1. Introduction
The application of biotechnology to achieve clean utilization of fossil fuels has emerged as a highly promising strategy in the field of bioenergy [
1]. This strategy aims to depolymerize complex organic macromolecules through the synergistic action of enzyme systems derived from microbial communities. For example, microbial coal gasification relies on multifunctional enzyme systems, such as hydrolases and oxidoreductases, produced by anaerobic microbial communities to convert the organic matter in coal into methane. Compared to traditional physical and chemical gasification methods, microbial gasification operates under mild conditions, is environmentally friendly, and has low energy consumption, thereby offering a promising novel pathway for the clean and efficient utilization of coal resources. However, owing to the highly cross-linked aromatic structures and abundant ether and C–C bonds in coal macromolecules, microorganisms often face issues, such as low substrate accessibility and limited catalytic efficiency. This results in low methane production rates that fail to meet industrial requirements.
Microbial coal gasification is inherently dependent on anaerobic microbial metabolism. The efficiency of this process is highly dependent on the activity of the functional microbial communities. Microorganisms are the core driving unit for the conversion of coal organic matter into gaseous fuels. Moreover, microorganisms are extremely sensitive to environmental conditions and are susceptible to factors, such as pH, temperature, nutrient concentration, redox potential, and the availability of trace elements. Therefore, optimizing and modifying coal properties may improve the efficiency of microbial coal gasification, thereby improving the reaction environment and enhancing functional microorganisms. Suitable strategies include increasing the contact area between coal and microorganisms via mechanical grinding and hydraulic fracturing [
2,
3], treating coal samples with oxidizing agents or surfactants to increase coal solubility [
4,
5], applying an electric field to the anaerobic fermentation system to improve electron transfer efficiency [
6,
7], and pretreating the degradation substrate using bacteria or fungi to enhance coal degradation efficiency [
8,
9]. Among these strategies, co-degradation technology has improved the stability of anaerobic degradation systems. Additives have a simpler structure than coal. Through anaerobic degradation, they dynamically regulate and stabilize the material exchange process within the reaction system. This reduces the environmental inhibition of microorganisms during the reaction. This inhibition is essentially caused by two factors: environmental conditions deviate from the microbial optimal metabolic range; and harmful substances accumulate that damage cell structures, inhibit enzymes, and interfere with metabolic pathways [
9,
10,
11]. This reduces community function and lowers methane yields.
Lignocellulosic biomass is abundantly available in nature and is an excellent renewable energy source. The biomass is categorized based on origin into agricultural waste (e.g., straw and grain husks), forestry residues (e.g., branches and sawdust), and industrial byproducts (e.g., pulp waste liquid and flax). However, the heterogeneous structure and recalcitrant bonds of lignocellulosic biomass severely hinder its industrial application [
12]. As one of the most abundant wastes produced annually in most developing countries, traditional disposal methods for lignocellulosic biomass include uncontrolled dumping, burning, composting, and landfilling [
13]. However, all these methods have negative environmental impacts, including greenhouse gas emissions, air pollution, and nutrient loss. Therefore, a rapid, efficient, and sustainable conversion process is crucial for reducing energy consumption and achieving sustainable development of natural resources. Lignocellulosic biomass is pretreated through physical, chemical, and biological pathways [
14,
15], such as crushing, organic solvents, high-temperature heating, and microbial pretreatment, to render it more amenable to hydrolysis. Furthermore, increasing attention has been paid to the use of hydrogen, methane, and numerous value-added chemicals derived from the depolymerization process [
16,
17].
Although previous research has shown that co-degrading lignocellulosic biomass like straw with coal can increase methane production [
18,
19,
20], few studies have examined coal biodegradation with sawdust—a forestry waste material rich in lignin and structurally similar to coal. On this basis, fewer have focused on microbial community changes and system stability. Unlike straw, sawdust contains more aromatic polymers and is harder to break down. This likely shifts microbial metabolic pathways and encourages the growth of species specialized in decomposing lignin. It is worth noting that sawdust acts as an excellent carbon source for microorganisms while also stabilizing fermentation pH, which helps maintain microbial activity [
21].
This study aims to fill this gap by examining how coal and sawdust together improve methane production under anaerobic conditions. We focus on how sawdust serves as both a carbon source and a buffer, and we optimize key process conditions to increase methane yield. Our work provides new ideas for converting hard-to-degrade carbon sources and supports a sustainable strategy for more efficient coal bioconversion.
4. Discussion
From the perspective of coal stoichiometry, the lack of hydrogen element limits the formation of biogenic coalbed methane. Biomass is rich in hydrogen element. Guo et al. [
26] found that the methane yield from co-fermentation of rice straw, sweet sorghum straw, wheat straw, and corn straw with coal was higher than that from fermentation of any single material. By comparing hydrogen production in co-fermentation and single fermentation, it was suggested that the increase in methane production may be attributed to enhanced biodegradation of coal rather than the supply of additional hydrogen. Guo et al. [
27] investigated the effects of different types of waste kitchen oil on biomethane production from lignite. Compared with anaerobic fermentation using lignite alone, the cumulative methane production from mixed anaerobic fermentation of waste kitchen oil and lignite increased by 377.86%. This was attributed to the fact that waste kitchen oil increased the content of volatile fatty acids associated with biomethane production. In another study, Guo et al. [
28] investigated how coal slime as an additive influences methane production. The aim was to improve methane yield from anaerobic digestion of chicken manure. The results showed that adding a suitable amount of coal slime increased the gene abundance of key enzymes involved in methane metabolism. This enhanced the initiation of fermentation. Based on the above studies, it has been demonstrated that co-fermentation of coal with other organic materials can enhance methane production. However, systematic studies on how organic matter enhances methane production from coal conversion remain limited.
This study investigated the effectiveness of co-fermentation of coal with sawdust. Compared to degradation of coal alone, the mixed degradation of coal and sawdust exhibited higher efficiency. Previous studies have indicated that co-digestion of lignocellulose-rich components (such as straw) with coal can increase the H/C ratio in the degradation substrate [
3,
29], thereby promoting biomethane production. However, in this study, methane production did not show a simple linear relationship with the amount of sawdust added, which is speculated to be related to the composition of the degradation matrix. The generally accepted optimal C/N ratio for anaerobic fermentation ranges from 20 to 30 [
30,
31]. Within this range, sufficient nitrogen source enables microorganisms to synthesize adequate enzymes and proteins, supporting their growth and metabolic activities and ensuring effective system degradation [
32,
33]. However, the addition of sawdust increased the C/N ratio beyond this range, which may lead to the accumulation of VFA, subsequently causing acidification [
34], impairing microbial cell function and unbalancing the anaerobic fermentation system [
35]. Surprisingly, in this study, compared with coal-alone degradation, the 4:1 group showed a significant increase in methane yield, up to 7.97 times higher (
Figure 1A). This may be because sawdust are easily degraded by microorganisms, providing immediate energy and carbon sources, which stimulated microbial activity and enabled timely degradation of the accumulated organic acids—resulting from the increased C/N ratio—into methane, thereby reducing acid inhibition. Nevertheless, although sawdust have a high H/C ratio, their individual degradation yielded very little methane, which may be related to the structure, polymerization degree, surface functional groups, pore volume, and specific surface area of lignocellulose [
36,
37].
Untargeted metabolomics was employed to investigate the changes in metabolites during coal degradation. The fermentation broth contained substantial amounts of soluble organic carbon, and the introduction of sawdust promoted the degradation of organic matter in the coal. This altered the dissolved organic matter in the fermentation broth (
Figure 2). Among these metabolites, alkanes, long-chain fatty acids, phenols, and low-molecular-weight aromatic hydrocarbons are important reactant [
38,
39]. This study focused on quantitative analysis of the characteristic organic compounds during the reaction. When sawdust was added, alcohols, phenols, aldehydes, and ketones exhibited systematic variations. During co-fermentation, the organic compounds in the broth became less diverse and structurally simpler, while the proportion of hydrocarbons increased. This implied that the addition of sawdust facilitated the conversion of organic matter into methane precursors. Under anaerobic conditions, microorganisms break down aromatic rings via reductase and other electron acceptors to obtain energy from carbon. [
36]. However, as the coal rank increases, the proportion of conjugated aromatic rings per structural unit in the coal matrix increases, leading to a decreased degradation rate of polycyclic aromatic hydrocarbons [
40,
41]. Lignin-derived aromatic compounds serve as carbon and energy sources for microorganisms. Furthermore, they may enhance the efficiency of converting lignin into value-added chemical products. During this process, benzene rings are cleaved to form monoaromatic and aliphatic compounds [
12,
42], which is consistent with the findings of this study. Elevated levels of ethylbenzene and xylene were detected in the mixed groups. This result indicates that adding sawdust accelerated the ring-opening and cleavage processes of aromatic hydrocarbons. Furthermore, fatty acids, lipids, and organic acids, which are key products of microbial fermentation and metabolic activity [
43,
44], were found at lower concentrations in the mixed groups than in the control groups. This reduction was most pronounced at a coal-to-sawdust ratio of 4:1. The observed differences in key enzyme activities likely resulted from the varying substrates. This difference enabled the mixed groups to utilize more target products via synergistic metabolism. In contrast, the experimental group accumulated metabolites due to a more restricted metabolic pathway.
In this study, the original sawdust and the solid products from the MX group served as the control. These were compared against the solid products from the experimental groups (MT and MM). The comparison revealed a significant decrease in the relative lignocellulose content after the reaction. Cellulose, hemicellulose, and lignin in sawdust are insoluble in water, diluted acids, and alkaline solutions at room temperature [
45]. This confirms that the consumption of lignocellulose in this experiment was related to anaerobic degradation (
Figure 3A). Sawdust addition introduced readily degradable organic matter. This organic matter stimulated the proliferation of functional microorganisms. These microorganisms are capable of producing cellulases and hemicellulases. To some extent, this alleviated the potential inhibitory effect of coal on microorganisms and created a suitable microenvironment for hydrolytic bacteria, such as
Lentimicrobium and
Desulfovibrio. After anaerobic degradation, the calorific value of the solid residues decreased to varying degrees compared with that of the original samples (
Figure 3B). This is due to the conversion of chemical energy in the degradation substrate via microbial metabolism into biological energy, chemical energy in gaseous products, and heat energy lost during energy conversion. Thus, the ratio of the calorific value of the degraded substrate to that of the products reflects the efficiency of microbial substrate utilization during degradation. The experimental data indicate that the addition of sawdust improves the conversion rate of the degradation substrate.
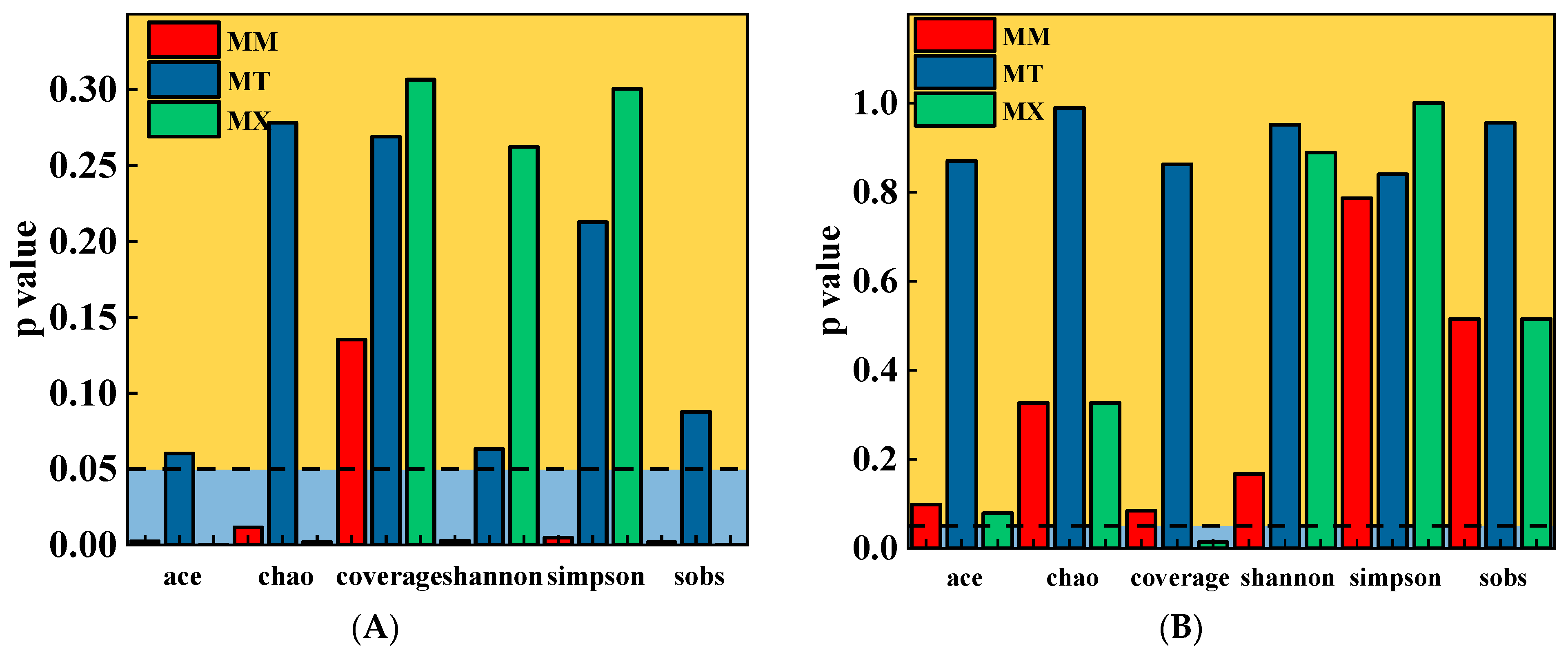
To some extent, changes in the microbial community structure corresponded with variations in the organic components in the fermentation broth; however, the response patterns of richness and diversity differed. In this study, the α-diversity indices of archaeal communities did not differ substantially among the experimental groups (
Table 5). This result indicated that coal-sawdust co-fermentation did not significantly alter archaeal species richness or community complexity. This stability suggests strong metabolic specialization and niche conservatism in the archaeal community. The addition of sawdust promoted the metabolism of certain functional bacteria, leading to increased bacterial richness and diversity in the MM group. However, as the woodchip content increased, the differences in community parameters between the MT and MX groups and the KB group decreased (
Table 4). This may be because excessively high proportions of sawdust caused the fermentation system to shift toward a “single-carbon source degradation” state. This resulted in functional redundancy, intensified intercommunity competition, a higher proportion of dominant species, and gradual simplification of the fermentation system, which was similar to the observations during pure coal degradation. This demonstrates that bacterial communities respond more sensitively to changes in the fermentation environment.
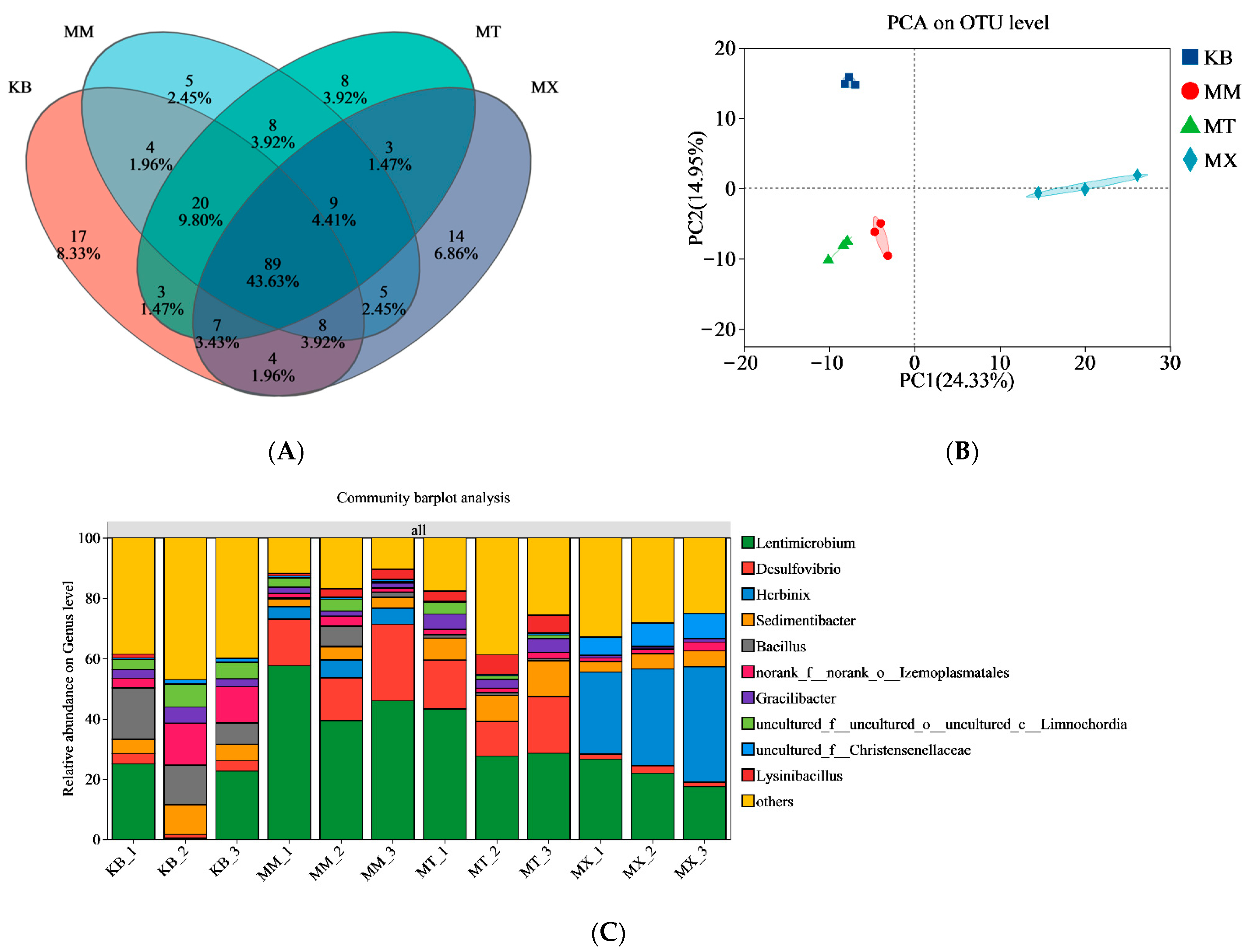
Changes in the substrate altered the niche partitioning of the bacterial taxa (
Figure 5). In the KB group, the bacterial community was complex, evenly distributed, and lacked dominant species, which could be attributed to the recalcitrant nature of coal [
46]. In contrast, as the proportion of sawdust in the substrate increased, functional microorganisms, particularly
Bacillus and
Clostridium, and thermophilic cellulose-degrading bacteria gradually became dominant in the fermentation system. These genera are closely associated with the formation of hydrogen and acetate [
47]. An increase in dominant genera improved the degradation efficiency of aromatic hydrocarbons and cellulose. Furthermore,
Desulfovibrio, which was present in the fermentation broth, often forms syntrophic relationships with methanogens. They oxidize carbohydrates and small-molecule organic acids via carboxylate transporter systems. This activity plays a key role in coal organic matter degradation and biomethane production [
48,
49].
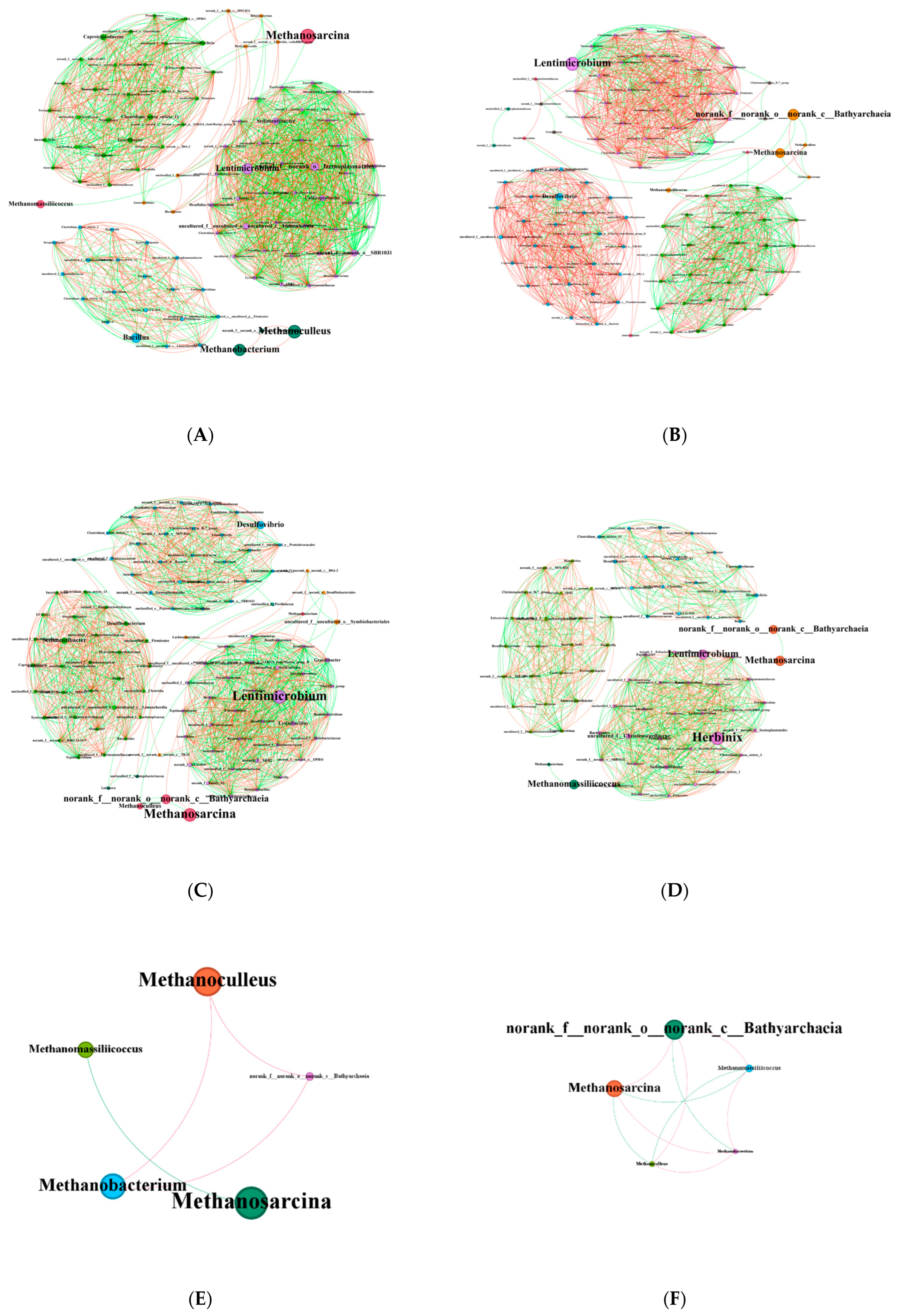
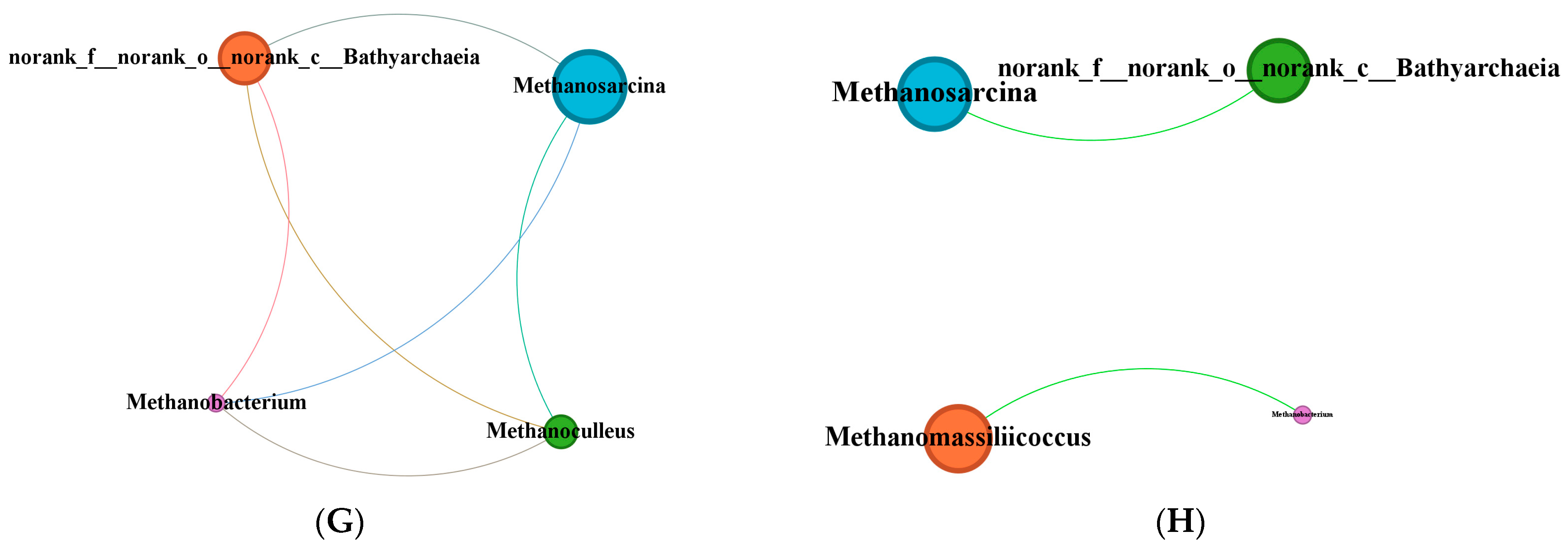
The KB group exhibited distinct differences from the other groups, whereas the addition of sawdust markedly weakened this specific selection pressure on the core archaeal taxa (
Figure 6), leading to more consistent community structures across the co-fermentation groups. This suggests that coal exerts a stronger selective pressure on archaea, whereas sawdust act as a buffer that mitigates this pressure and promotes niche release for certain taxa. The inclusion of sawdust increased the relative abundance of unclassified genera in the Bathyarchaeia. This group is capable of homoacetogenesis in the absence of methyl-coenzyme M reductase and links sulfur redox reactions to fermentation processes, thus indicating a potential syntrophic relationship with methylotrophic methanogens [
50,
51]. This aligns with the observed abundance of
Desulfovibrio and
Methanosarcina in this study. The relative abundance of
Methanosarcina remained consistent across groups, which may be attributed to its high metabolic versatility [
52]. Other archaea, such as the hydrogenotrophic
Methanoculleus and methylotrophic
Methanomassiliicoccus [
53], exhibited distinct distribution patterns. This indicates that varying coal-to-sawdust ratios may selectively enrich specific functional archaeal genera by altering substrate degradability.
The biological communities within the reactors exhibited clear niche-specific characteristics (
Figure 7). Analysis of the bacterial co-occurrence network revealed a compact “small-world” architecture with high microbial activity and strong interactions. A high average degree indicates strong co-occurrence relationships among microbial taxa during anaerobic degradation. As the proportion of sawdust in the substrate decreased, the community integration increased, with more complex and frequent species interactions. This likely stems from the recalcitrance and structural complexity of coal, which necessitates more intricate degradation pathways than those of sawdust. A high average eigenvector centrality indicates the presence of influential “core” species. These species form a hierarchical structure, which corresponds to the multi-stage reactions in anaerobic degradation. The bacterial communities in the experimental groups were more dependent than those in the control group on core species. This may imply reduced robustness but could also enable more efficient signal transduction. High graph density indicates tight internal connections and strong species interdependence. However, the highly integrated bacterial communities did not increase methane production. This may be attributed to the highly heterogeneous structure of coal, the non-stoichiometric nature of coal degradation reactions, and enzyme non-specificity. Although coal provides diverse degradation pathways, the concentration of bioavailable organic compounds in the liquid environment remains low. This limitation amplifies both functional redundancy and resource competition among microorganisms. Consequently, the overall efficiency of the system is reduced. In contrast, sawdust have a simpler structure and are more easily degraded, thereby releasing nutrients into the liquid environment. This explains why the graph density in the coal-containing groups was inversely correlated with sawdust content. Owing to substrate homogeneity, the MX group exhibited simpler degradation pathways, tighter bacterial connections, and higher graph densities than those of the coal-containing groups. Analysis of the archaeal communities indicated low diversity and high cohesion, with strong interactions among the archaeal genera. The addition of sawdust stimulated the emergence of functional archaea. This enhanced network complexity and stability while altering the dominant archaeal groups in the fermentation broth. Consequently, methanogenic pathways within the system were modified. These findings provide ecological insights into the assembly and functional dynamics of microbial communities in extreme environments.
This study investigated the enhancement of microbial methane production from low-rank coal through anaerobic co-degradation with sawdust. First, the experiments were carried out in small-scale batch reactors, so it remains uncertain whether the results would apply to continuous or large-scale operations. Second, the reliable supply of sawdust, its cost-effectiveness, and practical compatibility in real industrial settings need further assessment. Additionally, potential environmental effects—such as by-products and wastewater generated during co-degradation—are still not well understood, highlighting the need for broader sustainability analysis in future studies. Future work should focus on pilot-scale validation, optimization of substrate matching, and comprehensive energy-environment benefit evaluations to facilitate the transition of this technology toward industrial application.