Comparison of Habitat Selection Models Between Habitat Utilization Intensity and Presence–Absence Data: A Case Study of the Chinese Pangolin
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
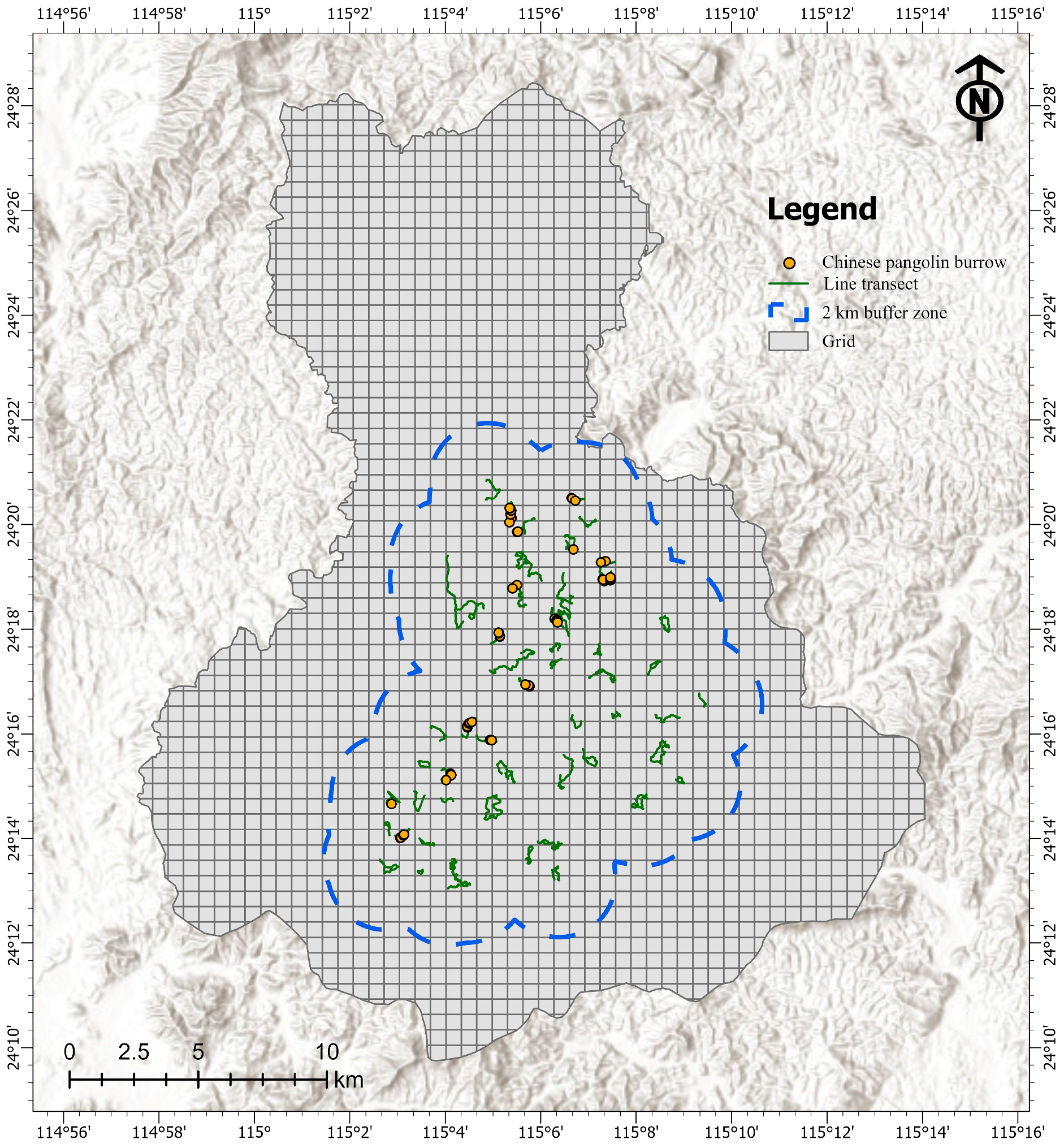
2.1. Study Area and Field Investigation
2.2. Environment Variable Acquisition and Screening
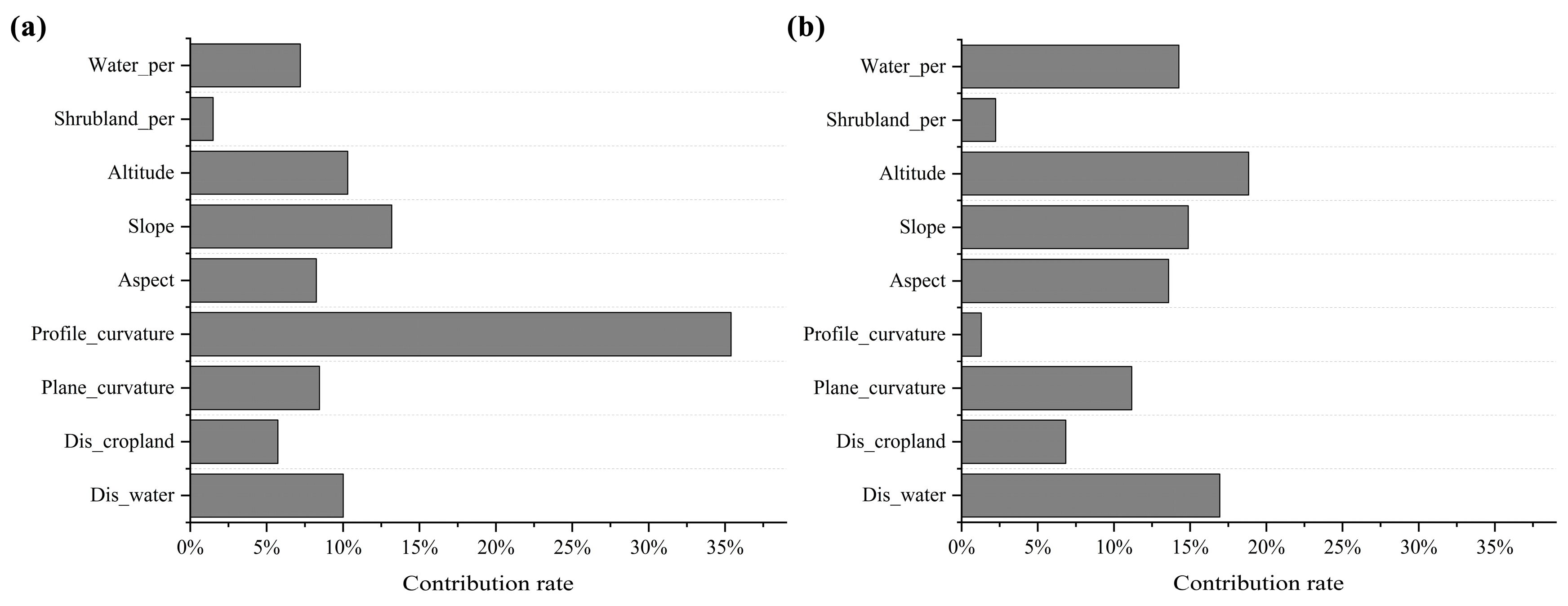
2.3. Model Fitting and Quantifying Variable Contribution Rate
3. Results
3.1. Differences Between Habitat Utilization Intensity and Presence–Absence Models
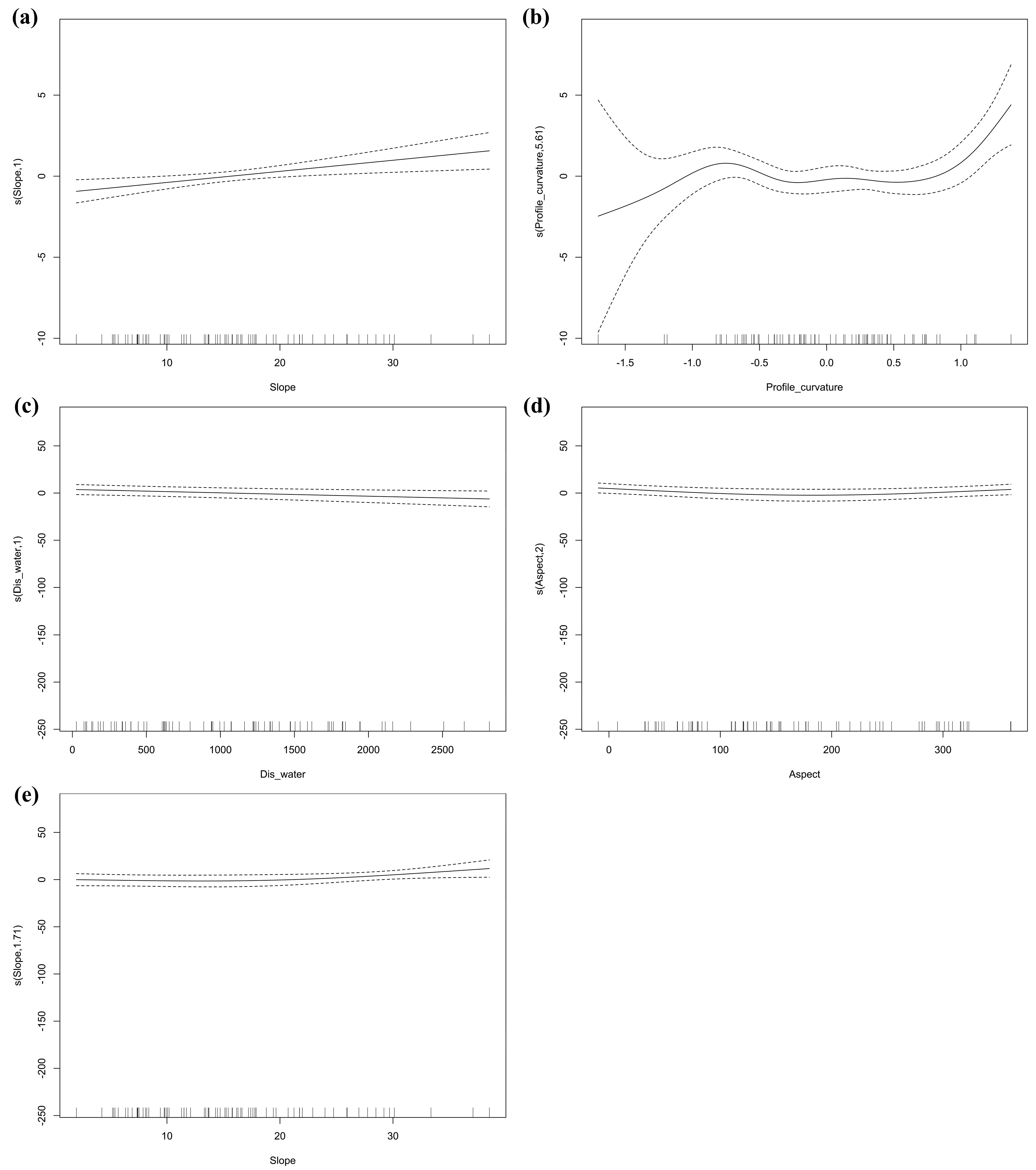
3.2. Response Differences of Environmental Variables
3.3. Applicability of k Gradient and Its Effects on R2, DE and AIC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Camaclang, A.E.; Maron, M.; Martin, T.G.; Possingham, H.P. Current practices in the identification of critical habitat for threatened species. Conserv. Biol. 2015, 29, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Volis, S.; Tojibaev, K. Defining critical habitat for plant species with poor occurrence knowledge and identification of critical habitat networks. Biodivers. Conserv. 2021, 30, 3603–3611. [Google Scholar] [CrossRef]
- Hirzel, A.H.; Le Lay, G.; Helfer, V.; Randin, C.; Guisan, A. Evaluating the ability of habitat suitability models to predict species presences. Ecol. Model. 2006, 199, 142–152. [Google Scholar] [CrossRef]
- Duflot, R.; Avon, C.; Roche, P.; Bergès, L. Combining habitat suitability models and spatial graphs for more effective landscape conservation planning: An applied methodological framework and a species case study. J. Nat. Conserv. 2018, 46, 38–47. [Google Scholar] [CrossRef]
- Ahmadi-Nedushan, B.; St-Hilaire, A.; Bérubé, M.; Robichaud, É.; Thiémonge, N.; Bobée, B. A review of statistical methods for the evaluation of aquatic habitat suitability for instream flow assessment. River Res. Appl. 2006, 22, 503–523. [Google Scholar] [CrossRef]
- Tulloch, A.I.; Sutcliffe, P.; Naujokaitis-Lewis, I.; Tingley, R.; Brotons, L.; Ferraz, K.M.P.; Possingham, H.; Guisan, A.; Rhodes, J.R. Conservation planners tend to ignore improved accuracy of modelled species distributions to focus on multiple threats and ecological processes. Biol. Conserv. 2016, 199, 157–171. [Google Scholar] [CrossRef]
- Engler, R.; Guisan, A.; Rechsteiner, L. An improved approach for predicting the distribution of rare and endangered species from occurrence and pseudo-absence data. J. Appl. Ecol. 2004, 41, 263–274. [Google Scholar] [CrossRef]
- Joseph, L.N.; Field, S.A.; Wilcox, C.; Possingham, H.P. Presence-absence versus abundance data for monitoring threatened species. Conserv. Biol. 2006, 20, 1679–1687. [Google Scholar] [CrossRef]
- Marshall, A.R.; Lovett, J.C.; White, P.C. Selection of line-transect methods for estimating the density of group-living animals: Lessons from the primates. Am. J. Primatol. 2008, 70, 452–462. [Google Scholar] [CrossRef]
- Hendrix, C.S.; Salehyan, I. No news is good news: Mark and recapture for event data when reporting probabilities are less than one. Int. Interact. 2015, 41, 392–406. [Google Scholar] [CrossRef]
- Sollmann, R.; Mohamed, A.; Samejima, H.; Wilting, A. Risky business or simple solution-relative abundance indices from camera-traps. Biol. Conserv. 2013, 159, 405–412. [Google Scholar] [CrossRef]
- Ma, M.J.; Li, J.; Dou, H.L.; Wang, K.; Yang, J.Z.; Wang, J.X.; Guo, R.P.; Wang, H.; Hua, Y. Diet composition and its seasonal variation for Chinese pangolin based on DNA macro-barcoding technology. Chin. J. Ecol. 2025, 44, 2089–2095. [Google Scholar]
- Chao, J.T.; Li, H.F.; Lin, C.C. The role of pangolins in ecosystems. In Pangolins; Academic Press: Cambridge, MA, USA, 2020; pp. 43–48. [Google Scholar]
- Meng, W.C. Ecological Role of Pangolins as a Burrow Excavator. Master’s Thesis, National Taiwan Normal University, Taiwan, China, 2023. [Google Scholar]
- Andersen, R. Nonparametric methods for modeling nonlinearity in regression analysis. Annu. Rev. Sociol. 2009, 35, 67–85. [Google Scholar] [CrossRef]
- Murase, H.; Nagashima, H.; Yonezaki, S.; Matsukura, R.; Kitakado, T. Application of a generalized additive model (GAM) to reveal relationships between environmental factors and distributions of pelagic fish and krill: A case study in Sendai Bay, Japan. ICES J. Mar. Sci. 2009, 66, 1417–1424. [Google Scholar] [CrossRef]
- Lin, J.S. Home range and burrow utilization in Formosan pangolin (Manis pentadactyla pentadactyla) at Luanshan, Taitung. Master’s Thesis, National Pingtung University of Science and Technology, Taiwan, China, 2011. [Google Scholar]
- Hawkins, D.M. The problem of overfitting. J. Chem. Inform. Comput. Sci. 2004, 44, 1–12. [Google Scholar] [CrossRef]
- Mosconi, F.; Julou, T.; Desprat, N.; Sinha, D.K.; Allemand, J.F.; Croquette, V.; Bensimon, D. Some nonlinear challenges in biology. Nonlinearity 2008, 21, T131. [Google Scholar] [CrossRef]
- Aschonitis, V.G.; Castaldelli, G.; Bartoli, M.; Fano, E.A. A review and synthesis of bivariate non-linear models to describe the relative variation of ecological, biological and environmental parameters. Environ. Model. Assess. 2015, 20, 169–182. [Google Scholar] [CrossRef]
- Lai, J.; Zou, Y.; Zhang, S.; Zhang, X.; Mao, L. glmm. hp: An R package for computing individual effect of predictors in generalized linear mixed models. J. Plant Ecol. 2022, 15, 1302–1307. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Bouchet, P.J.; Meeuwig, J.J.; Salgado Kent, C.P.; Letessier, T.B.; Jenner, C.K. Topographic determinants of mobile vertebrate predator hotspots: Current knowledge and future directions. Biol. Rev. 2015, 90, 699–728. [Google Scholar] [CrossRef]
- Beale, C.M.; Monaghan, P. Human disturbance: People as predation-free predators? J. Appl. Ecol. 2004, 41, 335–343. [Google Scholar] [CrossRef]
- Gulbransen, D.; Segrist, T.; Del Castillo, P.; Blumstein, D.T. The fixed slope rule: An inter-specific study. Ethology 2006, 112, 1056–1061. [Google Scholar] [CrossRef]
- Holt, R.D. Optimal foraging and the form of the predator isocline. Am. Nat. 1983, 122, 521–541. [Google Scholar] [CrossRef]
- Royston, P.; Altman, D.G.; Sauerbrei, W. Dichotomizing continuous predictors in multiple regression: A bad idea. Stat. Med. 2006, 25, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Collett, D. Modelling Binary Data; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Odvese, A.V.E. Comparing Discrete and Continuous Data: Concepts, Differences, and Applications; ScienceOpen Preprints: Berlin, Germany, 2024. [Google Scholar]
- Smith, T.J.; McKenna, C.M.A. Comparison of logistic regression pseudo R2 indices. Mult. Linear Regress. Viewp. 2013, 39, 17–26. [Google Scholar]
- Berg, A.; Meyer, R.; Yu, J. Deviance information criterion for comparing stochastic volatility models. J. Bus. Econ. Stat. 2004, 22, 107–120. [Google Scholar] [CrossRef]

| Edf | Ref.df | F | p-Value | R2 | Deviance Explained | |
|---|---|---|---|---|---|---|
| Habitat utilization intensity model | 0.488 | 65.30% | ||||
| s(Dis_water) | 1.000 | 1.000 | 2.785 | 0.101 | ||
| s(Dis_cropland) | 6.378 | 7.331 | 1.323 | 0.268 | ||
| s(Plane_curvature) | 2.749 | 3.412 | 1.366 | 0.197 | ||
| s(Profile_curvature) | 5.610 | 6.582 | 2.802 | 0.014 * | ||
| s(Aspect) | 2.136 | 2.657 | 1.808 | 0.193 | ||
| s(Slope) | 1.000 | 1.000 | 8.258 | 0.006 ** | ||
| s(Altitude) | 1.000 | 1.000 | 3.399 | 0.071 | ||
| s(Shrubland) | 1.000 | 1.000 | 0.076 | 0.784 | ||
| s(Water) | 2.959 | 3.565 | 1.860 | 0.107 | ||
| Presence–absence model | 0.585 | 63.70% | ||||
| s(Dis_water) | 1.000 | 1.000 | 6.077 | 0.014 * | ||
| s(Dis_cropland) | 1.000 | 1.000 | 0.232 | 0.630 | ||
| s(Plane_curvature) | 1.858 | 1.977 | 4.381 | 0.138 | ||
| s(Profile_curvature) | 1.000 | 1.000 | 0.326 | 0.568 | ||
| s(Aspect) | 2.000 | 2.000 | 7.306 | 0.026 * | ||
| s(Slope) | 1.709 | 1.909 | 5.377 | 0.043 * | ||
| s(Altitude) | 2.000 | 2.000 | 4.394 | 0.111 | ||
| s(Shrubland) | 1.000 | 1.000 | 1.758 | 0.185 | ||
| s(Water) | 1.666 | 1.885 | 1.874 | 0.440 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dou, H.; Gao, R.; Wu, F.; Gao, H. Comparison of Habitat Selection Models Between Habitat Utilization Intensity and Presence–Absence Data: A Case Study of the Chinese Pangolin. Biology 2025, 14, 976. https://doi.org/10.3390/biology14080976
Dou H, Gao R, Wu F, Gao H. Comparison of Habitat Selection Models Between Habitat Utilization Intensity and Presence–Absence Data: A Case Study of the Chinese Pangolin. Biology. 2025; 14(8):976. https://doi.org/10.3390/biology14080976
Chicago/Turabian StyleDou, Hongliang, Ruiqi Gao, Fei Wu, and Haiyang Gao. 2025. "Comparison of Habitat Selection Models Between Habitat Utilization Intensity and Presence–Absence Data: A Case Study of the Chinese Pangolin" Biology 14, no. 8: 976. https://doi.org/10.3390/biology14080976
APA StyleDou, H., Gao, R., Wu, F., & Gao, H. (2025). Comparison of Habitat Selection Models Between Habitat Utilization Intensity and Presence–Absence Data: A Case Study of the Chinese Pangolin. Biology, 14(8), 976. https://doi.org/10.3390/biology14080976




