Immunohistopathology of Cochleovestibular Schwannoma in Human Temporal Bone Specimens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
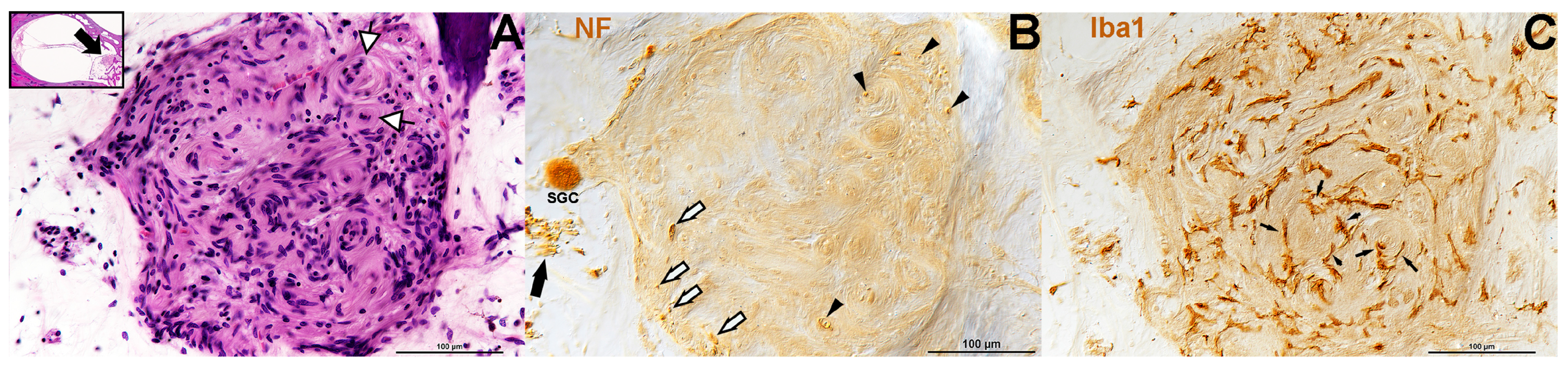
3. Results
| ID # | Nerve of Origin | Cochlear Nerve Invasion |
|---|---|---|
| 1 | Too large to determine | ✓ |
| 2 | Too large to determine (both divisions of vestibular and cochlear) | ✓ |
| 3 | Inferior vestibular and cochlear | ✓ |
| 4 | Superior division of vestibular | ✓ |
| 5 | Too large to determine (both divisions of vestibular and cochlear) | ✓ |
| 6 | Vestibular | ✓ |
| 7 | Cochlear | ✓ |
| 8 | Inferior division of vestibular | ✓ |
| 9 | Superior division of vestibular | no |
| 10 | Inferior division of vestibular | no |
| 11 | Superior division of vestibular | no |
| 12 | Too large to determine | ✓ |
| 13 | Intralabyrinthine to vestibular | ✓ |
| 14 | Cochlear | ✓ |
| 15 | Vestibular | no |
| 16 | Cochlear—intralabyrinthine | ✓ |
| 17 | Cochlear—intralabyrinthine | ✓ |
| 18 | Too large to determine (auditory, vestibular, and facial) | ✓ |
| 19 | Superior vestibular | no |
| 20 | Inferior vestibular | ✓ |
| 21 | Superior vestibular | ✓ |
| 22 | Superior vestibular | no |
| 23 | Superior vestibular | ✓ |
| 24 | Superior vestibular | no |
| 25 | Cochlear | ✓ |
| 26 | Superior vestibular | ✓ |
| 27 | Superior vestibular | ✓ |
| 28 | Too large to determine (both auditory and facial) | ✓ |
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABR | Auditory Brainstem Response |
| NF | Neurofilament |
| MPZ | Myelin Protein Zero |
| CVS | Cochleovestibular Schwannomas |
| NF2 | Neurofibromatosis Type 2 |
| SNHL | Sensorineural Hearing Loss |
| IAC | Internal Auditory Canal |
| H&E | Hematoxylin and Eosin |
| Iba1 | Ionized Calcium Binding Adaptor Molecule 1 |
| ROS | Reactive Oxygen Species |
| VEGF | Vascular Endothelial Growth Factor |
| DAB | Diaminobenzidine |
References
- Roosli, C.; Linthicum, F.H., Jr.; Cureoglu, S.; Merchant, S.N. What is the site of origin of cochleovestibular schwannomas? Audiol. Neurootol. 2012, 17, 121–125. [Google Scholar] [CrossRef]
- Gonzalez Revilla, A. Differential diagnosis of tumors at the cerebellopontile recess. Bull. Johns Hopkins Hosp. 1948, 83, 187–212. [Google Scholar]
- Halliday, J.; Rutherford, S.A.; McCabe, M.G.; Evans, D.G. An update on the diagnosis and treatment of vestibular schwannoma. Expert. Rev. Neurother. 2018, 18, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Carlson, M.L.; Smadbeck, J.B.; Link, M.J.; Klee, E.W.; Vasmatzis, G.; Schimmenti, L.A. Next Generation Sequencing of Sporadic Vestibular Schwannoma: Necessity of Biallelic NF2 Inactivation and Implications of Accessory Non-NF2 Variants. Otol. Neurotol. 2018, 39, e860–e871. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, S.R.; Messiaen, L.; Legius, E.; Pancza, P.; Avery, R.A.; Blakeley, J.O.; Babovic-Vuksanovic, D.; Ferner, R.; Fisher, M.J.; Friedman, J.M.; et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: An international consensus recommendation. Genet. Med. 2022, 24, 1967–1977. [Google Scholar] [CrossRef]
- Gupta, V.K.; Thakker, A.; Gupta, K.K. Vestibular Schwannoma: What We Know and Where We are Heading. Head Neck Pathol. 2020, 14, 1058–1066. [Google Scholar] [CrossRef]
- Seizinger, B.R.; Martuza, R.L.; Gusella, J.F. Loss of genes on chromosome 22 in tumorigenesis of human acoustic neuroma. Nature 1986, 322, 644–647. [Google Scholar] [CrossRef]
- Rouleau, G.A.; Seizinger, B.R.; Wertelecki, W.; Haines, J.L.; Superneau, D.W.; Martuza, R.L.; Gusella, J.F. Flanking markers bracket the neurofibromatosis type 2 (NF2) gene on chromosome 22. Am. J. Hum. Genet. 1990, 46, 323–328. [Google Scholar] [PubMed]
- Welling, D.B. Clinical manifestations of mutations in the neurofibromatosis type 2 gene in vestibular schwannomas (acoustic neuromas). Laryngoscope 1998, 108, 178–189. [Google Scholar] [CrossRef]
- Selters, W.A.; Brackmann, D.E. Acoustic tumor detection with brain stem electric response audiometry. Arch. Otolaryngol. 1977, 103, 181–187. [Google Scholar] [CrossRef]
- Eckermeier, L.; Pirsig, W.; Mueller, D. Histopathology of 30 non-operated acoustic schwannomas. Arch. Otorhinolaryngol. 1979, 222, 1–9. [Google Scholar] [CrossRef]
- Johnsson, L.G.; Hawkins, J.E., Jr.; Rouse, R.C. Sensorineural and vascular changes in an ear with acoustic neurinoma. Am. J. Otolaryngol. 1984, 5, 49–59. [Google Scholar] [CrossRef]
- Bozorg Grayeli, A.; Refass, A.; Smail, M.; Elgarem, H.; Kalamarides, M.; Bouccara, D.; Sterkers, O. Diagnostic value of auditory brainstem responses in cerebellopontine angle tumours. Acta Otolaryngol. 2008, 128, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Al-Asad, R.K.; Montigny, D.J.; O’Malley, J.T.; Welling, D.B.; Jung, D.H.; Eckhard, A.H.; Kempfle, J.S. Investigating cochlear cellular dynamics in neurofibromatosis type 2-associated schwannomatosis: A histopathological study. Front. Neurol. 2025, 16, 1650470. [Google Scholar] [CrossRef]
- Nadol, J.B., Jr.; Diamond, P.F.; Thornton, A.R. Correlation of hearing loss and radiologic dimensions of vestibular schwannomas (acoustic Neuromas). Am. J. Otol. 1996, 17, 312–316. [Google Scholar]
- Mahmud, M.R.; Khan, A.M.; Nadol, J.B., Jr. Histopathology of the inner ear in unoperated acoustic neuroma. Ann. Otol. Rhinol. Laryngol. 2003, 112, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.N.; McKenna, M.J. Neoplastic Growth. In Schuknecht’s Pathology of the Ear, 3rd ed.; Merchant, S.N., Nadol, J.B., Eds.; PMPH-USA: Shelton, CT, USA, 2010; pp. 492–511. [Google Scholar]
- Roosli, C.; Linthicum, F.H., Jr.; Cureoglu, S.; Merchant, S.N. Dysfunction of the cochlea contributing to hearing loss in acoustic neuromas: An underappreciated entity. Otol. Neurotol. 2012, 33, 473–480. [Google Scholar] [CrossRef]
- Dilwali, S.; Lysaght, A.; Roberts, D.; Barker, F.G.; McKenna, M.J.; Stankovic, K.M. Sporadic vestibular schwannomas associated with good hearing secrete higher levels of fibroblast growth factor 2 than those associated with poor hearing irrespective of tumor size. Otol. Neurotol. 2013, 34, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Dilwali, S.; Landegger, L.D.; Soares, V.Y.; Deschler, D.G.; Stankovic, K.M. Secreted Factors from Human Vestibular Schwannomas Can Cause Cochlear Damage. Sci. Rep. 2015, 5, 18599. [Google Scholar] [CrossRef]
- Eggink, M.C.; Frijns, J.H.M.; Sagers, J.E.; O’Malley, J.T.; Liberman, M.C.; Stankovic, K.M. Human vestibular schwannoma reduces density of auditory nerve fibers in the osseous spiral lamina. Hear. Res. 2022, 418, 108458. [Google Scholar] [CrossRef]
- Tesarova, M.; Peterkova, L.; Stastna, M.; Kolar, M.; Lacina, L.; Smetana, K., Jr.; Hynek, R.; Betka, J.; Vlasak, A.; Lukes, P.; et al. Tumor Biology and Microenvironment of Vestibular Schwannoma-Relation to Tumor Growth and Hearing Loss. Biomedicines 2023, 11, 32. [Google Scholar] [CrossRef]
- Neely, J.G. Gross and microscopic anatomy of the eighth cranial nerve in relationship to the solitary schwannoma. Laryngoscope 1981, 91, 1512–1531. [Google Scholar] [CrossRef]
- Feany, M.B.; Anthony, D.C.; Fletcher, C.D. Nerve sheath tumours with hybrid features of neurofibroma and schwannoma: A conceptual challenge. Histopathology 1998, 32, 405–410. [Google Scholar] [CrossRef]
- Wippold, F.J.; Lubner, M.; Perrin, R.J.; Lammle, M.; Perry, A. Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. AJNR Am. J. Neuroradiol. 2007, 28, 1633–1638. [Google Scholar] [CrossRef]
- Gompel, V.; Jamie, J.; Carlstrom, L.P.; Hadjipanayis, C.G.; Graffeo, C.S.; Patel, N.; Carlson, M.L.; Jacob, J.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guideline on Surgical Resection for the Treatment of Patients With Vestibular Schwannomas: Update. Neurosurgery 2025. [Google Scholar] [CrossRef] [PubMed]
- Corfas, G.; Velardez, M.O.; Ko, C.P.; Ratner, N.; Peles, E. Mechanisms and roles of axon-Schwann cell interactions. J. Neurosci. 2004, 24, 9250–9260. [Google Scholar] [CrossRef]
- Wechsler, J.; Lantieri, L.; Zeller, J.; Voisin, M.C.; Martin-Garcia, N.; Wolkenstein, P. Aberrant axon neurofilaments in schwannomas associated with phacomatoses. Virchows Arch. 2003, 443, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Neuroma, A. Acoustic Neuroma. NIH Consens. Statement 1991, 9, 1–24. [Google Scholar]
- Hung, G.; Colton, J.; Fisher, L.; Oppenheimer, M.; Faudoa, R.; Slattery, W.; Linthicum, F. Immunohistochemistry study of human vestibular nerve schwannoma differentiation. Glia 2002, 38, 363–370. [Google Scholar] [CrossRef]
- Ylikoski, J.; Palva, T.; Collan, Y. Eighth nerve in acoustic neuromas. Special reference to superior vestibular nerve function and histopathology. Arch. Otolaryngol. 1978, 104, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Ylikoski, J.; Collan, Y.; Palva, T.; Jauhiainen, T. Cochlear nerve in neurilemomas. Audiology and histopathology. Arch. Otolaryngol. 1978, 104, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Neely, J.G.; Armstrong, D.; Benson, J.; Neblett, C. “Onion bulb” formation associated with a solitary neoplasm of the eighth nerve sheath. Am. J. Otolaryngol. 1981, 2, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Neely, J.G.; Hough, J. Histologic findings in two very small intracanalicular solitary schwannomas of the eighth nerve. Ann. Otol. Rhinol. Laryngol. 1986, 95, 460–465. [Google Scholar] [CrossRef]
- Neely, J.G.; Hough, J.V. Histologic findings in two very small intracanalicular solitary schwannomas of the eighth nerve: II. “Onion bulbs”. Am. J. Otol. 1988, 9, 216–221. [Google Scholar]
- Forton, G.; Moeneclaey, L.; Declau, F.; Marquet, J. The involvement of the cochlear nerve in neurinomas of the eighth cranial nerve. Arch. Otorhinolaryngol. 1989, 246, 156–160. [Google Scholar] [CrossRef]
- Marquet, J.F.; Forton, G.E.; Offeciers, F.E.; Moeneclaey, L.L. The solitary schwannoma of the eighth cranial nerve. An immunohistochemical study of the cochlear nerve-tumor interface. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 1023–1025. [Google Scholar] [CrossRef]
- Hannan, C.J.; Raghunathan, A.; Van Gompel, J.J.; Pathmanaban, O. Pathology and tumor microenvironment of vestibular schwannoma. Handb. Clin. Neurol. 2025, 212, 47–57. [Google Scholar] [CrossRef]
- Nickl, V.; Ziebolz, D.; Rumpel, C.; Klein, D.; Nickl, R.; Rampeltshammer, E.; Monoranu, C.M.; Ernestus, R.I.; Matthies, C.; Lohr, M.; et al. Analysis of tumor microenvironment composition in vestibular schwannomas: Insights into NF2-associated and sporadic variations and their clinical correlations. Front. Oncol. 2024, 14, 1340184. [Google Scholar] [CrossRef]
- Nguyen, H.T.N.; Duhon, B.H.; Kuo, H.C.; Fisher, M.; Brickey, O.M.; Zhang, L.; Otero, J.J.; Prevedello, D.M.; Adunka, O.F.; Ren, Y. Matrix metalloproteinase 9: An emerging biomarker for classification of adherent vestibular schwannoma. Neurooncol. Adv. 2024, 6, vdae058. [Google Scholar] [CrossRef]
- Duhon, B.H.; Thompson, K.; Fisher, M.; Kaul, V.F.; Nguyen, H.T.; Harris, M.S.; Varadarajan, V.; Adunka, O.F.; Prevedello, D.M.; Kolipaka, A.; et al. Tumor biomechanical stiffness by magnetic resonance elastography predicts surgical outcomes and identifies biomarkers in vestibular schwannoma and meningioma. Sci. Rep. 2024, 14, 14561. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, J.T.; Burgess, B.J.; Jones, D.D.; Adams, J.C.; Merchant, S.N. Techniques of celloidin removal from temporal bone sections. Ann. Otol. Rhinol. Laryngol. 2009, 118, 435–441. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, J.T.; Nadol, J.B., Jr.; McKenna, M.J. Anti CD163+, Iba1+, and CD68+ Cells in the Adult Human Inner Ear: Normal Distribution of an Unappreciated Class of Macrophages/Microglia and Implications for Inflammatory Otopathology in Humans. Otol. Neurotol. 2016, 37, 99–108. [Google Scholar] [CrossRef]
- Escalona-Zapata, J.; Diez Nau, M.D. The nature of macrophages (foam cells) in neurinomas. Tissue culture study. Acta Neuropathol. 1978, 44, 71–75. [Google Scholar] [CrossRef]
- Rossi, M.L.; Jones, N.R.; Esiri, M.M.; Havas, L.; Nakamura, N.; Coakham, H.B. Mononuclear cell infiltrate, HLA-Dr expression and proliferation in 37 acoustic schwannomas. Histol. Histopathol. 1990, 5, 427–432. [Google Scholar]
- Gomez-Brouchet, A.; Delisle, M.B.; Cognard, C.; Bonafe, A.; Charlet, J.P.; Deguine, O.; Fraysse, B. Vestibular schwannomas: Correlations between magnetic resonance imaging and histopathologic appearance. Otol. Neurotol. 2001, 22, 79–86. [Google Scholar] [CrossRef]
- Park, C.K.; Kim, D.C.; Park, S.H.; Kim, J.E.; Paek, S.H.; Kim, D.G.; Jung, H.W. Microhemorrhage, a possible mechanism for cyst formation in vestibular schwannomas. J. Neurosurg. 2006, 105, 576–580. [Google Scholar] [CrossRef]
- Yokoo, H.; Oishi, T.; Isoda, K.; Nakazato, Y.; Toyokuni, S. Oxidative stress is related to the formation of Antoni B patterns and eosinophilic hyaline droplets in schwannomas. Neuropathology 2007, 27, 237–244. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Hogendoorn, P.C.; Briaire-de Bruyn, I.; Malessy, M.J.; van der Mey, A.G. Intratumoral hemorrhage, vessel density, and the inflammatory reaction contribute to volume increase of sporadic vestibular schwannomas. Virchows Arch. 2012, 460, 629–636. [Google Scholar] [CrossRef] [PubMed]
- de Vries, M.; Briaire-de Bruijn, I.; Malessy, M.J.; de Bruine, S.F.; van der Mey, A.G.; Hogendoorn, P.C. Tumor-associated macrophages are related to volumetric growth of vestibular schwannomas. Otol. Neurotol. 2013, 34, 347–352. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Jabaly, S.; Alhareth, F.; Jabaly, N.; Abboud, F.; Haddad, S. Sciatic schwannoma allegedly following repeated trauma: A case report and exploration of a potential association. Int. J. Surg. Case Rep. 2025, 134, 111794. [Google Scholar] [CrossRef] [PubMed]
- Judd, T.; Jones, T.; Thornberry, L. Schwannoma of the posterior tibial nerve: Case study. J. Am. Podiatr. Med. Assoc. 2014, 104, 539–543. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Schneider, A.B.; Ron, E.; Lubin, J.; Stovall, M.; Shore-Freedman, E.; Tolentino, J.; Collins, B.J. Acoustic neuromas following childhood radiation treatment for benign conditions of the head and neck. Neuro Oncol. 2008, 10, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kalisperati, P.; Spanou, E.; Pateras, I.S.; Korkolopoulou, P.; Varvarigou, A.; Karavokyros, I.; Gorgoulis, V.G.; Vlachoyiannopoulos, P.G.; Sougioultzis, S. Inflammation, DNA Damage, Helicobacter pylori and Gastric Tumorigenesis. Front. Genet. 2017, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Öcal, E. Understanding intracranial arachnoid cysts: A review of etiology, pathogenesis, and epidemiology. Child’s Nerv. Syst. 2023, 39, 73–78. [Google Scholar] [CrossRef]
- Davies, C.L.; Miron, V.E. Distinct origins, gene expression and function of microglia and monocyte-derived macrophages in CNS myelin injury and regeneration. Clin. Immunol. 2018, 189, 57–62. [Google Scholar] [CrossRef]
- Lund, H.; Pieber, M.; Parsa, R.; Grommisch, D.; Ewing, E.; Kular, L.; Han, J.; Zhu, K.; Nijssen, J.; Hedlund, E.; et al. Fatal demyelinating disease is induced by monocyte-derived macrophages in the absence of TGF-beta signaling. Nat. Immunol. 2018, 19, 1–7. [Google Scholar] [CrossRef]
- Belanger, E.; Henry, F.P.; Vallee, R.; Randolph, M.A.; Kochevar, I.E.; Winograd, J.M.; Lin, C.P.; Cote, D. In vivo evaluation of demyelination and remyelination in a nerve crush injury model. Biomed. Opt. Express 2011, 2, 2698–2708. [Google Scholar] [CrossRef]
- Hannan, C.J.; Lewis, D.; O’Leary, C.; Donofrio, C.A.; Evans, D.G.; Roncaroli, F.; Brough, D.; King, A.T.; Coope, D.; Pathmanaban, O.N. The inflammatory microenvironment in vestibular schwannoma. Neurooncol. Adv. 2020, 2, vdaa023. [Google Scholar] [CrossRef]
- Hannan, C.J.; Lewis, D.; O’Leary, C.; Donofrio, C.A.; Evans, D.G.; Stapleton, E.; Freeman, S.R.; Lloyd, S.K.; Rutherford, S.A.; Hammerbeck-Ward, C.; et al. Beyond Antoni: A Surgeon’s Guide to the Vestibular Schwannoma Microenvironment. J. Neurol. Surg. B Skull Base 2022, 83, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, C.K.; Cunnane, M.E.; McKenna, M.J.; Curtin, H.D.; Stankovic, K.M. Correlation Between Aspirin Intake and Reduced Growth of Human Vestibular Schwannoma: Volumetric Analysis. Otol. Neurotol. 2016, 37, 1428–1434. [Google Scholar] [CrossRef]
- Kandathil, C.K.; Dilwali, S.; Wu, C.C.; Ibrahimov, M.; McKenna, M.J.; Lee, H.; Stankovic, K.M. Aspirin intake correlates with halted growth of sporadic vestibular schwannoma in vivo. Otol. Neurotol. 2014, 35, 353–357. [Google Scholar] [CrossRef]
- Dilwali, S.; Kao, S.Y.; Fujita, T.; Landegger, L.D.; Stankovic, K.M. Nonsteroidal anti-inflammatory medications are cytostatic against human vestibular schwannomas. Transl. Res. 2015, 166, 1–11. [Google Scholar] [CrossRef]
- Parameswaran, N.; Patial, S. Tumor necrosis factor-alpha signaling in macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Di Stadio, A.; Dipietro, L.; Ralli, M.; Meneghello, F.; Minni, A.; Greco, A.; Stabile, M.R.; Bernitsas, E. Sudden hearing loss as an early detector of multiple sclerosis: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4611–4624. [Google Scholar] [CrossRef]
- Sagers, J.E.; Brown, A.S.; Vasilijic, S.; Lewis, R.M.; Sahin, M.I.; Landegger, L.D.; Perlis, R.H.; Kohane, I.S.; Welling, D.B.; Patel, C.J.; et al. Computational repositioning and preclinical validation of mifepristone for human vestibular schwannoma. Sci. Rep. 2018, 8, 5437. [Google Scholar] [CrossRef] [PubMed]
- Huynh, P.P.; Saba, E.S.; Hoerter, J.E.; Jiang, N. Steroid Efficacy on Audiologic Recovery in Patients With Sudden Sensorineural Hearing Loss and Vestibular Schwannoma: A Retrospective Review. Otol. Neurotol. 2023, 44, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, C.; Carlson, M.L. Improvement or Recovery From Sudden Sensorineural Hearing Loss With Steroid Therapy Does Not Preclude the Need for MRI to Rule Out Vestibular Schwannoma. Otol. Neurotol. 2019, 40, 674–680. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Allen, J.; Dhall, G.; Campian, J.L.; Clapp, D.W.; Fisher, M.J.; Jain, R.K.; Tonsgard, J.; Ullrich, N.J.; Thomas, C.; et al. Multicenter, prospective, phase II study of maintenance bevacizumab for children and adults with NF2-related schwannomatosis and progressive vestibular schwannoma. Neuro Oncol. 2023, 25, 1498–1506. [Google Scholar] [CrossRef]
- Ribatti, D. Immunosuppressive effects of vascular endothelial growth factor. Oncol. Lett. 2022, 24, 369. [Google Scholar] [CrossRef]
- Gregory, G.E.; Haley, M.J.; Jones, A.P.; Hannan, C.J.; Evans, D.G.; King, A.T.; Paszek, P.; Pathmanaban, O.N.; Couper, K.N.; Brough, D. Alternatively activated macrophages are associated with faster growth rate in vestibular schwannoma. Brain Commun. 2024, 6, fcae400. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Kannan, S.; Rutkowski, J.M. VEGFR-3 signaling in macrophages: Friend or foe in disease? Front. Immunol. 2024, 15, 1349500. [Google Scholar] [CrossRef]
- Lewis, D.; Donofrio, C.A.; O’Leary, C.; Li, K.L.; Zhu, X.; Williams, R.; Djoukhadar, I.; Agushi, E.; Hannan, C.J.; Stapleton, E.; et al. The microenvironment in sporadic and neurofibromatosis type II-related vestibular schwannoma: The same tumor or different? A comparative imaging and neuropathology study. J. Neurosurg. 2021, 134, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Zhao, H.; Ren, X.B. Relationship of VEGF/VEGFR with immune and cancer cells: Staggering or forward? Cancer Biol. Med. 2016, 13, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Motz, G.T.; Coukos, G. The parallel lives of angiogenesis and immunosuppression: Cancer and other tales. Nat. Rev. Immunol. 2011, 11, 702–711. [Google Scholar] [CrossRef]
- Cursiefen, C.; Chen, L.; Borges, L.P.; Jackson, D.; Cao, J.; Radziejewski, C.; D’Amore, P.A.; Dana, M.R.; Wiegand, S.J.; Streilein, J.W. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J. Clin. Investig. 2004, 113, 1040–1050. [Google Scholar] [CrossRef]
- O’Malley, J.T.; Merchant, S.N.; Burgess, B.J.; Jones, D.D.; Adams, J.C. Effects of fixative and embedding medium on morphology and immunostaining of the cochlea. Audiol. Neurootol. 2009, 14, 78–87. [Google Scholar] [CrossRef]
- de Vries, W.M.; Briaire-de Bruijn, I.H.; van Benthem, P.P.G.; van der Mey, A.G.L.; Hogendoorn, P.C.W. M-CSF and IL-34 expression as indicators for growth in sporadic vestibular schwannoma. Virchows Arch. 2019, 474, 375–381. [Google Scholar] [CrossRef]
- Fletcher, J.S.; Springer, M.G.; Choi, K.; Jousma, E.; Rizvi, T.A.; Dombi, E.; Kim, M.O.; Wu, J.; Ratner, N. STAT3 inhibition reduces macrophage number and tumor growth in neurofibroma. Oncogene 2019, 38, 2876–2884. [Google Scholar] [CrossRef] [PubMed]
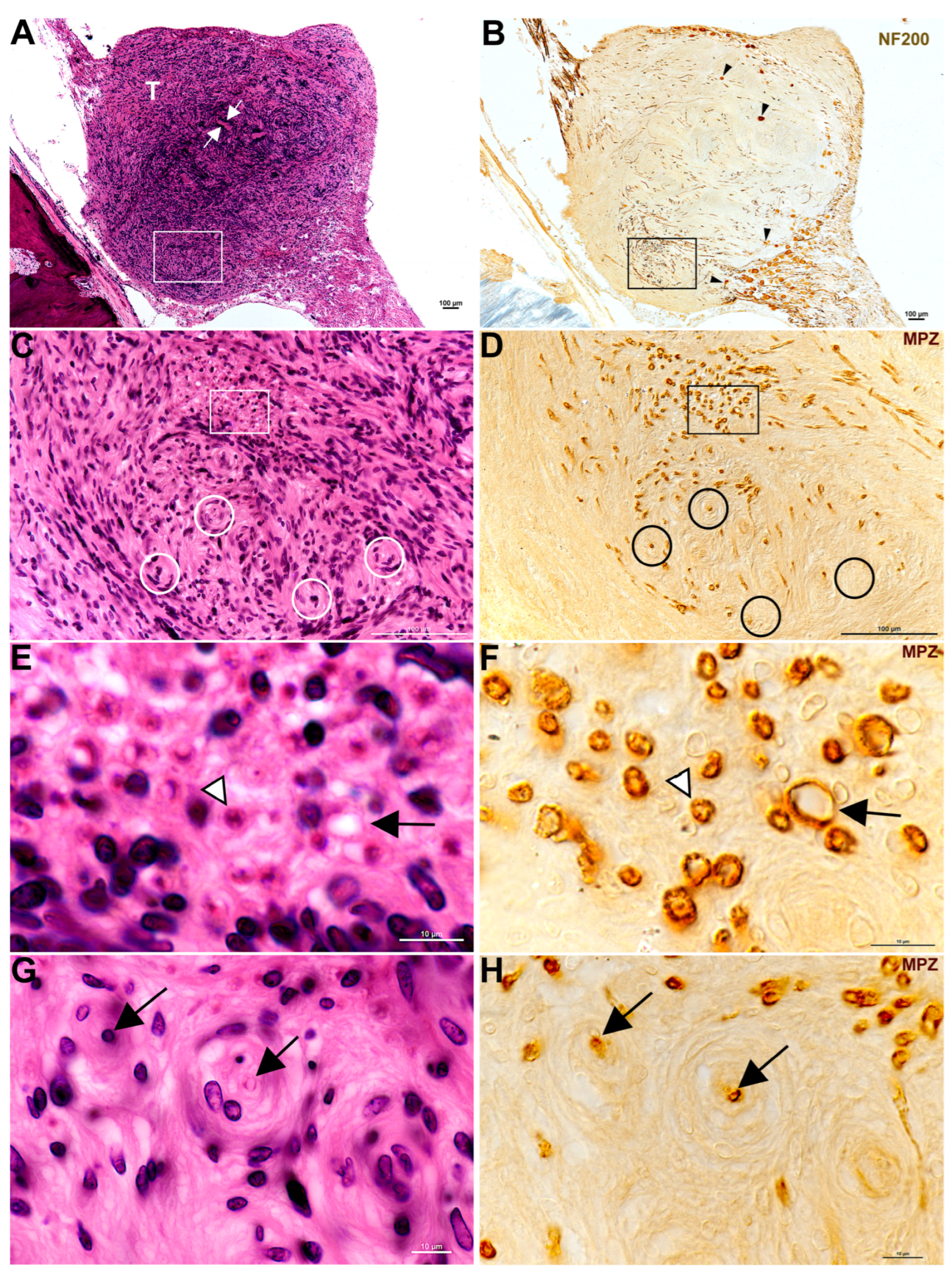
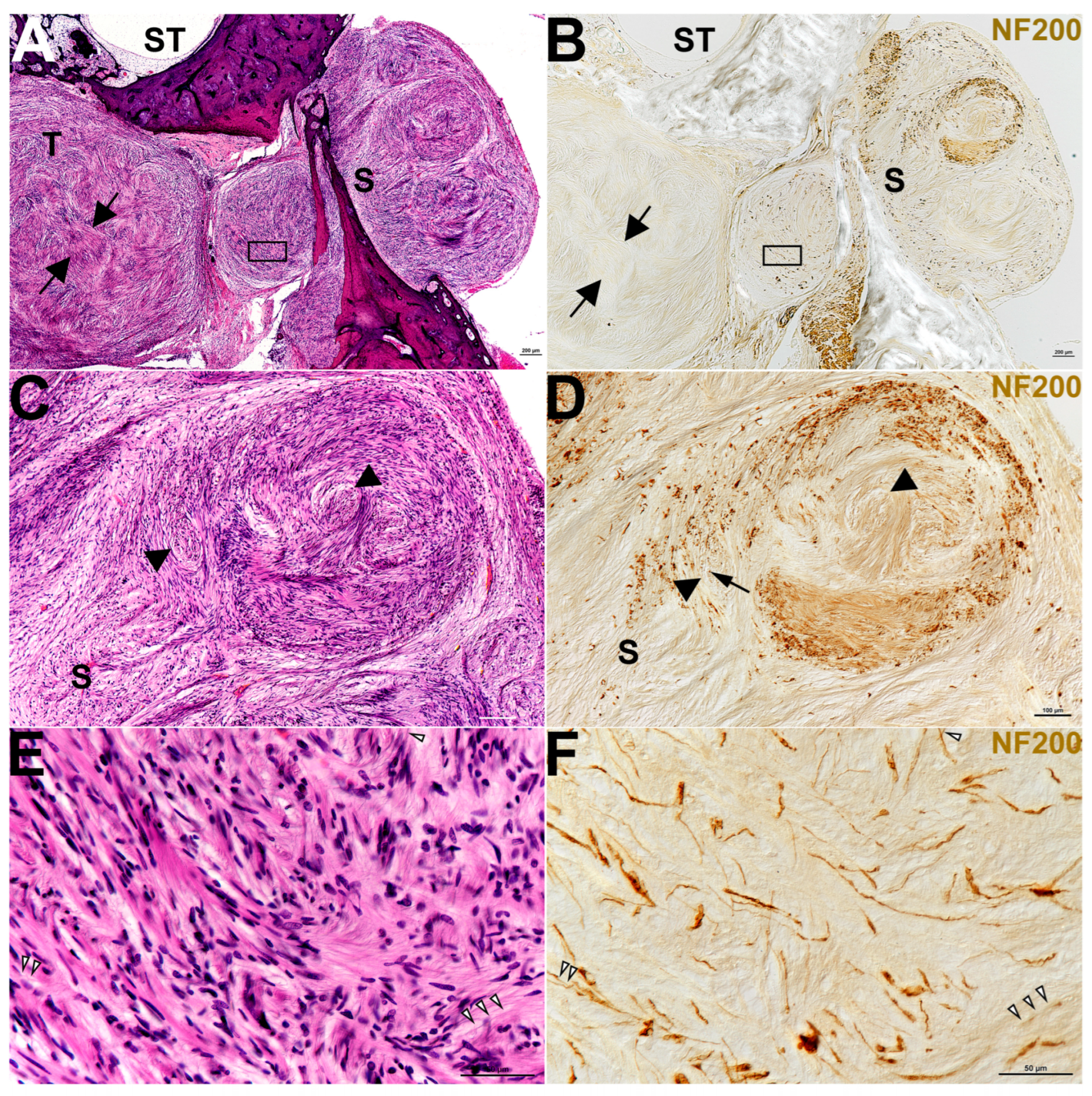
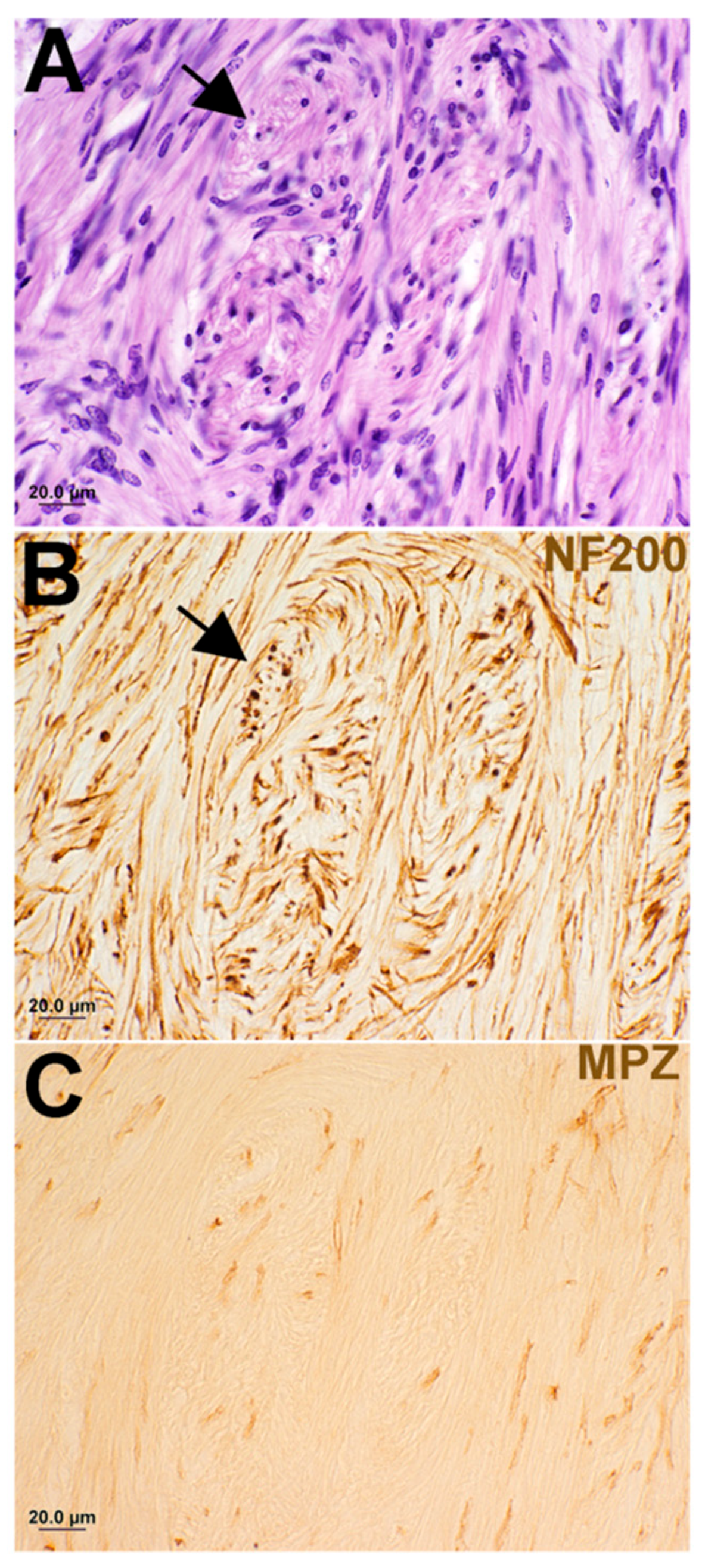
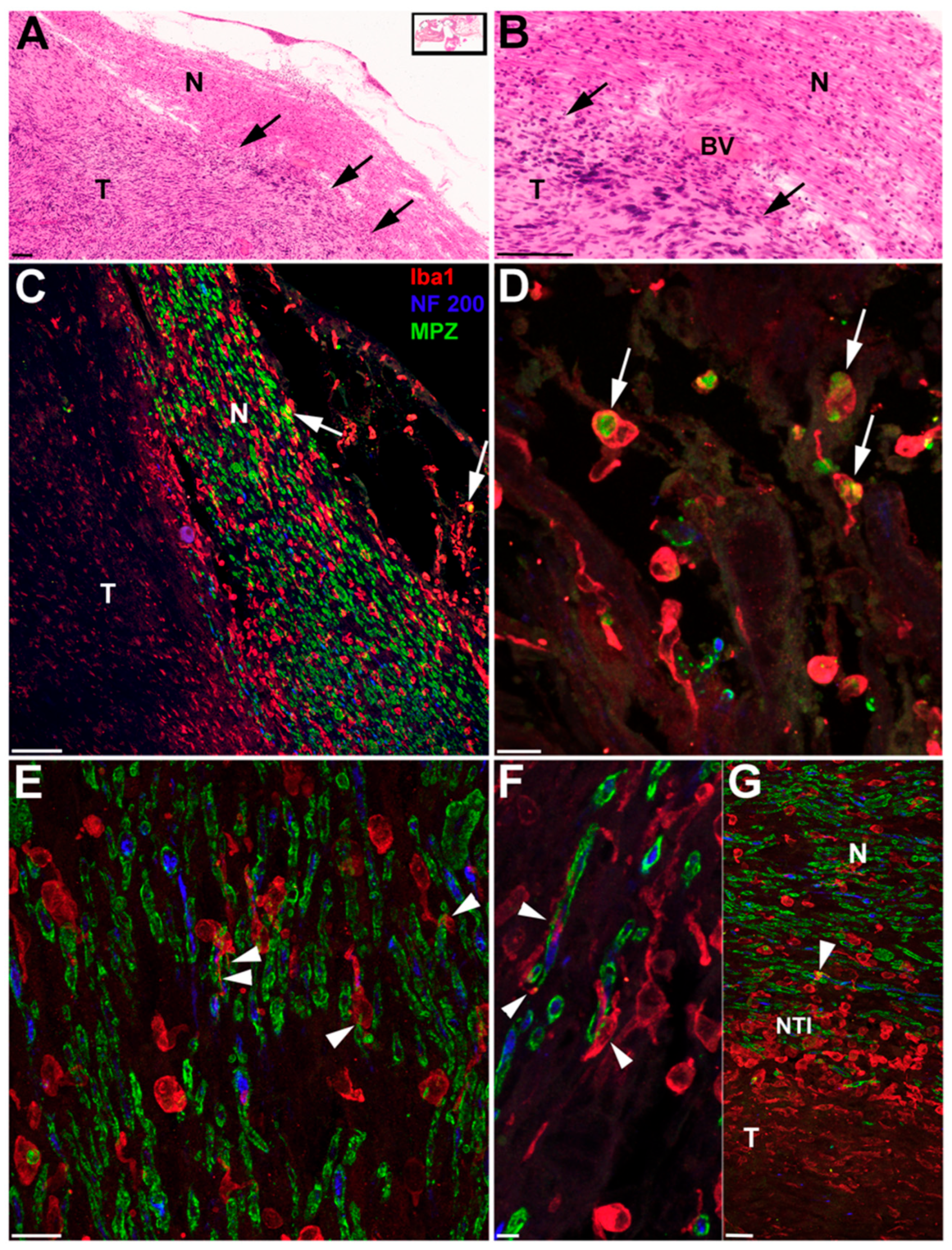
| ID # | Sex | Pattern of Involvement, Infiltrating (I)/ Pushing (p) | Tumor Type, Antoni A/ Antoni B | Tumor Size (mm) W × L × H | Occult/ Clinical | ABR | Speech Discrimination %, ear w/Tumor | Age at Death |
|---|---|---|---|---|---|---|---|---|
| 1 | F | I + p | A + B | 4.6 × 9.4 × 5.4 | Occult | NA | 80 | 74 |
| 2 | M | I + p | A + B | 10.0 × 11.0 × 8.0 | Clinical | Prolonged latencies waves I-III | 32 | 75 |
| 3 | F | I + p | A + B | 1.0 × 2.1 × 2.8 | Occult | NA | 56 | 94 |
| 4 | M | I + p | A + B | 4.9 × 6.7 × 8.0 | Occult | NA | NA | 81 |
| 5 | M | I + p | A + B | 5.3 × 8.4 × 6.6 | Occult | NA | NA | 74 |
| 6 | F | I + p | A + B | 15.0 × 17.0 × 7.0 | Clinical | NA | 0 | 100 |
| 7 | M | I + p | A + B | 1.0 × 1.1 × 1.0 | Occult | NA | 48 | 71 |
| 8 | F | I + p | A + B | 0.5 × 0.9 × 1.0 | Occult | NA | 100 | 74 |
| 9 | M | I + p | A + B | 1.2 × 1.9 × 1.2 | Occult | NA | 56 | 84 |
| 10 | M | I + p | A + B | 1.6 × 1.6 × 2.4 | Occult | NA | NA | 49 |
| 11 | F | I + p | A + B | 2.5 diameter | Occult | NA | 28 | 95 |
| 12 | F | I + p | A + B | Extensive | Clinical | Retro cochlear indicated | 56 | 43 |
| 13 | M | I + p | A + B | Extensive | Clinical | NA | 76 | 70 |
| 14 | M | I + p | B | 3.0 × 2.85 × 1.2 | Occult | NA | NA | 86 |
| 15 | F | I + p | A + B | 2.2 × 2.1 × 2.2 | Occult | NA | NA | 91 |
| 16 | M | I | Mostly B | 0.3 × 0.3 × 1.0 | Occult | NA | NA | 82 |
| 17 | F | I | B | 0.4 × 0.2 × 1.0 | Occult | NA | NA | 90 |
| 18 | M | I + p | A + B | 4.6 × 6.0 × 4.6 | Occult | NA | NA | 82 |
| 19 | F | I + p | A + B | 1.3 × 1.2 × 1.8 | Occult | NA | NA | 99 |
| 20 | M | I + p | A + B | 3.0 × 9.0 | NA | NA | NA | 44 |
| 21 | M | I + p | A + B | 6.5 × 14.0 | NA | NA | NA | 76 |
| 22 | M | I + p | A + B | 1.8 × 2.7 | NA | NA | NA | 67 |
| 23 | M | I + p | A + B | 1.8 × 5.6 | NA | NA | NA | 81 |
| 24 | M | I + p | A + B | 2.5 × 3.2 | NA | NA | NA | 68 |
| 25 | M | I + p | A + B | 5.0 × 4.0 | NA | NA | NA | 86 |
| 26 | M | I + p | A + B | 1.3 × 2.5 | NA | NA | NA | 77 |
| 27 | M | I + p | A + B | 2.6 × 5.2 | NA | NA | NA | 72 |
| 28 | M | I + p | A + B | 2.5 × 7.0 | NA | NA | NA | 72 |
| ID # | Slide #s with Positive Anti-NF Staining Within Tumor | Slide #s with Positive Anti-MPZ Staining Within Tumor | Slide #s with Evidence of Wallerian Degeneration Within Tumor |
|---|---|---|---|
| 1 | 122, 230 | 119, 223, 229 | 122, 230 |
| 2 | 102, 270, 352 | 103, 269, 353 | 102, 270 |
| 3 | 190, 320, 360 | 192, 322, 362 | 190, 360 |
| 4 | 40, 141, 303 | 151, 300 | 141, 303 |
| 5 | 170, 228 | 171, 231, 312 | 170 |
| 6 | 16, 150, 280 | 17, 149, 282 | 16, 150 |
| 7 | 112, 131, 149 | 113, 132, 151 | 112, 131 |
| 8 | 262, 283, 300 | 263, 284, 302 | 262, 283, 300 |
| 9 | 89, 109, 132 | 113, 134 | 109, 132 |
| 10 | 212, 262, 312 | 263, 310 | 262, 312 |
| 11 | 152, 192, 239 | 153, 193, 240 | 152, 192 |
| 12 | 110, 240, 330 | 109, 239, 332 | 110, 240, 330 |
| 13 | 130, 221 | 132, 222 | 221 |
| 14 | 262 | 264 | 262 |
| 15 | 205 | 207 | 205 |
| 16 | 239, 249 | 240 | 249 |
| 17 | 205 | 204 | 205 |
| 18 | 72, 178, 242 | 73, 179, 243 | 178 |
| 19 | 179 | 182 | 179 |
| 20 | 78, 162, 242 | 169, 247 | 78, 162, 246 |
| 21 | 446, 553, 717 | 452, 558, 725 | 446, 553, 717 |
| 22 | 285, 313, 336 | 338 | 285, 313, 336 |
| 23 | 64, 126, 171 | 73, 181 | 64, 126, 171 |
| 24 | 377, 412, 452 | 380, 417, 456 | 377, 412, 452 |
| 25 | 453 | 328, 458, 526 | |
| 26 | 68, 117, 192 | 77, 128, 195 | 68, 117, 192 |
| 27 | 359, 444, 493 | 365, 455, 486 | 359, 444, 493 |
| 28 | 24, 127, 248 | 37, 132, 265 | 248 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Malley, J.T.; Stemmer-Rachamimov, A.O.; Cureoglu, S.; McKenna, M.J.; Welling, D.B.; Quesnel, A.M. Immunohistopathology of Cochleovestibular Schwannoma in Human Temporal Bone Specimens. Biology 2025, 14, 1540. https://doi.org/10.3390/biology14111540
O’Malley JT, Stemmer-Rachamimov AO, Cureoglu S, McKenna MJ, Welling DB, Quesnel AM. Immunohistopathology of Cochleovestibular Schwannoma in Human Temporal Bone Specimens. Biology. 2025; 14(11):1540. https://doi.org/10.3390/biology14111540
Chicago/Turabian StyleO’Malley, Jennifer T., Anat O. Stemmer-Rachamimov, Sebahattin Cureoglu, Michael J. McKenna, D. Bradley Welling, and Alicia M. Quesnel. 2025. "Immunohistopathology of Cochleovestibular Schwannoma in Human Temporal Bone Specimens" Biology 14, no. 11: 1540. https://doi.org/10.3390/biology14111540
APA StyleO’Malley, J. T., Stemmer-Rachamimov, A. O., Cureoglu, S., McKenna, M. J., Welling, D. B., & Quesnel, A. M. (2025). Immunohistopathology of Cochleovestibular Schwannoma in Human Temporal Bone Specimens. Biology, 14(11), 1540. https://doi.org/10.3390/biology14111540







