduction and Survival: Implications for Population Demography. In The Biology of Deer; Brown, R.D., Ed.; Springer: New York, NY, USA, 1992; pp. 15–21. [Google Scholar]
- Houle, D. Comparing evolvability and variability of quantitative traits. Genetics 1992, 130, 195–204. [Google Scholar] [CrossRef]
- O’Neil, P. Natural selection on genetically correlated phenological characters in Lythrum salicaria L. (Lythraceae). Evolution 1997, 51, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Merilä, J.; Sheldon, B.C. Lifetime reproductive success and heritability in nature. Am. Nat. 2000, 155, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Jager, H.I. Individual variation in life history characteristics can influence extinction risk. Ecol. Model. 2001, 144, 61–76. [Google Scholar] [CrossRef]
- Ellegren, H.; Sheldon, B.C. Genetic basis of fitness differences in natural populations. Nature 2008, 452, 169–175. [Google Scholar] [CrossRef]
- Clutton-Brock, T.H.; Sheldon, B.C. Individuals and populations: The role of long-term, individual-based studies of animals in ecology and evolutionary biology. Trends Ecol. Evol. 2010, 25, 562–573. [Google Scholar] [CrossRef]
- Caswell, H. Beyond R0: Demographic models for variability of lifetime reproductive output. PLoS ONE 2011, 6, e20809. [Google Scholar] [CrossRef]
- Chambert, T.; Rotella, J.J.; Higgs, M.D.; Garrott, R.A. Individual heterogeneity in reproductive rates and cost of reproduction in a long-lived vertebrate. Ecol. Evol. 2013, 3, 2047–2060. [Google Scholar] [CrossRef] [PubMed]
- Vindenes, Y.; Langangen, Ø. Individual heterogeneity in life histories and eco-evolutionary dynamics. Ecol. Lett. 2015, 18, 417–432. [Google Scholar] [CrossRef]
- Vincenzi, S.; Mangel, M.; Jesensek, D.; Garza, J.C.; Crivelli, A.J. Within-and among-population variation in vital rates and population dynamics in a variable environment. Ecol. Appl. 2016, 26, 2086–2102. [Google Scholar] [CrossRef]
- Hansen, T.F. On the definition and measurement of fitness in finite populations. J. Theor. Biol. 2017, 419, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Paterson, J.T.; Rotella, J.J.; Link, W.A.; Garrott, R. Variation in the vital rates of an Antarctic marine predator: The role of individual heterogeneity. Ecology 2018, 99, 2385–2396. [Google Scholar] [CrossRef]
- Flatt, T. Life-history evolution and the genetics of fitness components in Drosophila melanogaster. Genetics 2020, 214, 3–48. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Parlato, E.H.; Frost, P.G. Incorporating individual variation in survival, reproduction and detection rates when projecting dynamics of small populations. Ecol. Model. 2021, 455, 109647. [Google Scholar] [CrossRef]
- Forsythe, A.B.; Day, T.; Nelson, W.A. Demystifying individual heterogeneity. Ecol. Lett. 2021, 24, 2282–2297. [Google Scholar] [CrossRef]
- Bonnet, T.; Morrissey, M.B.; De Villemereuil, P.; Alberts, S.C.; Arcese, P.; Bailey, L.D.; Boutin, S.; Brekke, P.; Brent, L.J.; Camenisch, G.; et al. Genetic variance in fitness indicates rapid contemporary adaptive evolution in wild animals. Science 2022, 376, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Arnqvist, G.; Rowe, L. Ecology, the pace-of-life, epistatic selection and the maintenance of genetic variation in life-history genes. Mol. Ecol. 2023, 32, 4713–4724. [Google Scholar] [CrossRef] [PubMed]
- Maynard Smith, J. The Evolution of Sex; Cambridge University Press: Cambridge, UK, 1976. [Google Scholar]
- Crow, J.F. An advantage of sexual reproduction in a rapidly changing environment. J. Hered. 1992, 83, 169–173. [Google Scholar] [CrossRef]
- Waxman, D.; Peck, J.R. Sex and adaptation in a changing environment. Genetics 1999, 153, 1041–1053. [Google Scholar] [CrossRef]
- Colegrave, N. Sex releases the speed limit on evolution. Nature 2002, 420, 664–666. [Google Scholar] [CrossRef]
- Becks, L.; Agrawal, A.F. The evolution of sex is favoured during adaptation to new environments. PLoS Biol. 2012, 10, e1001317. [Google Scholar] [CrossRef]
- Lively, C.M.; Morran, L. The ecology of sexual reproduction. J. Evol. Biol. 2014, 27, 1292–1303. [Google Scholar] [CrossRef]
- McDonald, M.J.; Rice, D.P.; Desai, M.M. Sex speeds adaptation by altering the dynamics of molecular evolution. Nature 2016, 531, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.C. Sex and Evolution; Princeton University Press: Princeton, NJ, USA, 1975. [Google Scholar]
- O’Donnell, D.; Armbruster, P. Evolutionary differentiation of fitness traits across multiple geographic scales in Aedes albopictus (Diptera: Culicidae). Ann. Entomol. Soc. Am. 2009, 102, 1135–1144. [Google Scholar] [CrossRef]
- Stanton-Geddes, J.; Shaw, R.G.; Tiffin, P. Interactions between soil habitat and geographic range location affect plant fitness. PLoS ONE 2012, 7, e36015. [Google Scholar] [CrossRef] [PubMed]
- Stanton-Geddes, J.; Tiffin, P.; Shaw, R.G. Role of climate and competitors in limiting fitness across range edges of an annual plant. Ecology 2012, 93, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- McKellar, A.E.; Kesler, D.C.; Mitchell, R.J.; Delaney, D.K.; Walters, J.R. Geographic variation in fitness and foraging habitat quality in an endangered bird. Biol. Conserv. 2014, 175, 52–64. [Google Scholar] [CrossRef]
- Benning, J.W.; Moeller, D.A. Maladaptation beyond a geographic range limit driven by antagonistic and mutualistic biotic interactions across an abiotic gradient. Evolution 2019, 73, 2044–2059. [Google Scholar] [CrossRef]
- Benning, J.W.; Moeller, D.A. Microbes, mutualism, and range margins: Testing the fitness consequences of soil microbial communities across and beyond a native plant’s range. New Phytol. 2021, 229, 2886–2900. [Google Scholar] [CrossRef]
- Brunot, M.; Morellet, N.; Balandier, M.; Marchand, P.; Gaillard, J.M.; Cargnelutti, B.; Bonnet, A.; Chaval, Y.; Pellerin, M.; Mark Hewison, A.J. Born in heterogenous landscapes: Birth timing, body mass and early development of roe deer fawns in contrasting habitats. J. Zool. 2025, 326, 277–288. [Google Scholar] [CrossRef]
- Bucciolini, G.L.; Morosinotto, C.; Brommer, J.; Vrezec, A.; Ericsson, P.; Nilsson, L.O.; Poprach, K.; Øien, I.J.; Karell, P. Lifetime fitness variation across the geographical range in a colour polymorphic species. Ecol. Evol. 2025, 15, e71051. [Google Scholar] [CrossRef] [PubMed]
- Carley, L.N.; Geber, M.A.; Morris, W.F.; Eckhart, V.M.; Moeller, D.A. Local adaptation is highest in populations with stable long-term growth. Ecol. Lett. 2025, 28, e70071. [Google Scholar] [CrossRef]
- Bayne, B.L.; Moore, M.N.; Widdows, J.; Livingstone, D.R.; Salkeld, P. Measurement of the responses of individuals to environmental stress and pollution: Studies with bivalve molluscs. Philos. Trans. R. Soc. B Biol. Sci. 1979, 286, 563–581. [Google Scholar] [CrossRef]
- Widdows, J.; Johnson, D. Physiological energetics of Mytilus edulis: Scope for growth. Mar. Ecol. Progr. Ser. 1988, 46, 113–121. [Google Scholar] [CrossRef]
- Sibly, R.M.; Calow, P. A life-cycle theory of responses to stress. Biol. J. Linn. Soc. 1989, 37, 101–116. [Google Scholar] [CrossRef]
- Maltby, L.; Naylor, C.; Calow, P. Effect of stress on a freshwater benthic detritivore: Scope for growth in Gammarus pulex. Ecotoxicol. Environ. Saf. 1990, 19, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Haag, W.R.; Berg, D.J.; Garton, D.W.; Farris, J.L. Reduced survival and fitness in native bivalves in response to fouling by the introduced zebra mussel (Dreissena polymorpha) in western Lake Erie. Can. J. Fish. Aquat. Sci. 1993, 50, 13–19. [Google Scholar] [CrossRef]
- Glazier, D.S. Springs as model systems for ecology and evolutionary biology: A case study of Gammarus minus Say (Amphipoda) in mid-Appalachian springs differing in pH and ionic content. In Studies in Crenobiology: The Biology of Springs and Springbrooks; Botosaneanu, L., Ed.; Backhuys: Leiden, The Netherlands, 1998; pp. 49–62. [Google Scholar]
- Hoffmann, A.A.; Hercus, M.J. Environmental stress as an evolutionary force. BioScience 2000, 50, 217–226. [Google Scholar] [CrossRef]
- Sibly, R.M.; Hone, J. Population growth rate and its determinants: An overview. Philos. Trans. R. Soc. B Biol. Sci. 2002, 357, 1153–1170. [Google Scholar] [CrossRef]
- Baillieul, M.; Smolders, R.; Blust, R. The effect of environmental stress on absolute and mass-specific scope for growth in Daphnia magna Strauss. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2005, 140, 364–373. [Google Scholar] [CrossRef]
- Parsons, P.A. Environments and evolution: Interactions between stress, resource inadequacy and energetic efficiency. Biol. Rev. 2005, 80, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Vila-Aiub, M.M.; Neve, P.; Powles, S.B. Fitness costs associated with evolved herbicide resistance alleles in plants. New Phytol. 2009, 184, 751–767. [Google Scholar] [CrossRef]
- Weitere, M.; Vohmann, A.; Schulz, N.; Linn, C.; Dietrich, D.; Arndt, H. Linking environmental warming to the fitness of the invasive clam Corbicula fluminea. Glob. Change Biol. 2009, 15, 2838–2851. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef]
- Kadri, A.; Julier, B.; Laouar, M.; Ben, C.; Badri, M.; Chedded, J.; Mouhouche, B.; Gentzbittel, L.; Abdelguerfi, A. Genetic determinism of reproductive fitness traits under drought stress in the model legume Medicago truncatula. Acta Physiol. Plant. 2017, 39, 227. [Google Scholar] [CrossRef]
- Sokolova, I.M. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr. Comp. Biol. 2013, 53, 597–608. [Google Scholar] [CrossRef]
- Sokolova, I.M. Bioenergetics in environmental adaptation and stress tolerance of aquatic ectotherms: Linking physiology and ecology in a multi-stressor landscape. J. Exp. Biol. 2021, 224, jeb236802. [Google Scholar] [CrossRef]
- Harris, B.N.; Josefson, C.C. Stress and Reproduction in Mammals. In Hormones and Reproduction of Vertebrates, Volume 5 Mammals; Norris, D.O., Lopez, K.H., Eds.; Academic Press: London, UK, 2024; pp. 169–197. [Google Scholar]
- de la Fuente, A.; Briscoe, N.J.; Kearney, M.R.; Williams, S.E.; Youngentob, K.N.; Marsh, K.J.; Cernusak, L.A.; Leahy, L.; Larson, J.; Krockenberger, A.K. Climate-induced physiological stress drives rainforest mammal population declines. Glob. Change Biol. 2025, 31, e70215. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.A.; Velotta, J.P.; Ghalambor, C.K. A systems approach to homeostasis: What euryhaline fish teach us about organismal stress responses. Integr. Comp. Biol. 2025, 65, 546–559. [Google Scholar] [CrossRef]
- Pieper, E.B.; Radich, J.A.; Randklev, C.R.; Berg, D.J.; Ramey, M.; Wright, R.A.; Abdelrahman, H.A.; Stoeckel, J.A. Scope for growth is optimized across a limited temperature range in an imperiled freshwater mussel. Hydrobiologia 2025, 852, 3445–3461. [Google Scholar] [CrossRef]
- Wild, K.H.; Huey, R.B.; Pianka, E.R.; Clusella-Trullas, S.; Gilbert, A.L.; Miles, D.B.; Kearney, M.R. Climate change and the cost-of-living squeeze in desert lizards. Science 2025, 387, 303–309. [Google Scholar] [CrossRef]
- Ten Caten, C.; Dallas, T. Population variability across geographical ranges: Perspectives and challenges. Proc. R. Soc. B Biol. Sci. 2025, 292, 20241644. [Google Scholar] [CrossRef]
- Van Valen, L.M. Group selection, sex, and fossils. Evolution 1975, 29, 87–94. [Google Scholar] [CrossRef]
- Bennett, P.M.; Owens, I.P. Variation in extinction risk among birds: Chance or evolutionary predisposition? Proc. R. Soc. B Biol. Sci. 1997, 264, 401–408. [Google Scholar] [CrossRef]
- McKinney, M.L. Extinction vulnerability and selectivity: Combining ecological and paleontological views. Annu. Rev. Ecol. Syst. 1997, 28, 495–516. [Google Scholar] [CrossRef]
- Purvis, A.; Jones, K.E.; Mace, G.M. Extinction. BioEssays 2000, 22, 1123–1133. [Google Scholar] [CrossRef]
- Cardillo, M.; Mace, G.M.; Gittleman, J.L.; Jones, K.E.; Bielby, J.; Purvis, A. The predictability of extinction: Biological and external correlates of decline in mammals. Proc. R. Soc. B Biol. Sci. 2008, 275, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Fritz, S.A.; Bininda-Emonds, O.R.; Purvis, A. Geographical variation in predictors of mammalian extinction risk: Big is bad, but only in the tropics. Ecol. Lett. 2009, 12, 538–549. [Google Scholar] [CrossRef]
- Miao, L.; Liu, X.; Brayard, A.; Korn, D.; Dai, X.; Song, H. Morphological complexity promotes origination and extinction rates in ammonoids. Curr. Biol. 2024, 34, 5587–5594. [Google Scholar] [CrossRef]
- Tamre, E.; Parsons, C. Selection by differential survival among marine animals in the Phanerozoic. J. Theor. Biol. 2024, 590, 111849. [Google Scholar] [CrossRef]
- Alzate, A.; Rozzi, R.; Velasco, J.A.; Robertson, D.R.; Zizka, A.; Tobias, J.A.; Hill, A.; Bacon, C.D.; Janzen, T.; Pellissier, L.; et al. Evolutionary age correlates with range size across plants and animals. Nat. Comm. 2025, 16, 7894. [Google Scholar] [CrossRef]
- Hanski, I. Dynamics of regional distribution: The core and satellite species hypothesis. Oikos 1982, 38, 210–221. [Google Scholar] [CrossRef]
- Diamond, J.M. “Normal” Extinctions of Isolated Populations. In Extinctions; Nitecki, M.H., Ed.; University of Chicago Press: Chicago, IL, USA, 1984; pp. 191–246. [Google Scholar]
- Glazier, D.S. Toward a predictive theory of speciation: The ecology of isolate selection. J. Theor. Biol. 1987, 126, 323–333. [Google Scholar] [CrossRef]
- Pimm, S.L.; Jones, H.L.; Diamond, J. On the risk of extinction. Am. Nat. 1988, 132, 757–785. [Google Scholar] [CrossRef]
- Gaston, K.J. Rarity; Springer: Dordrecht, The Netherlands, 1994. [Google Scholar]
- Gaston, K.J. The Structure and Dynamics of Geographic Ranges; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Levin, D.A. The Origin, Expansion, and Demise of Plant Species; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Jones, K.E.; Purvis, A.; Gittleman, J.L. Biological correlates of extinction risk in bats. Am. Nat. 2003, 161, 601–614. [Google Scholar] [CrossRef]
- Cardillo, M.; Huxtable, J.S.; Bromham, L. Geographic range size, life history and rates of diversification in Australian mammals. J. Evol. Biol. 2003, 16, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Cardillo, M.; Mace, G.M.; Jones, K.E.; Bielby, J.; Bininda-Emonds, O.R.; Sechrest, W.; Orme, C.D.L.; Purvis, A. Multiple causes of high extinction risk in large mammal species. Science 2005, 309, 1239–1241. [Google Scholar] [CrossRef]
- Koh, L.P.; Sodhi, N.S.; Brook, B.W. Ecological correlates of extinction proneness in tropical butterflies. Conserv. Biol. 2004, 18, 1571–1578. [Google Scholar] [CrossRef]
- Reynolds, J.D.; Webb, T.J.; Hawkins, L.A. Life history and ecological correlates of extinction risk in European freshwater fishes. Can. J. Fish. Aquat. Sci. 2005, 62, 854–862. [Google Scholar] [CrossRef]
- Jablonski, D.; Hunt, G. Larval ecology, geographic range, and species survivorship in Cretaceous mollusks: Organismic versus species-level explanations. Am. Nat. 2006, 168, 556–564. [Google Scholar] [CrossRef]
- Powell, M.G.; MacGregor, J. A geographic test of species selection using planktonic foraminifera during the Cretaceous/Paleogene mass extinction. Paleobiology 2011, 37, 426–437. [Google Scholar] [CrossRef]
- Heim, N.A.; Peters, S.E. Regional environmental breadth predicts geographic range and longevity in fossil marine genera. PLoS ONE 2011, 6, e18946. [Google Scholar] [CrossRef]
- Harnik, P.G.; Simpson, C.; Payne, J.L. Long-term differences in extinction risk among the seven forms of rarity. Proc. R. Soc. B Biol. Sci. 2012, 279, 4969–4976. [Google Scholar] [CrossRef]
- Tietje, M.; Kiessling, W. Predicting extinction from fossil trajectories of geographical ranges in benthic marine molluscs. J. Biogeogr. 2013, 40, 790–799. [Google Scholar] [CrossRef]
- Saupe, E.E.; Qiao, H.; Hendricks, J.R.; Portell, R.W.; Hunter, S.J.; Soberón, J.; Lieberman, B.S. Niche breadth and geographic range size as determinants of species survival on geological time scales. Glob. Ecol. Biogeogr. 2015, 24, 1159–1169. [Google Scholar] [CrossRef]
- Böhm, M.; Williams, R.; Bramhall, H.R.; McMillan, K.M.; Davidson, A.D.; Garcia, A.; Bland, L.M.; Bielby, J.; Collen, B. Correlates of extinction risk in squamate reptiles: The relative importance of biology, geography, threat and range size. Glob. Ecol. Biogeogr. 2016, 25, 391–405. [Google Scholar] [CrossRef]
- Hugueny, B. Age–area scaling of extinction debt within isolated terrestrial vertebrate assemblages. Ecol. Lett. 2017, 20, 591–598. [Google Scholar] [CrossRef]
- Chichorro, F.; Juslén, A.; Cardoso, P. A review of the relation between species traits and extinction risk. Biol. Conserv. 2019, 237, 220–229. [Google Scholar] [CrossRef]
- Casey, M.M.; Saupe, E.E.; Lieberman, B.S. The effects of geographic range size and abundance on extinction during a time of “sluggish” evolution. Paleobiology 2021, 47, 54–67. [Google Scholar] [CrossRef]
- Trubovitz, S.; Renaudie, J.; Lazarus, D.; Noble, P.J. Abundance does not predict extinction risk in the fossil record of marine plankton. Comm. Biol. 2023, 6, 554. [Google Scholar] [CrossRef]
- Brown, J.H.; Stevens, G.C.; Kaufman, D.M. The geographic range: Size, shape, boundaries, and internal structure. Annu. Rev. Ecol. Syst. 1996, 27, 597–623. [Google Scholar] [CrossRef]
- Lloyd, E.A.; Gould, S.J. Species selection on variability. Proc. Natl. Acad. Sci. USA 1993, 90, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Uchmański, J. What promotes persistence of a single population: An individual-based model. Ecol. Model. 1999, 115, 227–241. [Google Scholar] [CrossRef]
- Gould, S.J. The Structure of Evolutionary Theory; Harvard University Press: Cambridge, MA, USA, 2002. [Google Scholar]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Huxley, J.S. Evolution: The Modern Synthesis; George Allen and Unwin: London, UK, 1942. [Google Scholar]
- Brown, W.L., Jr. General adaptation and evolution. Syst. Zool. 1958, 7, 157–168. [Google Scholar] [CrossRef]
- Van Valen, L.M. Morphological variation and width of ecological niche. Am. Nat. 1965, 99, 377–390. [Google Scholar] [CrossRef]
- Babbel, G.R.; Selander, R.K. Genetic variability in edaphically restricted and widespread plant species. Evolution 1974, 28, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, I.F.; Baker, R.J.; Ramsey, P.R. Chromosomal evolution and the mode of speciation in three species of Peromyscus. Evolution 1978, 32, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Karron, J.D. A comparison of levels of genetic polymorphism and self-compatibility in geographically restricted and widespread plant congeners. Evol. Ecol. 1987, 1, 47–58. [Google Scholar] [CrossRef]
- Robbins, L.W.; Moulton, M.P.; Baker, R.J. Extent of geographic range and magnitude of chromosomal evolution. J. Biogeogr. 1983, 10, 533–541. [Google Scholar] [CrossRef]
- Hamrick, J.L.; Godt, M.W. Allozyme Diversity in Plant Species. In Plant Population Genetics, Breeding, and Genetic Resources; Brown, A.H.D., Clegg, M.T., Kahler, A.L., Weir, B.S., Eds.; Sinauer Associates: Sunderland, MA, USA, 1990; pp. 43–63. [Google Scholar]
- Soltis, P.S.; Soltis, D.E. Genetic variation in endemic and widespread plant species. Aliso 1991, 13, 215–223. [Google Scholar] [CrossRef]
- Frankham, R. Relationship of genetic variation to population size in wildlife. Conserv. Biol. 1996, 10, 1500–1508. [Google Scholar] [CrossRef]
- Gitzendanner, M.A.; Soltis, P.S. Patterns of genetic variation in rare and widespread plant congeners. Am. J. Bot. 2000, 87, 783–792. [Google Scholar] [CrossRef]
- Gibson, J.P.; Rice, S.A.; Stucke, C.M. Comparison of population genetic diversity between a rare, narrowly distributed species and a common, widespread species of Alnus (Betulaceae). Am. J. Bot. 2008, 95, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Soulé, M.E. Allozyme Variation: Its Determinants in Space and Time. In Molecular Evolution; Ayala, F.J., Ed.; Sinauer Associates: Sunderland, MA, USA, 1976; pp. 60–77. [Google Scholar]
- Ellstrand, N.C.; Elam, D.R. Population genetic consequences of small population size: Implications for plant conservation. Annu. Rev. Ecol. Syst. 1993, 24, 217–242. [Google Scholar] [CrossRef]
- Spielman, D.; Brook, B.W.; Frankham, R. Most species are not driven to extinction before genetic factors impact them. Proc. Natl. Acad. Sci. USA 2004, 101, 15261–15264. [Google Scholar] [CrossRef]
- Leimu, R.; Mutikainen, P.I.A.; Koricheva, J.; Fischer, M. How general are positive relationships between plant population size, fitness and genetic variation? J. Ecol. 2006, 94, 942–952. [Google Scholar] [CrossRef]
- Ouborg, N.J.; Van Treuren, R. Variation in fitness-related characters among small and large populations of Salvia pratensis. J. Ecol. 1995, 83, 369–380. [Google Scholar] [CrossRef]
- Buffalo, V. Quantifying the relationship between genetic diversity and population size suggests natural selection cannot explain Lewontin’s Paradox. Elife 2021, 10, e67509. [Google Scholar] [CrossRef]
- Reed, D.H.; Frankham, R. Correlation between fitness and genetic diversity. Conserv. Biol. 2003, 17, 230–237. [Google Scholar] [CrossRef]
- Neher, R.A.; Shraiman, B.I.; Fisher, D.S. Rate of adaptation in large sexual populations. Genetics 2010, 184, 467–481. [Google Scholar] [CrossRef]
- Edinger, E.N.; Risk, M.J. Preferential survivorship of brooding corals in a regional extinction. Paleobiology 1995, 21, 200–219. [Google Scholar] [CrossRef]
- Marquet, P.A.; Taper, M.L. On size and area: Patterns of mammalian body size extremes across landmasses. Evol. Ecol. 1998, 12, 127–139. [Google Scholar] [CrossRef]
- Purvis, A.; Gittleman, J.L.; Cowlishaw, G.; Mace, G.M. Predicting extinction risk in declining species. Proc. R. Soc. B Biol. Sci. 2000, 267, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Poulin, E.; Palma, A.T.; Féral, J.P. Evolutionary versus ecological success in Antarctic benthic invertebrates. Trends Ecol. Evol. 2002, 17, 218–222. [Google Scholar] [CrossRef]
- Reynolds, J.D.; Dulvy, N.K.; Goodwin, N.B.; Hutchings, J.A. Biology of extinction risk in marine fishes. Proc. R. Soc. B Biol. Sci. 2005, 272, 2337–2344. [Google Scholar] [CrossRef] [PubMed]
- Devictor, V.; Julliard, R.; Jiguet, F. Distribution of specialist and generalist species along spatial gradients of habitat disturbance and fragmentation. Oikos 2008, 117, 507–514. [Google Scholar] [CrossRef]
- Geiser, F.; Turbill, C. Hibernation and daily torpor minimize mammalian extinctions. Naturwissenschaften 2009, 96, 1235–1240. [Google Scholar] [CrossRef]
- Clavel, J.; Julliard, R.; Devictor, V. Worldwide decline of specialist species: Toward a global functional homogenization? Front. Ecol. Env. 2011, 9, 222–228. [Google Scholar] [CrossRef]
- Kemp, M.E.; Hadly, E.A. Extinction biases in Quaternary Caribbean lizards. Glob. Ecol. Biogeogr. 2015, 24, 1281–1289. [Google Scholar] [CrossRef]
- Tian, Y.; Jiang, Y.; Shao, W.; Wu, Y.; Liao, W. The impact of life-history traits on vulnerability to extinction of the oviparous species in reptiles. Integr. Zool. 2024, 20, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.W.; Lu, H.P.; Liao, P.C. A historical misstep: Niche shift to specialisation is pushing insular ginger towards an evolutionary dead end. Mol. Ecol. 2025, 34, e17765. [Google Scholar] [CrossRef]
- Moulatlet, G.M.; Merow, C.; Maitner, B.; Boyle, B.; Feng, X.; Frazier, A.E.; Hinojo-Hinojo, C.; Newman, E.A.; Roehrdanz, P.R.; Song, L.; et al. General laws of biodiversity: Climatic niches predict plant range size and ecological dominance globally. Proc. Natl. Acad. Sci. USA 2025, 122, e2517585122. [Google Scholar] [CrossRef] [PubMed]
- Sæther, B.E.; Bakke, Ø. Avian life history variation and contribution of demographic traits to the population growth rate. Ecology 2000, 81, 642–653. [Google Scholar] [CrossRef]
- Oli, M.K.; Dobson, F.S. The relative importance of life-history variables to population growth rate in mammals: Cole’s prediction revisited. Am. Nat. 2003, 161, 422–440. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, N.B.; Grant, A.; Perry, A.L.; Dulvy, N.K.; Reynolds, J.D. Life history correlates of density-dependent recruitment in marine fishes. Can. J. Fish. Aquat. Sci. 2006, 63, 494–509. [Google Scholar] [CrossRef]
- Gravel, S.; Bigman, J.S.; Pardo, S.A.; Wong, S.; Dulvy, N.K. Metabolism, population growth, and the fast-slow life history continuum of marine fishes. Fish Fish. 2024, 25, 349–361. [Google Scholar] [CrossRef]
- De Kort, H.; Prunier, J.G.; Ducatez, S.; Honnay, O.; Baguette, M.; Stevens, V.M.; Blanchet, S. Life history, climate and biogeography interactively affect worldwide genetic diversity of plant and animal populations. Nat. Comm. 2021, 12, 516. [Google Scholar] [CrossRef]
- Andrewartha, H.G.; Birch, L.C. The Distribution and Abundance of Animals; University of Chicago Press: Chicago, IL, USA, 1954. [Google Scholar]
- Bock, C.E.; Ricklefs, R.E. Range size and local abundance of some North American songbirds: A positive correlation. Am. Nat. 1983, 122, 295–299. [Google Scholar] [CrossRef]
- Glazier, D.S. Temporal variability of abundance and the distribution of species. Oikos 1986, 47, 309–314. [Google Scholar] [CrossRef]
- Spitzer, K.; Lepš, J. Determinants of temporal variation in moth abundance. Oikos 1988, 53, 31–36. [Google Scholar] [CrossRef]
- Pyron, M. Relationships between geographical range size, body size, local abundance, and habitat breadth in North American suckers and sunfishes. J. Biogeogr. 1999, 26, 549–558. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Cassey, P.; Gaston, K.J. Variations on a theme: Sources of heterogeneity in the form of the interspecific relationship between abundance and distribution. J. Anim. Ecol. 2006, 75, 1426–1439. [Google Scholar] [CrossRef]
- Borregaard, M.K.; Rahbek, C. Causality of the relationship between geographic distribution and species abundance. Q. Rev. Biol. 2010, 85, 3–25. [Google Scholar] [CrossRef]
- Fristoe, T.S.; Chytrý, M.; Dawson, W.; Essl, F.; Heleno, R.; Kreft, H.; Maurel, N.; Pergl, J.; Pyšek, P.; Seebens, H.; et al. Dimensions of invasiveness: Links between local abundance, geographic range size, and habitat breadth in Europe’s alien and native floras. Proc. Natl. Acad. Sci. USA 2021, 118, e2021173118. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J. Do faster-growing holoparasitic plant species exhibit broader niches and wider global distribtions? Plants 2025, 14, 831. [Google Scholar] [CrossRef]
- Arnold, A.J.; Fristrup, K. The theory of evolution by natural selection: A hierarchical expansion. Paleobiology 1982, 8, 113–129. [Google Scholar] [CrossRef]
- Vrba, E.S.; Eldredge, N. Individuals, hierarchies and processes: Towards a more complete evolutionary theory. Paleobiology 1984, 10, 146–171. [Google Scholar] [CrossRef]
- Vrba, E.S.; Gould, S.J. The hierarchical expansion of sorting and selection: Sorting and selection cannot be equated. Paleobiology 1986, 12, 217–228. [Google Scholar] [CrossRef]
- Simpson, C.; Halling, A.; Leventhal, S. Levels of selection and macroevolution in organisms, colonies, and species. Paleobiology 2024, 51, 62–70. [Google Scholar] [CrossRef]
- Darlington, P.J., Jr. Carabidae of mountains and islands: Data on the evolution of isolated faunas, and on atrophy of wings. Ecol. Monogr. 1943, 13, 37–61. [Google Scholar] [CrossRef]
- Williams, E.E. The ecology of colonization as seen in the zoogeography of anoline lizards on small islands. Q. Rev. Biol. 1969, 44, 345–389. [Google Scholar] [CrossRef]
- Pielou, E.C. Biogeography; Wiley: New York, NY, USA, 1979. [Google Scholar]
- Smith, J.M.B. Colonist ability, altitudinal range and origins of the flora of Mt Field, Tasmania. J. Biogeogr. 1981, 8, 249–261. [Google Scholar] [CrossRef]
- Kilroy, C. Diatom Communities in New Zealand Subalpine Mire Pools: Distribution, Ecology and Taxonomy of Endemic and Cosmopolitan Taxa. Ph.D. Dissertation, University of Canterbury, Canterbury, New Zealand, 2007. [Google Scholar]
- Verberk, W.C.; Van Der Velde, G.; Esselink, H. Explaining abundance–occupancy relationships in specialists and generalists: A case study on aquatic macroinvertebrates in standing waters. J. Anim. Ecol. 2010, 79, 589–601. [Google Scholar] [CrossRef]
- Hansen, G.J.; Vander Zanden, M.J.; Blum, M.J.; Clayton, M.K.; Hain, E.F.; Hauxwell, J.; Izzo, M.; Kornis, M.S.; McIntyre, P.B.; Mikulyuk, A.; et al. Commonly rare and rarely common: Comparing population abundance of invasive and native aquatic species. PLoS ONE 2013, 8, e77415. [Google Scholar] [CrossRef] [PubMed]
- Burness, G.P.; Diamond, J.; Flannery, T. Dinosaurs, dragons, and dwarfs: The evolution of maximal body size. Proc. Natl. Acad. Sci. USA 2001, 98, 14518–14523. [Google Scholar] [CrossRef] [PubMed]
- Okie, J.G.; Brown, J.H. Niches, body sizes, and the disassembly of mammal communities on the Sunda Shelf islands. Proc. Natl. Acad. Sci. USA 2009, 106, 19679–19684. [Google Scholar] [CrossRef]
- Millien, V.; Gonzalez, A. The maximal body mass–area relationship in island mammals. J. Biogeogr. 2011, 38, 2278–2285. [Google Scholar] [CrossRef]
- Bürger, R.; Lynch, M. Adaptation and Extinction in Changing Environments. In Environmental Stress, Adaptation and Evolution; Bijlsma, R., Loeschcke, V., Eds.; Birkhaüser Verlag: Basel, Switzerland, 1997; pp. 209–239. [Google Scholar]
- Wright, S. The roles of mutation, inbreeding, crossbreeding and selection in evolution. Proc. Sixth Int. Congr. Genet. 1932, 1, 356–366. [Google Scholar]
- Sibly, R.; Calow, P. An integrated approach to life-cycle evolution using selective landscapes. J. Theor. Biol. 1983, 102, 527–547. [Google Scholar] [CrossRef]
- Svensson, E.I.; Calsbeek, R. (Eds.) The Adaptive Landscape in Evolutionary Biology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]
- Fragata, I.; Blanckaert, A.; Louro, M.A.D.; Liberles, D.A.; Bank, C. Evolution in the light of fitness landscape theory. Trends Ecol. Evol. 2019, 34, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Bank, C. Epistasis and adaptation on fitness landscapes. Annu. Rev. Ecol. Evol. Syst. 2022, 53, 457–479. [Google Scholar] [CrossRef]
- Laughlin, D.C.; Messier, J. Fitness of multidimensional phenotypes in dynamic adaptive landscapes. Trends Ecol. Evol. 2015, 30, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Beausoleil, M.O.; Carrión, P.L.; Podos, J.; Camacho, C.; Rabadán-González, J.; Richard, R.; Lalla, K.; Raeymaekers, J.A.; Knutie, S.A.; De León, L.F.; et al. The fitness landscape of a community of Darwin’s finches. Evolution 2023, 77, 2533–2546. [Google Scholar] [CrossRef] [PubMed]
- Odum, E.P. The strategy of ecosystem development. Science 1969, 164, 262–270. [Google Scholar] [CrossRef]
- Singh, A. R-Reproductive Strategy. In Encyclopedia of Animal Cognition and Behavior; Vonk, J., Shackelford, T., Eds.; Springer: Cham, Switzerland, 2019; pp. 1–6. [Google Scholar]
- Wentzky, V.C.; Tittel, J.; Jäger, C.G.; Bruggeman, J.; Rinke, K. Seasonal succession of functional traits in phytoplankton communities and their interaction with trophic state. J. Ecol. 2020, 108, 1649–1663. [Google Scholar] [CrossRef]
- Glazier, D.S. The evolution of body size and selfhood: Size-scaling from selfless reproduction to enhanced self-preservation. J. Biosci. 2025, 50, 30. [Google Scholar] [CrossRef]
- Uchmański, J.; Grimm, V. Individual-based modelling in ecology: What makes the difference? Trends Ecol. Evol. 1996, 11, 437–441. [Google Scholar] [CrossRef]
- Łomnicki, A. Population Ecology of Individuals; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Uchmański, J. Density or variability: Is it time for a paradigm shift in ecology? Ecol. Quest. 2022, 33, 7–17. [Google Scholar] [CrossRef]
- DeAngelis, D.L.; Mooij, W.M. Individual-based modeling of ecological and evolutionary processes. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 147–168. [Google Scholar] [CrossRef]
- Grimm, V.; Railsback, S.F. Individual-Based Modeling and Ecology; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Birch, J. Natural selection and the maximization of fitness. Biol. Rev. 2016, 91, 712–727. [Google Scholar] [CrossRef]
- Basener, W.; Cordova, S.; Hössjer, O.; Sanford, J. Dynamical Systems and Fitness Maximization in Evolutionary Biology. In Handbook of the Mathematics of the Arts and Sciences; Sriraman, B., Ed.; Springer: Cham, Switzerland, 2021; pp. 2097–2169. [Google Scholar]
- Hancock, Z.B.; Cardinale, D.S. Back to the fundamentals: A reply to Basener and Sanford 2018. J. Math. Biol. 2024, 88, 54. [Google Scholar] [CrossRef]
- McShea, D.W.; Brandon, R.N. Biology’s First Law: The Tendency for Diversity and Complexity to Increase in Evolutionary Systems; University of Chicago Press: Chicago, IL, USA, 2010. [Google Scholar]
- McKinney, M.L.; McNamara, K.J. Heterochrony: The Evolution of Ontogeny; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Dobreva, M.P.; Camacho, J.; Abzhanov, A. Time to synchronize our clocks: Connecting developmental mechanisms and evolutionary consequences of heterochrony. J. Exp. Zool. B Mol. Dev. Evol. 2022, 338, 87–106. [Google Scholar] [CrossRef]
- Glazier, D.S. Beyond the ‘3/4-power law’: Variation in the intra- and interspecific scaling of metabolic rate in animals. Biol. Rev. 2005, 80, 611–662. [Google Scholar] [CrossRef]
- Glazier, D.S. The 3/4-power law is not universal: Evolution of isometric, ontogenetic metabolic scaling in pelagic animals. BioScience 2006, 56, 325–332. [Google Scholar] [CrossRef]
- DeLong, J.P.; Okie, J.G.; Moses, M.E.; Sibly, R.M.; Brown, J.H. Shifts in metabolic scaling, production, and efficiency across major evolutionary transitions of life. Proc. Natl. Acad. Sci. USA 2010, 107, 12941–12945. [Google Scholar] [CrossRef]
- White, C.R. There is no single p. Nature 2010, 464, 691–693. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, M.J. The evolution of stability in marine environments natural selection at the level of the ecosystem. Am. Nat. 1960, 94, 129–136. [Google Scholar] [CrossRef]
- Swenson, W.; Wilson, D.S.; Elias, R. Artificial ecosystem selection. Proc. Natl. Acad. Sci. USA 2000, 97, 9110–9114. [Google Scholar] [CrossRef]
- Borrelli, J.J.; Allesina, S.; Amarasekare, P.; Arditi, R.; Chase, I.; Damuth, J.; Holt, R.D.; Logofet, D.O.; Novak, M.; Rohr, R.P.; et al. Selection on stability across ecological scales. Trends Ecol. Evol. 2015, 30, 417–425. [Google Scholar] [CrossRef]
- Doolittle, W.F.; Inkpen, S.A. Processes and patterns of interaction as units of selection: An introduction to ITSNTS thinking. Proc. Natl. Acad. Sci. USA 2018, 115, 4006–4014. [Google Scholar] [CrossRef]
- Boyle, R.A.; Lenton, T.M. The evolution of biogeochemical recycling by persistence-based selection. Comm. Earth Environ. 2022, 3, 46. [Google Scholar] [CrossRef]
- Doolittle, W.F. Darwinizing Gaia: Natural Selection and Multispecies Community Evolution; MIT Press: Cambridge, MA, USA, 2024. [Google Scholar]
- Wilson, D.S. The Natural Selection of Populations and Communities; Benjamin/Cummings: Menlo Park, CA, USA, 1980. [Google Scholar]
- Roopnarine, P.D. Selection, evolution and persistence of paleoecological systems. Front. Earth Sci. 2025, 13, 1528448. [Google Scholar] [CrossRef]
- Pross, A.; Pascal, R. The origin of life: What we know, what we can know and what we will never know. Open Biol. 2013, 3, 120190. [Google Scholar] [CrossRef]
- Ruiz-Mirazo, K.; Briones, C.; de la Escosura, A. Prebiotic systems chemistry: New perspectives for the origins of life. Chem. Rev. 2014, 114, 285–366. [Google Scholar] [CrossRef] [PubMed]
- Gladyshev, G.P. Thermodynamics of the origin of life, evolution, and aging. Int. J. Nat. Sci. Rev. 2017, 2, 7. [Google Scholar] [CrossRef]
- Kunnev, D. Origin of life: The point of no return. Life 2020, 10, 269. [Google Scholar] [CrossRef]
- Kalambokidis, M.; Travisano, M. The eco-evolutionary origins of life. Evolution 2024, 78, 1–12. [Google Scholar] [CrossRef]
- Calow, P. Life Cycles: An Evolutionary Approach to the Physiology of Reproduction, Development and Aging; Chapman and Hall: London, UK, 1978. [Google Scholar]
- Thompson, D.W. On Growth and Form; Cambridge University Press: Cambridge, UK, 1917. [Google Scholar]
- Lotka, A.J. Elements of Physical Biology; Williams and Wilkins: Baltimore, MD, USA, 1925. [Google Scholar]
- Schrödinger, E. What Is Life? The Physical Aspect of the Living Cell; Cambridge University Press: Cambridge, UK, 1948. [Google Scholar]
- Alexander, R.M. The ideal and the feasible: Physical constraints on evolution. Biol. J. Linn. Soc. 1985, 26, 345–358. [Google Scholar] [CrossRef]
- Ginzburg, L.; Colyvan, M. Ecological Orbits: How Planets Move and Populations Grow; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Niklas, K.J. Biophysical and size-dependent perspectives on plant evolution. J. Exp. Bot. 2013, 64, 4817–4827. [Google Scholar] [CrossRef]
- Bejan, A. The Physics of Life: The Evolution of Everything; St. Martin’s Press: New York, NY, USA, 2016. [Google Scholar]
- Cockell, C.S. The laws of life. Phys. Today 2017, 70, 42–48. [Google Scholar] [CrossRef]
- Noble, D. Evolution viewed from physics, physiology and medicine. Interface Focus 2017, 7, 20160159. [Google Scholar] [CrossRef]
- Phillips, R. Schrödinger’s What is life? at 75. Cell Syst. 2021, 12, 465–476. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Marshall, D.J. Optimisation and constraint: Explaining metabolic patterns in biology. J. Exp. Biol. 2023, 226, jeb245426. [Google Scholar] [CrossRef]
- Duguid, C. Developing the structure of laws in biology. Biol. Philos. 2025, 40, 7. [Google Scholar] [CrossRef]
- Glazier, D.S. Resource Allocation Patterns. In Resource Allocation Theory Applied to Farm Animal Production; Rauw, W.M., Ed.; CAB International: Wallingford, UK, 2009; pp. 22–43. [Google Scholar]
- Van Valen, L.M. Three paradigms of evolution. Evol. Theor. 1989, 9, 1–17. [Google Scholar]
- Eldredge, N. Information, economics, and evolution. Annu. Rev. Ecol. Syst. 1986, 17, 351–369. [Google Scholar] [CrossRef]
- Yun, A.J.; Lee, P.Y.; Doux, J.D.; Conley, B.R. A general theory of evolution based on energy efficiency: Its implications for diseases. Med. Hypotheses 2006, 66, 664–670. [Google Scholar] [CrossRef]
- Donaldson-Matasci, M.C.; Bergstrom, C.T.; Lachmann, M. The fitness value of information. Oikos 2010, 119, 219–230. [Google Scholar] [CrossRef]
- McNamara, J.M.; Dall, S.R. Information is a fitness enhancing resource. Oikos 2010, 119, 231–236. [Google Scholar] [CrossRef]
- Schmidt, K.A.; Dall, S.R.; Van Gils, J.A. The ecology of information: An overview on the ecological significance of making informed decisions. Oikos 2010, 119, 304–316. [Google Scholar] [CrossRef]
- Brose, U.; Hirt, M.R.; Ryser, R.; Rosenbaum, B.; Berti, E.; Gauzens, B.; Hein, A.M.; Pawar, S.; Schmidt, K.; Wootton, K.; et al. Embedding information flows within ecological networks. Nat. Ecol. Evol. 2025, 9, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Calow, P. Homeostasis and fitness. Am. Nat. 1982, 120, 416–419. [Google Scholar] [CrossRef]
- Ellisen, L.W. Growth control under stress: mTOR regulation through the REDD1-TSC pathway. Cell Cycle 2005, 4, 1500–1502. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Sadoul, B.; Vijayan, M.M. Stress and growth. Fish Physiol. 2016, 35, 167–205. [Google Scholar]
- Texada, M.J.; Koyama, T.; Rewitz, K. Regulation of body size and growth control. Genetics 2020, 216, 269–313. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhu, J.K. Thriving under stress: How plants balance growth and the stress response. Dev. Cell 2020, 55, 529–543. [Google Scholar] [CrossRef]
- Ding, Y.; Shi, Y.; Yang, S. Regulatory networks underlying plant responses and adaptation to cold stress. Annu. Rev. Genet. 2024, 58, 43–65. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Saeed, U.; Rewitz, K.; Halberg, K.V. The integrative physiology of hormone signaling: Insights from insect models. Physiology 2025, 40, 343–362. [Google Scholar] [CrossRef]
- Verslues, P.E. Understanding and optimizing plant growth in water-limited environments: ‘Growth versus defence’ or ‘growth versus risk mitigation’? Philos. Trans. B 2025, 380, 20240232. [Google Scholar] [CrossRef]
- West, G.B.; Brown, J.H.; Enquist, B.J. A general model for ontogenetic growth. Nature 2001, 413, 628–631. [Google Scholar] [CrossRef]
- van der Meer, J. Metabolic theories in ecology. Trends Ecol. Evol. 2006, 21, 136–140. [Google Scholar] [CrossRef]
- Hou, C.; Zuo, W.; Moses, M.E.; Woodruff, W.H.; Brown, J.H.; West, G.B. Energy uptake and allocation during ontogeny. Science 2008, 322, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Glazier, D.S.; Butler, E.M.; Lombardi, S.A.; Deptola, T.J.; Reese, A.J.; Satterthwaite, E.V. Ecological effects on metabolic scaling: Amphipod responses to fish predators in freshwater springs. Ecol. Monogr. 2011, 81, 599–618. [Google Scholar] [CrossRef]
- Glazier, D.S.; Borrelli, J.J.; Hoffman, C.L. Effects of fish predators on the mass-related energetics of a keystone freshwater crustacean. Biology 2020, 9, 40. [Google Scholar] [CrossRef]
- Wilson, R.P.; Quintana, F.; Hobson, V.J. Construction of energy landscapes can clarify the movement and distribution of foraging animals. Proc. R. Soc. B Biol. Sci. 2012, 279, 975–980. [Google Scholar] [CrossRef]
- Shepard, E.L.; Wilson, R.P.; Rees, W.G.; Grundy, E.; Lambertucci, S.A.; Vosper, S.B. Energy landscapes shape animal movement ecology. Am. Nat. 2013, 182, 298–312. [Google Scholar] [CrossRef]
- Gallagher, A.J.; Creel, S.; Wilson, R.P.; Cooke, S.J. Energy landscapes and the landscape of fear. Trends Ecol. Evol. 2017, 32, 88–96. [Google Scholar] [CrossRef]
- Long, R.A.; Bowyer, R.T.; Porter, W.P.; Mathewson, P.; Monteith, K.L.; Findholt, S.L.; Dick, B.L.; Kie, J.G. Linking habitat selection to fitness-related traits in herbivores: The role of the energy landscape. Oecologia 2016, 181, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.G. Landscape ecology: The effect of pattern on process. Annu. Rev. Ecol. Syst. 1989, 20, 171–197. [Google Scholar] [CrossRef]
- Risser, P.G. Landscape Pattern and Its Effects on Energy and Nutrient Distribution. In Changing Landscapes: An Ecological Perspective; Zonneveld, I.S., Forman, R.T.T., Eds.; Springer: New York, NY, USA, 1990; pp. 45–56. [Google Scholar]
- Palola, P.; Pittman, S.J.; Collin, A.; Benkwitt, C.E.; Thomson, E.; Malhi, Y.; Graham, N.A.; Wedding, L.M. Nutrientscape ecology: A whole-system framework to support the understanding and management of coastal nutrient connectivity. Landsc. Ecol. 2025, 40, 48. [Google Scholar] [CrossRef]
- Day, K.J.; Hutchings, M.J.; John, E.A. The effects of spatial pattern of nutrient supply on yield, structure and mortality in plant populations. J. Ecol. 2003, 91, 541–553. [Google Scholar] [CrossRef]
- Klaus, V.H.; Boch, S.; Boeddinghaus, R.S.; Hölzel, N.; Kandeler, E.; Marhan, S.; Oelmann, Y.; Prati, D.; Regan, K.M.; Schmitt, B.; et al. Temporal and small-scale spatial variation in grassland productivity, biomass quality, and nutrient limitation. Plant Ecol. 2016, 217, 843–856. [Google Scholar] [CrossRef]
- Laundré, J.W.; Hernández, L.; Ripple, W.J. The landscape of fear: Ecological implications of being afraid. Open Ecol. J. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Brown, J.S.; Middleton, A.D.; Power, M.E.; Brashares, J.S. Landscapes of fear: Spatial patterns of risk perception and response. Trends Ecol. Evol. 2019, 34, 355–368. [Google Scholar] [CrossRef]
- Lind, J.; Cresswell, W. Determining the fitness consequences of antipredation behavior. Behav. Ecol. 2005, 16, 945–956. [Google Scholar] [CrossRef]
- Jaatinen, K.; Seltmann, M.W.; Öst, M. Context-dependent stress responses and their connections to fitness in a landscape of fear. J. Zool. 2014, 294, 147–153. [Google Scholar] [CrossRef]
- Tian, L.; Zhao, L.; Wu, X.; Hu, G.; Fang, H.; Zhao, Y.; Sheng, Y.; Chen, J.; Wu, J.; Li, W.; et al. Variations in soil nutrient availability across Tibetan grassland from the 1980s to 2010s. Geoderma 2019, 338, 197–205. [Google Scholar] [CrossRef]
- Palmer, M.S.; Gaynor, K.M.; Becker, J.A.; Abraham, J.O.; Mumma, M.A.; Pringle, R.M. Dynamic landscapes of fear: Understanding spatiotemporal risk. Trends Ecol. Evol. 2022, 37, 911–925. [Google Scholar] [CrossRef]
- Papastamatiou, Y.P.; Binder, B.M.; Boswell, K.M.; Malone, M.A.; Heithaus, M.R.; Huveneers, C.; Mourier, J.; Harborne, A.R. Dynamic energy landscapes of predators and the implications for modifying prey risk. Funct. Ecol. 2024, 38, 284–293. [Google Scholar] [CrossRef]
- Glazier, D.S. Does Death Drive the Size-Scaling of Life-History Traits? In Scaling in Biology: A New Synthesis; Enquist, B.J., O’Connor, M., Kempes, C., Eds.; Santa Fe Institute Press: Sante Fe, NM, USA, 2026; in press. [Google Scholar]
- Mills, S.K.; Beatty, J.H. The propensity interpretation of fitness. Philos. Sci. 1979, 46, 263–286. [Google Scholar] [CrossRef]
- Sober, E. The Nature of Selection: Evolutionary Theory in Philosophical Focus; University of Chicago Press: Chicago, IL, USA, 1984. [Google Scholar]
- Stearns, S.C. Life-history tactics: A review of the ideas. Q. Rev. Biol. 1976, 51, 3–47. [Google Scholar] [CrossRef]
- Dawkins, R. The Extended Phenotype; Oxford University Press: Oxford, UK, 1982. [Google Scholar]
- Murray, B.G., Jr. Population dynamics, genetic change, and the measurement of fitness. Oikos 1990, 59, 189–199. [Google Scholar] [CrossRef]
- Benton, T.G.; Grant, A. Evolutionary fitness in ecology: Comparing measures of fitness in stochastic, density-dependent environments. Evol. Ecol. Res. 2000, 2, 769–789. [Google Scholar]
- Ariew, A.; Lewontin, R.C. The confusions of fitness. Brit. J. Philos. Sci. 2004, 55, 347–363. [Google Scholar] [CrossRef]
- Orr, H.A. Fitness and its role in evolutionary genetics. Nat. Rev. Genet. 2009, 10, 531–539. [Google Scholar] [CrossRef]
- Wagner, G.P. The measurement theory of fitness. Evolution 2010, 64, 1358–1376. [Google Scholar] [CrossRef]
- Abrams, M. Measured, modeled, and causal conceptions of fitness. Front. Genet. 2012, 3, 196. [Google Scholar] [CrossRef]
- Hendry, A.P.; Schoen, D.J.; Wolak, M.E.; Reid, J.M. The contemporary evolution of fitness. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 457–476. [Google Scholar] [CrossRef]
- Suárez, M. The complex nexus of evolutionary fitness. Eur. J. Philos. Sci. 2022, 12, 9. [Google Scholar] [CrossRef]
- Fromhage, L.; Jennions, M.D.; Myllymaa, L.; Henshaw, J.M. Fitness as the organismal performance measure guiding adaptive evolution. Evolution 2024, 78, 1039–1053. [Google Scholar] [CrossRef] [PubMed]
- Spencer, H. Principles of Biology; Williams and Norgate: London, UK, 1880; Volume 1. [Google Scholar]
- Darwin, C. The Descent of Man, and Selection in Relation to Sex; Appleton: New York, NY, USA, 1872. [Google Scholar]
- Arnold, S.J.; Wade, M.J. On the measurement of natural and sexual selection: Theory. Evolution 1984, 38, 709–719. [Google Scholar] [CrossRef]
- Bell, G. Selection: The Mechanism of Evolution; Chapman & Hall: New York, NY, USA, 1997. [Google Scholar]
- Hallgrímsson, B.; Hall, B.K. (Eds.) Variation; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Pigliucci, M.; Kaplan, J. Making Sense of Evolution: The Conceptual Foundations of Evolutionary Biology; University of Chicago Press: Chicago, IL, USA, 2006. [Google Scholar]
- Godfrey-Smith, P. Darwinian Populations and Natural Selection; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Darlington, P.J., Jr. Zoogeography: The Geographical Distribution of Animals; Wiley: New York, NY, USA, 1957. [Google Scholar]
- Briggs, J.C. Zoogeography and evolution. Evolution 1966, 20, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Vermeij, G.J.; Dietl, G.P. Majority rule: Adaptation and the long-term dynamics of species. Paleobiology 2006, 32, 173–178. [Google Scholar] [CrossRef]
- Williams, G.C. Natural Selection: Domains, Levels, and Challenges; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Mayr, E. The objects of selection. Proc. Natl. Acad. Sci. USA 1997, 94, 2091–2094. [Google Scholar] [CrossRef]
- Bouchard, F. Causal processes, fitness, and the differential persistence of lineages. Philos. Sci. 2008, 75, 560–570. [Google Scholar] [CrossRef]
- Hoehn, K.B.; Harnik, P.G.; Roth, V.L. A framework for detecting natural selection on traits above the species level. Method Ecol. Evol. 2016, 7, 331–339. [Google Scholar] [CrossRef]
- Liow, L.H.; Simpson, C.; Bouchard, F.; Damuth, J.; Hallgrimsson, B.; Hunt, G.; McShea, D.W.; Powell, J.R.; Stenseth, N.C.; Stoller, M.K.; et al. Pioneering paradigms and magnificent manifestos—Leigh Van Valen’s priceless contributions to evolutionary biology. Evolution 2011, 65, 917–922. [Google Scholar] [CrossRef]
- Peters, R.H. Tautology in evolution and ecology. Am. Nat. 1976, 110, 971. [Google Scholar] [CrossRef]
- Maddox, J. Is Darwinism a thermodynamic necessity? Nature 1991, 350, 653. [Google Scholar] [CrossRef]
- Hall, C.A.S. The continuing importance of maximum power. Ecol. Model. 2004, 178, 107–113. [Google Scholar] [CrossRef]
- Tuomi, J. Structure and dynamics of Darwinian evolutionary theory. Syst. Biol. 1981, 30, 22–31. [Google Scholar] [CrossRef]
- Tuomi, J.; Vuorisalo, T.; Laihonen, P. Components of Selection: An Expanded Theory of Natural Selection. In Population Genetics and Evolution; de Jong, G., Ed.; Springer: Berlin, Germany, 1988; pp. 109–118. [Google Scholar]
- Campbell, R.; Robert, J.S. The structure of evolution by natural selection. Biol. Philos. 2005, 20, 673–696. [Google Scholar] [CrossRef]
- Boltzmann, L. Populare Schriften; Barth: Leipzig, Germany, 1905. [Google Scholar]
- Lotka, A.J. Contribution to the energetics of evolution. Proc. Natl. Acad. Sci. USA 1922, 8, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Van Valen, L.M. Evolution as a zero-sum game for energy. Evol. Theor. 1980, 4, 289–300. [Google Scholar]
- Odum, H.T.; Pinkerton, R.C. Time’s speed regulator: The optimum efficiency for maximum power output in physical and biological systems. Am. Sci. 1955, 43, 331–343. [Google Scholar]
- Odum, H.T. Environment, Power, and Society; Wiley: New York, NY, USA, 1971. [Google Scholar]
- Hall, C.A.S. Maximum Power: The Ideas and Applications of H.T. Odum; University of Colorado Press: Niwot, CO, USA, 1995. [Google Scholar]
- Vermeij, G.J. Power, competition, and the nature of history. Paleobiology 2019, 45, 517–530. [Google Scholar] [CrossRef]
- Vermeij, G.J. The Evolution of Power: A New Understanding of the History of Life; Princeton University Press: Princeton, NJ, USA, 2023. [Google Scholar]
- Hall, C.A.S.; McWhirter, T. Maximum power in evolution, ecology and economics. Philos. Trans. R. Soc. A 2023, 381, 20220290. [Google Scholar] [CrossRef]
- MacArthur, R.H. Geographical Ecology: Patterns in the Distribution of Species; Harper & Row: New York, NY, USA, 1972. [Google Scholar]
- Smith, C.C. When and how much to reproduce: The trade-off between power and efficiency. Am. Zool. 1976, 16, 763–774. [Google Scholar] [CrossRef]
- Sebens, K.P.; Sarà, G.; Carrington, E. Estimation of fitness from energetics and life-history data: An example using mussels. Ecol. Evol. 2018, 8, 5279–5290. [Google Scholar] [CrossRef]
- Molina-Montenegro, M.A.; Salgado-Luarte, C.; Oses, R.; Torres-Díaz, C. Is physiological performance a good predictor for fitness? Insights from an invasive plant species. PLoS ONE 2013, 8, e76432. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.A. Human adaptation and energetic efficiency. Hum. Ecol. 1979, 7, 53–74. [Google Scholar] [CrossRef]
- Mitchell, R. The evolution of thermophily in hot springs. Q. Rev. Biol. 1974, 49, 229–242. [Google Scholar] [CrossRef]
- Priede, I.G. Natural selection for energetic efficiency and the relationship between activity level and mortality. Nature 1977, 267, 610–611. [Google Scholar] [CrossRef] [PubMed]
- Antonovics, J. Concepts of Resource Allocation and Partitioning in Plants. In Limits to Action: The Allocation of Individual Behavior; Staddon, J.E.R., Ed.; Academic Press: New York, NY, USA, 1980; pp. 1–35. [Google Scholar]
- Bock, W.J. The definition and recognition of biological adaptation. Am. Zool. 1980, 20, 217–227. [Google Scholar] [CrossRef]
- Bonner, J.T. The Evolution of Complexity by Means of Natural Selection; Princeton University Press: Princeton, NJ, USA, 1988. [Google Scholar]
- Bonner, J.T. Why Size Matters: From Bacteria to Whales; Princeton University Press: Princeton, NJ, USA, 2006. [Google Scholar]
- Guderley, H.; Pörtner, H.O. Metabolic power budgeting and adaptive strategies in zoology: Examples from scallops and fish. Can. J. Zool. 2010, 88, 753–763. [Google Scholar] [CrossRef]
- Clauss, M.; Müller, D.W.; Codron, D. Within-niche pace of life acceleration as a fundamental evolutionary principle: A mammal pilot test case. Evol. Ecol. Res. 2019, 20, 385–401. [Google Scholar]
- Kepp, K.P. Survival of the cheapest: How proteome cost minimization drives evolution. Q. Rev. Biophys. 2020, 53, e7. [Google Scholar] [CrossRef]
- Martin, C. Waste not, want not. Curr. Biol. 2022, 32, R589–R590. [Google Scholar] [CrossRef]
- Watt, W.B. Power and efficiency as indexes of fitness in metabolic organization. Am. Nat. 1986, 127, 629–653. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; De Bari, B.; Dixon, J.A. Dissipative structures, organisms and evolution. Entropy 2020, 22, 1305. [Google Scholar] [CrossRef]
- Dobzhansky, T. On Some Fundamental Concepts of Darwinian Biology. In Evolutionary Biology; Dobzhansky, T., Hecht, M.K., Steere, W.C., Eds.; Plenum Press: New York, NY, USA, 1968; Volume 2, pp. 1–34. [Google Scholar]
- Ettinger, L.; Jablonka, E.; McLaughlin, P. On the adaptations of organisms and the fitness of types. Philos. Sci. 1990, 57, 499–513. [Google Scholar] [CrossRef]
- Pérez-González, S.; Luque, V.J. Evolutionary causes as mechanisms: A critical analysis. Hist. Philos. Life Sci. 2019, 41, 13. [Google Scholar] [CrossRef]
- Bourrat, P.; Deaven, K.; Villegas, C. Evolvability: Filling the explanatory gap between adaptedness and the long-term mathematical conception of fitness. Biol. Philos. 2024, 39, 15. [Google Scholar] [CrossRef]
- Southwood, T.R.E. The Croonian lecture, 1995. Natural communities: Structure and dynamics. Philos. Trans. R. Soc. B Biol. Sci. 1996, 351, 1113–1129. [Google Scholar] [CrossRef]
- Žliobaitė, I.; Fortelius, M. All sizes fit the Red Queen. Paleobiology 2020, 46, 478–494. [Google Scholar] [CrossRef]
- Dobzhansky, T. Evolution in the tropics. Am. Sci. 1950, 38, 209–221. [Google Scholar]
- Bonner, J.T. Randomness in Evolution; Princeton University Press: Princeton, NJ, USA, 2013. [Google Scholar]
- Loreau, M. From Populations to Ecosystems: Theoretical Foundations for a New Ecological Synthesis; Princeton University Press: Princeton, NJ, USA, 2010. [Google Scholar]
- Ghedini, G.; Loreau, M.; Marshall, D.J. Community efficiency during succession: A test of MacArthur’s minimization principle in phytoplankton communities. Ecology 2020, 101, e03015. [Google Scholar] [CrossRef]
- Huston, M.; Smith, T. Plant succession: Life history and competition. Am. Nat. 1987, 130, 168–198. [Google Scholar] [CrossRef]
- Smith, F.A.; Payne, J.L.; Heim, N.A.; Balk, M.A.; Finnegan, S.; Kowalewski, M.; Lyons, S.K.; McClain, C.R.; McShea, D.W.; Novack-Gottshall, P.M.; et al. Body size evolution across the Geozoic. Annu. Rev. Earth Planet. Sci. 2016, 44, 523–553. [Google Scholar] [CrossRef]
- Bazzaz, F.A. The physiological ecology of plant succession. Annu. Rev. Ecol. Syst. 1979, 10, 351–371. [Google Scholar] [CrossRef]
- Wilson, E.O. The nature of the taxon cycle in the Melanesian ant fauna. Am. Nat. 1961, 95, 169–193. [Google Scholar] [CrossRef]
- Greenslade, P.J.M. Island patterns in the Solomon Islands bird fauna. Evolution 1968, 22, 751–761. [Google Scholar] [CrossRef]
- Ricklefs, R.E.; Cox, G.W. Taxon cycles in the West Indian avifauna. Am. Nat. 1972, 106, 195–219. [Google Scholar] [CrossRef]
- Roughgarden, J.; Pacala, S. Taxon Cycle Among Anolis Lizard Populations: Review of Evidence. In Speciation and Its Consequences; Otte, D., Endler, J.A., Eds.; Sinauer Associates: Sunderland, MA, USA, 1989; pp. 403–432. [Google Scholar]
- Ricklefs, R.E.; Bermingham, E. The concept of the taxon cycle in biogeography. Glob. Ecol. Biogeogr. 2002, 11, 353–361. [Google Scholar] [CrossRef]
- Pepke, M.L.; Irestedt, M.; Fjeldså, J.; Rahbek, C.; Jønsson, K.A. Reconciling supertramps, great speciators and relict species with the taxon cycle stages of a large island radiation (Aves: Campephagidae). J. Biogeogr. 2019, 46, 1214–1225. [Google Scholar] [CrossRef]
- Osborne, T.R.; Lomolino, M.V.; Rundell, R.J. Flying snails: Immigrant selection and the taxon cycle in Pacific Island land snails. Front. Biogeogr. 2024, 16, e62005. [Google Scholar] [CrossRef]
- Howden, H.F. Expansion and Contraction Cycles, Endemism and Area: The Taxon Cycle Brought Full Circle. In Taxonomy, Phylogeny and Zoogeography of Beetles and Ants; Ball, G.E., Ed.; Springer: Dordrecht, The Netherlands, 1985; pp. 473–487. [Google Scholar]
- Steinbauer, M.J. A generalization of the taxon cycle. J. Biogeogr. 2017, 44, 1110–1112. [Google Scholar] [CrossRef]
- Slatyer, R.A.; Hirst, M.; Sexton, J.P. Niche breadth predicts geographical range size: A general ecological pattern. Ecol. Lett. 2013, 16, 1104–1114. [Google Scholar] [CrossRef]
- Cai, Q.; Welk, E.; Ji, C.; Fang, W.; Sabatini, F.M.; Zhu, J.; Zhu, J.; Tang, Z.; Attorre, F.; Campos, J.A.; et al. The relationship between niche breadth and range size of beech (Fagus) species worldwide. J. Biogeogr. 2021, 48, 1240–1253. [Google Scholar] [CrossRef]
- Alzate, A.; Onstein, R.E. Understanding the relationship between dispersal and range size. Ecol. Lett. 2022, 25, 2303–2323. [Google Scholar] [CrossRef]
- Olsen, K.; Svenning, J.C.; Balslev, H. Niche breadth predicts geographical range size and northern range shift in European dragonfly species (Odonata). Diversity 2022, 14, 719. [Google Scholar] [CrossRef]
- Hurtado, P.; Aragón, G.; Vicente, M.; Dalsgaard, B.; Krasnov, B.R.; Calatayud, J. Generalism in species interactions is more the consequence than the cause of ecological success. Nat. Ecol. Evol. 2024, 8, 1602–1611. [Google Scholar] [CrossRef] [PubMed]
- Dallas, T.A.; Ten Caten, C. Linking geographic distribution and niche through estimation of niche density. J. Anim. Ecol. 2025, 94, 1221–1230. [Google Scholar] [CrossRef]
- Granot, I.; Kulbicki, M.; Vigliola, L.; Belmaker, J. Population-level habitat breadth varies with richness in reef fishes. Glob. Ecol. Biogeogr. 2025, 34, e13948. [Google Scholar] [CrossRef]
- Glazier, D.S.; Eckert, S.E. Competitive ability, body size and geographical range size in small mammals. J. Biogeogr. 2002, 29, 81–92. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.