Using Citizen Science and Field Surveys to Document the Introduction, Establishment, and Rapid Spread of the Bare-Eyed Pigeon, Patagioenas corensis, on the Island of Saint-Martin, West Indies
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Study Species
2.3. Data Collection
2.4. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Habitat | PC1 | PC2 |
|---|---|---|
| Tree cover | 0.657 | −0.277 |
| Open habitat | 0.427 | 0.895 |
| Wetland habitat | −0.994 | −0.103 |
| Anthropomorphized habitat | 0.595 | −0.508 |
| Eigenvalues | 1.956 | 1.146 |
| Cumulative var (%) | 48.894 | 28.656 |
References
- Clavero, M.; Brotons, L.; Pons, P.; Sol, D. Prominent role of invasive species in avian biodiversity loss. Biol. Conserv. 2009, 142, 2043–2049. [Google Scholar] [CrossRef]
- Blackburn, T.M.; Delean, S.; Pyšek, P.; Cassey, P. On the island biogeography of aliens: A global analysis of the richness of plant and bird species on oceanic islands. Glob. Ecol. Biogeogr. 2016, 25, 859–868. [Google Scholar] [CrossRef]
- Bellard, C.; Rysman, J.-F.; Leroy, B.; Claud, C.; Mace, G.M. A global picture of biological invasion threat on islands. Nat. Ecol. Evol. 2017, 1, 1862–1869. [Google Scholar] [CrossRef] [PubMed]
- Soares, F.C.; Panisi, M.; Sampaio, H.; Soares, E.; Santana, A.; Buchanan, G.M.; Leal, A.I.; Palmeirim, J.M.; de Lima, R.F. Land-use intensification promotes non-native species in a tropical island bird assemblage. Anim. Conserv. 2020, 23, 573–584. [Google Scholar] [CrossRef]
- Naimi, B.; Capinha, C.; Ribeiro, J.; Rahbek, C.; Strubbe, D.; Reino, L.; Araújo, M.B. Potential for invasion of traded birds under climate and land-cover change. Glob. Chang. Biol. 2022, 28, 5654–5666. [Google Scholar] [CrossRef] [PubMed]
- Diagne, C.; Leroy, B.; Vaissière, A.-C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.-M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Zenni, R.D.; Essl, F.; García-Berthou, E.; McDermott, S.M. The economic costs of biological invasions around the world. NeoBiota 2021, 67, 1–9. [Google Scholar] [CrossRef]
- Soto, I.; Balzani, P.; Oficialdegui, F.J.; Molinero, C.; Kouba, A.; Ahmed, D.A.; Turbelin, A.J.; Hudgins, E.J.; Bodey, T.W.; Gojery, S.A.; et al. The wild cost of invasive feral animals worldwide. Sci. Total Environ. 2024, 912, 169281. [Google Scholar] [CrossRef] [PubMed]
- Lenzner, B.; Latombe, G.; Capinha, C.; Bellard, C.; Courchamp, F.; Diagne, C.; Dullinger, S.; Golivets, M.; Irl, S.D.H.; Kühn, I. What will the future bring for biological invasions on islands? An expert-based assessment. Front. Ecol. Evol. 2020, 8, 280. [Google Scholar] [CrossRef]
- Matos, J.; Little, A.; Broome, K.; Kennedy, E.; Méndez-Sánchez, F.; Latofski-Robles, M.; Irvine, R.; Gill, C.; Espinoza, A.; Howald, G.; et al. Connecting island communities on a global scale: Case studies in island biosecurity. West. N. Am. Nat. 2018, 78, 52. [Google Scholar] [CrossRef]
- Nowakowski, J.; Dulisz, B. The Red-vented Bulbul Pycnonotus cafer (Linnaeus, 1766)—A new invasive bird species breeding in Europe. Bioinvasions Rec. 2019, 8, 947–952. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Liao, J.-R.; Shiao, S.-F.; Ko, C.-C. Origin and potential expansion of the invasive Longan Lanternfly, Pyrops candelaria (Hemiptera: Fulgoridae) in Taiwan. Biology 2021, 10, 678. [Google Scholar] [CrossRef] [PubMed]
- Behm, J.E.; Busala, G.M.; Helmus, M.R. First records of three new lizard species and a range expansion of a fourth lizard species introduced to Aruba. Bioinvasions Rec. 2022, 11, 296–306. [Google Scholar] [CrossRef]
- Liu, Y.; Thomas, M.L.; Coupland, G.T.; Wang, P.; Zheng, D.; McKirdy, S.J. Info-gap theory to determine cost-effective eradication of invasive species. Sci. Rep. 2023, 13, 2744. [Google Scholar] [CrossRef]
- Russell, J.C.; Meyer, J.-Y.; Holmes, N.D.; Pagad, S. Invasive alien species on islands: Impacts, distribution, interactions and management. Environ. Conserv. 2017, 44, 359–370. [Google Scholar] [CrossRef]
- Larson, E.R.; Graham, B.M.; Achury, R.; Coon, J.J.; Daniels, M.K.; Gambrell, D.K.; Jonasen, K.L.; King, G.D.; LaRacuente, N.; Perrin-Stowe, T.I.N. From eDNA to citizen science: Emerging tools for the early detection of invasive species. Front. Ecol. Environ. 2020, 18, 194–202. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Shirk, J.; Bonter, D.; Bonney, R.; Crain, R.L.; Martin, J.; Phillips, T.; Purcell, K. The current state of citizen science as a tool for ecological research and public engagement. Front. Ecol. Environ. 2012, 10, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Gjerdrum, C.; Loch, J.; Fifield, D.A. The recent invasion of Cory’s Shearwaters into Atlantic Canada. Northeast. Nat. 2018, 25, 532–544. [Google Scholar] [CrossRef]
- Thibault, M.; Vidal, E.; Potter, M.A.; Dyer, E.; Brescia, F. The Red-vented Bulbul (Pycnonotus cafer): Serious pest or understudied invader? Biol. Invasions 2018, 20, 121–136. [Google Scholar] [CrossRef]
- Arazmi, F.N.; Ismail, N.A.; Daud, U.N.S.; Abidin, K.Z.; Nor, S.M.; Mansor, M.S. Spread of the invasive Javan myna along an urban–suburban gradient in Peninsular Malaysia. Urban. Ecosyst. 2022, 25, 1007–1014. [Google Scholar] [CrossRef]
- Fielding, R. Saint Martin/Sint Maarten and Saint Barthélemy. In Landscapes and Landforms of the Lesser Antilles; Allen, C.D., Ed.; Springer: Cham, Switzerland, 2017; pp. 45–59. [Google Scholar]
- Olson, D.M.; Dinerstein, E.; Wikramanayake, E.D.; Burgess, N.D.; Powell, G.V.N.; Underwood, E.C.; D’amico, J.A.; Itoua, I.; Strand, H.E.; Morrison, J.C.; et al. Terrestrial ecoregions of the world: A new map of life on earth. Bioscience 2001, 51, 933–938. [Google Scholar] [CrossRef]
- Baptista, L.F.; Trail, P.W.; Horblit, H.M.; Boesman, P.F.D. Bare-Eyed Pigeon (Patagioenas Corensis), version 1.0. Birds of World; Cornell Laboratory of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar] [CrossRef]
- Friedmann, H.; Foster, D.S. A contribution to the ornithology of northeastern Venezuela. Proc. U. S. Natl. Mus. 1950, 100, 411–538. [Google Scholar] [CrossRef]
- Verea, C.; Aponte, O.; Vargas, G.; Mendoza, A. Primer reporte de daño producido por la paloma ala blanca Patagioenas corensis (Columbiformes: Columbidae) en viñedos del área agrícola de Altagracia, estado Lara, Venezuela. Mem. Fund. Salle Cienc. Nat. 2011, 71, 141–145. [Google Scholar]
- Wells, J.V.; Wells, A.C.; Dean, R. Birds of Aruba, Bonaire, and Curacao: A Site and Field Guide; Cornell University Press: Ithaca, NY, USA, 2017; ISBN 1501712861. [Google Scholar]
- eBird Basic Dataset. Version: EBD_RelApr-2024; Cornell Lab of Ornithology: Ithaca, NY, USA, 2024. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2024; R Core Team: Vienna, Austria, 2024. [Google Scholar]
- QGIS Development Team. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2024. Available online: http://qgis.osgeo.org (accessed on 13 June 2024).
- Collins, D.P.; Carleton, S.A.; Coxen, C.L. Movement patterns of adult Interior Band-tailed pigeons (Patagioenas fasciata) in New Mexico. Wilson J. Ornithol. 2019, 131, 360–366. [Google Scholar] [CrossRef] [PubMed]
- McNair, D.B. Conservation implications of the current breeding distribution and abundance of the White-crowned Pigeon Patagioenas leucocephala at St. Croix, US Virgin Islands. Caribb. J. Sci. 2008, 44, 311–320. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. 2022. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 13 June 2024).
- Singmann, H.; Kellen, D. An introduction to mixed models for experimental psychology. In New Methods in Cognitive Psychology; Routledge: London, UK, 2019; pp. 4–31. [Google Scholar]
- Hector, A.; Von Felten, S.; Schmid, B. Analysis of variance with unbalanced data: An update for ecology & evolution. J. Anim. Ecol. 2010, 79, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Nagel, R.; Ruxton, G.D.; Morrissey, M.B. Classical tests, linear models and their extensions for the analysis of 2 × 2 contingency tables. Methods Ecol. Evol. 2024, 15, 843–855. [Google Scholar] [CrossRef]
- Johnson, P.C.D.; Barry, S.J.E.; Ferguson, H.M.; Müller, P. Power analysis for generalized linear mixed models in ecology and evolution. Methods Ecol. Evol. 2015, 6, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Zanaga, D.; Van De Kerchove, R.; Daems, D.; De Keersmaecker, W.; Brockmann, C.; Kirches, G.; Wevers, J.; Cartus, O.; Santoro, M.; Fritz, S. ESA WorldCover 10 m 2021 V200 2022; European Space Agency: Paris, France, 2022. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: London, UK, 2013; ISBN 0203771583. [Google Scholar]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: A package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Cambrone, C.; Bezault, E.; Cézilly, F. Efficiency of the call-broadcast method for detecting two Caribbean-endemic columbid game species. Eur. J. Wildl. Res. 2021, 67, 65. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community ecology package 2024. Available online: http://vegan.r-forge.r-project.org/ (accessed on 13 June 2024).
- Lockwood, J.L.; Welbourne, D.J.; Romagosa, C.M.; Cassey, P.; Mandrak, N.E.; Strecker, A.; Leung, B.; Stringham, O.C.; Udell, B.; Episcopio-Sturgeon, D.J.D. When pets become pests: The role of the exotic pet trade in producing invasive vertebrate animals. Front. Ecol. Environ. 2019, 17, 323–330. [Google Scholar] [CrossRef]
- Bruslund, S.; Leupen, B.; Shepherd, C.R.; Nelson, S.S. Online trade as a serious additional threat to the Critically Endangered Silvery Pigeon Columba argentina in Indonesia. Nat. Conserv. 2022, 46, 41. [Google Scholar] [CrossRef]
- IUCN SSC. IUCN SSC Pigeon and Dove Specialist Group: Position Statement on the Value of Ex Situ Populations in Conserving Threatened Columbiformes and Recommendations on Enhanced Use of CITES to Address Unsustainable Trade. 2023. Available online: https://www.iucn.org/ssc (accessed on 13 June 2024).
- Carrete, M.; Tella, J. Wild-bird trade and exotic invasions: A new link of conservation concern? Front. Ecol. Environ. 2008, 6, 207–211. [Google Scholar] [CrossRef]
- Prins, T.G.; Reuter, J.H.; Debrot, A.O.; Wattel, J.; Nijman, V. Checklist of the birds of Aruba, Curaçao and Bonaire, south Caribbean. Ardea 2009, 97, 137–268. [Google Scholar] [CrossRef]
- Mulhall, S.; Lill, A. What facilitates urban colonisation by Crested pigeons Ochyphaps lophotes? Corella 2011, 35, 73–81. [Google Scholar]
- Veech, J.A.; Small, M.F.; Baccus, J.T. The effect of habitat on the range expansion of a native and an introduced bird species. J. Biogeogr. 2011, 38, 69–77. [Google Scholar] [CrossRef]
- Bendjoudi, D.; Voisin, J.F.; Doumandji, S.; Merabet, A.; Benyounes, N.; Chenchouni, H. Rapid increase in numbers and change of land-use in two expanding Columbidae species (Columba palumbus and Streptopelia decaocto) in Algeria. Avian Res. 2015, 6, 18. [Google Scholar] [CrossRef]
- Luna, Á.; Romero-Vidal, P.; Hiraldo, F.; Tella, J. Cities favour the recent establishment and current spread of the Eurasian Collared Dove Streptopelia decaocto (Frivaldszky, 1838) in Dominican Republic. Bioinvasions Rec. 2018, 7, 95–99. [Google Scholar] [CrossRef]
- Hernández, F.; Brown, J.I.; Kaminski, M.; Harvey, M.G.; Lavretsky, P. Genomic evidence for rare hybridization and large demographic changes in the evolutionary histories of four North American dove species. Animals 2021, 11, 2677. [Google Scholar] [CrossRef]
- Crozariol, M.A.; Indiani, J. Um híbrido entre a pomba-galega (Patagioenas cayennensis) e pombão (Patagioenas picazuro)(Columbiformes: Columbidae). Atual. Ornitológicas 2010, 153, 6–7. [Google Scholar]
- Pasquon, K.; Jouannic, G.; Gargani, J.; Minh, C.T.D.; Crozier, D. Évolution de l’urbanisme et exposition au risque cyclonique à Saint-Martin de 1954 à 2017. VertigO-La Rev. Électronique Sci. L’Environ. 2022, 22, 1–33. [Google Scholar] [CrossRef]
- González-Lagos, C.; Cardador, L.; Sol, D. Invasion success and tolerance to urbanization in birds. Ecography 2021, 44, 1642–1652. [Google Scholar] [CrossRef]
- Wiley, J.W.; Wunderle, J.M. The effects of hurricanes on birds, with special reference to Caribbean islands. Bird Conserv. Int. 1993, 3, 319–349. [Google Scholar] [CrossRef]
- Gibbs, D.; Barnes, E.; Cox, J. Pigeons and Doves: A Guide to the Pigeons and Doves of the World; A&C Black: London, UK, 2001; Volume 13, ISBN 1873403607. [Google Scholar]
- Herrera, D.A.; Ault, T.R.; Carrillo, C.M.; Fasullo, J.T.; Li, X.; Evans, C.P.; Alessi, M.J.; Mahowald, N.M. Dynamical characteristics of drought in the Caribbean from observations and simulations. J. Clim. 2020, 33, 10773–10797. [Google Scholar] [CrossRef]
- Mazzamuto, M.V.; Wauters, L.A.; Koprowski, J.L. Exotic pet trade as a cause of biological invasions: The case of tree squirrels of the genus Callosciurus. Biology 2021, 10, 1046. [Google Scholar] [CrossRef] [PubMed]
- Kluever, B.M.; Avery, M.L.; Gawlik, D.E.; Hall, P.; Humphrey, J.S.; Pernas, T.; Ridgley, F. Eradication of African Sacred Ibis (Threskiornis aethiopicus) from South Florida, USA: A collaborative early detection and rapid response case study. Manag. Biol. Invasions 2023, 14, 123–132. [Google Scholar] [CrossRef]
- Cambrone, C.; Cézilly, F.; Wattier, R.; Eraud, C.; Bezault, E. Levels of genetic differentiation and gene flow between four populations of the Scaly-naped Pigeon, Patagioenas squamosa: Implications for conservation. Stud. Neotrop. Fauna Environ. 2021, 57, 349–361. [Google Scholar] [CrossRef]
- Cézilly, F.; Quinard, A.; Motreuil, S.; Pradel, R. Adult survival selection in relation to multilocus heterozygosity and body size in a tropical bird species, the Zenaida Dove, Zenaida aurita. Oecologia 2016, 180, 127–136. [Google Scholar] [CrossRef]
- Phillips, R.B.; Cooke, B.D.; Carrión, V.; Snell, H.L. Eradication of rock pigeons, Columba livia, from the Galápagos Islands. Biol. Conserv. 2012, 147, 264–269. [Google Scholar] [CrossRef]
- Bunbury, N.; Haverson, P.; Page, N.; Agricole, J.; Angell, G.; Banville, P.; Constance, A.; Friedlander, J.; Leite, L.; Mahoune, T.; et al. Five Eradications, Three Species, Three Islands: Overview, Insights and Recommendations from Invasive Bird Eradications in the Seychelles. In Proceedings of the Island Invasives Conference 2017, Gland, Switzerland, 10–14 July 2017; IUCN: Gland, Switzerland, 2019. [Google Scholar]
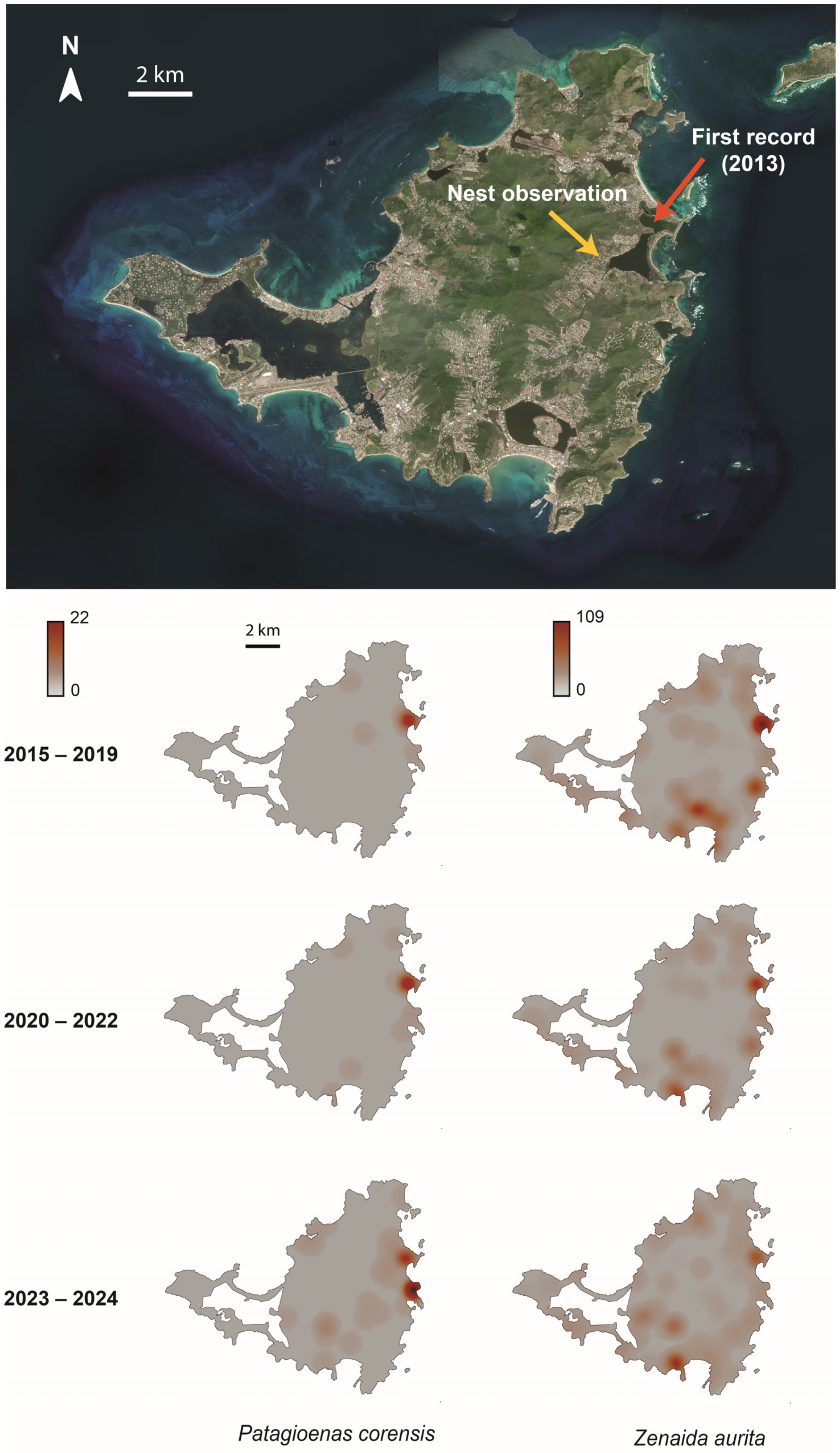
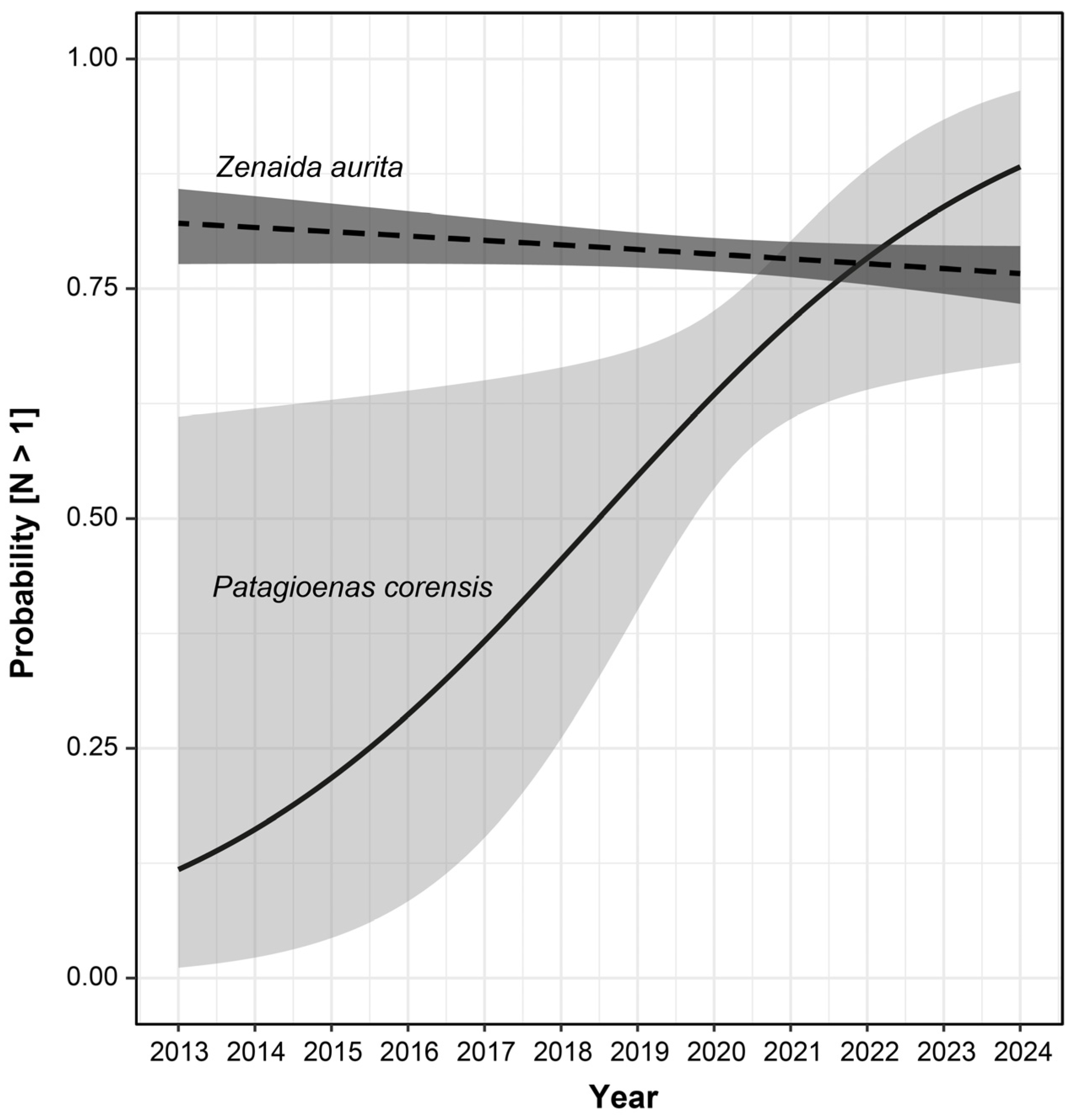

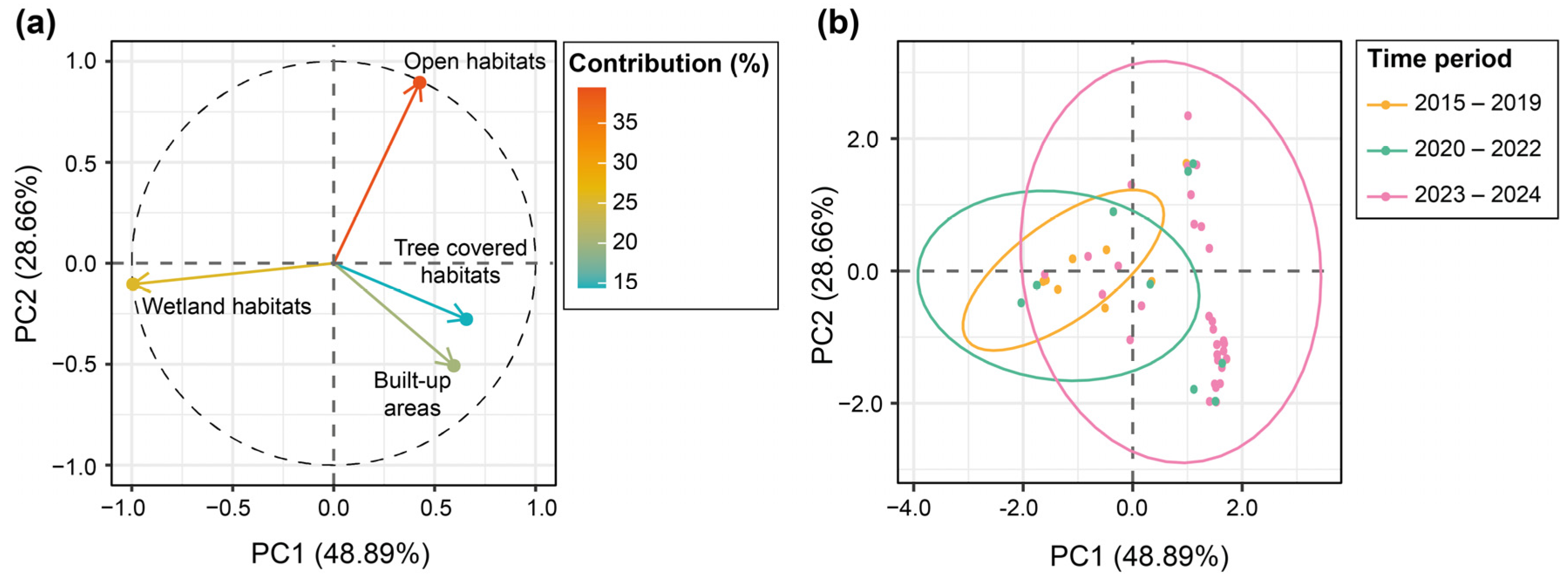

| Model | Parameter | Odds Ratio [95% CI] | X2 | p |
|---|---|---|---|---|
| (A) Y ~Year + Species + Year × Species | Year | 1.183 [1.016; 1.432] | 4.742 | 0.029 |
| Species | 2.148 [1.388; 3.323] | 6.817 | 0.009 | |
| Year × Species | 1.219 [1.047; 1.477] | 6.801 | 0.009 | |
| (B) Y ~Year (Zenaida aurita) | Year | 0.970 [0.935; 1.005] | 2.832 | 0.092 |
| (C) Y ~Year (Patagioenas corensis) | Year | 1.442 [1.066; 2.112] | 5.821 | 0.016 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cambrone, C.; Levesque, A.; Cézilly, F. Using Citizen Science and Field Surveys to Document the Introduction, Establishment, and Rapid Spread of the Bare-Eyed Pigeon, Patagioenas corensis, on the Island of Saint-Martin, West Indies. Biology 2024, 13, 585. https://doi.org/10.3390/biology13080585
Cambrone C, Levesque A, Cézilly F. Using Citizen Science and Field Surveys to Document the Introduction, Establishment, and Rapid Spread of the Bare-Eyed Pigeon, Patagioenas corensis, on the Island of Saint-Martin, West Indies. Biology. 2024; 13(8):585. https://doi.org/10.3390/biology13080585
Chicago/Turabian StyleCambrone, Christopher, Anthony Levesque, and Frank Cézilly. 2024. "Using Citizen Science and Field Surveys to Document the Introduction, Establishment, and Rapid Spread of the Bare-Eyed Pigeon, Patagioenas corensis, on the Island of Saint-Martin, West Indies" Biology 13, no. 8: 585. https://doi.org/10.3390/biology13080585
APA StyleCambrone, C., Levesque, A., & Cézilly, F. (2024). Using Citizen Science and Field Surveys to Document the Introduction, Establishment, and Rapid Spread of the Bare-Eyed Pigeon, Patagioenas corensis, on the Island of Saint-Martin, West Indies. Biology, 13(8), 585. https://doi.org/10.3390/biology13080585






