Linking Sleep Disorders to Atrial Fibrillation: Pathways, Risks, and Treatment Implications
Abstract
Simple Summary
Abstract
1. Introduction
2. Most Common Sleep Disorders
2.1. Insomnia
2.2. Sleep-Disordered Breathing
2.3. Obstructive Sleep Apnoea (OSA)
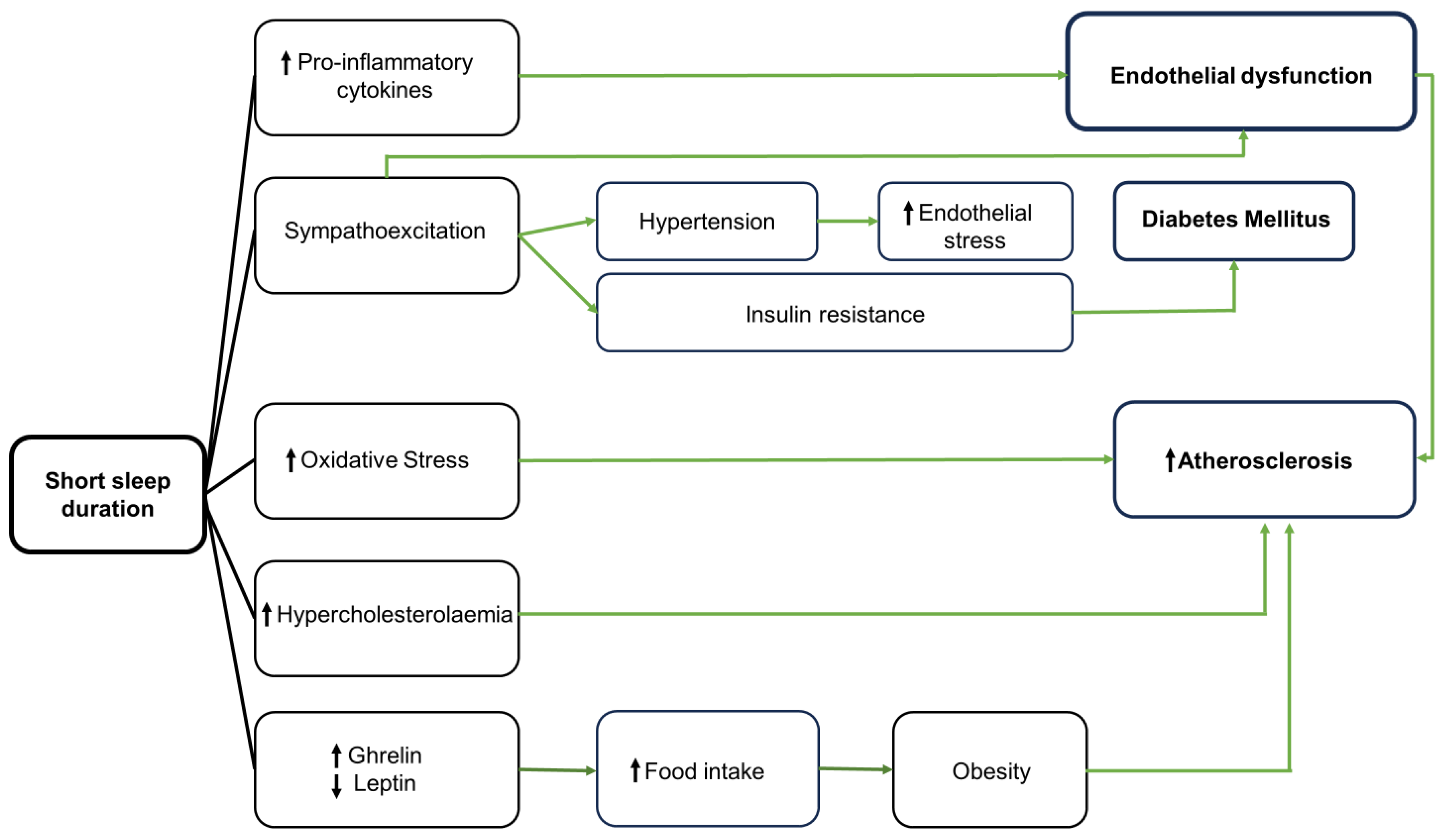
3. Pathophysiologic Mechanisms Linking Short Sleep Duration and Cardiovascular Diseases
3.1. Inflammation
3.2. Oxidative Stress and Endothelial Dysfunction
3.3. Autonomic Nervous System
3.4. Circadian Rhythms
3.5. Chronotype
4. Sleep and Atrial Fibrillation
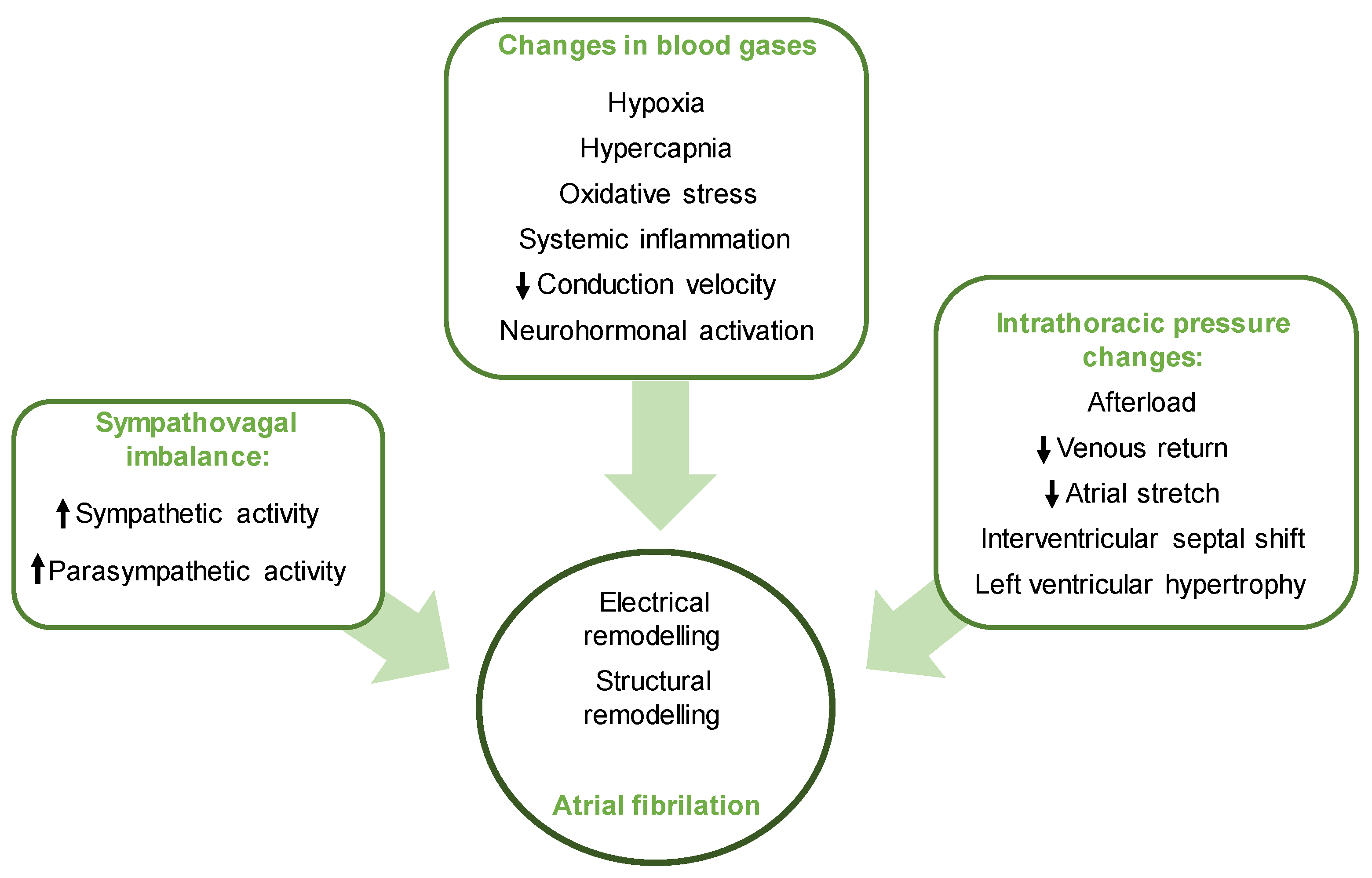
4.1. Sleep Disordered Breathing and AF
4.2. Insomnia and AF
4.3. Circadian Rhythm Disorders
4.4. Sleep Quality
4.5. Impact of Treating Sleep Disorders in AF
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Buysse, D.J. Sleep health: Can we define It? does it matter? Sleep 2024, 37, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Mai, E.; Buysse, D.J. Insomnia: Prevalence, Impact, Pathogenesis, Differential Diagnosis, and Evaluation. Sleep Med. Clin. 2008, 3, 167–174. [Google Scholar] [CrossRef]
- Nguyen, V.; George, T.; Brewster, G.S. Insomnia in Older Adults. Curr. Geriatr. Rep. 2019, 8, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased prevalence of sleep-disordered breathing in adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Evans, L.; Finn, L.; Palta, M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997, 20, 705–706. [Google Scholar] [CrossRef]
- Bailes, S.; Baltzan, M.; Alapin, I.; Fichten, C.S.; Libman, E. Diagnostic indicators of sleep apnea in older women and men: A prospective study. J. Psychosom. Res. 2005, 59, 365–373. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A. Pathophysiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef]
- Nadeem, R.; Molnar, J.; Madbouly, E.M.; Nida, M.; Aggarwal, S.; Sajid, H.; Naseem, J.; Loomba, R. Serum inflammatory markers in obstructive sleep apnea: A meta-analysis. J. Clin. Sleep Med. 2013, 9, 1003–1012. [Google Scholar] [CrossRef]
- Somers, V.K.; Dyken, M.E.; Clary, M.P.; Abboud, F.M. Sympathetic neural mechanisms in obstructive sleep apnea. J. Clin. Investig. 1995, 96, 1897–1904. [Google Scholar] [CrossRef]
- Maniaci, A.; Lavalle, S.; Parisi, F.M.; Barbanti, M.; Cocuzza, S.; Iannella, G.; Magliulo, G.; Pace, A.; Lentini, M.; Masiello, E.; et al. Impact of Obstructive Sleep Apnea and Sympathetic Nervous System on Cardiac Health: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2024, 11, 204. [Google Scholar] [CrossRef]
- Shiomi, T.; Guilleminault, C.; Stoohs, R.; Schnittger, I. Leftward shift of the interventricular septum and pulsus paradoxus in obstructive sleep apnea syndrome. Chest 1991, 100, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Manjavong, M.; Limpawattana, P.; Mairiang, P.; Anutrakulchai, S. Prevalence of insomnia and related impact. Int. J. Psychiatry Med. 2016, 51, 544–553. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Morin, C.M.; Belleville, G.; Bélanger, L.; Ivers, H. The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011, 34, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.H.; Brindle, R.C.; Buysse, D.J. Sleep and cardiovascular disease: Emerging opportunities for psychology. Am. Psychol. 2018, 73, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Goodday, R.H.; Percious, D.S.; Morrison, A.D.; Robertson, C.G. Obstructive sleep apnea syndrome: Diagnosis and management. J. Can. Dent. Assoc. 2001, 67, 652–658. [Google Scholar]
- Freedman, N. Treatment of obstructive sleep apnea syndrome. Clin. Chest Med. 2010, 31, 187–201. [Google Scholar] [CrossRef]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar]
- Chen, X.; Wang, R.; Zee, P.; Lutse, P.L.; Javaheri, S.; Alcántara, C.; Jackson, C.L.; Williams, M.A.; Redline, S. Racial/ethnic differences in sleep disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015, 38, 877–888. [Google Scholar] [CrossRef]
- Sajkov, D.; McEvoy, R.D. Obstructive Sleep Apnea and Pulmonary Hypertension. Prog. Cardiovasc. Dis. 2009, 51, 363–370. [Google Scholar] [CrossRef]
- Eisele, H.J.; Markart, P.; Schulz, R. Obstructive sleep apnea, oxidative stress, and cardiovascular disease: Evidence from human studies. Oxidative Med. Cell Longev. 2015, 2015, 608438. [Google Scholar] [CrossRef]
- Parish, J.M.; Somers, V.K. Obstructive sleep apnea and cardiovascular disease. Mayo Clin. Proc. 2004, 79, 1036–1046. [Google Scholar] [CrossRef] [PubMed]
- Stark, P.; Chang, E.Y. Sleep Apnea Combined with Pulmonary Hypertension in a Veteran Patient Population. J. Clin. Med. 2023, 12, 4634. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Dobrev, D. Sleep apnea and atrial fibrillation: Update 2020. Int. J. Cardiol. Heart Vasc. 2020, 31, 100681. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-de-la-Torre, M.; Lee, C.H.; Barbé, F. Obstructive sleep apnea and atrial fibrillation: We need to go step by step. J. Clin. Sleep Med. 2021, 17, 869–870. [Google Scholar] [CrossRef] [PubMed]
- Libby, P. Inflammation and cardiovascular disease mechanisms1–3. Am. J. Clin. Nutr. 2006, 83, 456–460. [Google Scholar] [CrossRef]
- Irwin, M.; Olmstead, R.; Carrol, J.E. Sleep Disturbance, Sleep Duration, and Inflammation. Biol. Psychiatry 2016, 80, 40–52. [Google Scholar] [CrossRef]
- Tobaldini, E.; Cogliati, C.; Fiorelli, E.M.; Nunziata, V.; Wu, M.A.; Prado, M.; Bevilacqua, M.; Trabattoni, D.; Porta, A.; Montano, N. One night on-call: Sleep deprivation affects cardiac autonomic control and inflammation in physicians. Eur. J. Intern. Med. 2013, 24, 664–670. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Papanicolaou, D.A.; Bixler, E.O.; Lotsikas, A.; Zachman, K.; Kales, A.; Prolo, P.; Wong, M.L.; Licinio, J.; Gold, P.W.; et al. Circadian interleukin-6 secretion and quantity and depth of sleep. J. Clin. Endocrinol. Metab. 1999, 84, 2603–2607. [Google Scholar] [CrossRef]
- Ridker, P.M. C-Reactive Protein and the Prediction of Cardiovascular Events Among Those at Intermediate Risk. Moving an Inflammatory Hypothesis Toward Consensus. J. Am. Coll. Cardiol. 2007, 49, 2129–2138. [Google Scholar] [CrossRef]
- Meier-Ewert, H.K.; Ridker, P.M.; Rifai, N.; Regan, M.M.; Price, N.J.; Dinges, D.F.; Mullington, J.M. Effect of sleep loss on C-Reactive protein, an inflammatory marker of cardiovascular risk. J. Am. Coll. Cardiol. 2004, 43, 678–683. [Google Scholar] [CrossRef]
- Münzel, T.; Camici, G.G.; Maack, C.; Bonetti, N.R.; Fuster, V.; Kovaci, J.C. Impact of Oxidative Stress on the Heart and Vasculature: Part 2 of a 3-Part Series. J. Am. Coll. Cardiol. 2017, 70, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Faraut, B.; Boudjeltia, K.Z.; Vanhamme, L.; Kerkhof, M. Immune, inflammatory and cardiovascular consequences of sleep restriction and recovery. Sleep Med. Rev. 2012, 16, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Boudjeltia, K.; Faraut, B.; Esposito, M.J.; Stenuit, P.; Dyzma, M.; van Antwerpen, P.; Brohée, D.; Vanhamme, L.; Moguilevsky, N.; Vanhaeverbeek, M.; et al. Temporal dissociation between myeloperoxidase (MPO)-modified LDL and MPO elevations during chronic sleep restriction and recovery in healthy young men. PLoS ONE 2011, 6, e28230. [Google Scholar] [CrossRef] [PubMed]
- Tosur, Z.; Green, D.; De Chavez, P.J.; Knutson, K.L.; Goldberger, J.J.; Zee, P.; Liu, K.; Kim, K.Y.; Carnethon, M.R. The association between sleep characteristics and prothrombotic markers in a population-based sample: Chicago Area Sleep Study. Sleep Med. 2014, 15, 973–978. [Google Scholar] [CrossRef]
- Sauvet, F.; Leftheriotis, G.; Gomez-Merino, D.; Langrume, C.; Drogou, C.; Van Beers, P.; Bourrilhon, C.; Florence, G.; Chennaoui, M. Effect of acute sleep deprivation on vascular function in healthy subjects. J. Appl. Physiol. 2010, 108, 68–75. [Google Scholar] [CrossRef]
- Chouchou, F.; Pichot, V.; Pépin, J.L.; Tamisier, R.; Celle, S.; Maudoux, D.; Garcin, A.; Lévy, P.; Barthélémy, J.C.; Roche, F. Sympathetic overactivity due to sleep fragmentation is associated with elevated diurnal systolic blood pressure in healthy elderly subjects: The PROOF-SYNAPSE study. Eur. Heart J. 2013, 34, 2122–2131. [Google Scholar] [CrossRef]
- Zhong, X.; Hilton, H.J.; Gates, G.J.; Jelic, S.; Stern, Y.; Bartels, M.N.; DeMeersman, R.E.; Basner, R.C. Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation. J. Appl. Physiol. 2005, 98, 2024–2032. [Google Scholar] [CrossRef]
- Tracey, K. The inflammatory reflex. Nature 2002, 420, 853–859. [Google Scholar] [CrossRef]
- Morris, C.J.; Aeschbach, D.; Scheer, F.A. Circadian system, sleep and endocrinology. Mol. Cell Endocrinol. 2012, 349, 91–104. [Google Scholar] [CrossRef]
- Baron, K.G.; Rei, K.J. Circadian misalignment and health. Int. Rev. Psych. 2014, 26, 139–154. [Google Scholar] [CrossRef]
- Fárková, E.; Novák, J.M.; Manková, D.; Kopřivová, J. Comparison of Munich Chronotype Questionnaire (MCTQ) and Morningness-Eveningness Questionnaire (MEQ) Czech version. Chronobiol. Int. 2020, 37, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, S.J. Psychometric properties of questionnaires for assessing chronotype. Chronobiol. Med. 2020, 2, 16–20. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Zoumakis, E.; Bixler, E.O.; Lin, H.M.; Follett, H.; Kales, A.; Chrousos, G.P. Adverse Effects of Modest Sleep Restriction on Sleepiness, Performance, and Inflammatory Cytokines. J. Clin. Endocrinol. Metabol. 2004, 89, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Gangwisch, J.E.; Heymsfield, S.B.; Boden-Albala, B.; Buijs, R.M.; Kreier, F.; Pickering, T.G.; Rundle, A.G.; Zammit, G.K.; Malaspina, D. Short sleep duration as a risk factor for hypertension: Analyses of the first National Health and Nutrition Examination Survey. Hypertension 2006, 47, 833–839. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Stranges, S.; Kandala, N.B.; Miller, M.A.; Taggart, F.M.; Kumari, M.; Ferrie, J.E.; Shipley, M.J.; Brunner, E.J.; Marmot, M.G. Gender-specific associations of short sleep duration with prevalent and incident hypertension: The whitehall II study. Hypertension 2007, 50, 693–700. [Google Scholar] [CrossRef]
- Knutson, K.L.; Van Cauter, E.; Rathouz, P.J.; Yan, L.L.; Hulley, S.B.; Liu, K.; Lauderdale, D.S. Association between sleep and blood pressure in midlife: The CARDIA sleep study. Arch. Intern. Med. 2009, 169, 1055–1061. [Google Scholar] [CrossRef]
- Kobayashi Frisk, M.; Hedner, J.; Grote, L.; Ekblom, Ö.; Arvidsson, D.; Bergström, G.; Börjesson, M.; Zou, D. Eveningness is associated with sedentary behavior and increased 10-year risk of cardiovascular disease: The SCAPIS pilot cohort. Sci. Rep. 2022, 12, 8203. [Google Scholar] [CrossRef]
- Yu, J.H.; Yun, C.H.; Ahn, J.H.; Suh, S.; Cho, H.J.; Lee, S.K.; Yoo, H.J.; Seo, J.A.; Kim, S.G.; Choi, K.M.; et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J. Clin. Endocrinol. Metab. 2015, 100, 1494–1502. [Google Scholar] [CrossRef]
- López-Olmeda, J.F.; Blanco-Vives, B.; Pujante, I.M.; Wunderink, Y.S.; Mancera, J.M.; Sánchez-Vázquez, F.J. Daily rhythms in the hypothalamus-pituitary-interrenal axis and acute stress responses in a teleost flatfish, solea senegalensis. Chronobiol. Int. 2013, 30, 530–539. [Google Scholar] [CrossRef]
- Morovatdar, N.; Ebrahimi, N.; Rezaee, R.; Poorzand, H.; Tork MA, B.; Sahebkar, A. Sleep duration and risk of atrial fibrillation: A systematic review. J. Atr. Fibrillation 2019, 11, 5–10. [Google Scholar] [CrossRef]
- Sunbul, M.; Kanar, B.; Kivrak, T.; Durmus, E.; Sari, I.; Tigen, K.; Cincin, A. Acute sleep deprivation in healthy adults is associated with increased arterial stiffness. Eur. Heart J. 2013, 34 (Suppl. S1), P4993. [Google Scholar] [CrossRef]
- AlDabal, L.; BaHammam, A.S. Metabolic, endocrine, and immune consequences of sleep deprivation. Open Respir. Med. J. 2011, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Açar, G.; Akçakoyun, M.; Sari, I.; Bulut, M.; Alizade, E.; Özkan, B.; Yazicioğlu, M.V.; Alici, G.; Avci, A.; Kargin, R.; et al. Acute sleep deprivation in healthy adults is associated with a reduction in left atrial early diastolic strain rate. Sleep Breath. 2013, 17, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Gadi, S.R.; Shah, N.R.; Stout, C.; Blackwell, J.N.; Cho, Y.; Koene, R.J.; Mehta, N.; Mazimba, S.; Darby, A.E.; et al. Atrial fibrillation and objective sleep quality by slow wave sleep. J. Atr. Fibrillation 2018, 11, 2031. [Google Scholar] [CrossRef] [PubMed]
- Ayas, N.T.; White, D.P.; Manson, J.A.E.; Stampfer, M.J.; Speizer, F.E.; Malhotra, A.; Hu, F.B. A prospective study of sleep duration and coronary heart disease in women. Arch. Intern. Med. 2023, 163, 205–209. [Google Scholar] [CrossRef]
- Linz, D.; Linz, B.; Hohl, M.; Böhm, M. Atrial arrhythmogenesis in obstructive sleep apnea: Therapeutic implications. Sleep Med. Rev. 2016, 26, 87–94. [Google Scholar] [CrossRef]
- Linz, D.; Schotten, U.; Neuberger, H.R.; Böhm, M.; Wirth, K. Negative tracheal pressure during obstructive respiratory events promotes atrial fibrillation by vagal activation. Heart Rhythm 2011, 8, 1436–1443. [Google Scholar] [CrossRef]
- Iwasaki, Y.K.; Kato, T.; Xiong, F.; Shi, Y.F.; Naud, P.; Maguy, A.; Mizuno, K.; Tardif, J.C.; Comtois, P.; Nattel, S. Atrial fibrillation promotion with long-term repetitive obstructive sleep apnea in a rat model. J. Am. Coll. Cardiol. 2014, 64, 2013–2023. [Google Scholar] [CrossRef]
- Dimitri, H.; Ng, M.; Brooks, A.G.; Kuklik, P.; Stiles, M.K.; Lau, D.H.; Antic, N.; Thornton, A.; Saint, D.A.; McEvoy, D.; et al. Atrial remodeling in obstructive sleep apnea: Implications for atrial fibrillation. Heart Rhythm 2012, 9, 321–327. [Google Scholar] [CrossRef]
- Nalliah, C.J.; Wong, G.R.; Lee, G.; Voskoboinik, A.; Kee, K.; Goldin, J.; Watts, T.; Linz, D.; Wirth, D.; Parameswaran, R.; et al. Sleep apnoea has a dose-dependent effect on atrial remodelling in paroxysmal but not persistent atrial fibrillation: A high-density mapping study. EP Eur. 2021, 23, 691–700. [Google Scholar] [CrossRef]
- Oliveira, M.; da Silva, M.N.; Geraldes, V.; Xavier, R.; Laranjo, S.; Silva, V.; Postolache, G.; Ferreira, R.; Rocha, I. Acute vagal modulation of electrophysiology of the atrial and pulmonary veins increases vulnerability to atrial fibrillation. Exp. Physiol. 2011, 96, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Jansen, H.J.; Bohne, L.J.; Gillis, A.M.; Rose, R.A. Atrial remodeling and atrial fibrillation in acquired forms of cardiovascular disease. Heart Rhythm 2020, 1, 147–159. [Google Scholar] [CrossRef]
- Nattel, S.; Heijman, J.; Zhou, L.; Dobrev, D. Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy: A Translational Perspective. Circ. Res. 2020, 127, 51–72. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Brooks, A.G.; Elliott, A.D.; Nalliah, C.J.; Hendriks JM, L.; Middeldorp, M.E.; Gallagher, C.; Mahajan, R.; Kalman, J.M.; McEvoy, R.D.; et al. Variability of Sleep Apnea Severity and Risk of Atrial Fibrillation: The VARIOSA-AF Study. J. Am. Coll. Cardiol. Clin. Electrophysiol. 2019, 5, 692–701. [Google Scholar] [CrossRef]
- Anter, E.; Di Biase, L.; Contreras-Valdes, F.M.; Gianni, C.; Mohanty, S.; Tschabrunn, C.M.; Viles-Gonzalez, J.F.; Leshem, E.; Buxton, A.E.; Kulbak, G.; et al. Atrial Substrate and Triggers of Paroxysmal Atrial Fibrillation in Patients with Obstructive Sleep Apnea. Circulation 2017, 10, e005407. [Google Scholar] [CrossRef]
- Lebek, S.; Pichler, K.; Reuthner, K.; Trum, M.; Tafelmeier, M.; Mustroph, J.; Camboni, D.; Rupprecht, L.; Schmid, C.; Maier, L.S.; et al. Enhanced CaMKII-Dependent Late INaInduces Atrial Proarrhythmic Activity in Patients with Sleep-Disordered Breathing. Circ. Res. 2020, 126, 603–615. [Google Scholar] [CrossRef]
- Christensen, M.A.; Dixit, S.; Dewland, T.A.; Whitman, I.R.; Nah, G.; Vittinghoff, E.; Mukamal, K.J.; Redline, S.; Robbins, J.A.; Newman, A.B.; et al. Sleep characteristics that predict atrial fibrillation. Heart Rhythm 2018, 15, 1289–1295. [Google Scholar] [CrossRef]
- James, S.M.; Honn, K.A.; Gaddameedhi, S.; Van Dongen, H.P.A. Shift Work: Disrupted Circadian Rhythms and Sleep—Implications for Health and Well-being. Curr. Sleep Med. Rep. 2017, 3, 104–112. [Google Scholar] [CrossRef]
- Jeyaraj, D.; Haldar, S.M.; Wan, X.; McCauley, M.D.; Ripperger, J.A.; Hu, K.; Lu, Y.; Eapen, B.L.; Sharma, N.; Ficker, E.; et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012, 483, 96–101. [Google Scholar] [CrossRef]
- Wong, C.X.; Modrow, M.F.; Sigona, K.; Tang, J.J.; Vittinghoff, E.; Hills, M.T.; McCall, D.; Sciarappa, K.; Pletcher, M.J.; Olgin, J.E.; et al. Preceding night sleep quality and atrial fibrillation episodes in the I-STOP-AFIB randomized trial. Clin. Electrophysiol. 2024, 10, 56–64. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S. Mechanisms of cardiovascular disease in obstructive sleep apnoea. J. Thorac. Dis. 2018, 10 (Suppl. S34), S4201–S4211. [Google Scholar] [CrossRef] [PubMed]
- Orrù, G.; Storari, M.; Scano, A.; Piras, V.; Taibi, R.; Viscuso, D. Obstructive Sleep Apnea, oxidative stress, inflammation and endothelial dysfunction- An overview of predictive laboratory biomarkers. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6939–6948. [Google Scholar] [CrossRef] [PubMed]
- Fung, M.M.; Peters, K.; Redline, S.; Ziegler, M.G.; Ancoli-Israel, S.; Barrett-Connor, E.; Stone, K.L. Osteoporotic Fractures in Men Research Group. Decreased slow wave sleep increases risk of developing hypertension in elderly men. Hypertension 2011, 58, 596–603. [Google Scholar] [CrossRef]
- Medic, G.; Wille, M.; Hemels, M.E.H. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef]
- Ghilotti, F.; Bellocco, R.; Trolle Lagerros, Y.; Thorson, A.; Theorell-Haglöw, J.; Åkerstedt, T.; Lindberg, E. Relationship between sleep characteristics and markers of inflammation in Swedish women from the general population. J. Sleep Res. 2021, 30, e13093. [Google Scholar] [CrossRef] [PubMed]
- Linz, D.; Mcevoy, R.D.; Cowie, M.R.; Somers, V.K.; Nattel, S.; Lévy, P.; Kalman, J.M.; Sanders, P. Associations of Obstructive Sleep Apnea With Atrial Fibrillation and Continuous Positive Airway Pressure Treatment: A Review. JAMA Cardiol. 2018, 3, 532–540. [Google Scholar] [CrossRef]
- Bradley, T.D.; Floras, J.S. Obstructive sleep apnoea and its cardiovascular consequences. Lancet 2009, 373, 82–93. [Google Scholar] [CrossRef]
- Hohl, M.; Linz, B.; Bohm, M.; Linz, D. Obstructive sleep apnea and atrial arrhythmogenesis. Curr. Cardiol. Rev. 2014, 10, 362–368. [Google Scholar] [CrossRef]
- Jean-Louis, G.; Brown, C.D.; Zizi, F.; Ogedegbe, G.; Boutin-Foster, C.; Gorga, J.; McFarlane, S.I. Cardiovascular disease risk reduction with sleep apnea treatment. Expert Rev. Cardiovasc. Ther. 2010, 8, 995–1005. [Google Scholar] [CrossRef]
- Congrete, S.; Bintvihok, M.; Thongprayoon, C.; Bathini, T.; Boonpheng, B.; Sharma, K.; Chokesuwattanaskul, R.; Srivali, N.; Tanawuttiwat, T.; Cheungpasitporn, W. Effect of obstructive sleep apnea and its treatment of atrial fibrillation recurrence after radiofrequency catheter ablation: A meta-analysis. J. Evid. Based Med. 2018, 11, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Avlonitou, E.; Kapsimalis, F.; Varouchakis, G.; Vardavas, C.I.; Behrakis, P. Adherence to CPAP therapy improves quality of life and reduces symptoms among obstructive sleep apnea syndrome patients. Sleep Breath. 2012, 16, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Shukla, G. Obstructive sleep apnea and stroke. J. Clin. Sleep Med. 2018, 14, 1819. [Google Scholar] [CrossRef] [PubMed]
- Tiyapun, N.; Sunkonkit, K.; Chaiwong, W.; Worasuthaneewan, R.; Theerakittikul, T. Factors influencing continuous positive airway pressure adherence in elderly with obstructive sleep apnea. J. Thorac. Dis. 2023, 15, 3488–3500. [Google Scholar] [CrossRef]
- Pattipati, M.; Gudavalli, G.; Zin, M.; Dhulipalla, L.; Kolack, E.; Karki, M.; Devarakonda, P.K.; Yoe, L. Continuous Positive Airway Pressure vs Mandibular Advancement Devices in the Treatment of Obstructive Sleep Apnea: Na Updated Systematic Review and Meta-Analysis. Cureus 2022, 14, e21759. [Google Scholar] [CrossRef]
- Belanche Monterde, A.; Zubizarreta-Macho, Á.; Lobo Galindo, A.B.; Albaladejo Martínez, A.; Montiel-Company, J.M. Mandibular advancement devices decrease systolic pressure during the day and night in patients with obstructive sleep apnea: A systematic review and meta-analysis. Sleep Breath. 2024, 28, 1037–1049. [Google Scholar] [CrossRef]
- Srijithesh, P.R.; Aghoram, R.; Goel, A.; Dhanya, J. Positional therapy for obstructive sleep apnoea. Cochrane Database Syst. Rev. 2019, 5, CD010990. [Google Scholar] [CrossRef]
- Wozniak, D.R.; Lasserson, T.J.; Smith, I. Educational, supportive and behavioural interventions to improve usage of continuous positive airway pressure machines in adults with obstructive sleep apnoea. Cochrane Database Syst. Rev. 2014, 8, CD007736. [Google Scholar] [CrossRef]
- Stafford, P.L.; Harmon, E.; Patel, P.; Walker, M.; Akoum, N.; Park, S.J.; Cho, Y.; Bilchick, K.; Mehta, N.; Mazimba, S.; et al. Positional obstructive sleep apnea in patients with atrial fibrillation. Sleep Breath. 2023, 27, 487–494. [Google Scholar] [CrossRef]
- Miller, J.D.; Aronis, K.N.; Chrispin, J.; Patil, K.D.; Marine, J.E.; Martin, S.S.; Blaha, M.J.; Blumenthal, R.S.; Calkins, H. Obesity, exercise, obstructive sleep apnea, and modifiable atherosclerotic cardiovascular disease risk factors in atrial fibrillation. J. Am. Coll. Cardiol. 2015, 66, 2899–2906. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Bansal, A.; Yanamaladoddi, V.R.; Sarvepalli, S.S.; Vemula, S.L.; Aramadaka, S.; Mannam, R. Atrial Fibrillation in Obstructive Sleep Apnea Patients: Mechanisms, Risk Factors, and Management Strategies. Cureus 2023, 15, e36282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Boyd, S.B.; Walters, A.S.; Song, Y.; Wang, L. Comparative effectiveness of maxillomandibular advancement and uvulopalatopharyngoplasty for the treatment of moderate to severe obstructive sleep apnea. J. Oral Maxillofac. Surg. 2013, 71, 743–751. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nastałek, P.; Polok, K.; Celejewska-Wójcik, N.; Kania, A.; Sładek, K.; Małczak, P.; Major, P. Impact of bariatric surgery on obstructive sleep apnea severity and continuous positive airway pressure therapy compliance-prospective observational study. Sci. Rep. 2021, 11, 5003. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peromaa-Haavisto, P.; HTuomilehto, J.K.ö.s.s.i.; Virtanen, J.; Luostarinen, M.; Pihlajamäki, J.; Käkelä, P.; Victorzon, M. Obstructive sleep apnea: The effect of bariatric surgery after 12 months. A prospective multicenter trial. Sleep Med. 2017, 35, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Shneerson, J.; Wright, J. Lifestyle modification for obstructive sleep apnoea. Cochrane Database Syst. Rev. 2001, 2001, CD002875. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Van Gelder, I.C.; Rienstra, M.; Bunting, K.V.; Casado-Arroyo, R.; Caso, V.; Crijns, H.J.; De Potter, T.J.; Dwight, J.; Guasti, L.; Hanke, T.; et al. 2024 ESC Guidelines for the management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) Developed by the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC), with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Endorsed by the European Stroke Organisation (ESO). Eur. Heart J. 2024; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Monahan, K.; Brewster, J.; Wang, L.; Parvez, B.; Goyal, S.; Roden, D.M.; Darbar, D. Relation of the severity of obstructive sleep apnea in response to anti-arrhythmic drugs in patients with atrial fibrillation or atrial flutter. Am. J. Cardiol. 2012, 110, 369–372. [Google Scholar] [CrossRef]
- Goyal, S.K.; Wang, L.; Upender, R.; Darbar, D.; Monahan, K. Severity of obstructive sleep apnea influences the effect of genotype on response to anti-arrhythmic drug therapy for atrial fibrillation. J. Clin. Sleep Med. 2014, 10, 503–507. [Google Scholar] [CrossRef]
- Chen, A.; Ju, C.; Mackenzie, I.S.; MacDonald, T.M.; Struthers, A.D.; Wei, L.; Man, K.K.C. Impact of beta-blockers on mortality and cardiovascular disease outcomes in patients with obstructive sleep apnoea: A population-based cohort study in target trial emulation framework. Lancet Reg. Health Eur. 2023, 33, 100715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, M.; Oliveira, M.; Laranjo, S.; Rocha, I. Linking Sleep Disorders to Atrial Fibrillation: Pathways, Risks, and Treatment Implications. Biology 2024, 13, 761. https://doi.org/10.3390/biology13100761
Ferreira M, Oliveira M, Laranjo S, Rocha I. Linking Sleep Disorders to Atrial Fibrillation: Pathways, Risks, and Treatment Implications. Biology. 2024; 13(10):761. https://doi.org/10.3390/biology13100761
Chicago/Turabian StyleFerreira, Monica, Mario Oliveira, Sergio Laranjo, and Isabel Rocha. 2024. "Linking Sleep Disorders to Atrial Fibrillation: Pathways, Risks, and Treatment Implications" Biology 13, no. 10: 761. https://doi.org/10.3390/biology13100761
APA StyleFerreira, M., Oliveira, M., Laranjo, S., & Rocha, I. (2024). Linking Sleep Disorders to Atrial Fibrillation: Pathways, Risks, and Treatment Implications. Biology, 13(10), 761. https://doi.org/10.3390/biology13100761





