Effect of Recombinant NGF Encapsulated in Chitosan on Rabbit Sperm Traits and Main Metabolic Pathways
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Semen Handling
- Control (Ctrl).
- Chitosan (ch).
- NGF + chitosan (rrβNGFch).
2.3. Capacitation and Acrosome Reaction of Sperm
2.4. Evaluation of Motility and Kinematic Traits
2.5. FACSscan Analysis of TrKA and p75NTR Receptors
2.6. Determination of Live, Apoptotic, and Necrotic Sperm
2.7. Protein Extraction and Quantification
2.8. Immunoblot Assay
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tessarollo, L. Pleiotropic functions of neurotrophins in development. Cytokine Growth Factor Rev. 1998, 9, 125–137. [Google Scholar] [CrossRef]
- Maranesi, M.; Boiti, C.; Zerani, M. Nerve Growth Factor (NGF) and Animal Reproduction. In Recent Advances in NGF and Related Molecules; Calzà, L., Aloe, L., Giardino, L., Eds.; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2021; Volume 1331. [Google Scholar] [CrossRef]
- Ratto, M.H.; Berland, M.A.; Silva, M.E.; Adams, G. New insights of the role of β-NGF in the ovulation mechanism of induced ovulating species. Reproduction 2019, 157, R199–R207. [Google Scholar] [CrossRef]
- Saeednia, S.; Bahadoran, H.; Amidi, F.; Asadi, M.H.; Naji, M.; Fallahi, P.; Nejad, N.A. Nerve growth factor in human semen: Effect of nerve growth factor on the normozoospermic men during cryopreservation process. Iran. J. Basic Med. Sci. 2015, 18, 292–299. [Google Scholar] [PubMed]
- Saeednia, S.; Shabani Nashtaei, M.; Bahadoran, H.; Aleyasin, A.; Amidi, F. Effect of nerve growth factor on sperm quality in asthenozoospermic men during cryopreservation. Reprod. Biol. Endocrinol. 2016, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Sun, Y.; Yi, K.; Ma, Y.; Sun, Y.; Zhang, W.; Zhou, X. Detection of nerve growth factor (NGF) and its specific receptor (TrkA) in ejaculated bovine sperm, and the effects of NGF on sperm function. Theriogenology 2010, 74, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Tanaka, A.; Watanabe, G.; Matsuda, H.; Taya, K. Effect of NGF on the motility and acrosome reaction of golden hamster spermatozoa in vitro. J. Reprod. Dev. 2010, 56, 437–443. [Google Scholar] [CrossRef]
- Harper, G.; Barde, Y.; Burnstock, G.; Carstairs, J.; Dennison, M.; Suda, K. Guinea pig prostate is a rich source of nerve growth factor. Nature 1979, 279, 160. [Google Scholar] [CrossRef]
- Harper, G.; Thoenen, H. The distribution of nerve growth factor in the male sex organs of mammals. J. Neurochem. 1980, 34, 893–903. [Google Scholar] [CrossRef]
- Sheets, R.; Rajaboina, B.; Bromberg, C.E.; Curtin, L.P.; Haddock, M.L.; Stafford, P.; Thomas, T.C.; Scheck, A.C. Freezing diluted bovine serum albumin standards does not significantly affect standard curves. Biotechniques 2025, 77, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Ayer-LeLievre, C.; Olson, L.; Ebendal, T.; Hallböök, F.; Persson, H. Nerve growth factor mRNA and protein in the testis and epididymis of mouse and rat. Proc. Natl. Acad. Sci. USA 1988, 85, 2628–2632. [Google Scholar] [CrossRef]
- Hofmann, H.D.; Unsicker, K. The seminal vesicle of the bull: A new and very rich source of nerve growth factor. Eur. J. Biochem 1982, 128, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.; Niño, A.; Guerra, M.; Letelier, C.; Valderrama, X.; Adams, G.; Ratto, M. Is an ovulation-inducing factor (OIF) present in the seminal plasma of rabbits? Anim. Reprod. Sci. 2011, 127, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.P.; Glanville, R.; Thoenen, H. The purification of nerve growth factor from bovine seminal plasma. Biochemical characterization and partial amino acid sequence. J. Biol. Chem. 1982, 257, 8541–8548. [Google Scholar] [CrossRef] [PubMed]
- Druart, X.; Rickard, J.; Mactier, S.; Kohnke, P.; Kershaw-Young, C.; Bathgate, R.; de Graaf, S.; Gibb, Z.; Crossett, B.; Druart, N.; et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteomic 2013, 91, 13–22. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, V.K.; Singh, S.; Hariprasad, G.R.; Mal, G.; Srinivasan, A.; Yadav, S. Proteomic identification of camel seminal plasma: Purification of β-nerve growth factor. Anim. Reprod. Sci. 2013, 136, 289–295. [Google Scholar] [CrossRef]
- Bothwell, M. Recent advances in understanding context-dependent mechanisms controlling neurotrophin signaling and function. F1000Research 2019, 8, 1658. [Google Scholar] [CrossRef]
- Salas, C.; Julio-Pieper, M.; Valladares, M.; Pommer, R.; Vega, M.; Mastronardi, C.; Kerr, B.; Ojeda, S.R.; Lara, H.E.; Romero, C. Nerve growth Factor-Dependent activation of TRKA receptors in the human ovary results in synthesis of Follicle-Stimulating hormone receptors and estrogen secretion. J. Clin. Endocrinol. Metab. 2006, 91, 2396–2403. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Cotozzolo, E.; Pistilli, A.; Rende, M.; Bartolini, D.; Di Sante, G.; Menchetti, L.; Dal Bosco, A.; Stabile, A.M. The effect of interaction ngf/p75ntr in sperm cells: A rabbit model. Cells 2022, 11, 1035. [Google Scholar] [CrossRef]
- Stabile, A.M.; Pistilli, A.; Moretti, E.; Bartolini, D.; Ruggirello, M.; Rende, M.; Castellini, C.; Mattioli, S.; Ponchia, R.; Tripodi, S.A.; et al. Possible role of nerve growth factors and their receptors in human sperm pathology. Biomedicines 2023, 11, 3345. [Google Scholar] [CrossRef]
- García-García, R.; Arias-Álvarez, M.; Sánchez-Rodríguez, A.; Lorenzo, P.; Rebollar, P. Role of nerve growth factor in the reproductive physiology of female rabbits: A review. Theriogenology 2020, 150, 321–328. [Google Scholar] [CrossRef]
- Quiroga, A.C.; Gimeno-Martos, S.; Lorenzo, P.L.; Álvarez, M.A.; Rebollar, P.G.; García-García, R.M. Microencapsulated rrβNGF as an alternative ovulation induction method in rabbits. BMC Vet. Res. 2025, 21, 133. [Google Scholar] [CrossRef] [PubMed]
- Viudes-De-Castro, M.P.; Moce, E.; Lavara, R.; Marco-Jiménez, F.; Vicente, J.S. Aminopeptidase activity in seminal plasma and effect of dilution rate on rabbit reproductive performance after insemination with an extender supplemented with buserelin acetate. Theriogenology 2014, 81, 1223–1228. [Google Scholar] [CrossRef] [PubMed]
- Cocchia, N.; Pasolini, M.P.; Mancini, R.; Albrizio, M.; De Rosa, A.; Esposito, L.; Prisco, M.; Lorizio, R.; Tortora, G.; Gasparrini, B. Effect of SOD (superoxide dismutase) protein supplementation in semen extenders on motility, viability, acrosome status and ERK protein phosphorylation of chilled stallion spermatozoa. Theriogenology 2011, 75, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Casares-Crespo, L.; Fernández-Serrano, P.; Vicente, J.S.; Marco-Jiménez, F. Insemination extender supplementation with bestatin and EDTA has no effect on rabbit reproductive performance. Theriogenology 2018, 105, 61–65. [Google Scholar] [CrossRef]
- Hashem, N.M.; González-Bulnes, A. State-of-the-art and prospective of nanotechnologies for smart reproductive management of farm animals. Animals 2020, 10, 840. [Google Scholar] [CrossRef]
- Desai, N.; Rana, D.; Salave, S.; Gupta, R.; Patel, P.; Karunakaran, B.; Sharma, A.; Giri, J.; Benival, D.; Kommineni, N. Chitosan: A potential biopolymer in drug delivery and biomedical applications. Pharmaceutics 2023, 15, 1313. [Google Scholar] [CrossRef]
- Sanchez-Rodriguez, A.; Arias-Alvarez, M.; Timón, P.; Bautista, J.M.; Rebollar, P.G.; Lorenzo, P.L.; Garcia-Garcia, R.M. Characterization of β-nerve growth factor-TrkA system in male reproductive tract of rabbit and the relationship between β-NGF and testosterone levels with seminal quality during sexual maturation. Theriogenology 2019, 126, 206–213. [Google Scholar] [CrossRef]
- Castellini, C.; Mattioli, S.; Bosco, A.D.; Cotozzolo, E.; Mancinelli, A.C.; Rende, M.; Stabile, A.M.; Pistilli, A. Nerve growth factor receptor role on rabbit sperm storage. Theriogenology 2020, 153, 54–61. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengibar, M.; Harris, R.; Panos, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar] [CrossRef]
- Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 1985. [Google Scholar] [CrossRef]
- Gimeno-Martos, S.; Bosa, L.; Lorenzo, P.L.; Arias-Álvarez, M.; Castellini, C.; García-Rebollar, P.; García-García, R.M. Influence of free and microencapsulated recombinant rabbit nerve growth factor with chitosan on rabbit sperm quality parameters. Reprod. Domest. Anim. 2024, 59 (Suppl. S3), e14636. [Google Scholar] [CrossRef]
- Li, C.; Zheng, L.; Wang, C.; Zhou, X. Absence of nerve growth factor and comparison of tyrosine kinase receptor a level in mature spermatozoa from oligoasthenozoospermic, asthenozoospermic and fertile men. Clin. Chim. Acta 2010, 411, 1482–1486. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 14; StataCorp LP: College Station, TX, USA, 2015. [Google Scholar]
- Sari, L.M.; Zampini, R.; Argañaraz, M.E.; Carretero, M.I.; Fumuso, F.G.; Barraza, D.E.; Ratto, M.; Apichela, S.A. Expression of β-NGF and high-affinity NGF receptor (TrkA) in llama (*Lama glama*) male reproductive tract and spermatozoa. Mol. Reprod. Dev. 2018, 85, 934–944. [Google Scholar] [CrossRef] [PubMed]
- Ferraguti, G.; Fanfarillo, F.; Tarani, L.; Blaconà, G.; Tarani, F.; Barbato, C.; Minni, A.; Ralli, M.; Francati, S.; Greco, A.; et al. NGF and the male reproductive system: Potential clinical applications in infertility. Int. J. Med. Sci. 2022, 23, 13127. [Google Scholar] [CrossRef] [PubMed]
- Bruno, F.; Arcuri, D.; Vozzo, F.; Malvaso, A.; Montesanto, A.; Maletta, R. Expression and signaling pathways of nerve growth factor (NGF) and pro-NGF in breast cancer: A systematic review. Curr. Oncol. 2022, 29, 8103–8120. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.J.; Jo, J.H.; Uwamahoro, C.; Jang, S.I.; Jung, E.J.; Bae, J.W.; Moon, J.; Kim, D.H.; Yi, J.K.; Ha, J.J.; et al. Role of PI3K/AKT signaling pathway during capacitation. Theriogenology 2025, 235, 94–102. [Google Scholar] [CrossRef]
- Jaldety, Y.; Breitbart, H. ERK1/2 mediates sperm acrosome reaction through elevation of intracellular calcium concentration. Zygote 2015, 23, 652–661. [Google Scholar] [CrossRef]
- Volonté, C.; Ross, A.H.; Greene, L.A. Association of a purine-analogue-sensitive protein kinase activity with p75 nerve growth factor receptors. Mol. Biol. Cell 1993, 4, 71–78. [Google Scholar] [CrossRef][Green Version]
| TREATMENT | RMSE | ||||||
|---|---|---|---|---|---|---|---|
| Control | Chitosan | rrβNGFch | |||||
| Time of storage | 30′ | 2 h | 30′ | 2 h | 30′ | 2 h | |
| Live cells (%) | 71.59 b | 69.80 a | 72.12 b | 69.37 a | 73.06 b | 69.23 a | 4.38 |
| Motility rate (%) | 82.5 b | 78.0 a | 88.1 b | 71.5 a | 84.8 b | 70.5 a | 9.90 |
| VCL (µm/s) | 95.5 b | 84.4 a | 101.4 b | 91.9 a | 103.5 b | 89.5 a | 10.61 |
| LIN (%) | 64.6 | 57.1 | 58.8 | 47.7 | 58.2 | 50.1 | 12.63 |
| ALH (µm) | 3.08 | 3.02 | 3.41 | 2.95 | 3.47 | 2.99 | 0.35 |
| Apoptotic sperm (%) | 6.31 A | 7.87 B | 6.17 A | 7.68 B | 5.79 A | 7.16 B | 0.66 |
| Capacitated sperm (%) | 5.25 a | 9.18 b | 5.50 a | 9.4 b | 12.31 c | 14.06 d | 2.70 |
| Acrosome-reacted sperm (%) | 5.62 a | 7.875 b | 6.25 a | 7.75 b | 11.50 c | 10.43 d | 1.20 |
| p75NTR (%) | 26.07 a | 29.86 b | 26.12 a | 29.87 b | 29.51 a | 31.09 b | 2.35 |
| TrKA (%) | 91.28 b | 88.80 a | 91.37 b | 88.87 a | 92.46 b | 88.80 a | 4.01 |
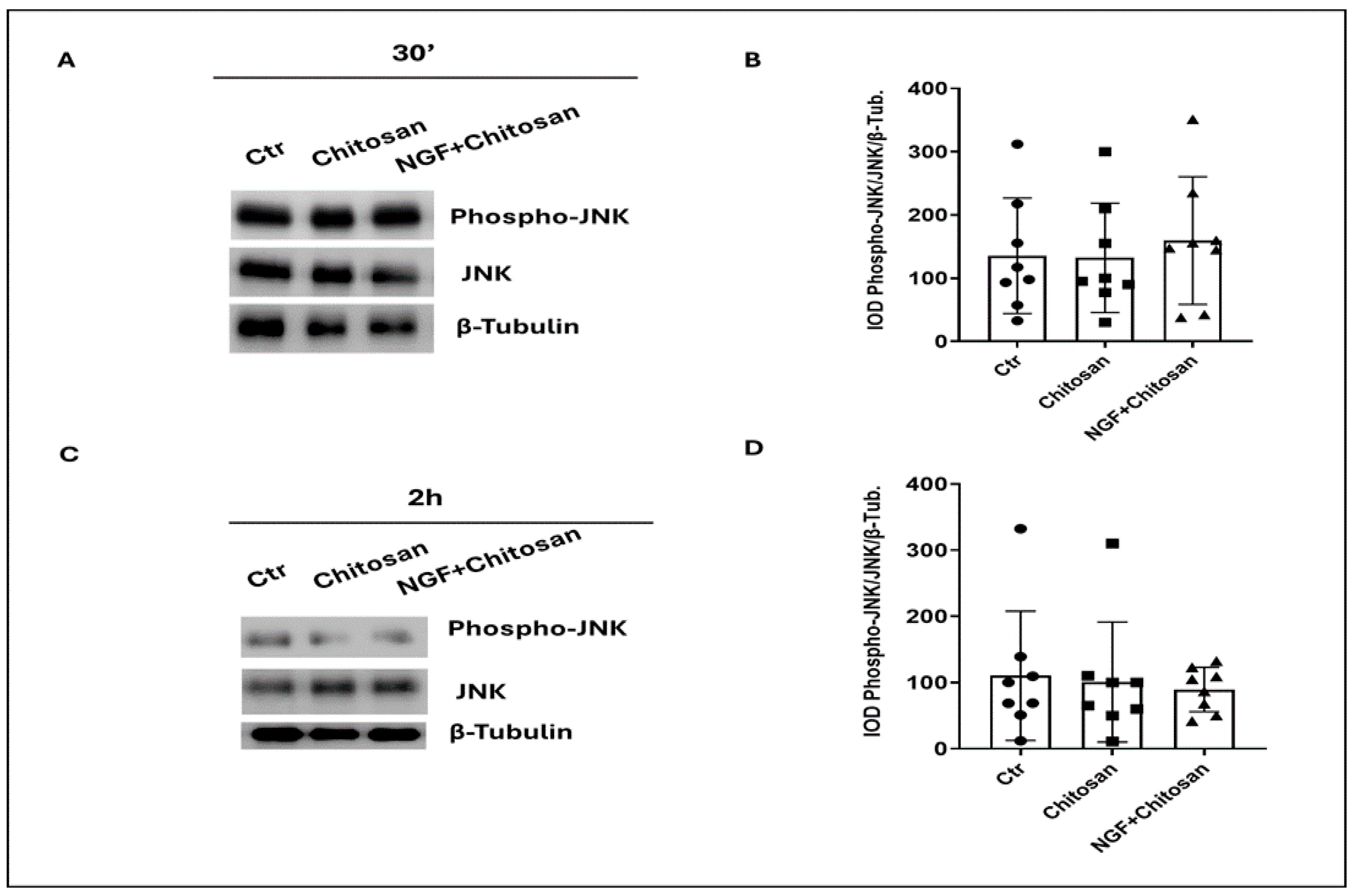
| JNK (a.u.) | 135.4 b | 110.2 a | 132.1 b | 100.7 a | 159.3 b | 89.4 a | 75.68 |
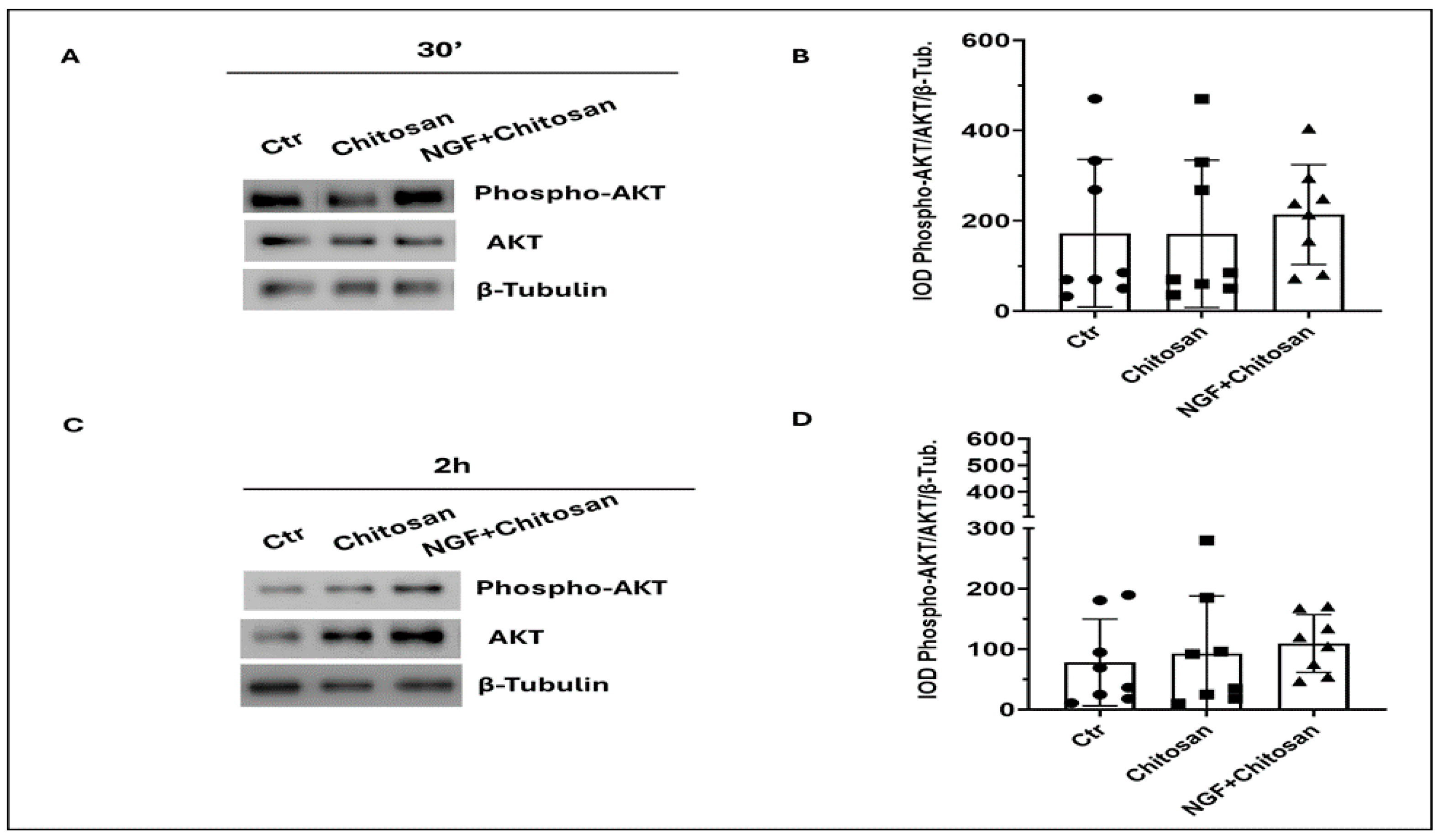
| AKT (a.u.) | 172.3 b | 103.1 a | 171.1 b | 92.1 a | 213.8 b | 95.8 a | 84.62 |
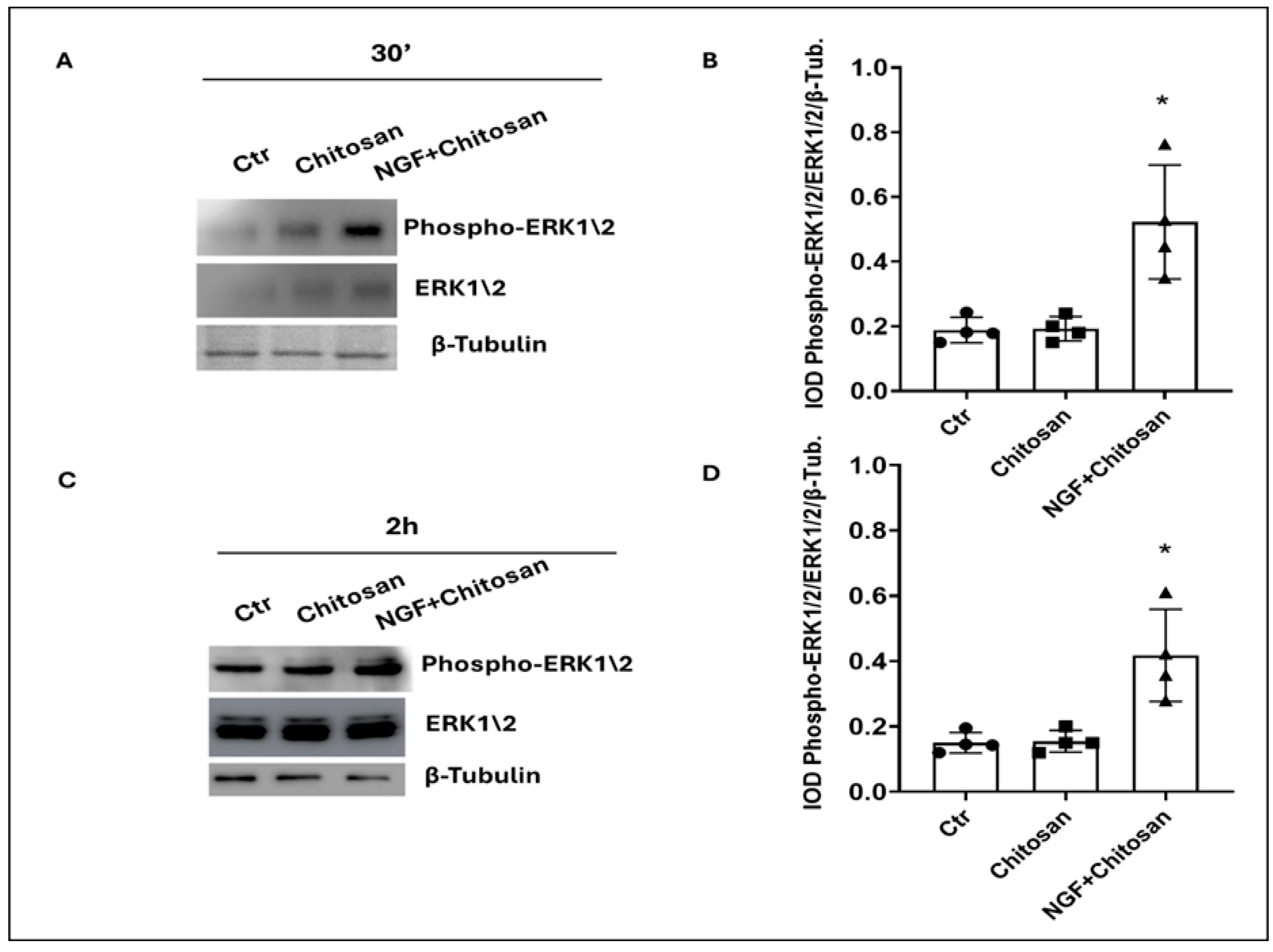
| ERK 1/2 (a.u.) | 0.18 a | 0.15 a | 0.20 a | 0.15 a | 0.52 b | 0.41 b | 0.08 |
| JNK | ERK 1/2 | AKT | p75NTR | Capacitated Sperm | Acrosome-Reacted Sperm | |
|---|---|---|---|---|---|---|
| ERK 1/2 | 0.126 | |||||
| AKT | 0.422 * | 0.254 | ||||
| p75NTR | −0.091 | 0.076 | −0.161 | |||
| Capacitated sperm | −0.228 | 0.429 * | −0.134 | 0.405 * | ||
| Acrosome-reacted sperm | −0.072 | 0.515 ** | −0.055 | 0.360 * | 0.647 ** | |
| Apoptotic sperm | −0.184 | 0.396 * | −0.218 | 0.842 ** | 0.374 * | 0.354 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bosa, L.; Mattioli, S.; Stabile, A.M.; Bartolini, D.; Tognoloni, A.; Pistilli, A.; Ruggirello, M.; Rende, M.; Gimeno-Martos, S.; Jordán-Rodríguez, D.; et al. Effect of Recombinant NGF Encapsulated in Chitosan on Rabbit Sperm Traits and Main Metabolic Pathways. Biology 2025, 14, 974. https://doi.org/10.3390/biology14080974
Bosa L, Mattioli S, Stabile AM, Bartolini D, Tognoloni A, Pistilli A, Ruggirello M, Rende M, Gimeno-Martos S, Jordán-Rodríguez D, et al. Effect of Recombinant NGF Encapsulated in Chitosan on Rabbit Sperm Traits and Main Metabolic Pathways. Biology. 2025; 14(8):974. https://doi.org/10.3390/biology14080974
Chicago/Turabian StyleBosa, Luigia, Simona Mattioli, Anna Maria Stabile, Desirée Bartolini, Alessia Tognoloni, Alessandra Pistilli, Mariangela Ruggirello, Mario Rende, Silvia Gimeno-Martos, Daniela Jordán-Rodríguez, and et al. 2025. "Effect of Recombinant NGF Encapsulated in Chitosan on Rabbit Sperm Traits and Main Metabolic Pathways" Biology 14, no. 8: 974. https://doi.org/10.3390/biology14080974
APA StyleBosa, L., Mattioli, S., Stabile, A. M., Bartolini, D., Tognoloni, A., Pistilli, A., Ruggirello, M., Rende, M., Gimeno-Martos, S., Jordán-Rodríguez, D., Arias-Álvarez, M., García Rebollar, P., García-García, R. M., & Castellini, C. (2025). Effect of Recombinant NGF Encapsulated in Chitosan on Rabbit Sperm Traits and Main Metabolic Pathways. Biology, 14(8), 974. https://doi.org/10.3390/biology14080974








