Beneficial Effects of Tyrosol and Oleocanthal from Extra Virgin Olive Oil on Liver Health: Insights into Their Mechanisms of Action
Abstract
Simple Summary
Abstract
1. Introduction
2. The Phenolic Compounds of EVOO
2.1. Oleocanthal
2.2. Tyrosol and Other Phenolic Compounds
3. The Effects of Tyrosol and Oleocanthal on Steatotic Liver Disease
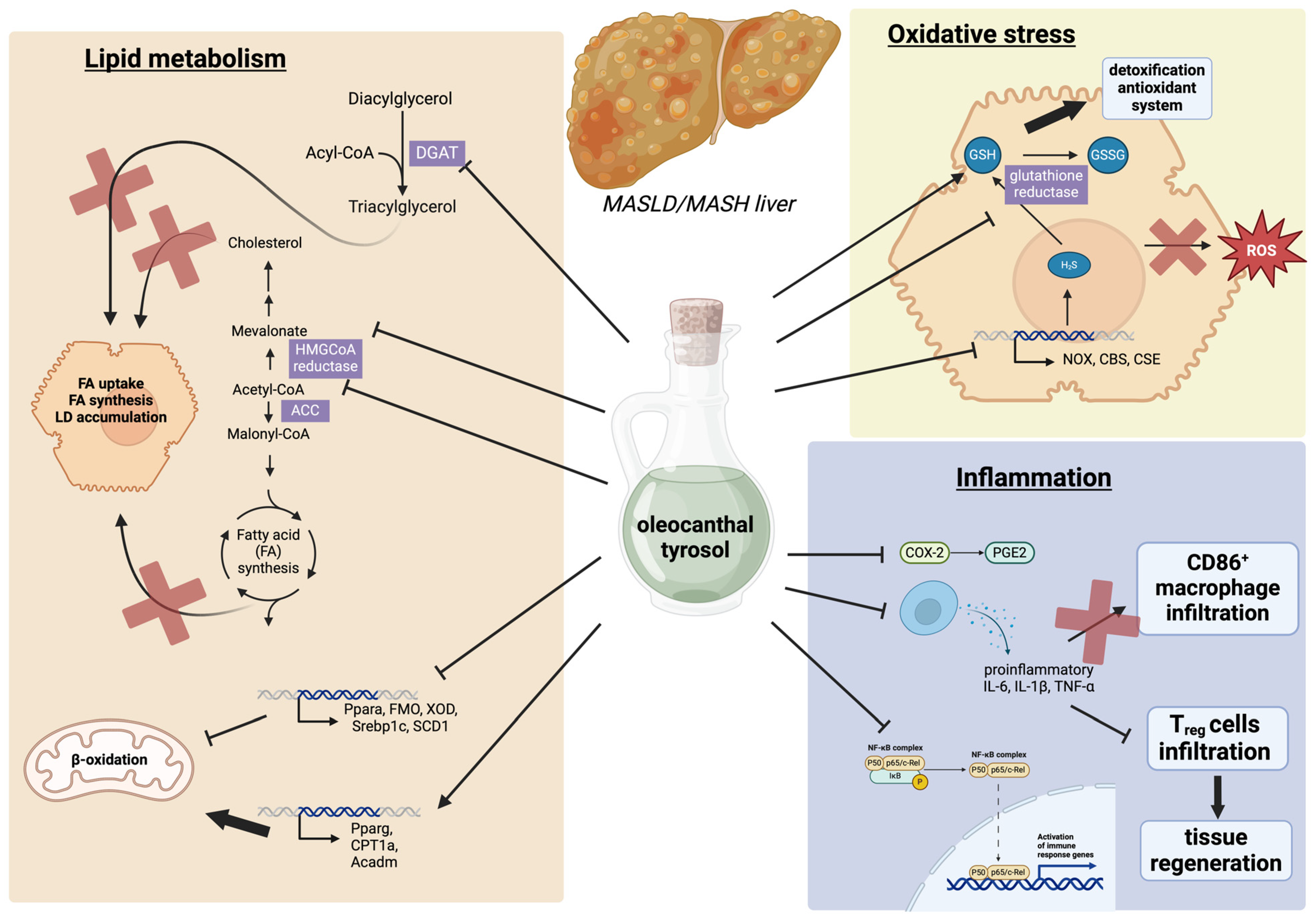
3.1. The Effects of Tyrosol and Oleocanthal on Lipid Metabolism
3.2. The Effect of Tyrosol and Oleocanthal on Oxidative Stress
3.3. The Effects of Tyrosol and Oleocanthal on Hepatic Inflammation
3.4. Clinical Trials Assessing the Beneficial Effects of EVOO Consumption on MASLD
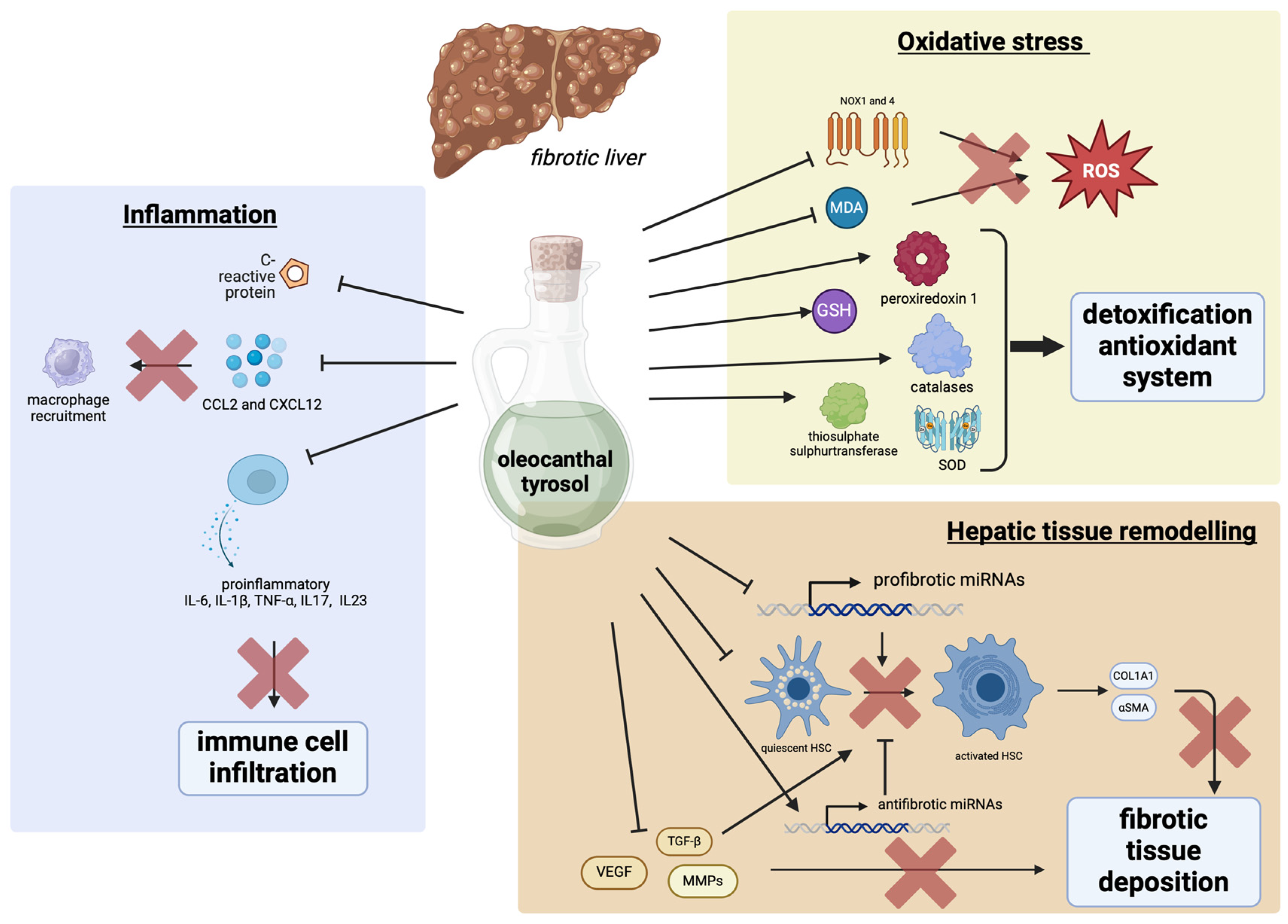
4. The Effects of Tyrosol and Oleocanthal on Hepatic Fibrosis
4.1. Tyrosol and Oleocanthal Effects on Tissue Remodeling
4.2. Tyrosol and Oleocanthal Effects on Fibrosis-Induced Oxidative Stress
4.3. Tyrosol and Oleocanthal Effects on Inflammation Induced by Fibrosis
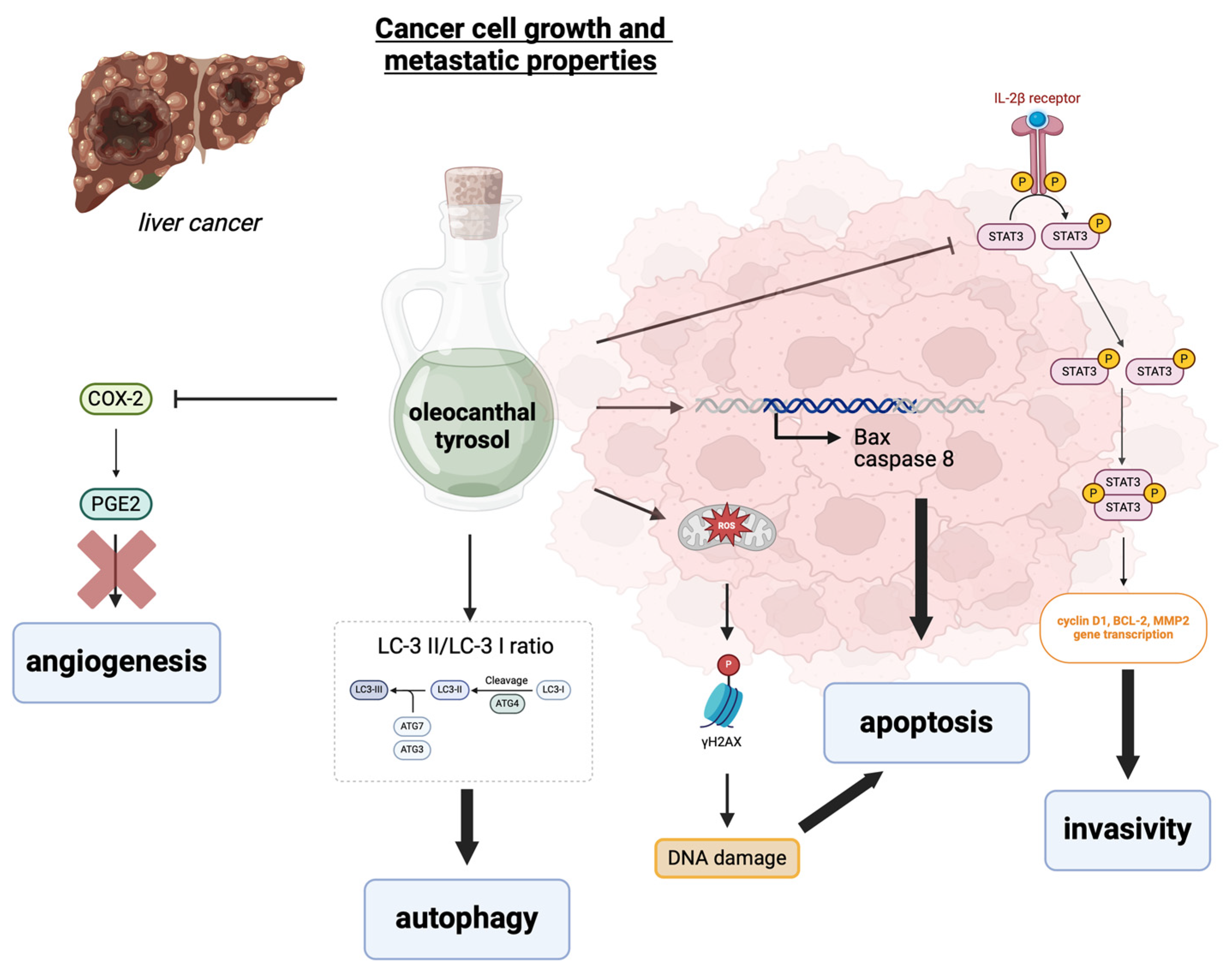
5. The Effects of Tyrosol and Oleocanthal on Liver Cancer
6. Conclusions
Funding
Conflicts of Interest
References
- Sayaf, K.; Gabbia, D.; Russo, F.P.; De Martin, S. The Role of Sex in Acute and Chronic Liver Damage. Int. J. Mol. Sci. 2022, 23, 10654. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Nagalli, S. Chronic Liver Disease. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2024. [Google Scholar]
- Ortega-Alonso, A.; Andrade, R.J. Chronic Liver Injury Induced by Drugs and Toxins. J. Dig. Dis. 2018, 19, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; De Martin, S. Targeting the Adipose Tissue–Liver–Gut Microbiota Crosstalk to Cure MASLD. Biology 2023, 12, 1471. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Ong, K.L.; Aali, A.; Ababneh, H.S.; Abate, Y.H.; Abbafati, C.; Abbasgholizadeh, R.; Abbasian, M.; Abbasi-Kangevari, M.; Abbastabar, H.; et al. Global Burden of 288 Causes of Death and Life Expectancy Decomposition in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Soto-Alarcon, S.A.; Valenzuela, R.; Valenzuela, A.; Videla, L.A. Liver Protective Effects of Extra Virgin Olive Oil: Interaction between Its Chemical Composition and the Cell-Signaling Pathways Involved in Protection. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 75–84. [Google Scholar] [CrossRef]
- Ma, Y.; Ding, X.; Gu, J.; Zhou, S.; Jiang, Y. Effects of Olive Oil on Hepatic Steatosis and Liver Enzymes: A Systematic Review. J. Funct. Foods 2023, 109, 105815. [Google Scholar] [CrossRef]
- González-Rodríguez, M.; Ait Edjoudi, D.; Cordero-Barreal, A.; Farrag, M.; Varela-García, M.; Torrijos-Pulpón, C.; Ruiz-Fernández, C.; Capuozzo, M.; Ottaiano, A.; Lago, F.; et al. Oleocanthal, an Antioxidant Phenolic Compound in Extra Virgin Olive Oil (EVOO): A Comprehensive Systematic Review of Its Potential in Inflammation and Cancer. Antioxidants 2023, 12, 2112. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, J.; Li, X.; Chen, P.; Chen, F.; Pan, Y.; Deng, Z.; Li, J.; Liu, R.; Luo, T. Tyrosol Regulates Hepatic Lipid Metabolism in High-Fat Diet-Induced NAFLD Mice. Food Funct. 2024, 15, 3752–3764. [Google Scholar] [CrossRef]
- Rodríguez-López, P.; Lozano-Sánchez, J.; Borras-Linares, I.; Emanuelli, T.; Menendez, J.A.; Segura-Carretero, A. Chapter 10—Polyphenols in Olive Oil: The Importance of Phenolic Compounds in the Chemical Composition of Olive Oil. In Olives and Olive Oil in Health and Disease Prevention, 2nd ed.; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2021; pp. 111–122. ISBN 978-0-12-819528-4. [Google Scholar]
- Segura-Carretero, A.; Menéndez-Menéndez, J.; Fernández-Gutiérrez, A. Chapter 19—Polyphenols in Olive Oil: The Importance of Phenolic Compounds in the Chemical Composition of Olive Oil. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 167–175. ISBN 978-0-12-374420-3. [Google Scholar]
- Kalogeropoulos, N.; Kaliora, A.C. Effect of Fruit Maturity on Olive Oil Phenolic Composition and Antioxidant Capacity. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Washington, DC, USA, 2015; pp. 123–145. ISBN 978-1-63067-041-2. [Google Scholar]
- Rodríguez-López, P.; Lozano-Sanchez, J.; Borrás-Linares, I.; Emanuelli, T.; Menéndez, J.A.; Segura-Carretero, A. Structure–Biological Activity Relationships of Extra-Virgin Olive Oil Phenolic Compounds: Health Properties and Bioavailability. Antioxidants 2020, 9, 685. [Google Scholar] [CrossRef]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, E.; Castellote, A.I.; Lamuela-Raventós, R.M.; De la Torre, M.C.; López-Sabater, M.C. The Effects of Harvest and Extraction Methods on the Antioxidant Content (Phenolics, α-Tocopherol, and β-Carotene) in Virgin Olive Oil. Food Chem. 2002, 78, 207–211. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Carpena, M.; Lourenço-Lopes, C.; Gallardo-Gomez, M.; Lorenzo, J.M.; Barba, F.J.; Prieto, M.A.; Simal-Gandara, J. Bioactive Compounds and Quality of Extra Virgin Olive Oil. Foods 2020, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Celano, R.; Piccinelli, A.L.; Pugliese, A.; Carabetta, S.; di Sanzo, R.; Rastrelli, L.; Russo, M. Insights into the Analysis of Phenolic Secoiridoids in Extra Virgin Olive Oil. J. Agric. Food Chem. 2018, 66, 6053–6063. [Google Scholar] [CrossRef]
- Lozano-Castellón, J.; López-Yerena, A.; Olmo-Cunillera, A.; Jáuregui, O.; Pérez, M.; Lamuela-Raventós, R.M.; Vallverdú-Queralt, A. Total Analysis of the Major Secoiridoids in Extra Virgin Olive Oil: Validation of an UHPLC-ESI-MS/MS Method. Antioxidants 2021, 10, 540. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants 2018, 7, 170. [Google Scholar] [CrossRef]
- Servili, M.; Baldioli, M.; Selvaggini, R.; Macchioni, A.; Montedoro, G. Phenolic Compounds of Olive Fruit: One- and Two-Dimensional Nuclear Magnetic Resonance Characterization of Nüzhenide and Its Distribution in the Constitutive Parts of Fruit. J. Agric. Food Chem. 1999, 47, 12–18. [Google Scholar] [CrossRef]
- Sánchez de Medina, V.; Riachy, M.E.; Priego-Capote, F.; Luque de Castro, M.D. Mass Spectrometry to Evaluate the Effect of the Ripening Process on Phenols of Virgin Olive Oils. Eur. J. Lipid Sci. Technol. 2013, 115, 1053–1061. [Google Scholar] [CrossRef]
- Cui, W.; Shen, X.; Agbas, E.; Tompkins, B.; Cameron-Carter, H.; Staudinger, J.L. Phosphorylation Modulates the Coregulatory Protein Exchange of the Nuclear Receptor Pregnane X Receptor. J. Pharmacol. Exp. Ther. 2020, 373, 370–380. [Google Scholar] [CrossRef]
- Mateos, R.; Sarria, B.; Bravo, L. Nutritional and Other Health Properties of Olive Pomace Oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 3506–3521. [Google Scholar] [CrossRef]
- Mateos, R.; Cert, A.; Pérez-Camino, M.C.; García, J.M. Evaluation of Virgin Olive Oil Bitterness by Quantification of Secoiridoid Derivatives. J. Am. Oil Chem. Soc. 2004, 81, 71–75. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Ryan, D.; Servili, M.; Taticchi, A.; Esposto, S.; Robards, K. Biosynthesis and Biotransformations of Phenol-Conjugated Oleosidic Secoiridoids from Olea europaea L. Nat. Prod. Rep. 2008, 25, 1167–1179. [Google Scholar] [CrossRef]
- Abbattista, R.; Losito, I.; Castellaneta, A.; De Ceglie, C.; Calvano, C.D.; Cataldi, T.R.I. Insight into the Storage-Related Oxidative/Hydrolytic Degradation of Olive Oil Secoiridoids by Liquid Chromatography and High-Resolution Fourier Transform Mass Spectrometry. J. Agric. Food Chem. 2020, 68, 12310–12325. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Montedoro, G.; Servili, M.; Baldioli, M.; Selvaggini, R.; Miniati, E.; Macchioni, A. Simple and Hydrolyzable Compounds in Virgin Olive Oil. 3. Spectroscopic Characterizations of the Secoiridoid Derivatives. J. Agric. Food Chem. 1993, 41, 2228–2234. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J.; Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Oleocanthal: A Naturally Occurring Anti-Inflammatory Agent in Virgin Olive Oil. In Olive Oil-Constituents, Quality, Health Properties and Bioconversions; IntechOpen: London, UK, 2012; ISBN 978-953-307-921-9. [Google Scholar]
- Andrewes, P.; Busch, J.L.H.C.; de Joode, T.; Groenewegen, A.; Alexandre, H. Sensory Properties of Virgin Olive Oil Polyphenols: Identification of Deacetoxy-Ligstroside Aglycon as a Key Contributor to Pungency. J. Agric. Food Chem. 2003, 51, 1415–1420. [Google Scholar] [CrossRef]
- des Gachons, C.P.; Uchida, K.; Bryant, B.; Shima, A.; Sperry, J.B.; Dankulich-Nagrudny, L.; Tominaga, M.; Smith, A.B.; Beauchamp, G.K.; Breslin, P.A.S. Unusual Pungency from Extra-Virgin Olive Oil Is Attributable to Restricted Spatial Expression of the Receptor of Oleocanthal. J. Neurosci. 2011, 31, 999–1009. [Google Scholar] [CrossRef]
- Monti, M.C.; Margarucci, L.; Riccio, R.; Casapullo, A. Modulation of Tau Protein Fibrillization by Oleocanthal. J. Nat. Prod. 2012, 75, 1584–1588. [Google Scholar] [CrossRef]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; El Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal Enhances Amyloid-β Clearance from the Brains of TgSwDI Mice and in Vitro across a Human Blood-Brain Barrier Model. ACS Chem. Neurosci. 2015, 6, 1849–1859. [Google Scholar] [CrossRef]
- Francisco, V.; Ruiz-Fernández, C.; Lahera, V.; Lago, F.; Pino, J.; Skaltsounis, L.; González-Gay, M.A.; Mobasheri, A.; Gómez, R.; Scotece, M.; et al. Natural Molecules for Healthy Lifestyles: Oleocanthal from Extra Virgin Olive Oil. J. Agric. Food Chem. 2019, 67, 3845–3853. [Google Scholar] [CrossRef]
- López-Yerena, A.; Vallverdú-Queralt, A.; Mols, R.; Augustijns, P.; Lamuela-Raventós, R.M.; Escribano-Ferrer, E. Absorption and Intestinal Metabolic Profile of Oleocanthal in Rats. Pharmaceutics 2020, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Edgecombe, S.C.; Stretch, G.L.; Hayball, P.J. Oleuropein, an Antioxidant Polyphenol from Olive Oil, Is Poorly Absorbed from Isolated Perfused Rat Intestine. J. Nutr. 2000, 130, 2996–3002. [Google Scholar] [CrossRef] [PubMed]
- Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Halabalaki, M. Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies. Nutrients 2022, 14, 3773. [Google Scholar] [CrossRef] [PubMed]
- López de las Hazas, M.-C.; Piñol, C.; Macià, A.; Romero, M.-P.; Pedret, A.; Solà, R.; Rubió, L.; Motilva, M.-J. Differential Absorption and Metabolism of Hydroxytyrosol and Its Precursors Oleuropein and Secoiridoids. J. Funct. Foods 2016, 22, 52–63. [Google Scholar] [CrossRef]
- Nikou, T.; Karampetsou, K.V.; Koutsoni, O.S.; Skaltsounis, A.-L.; Dotsika, E.; Halabalaki, M. Pharmacokinetics and Metabolism Investigation of Oleocanthal. J. Nat. Prod. 2024, 87, 530–543. [Google Scholar] [CrossRef]
- Lennernäs, H. Human Jejunal Effective Permeability and Its Correlation with Preclinical Drug Absorption Models. J. Pharm. Pharmacol. 1997, 49, 627–638. [Google Scholar] [CrossRef]
- Lee, H.; Im, S.W.; Jung, C.H.; Jang, Y.J.; Ha, T.Y.; Ahn, J. Tyrosol, an Olive Oil Polyphenol, Inhibits ER Stress-Induced Apoptosis in Pancreatic β-Cell through JNK Signaling. Biochem. Biophys. Res. Commun. 2016, 469, 748–752. [Google Scholar] [CrossRef]
- Kim, Y.-Y.; Lee, S.; Kim, M.-J.; Kang, B.-C.; Dhakal, H.; Choi, Y.-A.; Park, P.-H.; Choi, H.; Shin, T.-Y.; Choi, H.G.; et al. Tyrosol Attenuates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting the Inflammatory Response and Maintaining the Alveolar Capillary Barrier. Food Chem. Toxicol. 2017, 109, 526–533. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA); Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Safety of Hydroxytyrosol as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, e04728. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the Substantiation of Health Claims Related to Polyphenols in Olive and Protection of LDL Particles from Oxidative Damage (ID 1333, 1638, 1639, 1696, 2865), Maintenance of Normal Blood HDL Cholesterol Concentrations (ID 1639), Maintenance of Normal Blood Pressure (ID 3781), “Anti-Inflammatory Properties” (ID 1882), “Contributes to the Upper Respiratory Tract Health” (ID 3468), “Can Help to Maintain a Normal Function of Gastrointestinal Tract” (3779), and “Contributes to Body Defences against External Agents” (ID 3467) Pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2033. [Google Scholar] [CrossRef]
- Lee, D.-H.; Kim, Y.-J.; Kim, M.J.; Ahn, J.; Ha, T.-Y.; Lee, S.H.; Jang, Y.J.; Jung, C.H. Pharmacokinetics of Tyrosol Metabolites in Rats. Molecules 2016, 21, 128. [Google Scholar] [CrossRef] [PubMed]
- Weinbrenner, T.; Fitó, M.; Farré Albaladejo, M.; Saez, G.T.; Rijken, P.; Tormos, C.; Coolen, S.; De La Torre, R.; Covas, M.I. Bioavailability of Phenolic Compounds from Olive Oil and Oxidative/Antioxidant Status at Postprandial State in Healthy Humans. Drugs Exp. Clin. Res. 2004, 30, 207–212. [Google Scholar] [PubMed]
- Visioli, F.; Galli, C.; Bornet, F.; Mattei, A.; Patelli, R.; Galli, G.; Caruso, D. Olive Oil Phenolics Are Dose-Dependently Absorbed in Humans. FEBS Lett. 2000, 468, 159–160. [Google Scholar] [CrossRef]
- D’Angelo, S.; Manna, C.; Migliardi, V.; Mazzoni, O.; Morrica, P.; Capasso, G.; Pontoni, G.; Galletti, P.; Zappia, V. Pharmacokinetics and Metabolism of Hydroxytyrosol, a Natural Antioxidant from Olive Oil. Drug Metab. Dispos. 2001, 29, 1492–1498. [Google Scholar] [PubMed]
- Miró-Casas, E.; Covas, M.-I.; Fitó, M.; Farré-Albadalejo, M.; Marrugat, J.; de la Torre, R. Tyrosol and Hydroxytyrosol Are Absorbed from Moderate and Sustained Doses of Virgin Olive Oil in Humans. Eur. J. Clin. Nutr. 2003, 57, 186–190. [Google Scholar] [CrossRef]
- Maardh, G.; Vallee, B.L. Human Class I Alcohol Dehydrogenases Catalyze the Interconversion of Alcohols and Aldehydes in the Metabolism of Dopamine. Biochemistry 1986, 25, 7279–7282. [Google Scholar] [CrossRef]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics and Bioavailability of Hydroxytyrosol Are Dependent on the Food Matrix in Humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A Multi-Society Delphi Consensus Statement on New Fatty Liver Disease Nomenclature. J. Hepatol. 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Younossi, Z.M.; AlQahtani, S.A.; Funuyet-Salas, J.; Romero-Gómez, M.; Yilmaz, Y.; Keklikkiran, C.; Alswat, K.; Yu, M.-L.; Liu, C.-J.; Fan, J.-G.; et al. The Impact of Stigma on Quality of Life and Liver Disease Burden Among Patients with Nonalcoholic Fatty Liver Disease. JHEP Rep. 2024, 6, 101066. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Golabi, P.; Paik, J.M.; Henry, A.; Van Dongen, C.; Henry, L. The Global Epidemiology of Nonalcoholic Fatty Liver Disease (NAFLD) and Nonalcoholic Steatohepatitis (NASH): A Systematic Review. Hepatology 2023, 77, 1335–1347. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The Diagnosis and Management of Nonalcoholic Fatty Liver Disease: Practice Guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.D.; Targher, G. NAFLD: A Multisystem Disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [PubMed]
- Cobbina, E.; Akhlaghi, F. Non-Alcoholic Fatty Liver Disease (NAFLD)-Pathogenesis, Classification, and Effect on Drug Metabolizing Enzymes and Transporters. Drug Metab. Rev. 2017, 49, 197–211. [Google Scholar] [CrossRef]
- Priore, P.; Siculella, L.; Gnoni, G.V. Extra Virgin Olive Oil Phenols Down-Regulate Lipid Synthesis in Primary-Cultured Rat-Hepatocytes. J. Nutr. Biochem. 2014, 25, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Burò, I.; Consoli, V.; Castellano, A.; Vanella, L.; Sorrenti, V. Beneficial Effects of Standardized Extracts from Wastes of Red Oranges and Olive Leaves. Antioxidants 2022, 11, 1496. [Google Scholar] [CrossRef]
- Chandramohan, R.; Pari, L. Antihyperlipidemic Effect of Tyrosol, a Phenolic Compound in Streptozotocin-Induced Diabetic Rats. Toxicol. Mech. Methods 2021, 31, 507–516. [Google Scholar] [CrossRef]
- Zhan, X.; He, M.; Pei, J.; Fan, W.; Mwangi, C.N.; Zhang, P.; Chai, X.; Jiang, M. Natural Phenylethanoid Supplementation Alleviates Metabolic Syndrome in Female Mice Induced by High-Fructose Diet. Front. Pharmacol. 2022, 13, 850777. [Google Scholar] [CrossRef]
- Tincopa, M.A.; Anstee, Q.M.; Loomba, R. New and Emerging Treatments for Metabolic Dysfunction-Associated Steatohepatitis. Cell Metab. 2024, 36, 912–926. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular Mechanism of PPARα Action and Its Impact on Lipid Metabolism, Inflammation and Fibrosis in Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef]
- Gabbia, D.; Cannella, L.; De Martin, S. The Role of Oxidative Stress in NAFLD–NASH–HCC Transition—Focus on NADPH Oxidases. Biomedicines 2021, 9, 687. [Google Scholar] [CrossRef]
- Sarna, L.K.; Sid, V.; Wang, P.; Siow, Y.L.; House, J.D.; O, K. Tyrosol Attenuates High Fat Diet-Induced Hepatic Oxidative Stress: Potential Involvement of Cystathionine β-Synthase and Cystathionine γ-Lyase. Lipids 2016, 51, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Vergani, L.; Vecchione, G.; Baldini, F.; Grasselli, E.; Voci, A.; Portincasa, P.; Ferrari, P.F.; Aliakbarian, B.; Casazza, A.A.; Perego, P. Polyphenolic Extract Attenuates Fatty Acid-Induced Steatosis and Oxidative Stress in Hepatic and Endothelial Cells. Eur. J. Nutr. 2018, 57, 1793–1805. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, S.; Han, Z.; Du, J.; Liu, X.; Zhu, Z.; Zheng, L.; Han, S.; Shi, H.; Wang, X.; et al. Tyrosol Ameliorates Liver Inflammatory Response in a Mouse Model of Nonalcoholic Fatty Liver Disease (NFALD) by Regulating JAK1/STAT3. Nat. Product. Commun. 2022, 17, 1934578X221111033. [Google Scholar] [CrossRef]
- Barbagallo, I.; Li Volti, G.; Raffaele, M.; Distefano, A.; Palmeri, R.; Parafati, L.; Licari, M.; Zingales, V.; Avola, R.; Vanella, L. The Effects of Olive Leaf Extract from a Sicilian Cultivar in an Experimental Model of Hepatic Steatosis. Rend. Fis. Acc. Lincei 2017, 28, 643–650. [Google Scholar] [CrossRef]
- D’Amore, S.; Vacca, M.; Cariello, M.; Graziano, G.; D’Orazio, A.; Salvia, R.; Sasso, R.C.; Sabbà, C.; Palasciano, G.; Moschetta, A. Genes and miRNA Expression Signatures in Peripheral Blood Mononuclear Cells in Healthy Subjects and Patients with Metabolic Syndrome after Acute Intake of Extra Virgin Olive Oil. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 1671–1680. [Google Scholar] [CrossRef]
- Gabbia, D.; Sayaf, K.; Zanotto, I.; Colognesi, M.; Frion-Herrera, Y.; Carrara, M.; Russo, F.P.; De Martin, S. Tyrosol Attenuates NASH Features by Reprogramming the Hepatic Immune Milieu. Eur. J. Pharmacol. 2024, 969, 176453. [Google Scholar] [CrossRef]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene Expression Changes in Mononuclear Cells in Patients with Metabolic Syndrome after Acute Intake of Phenol-Rich Virgin Olive Oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef]
- Álvarez-Amor, L.; Sierra, A.L.; Cárdenas, A.; López-Bermudo, L.; López-Beas, J.; Andújar, E.; Pérez-Alegre, M.; Gallego-Durán, R.; Varela, L.M.; Martin-Montalvo, A.; et al. Extra Virgin Olive Oil Improved Body Weight and Insulin Sensitivity in High Fat Diet-Induced Obese LDLr−/−.Leiden Mice without Attenuation of Steatohepatitis. Sci. Rep. 2021, 11, 8250. [Google Scholar] [CrossRef]
- Di Daniele, N.; Noce, A.; Vidiri, M.F.; Moriconi, E.; Marrone, G.; Annicchiarico-Petruzzelli, M.; D’Urso, G.; Tesauro, M.; Rovella, V.; De Lorenzo, A. Impact of Mediterranean Diet on Metabolic Syndrome, Cancer and Longevity. Oncotarget 2016, 8, 8947–8979. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, X.; Yi, D.; Qiu, F.; Wu, L.; Tang, Y.; Wang, N. Mediterranean Diet Affects the Metabolic Outcome of Metabolic Dysfunction-Associated Fatty Liver Disease. Front. Nutr. 2023, 10, 1225946. [Google Scholar] [CrossRef]
- Seidita, A.; Soresi, M.; Giannitrapani, L.; Di Stefano, V.; Citarrella, R.; Mirarchi, L.; Cusimano, A.; Augello, G.; Carroccio, A.; Iovanna, J.L.; et al. The Clinical Impact of an Extra Virgin Olive Oil Enriched Mediterranean Diet on Metabolic Syndrome: Lights and Shadows of a Nutraceutical Approach. Front. Nutr. 2022, 9, 980429. [Google Scholar] [CrossRef] [PubMed]
- Hassani Zadeh, S.; Mansoori, A.; Hosseinzadeh, M. Relationship between Dietary Patterns and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. J. Gastroenterol. Hepatol. 2021, 36, 1470–1478. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Pintó, X.; Fanlo-Maresma, M.; Corbella, E.; Corbella, X.; Mitjavila, M.T.; Moreno, J.J.; Casas, R.; Estruch, R.; Corella, D.; Bulló, M.; et al. A Mediterranean Diet Rich in Extra-Virgin Olive Oil Is Associated with a Reduced Prevalence of Nonalcoholic Fatty Liver Disease in Older Individuals at High Cardiovascular Risk. J. Nutr. 2019, 149, 1920–1929. [Google Scholar] [CrossRef]
- Assy, N.; Nassar, F.; Nasser, G.; Grosovski, M. Olive Oil Consumption and Non-Alcoholic Fatty Liver Disease. World J. Gastroenterol. 2009, 15, 1809–1815. [Google Scholar] [CrossRef]
- Priore, P.; Cavallo, A.; Gnoni, A.; Damiano, F.; Gnoni, G.V.; Siculella, L. Modulation of Hepatic Lipid Metabolism by Olive Oil and Its Phenols in Nonalcoholic Fatty Liver Disease. IUBMB Life 2015, 67, 9–17. [Google Scholar] [CrossRef]
- Kaliora, A.C.; Gioxari, A.; Kalafati, I.P.; Diolintzi, A.; Kokkinos, A.; Dedoussis, G.V. The Effectiveness of Mediterranean Diet in Nonalcoholic Fatty Liver Disease Clinical Course: An Intervention Study. J. Med. Food 2019, 22, 729–740. [Google Scholar] [CrossRef]
- Marin-Alejandre, B.A.; Abete, I.; Cantero, I.; Monreal, J.I.; Elorz, M.; Herrero, J.I.; Benito-Boillos, A.; Quiroga, J.; Martinez-Echeverria, A.; Uriz-Otano, J.I.; et al. The Metabolic and Hepatic Impact of Two Personalized Dietary Strategies in Subjects with Obesity and Nonalcoholic Fatty Liver Disease: The Fatty Liver in Obesity (FLiO) Randomized Controlled Trial. Nutrients 2019, 11, 2543. [Google Scholar] [CrossRef]
- Akbulut, U.E.; Isik, I.A.; Atalay, A.; Eraslan, A.; Durmus, E.; Turkmen, S.; Yurttas, A.S. The Effect of a Mediterranean Diet vs. a Low-Fat Diet on Non-Alcoholic Fatty Liver Disease in Children: A Randomized Trial. Int. J. Food Sci. Nutr. 2022, 73, 357–366. [Google Scholar] [CrossRef]
- Patti, A.M.; Carruba, G.; Cicero, A.F.G.; Banach, M.; Nikolic, D.; Giglio, R.V.; Terranova, A.; Soresi, M.; Giannitrapani, L.; Montalto, G.; et al. Daily Use of Extra Virgin Olive Oil with High Oleocanthal Concentration Reduced Body Weight, Waist Circumference, Alanine Transaminase, Inflammatory Cytokines and Hepatic Steatosis in Subjects with the Metabolic Syndrome: A 2-Month Intervention Study. Metabolites 2020, 10, 392. [Google Scholar] [CrossRef]
- Ruiz-García, I.; Ortíz-Flores, R.; Badía, R.; García-Borrego, A.; García-Fernández, M.; Lara, E.; Martín-Montañez, E.; García-Serrano, S.; Valdés, S.; Gonzalo, M.; et al. Rich Oleocanthal and Oleacein Extra Virgin Olive Oil and Inflammatory and Antioxidant Status in People with Obesity and Prediabetes. The APRIL Study: A Randomised, Controlled Crossover Study. Clin. Nutr. 2023, 42, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Sayaf, K.; Zanotto, I.; Gabbia, D.; Alberti, D.; Pasqual, G.; Zaramella, A.; Fantin, A.; De Martin, S.; Russo, F.P. Sex Drives Functional Changes in the Progression and Regression of Liver Fibrosis. Int. J. Mol. Sci. 2023, 24, 16452. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Hepatic Fibrosis and Cancer: The Silent Threats of Metabolic Syndrome. Diabetes Metab. J. 2024, 48, 161–169. [Google Scholar] [CrossRef]
- Berumen, J.; Baglieri, J.; Kisseleva, T.; Mekeel, K. Liver Fibrosis: Pathophysiology and Clinical Implications. WIREs Mech. Dis. 2021, 13, e1499. [Google Scholar] [CrossRef] [PubMed]
- Böttcher, K.; Pinzani, M. Pathophysiology of Liver Fibrosis and the Methodological Barriers to the Development of Anti-Fibrogenic Agents. Adv. Drug Deliv. Rev. 2017, 121, 3–8. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Gabbia, D.; Carpi, S.; Sarcognato, S.; Cannella, L.; Colognesi, M.; Scaffidi, M.; Polini, B.; Digiacomo, M.; Esposito Salsano, J.; Manera, C.; et al. The Extra Virgin Olive Oil Polyphenol Oleocanthal Exerts Antifibrotic Effects in the Liver. Front. Nutr. 2021, 8, 715183. [Google Scholar] [CrossRef]
- Wang, H.; Sit, W.-H.; Tipoe, G.L.; Wan, J.M.-F. Differential Protective Effects of Extra Virgin Olive Oil and Corn Oil in Liver Injury: A Proteomic Study. Food Chem. Toxicol. 2014, 74, 131–138. [Google Scholar] [CrossRef]
- Kutlu, T.; Özkan, H.; Güvenç, M. Tyrosol Retards Induction of Fibrosis in Rats. J. Food Biochem. 2021, 45, e13965. [Google Scholar] [CrossRef]
- Gabbia, D.; Carpi, S.; Sarcognato, S.; Zanotto, I.; Sayaf, K.; Colognesi, M.; Polini, B.; Digiacomo, M.; Macchia, M.; Nieri, P.; et al. The Phenolic Compounds Tyrosol and Hydroxytyrosol Counteract Liver Fibrogenesis via the Transcriptional Modulation of NADPH Oxidases and Oxidative Stress-Related miRNAs. Biomed. Pharmacother. 2023, 157, 114014. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Anwar, S.; Yousif Babiker, A.; Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Antioxidant, Anti-Inflammatory and Hepatoprotective Effects of Olive Fruit Pulp Extract: In Vivo and in Vitro Study. J. Taibah Univ. Sci. 2020, 14, 1660–1670. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the Global Cancer Incidence and Mortality in 2018: GLOBOCAN Sources and Methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Paul, B.; Lewinska, M.; Andersen, J.B. Lipid Alterations in Chronic Liver Disease and Liver Cancer. JHEP Rep. 2022, 4, 100479. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.S.; Callahan, K.E.; Jones, P.D.; Morris, C.; Ransdell, J.M.; Kwon, D.; Brown, C.P.; Kobetz, E.N. Liver Cancer: A Leading Cause of Cancer Death in the United States and the Role of the 1945–1965 Birth Cohort by Ethnicity. JHEP Rep. 2019, 1, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Danpanichkul, P.; Suparan, K.; Ng, C.H.; Dejvajara, D.; Kongarin, S.; Panpradist, N.; Chaiyakunapruk, N.; Muthiah, M.D.; Chen, V.L.; Huang, D.Q.; et al. Global and Regional Burden of Alcohol-Associated Liver Disease and Alcohol Use Disorder in the Elderly. JHEP Rep. 2024, 6, 101020. [Google Scholar] [CrossRef]
- Emma, M.R.; Augello, G.; Di Stefano, V.; Azzolina, A.; Giannitrapani, L.; Montalto, G.; Cervello, M.; Cusimano, A. Potential Uses of Olive Oil Secoiridoids for the Prevention and Treatment of Cancer: A Narrative Review of Preclinical Studies. Int. J. Mol. Sci. 2021, 22, 1234. [Google Scholar] [CrossRef]
- Infante, R.; Infante, M.; Pastore, D.; Pacifici, F.; Chiereghin, F.; Malatesta, G.; Donadel, G.; Tesauro, M.; Della-Morte, D. An Appraisal of the Oleocanthal-Rich Extra Virgin Olive Oil (EVOO) and Its Potential Anticancer and Neuroprotective Properties. Int. J. Mol. Sci. 2023, 24, 17323. [Google Scholar] [CrossRef]
- Beauchamp, G.K.; Keast, R.S.J.; Morel, D.; Lin, J.; Pika, J.; Han, Q.; Lee, C.-H.; Smith, A.B.; Breslin, P.A.S. Ibuprofen-like Activity in Extra-Virgin Olive Oil. Nature 2005, 437, 45–46. [Google Scholar] [CrossRef]
- Han, Y.-M.; Lee, Y.-J.; Jang, Y.-N.; Kim, H.-M.; Seo, H.S.; Jung, T.W.; Jeong, J.H. Aspirin Improves Nonalcoholic Fatty Liver Disease and Atherosclerosis through Regulation of the PPARδ-AMPK-PGC-1α Pathway in Dyslipidemic Conditions. Biomed. Res. Int. 2020, 2020, 7806860. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Hsu, Y.-C.; Ho, H.J.; Lin, J.-T.; Chen, Y.-J.; Wu, C.-Y. Daily Aspirin Associated with a Reduced Risk of Hepatocellular Carcinoma in Patients with Non-Alcoholic Fatty Liver Disease: A Population-Based Cohort Study. eClinicalMedicine 2023, 61, 102065. [Google Scholar] [CrossRef]
- Khanal, P.; Oh, W.-K.; Yun, H.J.; Namgoong, G.M.; Ahn, S.-G.; Kwon, S.-M.; Choi, H.-K.; Choi, H.S. P-HPEA-EDA, a Phenolic Compound of Virgin Olive Oil, Activates AMP-Activated Protein Kinase to Inhibit Carcinogenesis. Carcinogenesis 2011, 32, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Pei, T.; Meng, Q.; Han, J.; Sun, H.; Li, L.; Song, R.; Sun, B.; Pan, S.; Liang, D.; Liu, L. (-)-Oleocanthal Inhibits Growth and Metastasis by Blocking Activation of STAT3 in Human Hepatocellular Carcinoma. Oncotarget 2016, 7, 43475–43491. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, A.; Balasus, D.; Azzolina, A.; Augello, G.; Emma, M.R.; Di Sano, C.; Gramignoli, R.; Strom, S.C.; McCubrey, J.A.; Montalto, G.; et al. Oleocanthal Exerts Antitumor Effects on Human Liver and Colon Cancer Cells through ROS Generation. Int. J. Oncol. 2017, 51, 533–544. [Google Scholar] [CrossRef] [PubMed]
- De Stefanis, D.; Scimè, S.; Accomazzo, S.; Catti, A.; Occhipinti, A.; Bertea, C.M.; Costelli, P. Anti-Proliferative Effects of an Extra-Virgin Olive Oil Extract Enriched in Ligstroside Aglycone and Oleocanthal on Human Liver Cancer Cell Lines. Cancers 2019, 11, 1640. [Google Scholar] [CrossRef] [PubMed]
- Bahrani, H.M.H.; Ghobeh, M.; Homayouni Tabrizi, M. The Anticancer, Anti-Oxidant, and Antibacterial Activities of Chitosan-Lecithin-Coated Parthenolide/Tyrosol Hybrid Nanoparticles. J. Biomater. Sci. Polym. Ed. 2023, 34, 1603–1617. [Google Scholar] [CrossRef]
- Pang, K.-L.; Chin, K.-Y. The Biological Activities of Oleocanthal from a Molecular Perspective. Nutrients 2018, 10, 570. [Google Scholar] [CrossRef]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant Activity of Hydroxytyrosol and Vitamin E Reduces Systemic Inflammation in Children with Paediatric NAFLD. Dig. Liver Dis. 2021, 53, 1154–1158. [Google Scholar] [CrossRef]
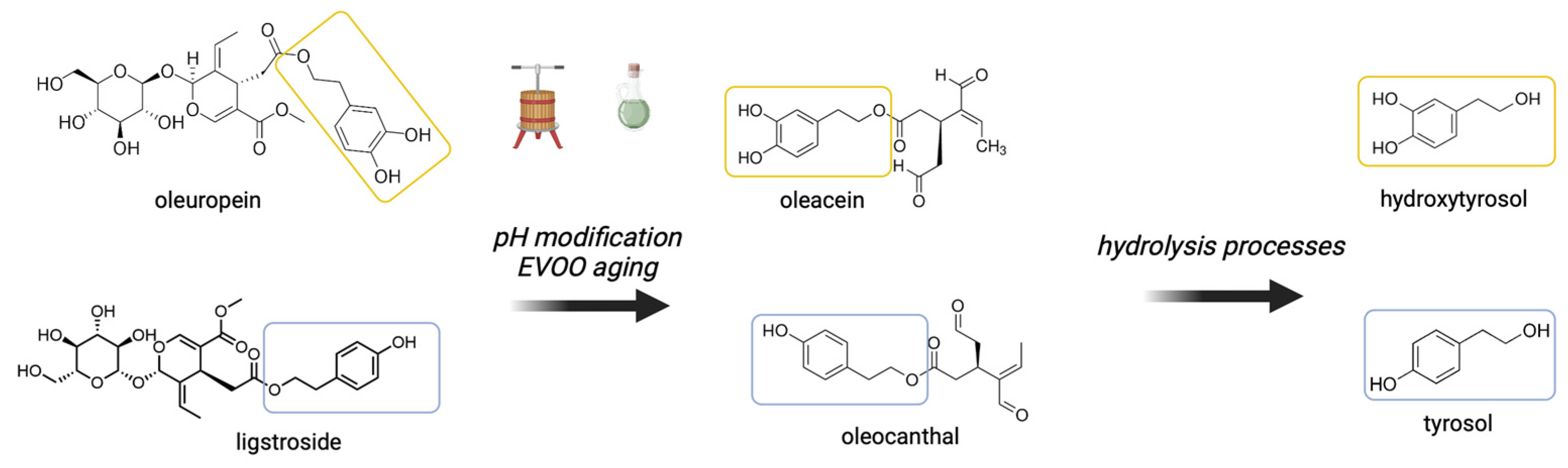
| Compound Name | Chemical Structure |
|---|---|
| oleuropein | 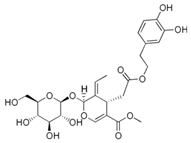 |
| ligstroside | 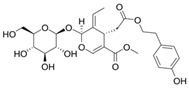 |
| oleacein | 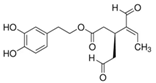 |
| oleocanthal | 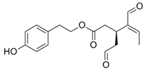 |
| hydroxytyrosol | 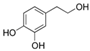 |
| tyrosol | 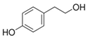 |
| Study Refs. | Study Type | Study Population | NAFLD Diagnosis | Treatment | Results |
|---|---|---|---|---|---|
| [82] | non-randomized, open-label, prospective intervention study | 44 patients with untreated NAFLD without fibrosis (>18 years of age) and BMI > 25 kg/m2 | abdominal ultrasonography and elastography stiffness (<75 kPa) | 24 weeks of traditional Mediterranean diet, with increased EVOO, vegetables, whole grains, fruits, fish, and legumes | improved steatosis, decreased blood pressure, fasting glucose, and glycated hemoglobin, decreased C-reactive protein (CRP), and oxidized low-density lipoprotein levels |
| [83] | randomized study—FLiO: Fatty Liver in Obesity | 98 overweight or obese patients with NAFLD (40–80 years of age) | abdominal ultrasonography | 6 months of two energy-restricted diets (30% energy restriction): one standard restricted diet and MetDiet with increased natural antioxidant like EVOO | greater reduction in body weight, total fat mass, and liver fat compared to other type of diet after 6 months of follow-up in the Mediterranean diet group |
| [84] | single-blind randomized trial | 45 pediatric patients with NAFLD (9–17 years of age) | abdominal ultrasonography | 12 weeks of Mediterranean diet (high intake of EVOO, vegetables, fruits, cereals, nuts, and legumes) vs. low-fat diet | improved hepatic steatosis, insulin resistance, and levels of liver enzymes (ALT) |
| [85] | non-randomized, open-label, intervention study | 23 subjects with metabolic syndrome and hepatic steatosis (18–70 years of age) | hepatic steatosis (by fatty liver index, FLI), abdominal fat distribution (by ultrasound) | 4 large spoons daily of EVOO rich in oleocanthal (which corresponded to 32 g of EVOO) during their main meals, e.g., at lunch and dinner, for a period of 60 days. | reduction in body weight, waist circumference, body mass index, ALT and fatty liver index, IL6, IL17A, TNF-α, and IL-1β, and increasing IL10 |
| Compound | Biological Activity | Mechanism of Action | Study Type | Ref. |
|---|---|---|---|---|
| Tyrosol | Reduced fatty acid synthesis, de novo lipogenesis, and TG synthesis | Inhibition of acetyl-CoA carboxylase (ACC) and diacylglycerol acyltransferase (DGAT) | In vitro (primary cultured rat hepatocytes) | [59] |
| Regulation of lipid metabolism | Increase in liver spermidine, taurine, linoleic acid, malic acid, and eicosapentaenoic acid Upregulation of Pparα, Cpt1a, Acadm Downregulation of Scd1 and Srebp-1c | In vivo (high-fat-diet-fed mice) | [10] | |
| Increased lipid oxidation and inhibition of de novo lipogenesis | Reduction in total cholesterol insulin (INS), uric acid, low-density lipoprotein cholesterol (LDL-C), and aspartate aminotransferase (ALT), Reduction in TNF-α, flavin monooxygenase 3 (FMO3), and xanthine oxidase (XOD) Reduction in the detrimental accumulation of hepatic trimethylamine N-oxide (TMAO) | In vivo (high-fructose-fed mice) | [62] | |
| Antioxidant effect and inhibition of H2S biosynthesis | Modulation of hepatic glutathione, decrease in the GSH:GSSG ratio associated with liver injury, upregulation of cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) | In vivo (high-fat-diet-fed mice) | [66] | |
| Reduced mitochondrial β-oxidation, FA uptake, and lipid accumulation | Downregulation of Ppara and upregulation of Pparg and Cpt1; decreased ROS production | In vitro (steatotic hepatocytes) | [67] | |
| Anti-inflammatory effect | Reduction in the up-regulation of JAK1 and STAT3 and decrease in IL-6, TNF-α, and IL-10 | In vivo (high-fat-diet-fed mice) | [68] | |
| Improvement in inflammation, degeneration, and fibrosis | Reduction in α-SMA and hepatocyte apoptosis; increase in glutathione (GSH) level, glutathione peroxidase (GSH.Px), and catalase (CAT) | In vivo (thioacethamide-treated rats) | [94] | |
| Antifibrotic and antioxidant effect | Modulation of two oxidative-stress-related miR-181–5p and miR-29b-3p; downregulation of NOX1 and NOX4 | In vivo (CCl4-treated fibrotic mice) | [95] | |
| Hypocholesterolemic effect | Inhibition of 3-hydroxy 3-methylglutaryl coenzyme A reductase (HMG-CoA reductase) | In vitro (steatotic HepG2 cells) | [60] | |
| Antihyperlipidemic effect | Inhibition of HMG-CoA reductase; increase in plasma lipoprotein lipase and lecithin cholesterol acyltransferase | In vivo (streptozotocin-induced diabetic rats) | [61] | |
| Improvement in the three hallmarks of MASH: steatosis, inflammation, and fibrosis | Decreased accumulation of CD86+ macrophages, restoration of levels of CD4+ CD8− T cells, increase in CD4+ FoxP3+ Treg cells, involved in regenerative pathways downregulation of NOX1, TGF-β1 and IL6 | In vivo (high-fat-, high-fructose-diet-fed mice treated with CCL4) | [71] | |
| Oleocanthal | Regulation of lipid metabolism Reduction of fatty acid synthesis and TG synthesis | Inhibition of acetyl-CoA carboxylase (ACC), enzyme that catalyzes one step of de novo lipogenesis, and diacylglycerol acyltransferase (DGAT) | ||
| Antifibrotic and antioxidant effect | Modulation of miR-221-3p and miR-181-5p, upregulation of antifibrotic miR-29b-3p and miR-101b-3p, downregulation of VEGFA, MMP2, MMP3, MMP7, NOX1, and NOX4 | In vitro/in vivo (activated LX2 cells and CCl4-treated fibrotic mice) | [92] | |
| Anticancer effect | Activation of the AMPK pathway and inhibition of COX-2 | In vitro (cancer cell model) | [106] | |
| Anticancer effect and inhibition of metastatic capacity | Inhibition of the STAT3 transcription pathway, downregulation of Cyclin D1, BCL-2, and MMP2 | In vitro (HCC cell model) | [107] | |
| Anticancer effect | mitochondrial depolarization and increased expression of γ phosphorylated form of the histone H2AX (γH2AX), | In vitro (HCC cell model) | [108] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabbia, D. Beneficial Effects of Tyrosol and Oleocanthal from Extra Virgin Olive Oil on Liver Health: Insights into Their Mechanisms of Action. Biology 2024, 13, 760. https://doi.org/10.3390/biology13100760
Gabbia D. Beneficial Effects of Tyrosol and Oleocanthal from Extra Virgin Olive Oil on Liver Health: Insights into Their Mechanisms of Action. Biology. 2024; 13(10):760. https://doi.org/10.3390/biology13100760
Chicago/Turabian StyleGabbia, Daniela. 2024. "Beneficial Effects of Tyrosol and Oleocanthal from Extra Virgin Olive Oil on Liver Health: Insights into Their Mechanisms of Action" Biology 13, no. 10: 760. https://doi.org/10.3390/biology13100760
APA StyleGabbia, D. (2024). Beneficial Effects of Tyrosol and Oleocanthal from Extra Virgin Olive Oil on Liver Health: Insights into Their Mechanisms of Action. Biology, 13(10), 760. https://doi.org/10.3390/biology13100760






