Oxidative Stress Markers and Na,K-ATPase Enzyme Kinetics Are Altered in the Cerebellum of Zucker Diabetic Fatty fa/fa Rats: A Comparison with Lean fa/+ and Wistar Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Model
2.2. Determination of Parameters of Oxidative Stress in Plasma and Cerebellar Tissue
2.3. Isolation of Plasma Membrane Fraction of Cerebellum
2.4. Kinetic Parameters of Na,K-ATPase Enzyme
2.5. Statistical Analyses
3. Results
3.1. Main Characteristics of the Laboratory Rats
3.2. Parameters of Oxidative Stress and Antioxidant Status in Blood Plasma and Cerebellum
3.3. Na,K-ATPase Enzyme Kinetic in the Cerebellum
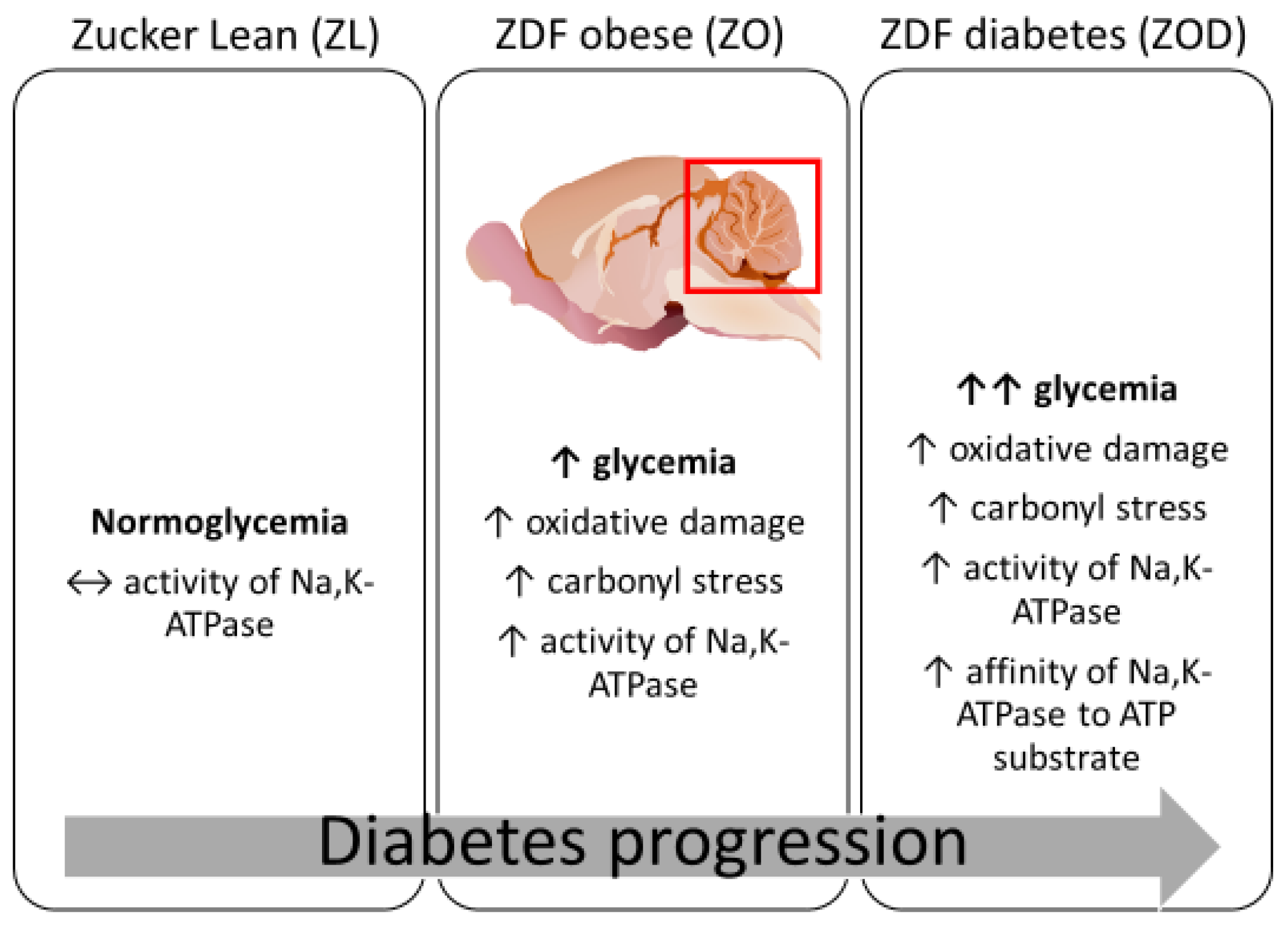
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ogurtsova, K.; Da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global Estimates for the Prevalence of Diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Moheet, A.; Mangia, S.; Seaquist, E.R. Impact of Diabetes on Cognitive Function and Brain Structure. Ann. N. Y. Acad. Sci. 2015, 1353, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Madhusudhanan, J.; Suressh, G.; Devanathan, V. Neurodegeneration in Type 2 Diabetes: Alzheimer’s as a Case Study. Brain Behav. 2020, 10, e01577. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Corral, S.; Tan, D.-X.; Manchester, L.; Reiter, R.J. Diabetes and Alzheimer Disease, Two Overlapping Pathologies with the Same Background: Oxidative Stress. Oxidative Med. Cell. Longev. 2015, 2015, 985845. [Google Scholar] [CrossRef] [PubMed]
- Geijselaers, S.L.C.; Sep, S.J.S.; Stehouwer, C.D.A.; Biessels, G.J. Glucose Regulation, Cognition, and Brain MRI in Type 2 Diabetes: A Systematic Review. Lancet Diabetes Endocrinol. 2015, 3, 75–89. [Google Scholar] [CrossRef]
- Veselov, I.M.; Vinogradova, D.V.; Maltsev, A.V.; Shevtsov, P.N.; Spirkova, E.A.; Bachurin, S.O.; Shevtsova, E.F. Mitochondria and Oxidative Stress as a Link between Alzheimer’s Disease and Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 14450. [Google Scholar] [CrossRef]
- Zhang, D.; Qi, F.; Gao, J.; Yan, X.; Wang, Y.; Tang, M.; Zhe, X.; Cheng, M.; Wang, M.; Xie, Q.; et al. Altered Cerebellar-Cerebral Circuits in Patients With Type 2 Diabetes Mellitus. Front. Neurosci. 2020, 14, 571210. [Google Scholar] [CrossRef]
- Vrbjar, N.; Jasenovec, T.; Kollarova, M.; Snurikova, D.; Chomova, M.; Radosinska, D.; Shawkatova, I.; Tothova, L.; Radosinska, J. Na,K-ATPase Kinetics and Oxidative Stress in Kidneys of Zucker Diabetic Fatty (fa/fa) Rats Depending on the Diabetes Severity—Comparison with Lean (fa/+) and Wistar Rats. Biology 2022, 11, 1519. [Google Scholar] [CrossRef]
- Ottlecz, A.; Garcia, C.A.; Eichberg, J.; Fox, D.A. Alterations in Retinal Na+, K+-ATPase in Diabetes: Streptozotocin-Induced and Zucker Diabetic Fatty Rats. Curr. Eye Res. 1993, 12, 1111–1121. [Google Scholar] [CrossRef]
- Zhang, X.; Lee, W.; Bian, J.-S. Recent Advances in the Study of Na+/K+-ATPase in Neurodegenerative Diseases. Cells 2022, 11, 4075. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.N.; Triller, A.; Melki, R. Cell Biology and Dynamics of Neuronal Na+/K+-ATPase in Health and Diseases. Neuropharmacology 2020, 169, 107461. [Google Scholar] [CrossRef] [PubMed]
- Talaei, F.; Van Praag, V.M.; Shishavan, M.H.; Landheer, S.W.; Buikema, H.; Henning, R.H. Increased Protein Aggregation in Zucker Diabetic Fatty Rat Brain: Identification of Key Mechanistic Targets and the Therapeutic Application of Hydrogen Sulfide. BMC Cell Biol. 2014, 15, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Winocur, G.; Greenwood, C.E.; Piroli, G.G.; Grillo, C.A.; Reznikov, L.R.; Reagan, L.P.; McEwen, B.S. Memory Impairment in Obese Zucker Rats: An Investigation of Cognitive Function in an Animal Model of Insulin Resistance and Obesity. Behav. Neurosci. 2005, 119, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Oltman, C.L.; Coppey, L.J.; Gellett, J.S.; Davidson, E.P.; Lund, D.D.; Yorek, M.A. Progression of Vascular and Neural Dysfunction in Sciatic Nerves of Zucker Diabetic Fatty and Zucker Rats. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E113–E122. [Google Scholar] [CrossRef]
- Almon, R.; Wang, X.; DuBois, D.C.; Sukumaran, S.; Ayyar, V.; Jusko, W.J. Variability in Zucker Diabetic Fatty Rats: Differences in Disease Progression in Hyperglycemic and Normoglycemic Animals. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 531–541. [Google Scholar] [CrossRef]
- Kollarova, M.; Chomova, M.; Radosinska, D.; Tothova, L.; Shawkatova, I.; Radosinska, J. ZDF (fa/fa) Rats Show Increasing Heterogeneity in Main Parameters during Ageing, as Confirmed by Biometrics, Oxidative Stress Markers and MMP Activity. Exp. Physiol. 2022, 107, 1326–1338. [Google Scholar] [CrossRef]
- Ito, D.; Cao, P.; Kakihana, T.; Sato, E.; Suda, C.; Muroya, Y.; Ogawa, Y.; Hu, G.; Ishii, T.; Ito, O.; et al. Chronic Running Exercise Alleviates Early Progression of Nephropathy with Upregulation of Nitric Oxide Synthases and Suppression of Glycation in Zucker Diabetic Rats. PLoS ONE 2015, 10, e0138037. [Google Scholar] [CrossRef]
- Jørgensen, P.L.; Skou, J.C.; Solomonson, L.P. Purification and Characterization of (Na++ K+-ATPase. II. Preparation by Zonal Centrifugation of Highly Active (Na++ K+-ATPase from the Outer Medulla of Rabbit Kidneys. Biochim. Biophys. Acta BBA Biomembr. 1971, 233, 381–394. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Kodl, C.T.; Seaquist, E.R. Cognitive Dysfunction and Diabetes Mellitus. Endocr. Rev. 2008, 29, 494–511. [Google Scholar] [CrossRef]
- Jacobs, H.I.L.; Hopkins, D.A.; Mayrhofer, H.C.; Bruner, E.; Van Leeuwen, F.W.; Raaijmakers, W.; Schmahmann, J.D. The Cerebellum in Alzheimer’s Disease: Evaluating Its Role in Cognitive Decline. Brain 2018, 141, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sickmann, H.M.; Waagepetersen, H.S.; Schousboe, A.; Benie, A.J.; Bouman, S.D. Obesity and Type 2 Diabetes in Rats Are Associated with Altered Brain Glycogen and Amino-Acid Homeostasis. J. Cereb. Blood Flow. Metab. 2010, 30, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, R.A.; Desai, S.; Moitra, P.; Salis, S.; Agashe, S.; Battalwar, R.; Mehta, A.; Madan, J.; Kalita, S.; Udipi, S.A.; et al. Hyperinsulinemia: An Early Biomarker of Metabolic Dysfunction. Front. Clin. Diabetes Healthc. 2023, 4, 1159664. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.; Teixeira-de-Lemos, E.; Pinto, F.; Parada, B.; Mega, C.; Vala, H.; Pinto, R.; Garrido, P.; Sereno, J.; Fernandes, R.; et al. Effects of Sitagliptin Treatment on Dysmetabolism, Inflammation, and Oxidative Stress in an Animal Model of Type 2 Diabetes (ZDF Rat). Mediat. Inflamm. 2010, 2010, 592760. [Google Scholar] [CrossRef]
- Gallardo-Villanueva, P.; Fernández-Marcelo, T.; Villamayor, L.; Valverde, A.M.; Ramos, S.; Fernández-Millán, E.; Martín, M.A. Synergistic Effect of a Flavonoid-Rich Cocoa–Carob Blend and Metformin in Preserving Pancreatic Beta Cells in Zucker Diabetic Fatty Rats. Nutrients 2024, 16, 273. [Google Scholar] [CrossRef]
- Moreno, F.; Méndez, L.; Raner, A.; Miralles-Pérez, B.; Romeu, M.; Ramos-Romero, S.; Torres, J.L.; Medina, I. Dietary Marine Oils Selectively Decrease Obesogenic Diet-Derived Carbonylation in Proteins Involved in ATP Homeostasis and Glutamate Metabolism in the Rat Cerebellum. Antioxidants 2024, 13, 103. [Google Scholar] [CrossRef]
- Raza, H.; John, A.; Howarth, F.C. Increased Oxidative Stress and Mitochondrial Dysfunction in Zucker Diabetic Rat Liver and Brain. Cell. Physiol. Biochem. 2015, 35, 1241–1251. [Google Scholar] [CrossRef]
- Wang, X.; Michaelis, E.K. Selective Neuronal Vulnerability to Oxidative Stress in the Brain. Front. Aging Neurosci. 2010, 2, 12. [Google Scholar] [CrossRef]
- Ramalingam, M.; Kim, S.-J. Mechanisms of Action of Brain Insulin against Neurodegenerative Diseases. J. Neural. Transm. 2014, 121, 611–626. [Google Scholar] [CrossRef]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; De Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH Oxidase 1 Plays a Key Role in Diabetes Mellitus–Accelerated Atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Pickering, R.J.; Rosado, C.J.; Sharma, A.; Buksh, S.; Tate, M.; De Haan, J.B. Recent Novel Approaches to Limit Oxidative Stress and Inflammation in Diabetic Complications. Clin. Transl. Immunol. 2018, 7, e1016. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Nie, Y.; Chaudhry, M.; Bai, F.; Chuang, J.; Sodhi, K.; Shapiro, J.I. The Redox-Sensitive Na/K-ATPase Signaling in Uremic Cardiomyopathy. Int. J. Mol. Sci. 2020, 21, 1256. [Google Scholar] [CrossRef] [PubMed]
- Namazi, G.; Asa, P.; Sarrafzadegan, N.; Pourfarzam, M. Decreased Na+/K+-ATPase Activity and Altered Susceptibility to Peroxidation and Lipid Composition in the Erythrocytes of Metabolic Syndrome Patients with Coronary Artery Disease. Ann. Nutr. Metab. 2019, 74, 140–148. [Google Scholar] [CrossRef]
- Chakraborty, H.; Sen, P.; Sur, A.; Chatterjee, U.; Chakrabarti, S. Age-Related Oxidative Inactivation of Na+, K+-ATPase in Rat Brain Crude Synaptosomes. Exp. Gerontol. 2003, 38, 705–710. [Google Scholar] [CrossRef]
- Petrushanko, I.; Bogdanov, N.; Bulygina, E.; Grenacher, B.; Leinsoo, T.; Boldyrev, A.; Gassmann, M.; Bogdanova, A. Na-K-ATPase in Rat Cerebellar Granule Cells Is Redox Sensitive. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 290, R916–R925. [Google Scholar] [CrossRef]
- Attwell, D.; Laughlin, S.B. An Energy Budget for Signaling in the Grey Matter of the Brain. J. Cereb. Blood Flow. Metab. 2001, 21, 1133–1145. [Google Scholar] [CrossRef]
- Erecińska, M.; Silver, I.A. Ions and Energy in Mammalian Brain. Prog. Neurobiol. 1994, 43, 37–71. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Sagrillo, M.R.; Da Silva, A.S.; Stefani, L.M.; Monteiro, S.G. The Use of Tucumã Oil (Astrocaryum Vulgare) in Alloxan-Induced Diabetic Mice: Effects on Behavior, Oxidant/Antioxidant Status, and Enzymes Involved in Brain Neurotransmission. Mol. Cell Biochem. 2017, 436, 159–166. [Google Scholar] [CrossRef]
- Chellammal, H.S.J.; Hasan, M.H.; Kshirsagar, R.P.; Musukula, V.K.R.; Ramachandran, D.; Diwan, P.V. Metformin Inhibits Cardiometabolic Syndrome Associated Cognitive Deficits in High Fat Diet Rats. J. Diabetes Metab. Disord. 2022, 21, 1415–1426. [Google Scholar] [CrossRef]
- Stefanello, N.; Schmatz, R.; Pereira, L.B.; Rubin, M.A.; Da Rocha, J.B.T.; Facco, G.; Pereira, M.E.; Mazzanti, C.M.D.A.; Passamonti, S.; Rodrigues, M.V.; et al. Effects of Chlorogenic Acid, Caffeine, and Coffee on Behavioral and Biochemical Parameters of Diabetic Rats. Mol. Cell Biochem. 2014, 388, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Vér, Á.; Csermely, P.; Bányász, T.; Kovács, T.; Somogyi, J. Alterations in the Properties and Isoform Ratios of Brain Na+/K+-ATPase in Streptozotocin Diabetic Rats. Biochim. Biophys. Acta BBA Biomembr. 1995, 1237, 143–150. [Google Scholar] [CrossRef]
- Bojorge, G.; Deloresarnaiz, G. Insulin Modifies Na+, K+-ATPase Activity of Synaptosomal Membranes and Whole Homogenates Prepared from Rat Cerebral Cortex. Neurochem. Int. 1987, 11, 11–16. [Google Scholar] [CrossRef]
- Brodsky, J.L. Insulin Activation of Brain Na(+)-K(+)-ATPase Is Mediated by Alpha 2-Form of Enzyme. Am. J. Physiol. Cell Physiol. 1990, 258, C812–C817. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, E.; Velázquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the Brain: Its Pathophysiological Implications for States Related with Central Insulin Resistance, Type 2 Diabetes and Alzheimer’s Disease. Front. Endocrinol. 2014, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Pacak, K.; McCarty, R.; Palkovits, M.; Cizza, G.; Kopin, I.J.; Goldstein, D.S.; Chrousos, G.P. Decreased Central and Peripheral Catecholaminergic Activation in Obese Zucker Rats. Endocrinology 1995, 136, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Mayanil, C.S.K.; Kazmi, S.M.I.; Baquer, N.Z. Na+, K+-ATPase and Mg2+-ATPase Activities in Different Regions of Rat Brain During Alloxan Diabetes. J. Neurochem. 1982, 39, 903–908. [Google Scholar] [CrossRef]
- Nwanna, E.E.; Ibukun, E.O.; Oboh, G. Eggplant (Solanum Spp.) Supplemented Fruits Diet Modulated the Activities of Ectonucleoside Triphosphate Diphosphohydrolase (ENTPdase), Monoamine Oxidase (MAO), and Cholinesterases (AChE/BChE) in the Brain of Diabetic Wistar Male Rats. J. Food Biochem. 2019, 43, e12910. [Google Scholar] [CrossRef]
- Philbert, S.A.; Xu, J.; Scholefield, M.; Church, S.J.; Unwin, R.D.; Cooper, G.J.S. Contrasting Sodium and Potassium Perturbations in the Hippocampus Indicate Potential Na+/K+-ATPase Dysfunction in Vascular Dementia. Front. Aging Neurosci. 2022, 14, 822787. [Google Scholar] [CrossRef]
- Ma, W.-X.; Tang, J.; Lei, Z.-W.; Li, C.-Y.; Zhao, L.-Q.; Lin, C.; Sun, T.; Li, Z.-Y.; Jiang, Y.-H.; Jia, J.-T.; et al. Potential Biochemical Mechanisms of Brain Injury in Diabetes Mellitus. Aging Dis. 2020, 11, 978. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, W.; Song, Y.; Zhang, B.; Li, F.; Liu, Y. Blockade of Store-Operated Calcium Entry Alleviates High Glucose-Induced Neurotoxicity via Inhibiting Apoptosis in Rat Neurons. Chem. Biol. Interact. 2016, 254, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Chauhan, A.; Brown, W.T.; Chauhan, V. Increased Activities of Na+/K+-ATPase and Ca2+/Mg2+-ATPase in the Frontal Cortex and Cerebellum of Autistic Individuals. Life Sci. 2009, 85, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Scarpini, E.; Bianchi, R.; Moggio, M.; Sciacco, M.; Fiori, M.G.; Scarlato, G. Decrease of Nerve Na+,K+-ATPase Activity in the Pathogenesis of Human Diabetic Neuropathy. J. Neurol. Sci. 1993, 120, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.V.; Lin, C.S.-Y.; Kiernan, M.C. Activity-Dependent Excitability Changes Suggest Na+/K+ Pump Dysfunction in Diabetic Neuropathy. Brain 2008, 131, 1209–1216. [Google Scholar] [CrossRef]
- Leong, S.F.; Leung, T.K.C. Diabetes Induced by Streptozotocin Causes Reduced Na?K ATPase in the Brain. Neurochem. Res. 1991, 16, 1161–1165. [Google Scholar] [CrossRef]
- Phillips, F.C.; Cleary, M.P. Metabolic Measurements among Homozygous (fa/fa) Obese, Heterozygous (fa/fa) Lean and Homozygous (fa/fa) Lean Zucker Rat Pups at 17 Days of Age. J. Nutr. 1994, 124, 1230–1237. [Google Scholar] [CrossRef]
- Koerber-Rosso, I.; Brandt, S.; Von Schnurbein, J.; Fischer-Posovszky, P.; Hoegel, J.; Rabenstein, H.; Siebert, R.; Wabitsch, M. A Fresh Look to the Phenotype in Mono-Allelic Likely Pathogenic Variants of the Leptin and the Leptin Receptor Gene. Mol. Cell. Pediatr. 2021, 8, 10. [Google Scholar] [CrossRef]
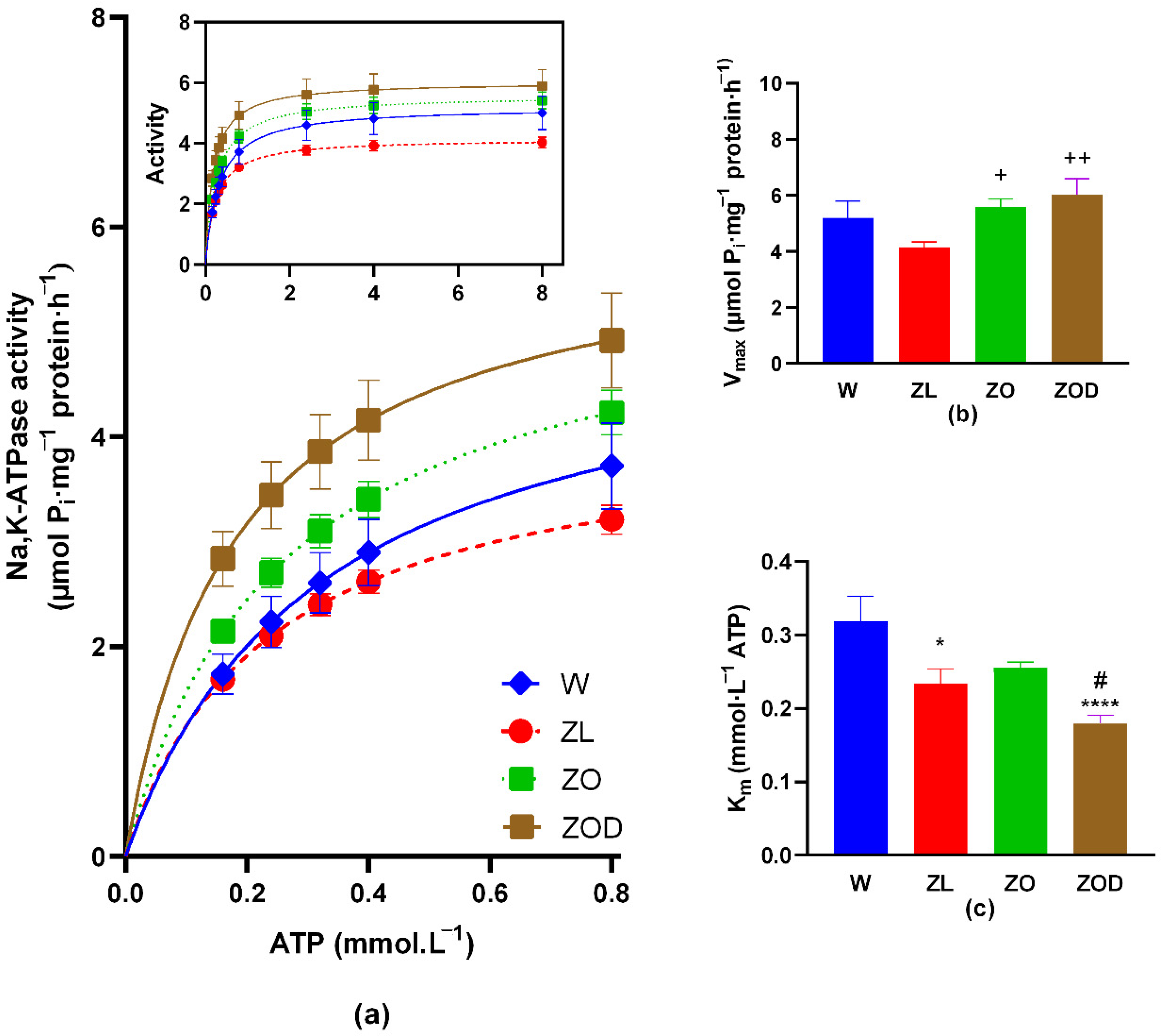
| W (n = 12) | ZL (n = 12) | ZO (n = 8) | ZOD (n = 8) | |
|---|---|---|---|---|
| BW (g) | 488 ± 27 | 413 ± 10 * | 628 ± 8 ****,++++ | 528 ± 17 +++,## |
| Brain weight (g) | 2.07 ± 0.02 | 1.96 ± 0.03 * | 1.87 ± 0.03 *** | 1.87 ± 0.02 *** |
| CW (g) | 0.29 ± 0.009 | 0.25 ± 0.009 * | 0.24 ± 0.011 ** | 0.24 ± 0.006 ** |
| CW/BW × 105 | 56.1 ± 1.0 | 61.1 ± 2.2 | 38.0 ± 2.0 ****,++++ | 45.3 ± 1.9 **,++++ |
| Glucose (mmol·L−1) | 7.2 ± 0.1 | 6.7 ± 0.1 | 8.8 ± 0.2 + | 18.3 ± 1.2 ****,++++,#### |
| Insulin (ng·mL−1) | 7.9 ± 1.8 | 1.6 ± 0.08 ** | 19.3 ± 2.1 ****,++++ | 8.7 ± 1.0 ++,### |
| HOMA-IR | 2.77 ± 0.59 | 0.47 ± 0.02 ** | 7.49 ± 0.77 ****,++++ | 6.87 ± 0.63 ****,++++ |
| HOMA-IS | 20.55 ± 5.15 | 54.39 ± 2.75 **** | 3.15 ± 0.24 *,++++ | 3.82 ± 0.36 *,++++ |
| HOMA-β | 1.18 ± 0.23 | 0.27 ± 0.02 ** | 2.51 ± 0.31 ***,++++ | 0.57 ± 0.09 #### |
| Cerebellum | W (n = 11–12) | ZL (n = 10–12) | ZO (n = 7–8) | ZOD (n = 7–8) |
|---|---|---|---|---|
| TBARSs (µmol·L−1) | 34.20 ± 4.23 | 33.94 ± 3.57 | 60.17 ± 3.87 ***,+++ | 57.44 ± 2.33 ***,+++ |
| AOPPs (µmol·L−1) | 71.81 ± 7.74 | 89.74 ± 16.40 | 140.0 ± 10.07 ** | 127.2 ± 10.11 * |
| Fructosamine (mmol·L−1) | 0.91 ± 0.14 | 0.87 ± 0.09 | 2.23 ± 0.52 **,++ | 1.37 ± 0.19 |
| AGE-Fl (AU) | 506 ± 8.22 | 489 ± 18.91 | 584 ± 9.09 **,++ | 613 ± 16.80 ****,++++ |
| FRAP (µmol·L−1) | 118.3 ± 17.12 | 228.3 ± 34.37 * | 243.1 ± 35.16 * | 176.7 ± 34.38 |
| GSH/GSSG ratio | 1.73 ± 0.05 | 1.60 ± 0.05 | 1.58 ± 0.11 | 1.44 ± 0.04 * |
| Plasma | W (n = 15) | ZL (n = 15) | ZO (n = 7–8) | ZOD (n = 7–8) |
| TBARSs (µmol·L−1) | 10.00 ± 0.19 | 10.95 ± 0.23 | 20.69 ± 1.10 ****,++++ | 14.85 ± 1.13 ****,+++,#### |
| AOPPs (µmol·L−1) | 128.1 ± 9.53 | 286.8 ± 33.26 | 2135 ± 388 ****,++++ | 3427 ± 617.1 ****,++++,# |
| Fructosamine (mmol·L−1) | 1.11 ± 0.05 | 1.42 ± 0.08 | 13.77 ± 2.27 ****,++++ | 7.64 ± 0.79 ****,++++,### |
| FRAP (µmol·L−1) | 397.9 ± 19.63 | 473.5 ± 19.71 | 2917 ± 662 ****,++++ | 1617 ± 200 **,++,## |
| GSH/GSSG ratio | 11.25 ± 0.55 | 11.13 ± 0.44 | 9.16 ± 0.17 *,+ | 9.77 ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radosinska, D.; Gaal Kovalcikova, A.; Gardlik, R.; Chomova, M.; Snurikova, D.; Radosinska, J.; Vrbjar, N. Oxidative Stress Markers and Na,K-ATPase Enzyme Kinetics Are Altered in the Cerebellum of Zucker Diabetic Fatty fa/fa Rats: A Comparison with Lean fa/+ and Wistar Rats. Biology 2024, 13, 759. https://doi.org/10.3390/biology13100759
Radosinska D, Gaal Kovalcikova A, Gardlik R, Chomova M, Snurikova D, Radosinska J, Vrbjar N. Oxidative Stress Markers and Na,K-ATPase Enzyme Kinetics Are Altered in the Cerebellum of Zucker Diabetic Fatty fa/fa Rats: A Comparison with Lean fa/+ and Wistar Rats. Biology. 2024; 13(10):759. https://doi.org/10.3390/biology13100759
Chicago/Turabian StyleRadosinska, Dominika, Alexandra Gaal Kovalcikova, Roman Gardlik, Maria Chomova, Denisa Snurikova, Jana Radosinska, and Norbert Vrbjar. 2024. "Oxidative Stress Markers and Na,K-ATPase Enzyme Kinetics Are Altered in the Cerebellum of Zucker Diabetic Fatty fa/fa Rats: A Comparison with Lean fa/+ and Wistar Rats" Biology 13, no. 10: 759. https://doi.org/10.3390/biology13100759
APA StyleRadosinska, D., Gaal Kovalcikova, A., Gardlik, R., Chomova, M., Snurikova, D., Radosinska, J., & Vrbjar, N. (2024). Oxidative Stress Markers and Na,K-ATPase Enzyme Kinetics Are Altered in the Cerebellum of Zucker Diabetic Fatty fa/fa Rats: A Comparison with Lean fa/+ and Wistar Rats. Biology, 13(10), 759. https://doi.org/10.3390/biology13100759







