Aphid Nymphs Experiencing Diurnal Temperature Fluctuation Alter the Toxicity of Adults Depending on the Role of the Insecticide Temperature Coefficients
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
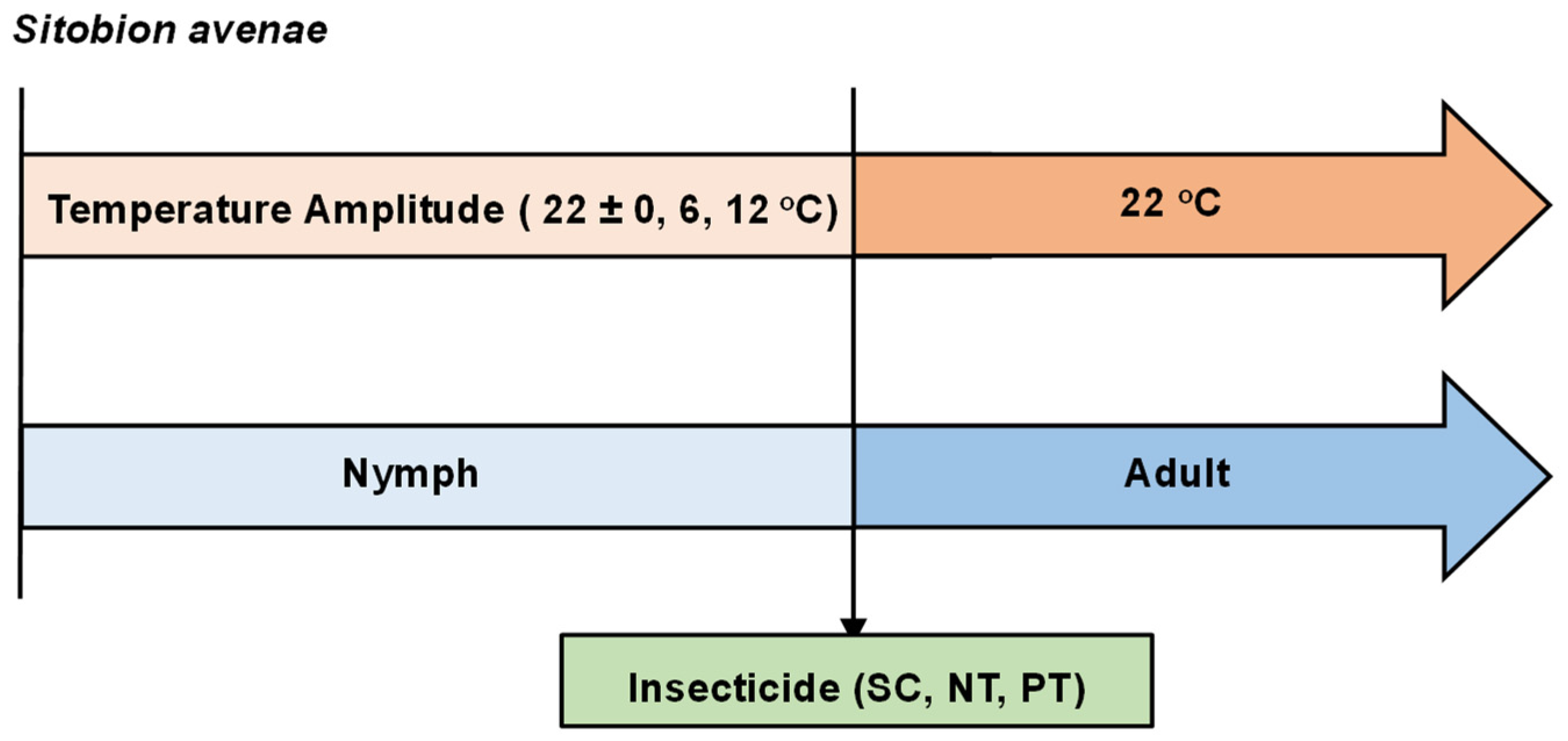
2.1. Insects
2.2. Temperature Design
2.3. Insecticide Concentration
2.4. Test Procedure
2.5. Statistical Analysis
3. Results
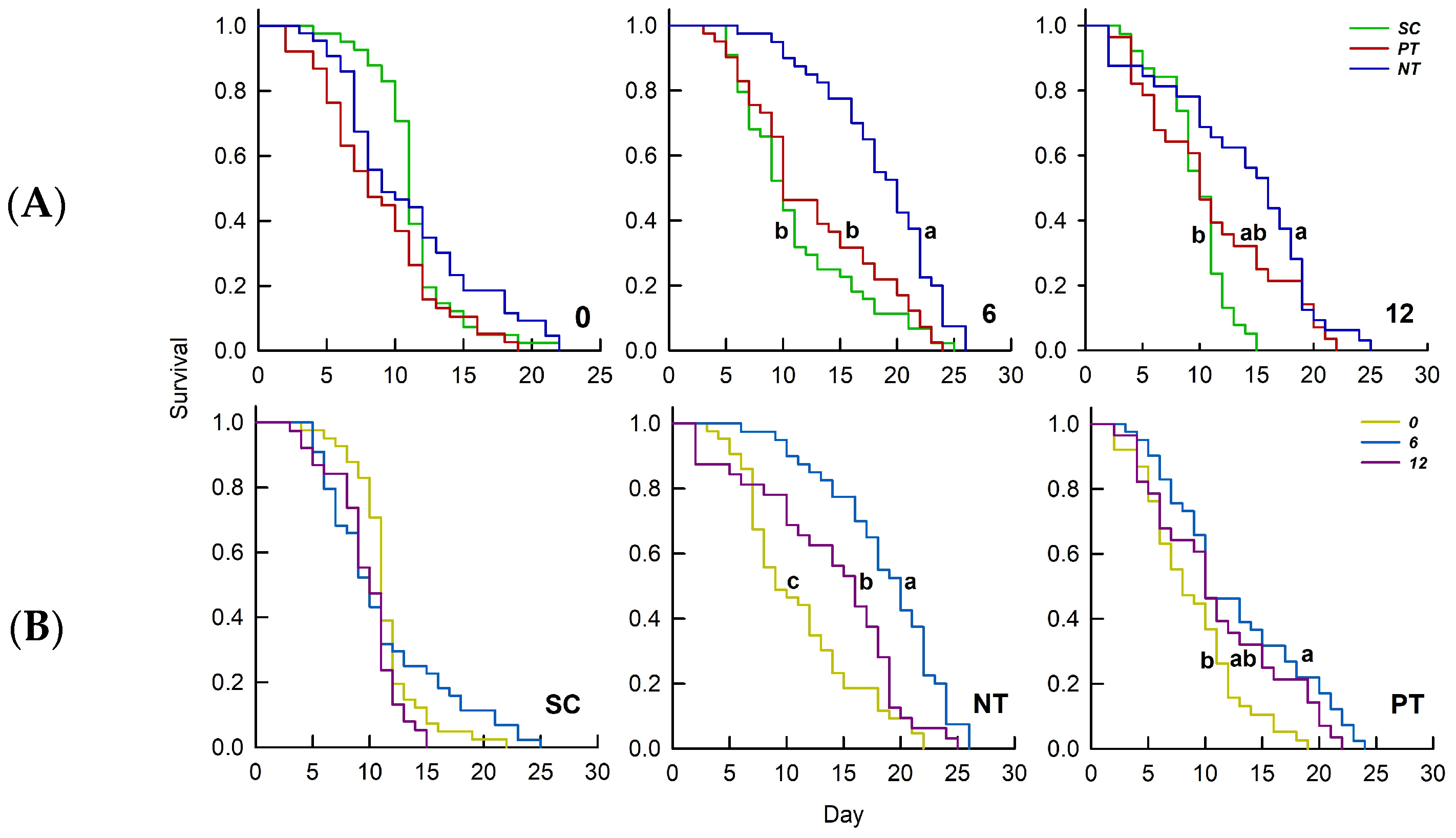
3.1. Effects of Temperature Amplitude and Insecticide on Survival
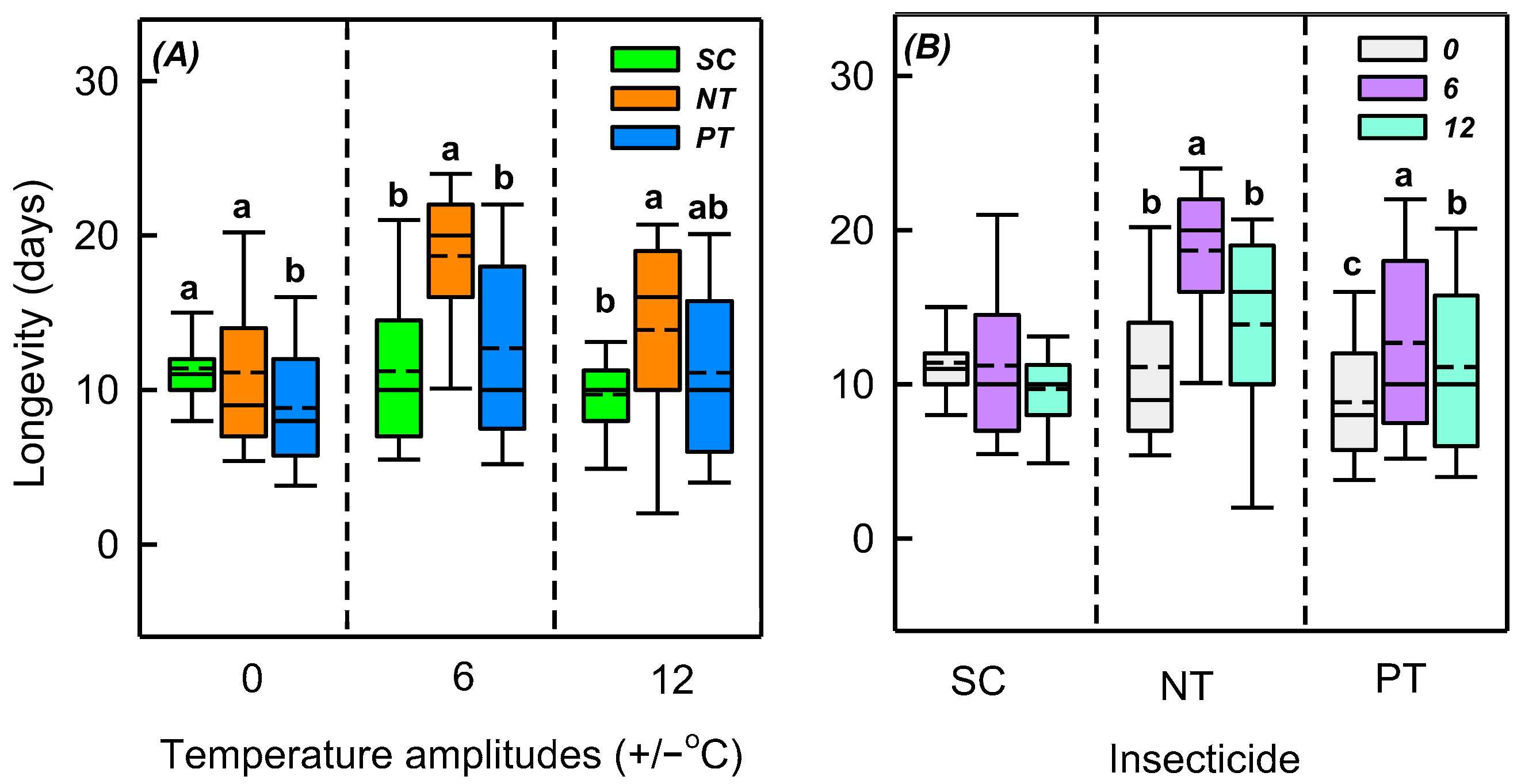
3.2. Effects of Temperature Amplitude and Insecticide on Longevity
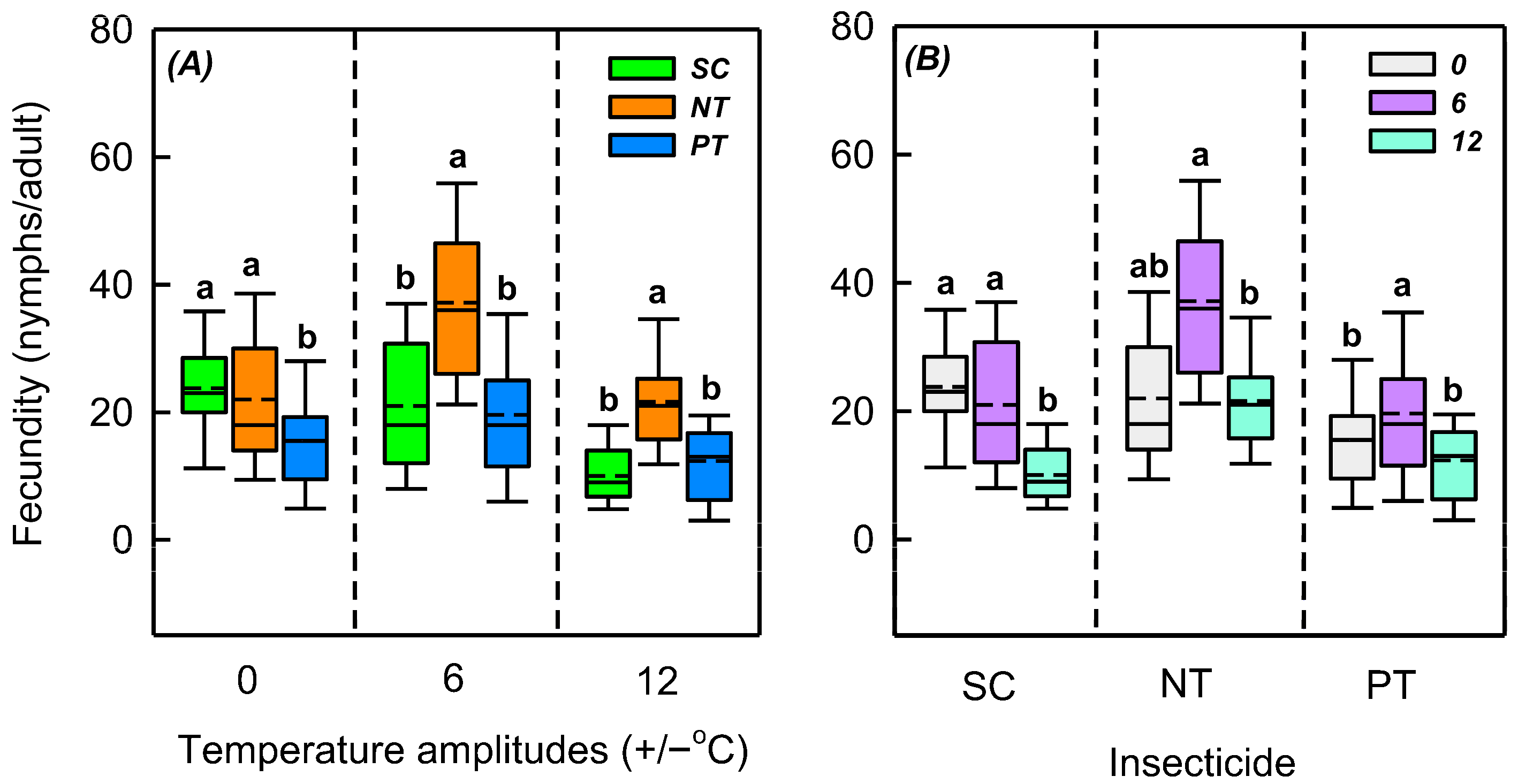
3.3. Effects of Temperature Amplitude and Insecticide on Fecundity
3.4. Effects of Temperature Amplitude and Insecticide on Early Fecundity
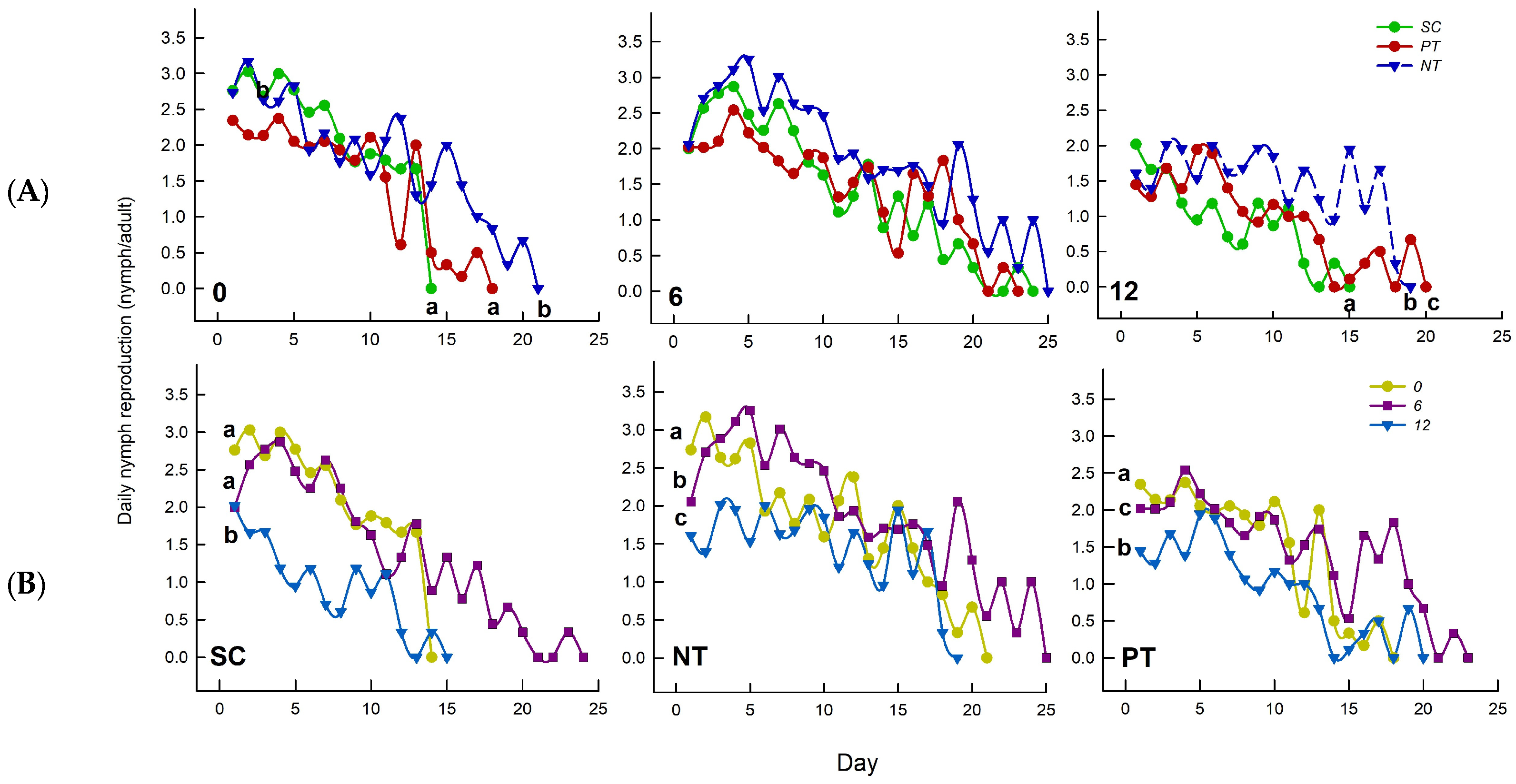
3.5. Effects of Temperature Amplitude and Insecticide on Daily Nymph Reproduction
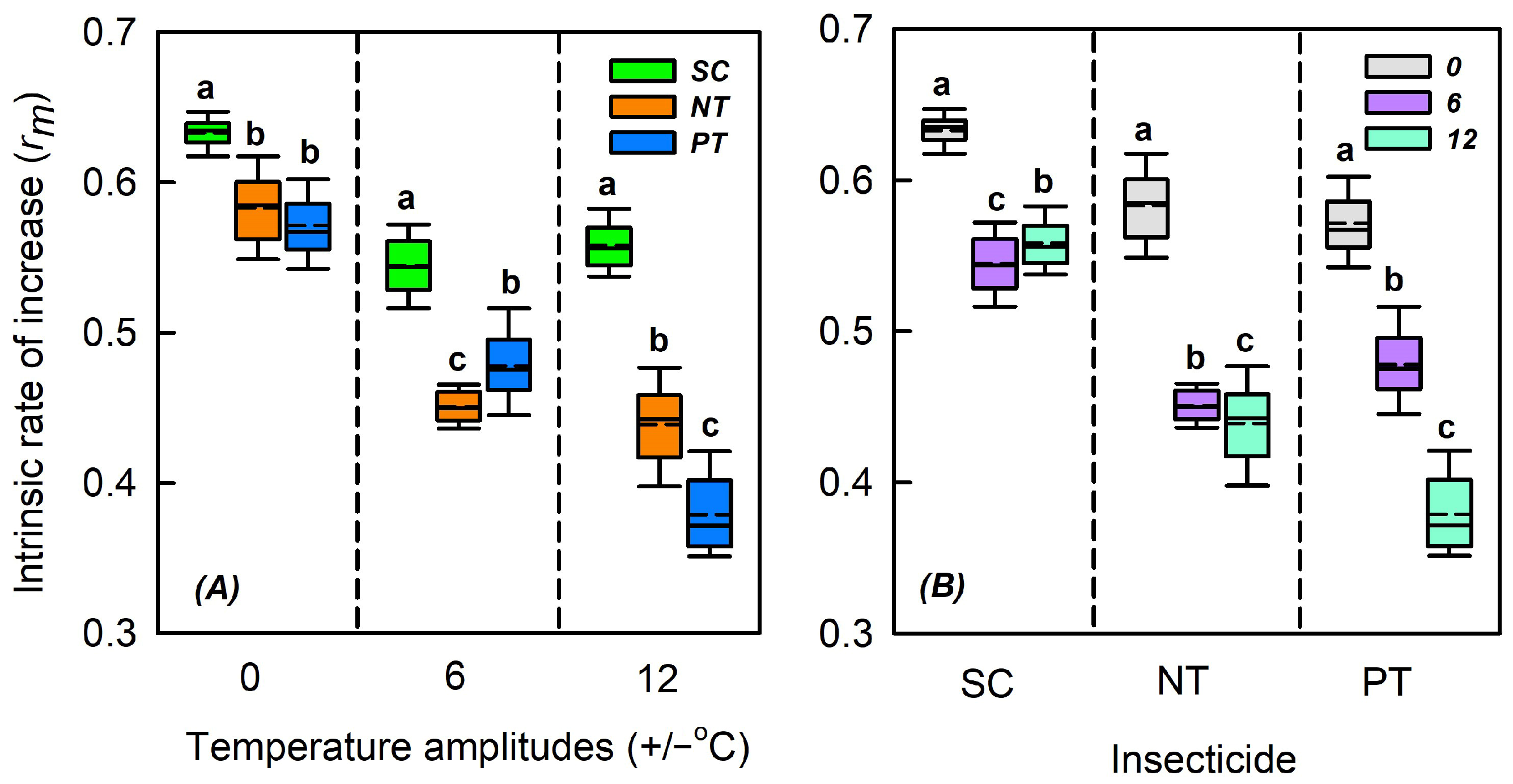
3.6. Effects of Temperature Amplitude and Insecticide on the Intrinsic Rate of Increase
4. Discussion
4.1. Effects of Insecticides
4.2. Effects of Temperature Amplitudes
4.3. Interaction Effects of Insecticides and Temperature Amplitudes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Körner, C.; Basler, D. Phenology under global warming. Science 2010, 327, 1461–1462. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Sesterhenn, T.M.; Stieha, C. Will climate change reduce the effects of a insecticide on amphibians? Partitioning effects on exposure and susceptibility to contaminants. Glob. Change Biol. 2011, 17, 657–666. [Google Scholar] [CrossRef]
- Stoks, R.; Tüzün, N. Editorial overview: Global change: Coping with the complexity of interacting stressors, interacting responses, and their feedback loops. Curr. Opin. Insect Sci. 2022, 53, 100949. [Google Scholar] [CrossRef]
- Noyes, P.D.; Lema, S.C. Forecasting the impacts of chemical pollution and climate change interactions on the health of wildlife. Curr. Zool. 2015, 61, 669–689. [Google Scholar] [CrossRef]
- Xing, K.; Mo, Z.F.; Xu, Z.P.; Wei, J.J.; Cheng, H.; Zhao, F. Multigenerational responses of wheat aphids to multiple stresses: Interactions between sublethal thermal stress types and a low insecticide dose. Entomol. Gen. 2024, 44, 553–562. [Google Scholar] [CrossRef]
- de Beeck, L.O.; Verheyen, J.; Stoks, R. Competition magnifies the impact of a pesticide in a warming world by reducing heat tolerance and increasing autotomy. Environ. Pollut. 2018, 233, 226–234. [Google Scholar] [CrossRef]
- Thompson, R.M.; Beardall, J.; Beringer, J.; Grace, M.; Sardina, P. Means and extremes: Building variability into community-level climate change experiments. Ecol. Lett. 2013, 16, 799–806. [Google Scholar] [CrossRef]
- Willming, M.M.; Qin, G.; Maul, J.D. Effects of environmentally realistic daily temperature variation on insecticide toxicity to aquatic invertebrates. Environ. Toxicol. Chem. 2013, 32, 2738–2745. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.Q.; Zhang, B.; Zhang, S.T.; Zheng, C.Y.; Wan, F.H. Combined effects of high temperature and acetamiprid on life table parameters of the F1 offspring of the treated Frankliniella occidentalis (Thysanoptera: Thripidae). Biol. Environ. Sci. 2015, 58, 767–775. [Google Scholar] [CrossRef]
- Delnat, V.; Tran, T.T.; Verheyen, J.; Dinh, K.V.; Janssens, L.; Stoks, R. Temperature variation magnifies chlorpyrifos toxicity differently between larval and adult mosquitoes. Sci. Total Environ. 2019, 690, 1237–1244. [Google Scholar] [CrossRef]
- Tran, T.T.; Janssens, L.; Dinh, K.V.; Stoks, R. An adaptive transgenerational effect of warming but not of insecticide exposure determines how a insecticide and warming interact for antipredator behaviour. Environ. Pollut. 2019, 245, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Delnat, V.; Verborgt, J.; Janssens, L.; Stoks, R. Daily temperature variation lowers the lethal and sublethal impact of a insecticide pulse due to a higher degradation rate. Chemosphere 2021, 263, 128114. [Google Scholar] [CrossRef] [PubMed]
- Janssens, L.; Tüzün, N.; Stoks, R. Testing the time-scale dependence of delay interactions: A heat wave during the egg stage shapes how a insecticide interacts with a successive heat wave in the larval stage. Environ. Pollut. 2017, 230, 351–359. [Google Scholar] [CrossRef]
- Cheng, J.; Huang, L.J.; Zhu, Z.F.; Jiang, L.B.; Ge, L.Q.; Wu, J.C. Heat-dependent fecundity enhancement observed in Nilaparvata lugens (Hemiptera: Delphacidae) after treatment with triazophos. Environ. Entomol. 2014, 43, 474–481. [Google Scholar] [CrossRef]
- Muturi, E.J.; Alto, B.W. Larval environmental temperature and insecticide exposure alter Aedes aegypti competence for arboviruses. Vector-Borne Zoonotic Dis. 2011, 11, 1157–1163. [Google Scholar] [CrossRef]
- Barbosa, M.; Inocentes, N.; Soares, A.M.V.M.; Oliveira, M. Synergy effects of fluoxetine and variability in temperature lead to proportionally greater fitness costs in Daphnia: A multigenerational test. Aquat. Toxicol. 2017, 193, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Miner, B.E.; Meester, L.D.; Pfrender, M.E.; Lampert, W.; Hairston, N.G. Linking genes to communities and ecosystems: Daphnia as an ecogenomic model. Proc. Biol. Sci. 2012, 279, 1873–1882. [Google Scholar] [CrossRef]
- Overgaard, J.; Hoffmann, A.A.; Kristensen, T.N. Assessing population and environmental effects on thermal resistance in Drosophila melanogaster using ecologically relevant assays. J. Therm. Biol. 2011, 36, 409–416. [Google Scholar] [CrossRef]
- Bozinovic, F.; Bastías, D.A.; Boher, F.; Clavijo-Baquet, S.; Estay, S.A.; Angilletta, M.J. The mean and variance of environmental temperature interact to determine physiological tolerance and fitness. Physiol. Biochem. Zool. 2011, 84, 543–552. [Google Scholar] [CrossRef]
- Stahlschmidt, Z.R.; Whitlock, J.; Vo, C.; Evalen, P.; Bui, D. Pesticides in a warmer world: Effects of glyphosate and warming across insect life stages. Environ. Pollut. 2022, 307, 119508. [Google Scholar] [CrossRef]
- Xing, K.; Zhang, S.M.; Jia, M.Q.; Zhao, F. Response of wheat aphid to insecticides is influenced by the interaction between temperature amplitudes and insecticide characteristics. Front. Physiol. 2023, 14, 1188917. [Google Scholar] [CrossRef] [PubMed]
- Xing, K.; Yan, Y.L.; Wei, N.; Zhao, F. The impact of diurnal temperature fluctuations and sublethal insecticide exposure on the life history traits and population parameters of the English grain aphid Sitobion miscanthi. J. Plant Prot. 2025, 52, 113–122. [Google Scholar] [CrossRef]
- George, K.S.; Gair, R. Crop loss assessment on winter wheat attacked by the grain aphid, Sitobion avenae (F.). Plant Pathol. 1979, 28, 143–149. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.A.; Liu, E.L.; Gao, H.F.; Zhang, Y.H.; Li, X.R. Research status of wheat aphid resistance to insecticides in China. J. Environ. Entomol. 2022, 44, 626–635. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, W.; Hoffmann, A.A.; Ma, C.S. Night warming on hot days produces novel impacts on development, survival and fecundity in a small arthropod. J. Anim. Ecol. 2014, 83, 769–778. [Google Scholar] [CrossRef]
- Xing, K.; Sun, D.B.; Zhang, J.; Zhao, F. Wide diurnal temperature amplitude and high population density can positively affect the life history of Sitobion avenae (Hemiptera: Aphididae). J. Insect Sci. 2021, 21, 6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, L.; Ma, C.S. Sporadic short temperature events cannot be neglected in predicting impacts of climate change on small insects. J. Insect Physiol. 2019, 112, 48–56. [Google Scholar] [CrossRef] [PubMed]
- James, D.G. A neonicotinoid insecticide at a rate found in nectar reduces longevity but not oogenesis in monarch butterflies, Danaus plexippus (L.). (Lepidoptera: Nymphalidae). Insects 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Saeed, N.; Tonina, L.; Battisti, A.; Mori, N. Temperature alters the response to insecticides in Drosophila suzukii (Diptera: Drosophilidae). J. Econ. Entomol. 2018, 111, 1306–1312. [Google Scholar] [CrossRef]
- Lu, Y.H.; Zheng, X.S.; Gao, X.W. Sublethal effects of imidacloprid on the fecundity, longevity, and enzyme activity of Sitobion avenae (Fabricius) and Rhopalosiphum padi (Linnaeus). Bull. Entomol. Res. 2016, 106, 551–559. [Google Scholar] [CrossRef]
- Harwood, A.D.; You, J.; Lydy, M.J. Temperature as a toxicity identification evaluation tool for pyrethroid insecticides: Toxicokinetic confirmation. Environ. Toxicol. Chem. 2009, 28, 1051–1058. [Google Scholar] [CrossRef]
- Potter, K.A.; Davidowitz, G.; Woods, H.A. Cross-stage consequences of egg temperature in the insect Manduca sexta. Funct. Ecol. 2011, 25, 548–556. [Google Scholar] [CrossRef]
- Metcalfe, N.B.; Monaghan, P. Compensation for a bad start: Grow now, pay later? Trends Ecol. Evol. 2001, 16, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.W.; Monaghan, J.R.; Smith, M.D.; Pasch, B.; Stier, A.C.; Michonneau, F. The influence of fundamental traits on mechanisms controlling appendage regeneration. Biol. Rev. Camb. Philos. Soc. 2012, 87, 330–345. [Google Scholar] [CrossRef]
- Xing, K.; Hoffmann, A.A.; Zhao, F.; Ma, C.S. Wide diurnal temperature variation inhibits larval development and adult fecundity in the diamondback moth. J. Therm. Biol. 2019, 84, 8–15. [Google Scholar] [CrossRef]
- Xing, K.; Zhang, S.M.; Zhao, F.; Zhang, J.Z. Study on physiological and ecological phenotypes of the wheat aphid, Sitobion avenae (Fabricius) and its correlation under temperature amplitudes. J. Environ. Entomol. 2022, 44, 367–375. [Google Scholar] [CrossRef]
- Asin, L.; Pons, X. Effect of high temperature on the growth and fecundity of corn aphids (Homoptera: Aphididae) and implications for their population dynamics on the northeastern Iberian peninsula. Environ. Entomol. 2001, 30, 1127–1134. [Google Scholar] [CrossRef]
- Mutamiswa, R.; Tarusikirwa, V.; Nyamukondiwa, C.; Chidawanyika, F. Fluctuating environments impact thermal tolerance in an invasive insect species Bactrocera dorsalis (Diptera: Tephritidae). J. Appl. Entomol. 2020, 144, 885–896. [Google Scholar] [CrossRef]
- Verheyen, J.; Stoks, R. Current and future daily temperature fluctuations make a insecticide more toxic: Contrasting effects on life history and physiology. Environ. Pollut. 2019, 248, 209–218. [Google Scholar] [CrossRef]
- Xing, K.; Cao, J.J.; Hao, B.; Zhao, F. Diurnal temperature amplitude affects diamondback moth adult and next-generation traits. J. Appl. Entomol. 2022, 147, 63–71. [Google Scholar] [CrossRef]
- Hoffmann, A.A.; Sørensen, J.G.; Loeschcke, V. Adaptation of Drosophila to temperature extremes: Bringing together quantitative and molecular approaches. J. Therm. Biol. 2003, 28, 175–216. [Google Scholar] [CrossRef]
- DiDomenico, B.J.; Bugaisky, G.E.; Lindquist, S. Heat shock and recovery are mediated by different translational mechanisms. Proc. Natl. Acad. Sci. USA 1982, 79, 6181–6185. [Google Scholar] [CrossRef] [PubMed]
- Liess, M.; Foit, K.; Knillmann, S.; Schäfer, R.B.; Liess, H.D. Predicting the synergy of multiple stress effects. Sci. Rep. 2016, 6, 32965. [Google Scholar] [CrossRef]
- Dixon, A.F.G. Aphid ecology: Life cycles, polymorphism, and population regulation. Ann. Rev. Eco. Syst. 1977, 8, 29–53. [Google Scholar] [CrossRef]
- Dean, G.J.W. Distribution of aphids in spring cereals. J. Appl. Ecol. 1973, 10, 447–462. [Google Scholar] [CrossRef]
- Acreman, S.J.; Dixon, A.F.G. The effects of temperature and host quality on the rate of increase of the grain aphid (Sitobion avenae) on wheat. Ann. Appl. Biol. 1989, 115, 3–9. [Google Scholar] [CrossRef]
- Verheyen, J.; Delnat, V.; Theys, C. Daily temperature fluctuations can magnify the toxicity of pesticides. Curr. Opin. Insect. Sci. 2022, 51, 100919. [Google Scholar] [CrossRef] [PubMed]
- Musser, F.R.; Shelton, A.M. The influence of post-exposure temperature on the toxicity of insecticides to Ostrinia nubilalis (Lepidoptera: Crambidae). Pest Manag. Sci. 2005, 61, 508–510. [Google Scholar] [CrossRef]
- Van den Brink, P.J.; Boxall, A.B.A.; Maltby, L.; Brooks, B.W.; Rudd, M.A.; Backhaus, T. Toward sustainable environmental quality: Priority research questions for Europe. Environ. Toxicol. Chem. 2018, 37, 2281–2295. [Google Scholar] [CrossRef]

| Trait | Source | Z | df | p |
|---|---|---|---|---|
| Survivorship | Temperature amplitudes (TA) | 5.226 | 2 | 0.073 |
| Insecticide treatments (IT) | 14.027 | 2 | <0.001 | |
| TA × IT | 14.563 | 4 | 0.006 | |
| Daily nymph reproduction | Temperature amplitudes (TA) | 46.738 | 2 | <0.001 |
| Insecticide treatments (IT) | 24.086 | 2 | <0.001 | |
| TA × IT | 1.209 | 4 | 0.342 |
| Trait | Source | χ2 | df | p |
|---|---|---|---|---|
| Longevity | Temperature amplitudes (TA) | 36.353 | 2 | <0.001 |
| Insecticide treatments (IT) | 42.078 | 2 | <0.001 | |
| TA × IT | 26.739 | 4 | <0.001 | |
| Fecundity | Temperature amplitudes (TA) | 68.830 | 2 | <0.001 |
| Insecticide treatments (IT) | 74.899 | 2 | <0.001 | |
| TA × IT | 42.621 | 4 | <0.001 | |
| Early fecundity | Temperature amplitudes (TA) | 15.362 | 2 | <0.001 |
| Insecticide treatments (IT) | 42.405 | 2 | <0.001 | |
| TA × IT | 40.831 | 4 | <0.001 | |
| Intrinsic rate of increase (rm) | Temperature amplitudes (TA) | 6015.801 | 2 | <0.001 |
| Insecticide treatments (IT) | 3597.393 | 2 | <0.001 | |
| TA × IT | 986.620 | 4 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Gao, B.; Cheng, X.; Liu, Y.-W.; Xing, K.; Zhao, F. Aphid Nymphs Experiencing Diurnal Temperature Fluctuation Alter the Toxicity of Adults Depending on the Role of the Insecticide Temperature Coefficients. Biology 2025, 14, 1543. https://doi.org/10.3390/biology14111543
Liu B, Gao B, Cheng X, Liu Y-W, Xing K, Zhao F. Aphid Nymphs Experiencing Diurnal Temperature Fluctuation Alter the Toxicity of Adults Depending on the Role of the Insecticide Temperature Coefficients. Biology. 2025; 14(11):1543. https://doi.org/10.3390/biology14111543
Chicago/Turabian StyleLiu, Biao, Bo Gao, Xu Cheng, Yun-Wei Liu, Kun Xing, and Fei Zhao. 2025. "Aphid Nymphs Experiencing Diurnal Temperature Fluctuation Alter the Toxicity of Adults Depending on the Role of the Insecticide Temperature Coefficients" Biology 14, no. 11: 1543. https://doi.org/10.3390/biology14111543
APA StyleLiu, B., Gao, B., Cheng, X., Liu, Y.-W., Xing, K., & Zhao, F. (2025). Aphid Nymphs Experiencing Diurnal Temperature Fluctuation Alter the Toxicity of Adults Depending on the Role of the Insecticide Temperature Coefficients. Biology, 14(11), 1543. https://doi.org/10.3390/biology14111543





